Advanced pharmaceutical bulletin. 10(1):141-145.
doi: 10.15171/apb.2020.019
Short Communication
Hollow Alginate-Poly-L-Lysine-Alginate Microspheres Promoted an Epithelial-Mesenchymal Transition in Human Colon Adenocarcinoma Cells
Shirin Saberianpour 1, 2  , Arezoo Rezaie Nezhad Zamani 2, Abbas Karimi 1
, Arezoo Rezaie Nezhad Zamani 2, Abbas Karimi 1  , Mahdi Ahmadi 2
, Mahdi Ahmadi 2  , Neda Khatami 3, Ayda Pouyafar 2, Reza Rahbarghazi 1, 4, *
, Neda Khatami 3, Ayda Pouyafar 2, Reza Rahbarghazi 1, 4, *  , Mohammad Nouri 1, *
, Mohammad Nouri 1, * 
Author information:
1Department of Molecular Medicine, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
2Stem Cell Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
3Chemical Engineering Faculty, Sahand University of Technology, Tabriz, Iran.
4Department of Applied Cell Sciences, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
Abstract
Purpose:
Today, there is an urgent need to develop a three-dimentional culture systems mimicking native in vivo condition in order to screen potency of drugs and possibly any genetic alterations in tumor cells. Due to the existence of limitations in animal models, the development of three dimensional systems is highly recommended. To this end, we encapsulated human colon adenocarcinoma cell line HT29 with alginate-poly-L-lysine (Alg-PLL) microspheres and the rate of epithelial-mesenchymal transition was monitored.
Methods:
Cells were randomly divided into three groups; control, alginate and Alg-PLL. To encapsulate cells, we mixed HT-29 cells (1 × 106 ) with 1 mL of 0.05% PLL and 1% Alg mixture and electrosprayed into CaCl2 solution by using a high-voltage power. Cells from all groups were maintained at 37˚C in a humidified atmosphere containing 5% CO2 for 7 days. Cell viability was assessed by MTT assay. To monitor the stemness feature, we measured the transcription of genes such as Snail, Zeb, and Vimentin by using real-time PCR analysis.
Results:
Addition of PLL to Alg in a hallowed state increased the cell survival rate compared to the control and Alg groups (P<0.05). Cells inside Alg-PLL tended to form microcellular aggregates while in Alg microspheres an even distribution of HT-29 cells was found. Real-time PCR analysis showed the up-regulation of Snail, Zeb, and Vimentin in Alg-PLL microspheres compared to the other groups, showing the acquisition of stemness feature (P<0.05).
Conclusion:
This study showed that hallow Alg-PLL microspheres increased the epithelialmesenchymal transition rate after 7 days in in vitro condition. Such approaches could be touted as appropriate in vitro models for drug screening.
Keywords: Alginate, Epithelial-mesenchymal transition, Human colon adenocarcinoma cell line HT29, Poly-L-lysine, Microspheres
Copyright and License Information
© 2020 The Author (s)
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Up to the present time, many attempts have been made to investigate the type and entity of extracellular matrix (ECM) on cancer cells dynamics by using two (2D) and three dimensional (3D) substrates.
1
There are numerous peptides and proteins playing as modulators of cancer cells behavior and even could act as growth factors during the development and progression of various tumor types.
2
It was shown that distinct ECM could transmit survival signals between cancer cells.
3
In this regard, it was elucidated that these proteins had potential to dictate polarity and phenotype acquisition to the neoplastic cells by triggering a phenomenon termed as epithelial-to-mesenchymal transmission (EMT), causing tumor resistance to the anticancer agents and therapies.
4
This phenotypic switching increases the number of cancer progenitor cells so-called cancer stem cells (CSCs).
5
EMT is regulated by a number of functional proteins inside cancer cells that act as polyoleptic agents such as ZEB, Snail, and Vimentin, etc. The activity of these genes performs an important milieu for the expression of markers associated with mesenchymal cells phenotype.
6
In recent years, the microencapsulation technique has been used extensively to create a 3D microenvironment in the field of cancer biology and tissue engineering.
7
Basically, alginate (Alg), a natural polysaccharide, and to less extent other substrates such as poly-l-lysine (PLL), are extensively used for the preparation of microspheres.
8
Alg is potential to cross-link with different substrates in the presence of calcium and barium ions and functions as the basic scaffold to create a membrane shell protecting cells from external injuries.
9
Based on the scientific literature, the main reason for the use of PLL is to increase resistance around the center of the microspheres.
10
This resistance is correlated with the reduction of swelling rate and membrane shell pore size.
11
The main goal of this study was to investigate the occurrence of EMT in the human colorectal adenocarcinoma cell line (HT-29) after being encapsulated by the mixture of Alg and PLL. The cells were maintained inside the microspheres containing Alg alone and the mixture of Alg and PLL. The advantage of this method can be related to create a 3D environment for human cancer cells and study the possibility of EMT in HT-29 cells inside the Alg-PLL microspheres.
Materials and Methods
Cell culture
Human colorectal adenocarcinoma cell line; HT-29, was purchased from Iranian Cell Bank (Pasteur, Iran). Cells were grown in high glucose-content Dulbecco’s Modified Eagle Medium (DMEM/HG; Cat no: 31600-083; Gibco; Carlsbad; CA; USA) with 10% v/v fetal bovine serum (FBS; Cat no: 10270; Gibco; Carlsbad, USA) and 1% v/v penicillin-streptomycin (Pen-Strep; Cat no: 10378016; USA) in culture flasks. Cells were sub-cultured at 70%-80% confluence by using 0.25% Trypsin and 1 mM EDTA solutions (Cat No: 25200-056; Gibco; USA) solution. Cells were subjected to the current experiments at passages three to six.
Microencapsulation of HT-29 cells using Algand the PLL mixture
To explore the potency of ECM type on the stemness and multipotentiality of HT-29 cells, we encapsulated cells with the combination of Alg (1% w/v; Cat no: A2033; Sigma-Aldrich; Germany) and PLL (0.05% w/v; Cat no: P8920; Sigma-Aldrich; Germany) by using electrospray device (FnM, Hu35p oc, Nigh voltage power supply). To this end, we classified the cells into three different groups as follows; Control: HT-29 cells were expanded on the conventional plastic surface; Alg group: HT-29 cells were encapsulated by 1% Alg solution and Alg + PLL group: cells were enclosed by the mixture of Alg and PLL. To encapsulate cells, 1 × 106 HT-29 cells were mixed with 1 ml of 1% Alg and transferred into a sterile syringe with 26G-gauge needle. Thereafter, the cells suspension was poured into a syringe pump connected to an electrospray device, adjusted to a voltage of 8 kV. Dropping the cell mixture into a 1% CaCl2 (Cat no: 1.02382.1000; Darmstadt; Germany) solution contributed to the formation of microcapsules. To exclude CaCl2, we washed the microcapsules by CF-KRH solution (0.48% HEPES, 0.79% NaCl, 0.35% KCl, 0.1% Glucose). In Alg + PLL group, we added PLL to the Alg backbone. To remove the Alg from Alg + PLL microspheres, we treated these microcapsules with 0.02% EDTA (Cat no: 60-00-4; Sigma-Aldrich, Germany) for 5 min at 37°C by using CF-KRH solution. Cells from all groups were incubated at 37°C in a humidified atmosphere containing 5% CO2 for 7 days. Seven days after encapsulation, the cells were released from microcapsules by incubating cells with 0.01% sodium citrate (Cat no: 6132-04-3; Merck, Darmstadt; Germany). Electrospraying procedure was carried out under sterile condition.
Cell Survival detection by MTT assay
The survival of HT-29 cells from each group was evaluated by MTT assay. For this propose, 3000 cells were mixed with 100 μL of MTT solution (Cat no: 298-93-1; Sigma-Aldrich; Germany) and poured into each well of 96-well plates and kept at 37°C conditions for 4 hours. Then, 50 μL of dimethyl sulfide (DMSO; Merck, Darmstadt, Germany) solution were added into each well and plates gently agitated for 15 min. Finally, the optical density of each well was read by using an ELISA reader (Model: Ex808, BioTek, USA) at a wavelength of 570 nm.
Real-time PCR
HT-29 cells were collected from each group and total RNA content isolated by RNA extraction kit (Cat no: FABRK001; Yekta Tajhiz Azma, Iran). The purity of isolated RNAs was evaluated using a Thermo Scientific NanodropTM 1000 system. Subsequently, RNAs were converted cDNA by using cDNA synthetase (Bioneer). In this study, we monitored the transcription of three genes Snail, Vimentin, and Zeb by appropriate primers designated by Oligo 7 software (version 7.60) (Table 1). The level of gene expression was measured by SYBR Green and Mic Real-Time PCRSystem. The expression of each gene was normalized to the housekeeping gene GAPDH.
Table 1.
Primer list
|
Gene
|
Sequences (5' -> 3')
|
Accession No.
|
Temperature (°C)
|
Length (bp)
|
| VIM |
F:CAGATGCGGTGAAATGGAAGAGAA |
NM_003380.5 |
61 |
174 |
|
|
R:TAGGTGGCAATCTCAATGTCAA |
|
|
|
| ZEB1 |
F:CTGGAGAAAAGCCCTATCAATGT |
NM_001323671.2 |
59.7 |
244 |
|
|
R: CTGTCTTCATCCTCTTCCCTTGT |
|
|
|
| SNAI1 |
F: TAGCGAGTGGTTCTTCTGCG |
NM_005985.4 |
60 |
164 |
|
|
R: AGGGCTGCTGGAAGGTAAAC |
|
|
|
| GAPDH |
F:CCTGCACCACCAACTGCTTA |
NM_001289745.3 |
60 |
95 |
|
|
R: AGTGATGGCATGGACTGTGG |
|
|
|
Results and Discussion
The transition to invasive and metastatic subpopulations consists of changes in tumor cells function.
12
To attain these characteristics, morphogenetic transformations, known as EMT, is in an active state that maintains the balance between epithelial phenotype and stemness feature.
13
The application of 3D culture system could mimic to some extents the in vivo conditions.
14
Cell morphology and appearance
According to our data, HT-29 cells acquired a round shape morphology and were evenly distributed inside Alg microspheres (Figure 1). To elucidate the role of PLL on cell dynamic, we removed Alg from Alg + PLL microspheres core by using EDTA solution (a hallowed structure) while the membrane shell remained and consisted of PLL and Alg. In hallowed microspheres, HT-29 cell tended to form microaggregates after 7 days (Figure 1). The data showed that encapsulation of HT-29 cells with microspheres consisted of Alg distributed cells evenly over 7 days while Alg removal and enclose of cells with Alg + PLL membrane contributed to the formation of cellular microaggregates.
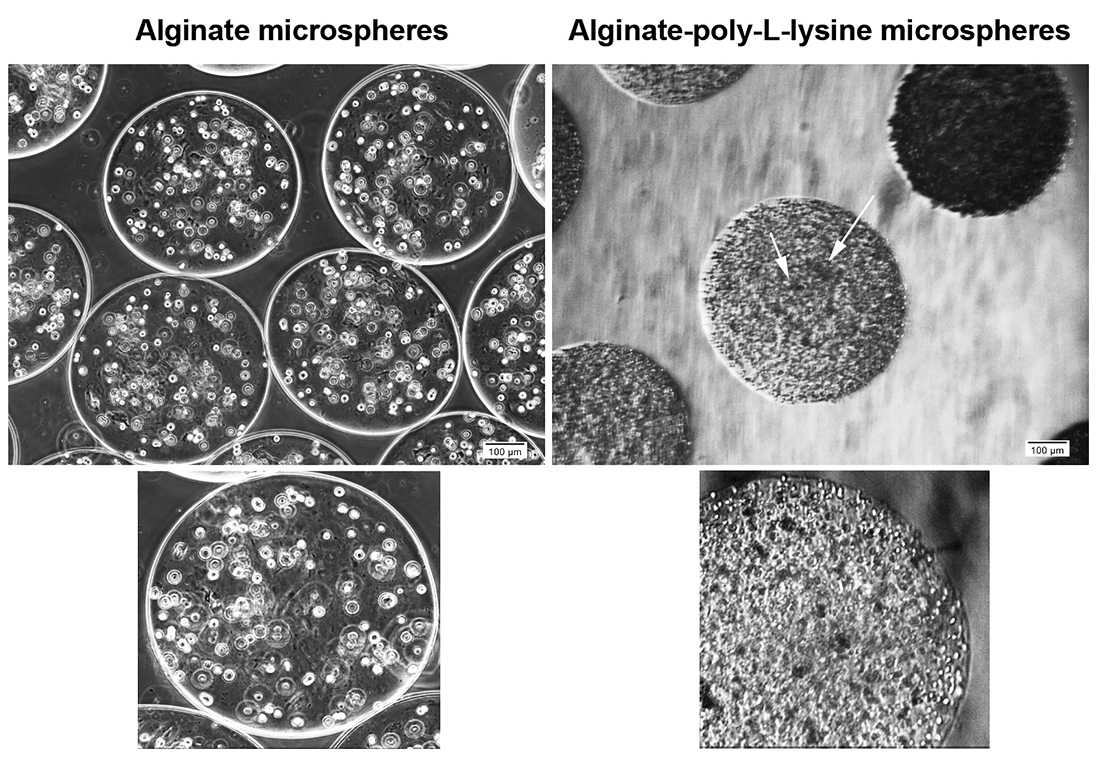
Figure 1.
Bright-field microscopic imaging of HT29 cells after 7-day encapsulation with alginate and alginate-poly-l-lysine. As shown, cells are evenly distributed inside the alginate microspheres while these cells tend to form cellular microaggregates in the matrix of alginate-poly-l-lysine (arrow = cellular microaggregates).
.
Bright-field microscopic imaging of HT29 cells after 7-day encapsulation with alginate and alginate-poly-l-lysine. As shown, cells are evenly distributed inside the alginate microspheres while these cells tend to form cellular microaggregates in the matrix of alginate-poly-l-lysine (arrow = cellular microaggregates).
PLL + Alg encapsulation increased HT-29 cells survival rate
MTT assay showed an increased HT-29 cells survival rate after being encapsulated with Alg alone or Alg+ PLL microspheres compared to the control HT-29 cells expanded on the plastic surface (Figure 2a). Compared to the control, an approximately 3-fold increase was recorded in the survival rate for HT-29 cells inside Alg + PLL microspheres (P < 0.0001) while lower survival rate was evident in Alg group (P < 0.05). It seems that the addition of PLL to Alg backbone had the potential to increase the HT-29 cell survival rate (P < 0.0001; Figure 2a). These data showed that the encapsulation of human adenocarcinoma HT-29 cells with Alg and especially with the combination of Alg + PLL promoted cell survival rate. Based on the previously published data, it seems that the addition of PLL to Alg and development of hallow environment could reduce membrane shell pore size and decrease Alg swelling rate while increasing cell-to-cell interaction in 3D condition.
15
Due to the existence of negative charges in Alg microspheres, the juxtacrine interaction of tumor cells would decrease.
16
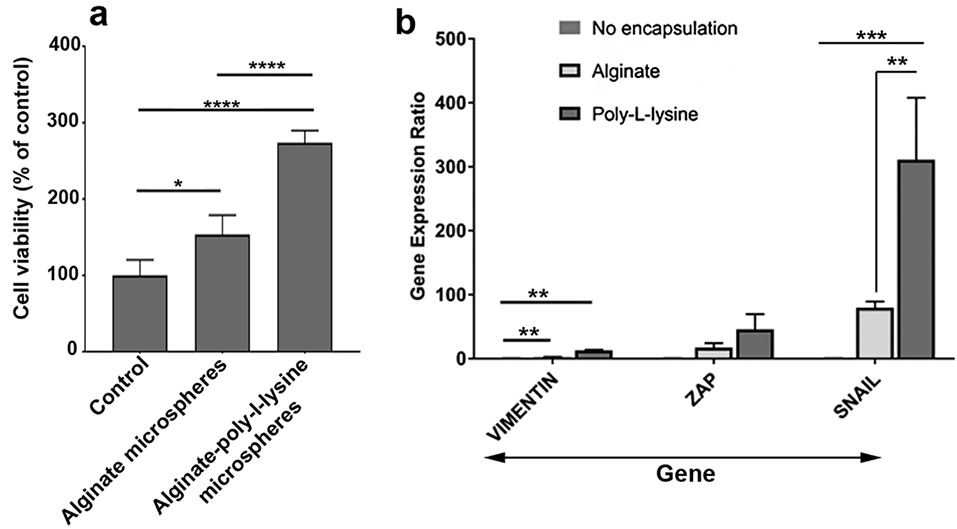
Figure 2.
Measuring cell viability after 7-day encapsulation with alginate and alginate-ploy-l-lysine (a). Compared to the control, the viability of cells was increased inside the alginate and alginate-poly-l-lysine microspheres. Based on the results, the addition of poly-l-lysine to alginate increased cell survival (n = 3). Real-time PCR assay showed the expression of all genes Vimentin, Zeb and Snail compared to the control and alginate group (n = 3). One-way ANOVA and Tukey post hoc analysis. * P < 0.05; ** P < 0.01; *** P < 0.001 and **** P < 0.0001.
.
Measuring cell viability after 7-day encapsulation with alginate and alginate-ploy-l-lysine (a). Compared to the control, the viability of cells was increased inside the alginate and alginate-poly-l-lysine microspheres. Based on the results, the addition of poly-l-lysine to alginate increased cell survival (n = 3). Real-time PCR assay showed the expression of all genes Vimentin, Zeb and Snail compared to the control and alginate group (n = 3). One-way ANOVA and Tukey post hoc analysis. * P < 0.05; ** P < 0.01; *** P < 0.001 and **** P < 0.0001.
Microencapsulation increased EMT rate
To monitor the potency of encapsulation on EMT and stemness acquisition, we also performed real-time PCR analysis.
17
Data showed that the expression of three genes Snail, Vimentin and Zeb were increased 7 days after encapsulation of HT-29 cells with Alg and PLL compared to the control cells expanded on the plastic surface (P < 0.05; Figure 2b). Of note, the use of Alg microspheres loaded with PLL could significantly up-regulate all genes (Figure 2b). These genes participate in the EMT process. Consistent with these results, one could hypothesize that the development of a 3D niche consisted of distinct peptides such as PLL could induce the stemness feature and multipotentiality in cancer cells. Consistent with our result, it was demonstrated that soluble PLL could induce necrosis and apoptosis in tumor cells by recruiting monocytes and inflammatory cells. Various facts highlighted the emergence of CSCs in response to an insulting condition such as immune-related activity.
18
Physical contact is essential for cells bioactivities such as proliferation, cell migration, and differentiation.
19
These events were promptly facilitated by neighboring scaffolds.
20
Even, the type of ECM participates in the regulation of cell expansion, migration, and survival. The entity of ECM could activate mechanical receptors on the surface of cancer cells which further activates downstream signaling pathways.
21
In this regard, due to the existence of cationic amino acid, profound cellular connectivity and cellular aggregation are provided by PLL.
22
Cellular adhesion was also stimulated via the strong electrostatic bond between positively charged PLL and the negative charge of cell surface.
23
Qi et al previously showed that the combination of PLL with graphene oxide not only could increase the proliferation of mesenchymal stem cells but also dictated trans-differentiation into osteoblast-like cells.
24
The stimulatory effect of PLL could be related in part to its physicochemical activity. It is postulated that the existence of positive charges on the PLL generates an electrostatic interaction with negative elements of the cell membrane.
25
In support of this statement, in vitro culture of hematopoietic stems cells on PLL substrate increased the number of cells entering S phase.
25
Conclusion
Based on the entity and composition of ECM, the phenotype of tumor cells could be changed in the 3D milieu. Therefore, the use of Alg-based components could be advised to the promotion of specific and distinct cell type in in vitro and in vivo milieu.
26
The current experiment highlighted the stimulatory effect of PLL on EMT and phenotype shifting.
Ethical Issues
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
We would like to thank the personnel of the Stem Cell Research Center for guidance and help. This work was financially supported by a grant from Tabriz University of Medical Sciences.
References
- Kim M-C, Silberberg YR, Abeyaratne R, Kamm RD, Asada HH. Computational modeling of three-dimensional ecm-rigidity sensing to guide directed cell migration. Proc Natl Acad Sci USA 2018; 115(3):E390-9. doi: 10.1073/pnas.1717230115 [Crossref] [ Google Scholar]
- van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018; 19(4):213. doi: 10.1038/nrm.2017.125 [Crossref] [ Google Scholar]
- Schwager SC, Taufalele PV, Reinhart-King CA. Cell–cell mechanical communication in cancer. Cell Mol Bioeng 2019; 12(1):1-14. doi: 10.1007/s12195-018-00564-x [Crossref] [ Google Scholar]
- Otsuki Y, Saya H, Arima Y. Prospects for new lung cancer treatments that target emt signaling. Dev Dyn 2018; 247(3):462-72. doi: 10.1002/dvdy.24596 [Crossref] [ Google Scholar]
- Degirmenci B, Hausmann G, Valenta T, Basler K. Wnt ligands as a part of the stem cell niche in the intestine and the liver. Prog Mol Biol Transl Sci 2018; 153:1-19. doi: 10.1016/bs.pmbts.2017.11.011 [Crossref] [ Google Scholar]
- Heng WS, Gosens R, Kruyt FA. Lung cancer stem cells: Origin, features, maintenance mechanisms and therapeutic targeting. Biochem Pharmacol 2019; 160:121-33. doi: 10.1016/j.bcp.2018.12.010 [Crossref] [ Google Scholar]
- Liaw CY, Ji S, Guvendiren M. Engineering 3d hydrogels for personalized in vitro human tissue models. Adv Healthc Mater 2018; 7(4):1701165. doi: 10.1002/adhm.201701165 [Crossref] [ Google Scholar]
- Yuan Y, Shi X, Gan Z, Wang F. Modification of porous plga microspheres by poly-l-lysine for use as tissue engineering scaffolds. Colloids Surf B Biointerfaces 2018; 161(1):162-8. doi: 10.1016/j.colsurfb.2017.10.044 [Crossref] [ Google Scholar]
-
Ma M, Anderson DG, Langer RS, Veiseh O, Doloff JC, Chen D, et al. Multi-layer hydrogel capsules for encapsulation of cells and cell aggregates. US Patent. 14/776, 639; 2019.
- Huang J, Zhuang W, Ge L, Wang K, Wang Z, Niu H. Improving biocatalytic microenvironment with biocompatible ε-poly-l-lysine for one step gluconic acid production in low ph enzymatic systems. Process Biochem 2019; 76:118-127. doi: 10.1016/j.procbio.2018.10.018 [Crossref] [ Google Scholar]
- Habib A, Sathish V, Mallik S, Khoda B. 3d printability of alginate-carboxymethyl cellulose hydrogel. Materials 2018; 11(3):454. doi: 10.3390/ma11030454 [Crossref] [ Google Scholar]
- Krstic M, Kolendowski B, Cecchini MJ, Postenka CO, Hassan HM, Andrews J. Tbx3 promotes progression of pre‐invasive breast cancer cells by inducing emt and directly up‐regulating slug. J Pathol 2019; 248(2):191-203. doi: 10.1002/path.5245 [Crossref] [ Google Scholar]
- Bates RC. Colorectal cancer progression: Integrin alphavbeta6 and the epithelial-mesenchymal transition (emt). Cell Cycle 2005; 4(10):1350-2. doi: 10.4161/cc.4.10.2053 [Crossref] [ Google Scholar]
- Jain A, Hasan J, Desingu PA, Sundaresan NR, Chatterjee K. Engineering an in vitro organotypic model for studying cardiac hypertrophy. Colloids Surf B Biointerfaces 2018; 165:355-62. doi: 10.1016/j.colsurfb.2018.02.036 [Crossref] [ Google Scholar]
- Cañibano-Hernández A, Saenz del Burgo L, Espona-Noguera A, Orive G, Hernández RM, Ciriza JS. Alginate microcapsules incorporating hyaluronic acid recreate closer in vivo environment for mesenchymal stem cells. Mol Pharm 2017; 14(7):2390-9. doi: 10.1021/acs.molpharmaceut.7b00295 [Crossref] [ Google Scholar]
- Kim BJ, Cho H, Park JH, Mano JF, Choi IS. Strategic advances in formation of cell‐in‐shell structures: From syntheses to applications. Adv Mater 2018; 30(14):1706063. doi: 10.1002/adma.201706063 [Crossref] [ Google Scholar]
- Turini S, Bergandi L, Gazzano E, Prato M, Aldieri E. Epithelial to mesenchymal transition in human mesothelial cells exposed to asbestos fibers: Role of tgf-β as mediator of malignant mesothelioma development or metastasis via emt event. Int J Mol Sci 2019; 20(1):150. doi: 10.3390/ijms20010150 [Crossref] [ Google Scholar]
- Musetti S, Huang L. Nanoparticle-mediated remodeling of the tumor microenvironment to enhance immunotherapy. ACS Nano 2018; 12(12):11740-55. doi: 10.1021/acscentsci.8b00812 [Crossref] [ Google Scholar]
- Witjas FM, van den Berg BM, van den Berg CW, Engelse MA, Rabelink TJ. Concise review: The endothelial cell extracellular matrix regulates tissue homeostasis and repair. Stem Cells Transl Med 2019; 8(4):375-382. doi: 10.1002/sctm.18-0155 [Crossref] [ Google Scholar]
- Hozumi T, Kageyama T, Ohta S, Fukuda J, Ito T. Injectable hydrogel with slow degradability composed of gelatin and hyaluronic acid cross-linked by schiff’s base formation. Biomacromolecules 2018; 19(2):288-97. doi: 10.1021/acs.biomac.7b01133 [Crossref] [ Google Scholar]
- Northcott JM, Dean IS, Mouw JK, Weaver VM. Feeling stress: the mechanics of cancer progression and aggression. Front Cell Dev Biol 2018; 6:17. doi: 10.3389/fcell.2018.00017 [Crossref] [ Google Scholar]
- Lih E, Park W, Park KW, Chun SY, Kim H, Joung YK. A bioinspired scaffold with anti-inflammatory magnesium hydroxide and decellularized extracellular matrix for renal tissue regeneration. ACS Cent Sci 2019; 5(3):458-67. doi: 10.1021/acscentsci.8b00812 [Crossref] [ Google Scholar]
- Ren KF, Hu M, Zhang H, Li BC, Lei WX, Chen JY. Layer-by-layer assembly as a robust method to construct extracellular matrix mimic surfaces to modulate cell behavior. Prog Polym Sci 2019; 92:1-34. doi: 10.1016/j.progpolymsci.2019.02.004 [Crossref] [ Google Scholar]
- Qi W, Zhang X, Wang H. Self-assembled polymer nanocomposites for biomedical application. Curr Opin Colloid Interface Sci 2018; 35:36-41. doi: 10.1016/j.cocis.2018.01.003 [Crossref] [ Google Scholar]
- Park K-S, Ahn J, Kim JY, Park H, Kim HO, Lee SH. Poly-l-lysine increases the ex vivo expansion and erythroid differentiation of human hematopoietic stem cells, as well as erythroid enucleation efficacy. Tissue Eng Part A 2014; 20:1072-80. doi: 10.1089/ten.TEA.2013.0193 [Crossref] [ Google Scholar]
- Nemati S, Rezabakhsh A, Khoshfetrat AB, Nourazarian A, Biray Avci Ç, Goker Bagca B. Alginate-gelatin encapsulation of human endothelial cells promoted angiogenesis in in vivo and in vitro milieu. Biotechnol Bioeng 2017; 114(12):2920-30. doi: 10.1002/bit.26395 [Crossref] [ Google Scholar]