Advanced pharmaceutical bulletin. 10(4):656-661.
doi: 10.34172/apb.2020.079
Short Communication
Impact of Tablet Shape on Drug Dissolution Rate Through Immediate Released Tablets
Fatima Molavi 1  , Hamed Hamishehkar 2, *
, Hamed Hamishehkar 2, *  , Ali Nokhodchi 3, *
, Ali Nokhodchi 3, * 
Author information:
1Biotechnology Research Center, Student Research Committee and Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran.
2Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
3Pharmaceutics Research Laboratory, School of Life Sciences, University of Sussex, Falmer, Brighton BN1 9QJ, United Kingdom.
Abstract
Purpose:
The aim of this study was to evaluate the influence of the geometric shape on the dissolution rate of the domperidone, a drug model for immediate release dosage form. In this regard, a lack of sufficient information about the effective dissolution rate of the drugs regarding their shapes has made this issue an interesting subject for researchers.
Methods:
For this purpose, three tablet shapes, namely flat and biconvex both in a round and oblong shapes, with different four sizes were modelled for the preparation of domperidone tablet. In vitro dissolution test was accomplished using a USP dissolution apparatus II. The drug dissolution rate was assessed by calculating various dissolution parameters; e.g., dissolution efficiency (DE), mean dissolution rate (MDR), mean dissolution time (MDT), and difference and similarity factors (f1 and f2 ).
Results:
Regarding the disintegration time, the larger tablets showed a faster disintegration time. When the size of the tablets was smaller, the amount of released drug was significantly decreased. In addition, #9 tablets with a flat or biconvex geometry had obvious effects on the DE values. Generally, biconvex tablets had higher DE percentage than the flat tablets.
Conclusion:
Noticeable differences in dissolution parameters by considering the different geometric shapes play an important role in the drug release kinetics which makes a significant effect on quick onset of action in oral administration.
Keywords: Dissolution modeling, Tablet, Drug release, Domperidone, Geometric properties
Copyright and License Information
© 2020 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Dissolution rate studies play a key role in the development of pharmaceutical dosage forms, in vitro and in vivo correlation (IVIVC) assessment,
1
registration, and quality control of various dosage forms.
2
The dissolution methods for individual drugs are determined by the solubility of the active substance,
3
the dosage form characteristics,
4
and the intended route of administration such as oral solid dosage form. Improving the dissolution rates of drugs by different techniques is an increasing demand of pharmaceutical industries and can be achieved by various methods including modifying physicochemical properties by preparation of nano-sized drug particles,
5,6
solid dispersions approach,
7
particle design
8
and adsorption onto pharmaceutical diluents.
9
It has been proved that the in vitro dissolution rate is proportional to in vivo absorption rate data.
1,10
Therefore, the prediction of drug dissolution is extremely important for formulators in pharmaceutical industries. Attracting appearance for marketing aspects and production of various tablet shapes for pediatric compliance have led to the creation of various tablet shapes.
11
In addition, tablet shape is an important subject for pharmaceutical industrials because of its influence in product development, process operating conditions
12-14
and marketing issues. Domperidone, a drug model for immediate release dosage form was selected according to its biopharmaceutical classification system that classified to class II, suggesting that the release of these type of drugs from dosage forms is easily controlled by formulation composition.
15
Therefore, we can easily study and evaluate dissolution manner related to various tablet shapes. The objective of the present study was to investigate the impact of different tablet shapes on dissolution rate. By considering that the formulation composition and hardness, two main affecting factors on dissolution rate are the same in the tablets with various shapes. In addition as the novelty of the study, the results of this project will be able to guide pharmaceutical industry formulators to simulate the drug release pattern of generic formulations or adjust it according to the pharmacopoeia requirements.
Materials and Methods
Materials
Domperidone maleate (M/S Vasudha Pharma Chem Limited, India) was kindly donated from Zahravi Pharmaceutical Co. (Tabriz, Iran). Lactose monohydrate, Avicel® PH-101, pregelatinized starch, and magnesium stearate were provided from DMV (Germany), Mingtai (Taiwan), Colorcon (USA), respectively. Hydrochloric acid and polysorbate 20 were purchased from Merck Chemicals Co. (Germany). Colloidal silicon dioxide was provided from Kirsch pharma (Germany).
Methods
Formulation and tablet Preparation
To prepare domperidone maleate tablets wet granulation method was employed. The required quantities of the ingredients were weighed and blended using the tumbling method to provide a homogenous granule mixture as summarized in Table 1. The granules were compressed on different punch and die shapes i.e. round #6 biconvex, round #6 flat, round #9 biconvex, round #9 flat, oblong #12 flat, round #11 flat (Figure 1). Tablets were pressed by a rotary compression machine (ERWEKA AR 400, Germany). Fifty tablets in each shape were manufactured containing different weights but the same powder composition with approximately equal hardness about 8-9 kPa.

Figure 1.
Domperidone tablets with different shapes: a) Round #6 flat, b) Round #6 biconvex, c) Round #9 flat, d) Round #9 biconvex, e) Round #11 flat, and f) Oblong #12 biconvex.
.
Domperidone tablets with different shapes: a) Round #6 flat, b) Round #6 biconvex, c) Round #9 flat, d) Round #9 biconvex, e) Round #11 flat, and f) Oblong #12 biconvex.
Table 1.
Formulation of domperidone maleate
|
Composition
|
Percentage (%)
|
Function in the formulation
|
| Domperidone maleate |
12.50 |
Active ingredient |
| Lactose monohydrate |
51.83 |
Diluent |
| Avicel PH-101 |
24.55 |
Diluent and disintegrant |
| Pregelatinized starch |
6.88 |
Diluent |
| Polysorbate 20 |
0.96 |
Solubilizing agent |
| Colloidal silicon dioxide |
0.29 |
Glidant |
| Magnesium stearate |
2.95 |
Lubricant |
| Total quantity |
100.0 |
- |
Tablet characterization
Thickness, diameter and hardness were characterized using a hardness tester (Model, ERWEKA, Germany). Assay and content uniformity of tablets also were assessed according to British Pharmacopoeia (BP 2015).
Values of the total surface area of tablets either flat or biconvex are calculated by addition of surface on both sides of the tablet (SA) of radius (πr2) and spherical cap surface areas (2πRh). Where h is the height, R is the spherical cap radius and r is the base radius.
Dissolution study
Dissolution test for domperidone tablets in the BP, briefly is described in Table 2. The area under the dissolution curve up to the time, t, is defined as the dissolution efficiency (DE).
Table 2.
Dissolution test conditions for domperidone tablets according to BP (2015)
|
Apparatus
|
Paddle
|
| Medium |
HCl 0.1 N |
| Speed |
50 rpm |
| Procedure |
UV (λ=286nm) |
| Time |
2, 5, 8, 10, 15, 20, 30, and 45 minutes |
Where y is the percentage of domperidone dissolved at time t.
16
The mean dissolution time (MDT) is another parameter to explain the drug dissolution rate from a solid state of a dosage form.
Where i is the total number of dissolution sample times, ΔMi is the added amount of drug dissolved between ti and t-1; and ti is the midpoint time between two samples in the t and t-1.
Another parameter that represents the dissolution rate is the mean dissolution rate (MDR) as an independent metric. It can be calculated according to the following equation, in addition showing the mean percentage of drug dissolved to time.
Where n is the number of sample time points, Δt is the midpoint time.
17
The similarity factor f2 and the difference factor f1 were calculated according to the following equations:
Where Rt and Tt are the refrence profile and the test profile of cumulative percentage of drug dissolved at time point t, respectively. To consider dissolution profiles as similar and bioequivalent, the value of f2 and f1 should be between 50 through 100 and lower than 10, respectively.
18
To study drug-release kinetics, several dissolution models such as zero order, first order, Higuchi, Hixson–Crowell, Weibull, and Korsmeyer and Peppas has well-known.
19,20
The accurate fitting model was selected based on R-squared (RSQ) and mean percent error (E) from drug release data.
21
Statistical analysis
Independent comparison models and ANOVA based on statistical methods has been performed to compare dissolution profiles. In this procedure, Tukey test was applied as independent analysis model, dominating the multiple comparison tests.
Results and Discussion
Domperidone tablets were prepared through wet granulation technique using scale-up equipment to reach an acceptable appearance and same hardness. Table 3 shows the characteristics of the appearance of tablets with the same formulation. The results have shown that the hardness of tablets is almost similar. Tablet shapes and dissolution rate are considered as independent and dependent variables, respectively. The results of the assay and content uniformity of tablets have been reported as acceptable. The result of active pharmaceutical ingredient assay was 98-102% and the acceptance value (AV) for content uniformity test was around 4-5, where AV≤15 is considered as suitable.
Table 3.
Description for different tablets
|
Appearance Shape
|
Weight (mg)
|
Thickness (mm)
|
Diameter (mm)
|
Area (mm
2
)
|
Hardness (kp
a
)
|
| Round #6 biconvex |
102 ± 0.6 |
3.08 ± 0.01 |
6.04 ± 0.02 |
113.84 |
9.1 ± 0.1 |
| Round #6 flat |
102 ± 0.1 |
2.70 ± 0.01 |
6.06 ± 0.01 |
95.56 |
8.8 ± 0.3 |
| Round #9 flat |
250 ± 0.7 |
3.09 ± 0.01 |
9.02 ± 0.01 |
214.88 |
9.9 ± 0.4 |
| Round #9 biconvex |
251 ± 1.4 |
3.70 ± 0.05 |
9.06 ± 0.06 |
231.84 |
8.5 ± 0.8 |
| Round #11 flat |
394 ± 2.2 |
3.05 ± 0.02 |
11.06 ± 0.02 |
321.59 |
8.7 ± 0.6 |
| Oblong #12 biconvex |
253.8 ± 0.7 |
3.83 ± 0.01 |
12.16 × 6.15 ± 0.0 |
>400 |
6.9± 0.5 |
Data was presented as mean ± standard deviation (n=6).
akp, kilopond.
Model-independent approaches
The aim of this study was to characterize the different geometric types of tablets on domperidone release. To evaluate dissolution behavior and to describe the relationship between drug release behaviors, the main dissolution parameters i.e., DE%, MDT, MDR, and disintegration time were studied. By comparing the results, the dissolution test parameters are completely changed by changing the appearance of the tablets. The results, as shown in Table 4, indicated that larger tablets had faster disintegration time. The oblong #12 biconvex tablets had the fastest disintegration time, which would be desired in its pharmacological performance. In addition, biconvex tablets demonstrated a faster drug release than flat tablets. This fact is more significant in larger tablets probably because of providing more surface area for tablets. The results of other time-dependent parameters (MDT and MDR) were well matched with the results of disintegration time. The high disintegration time and low DE% in round #6 tablets matrix can be attributed to its low surface area. As the formulation of tablets is the same, according to the Noyes and Whitney equation
22
a low surface area between the solute and the solvent leads to a reduction in the amount of dissolved substance. In fact, the smaller size of tablets leads to significantly decreased percent of DE. In addition, flat and biconvex (#9) tablets showed high DE values and comparing between flat and biconvex tablets, biconvex tablets exhibited a higher DE value. Statistical analysis was carried out using one-way ANOVA based on DE data, this result was insignificant at the p = 0.319 level for #6 flat and biconvex tablets. It revealed that the release profiles of these shapes are truly similar. Besides, p-value for #6 flat and #9 flat tablets is less than 0.003, suggesting that the size of tablets is a very important parameter in dissolution behavior. In addition, by comparing drug dissolution between #9 flat-faced and biconvex tablets, at the P = 0.0001 level, signifying that by increasing in the size of tablets, the difference between flat-faced tablets and biconvex tablets becomes more significant. Furthermore, different types of tablets have shown different initial release which is important in immediate-release tablets to start the biological effect as early as the drug was taken.
Table 4.
Dissolution parameters
|
Sample
|
Disintegration time (min)
|
DE (%)
|
MDT
|
MDR
|
| Round #6 biconvex |
15:20”± 01:30” |
73.71± 1.94 |
18.85 ± 1.40 |
1.36 ± 0.07 |
| Round #6 flat |
14:05”± 01:00” |
73.80± 1.89 |
18.78 ± 1.37 |
1.11 ± 0.08 |
| Round #9 flat |
7:19” ± 01:30” |
88.59± 1.49 |
8.44 ± 1.04 |
2.36 ± 0.20 |
| Round #9 biconvex |
3:52” ± 01:30”’ |
91.95± 2.14 |
6.10 ± 1.47 |
3.49 ± 0.91 |
| Round #11 flat |
6:03” ± 01:00” |
88.75± 1.94 |
8.36 ± 1.38 |
2.38 ± 0.34 |
| Oblong #12 biconvex |
1:40” ± 00:30” |
90.54± 1.81 |
7.14 ± 1.30 |
4.07 ± 0.21 |
DE, Dissolution efficiency; MDR, Mean dissolution rate; MDT, Mean dissolution time.
Data was presented as mean ± standard deviation (n=6).
As the size of the tablets increased, the burst release was faster. It reveals that in addition to the effect of hardness of the tablet on the disintegration and the dissolution rate of the tablet, the shape of the tablet also has a significant effect which makes the quick onset of action in oral administration. A similar study performed on metronidazole sustained dosage forms also presented that surface area can be used as a factor to estimate the drug release profile.
23
A study on the effect of different geometrical shapes i.e. triangular, cylindrical and half-spherical on theophylline release indicated that the highest releases were observed for triangular erodible tablets in 1:1 drug/polymer ratio and for half-spherical tablets with 1:0.5 drug/polymer ratio.
24
The results of this study proved that the geometric shape has an effect on the diffusion and release kinetics. The results obtained from the analysis of drug release of the different geometric shapes of tablets are shown in Figure 2. It reveals that there has been a different dissolution profile among the products. Burst release plays the main role in the successful therapeutic performance of anti-vomiting dosage forms. Tablets in various shapes provide different burst releases. The order of domperidone burst release from various tablets were Round #6 flat < Round #6 biconvex <Round #9 flat < Round #11 flat < Round #9 biconvex < Oblong #12 biconvex. It is concluded that a decrease in the size of tablets significantly reduces the drug burst release. Interestingly, biconvexity improved the burst release and helped fulfilment of complete drug release from tablet dosage forms. Statistical approaches, f1 (the difference factor) and f2 (the similarity factor) were used to compare the dissolution profiles. Table 5 presents the experimental data for the comparison of the individual dissolution profiles. As can be seen, tablet size has a key role in the drug release behavior of tablets. The varying surface area can change the dissolution rate of non-flat geometry of solid dosage forms.
25
Although comparing round #6 biconvex and round #6 flat tablets demonstrated that being flat and biconvex in small tablets is not important for drug dissolution, but this comparison for #9 tablets indicated the opposite results. This observation confirmed that the drug release behavior are affected by being flat and biconvex in larger tablets.
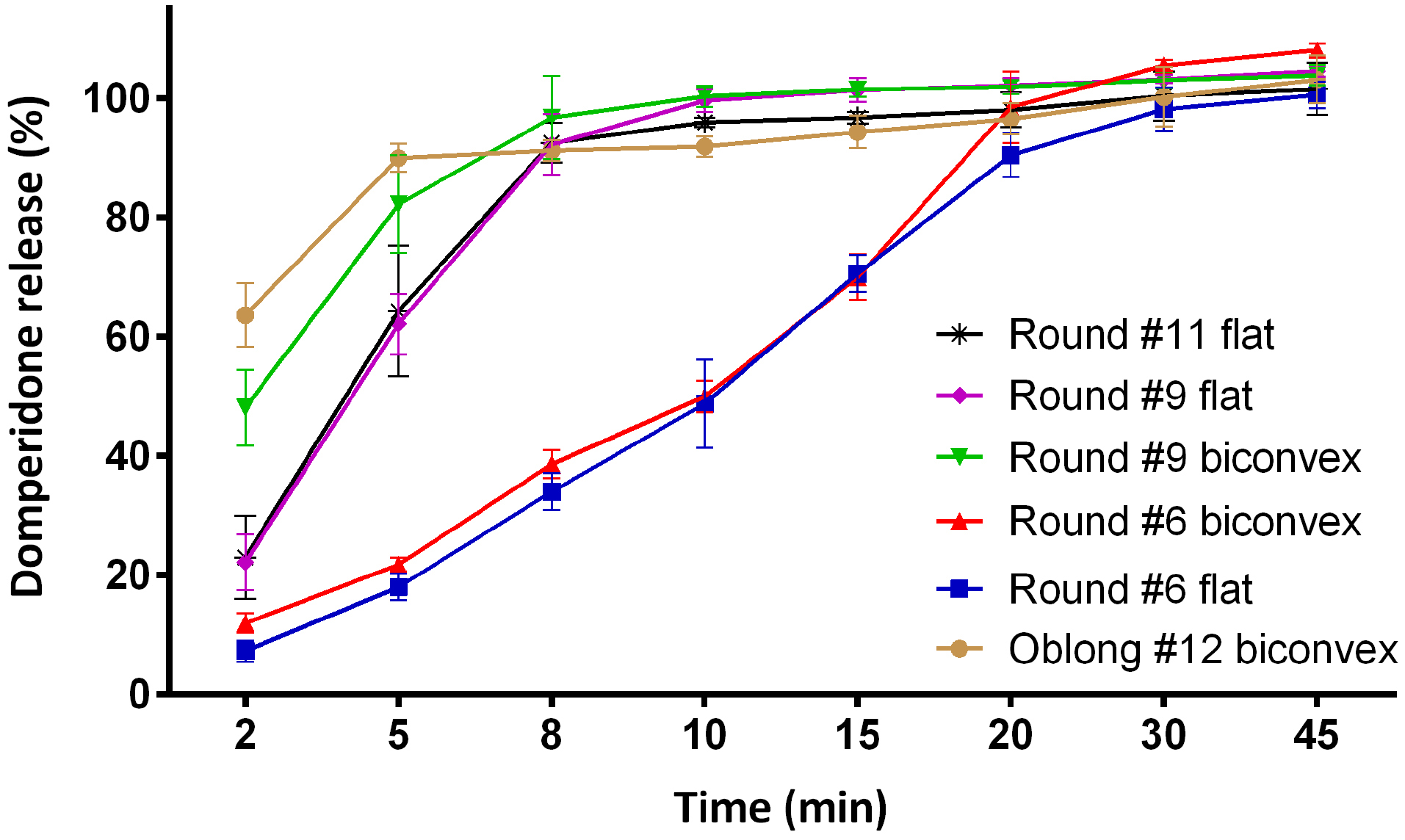
Figure 2.
Dissolution profiles of different shapes of tablets. Values were presented in mean with standard deviation (n=6).
.
Dissolution profiles of different shapes of tablets. Values were presented in mean with standard deviation (n=6).
Table 5.
Domperidone release profile comparison between different shape tablets through difference (f1) and similarity (F2) factors
|
Tablet shape
|
f
1
(%)
|
f
2
(%)
|
| Round #6 biconvex vs. Round #6 flat |
7.49 |
62.97 |
| Round #9 flat vs. Round #9 biconvex |
7.62 |
46.47 |
| Round #9 flat vs. Round #6 flat |
31.93 |
23.40 |
Model-dependent approaches
Mathematical models based on statistical analysis was used to characterize the dissolution profiles.
26
Model dependent approaches due to the complexity ofin vitro methods have received considerable attention. The minimum errors and RSQ around 100 between the fitted and the actual data are acceptance conditions to apply dissolution models. Here, only Korsmeyer-Peppas model describes accurately the release data of round #6 flat, round #6 biconvex, and round #9 flat domperidone tablets with RSQ of 0.97, 0.99, and 1 with an error of 26.34, 30.00, and 0.00%, respectively. Other shapes cannot be defined by any of the models as the large burst release (60%) happened in the first minutes. Korsmeyer-Peppas model is expected to be successfully applied to the analysis of drug release kinetics from homogenous and dissolving tablet matrix in 60% of the initial release. To the best of our knowledge, these models were justified theoretically via unification of the Fick’s first law of diffusion and the Noyes- Whitney law of dissolution. As result, according to Korsmeyer-Peppas model, the release mechanism for small flat tablets, #6 and relatively #9, in addition to diffusion, erosion of tablets can also be considered in the first minutes; as these results match those observed in the disintegration study.
Conclusion
Since the emerge of the Noyes-Whitney equation in 1897, dissolution research has been initiated and still now it is a critical issue in the chemistry.
27
In this regard, the dissolution test is an essential component of drug development in the pharmaceutical industry. This research evaluated the effect of six different tablet shapes on the dissolution rate. The current study indicated that by decreasing the size of tablets, the drug burst release was significantly decreased. Although the present study is based on a small sample of tablet shapes, the findings indicate that the initial burst and complete release occurred in large, biconvex tablets. Further research regarding the role of tablet shapes would be worthwhile.
Ethical Issues
Not applicable.
Acknowledgments
The research reported in this publication was supported by Elite Researcher Grant Committee under award number 958777 from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran.
Conflict of Interest
The authors confirm that this article has no conflict of interest.
References
- Cardot JM, Beyssac E, Alric M. In vitro–in vivo correlation: importance of dissolution in IVIVC. Dissolut Technol 2007; 14(1):15-9. doi: 10.14227/DT140107P15 [Crossref] [ Google Scholar]
-
Lee SL, Raw AS, Yu L. Dissolution testing. In: Krishna R, Yu L, eds. Biopharmaceutics Applications in Drug Development. Boston, MA: Springer; 2008. p. 47-74. 10.1007/978-0-387-72379-2_3
- Bredael GM, Liang S, Hahn D. A strategy for quality control dissolution method development for immediate-release solid oral dosage forms. Dissolut Technol 2015; 22(3):10-6. doi: 10.14227/DT220315P10 [Crossref] [ Google Scholar]
- Siewert M, Dressman J, Brown CK, Shah VP, Aiache JM, Aoyagi N. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. Dissolut Technol 2003; 10(1):6-15. doi: 10.14227/DT100103P6 [Crossref] [ Google Scholar]
- Kaur A, Gupta S, Tyagi A, Sharma RK, Ali J, Gabrani R. Development of nanoemulsion based gel loaded with phytoconstituents for the treatment of urinary tract infection and in vivo biodistribution studies. Adv Pharm Bull 2017; 7(4):611-9. doi: 10.15171/apb.2017.073 [Crossref] [ Google Scholar]
- Ravouru N, Venna RSA, Penjuri SCB, Damineni S, Kotakadi VS, Poreddy SR. Fabrication and characterization of gliclazide nanocrystals. Adv Pharm Bull 2018; 8(3):419-27. doi: 10.15171/apb.2018.049 [Crossref] [ Google Scholar]
- Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull 2015; 5(3):305-13. doi: 10.15171/apb.2015.043 [Crossref] [ Google Scholar]
- Panzade P, Shendarkar G, Shaikh S, Balmukund Rathi P. Pharmaceutical cocrystal of piroxicam: design, formulation and evaluation. Adv Pharm Bull 2017; 7(3):399-408. doi: 10.15171/apb.2017.048 [Crossref] [ Google Scholar]
- Weerapol Y, Limmatvapirat S, Nunthanid J, Konthong S, Suttiruengwong S, Sriamornsak P. Development and characterization of nifedipine-amino methacrylate copolymer solid dispersion powders with various adsorbents. Asian J Pharm Sci 2017; 12(4):335-43. doi: 10.1016/j.ajps.2017.01.002 [Crossref] [ Google Scholar]
- Modi NB, Lam A, Lindemulder E, Wang B, Gupta SK. Application of in vitro-in vivo correlations (IVIVC) in setting formulation release specifications. Biopharm Drug Dispos 2000; 21(8):321-6. doi: 10.1002/bdd.248 [Crossref] [ Google Scholar]
- Stegemann S. Colored capsules-a contribution to drug safety. Pharm Ind 2005; 67(9):1088-95. [ Google Scholar]
- Suzzi D, Toschkoff G, Radl S, Machold D, Fraser SD, Glasser BJ. DEM simulation of continuous tablet coating: effects of tablet shape and fill level on inter-tablet coating variability. Chem Eng Sci 2012; 69(1):107-21. doi: 10.1016/j.ces.2011.10.009 [Crossref] [ Google Scholar]
- Davies PN, Worthington HE, Podczeck F, Newton JM. The determination of the mechanical strength of tablets of different shapes. Eur J Pharm Biopharm 2007; 67(1):268-76. doi: 10.1016/j.ejpb.2007.01.014 [Crossref] [ Google Scholar]
- Desai PM, Anbalagan P, Koh CJN, Heng PWS, Liew CV. Evaluation of tablet punch configuration on mitigating capping by a quality by design approach. Drug Deliv Transl Res 2018; 8(6):1635-43. doi: 10.1007/s13346-017-0425-0 [Crossref] [ Google Scholar]
- Arrunátegui LB, Silva-Barcellos NM, Bellavinha KR, da Silveira Ev L, de Souza J. Biopharmaceutics classification system: importance and inclusion in biowaiver guidance. Braz J Pharm Sci 2015; 51(1):143-54. doi: 10.1590/s1984-82502015000100015 [Crossref] [ Google Scholar]
- Chaturvedi M, Kumar M, Pathak K, Bhatt S, Saini V. Surface solid dispersion and solid dispersion of meloxicam: comparison and product development. Adv Pharm Bull 2017; 7(4):569-77. doi: 10.15171/apb.2017.068 [Crossref] [ Google Scholar]
- Shojaee S, Nokhodchi A, Maniruzzaman M. Evaluation of the drug solubility and rush ageing on drug release performance of various model drugs from the modified release polyethylene oxide matrix tablets. Drug Deliv Transl Res 2017; 7(1):111-24. doi: 10.1007/s13346-016-0344-5 [Crossref] [ Google Scholar]
- Kuang C, Sun Y, Li B, Fan R, Zhang J, Yao Y. Preparation and evaluation of duloxetine hydrochloride enteric-coated pellets with different enteric polymers. Asian J Pharm Sci 2017; 12(3):216-26. doi: 10.1016/j.ajps.2016.08.007 [Crossref] [ Google Scholar]
- Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci 2001; 13(2):123-33. doi: 10.1016/s0928-0987(01)00095-1 [Crossref] [ Google Scholar]
- Siepmann J, Siepmann F. Mathematical modeling of drug delivery. Int J Pharm 2008; 364(2):328-43. doi: 10.1016/j.ijpharm.2008.09.004 [Crossref] [ Google Scholar]
- Barzegar-Jalali M, Adibkia K, Valizadeh H, Siahi Shadbad MR, Nokhodchi A, Omidi Y. Kinetic analysis of drug release from nanoparticles. J Pharm Pharm Sci 2008; 11(1):167-77. doi: 10.18433/j3d59t [Crossref] [ Google Scholar]
- Noyes AA, Whitney WR. The rate of solution of solid substances in their own solutions. J Am Chem Soc 1897; 19(12):930-4. doi: 10.1021/ja02086a003 [Crossref] [ Google Scholar]
- Gökçe EH, Ozyazici M, Ertan G. The effect of geometric shape on the release properties of metronidazole from lipid matrix tablets. J Biomed Nanotechnol 2009; 5(4):421-7. doi: 10.1166/jbn.2009.1052 [Crossref] [ Google Scholar]
- Karasulu HY, Ertan G, Köse T. Modeling of theophylline release from different geometrical erodible tablets. Eur J Pharm Biopharm 2000; 49(2):177-82. doi: 10.1016/s0939-6411(99)00082-x [Crossref] [ Google Scholar]
-
Qiu Y, Chen Y, Zhang G, Liu L, Porter W. Developing Solid Oral Dosage Forms: Pharmaceutical Theory and Practice. Elsevier Science; 2009.
- Ahuja N, Katare OP, Singh B. Studies on dissolution enhancement and mathematical modeling of drug release of a poorly water-soluble drug using water-soluble carriers. Eur J Pharm Biopharm 2007; 65(1):26-38. doi: 10.1016/j.ejpb.2006.07.007 [Crossref] [ Google Scholar]
- Dokoumetzidis A, Macheras P. A century of dissolution research: from Noyes and Whitney to the biopharmaceutics classification system. Int J Pharm 2006; 321(1-2):1-11. doi: 10.1016/j.ijpharm.2006.07.011 [Crossref] [ Google Scholar]