Advanced pharmaceutical bulletin. 10(3):379-388.
doi: 10.34172/apb.2020.046
Research Article
Integrated Quality by Design Approach for Developing Nanolipidic Drug Delivery Systems of Olmesartan Medoxomil with Enhanced Antihypertensive Action
Jagdish Kumar Arun 1  , Rajeshwar Vodeti 1, 2, *
, Rajeshwar Vodeti 1, 2, *  , Birendra Shrivastava 1, Vasudha Bakshi 2
, Birendra Shrivastava 1, Vasudha Bakshi 2
Author information:
1School of Pharmaceutical Sciences, Jaipur National University, Jagatpura, Jaipur, Rajasthan-302 017, India.
2School of Pharmacy, ANURAG Group of Institutions, Venkatapur (V), Ghatkesar (M) Medchal (Dist.), Hyderabad, Telangana-500 038, India.
Abstract
Purpose:
The present work endeavors to report a systematic approach of developing the lipidic self-nanoemulsifying formulation of olmesartan medoxomil (OMT) on the principles of Quality by Design (QbD).
Methods: For preparing the self-nanoemulsifying formulation, a mixture of oil, surfactant and cosurfactant were used as vehicles. The excipients were selected after screening by solubility as well as pseudoternary phase titration studies. Mixture design was adopted for systematic optimization of the composition of nanolipidic formulations, which were evaluated for smaller globule size, stable zeta potential and lower values of polydispersity index. The optimized liquid self-nanoemulsifying formulation was identified using numerical and graphical optimization techniques, followed by validation of the experimental model. Solidification of self-nanoemulsifying formulation was carried out using porous carriers, and then optimized on the basis of oil adsorption potential, powder flow property and drug release performance. Pharmacokinetic study was performed in male Wistar rats for evaluating the drug absorption parameters. All the experimental data obtained were subjected to statistical analysis using oneway ANOVA followed by post hoc analysis using Student’s t test.
Results: The optimized liquid self-nanoemulsifying formulation showed globule size <100 nm, emulsification efficiency <5 minutes andin vitro drug release >85% within in 30 minutes. Further, the solid SNEDDS formulation was effectively formulated using Neusilin US2 with maximum oil adsorption capacity and good micromeritic properties. Pharmacokinetic evaluation indicated 4 to 5-folds increase (P <0.05) in the values of Cmax, AUC, and reduction in Tmax from the nanoformulations vis-à-vis the marketed formulation.
Conclusion: Overall, the developed nanolipidic formulation of olmesartan indicated superior efficacy in augmenting the drug dissolution and absorption performance.
Keywords: Bioavailability, Nanoemulsion, Optimization, Experimental design, Pharmacokinetics
Copyright and License Information
© 2020 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Quality by Design (QbD), a concept proposed by American quality expert, J.M. Juran for systematic planning and execution of manufacturing operations for avoiding quality crisis.1,2+ Later, United States Food and Drug Administration (USFDA) adopted the Juran’s concept of QbD into pharmaceutical drug product development. QbD approach provides a platform for systematic development of the drug products with predefined objectives for rational selection of the product and process parameters influencing the end-product quality. Hence, the product development as per QbD paradigm provides enhanced product and process understanding, and robust performance.3-5
Olmesartan medoxomil (OMT), a potential antihypertensive agent, inhibits the synthesis of angiotensin II by blocking the angiotensin type I receptor. Despite its efficacy, it shows poor aqueous solubility and high lipophilicity, as the major rate-limiting factors for low and inconsistent oral bioavailability.6,7 Several literature reports have demonstrated the application different solubility enhancement technologies for improving biopharmaceutical performance of the drug.8-12 However, beyond solubility, many other rate-limiting factors tend to affect the oral bioavailability of OMT. Some of them are first-pass effect and terminal metabolism by gut enzymes. Thus, a novel formulation strategy used must address the aforementioned challenges for getting maximal therapeutic benefits of the drug.
Lately, self-nanoemulsifying drug delivery systems (SNEDDS) have attracted much interest of researchers as one of the extensively investigated formulation approaches for number of BCS class II and IV drugs.13 Constituted of a blend of oils, emulgents and cosolvents, SNEDDSs have unparalleled potential not only for augmenting the solubility of the drugs but also by overcoming the biological metabolism of drugs. Overall, this ultimately increases the oral bioavailability of the drugs to several folds.14,15 Besides, SNEDDS provide added advantage for ease of preparation, scale-up and prolonged stability for months.
The present research work, therefore, was undertaken as per the QbD framework, in order to carry out the experimental design-guided development, optimization and evaluation of the nanoformulation of OMT. Besides, the optimized liquid nanoformulation formulation was converted into solid, which was further optimized on the basis of desirable formulation attributes.
Materials and Methods
OMT was supplied from M/s Cipla Pharmaceuticals (Baddi, India). Various lipids used for formulation development were obtained from M/s Gattefosse (Cedex, France) and M/s Abitec Inc. (Janesville, USA). The emulgents and cosolvents used were also purchased from M/s Fischer Scientific and M/s SD Fine Chemicals (Hayana, India). The porous carriers used in the study were provided by M/s Evonik GmbH (Dresden, Germany) and M/s Gangwal Chemicals (Mumbai, India).
Target product profile (TPP)
TPP includes the entire drug product development plan enlisting the key quality attributes of the drug product, which have direct link with patient safety and efficacy.16,17 TPP was defined for target liquid SNEDDS formulation of OMT, in order to augment the oral bioavailability of the drug. The elements of typical TPP include type of formulation, strength, delivery route, drug release and pharmacokinetics, stability and packaging attributes.
Critical quality attributes (CQAs)
Out of the TPPs, some important quality attributes were selected as the CQAs on the basis of their criticality of influence on patients benefit. For the target SNEDDS formulation, we earmarked viz. particle size (PS, indicator of formulation stability and drug absorption potential through GI tract), time for emulsification (ET, crucial for rapid drug dissolution in the gastrointestinal fluid), mean time for dissolution (TD) and dissolution efficiency (DE) (pivotal for drug release from the formulation).
Formulation quality risk management
Fish-bone diagram was drawn by highlighting a list of causal factors such as drug substance, excipients, machines, measurement techniques and environment milieu, and their sub-causes on the CQAs of SNEDDS. Further, a risk matrix was created by giving green, yellow and red color coding to portray low, medium and high level of risk, respectively, for each of the formulation and/or process attributes.18,19
Screening of formulation excipients
Solubility of the drug was estimated in various lipids like Peceol, Maisine 35-1, Capmul MCM, ethyl oleate, Lauroglycol 90 and Lauroglycol FCC. Also, solubility evaluation was carried out in various surfactants such as Tween 20, Tween 80, Acrysol K-140, Acrysol EL-135, and cosolvent, Transcutol HP. Excess quantity of drug was added to each of the excipients and kept on a mechanical shaker (Rivotek, Riviera Glass Pvt. Ltd., Mumbai, India) with water bath maintained at 37 ± 0.5°C for 72 hours. Vials were examined time to time for solubilization of the drug and subsequently drug was added if required. All the excipients were transferred into centrifuge tubes (Spinwin MC02, Tarsons Pvt Ltd., Kolkata, India) for separation of the undissolved drug, while actual amount of drug solubilized was analyzed spectrophotometrically (UV-Vis Spectrophotometer, Labindia Ltd., Mumbai, India) at 243 nm (i.e., absorption maxima of the drug) from the supernatant fraction.20
Aqueous titration procedure was used for constructing the pseudoternary phase diagrams. Lipid with maximum drug solubilization capacity was titrated with surfactant/cosolvent mixtures (Smix) in the ratio between 1:9 and 9:1. Various Smix ratios explored were 1:0, 1:1, 2:1 and 3:1, thus selecting the ratio with maximal region for nanoemulsion formation.21
Preparation of liquid SNEDDS formulation
The preparation of liquid SNEDDS was attempted using simple admixture method, where selected oil, surfactant and cosolvent were added one by one to the clean and dry culture tubes. The contents were thoroughly mixed under mild heating at 40 ± 2oC with the help of vortex mixer (Cyclomixer CM 101, Remi Elektrotechnik Ltd., Mumbai, India) in order to achieve a homogeneous mixture of the excipients. Then, accurately weighed amount of the drug was added to the excipient mixture and thoroughly mixed to solubilize it.22 The formulations were stored in cool and dry place till further analysis.
Systematic formulation optimization studies
Experimental designs are the important elements of QbD, which helps in optimizing the formulation composition and process parameters. Among the various design available, mixture response surface designs are very useful in optimizing the nanoemulsion where sum total of all the components are equal to 100%.23,24 Hence, 3-factor and 3-level containing D-optimal response surface design with mixture components was adopted for optimizing the SNEDDS composition. Design Expert® 9.0.1 (M/s Stat-Ease Inc., Minneapolis, USA) was used for generating the experimental trials, where quantities of Capmul MCM, Tween 80 and Transcutol HP were taken as the factors. A total of 16 trial formulations were prepared including five replicates of the center point trial, thus evaluated for the formulation CQAs.
Characterization of the liquid SNEDDS formulations Globule size measurement
The SNEDDS formulations were diluted with distilled water and globule size was measured by dynamic light scattering technique using Zetasizer ZS-90 (Malvern Instruments, Worcestershire, UK). Mean globule size of nanoemulsion formed after dilution as Z-average diameter was noted.
Emulsification time determination
The SNEDDS formulations were diluted with distilled water and time required for nanoemulsion formation was noted.
Transmission electron microscopy (TEM)
TEM imaging of the optimized liquid SNEDDS formulation was carried out after 100-fold dilution, followed by negative staining with 1% phosphotungstic acid solution on a gold plate. Then, images were captured under electron microscope (JEM-2100 F, Jeol, Tokyo, Japan) for morphological characterization of the emulsion globules.
Drug release evaluation
It was conducted using dialysis bag (MW 1 kDa) diffusion method, where the SNEDDS formulations were exposed to simulated gastric and simulated intestinal fluids (250 mL), both containing 0.5% sodium lauryl sulfate for the period of 1 h and 2 h, respectively.22,25,26 SNEDDS loaded with 20 mg drug were diluted in 1 ml of SGF and filled in the dialysis bags. The blank formulations were also run for each of the studied the formulations to nullify the interference of excipients with drug during analysis. Similarly, the drug release from pure drug suspension was also performed for comparative evaluation with the SNEDDS formulations.27 The samples were collected at periodic time intervals and an equal volume of fresh medium was added. The collected samples were analyzed using HPLC (Alliance 2695, Waters, Massachusetts, USA) method of the drug and cumulative amount of drug release versus time was calculated. The chromatographic analysis of the drug was performed using the reported procedure on a C18 column using acetonitrile and water (containing 0.1% orthophosphoric acid, pH 3.5) in 40:60 (v/v) as mobile phase at a flow rate of 1.0 mL/min with UV detection at 243 nm.28 The obtained drug release data was then subjected to modeling to fit with various kinetic models as reported in literature and best suited model governing the mechanism of drug release from the SNEDDS formulation was identified.29,30
Modelization, data analysis and optimum search
The modelization of experimental data was conducted by linear regression analysis. Best fit model was selected by virtue of its statistical validity for the model terms and evaluating parameters like good correlation coefficient, lower residual sum of square and lower lack of fit.31,32 Moreover, the developed mathematical equation was observed for the interaction terms and their net resultant influence on the studied CQAs. Various model plots as diagnostic tools of the design were analyzed to check the feasibility and suitability of the data. In addition, 3D- and 2D-plots were studied for analyzing the factor-response relationships. The optimum formulation was identified by numerical and graphical search techniques. Validation was performed by comparing the closeness between the predicted values with experimental values of the CQAs.33
Evaluation of the optimized liquid SNEDDS formulation
The optimized SNEDDS formulation was subjected to size measurement and comparative in vitro drug release analysis with the marketed immediate release tablet formulation (Olmecip, M/s Cipla Ltd., Mumbai, India) and pure drug suspension.
Development and evaluation of the solid SNEDDS formulation
As widely reported in the literature, adsorption technique was employed for preparing the solid SNEDDS. The optimized liquid SNEDDS formulation was subjected wet mixing with Aerosil 200 in a mortar-pestle, and passed through 30 mesh sieve to obtain the uniform powder mass. The weight quantity of carrier material required for adsorption of the oily formulation was noted and powder was subjected to the micromeritic characterization.
Pharmacokinetic evaluation studies
The pharmacokinetic evaluation of the SNEDDS was conducted in unisex Wistar rats under fasting condition. The rats were randomly distributed into four groups, with six animals in each group. Group I and II rats were given with solid and liquid SNEEDS formulations, while group III and IV rats were administered with marketed formulation and pure drug suspension, respectively. All the formulations were administered with the animal dose of the drug (20 mg) equivalent to the body weight of rats. After dosing, blood samples (~0.2 mL) were withdrawn by puncturing the tail vein and centrifuged to collect plasma for HPLC analysis. The chromatographic analysis of the drug was performed using the reported procedure on a C18 column using acetonitrile and water (containing 0.1% orthophosphoric acid, pH 3.5) in 40:60 (v/v) as mobile phase at a flow rate of 1.0 mL/min with UV detection at 243 nm.28 The developed method was translated to bioanalytical level for estimation of the drug in rat plasma as per the reported procedure.32,34,35 All the blood samples were analysed using the HPLC method and plasma concentration of the drug at various time points were measured. Various pharmacokinetic parameters were calculated using non-compartmental analysis method for different groups of treatments.
Statistical data analysis
All experimental data obtained were subjected to statistical analysis using ANOVA, followed by post-hoc analysis using Student’s t test at 5% level of significance.
Accelerated stability study
The optimized liquid and solid SNEDDS formulations were subjected to accelerated stability study for six months using a stability chamber (TH 200G, Thermolab, Thane, India). Both the formulations were filled in capsules and packed in a glass bottle with cotton plug and kept in the stability chamber maintained at 40°C temperature and 75% relative humidity. The samples were removed from stability at 0, 1, 2, 3 and 6 months time periods, and evaluated for emulsification efficiency, globule size, zeta potential and drug release in 15 minutes.
Results and Discussion
Target product profile (TPP)
Supplementary data Table S1 gives account of TPP elements of the liquid SNEDDS of OMT, which includes formulation type, clinical dose, drug release nature, intended route of delivery, pharmacokinetics, packaging and stability requirements.
Critical quality attributes (CQAs)
Out of TPP elements, the key formulation attributes were identified as the CQAs for liquid SNEDDS. Supplementary data Table S2 gives account on rational justification for selection of CQAs and their influence on the biopharmaceutical performance of the liquid SNEDDS.
Excipients screening
Figure 1 shows the bar chart depicting the drug solubility in various lipids, which were found to be in the order: Lauroglycol FCC < Lauroglycol 90 < Maisine 35-1 < ethyl oleate < Peceol < Capmul MCM. Capmul MCM was chosen as the lipid with maximum solubility for the drug. Similarly, Figure 2shows the bar chart depicting the solubility of drug in surfactants and cosolvents, which were found to be in the order: Acrysol EL-135 < Acrysol K-140 < Tween 20 < Tween 80 < Transcutol HP.
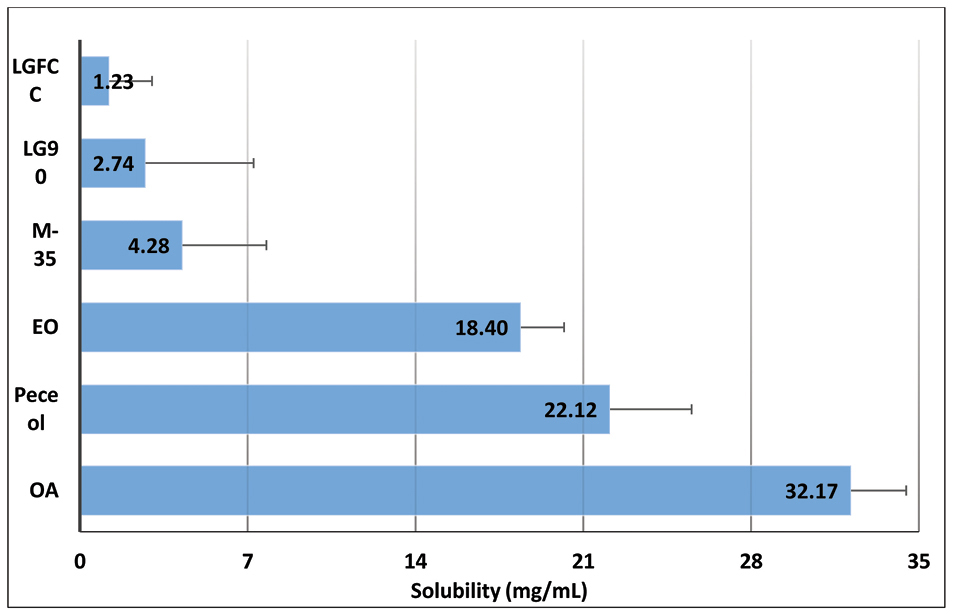
Figure 1.
Equilibrium solubility data of OMT in lipids. Data expressed as mean ± SD (n=3). OMT: Olmesartan medoxomil; OA: Oleic acid, EO: Ethyl oleate, M-35: Maisine 35-1, LG90: Lauroglycol 90, LGFCC: Lauroglycol FCC.
.
Equilibrium solubility data of OMT in lipids. Data expressed as mean ± SD (n=3). OMT: Olmesartan medoxomil; OA: Oleic acid, EO: Ethyl oleate, M-35: Maisine 35-1, LG90: Lauroglycol 90, LGFCC: Lauroglycol FCC.
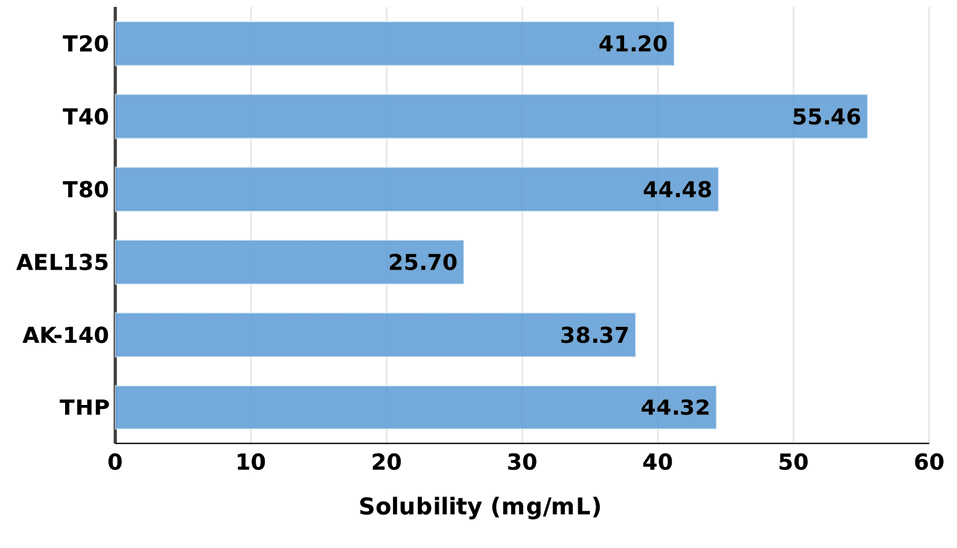
Figure 2.
Equilibrium solubility data of OMT in surfactants and cosolvents. Data expressed as mean ± SD (n=3). OMT: Olmesartan medoxomil; THP: Transcutol HP; AK-140: Acrysol K-140, AEL135: Acrysol 135, T80: Tween 80; T40: Tween 40, T20: Tween 20.
.
Equilibrium solubility data of OMT in surfactants and cosolvents. Data expressed as mean ± SD (n=3). OMT: Olmesartan medoxomil; THP: Transcutol HP; AK-140: Acrysol K-140, AEL135: Acrysol 135, T80: Tween 80; T40: Tween 40, T20: Tween 20.
Pseudoternary phase diagrams
Figure 3 depicts the images showing pseudoternary phase diagrams for Capmul MCM titrated with Tween 80-Transcutol HP (Smix) at 1:0, 1:1, 2:1 and 3:1 ratios. In all Smix combinations, we observed good nanoemulsion region indicating good emulsification characteristics of the selected surfactant and cosolvent. Increasing the amount of surfactant in Smix also showed gradual increase in the area of nanoemulsion region. Among the studies Smix combinations, 1:1 ratio was chosen for the formulation of SNEDDS.
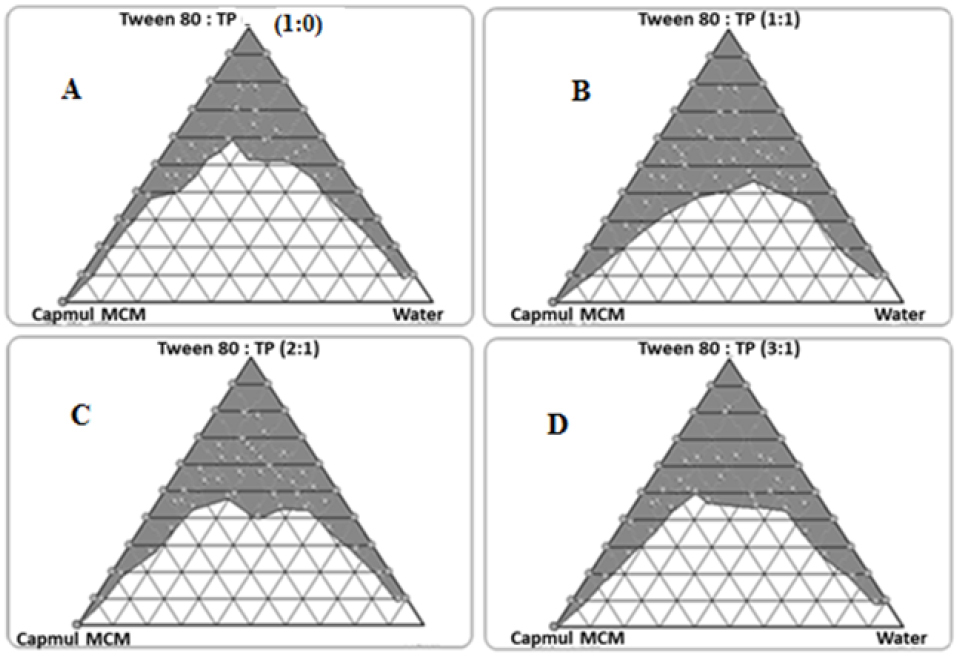
Figure 3.
Pseudoternary phase diagrams depicting nanoemulsion region with Smix ratios (A) 1:0, (B) 1:1, (C) 2:1 and (D) 3:1
.
Pseudoternary phase diagrams depicting nanoemulsion region with Smix ratios (A) 1:0, (B) 1:1, (C) 2:1 and (D) 3:1
Characterization of the liquid SNEDDS
Supplementary data Table S3 summarizes the values of CQAs, as the physical and dissolution parameters of the prepared liquid SNEDDS prepared as per the mixture experimental design.
Globule size measurement
All the formulations showed globule size in the range between 45 and 99 nm. The smaller size less than 100 nm thus confirmed the nano lipidic nature of the prepared formulation, which are inconsonance with the literature reports.36
Emulsification time determination
All the formulations showed emulsification time in the range between 103 and 184 seconds. This confirmed good self-emulsification efficiency of the prepared nano lipidic formulation, ostensibly ascribed to the characteristic nature of the excipients.37
Transmission electron microscopy (TEM)
The inset of Figure 3AillustratesTEM images of the liquid SNEDDS formulations with dark spots as the nanoemulsion globules. The result observed is inconsonance with the literature reports.36,37
Drug release performance
Figure 4 portrays the release profiles of OMT from liquid SNEDDS formulations. More than 90% drug was released from all the SNEDDS formulations within 1 h without any lag-phase. While, drug release profile from pure drug suspension showed maximum of 42% drug release in 1 hour. Further, the dissolution was continued for 3 hours, in case of pure drug suspension also indicated incomplete drug release. The net release of drug was up by 2.5-fold by SNEDDS formulation as compared to the suspension of pure drug. Besides, the calculation of mean time of dissolution indicated values ranging between 3.2 and 18.6 minutes, while efficiency of dissolution indicated values between 20% and 34%, respectively for the SNEDDS. These results further confirmed faster drug release characteristics of the nanolipidic formulation of OMT.20,38
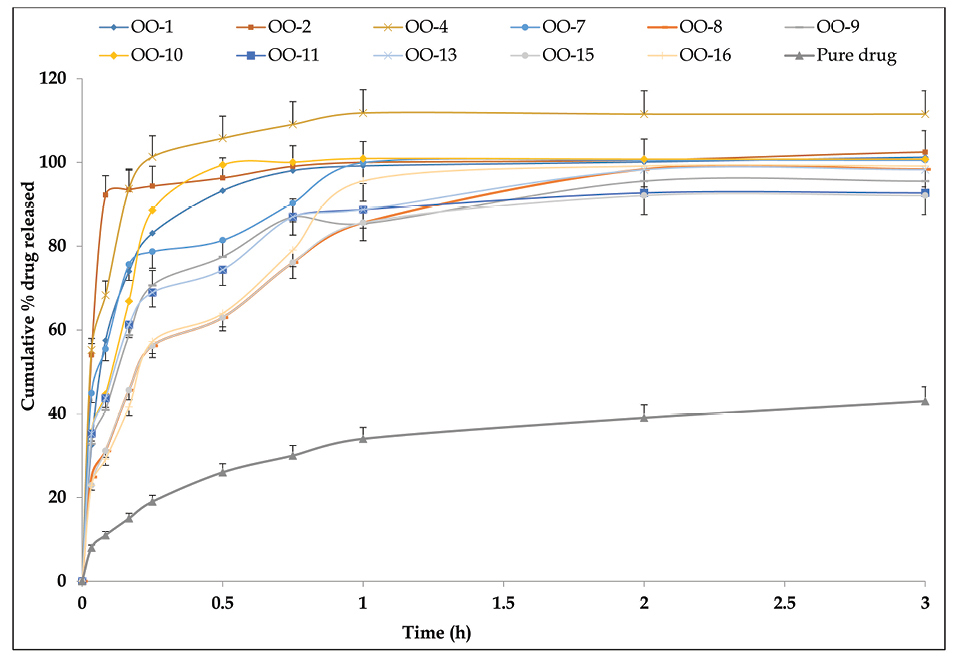
Figure 4.
Cumulative drug release profile of OMT from different liquid SNEDDS in dialysis membrane with molecular cut-off weight of 1 kDa. Data expressed as mean±SD (n=3). OMT: Olmesartan medoxomil; SNEDDS: Self-nanoemulsifying drug delivery systems..
.
Cumulative drug release profile of OMT from different liquid SNEDDS in dialysis membrane with molecular cut-off weight of 1 kDa. Data expressed as mean±SD (n=3). OMT: Olmesartan medoxomil; SNEDDS: Self-nanoemulsifying drug delivery systems..
Modeling and optimization data analysis
The coefficients of each model term generated as per the Eq. (1) for CQAs has been listed in Supplementary data Table S4.
Y = β1X1 + β2X2 + β3X3 + β4X1X2 + β5X2X3 + β8X1X2(X1-X2) + βnXnXm(Xn-Xm) Eq. (1)
where, Y indicates the CQA; β1 to β5 are the coefficient values of various model terms; X1, X2, X3 indicates the CMAs used for optimization studies
The magnitude of polynomial coefficients of the model terms in the polynomial equations of the CQAs confirmed factor interaction effects. High values of correlation coefficients ranging from 0.9414 and 0.9998, and insignificant values of lack of fit indicated goodness in the model fitting.
Response surface analysis
The understanding of the dependence, inter-dependence and co-variation among the variables studied and their interactions is facilitated by constructing response surface diagrams to map the responses over the entire experimental domain.39 The 3D- and 2D-plot for various CQAs are shown in Figure 5.
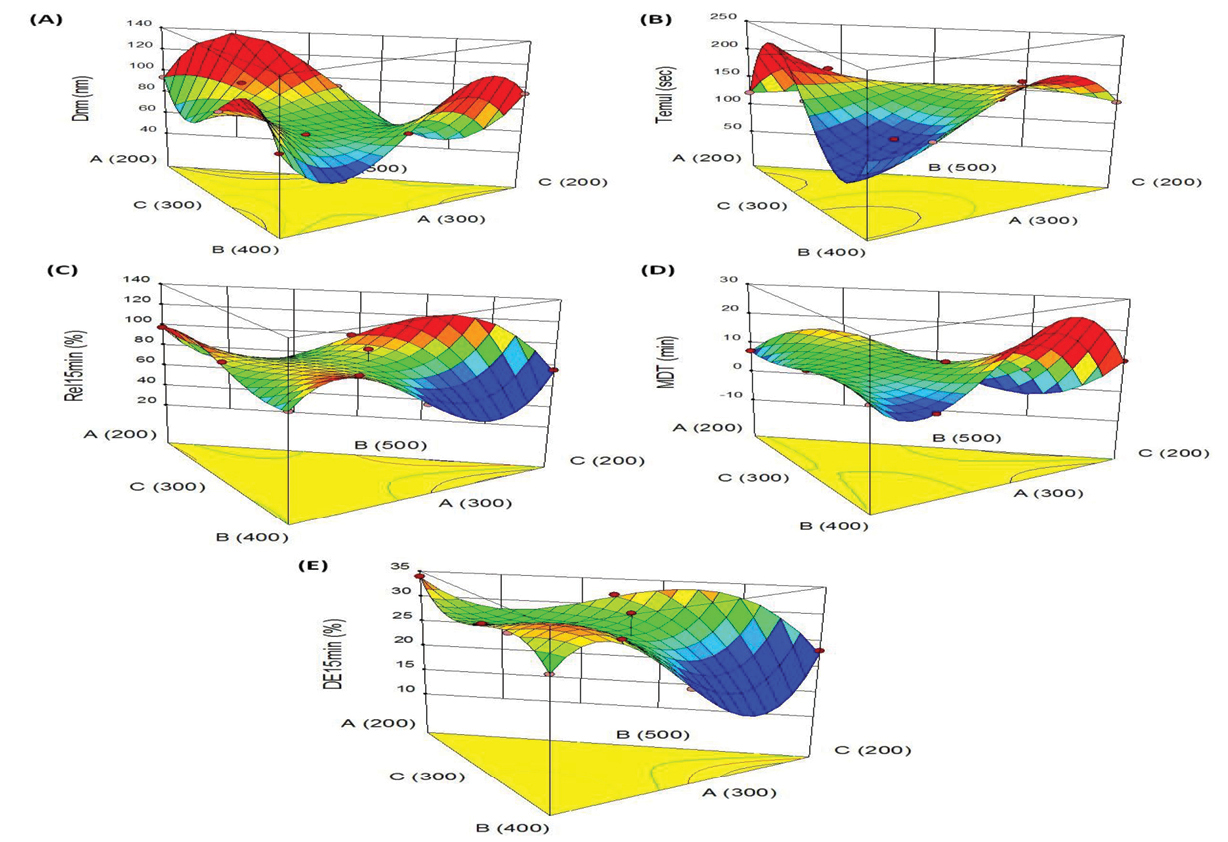
Figure 5.
3D-Response surface plots indicating the relationship between the factors A, B and C on the response variables, (A) particle size, (B) time required for emulsification, (C) drug release in 15 min, (D) mean dissolution time, (E) dissolution efficiency in 15 min. Dnm: Globule size, Temul: Emulsification efficiency, Rel15min: Cumulative %drug release in 15 minutes, MDT: Mean dissolution time, DE15min: Dissolution efficiency in 15 minutes.
.
3D-Response surface plots indicating the relationship between the factors A, B and C on the response variables, (A) particle size, (B) time required for emulsification, (C) drug release in 15 min, (D) mean dissolution time, (E) dissolution efficiency in 15 min. Dnm: Globule size, Temul: Emulsification efficiency, Rel15min: Cumulative %drug release in 15 minutes, MDT: Mean dissolution time, DE15min: Dissolution efficiency in 15 minutes.
Figure 5A illustrates the 3D graph indicating influence of Capmul MCM, Tween 80 and Transcutol HP on particle size. Among the CMAs, Tween 80 shows curvilinear trend on size, where high level of Tween 80 helps in reducing the size. However, increase in Capmul MCM and Transcutol HP levels yielded linear escalation in the size from minimum to maximum levels. The response surface graph shown in Figure 5B is for time of emulsification. This portrays a “saddle shaped” structure, revealing significant influence of Capmul MCM and Transcutol HP on time, whereas Tween 80 shows quite negligible effect.
Figure 5C depicts the inverted “saddle shaped” 3D graph for drug release behaviour. This shows a sharp dip in drug release profile upon increase in the concentrations of Capmul MCM and Tween 80 while the effect of Transcutol HP on Rel15min shows quite negligible influence.
The typical response surface plots for MDT depicted in Figure 5D shows the prominent influence of Capmul MCM and Transcutol HP. The curvilinear trend can be observed for MDT upon increasing the levels of both of these constituents. However, the effect of Tween 80 on MDT was found to be quite negligible.
Like drug release, analogous 3D plot was revealed for the influence efficiency of dissolution. Figure 5E depicts the maximal values of DE15min at lower levels of Capmul, high levels of Tween and Transcutol. As the prepared formulations exhibited quite higher values of Rel15min, the values of DE15min also show an increasing trend in close proximity with the faster drug release characteristics.
Selection of the optimum SNEDDS formulation
The optimum formulation was identified out on the basis of various goals, i.e., smaller the particle size and lesser the time required for emulsification, and faster the drug release profile, higher the mean dissolution time and efficiency for dissolution. The desirable region was also selected using overlay plotting, as shown in Figure 6. The optimized formulation contained, i.e., Capmul MCM (393 mg): Tween 80 (423 mg): Transcutol HP (184 mg), as indicated by flagged point in the figure, along with predicted values of various CQAs.
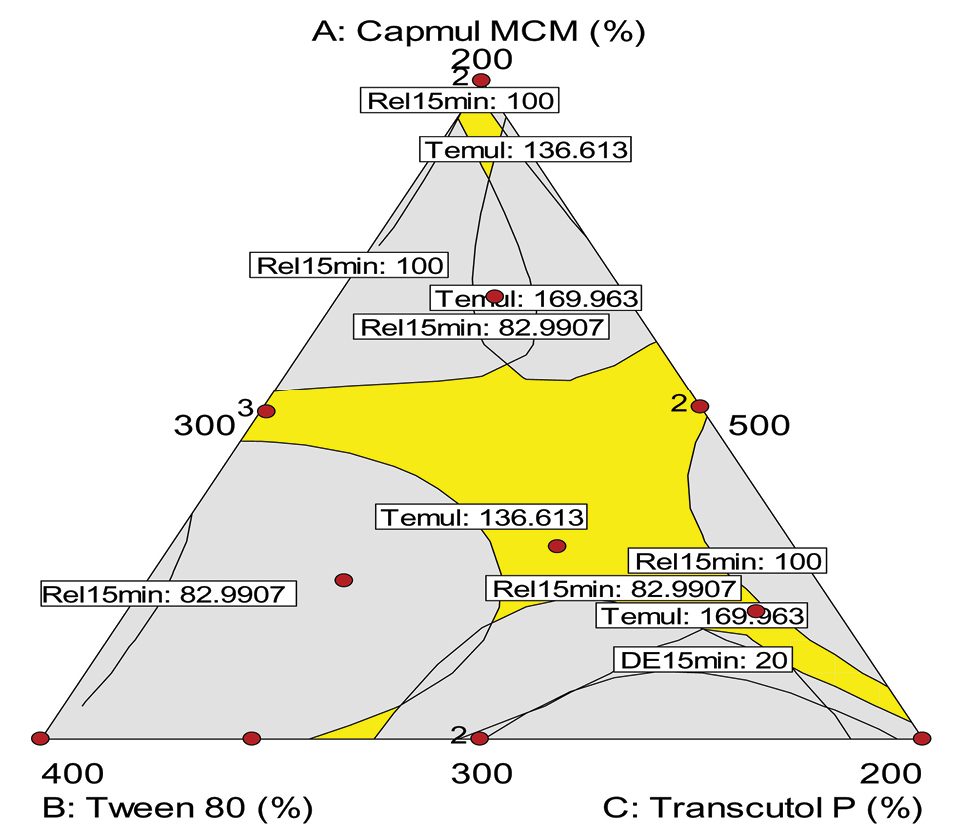
Figure 6.
Design space overlay plot for optimized liquid SNEDDS of OMT. OMT: Olmesartan medoxomil; SNEDDS: Self-nanoemulsifying drug delivery systems, Dnm: Globule size, Temul: Emulsification efficiency, Rel15min: Cumulative %drug release in 15 minutes, MDT: Mean dissolution time, DE15min: Dissolution efficiency in 15 minutes.
.
Design space overlay plot for optimized liquid SNEDDS of OMT. OMT: Olmesartan medoxomil; SNEDDS: Self-nanoemulsifying drug delivery systems, Dnm: Globule size, Temul: Emulsification efficiency, Rel15min: Cumulative %drug release in 15 minutes, MDT: Mean dissolution time, DE15min: Dissolution efficiency in 15 minutes.
Evaluation of the optimized formulation
Globule size and zeta potential
Figure 7A depicts globule size distribution of the optimized liquid SNEDDS with mean globule 64.2. The obtained size in the nanometric range fulfilled the target particle size of the developed formulation.
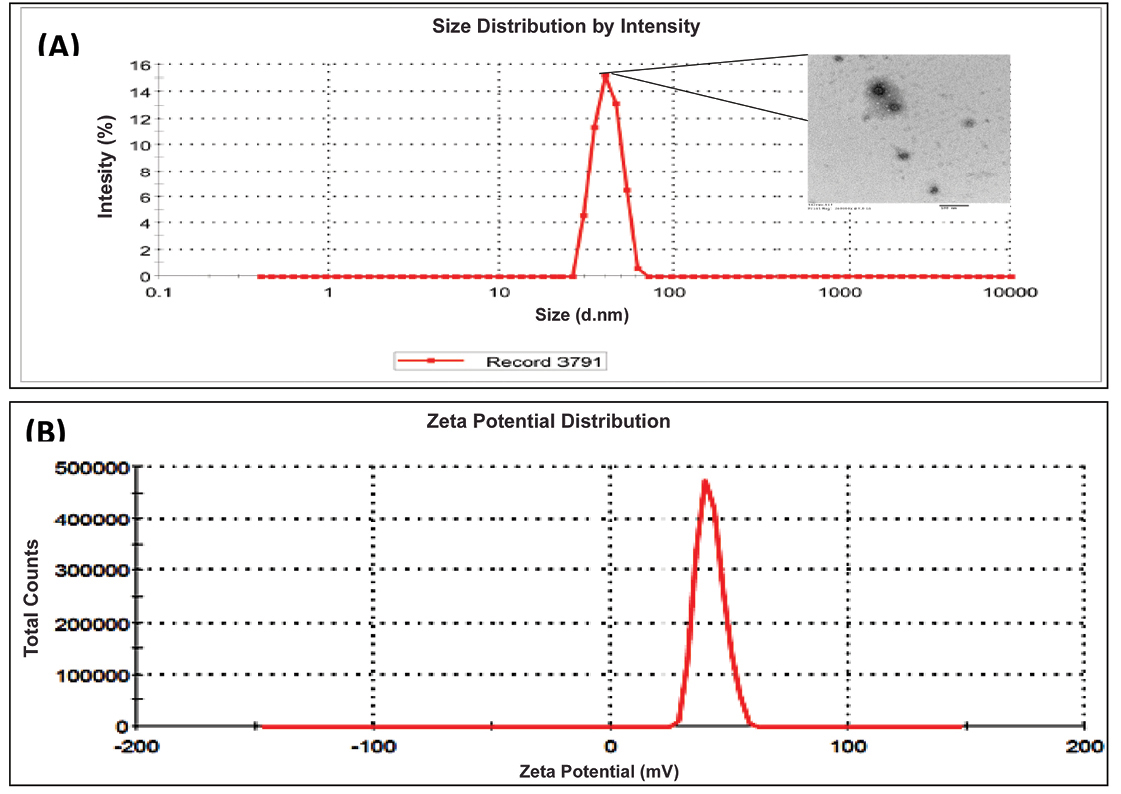
Figure 7.
(A) Globule size and TEM image (B) Zeta potential distribution of optimized liquid SNEDDS [SNEDDS: Self-nanoemulsifying drug delivery systems]
.
(A) Globule size and TEM image (B) Zeta potential distribution of optimized liquid SNEDDS [SNEDDS: Self-nanoemulsifying drug delivery systems]
Zeta potential
The magnitudes of zeta potential of the optimized liquid SNEDDS was found to be -25.4 mV (Figure 7B). The obtained negative magnitude of zeta potential fulfilled the requirement for the developed formulation.
Evaluation of solid SNEDDS
Micromeritic characterisation
Supplementary data Table S5enlists the micromeritic properties of solid SNEDDS, where true density ranged from 1.29 to 1.67 g.cm-3, bulk density ranged from 0.20 and 0.27 g.cm-3, and tapped density ranged from 0.21 and 0.49 g.cm-3, respectively. Based on these micromeritic properties, the best porous carrier was selected.40,41
Flow and compaction properties
The flow and compaction parameters of solid SNEDDS are enlisted in Supplementary data Table S6. Solid SNEDDS formulations showed good flow behavior, as indicated by angle of repose ranged from 22.6 to 39.3 degree, and Carr’s index ranged 17.2 from 39.5. All these parameters construed good overall flow behaviour of the carriers used for preparing solid SNEDDS, which can provide desired compaction and compression ability.40,42
Drug release evaluation
The rate and extent of drug release from the solid SNEDDS formulations was found to be analogous with the liquid SNEDDS (P < 0.05). Moreover, both the formulations exhibited immediate drug release nature (>85%) within 30 minutes and complete drug release in 60 minutes, thus fulfilled the expected drug release performance. The obtained data for the release analysis of liquid and solid SNEDDS are inconsonance with the literature reports published by other authors.38,42 No statistically significant difference (P > 0.05), however, was observed in the drug release profile of liquid and solid SEDDS. Additionally, release characterization of the pure drug showed incomplete drug release only upto 43% into the dissolution medium, which can be ascribed to the poor aqueous solubility of the drug as reported in literature.22,43
Drug release kinetics modeling
The release kinetic analysis of the obtained drug release data indicated best model fitting as per first-order model by Fickian diffusion mechanism, which can be attributed to the fluidic nature of the prepared formulation. The observed findings are inconsonance with the literature reports.38,44
In vivo pharmacokinetic studies
The mean plasma concentration with respect to time profile of the drug obtained after oral administration of the different treatment formulations is shown in Figure 8. The plasma concentration data of liquid SNEDDS showed nearly 5.58 and 4.21-folds extension in Cmax and AUC values, and 0.54-folds drop in Tmax values, while solid SNEDDS revealed nearly 5.06 and 3.87-folds up in Cmax and AUC values, and 0.31-fold drop in Tmax values, respectively. These results ratify distinct progress in the oral bioavailability of drug from SNEDDS formulations vis-à-vis pure drug (P < 0.0001), plausibly owing to the superior drug absorption characteristics as a function of avoidance of hepatic first-pass effect, improvement in the aqueous drug solubility, dissolution rate and intestinal drug permeability.45-48 Moreover, the pharmacokinetic parameters for the solid SNEDDS revealed quite analogous results with those of the liquid SNEDDS (P > 0.05). This demonstrated that adsorption of the liquid SNEDDS onto the solidifying carriers showed no significant change in the bioavailability parameters, except a mild extension in the values of Tmax and AUC for solid SNEDDS as compared to the liquid SNEDDS. The observed findings were quite inconsonance with the formulation characteristics reported in literature.43
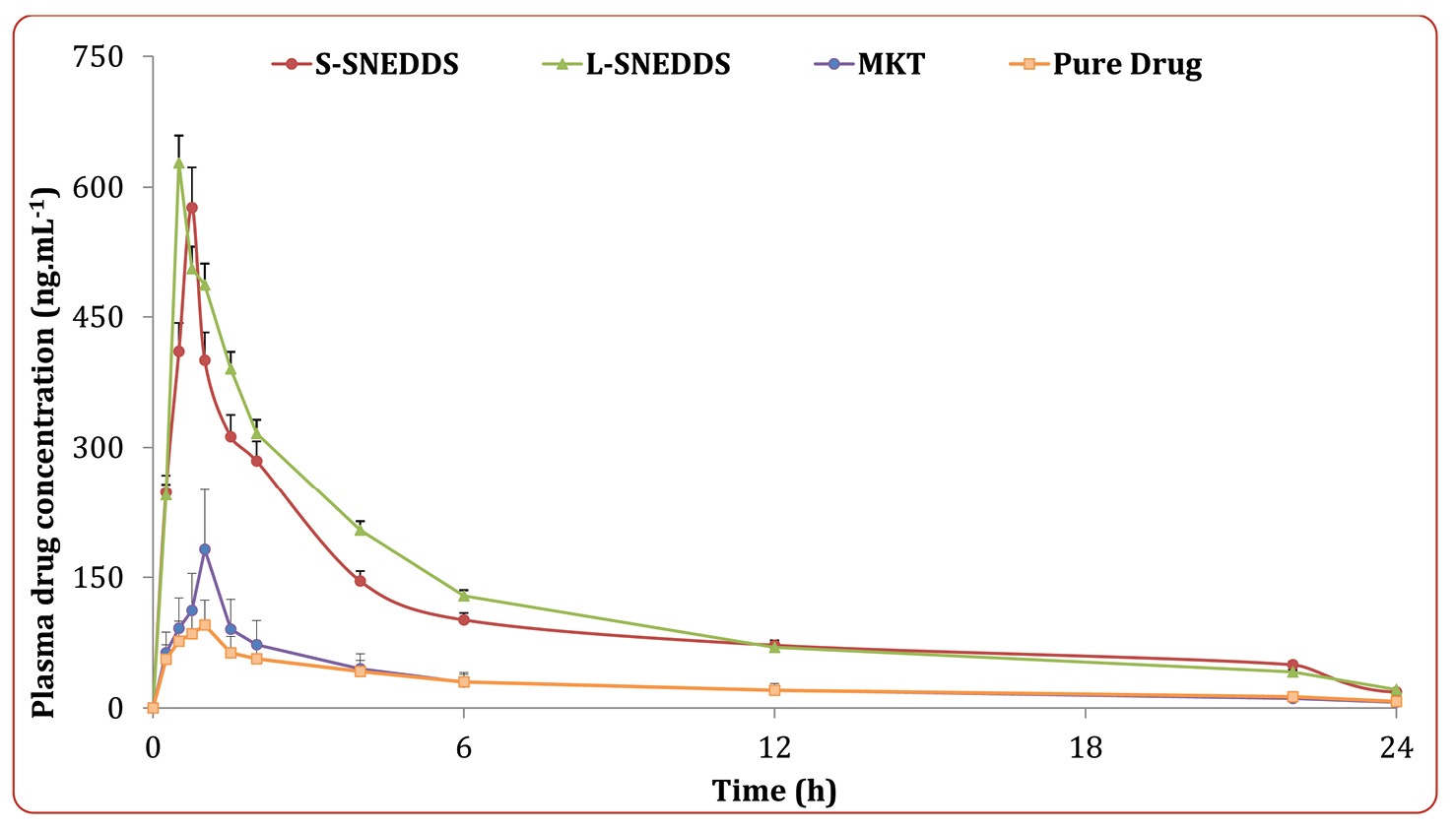
Figure 8.
Plasma concentration versus time profile of OMT from the optimized solid SNEDDS, liquid SNEDDS, MKT formulation and pure drug suspension; while the inset depicts percent alteration in various parameters from the treatment formulations vis-à-vis the pure drug. Data is represented as mean ± 1SD (n=6). OMT: Olmesartan medoxomil; L-SNEDDS: Liquid Self-nanoemulsifying drug delivery systems; S-SNEDDS: Solid Self-nanoemulsifying drug delivery systems; MKT: Market formulation.
.
Plasma concentration versus time profile of OMT from the optimized solid SNEDDS, liquid SNEDDS, MKT formulation and pure drug suspension; while the inset depicts percent alteration in various parameters from the treatment formulations vis-à-vis the pure drug. Data is represented as mean ± 1SD (n=6). OMT: Olmesartan medoxomil; L-SNEDDS: Liquid Self-nanoemulsifying drug delivery systems; S-SNEDDS: Solid Self-nanoemulsifying drug delivery systems; MKT: Market formulation.
Accelerated stability study
Supplementary data Table S7 provides the values of various physical and dissolution parameters of the liquid and solid SNEDDS formulations. Statistically insignificant (P > 0.05) difference was observed in the parameters, i.e., emulsification time, globule size, zeta potential and drug release during the six-month evaluation period. This construed highly stable and robust nature of the prepared formulations even under stress conditions of high temperature and humidity.
Conclusion
The present research work ratifies the successful development of SNEDDS formulations of OMT. Use of QbD approach was very useful in optimizing the formulating composition and identifying the best formulation with CQAs matching the desired limits. As envisioned, the SNEDDS exhibited good in vitro and in vivo performance. Thus, it can be concluded that SNEDDS developed using porous carriers has industrial importance with respect to its applicability for other drugs having oral bioavailability challenges.
Ethical Issues
All the animal studies performed in the preset work were carried out prior approval of the study protocol (Reference No. I/IAEC/AGI/025/2018) from the Institutional Ethics Committee, Anurag Group of Institutions, Hyderabad, India. All the studies were carried out as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
Conflict of Interest
Authors declare no conflict of interest.
Supplementary Materials
Supplementary file 1 contains Tables S1-S7.
(pdf)
References
-
Beg S, Hasnain MS, Rahman M, Swain S. Introduction to quality by design (QbD): fundamentals, principles, and applications. In: Beg S, Hasnain MS, eds. Pharmaceutical Quality by Design. New York: Academic Press; 2019. p. 1-17. 10.1016/B978-0-12-815799-2.00001-0
-
Beg S, Hasnain MS. Pharmaceutical Quality by Design: Principles and Applications. New York: Academic Press; 2019.
- Beg S, Rahman M, Panda SS. Pharmaceutical QbD: Omnipresence in the product development lifecycle. Eur Pharm Rev 2017; 22(1):58-64. [ Google Scholar]
- Lionberger RA, Lee SL, Lee L, Raw A, Yu LX. Quality by design: concepts for ANDAs. AAPS J 2008; 10(2):268-76. doi: 10.1208/s12248-008-9026-7 [Crossref] [ Google Scholar]
- Beg S, Rahman M, Kohli K. Quality-by-design approach as a systematic tool for the development of nanopharmaceutical products. Drug Discov Today 2019; 24(3):717-25. doi: 10.1016/j.drudis.2018.12.002 [Crossref] [ Google Scholar]
- Tocci G, Volpe M. Olmesartan medoxomil for the treatment of hypertension in children and adolescents. Vasc Health Risk Manag 2011; 7:177-81. doi: 10.2147/vhrm.s11672 [Crossref] [ Google Scholar]
- von Bergmann K, Laeis P, Püchler K, Sudhop T, Schwocho LR, Gonzalez L. Olmesartan medoxomil: influence of age, renal and hepatic function on the pharmacokinetics of olmesartan medoxomil. J Hypertens Suppl 2001; 19(1):S33-40. doi: 10.1097/00004872-200106001-00005 [Crossref] [ Google Scholar]
- Patel J, Dhingani A, Tilala J, Raval M, Sheth N. Formulation and development of self-nanoemulsifying granules of olmesartan medoxomil for bioavailability enhancement. Particul Sci Technol 2014; 32(3):274-90. doi: 10.1080/02726351.2013.855686 [Crossref] [ Google Scholar]
- Prajapati ST, Joshi HA, Patel CN. Preparation and characterization of self-microemulsifying drug delivery system of olmesartan medoxomil for bioavailability improvement. J Pharm (Cairo) 2013; 2013:728425. doi: 10.1155/2013/728425 [Crossref] [ Google Scholar]
- Sruthy PN, Anoop KR. Formulation and evaluation of olmesartan medoxomil floating tablets. Int J Pharm Pharm Sci 2013; 5(3):691-6. [ Google Scholar]
- Thakkar HP, Patel BV, Parmar MP, Chauhan NP, Patel AA. Studies on inclusion complex as potential systems for enhancement of oral bioavailability of olmesartan medoxomil. Chron Young Sci 2012; 3(2):129-36. doi: 10.4103/2229-5186.98685 [Crossref] [ Google Scholar]
- Thakkar HP, Patel BV, Thakkar SP. Development and characterization of nanosuspensions of olmesartan medoxomil for bioavailability enhancement. J Pharm Bioallied Sci 2011; 3(3):426-34. doi: 10.4103/0975-7406.84459 [Crossref] [ Google Scholar]
- Singh B, Beg S, Khurana RK, Sandhu PS, Kaur R, Katare OP. Recent advances in self-emulsifying drug delivery systems (SEDDS). Crit Rev Ther Drug Carrier Syst 2014; 31(2):121-85. doi: 10.1615/critrevtherdrugcarriersyst.2014008502 [Crossref] [ Google Scholar]
- Hussain A, Samad A, Singh SK, Beg S. Self-emulsifying systems for oral bioavailability enhancement. Recent Pat Nanomed 2015; 5(2):71-7. doi: 10.2174/1877912305666150616221856 [Crossref] [ Google Scholar]
- Beg S, Saini S, Bandopadhyay S, Katare OP, Singh B. QbD-driven development and evaluation of nanostructured lipid carriers (NLCs) of Olmesartan medoxomil employing multivariate statistical techniques. Drug Dev Ind Pharm 2018; 44(3):407-20. doi: 10.1080/03639045.2017.1395459 [Crossref] [ Google Scholar]
- Beg S, Choudhry H, Zamzami MA, Alharbi KS, Rahman M, Singh B. Nanocolloidal lipidic carriers of olmesartan medoxomil surface-tailored with Concavalin-A for lectin receptor targeting. Nanomedicine (Lond) 2018; 13(24):3107-28. doi: 10.2217/nnm-2018-0188 [Crossref] [ Google Scholar]
- Singh B, Beg S. Quality by design in product development life cycle. Chronicle Pharmabiz 2013; 22:72-9. [ Google Scholar]
- Negi P, Singh B, Sharma G, Beg S, Raza K, Katare OP. Phospholipid microemulsion-based hydrogel for enhanced topical delivery of lidocaine and prilocaine: QbD-based development and evaluation. Drug Deliv 2016; 23(3):951-67. doi: 10.3109/10717544.2014.923067 [Crossref] [ Google Scholar]
- Garg V, Singh H, Bhatia A, Raza K, Singh SK, Singh B. Systematic development of transethosomal gel system of piroxicam: formulation optimization, in vitro evaluation, and ex vivo assessment. AAPS PharmSciTech 2017; 18(1):58-71. doi: 10.1208/s12249-016-0489-z [Crossref] [ Google Scholar]
- Beg S, Sandhu PS, Batra RS, Khurana RK, Singh B. QbD-based systematic development of novel optimized solid self-nanoemulsifying drug delivery systems (SNEDDS) of lovastatin with enhanced biopharmaceutical performance. Drug Deliv 2015; 22(6):765-84. doi: 10.3109/10717544.2014.900154 [Crossref] [ Google Scholar]
- Beg S, Swain S, Singh HP, Patra Ch N, Rao ME. Development, optimization, and characterization of solid self-nanoemulsifying drug delivery systems of valsartan using porous carriers. AAPS PharmSciTech 2012; 13(4):1416-27. doi: 10.1208/s12249-012-9865-5 [Crossref] [ Google Scholar]
- Beg S, Sharma G, Thanki K, Jain S, Katare OP, Singh B. Positively charged self-nanoemulsifying oily formulations of olmesartan medoxomil: systematic development, in vitro, ex vivo and in vivo evaluation. Int J Pharm 2015; 493(1-2):466-82. doi: 10.1016/j.ijpharm.2015.07.048 [Crossref] [ Google Scholar]
- Bhoop BS, Beg S, Raza K. Developing “optimized” drug products employing “designed” experiments. Chemical Industry Digest 2013; 23:70-6. [ Google Scholar]
-
Beg S, Swain S, Rahman M, Hasnain MS, Imam SS. Application of design of experiments (DoE) in pharmaceutical product and process optimization. In: Beg S, Hasnain MS, eds. Pharmaceutical Quality by Design. New York: Academic Press; 2019. p. 43-64. doi: 10.1016/B978-0-12-815799-2.00003-4.
- Beg S, Sharma G, Katare OP, Singh B. Optimized positively charged self-nanoemulsifying systems of candesartan cilexetil with enhanced bioavailability potential. J Nanomed Nanotechnol 2013; 4(6):297. [ Google Scholar]
- Jain A, Kaur R, Beg S, Kushwah V, Jain S, Singh B. Novel cationic supersaturable nanomicellar systems of raloxifene hydrochloride with enhanced biopharmaceutical attributes. Drug Deliv Transl Res 2018; 8(3):670-92. doi: 10.1007/s13346-018-0514-8 [Crossref] [ Google Scholar]
- Eskandani M, Nazemiyeh H. Self-reporter shikonin-Act-loaded solid lipid nanoparticle: formulation, physicochemical characterization and geno/cytotoxicity evaluation. Eur J Pharm Sci 2014; 59:49-57. doi: 10.1016/j.ejps.2014.04.009 [Crossref] [ Google Scholar]
- Beg S, Sharma G, Katare OP, Lohan S, Singh B. Development and validation of a stability-indicating liquid chromatographic method for estimating olmesartan medoxomil using Quality by Design. J Chromatogr Sci 2015; 53(7):1048-59. doi: 10.1093/chromsci/bmu165 [Crossref] [ Google Scholar]
- Vandghanooni S, Eskandani M, Barar J, Omidi Y. AS1411 aptamer-decorated cisplatin-loaded poly(lactic-co-glycolic acid) nanoparticles for targeted therapy of miR-21-inhibited ovarian cancer cells. Nanomedicine (Lond) 2018; 13(21):2729-58. doi: 10.2217/nnm-2018-0205 [Crossref] [ Google Scholar]
- Hamishehkar H, Bahadori MB, Vandghanooni S, Eskandani M, Nakhlband A, Eskandani M. Preparation, characterization and anti-proliferative effects of sclareol-loaded solid lipid nanoparticles on A549 human lung epithelial cancer cells. J Drug Deliv Sci Technol 2018; 45:272-80. doi: 10.1016/j.jddst.2018.02.017 [Crossref] [ Google Scholar]
-
Singh B, Saini S, Lohan S, Beg S. Chapter 3 - Systematic development of nanocarriers employing quality by design paradigms. In: Mishra V, Kesharwani P, Mohd Amin MCI, Iyer A, eds. Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes. New York: Academic Press; 2017. p. 110-48. doi: 10.1016/B978-0-12-809717-5.00003-8.
- Khurana RK, Bansal AK, Beg S, Burrow AJ, Katare OP, Singh KK. Enhancing biopharmaceutical attributes of phospholipid complex-loaded nanostructured lipidic carriers of mangiferin: systematic development, characterization and evaluation. Int J Pharm 2017; 518(1-2):289-306. doi: 10.1016/j.ijpharm.2016.12.044 [Crossref] [ Google Scholar]
- Beg S, Jain S, Kushwah V, Bhatti GK, Sandhu PS, Katare OP. Novel surface-engineered solid lipid nanoparticles of rosuvastatin calcium for low-density lipoprotein-receptor targeting: a Quality by Design-driven perspective. Nanomedicine (Lond) 2017; 12(4):333-56. doi: 10.2217/nnm-2016-0336 [Crossref] [ Google Scholar]
- Beg S, Jain A, Kaur R, Panda SS, Katare OP, Singh B. QbD-driven development and validation of an efficient bioanalytical UPLC method for estimation of olmesartan medoxomil. J Liq Chromatogr Relat Technol 2016; 39(13):587-97. doi: 10.1080/10826076.2016.1206023 [Crossref] [ Google Scholar]
- Beg S, Panda SS, Singh B. Development and validation of high-performance liquid chromatographic method for estimation of olmesartan medoxomil in rat lymph. Anal Chem Lett 2018; 8(5):704-12. doi: 10.1080/22297928.2018.1487333 [Crossref] [ Google Scholar]
- Tripathi CB, Beg S, Kaur R, Shukla G, Bandopadhyay S, Singh B. Systematic development of optimized SNEDDS of artemether with improved biopharmaceutical and antimalarial potential. Drug Deliv 2016; 23(9):3209-23. doi: 10.3109/10717544.2016.1162876 [Crossref] [ Google Scholar]
- Beg S, Katare OP, Singh B. Formulation by design approach for development of ultrafine self-nanoemulsifying systems of rosuvastatin calcium containing long-chain lipophiles for hyperlipidemia management. Colloids Surf B Biointerfaces 2017; 159:869-79. doi: 10.1016/j.colsurfb.2017.08.050 [Crossref] [ Google Scholar]
- Beg S, Sandhu PS, Batra RS, Khurana RK, Singh B. QbD-based systematic development of novel optimized solid self-nanoemulsifying drug delivery systems (SNEDDS) of lovastatin with enhanced biopharmaceutical performance. Drug Deliv 2015; 22(6):765-84. doi: 10.3109/10717544.2014.900154 [Crossref] [ Google Scholar]
- Singh B, Kumar R, Ahuja N. Optimizing drug delivery systems using systematic “design of experiments” Part I: fundamental aspects. Crit Rev Ther Drug Carrier Syst 2005; 22(1):27-105. doi: 10.1615/critrevtherdrugcarriersyst.v22.i1.20 [Crossref] [ Google Scholar]
- Beg S, Jena SS, Patra Ch N, Rizwan M, Swain S, Sruti J. Development of solid self-nanoemulsifying granules (SSNEGs) of ondansetron hydrochloride with enhanced bioavailability potential. Colloids Surf B Biointerfaces 2013; 101:414-23. doi: 10.1016/j.colsurfb.2012.06.031 [Crossref] [ Google Scholar]
- Garg B, Katare OP, Beg S, Lohan S, Singh B. Systematic development of solid self-nanoemulsifying oily formulations (S-SNEOFs) for enhancing the oral bioavailability and intestinal lymphatic uptake of lopinavir. Colloids Surf B Biointerfaces 2016; 141:611-22. doi: 10.1016/j.colsurfb.2016.02.012 [Crossref] [ Google Scholar]
- Beg S, Katare OP, Saini S, Garg B, Khurana RK, Singh B. Solid self-nanoemulsifying systems of olmesartan medoxomil: Formulation development, micromeritic characterization, in vitro and in vivo evaluation. Powder Technol 2016; 294:93-104. doi: 10.1016/j.powtec.2016.02.023 [Crossref] [ Google Scholar]
- Beg S, Alam MN, Ahmad FJ, Singh B. Chylomicron mimicking nanocolloidal carriers of rosuvastatin calcium for lymphatic drug targeting and management of hyperlipidemia. Colloids Surf B Biointerfaces 2019; 177:541-9. doi: 10.1016/j.colsurfb.2019.02.039 [Crossref] [ Google Scholar]
- Bandyopadhyay S, Beg S, Katare OP, Sharma G, Singh B. QbD-oriented development of self-nanoemulsifying drug delivery systems (SNEDDS) of valsartan with improved biopharmaceutical performance. Curr Drug Deliv 2015; 12(5):544-63. doi: 10.2174/1567201812666150227125639 [Crossref] [ Google Scholar]
- Singh B, Khurana L, Bandyopadhyay S, Kapil R, Katare OO. Development of optimized self-nano-emulsifying drug delivery systems (SNEDDS) of carvedilol with enhanced bioavailability potential. Drug Deliv 2011; 18(8):599-612. doi: 10.3109/10717544.2011.604686 [Crossref] [ Google Scholar]
- Singh B, Singh R, Bandyopadhyay S, Kapil R, Garg B. Optimized nanoemulsifying systems with enhanced bioavailability of carvedilol. Colloids Surf B Biointerfaces 2013; 101:465-74. doi: 10.1016/j.colsurfb.2012.07.017 [Crossref] [ Google Scholar]
- Akhtar N, Talegaonkar S, Khar RK, Jaggi M. Self-nanoemulsifying lipid carrier system for enhancement of oral bioavailability of etoposide by P-glycoprotein modulation: in vitro cell line and in vivo pharmacokinetic investigation. J Biomed Nanotechnol 2013; 9(7):1216-29. doi: 10.1166/jbn.2013.1613 [Crossref] [ Google Scholar]
- Feng Y, Sun C, Yuan Y, Zhu Y, Wan J, Firempong CK. Enhanced oral bioavailability and in vivo antioxidant activity of chlorogenic acid via liposomal formulation. Int J Pharm 2016; 501(1-2):342-9. doi: 10.1016/j.ijpharm.2016.01.081 [Crossref] [ Google Scholar]