Advanced pharmaceutical bulletin. 11(1):10-27.
doi: 10.34172/apb.2021.002
Review Article
A Review on Recent Trends in Green Synthesis of Gold Nanoparticles for Tuberculosis
Arti Gupta 1, *  , Sonia Pandey 1
, Sonia Pandey 1  , Jitendra Singh Yadav 2
, Jitendra Singh Yadav 2
Author information:
1Uka Tarsadia University, Maliba Pharmacy College, Gopal Vidhya Nagar, Bardoli, Gujarat, India.
2Shree Naranjibhai Lalbhai Patel College of Pharmacy, Umrakh, Gujarat, India.
Abstract
Tuberculosis (TB) is a contagious disease that has affected mankind. The anti-TB treatment has been used from ancient times to control symptoms of this disease but these medications produced some serious side effects. Herbal products have been successfully used for the treatment of TB. Gold is the most biocompatible metal among all available for biomedical purposes so Gold nanoparticles (GNPs) have sought attention as an attractive biosynthesized drug to be studied in recent years for bioscience research. GNPs are used as better catalysts and due to unique small size, physical resemblance to physiological molecules, biocompatibility and non-cytotoxicity extensively used for various applications including drug and gene delivery. Greenly synthesized GNPs have much more potential in different fields because phytoconstituents used in GNP synthesis itself act as reducing and capping agents and produced more stabilized GNPs. This review is devoted to a discussion on GNPs synthesis with herbs for TB. The main focus is on the role of the natural plant bio-molecules involved in the bioreduction of metal salts during the GNPs synthesis with phytoconstituents used as antitubercular agents.
Keywords: Green synthesis, Gold nanoparticles, Tuberculosis, Phytoconstituents
Copyright and License Information
© 2021 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Tuberculosis (TB) is a bacterial infectious disease caused by Mycobacterium tuberculosis, one of the oldest bacterial diseases. TB is still affecting and posing major health, social and economic burdens at the global level. However, low and middle-income countries are mainly affected. If the disease would not be managed efficiently then TB will be resurged due to some other diseases like HIV infection as well as multiple drug-resistant tuberculosis (MDR-TB) by considering these facts in 1993, the World Health Organization (WHO) took an unprecedented step and declared TB a global emergency.1,2 Synthetic anti-TB drugs are a two-edged sword while they destroy pathogenic M. tuberculosis they also select for drug-resistant bacteria against which those drugs are then ineffective1. TB either kills the infected individual or renders him/her incapable of assuming normal functions. Upon gaining entry into a new host, M. tuberculosis may result in an active infection or remain latent.3 TB is spread via various sources like infectious aerosols from an infected person. TB infections and their development are represented in Figure 1.
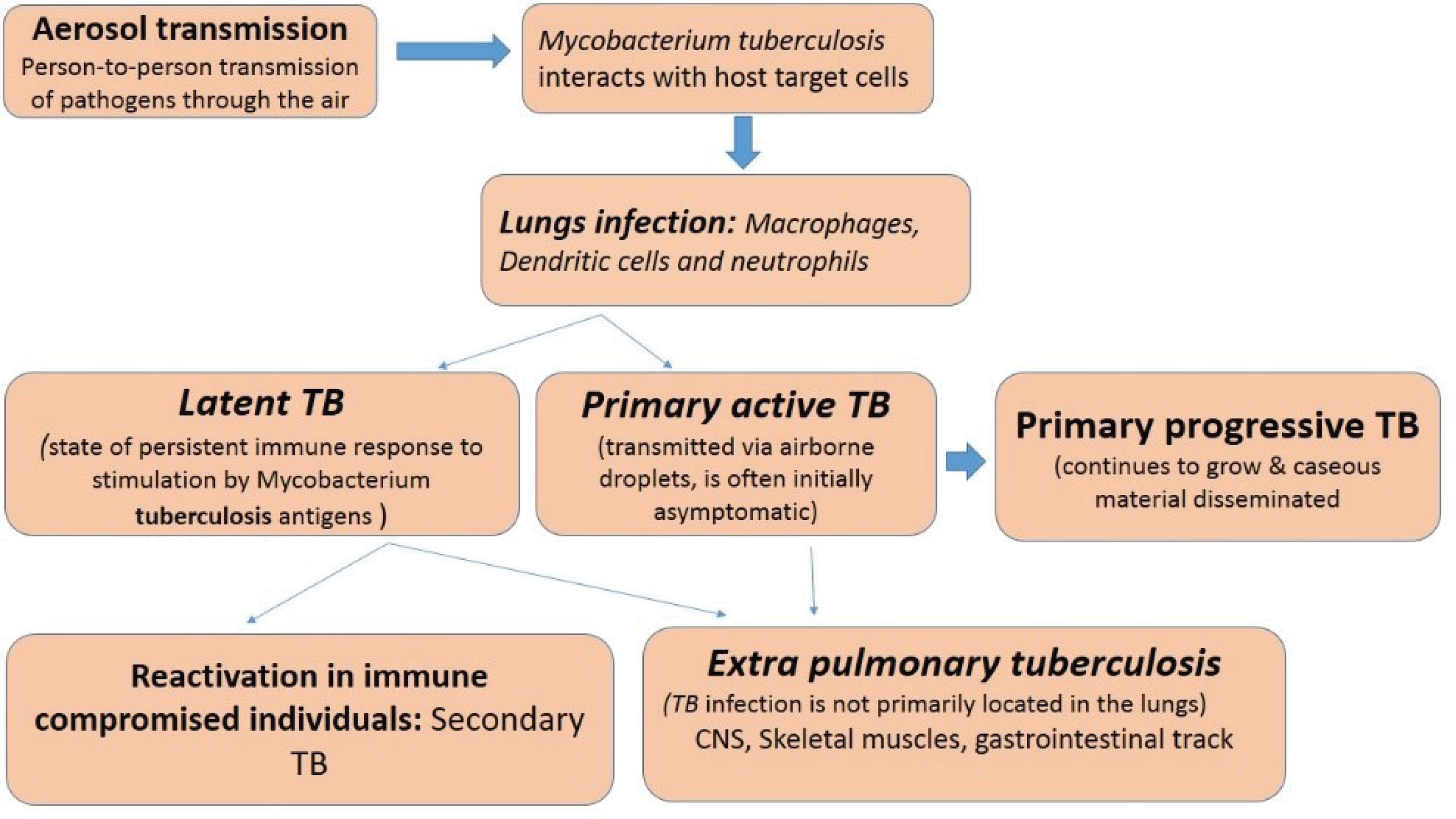
Figure 1.
Tuberculosis infection and development.
.
Tuberculosis infection and development.
Wide ranges of phytoconstituents having the desired pharmacological effect on the body were responsible for anti-tubercular activity includes alkaloids4-6 glycosides7-9 glycoterpenoids,10 diterpenoids glycosides,11 tannins,12 phenolics and amides13-18 xanthones19-23 quinones,24 sterol25-28 triterpenoids.29-37 Terpenoids are scope for compounds that can be developed as future anti-mycobacterial drugs. It has been reported that ursolic and oleanolic acids are not so toxic and possess antimicrobial activity against some multi-resistant bacteria.34,38-41
Various antimycobacterial chemical compounds have also been isolated from plants, including ellagitannin punicalagin, allicin, and these compounds offered various clues for effective management of the disease to lessen the global burden of TB and drug-resistant M. tuberculosis strains.42 In this review, the author has emphasized the green synthesis of gold nanoparticles (GNPs) with herbs for TB (Antimicrobial and antibacterial activity). The main focus is on the role of the natural plant bio-molecules involved in the bioreduction of metal salts during the GNPs synthesis with phytoconstituents used as antitubercular agents. The plants having phytoconstituents acting as antitubercular agents discussed in Table 1.
Table 1.
List of plants containing phytoconstituents having anti tubercular activity
|
Botanical/family name
|
Phytoconstituents
|
References |
|
Acalypha indica (Euphorbiaceae) |
Kaempferol, acalyphamide and other amides, quinone, sterols, cyanogenic glycoside |
43-47
|
|
Allium cepa (Liliaceae) |
Antibacterial substances (subterranean) allicin, ajoene indole alkaloids, steroidal triterpenes |
44,48-50 |
|
Allium sativum (Liliaceae) |
Sulphur containing amino acids known as alliin |
51,52-55 |
|
Adhatoda vasica ( Acanthaceae) |
Vasicine acetate and 2-acetyl benzylamine, bromhexine and ambroxol, semi-synthetic derivatives of vasicine |
56,57
|
|
Aloe vera (Liliaceae) |
Anthraquinone glycosides (aloin), |
44,58
|
|
Berberis Hispanica ( Berberidaceae ) |
- |
59
|
|
Byrsonima crassa
( Malpighiaceae) |
Triterpenes:α-amyrin, β-amyrin and their acetates, lupeol, oleanolic acid,ursolic acid and α-amyrinone alkane dotriacontane, triterpenoids as bassic acid |
37,60
|
|
Buddleja saligna (Scrophulariaceae) |
Non-cytotoxic triterpenoids oleanolic |
61-63
|
|
Baccharis patagonica (Asteraceae) |
Oleanolic acid |
31
|
|
Clavijap rocera (Theophrastaceae) |
Oleanane triterpenoid (aegicerin) |
64
|
|
Canscora decussate (Gentianaceae) |
β-amyrin, friedelin, genianine, mangiferin, xanthones |
20,65
|
|
Colebrookea oppositifolia (Lamiaceae) |
dinor-cis-labdane diterpene and flavonoids |
66
|
|
Chuquiragau licina
|
Lupeol |
31
|
|
Caesalpinia pulcherrima (Rosaceae) |
Furanoditerpenoids (6β-benzoyl-7β-hydroxyvouacapen-5α-ol, 6β-cinnamoyl-7β-hydroxyvouacapen-5α-ol) Flavonoid (myricitroside) |
67
|
|
Flacourtia ramontchii (Flacourtiaceae) |
Phenolic glucoside ester, (−)-flacourtin, ramontoside, β-sitosterol and its β- D-glucopyranoside |
1,65,68
|
| Junellia tridens (Verbenaceae) |
Oleanonic acid |
31
|
|
Kalanchoe integra, (Crassulaceae) |
Triterpenoids- friedelin, taraxerol and glutinol and a mixture of long chain hydrocarbons Hypotensive, antiarrhythmic |
59
|
|
Leysera gnaphalodes
(Asteraceae) |
Non-cytotoxic triterpenoids oleanolic |
62,39
|
|
Mallotus philippensis (Euphorbiaceae) |
Phloroglucinol derivatives; rottlerin, isorottlerin, isoallorottlerin |
68,69
|
|
Mimosa pudica, (Mimosaceae) |
Mimosine and turgorin |
68,70
|
|
Trichosanthes dioica (Cucurbitaceae) |
Amino acids, nicotinic acid, riboflavin, vitamin C, thiamine, 5-hydroxytryptamine |
70
|
|
Tinospora cordifolia (Menispermaceae) |
Alkaloids, carbohydrates, flavonoids, glycosides, lignin, saponins, terpenes, tannins, steroids |
71-74
|
|
Morinda citrifolia (Rubiaceae) |
Scopoletin, Anthraquinone salizarin and its glycosides, nordamnacanthol. Ursolic acid and β- sitosterol asperuloside and caproic acid |
75,76
|
|
Myrtus communis (Myrtaceae) |
Phenolic compounds |
77
|
|
Ocimum sanctum (Labiatae) |
Essential oil |
78-82
|
|
Prunus armeniaca (Rosaceae) |
Flavonoid glycosides, polyphenols, sterol derivatives, carotenoids, cynogenic glycosides and volatile compounds |
83,84,65
|
|
Piper species, Piper regnellii (Piperaceae) |
Piperine, neolignans, eupomatenoid-5, Aristolactams, dioxoaporphines, lignans, longamide, pluviatilol, methyl pluviatilol (fargesin), sesamin. |
85-87
|
|
Rumex hastatus (Polygonaceae) |
Naphthalene acylglucosides, rumexneposides. |
88
|
|
Salvia hypargeia (Lamiaceae) |
Diterpenoids (Labdane), hypargenin |
89-92
|
|
Senecio chionophilus (Asteraceae) |
Sesqui terpenoids (oxofuranoeremophilane) |
93,94
|
|
Vitex trifolia (Verbenaceae) |
Diterpenoids (halimane and labdane) |
1,95
|
|
Vitex negundo (Verbenaceae) |
Iridoid glycosides, isomeric flavanones and flavonoids |
96,97
|
|
Juniperus communis (Cuppressaceae) |
Isocupressic acid, communic acid and deoxypodophyllotoxin |
98,99
|
|
Monoterpenoids
|
| Cymbopogon (lemon grass). |
Citronellol, nero, dehydro costuslactone |
100
|
|
Sesquiterpenes
|
|
Saussurea lappa (Compositae) |
Costunolide |
101
|
|
Magnolia grandiflora (Magnoliaceae) |
Parthenolide |
101
|
|
Ambrosia artemisiifolia (Asteraceae) |
11bH-dihydroparthenolide |
101
|
|
Ambrosia confertiflora (Asteraceae) |
Santamarine |
101
|
|
Sonchus hierrensis (Asteraceae) |
Santamarine |
101
|
|
Ambrosia confertiflora (Asteraceae) |
Reynosin |
101
|
|
Artemisia ramose (Compositae) |
Santonin |
101
|
|
Podachenium eminens (Asteraceae) |
7-hydroxydehydrocostuslactone |
102
|
| Zaluzania triloba (Compositae]) |
Zaluzanin C |
101
|
|
Diterpenes
|
|
Tetradenia riparia (Lamiaceae) |
Sandaracopimara-8(14)-15-diene-7a,18-dio |
103
|
|
Juniperus excels (Cupressaceae) |
Sandracopimaric acid, juniperexcelsic acid |
104
|
|
Salvia multicaulis (Lamiaceae) |
12-demethylmulticauline, multicaulin, 12-demethylmultiorthoquinone, multiorthoquinone, 12-methyl-5-dehydrohorminone, 2-methyl-5-dehydroacetylhorminone, salvipimarone |
90
|
|
Azorella madreporica (Apiaceae) |
9,12-cyclomulin-13-ol |
105
|
|
Triterpenes
|
|
Ajuga remot a (Lamiaceae) |
Ergosterol-5,8-endoperoxide |
106
|
|
Melia volkensii (Meliaceae) |
6b-hydroxykulactone, kulonate |
106
|
|
Borrichia frutescens (Asteraceae) |
(24R)-24,25-epoxycycloartan-3-one, (3b,24R)-24,25-epoxycycloartan-3-ol, (3b,24R)-24,25-epoxycycloartan-3-ol acetate, (23R)-3-oxolanosta-8,24-dien-23-o |
107
|
|
Sarmienta scandens (Gesneriaceae) |
Zeorin, 7b-acetyl-22-hydroxyhopane, 7b,22-dihydroxyhopane, |
31
|
|
Baccharis patagonica (Asteraceae) |
Oleanolic acid, erythodio |
31
|
|
Junellia tridens (Verbenaceae) |
3-epioleanolic acid, oleanonic acid |
108
|
|
Chuquiraga ulicina ( Asteraceae) |
lupeol acetate, lupenone, 3-hydroxynorlupen-2-one, 3-acetoxynorlupen-2-one |
31
|
|
Acaena pinnatifida (Rosaceae) |
Pomolic acid, pomolic acid acetate, tormentic acid, 2-epi-tormentic acid, euscaphic acid, niga-ichigoside F1 aglycone |
31
|
To avoid the adverse effect of recently used synthetic anti-TB drug109 natural products including plants, animals, and minerals have been the basis of treatment of human diseases 1. Studies showed that males with above 35 years of age of the patients, female, HIV-infected, older, and Asian-born patients are more prone to the major adverse effect of recent anti-TB drugs.110
Owing to the diversity of different natural active components such as plants, marine algae and types of metal salts and their ability to alter the composition of a reaction mixture through exposure to changes in the temperature, pH, and presence of various additives of biological origin (bio-matrices) which allows to produce nanoparticles of various metals with a defined size and shape.111 It is well established that biologically synthesized metal nanoparticles had various proved, biomedical applications like targeted delivery of cancer drugs, molecular imaging, wastewater treatment, cosmetics, as antiseptics, bio-sensors, antimicrobials, catalysts, optical fibers, agricultural, bio-labeling and in other areas is proved to be much safer, environment-friendly and cost-effective method of synthesis.111-113 Due to the diverse applications of Nanoparticles, several green approaches have been explored for synthesizing nanoparticles using different natural sources such as plants, marine algae, all these having immense tolerance to metal salts and have good ability to secrete extracellular enzymes for reduction of metals to consecutive nanoparticles.113-115 Gold is the most biocompatible metal nanoparticles are used in therapeutics and diagnostics in recent days to be studied in the recent field of bioscience.115-119 The biosynthesized GNPs were found to be better catalysts without using synthetic surfactant or capping agent at a low and definite concentration120 GNPs provide non-toxic carriers for drug and gene delivery applications. With these systems, the gold core imparts stability to the assembly, while the monolayer allows tuning of surface properties such as charge and hydrophobicity. An additional attractive feature of GNPs is their interaction with thiols, providing an effective and selective means of controlled intracellular release.121
By controlling shape like nanospheres, nanorods, nanoshells, nanocages and structure of GNPs the surface plasmon resonance peaks of gold nanostructures can be tuned from the visible to the near-infrared region (solid vs. hollow). A combination of this optical tunability with the inertness of gold makes gold nanostructures well suited for various biomedical applications.122 The principle application of GNPs in the biomedical field is sensors,123-125 antimicrobials,126-128 catalysts,129-131 electronics,132,133 optical fibers,134,135 agricultural,136-138 bio-labelling139 development of specific scaffolds, conjugates to biomedical diagnostics and analytics, photothermal and photodynamic therapies, and delivery of target molecules.140-142 Different shapes (nanosphere, nanobelt, branched, nanocage, nanoshell, nanocubes, nanorod, nanostar, and nanocluster) of GNPs are represented in Figure 2 and their applications are discussed in Table 2.
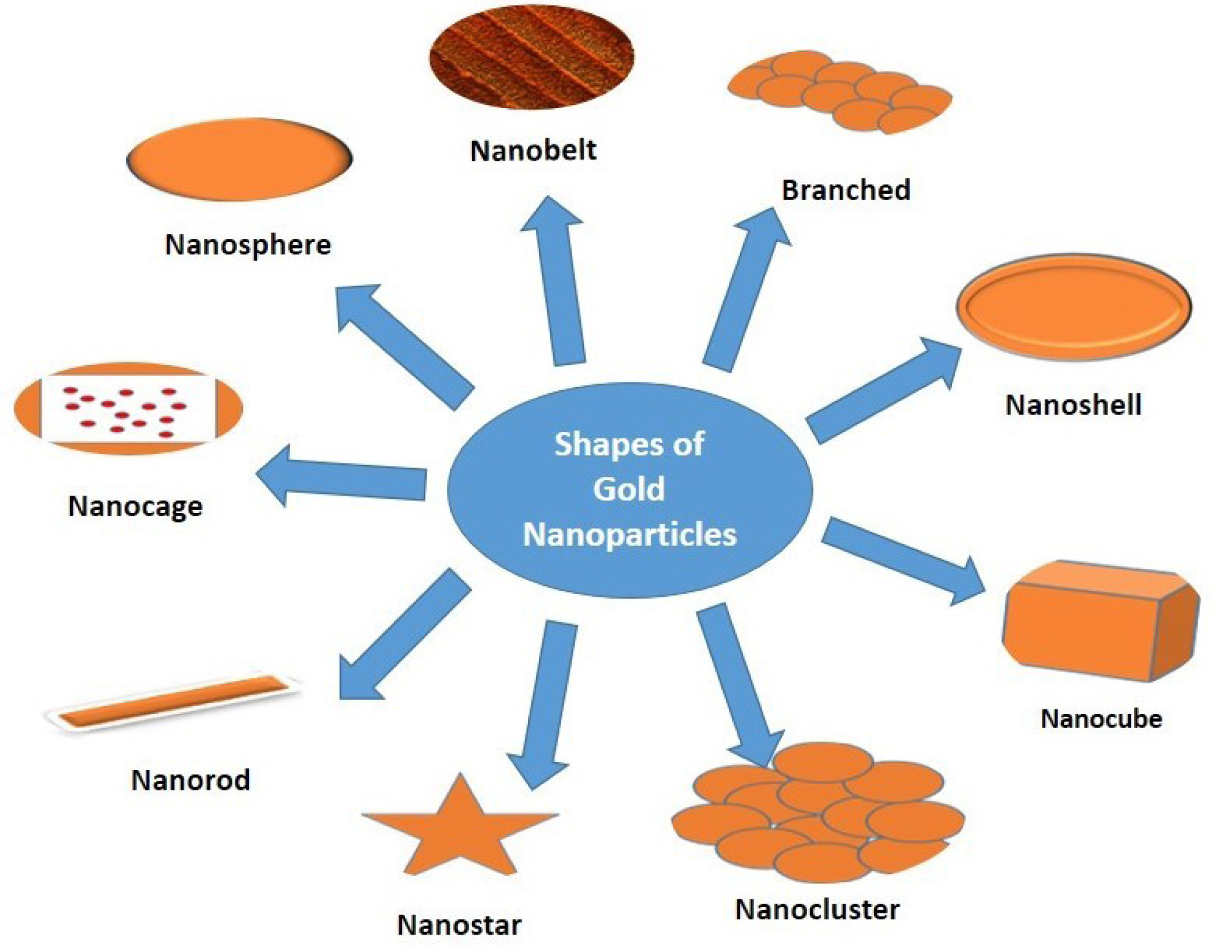
Figure 2.
Different shapes of gold nanoparticles.
.
Different shapes of gold nanoparticles.
Table 2.
Shapes of gold nanoparticles and their applications
|
Shape
|
Size
|
Applications
|
| Nano rod |
2-5 nm |
Photothermal Tumor Therapy, gas sensors139,143 |
| Nano sphere |
10-200 nm |
(i) The development of an ultrasensitive nanoscale optical biosensor based on LSPR wavelength-shift spectroscopy and (ii) The SERS detection of an anthrax biomarker 144 Nanospheres used as targeted drug delivery on tumor and brain144,145 |
| Nano star |
46-76 nm |
Inkjet printing technology,146 SERS sensor for Hg2+ detection147 |
| Nano clusters |
∼1.4 nm |
Potential for cancer therapy,148 biological labelling applications149 |
| Nano cube |
50 nm |
Biomedical Applications150 |
| Branched particle |
90 nm |
Nanostars have been predicted and demonstrated to shine brighter than any other shapes, thus opening new avenues for highly sensitive detection or biolabelling, among other applications.151 |
| Nanocage |
36 nm nanocage |
Photothermal cancer treatment, applications in nanobioelectronics,152 Biomedical Applications.150 |
| Nanobelt |
Thickness: 80 nm
With: 20 nm
Lenth: 0.15 nm |
One-dimensional nano-scale sensors, transducers, and resonators.153 |
| Nanoshell |
≥100 nm |
Fluorescent diagnostic labels, catalysis, avoiding photo degradation, enhancing photoluminescence, creating photonic crystals, preparation of bio conjugates, chemical and colloidal stability.154 |
Green synthesis of gold nanoparticle
In the late 1990s, the development of non-toxic methods has embraced the principles of green chemistry.155 Green synthesis of metal nanoparticles has received widespread attention in the past decade due to its ability to meet environmental and economic goals simultaneously without using the chemical and cost-effective too. Green synthesis common approaches for GNPs have been shown in Figure 3. For the green synthesis of GNPs, the antioxidant components of the studied plant extracts are responsible for the reduction of metal salts, leading to the growth and stabilization of the GNPs.156
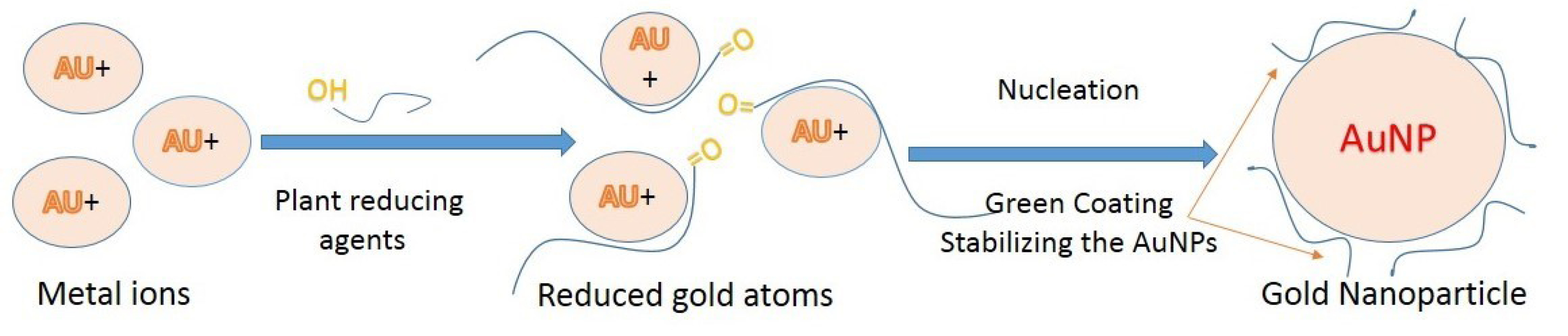
Figure 3.
Green gold nanoparticles synthesis using plant/plants extract.
.
Green gold nanoparticles synthesis using plant/plants extract.
Medicinal herbs having phytochemicals like as alcohols, phenols, proteins, terpenes, alkaloids, saponins, etc which can act as reducing as well as capping agents in the GNPs biosynthesis.157,158
Role of natural constituents for the green synthesis of GNPs
The triterpenes skeletons like cucurbitanes, cycloartanes, dammaranes, euphanes, friedelanes, holostanes, hopanes, isomalabaricanes, lanostanes, lupanes, oleananes, protostanes, tirucallanes, and ursanes159 are of interest ranging from primarily structural (cholesterol in cell membranes) to functional (carotenoids in photosynthesis, retinal in vision, quinones in electron transfer).160 Terpenoids play a crucial role in the reduction process of metal ions into nanoparticles, like eugenol the main terpenoid present in many plants.111
GNPs of Schinus molle L extract contain phenol, which shows that the differences in transmittance. Purified phenolics like gallic and protocatechuic acid act as reducing and capping agents in GNP synthesis because of the involvement of functional groups present in this phenolic compounds.161-163 These findings can help to determine the mechanism of metal nanoparticles by using crude extracts formation and stabilization. Cinnamomum verum extract contains polyols like (flavones and terpenoids) and polysaccharides, both contents act as reducing agent in metal ion synthesis.164 Flavonoids belong to the group of polyphenolic compounds that comprise several subgroups: anthocyanins, isoflavonoids, flavonols, chalcones, flavones, and flavanones, which can actively participate in the reduction and chelation of metal ions into nanoparticles. Literature established that reactive hydrogen atom release from tautomeric transformations of flavonoids from the enol-form to the keto-form can reduce metal ions to form nanoparticles. For example, flavonoids luteolin and rosmarinic acid present in Ocimum basilicum extracts it is the transform from the enol- to the keto-form.111 Apiin glycoside obtained from Lawsonia inermis used for the synthesis of anisotropic gold and quasi-spherical silver nanoparticle.165 The oxygen atoms belonging to 3-hydroxy and 4-oxo, and the 5-hydroxy and 4-oxo groups, are the preferred potential sites for chelation on quercetin.166
Many proteins contain active sites for metal ion accumulation and reduction where GNPs can form and be stabilized. In the process of nanoparticles formation, Protein donates electrons to react with metal ions and their subsequent stabilization that leads to the formation of nanoparticles.167 Some low molecular weight protein bands present in the soya bean extract, this may have been used up in biosynthesis of GNPs.168
The compounds present in the extracts can act as reducing as well as stabilizing agents and render more biocompatibility to the green synthesis of GNPs.169 High cost, use of environmentally hazardous chemicals, non-availability and presence of toxic capping agents limit the use of various physical and chemical methods.170-171 These limitations contributed the need for the development of new methods and materials for the production of nanoparticles based on the principles of ‘‘Green synthesis’’. The emphasis in this approach is on the synthesis and application of the nanoparticles for a maximum societal benefit, with minimal impact on the ecosystem.172
In Table 3 some part of plants which have been exploited by researchers for making AuNPs from the last decades have been summarised.
Table 3.
List of synthesized gold nanoparticles using whole, parts or extracts of different plants
|
Extract of plants
|
Part/ bomolecule
|
Size and shape
|
References |
|
Allium cepa L. |
Vitamin C |
~100 nm |
173
|
|
Anacardium occidentale L. |
Polyols and proteins |
Hexagonal |
174
|
|
Azadirachta indica
|
Nimbin, Azadirone, Azadirachtins |
2-100 nm |
175
|
|
Camellia sinensis
|
Polyphenolic compounds |
25 nm |
176
|
|
Chenopodium album
|
Oxalic acid |
12 nm,10 nm |
177
|
|
Justicia gendarussa
|
Polyphenol and flavonoid |
27 nm |
178
|
|
Macrotyloma uniflorum (Lam) |
Aqueous extract |
14-17 nm |
179
|
|
Mentha piperita L |
Menthol |
90 nm, 150 nm |
180
|
|
Mirabilis jalapa L. |
Polyols |
100 nm |
181
|
|
Swietenia mahogany
|
Polyhydroxy limonoids |
- |
182
|
|
Sapindus mukorossi
|
Fruit pericarp |
9 nm-19 nm |
183
|
|
Prunus domestica
|
Fruit extract |
14 nm-26 nm |
184
|
|
Magnolia kobus
|
Leaf extract |
5 nm-300 nm |
185
|
|
Coleus amboinicus lour
|
Leaf extract |
9.05 nm-31.95 nm |
186
|
|
Cassia auriculata
|
Leaf extract |
15 nm-25 nm |
187
|
|
Abelmoschus esculentus
|
Seed, aqueous extract |
45 nm-75 nm |
188
|
|
Zingiber officinale
|
Rhizome extract |
5 nm-15 nm |
189
|
|
Rosa hybrid Petal
|
Petal extract |
Petal 10 nm |
190
|
|
Cicer arietinum
|
Been |
Gold prisms (∼25 nm thick) |
191
|
|
Sugar beet
|
Pulp |
Nanowire |
192
|
|
Nyctanthes arbortristis
|
Flower |
19.8 ± 5.0 nm |
193
|
|
Gnidia glauca
|
Flower |
50 nm-150 nm |
170
|
|
Mangifera indica
|
Peel extract |
6.03-18 nm; spherical |
136
|
|
Gymnocladus assamicus
|
pod extract |
4-22 nm; hexagonal, pentagonal and triangular |
194
|
|
Cacumen platycladi
|
--- |
Variable |
195
|
|
Coriandrum sativum
|
Leaf |
6.75-57.91 nm; spherical |
196
|
|
Nerium oleander
|
Leaf extract |
2-10 nm; spherical |
197
|
|
Butea monosperma
|
- |
10-100 nm; spherical, triangular |
198
|
|
Pea nut
|
-- |
110 to 130 nm; variable |
199
|
|
Hibiscus cannabinus
|
Stem extract |
10-13 nm; spherical |
200
|
|
Sesbania grandiflora
|
Leaf extract |
7-34 nm; spherical |
201
|
|
Salix alba
|
Leaf extract |
50-80 nm |
202
|
|
Eucommia ulmoides
|
Bark |
Spherical |
203
|
|
Galaxaura elongata
|
Powder or extract |
3.85-77.13 nm; spherical, triangular, and hexagonal |
204
|
|
Ocimum sanctum
|
Leaf extract |
30 nm; hexagonal |
205
|
|
Torreya nucifera
|
--- |
10-125 nm; spherical |
206
|
|
Olea europaea
|
Leaf extracts |
50-100 nm; triangular, hexagonal |
207
|
|
Rosa indica
|
Rose petals |
3-15 nm; spherical |
208
|
|
Pistacia integerrima
|
Galls extract |
20-200 nm |
209
|
|
Terminalia arjuna
|
Fruit |
60 nm, spherical |
118
|
|
Euphorbia hirta
|
Leaf extract |
6-71 nm, spherical |
210
|
|
Morinda citrifolia
|
Root extract |
12.17-38.26 nm, spherical |
211
|
|
Zizyphus mauritiana
|
Extract |
20-40 nm, spherical |
212
|
Role of microorganisms for the green synthesis of GNPs
A variety of microorganisms are interacted with inorganic metals like gold, zinc, and silver and are known to use in bioleaching of minerals.213 Microbial cells treated with gold nanostructures synthesize by gold salts which are then isolated and purified using various techniques to obtain GNPs. Table 4 reflects a variety of microbes along with their genus which was used to make GNPs of different size ranges.
Table 4.
List of microorganisms which have been used for synthesis of GNPs
|
Microorganism
|
Genus
|
Size
|
References |
|
Pseudomonas fluorescens
|
Bacterium
|
50 nm–70 nm |
214
|
|
Shewanella algae
|
Bacterium
|
10 nm–20 nm |
215
|
|
Geobacillus stearothermophilus
|
Bacterium
|
- |
216
|
|
Escherichia coli DH5 α
|
Bacterium
|
- |
217
|
|
Marinobacter Pelagius
|
Bacterium
|
10 nm |
218
|
|
Stenotrophomonas maltophilia
|
Bacterium
|
40 nm |
219
|
|
Rhodopseudomonas capsulate
|
Bacterium
|
10 nm–20 nm |
220
|
|
Micrococcus luteus
|
Bacterium
|
- |
221
|
|
Yarrowia lipolytica
|
Marine Yeast
|
- |
222
|
|
Acanthella elongate
|
Sponge
|
7 nm–20 nm |
223
|
|
Stoechospermum marginatum
|
Algae
|
18.7 nm–93.7 nm |
224
|
|
Sargassum wightii Greville
|
Algae
|
8 nm–12 nm |
225
|
|
Streptomyces viridogens
|
Bacterium
|
18 nm–20 nm |
226
|
|
Candida albicans
|
Fungi
|
20 nm–80 nm |
227
|
|
Aspergillus fischeri
|
Fungi
|
50 nm spherical shaped |
112
|
|
Acanthophora spicifera
|
Algae |
- |
228
|
|
Chlorella pyrenoidusa
|
Algae |
- |
229
|
|
Kappaphycus alvarezii
|
Algae |
- |
230
|
|
Galaxaura elongata
|
Marine alga |
|
203
|
|
Tetraselmis kochinensis
|
Algae |
5–35 nm |
231
|
|
Sargassum myriocystum
|
Algae |
15 nm |
232
|
|
Stoechospermum marginatum
|
Algae |
- |
223
|
|
Laminaria japonica
|
Aqueous of extract Brown algae |
- |
233
|
Role of biomolecules for the green synthesis of GNPs
Biomolecules produced by living organisms to catalyze biological functions, such as nucleic acids, amino acids, lipids, and carbohydrates, possess hydroxyl and carbonyl functional groups in their structure which can reduce Au3+ ions to Au0 neutral atoms. These Au0 neutral atoms are then capped to form stabilized GNPs.234 This method can use for the biosafety of the reactants in GNPs synthesis. In Table 5 various biomolecules with type and size have been discussed.
Table 5.
List of various biomolecules involved in synthesis of AuNPs
|
Biomolecule
|
Type
|
Size (diameter)
|
References |
| Linoleic acid |
Fatty acid |
10 nm |
235
|
| Tannic acid |
Fatty acid |
8 nm–12 nm |
178
|
| NADPH-dependent enzyme |
Enzyme |
25 nm |
236
|
| Aminodextran |
Polysaccharide |
18 nm–14 nm |
237
|
| Chitosan |
Polysaccharide |
- |
238
|
| Glucose |
Carbohydrate |
22 nm–38 nm |
239
|
| Sucrose, Raffinose |
Carbohydrate |
4 nm–16 nm, 30 nm–48 nm |
238
|
| Dextrose-encapsulated |
Carbohydrate |
25 nm, 60 nm, 120 nm |
240
|
| Starch |
Polysaccharide |
11 nm–15 nm |
241
|
| Bovine serum albumin |
Protein |
- |
242
|
| Serrapeptase |
Protein |
20 nm -200 nm |
243
|
| Trypsin |
Enzyme |
- |
244
|
| Glycosaminoglycans |
Mucopolysaccharides |
- |
245
|
| Serratiopeptidase |
Enzyme |
- |
246
|
| DNA |
Nucleotide |
45 nm–80 nm |
247
|
| Aspartate |
Amino acid |
30 nm |
248
|
| Phospholipid |
Lipids |
05 nm |
249
|
Bioreactors for green synthesis of gold nanoparticles
Phytomining is the approach through which plants can reduce metal ions both on their surface and in various organs and tissues remote from the ion penetration site. The metals like copper, gold, silver, platinum, iron, and many others accumulated by the plants can be recovered after harvesting methods. For example, Brassica juncea and Medicago sativa, both the plant accumulate 50 nm silver nanoparticles (13.6% of their weight) when grown on silver nitrate as a substrate whereas M. sativa accumulate 4 nm gold icosahedra,250 and Iris pseudacorus (yellow iris) accumulate 2 nm semi-spherical copper particles when grown on substrates containing salts of the respective metals. Few approaches have been demonstrated in which different varieties of plant extracts have been used in combination with different varieties of acids and salts of metals.170,251
Factors affecting the formation of metal nanoparticles in plants
Various limitations of nanoparticle synthesis by phytoconstituents are observed and it needed to be resolved carefully before industrial manufacture. The prime limitation is the intricacy in the identification of the phytoconstituents present in plants responsible for the NPs synthesis and therapeutic activity. The amount of reducing agent needs to be controlled because it hampers the reduction rate which results in the formation of large aggregated nanoparticles. Simultaneously the process parameter like thermal heating must be under controlled because during synthesis it can damage and denature various active molecules like sugars, and proteins resulting in the loss of activity. The reaction rate can be optimized by controlling the reduction reaction by varying the concentration of phytochemicals carefully. All the factors affecting the green synthesis of metal nanoparticles are presented in Figure 4.
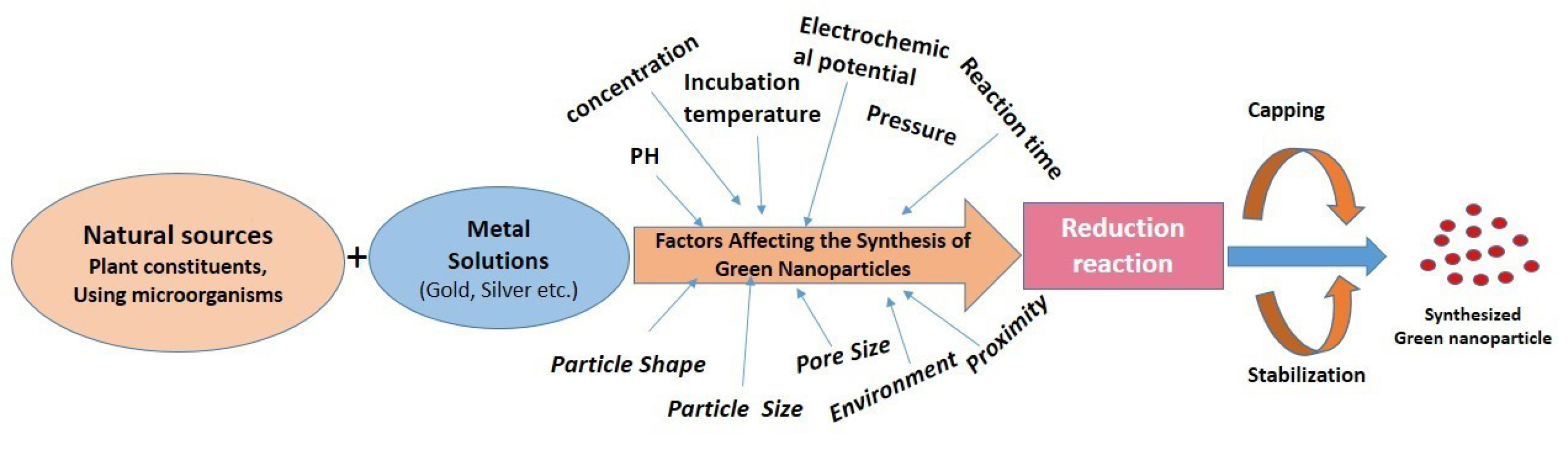
Figure 4.
Factors affecting the formation of metal nanoparticles in plants.
.
Factors affecting the formation of metal nanoparticles in plants.
To improve the efficacy, size and morphology of nanoparticles synthesized from biological sources by microorganisms several parameters need to be monitored like microorganism type, growth medium, growth stage (phase), synthesis conditions, reaction mixture pH, substrate concentrations, size, shape, incubation temperature and reaction time. The reduction process and stability of the biologically synthesized nanoparticles have a major concern and have to be controlled to improve the efficacy of the biologically synthesized nanoparticles. Major limitations in biologically synthesized nanoparticles are, the reduction process is quite slow and stable due to the decomposition of microorganisms over time.111,157,252-254
Nanoparticle aggregation is high at highly acidic pH over the reduction process and nucleation of reduced atoms. This may be related to the fact that a larger number of functional groups that bind and nucleate tetra-chloroauric acid ions become accessible at acidic pH.115,255-257 Efficiency and reaction rate of metal nanoparticle synthesis increases as an increase in the temperature. High temperatures required for crystal particle formation (nucleation rate is higher as increases the temperature). Interaction of phytochemicals with the nanoparticle surface may alter by elevated temperatures.258-263 Morphological diversity of the nanoparticles: triangles, hexagons, pentagons, cubes, spheres, ellipsoids, nanowires, and nanorods may occur due to the variation in concentration and composition of bioactive compounds present in plants.252,264
Green synthesis of gold nanoparticles for tuberculosis
Apart from diversified biomedical applications, GNPs have been reported for antimicrobial activity against food and agriculture pathogens.199 Inherent property of antibacterial and antimicrobial265 activity of GNPs along with the entrapped plant extract, facilitate the early recovery from TB. The proposed mechanism for antibacterial activity of GNPs is that it increases gene expression in the redox process which leads to the death of bacteria and fungi. The nano size, surface area and photo thermic nature of GNPs directly influenced the antimicrobial activity.266 Another excepted mechanism is that intracellularly GNPs attached to the sulfur base present in cells in the form of thiol group in enzymes which leads the disturbance of respiratory chain suddenly by the generation of a large number of free radicals leading to death. On the contrary, the GNPs decrease ATP activities wherein they reduce the t RNA and ribosomal interaction. GNPs also block the transmembrane hydrogen efflux however lesser concentration of GNPs can inhibit bacterial growth about 250-fold. Due to the smaller size of GNPs then bacterial cells, they stick on the cell wall of pathogens and delay cell process, causing death. Some report shows a different mechanism when compared to other metal nanoparticles. GNPs due to the charge difference on the cell wall and nanoparticle surfaces it attracts bacterial DNA. On the other side, GNPs show the varied activity of gram-positive and gram-negative bacteria, which are classified based on the thick layer called peptidoglycan. Peptidoglycan generally consists of two joined amino sugars, N-acetylglucosamine and N-acetylmuramic acid (NAM), with a pentapeptide coming off the NAM forming an inflexible structure to diffuse the GNPs. Therefore, the peptidoglycan is very strong in gram-positive bacteria that penetrate GNPs across cell wall whereas gram-negative bacteria contain a thin layer which easily undergoes cell death. The anti-microbial activity also assisted by the concentration of capping agents and purification methods apart from the size and peptidoglycan thickness. In green synthesized GNPs the antimicrobial activity may be due to the synergistic effects of GNPS with plant extracts.267
The biophysical interactions between bacteria and nanoparticle occur through aggregation biosorption and cellular uptake that can damage the membrane and produce toxicity.268 The mechanism of antibacterial activity of the GNPs is attributed to the generation of reactive oxygen species that causes an increase of the oxidative stress of microbial cells and the release of intracellular lactate dehydrogenase enzyme into extracellular medium in form of vacuole formation as an indication of potent activity.269-271 Such effect was enhanced and exaggerated by photothermal degeneration in a combined approach, GNPs-laser, which causes quick loss of cell membrane integrity.272
GNPs have advantages over other metal nanoparticles because they are chemically inert, biocompatible nature and not producing cytotoxicity. Gold is used internally in humans for the last 50 years.273
Physical properties of the nanoparticle may differ from their corresponding parent materials by decreasing the size of nanoparticles and this relation offered many opportunities for many scientific breakthroughs. GNPs produced good antibacterial activity. It had been shown their best result when particles aggregation is not observed at high levels. GNPs with the same shape and size exhibited different inhibitory effects by changing surface modifications agents.265 It can also use in targeted molecular imaging in living subjects.274
Recentely Gupta et al reported that the GNPs of ethanolic and hydroalcoholic exhibited anti-tubercular activity only at MIC 2.5 µg/mL and 20 µg/mL, respectively while ethanolic and hydroalcoholic extracts showed activity at much higher concentrations 50 µg/mL and 75 µg/mL, respectively.275 GNPs from Nigella arvensis (NA-GNPs) leaf extract were evaluated for antibacterial, antioxidant, cytotoxicity and catalytic activities and Chahardodli et al observed that NA-GNPs showed excellent cytotoxicity effects against H1299 and MCF-7 cancer cell lines with an IC50 value of 10 and 25 μg/mL, respectively and catalytic activity of NA-GNPs against methylene blue was 44%.276 Cheng et al synthesize GNPs using Chenopodium formosanum shell extract and concluded that GNPs possessed potent antibacterial activity against Escherichia coli and Staphylococcus aureus.277 Sunderam et al278 reported that green synthesized GNPs of Anacardium occidentale leaves extract, data presents good antibacterial effect against Escherichia coli and Bacillus subtilis and exhibited 74.47% viability on PBMC and 23.56% viability on MCF-7 cell lines at a maximum concentration of 100 µg/mL.278 Katas et al279 reported that the concentration of chitosan needed to synthesize antibacterial chitosan-GNPs with Lignosus rhinocerotis (LRE) was lower than those without LRE, suggesting that the addition of LRE as reducing agent resulted in higher antibacterial activity. Thus, chitosan as a stabilizing or capping agent and LRE as a reducing agent for the production of GNPs improved antibacterial activity of their resultant nanoparticles.276-279 Veena et al280 developed the green synthesis of Vitex negundo GNPs from leaf extracts and results exhibited strong antibacterial activity against gram-negative strains and moderate activity against gram-positive strains.280 The overview of the review is presented in Figure 5.
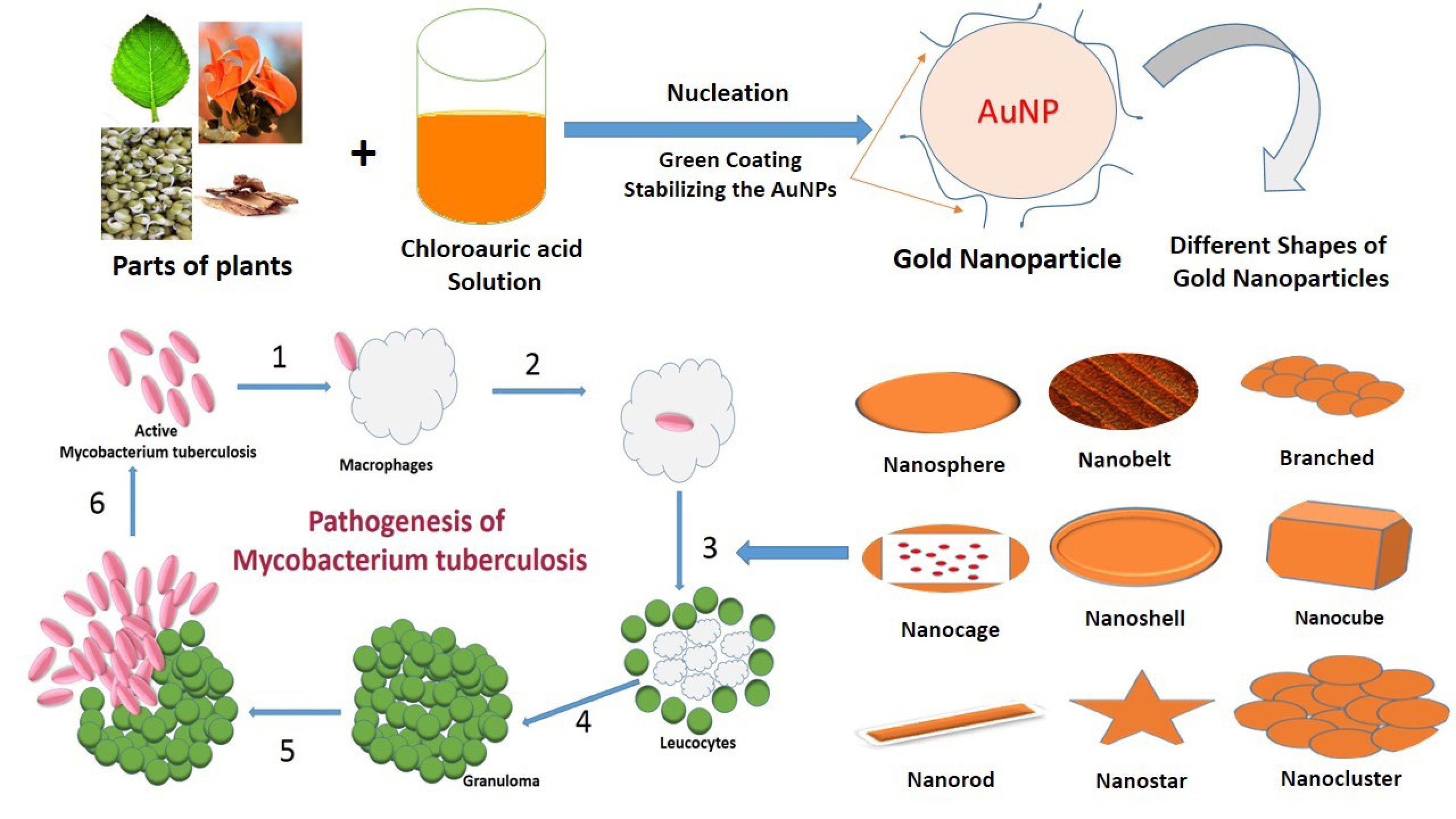
Figure 5.
The summary of the review.
.
The summary of the review.
Conclusion
The study of green synthesis of GNPs is a quickly evolving field in nanotechnology for TB. The present review summarises exhaustive literature for plants containing phytoconstituents having antitubercular activity along with the understanding of the synthesis of GNPs not only using plant extracts but biomolecules, microorganism, and various bioreactors. A detailed study is needed to give a lucid mechanism of biosynthesis of GNPs using biomolecules; microorganism present in different plant extracts which will be valuable to improve the properties of GNPs for TB treatment. With green chemical syntheses of these nanomaterials, researchers will able to conduct in-depth studies investigating biomedical applications without further biocompatibility preparations. In the coming years, the green chemistry procedure which utilizes plants their constituents, microorganisms, and biomolecules for nanoparticle preparation for TB has used as an alternative to conventional physicochemical methods since it is facile, rapid, cost-effective, and eco-friendly.
Ethical Issues
Not applicable.
Conflict of Interest
Authors declare no conflict of interest in this study.
References
- Arya V. A review on anti-tubercular plants. Int J PharmTech Res 2011; 3(2):872-80. [ Google Scholar]
-
Johnson R. Understanding the Mechanisms of Drug Resistance in Enhancing Rapid Molecular Detection of Drug Resistance in Mycobacterium tuberculosis [thesis]. Stellenbosch: University of Stellenbosch; 2007.
- Tuyiringire N, Tusubira D, Munyampundu JP, Tolo CU, Muvunyi CM, Ogwang PE. Application of metabolomics to drug discovery and understanding the mechanisms of action of medicinal plants with anti-tuberculosis activity. Clin Transl Med 2018; 7(1):29. doi: 10.1186/s40169-018-0208-3 [Crossref] [ Google Scholar]
- Khan EM, Haque I, Pandey R, Mishra SK, Sharma AK. Tuberculosis of the thyroid gland: a clinicopathological profile of four cases and review of the literature. Aust N Z J Surg 1993; 63(10):807-10. doi: 10.1111/j.1445-2197.1993.tb00345.x [Crossref] [ Google Scholar]
- Choi TA, Czerwonka R, Fröhner W, Krahl MP, Reddy KR, Franzblau SG. Synthesis and activity of carbazole derivatives against Mycobacterium tuberculosis. ChemMedChem 2006; 1(8):812-5. doi: 10.1002/cmdc.200600002 [Crossref] [ Google Scholar]
- Sinha N, Jain S, Tilekar A, Upadhayaya RS, Kishore N, Jana GH. Synthesis of isonicotinic acid N’-arylidene-N-[2-oxo-2-(4-aryl-piperazin-1-yl)-ethyl]-hydrazides as antituberculosis agents. Bioorg Med Chem Lett 2005; 15(6):1573-6. doi: 10.1016/j.bmcl.2005.01.073 [Crossref] [ Google Scholar]
- Barnes CC, Smalley MK, Manfredi KP, Kindscher K, Loring H, Sheeley DM. Characterization of an anti-tuberculosis resin glycoside from the prairie medicinal plant Ipomoea leptophylla. J Nat Prod 2003; 66(11):1457-62. doi: 10.1021/np030197j [Crossref] [ Google Scholar]
- Kataev VE, Strobykina I, Andreeva OV, Garifullin BF, Sharipova RR, Mironov VF. [Synthesis and antituberculosis activity of the derivatives of glycoside steviolbioside from the plant Stevia rebaudiana and diterpenoid isosteviol containing hydrazone, hydrazide and pyridinoyl moieties]. Bioorg Khim 2011; 37(4):542-51. doi: 10.1134/s1068162011030095 [Crossref] [ Google Scholar]
- Sharipova RR, Strobykina IY, Mordovskoi GG, Chestnova RV, Mironov VF, Kataev VE. Antituberculosis activity of glycosides from Stevia rebaudiana and hybrid compounds of steviolbioside and pyridinecarboxylic acid hydrazides. Chem Nat Compd 2011; 46(6):902-5. doi: 10.1007/s10600-011-9779-6 [Crossref] [ Google Scholar]
- Garifullin BF, Strobykina IY, Sharipova RR, Kravchenko MA, Andreeva OV, Bazanova OB. Synthesis and antituberculosis activity of the first macrocyclic glycoterpenoids comprising glucosamine and diterpenoid isosteviol. Carbohydr Res 2016; 431:15-24. doi: 10.1016/j.carres.2016.05.007 [Crossref] [ Google Scholar]
- Ibrahim MA, Rodenburg DL, Alves K, Fronczek FR, McChesney JD, Wu C. Minor diterpene glycosides from the leaves of Stevia rebaudiana. J Nat Prod 2014; 77(5):1231-5. doi: 10.1021/np4009656 [Crossref] [ Google Scholar]
- Bartzoka ED, Lange H, Poce G, Crestini C. Stimuli-Responsive Tannin-Fe(III) Hybrid Microcapsules Demonstrated by the Active Release of an Anti-Tuberculosis Agent. ChemSusChem 2018; 11(22):3975-91. doi: 10.1002/cssc.201801546 [Crossref] [ Google Scholar]
- Hussain K, Ismail Z, Sadikun A, Ibrahim P. Antioxidant, anti-TB activities, phenolic and amide contents of standardised extracts of Piper sarmentosum Roxb. Nat Prod Res 2009; 23(3):238-49. doi: 10.1080/14786410801987597 [Crossref] [ Google Scholar]
- Awouafack MD, Kouam SF, Hussain H, Ngamga D, Tane P, Schulz B. Antimicrobial prenylated dihydrochalcones from Eriosema glomerata. Planta Med 2008; 74(1):50-4. doi: 10.1055/s-2007-993782 [Crossref] [ Google Scholar]
- Cardoso CAL, Coelho RG, Honda NK, Pott A, Pavan FR, Leite CQ. Phenolic compounds and antioxidant, antimicrobial and antimycobacterial activities of Serjania erecta Radlk (Sapindaceae). Braz J Pharm Sci 2013; 49(4):775-82. doi: 10.1590/s1984-82502013000400017 [Crossref] [ Google Scholar]
- Coelho RG, Honda NK, Vieira Mdo C, Brum RL, Pavan FR, Leite CQ. Chemical composition and antioxidant and antimycobacterial activities of Bromelia balansae (Bromeliaceae). J Med Food 2010; 13(5):1277-80. doi: 10.1089/jmf.2009.0032 [Crossref] [ Google Scholar]
- Trevizan LNF, Nascimento KFD, Santos JA, Kassuya CAL, Cardoso CAL, Vieira MDC. Anti-inflammatory, antioxidant and anti-Mycobacterium tuberculosis activity of viridiflorol: the major constituent of Allophylus edulis (A St-Hil, A Juss & Cambess) Radlk. J Ethnopharmacol 2016; 192:510-5. doi: 10.1016/j.jep.2016.08.053 [Crossref] [ Google Scholar]
- Sinsimer D, Huet G, Manca C, Tsenova L, Koo MS, Kurepina N. The phenolic glycolipid of Mycobacterium tuberculosis differentially modulates the early host cytokine response but does not in itself confer hypervirulence. Infect Immun 2008; 76(7):3027-36. doi: 10.1128/iai.01663-07 [Crossref] [ Google Scholar]
- Suksamrarn S, Suwannapoch N, Phakhodee W, Thanuhiranlert J, Ratananukul P, Chimnoi N. Antimycobacterial activity of prenylated xanthones from the fruits of Garcinia mangostana. Chem Pharm Bull (Tokyo) 2003; 51(7):857-9. doi: 10.1248/cpb.51.857 [Crossref] [ Google Scholar]
- Ghosal S, Biswas K, Chaudhuri RK. Chemical constituents of Gentianaceae XXIV: anti-Mycobacterium tuberculosis activity of naturally occurring xanthones and synthetic analogs. J Pharm Sci 1978; 67(5):721-2. doi: 10.1002/jps.2600670546 [Crossref] [ Google Scholar]
- Chen JJ, Peng CF, Huang HY, Chen IS. Benzopyrans, biphenyls and xanthones from the root of Garcinia linii and their activity against Mycobacterium tuberculosis. Planta Med 2006; 72(5):473-7. doi: 10.1055/s-2005-916253 [Crossref] [ Google Scholar]
- Pickert M, Schaper KJ, Frahm AW. Substituted xanthones as antimycobacterial agents, Part 2: antimycobacterial activity. Arch Pharm (Weinheim) 1998; 331(5):193-7. doi: 10.1002/(sici)1521-4184(199805)331:5<193::aid-ardp193>3.0.co;2-s [Crossref] [ Google Scholar]
- Szkaradek N, Stachura K, Waszkielewicz AM, Cegła M, Szneler E, Marona H. Synthesis and antimycobacterial assay of some xanthone derivatives. Acta Pol Pharm 2008; 65(1):21-8. [ Google Scholar]
- Tran T, Saheba E, Arcerio AV, Chavez V, Li QY, Martinez LE. Quinones as antimycobacterial agents. Bioorg Med Chem 2004; 12(18):4809-13. doi: 10.1016/j.bmc.2004.07.015 [Crossref] [ Google Scholar]
- Podust LM, Poulos TL, Waterman MR. Crystal structure of cytochrome P450 14alpha -sterol demethylase (CYP51) from Mycobacterium tuberculosis in complex with azole inhibitors. Proc Natl Acad Sci U S A 2001; 98(6):3068-73. doi: 10.1073/pnas.061562898 [Crossref] [ Google Scholar]
- Bouic PJ, Lamprecht JH. Plant sterols and sterolins: a review of their immune-modulating properties. Altern Med Rev 1999; 4(3):170-7. [ Google Scholar]
- Bellamine A, Mangla AT, Nes WD, Waterman MR. Characterization and catalytic properties of the sterol 14alpha-demethylase from Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 1999; 96(16):8937-42. doi: 10.1073/pnas.96.16.8937 [Crossref] [ Google Scholar]
- Woldemichael GM, Franzblau SG, Zhang F, Wang Y, Timmermann BN. Inhibitory effect of sterols from Ruprechtia triflora and diterpenes from Calceolaria pinnifolia on the growth of Mycobacterium tuberculosis. Planta Med 2003; 69(7):628-31. doi: 10.1055/s-2003-41109 [Crossref] [ Google Scholar]
- Jiménez-Arellanes A, Meckes M, Torres J, Luna-Herrera J. Antimycobacterial triterpenoids from Lantana hispida (Verbenaceae). J Ethnopharmacol 2007; 111(2):202-5. doi: 10.1016/j.jep.2006.11.033 [Crossref] [ Google Scholar]
- Akihisa T, Franzblau SG, Ukiya M, Okuda H, Zhang F, Yasukawa K. Antitubercular activity of triterpenoids from Asteraceae flowers. Biol Pharm Bull 2005; 28(1):158-60. doi: 10.1248/bpb.28.158 [Crossref] [ Google Scholar]
- Wächter GA, Valcic S, Flagg ML, Franzblau SG, Montenegro G, Suarez E. Antitubercular activity of pentacyclic triterpenoids from plants of Argentina and Chile. Phytomedicine 1999; 6(5):341-5. doi: 10.1016/s0944-7113(99)80056-7 [Crossref] [ Google Scholar]
- Olugbuyiro JA, Moody JO, Hamann MT. AntiMtb activity of triterpenoid-rich fractions from Spondias mombin L. Afr J Biotechnol 2009; 8(9):1807-9. doi: 10.5897/ajb2009.000-9258 [Crossref] [ Google Scholar]
- Jiménez A, Meckes M, Alvarez V, Torres J, Parra R. Secondary metabolites from Chamaedora tepejilote (Palmae) are active against Mycobacterium tuberculosis. Phytother Res 2005; 19(4):320-2. doi: 10.1002/ptr.1664 [Crossref] [ Google Scholar]
- Ge F, Zeng F, Liu S, Guo N, Ye H, Song Y. In vitro synergistic interactions of oleanolic acid in combination with isoniazid, rifampicin or ethambutol against Mycobacterium tuberculosis. J Med Microbiol 2010; 59(Pt 5):567-72. doi: 10.1099/jmm.0.014837-0 [Crossref] [ Google Scholar]
- Weigenand O, Hussein AA, Lall N, Meyer JJ. Antibacterial activity of naphthoquinones and triterpenoids from Euclea natalensis root bark. J Nat Prod 2004; 67(11):1936-8. doi: 10.1021/np030465d [Crossref] [ Google Scholar]
- Truong NB, Pham CV, Doan HT, Nguyen HV, Nguyen CM, Nguyen HT. Antituberculosis cycloartane triterpenoids from Radermachera boniana. J Nat Prod 2011; 74(5):1318-22. doi: 10.1021/np200022b [Crossref] [ Google Scholar]
- Higuchi CT, Pavan FR, Leite CQ, Sannomiya M, Vilegas W, de Andrade Leite SR. Triterpenes and antitubercular activity of Byrsonima crassa. Quim Nova 2008; 31(7):1719-21. doi: 10.1590/s0100-40422008000700023 [Crossref] [ Google Scholar]
- Jiménez-Arellanes A, Luna-Herrera J, Cornejo-Garrido J, López-García S, Castro-Mussot ME, Meckes-Fischer M. Ursolic and oleanolic acids as antimicrobial and immunomodulatory compounds for tuberculosis treatment. BMC Complement Altern Med 2013; 13:258. doi: 10.1186/1472-6882-13-258 [Crossref] [ Google Scholar]
- Wolska KI, Grudniak AM, Fiecek B, Kraczkiewicz-Dowjat A, Kurek A. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Cent Eur J Biol 2010; 5(5):543-53. doi: 10.2478/s11535-010-0045-x [Crossref] [ Google Scholar]
- Tanachatchairatana T, Bremner JB, Chokchaisiri R, Suksamrarn A. Antimycobacterial activity of cinnamate-based esters of the triterpenes betulinic, oleanolic and ursolic acids. Chem Pharm Bull (Tokyo) 2008; 56(2):194-8. doi: 10.1248/cpb.56.194 [Crossref] [ Google Scholar]
- Aparecida Resende F, de Andrade Barcala CA, da Silva Faria MC, Kato FH, Cunha WR, Tavares DC. Antimutagenicity of ursolic acid and oleanolic acid against doxorubicin-induced clastogenesis in Balb/c mice. Life Sci 2006; 79(13):1268-73. doi: 10.1016/j.lfs.2006.03.038 [Crossref] [ Google Scholar]
- Chinsembu KC. Tuberculosis and nature’s pharmacy of putative anti-tuberculosis agents. Acta Trop 2016; 153:46-56. doi: 10.1016/j.actatropica.2015.10.004 [Crossref] [ Google Scholar]
- Robles-Zepeda RE, Coronado-Aceves EW, Velázquez-Contreras CA, Ruiz-Bustos E, Navarro-Navarro M, Garibay-Escobar A. In vitro anti-mycobacterial activity of nine medicinal plants used by ethnic groups in Sonora, Mexico. BMC Complement Altern Med 2013; 13:329. doi: 10.1186/1472-6882-13-329 [Crossref] [ Google Scholar]
- Gupta R, Thakur B, Singh P, Singh HB, Sharma VD, Katoch VM. Anti-tuberculosis activity of selected medicinal plants against multi-drug resistant Mycobacterium tuberculosis isolates. Indian J Med Res 2010; 131:809-13. [ Google Scholar]
- Chidambaram S, Swaminathan R. Determination of anti-tubercular activity of four Indian medicinal plants against Mycobacterium tuberculosis by broth micro dilution method. Int J Pharm Sci Res 2013; 4(10):3932-7. [ Google Scholar]
- Sinha T, Bandyopadhyay A. Ethno-pharmacological importance and valuable phytochemicals of Acalypha indica (L) a review. Int J Res Pharm Sci 2012; 3(3):360-8. [ Google Scholar]
- Ayyanar M, Ignacimuthu S. Medicinal uses and pharmacological actions of five commonly used Indian medicinal plants: a mini-review. Iran J Pharmacol Ther 2008; 7(1):107-14. [ Google Scholar]
- Kim JH. Anti-bacterial action of onion (Allium cepa L) extracts against oral pathogenic bacteria. J Nihon Univ Sch Dent 1997; 39(3):136-41. doi: 10.2334/josnusd1959.39.136 [Crossref] [ Google Scholar]
- Gibbons S. Phytochemicals for bacterial resistance--strengths, weaknesses and opportunities. Planta Med 2008; 74(6):594-602. doi: 10.1055/s-2008-1074518 [Crossref] [ Google Scholar]
- Mohamad S, Zin NM, Wahab HA, Ibrahim P, Sulaiman SF, Zahariluddin AS. Antituberculosis potential of some ethnobotanically selected Malaysian plants. J Ethnopharmacol 2011; 133(3):1021-6. doi: 10.1016/j.jep.2010.11.037 [Crossref] [ Google Scholar]
- Ratnakar P, Murthy PS. Purification and mechanism of action of antitubercular principle from garlic (Allium sativum) active against isoniazid susceptible and resistant Mycobacterium tuberculosis H 37 R v. Indian J Clin Biochem 1995; 10(1):34-8. doi: 10.1007/bf02873666 [Crossref] [ Google Scholar]
- Hannan A, Ikram Ullah M, Usman M, Hussain S, Absar M, Javed K. Anti-mycobacterial activity of garlic (Allium sativum) against multi-drug resistant and non-multi-drug resistant Mycobacterium tuberculosis. Pak J Pharm Sci 2011; 24(1):81-5. [ Google Scholar]
- Ratnakar P, Suryanarayana Murthy P. Preliminary studies on the antitubercular activity and the mechanism of action of the water extract of garlic (Allium sativum) and its two partially purified proteins (Garlic defensins?). Indian J Clin Biochem 1996; 11(1):37-41. doi: 10.1007/bf02868409 [Crossref] [ Google Scholar]
- Dini C, Fabbri A, Geraci A. The potential role of garlic (Allium sativum) against the multi-drug resistant tuberculosis pandemic: a review. Ann Ist Super Sanita 2011; 47(4):465-73. doi: 10.4415/ann_11_04_18 [Crossref] [ Google Scholar]
- Murthy PS, Ratnakar P, Gadre DV, Talwar V, Gupta HC, Gupta RL. Trifluoperazine and CEF-allicin from garlic (Allium sativum) as potential new antitubercular drugs active against drug resistant Mycobacterium tuberculosis. Indian J Clin Biochem 1997; 12(Suppl 1):72-5. doi: 10.1007/bf02873066 [Crossref] [ Google Scholar]
- Ignacimuthu S, Shanmugam N. Antimycobacterial activity of two natural alkaloids, vasicine acetate and 2-acetyl benzylamine, isolated from Indian shrub Adhatoda vasica Ness leaves. J Biosci 2010; 35(4):565-70. doi: 10.1007/s12038-010-0065-8 [Crossref] [ Google Scholar]
- Grange JM, Snell NJ. Activity of bromhexine and ambroxol, semi-synthetic derivatives of vasicine from the Indian shrub Adhatoda vasica, against Mycobacterium tuberculosis in vitro. J Ethnopharmacol 1996; 50(1):49-53. doi: 10.1016/0378-8741(95)01331-8 [Crossref] [ Google Scholar]
- Grindlay D, Reynolds T. The Aloe vera phenomenon: a review of the properties and modern uses of the leaf parenchyma gel. J Ethnopharmacol 1986; 16(2-3):117-51. doi: 10.1016/0378-8741(86)90085-1 [Crossref] [ Google Scholar]
- Haouat AC, Haggoud A, David S, Ibnsouda S, Iraqui M. In vitro evaluation of the antimycobacterial activity and fractionation of Berberis hispanica root bark. J Pure Appl Microbiol 2014; 8(2):917-25. [ Google Scholar]
- Higuchi CT, Sannomiya M, Pavan FR, Leite SR, Sato DN, Franzblau SG. Byrsonima fagifolia Niedenzu apolar compounds with antitubercular activity. Evid Based Complement Alternat Med 2011; 2011:128349. doi: 10.1093/ecam/nen077 [Crossref] [ Google Scholar]
- Singh A, Venugopala KN, Khedr MA, Pillay M, Nwaeze KU, Coovadia Y. Antimycobacterial, docking and molecular dynamic studies of pentacyclic triterpenes from Buddleja saligna leaves. J Biomol Struct Dyn 2017; 35(12):2654-64. doi: 10.1080/07391102.2016.1227725 [Crossref] [ Google Scholar]
- Bamuamba K, Gammon DW, Meyers P, Dijoux-Franca MG, Scott G. Anti-mycobacterial activity of five plant species used as traditional medicines in the Western Cape province (South Africa). J Ethnopharmacol 2008; 117(2):385-90. doi: 10.1016/j.jep.2008.02.007 [Crossref] [ Google Scholar]
- Wolska KI, Grudniak AM, Fiecek B, Kraczkiewicz-Dowjat A, Kurek A. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Cent Eur J Biol 2010; 5(5):543-53. doi: 10.2478/s11535-010-0045-x [Crossref] [ Google Scholar]
- Rojas R, Caviedes L, Aponte JC, Vaisberg AJ, Lewis WH, Lamas G. Aegicerin, the first oleanane triterpene with wide-ranging antimycobacterial activity, isolated from Clavija procera. J Nat Prod 2006; 69(5):845-6. doi: 10.1021/np050554l [Crossref] [ Google Scholar]
- Shashidhar M, Sandhya MS, Pankaj P, Suhasini B. Herbal drugs as anti-tuberculosis agents. Int J Ayurvedic Herb Med 2015; 5(4):1895-900. [ Google Scholar]
- Chinchansure AA, Arkile M, Shinde DR, Sarkar D, Joshi SP. A New Dinor-cis-Labdane Diterpene and Flavonoids with Antimycobacterium Activity from Colebrookea oppositifolia. Planta Med Lett 2016; 3(1):e20-e4. doi: 10.1055/s-0042-102200 [Crossref] [ Google Scholar]
- Promsawan N, Kittakoop P, Boonphong S, Nongkunsarn P. Antitubercular cassane furanoditerpenoids from the roots of Caesalpinia pulcherrima. Planta Med 2003; 69(8):776-7. doi: 10.1055/s-2003-42782 [Crossref] [ Google Scholar]
- Gupta VK, Shukla C, Bisht GR, Saikia D, Kumar S, Thakur RL. Detection of anti-tuberculosis activity in some folklore plants by radiometric BACTEC assay. Lett Appl Microbiol 2011; 52(1):33-40. doi: 10.1111/j.1472-765X.2010.02963.x [Crossref] [ Google Scholar]
- Hong Q, Minter DE, Franzblau SG, Arfan M, Amin H, Reinecke MG. Anti-tuberculosis compounds from Mallotus philippinensis. Nat Prod Commun 2010; 5(2):211-7. [ Google Scholar]
- Gautam R, Saklani A, Jachak SM. Indian medicinal plants as a source of antimycobacterial agents. J Ethnopharmacol 2007; 110(2):200-34. doi: 10.1016/j.jep.2006.12.031 [Crossref] [ Google Scholar]
- Sinha K, Mishra NP, Singh J, Khanuja SP. Tinospora cordifolia (Guduchi): a reservoir plant for therapeutic applications: a review. Indian J Tradit Knowl 2004; 3(3):257-70. [ Google Scholar]
- Singh K, Panghal M, Kadyan S, Chaudhary U, Yadav JP. Nanotechnology Antibacterial activity of synthesized silver nanoparticles from Tinospora cordifolia against multi drug resistant strains of Pseudomonas aeruginosa isolated from burn patients. J Nanomed Nanotechnol 2014; 5(2):192. doi: 10.4172/2157-7439.1000192 [Crossref] [ Google Scholar]
- Jeyachandran R, Xavier TF, Anand SP. Antibacterial activity of stem extracts of Tinospora cordifolia (Willd) Hook f & Thomson. Anc Sci Life 2003; 23(1):40-3. [ Google Scholar]
- Rose MF, Noorulla KM, Asma M, Kalaichelvi R, Vadivel K, Thangabalan B. Invitro antibacterial activity of methanolic root extract of Tinospora cordifolia (Willd). International Journal of Pharma Research and Development 2007; 2(5):1-5. [ Google Scholar]
- Mittal J, Sharma MM, Batra A. Tinospora cordifolia: a multipurpose medicinal plant-A. J Med Plants Stud 2014; 2(2):32-47. [ Google Scholar]
- Mauliku N, Hendro W, Saputro S, Kristina T. Anti-tubercular activity of extract and coumpounds of noni (Morinda citrifolia Linn). Int J Pharm Pharm Sci 2017; 9(12):105-9. doi: 10.22159/ijpps.2017v9i12.19841 [Crossref] [ Google Scholar]
- Zanetti S, Cannas S, Molicotti P, Bua A, Cubeddu M, Porcedda S. Evaluation of the antimicrobial properties of the essential oil of Myrtus communis L against clinical strains of Mycobacterium spp. Interdiscip Perspect Infect Dis 2010; 2010. doi: 10.1155/2010/931530 [Crossref]
- Ubaid RS, Anantrao KM, Jaju JB, Mateenuddin M. Effect of Ocimum sanctum (OS) leaf extract on hepatotoxicity induced by antitubercular drugs in rats. Indian J Physiol Pharmacol 2003; 47(4):465-70. [ Google Scholar]
- Mondal S, Mirdha BR, Mahapatra SC. The science behind sacredness of Tulsi (Ocimum sanctum Linn). Indian J Physiol Pharmacol 2009; 53(4):291-306. [ Google Scholar]
- Joshi C, Magar N. Antibiotic activity of some Indian medicinal plants. J Sci Ind Res 1952; 11:261. [ Google Scholar]
- Bhatia A, Kumar A, Goel A, Gupta A, Rahal A. Antibacterial activity of hot aqueous extract of Ocimum sanctum leaves against common bacterial pathogens of animals. Pharma Sci Monit 2013; 4(3 Suppl 1):279-85. [ Google Scholar]
- Kumar A, Rahal A, Chakraborty S, Tiwari R, Latheef SK, Dhama K. Ocimum sanctum (Tulsi): a miracle herb and boon to medical science-a Review. Int J Agron Plant Prod 2013; 4(7):1580-9. [ Google Scholar]
- Sehgal J, Siddheswaran P, Senthil Kumar KL, Karthiyayini T. Anti-tubercular activity of fruits of Prunus armeniaca (L). Int J Pharma Bio Sci 2010; 1(2).
- Rashid F, Ahmed R, Mahmood A, Ahmad Z, Bibi N, Kazmi SU. Flavonoid glycosides from Prunus armeniaca and the antibacterial activity of a crude extract. Arch Pharm Res 2007; 30(8):932-7. doi: 10.1007/bf02993959 [Crossref] [ Google Scholar]
- Sharma S, Kalia NP, Suden P, Chauhan PS, Kumar M, Ram AB. Protective efficacy of piperine against Mycobacterium tuberculosis. Tuberculosis (Edinb) 2014; 94(4):389-96. doi: 10.1016/j.tube.2014.04.007 [Crossref] [ Google Scholar]
- Scodro RB, Pires CT, Carrara VS, Lemos CO, Cardozo-Filho L, Souza VA. Anti-tuberculosis neolignans from Piper regnellii. Phytomedicine 2013; 20(7):600-4. doi: 10.1016/j.phymed.2013.01.005 [Crossref] [ Google Scholar]
- Kumar V, Poonam Poonam, Prasad AK, Parmar VS. Naturally occurring aristolactams, aristolochic acids and dioxoaporphines and their biological activities. Nat Prod Rep 2003; 20(6):565-83. doi: 10.1039/b303648k [Crossref] [ Google Scholar]
- Liang HX, Dai HQ, Fu HA, Dong XP, Adebayo AH, Zhang LX. Bioactive compounds from Rumex plants. Phytochem Lett 2010; 3(4):181-4. doi: 10.1016/j.phytol.2010.05.005 [Crossref] [ Google Scholar]
- Topçu G, Gören AC. Biological activity of diterpenoids isolated from Anatolian Lamiaceae plants. Rec Nat Prod 2007; 1(1):1-16. [ Google Scholar]
- Ulubelen A, Evren N, Tuzlaci E, Johansson C. Diterpenoids from the roots of Salvia hypargeia. J Nat Prod 1988; 51(6):1178-83. doi: 10.1021/np50060a021 [Crossref] [ Google Scholar]
- Habibi Z, Eftekhar F, Samiee K, Rustaiyan A. Structure and antibacterial activity ofp6 new labdane diterpenoid from Salvia leriaefolia. J Nat Prod 2000; 63(2):270-1. doi: 10.1021/np990287h [Crossref] [ Google Scholar]
- Aşkun T, Hüsnü Can Başer K, Tümen G, Kürkçüoğlu M. Characterization of essential oils of some Salvia species and their antimycobacterial activities. Turk J Biol 2010; 34:89-95. doi: 10.3906/biy-0809-2 [Crossref] [ Google Scholar]
- Gu JQ, Wang Y, Franzblau SG, Montenegro G, Timmermann BN. Constituents of Senecio chionophilus with potential antitubercular activity. J Nat Prod 2004; 67(9):1483-7. doi: 10.1021/np049831z [Crossref] [ Google Scholar]
- Hong Q, Minter DE, Franzblau SG, Reinecke MG. Anti-tuberculosis compounds from two Bolivian medicinal plants, Senecio mathewsii and Usnea florida. Nat Prod Commun 2008; 3(9):1377-84. doi: 10.1177/1934578x0800300901 [Crossref] [ Google Scholar]
- Tiwari N, Thakur J, Saikia D, Gupta MM. Antitubercular diterpenoids from Vitex trifolia. Phytomedicine 2013; 20(7):605-10. doi: 10.1016/j.phymed.2013.01.003 [Crossref] [ Google Scholar]
- Ladda PL, Naikwade NS, Magdum CS. Antimycobacterial and antimicrobial activity of leaf extracts of Vitex negundo Linn. Res J Pharmacogn Phytochem 2010; 2(2):166-8. [ Google Scholar]
- Tandon VR, Khajuria V, Kapoor B, Kour D, Gupta S. Hepatoprotective activity of Vitex negundo leaf extract against anti-tubercular drugs induced hepatotoxicity. Fitoterapia 2008; 79(7-8):533-8. doi: 10.1016/j.fitote.2008.05.005 [Crossref] [ Google Scholar]
- Gordien AY, Gray AI, Franzblau SG, Seidel V. Antimycobacterial terpenoids from Juniperus communis L (Cuppressaceae). J Ethnopharmacol 2009; 126(3):500-5. doi: 10.1016/j.jep.2009.09.007 [Crossref] [ Google Scholar]
- Carpenter CD, O’Neill T, Picot N, Johnson JA, Robichaud GA, Webster D. Anti-mycobacterial natural products from the Canadian medicinal plant Juniperus communis. J Ethnopharmacol 2012; 143(2):695-700. doi: 10.1016/j.jep.2012.07.035 [Crossref] [ Google Scholar]
- Cantrell CL, Rajab MS, Franzblau SG, Fronczek FR, Fischer NH. Antimycobacterial ergosterol-5,8-endoperoxide from Ajuga remota. Planta Med 1999; 65(8):732-4. doi: 10.1055/s-1999-14053 [Crossref] [ Google Scholar]
- Fischer NH, Lu T, Cantrell CL, Castañeda-Acosta J, Quijano L, Franzblau SG. Antimycobacterial evaluation of germacranolides in honour of professor GH Neil Towers 75th birthday. Phytochemistry 1998; 49(2):559-64. doi: 10.1016/S0031-9422(98)00253-2 [Crossref] [ Google Scholar]
- Cantrell CL, Nuñez IS, Castañeda-Acosta J, Foroozesh M, Fronczek FR, Fischer NH. Antimycobacterial activities of dehydrocostus lactone and its oxidation products. J Nat Prod 1998; 61(10):1181-6. doi: 10.1021/np970333i [Crossref] [ Google Scholar]
- van Puyvelde L, Ntawukiliyayo JD, Portaels F, Hakizamungu E. In vitro inhibition of mycobacteria by Rwandese medicinal plants. Phytother Res 1994; 8(2):65-9. doi: 10.1002/ptr.2650080202 [Crossref] [ Google Scholar]
- Topçu G, Erenler R, Cakmak O, Johansson CB, Celik C, Chai HB. Diterpenes from the berries of Juniperus excelsa. Phytochemistry 1999; 50(7):1195-9. doi: 10.1016/s0031-9422(98)00675-x [Crossref] [ Google Scholar]
- Wächter GA, Franzblau SG, Montenegro G, Suarez E, Fortunato RH, Saavedra E. A new antitubercular mulinane diterpenoid from Azorella madreporica Clos. J Nat Prod 1998; 61(7):965-8. doi: 10.1021/np980066w [Crossref] [ Google Scholar]
- Cantrell CL, Rajab MS, Franzblau SG, Fischer NH. Antimycobacterial triterpenes from Melia volkensii. J Nat Prod 1999; 62(4):546-8. doi: 10.1021/np980288u [Crossref] [ Google Scholar]
- Cantrell CL, Lu T, Fronczek FR, Fischer NH, Adams LB, Franzblau SG. Antimycobacterial cycloartanes from Borrichia frutescens. J Nat Prod 1996; 59(12):1131-6. doi: 10.1021/np960551w [Crossref] [ Google Scholar]
- Caldwell CG, Franzblau SG, Suarez E, Timmermann BN. Oleanane triterpenes from Junellia tridens. J Nat Prod 2000; 63(12):1611-4. doi: 10.1021/np0002233 [Crossref] [ Google Scholar]
- Prasad R, Singh A, Gupta N. Adverse drug reactions in tuberculosis and management. Indian J Tuberc 2019; 66(4):520-32. doi: 10.1016/j.ijtb.2019.11.005 [Crossref] [ Google Scholar]
- Ghosh S, Malik S, Gupta A, Chaudhary R. A prospective, observational cohort study to elicit adverse effects of anti-tuberculosis drugs among patient treated for active tuberculosis. J Pharm Res 2010; 3:10-6. [ Google Scholar]
- Makarov VV, Love AJ, Sinitsyna OV, Makarova SS, Yaminsky IV, Taliansky ME. “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Naturae 2014; 6(1):35-44. [ Google Scholar]
- Banerjee K, Rai VR. Study on green synthesis of gold nanoparticles and their potential applications as catalysts. J Clust Sci 2016; 27(4):1307-15. doi: 10.1007/s10876-016-1001-3 [Crossref] [ Google Scholar]
- Banerjee K, Rai VR. A review on mycosynthesis, mechanism, and characterization of silver and gold nanoparticles. BioNanoScience 2018; 8(1):17-31. doi: 10.1007/s12668-017-0437-8 [Crossref] [ Google Scholar]
- Ramakrishna M, Babu DR, Gengan RM, Chandra S, Rao GN. Green synthesis of gold nanoparticles using marine algae and evaluation of their catalytic activity. J Nanostructure Chem 2016; 6(1):1-13. doi: 10.1007/s40097-015-0173-y [Crossref] [ Google Scholar]
- Ghodake GS, Deshpande NG, Lee YP, Jin ES. Pear fruit extract-assisted room-temperature biosynthesis of gold nanoplates. Colloids Surf B Biointerfaces 2010; 75(2):584-9. doi: 10.1016/j.colsurfb.2009.09.040 [Crossref] [ Google Scholar]
- Ghodake G, Eom CY, Kim SW, Jin E. Biogenic nano-synthesis; towards the efficient production of the biocompatible gold nanoparticles. Bull Korean Chem Soc 2010; 31(10):2771-5. doi: 10.5012/bkcs.2010.31.10.2771 [Crossref] [ Google Scholar]
- Bogireddy NKR, Hoskote Anand KK, Mandal BK. Gold nanoparticles — synthesis by Sterculia acuminata extract and its catalytic efficiency in alleviating different organic dyes. J Mol Liq 2015; 211:868-75. doi: 10.1016/j.molliq.2015.07.027 [Crossref] [ Google Scholar]
- Kumar KM, Mandal BK, Sinha M, Krishnakumar V. Terminalia chebula mediated green and rapid synthesis of gold nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 2012; 86:490-4. doi: 10.1016/j.saa.2011.11.001 [Crossref] [ Google Scholar]
- Nellore J, Pauline PC, Amarnath K, Biostructures Biostructures. Biogenic synthesis by Sphearanthus amaranthoids; towards the efficient production of the biocompatible gold nanoparticles. Digest J Nanomater Biostruct 2012; 7(1):123-33. [ Google Scholar]
- Guo Q, Guo Q, Yuan J, Zeng J. Biosynthesis of gold nanoparticles using a kind of flavonol: dihydromyricetin. Colloids Surf A Physicochem Eng Asp 2014; 441:127-32. doi: 10.1016/j.colsurfa.2013.08.067 [Crossref] [ Google Scholar]
- Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev 2008; 60(11):1307-15. doi: 10.1016/j.addr.2008.03.016 [Crossref] [ Google Scholar]
- Hu M, Chen J, Li ZY, Au L, Hartland GV, Li X. Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chem Soc Rev 2006; 35(11):1084-94. doi: 10.1039/b517615h [Crossref] [ Google Scholar]
- Sugunan A, Thanachayanont C, Dutta J, Hilborn JG. Heavy-metal ion sensors using chitosan-capped gold nanoparticles. Sci Technol Adv Mater 2005; 6(3-4):335-40. doi: 10.1016/j.stam.2005.03.007 [Crossref] [ Google Scholar]
- Li B, Du Y, Dong S. DNA based gold nanoparticles colorimetric sensors for sensitive and selective detection of Ag(I) ions. Anal Chim Acta 2009; 644(1-2):78-82. doi: 10.1016/j.aca.2009.04.022 [Crossref] [ Google Scholar]
- Souza GR, Christianson DR, Staquicini FI, Ozawa MG, Snyder EY, Sidman RL. Networks of gold nanoparticles and bacteriophage as biological sensors and cell-targeting agents. Proc Natl Acad Sci U S A 2006; 103(5):1215-20. doi: 10.1073/pnas.0509739103 [Crossref] [ Google Scholar]
- Li X, Robinson SM, Gupta A, Saha K, Jiang Z, Moyano DF. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 2014; 8(10):10682-6. doi: 10.1021/nn5042625 [Crossref] [ Google Scholar]
- Rai A, Prabhune A, Perry CC. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J Mater Chem 2010; 20(32):6789-98. doi: 10.1039/c0jm00817f [Crossref] [ Google Scholar]
- Hernández-Sierra JF, Ruiz F, Pena DC, Martínez-Gutiérrez F, Martínez AE, Guillén Ade J. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine 2008; 4(3):237-40. doi: 10.1016/j.nano.2008.04.005 [Crossref] [ Google Scholar]
- Corma A, Garcia H. Supported gold nanoparticles as catalysts for organic reactions. Chem Soc Rev 2008; 37(9):2096-126. doi: 10.1039/b707314n [Crossref] [ Google Scholar]
- Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 2004; 104(1):293-346. doi: 10.1021/cr030698+ [Crossref] [ Google Scholar]
- Haruta M. Catalysis of gold nanoparticles deposited on metal oxides. Cattech 2002; 6(3):102-15. doi: 10.1023/A:1020181423055 [Crossref] [ Google Scholar]
- Huang D, Liao F, Molesa S, Redinger D, Subramanian V. Plastic-compatible low resistance printable gold nanoparticle conductors for flexible electronics. J Electrochem Soc 2003; 150(7):G412-G7. doi: 10.1149/1.1582466 [Crossref] [ Google Scholar]
- Luechinger NA, Athanassiou EK, Stark WJ. Graphene-stabilized copper nanoparticles as an air-stable substitute for silver and gold in low-cost ink-jet printable electronics. Nanotechnology 2008; 19(44):445201. doi: 10.1088/0957-4484/19/44/445201 [Crossref] [ Google Scholar]
- Danckwerts M, Novotny L. Optical frequency mixing at coupled gold nanoparticles. Phys Rev Lett 2007; 98(2):026104. doi: 10.1103/PhysRevLett.98.026104 [Crossref] [ Google Scholar]
- Cheng SF, Chau LK. Colloidal gold-modified optical fiber for chemical and biochemical sensing. Anal Chem 2003; 75(1):16-21. doi: 10.1021/ac020310v [Crossref] [ Google Scholar]
- Yang N, WeiHong L, Hao L. Biosynthesis of Au nanoparticles using agricultural waste mango peel extract and its in vitro cytotoxic effect on two normal cells. Mater Lett 2014; 134:67-70. doi: 10.1016/j.matlet.2014.07.025 [Crossref] [ Google Scholar]
- Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW. Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot 2012; 35:64-70. doi: 10.1016/j.cropro.2012.01.007 [Crossref] [ Google Scholar]
- Green M, Harwood H, Barrowman C, Rahman P, Eggeman A, Festry F. A facile route to CdTe nanoparticles and their use in bio-labelling. J Mater Chem 2007; 17(19):1989-94. doi: 10.1039/b615871d [Crossref] [ Google Scholar]
- von Maltzahn G, Park JH, Agrawal A, Bandaru NK, Das SK, Sailor MJ. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res 2009; 69(9):3892-900. doi: 10.1158/0008-5472.can-08-4242 [Crossref] [ Google Scholar]
- Cabuzu D, Cirja A, Puiu R, Grumezescu AM. Biomedical applications of gold nanoparticles. Curr Top Med Chem 2015; 15(16):1605-13. doi: 10.2174/1568026615666150414144750 [Crossref] [ Google Scholar]
- Dykman L, Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev 2012; 41(6):2256-82. doi: 10.1039/c1cs15166e [Crossref] [ Google Scholar]
- Tiwari PM, Vig K, Dennis VA, Singh SR. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials (Basel) 2011; 1(1):31-63. doi: 10.3390/nano1010031 [Crossref] [ Google Scholar]
- Vayssieres L, Beermann N, Lindquist SE, Hagfeldt A. Controlled aqueous chemical growth of oriented three-dimensional crystalline nanorod arrays: application to iron (III) oxides. Chem Mater 2001; 13(2):233-5. doi: 10.1021/cm001202x [Crossref] [ Google Scholar]
- Zhang X, Yonzon CR, Van Duyne RP. Nanosphere lithography fabricated plasmonic materials and their applications. J Mater Res 2006; 21(5):1083-92. doi: 10.1557/jmr.2006.0136 [Crossref] [ Google Scholar]
- Cao CY, Guo W, Cui ZM, Song WG, Cai W. Microwave-assisted gas/liquid interfacial synthesis of flowerlike NiO hollow nanosphere precursors and their application as supercapacitor electrodes. J Mater Chem 2011; 21(9):3204-9. doi: 10.1039/c0jm03749d [Crossref] [ Google Scholar]
- Borzenkov M, Määttänen A, Ihalainen P, Collini M, Cabrini E, Dacarro G. Fabrication of inkjet-printed gold nanostar patterns with photothermal properties on paper substrate. ACS Appl Mater Interfaces 2016; 8(15):9909-16. doi: 10.1021/acsami.6b02983 [Crossref] [ Google Scholar]
- Ma W, Sun M, Xu L, Wang L, Kuang H, Xu C. A SERS active gold nanostar dimer for mercury ion detection. Chem Commun (Camb) 2013; 49(44):4989-91. doi: 10.1039/c3cc39087j [Crossref] [ Google Scholar]
- Zharov VP, Galitovskaya EN, Johnson C, Kelly T. Synergistic enhancement of selective nanophotothermolysis with gold nanoclusters: potential for cancer therapy. Lasers Surg Med 2005; 37(3):219-26. doi: 10.1002/lsm.20223 [Crossref] [ Google Scholar]
- Lin CA, Yang TY, Lee CH, Huang SH, Sperling RA, Zanella M. Synthesis, characterization, and bioconjugation of fluorescent gold nanoclusters toward biological labeling applications. ACS Nano 2009; 3(2):395-401. doi: 10.1021/nn800632j [Crossref] [ Google Scholar]
- Chen J, Wiley B, Li ZY, Campbell D, Saeki F, Cang H. Gold nanocages: engineering their structure for biomedical applications. Adv Mater 2005; 17(18):2255-61. doi: 10.1002/adma.200500833 [Crossref] [ Google Scholar]
- Guerrero-Martínez A, Barbosa S, Pastoriza-Santos I, Liz-Marzán LM. Nanostars shine bright for you: colloidal synthesis, properties and applications of branched metallic nanoparticles. Curr Opin Colloid Interface Sci 2011; 16(2):118-27. doi: 10.1016/j.cocis.2010.12.007 [Crossref] [ Google Scholar]
- Katz E, Willner I. Biomolecule-functionalized carbon nanotubes: applications in nanobioelectronics. Chemphyschem 2004; 5(8):1084-104. doi: 10.1002/cphc.200400193 [Crossref] [ Google Scholar]
- Wang ZL. Functional oxide nanobelts: materials, properties and potential applications in nanosystems and biotechnology. Annu Rev Phys Chem 2004; 55:159-96. doi: 10.1146/annurev.physchem.55.091602.094416 [Crossref] [ Google Scholar]
- Kalele S, Gosavi SW, Urban J, Kulkarni SK. Nanoshell particles: synthesis, properties and applications. Curr Sci 2006; 91(8):1038-52. doi: 10.2307/24093981 [Crossref] [ Google Scholar]
-
Anastas PT, Warner JC. Green Chemistry: Theory and Practice. New York: Oxford University Press; 1998.
- Ismail EH, Saqer AMA, Assirey E, Naqvi A, Okasha RM. Successful green synthesis of gold nanoparticles using a Corchorus olitorius extract and their antiproliferative effect in cancer cells. Int J Mol Sci 2018; 19(9). doi: 10.3390/ijms19092612 [Crossref]
- Ovais M, Khalil AT, Islam NU, Ahmad I, Ayaz M, Saravanan M. Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl Microbiol Biotechnol 2018; 102(16):6799-814. doi: 10.1007/s00253-018-9146-7 [Crossref] [ Google Scholar]
- Koperuncholan M. Bioreduction of chloroauric acid (HAuCl4) for the synthesis of gold nanoparticles (GNPs): a special empathies of pharmacological activity. Int J Phytopharm 2015; 5(4):72-80. doi: 10.7439/ijpp.v5i4.2503 [Crossref] [ Google Scholar]
- Jesus JA, Lago JH, Laurenti MD, Yamamoto ES, Passero LF. Antimicrobial activity of oleanolic and ursolic acids: an update. Evid Based Complement Alternat Med 2015; 2015:620472. doi: 10.1155/2015/620472 [Crossref] [ Google Scholar]
- Oldfield E, Lin FY. Terpene biosynthesis: modularity rules. Angew Chem Int Ed Engl 2012; 51(5):1124-37. doi: 10.1002/anie.201103110 [Crossref] [ Google Scholar]
- Mares-Briones F, Rosas G. Structure and stability of gold nanoparticles synthesized using Schinus molle L extract. J Clust Sci 2017; 28(4):1995-2003. doi: 10.1007/s10876-017-1197-x [Crossref] [ Google Scholar]
- Raja S, Ramesh V, Thivaharan V. Antibacterial and anticoagulant activity of silver nanoparticles synthesised from a novel source–pods of Peltophorum pterocarpum. J Ind Eng Chem 2015; 29:257-64. doi: 10.1016/j.jiec.2015.03.033 [Crossref] [ Google Scholar]
- Bursal E, Gülçin İ. Polyphenol contents and in vitro antioxidant activities of lyophilised aqueous extract of kiwifruit (Actinidia deliciosa). Food Res Int 2011; 44(5):1482-9. doi: 10.1016/j.foodres.2011.03.031 [Crossref] [ Google Scholar]
- Sathishkumar M, Sneha K, Won SW, Cho CW, Kim S, Yun YS. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf B Biointerfaces 2009; 73(2):332-8. doi: 10.1016/j.colsurfb.2009.06.005 [Crossref] [ Google Scholar]
- Kasthuri J, Veerapandian S, Rajendiran N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf B Biointerfaces 2009; 68(1):55-60. doi: 10.1016/j.colsurfb.2008.09.021 [Crossref] [ Google Scholar]
- Leopoldini M, Russo N, Chiodo S, Toscano M. Iron chelation by the powerful antioxidant flavonoid quercetin. J Agric Food Chem 2006; 54(17):6343-51. doi: 10.1021/jf060986h [Crossref] [ Google Scholar]
- Li H, Liang R, Turner DH, Rothberg LJ, Duan S. Selective quenching of fluorescence from unbound oligonucleotides by gold nanoparticles as a probe of RNA structure. RNA 2007; 13(11):2034-41. doi: 10.1261/rna.138807 [Crossref] [ Google Scholar]
- Shukla R, Nune SK, Chanda N, Katti K, Mekapothula S, Kulkarni RR. Soybeans as a phytochemical reservoir for the production and stabilization of biocompatible gold nanoparticles. Small 2008; 4(9):1425-36. doi: 10.1002/smll.200800525 [Crossref] [ Google Scholar]
- Dumur F, Guerlin A, Dumas E, Bertin D, Gigmes D, Mayer CR. Controlled spontaneous generation of gold nanoparticles assisted by dual reducing and capping agents. Gold Bull 2011; 44(2):119-37. doi: 10.1007/s13404-011-0018-5 [Crossref] [ Google Scholar]
- Edison TJI, Sethuraman MG. Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Process Biochem 2012; 47(9):1351-7. doi: 10.1016/j.procbio.2012.04.025 [Crossref] [ Google Scholar]
- Ghosh S, Patil S, Ahire M, Kitture R, Gurav DD, Jabgunde AM. Gnidia glauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential. J Nanobiotechnology 2012; 10:17. doi: 10.1186/1477-3155-10-17 [Crossref] [ Google Scholar]
- Dahl JA, Maddux BL, Hutchison JE. Toward greener nanosynthesis. Chem Rev 2007; 107(6):2228-69. doi: 10.1021/cr050943k [Crossref] [ Google Scholar]
- Parida UK, Bindhani BK, Nayak P. Green synthesis and characterization of gold nanoparticles using onion (Allium cepa) extract. World J Nano Sci Eng 2011; 1(4):93-8. doi: 10.4236/wjnse.2011.14015 [Crossref] [ Google Scholar]
- Sheny DS, Mathew J, Philip D. Phytosynthesis of Au, Ag and Au-Ag bimetallic nanoparticles using aqueous extract and dried leaf of Anacardium occidentale. Spectrochim Acta A Mol Biomol Spectrosc 2011; 79(1):254-62. doi: 10.1016/j.saa.2011.02.051 [Crossref] [ Google Scholar]
- Thirumurugan A, Jiflin G, Rajagomathi G, Tomy N, Ramachandran S, Jaiganesh R. Biotechnological synthesis of gold nanoparticles of Azadirachta indica leaf extract. Int J Biol Technol 2010; 1(1):75-7. [ Google Scholar]
- Boruah SK, Boruah PK, Sarma P, Medhi C, Medhi OK. Green synthesis of gold nanoparticles using Camellia sinensis and kinetics of the reaction. Adv Mater Lett 2012; 3(6):481-6. doi: 10.5185/amlett.2012.icnano.103 [Crossref] [ Google Scholar]
- Dwivedi AD, Gopal K. Plant-mediated biosynthesis of silver and gold nanoparticles. J Biomed Nanotechnol 2011; 7(1):163-4. doi: 10.1166/jbn.2011.1250 [Crossref] [ Google Scholar]
- Fazaludeen MF, Manickam C, Ashankyty IM, Ahmed MQ, Beg Q. Synthesis and characterizations of gold nanoparticles by Justicia gendarussa Burm F leaf extract. J Microbiol Biotechnol Res 2012; 2(1):23-34. [ Google Scholar]
- Aromal SA, Vidhu VK, Philip D. Green synthesis of well-dispersed gold nanoparticles using Macrotyloma uniflorum. Spectrochim Acta A Mol Biomol Spectrosc 2012; 85(1):99-104. doi: 10.1016/j.saa.2011.09.035 [Crossref] [ Google Scholar]
- MubarakAli D, Thajuddin N, Jeganathan K, Gunasekaran M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf B Biointerfaces 2011; 85(2):360-5. doi: 10.1016/j.colsurfb.2011.03.009 [Crossref] [ Google Scholar]
- Vankar PS, Bajpai D. Preparation of gold nanoparticles from Mirabilis jalapa flowers. Indian J Biochem Biophys 2010; 47(3):157-60. [ Google Scholar]
- Mondal S, Roy N, Laskar RA, Sk I, Basu S, Mandal D. Biogenic synthesis of Ag, Au and bimetallic Au/Ag alloy nanoparticles using aqueous extract of mahogany (Swietenia mahogani JACQ) leaves. Colloids Surf B Biointerfaces 2011; 82(2):497-504. doi: 10.1016/j.colsurfb.2010.10.007 [Crossref] [ Google Scholar]
- Reddy V, Torati RS, Oh S, Kim C. Biosynthesis of gold nanoparticles assisted by Sapindus mukorossi Gaertn Fruit pericarp and their catalytic application for the reduction of p-nitroaniline. Ind Eng Chem Res 2013; 52(2):556-64. doi: 10.1021/ie302037c [Crossref] [ Google Scholar]
- Dauthal P, Mukhopadhyay M. Prunus domestica fruit extract-mediated synthesis of gold nanoparticles and its catalytic activity for 4-nitrophenol reduction. Ind Eng Chem Res 2012; 51(40):13014-20. doi: 10.1021/ie300369g [Crossref] [ Google Scholar]
- Song JY, Jang H-K, Kim BS. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem 2009; 44(10):1133-8. doi: 10.1016/j.procbio.2009.06.005 [Crossref] [ Google Scholar]
- Narayanan KB, Sakthivel N. Phytosynthesis of gold nanoparticles using leaf extract of Coleus amboinicus Lour. Mater Charact 2010; 61(11):1232-8. doi: 10.1016/j.matchar.2010.08.003 [Crossref] [ Google Scholar]
- Kumar VG, Gokavarapu SD, Rajeswari A, Dhas TS, Karthick V, Kapadia Z. Facile green synthesis of gold nanoparticles using leaf extract of antidiabetic potent Cassia auriculata. Colloids Surf B Biointerfaces 2011; 87(1):159-63. doi: 10.1016/j.colsurfb.2011.05.016 [Crossref] [ Google Scholar]
- Jayaseelan C, Ramkumar R, Rahuman AA, Perumal P. Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity. Ind Crops Prod 2013; 45:423-9. doi: 10.1016/j.indcrop.2012.12.019 [Crossref] [ Google Scholar]
- Kumar KP, Paul W, Sharma CP. Green synthesis of silver nanoparticles with Zingiber officinale extract and study of its blood compatibility. BioNanoScience 2012; 2(3):144-52. doi: 10.1007/s12668-012-0044-7 [Crossref] [ Google Scholar]
- Noruzi M, Zare D, Khoshnevisan K, Davoodi D. Rapid green synthesis of gold nanoparticles using Rosa hybrida petal extract at room temperature. Spectrochim Acta A Mol Biomol Spectrosc 2011; 79(5):1461-5. doi: 10.1016/j.saa.2011.05.001 [Crossref] [ Google Scholar]
- Ghule K, Ghule AV, Liu JY, Ling YC. Microscale size triangular gold prisms synthesized using Bengal gram beans (Cicer arietinum L) extract and HAuCl4x3H20: a green biogenic approach. J Nanosci Nanotechnol 2006; 6(12):3746-51. doi: 10.1166/jnn.2006.608 [Crossref] [ Google Scholar]
- Castro L, Blázquez ML, Muñoz JA, González F, García-Balboa C, Ballester A. Biosynthesis of gold nanowires using sugar beet pulp. Process Biochem 2011; 46(5):1076-82. doi: 10.1016/j.procbio.2011.01.025 [Crossref] [ Google Scholar]
- Das RK, Gogoi N, Bora U. Green synthesis of gold nanoparticles using Nyctanthes arbortristis flower extract. Bioprocess Biosyst Eng 2011; 34(5):615-9. doi: 10.1007/s00449-010-0510-y [Crossref] [ Google Scholar]
- Tamuly C, Hazarika M, Bordoloi M. Biosynthesis of Au nanoparticles by Gymnocladus assamicus and its catalytic activity. Mater Lett 2013; 108:276-9. doi: 10.1016/j.matlet.2013.07.020 [Crossref] [ Google Scholar]
- Zhan G, Huang J, Lin L, Lin W, Emmanuel K, Li Q. Synthesis of gold nanoparticles by Cacumen Platycladi leaf extract and its simulated solution: toward the plant-mediated biosynthetic mechanism. J Nanopart Res 2011; 13(10):4957. doi: 10.1007/s11051-011-0476-y [Crossref] [ Google Scholar]
- Narayanan KB, Sakthivel N. Coriander leaf mediated biosynthesis of gold nanoparticles. Mater Lett 2008; 62(30):4588-90. doi: 10.1016/j.matlet.2008.08.044 [Crossref] [ Google Scholar]
- Tahir K, Nazir S, Li B, Khan AU, Khan ZUH, Gong PY. Nerium oleander leaves extract mediated synthesis of gold nanoparticles and its antioxidant activity. Mater Lett 2015; 156:198-201. doi: 10.1016/j.matlet.2015.05.062 [Crossref] [ Google Scholar]
- Patra S, Mukherjee S, Barui AK, Ganguly A, Sreedhar B, Patra CR. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater Sci Eng C Mater Biol Appl 2015; 53:298-309. doi: 10.1016/j.msec.2015.04.048 [Crossref] [ Google Scholar]
- Raju D, Vishwakarma RK, Khan BM, Mehta UJ, Ahmad A. Biological synthesis of cationic gold nanoparticles and binding of plasmid DNA. Mater Lett 2014; 129:159-61. doi: 10.1016/j.matlet.2014.05.021 [Crossref] [ Google Scholar]
- Bindhu MR, Vijaya Rekha P, Umamaheswari T, Umadevi M. Antibacterial activities of Hibiscus cannabinus stem-assisted silver and gold nanoparticles. Mater Lett 2014; 131:194-7. doi: 10.1016/j.matlet.2014.05.172 [Crossref] [ Google Scholar]
- Das J, Paul Das M, Velusamy P. Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochim Acta A Mol Biomol Spectrosc 2013; 104:265-70. doi: 10.1016/j.saa.2012.11.075 [Crossref] [ Google Scholar]
- Islam NU, Jalil K, Shahid M, Rauf A, Muhammad N, Khan A. Green synthesis and biological activities of gold nanoparticles functionalized with Salix alba. Arab J Chem 2019; 12(8):2914-25. doi: 10.1016/j.arabjc.2015.06.025 [Crossref] [ Google Scholar]
- Guo M, Li W, Yang F, Liu H. Controllable biosynthesis of gold nanoparticles from a Eucommia ulmoides bark aqueous extract. Spectrochim Acta A Mol Biomol Spectrosc 2015; 142:73-9. doi: 10.1016/j.saa.2015.01.109 [Crossref] [ Google Scholar]
- Abdel-Raouf N, Al-Enazi NM, Ibraheem IBM. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab J Chem 2017; 10:S3029-S39. doi: 10.1016/j.arabjc.2013.11.044 [Crossref] [ Google Scholar]
- Philip D, Unni C. Extracellular biosynthesis of gold and silver nanoparticles using Krishna tulsi (Ocimum sanctum) leaf. Physica E Low Dimens Syst Nanostruct 2011; 43(7):1318-22. doi: 10.1016/j.physe.2010.10.006 [Crossref] [ Google Scholar]
- Kalpana D, Pichiah PBT, Sankarganesh A, Park WS, Lee SM, Wahab R. Biogenesis of gold nanoparticles using plant powders and assessment of in vitro cytotoxicity in 3T3-L1 cell line. J Pharm Innov 2013; 8(4):265-75. doi: 10.1007/s12247-013-9166-x [Crossref] [ Google Scholar]
- Khalil MMH, Ismail EH, El-Magdoub F. Biosynthesis of Au nanoparticles using olive leaf extract: 1st nano updates. Arab J Chem 2012; 5(4):431-7. doi: 10.1016/j.arabjc.2010.11.011 [Crossref] [ Google Scholar]
- Jha AK, Prasad K. Rose (Rosa sp) petals assisted green synthesis of gold nanoparticles. J Bionanoscience 2013; 7(3):245-50. doi: 10.1166/jbns.2013.1139 [Crossref] [ Google Scholar]
- Islam NU, Jalil K, Shahid M, Muhammad N, Rauf A. Pistacia integerrima gall extract mediated green synthesis of gold nanoparticles and their biological activities. Arab J Chem 2019; 12(8):2310-9. doi: 10.1016/j.arabjc.2015.02.014 [Crossref] [ Google Scholar]
- Annamalai A, Christina VLP, Sudha D, Kalpana M, Lakshmi PTV. Green synthesis, characterization and antimicrobial activity of Au NPs using Euphorbia hirta L leaf extract. Colloids Surf B Biointerfaces 2013; 108:60-5. doi: 10.1016/j.colsurfb.2013.02.012 [Crossref] [ Google Scholar]
- Suman TY, Rajasree SR, Ramkumar R, Rajthilak C, Perumal P. The green synthesis of gold nanoparticles using an aqueous root extract of Morinda citrifolia L. Spectrochim Acta A Mol Biomol Spectrosc 2014; 118:11-6. doi: 10.1016/j.saa.2013.08.066 [Crossref] [ Google Scholar]
- Sadeghi B. Zizyphus mauritiana extract-mediated green and rapid synthesis of gold nanoparticles and its antibacterial activity. J Nanostructure Chem 2015; 5(3):265-73. doi: 10.1007/s40097-015-0157-y [Crossref] [ Google Scholar]
- Bosecker K. Bioleaching: metal solubilization by microorganisms. FEMS Microbiol Rev 1997; 20(3-4):591-604. doi: 10.1016/s0168-6445(97)00036-3 [Crossref] [ Google Scholar]
- Rajasree SRR, Suman TY. Extracellular biosynthesis of gold nanoparticles using a gram negative bacterium Pseudomonas fluorescens. Asian Pac J Trop Dis 2012; 2:S796-S9. doi: 10.1016/S2222-1808(12)60267-9 [Crossref] [ Google Scholar]
- Konishi Y, Tsukiyama T, Tachimi T, Saitoh N, Nomura T, Nagamine S. Microbial deposition of gold nanoparticles by the metal-reducing bacterium Shewanella algae. Electrochim Acta 2007; 53(1):186-92. doi: 10.1016/j.electacta.2007.02.073 [Crossref] [ Google Scholar]
- Mohammed Fayaz A, Girilal M, Rahman M, Venkatesan R, Kalaichelvan PT. Biosynthesis of silver and gold nanoparticles using thermophilic bacterium Geobacillus stearothermophilus. Process Biochem 2011; 46(10):1958-62. doi: 10.1016/j.procbio.2011.07.003 [Crossref] [ Google Scholar]
- Du L, Jiang H, Liu X, Wang E. Biosynthesis of gold nanoparticles assisted by Escherichia coli DH5α and its application on direct electrochemistry of hemoglobin. Electrochem commun 2007; 9(5):1165-70. doi: 10.1016/j.elecom.2007.01.007 [Crossref] [ Google Scholar]
- Sharma N, Pinnaka AK, Raje M, Fnu A, Bhattacharyya MS, Choudhury AR. Exploitation of marine bacteria for production of gold nanoparticles. Microb Cell Fact 2012; 11:86. doi: 10.1186/1475-2859-11-86 [Crossref] [ Google Scholar]
- Nangia Y, Wangoo N, Goyal N, Shekhawat G, Suri CR. A novel bacterial isolate Stenotrophomonas maltophilia as living factory for synthesis of gold nanoparticles. Microb Cell Fact 2009; 8:39. doi: 10.1186/1475-2859-8-39 [Crossref] [ Google Scholar]
- He S, Guo Z, Zhang Y, Zhang S, Wang J, Gu N. Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulata. Mater Lett 2007; 61(18):3984-7. doi: 10.1016/j.matlet.2007.01.018 [Crossref] [ Google Scholar]
- Arunkumar P, Thanalakshmi M, Kumar P, Premkumar K. Micrococcus luteus mediated dual mode synthesis of gold nanoparticles: involvement of extracellular α-amylase and cell wall teichuronic acid. Colloids Surf B Biointerfaces 2013; 103:517-22. doi: 10.1016/j.colsurfb.2012.10.051 [Crossref] [ Google Scholar]
- Agnihotri M, Joshi S, Kumar AR, Zinjarde S, Kulkarni S. Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Mater Lett 2009; 63(15):1231-4. doi: 10.1016/j.matlet.2009.02.042 [Crossref] [ Google Scholar]
- Inbakandan D, Venkatesan R, Ajmal Khan S. Biosynthesis of gold nanoparticles utilizing marine sponge Acanthella elongata (Dendy, 1905). Colloids Surf B Biointerfaces 2010; 81(2):634-9. doi: 10.1016/j.colsurfb.2010.08.016 [Crossref] [ Google Scholar]
- Arockiya Aarthi Rajathi F, Parthiban C, Ganesh Kumar V, Anantharaman P. Biosynthesis of antibacterial gold nanoparticles using brown alga, Stoechospermum marginatum (kützing). Spectrochim Acta A Mol Biomol Spectrosc 2012; 99:166-73. doi: 10.1016/j.saa.2012.08.081 [Crossref] [ Google Scholar]
- Singaravelu G, Arockiamary JS, Kumar VG, Govindaraju K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf B Biointerfaces 2007; 57(1):97-101. doi: 10.1016/j.colsurfb.2007.01.010 [Crossref] [ Google Scholar]
- Balagurunathan R, Radhakrishnan M, Rajendran RB, Velmurugan D. Biosynthesis of gold nanoparticles by actinomycete Streptomyces viridogens strain HM10. Indian J Biochem Biophys 2011; 48(5):331-5. [ Google Scholar]
- Chauhan A, Zubair S, Tufail S, Sherwani A, Sajid M, Raman SC. Fungus-mediated biological synthesis of gold nanoparticles: potential in detection of liver cancer. Int J Nanomedicine 2011; 6:2305-19. doi: 10.2147/ijn.s23195 [Crossref] [ Google Scholar]
- Swaminathan S, Murugesan S, Damodarkumar S, Dhamotharan R, Bhuvaneshwari SJ. Synthesis and characterization of gold nanoparticles from alga Acanthophora specifera (VAHL) boergesen. Int J Nanosci Nanotechnol 2011; 2:85-94. [ Google Scholar]
- Oza G, Pandey S, Mewada A, Kalita G, Sharon M. Facile biosynthesis of gold nanoparticles exploiting optimum pH and temperature of fresh water algae Chlorella pyrenoidusa. Adv Appl Sci Res 2012; 3(3):1405-12. [ Google Scholar]
- Rajasulochana P, Dhamotharan R, Murugakoothan P, Murugesan S, Krishnamoorthy P. Biosynthesis and characterization of gold nanoparticles using the alga Kappaphycus alvarezii. Int J Nanosci 2010; 09(05):511-6. doi: 10.1142/s0219581x10007149 [Crossref] [ Google Scholar]
- Senapati S, Syed A, Moeez S, Kumar A, Ahmad A. Intracellular synthesis of gold nanoparticles using alga Tetraselmis kochinensis. Mater Lett 2012; 79:116-8. doi: 10.1016/j.matlet.2012.04.009 [Crossref] [ Google Scholar]
- Stalin Dhas T, Ganesh Kumar V, Stanley Abraham L, Karthick V, Govindaraju K. Sargassum myriocystum mediated biosynthesis of gold nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 2012; 99:97-101. doi: 10.1016/j.saa.2012.09.024 [Crossref] [ Google Scholar]
- Ghodake G, Lee DS. Optoelectronics Biological synthesis of gold nanoparticles using the aqueous extract of the brown algae Laminaria japonica. J Nanoelectron Optoelectron 2011; 6(3):268-71. doi: 10.1166/jno.2011.1166 [Crossref] [ Google Scholar]
- Shah M, Badwaik V, Kherde Y, Waghwani HK, Modi T, Aguilar ZP. Gold nanoparticles: various methods of synthesis and antibacterial applications. Front Biosci (Landmark Ed) 2014; 19:1320-44. doi: 10.2741/4284 [Crossref] [ Google Scholar]
- Das R, Nath SS, Bhattacharjee R. Preparation of linoleic acid capped gold nanoparticles and their spectra. Physica E Low Dimens Syst Nanostruct 2010; 43(1):224-7. doi: 10.1016/j.physe.2010.07.008 [Crossref] [ Google Scholar]
- Narayanan KB, Sakthivel N. Facile green synthesis of gold nanostructures by NADPH-dependent enzyme from the extract of Sclerotium rolfsii. Colloids Surf A Physicochem Eng Asp 2011; 380(1-3):156-61. doi: 10.1016/j.colsurfa.2011.02.042 [Crossref] [ Google Scholar]
- Morrow BJ, Matijević E, Goia DV. Preparation and stabilization of monodisperse colloidal gold by reduction with aminodextran. J Colloid Interface Sci 2009; 335(1):62-9. doi: 10.1016/j.jcis.2009.02.053 [Crossref] [ Google Scholar]
- Du Y, Luo XL, Xu JJ, Chen HY. A simple method to fabricate a chitosan-gold nanoparticles film and its application in glucose biosensor. Bioelectrochemistry 2007; 70(2):342-7. doi: 10.1016/j.bioelechem.2006.05.002 [Crossref] [ Google Scholar]
- Katti KK, Kattumuri V, Bhaskaran S, Katti KV, Kannan R. Facile and general method for synthesis of sugar coated gold nanoparticles. Int J Green Nanotechnol Biomed 2009; 1(1):B53-b9. doi: 10.1080/19430850902983848 [Crossref] [ Google Scholar]
- Badwaik VD, Vangala LM, Pender DS, Willis CB, Aguilar ZP, Gonzalez MS. Size-dependent antimicrobial properties of sugar-encapsulated gold nanoparticles synthesized by a green method. Nanoscale Res Lett 2012; 7(1):623. doi: 10.1186/1556-276x-7-623 [Crossref] [ Google Scholar]
- Engelbrekt C, Sørensen KH, Zhang J, Welinder AC, Jensen PS, Ulstrup J. Green synthesis of gold nanoparticles with starch–glucose and application in bioelectrochemistry. J Mater Chem 2009; 19(42):7839-47. doi: 10.1039/b911111e [Crossref] [ Google Scholar]
- Basu N, Bhattacharya R, Mukherjee P. Protein-mediated autoreduction of gold salts to gold nanoparticles. Biomed Mater 2008; 3(3):034105. doi: 10.1088/1748-6041/3/3/034105 [Crossref] [ Google Scholar]
- Ravindra P. Protein-mediated synthesis of gold nanoparticles. Mater Sci Eng B 2009; 163(2):93-8. doi: 10.1016/j.mseb.2009.05.013 [Crossref] [ Google Scholar]
- Yuan H, Khoury CG, Hwang H, Wilson CM, Grant GA, Vo-Dinh T. Gold nanostars: surfactant-free synthesis, 3D modelling, and two-photon photoluminescence imaging. Nanotechnology 2012; 23(7):075102. doi: 10.1088/0957-4484/23/7/075102 [Crossref] [ Google Scholar]
- Kemp MM, Kumar A, Mousa S, Park TJ, Ajayan P, Kubotera N. Synthesis of gold and silver nanoparticles stabilized with glycosaminoglycans having distinctive biological activities. Biomacromolecules 2009; 10(3):589-95. doi: 10.1021/bm801266t [Crossref] [ Google Scholar]
- Venkatpurwar VP, Pokharkar VB. Biosynthesis of gold nanoparticles using therapeutic enzyme: in-vitro and in-vivo efficacy study. J Biomed Nanotechnol 2010; 6(6):667-74. doi: 10.1166/jbn.2010.1163 [Crossref] [ Google Scholar]
- Sohn JS, Kwon YW, Jin JI, Jo BW. DNA-templated preparation of gold nanoparticles. Molecules 2011; 16(10):8143-51. doi: 10.3390/molecules16108143 [Crossref] [ Google Scholar]
- Shao Y, Jin Y, Dong S. Synthesis of gold nanoplates by aspartate reduction of gold chloride. Chem Commun (Camb) 2004(9):1104-5. doi: 10.1039/b315732f [Crossref]
- He P, Urban MW. Phospholipid-stabilized Au-nanoparticles. Biomacromolecules 2005; 6(3):1224-5. doi: 10.1021/bm0501961 [Crossref] [ Google Scholar]
- Armendariz V, Herrera I, Peralta-Videa JR, Jose-Yacaman M, Troiani H, Santiago P. Size controlled gold nanoparticle formation by Avena sativa biomass: use of plants in nanobiotechnology. J Nanopart Res 2004; 6(4):377-82. doi: 10.1007/s11051-004-0741-4 [Crossref] [ Google Scholar]
- Khan M, Khan M, Adil SF, Tahir MN, Tremel W, Alkhathlan HZ. Green synthesis of silver nanoparticles mediated by Pulicaria glutinosa extract. Int J Nanomedicine 2013; 8:1507-16. doi: 10.2147/ijn.s43309 [Crossref] [ Google Scholar]
- Raveendran P, Fu J, Wallen SL. Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc 2003; 125(46):13940-1. doi: 10.1021/ja029267j [Crossref] [ Google Scholar]
- Selvakannan P, Mandal S, Phadtare S, Gole A, Pasricha R, Adyanthaya SD. Water-dispersible tryptophan-protected gold nanoparticles prepared by the spontaneous reduction of aqueous chloroaurate ions by the amino acid. J Colloid Interface Sci 2004; 269(1):97-102. doi: 10.1016/s0021-9797(03)00616-7 [Crossref] [ Google Scholar]
- Willett RL, Baldwin KW, West KW, Pfeiffer LN. Differential adhesion of amino acids to inorganic surfaces. Proc Natl Acad Sci U S A 2005; 102(22):7817-22. doi: 10.1073/pnas.0408565102 [Crossref] [ Google Scholar]
- Gan PP, Li SFY. Potential of plant as a biological factory to synthesize gold and silver nanoparticles and their applications. Reviews in Environmental Science and Bio/Technology 2012; 11(2):169-206. doi: 10.1007/s11157-012-9278-7 [Crossref] [ Google Scholar]
- Asgari F, Majd A, Jonoubi P, Najafi F. Effects of silicon nanoparticles on molecular, chemical, structural and ultrastructural characteristics of oat (Avena sativa L). Plant Physiol Biochem 2018; 127:152-60. doi: 10.1016/j.plaphy.2018.03.021 [Crossref] [ Google Scholar]
- Sathishkumar M, Sneha K, Won SW, Cho CW, Kim S, Yun YS. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf B Biointerfaces 2009; 73(2):332-8. doi: 10.1016/j.colsurfb.2009.06.005 [Crossref] [ Google Scholar]
- Bankar A, Joshi B, Kumar AR, Zinjarde S. Banana peel extract mediated synthesis of gold nanoparticles. Colloids Surf B Biointerfaces 2010; 80(1):45-50. doi: 10.1016/j.colsurfb.2010.05.029 [Crossref] [ Google Scholar]
- Patra JK, Baek KH. Green nanobiotechnology: factors affecting synthesis and characterization techniques. J Nanomater 2014; 2014:417305. doi: 10.1155/2014/417305 [Crossref] [ Google Scholar]
- Vijayaraghavan K, Ashokkumar T. Plant-mediated biosynthesis of metallic nanoparticles: a review of literature, factors affecting synthesis, characterization techniques and applications. J Environ Chem Eng 2017; 5(5):4866-83. doi: 10.1016/j.jece.2017.09.026 [Crossref] [ Google Scholar]
- Lukman AI, Gong B, Marjo CE, Roessner U, Harris AT. Facile synthesis, stabilization, and anti-bacterial performance of discrete Ag nanoparticles using Medicago sativa seed exudates. J Colloid Interface Sci 2011; 353(2):433-44. doi: 10.1016/j.jcis.2010.09.088 [Crossref] [ Google Scholar]
- Guo JZ, Cui H, Zhou W, Wang W. Ag nanoparticle-catalyzed chemiluminescent reaction between luminol and hydrogen peroxide. J Photochem Photobiol A Chem 2008; 193(2-3):89-96. doi: 10.1016/j.jphotochem.2007.04.034 [Crossref] [ Google Scholar]
- Wu W, Huang J, Wu L, Sun D, Lin L, Zhou Y. Two-step size- and shape-separation of biosynthesized gold nanoparticles. Sep Purif Technol 2013; 106:117-22. doi: 10.1016/j.seppur.2013.01.005 [Crossref] [ Google Scholar]
- Haverkamp RG, Marshall AT. The mechanism of metal nanoparticle formation in plants: limits on accumulation. J Nanopart Res 2009; 11(6):1453-63. doi: 10.1007/s11051-008-9533-6 [Crossref] [ Google Scholar]
- Zhang Y, Shareena Dasari TP, Deng H, Yu H. Antimicrobial activity of gold nanoparticles and ionic gold. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2015; 33(3):286-327. doi: 10.1080/10590501.2015.1055161 [Crossref] [ Google Scholar]
- Xu JW, Yao K, Xu ZK. Nanomaterials with a photothermal effect for antibacterial activities: an overview. Nanoscale 2019; 11(18):8680-91. doi: 10.1039/c9nr01833f [Crossref] [ Google Scholar]
- Shamaila S, Zafar N, Riaz S, Sharif R, Nazir J, Naseem S. Gold nanoparticles: an efficient antimicrobial agent against enteric bacterial human pathogen. Nanomaterials (Basel) 2016; 6(4):?. doi: 10.3390/nano6040071 [Crossref] [ Google Scholar]
- Zhou Y, Kong Y, Kundu S, Cirillo JD, Liang H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J Nanobiotechnology 2012; 10:19. doi: 10.1186/1477-3155-10-19 [Crossref] [ Google Scholar]
- Brewer M, Zhang T, Dong W, Rutherford M, Tian ZR. Future approaches of nanomedicine in clinical science. Med Clin North Am 2007; 91(5):963-1016. doi: 10.1016/j.mcna.2007.05.006 [Crossref] [ Google Scholar]
- Mishra A, Mehdi SJ, Irshad M, Ali A, Sardar M, Moshahid M. Effect of biologically synthesized silver nanoparticles on human cancer cells. Sci Adv Mater 2012; 4(12):1200-6. doi: 10.1166/sam.2012.1414 [Crossref] [ Google Scholar]
- Zhang LW, Monteiro-Riviere NA. Use of confocal microscopy for nanoparticle drug delivery through skin. J Biomed Opt 2013; 18(6):061214. doi: 10.1117/1.jbo.18.6.061214 [Crossref] [ Google Scholar]
- Umamaheswari K, Baskar R, Chandru K, Rajendiran N, Chandirasekar S. Antibacterial activity of gold nanoparticles and their toxicity assessment. BMC Infect Dis 2014; 14(Suppl 3):P64. doi: 10.1186/1471-2334-14-S3-P64 [Crossref] [ Google Scholar]
- Tomar A, Garg G. Short review on application of gold nanoparticles. Glob J Pharmacol 2013; 7(1):34-8. doi: 10.5829/idosi.gjp.2013.7.1.66173 [Crossref] [ Google Scholar]
- Cai W, Gao T, Hong H, Sun J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol Sci Appl 2008; 1:17-32. doi: 10.2147/nsa.s3788 [Crossref] [ Google Scholar]
- Gupta A, Pandey S, Variya B, Shah S, Yadav JS. Green synthesis of gold nanoparticles using different leaf extracts of Ocimum gratissimum Linn for anti-tubercular activity. Current Nanomedicine 2019; 9(2):146-57. doi: 10.2174/2468187308666180807125058 [Crossref] [ Google Scholar]
- Chahardoli A, Karimi N, Sadeghi F, Fattahi A. Green approach for synthesis of gold nanoparticles from Nigella arvensis leaf extract and evaluation of their antibacterial, antioxidant, cytotoxicity and catalytic activities. Artif Cells Nanomed Biotechnol 2018; 46(3):579-88. doi: 10.1080/21691401.2017.1332634 [Crossref] [ Google Scholar]
- Chen MN, Chan CF, Huang SL, Lin YS. Green biosynthesis of gold nanoparticles using Chenopodium formosanum shell extract and analysis of the particles’ antibacterial properties. J Sci Food Agric 2019; 99(7):3693-702. doi: 10.1002/jsfa.9600 [Crossref] [ Google Scholar]
- Sunderam V, Thiyagarajan D, Lawrence AV, Mohammed SSS, Selvaraj A. In-vitro antimicrobial and anticancer properties of green synthesized gold nanoparticles using Anacardium occidentale leaves extract. Saudi J Biol Sci 2019; 26(3):455-9. doi: 10.1016/j.sjbs.2018.12.001 [Crossref] [ Google Scholar]
- Katas H, Lim CS, Nor Azlan AYH, Buang F, Mh Busra MF. Antibacterial activity of biosynthesized gold nanoparticles using biomolecules from Lignosus rhinocerotis and chitosan. Saudi Pharm J 2019; 27(2):283-92. doi: 10.1016/j.jsps.2018.11.010 [Crossref] [ Google Scholar]
- Veena S, Devasena T, Sathak SSM, Yasasve M, Vishal LA. Green synthesis of gold nanoparticles from Vitex negundo leaf extract: characterization and in vitro evaluation of antioxidant–antibacterial activity. J Clust Sci 2019; 30(6):1591-7. doi: 10.1007/s10876-019-01601 [Crossref] [ Google Scholar]