Advanced pharmaceutical bulletin. 10(4):542-555.
doi: 10.34172/apb.2020.065
Review Article
The Evaluation of Effective Drugs for the Treatment of Non-Alcoholic Fatty Liver Disease: A Systematic Review and Network Meta-Analysis
Ramin Jalili 1  , Mohammad Hossein Somi 2, Hossein Hosseinifard 3, Fatemeh Salehnia 3, Morteza Ghojazadeh 3, Nima Makhdami 4, Masoud Shirmohammadi 5, *
, Mohammad Hossein Somi 2, Hossein Hosseinifard 3, Fatemeh Salehnia 3, Morteza Ghojazadeh 3, Nima Makhdami 4, Masoud Shirmohammadi 5, * 
Author information:
1Department of Internal Medicine, Tabriz University of Medical Sciences, Tabriz, Iran.
2Liver and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
3Research Center for Evidence Based Medicine (RCEBM), Tabriz University of Medical Sciences, Tabriz, Iran.
4St Joseph’s Hospital, Hamilton, Canada.
5Department of Gastroenterology, Liver, and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Abstract
Purpose:
Non-alcoholic fatty liver disease (NAFLD) and steatohepatitis are two forms of fatty liver disease with benign and malignant nature, respectively. These two conditions can cause an increased risk of liver cirrhosis and hepatocellular carcinoma. Given the importance and high prevalence of NAFLD, it is necessary to investigate the results of different studies in related scope to provide a clarity guarantee of effectiveness. Therefore, this systematic review and meta-analysis aim to study the efficacy of various medications used in the treatment of NAFLD.
Methods:
A systematic search of medical databases identified 1963 articles. After exclusion of duplicated articles and those which did not meet our inclusion criteria, eta-analysis was performed on 84 articles. Serum levels of alanine aminotransferase (ALT), aspartate amino transferase (AST) were set as primary outcomes and body mass index (BMI), hepatic steatosis, and NAFLD activity score (NAS) were determined as secondary outcomes.
Results:
Based on the P-score of the therapeutic effects on the non-alcoholic steatohepatitis (NASH), we observed the highest efficacy for atorvastatin, tryptophan, orlistat, omega-3 and obeticholic acid for reduction of ALT, AST, BMI, steatosis and NAS respectively.
Conclusion:
This meta-analysis showed that atorvastatin. life-style modification, weight loss, and BMI reduction had a remarkable effect on NAFLD-patients by decreasing aminotransferases.
Keywords: Non-alcoholic fatty liver disease, Therapeutic, Systematic review, Network meta-analysis
Copyright and License Information
© 2020 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
The non-alcoholic fatty liver disease (NAFLD) covers a broad spectrum of liver disease ranging from pure fatty liver to non-alcoholic steatohepatitis (NASH) and finally cirrhosis. It occurs in patients without a significant alcohol intake.
1
Although the pathogenesis of NAFLD is not well-understood, some studies support the “two-hit theory.” While the “first hit” involves insulin resistance and results in the collection of fat in the liver, the “second hit” consists of oxidative stress resulting in lipid peroxidation, hepatocellular degeneration, cell death, hepatic stellate cell (HSC) activation, and fibrogenesis.
2,3
End-stage liver disease and hepatocellular carcinoma secondary to NAFLD are the second leading cause of liver transplantation in the United States. This is an alarming trend that NAFLD is replacing hepatitis C as the most common indication for liver transplantation during the future decades.
4-6
As a result of obesity epidemics, the prevalence of NAFLD, as a common liver disease, has dramatically increased. Estimations reveal an incidence of up to 20%-30% for NAFLD in Western and 5-18% in Asian countries.
7
Due to the inappropriate diet and sedentary lifestyle, the increased prevalence of NAFLD throughout the world is considered a common clinical concern over time.
8
Weight loss, achieved either by a hypocaloric diet alone or in combination with increased physical activity, reduces hepatic steatosis a combination of a hypocaloric diet (daily caloric reduction of 500-1000 kcal), and moderate-intensity exercise is presumably to provide the best likelihood of improving steatosis. However, a more significant weight loss (7%-10%) is required to improve the majority of the histopathological features of NASH.
9
Although NAFLD is associated with components of metabolic syndrome, the presence of an increasing number of metabolic disorders, including insulin resistance, type 2 diabetes, hypertension dyslipidemia, and visceral obesity, seems to increase the risk of progressive fatty liver disease.
10-12
Therefore, NAFLD-patients are at the highest risk for adverse outcomes because of multiple risk factors such as type-2 diabetes mellitus (T2DM) and hypertension.
13
The current standard of care (SOC) for managing NAFLD mainly includes caloric restriction of 25–30 kcal/kg/day ideal body weight and moderate physical activity.
14
Although there are no well-established guidelines recommendations for managing NAFLD,
15
medications that are mainly prescribed include antioxidants (e.g. vitamins E and C, betaine), insulin-sensitizing agents (thiazolidinediones and metformin), lipid-lowering agents (statins, orlistat, probucol), choleretic agents such as ursodeoxycholic acid (UCDA), and medications with anti-inflammatory (pentoxifylline) or anti-fibrotic (angiotensin-receptor blockers) potential.
16
Given the enormous variety of prescribed drugs in clinical trials for the treatment of NAFLD, multi-drug combination studies and many not-investigated drugs in head-to-head trials, we have aimed to evaluate the effectiveness of most commonly used medications on reducing liver transaminases (alanine aminotransferase (ALT), aspartate amino transferase (AST), body mass index (BMI), NAFLD activity score (NAS) and steatosis in patients with NAFLD in a systematic review and network meta-analysis.
Materials and Methods
Search methodology
This systematic review and network meta-analysis of randomized clinical trials evaluate the efficacy of NAFLD treatments. Medline, Scopus, Web of Science, Embase, and Cochran Library databases were searched for clinical trials that examined the effects of therapeutic interventions in NAFLD patients. We used the following keywords in our database search: NAFLD, non-alcoholic fatty liver disease, fatty liver, fatty liver disease, NASH, hepatic steatosis, and steatohepatitis by combining the OR, AND and NOT operators. Table 1, illustrates the abbreviations that are used throughout this paper to describe the different studied medications.
Table 1.
Medication name and their abbreviations used in this manuscript
|
Medication name
|
Abbreviation
|
Medication name
|
Abbreviation
|
| Metformin |
Met |
Ipragliflozin |
Lpra |
| Melatonin |
Mela |
Losartan |
Losa |
| Liraglutide |
Lira |
Insulin |
Insu |
| L-carnitine |
L-Car |
Gliclazide |
Glicl |
| Sitagliptin |
Sita |
Rosiglitazone |
Rosi |
| Silymarin |
Silly |
Resveratrol |
Resv |
| Probucol |
Probu |
Pentoxifylline |
Pant |
| Probiotic |
Prob |
Orlistat |
Orli |
| Placebo |
Plac |
Omega-3 Acid |
Omeg3 |
| Pioglitazone |
Piog |
Obeticholic |
Obet |
| Fenofibrate |
Feno |
Exenatide |
Exen |
| Ezetimbe |
Ezet |
Colesevelam |
Coles |
| Bicycline |
Bicy |
Atorvastatin |
Atro |
| Betaine |
Beta |
Vitamin- E |
Vit-E |
| Anti-Oxidants |
Antiox |
Vildagliptin |
Vida |
| Ursodeoxycholic acid |
UDCA |
Tocotrienol |
Toco |
| Tryptophan |
Tryp |
Telmisartan |
Telm |
| Symbiotic |
Synb |
Standard of Care |
SOC |
Manual search also performed to prevent the loss of published articles in the study sources. Correspondence was communicated with the authors of selected articles in order to obtain unpublished articles as well.
Inclusion, exclusion and eligibility criteria
We included publications that: had studied the various medical interventions for the treatment of NAFLD, were published in English language, were published to January 2019 and also paperers which were presented in different conferences.
We excluded: articles that were published in languages other than English, animal studies, research studies with no apparent sample size and, low quality research works.
Additionally, we only included clinical trials that were conducted on patients with NAFLD and had compared an active drug with another active drug or placebo.
Methodological quality
Select studies
The selected articles were processed in 3 states by the subject experts: The titles of all review and incompatible articles were excluded. Abstracts and full texts of the articles were studied.
The selected studies were evaluated by the two experts for recognizing the risk of bias using Joanna Briggs Institute tool for critically appraising the clinical trials; the controversies between them were resolved by a third expert.
17
The critical appraisal form of the select studies is shown in Table S1.
18-101
The extracted information was summarized in the data extraction table (Table 2). Extracted data were included: first author, publication year, country, sample size, and number of participants over. Finally, the Endnote X5 software was used to manage the references.
Table 2.
Critical appraisal results of eligible studies
|
Study
|
Q1
|
Q2
|
Q3
|
Q4
|
Q5
|
Q6
|
Q7
|
Q8
|
Q9
|
Q10
|
Q11
|
Q12
|
Q13
|
|
Marchesini et al (2001)
18
|
Y |
N |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Athyros et al (2006)
19
|
Y |
N |
Y |
N |
N |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Ratziu et al (2008)
20
|
Y |
N |
Y |
Y |
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Hatzitolios et al (2004)
21
|
Y |
N |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Fan et al (2013)
22
|
Y |
N |
Y |
N |
N |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Capanni et al (2006)
23
|
Y |
N |
Y |
Y |
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Solhi et al (2014)
24
|
Y |
N |
U |
U |
U |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Khoshbaten et al (2010)
25
|
Y |
N |
Y |
Y |
N |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Lindor et al (2004)
26
|
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Zein et al (2011)
27
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Mumtaz et al (2017)
28
|
Y |
N |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Shahebrahimi et al (2017)
29
|
Y |
N |
Y |
Y |
N |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Wong et al (2017)
30
|
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Oscarsson et al (2018)
31
|
Y |
N |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Ito et al (2017)
32
|
Y |
N |
Y |
N |
N |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Asghari et al (2018)
33
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Celinski et al (2014)
34
|
Y |
N |
Y |
U |
U |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Neuschwander-Tetri et al (2015)
35
|
Y |
Y |
Y |
Y |
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Abdelmalek et al (2009)
36
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Alam et al (2016)
37
|
Y |
N |
Y |
N |
N |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Aller et al (2011)
38
|
Y |
N |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Lonardo et al (2015)
39
|
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Armstrong et al (2016)
40
|
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Balas et al (2007)
41
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Baniasadi et al (2015)
42
|
Y |
N |
Y |
Y |
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Belfort et al (2006)
43
|
Y |
N |
Y |
Y |
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Chachay et al (2014)
44
|
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Xu et al (2015)
45
|
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Cui et al (2016)
46
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Cusi et al (2016)
47
|
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Deng et al (2017)
48
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Ebrahimi et al (2016)
49
|
Y |
N |
Y |
Y |
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Ekhlasi et al (2017)
50
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Eslamparast et al (2014)
51
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Faghihzadeh et al (2014)
52
|
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Feng et al (2017)
53
|
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Garinis et al (2010)
54
|
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Hajiaghamohammadi et al (2012)
55
|
Y |
N |
Y |
U |
U |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Han et al (2014)
56
|
Y |
N |
Y |
U |
U |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Hannah and Harrison (2003)
57
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Harrison et al (2009)
58
|
Y |
N |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Haukeland (2009)
59
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Heebøll et al (2016)
60
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Hirataet al (2013)
61
|
Y |
N |
Y |
N |
N |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Hussain et al (2016)
62
|
Y |
N |
Y |
U |
U |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Khoo et al (2017)
63
|
Y |
N |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Le et al (2012)
64
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Lee et al (2008)
65
|
Y |
Y |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Liechti et al (2012)
66
|
Y |
N |
Y |
Y |
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Le and Wang (2017)
67
|
Y |
N |
Y |
N |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Loomba (2015)
68
|
Y |
Y |
Y |
Y |
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
McPherson et al (2017)
69
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Mendez-Sanchez et al (2004)
70
|
Y |
N |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Merat et al (2003)
71
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Mofidi et al (2017)
72
|
Y |
N |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
van Wagner et al (2011)
73
|
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Dagan et al (2006)
74
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Wah Kheong et al (2017)
75
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Wong et al (2013)
76
|
Y |
N |
Y |
U |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Tiikkainen et al (2004)
77
|
Y |
N |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Zhu et al (2008)
78
|
Y |
N |
Y |
U |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Mudaliar et al (2013)
79
|
Y |
Y |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Nelson et al (2009)
80
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Nogueira et al (2016)
81
|
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Omer et al (2009-10)
82
|
Y |
N |
Y |
N |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Pakravan et al (2017)
83
|
Y |
N |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Parikh et al (2016)
84
|
Y |
N |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Ratziu et al (2016)
85
|
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Shenoy et al (2014)
96
|
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Shang et al (2008)
95
|
Y |
N |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Sofi et al (2010)
94
|
Y |
N |
Y |
U |
U |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Takeshita et al (2014)
93
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Shibuya et al (2017)
92
|
Y |
N |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Chen et al (2015)
91
|
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Sofer (2011)
100
|
Y |
N |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Sharma et al (2012)
90
|
Y |
N |
Y |
N |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Harrison et al (2014)
89
|
Y |
N |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Sanyal et al (2010)
88
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N |
Y |
Y |
Y |
|
Santos et al (2003)
87
|
Y |
N |
U |
Y |
Y |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Razavizade et al (2013)
86
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Hameed et al (2017)
97
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Hosseinpour-Arjmand et al (2018)
98
|
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Kuchay et al (2018)
99
|
Y |
N |
U |
N |
N |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Abenavoli et al (2017)
101
|
Y |
N |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Total % |
100 |
30.9 |
96.4 |
79.7 |
77.3 |
51.1 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
Y = Yes, N = No, U = Unclear; JBI critical appraisal checklist for randomized controlled trials: Q1 = Was true randomization used for assignment of participants to treatment groups?; Q2 = Was allocation to treatment groups concealed?; Q3 = Were treatment groups similar at baseline?; Q4 = Were participants blind to treatment assignment?; Q5 = Were those delivering treatment blind to treatment assignment?; Q6 = Were outcome assessors blind to treatment assignment?; Q7 = Were treatment groups treated identically other than the intervention of interest?; Q8 = Was follow-up complete, and if not, were strategies to address incomplete follow-up utilized?; Q9 = Were participants analyzed in the groups to which they were randomized?; Q10 = Were outcomes measured in the same way for treatment groups?; Q11 = Were outcomes measured in a reliable way?; Q12 = Was appropriate statistical analysis used?; Q13 = Was the trial design appropriate, and any deviations from the standard RCT design (individual randomization, parallel groups) accounted for in the conduct and analysis of the trial?
Publication bias
The funnel plot (Figure 1) illustrates the publication bias of the studies that have been entered into the network meta-analysis. This symmetry shows that there has been evidence of publication bias in this meta-analysis.

Figure 1.
Funnel plot of selected articles showing degrees of publication bias.
.
Funnel plot of selected articles showing degrees of publication bias.
Primary and secondary outcomes in the study
The primary outcome measure in this study was the mean changes in ALT and AST levels.
Changes in BMI, NAS, and steatosis were considered as secondary outcomes.
Statistical methods
Investigation of stability and similarity assumptions in intervention network:
To assess the similarity hypothesis, the baseline characteristics of the participants were assessed, and trials with no similarity hypothesis were included in the network meta-analysis.
The heterogeneity between studies was analyzed via the Cochran Q test and I-square statistic.
Rankings of treatments P-scores were used to rank for each treatment. P-scores are calculated on the basis of defect estimation and standard error estimates in the grid. The wake score is between zero and one, and the closer the wake of a cure to a cure, the better the cure. We have utilized the GRADE approach to calculate the level of evidence; the level of evidence has been categorized to very low, low, moderate and high taking bias, inconsistency, indirectness and imprecision into account.
Results and Discussion
Search results and basic characteristics of studies entered into a meta-analysis of networks. The data extraction is represented in Table S1.
After excluding duplicate papers, and papers that did not meet the inclusion criteria we entered 84 to the final meta-analysis. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram (Figure 2) illustrates the number of the articles screened for final number of analyzed articles.
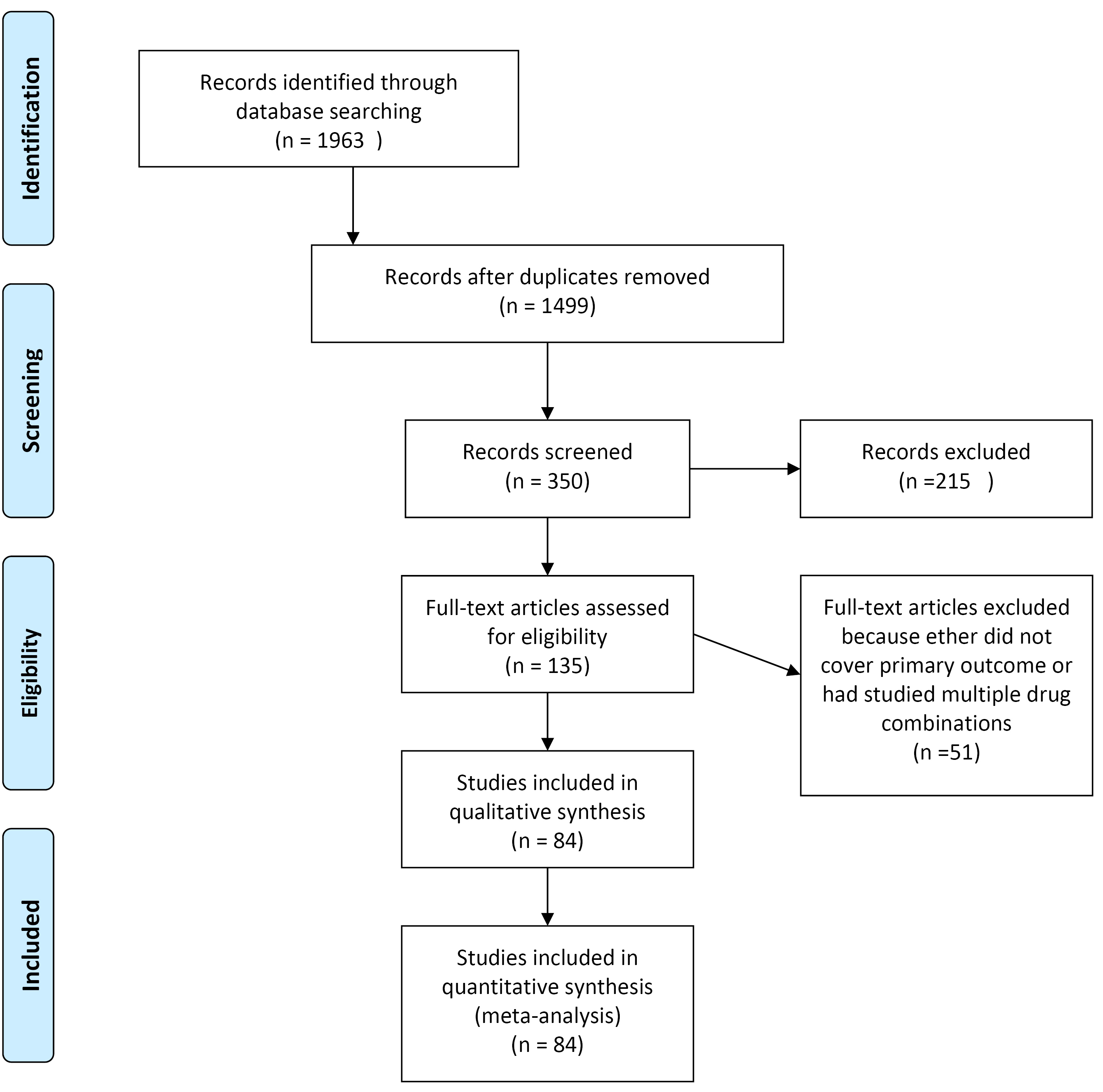
Figure 2.
PRISMA flow diagram.
.
PRISMA flow diagram.
Network meta-analysis results for primary outcomes
Comparison of the effects of treatments on ALT
After selecting studies, 84 studies were included in the meta-analysis ALT outcome. Heterogeneity was not significant between studies (Q-value = 52.83, P value = 0.002, I-square = 55.9). Figure 3 shows the network of interventions introduced into the network meta-analysis for ALT outcome.
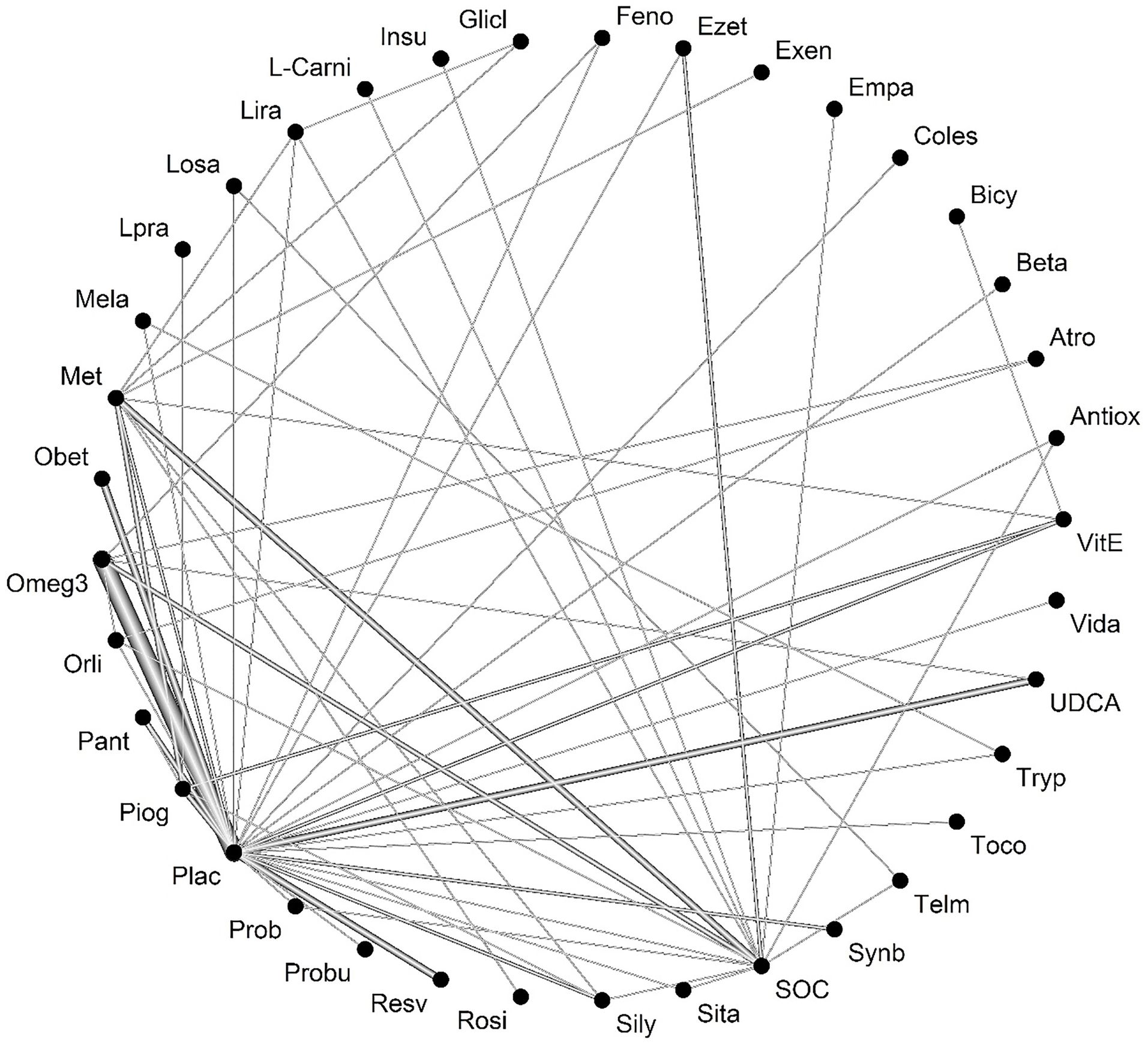
Figure 3.
Network of interventions introduced into the network meta-analysis for ALT outcome.
Legend: Feno: fenofibrate, Ezet: ezetimbe, Exen: exenatide, Coles: Colesevelam, Bicy: bicycline, Beta: betaine, Atrp: atrovastatin, Antiox: anti-oxidants, Vit-E: vitamin- E, Vida:vidagliptin, UDCA: Ursodeoxycholic acid, Tryp: tryptophan, Toco: Tocotrienol, Telm: telmisartan, Synb:symbiotic, SOC: Standard of Care, Sita: sitagliptin, Silly:silymarin, Rosi: rosiglitazone, Resv: Resveratrol, Probu: probucol, Prob: probiotic, Plac:placebo, Piog: pioglitazone, Pant: Pentoxifylline, Orli: Orlistat, Omeg3: Omega-3, Obet: obeticholic acid, Met: metformin, Mela: Melatonin, Lpra: Ipragliflozin, Losa; losartan, Lira: liraglutide, L-Car: L-carnitine, Insu: insulin, Glicl: gliclazide
.
Network of interventions introduced into the network meta-analysis for ALT outcome.
Legend: Feno: fenofibrate, Ezet: ezetimbe, Exen: exenatide, Coles: Colesevelam, Bicy: bicycline, Beta: betaine, Atrp: atrovastatin, Antiox: anti-oxidants, Vit-E: vitamin- E, Vida:vidagliptin, UDCA: Ursodeoxycholic acid, Tryp: tryptophan, Toco: Tocotrienol, Telm: telmisartan, Synb:symbiotic, SOC: Standard of Care, Sita: sitagliptin, Silly:silymarin, Rosi: rosiglitazone, Resv: Resveratrol, Probu: probucol, Prob: probiotic, Plac:placebo, Piog: pioglitazone, Pant: Pentoxifylline, Orli: Orlistat, Omeg3: Omega-3, Obet: obeticholic acid, Met: metformin, Mela: Melatonin, Lpra: Ipragliflozin, Losa; losartan, Lira: liraglutide, L-Car: L-carnitine, Insu: insulin, Glicl: gliclazide
Based on the ratings given the therapeutic effect of ALT on the follow-up score, it is evident that treatment with Atorvastatin had the most significant impact on ALT compared to other investigated drugs. Table 3 shows the treatment ratings by use of P-score for ALT.
Table 3.
Treatment ratings by use of P-score for ALT
|
Rank
|
Drug
|
P-score (fixed)
|
P-score (random)
|
RANK
|
DRUG
|
P-score(fixed)
|
P-score(random)
|
| 1 |
Atro |
0.913 |
0.8787 |
20 |
Empa |
0.5179 |
0.5189 |
| 2 |
Coles |
0.8828 |
0.8622 |
21 |
Toco |
0.4967 |
0.5027 |
| 3 |
Ezet |
0.7976 |
0.8612 |
22 |
Prob |
0.5459 |
0.5004 |
| 4 |
SOC |
0.8719 |
0.8323 |
23 |
Pant |
0.4512 |
0.4393 |
| 5 |
Glicl |
0.8714 |
0.8273 |
24 |
Tryp |
0.4256 |
0.4388 |
| 6 |
Sita |
0.8243 |
0.7925 |
25 |
Sily |
0.3156 |
0.3646 |
| 7 |
Plac |
0.8153 |
0.7865 |
26 |
Obet |
0.3073 |
0.3542 |
| 8 |
Feno |
0.8006 |
0.7256 |
27 |
Bicy |
0.2251 |
0.3061 |
| 9 |
Omeg3 |
0.773 |
0.7243 |
28 |
Piog |
0.2948 |
0.2823 |
| 10 |
Insu |
0.6554 |
0.6368 |
29 |
Beta |
0.2371 |
0.2633 |
| 11 |
Telm |
0.6556 |
0.6324 |
30 |
Lpra |
0.2427 |
0.2578 |
| 12 |
UDCA |
0.6985 |
0.6072 |
31 |
Exen |
0.1934 |
0.2575 |
| 13 |
Losa |
0.6211 |
0.6029 |
32 |
Synb |
0.3278 |
0.2498 |
| 14 |
VitE |
0.5289 |
0.5824 |
33 |
Orli |
0.1487 |
0.2049 |
| 15 |
Mela |
0.5741 |
0.5652 |
34 |
Vida |
0.1102 |
0.1504 |
| 16 |
Resv |
0.5831 |
0.5592 |
35 |
Rosi |
0.0979 |
0.1464 |
| 17 |
Antiox |
0.5688 |
0.5568 |
36 |
L-Carni |
0.0875 |
0.1303 |
| 18 |
Lira |
0.5249 |
0.5513 |
37 |
Probu |
0.0002 |
0.0015 |
| 19 |
Met |
0.5141 |
0.5462 |
|
|
|
|
The forest plot has also combined preliminary results with studies performed on the network meta-analysis in comparison to the placebo treatment (Figure 4). The level of evidence for the impact of atorvastatin vs. placebo on ALT levels has been calculated as low using GRADE approach.
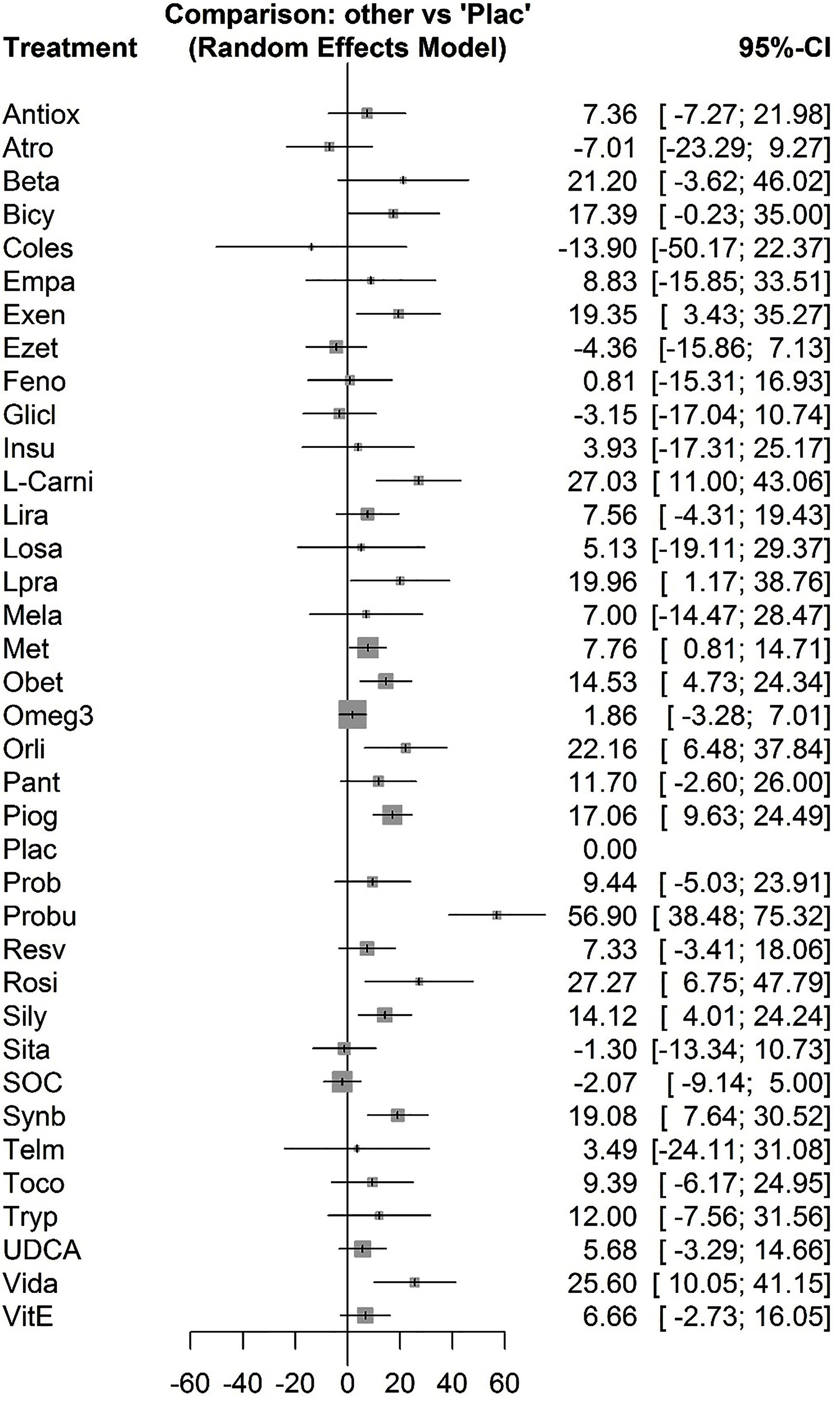
Figure 4.
Combined results of netwrok meta-analysis with placebo.
.
Combined results of netwrok meta-analysis with placebo.
Comparison of treatment effects on AST
After selecting studies, 71 cases were included in network meta-analysis for AST.
Heterogenicity was significant between studies (Q-value = 261.46, P value <0.001, I-square = 86.4). Figure 5 represents the network of interventions introduced into the network meta-analysis for the AST outcome. Based on the ratings given the therapeutic effect on AST, it was found that treatment with tryptophan had the best change over AST in comparison to the other investigated drugs.
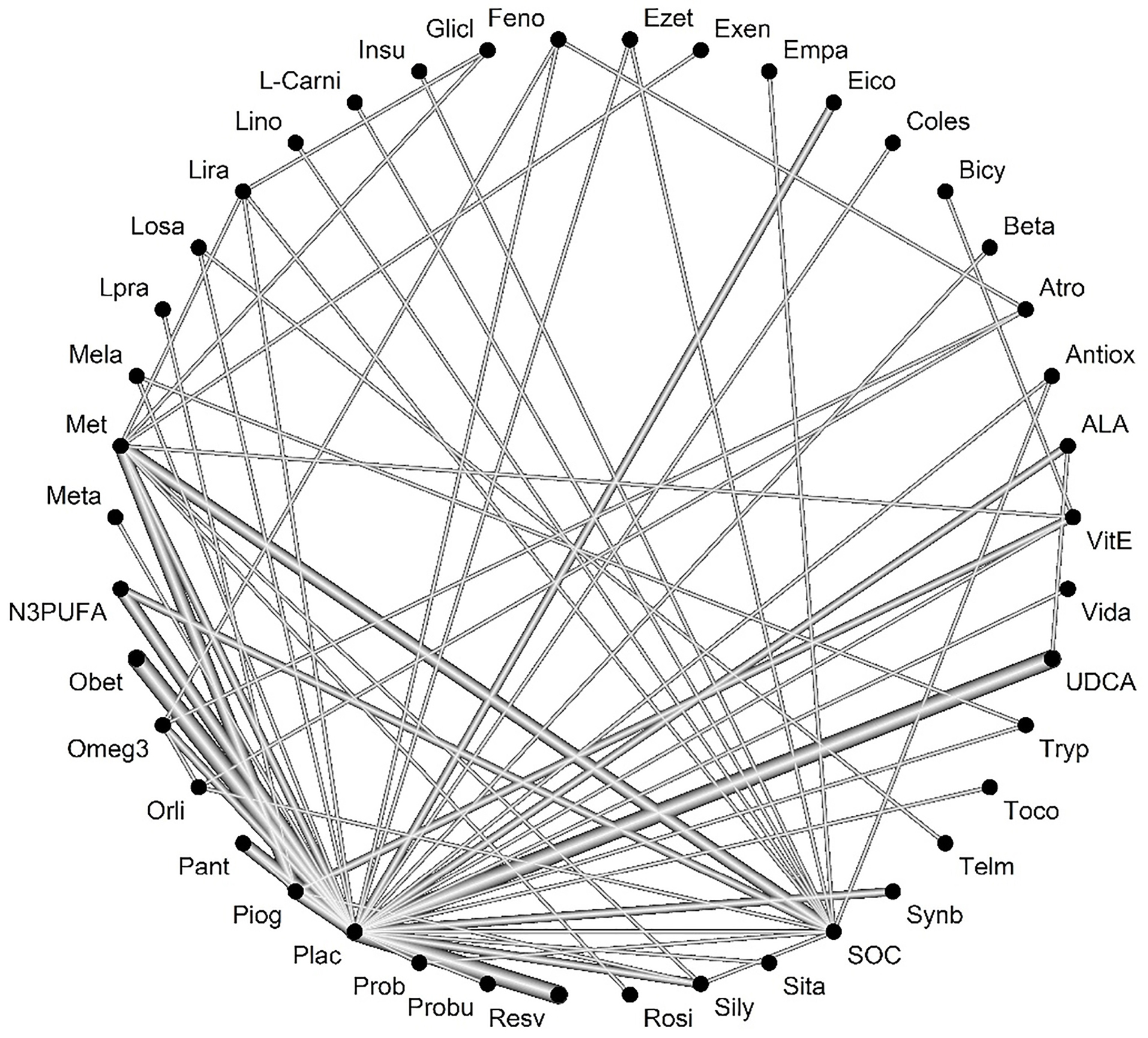
Figure 5.
Network of interventions introduced into the network meta-analysis for AST outcome.
Legend: Feno:fenofibrate, Ezet: ezetimbe, Exen: exenatide, Coles: Colesevelam, Bicy: bicycline, Beta:betaine, Atrp: atrovastatin, Antiox: anti-oxidants, Vit-E: vitamin- E, Vida:vidagliptin, UDCA: Ursodeoxycholic acid, Tryp: tryptophan, Toco: Tocotrienol, Telm: telmisartan, Synb:symbiotic, SOC: Standard of Care, Sita: sitagliptin, Silly:silymarin, Rosi: rosiglitazone, Resv: Resveratrol, Probu: probucol, Prob: probiotic, Plac:placebo, Piog: pioglitazone, Pant: Pentoxifylline, Orli: Orlistat, Omeg3: Omega-3, Obet: obeticholic acid, Met: metformin, Mela: Melatonin, Lpra: Ipragliflozin, Losa; losartan, Lira: liraglutide, L-Car: L-carnitine, Insu: insulin, Glicl: gliclazide
.
Network of interventions introduced into the network meta-analysis for AST outcome.
Legend: Feno:fenofibrate, Ezet: ezetimbe, Exen: exenatide, Coles: Colesevelam, Bicy: bicycline, Beta:betaine, Atrp: atrovastatin, Antiox: anti-oxidants, Vit-E: vitamin- E, Vida:vidagliptin, UDCA: Ursodeoxycholic acid, Tryp: tryptophan, Toco: Tocotrienol, Telm: telmisartan, Synb:symbiotic, SOC: Standard of Care, Sita: sitagliptin, Silly:silymarin, Rosi: rosiglitazone, Resv: Resveratrol, Probu: probucol, Prob: probiotic, Plac:placebo, Piog: pioglitazone, Pant: Pentoxifylline, Orli: Orlistat, Omeg3: Omega-3, Obet: obeticholic acid, Met: metformin, Mela: Melatonin, Lpra: Ipragliflozin, Losa; losartan, Lira: liraglutide, L-Car: L-carnitine, Insu: insulin, Glicl: gliclazide
Table 4 shows the treatment ratings by use of the P-score. The level of evidence for impact of tryptophan vs. placebo on AST levels has been calculated as low using GRADE approach.
Table 4.
Treatment ratings by use of the P- score on AST
|
Rank
|
Drug
|
P-score (fixed)
|
P-score (random)
|
Rank
|
Drug
|
P-score (fixed)
|
P-score (random)
|
| 1 |
Tryp |
0.9533 |
0.9181 |
20 |
Met |
0.5205 |
0.516 |
| 2 |
Meta |
0.952 |
0.9176 |
21 |
Rosi |
0.5131 |
0.509 |
| 3 |
Coles |
0.875 |
0.8413 |
22 |
Ezet |
0.373 |
0.4945 |
| 4 |
Mela |
0.8726 |
0.8144 |
23 |
Toco |
0.4676 |
0.4892 |
| 5 |
Atro |
0.8426 |
0.7784 |
24 |
Empa |
0.3669 |
0.4191 |
| 6 |
Telm |
0.799 |
0.7624 |
25 |
VitE |
0.2622 |
0.3369 |
| 7 |
Sita |
0.7806 |
0.7285 |
26 |
Prob |
0.4346 |
0.3234 |
| 8 |
Losa |
0.7278 |
0.7099 |
27 |
Lpra |
0.2579 |
0.3122 |
| 9 |
Plac |
0.682 |
0.6759 |
28 |
Piog |
0.2909 |
0.3104 |
| 10 |
SOC |
0.6417 |
0.667 |
29 |
Pant |
0.265 |
0.3062 |
| 11 |
Glicl |
0.8012 |
0.6637 |
30 |
Exen |
0.2447 |
0.3046 |
| 12 |
UDCA |
0.65 |
0.6577 |
31 |
Obet |
0.178 |
0.2871 |
| 13 |
Feno |
0.759 |
0.6347 |
32 |
Orli |
0.1675 |
0.2749 |
| 14 |
Omeg3 |
0.6874 |
0.6301 |
33 |
Bicy |
0.1107 |
0.2111 |
| 15 |
Resv |
0.4763 |
0.6035 |
34 |
Synb |
0.3962 |
0.2061 |
| 16 |
Antiox |
0.669 |
0.5915 |
35 |
Beta |
0.1376 |
0.1852 |
| 17 |
Insu |
0.5092 |
0.5364 |
36 |
L-Carni |
0.0808 |
0.1574 |
| 18 |
Lira |
0.7011 |
0.5336 |
37 |
Vida |
0.0847 |
0.1555 |
| 19 |
Sily |
0.4669 |
0.5254 |
38 |
Probu |
0.0015 |
0.011 |
Network meta-analysis results for secondary outcomes
Comparison of the effects of the treatment on BMI
After selecting the studies, 48 cases were included in the meta-analysis. Heterogeneity was not significant between studies (Q-value = 15.98, P value = 0.31, I-square = 0.00).
The network of interventions introduced into the network meta-analysis for BMI outcomes is demonstrated in Figure 6.
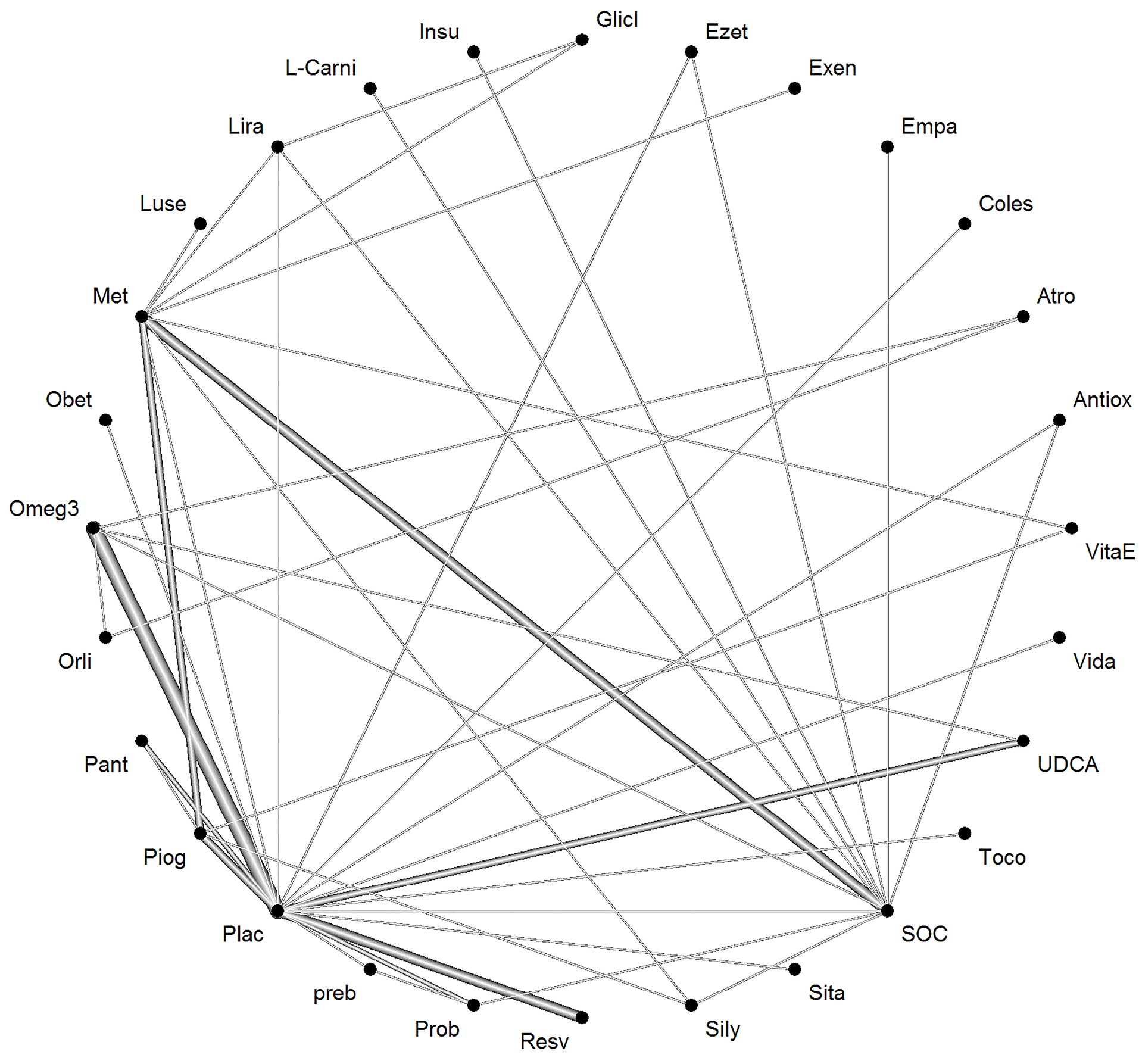
Figure 6.
Network of interventions introduced into the network meta-analysis for BMIoutcome.
Legend: Feno:fenofibrate, Ezet: ezetimbe, Exen: exenatide, Coles: Colesevelam, Bicy: bicycline, Beta:betaine, Atrp: atrovastatin, Antiox: anti-oxidants, Vit-E: vitamin- E, Vida:vidagliptin, UDCA: Ursodeoxycholic acid, Tryp: tryptophan, Toco: Tocotrienol, Telm: telmisartan, Synb:symbiotic, SOC: Standard of Care, Sita: sitagliptin, Silly:silymarin, Rosi: rosiglitazone, Resv: Resveratrol, Probu: probucol, Prob: probiotic, Plac:placebo, Piog: pioglitazone, Pant: Pentoxifylline, Orli: Orlistat, Omeg3: Omega-3, Obet: obeticholic acid, Met: metformin, Mela: Melatonin, Lpra: Ipragliflozin, Losa; losartan, Lira: liraglutide, L-Car: L-carnitine, Insu: insulin, Glicl: gliclazide
.
Network of interventions introduced into the network meta-analysis for BMIoutcome.
Legend: Feno:fenofibrate, Ezet: ezetimbe, Exen: exenatide, Coles: Colesevelam, Bicy: bicycline, Beta:betaine, Atrp: atrovastatin, Antiox: anti-oxidants, Vit-E: vitamin- E, Vida:vidagliptin, UDCA: Ursodeoxycholic acid, Tryp: tryptophan, Toco: Tocotrienol, Telm: telmisartan, Synb:symbiotic, SOC: Standard of Care, Sita: sitagliptin, Silly:silymarin, Rosi: rosiglitazone, Resv: Resveratrol, Probu: probucol, Prob: probiotic, Plac:placebo, Piog: pioglitazone, Pant: Pentoxifylline, Orli: Orlistat, Omeg3: Omega-3, Obet: obeticholic acid, Met: metformin, Mela: Melatonin, Lpra: Ipragliflozin, Losa; losartan, Lira: liraglutide, L-Car: L-carnitine, Insu: insulin, Glicl: gliclazide
Based on the given ratings related to therapeutic effects on BMI, it was found that treatment with Orlistat had the best efficiency in BMI over the other drugs studied. Table 5 shows the treatment ratings by use of the P-score. The level of evidence for the impact of orlistat vs. placebo on BMI has been calculated as low using GRADE approach.
Table 5.
Treatment ratings by use of the P-score
|
Rank
|
Drug
|
P-score (fixed)
|
P-score (random)
|
Rank
|
Drug
|
P-score (fixed)
|
P-score(random)
|
| 1 |
Orli |
0.989 |
0.989 |
15 |
preb |
0.4783 |
0.4783 |
| 2 |
Vida |
0.892 |
0.892 |
16 |
Obet |
0.4507 |
0.4507 |
| 3 |
Exen |
0.8776 |
0.8776 |
17 |
Sily |
0.4427 |
0.4427 |
| 4 |
Antiox |
0.8236 |
0.8236 |
18 |
Omeg3 |
0.401 |
0.401 |
| 5 |
Empa |
0.7142 |
0.7142 |
19 |
Piog |
0.384 |
0.384 |
| 6 |
Lira |
0.7093 |
0.7093 |
20 |
Coles |
0.3758 |
0.3758 |
| 7 |
Insu |
0.6821 |
0.6821 |
21 |
Toco |
0.3401 |
0.3401 |
| 8 |
L-Carni |
0.6109 |
0.6109 |
22 |
VitaE |
0.3401 |
0.3401 |
| 9 |
Ezet |
0.6033 |
0.6033 |
23 |
Atro |
0.3106 |
0.3106 |
| 10 |
Prob |
0.5963 |
0.5963 |
24 |
UDCA |
0.2293 |
0.2293 |
| 11 |
SOC |
0.5942 |
0.5942 |
25 |
Resv |
0.2165 |
0.2165 |
| 12 |
Luse |
0.5689 |
0.5689 |
26 |
Sita |
0.1755 |
0.1755 |
| 13 |
Met |
0.5285 |
0.5285 |
27 |
Plac |
0.1516 |
0.1516 |
| 14 |
Pant |
0.4962 |
0.4962 |
28 |
Glicl |
0.0178 |
0.0178 |
Comparison of the effects of the treatment on steatosis
After selecting the studies, 14 studies were included in the meta-analysis. Heterogenicity was not significant between studies (Q-value = 0.43, P value = 0.51, I-square = 0.00).
Based on the given ratings associated with the therapeutic effects of steatosis, it was concluded that treatment with Omega-3 had the best efficiency over steatosis in comparison to the other investigated drugs. Table 6 shows the ratings of treatments using the P-score. The forest plot has also been shown to combine preliminary results with studies performed on the meta-analysis of networks compared with placebo treatment (Figure 7). The level of evidence for impact of Omega-3 vs. placebo on steatosis has been calculated as low using GRADE approach.
Table 6.
Ratings of treatments using the P- score for steatosis
|
Rank
|
Intervention
|
P-score (fixed)
|
P-score (random)
|
| 1 |
Omeg3 |
0.979 |
0.979 |
| 2 |
Plac |
0.8906 |
0.8906 |
| 3 |
UDCA |
0.7794 |
0.7794 |
| 4 |
Coles |
0.7403 |
0.7403 |
| 5 |
Pent |
0.6891 |
0.6891 |
| 6 |
VitE |
0.6728 |
0.6728 |
| 7 |
Lira |
0.5653 |
0.5653 |
| 8 |
Obet |
0.5233 |
0.5233 |
| 9 |
Piog |
0.5173 |
0.5173 |
| 10 |
Bicy |
0.4231 |
0.4231 |
| 11 |
Met |
0.2247 |
0.2247 |
| 12 |
Ezet |
0.1765 |
0.1765 |
| 13 |
SOC |
0.1765 |
0.1765 |
| 14 |
Insu |
0.1389 |
0.1389 |
| 15 |
L-Carni |
0.0033 |
0.0033 |
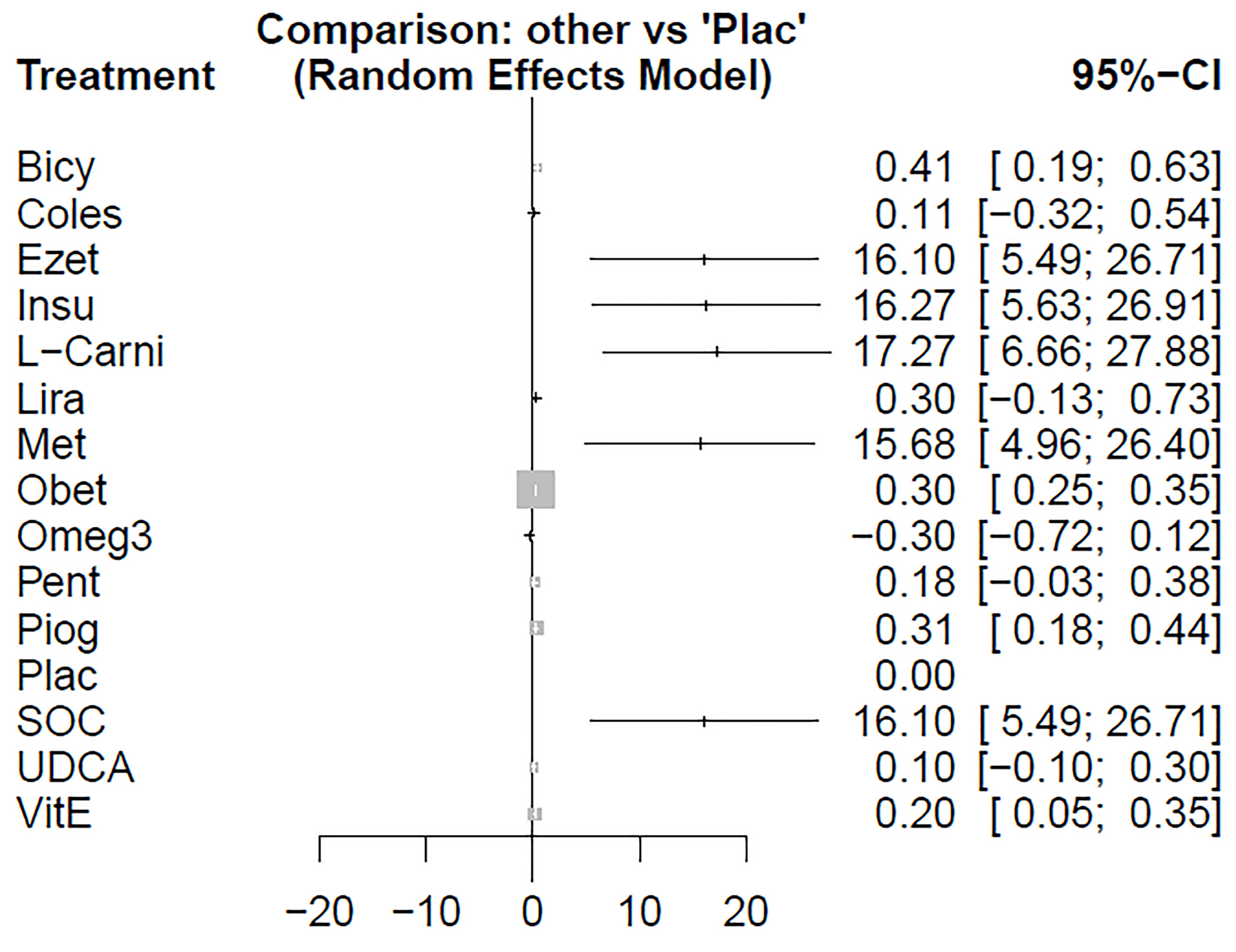
Figure 7.
Forest plot of comparison of medication for steatosis.
Beta:betaine, Atrp: atrovastatin, Antiox: anti-oxidants, Vit-E: vitamin- E, Vida: vidagliptin, UDCA: Ursodeoxycholic acid, Tryp: tryptophan, Toco: Tocotrienol, Telm: telmisartan, Synb:symbiotic, SOC: Standard of Care, Sita: sitagliptin, Silly:silymarin, Rosi: rosiglitazone, Resv: Resveratrol, Probu: probucol, Prob: probiotic, Plac:placebo, Piog: pioglitazone, Pant: Pentoxifylline, Orli: Orlistat, Omeg3: Omega-3, Obet: obeticholic acid, Met: metformin, Mela: Melatonin, Lpra: Ipragliflozin, Losa; losartan, Lira: liraglutide, L-Car: L-carnitine, Insu: insulin, Glicl: gliclazide
.
Forest plot of comparison of medication for steatosis.
Beta:betaine, Atrp: atrovastatin, Antiox: anti-oxidants, Vit-E: vitamin- E, Vida: vidagliptin, UDCA: Ursodeoxycholic acid, Tryp: tryptophan, Toco: Tocotrienol, Telm: telmisartan, Synb:symbiotic, SOC: Standard of Care, Sita: sitagliptin, Silly:silymarin, Rosi: rosiglitazone, Resv: Resveratrol, Probu: probucol, Prob: probiotic, Plac:placebo, Piog: pioglitazone, Pant: Pentoxifylline, Orli: Orlistat, Omeg3: Omega-3, Obet: obeticholic acid, Met: metformin, Mela: Melatonin, Lpra: Ipragliflozin, Losa; losartan, Lira: liraglutide, L-Car: L-carnitine, Insu: insulin, Glicl: gliclazide
Comparison of the treatment effects on the NAS
After selecting studies, 15 cases were included in the meta-analysis. Heterogeneity was not significant between studies (Q-value = 1.15, P value = 0.28, I-square = 12.8).
The network of interventions introduced into the network meta-analysis for the outcome of NAS are shown in Figure 8.
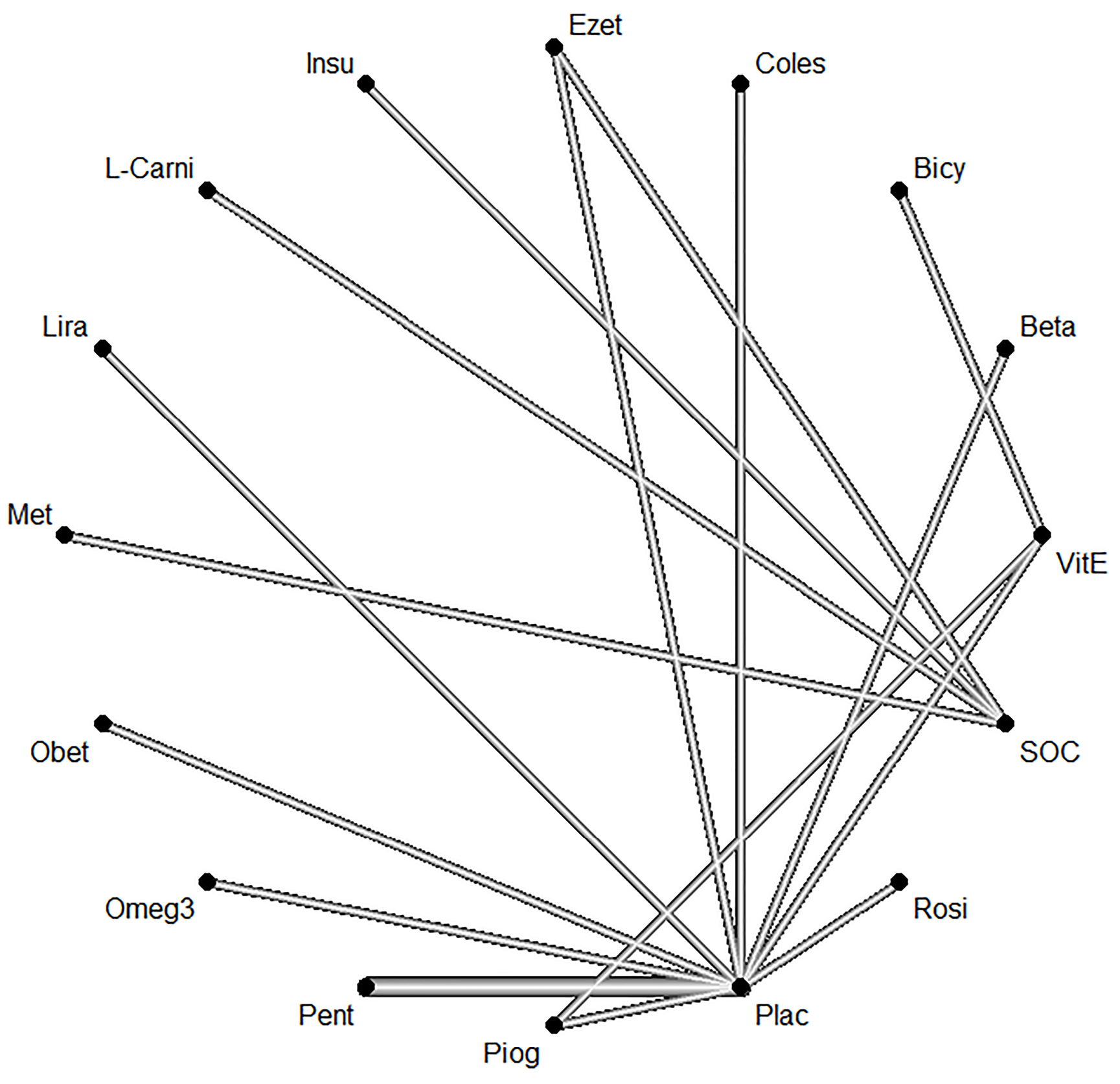
Figure 8.
Network of interventions introduced into the network meta-analysis for NAS
Legend: Feno:fenofibrate, Ezet: ezetimbe, Exen: exenatide, Coles: Colesevelam, Bicy: bicyclineBeta:betaine, Atrp: atrovastatin, Antiox: anti-oxidants, Vit-E: vitamin- E, Vida:vidagliptin, UDCA: Ursodeoxycholic acid, Tryp: tryptophan, Toco: Tocotrienol, Telm: telmisartan, Synb:symbiotic, SOC: Standard of Care, Sita: sitagliptin, Silly:silymarin, Rosi: rosiglitazone, Resv: Resveratrol, Probu: probucol, Prob: probiotic, Plac:placebo, Piog: pioglitazone, Pant: Pentoxifylline, Orli: Orlistat, Omeg3: Omega-3, Obet: obeticholic acid, Met: metformin, Mela: Melatonin, Lpra: Ipragliflozin, Losa; losartan, Lira: liraglutide, L-Car: L-carnitine, Insu: insulin, Glicl: gliclazide
.
Network of interventions introduced into the network meta-analysis for NAS
Legend: Feno:fenofibrate, Ezet: ezetimbe, Exen: exenatide, Coles: Colesevelam, Bicy: bicyclineBeta:betaine, Atrp: atrovastatin, Antiox: anti-oxidants, Vit-E: vitamin- E, Vida:vidagliptin, UDCA: Ursodeoxycholic acid, Tryp: tryptophan, Toco: Tocotrienol, Telm: telmisartan, Synb:symbiotic, SOC: Standard of Care, Sita: sitagliptin, Silly:silymarin, Rosi: rosiglitazone, Resv: Resveratrol, Probu: probucol, Prob: probiotic, Plac:placebo, Piog: pioglitazone, Pant: Pentoxifylline, Orli: Orlistat, Omeg3: Omega-3, Obet: obeticholic acid, Met: metformin, Mela: Melatonin, Lpra: Ipragliflozin, Losa; losartan, Lira: liraglutide, L-Car: L-carnitine, Insu: insulin, Glicl: gliclazide
Based on the given ratings pertinent to the therapeutic effects on NAS, it was observed that treatment with betaine had the best efficiency over NAS compared to the other investigated drugs. The forest plot has also been shown to combine preliminary results with studies performed on the meta-analysis of networks compared with placebo treatment (Figure 9). The level of evidence for impact of betaine vs. placebo on NAS has been calculated as low using GRADE approach (Table 7).
Table 7.
The level of evidence for each first identified intervention on primary and secondary outcomes vs. placebo
|
Certainty assessment
|
Effect
|
Certainty
|
|
Outcome
|
Study design
|
Risk of bias
|
Inconsistency
|
Indirectness
|
Imprecision
|
Other considerations
|
Relative (95% CI)
|
Absolute (95% CI)
|
| ALT(Atro vs Plac) |
Randomized trials |
Not serious |
Serious |
Serious |
Not serious |
None |
- |
MD 7.01
(23.22 to 9.27)
|
⨁⨁OO
LOW
|
| AST (Tryp vs Plac) |
Randomized trials |
Not serious |
Serious |
Serious |
Not serious |
None |
- |
16
(34.11 to 2.11)
|
⨁⨁OO
LOW
|
| BMI (Orli vs Plac) |
Randomized trials |
Not serious |
Serious |
Serious |
Not serious |
None |
- |
4.47
(5.91 to 3.02)
|
⨁⨁OO
LOW
|
| Steatosis (Omeg3 vs Plac) |
Randomized trials |
Not serious |
Serious |
Serious |
Not serious |
None |
- |
MD 0.3
(0.72 to 0.12)
|
⨁⨁OO
LOW
|
| NAS (Met vs Plac) |
Randomized trials |
Not serious |
Serious |
Serious |
Not serious |
None |
- |
MD 0.32
(2.23 lower to 1.59 higher)
|
⨁⨁OO
LOW
|
CI: Confidence interval; MD: Mean difference
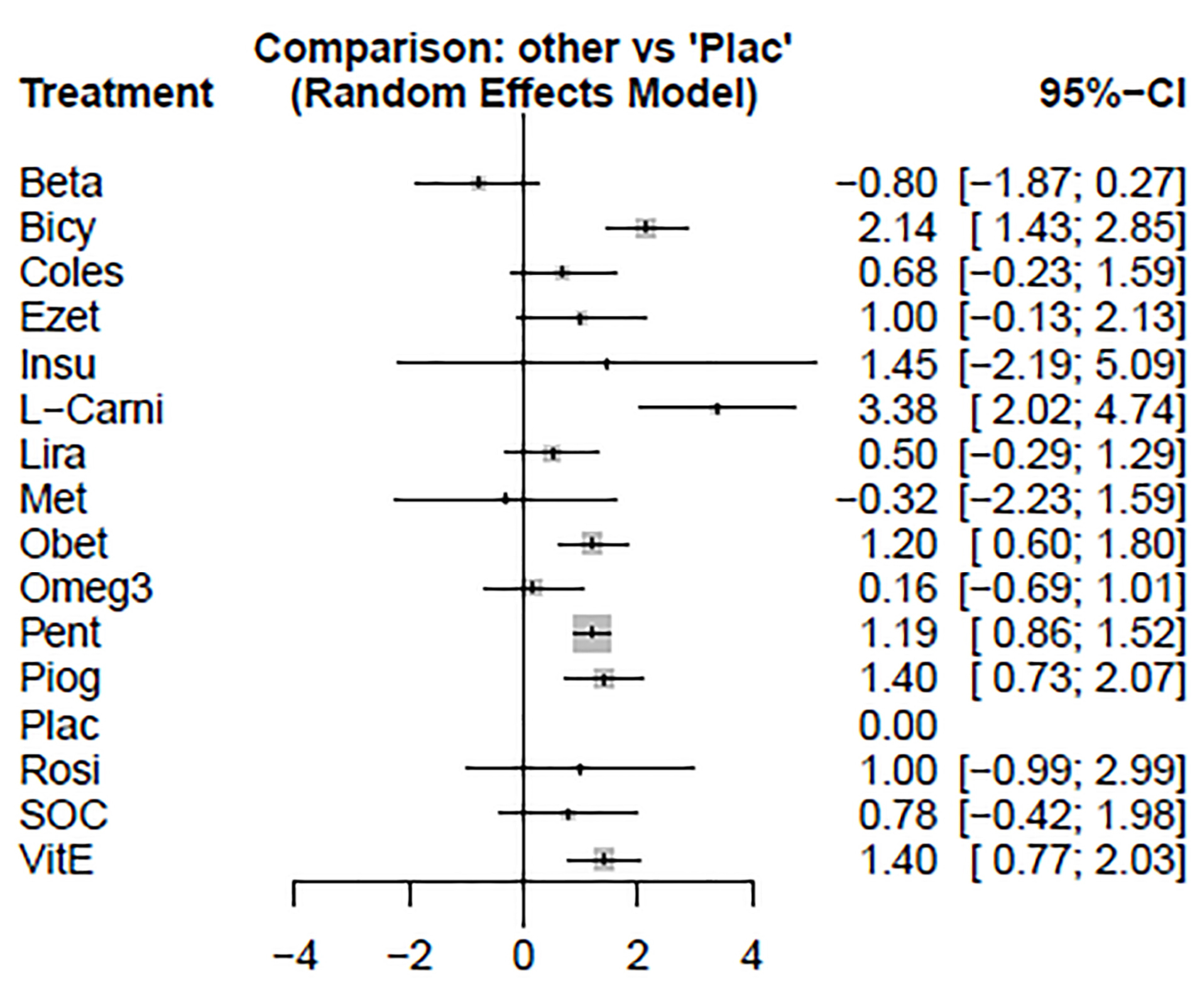
Figure 9.
Preliminary results with studies performed on the meta-analysis of networks compared with placebo treatment on NAS.
Legend: Feno:fenofibrate, Ezet: ezetimbe, Exen: exenatide, Coles: Colesevelam, Bicy: bicycline, Beta:betaine, Atrp: atrovastatin, Antiox: anti-oxidants, Vit-E: vitamin- E, Vida:vidagliptin, UDCA: Ursodeoxycholic acid, Tryp: tryptophan, Toco: Tocotrienol, Telm: telmisartan, Synb:symbiotic, SOC: Standard of Care, Sita: sitagliptin, Silly:silymarin, Rosi: rosiglitazone, Resv: Resveratrol, Probu: probucol, Prob: probiotic, Plac:placebo, Piog: pioglitazone, Pant: Pentoxifylline, Orli: Orlistat, Omeg3: Omega-3, Obet: obeticholic acid, Met: metformin, Mela: Melatonin, Lpra: Ipragliflozin, Losa; losartan, Lira: liraglutide, L-Car: L-carnitine, Insu: insulin, Glicl: gliclazide.
.
Preliminary results with studies performed on the meta-analysis of networks compared with placebo treatment on NAS.
Legend: Feno:fenofibrate, Ezet: ezetimbe, Exen: exenatide, Coles: Colesevelam, Bicy: bicycline, Beta:betaine, Atrp: atrovastatin, Antiox: anti-oxidants, Vit-E: vitamin- E, Vida:vidagliptin, UDCA: Ursodeoxycholic acid, Tryp: tryptophan, Toco: Tocotrienol, Telm: telmisartan, Synb:symbiotic, SOC: Standard of Care, Sita: sitagliptin, Silly:silymarin, Rosi: rosiglitazone, Resv: Resveratrol, Probu: probucol, Prob: probiotic, Plac:placebo, Piog: pioglitazone, Pant: Pentoxifylline, Orli: Orlistat, Omeg3: Omega-3, Obet: obeticholic acid, Met: metformin, Mela: Melatonin, Lpra: Ipragliflozin, Losa; losartan, Lira: liraglutide, L-Car: L-carnitine, Insu: insulin, Glicl: gliclazide.
Based on our search of medical databases and although there is a meta-analysis conducted on the topic of effective medication on NAFLD conducted by Sridharan et al our research is the first network meta-analysis that has been conducted on this topic among adult population so far. Additionally, our systematic review and network meta-analysis is different than the previous work as this article has also included children’s studies that could potentially have confounding factors as NAFLD in children ma arise from genetic origins.
103
Another major difference that is noted in the conduct of the present stud and the research by Sridharan et.al, is that despite our study, authors in this comparable study has different set of primary and secondary outcomes. As mentioned in the methods and materials section, we have set our primary outcomes for changes in ALT, AST and secondary outcomes include change in BMI, NAS and hepatic steatosis whereas, Sridharan and colleagues has put overall response rate to medication and AST, ALT, NAS, BMI, lipid profiles were set as secondary outcome.
We have implicated the primary impact of different medical therapies and their effect on liver transaminases, as our primary outcome of NAFLD. We showed that atorvastatin, colesevelam and ezetimibe had the best outcome compared to the SOC on ALT, and tryptophan and melatonine had the best impact on lowering AST while in the study of Sridharan and colleagues, the best medication to reduce ALT is reported to be with vitamin D followed by gemfibrozil and ipragliflozin.
104
Based on the given ratings pertinent to the therapeutic effects on NAS, it was observed that treatment with Betaine had the best efficiency over NAS compared to the other investigated drugs.
Sridharan et al have determined the better efficacy for elafibranor.
104
While Sridharan et al had reported the best outcome from telmisartan, in the current investigation, Telmisartan had lower efficacy than SOC on.
104
High-quality clinical trials are needed to prove the efficacy of atorvastatin in NAFLD.
As we expected, life-style modification shown to be effective in reducing aminotransferases. Alongside the use of medicinal compound. Of these, orlistat was the first effective drug followed by liraglutide. Given to the fact that treatment of NAFLD can be associated with complications including NAS, we illustrated that betaine, metformin, omega 3, liraglutide and colesevelam were most effective drugs in reduction of NAS. Of these, efficacy of betaine and liraglutide had similarities to the results of previous meta-analyses. Omega 3 were shown to be best therapeutic choice in hepatic steatosis.
In previous meta-analysis, pentoxifylline was one of the drugs that had a remarkable effect on reducing liver fibrosis. Compared to our findings, although pentoxifylline were a weaker agent in reducing NAS, it worked well.
104
According to the guidelines of American Association for Liver Diseases, vitamin E administered at a daily dose of 800 IU/day improves liver histology in nondiabetic adults with biopsy-proven NASH and therefore may be considered for this patient population.
9
Based on the results of our meta-analysis, vitamin E had no significant impact on the reduction of aminotransferases compared to SOC in the context of decline hepatic steatosis, nevertheless, has not been shown to be effective in reducing NAS.
We observed no obvious effect for polyunsaturated fatty acids (PUFA) for both primary and secondary outcomes, therefore, its administration in NAFLD is not recommended.
Ultimately for NAS, our meta-analysis revealed the best response from Betaine. It is also recommended to include the betaine in larger clinical trials to further study its anti-steatotic properties.
We encountered some limitation in performing current systematic review, heterogenicity of studies and the lack of studies that had investigated the combined medications are some of them.
It is suggested to do other meta-analyses to update previous studies, which had reduced the amount of drug diversity. A general consensus about some drugs and patient’s treatment process is needed in prospective studies as well.
Conclusion
All in all, our study shows a higher efficacy for the reduction in liver transaminases for atorvastatin. Although lifestyle modification, weight loss and BMI reduction are all effective in improving the primary and secondary outcomes, our network meta-analysis showed the greater efficacy for tryptophan, orlistat, omega-3 and betaine to improve AST, BMI, steatosis and NAS. Considering these findings, we recommend randomized clinical trials to examine these medical modalities with placebo and each other.
Ethical Issues
This systematic review and meta-analysis has been evaluated and approved by regional ethics at Tabriz university of Medical Sciences, Faculty of Medicine. Additionally, all ethical considerations were meet in all levels of systematic search according to systematic review and meta-analysis
Conflict of Interests
None to declare.
Acknowledgments
This research was financially supported by the Tabriz University of Medical Sciences, Tabriz, Iran. The authors would like to thank the Tabriz University of Medical Sciences for providing the expertise that greatly assisted.
Supplementary Materials
Supplementary file 1 contains Table S1.
(pdf)
References
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002; 346(16):1221-31. doi: 10.1056/NEJMra011775 [Crossref] [ Google Scholar]
- Brunt EM. Nonalcoholic steatohepatitis: pathologic features and differential diagnosis. Semin Diagn Pathol 2005; 22(4):330-8. doi: 10.1053/j.semdp.2006.04.002 [Crossref] [ Google Scholar]
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 2006; 43(2 Suppl 1):S99-s112. doi: 10.1002/hep.20973 [Crossref] [ Google Scholar]
- Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148(3):547-55. doi: 10.1053/j.gastro.2014.11.039 [Crossref] [ Google Scholar]
- Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011; 141(4):1249-53. doi: 10.1053/j.gastro.2011.06.061 [Crossref] [ Google Scholar]
- Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology 2017; 152(5):1090-9. doi: 10.1053/j.gastro.2017.01.003 [Crossref] [ Google Scholar]
- Anstee QM, McPherson S, Day CP. How big a problem is non-alcoholic fatty liver disease?. BMJ 2011; 343:d3897. doi: 10.1136/bmj.d3897 [Crossref] [ Google Scholar]
- Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin Liver Dis 2016; 20(2):205-14. doi: 10.1016/j.cld.2015.10.001 [Crossref] [ Google Scholar]
- Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67(1):328-57. doi: 10.1002/hep.29367 [Crossref] [ Google Scholar]
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64(1):73-84. doi: 10.1002/hep.28431 [Crossref] [ Google Scholar]
- Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol 2015; 62(1 Suppl):S47-64. doi: 10.1016/j.jhep.2014.12.012 [Crossref] [ Google Scholar]
- Caldwell S, Argo C. The natural history of non-alcoholic fatty liver disease. Dig Dis 2010; 28(1):162-8. doi: 10.1159/000282081 [Crossref] [ Google Scholar]
- Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011; 9(6):524-30. doi: 10.1016/j.cgh.2011.03.020 [Crossref] [ Google Scholar]
- Grattagliano I, Portincasa P, Palmieri VO, Palasciano G. Managing nonalcoholic fatty liver disease: recommendations for family physicians. Can Fam Physician 2007; 53(5):857-63. [ Google Scholar]
- Arab JP, Candia R, Zapata R, Muñoz C, Arancibia JP, Poniachik J. Management of nonalcoholic fatty liver disease: an evidence-based clinical practice review. World J Gastroenterol 2014; 20(34):12182-201. doi: 10.3748/wjg.v20.i34.12182 [Crossref] [ Google Scholar]
- Mouzaki M, Allard J. Non-alcoholic steatohepatitis: the therapeutic challenge of a global epidemic. Ann Gastroenterol 2012; 25(3):207-17. [ Google Scholar]
- Pathak S, Pandanaboyana S, Daniels I, Smart N, Prasad KR. Obesity and colorectal liver metastases: mechanisms and management. Surg Oncol 2016; 25(3):246-51. doi: 10.1016/j.suronc.2016.05.021 [Crossref] [ Google Scholar]
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet 2001; 358(9285):893-4. doi: 10.1016/s0140-6736(01)06042-1 [Crossref] [ Google Scholar]
- Athyros VG, Mikhailidis DP, Didangelos TP, Giouleme OI, Liberopoulos EN, Karagiannis A. Effect of multifactorial treatment on non-alcoholic fatty liver disease in metabolic syndrome: a randomised study. Curr Med Res Opin 2006; 22(5):873-83. doi: 10.1185/030079906x104696 [Crossref] [ Google Scholar]
- Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology 2008; 135(1):100-10. doi: 10.1053/j.gastro.2008.03.078 [Crossref] [ Google Scholar]
- Hatzitolios A, Savopoulos C, Lazaraki G, Sidiropoulos I, Haritanti P, Lefkopoulos A. Efficacy of omega-3 fatty acids, atorvastatin and orlistat in non-alcoholic fatty liver disease with dyslipidemia. Indian J Gastroenterol 2004; 23(4):131-4. [ Google Scholar]
- Fan H, Pan Q, Xu Y, Yang X. Exenatide improves type 2 diabetes concomitant with non-alcoholic fatty liver disease. Arq Bras Endocrinol Metabol 2013; 57(9):702-8. doi: 10.1590/s0004-27302013000900005 [Crossref] [ Google Scholar]
- Capanni M, Calella F, Biagini MR, Genise S, Raimondi L, Bedogni G. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Aliment Pharmacol Ther 2006; 23(8):1143-51. doi: 10.1111/j.1365-2036.2006.02885.x [Crossref] [ Google Scholar]
- Solhi H, Ghahremani R, Kazemifar AM, Hoseini Yazdi Z. Silymarin in treatment of non-alcoholic steatohepatitis: a randomized clinical trial. Caspian J Intern Med 2014; 5(1):9-12. [ Google Scholar]
- Khoshbaten M, Aliasgarzadeh A, Masnadi K, Tarzamani MK, Farhang S, Babaei H. N-acetylcysteine improves liver function in patients with non-alcoholic Fatty liver disease. Hepat Mon 2010; 10(1):12-6. [ Google Scholar]
- Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology 2004; 39(3):770-8. doi: 10.1002/hep.20092 [Crossref] [ Google Scholar]
- Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology 2011; 54(5):1610-9. doi: 10.1002/hep.24544 [Crossref] [ Google Scholar]
- Mumtaz A, Ashfaq UA, Ul Qamar MT, Anwar F, Gulzar F, Ali MA. MPD3: a useful medicinal plants database for drug designing. Nat Prod Res 2017; 31(11):1228-36. doi: 10.1080/14786419.2016.1233409 [Crossref] [ Google Scholar]
- Shahebrahimi K, Zulnoorian S, Almasi A, Sharifi A, Keshvarz A, Farshchian N. A comparison of the therapeutic effects of metformin, pioglitazone and vitamin e in patients with non-alcoholic fatty liver. Journal of Babol University of Medical Sciences 2017; 19(9):32-8. doi: 10.22088/jbums.19.9.32 [Crossref] [ Google Scholar]
- Wong VW, Chan WK, Chitturi S, Chawla Y, Dan YY, Duseja A. Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 1: Definition, risk factors and assessment. J Gastroenterol Hepatol 2018; 33(1):70-85. doi: 10.1111/jgh.13857 [Crossref] [ Google Scholar]
- Oscarsson J, Önnerhag K, Risérus U, Sundén M, Johansson L, Jansson PA. Effects of free omega-3 carboxylic acids and fenofibrate on liver fat content in patients with hypertriglyceridemia and non-alcoholic fatty liver disease: a double-blind, randomized, placebo-controlled study. J Clin Lipidol 2018; 12(6):1390-403 e4. doi: 10.1016/j.jacl.2018.08.003 [Crossref] [ Google Scholar]
- Ito D, Shimizu S, Inoue K, Saito D, Yanagisawa M, Inukai K. Comparison of ipragliflozin and pioglitazone effects on nonalcoholic fatty liver disease in patients with type 2 diabetes: a randomized, 24-week, open-label, active-controlled trial. Diabetes Care 2017; 40(10):1364-72. doi: 10.2337/dc17-0518 [Crossref] [ Google Scholar]
- Asghari S, Rafraf M, Farzin L, Asghari-Jafarabadi M, Ghavami SM, Somi MH. Effects of pharmacologic dose of resveratrol supplementation on oxidative/antioxidative status biomarkers in nonalcoholic fatty liver disease patients: a randomized, double-blind, placebo-controlled trial. Adv Pharm Bull 2018; 8(2):307-17. doi: 10.15171/apb.2018.036 [Crossref] [ Google Scholar]
- Celinski K, Konturek PC, Slomka M, Cichoz-Lach H, Brzozowski T, Konturek SJ. Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease--14 months follow up. J Physiol Pharmacol 2014; 65(1):75-82. [ Google Scholar]
- Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015; 385(9972):956-65. doi: 10.1016/s0140-6736(14)61933-4 [Crossref] [ Google Scholar]
- Abdelmalek MF, Sanderson SO, Angulo P, Soldevila-Pico C, Liu C, Peter J. Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology 2009; 50(6):1818-26. doi: 10.1002/hep.23239 [Crossref] [ Google Scholar]
- Alam S, Mustafa G, Alam M, Ahmad N. Insulin resistance in development and progression of nonalcoholic fatty liver disease. World J Gastrointest Pathophysiol 2016; 7(2):211-7. doi: 10.4291/wjgp.v7.i2.211 [Crossref] [ Google Scholar]
- Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci 2011; 15(9):1090-5. [ Google Scholar]
- Lonardo A, Bellentani S, Argo CK, Ballestri S, Byrne CD, Caldwell SH. Epidemiological modifiers of non-alcoholic fatty liver disease: focus on high-risk groups. Dig Liver Dis 2015; 47(12):997-1006. doi: 10.1016/j.dld.2015.08.004 [Crossref] [ Google Scholar]
- Armstrong MJ, Hull D, Guo K, Barton D, Hazlehurst JM, Gathercole LL. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol 2016; 64(2):399-408. doi: 10.1016/j.jhep.2015.08.038 [Crossref] [ Google Scholar]
- Balas B, Belfort R, Harrison SA, Darland C, Finch J, Schenker S. Pioglitazone treatment increases whole body fat but not total body water in patients with non-alcoholic steatohepatitis. J Hepatol 2007; 47(4):565-70. doi: 10.1016/j.jhep.2007.04.013 [Crossref] [ Google Scholar]
- Baniasadi N, Salajegheh F, Pardakhty A, Seyedmirzaee SM, Hayatbakhsh MM, Nikpoor AR. Effects of pentoxifylline on non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled trial in Iran. Hepat Mon 2015; 15(11):e32418. doi: 10.5812/hepatmon.32418 [Crossref] [ Google Scholar]
- Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006; 355(22):2297-307. doi: 10.1056/NEJMoa060326 [Crossref] [ Google Scholar]
- Chachay VS, Macdonald GA, Martin JH, Whitehead JP, O’Moore-Sullivan TM, Lee P. Resveratrol does not benefit patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2014; 12(12):2092-103. doi: 10.1016/j.cgh.2014.02.024 [Crossref] [ Google Scholar]
- Xu R, Tao A, Zhang S, Deng Y, Chen G. Association between vitamin E and non-alcoholic steatohepatitis: a meta-analysis. Int J Clin Exp Med 2015; 8(3):3924-34. [ Google Scholar]
- Cui JY, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R. Sitagliptin versus placebo in the treatment of non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol 2016; 64(2):S192-S3. doi: 10.1016/S0168-8278(16)00137-9 [Crossref] [ Google Scholar]
- Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med 2016; 165(5):305-15. doi: 10.7326/m15-1774 [Crossref] [ Google Scholar]
- Deng XL, Ma R, Zhu HX, Zhu J. Short article: A randomized-controlled study of sitagliptin for treating diabetes mellitus complicated by nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 2017; 29(3):297-301. doi: 10.1097/meg.0000000000000780 [Crossref] [ Google Scholar]
- Ebrahimi-Mameghani M, Jamali H, Mahdavi R, Kakaei F, Abedi R, Kabir-Mamdooh B. Conjugated linoleic acid improves glycemic response, lipid profile, and oxidative stress in obese patients with non-alcoholic fatty liver disease: a randomized controlled clinical trial. Croat Med J 2016; 57(4):331-42. doi: 10.3325/cmj.2016.57.331 [Crossref] [ Google Scholar]
- Ekhlasi G, Zarrati M, Agah S, Hosseini AF, Hosseini S, Shidfar S. Effects of symbiotic and vitamin E supplementation on blood pressure, nitric oxide and inflammatory factors in non-alcoholic fatty liver disease. EXCLI J 2017; 16:278-90. doi: 10.17179/excli2016-846 [Crossref] [ Google Scholar]
- Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr 2014; 99(3):535-42. doi: 10.3945/ajcn.113.068890 [Crossref] [ Google Scholar]
- Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res 2014; 34(10):837-43. doi: 10.1016/j.nutres.2014.09.005 [Crossref] [ Google Scholar]
- Feng W, Gao C, Bi Y, Wu M, Li P, Shen S. Randomized trial comparing the effects of gliclazide, liraglutide, and metformin on diabetes with non-alcoholic fatty liver disease. J Diabetes 2017; 9(8):800-9. doi: 10.1111/1753-0407.12555 [Crossref] [ Google Scholar]
- Garinis GA, Fruci B, Mazza A, De Siena M, Abenavoli S, Gulletta E. Metformin versus dietary treatment in nonalcoholic hepatic steatosis: a randomized study. Int J Obes (Lond) 2010; 34(8):1255-64. doi: 10.1038/ijo.2010.40 [Crossref] [ Google Scholar]
- Hajiaghamohammadi AA, Ziaee A, Oveisi S, Masroor H. Effects of metformin, pioglitazone, and silymarin treatment on non-alcoholic Fatty liver disease: a randomized controlled pilot study. Hepat Mon 2012; 12(8):e6099. doi: 10.5812/hepatmon.6099 [Crossref] [ Google Scholar]
- Han Y, Shi JP, Ma AL, Xu Y, Ding XD, Fan JG. Randomized, vitamin E-controlled trial of bicyclol plus metformin in non-alcoholic fatty liver disease patients with impaired fasting glucose. Clin Drug Investig 2014; 34(1):1-7. doi: 10.1007/s40261-013-0136-3 [Crossref] [ Google Scholar]
- Hannah WN Jr, Harrison SA. Lifestyle and dietary interventions in the management of nonalcoholic fatty liver disease. Dig Dis Sci 2016; 61(5):1365-74. doi: 10.1007/s10620-016-4153-y [Crossref] [ Google Scholar]
- Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology 2009; 49(1):80-6. doi: 10.1002/hep.22575 [Crossref] [ Google Scholar]
- Haukeland JW. NAFLD - Diagnisos and management. Scandinavian Journal of Gastroenterology 2009; 44:11-2. doi: 10.1080/00365520903083554 [Crossref] [ Google Scholar]
- Heebøll S, Kreuzfeldt M, Hamilton-Dutoit S, Kjær Poulsen M, Stødkilde-Jørgensen H, Møller HJ. Placebo-controlled, randomised clinical trial: high-dose resveratrol treatment for non-alcoholic fatty liver disease. Scand J Gastroenterol 2016; 51(4):456-64. doi: 10.3109/00365521.2015.1107620 [Crossref] [ Google Scholar]
- Hirata T, Tomita K, Kawai T, Yokoyama H, Shimada A, Kikuchi M. Effect of telmisartan or losartan for treatment of nonalcoholic fatty liver disease: Fatty Liver Protection Trial by Telmisartan or Losartan Study (FANTASY). Int J Endocrinol 2013; 2013:587140. doi: 10.1155/2013/587140 [Crossref] [ Google Scholar]
- Hussain M, Majeed Babar MZ, Hussain MS, Akhtar L. Vildagliptin ameliorates biochemical, metabolic and fatty changes associated with non alcoholic fatty liver disease. Pak J Med Sci 2016; 32(6):1396-401. doi: 10.12669/pjms.326.11133 [Crossref] [ Google Scholar]
- Khoo J, Hsiang J, Taneja R, Law NM, Ang TL. Comparative effects of liraglutide 3 mg vs structured lifestyle modification on body weight, liver fat and liver function in obese patients with non-alcoholic fatty liver disease: a pilot randomized trial. Diabetes Obes Metab 2017; 19(12):1814-7. doi: 10.1111/dom.13007 [Crossref] [ Google Scholar]
- Le TA, Chen J, Changchien C, Peterson MR, Kono Y, Patton H. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 2012; 56(3):922-32. doi: 10.1002/hep.25731 [Crossref] [ Google Scholar]
- Lee YM, Sutedja DS, Wai CT, Dan YY, Aung MO, Zhou L. A randomized controlled pilot study of Pentoxifylline in patients with non-alcoholic steatohepatitis (NASH). Hepatology International 2008; 2(2):196-201. doi: 10.1007/s12072-008-9058-1 [Crossref] [ Google Scholar]
- Liechti F, Dufour JF. Treatment of NASH with ursodeoxycholic acid: cons. Clin Res Hepatol Gastroenterol 2012; 36 Suppl 1:S46-52. doi: 10.1016/s2210-7401(12)70021-9 [Crossref] [ Google Scholar]
- Yu L, Yuan M, Wang L. The effect of omega-3 unsaturated fatty acids on non-alcoholic fatty liver disease: a systematic review and meta-analysis of RCTs. Pak J Med Sci 2017; 33(4):1022-8. doi: 10.12669/pjms.334.12315 [Crossref] [ Google Scholar]
- Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 2015; 61(4):1239-50. doi: 10.1002/hep.27647 [Crossref] [ Google Scholar]
- McPherson S, Wilkinson N, Tiniakos D, Wilkinson J, Burt AD, McColl E. A randomised controlled trial of losartan as an anti-fibrotic agent in non-alcoholic steatohepatitis. PLoS One 2017; 12(4):e0175717. doi: 10.1371/journal.pone.0175717 [Crossref] [ Google Scholar]
- Méndez-Sánchez N, González V, Chávez-Tapia N, Ramos MH, Uribe M. Weight reduction and ursodeoxycholic acid in subjects with nonalcoholic fatty liver disease A double-blind, placebo-controlled trial. Ann Hepatol 2004; 3(3):108-12. [ Google Scholar]
- Merat S, Malekzadeh R, Sohrabi MR, Sotoudeh M, Rakhshani N, Sohrabpour AA. Probucol in the treatment of non-alcoholic steatohepatitis: a double-blind randomized controlled study. J Hepatol 2003; 38(4):414-8. doi: 10.1016/s0168-8278(02)00441-5 [Crossref] [ Google Scholar]
- Mofidi F, Poustchi H, Yari Z, Nourinayyer B, Merat S, Sharafkhah M. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. Br J Nutr 2017; 117(5):662-8. doi: 10.1017/s0007114517000204 [Crossref] [ Google Scholar]
- Van Wagner LB, Koppe SW, Brunt EM, Gottstein J, Gardikiotes K, Green RM. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Ann Hepatol 2011; 10(3):277-86. [ Google Scholar]
- Dagan SS, Zelber-Sagi S, Zilberman-Schapira G, Webb M, Buch A, Keidar A. Probiotics do not improve hepatic outcomes after laparoscopic sleeve gastrectomy surgery: A randomized clinical trial. Hepatology 2016; 64(1):567A. [ Google Scholar]
- Wah Kheong C, Nik Mustapha NR, Mahadeva S. A randomized trial of silymarin for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2017; 15(12):1940-9. doi: 10.1016/j.cgh.2017.04.016 [Crossref] [ Google Scholar]
- Wong VW, Won GL, Chim AM, Chu WC, Yeung DK, Li KC. Treatment of nonalcoholic steatohepatitis with probiotics A proof-of-concept study. Ann Hepatol 2013; 12(2):256-62. [ Google Scholar]
- Tiikkainen M, Häkkinen AM, Korsheninnikova E, Nyman T, Mäkimattila S, Yki-Järvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes 2004; 53(8):2169-76. doi: 10.2337/diabetes.53.8.2169 [Crossref] [ Google Scholar]
- Zhu FS, Liu S, Chen XM, Huang ZG, Zhang DW. Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. World J Gastroenterol 2008; 14(41):6395-400. doi: 10.3748/wjg.14.6395 [Crossref] [ Google Scholar]
- Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013; 145(3):574-82. doi: 10.1053/j.gastro.2013.05.042 [Crossref] [ Google Scholar]
- Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: a randomized placebo-controlled trial. J Clin Gastroenterol 2009; 43(10):990-4. doi: 10.1097/MCG.0b013e31819c392e [Crossref] [ Google Scholar]
- Nogueira MA, Oliveira CP, Ferreira Alves VA, Stefano JT, Rodrigues LS, Torrinhas RS. Omega-3 polyunsaturated fatty acids in treating non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled trial. Clin Nutr 2016; 35(3):578-86. doi: 10.1016/j.clnu.2015.05.001 [Crossref] [ Google Scholar]
- Omer Z, Cetinkalp S, Akyildiz M, Yilmaz F, Batur Y, Yilmaz C. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 2010; 22(1):18-23. doi: 10.1097/MEG.0b013e32832e2baf [Crossref] [ Google Scholar]
- Pakravan H, Ahmadian M, Fani A, Aghaee D, Brumanad S, Pakzad B. The effects of melatonin in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Adv Biomed Res 2017; 6:40. doi: 10.4103/2277-9175.204593 [Crossref] [ Google Scholar]
- Parikh P, Ingle M, Patel J, Bhate P, Pandey V, Sawant P. An open-label randomized control study to compare the efficacy of vitamin e versus ursodeoxycholic acid in nondiabetic and noncirrhotic Indian NAFLD patients. Saudi J Gastroenterol 2016; 22(3):192-7. doi: 10.4103/1319-3767.182451 [Crossref] [ Google Scholar]
- Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 2016; 150(5):1147-59. doi: 10.1053/j.gastro.2016.01.038 [Crossref] [ Google Scholar]
- Razavizade M, Jamali R, Arj A, Matini SM, Moraveji A, Taherkhani E. The effect of pioglitazone and metformin on liver function tests, insulin resistance, and liver fat content in nonalcoholic fatty liver disease: a randomized double blinded clinical trial. Hepat Mon 2013; 13(5):e9270. doi: 10.5812/hepatmon.9270 [Crossref] [ Google Scholar]
- Santos V, Lanzoni VP, Szejnfeld J, Shigueoka D, Parise ER. A randomized double-blind study of the short-time treatment of obese patients with nonalcoholic fatty liver disease with ursodeoxycholic acid. Braz J Med Biol Res 2003; 36(6):723-9. doi: 10.1590/s0100-879x2003000600007 [Crossref] [ Google Scholar]
- Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010; 362(18):1675-85. doi: 10.1056/NEJMoa0907929 [Crossref] [ Google Scholar]
- Harrison SA, Chalasani NP, Lawitz E, Marri S, Noureddin M, Sanyal AJ. Early phase 1 clinical trial results of GR-MD-02, a galectin-3 inhibitor, in patients having non-alcoholic steatohepatitis (NASH) with advanced fibrosis. Hepatology 2014; 60:225A-6A. doi: 10.1002/hep.27457 [Crossref] [ Google Scholar]
- Sharma BC, Kumar A, Garg V, Reddy RS, Sakhuja P, Sarin SK. A randomized controlled trial comparing efficacy of pentoxifylline and pioglitazone on metabolic factors and liver histology in patients with non-alcoholic steatohepatitis. J Clin Exp Hepatol 2012; 2(4):333-7. doi: 10.1016/j.jceh.2012.10.010 [Crossref] [ Google Scholar]
- Chen S, Zhao X, Ran L, Wan J, Wang X, Qin Y. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Dig Liver Dis 2015; 47(3):226-32. doi: 10.1016/j.dld.2014.11.015 [Crossref] [ Google Scholar]
- Shibuya T, Fushimi N, Kawai M, Yoshida Y, Hachiya H, Ito S. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: a prospective randomized controlled pilot study. Diabetes Obes Metab 2018; 20(2):438-42. doi: 10.1111/dom.13061 [Crossref] [ Google Scholar]
- Takeshita Y, Takamura T, Honda M, Kita Y, Zen Y, Kato K. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: a randomised controlled trial. Diabetologia 2014; 57(5):878-90. doi: 10.1007/s00125-013-3149-9 [Crossref] [ Google Scholar]
- Sofi F, Giangrandi I, Cesari F, Corsani I, Abbate R, Gensini GF. Effects of a 1-year dietary intervention with n-3 polyunsaturated fatty acid-enriched olive oil on non-alcoholic fatty liver disease patients: a preliminary study. Int J Food Sci Nutr 2010; 61(8):792-802. doi: 10.3109/09637486.2010.487480 [Crossref] [ Google Scholar]
- Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin 2008; 29(6):698-706. doi: 10.1111/j.1745-7254.2008.00807.x [Crossref] [ Google Scholar]
- Shenoy KT, Balakumaran LK, Mathew P, Prasad M, Prabhakar B, Sood A. Metadoxine versus placebo for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. J Clin Exp Hepatol 2014; 4(2):94-100. doi: 10.1016/j.jceh.2014.03.041 [Crossref] [ Google Scholar]
- Hameed B, Terrault NA, Gill RM, Loomba R, Chalasani N, Hoofnagle JH. Clinical and metabolic effects associated with weight changes and obeticholic acid in non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2018; 47(5):645-56. doi: 10.1111/apt.14492 [Crossref] [ Google Scholar]
- Hosseinpour-Arjmand S, Hamedi-Shahraki S, Ebrahimi-Mameghani M, Amirkhizi F. The Effect of alpha-lipoic acid on liver function and metabolic markers in obese patients with non-alcoholic fatty liver disease: a double - blind randomized controlled trial. Iran Red Crescent Med J 2018; 20(3):e65925. doi: 10.5812/ircmj.65925 [Crossref] [ Google Scholar]
- Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial). Diabetes Care 2018; 41(8):1801-8. doi: 10.2337/dc18-0165 [Crossref] [ Google Scholar]
- Sofer E, Boaz M, Matas Z, Mashavi M, Shargorodsky M. Treatment with insulin sensitizer metformin improves arterial properties, metabolic parameters, and liver function in patients with nonalcoholic fatty liver disease: a randomized, placebo-controlled trial. Metabolism 2011; 60(9):1278-84. doi: 10.1016/j.metabol.2011.01.011 [Crossref] [ Google Scholar]
- Abenavoli L, Greco M, Milic N, Accattato F, Foti D, Gulletta E. Effect of Mediterranean diet and antioxidant formulation in non-alcoholic fatty liver disease: a randomized study. Nutrients 2017; 9(8). doi: 10.3390/nu9080870 [Crossref]
- Alam S, Kabir J, Das U, Hasan N, Alam AK. Effect of telmisartan on histological activity and fibrosis of non alcoholic steatohepatitis patient a one year randomized control trial. Hepatol Int 2015; 9(1):S116. doi: 10.1007/s12072-015-9609-1 [Crossref] [ Google Scholar]
- Nobili V, Alisi A, Valenti L, Miele L, Feldstein AE, Alkhouri N. NAFLD in children: new genes, new diagnostic modalities and new drugs. Nat Rev Gastroenterol Hepatol 2019; 16(9):517-30. doi: 10.1038/s41575-019-0169-z [Crossref] [ Google Scholar]
- Sridharan K, Sivaramakrishnan G, Sequeira RP, Elamin A. Pharmacological interventions for non-alcoholic fatty liver disease: a systematic review and network meta-analysis. Postgrad Med J 2018; 94(1116):556-65. doi: 10.1136/postgradmedj-2018-135967 [Crossref] [ Google Scholar]