Advanced pharmaceutical bulletin. 10(1):13-19.
doi: 10.15171/apb.2020.002
Mini Review
An Overview of the Cellular Mechanisms of Flavonoids Radioprotective Effects
Mahdi Mashhadi Akbar Boojar 1, 2, * 
Author information:
1Student Research Committee, Baqiyatallah University of Medical Sciences, Tehran, Iran.
2Department of Pharmacology and Toxicology, Faculty of Pharmacy, Baqiyatallah University of Medical Sciences, Tehran, Iran.
*
Corresponding Author: Mahdi Mashhadi Akbar Boojar, Tel: +98 21 82455414, Fax: +98 21 82455396, Email:
mahdimashhadi@yahoo.com
Abstract
Considering the remarkable application of radiotherapy in the treatment and diagnosis of various diseases and even nuclear war, it is important to protect healthy tissues and people at risk from the radiation. Currently, there is no ideal and safe radioprotective agent available and we are seeing a great effort to find these agents from natural sources. Phenolic compounds, as well as flavonoid, are presented widely as the second metabolite in plants and they have been considered for investigation according to their benefits for human health, healing and preventing many disorders. The major bioactive benefits of flavonoids include antioxidant, anti-inflammatory, anti-tumor, anti-aging, anti-bacterial and viral, neuroprotection and radioprotective effects. Their lower toxicity and oral administration have made it suitable for radiotherapy patient, radiation, military forces, and even the general public. This review attempts to provide a summary of the main molecular mechanisms involved in flavonoid radio-protective effects. Data of these studies will provide a comprehensive perspective to flavonoids and can help to optimize their effects in radioprotection procedures.
Keywords: Ionizing radiation, Radio-protector, Flavonoids
Copyright and License Information
© 2020 The Author (s)
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
During recent years, along with the development of science and technology, the ionizing radiations are widely used in various fields, including medical diagnosis, therapeutic interventions, and agricultural and industrial sciences.
1
Hence, humans have been exposed to high levels of radiations in comparison to the past.
2
In addition, increasing the likelihood of terrorist attacks on power plants and reactors and even unintentional incidents and accidental leakage of radioactive substances have always been a real concern.
3
Radiation therapy is one of the most common and alternative surgical methods used to treatment of cancer alone or in combination with chemotherapy. Healthy tissue damage and the risk of developing new malignancies are one of the major challenges of radiotherapy that can be prevented by means of radiation protection (or radiological protection) and increased resistance of natural cells to the event.
4,5
Ionizing radiation beams are responsible for the excessive generation of free radicals in the human body and these radicals play a serious role in the pathogenesis of various diseases, as they are capable of inducing damage to cellular structures and DNA, oxidizing proteins, or inducing peroxidation of lipids.
6
These destructive effects can lead to protein dysfunction in the hematopoietic system and immune cells, accelerated aging and expanded cellular degeneration.
7
Therefore, the importance of protecting human beings from radiation in radiotherapy and individuals at risk, especially the military, has increased.
8
Flavonoids are a group of polyphenolic compounds that so far have been identified more than 9000 of them in different species in many plants.
9
Since the early 1920s, the bioavailability and other biological effects of flavonoid derivatives have been noted, including the elimination of free radicals, increased resistance to beams, reduced inflammation, anti-tumor effects, and decreased senescence process of cells.
10-12
Currently, many studies focus on the effects of radioprotective substances. Therefore, it is necessary to examine the radiation protection mechanisms of flavonoids and their proper use in the prevention of possible damages before and after exposure to radiation hazards.
Materials and Methods
In this review study, related and considerable research papers and review articles of online databases include PubMed, Scopus, Google Scholar and Web of Science which recently published by reliable publishers such as Elsevier, Springer and PLoS One, up to April 2019 were collected and discussed. The literature was searched using the following keywords: Ionizing radiation, Radio-protector, Flavonoids.
The biological damages induced by ionizing radiation
Naturally, ionizing radiation appears in various forms, such as X-ray, alpha, beta, gamma, and neutron.
13
Human exposure to these beams can lead to a series of biochemical and pathological changes and ultimately cause injury or necrosis of the target organ.
14
Current studies show that the damage caused by these beams directly or indirectly affects the human body. Generally, direct damage occurs when large biological molecules in the living organism interact with the radiation physically and acute biological destruction happens.
15
Indirect damage is mainly due to the production of free radicals that are generated from water molecules in the body.
16
The ionizing radiation leads to the production of free radicals such as hydroxyl and superoxide radicals that can interfere with the body’s biological macro-molecules and cause significant changes in their structure and function, which, in turn, causes serious injuries in response to the beams.
17
Damage to the DNA and the immune and hematopoietic systems are the major injuries that the ionizing radiation brings to the living organism. Damage to cellular DNA is involved in the loss of biological information and degradation of normal cellular function, which can consequently lead to the death of cells or escape from the regulating mechanisms of the cell cycle and finally development of cancer.
18
Immune system and hematopoietic cells are highly susceptible to ionizing radiation. Ionizing radiation can reduce the number of immune cells and weaken their specific and non-specific functions.
19
In the hematopoietic system, these beams can also reduce red blood cells during the development of bone marrow suppression.
18,20
Classification of flavonoids
Flavonoids are the major group of polyphenolic compounds with phenyl chromium backbone. These compounds generally have two aromatic rings having a phenolic hydroxyl substituent (ring A and B) attached to the carbon moiety.
21
They are containing 15 carbon atoms forming a C6C3C6 structure shown in Figure 1.
22
These compounds are found extensively in the stem or trunk and also in fruits and generally in the form of secondary metabolites in plants.
23
Flavonoids are classified according to the chemical features of carbon bonds and the number and position of hydroxyl groups in more than 10 different categories, among which flavones, flavonones, flavonols, flavononols, isoflavones, flavanoles (catechins) and anthocyanidins are more important than others, and in continuation will be discussed.
24
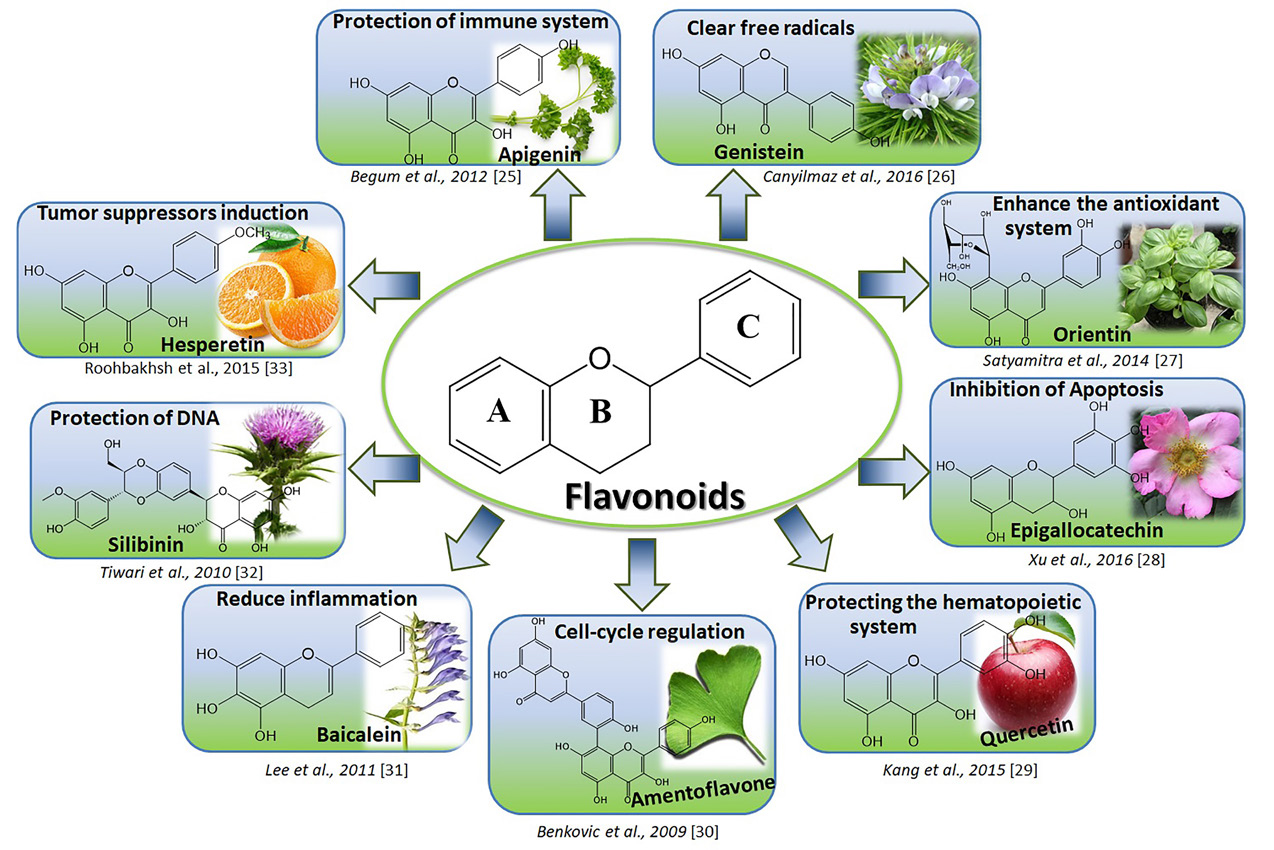
Figure 1.
The backbone of the flavonoids chemical structure and the main molecular mechanisms in the radiation protection effects of nine well-known flavonoids.
25-33
.
The backbone of the flavonoids chemical structure and the main molecular mechanisms in the radiation protection effects of nine well-known flavonoids.
25-33
Cellular and molecular mechanisms of flavonoids in radio-protection
Natural radio-protectives are non-toxic or low toxic products obtained from natural compounds that can be used before or after exposure to ionizing radiation to reduce radiation damage.
34
The usefulness of flavonoids in various cases, such as cytoprotective properties, antioxidant, and free radical scavenging effects, antiviral and antibacterial effects, anti-tumor properties in the prevention and treatment of cancer, and their anti-inflammatory effects have been proven in various cellular and animal studies.
35-37
For example, administration of different flavonoids in mice has significantly increased the survival of 30 days and decreased apoptosis-necrosis induced by ionizing radiation.
38,39
In general, the main mechanisms of radiation protection for flavonoids include DNA protection, antioxidant activity, immune and hematopoietic systems protection, and inflammation reduction, which are discussed below.
Prevention of DNA damage and genotoxicity
DNA is a major target of ionizing radiation. Secondary DNA damage due to the exposure to ionizing radiation can cause many harmful effects on human life. Therefore, reducing the destructive effects of ionizing radiation on DNA is an important problem in protecting the body from ionizing radiation.
40
Alkaline comet assay to detect the effects of Baicalein on the radiation exposure induced DNA damage showed that the administration of this flavonoid would have a significant protective effect before exposure to the radiation.
41
Micronucleus assay in rat blood cells exposed to radiation also indicated a reduction in damage to the bone marrow. It has been shown that treating mice lymphocytes exposed to 3 Gy of ionizing radiation with Silibinin reduces DNA damage and microcrystalline formation. Oral administration of Silibinin to mice before the exposure of the animal body to radiation (7.5 Gy), significantly attenuated the death caused by these beams and also ameliorated the blood cells injuries.
32
The information obtained from the measurement of cytokinesis-block micronucleus in assessing of Apigenin radio-protector effects on human lymphocytes suggests a reduction in the injuries to the chromosome from ionizing radiation.
25
This flavonoid significantly reduces the rate of micronuclei (MN) formation in a dose-dependent manner.
Xu Ping and colleagues assessed the radiation protection effects of the Guipi pill flavonoids in mice and showed that these flavonoids increased significantly the thirty-day survival rate by administration eight days prior to exposure to ionizing radiation of at least 8% in these animals.
42
It has been shown that these flavonoids increase the number of white blood cells and protect bone marrow DNA content.
43
In addition, flavonoids from the families Ocimum (orientin), naringin, procyanidin, the isolated flavonoids from propolis, and Gentianella flavonoids can also effectively reduce the genetic toxicity of the beams and protect DNA from the radiation damage.
30,44-46
Free radical clearing ability and antioxidant effects
Ionizing radiations can produce a large number of free radicals by affecting the aquatic environment of living organisms. These radicals can reduce the activity of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT), causing lipid peroxidation and an increase in malondialdehyde (MDA) index, which have great potential for damaging the cell membrane and DNA content.
6,41
Flavonoids can effectively clear oxygen-derived free radicals and remove the indirect effects of ionizing radiations on cells in the human body.
47
In many animal studies, these effects have been able to diminish the deaths from radiation received.
48
It has been shown that Humuluslupulus flavonoids can induce the activity of SOD, GSH-Px, and CAT and reduce the MDA content in mice exposed to radiation. These flavonoids can also increase the number of blood leukocytes and show a protective effect on the immune system of the mice.
49
A comparative study of the human keratinocyte cell line HaCaT indicated the high ability of quercetin and the next genistein to purify the free radicals of hydroxyl, induced by UVB rays. In addition, the treatment of the cells with the flavonoids mentioned above before contact with the radiation increased the activity of SOD and reduced the levels of tumor necrosis factor (TNFα), reactive oxygen species (ROS) and MDA.
50
In a similar study, a significant increase in total antioxidant capacity and elevated SOD activity in the PC12 clone, developed from a pheochromocytoma tumor of the rat adrenal medulla treated with quercetin was quite significant compared to control cells.
51
It has been demonstrated that the breviscapine flavonoids collection (derived from Chinese herb Erigeron breviscapus) effectively neutralizes radiated free radicals and subsequently increases the total intracellular antioxidant capacity by attenuation of lipid peroxidation, thereby contributing to the cytoprotective role from ionizing radiation.
52
Ping et al evaluated the effects of amentoflavone radiation protection by measuring cell viability, apoptosis, and ROS levels after exposure to 8 Gy gamma rays from
60
Co in guinea pig pulmonary fibroblast cells. This study confirmed that treatment with this flavonoid in the 24-hour period before exposure significantly inhibited apoptosis and reduced levels of ROS.
39
Also, another similar study showed that administration of 200 mg/kg of genistein at one hour prior to X-ray exposure improved bone marrow function by up to 44% and elevated the 30-day life expectancy index in mice.
53
Protective effects on the immune system
The immune system is extremely sensitive to harmful radiation. Exposure to ionizing radiation can lead to functional impairment of the immune system and even death resulting from a decrease in the number of immune cells. Also, ionizing radiation can cause problems in producing antibodies and impair the regulation of cytokine network.
19
Among bio-flavonoids, isoflavones, which are often found in Fabaceae (i.e., Leguminosae, or bean) family, have a greater role in protecting the immune system against ionizing radiation.
43
It has been shown that treatment of mice with only 4 Gy to ionizing radiation is sufficient to reduce the immune function of the thymus and the spleen. Mice receiving soy isoflavones significantly improved lymphocyte function index, decreased apoptosis, reduced cells in the G0 or G1 phase and increased the presence of cells in G2 and post-irradiation mitotic cells. In addition, a significant increase in the macrophage recognition and phagocytic ability and serum levels of immunoglobulin, IgA, IgG, and IgM were also observed.
54
The flavonoids from buckwheat (especially Tartary buckwheat) also have the same effects on the protection of immune cells, especially T lymphocytes.
43
The literature review reveals that quercetin, apigenin, hesperidin, and rutin can significantly enhance lymphocyte proliferation and secretion of key immune system cytokines and remarkably reduce the damage to peripheral blood lymphocytes.
55-58
It has been demonstrated that administrating resveratrol to workers who work in an environment with a high potential for electromagnetic hazards can help to reconstruct the expression and function of the nuclear factor-kappa B (NF-kappa B), IL-6, and ultimately prevent the deterioration of the immune system.
59
Protective effects on the hematopoietic system
The human hematopoietic system cells are highly sensitive to radiation due to the high volume of proliferation and cell division. Ionizing radiations, target all bone marrow stem cells.
60
Hence, protecting hematopoietic stem cells is an important factor in preventing radiation damages. There have been several new findings demonstrate that flavonoids can protect bloodstream organs from radiation damage and improve their repair to increase body resistance to radiation injuries.
61
Synthetic derivatives of tetrahydroxyflavone (such as fisetin) have been shown to possess a more potent biological effect than their natural source. It has been proved that the treatment of mice exposed to gamma rays from
60
Co with these flavonoids, largely contributes to the regeneration and function improvement of hematopoiesis.
62
Previous studies have shown similar effects of quercetin, genistein, and soluble derivatives in propolis to prevent the reduction of white and red blood cells, platelets and hemoglobin, as well as damage to leukocyte DNA from ionizing radiation.
63-65
The pivotal part of these effects seems to be due to increased production of granulocyte colony-stimulating factors as a key factor in the recovery of stem cells.
43
It has been confirmed that administration of genistein nanoparticles suspensions in mice reduces the death of hematopoietic stem cells from 43% to 77% and decreases suppression of pro-inflammatory factors such as IL-6 and COX-2 in mice bone marrow and spleen cells.
43
Reducing effects on inflammation
Major radiation exposure can lead to severe inflammatory reactions. Inflammatory cytokines can cause damage or necrosis in any tissues of the body. Such injuries are more common in the lung and kidneys.
66
Soybean isoflavones have promising protective effects both before and after exposure to radiation in the lung tissue by reducing the infiltration of macrophages and neutrophils in alveolar and bronchial surfaces and ultimately reducing fibrosis.
67
Oral administration of Astragalus complanatus plant flavonoids after exposing mice to 10 Gy resulted in a significant reduction in the serum transforming growth factor β, TNF-α and IL-6 levels, and consequently reduced inflammatory damage in the exposed mice.
68
Baicalein, as the most active flavonoid in Paeonia lactiflora (Chinese peony or common garden peony), can suppress inflammatory responses from radiation by modulating the NF-kB and increasing the activation of FOXO transcription factors, CAT and SOD in mouse kidney. In addition, this compound inhibits the phosphorylation of mitogen-activated protein kinase (MAPK) and Akt induced by the beam, that amplify NF-kB kinases.
31
It has recently been shown that administration of quercetin and hesperidin in mice can significantly prevent intestinal damage by decreasing TNF-α levels and enhancing IL-10.
69
Flavonoids and apoptosis
In the most cellular and molecular studies of cancerous cells, flavonoids have been characterized as an apoptosis inducer by various mechanisms.
36
Some pro-apoptotic pathways such as BAD, BID, Bax, caspases 3, 8, and 9, tumor suppressors including p53, cell-cycle inhibitors such as some cyclin-dependent kinases and ceramide activator cascades and its messengers in tumor cells have been enhanced by flavonoids.
70-72,10
In contrast to the inhibitors of apoptosis, such as phosphoinositide-3-kinase, along with protein kinase B, MAPK, metalloproteinases 2 and 9, and many growth factors that promote tumor cell proliferation and differentiation, were suppressed by flavonoids.
73-75
However, the effects of flavonoids in healthy and non-cancerous cells have been largely inconsistent with tumor cells. The protective effects of flavonoids on neural cells, liver, kidney, heart, skin and immune and hematopoietic systems have been discussed in numerous investigations.
76-79
Flavonoids distinctions between these two groups of cells have created a great promise in applying them for radioprotection and other beneficial effects.
Conclusion
According to the previous studies in this field, flavonoids as natural radio-protector in radiotherapy applications for the protection of healthy cells, unexpected radiation accidents and also in terrorist incidents can be effective for reducing the harmful effects of radiations. Protecting the DNA, immune and hematopoietic systems, clearing free radicals, strengthening the immune response and antioxidant properties of flavonoids, justify these abilities. Although generally, these compounds in mild doses are often not toxic to healthy cells, they have considerable toxicity on tumor cells by induction of programmed cell death. If future clinical trials confirm the radio-sensitizer effect of these compounds to the malignant tissues as well as the protective properties of radiation on healthy tissues, they can also be used as a radio-protector for safety and efficiency in advanced radiation therapy.
Ethical Issues
Not applicable.
Conflict of Interest
None.
References
- Varghese JJ, Hansen ME, Benoit D, Ovitt C. A historical review of salivary gland radioprotection. Int J Radiat Oncol Biol Phys 2017; 99(2):E129-30. doi: 10.1016/j.ijrobp.2017.06.909 [Crossref] [ Google Scholar]
- Tang Q, Zhao F, Yu X, Wu L, Lu Z, Yan S. The role of radioprotective spacers in clinical practice: a review. Quant Imaging Med Surg 2018; 8(5):514-24. doi: 10.21037/qims.2018.06.06 [Crossref] [ Google Scholar]
- Baniyaghoobi F, Aliyari SH, Sharififar S, Pishgooie AH. Radiation accidents and how to deal with it. Military Caring Sciences Journal 2014; 1(1):43-51. doi: 10.18869/acadpub.mcs.1.1.43 [Crossref] [ Google Scholar]
- Wallis CJ, Cheung P, Herschorn S, Saskin R, Su J, Klotz LH. Complications following surgery with or without radiotherapy or radiotherapy alone for prostate cancer. Br J Cancer 2015; 112(6):977-82. doi: 10.1038/bjc.2015.54 [Crossref] [ Google Scholar]
- Johnke RM, Sattler JA, Allison RR. Radioprotective agents for radiation therapy: future trends. Future Oncol 2014; 10(15):2345-57. doi: 10.2217/fon.14.175 [Crossref] [ Google Scholar]
- Poprac P, Jomova K, Simunkova M, Kollar V, Rhodes CJ, Valko M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol Sci 2017; 38(7):592-607. doi: 10.1016/j.tips.2017.04.005 [Crossref] [ Google Scholar]
- Sen S, Chakraborty R, Sridhar C, Reddy YS, De B. Free radicals, antioxidants, diseases and phytomedicines: current status and future prospect. Int J Pharm Sci Rev Res 2010; 3(1):91-100. [ Google Scholar]
- Greenberger JS. Radioprotection. In Vivo 2009; 23(2):323-36. [ Google Scholar]
- Xiao ZP, Peng ZY, Peng MJ, Yan WB, Ouyang YZ, Zhu HL. Flavonoids health benefits and their molecular mechanism. Mini Rev Med Chem 2011; 11(2):169-77. doi: 10.2174/138955711794519546 [Crossref] [ Google Scholar]
- Mashhadi Akbar Boojar M, Hassanipour M, Ejtemaei Mehr S, Mashhadi Akbar Boojar M, Dehpour AR. New aspects of silibinin stereoisomers and their 3-O-galloyl derivatives on cytotoxicity and ceramide metabolism in Hep G2 hepatocarcinoma cell line. Iran J Pharm Res 2016; 15(3):421-33. [ Google Scholar]
- Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci 2016; 5:e47. doi: 10.1017/jns.2016.41 [Crossref] [ Google Scholar]
- Banjarnahor SD, Artanti N. Antioxidant properties of flavonoids. Med J Indones 2015; 23(4):239-44. doi: 10.13181/mji.v23i4.1015 [Crossref] [ Google Scholar]
- Flakus FN. Detecting and Measuring Ionizing Radiation- A Short History. IAEA bulletin 1982; 23(4):31-6. [ Google Scholar]
- Bréchignac F, Oughton D, Mays C, Barnthouse L, Beasley JC, Bonisoli-Alquati A. Addressing ecological effects of radiation on populations and ecosystems to improve protection of the environment against radiation: Agreed statements from a Consensus Symposium. J Environ Radioact 2016; 158-159:21-9. doi: 10.1016/j.jenvrad.2016.03.021 [Crossref] [ Google Scholar]
- Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem 2015; 30(1):11-26. doi: 10.1007/s12291-014-0446-0 [Crossref] [ Google Scholar]
- Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett 2012; 327(1-2):48-60. doi: 10.1016/j.canlet.2011.12.012 [Crossref] [ Google Scholar]
- Sankaranarayanan K. Estimation of the genetic risks of exposure to ionizing radiation in humans: current status and emerging perspectives. J Radiat Res 2006; 47 Suppl B:B57-66. doi: 10.1269/jrr.47.b57 [Crossref] [ Google Scholar]
- Zhang XD, Ma C, Sun X, Zhang K. Research progress of natural drug antiradiation effect. Chinese Journal of Radiological Health 2004; 13:228-30. [ Google Scholar]
- Norval M. Effects of solar radiation on the human immune system. J Photochem Photobiol B 2001; 63(1-3):28-40. doi: 10.1016/s1011-1344(01)00200-7 [Crossref] [ Google Scholar]
- Gläser K, Rohland M, Kleine-Ostmann T, Schrader T, Stopper H, Hintzsche H. Effect of radiofrequency radiation on human hematopoietic stem cells. Radiat Res 2016; 186(5):455-65. doi: 10.1667/rr14405.1 [Crossref] [ Google Scholar]
- Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010; 2(12):1231-46. doi: 10.3390/nu2121231 [Crossref] [ Google Scholar]
- Ghasemzadeh A, Ghasemzadeh N. Flavonoids and phenolic acids: role and biochemical activity in plants and human. J Med Plant Res 2011; 5(31):6697-703. doi: 10.5897/JMPR11.1404 [Crossref] [ Google Scholar]
- Williams CA, Grayer RJ. Anthocyanins and other flavonoids. Nat Prod Rep 2004; 21(4):539-73. doi: 10.1039/b311404j [Crossref] [ Google Scholar]
- Brodowska KM. Natural flavonoids: classification, potential role, and application of flavonoid analogues. Eur J Biol Res 2017; 7(2):108-23. [ Google Scholar]
- Begum N, Prasad NR, Kanimozhi G, Hasan AQ. Apigenin ameliorates gamma radiation-induced cytogenetic alterations in cultured human blood lymphocytes. Mutat Res 2012; 747(1):71-6. doi: 10.1016/j.mrgentox.2012.04.001 [Crossref] [ Google Scholar]
- Canyilmaz E, Uslu GH, Bahat Z, Kandaz M, Mungan S, Haciislamoglu E. Comparison of the effects of melatonin and genistein on radiation-induced nephrotoxicity: results of an experimental study. Biomed Rep 2016; 4(1):45-50. doi: 10.3892/br.2015.547 [Crossref] [ Google Scholar]
- Satyamitra M, Mantena S, Nair CK, Chandna S, Dwarakanath BS, Uma Devi P. The antioxidant flavonoids, orientin and vicenin enhance repair of radiation-induced damage. SAJ Pharmacy and Pharmacology 2014; 1(1):1-9. doi: 10.18875/2375-2262.1.105 [Crossref] [ Google Scholar]
- Xu P, Cai X, Zhang W, Li Y, Qiu P, Lu D. Flavonoids of Rosa roxburghii Tratt exhibit radioprotection and anti-apoptosis properties via the Bcl-2(Ca(2+))/Caspase-3/PARP-1 pathway. Apoptosis 2016; 21(10):1125-43. doi: 10.1007/s10495-016-1270-1 [Crossref] [ Google Scholar]
- Kang J, Yoon SH, Rho JK, Choi DS, Jang BS, Park SH. Radioprotective effect of quercetin post-treatment against γ-irradiation-induced hepatocellular and hematopoiectic system damage in mice. J Korean Soc Food Sci Nutr 2015; 44(7):970-4. doi: 10.3746/jkfn.2015.44.7.970 [Crossref] [ Google Scholar]
- Benkovic V, Knezevic AH, Orsolic N, Basic I, Ramic S, Viculin T. Evaluation of radioprotective effects of propolis and its flavonoid constituents: in vitro study on human white blood cells. Phytother Res 2009; 23(8):1159-68. doi: 10.1002/ptr.2774 [Crossref] [ Google Scholar]
- Lee EK, Kim JM, Choi J, Jung KJ, Kim DH, Chung SW. Modulation of NF-kappaB and FOXOs by baicalein attenuates the radiation-induced inflammatory process in mouse kidney. Free Radic Res 2011; 45(5):507-17. doi: 10.3109/10715762.2011.555479 [Crossref] [ Google Scholar]
- Tiwari P, Kumar A, Ali M, Mishra KP. Radioprotection of plasmid and cellular DNA and Swiss mice by silibinin. Mutat Res 2010; 695(1-2):55-60. doi: 10.1016/j.mrgentox.2009.11.007 [Crossref] [ Google Scholar]
- Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci 2015; 124:64-74. doi: 10.1016/j.lfs.2014.12.030 [Crossref] [ Google Scholar]
- Dobrzynska MM. Resveratrol as promising natural radioprotector A review. Rocz Panstw Zakl Hig 2013; 64(4):255-62. [ Google Scholar]
- Hoensch HP, Oertel R. The value of flavonoids for the human nutrition: short review and perspectives. Clin Nutr Exp 2015; 3:8-14. doi: 10.1016/j.yclnex.2015.09.001 [Crossref] [ Google Scholar]
- Ghasemi S, Lorigooini Z. A review of significant molecular mechanisms of flavonoids in prevention of prostate cancer. J Chem Pharm Sci 2016; 9(4):3388-94. [ Google Scholar]
- Sarbu LG, Bahrin LG, Babii C, Stefan M, Birsa ML. Synthetic flavonoids with antimicrobial activity: a review. J Appl Microbiol 2019; 127(5):1282-90. doi: 10.1111/jam.14271 [Crossref] [ Google Scholar]
- Ping X, Junqing J, Junfeng J, Enjin J. Radioprotective effects of troxerutin against gamma irradiation in V79 cells and mice. Asian Pac J Cancer Prev 2011; 12(10):2593-6. [ Google Scholar]
- Xu P, Zhang WB, Cai XH, Lu DD, He XY, Qiu PY. Flavonoids of Rosa roxburghii Tratt act as radioprotectors. Asian Pac J Cancer Prev 2014; 15(19):8171-5. doi: 10.7314/apjcp.2014.15.19.8171 [Crossref] [ Google Scholar]
- Hall J, Angèle S. Radiation, DNA damage and cancer. Mol Med Today 1999; 5(4):157-64. [ Google Scholar]
- Gandhi NM. Baicalein protects mice against radiation-induced DNA damages and genotoxicity. Mol Cell Biochem 2013; 379(1-2):277-81. doi: 10.1007/s11010-013-1649-z [Crossref] [ Google Scholar]
- Xu P, Jia JQ, Jiang EJ, Kang LP, Wu KL. Protective effect of an extract of Guipi Pill against radiation-induced damage in mice. Chin J Integr Med 2012; 18(7):490-5. doi: 10.1007/s11655-012-1097-8 [Crossref] [ Google Scholar]
- Li YN, Zhang WB, Zhang JH, Xu P, Hao MH. Radioprotective effect and other biological benefits associated with flavonoids. Trop J Pharm Res 2016; 15(5):1099-108. doi: 10.4314/tjpr.v15i5.28 [Crossref] [ Google Scholar]
- Jagetia GC, Venkatesha VA, Reddy TK. Naringin, a citrus flavonone, protects against radiation-induced chromosome damage in mouse bone marrow. Mutagenesis 2003; 18(4):337-43. doi: 10.1093/mutage/geg001 [Crossref] [ Google Scholar]
- Hosseinimehr SJ, Mahmoudzadeh A, Ahmadi A, Mohamadifar S, Akhlaghpoor S. Radioprotective effects of hesperidin against genotoxicity induced by gamma-irradiation in human lymphocytes. Mutagenesis 2009; 24(3):233-5. doi: 10.1093/mutage/gep001 [Crossref] [ Google Scholar]
- Moon HI, Jeong MH, Jo WS. Protective activity of C-geranylflavonoid analogs from Paulownia tomentosa against DNA damage in 137Cs irradiated AHH-1 cells. Nat Prod Commun 2014; 9(9):1295-8. [ Google Scholar]
-
Ma X, Deng D, Chen W. Inhibitors and Activators of SOD, GSH‐Px, and CAT. Enzyme Inhibitors and Activators; 2017. p. 207. doi: 10.5772/65936.
- Gvilava I, Ormotsadze G, Chkhikvishvili I, Giorgobiani M, Kipiani NV, Sanikidze T. [Radioprotective activity of polymetoxy-lated flavonoids of citrus extract]. Georgian Med News 2018(285):119-24.
- Landauer MR, Harvey AJ, Kaytor MD, Day RM. Mechanism and therapeutic window of a genistein nanosuspension to protect against hematopoietic-acute radiation syndrome. J Radiat Res 2019; 60(3):308-17. doi: 10.1093/jrr/rrz014 [Crossref] [ Google Scholar]
- Zhu X, Li N, Wang Y, Ding L, Chen H, Yu Y. Protective effects of quercetin on UVB irradiationinduced cytotoxicity through ROS clearance in keratinocyte cells. Oncol Rep 2017; 37(1):209-18. doi: 10.3892/or.2016.5217 [Crossref] [ Google Scholar]
- Bao D, Wang J, Pang X, Liu H. Protective effect of quercetin against oxidative stress-induced cytotoxicity in rat pheochromocytoma (PC-12) cells. Molecules 2017; 22(7). doi: 10.3390/molecules22071122 [Crossref]
- He X, Long W, Dong H, Wang C, Chu X, Zheng Q. Evaluation of the protective effects of 13 traditional Chinese medicine compounds on ionizing radiation injury: bupleurum, shenmai, and breviscapine as candidate radioprotectors. RSC Adv 2017; 7(37):22640-8. doi: 10.1039/C7RA01108C [Crossref] [ Google Scholar]
- Grebeniuk AN, Basharin VA, Tarumov RA, Aksenova NV, Nazarov VB, Kovtun V. [The experimental evaluation of antiradiation effectiveness of genistein by survival rates and bone marrow hemopoiesis of mice irradiated by X-rays]. Radiats Biol Radioecol 2013; 53(5):468-74. [ Google Scholar]
- Liu L, Jin H, Wang XY, Xu ZQ, Nan WK, Li PB. [Effects of soybean isoflavones on the cell cycles, the cell apoptosis and the proliferation of spleen in radiated mice]. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2006; 22(4):497-500. [ Google Scholar]
- Zbikowska HM, Antosik A, Szejk M, Bijak M, Olejnik AK, Saluk J. Does quercetin protect human red blood cell membranes against gamma-irradiation?. Redox Rep 2014; 19(2):65-71. doi: 10.1179/1351000213y.0000000074 [Crossref] [ Google Scholar]
- Sunada S, Fujisawa H, Cartwright IM, Maeda J, Brents CA, Mizuno K. Monoglucosyl-rutin as a potential radioprotector in mammalian cells. Mol Med Rep 2014; 10(1):10-4. doi: 10.3892/mmr.2014.2181 [Crossref] [ Google Scholar]
- Katoch O, Kaushik S, Kumar MS, Agrawala PK, Misra K. Radioprotective property of an aqueous extract from Valeriana wallichii. J Pharm Bioallied Sci 2012; 4(4):327-32. doi: 10.4103/0975-7406.103272 [Crossref] [ Google Scholar]
- Hien TV, Huong NB, Hung PM, Duc NB. Radioprotective effects of vitexina for breast cancer patients undergoing radiotherapy with cobalt-60. Integr Cancer Ther 2002; 1(1):38-43. doi: 10.1177/153473540200100103 [Crossref] [ Google Scholar]
- Zhang D, Zhang Y, Zhu B, Zhang H, Sun Y, Sun C. Resveratrol may reverse the effects of long-term occupational exposure to electromagnetic fields on workers of a power plant. Oncotarget 2017; 8(29):47497-506. doi: 10.18632/oncotarget.17668 [Crossref] [ Google Scholar]
- Shao L, Luo Y, Zhou D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid Redox Signal 2014; 20(9):1447-62. doi: 10.1089/ars.2013.5635 [Crossref] [ Google Scholar]
- Yahyapour R, Shabeeb D, Cheki M, Musa AE, Farhood B, Rezaeyan A. Radiation protection and mitigation by natural antioxidants and flavonoids: implications to radiotherapy and radiation disasters. Curr Mol Pharmacol 2018; 11(4):285-304. doi: 10.2174/1874467211666180619125653 [Crossref] [ Google Scholar]
- Liu C, Liu J, Hao Y, Gu Y, Yang Z, Li H. 6,7,3’,4’-Tetrahydroxyisoflavone improves the survival of whole-body-irradiated mice via restoration of hematopoietic function. Int J Radiat Biol 2017; 93(8):793-802. doi: 10.1080/09553002.2017.1321808 [Crossref] [ Google Scholar]
- Benković V, Knezević AH, Dikić D, Lisicić D, Orsolić N, Basić I. Radioprotective effects of quercetin and ethanolic extract of propolis in gamma-irradiated mice. Arh Hig Rada Toksikol 2009; 60(2):129-38. doi: 10.2478/10004-1254-60-2009-1908 [Crossref] [ Google Scholar]
- Benković V, Kopjar N, Horvat Knezevic A, Dikić D, Basić I, Ramić S. Evaluation of radioprotective effects of propolis and quercetin on human white blood cells in vitro. Biol Pharm Bull 2008; 31(9):1778-85. doi: 10.1248/bpb.31.1778 [Crossref] [ Google Scholar]
- Song L, Ma L, Cong F, Shen X, Jing P, Ying X. Radioprotective effects of genistein on HL-7702 cells via the inhibition of apoptosis and DNA damage. Cancer Lett 2015; 366(1):100-11. doi: 10.1016/j.canlet.2015.06.008 [Crossref] [ Google Scholar]
- Karkanitsa LV, Komarovskaya ME, Krivenko SI. Abrogation of radiation injury to human hematopoietic stem cells with tumor necrosis factor-α. Stem Cells 1997; 15(S1):95-102. doi: 10.1002/stem.5530150714 [Crossref] [ Google Scholar]
- Abernathy LM, Fountain MD, Rothstein SE, David JM, Yunker CK, Rakowski J. Soy isoflavones promote radioprotection of normal lung tissue by inhibition of radiation-induced activation of macrophages and neutrophils. J Thorac Oncol 2015; 10(12):1703-12. doi: 10.1097/jto.0000000000000677 [Crossref] [ Google Scholar]
- Wang J, Xu HW, Li BS, Zhang J, Cheng J. Preliminary study of protective effects of flavonoids against radiation-induced lung injury in mice. Asian Pac J Cancer Prev 2012; 13(12):6441-6. doi: 10.7314/apjcp.2012.13.12.6441 [Crossref] [ Google Scholar]
- Guven B, Can M, Piskin O, Aydin BG, Karakaya K, Elmas O. Flavonoids protect colon against radiation induced colitis. Regul Toxicol Pharmacol 2019; 104:128-32. doi: 10.1016/j.yrtph.2019.03.006 [Crossref] [ Google Scholar]
- Bevara GB, Naveen Kumar AD, Koteshwaramma KL, Badana A, Kumari S, Malla RR. C-glycosyl flavone from Urginea indica inhibits proliferation & angiogenesis & induces apoptosis via cyclin-dependent kinase 6 in human breast, hepatic & colon cancer cell lines. Indian J Med Res 2018; 147(2):158-68. doi: 10.4103/ijmr.IJMR_51_16 [Crossref] [ Google Scholar]
- Mashhadi Akbar Boojar M, Mashhadi Akbar Boojar M, Golmohammad S, Bahrehbar I. Data on cell survival, apoptosis, ceramide metabolism and oxidative stress in A-494 renal cell carcinoma cell line treated with hesperetin and hesperetin-7-O-acetate. Data Brief 2018; 20:596-601. doi: 10.1016/j.dib.2018.08.065 [Crossref] [ Google Scholar]
- Banjerdpongchai R, Wudtiwai B, Khaw-On P, Rachakhom W, Duangnil N, Kongtawelert P. Hesperidin from Citrus seed induces human hepatocellular carcinoma HepG2 cell apoptosis via both mitochondrial and death receptor pathways. Tumour Biol 2016; 37(1):227-37. doi: 10.1007/s13277-015-3774-7 [Crossref] [ Google Scholar]
- Fernando W, Rupasinghe HP, Hoskin DW. Regulation of hypoxia-inducible factor-1α and vascular endothelial growth factor signaling by plant flavonoids. Mini Rev Med Chem 2015; 15(6):479-89. doi: 10.2174/1389557515666150414152933 [Crossref] [ Google Scholar]
- Yao X, Jiang W, Yu D, Yan Z. Luteolin inhibits proliferation and induces apoptosis of human melanoma cells in vivo and in vitro by suppressing MMP-2 and MMP-9 through the PI3K/AKT pathway. Food Funct 2019; 10(2):703-12. doi: 10.1039/c8fo02013b [Crossref] [ Google Scholar]
- Chae HS, Xu R, Won JY, Chin YW, Yim H. Molecular targets of genistein and its related flavonoids to exert anticancer effects. Int J Mol Sci 2019; 20(10). doi: 10.3390/ijms20102420 [Crossref]
- Miltonprabu S, Tomczyk M, Skalicka-Woźniak K, Rastrelli L, Daglia M, Nabavi SF. Hepatoprotective effect of quercetin: from chemistry to medicine. Food Chem Toxicol 2017; 108(Pt B):365-74. doi: 10.1016/j.fct.2016.08.034 [Crossref] [ Google Scholar]
- Castro-Vazquez L, Alañón ME, Rodríguez-Robledo V, Pérez-Coello MS, Hermosín-Gutierrez I, Díaz-Maroto MC. Bioactive flavonoids, antioxidant behaviour, and cytoprotective effects of dried grapefruit peels (Citrus paradisi Macf). Oxid Med Cell Longev 2016; 2016:8915729. doi: 10.1155/2016/8915729 [Crossref] [ Google Scholar]
- Mashhadi Akbar Boojar M, Mashhadi Akbar Boojar M, Golmohammad S. An overview of the anti-tumor effects of silibinin. Razi Journal of Medical Sciences 2019; 25(12):116-29. [ Google Scholar]
- Mashhadi Akbar Boojar M, Mashhadi Akbar Boojar M, Golmohammad S, Nikkhah Yazdi M. Ceramide generation as a novel biological mechanism for chemo-preventive and cytotoxic effects of hesperidin on HT-144 melanoma cells. Beni-Suef Univ J Basic Appl Sci 2018; 7(4):640-5. doi: 10.1016/j.bjbas.2018.07.008 [Crossref] [ Google Scholar]