Advanced pharmaceutical bulletin. 10(1):56-64.
doi: 10.15171/apb.2020.007
Research Article
Kinetics Analysis and Susceptibility Coefficient of the Pathogenic Bacteria by Titanium Dioxide and Zinc Oxide Nanoparticles
Mahmood Alizadeh-Sani 1, 2  , Hamed Hamishehkar 3
, Hamed Hamishehkar 3  , Arezou Khezerlou 2
, Arezou Khezerlou 2  , Mohammad Maleki 4, Maryam Azizi-Lalabadi 2, Vahid Bagheri 5
, Mohammad Maleki 4, Maryam Azizi-Lalabadi 2, Vahid Bagheri 5  , Payam Safaei 1, Taher Azimi 6, 7
, Payam Safaei 1, Taher Azimi 6, 7  , Mohammad Hashemi 8, Ali Ehsani 9, *
, Mohammad Hashemi 8, Ali Ehsani 9, * 
Author information:
1Student’s Scientific Research Center, Food Safety and Hygiene Division, Environmental Health Department, School of Public Health, Tehran University Of Medical Sciences, Tehran, Iran.
2Students Research Committee, Department of Food Sciences and Technology, Faculty of Nutrition and Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
3Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
4Department of Food Hygiene and Aquaculture, Ferdowsi university of Mashhad, Mashhad, Iran.
5Department of Food Science and Technology, Faculty of Agriculture, University of Tabriz, Tabriz, Iran.
6Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
7Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
8Department of Nutrition, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
9Nutrition Research Center, Department of Food Sciences and Technology, Faculty of Nutrition and Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
*
Corresponding Author: Ali Ehsani, Tel: +984133357581-3, Fax: +984133340634, Email:
ehsani@tbzmed.ac.ir
Abstract
Purpose:
The increase of bacterial resistance to common antibacterial agents is one of the major problems of health care systems and hospital infection control programs. In this study, antimicrobial activity of titanium dioxide (TiO2 ) and zinc oxide (ZnO) nanoparticles (NPs) was investigated against E. coli, Salmonella enteritidis, Listeria monocytogenes, and Staphylococcus aureus pathogenic bacteria by determining sensitivity coefficient and kinetics of bacterial death.
Methods:
Antimicrobial tests were performed with ~106 CFU/mL of each bacterium at baseline. At first, minimum inhibitory concentration (MIC) was concluded by the dilution method and then, death kinetic and susceptibility coefficient of NPs suspensions was determined at 0 to 360 min. treatment time.
Results:
The results of this study revealed that, the highest susceptibility was observed for L. monocytogenes (Z=0.025 mL/μg) to TiO2 NPs, whereas the lowest susceptibility was obtained in the reaction of ZnO NPs with S. enteritidis (Z=0.0033 mL/μg). The process of bacterial death in NPs suspension was assumed to follow first-degree kinetic and the survival ratio of bacteria decreased by the increase in treatment time. An increase in the concentration of NPs was seen to enhance the bactericidal action.
Conclusion:
Results showed that L. monocytogenes had higher sensitivity compared to S. enteritidis. The results of this study also demonstrated that TiO2 NPs have a strong antimicrobial effect in comparison with ZnO NPs and it could be employed to aid the control of pathogenic bacteria.
Keywords: Pathogenic bacteria, ZnO, TiO2, Kinetics, Susceptibility coefficient
Copyright and License Information
© 2020 The Author (s)
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Nowadays, new approaches are required to control harmful microorganisms. A broad spectrum of microorganisms is balanced with the human environment and food products; however, the uncontrolled growth of microorganisms can cause serious problems.
1-3
Food poisoning, foodborne and hospital infections are some of the oldest health care problems with challenging control programs.
4-6
Recent studies have shown that some metals/oxides such as CaO, MgO, as well as many nanoparticles (NPs) such as Ag, zinc oxide (ZnO), CuO, and titanium dioxide (TiO2) are known to have marked antibacterial activities.
7-9
Nanoparticles have received considerable attention as antimicrobial agents, which can substitute common antimicrobial agents, such as antibiotics and chlorine disinfectant in order to control spoilage and the spread of pathogenic bacteria in food and in the environment such as hospitals.
10,11
In recent years, nanotechnology has been increasingly applied in different fields, especially in medical, food and pharmaceutical domains.
12-14
Among various NPs, TiO2 and ZnO are widely used due to their strong antimicrobial effects. These materials, when synthesized on a nanoscale, exhibit strong antimicrobial effects because of an increase in surface-to-volume ratio and their specific surface area.
15,16
It has been suggested that these nanomaterials react with proteins especially with -SH groups; consequently, which leads to protein inactivation.
11,15
Several studies have reported the antimicrobial effects of various NPs such as silver and copper against E. coli and Bacillus subtilis,
17
silver NPs against multidrug-resistant bacteria
18
and TiO2 NPs against pathogenic bacteria.
8,19
According to the results obtained by various tests such as proteomics research, transmission electron microscopy (TEM) and scanning electron microscopy, it is proposed that the mechanism of antimicrobial activity varies from one nanoparticle to another, because NPs react with important elements of bacterial membrane and cell wall, which cause structural change and damage, destruction of the proton motive force, and finally cell death.
11,20,21
Nano-antimicrobial agents can be used in coating surfaces in order to produce antimicrobial characteristics in food and in medical devices and water treatment filters.
22
The effectiveness of nanomaterials’ antimicrobial activity is determined by experimental techniques that measure microorganism viability after exposure. In industrial functions of antimicrobial agents, numerical and mathematical models and quantitative parameters are essential for design optimization, performance assessment and life-time prediction of antimicrobial techniques. Therefore, for the application of nanomaterials in commercial and industrial scales, the numerical models and quantitative parameters are necessary to determine the efficiency, design optimization, and survival rate prediction of antimicrobial agents.
17,23
As two of quantitative parameters, susceptibility coefficient and death kinetic have been applied in numerical models to evaluate the antimicrobial effects of nanomaterials against foodborne microorganisms.
24,25
The main objective of this study was to evaluate the antimicrobial activity of TiO2 and ZnO NPs against E. coli, S. enteritidis, L. monocytogenes, and S. aureus pathogenic bacteria, and also to determine the susceptibility coefficient and death kinetic using predictive modeling of microbial growth.
Materials and Methods
This study was conducted as an empirical research in the laboratory. In order to investigate the antibacterial activity, commercial ZnO and TiO2 with the purity of 99% were purchased from US Research Nanomaterials, Inc., Houston, U.S.A. Pathogenic bacterial strains E. coli (ATCC-25922), Salmonella enteritidis (ATCC-49221), Listeria monocytogenes (ATCC-13932) and Staphylococcus aureus (ATCC-33591) were obtained from Iranian Biological and Genetic Resources Center, Tehran, Iran. All the applied reagents were of analytical grade.
Preparation of nanoparticles suspension
The TiO2 and ZnO NPs suspensions were prepared in various concentrations (0.25, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 7, 8, 9 and 10 mg/mL) of each nanoparticle in sterile distilled water. Then, an ultrasonic probe sonicator (UCD-1200, Bio-Base, Shandong, China) was applied for 15 min. with 150W, and Ultra-Turrax (IKA, Germany) homogenizer was used for 10 min. with 10 000 rpm in order to well-disperse NPs.
8
Bacterial culture and preparation of bacterial suspension
Standard bacterial strains included gram-negative bacteria (E. coli and S. enteritidis) and gram-positive bacteria (L. monocytogenes and S. aureus), were exploited as tested bacteria and kept under freeze-dried conditions. Then, standard strains of the bacteria were cultured at 37°C for 24 h on a Trypticase Soy agar (TSA) medium (Merck, Germany). After incubation, the bacterial single-colonies were collected from medium by sterile loop, and used to prepare bacterial suspensions.
To prepare a bacterial suspension, at first, bacterial single-colonies were added to 1 mL phosphate buffer saline (PBS), its turbidity was then adjusted to 0.5 McFarland standard with ~1.5 × 108 CFU/mL and diluted to the desired bacterial density (~1.5 × 106 CFU/mL). The turbidity approved by measuring the absorbance of bacterial suspension using an ultraviolet (UV) spectrophotometer (UNICO-2100, Northbrook, Illinois, USA), in ranging of 0.08 to 0.1 at 625 nm.
8,26
Kinetics of bacterial death
At first, to kinetic of bacterial death, minimum inhibitory concentration (MIC) for each of the bacteria in contact with NPs suspension was determined as previously described with some modification.
27-29
MIC is generally defined as the lowest concentration of an antimicrobial agent that inhibits the visible growth of a microorganism after overnight incubation. Then, the suspensions of the desired microorganisms (20 μL) with different concentrations of ZnO/TiO2 NPs (0.25, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 mg/mL) (20 μL) were added to microplates containing 160 μL broth neutralizing medium (Trypticase Soy broth). The microplates containing NPs and bacteria were incubated for approximately 24 h in a shaking incubator (100 rpm, temperature of 37°C).
Two concentrations of bacterial suspension (1 and 2 × MIC) were added to the medium containing NPs and were then incubated at 37°C for 24 h. At the desired time (from 0 to 360 min), a bacterial/nanoparticle suspension was sampled and spread on TSA plates. The number of colonies were counted and recorded for each time, bacteria and concentration.
Survival rates (N/N0) were calculated by dividing the number of colonies at the time of the sampling (N) by the number of colonies at the time when they had no contact with ZnO/TiO2 NPs suspensions (N0). To study the kinetic of bacterial death, the kinetic of first-degree death was used. The general form of this kinetic is as follows:
7,30-32
Where k is the death rate constant, N0, the number of initial bacterial colonies, and N is the number of bacterial colonies at the time t. In this study, the sensitivity of NPs (Z) based on mL/μg, is obtained by the following equation:
17,32
In this equation N is the bacterial colony-forming units (CFUs) on the agar plate containing NPs, N0 is the CFUs on the agar plate, and C is the concentration of NPs (mg/mL). By the use of Z and C values, the survival fraction (N/N0) can be predicted. A higher Z value means that the bacteria are more sensitive to NPs, which also indicates that NPs have more antimicrobial susceptibility (stronger antimicrobial properties).
Statistical analysis
Statistical analysis was carried out using SPSS software (version 16.0, IBM; Armonk, USA), with Descriptive statistics and Independent-Samples t test analysis. Differences were determined to be significant at P ˂ 0.05. All tests of the present study were performed in triplicate.
Results and Discussion
The crystalline state and the presence of impurities in NPs were identified by X-ray diffraction (XRD) and TEM (Figures 1 and 2). TEM results showed that NPs have a hexagonal and cubic (ZnO) andtetragonal (TiO2) shape and the diameter of particles was about ~20 nm and 10~25 nm for ZnO and TiO2 NPs, respectively. The specific surface area, by measuring the adsorption isotherms of N2 at −196°C (BET; Belsorp-mini), was approximate ~90 m2/g and ~185 m2/g for ZnO and TiO2 NPs, respectively.
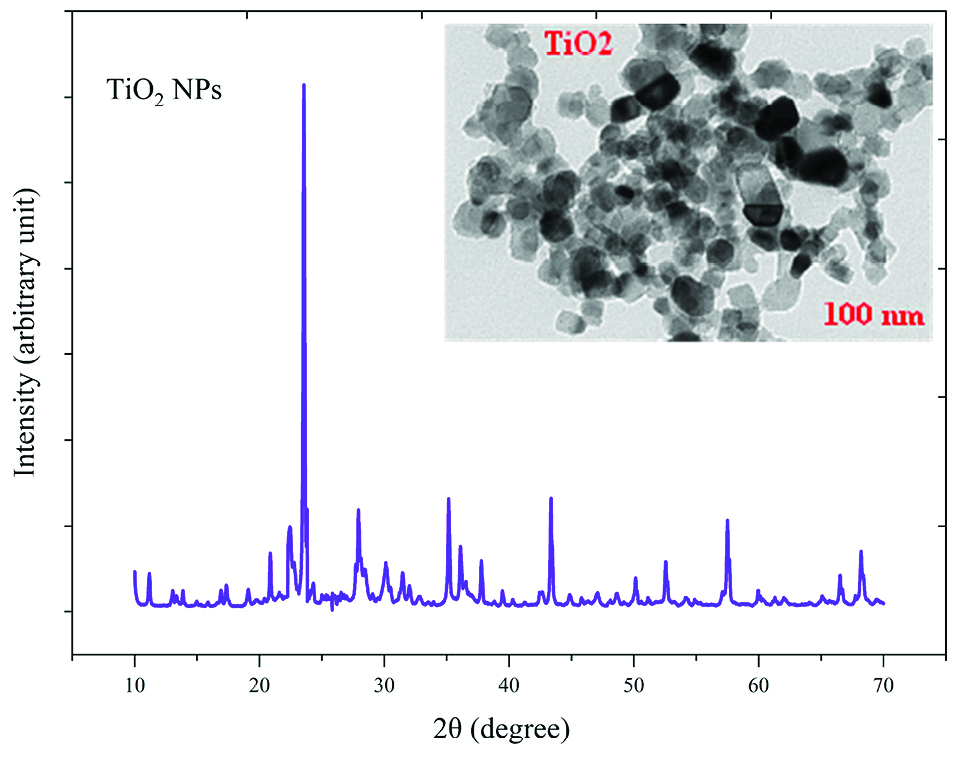
Figure 1.
X-Ray diffraction (X-RD) spectra and Transmission Electron Microscopy (TEM) image of TiO2 nanoparticles.
.
X-Ray diffraction (X-RD) spectra and Transmission Electron Microscopy (TEM) image of TiO2 nanoparticles.
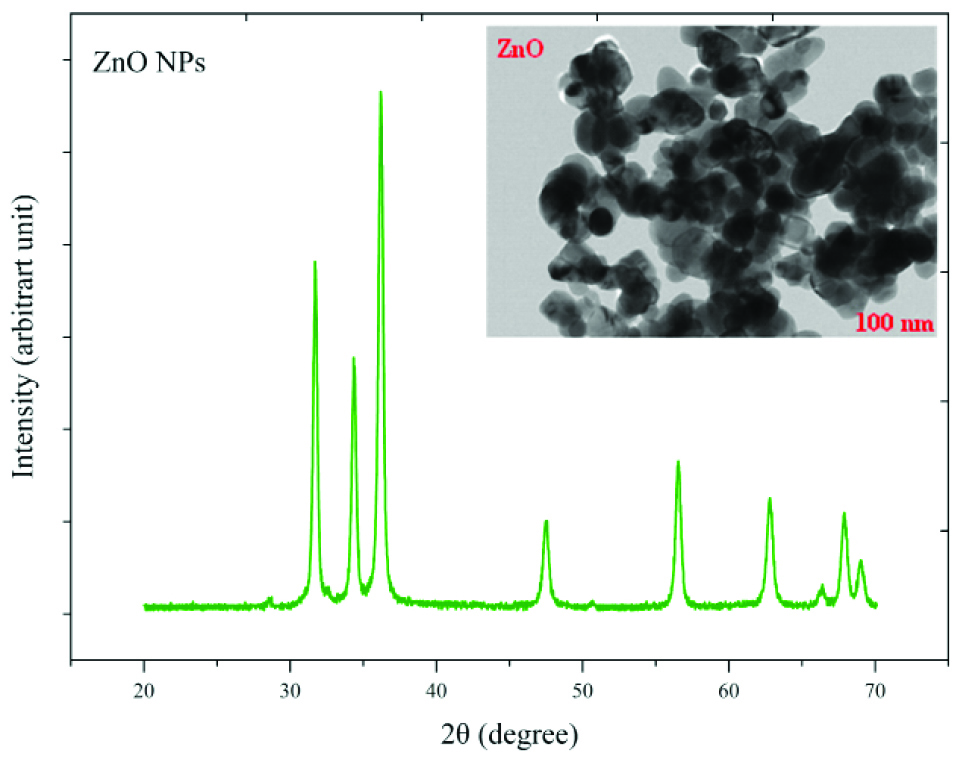
Figure 2.
X-Ray diffraction (X-RD) spectra and Transmission Electron Microscopy (TEM) image of ZnO nanoparticles.
.
X-Ray diffraction (X-RD) spectra and Transmission Electron Microscopy (TEM) image of ZnO nanoparticles.
According to the obtained results in Table 1, that shows the antimicrobial activity of ZnO and TiO2 NPs suspensions against the tested bacteria, MIC values for gram-negative and gram-positive bacteria to ZnO NPs were found to be ~2.5-3 mg/mL and ~1.5-2 mg/mL, respectively. Also, MIC values for gram-negative bacteria to TiO2 NPs were found to be ~2-2.5 mg/mL and for Gram-positive bacteria was ~1-1.5 mg/mL.
Table 1.
MIC of TiO2 and ZnO NPs against pathogenic bacteria
|
Bacteria strains
|
MIC (mg/mL)
|
|
ZnOTiO
2
|
|
Mean±SD
|
Mean±SD
|
|
E. coli
|
2.50±0.18 |
2.00±0.33 |
|
S. enteritidis
|
3.00±0.21 |
2.50±0.17 |
|
S. aureus
|
2.00±0.17 |
1.50±0.19 |
|
L. monocytogenes
|
1.50±0.15 |
1.00±0.14 |
MIC: minimum inhibition concentration; TiO2 NPs: titanium dioxide nanoparticles; ZnO NPs: zinc oxide nanoparticles; SD: standard deviation.
The sensitivity coefficient of each bacterium to NPs suspension at each sampling period was calculated. The result of the mean of the sensitivity coefficient is shown in Table 2. The sensitivity coefficient of gram-positive bacteria to both NPs has increased by increasing the concentration of NPs from 1 to 2 MIC values, but the average sensitivity coefficient for gram-negative bacteria, especially S. enteritidis, has decreased by increasing the concentrations of both NPs. The changes of the bacterial cells in contact with NPs suspension at different times and the kinetics of their death were calculated. The changes in the populations of bacteria and the sensitivity coefficient are shown in Figures 3-6, respectively. The kinetics of bacterial death showed that the survival ratio of each bacteria decreased by increasing concentrations of NPs. The population of bacteria decreased linearly over time. The sensitivity coefficient of both NPs has increased by an increase in contact time for S. aureus and L. monocytogenes. As shown in Table 2, the sensitivity coefficient values for TiO2 NPs are always higher than ZnO NPs. The kinetic results indicated that the population of bacteria declined when the concentration of NPs increased. At a concentration of one-time the MIC value, the re-growth of bacteria was observed. But with increasing concentrations of twice the MIC value, after reducing the population of the bacteria, there was no re-growth.
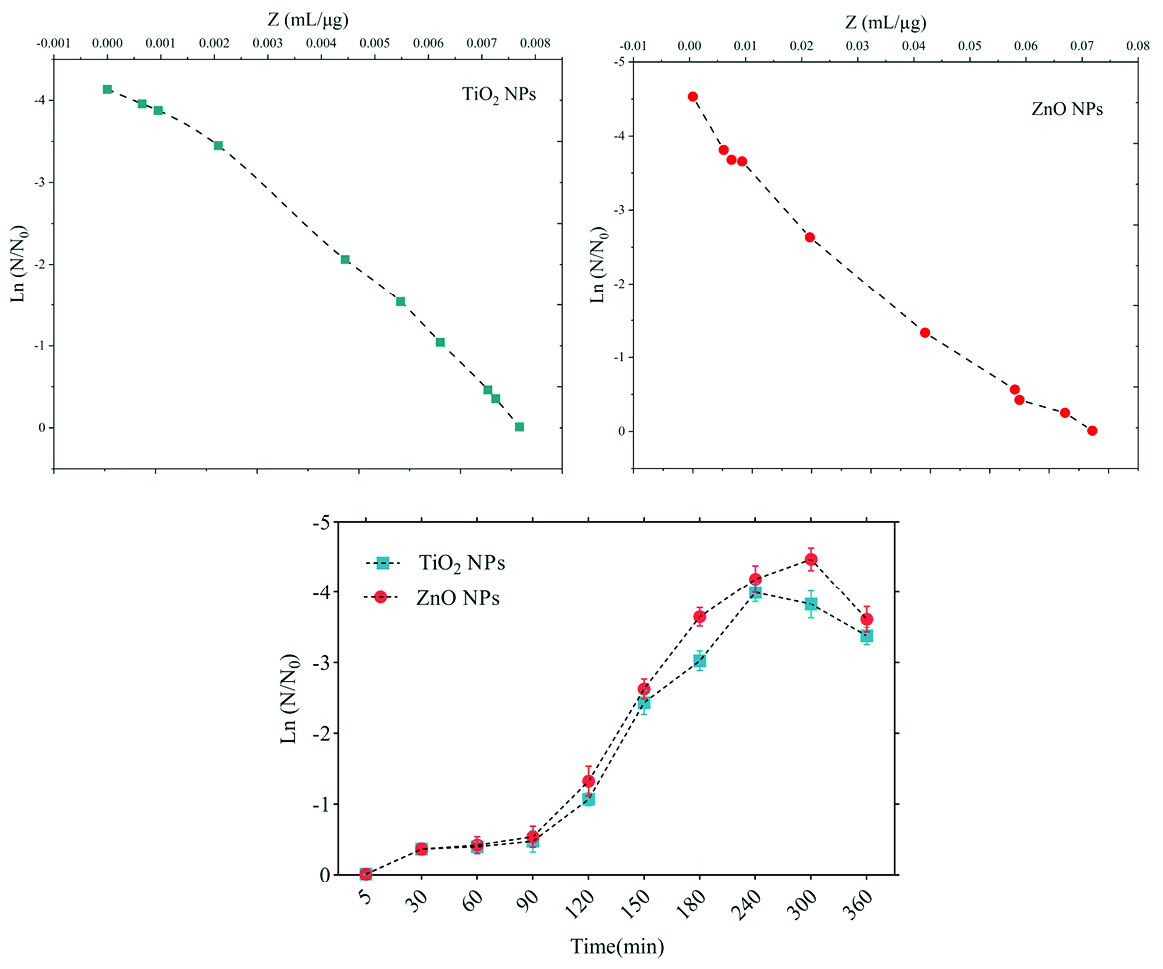
Figure 3.
Death kinetics (bacterial population changes) and sensitivity coefficient of Listeria monocytogenes bacterium to time.
.
Death kinetics (bacterial population changes) and sensitivity coefficient of Listeria monocytogenes bacterium to time.
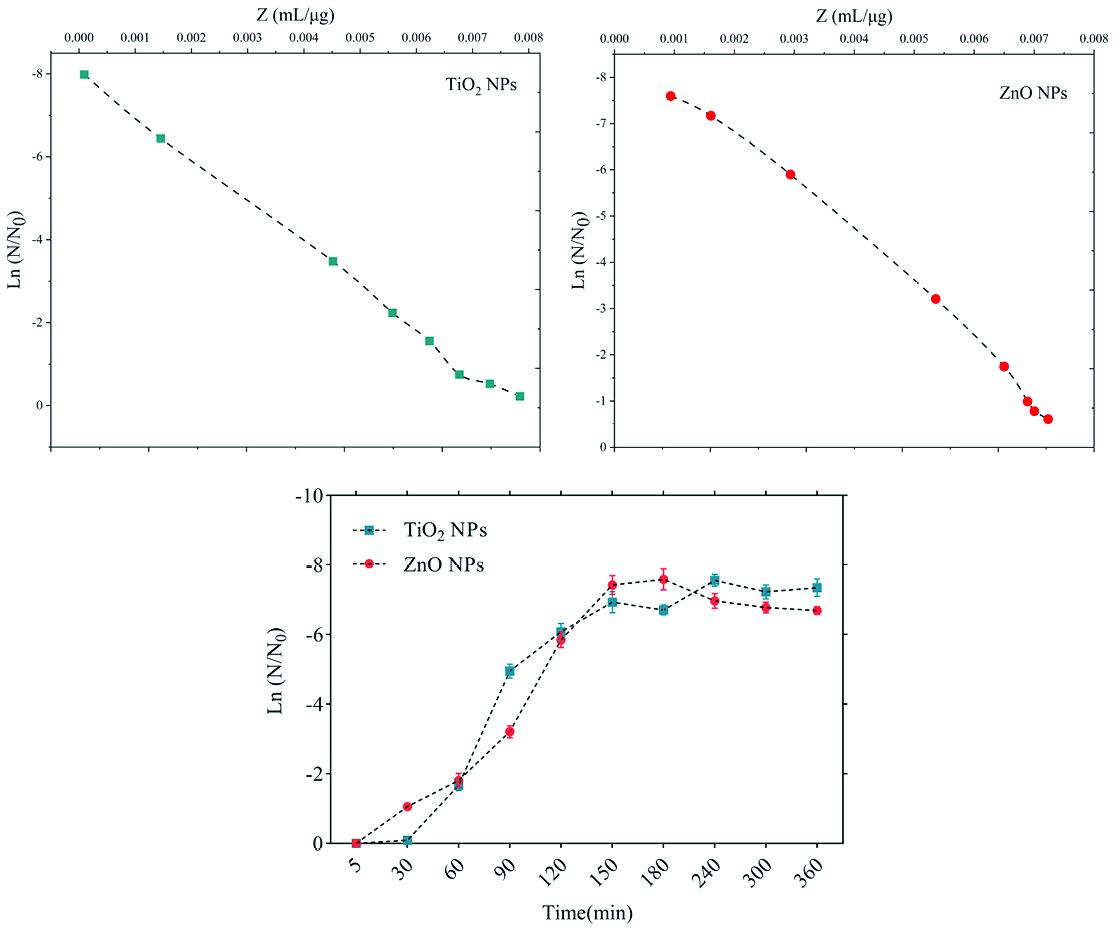
Figure 4.
Death kinetics (bacterial population changes) and sensitivity coefficient of Staphylococcus aureus bacterium to time.
.
Death kinetics (bacterial population changes) and sensitivity coefficient of Staphylococcus aureus bacterium to time.
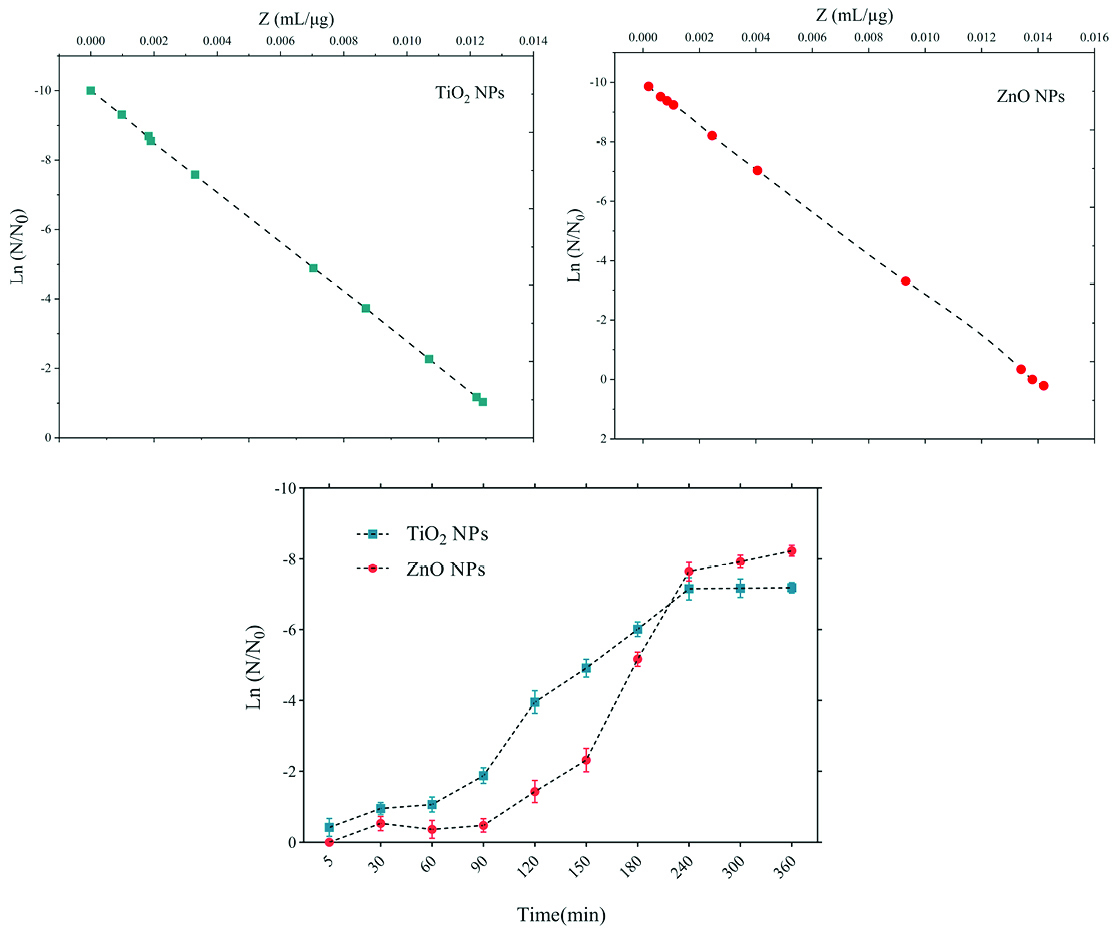
Figure 5.
Death kinetics (bacterial population changes) and sensitivity coefficient of E. coli bacterium to time.
.
Death kinetics (bacterial population changes) and sensitivity coefficient of E. coli bacterium to time.
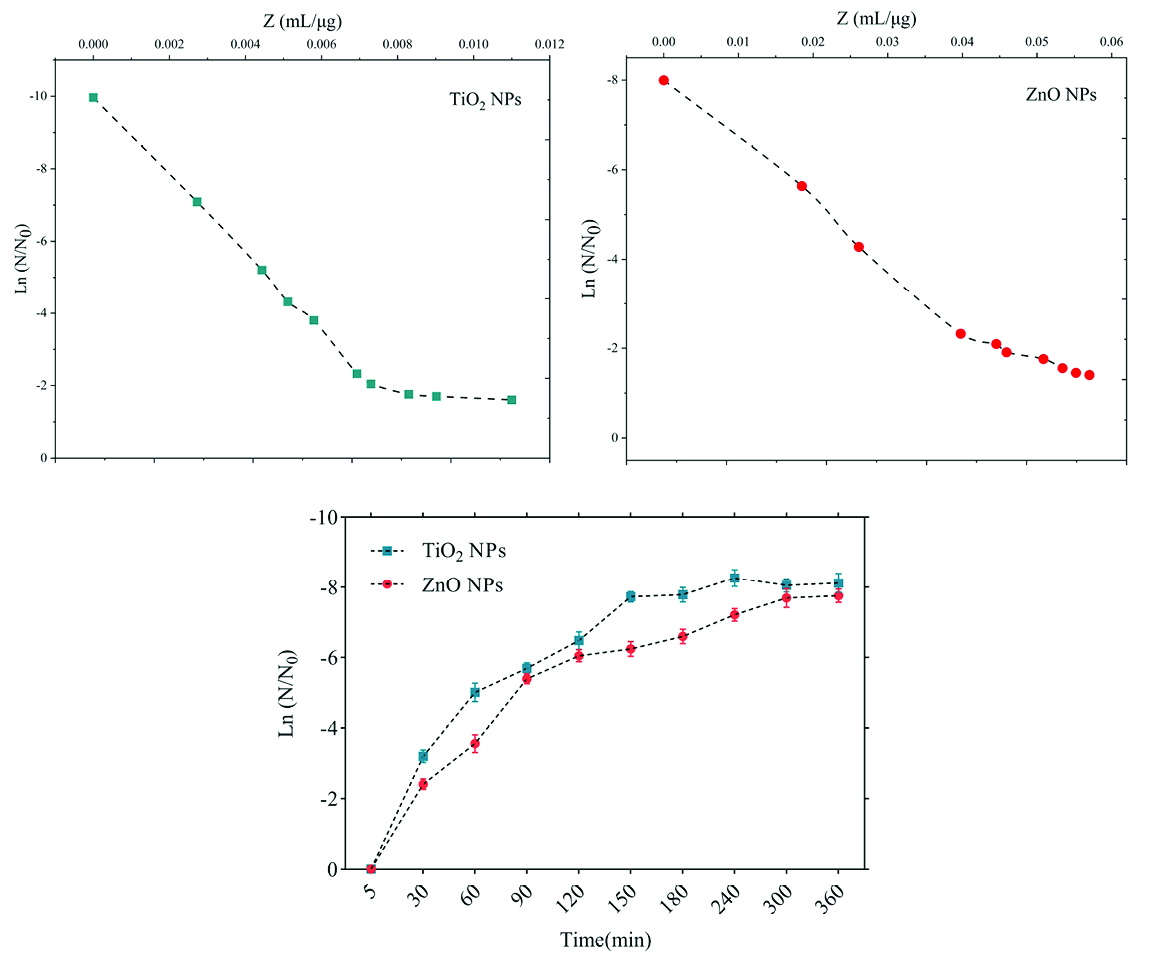
Figure 6.
Death kinetics (bacterial population changes) and sensitivity coefficient of Salmonella enteritidis bacterium to time.
.
Death kinetics (bacterial population changes) and sensitivity coefficient of Salmonella enteritidis bacterium to time.
Table 2.
The sensitivity coefficient for each of the pathogenic bacteria and nanoparticles studied
|
Bacteria strains
|
Z value (mL/µg)
|
P
value
|
|
TiO
2
ZnO
|
|
Mean±SD
|
Mean±SD
|
|
L. monocytogenes
|
0.025±0.0052 |
0.019±0.0042 |
0.033* |
|
S. aureus
|
0.016±0.0041 |
0.012±0.0033 |
0.044* |
|
E. coli
|
0.0057±0.0010 |
0.0048±0.0010 |
0.078 |
|
S. enteritidis
|
0.0045±0.0010 |
0.0033±0.0010 |
0.086 |
MIC: minimum inhibition concentration; TiO2 NPs: titanium dioxide nanoparticles; ZnO NPs: zinc oxide nanoparticles; SD: standard deviation.
* P value reported based on ANOVA test (significant at level of P value <0.05.)
The average sizes of ZnO and TiO2 NPs were ~20 nm and 10~25 nm, respectively. Reducing the particle size of NPs can change their structural and physicochemical properties, also their toxicity would increase due to their access to biological organisms.
7,9,11
In the XRD profile of ZnO and TiO2 NPs, a peak of oxygen was observed. TiO2 and ZnO NPs both contain high atom density, known to be highly reactive NPs.
33,34
The specific surface area of TiO2 NPs is approximately 2.05 times greater than the specific surface area of ZnO NPs. The decrease in size and the increase in specific surface area, as the factors in increasing the number of reactive groups on the particle surface, were considered as the most important factors for the increase in the toxicity of NPs and increased antimicrobial activity.
33
Probably, the increase in reactive groups which act as the active sites for the formation of reactive oxygen species (ROS) including superoxide, hydrogen peroxide, and radical hydroxyl cause oxidative stress.
9,11
The results showed that gram-negative bacteria were more resistant to NPs compared to gram-positive bacteria. One of the reasons for the lower sensitivity of Gram-negative bacteria can be the fact that external membranes of gram-negative bacteria, such as Salmonella and Escherichia coli, are predominantly composed of a strong lipopolysaccharide layer (LPS), which is considered to be a permanent barrier against NPs.
35
The results obtained by Yoon et al proved that B. subtilis was more sensitive than E. coli.
17
In the present study, gram-positive bacteria were more susceptible to TiO2 NPs rather than ZnO NPs (1 and 1.5 mg/mL inhibitory concentrations versus 2.5 and 3 mg/mL). In addition, the first-degree equation had been used to describe the kinetic of bacterial growth. As seen in Figures 3-6, when the logarithmic ratio of surviving bacteria and the time of exposure are plotted as vertical and horizontal axes, it can be seen that the population of the tested bacteria decreases linearly. The results obtained by Sawai et al are consistent with the results of this section of the present study.
30
In colony counting, when the bacteria survival ratio is negative over time, it can be concluded that NPs have strong antimicrobial activity.
30,31,36
As the kinetic charts of bacterial death (Figures 3-6) are deduced to a specific time (common to both concentrations of NPs), the survival ratio decreased rapidly with increasing nanoparticle concentrations. In addition, the antibacterial activity of NPs suspension increased with concentration increase. This result can be associated with higher toxicity at higher concentrations of NPs.
15
Of course, this cannot indicate the linear relationship between the concentration and antimicrobial potential of NPs, since at higher concentrations there is a potential for bacterial compatibility with NPs. As the contact time increases, with the increase in the concentration of NPs, the survival ratio of the bacteria reduces. In other words, the antibacterial activity of NPs has increased with increasing contact time and concentration of NPs against tested bacteria, which could indicate the broad spectrum of antimicrobial properties of these NPs.
15,37
Although, their antimicrobial activity against other microorganisms is required. By increasing the concentration of NPs by one-fold to twice the MIC value, the value of the Z parameter for Gram-negative bacteria reduced, which can be due to the compatibility resistance bacteria at higher concentrations.
36
The slope of log N/N0 ratio in Gram-negative bacteria was lower compared to Gram-positive bacteria by increasing the concentration of NPs, which could be due to the resistance of these bacteria to NPs investigated in this study.
32
Shahverdi et al concluded in their study that a gram-positive bacterium (S. aureus) had a higher sensitivity to chemicals than to antibiotics because of the differences in its cell wall composition compared to E. coli.
38
At each concentration of NPs, the sensitivity coefficient was determined using equation (2), and the Z value was calculated for a range of NPs concentration and contact time. The results of the over-time sensitivity analysis and the concentration of NPs showed that the magnitude of the sensitivity of both NPs increased by increasing contact time. Although the magnitude of the sensitivity of both NPs is very close, the value of this parameter has always been higher for TiO2 NPs than ZnO NPs. As can be seen in diagrams and Table 2, at a time interval of 300 and 360 minutes, the TiO2 NPs sensitivity coefficient was higher. The values of the sensitivity coefficient of gram-positive bacteria for ZnO NPs were lower than that of TiO2 NPs, which indicates that L. monocytogenes and S. aureus are more sensitive to TiO2 NPs.
Various studies have reported that Gram-negative bacteria are usually more resistant to antimicrobial compounds. This resistance can be attributed to a more complex cell wall of gram-negative bacteria than gram-positive bacteria.
35,39
Antimicrobial materials such as NPs have been taken into account because of their strong antimicrobial effects in low concentrations against microorganisms.
40,41
Usually, all of the metal NPs have the ability to reduce or remove the microorganisms by two main mechanisms: (a) free metal ion toxicity arising from dissolution of metals from the surface of NPs and (b) oxidative stress via generation of ROS, by using hydrogen peroxide (H2O2) and organic hydroperoxides, on the surface of NPs.
11,35,42
In fact, NPs can affect the survival of bacteria by agglomeration on the surface of bacteria and changing the structure of lipids, peptidoglycan, proteins, and their DNA.
11
But there may be a diversity in the effects of NPs on specific types of bacteria. For example, Alizadeh-Sani et al showed the antimicrobial effects of TiO2 NPs against S. aureus, L. monocytogenes, E. coli, P. fluorescence, and S. enteritidis.
8
In another study, Ruparelia et al demonstrated that copper NPs had a great impact as an antimicrobial agent against E. coli, B. subtilis, and S. aureus than TiO2 NPs.
43
Fu et al investigated the antibacterial activity of TiO2 nanocomposites against, Escherichia coli (DH 5α) and Bacillus megaterium (QM B1551). The results of their study showed that TiO2 NPs had good inhibitory effects, especially versus B. megaterium (as a gram-positive bacteria).
44
TiO2, as a metal oxide, is known as one of the most widely used semiconductor NPs with specific hydrophilic and photocatalytic properties, which leads to antimicrobial, and UV protecting characteristics.
8,45
These properties are obtained without the use of chemicals and only by using sunlight and water.
46
TiO2 NPs can produce active oxygen species when exposed to sunlight. Moreover, the antimicrobial activity of TiO2 NPs is related to its crystal structure, the kind of artificial light, UV light intensity, shape, and size, as well as the production of ROS active radical species, hydrogen peroxide, superoxide radical, and hydroxyl radical.
11,35,47
These active species destroy the outer membrane of the bacteria, namely phospholipids, proteins, and lipopolysaccharides, and finally damage the bacteria. In addition, several studies reported the antibacterial activities of ZnO NPs against foodborne pathogens including E. coli, L. monocytogenes, Salmonella, and S. aureus.
48
The antimicrobial effects of ZnO NPs are usually related to the photocatalytic activity of H2O2. Even both Zn+2 and ZnO particles have antibacterial activities. The antimicrobial activities of ZnO NPs at nanoscale would yield affordable and safe innovative strategies.
15,49
Regarding these phenomena and cell responses, the direct interaction between ZnO NPs and cell surfaces can be considered as a reasonable mechanism of ZnO NPs bacterial inactivation.
9,11,35,49
In comparison with large particles, NPs have more evident antimicrobial activities. The small size (<100 nm) and the high surface-to-volume ratio of NPs facilitate the prerequisite interaction with the microorganisms. As mentioned earlier, antimicrobial activity of NPs such as TiO2 and ZnO is usually attributed to its crystal structure, size, shape, and surface area.
9,11,35
Oxidative stress caused by ROS is particularly the mechanism proposed for NPs. As a result, ROS causes site-specific DNA damage and eventually results in cell death. Although the certain antimicrobial mechanism of NPs has not been well understood, it was revealed that the antimicrobial activity of these NPs has been associated with the following mechanisms: the release of antimicrobial ions, damaging the integrity of bacterial cell in result of interaction of NPs with microorganisms, and the formation of radicals through light irradiation.
9,11,35,48,50
On the other hand, despite significant potential NPs as antimicrobial compounds, the toxicity of NPs at high concentrations restricts their use in human’s application. Consequently, further studies should be carried out in the future.
Conclusion
In this study, the kinetic of bacterial death of NPs (TiO2 and ZnO) and the susceptibility coefficient were determined and used to evaluate their antimicrobial activity against L. monocytogenes, S. aureus, S. enteritidis, and E. coli pathogenic bacteria. Universally, our observations showed that Gram-positive bacterial strains are more sensitive in comparison with gram-negative bacteria against the tested NPs. The effect of NPs suspension on death kinetic of bacteria showed that the survival ratio of bacteria decreased almost linearly with increasing NPs concentration and contact time. The slope (negative) of the survival curve indicates a significant antimicrobial activity of NPs. The sensitivity coefficient and death kinetic can be used to evaluate the antimicrobial effects of various antimicrobial agents. For future studies, the focus should be on the relationship between the antimicrobial activity of these NPs in different sizes with death kinetics and the sensitivity coefficient.
Ethical Issues
Not applicable.
Conflict of Interest
The authors declare no conflict of interests.
Acknowledgments
This study was conducted in Tabriz University of Medical Sciences, Tabriz, Iran.
References
- Eriksen HM, Iversen BG, Aavitsland P. Prevalence of nosocomial infections in hospitals in Norway, 2002 and 2003. J Hosp Infect 2005; 60(1):40-5. doi: 10.1016/j.jhin.2004.09.038 [Crossref] [ Google Scholar]
- Hadi M, Shokoohi R, Ebrahimzadeh Namvar AM, Karimi M, Solaimany Aminabad M. Antibiotic resistance of isolated bacteria from urban and hospital wastewaters in Hamadan City. Iran J Health Environ 2011; 4(1):105-14. [ Google Scholar]
- Shariati A, Fallah F, Pormohammad A, Taghipour A, Safari H, Chirani AS. The possible role of bacteria, viruses, and parasites in initiation and exacerbation of irritable bowel syndrome. J Cell Physiol 2019; 234(6):8550-69. doi: 10.1002/jcp.27828 [Crossref] [ Google Scholar]
- Baş M, Şafak Ersun A, Kıvanç G. The evaluation of food hygiene knowledge, attitudes, and practices of food handlers’ in food businesses in Turkey. Food Control 2006; 17(4):317-22. doi: 10.1016/j.foodcont.2004.11.006 [Crossref] [ Google Scholar]
- Lund BM, O’Brien SJ. Microbiological safety of food in hospitals and other healthcare settings. J Hosp Infect 2009; 73(2):109-20. doi: 10.1016/j.jhin.2009.05.017 [Crossref] [ Google Scholar]
- Nasser A, Moradi M, Jazireian P, Safari H, Alizadeh-Sani M, Pourmand MR. Staphylococcus aureus versus neutrophil: Scrutiny of ancient combat. Microb Pathog 2019; 131:259-69. doi: 10.1016/j.micpath.2019.04.026 [Crossref] [ Google Scholar]
- Ohira T, Yamamoto O, Iida Y, Nakagawa ZE. Antibacterial activity of ZnO powder with crystallographic orientation. J Mater Sci Mater Med 2008; 19(3):1407-12. doi: 10.1007/s10856-007-3246-8 [Crossref] [ Google Scholar]
- Alizadeh-Sani M, Ehsani A, Hashemi M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int J Food Microbiol 2017; 251:8-14. doi: 10.1016/j.ijfoodmicro.2017.03.018 [Crossref] [ Google Scholar]
- Hajipour MJ, Fromm KM, Ashkarran AA, Jimenez de Aberasturi D, de Larramendi IR, Rojo T. Antibacterial properties of nanoparticles. Trends Biotechnol 2012; 30(10):499-511. doi: 10.1016/j.tibtech.2012.06.004 [Crossref] [ Google Scholar]
- Maleki Dizaj S, Mennati A, Jafari S, Khezri K, Adibkia K. Antimicrobial activity of carbon-based nanoparticles. Adv Pharm Bull 2015; 5(1):19-23. doi: 10.5681/apb.2015.003 [Crossref] [ Google Scholar]
- Khezerlou A, Alizadeh-Sani M, Azizi-Lalabadi M, Ehsani A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb Pathog 2018; 123:505-26. doi: 10.1016/j.micpath.2018.08.008 [Crossref] [ Google Scholar]
- Vargas-Reus MA, Memarzadeh K, Huang J, Ren GG, Allaker RP. Antimicrobial activity of nanoparticulate metal oxides against peri-implantitis pathogens. Int J Antimicrob Agents 2012; 40(2):135-9. doi: 10.1016/j.ijantimicag.2012.04.012 [Crossref] [ Google Scholar]
- Piran P, Kafil HS, Ghanbarzadeh S, Safdari R, Hamishehkar H. Formulation of menthol-loaded nanostructured lipid carriers to enhance its antimicrobial activity for food preservation. Adv Pharm Bull 2017; 7(2):261-8. doi: 10.15171/apb.2017.031 [Crossref] [ Google Scholar]
- Shirdel M, Tajik H, Moradi M. Combined activity of colloid nanosilver and Zataria multi flora Boiss essential oil-mechanism of action and biofilm removal activity. Adv Pharm Bull 2017; 7(4):621-8. doi: 10.15171/apb.2017.074 [Crossref] [ Google Scholar]
- Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro Lett 2015; 7(3):219-42. doi: 10.1007/s40820-015-0040-x [Crossref] [ Google Scholar]
- Jin T, Sun D, Su JY, Zhang H, Sue HJ. Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella enteritidis, and Escherichia coli O157:H7. J Food Sci 2009; 74(1):M46-52. doi: 10.1111/j.1750-3841.2008.01013.x [Crossref] [ Google Scholar]
- Yoon KY, Hoon Byeon J, Park JH, Hwang J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci Total Environ 2007; 373(2-3):572-5. doi: 10.1016/j.scitotenv.2006.11.007 [Crossref] [ Google Scholar]
- Lara HH, Ayala-Núñez NV, Ixtepan Turrent LdC, Rodríguez Padilla C. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J Microbiol Biotechnol 2010; 26(4):615-21. doi: 10.1007/s11274-009-0211-3 [Crossref] [ Google Scholar]
- Alizadeh-Sani M, Khezerlou A, Ehsani A. Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil. Ind Crops Prod 2018; 124:300-15. doi: 10.1016/j.indcrop.2018.08.001 [Crossref] [ Google Scholar]
- Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res 2006; 5(4):916-24. doi: 10.1021/pr0504079 [Crossref] [ Google Scholar]
- Abdelhamid HN, Wu HF. Proteomics analysis of the mode of antibacterial action of nanoparticles and their interactions with proteins. TrAC Trends Anal Chem 2015; 65:30-46. doi: 10.1016/j.trac.2014.09.010 [Crossref] [ Google Scholar]
- Beyth N, Houri-Haddad Y, Domb A, Khan W, Hazan R. Alternative antimicrobial approach: nano-antimicrobial materials. Evid Based Complement Alternat Med 2015; 2015:246012. doi: 10.1155/2015/246012 [Crossref] [ Google Scholar]
- Stark WJ, Stoessel PR, Wohlleben W, Hafner A. Industrial applications of nanoparticles. Chem Soc Rev 2015; 44(16):5793-805. doi: 10.1039/c4cs00362d [Crossref] [ Google Scholar]
- Beggs CB, Noakes CJ, Sleigh PA, Fletcher LA, Kerr KG. Methodology for determining the susceptibility of airborne microorganisms to irradiation by an upper-room UVGI system. J Aerosol Sci 2006; 37(7):885-902. doi: 10.1016/j.jaerosci.2005.08.002 [Crossref] [ Google Scholar]
- Seil JT, Webster TJ. Antimicrobial applications of nanotechnology: methods and literature. Int J Nanomedicine 2012; 7:2767-81. doi: 10.2147/ijn.s24805 [Crossref] [ Google Scholar]
- Zapata A, Ramirez-Arcos S. A comparative study of McFarland turbidity standards and the Densimat photometer to determine bacterial cell density. Curr Microbiol 2015; 70(6):907-9. doi: 10.1007/s00284-015-0801-2 [Crossref] [ Google Scholar]
- Xu K, Wang JX, Kang XL, Chen JF. Fabrication of antibacterial monodispersed Ag–SiO2 core–shell nanoparticles with high concentration. Mater Lett 2009; 63(1):31-3. doi: 10.1016/j.matlet.2008.08.039 [Crossref] [ Google Scholar]
- Marshall SA, Jones RN, Wanger A, Washington JA, Doern GV, Leber AL. Proposed MIC quality control guidelines for National Committee for Clinical Laboratory Standards susceptibility tests using seven veterinary antimicrobial agents: ceftiofur, enrofloxacin, florfenicol, penicillin G-novobiocin, pirlimycin, premafloxacin, and spectinomycin. J Clin Microbiol 1996; 34(8):2027-9. [ Google Scholar]
-
NCCLS. Quality Assurance of Commercially Prepared Microbiological Culture Media. 2nd ed. Wayne, PA: NCCLS; 1996.
- Sawai J, Himizu K, Yamamoto O. Kinetics of bacterial death by heated dolomite powder slurry. Soil Biol Biochem 2005; 37(8):1484-9. doi: 10.1016/j.soilbio.2005.01.011 [Crossref] [ Google Scholar]
- Sawai J, Shiga H, Kojima H. Kinetic analysis of the bactericidal action of heated scallop-shell powder. Int J Food Microbiol 2001; 71(2-3):211-8. doi: 10.1016/s0168-1605(01)00619-5 [Crossref] [ Google Scholar]
- Sawai J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J Microbiol Methods 2003; 54(2):177-82. doi: 10.1016/S0167-7012(03)00037-X [Crossref] [ Google Scholar]
- Wei X, Zhu G, Fang J, Chen J. Synthesis, characterization, and photocatalysis of well-dispersible phase-pure anatase TiO2 nanoparticles. Int J Photoenergy 2013; 2013:726872. doi: 10.1155/2013/726872 [Crossref] [ Google Scholar]
- Kumar SS, Venkateswarlu P, Rao VR, Rao GN. Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int Nano Lett 2013; 3(1):30. doi: 10.1186/2228-5326-3-30 [Crossref] [ Google Scholar]
- Azam A, Ahmed AS, Oves M, Khan MS, Habib SS, Memic A. Antimicrobial activity of metal oxide nanoparticles against gram-positive and gram-negative bacteria: a comparative study. Int J Nanomedicine 2012; 7:6003-9. doi: 10.2147/ijn.s35347 [Crossref] [ Google Scholar]
- Sawai J, Shiga H, Kojima H. Kinetic analysis of death of bacteria in CaO powder slurry. Int Biodeterior Biodegradation 2001; 47(1):23-6. doi: 10.1016/S0964-8305(00)00115-3 [Crossref] [ Google Scholar]
- Li Y, Ma M, Wang X, Wang X. Inactivated properties of activated carbon-supported TiO2 nanoparticles for bacteria and kinetic study. J Environ Sci (China) 2008; 20(12):1527-33. doi: 10.1016/s1001-0742(08)62561-9 [Crossref] [ Google Scholar]
- Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine 2007; 3(2):168-71. doi: 10.1016/j.nano.2007.02.001 [Crossref] [ Google Scholar]
- Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 2001; 9(1):34-9. doi: 10.1016/s0966-842x(00)01913-2 [Crossref] [ Google Scholar]
- Guan Y, Chen J, Qi X, Chen G, Peng F, Sun R. Fabrication of biopolymer hydrogel containing Ag nanoparticles for antibacterial property. Ind Eng Chem Res 2015; 54(30):7393-400. doi: 10.1021/acs.iecr.5b01532 [Crossref] [ Google Scholar]
- Kaur G, Kumar S, Kant R, Bhanjana G, Dilbaghi N, Guru SK. One-step synthesis of silver metallosurfactant as an efficient antibacterial and anticancer material. RSC Adv 2016; 6(62):57084-97. doi: 10.1039/C6RA09677H [Crossref] [ Google Scholar]
- Besinis A, De Peralta T, Handy RD. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology 2014; 8(1):1-16. doi: 10.3109/17435390.2012.742935 [Crossref] [ Google Scholar]
- Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater 2008; 4(3):707-16. doi: 10.1016/j.actbio.2007.11.006 [Crossref] [ Google Scholar]
- Fu G, Vary PS, Lin C-T. Anatase TiO2 nanocomposites for antimicrobial coatings. J Phys Chem B 2005; 109(18):8889-98. doi: 10.1021/jp0502196 [Crossref] [ Google Scholar]
- Han K, Yu M. Study of the preparation and properties of UV-blocking fabrics of a PET/TiO2 nanocomposite prepared by in situ polycondensation. J Appl Polym Sci 2006; 100(2):1588-93. doi: 10.1002/app.23312 [Crossref] [ Google Scholar]
- Zhou JJ, Wang SY, Gunasekaran S. Preparation and characterization of whey protein film incorporated with TiO2 nanoparticles. J Food Sci 2009; 74(7):N50-6. doi: 10.1111/j.1750-3841.2009.01270.x [Crossref] [ Google Scholar]
- Allahverdiyev AM, Abamor ES, Bagirova M, Rafailovich M. Antimicrobial effects of TiO(2) and Ag(2)O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol 2011; 6(8):933-40. doi: 10.2217/fmb.11.78 [Crossref] [ Google Scholar]
- Jones N, Ray B, Ranjit KT, Manna AC. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett 2008; 279(1):71-6. doi: 10.1111/j.1574-6968.2007.01012.x [Crossref] [ Google Scholar]
- Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, Casey PS. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ Sci Technol 2007; 41(24):8484-90. doi: 10.1021/es071445r [Crossref] [ Google Scholar]
- Azizi-Lalabadi M, Ehsani A, Alizadeh-Sani M, Khezerlou A, Mirzanajafi-Zanjani M, Divband B. Nanoparticles and zeolites: Antibacterial effects and their mechanism against pathogens. Curr Pharm Biotechnol 2019. doi: 10.2174/1573397115666190708120040 [Crossref]