Advanced pharmaceutical bulletin. 10(2):203-212.
doi: 10.34172/apb.2020.024
Review Article
Pharmaceutical Co-Crystallization: Regulatory Aspects, Design, Characterization, and Applications
Abdul Raheem Thayyil *  , Thimmasetty Juturu
, Thimmasetty Juturu  , Shashank Nayak
, Shashank Nayak  , Shwetha Kamath
, Shwetha Kamath 
Author information:
Faculty of Industrial Pharmacy, Bapuji Pharmacy College, SS layout, Shamnur road, Davanagere-577004, Karnataka, India. Introduction
Abstract
Pharmaceutical co-crystals are novel class of pharmaceutical substances, which possess an apparent probability of advancement of polished physical properties offering stable and patentable solid forms. These multi-component crystalline forms influence pertinent physicochemical parameters like solubility, dissolution rate, chemical stability, physical stability, etc. which in turn result in the materials with superior properties to those of the free drug. Co-crystallization is a process by which the molecular interactions can be altered to optimize the drug properties. Co-crystals comprise a multicomponent system of active pharmaceutical ingredient (API) with a stoichiometric amount of a pharmaceutically acceptable coformer incorporated in the crystal lattice. By manufacturing pharmaceutical co-crystals, the physicochemical properties of a drug can be improved thus multicomponent crystalline materials have received renewed interest in the current scenario due to the easy administration in the pharmaceutical industry. There is an immense amount of literature available on co-crystals. However, there is a lack of an exhaustive review on a selection of coformers and regulations on co-crystals. The review has made an attempt to bridge this gap. The review also describes the methods used to prepare co-crystals with their characterization. Brief description on the pharmaceutical applications of co-crystals has also been incorporated here. Efforts are made to include reported works on co-crystals, which further help to understand the concept of co-crystals in depth.
Keywords: Co-crystals, Evaluation of co-crystals, Hansen solubility parameter, Liquid assisted grinding, Preparation of co-crystals, Solvent evaporation technique
Copyright and License Information
© 2020 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
A multi-component crystalline system is not new but recently the term co-crystal has gained momentum in the glossary of the pharmaceutical world. The physicochemical properties of a drug like melting point, hygroscopicity, solubility, dissolution, and stability are improved for better drug bioavailability, which leads to gain better therapeutic effect of the drug with reduced adverse effects. Solubility as well as dissolution is some of the mechanical characteristics of an active pharmaceutical ingredient (API) that should be thoroughly studied in pharmaceutical drug development.
1-3
Pharmaceutical co-crystallization is a novel technique that can be employed to get alternatives of salts, solvates, and polymorphs for the modification of API during the process of dosage form design.
4,5
Making pharmaceutical co-crystals allows the modifications to be made to a crystalline form of an API, which in turn results in an API with altered physicochemical properties without compromising its intended biological properties.
6,7
The co-crystals can be dissociated into its components by dilution of the co-crystal solution while recent studies also revealed the possibility of co-crystal dissociation by pH-dependent mechanism.
8
The co-crystals are held, by supramolecular heterosynthons that occur between the functional groups like carboxylic acid–aromatic nitrogen, carboxylic acid–amide and alcohol–pyridine, with non-covalent forces, often including hydrogen bonding.
9,10
Other forces involved in co-crystallization are ionic and Van der Waals forces, lipophilic-lipophilic interactions and pi-pi interactions.
11
The co-crystals are different from that of salts due to less extent of proton transfer between drug and coformer. Salt is formed by transferring the proton when the p Ka difference between the partners is sufficiently large.
12
Pharmaceutical co-crystals could be prepared by different methods like solvent evaporation, anti-solvent addition, crystallization from the melt, solid state grinding, etc.
13-16
Eddleston et al have used freeze-drying as an approach for the formulation of novel multicomponent crystal forms.
17
There are limited reports on patents of co-crystals but are expected to grow due to the tremendous improvement in the regulations of co-crystals made by various regulatory authorities across the world.
18
United States Food and Drug Administration (USFDA) and European Medicine Agency (EMA) are the current two regulatory agencies that regulate the approaches for controlling the quality of pharmaceutical co-crystals. USFDA defined co-crystals as “Crystalline materials composed of two or more molecules within the same crystal lattice”.
19
According to the FDA, co-crystals are taken into account as a drug product intermediate (DPI) that supposes to strengthen the efficiency of an API. EMA defined co-crystal as “Homogenous crystalline structures made up of two or more components in a definite stoichiometric ratio where the arrangement in the crystal lattice is not based on ionic bonds”.
20
The regulations might impact the traits of pharmaceutical co-crystals and their formulations.
6
Selection ofcoformers
Coformer with its drug compatibility should be studied prior to its pharmaceutical co-crystal development and are the main challenges to be solved. Coformer screening is the tool used for selecting the coformer that can be formulated as a co-crystal with the drug. The superior candidate is then studied for its physicochemical and pharmacological properties prior to its development to a suitable dosage form. Usually, the coformers are selected from the substances which are approved as generally recognized as safe (GRAS) list by USFDA, these coformers do not affect the pharmacological activities of an API.
21
PKabased model
Proton transfer is the phenomenon that occurs in the case of salts. The equation involved in the prediction of co-crystal formation is ΔpK a=[pK a(base) - pK a(acid)].
22
The transfer of proton can be seen if the difference in the pK a value is more than 3. If the ΔpK a value is less than zero, then co-crystal might be formed and the higher value that is more than 3 results in the formation of salts. If the ΔpK a is in between 0-3, then either co-crystal or salt can be expected.
23
For example, succinic acid (pK a 4.2) forms co-crystal with urea base (pK a 0.1) while the salt is formed by using L-lysine base (pKa 9.5).
24
Cambridge structural database
Cambridge structural database (CSD) can incorporate to assess the intermolecular hydrogen bonding possibility between different molecules.
16
CSD single crystal x-ray crystallography can be employed for characterizing the crystal structure of a compound. The resolved structure can be saved in CSD and information can be searched, retrieved, and utilized from the database at any time. ‘Atoms’ and ‘powder cell’ are two examples of the software which can be used to visualize the structure by the information obtained from the CSD.
12
Hansen solubility parameter (HSP)
The prediction of miscibility of a drug and coformer, co-crystal formation, is possible by using HSP. In the HSP, the group contribution method is commonly used to determine the HSP since it only requires the structure of the compound.
25,26
Fedors method, Hoy’s method, and Van Krevelen’s method are the common group contribution methods employed in the calculation of HSP.
27,28
The theoretical prediction or possibility of the co-crystal formulation is suggested by the scientists Krevelen and Greenhalgh. According to Krevelen, if the deviation in the solubility parameter value of the partners is ≤ 5MPa1/2, then co-crystals might be formed. Greenhalgh suggests the formation of co-crystals if the difference is ≤ 7 MPa1/2.
22,29
In addition to this, Salem et al have recently contributed cut-off value 8.18 MPa1/2, which is more dependable due to the relaxation of the cut-off value compared to the previous values.
30
Hydrogen bonding
From the various studies, it is found that the hydrogen bond donors and acceptors of the partners shall make hydrogen bond. Moreover, the best hydrogen bond donors and acceptors interact within the crystal structure cause to the development of co-crystals.
31
The formation of hydrogen bonding can be confirmed by FTIR spectroscopy.
Supramolecular Synthon Approach
Bolla and Nangia have used the supramolecular synthon approach for screening the coformers for a sulfa drug; acetazolamide.
32
Supramolecular synthons are further divided into two groups namely supramolecular homosynthons and supramolecular heterosynthons. The former are identical functional groups like two carboxylic acid groups whereas the later consist of different functional groups like carboxylic acid and amide group.
33
The most commonly used supramolecular synthons are shown in Figure 1.
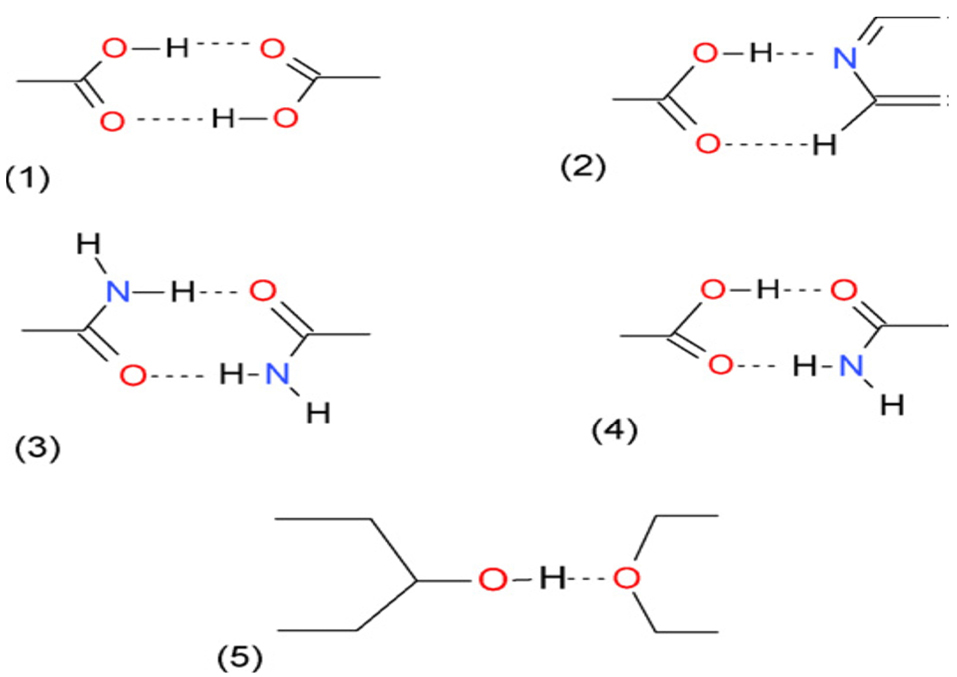
Figure 1.
Common supra molecular synthons in crystal engineering: (1) Carboxylic acid diamer (Homosynthon), (2) Carboxylic acid-pyridine (Heterosynthon), (3) Amide diamer (Homosynthon), (4) Carboxylic acid-amide (Heterosynthon), (5) Alcohol-ether (Heterosynthon).
34
.
Common supra molecular synthons in crystal engineering: (1) Carboxylic acid diamer (Homosynthon), (2) Carboxylic acid-pyridine (Heterosynthon), (3) Amide diamer (Homosynthon), (4) Carboxylic acid-amide (Heterosynthon), (5) Alcohol-ether (Heterosynthon).
34
Binary and ternary phase diagrams
These phase diagrams illustrate the solubility of either API-coformer (Binary) or API-coformer-solvent (Ternary). DSC analysis can be employed for the construction of binary phase diagram. A ‘W’ shaped diagram will obtain in case of cocrystal formation rather than a ‘V’ shaped diagram, which is found when eutectic mixture is formed between the API and coformer.
35
Yamashita et al carried out the coformer screening of salts and co-crystals based on binary phase diagram.
36
Ternary phase diagram (TPD) is a solute-solute-solvent triangular phase diagram that is used for coformer screening in the solution co-crystallization.
37
Hong et al have used TPD for the successful preparation of myricetin co-crystals.
38
Conductor-like screening model for real solvents (CSMO-RS)
CSMO-RS is a computational screening technique where the enthalpy of the API and coformer are calculated and can be implemented for coformer ranking. The excess enthalpy of the API-coformer complex than that of the pure components reveals the possibility of cocrystal formation.
35
Abramov et al have successfully implemented CSMO-RS theory for the screening of coformers using COSMOthermsoftware.
39
Regulation of pharmaceutical co-crystals
The regulation of pharmaceutical co-crystals and their formulations may affect the development and quality control strategies. According to the USFDA, co-crystals are defined as “Solids which are crystalline materials composed of two or more molecules in the same crystal lattice”.
23,40
USFDA was the first agency to give guidance on the regulatory classification of pharmaceutical co-crystals. Co-crystals are classified as DPIs which are expected to improve the physicochemical properties of a drug.
41
The extent of proton transfer should be proved in the substances claimed as cocrystals. Cocrystals should undergo dissociation to regenerate the drug prior to its location for the therapeutic activity. The guidance gives the data required for the submission as well as its implications of the classification for the new drug applications and abbreviated new drug applications. The recommendations of USFDA do not apply to the already existing materials like complexes, polymers, salts or other non-crystalline forms. The guidance applies only to those materials which have not been determined previously like the pharmaceutical co-crystals.
Recently, the co-crystals are included in the category of ‘new active substances’
24
and are defined as “The different salts, esters, ethers, isomers, mixtures of isomers, complexes or derivatives of an active substance shall be considered to be the same active substance unless they differ significantly in properties with regard to safety and/or efficacy”. From a scientific point of view, solvates including hydrates can be considered as a subgroup of co-crystals however the regulatory context may sometimes differ. Multiple phase materials which are obtained by co-precipitation or physical mixing are not considered as co-crystals by EMA.
19
According to EMA, the procedure adopted for documentation of co-crystal and salts can be used due to conceptual similarities. In case of any complex coformer usage, additional documentation may be required with the scientific procedure. The comparison of co-crystal parameters of USFDA and EMA based on regulatory status is shown in Table 1.
Table 1.
Comparison of co-crystal parameters of USFDA and EMA based on regulatory status
19,20,42
|
Co-crystal Parameters
|
USFDA
|
EMA
|
| Definition |
Solids that are crystalline materials composed of two or more molecules in the same crystal lattice. |
Homogenous (single phase) crystalline structures made up of two or more components in a definite stoichiometric ratio where the arrangement in the crystal lattice is not based on ionic bonds (as with salts). |
| Regulatory status |
DPI, not regarded as a new API |
New Active Substance status depends upon demonstration of efficacy and/or safety |
| Regulatory regard |
Similar to polymorph of the same API |
Similar to salts of the same API |
| Coformers |
Neutral guest compound (excipient) |
Non-active components/Reagents (excipient) |
| Chemical interactions |
Nonionic |
Nonionic |
| Similarity with API |
Yes |
Depends upon demonstration of efficacy and/or safety |
| US-Drug master files (DMF)/EMA-Active substance master file (ASMF) |
Not feasible being DPI |
Can be filed |
| Applicable Good manufacturing practice (GMP) regulations/guide |
cGMP for drug product |
Part II of EU GMP Guide (active substances) and ICH Q7 and in rare cases Part I of EU GMP Guide (finished drug product) |
After a thorough look in to the regulatory status, the coformers can be screened based on the method explained in the previous section and the preparation of the cocrystals can be achieved by the techniques described in ‘Preparation of co-crystal section’.
Preparation of co-crystals
Various methods employed in the preparation of co-crystals are shown in Figure 2.
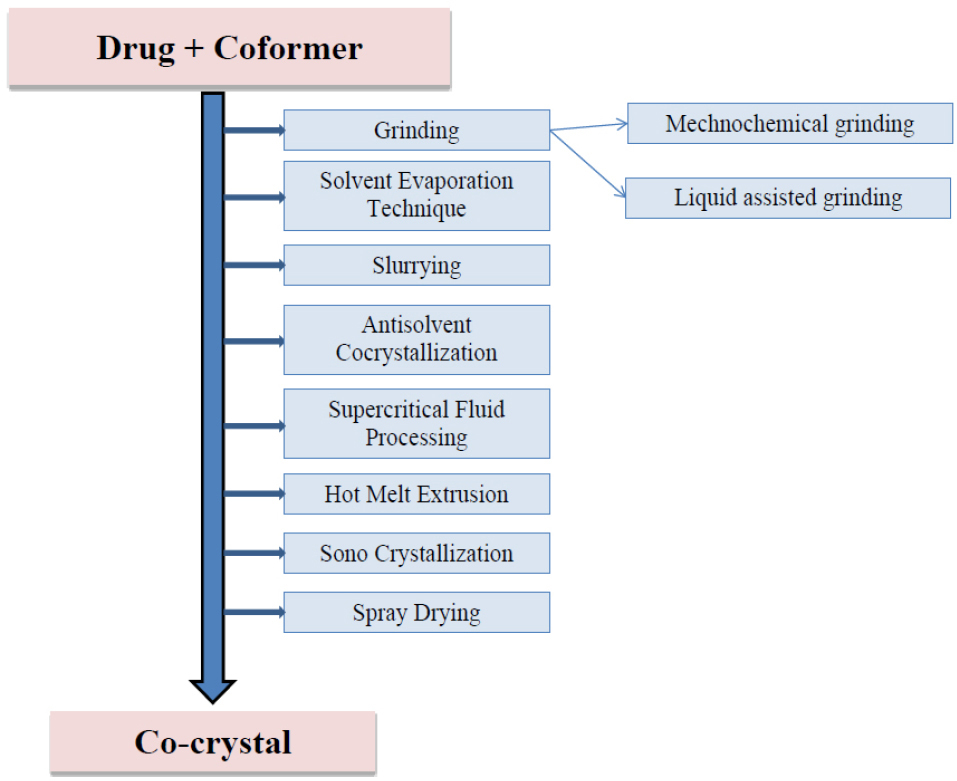
Figure 2.
Methods of preparation of co-crystals.
.
Methods of preparation of co-crystals.
Grinding method
Mechanochemical grinding
It is also known as solid-state grinding or dry grinding.
9
The drug and coformers are mixed and crushed either by using mortar and pestle or by mill such as ball mill.
43
There may be a chance of failure in the formation of co-crystals by this method due to the inability to form crystal arrangement by poor grinding.
12
The heat produced during the grinding process may affect the stability of the material. Therefore high melting point drugs are usually employed for co-crystallization using mechanochemical grinding. Quashie et al have employed mechanochemical synthesis successfully for the preparation of co-crystals of sulfamethoxazole by using 8-hydroxy-7-iodoquinoline-5-sulfonic acid as a coformer.
44
Zaini et al have confirmed the formation of sulfamethoxazole-trimethoprim co-crystal by the milling process.
13
Liquid-assisted grinding
Liquid-assisted grinding (LAG) is an alteration of solid-state grinding method with additional small quantity of solvent in the process of development of co-crystal. Here the added solvent acts as a catalyst for the formation of co-crystals.
45
This method is advantageous when compared to the solvent evaporation technique due to its minimum time consumption and less requirement of the solvent. Thenge et al used LAG for the preparation of diacereinco-crystals from urea and tartaric acid. Both the coformer and drug were taken separately in 1: 1 ratio in a mortar and pestle and ground for 90 minutes by adding a few drops of ethanol approximately 10% of the total weight.
46
Solvent evaporation technique
Solvent evaporation is the most common and reliable method used in the preparation of co-crystals. In this technique, the drug and coformer are selected in a proper ratio and dissolved in a common solvent. The solvent is then evaporated at room temperature to get co-crystals. The solubility of drugs and coformer play a great role in the selection of a common solvent. The functional group of drug and coformer undergo intermolecular interaction such as H-bonding and form co-crystal. The requirement of the more quantity of solvent compared to LAG is the main drawback of this method.
12
Mounika et al prepared fexofenadine-tartaric acid co-crystals by various techniques and concluded that among various methods, the solvent evaporation technique is simple and there is an improvement in the stability and solubility of the drug.
47
Multidrug co-crystals like sulfadimidine–aspirin can also be prepared by this technique.
48
Savjani and Pathak prepared acyclovir co-crystals by solvent evaporation, wet grinding, and antisolvent addition. They found that the formation of co-crystals by the solvent evaporation method is better than the other methods used.
10
Slurrying
In the slurry co-crystallization, a solvent is added to the API along with its suitable coformer. The solvent is selected according to the solubility of the API and coformer in the solvent. Addition of the coformer to the solution followed by stirring is done for the co-crystals formation.
12
The solvent is then evaporated at room temperature to obtain co-crystals and can be subjected to PXRD for its characterization.
14,49-51
Antisolvent crystallization
Antisolvent crystallization is another method to prepare high-quality co-crystals.
52
During this process, a second liquid such as organic solvent or buffer is inserted to the drug coformer medium in order to achieve supersaturation. The added liquid should be miscible with solvent so as to precipitate the co-crystal.
9
The drawback of this method is requirement of large volume of solvent for the preparation.
16
Supercritical fluid processing
CO2 is the most used supercritical fluid in the preparation of co-crystals due to its penetration ability through the solids. The drug and coformer are added to a stainless steel vessel by dissolving in CO2 and rapid expansion of the CO2 by gradually decreasing the pressure leads to the formation of co-crystals.
40
The limited solubility of the drug and coformer in the supercritical fluid and less purity of the co-crystals are the main disadvantages of this method.
9
Hot melt extrusion
Hot melt extrusion can be employed for the preparation of co-crystals as a continuous manufacturing process in a single step.
53
In this method, the co-crystals are prepared by providing heat energy with high intense mixing to the drug and coformer to become miscible in the molten stage.
22
The criteria for this method are the drug and coformer, which should be miscible at the molten stage.
16
Sonocrystallization
Sonoreactor can be used for the preparation of co-crystals. In this method, drugs and coformer are dissolved in a common solvent and kept for sonication at a constant temperature.
54
Cold water is provided in order to maintain a constant temperature of sonicator.
22
The air bubbles or voids are produced due to the high energy, that makes size reduction and supersaturation, causes crystallization.
40
Further evaporation of the solvent accelerates the formation co-crystals.
Spray drying
Spray dryers can be used for preparing the co-crystals. The partners are dissolved in a common evaporating solvent and sprayed to a hot stream of air for evaporation of the solvent to yield good co-crystals.
22,54
Once after the preparation of the co-crystals, a rigorous scrutiny has been carried out for the confirmation and purity of the prepared cocrystals. The following section covers some of the characterization techniques that can be adopted for the assessment of co-crystals.
Characterization of co-crystals
The different instrumental analytical techniques used in the characterization of the cocrystals are shown in Figure 3.
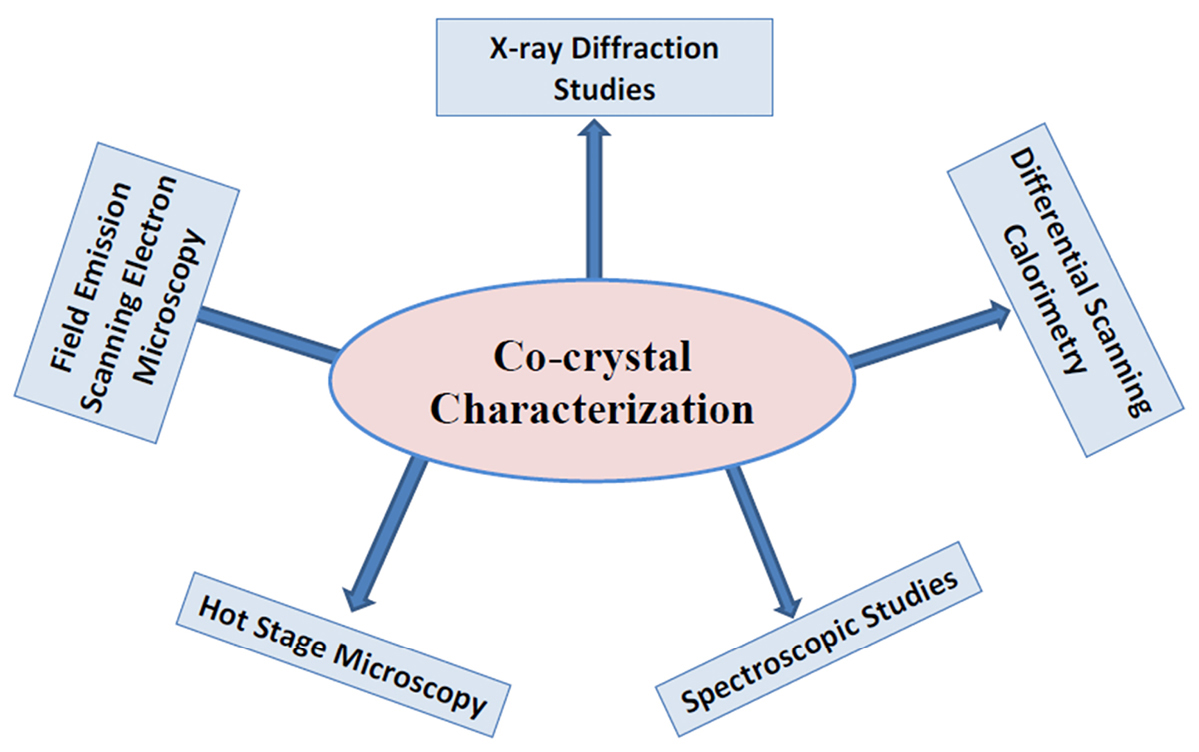
Figure 3.
Methods for characterization of co-crystals.
.
Methods for characterization of co-crystals.
X-ray diffraction (XRD) studies - single crystalline and powder XRD
This analytical tool is employed for phase identification of unit cells associated with the co-crystal. Complete structural information of co-crystals can be possible to derive from single and powder X-ray crystallography.
55
Powder XRD is regularly utilized in the identification of various co-crystals by detecting changes in the crystal lattice since different characteristic peaks are associated with different co-crystals while single crystal XRD is mainly employed for structural recognition by using software such as ‘DIFFRAC.SUITE TOPAS’. Difficulty in the procurement of a single crystal is the main problem associated with single crystal XRD.
22
For powder XRD, the sample is triturated to get a homogenous fine powder. The sample should obey Bragg’s law (n λ=2d sin θ) for analysis.
14,56
Differential scanning calorimetry
Differential scanning calorimetry (DSC) is widely used for the characterization of co-crystals in the pharmaceutical field. In this technique, the co-crystal and pure components are heated at a controlled rate and the obtained thermogram is scrutinized for checking the possibility of co-crystal formation.
9
In this process, eutectic melt generated at slow heating rates recrystallizes to the co-crystal form and then melts, irrespective of the ratio of drug and coformer.
57
The thermogram obtained by the DSC scan is used for screening of co-crystals as it allows the co-crystal detection. The thermogram of co-crystals shows a different exothermic peak from that of the pure thermogram of drug and coformer followed by an endothermic peak. The melting point and heat of fusion detected for co-crystals will be different from that of the pure components. If a physical mixture that cannot form co-crystals is heated, then only a single endothermic peak associated with eutectic melting can be seen in thermogram.
40,58
Spectroscopy – vibrational, nuclear magnetic resonance
In vibrational spectroscopy (infrared and Raman), the energy absorbed or scattered by the chemical bonds in the co-crystals will be different from that of the pure components, leads to the identification of the structural behavior of co-crystals. In infrared spectroscopy, co-crystals show a different spectrum of bands from the pure drug and coformer due to the formation of hydrogen bonding between them. A clear difference is seen in the bands of functional groups which have undergone hydrogen bonding. For example, a neutral carboxylic acid (COOH) group shows a stronger and weaker tension band of C=O at about 1700 cm-1 and 1200 cm-1, respectively, and carboxylic anion (COO-) shows a weak tension band in between 1000-1400 cm-1 due to the formation of salts. If the H-bonding occurs between OH….N, then two broad zones will be witnessed at about 2450 cm-1 and 1950 cm-1.
40
Solid-state nuclear magnetic resonanceis widely used for characterization of pharmaceutical co-crystals due to their ability to provide structural information of co-crystals.
59
This method is also used to distinguish co-crystals and salts since it can detect the degree of proton transfer.
22
One of the main disadvantages of this method is the low sensitivity of the instrument.
60
Field emission scanning electron microscopy (FESEM)
FESEM or topography is used to study the surface morphology of co-crystals. Micrographs of components and co-crystals obtained in the FESEM studies are utilized for the comparison. In the field emission electron microscope, heat energy is not used so-called “cold” source is employed. A strong electric field is utilized to emit the electrons from the surface of the conductor. A tungsten filament with a thin and sharp needle (tip diameter 10–100 nm) is employed as a cathode. The field emission source is attached with a scanning electron microscope for the capture of micrographs of co-crystals.
12,61-63
Hot Stage Microscopy
A combination of microscopy and thermal analysis is included in the hot stage microscopystudy. The physicochemical characteristic of a solid form is studied as a function of temperature and time. The changes occurred while heating the co-crystal sample placed on a glass slide are clearly observed under the microscope for assessing the changes such as melting point, melting range, and crystalline transformation.
40
Applications of co-crystals in pharmaceutical industry
When compared to other solid-state manipulation methods of a drug such as complexation, solid dispersion, micellar solubilization, cosolvency, etc, co-crystals gained tremendous advantages due to their ease of preparation in the pharmaceutical field.
64,65
As per the guidelines of USFDA and EMA, all the co-crystals prepared by various coformers can be patented separately, though still there are unclearness lasts. In the recent years, a tremendous increase in the patent application of cocrystals has been found in WIPO patent database.
37
Four marketed products of co-crystals have been noticed during 2014 to 2017.
66
The co-crystallization technique can be used for those drugs which are weakly ionized in nature.
22
Moreover, co-crystals can act as crystallization inhibitor and thereby super saturation can be maintained for a long time during dissolution, which in turn helps to achieve better bioavailability and controlled release of the drug.
2,37,67
Further the bioavailability of API in cocrystal form can be enhanced by preparing nanosized co-crystals.
34
But the preparation of nano co-crystals are challenging due to their phase instability of co-crystals in aqueous medium.
68
Recently multi-drug co-crystal (MDC) is also gaining attraction among pharmaceutical scientists.
69
MDC have synergistic effects, increased solubility, bioavailability, and potential to stabilize unstable components via intermolecular interactions.
48
Co-crystals also used for the in process separation and purification of the API.
37
Rajput et al investigated on various new solid forms of etravirine (anti-HIV drug) and found improved solubility and stability of etravirine co-crystals when compared to salts. It was concluded that the co-crystals approach is a better option for improving the solubility of API compared to salt formation.
70
Nutraceuticals, which are having good health benefits can also be used as coformers for better-combined health benefits along with the API.
69,71
By using the coformers such as saccharin sodium, the bitter taste of the API can be modified thereby co-crystallization technique can be utilized in case of fast dissolving tablets. Though there are plenty of co-crystals available in the literature, some of the reported cocrystals are presented, based on the method of preparation, in Table 2.
Table 2.
Some reported co-crystals with their coformers and method of preparation
|
Drug
|
Coformer
|
Method of preparation
|
|
Acyclovir
10
|
Tartaric acid, malonic acid, adipic acid |
Solvent evaporation |
|
Aripiprazole
72
|
Orcinol |
|
|
Fexofenadine
47
|
Tartaric acid |
|
|
Ibuprofen
73,74
|
Nicotinamide |
|
|
Indomethacin
75,76
|
Saccharin |
|
|
Itraconazole
77
|
Succinic acid |
|
|
Myricetin
78-81
|
Isonicotinamide, caffeine, proline, nicotinamide, 4-cyano pyridine |
|
|
Valsartan
82
|
Succinic acid |
|
|
Aceclofenac
83
|
Nicotinamide |
Neat grinding |
|
Aripiprazole
72
|
Orcinol |
|
|
Etodolac
84
|
Salicylic acid, benzoic acid, malonic acid, cinnamic acid, tartaric acid, PABA, hippuric acid, ferulic acid, maleic acid, glutaric acid |
|
|
Acemetacin
85
|
Isonicotinamide, picolinamide, caprolactam |
Cooling crystallization |
|
Chloral hydrate
86
|
Betain |
|
|
Darunavir
87
|
Succinic acid |
|
|
Atorvastatin calcium
88
|
Aspartame |
Slurry method |
|
Baicalein
89
|
Nicotinamide |
High-pressure homogenization |
|
Caffeine
90-92
|
Oxalic acid, glutaric acid |
Twin screw extrusion, spray congealing, cooling crystallization |
|
Carbamazepine
90
|
Saccharin |
Twin screw extrusion |
|
Nicotinamide
90
|
Trance Cinnamic acid |
|
|
Furosemide
91
|
Anthranilamide, 4-toluamide, 2-picolinic acid, isoniazid, theophylline, 2,3,5,6-tetramethyl pyrazine, 2-picolinamide, pyrazine, piperazine |
Liquid-assisted grinding |
|
Gliclazide
92
|
Sebacic acid, α-hydroxyacetic acid |
|
|
Entacapone
93
|
Theophylline, nicotinamide, Acetamide isonicotinamide, pyrazinamide, isoniazid |
|
|
Salicylic acid
94
|
Nicotinic acid, DL- phenylalanine, 6-hydroxy nicotinic acid, benzamide, isonicotinamide |
|
|
Piroxicam
95
|
Sodium acetate, saccharin sodium, urea, nicotinamide, resorcinol |
Dry grinding |
|
Diacerin
46
|
Urea, tartaric acid |
Solvent drop grinding |
|
Fenofibrate
96
|
Nicotinamide |
|
|
Quercetin
97
|
Caffeine, nicotinamide |
Electrospray technique |
|
Exemestane
98
|
Maleic acid |
Slurry crystallization |
|
Isoniazide
99
|
Vanillic acid, ferulic acid, caffeic acid, resorcinol |
|
|
Megestrol acetate
98
|
Saccharin |
|
|
P-coumaric acid
100
|
Nicotinamide |
|
|
Mefenamic acid
101
|
Nicotinamide |
Co-milling |
Conclusion
Pharmaceutical co-crystals are a novel class of pharmaceutical substances which are proposing an apparent probability of advancement of polished physical properties offering new stable and patentable solid forms. These multi-component crystalline forms influence pertinent physicochemical parameters like solubility, dissolution rate, chemical stability, physical stability, etc. which in turn results in the materials with superior properties to those of the free drug. This review offers an insight to regulatory prospects with a standard description of various methods that can be employed in the preparation of co-crystals followed by their characterization. Prior to the preparation of co-crystals, it is advisable to perform the screening of the coformers and prediction of co-crystal formation to avoid unexpected results. As of our understanding, the intermolecular interaction that defines the formation of co-crystals could become a strengthening tool for assisting co-crystal based dosage form design. It is expected that, a drastic adaptation of the co-crystallization technique to prepare pharmaceutically useful co-crystals in the coming years with well elaborated regulatory frame work.
Ethical Issues
Not applicable
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors are thankful to Advance Research Wing, Rajiv Gandhi University of Health Sciences, Karnataka, for the financial support and Bapuji Pharmacy College, Davangere for providing all the library facilities.
References
-
Wouters J, Quéré L. Pharmaceutical Salts and Co-crystals. Cambridge: The Royal Society of Chemistry; 2012.
- Brittain HG. Cocrystal systems of pharmaceutical interest: 2010. Cryst Growth Des 2012; 12(2):1046-54. doi: 10.1021/cg201510n [Crossref] [ Google Scholar]
- Peltonen L. Practical guidelines for the characterization and quality control of pure drug nanoparticles and nano-cocrystals in the pharmaceutical industry. Adv Drug Deliv Rev 2018; 131:101-15. doi: 10.1016/j.addr.2018.06.009 [Crossref] [ Google Scholar]
- Blagden N, de Matas M, Gavan PT, York P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv Drug Deliv Rev 2007; 59(7):617-30. doi: 10.1016/j.addr.2007.05.011 [Crossref] [ Google Scholar]
- Sekhon BS. Pharmaceutical co-crystals - An update. Int Bull Drug Res 2010; 1(2):24-39. [ Google Scholar]
- Izutsu KI, Koide T, Takata N, Ikeda Y, Ono M, Inoue M. Characterization and quality control of pharmaceutical cocrystals. Chem Pharm Bull (Tokyo) 2016; 64(10):1421-30. doi: 10.1248/cpb.c16-00233 [Crossref] [ Google Scholar]
- Stahly GP. Stahly GPDiversity in single-and multiple-component crystalsThe search for and prevalence of polymorphs and cocrystals. Cryst Growth Des 2007; 7(6):1007-26. doi: 10.1021/cg060838j [Crossref] [ Google Scholar]
- Lange L, Lehmkemper K, Sadowski G. Predicting the aqueous solubility of pharmaceutical cocrystals as a function of pH and temperature. Cryst Growth Des 2016; 16(5):2726-40. doi: 10.1021/acs.cgd.6b00024 [Crossref] [ Google Scholar]
- Douroumis D, Ross SA, Nokhodchi A. Advanced methodologies for cocrystal synthesis. Adv Drug Deliv Rev 2017; 117:178-95. doi: 10.1016/j.addr.2017.07.008 [Crossref] [ Google Scholar]
- Savjani JK, Pathak C. Improvement of physicochemical parameters of acyclovir using cocrystallization approach. Braz J Pharm Sci 2016; 52(4):727-4. doi: 10.1590/S1984-82502016000400017 [Crossref] [ Google Scholar]
- Stoler E, Warner JC. Non-covalent derivatives: cocrystals and eutectics. Molecules 2015; 20(8):14833-48. doi: 10.3390/molecules200814833 [Crossref] [ Google Scholar]
- Kotak U, Prajapati VD, Solanki HK, Jani GK, Jha P. Co-crystallization technique its rationale and recent progress. World J Pharm Pharm Sci 2015; 4(4):1484-508. [ Google Scholar]
- Zaini E, Sumirtapura YC, Soewandhi SN, Halim A, Uekusa H, Fujii K. Cocrystalline phase transformation of binary mixture of trimethoprim and sulfamethoxazole by slurry technique. Asian J Pharm Clin Res 2010; 3(4):26-9. [ Google Scholar]
- Patole T, Deshpande A. Co-crystallization-a technique for solubility enhancement. Int J Pharm Sci Res 2014; 5(9):3566-76. doi: 10.13040/ijpsr.0975-8232.5(9).3566-76 [Crossref] [ Google Scholar]
- Ghadi R, Ghuge A, Ghumre S, Waghmare N, Kadam VJ. Co-crystals: emerging approach in pharmaceutical design. Indo Am J Pharm Res 2014; 4(7):3881-93. [ Google Scholar]
- Savjani JK. Co‐crystallization: an approach to improve the performance characteristics of active pharmaceutical ingredients. Asian J Pharm 2015; 9(3):147-51. doi: 10.4103/0973-8398.160309 [Crossref] [ Google Scholar]
- Eddleston MD, Patel B, Day GM, Jones W. Cocrystallization by freeze-drying: preparation of novel multicomponent crystal forms. Cryst Growth Des 2013; 13(10):4599-606. doi: 10.1021/cg401179s [Crossref] [ Google Scholar]
- Schultheiss N, Newman A. Pharmaceutical cocrystals and their physicochemical properties. Cryst Growth Des 2009; 9(6):2950-67. doi: 10.1021/cg900129f [Crossref] [ Google Scholar]
-
Regulatory classification of pharmaceutical co-crystals guidance for industry. Available from: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm. Accessed 10 December 2018.
-
Reflection paper on the use of cocrystals of active substances in medicinal products. Available from: https://www.ema.europa.eu/documents/scientific-guideline/reflection-paper-use-cocrystals-active-substances-medicinal-products_en.pdf. Accessed 10 December 2018.
- Chandel N, Gupta V, Pandey A, Saxena S, Choudhary S. Co-crystalization of aceclofenac and paracetamol and their characterization. Int J Pharm Life Sci 2011; 2(8):1020-8. [ Google Scholar]
- Kumar S, Nanda A. Pharmaceutical cocrystals: an overview. Indian J Pharm Sci 2017; 79(6):858-71. doi: 10.4172/pharmaceutical-sciences.1000302 [Crossref] [ Google Scholar]
- Korotkova EI, Kratochvíl B. Pharmaceutical cocrystals. Procedia Chem 2014; 10:473-6. doi: 10.1016/j.proche.2014.10.079 [Crossref] [ Google Scholar]
- Sekhon BS. Pharmaceutical co-crystals-a review. Ars Pharm 2009; 50(2):99-117. [ Google Scholar]
-
James KC. Solubility and Related Properties. Vol. 18. New York: Marcel Dekker, Inc; 1986.
-
Barton AFM. CRC Handbook of Solubility Parameters and other Cohesion Parameters. 2nd ed. CRC Press LLC; 1991.
- Fedors RF. A method for estimating both the solubility parameters and molar volumes of liquids. Polym Eng Sci 1974; 14(2):147-54. doi: 10.1002/pen.760140211 [Crossref] [ Google Scholar]
- Hoy KL. New values of the solubility parameters from vapor pressure data. J Paint Technol 1970; 42(541):76-118. [ Google Scholar]
- Gaikwad ER, Khabade SS, Sutar TB, Bhat MR, Payghan SA. Three-dimensional hansen solubility parameters as predictors of miscibility in cocrystal formation. Asian J Pharm 2017; 11(4):302-18. doi: 10.22377/ajp.v11i04.1627 [Crossref] [ Google Scholar]
- Salem A, Nagy S, Pál S, Széchenyi A. Reliability of the Hansen solubility parameters as co-crystal formation prediction tool. Int J Pharm 2019; 558:319-27. doi: 10.1016/j.ijpharm.2019.01.007 [Crossref] [ Google Scholar]
- Yadav S, Gupta PC, Sharma N, Kumar J. Cocrystals: an alternative approach to modify physicochemical properties of drugs. Int J Pharm Chem Biol Sci 2015; 5(2):427-36. [ Google Scholar]
- Bolla G, Nangia A. Binary and ternary cocrystals of sulfa drug acetazolamide with pyridine carboxamides and cyclic amides. IUCrJ 2016; 3(Pt 2):152-60. doi: 10.1107/s2052252516000543 [Crossref] [ Google Scholar]
- Fukte SR, Wagh MP, Rawat S. Coformer selection: an important tool in cocrystal formation. Int J Pharm Pharm Sci 2014; 6(7):9-14. [ Google Scholar]
- Peltonen L. Practical guidelines for the characterization and quality control of pure drug nanoparticles and nano-cocrystals in the pharmaceutical industry. Adv Drug Deliv Rev 2018; 131:101-15. doi: 10.1016/j.addr.2018.06.009 [Crossref] [ Google Scholar]
- Sathisaran I, Dalvi SV. Engineering cocrystals of poorly water-soluble drugs to enhance dissolution in aqueous medium. Pharmaceutics 2018; 10(3). doi: 10.3390/pharmaceutics10030108 [Crossref]
- Yamashita H, Hirakura Y, Yuda M, Terada K. Coformer screening using thermal analysis based on binary phase diagrams. Pharm Res 2014; 31(8):1946-57. doi: 10.1007/s11095-014-1296-4 [Crossref] [ Google Scholar]
- Karimi-Jafari M, Padrela L, Walker GM, Croker DM. Creating cocrystals: a review of pharmaceutical cocrystal preparation routes and applications. Cryst Growth Des 2018; 18(10):6370-87. doi: 10.1021/acs.cgd.8b00933 [Crossref] [ Google Scholar]
- Hong C, Xie Y, Yao Y, Li G, Yuan X, Shen H. A novel strategy for pharmaceutical cocrystal generation without knowledge of stoichiometric ratio: myricetin cocrystals and a ternary phase diagram. Pharm Res 2015; 32(1):47-60. doi: 10.1007/s11095-014-1443-y [Crossref] [ Google Scholar]
- Abramov YA, Loschen C, Klamt A. Rational coformer or solvent selection for pharmaceutical cocrystallization or desolvation. J Pharm Sci 2012; 101(10):3687-97. doi: 10.1002/jps.23227 [Crossref] [ Google Scholar]
- Karagianni A, Malamatari M, Kachrimanis K. Pharmaceutical cocrystals: new solid phase modification approaches for the formulation of APIs. Pharmaceutics 2018; 10(1). doi: 10.3390/pharmaceutics10010018 [Crossref]
- Aitipamula S, Banerjee R, Bansal AK, Biradha K, Cheney ML, Choudhury AR. Polymorphs, salts, and cocrystals: what’s in a name?. Cryst Growth Des 2012; 12(5):2147-52. doi: 10.1021/cg3002948 [Crossref] [ Google Scholar]
-
US Food and Drug Administration. Guidance for Industry: Regulatory Classification of Pharmaceutical Co-crystals. Available from: https://www.fda.gov/media/81824/download. Accessed 10 December 2018.
- Friščić T, Jones W. Recent advances in understanding the mechanism of cocrystal formation via grinding. Cryst Growth Des 2009; 9(3):1621-37. doi: 10.1021/cg800764n [Crossref] [ Google Scholar]
- Quashie A, Kingsford-Adaboh R, Gadzekpo VPY. Mechano synthesis of co-crystals of Sulfamethoxazole and 8-Hydroxy-7-Iodoquinoline-5-Sulfonic acid based on Green Chemistry principles. Sci Dev 2017; 1(1):88-97. [ Google Scholar]
- Gadade DD, Pekamwar SS. Pharmaceutical cocrystals: regulatory and strategic aspects, design and development. Adv Pharm Bull 2016; 6(4):479-94. doi: 10.15171/apb.2016.062 [Crossref] [ Google Scholar]
- Thenge RR, Patond VB, Adhao VS, Ajmire PV, Barde LN, Mahajan NM. Preparation and characterization of co-crystals of diacerein. Indones J Pharm 2017; 28(1):34-41. doi: 10.14499/indonesianjpharm28iss1pp34 [Crossref] [ Google Scholar]
- Mounika P, Raj SV, Divya G, Gowramma A, Vijayamma G. Preparation and characterization of novel co-crystal forms of fexofenadine. Int J Innov Pharm Res 2015; 6(1):458-63. [ Google Scholar]
- Thipparaboina R, Kumar D, Chavan RB, Shastri NR. Multidrug co-crystals: towards the development of effective therapeutic hybrids. Drug Discov Today 2016; 21(3):481-90. doi: 10.1016/j.drudis.2016.02.001 [Crossref] [ Google Scholar]
- Chandramouli Y, Gandhimathi R, Yasmeen BR, Vikram A, Mahitha B, Imroz SM. Review on cocrystal as an approach with newer implications in pharmaceutical field. Int J Med Chem Anal 2012; 2(2):91-100. [ Google Scholar]
- Sanjay AN, Manohar SD, Bhanudas SR. Pharmaceutical cocrystallization: a review. J Adv Pharm Educ Res 2014; 4(4):388-96. [ Google Scholar]
- Yadav AV, Shete AS, Dabke AP, Kulkarni PV, Sakhare SS. Co-crystals: a novel approach to modify physicochemical properties of active pharmaceutical ingredients. Indian J Pharm Sci 2009; 71(4):359-70. doi: 10.4103/0250-474x.57283 [Crossref] [ Google Scholar]
- Raza SA, Schacht U, Svoboda V, Edwards DP, Florence AJ, Pulham CR. Rapid Continuous Antisolvent Crystallization of Multicomponent Systems. Cryst Growth Des 2018; 18(1):210-8. doi: 10.1021/acs.cgd.7b01105 [Crossref] [ Google Scholar]
- Gajda M, Nartowski KP, Pluta J, Karolewicz B. Continuous, one-step synthesis of pharmaceutical cocrystals via hot melt extrusion from neat to matrix-assisted processing - State of the art. Int J Pharm 2019; 558:426-40. doi: 10.1016/j.ijpharm.2019.01.016 [Crossref] [ Google Scholar]
- Chaudhari S, Nikam S, Khatri N, Wakde S. Co-crystals: a review. J Drug Deliv Ther 2018; 8(6S):350-8. doi: 10.22270/jddt.v8i6-s.2194 [Crossref] [ Google Scholar]
- Lapidus SH, Stephens PW, Arora KK, Shattock TR, Zaworotko MJ. A comparison of cocrystal structure solutions from powder and single crystal techniques. Cryst Growth Des 2010; 10(10):4630-7. doi: 10.1021/cg1009237 [Crossref] [ Google Scholar]
- Aitipamula S, Vangala VR. X-ray crystallography and its role in understanding the physicochemical properties of pharmaceutical cocrystals. J Indian Inst Sci 2017; 97(2):227-43. doi: 10.1007/s41745-017-0026-4 [Crossref] [ Google Scholar]
- Mohammad MA, Alhalaweh A, Velaga SP. Hansen solubility parameter as a tool to predict cocrystal formation. Int J Pharm 2011; 407(1-2):63-71. doi: 10.1016/j.ijpharm.2011.01.030 [Crossref] [ Google Scholar]
- Saganowska P, Wesolowski M. DSC as a screening tool for rapid co-crystal detection in binary mixtures of benzodiazepines with co-formers. J Therm Anal Calorim 2018; 133(1):785-95. doi: 10.1007/s10973-017-6858-3 [Crossref] [ Google Scholar]
- Vogt FG, Clawson JS, Strohmeier M, Edwards AJ, Pham TN, Watson SA. Solid-state NMR analysis of organic cocrystals and complexes. Cryst Growth Des 2009; 9(2):921-37. doi: 10.1021/cg8007014 [Crossref] [ Google Scholar]
- Zhao L, Hanrahan MP, Chakravarty P, DiPasquale AG, Sirois LE, Nagapudi K. Characterization of pharmaceutical cocrystals and salts by dynamic nuclear polarization-enhanced solid-state NMR spectroscopy. Cryst Growth Des 2018; 18(4):2588-601. doi: 10.1021/acs.cgd.8b00203 [Crossref] [ Google Scholar]
-
Yao H, Kimura K. Field emission scanning electron microscopy for structural characterization of 3D gold nanoparticle superlattices. In: Méndez-Vilas A, Díaz J, eds. Modern Research and Educational Topics in Microscopy. Formatex; 2007. p. 568-75.
- Gnanamoorthy P, Karthikeyan V, Prabu VA. Field Emission Scanning Electron Microscopy (FESEM) characterisation of the porous silica nanoparticulate structure of marine diatoms. J Porous Mater 2014; 21(2):225-33. doi: 10.1007/s10934-013-9767-2 [Crossref] [ Google Scholar]
- Perera Y, Cano J, Martínez S, Quercia Bianchi G, Blanco A. Characterization of Nano-Cement phases by field Emission Scanning electron Microscopy (FESEM). Acta Microsc 2007; 16(1-2):206-7. [ Google Scholar]
- Rekdal M, Pai A, Choudhari R, Badamane Sathyanarayana M. Applications of co-crystals in pharmaceutical drugs. Sys Rev Pharm 2018; 9(1):55-7. doi: 10.5530/srp.2018.1.11 [Crossref] [ Google Scholar]
-
Leusen FJJ, Kendrick J. Pharmaceutical Salts and Co-crystals. RCS Publishing; 2012. p. 44-61.
- Kavanagh ON, Croker DM, Walker GM, Zaworotko MJ. Pharmaceutical cocrystals: from serendipity to design to application. Drug Discov Today 2019; 24(3):796-804. doi: 10.1016/j.drudis.2018.11.023 [Crossref] [ Google Scholar]
- Dai XL, Chen JM, Lu TB. Pharmaceutical cocrystallization: an effective approach to modulate the physicochemical properties of solid-state drugs. CrystEngComm 2018; 20(36):5292-316. doi: 10.1039/C8CE00707A [Crossref] [ Google Scholar]
- Emami S, Siahi-Shadbad M, Adibkia K, Barzegar-Jalali M. Recent advances in improving oral drug bioavailability by cocrystals. Bioimpacts 2018; 8(4):305-20. doi: 10.15171/bi.2018.33 [Crossref] [ Google Scholar]
- Thakuria R, Sarma B. Drug-drug and drug-nutraceutical cocrystal/salt as alternative medicine for combination therapy: a crystal engineering approach. Crystals 2018; 8(2):101. doi: 10.3390/cryst8020101 [Crossref] [ Google Scholar]
- Rajput L, Sanphui P, Desiraju GR. New solid forms of the anti-HIV drug etravirine: salts, cocrystals, and solubility. Cryst Growth Des 2013; 13(8):3681-90. doi: 10.1021/cg4007058 [Crossref] [ Google Scholar]
- Sinha AS, Maguire AR, Lawrence SE. Cocrystallization of nutraceuticals. Cryst Growth Des 2015; 15(2):984-1009. doi: 10.1021/cg501009c [Crossref] [ Google Scholar]
- Cho MY, Kim P, Kim GY, Lee JY, Song KH, Lee MJ. Preparation and characterization of aripiprazole cocrystals with coformers of multihydroxybenzene compounds. Cryst Growth Des 2017; 17(12):6641-52. doi: 10.1021/acs.cgd.7b01281 [Crossref] [ Google Scholar]
- Chow SF, Chen M, Shi L, Chow AH, Sun CC. Simultaneously improving the mechanical properties, dissolution performance, and hygroscopicity of ibuprofen and flurbiprofen by cocrystallization with nicotinamide. Pharm Res 2012; 29(7):1854-65. doi: 10.1007/s11095-012-0709-5 [Crossref] [ Google Scholar]
- Yuliandra Y, Zaini E, Syofyan S, Pratiwi W, Putri LN, Pratiwi YS. Cocrystal of ibuprofen-nicotinamide: solid-state characterization and in vivo analgesic activity evaluation. Sci Pharm 2018; 86(2). doi: 10.3390/scipharm86020023 [Crossref]
- Basavoju S, Bostrom D, Velaga SP. Indomethacin-saccharin cocrystal: design, synthesis and preliminary pharmaceutical characterization. Pharm Res 2008; 25(3):530-41. doi: 10.1007/s11095-007-9394-1 [Crossref] [ Google Scholar]
- Moradiya HG, Islam MT, Scoutaris N, Halsey SA, Chowdhry BZ, Douroumis D. Continuous manufacturing of high quality pharmaceutical cocrystals integrated with process analytical tools for in-line process control. Cryst Growth Des 2016; 16(6):3425-34. doi: 10.1021/acs.cgd.6b00402 [Crossref] [ Google Scholar]
- Shete A, Murthy S, Thorat B, Yadav A, Sajane S, Sakhare S. Studies on effect of hydrophilic polymers on physicochemical properties of itraconazole cocrystals. Future J Pharm Sci 2017; 3:95-102. doi: 10.1016/j.fjps.2017.04.005 [Crossref] [ Google Scholar]
- Ren S, Liu M, Hong C, Li G, Sun J, Wang J. The effects of pH, surfactant, ion concentration, coformer, and molecular arrangement on the solubility behavior of myricetin cocrystals. Acta Pharm Sin B 2019; 9(1):59-73. doi: 10.1016/j.apsb.2018.09.008 [Crossref] [ Google Scholar]
- Hong C, Xie Y, Yao Y, Li G, Yuan X, Shen H. A novel strategy for pharmaceutical cocrystal generation without knowledge of stoichiometric ratio: myricetin cocrystals and a ternary phase diagram. Pharm Res 2015; 32(1):47-60. doi: 10.1007/s11095-014-1443-y [Crossref] [ Google Scholar]
- Liu M, Hong C, Yao Y, Shen H, Ji G, Li G. Development of a pharmaceutical cocrystal with solution crystallization technology: Preparation, characterization, and evaluation of myricetin-proline cocrystals. Eur J Pharm Biopharm 2016; 107:151-9. doi: 10.1016/j.ejpb.2016.07.008 [Crossref] [ Google Scholar]
- Liu M, Hong C, Li G, Ma P, Xie Y. The generation of myricetin-nicotinamide nanococrystals by top down and bottom up technologies. Nanotechnology 2016; 27(39):395601. doi: 10.1088/0957-4484/27/39/395601 [Crossref] [ Google Scholar]
- Thomas JE, Nayak UY, Jagadish PC, Koteshwara KB. Design and characterization of valsartan co-crystals to improve its aqueous solubility and dissolution behavior. Res J Pharm Technol 2017; 10(1):26-30. doi: 10.5958/0974-360X.2017.00007.5 [Crossref] [ Google Scholar]
- Sevukarajan M, Thanuja B, Sodanapalli R, Nair R. Synthesis and characterization of a pharmaceutical co-crystal: (aceclofenac: nicotinamide). J Pharm Sci Res 2011; 3(6):1288-93. [ Google Scholar]
- Gadade DD, Pekamwar SS, Lahoti SR, Patni SD, Sarode MC. Cocrystallization of etodolac: prediction of cocrystallization, synthesis, solid state characterization and in vitro drug release. Marmara Pharm J 2017; 21(1):78-88. doi: 10.12991/marupj.259884 [Crossref] [ Google Scholar]
- Sanphui P, Bolla G, Nangia A, Chernyshev V. Acemetacin cocrystals and salts: structure solution from powder X-ray data and form selection of the piperazine salt. IUCrJ 2014; 1(Pt 2):136-50. doi: 10.1107/s2052252514004229 [Crossref] [ Google Scholar]
- O’ Nolan D, Perry ML, Zaworotko MJ. Chloral hydrate polymorphs and cocrystal revisited: solving two pharmaceutical cold cases. Cryst Growth Des 2016; 16(4):2211-7. doi: 10.1021/acs.cgd.6b00032 [Crossref] [ Google Scholar]
- Bagde SA, Upadhye KP, Dixit GR, Bakhle SS. Formulation and evaluation of co-crystals of poorly water soluble drug. Int J Pharm Sci Res 2016; 7(12):4988-97. doi: 10.13040/IJPSR.0975-8232.7(12).4988-97 [Crossref] [ Google Scholar]
- Gozali D, Megantara S, Levita J, Bahti HH, Soewandhi SN, Abdassah M. Virtual screening of coformers for atorvastatin co-crystallization and the characterizations of the co-crystals. Int J Pharm Sci Res 2016; 7(4):1450-5. doi: 10.13040/IJPSR.0975-8232.7 [Crossref] [ Google Scholar]
- Pi J, Wang S, Li W, Kebebe D, Zhang Y, Zhang B. A nano-cocrystal strategy to improve the dissolution rate and oral bioavailability of baicalein. Asian J Pharm Sci 2019; 14(2):154-64. doi: 10.1016/j.ajps.2018.04.009 [Crossref] [ Google Scholar]
- Daurio D, Medina C, Saw R, Nagapudi K, Alvarez-Núñez F. Application of twin screw extrusion in the manufacture of cocrystals, part I: four case studies. Pharmaceutics 2011; 3(3):582-600. doi: 10.3390/pharmaceutics3030582 [Crossref] [ Google Scholar]
- Duarte Í, Andrade R, Pinto JF, Temtem M. Thermal analysis, X-ray powder diffraction and electron microscopy data related with the production of 1:1 Caffeine:Glutaric Acid cocrystals. Data Brief 2016; 8:247-50. doi: 10.1016/j.dib.2016.04.074 [Crossref] [ Google Scholar]
- Yu ZQ, Chow PS, Tan RBH. Operating regions in cooling cocrystallization of caffeine and glutaric acid in acetonitrile. Cryst Growth Des 2010; 10(5):2382-7. doi: 10.1021/cg100198u [Crossref] [ Google Scholar]
- Bommaka MK, Mannava MKC, Suresh K, Gunnam A, Nangia A. Entacapone: improving aqueous solubility, diffusion permeability, and cocrystal stability with theophylline. Cryst Growth Des 2018; 18(10):6061-9. doi: 10.1021/acs.cgd.8b00921 [Crossref] [ Google Scholar]
- Zhou Z, Chan HM, Sung HH, Tong HH, Zheng Y. Identification of new cocrystal systems with stoichiometric diversity of salicylic acid using thermal methods. Pharm Res 2016; 33(4):1030-9. doi: 10.1007/s11095-015-1849-1 [Crossref] [ Google Scholar]
- Panzade P, Shendarkar G, Shaikh S, Balmukund Rathi P. Pharmaceutical cocrystal of piroxicam: design, formulation and evaluation. Adv Pharm Bull 2017; 7(3):399-408. doi: 10.15171/apb.2017.048 [Crossref] [ Google Scholar]
- Shewale S, Shete AS, Doijad RC, Kadam SS, Patil VA, Yadav AV. Formulation and solid state characterization of nicotinamide-based co-crystals of fenofibrate. Indian J Pharm Sci 2015; 77(3):328-34. doi: 10.4103/0250-474x.159669 [Crossref] [ Google Scholar]
- Patil S, Chaudhari K, Kamble R. Electrospray technique for cocrystallization of phytomolecules. J King Saud Univ Sci 2018; 30(1):138-41. doi: 10.1016/j.jksus.2017.04.001 [Crossref] [ Google Scholar]
- Shiraki K, Takata N, Takano R, Hayashi Y, Terada K. Dissolution improvement and the mechanism of the improvement from cocrystallization of poorly water-soluble compounds. Pharm Res 2008; 25(11):2581-92. doi: 10.1007/s11095-008-9676-2 [Crossref] [ Google Scholar]
- Swapna B, Maddileti D, Nangia A. Cocrystals of the tuberculosis drug isoniazid: polymorphism, isostructurality, and stability. Cryst Growth Des 2014; 14(11):5991-6005. doi: 10.1021/cg501182t [Crossref] [ Google Scholar]
- Bevill MJ, Vlahova PI, Smit JP. Polymorphic Cocrystals of nutraceutical compound p-coumaric acid with nicotinamide: characterization, relative solid-state stability, and conversion to alternate stoichiometries. Cryst Growth Des 2014; 14(3):1438-48. doi: 10.1021/cg4019037 [Crossref] [ Google Scholar]
- Utami D, Nugrahani I, Ibrahim S. Formation and characterization of mefenamic acid-nicotinamide cocrystal during co-milling based on X-ray powder diffraction analysis. J Appl Pharm Sci 2016; 6(10):75-81. doi: 10.7324/JAPS.2016.601010 [Crossref] [ Google Scholar]