Advanced pharmaceutical bulletin. 11(3):399-413.
doi: 10.34172/apb.2021.048
Review Article
A Review on Newer Ocular Drug Delivery Systems with an Emphasis on Glaucoma
Maneesha Peter  , Rajitha Panonnummal *
, Rajitha Panonnummal * 
Author information:
Amrita School of Pharmacy, Amrita Institute of Medical Science & Research Centre, Amrita Vishwa Vidyapeetham, Kochi-682041, India.
Abstract
Glaucoma is an irreversible condition resulting from the increase in intraocular pressure (IOP); which leads to permanent loss of vision with the destruction of retinal ganglion cells (RGCs). The IOP elevations are controlled in normal by the physiological flow of aqueous humour. A population with age above 40 is more susceptible to glaucoma. Other factors like gender, genetics, race etc. plays major roles in the development of the disease. Current treatment methods available for the disease includes drugs come under the classes of beta receptor blockers, carbonic anhydrase inhibitors, cholinergic agonists, prostaglandins etc. N-methyl-D-aspartate (NMDA) antagonists, inducible nitric oxide synthase (iNOS) inhibition, cytoskeletal agents, Rho-kinase inhibitors etc are few novel targets sites which are in research focus for the treatment of the disease. Developments in nanomedicine are also being evaluated for their potential in treating the growing glaucomatous population. Nanosystems are suggested to avoid the difficulties in tackling the various ocular barriers to a limit, help to decrease the instillation frequency of topical medication and can provide drug delivery in a sustained or controlled manner. This review focuses on the current and emerging treatment methods for glaucoma along with some of the nanoformulations for ocular drug delivery.
Keywords: Glaucoma, Intraocular pressure, Aqueous humour, Nanoformulations
Copyright and License Information
©2021 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Eye forms an integral component of the sensory system with highly advanced anatomy and physiology. The different segments of the eye work in harmony to facilitate vision. Human eye is distinctively categorized into an anterior chamber, posterior chamber and the vitreous cavity as depicted in Figure 1A. The outer layers of the eye consist of the sclera and cornea. The former maintains the structural configuration and attaches eye to the extrinsic muscles. The cornea is noted as a clear membrane that extends along with the sclera and manifests as the outer fibrous layers of the eye. The vascular layers are formed by the choroid, ciliary body and iris.
1,2
Choroid operates by absorbing the light rays which fall onto the retina. Ciliary bodies are innervated with a system of parasympathetic nerves and it also renders the attachment to the suspensory ligaments. Contractions and relaxations of the ciliary muscles assist in the bending and entry of the light rays, so that it can be further focused onto the retina. Aqueous humour, which is released into the anterior segment of the eye, is produced by the ciliary body. The watery fluid generated from the ciliary body is filtered by the posterior chambers and drained out through the cells of the trabecular meshwork.
3,4
Lying behind the cornea and in front of the lens is the iris, which divides the eye into anterior and posterior chambers. Iris is composed of circular and radial muscle along with an aperture in the middle, named as pupil.
5
Pupil can change its size depending on the intensity of the light entering into the eye. Behind the pupil lies the lens, which is biconvex and is orchestrated by the suspensory ligaments.
6,7
It is followed by the innermost layer, retina. It is constituted by a multitude of neurons. Retina accommodates rods and cones which forms the light-sensitive part. Blood supply to the eye is enabled by the ciliary arteries and the central arteries of retina. The jelly-like, vitreous body is formed beyond the lens, helps to maintain the structure of the eye and avert the walls of eyeball from collapsing.
8,9
Along with these central regions that control sight, eye is equipped with certain accessory structures like the eyebrows, eyelids and eyelashes that protect the eye from injuries and high luminescence. Along with them, the conjunctiva lines the eyelids and acts as a protectant of the cornea. Lacrimal glands secrete tears composed of salts, water and lysozyme which wipes and nourishes the front of the eye.
3,10
The optic nerve emerges from the retina, converges to the side of macula lutea, passes through the optic foramen and meets at the optic chiasma. As the light rays entered into the eye, the pupil size is varied. The entry of bright light constricts the pupils and the pupils dilate when less intense light is detected. Pupils restrict the entry of high amounts of light and thereby prevent the damage to the retina. Also, the eyeball moves inside (convergence) so that the nearer objects are easily visible.
3
In order to view a distant object, the convergence produced by the eyeball is less. Brighter lights are required to impart the stimulation of cones and thereby the perception of vision. The lights rays which entered into the eye introduce reactions which are converted into nerve impulses and transmitted through the optic nerve, to aid vision.
4
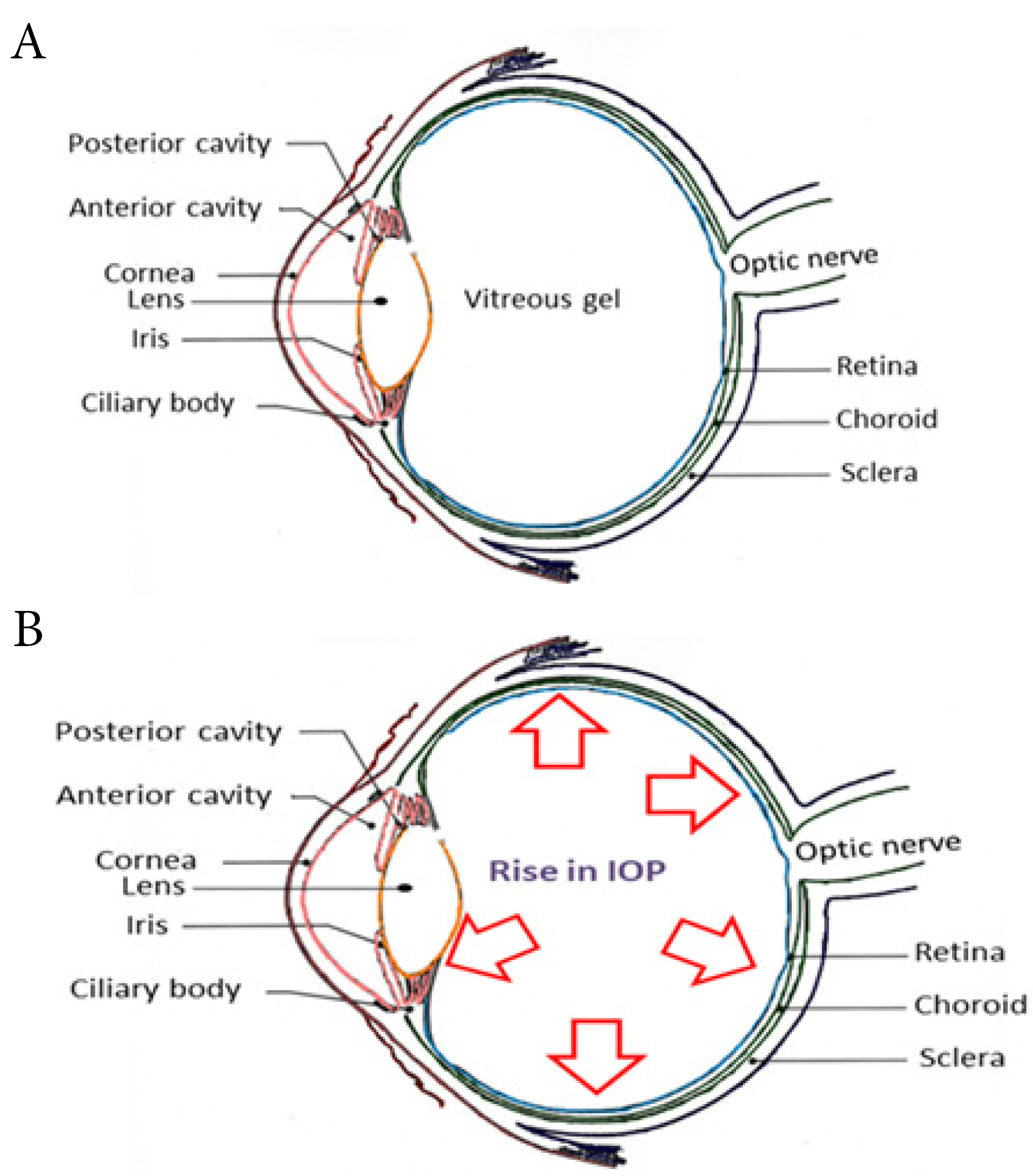
Figure 1.
Schematic representation of (A) Healthy eye & (B) Eye affected with glaucoma
.
Schematic representation of (A) Healthy eye & (B) Eye affected with glaucoma
Glaucoma is an irrevocable condition that emerges as a result of the increase in intraocular pressure (IOP) (more than 20 mm Hg) from the normal pressure of 10-20 mm Hg (Figure 1B) along with the regression of retinal ganglion cell (RGC) layers.
11
The IOP elevations in glaucoma occur as a result of increased resistance to aqueous humour outflow and are recognized as one of the paramount inducers of detrimental effect on vision.
12,13
Glaucoma is divided into primary, secondary, congenital, pigmentary and normal-tension glaucoma (Table 1).
14
Table 1.
Different categories of glaucoma
|
Type
|
Definition
|
| Primary glaucoma |
Arises due to an unknown cause |
| Secondary glaucoma |
Due to any defined cause for the increase in intraocular pressure, e.g. eye injury, inflammation etc. |
| Congenital glaucoma |
A congenital defect which may be inherited |
| Pigmentary glaucoma |
Pigments of the iris flake off and clog the drain outlet of the aqueous humour |
| Normal-tension glaucoma |
Damage to the optic nerve even when the pressure is normal |
Glaucoma is again subdivided based on the iridocorneal angle into:
i) Open-angle glaucoma/Wide-angle glaucoma
ii) Closed-angle glaucoma/Narrow-angle glaucoma
When the iridocorneal angle is intact but the flow of the aqueous humour through the trabecular meshwork is disturbed due to any degeneration or disturbance in the meshwork system, it is termed as open-angle glaucoma. The condition is asymptomatic and may be chronic. Closed-angle glaucoma is defined as a state when the iridocorneal angle is closed and the flow of aqueous humour from posterior to anterior chamber is disrupted. The condition is manifested with pain and pressure build-up in the eye.
15,16
Pathophysiology
Normally the aqueous humour produced by the ciliary body is drained out through the trabecular meshwork and the Schlemm’s canal. Some amount of the aqueous fluid also passes through the uveoscleral pathway; this decreases the build-up of pressure inside the eye. But in glaucomatous condition, the systematic outflow of the aqueous humour is altered due to any insult in the trabecular meshwork or any changes in the iridocorneal angle. This may result in the obstruction of the aqueous humour outflow with pressure build-up and damages the optic
nerve head (Figure 2). Studies also indicate that the presence of mutant form of myocilin (myocilin is a protein secreted into the aqueous humour and its function is not clear) deposits in the trabecular system initiates a cascade of toxic events that may result in higher IOP.
16,17
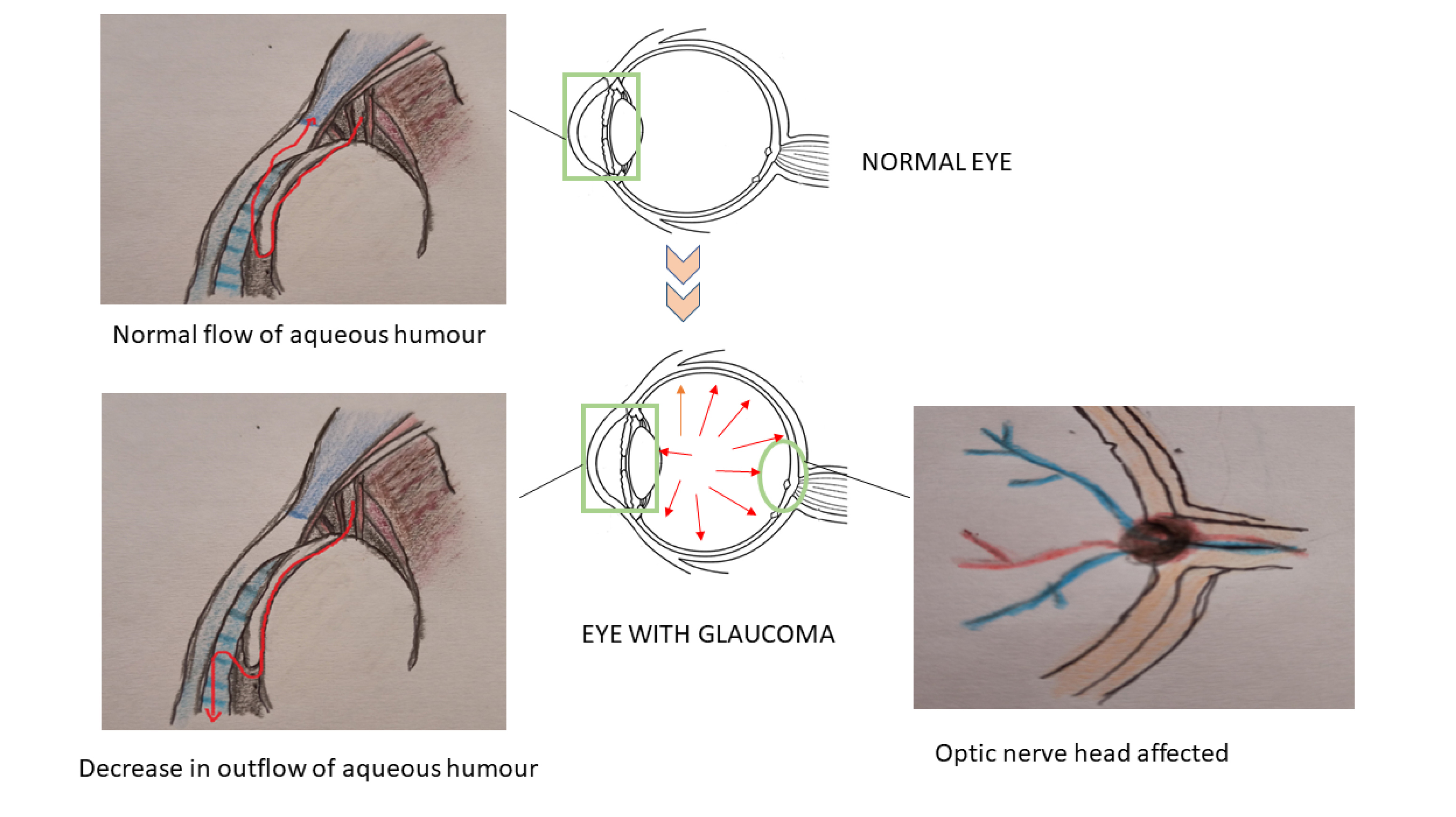
Figure 2.
Schematic representation of glaucoma and its main defects
.
Schematic representation of glaucoma and its main defects
Damage to the RGCs is reported to be involved in the development of glaucoma. The altered appearance of the optic disc also speaks for the same. An escalation in the IOP, causes the fluid to move from the vitreous cavity to the extracellular spaces, thereby resulting in rapid necrosis of the neuronal bunches. Deprivation of the visual field is due to the trauma to the optic nerve head (ONH). The elevated IOP also damages the RGCs as revealed by the depletion of the rate of the axonal transport. The glial cells in the ONH are activated due to high IOP and this leads to the remodelling of the extracellular matrix (ECM) and its characteristic degradation. These alterations result in the amplification of tension on the RGC axon.
18,19
An imbalance between the generation and expulsion of the free radicals result in a state denominated as oxidative stress.
20,21
It affects the vascular flux of the optic nerves, produces injury to the trabecular meshwork and initiate an aberrant immune response with glial cells dysfunction. Activation of the apoptotic pathway by reactive oxygen species further contribute to the death of RGC.
22
Decreased amount of oxygen in the retina stimulates hypoxia-inducible factor 1ɑ which in turn exerts protective effects on RGC. Free radicals also activate biological pathways of NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), IL-1 (interleukin 1), ELAM- 1 (endothelial cell leukocyte adhesion molecule-1), TNF-ɑ (tumor necrosis factor-α) etc contribute to neuronal death.
23,24
Risk factors
Distinguishing glaucomatous population is a tough endeavour as the illness is identified only after the diminution of a notable percent of RGCs. Evidences revealed that a senile community, Africans, Americans, familial history of glaucoma, raised IOP, reduced diastolic perfusion pressure and myopia as cardinal risk factors for glaucoma.
Age
Age-related susceptibility can be perceived as a supreme influencer in the rise of the glaucomatous population. Patients aged 40 and above are more prone to developing glaucoma.
25,26
Gender
As per reports, the female population is more prone to get affected by glaucoma, particularly angle-closure glaucoma.
27
Familial history
Research works revealed that the persons having a first degree relative with glaucoma there is four times risk for getting the disease when compared with persons without such a relative.
26
Genetics
GLC1A is a gene coding for a molecule that is available in the aqueous outflow channels. Mutation in these passages fosters an occlusion in the outflow leading to a higher IOP. Both the trabecular meshwork and ciliary bodies monitor the IOP and contain myocilin fraction. MYOC locus in the GLC1A codes for myocilin and mutations in this locus is considered as a contributor factor for 3%-5% of adult-onset primary open-angle glaucoma.
26
Ethnicity
Glaucoma is being considered as one of the significant causes of blindness among African Americans, Caucasians etc.
26
Treatment
Drug therapy, laser and surgical methods are also utilized to reduce IOP. The judgment of the correct therapy for the treatment of the disease is influenced by certain criteria like IOP levels, stage and advancement of diseases, treatment methodologies followed etc. Along with all these factors, satisfaction with the therapy provided and patient compliance forms the basis for choosing the right treatment. The conventional therapy employed mainly relies on topical administration of the drugs as eye drops or ointments.
Beta-blockers
β blockers act by competitively inhibiting adrenergic β receptors and thereby decreasing the secretion of aqueous humour. Topical β blockers are either non-selective or β1selective in performance. Non-selective β blockers suppress the action of adrenergic agonist on both β1 and β2 receptors.
28,29
Topical carbonic anhydrase inhibitors
Dorzolamide, Brinzolamide etc. lower the IOP by minimizing the aqueous humour production by hindering the sodium pump and is often used as adjuvant in therapy. The adverse events reported with the instillation of topical carbonic anhydrase inhibitors are burning and stinging sensation, bad taste in the mouth and conjunctivitis.
30-32
Cholinergic agonists
Parasympathomimetic agents like pilocarpine are rarely used as first-line therapy. They act on the iris, reduce contraction of the ciliary muscle and improve the IOP.
31,32
ɑ2 agonists
Brimonidine is a highly selective and potent ɑ2 adrenergic receptor agonist that lowers IOP by promoting uveoscleral outflow of aqueous humour and by decreasing its production. Studies showed that brimonidine 0.2% can significantly decrease IOP in patients with uncontrolled primary open-angle glaucoma when used as an adjuvant along with dorzolamide and is also having neuroprotective effect.
33,34
Prostaglandins
Prostaglandins act by relaxing the muscles in the inner segments of the eye, thereby enabling efficient outflow of aqueous humour and thus prevent the escalation of ocular pressure. Possible side effects associated with the use of prostaglandins include stinging and burning, eye colour change etc.
33-35
Rho-kinase inhibitors
A newly developed agent in the topical treatment for glaucoma is the Rho-kinase inhibitors. The enzyme, Rho kinase regulates the calcium-independent contractions of smooth muscles. Reports stated that these enzymes can affect the cytoskeletal aspects like morphology, cell adhesion etc. Rho-kinase inhibitors can reduce the IOP by increasing the flow of aqueous humour through the modification of the structure of the trabecular meshwork system.
31-35
Due to the asymptomatic and chronic nature of the disease, patient compliance is necessary to prevent the progress of the disease and to ensure medication adherence for long periods.
30
Treatment often involves multiple drug therapies with the potential for adverse interactions including the development of ocular surface disorders. The justification for non-adherence to the treatment partly lies in the discomfort of eyes by the use of these medications. Laser treatment and trabeculectomy are considered as alternatives to drug therapy.
36
Argon laser trabeculoplasty & micro pulse laser trabeculoplasty are few examples of this. Other relatively newer techniques employed are viscocanalostomy and high frequency deep sclerectomy.
37,38
Barriers to treatment
Tear
To counteract the irritations produced on the eye, lacrimal glands secrete lacrimal fluid or tears and the mean volume of production is about 7 to 9 mL. On installation of eye drops into the eye, the instantaneous increase in tear volume causes excessive medication to spill out.
39,40
Cornea
The corneal epithelium acts as a powerful barrier for the drug to pass. The epithelium is a highly lipophilic tissue and makes is difficult for the hydrophilic drug moieties to cross this barrier.
39-41
Conjunctiva
The conjunctiva is composed of 5-15 sheets of stratified squamous epithelium cell layers. It is supplied with lot of the nerves, lymphatic and blood vessels. The permeability of drug to the conjunctiva is higher when compared with that of sclera and cornea.
42-44
Blood retinal barrier (BRB)
BRB functions as a barrier between the blood and the retina and contains tight junctions. As a result of the anatomical position of the BRB, it effectively limits the transportation of molecules from the choroidal blood circulation to the posterior segment of the eye.
40,43
On topical application, the primary aspect to be noted is that the drug needs to be penetrated. Lipophilic moieties and unionized fractions are better penetrated compared to a hydrophilic agent or an ionized drug. The drug needs to be delivered to the target site using a vehicle & the vehicle used influences the retention time of the agent in the conjunctival cul-de-sac.
45
Elevation of retention time increases the trans corneal diffusion and lowers the systematic level of the drug. The viscosity of ophthalmic products can be made adequate by the inclusion of compounds such as hydroxyl propyl methylcellulose, polyvinyl alcohol etc. These polymers increase the residence time of the drug in the conjunctival cul-de-sac and offers a slower clearance resulting in enhanced absorption of the drug moiety.
46
Novel therapeutic targets in glaucoma
Even in this era of modern medicine, glaucoma still remains as a major cause of blindness worldwide. Therefore, newer therapeutic strategies targeting the alternative pathways involved in disease genesis and progression need to be identified.
NMDA antagonists
Stimulation of the glutamate N-methyl-D-aspartate (NMDA) receptor triggers the opening of ion channels and permits the entry of extracellular calcium and sodium. Extracellular calcium acts as a secondary messenger to initiate neuronal cell death. To suppress excitotoxicity mediated cell death, efficient removal of synaptic glutamate is essential.
47
The synapses are encircled by Muller cells and astrocytes which transport glutamate (extracellular) into the glial cells. Glutamine synthetase converts glutamate to glutamine (non-toxic) which is taken up by the neuronal cells and is again converted to glutamate in the presence of glutaminase thereby preventing the glutamate toxicity by maintaining the physiological neurotransmitter stores.
48,49
Cytoskeletal agents
Alterations to the trabecular meshwork cytoskeleton can change the local geometric outflow pathway and consequently alter the aqueous outflow. Compounds having the property to causes cytoskeletal alterations are found to effectively lower IOP. For example, cytochalasin, prevent the elongation of actin filaments, causing distension of the trabecular meshwork, rupture the inner walls of the Schlemm’s canal and increase the aqueous humour outflow.
47,48
Matrix metalloproteinase inducers
These agents are capable to catalyse the hydrolysis of ECM proteins; as higher concentration of ECM in the trabecular meshwork causes resistance for the aqueous humour outflow.
47,49
iNOS inhibition
Nitric oxide synthases (NOSs) are enzymes that catalyse the synthesis of nitric oxides. An elevated level of iNOS (inducible-NOS 2) is observed in the glaucomatous population. These findings point out the accumulation of toxic amounts of NO, leading to the development of peroxy radicals; which is capable to induce inflammations and neuronal damage. Therefore inhibition of iNOS is an important strategy to reduce inflammation and neural damage; hence it serves as a potential tool in the treatment of glaucoma.
31,49
Neuroprotectives in glaucoma
The complexity of mechanisms which transform to the formation of neuronal damage in glaucoma has to be dealt with utmost perceptiveness. Neurons in the visual pathway are deprived due to the progressive damage caused by the rise in ocular tension.
50
RGCs are the primary victims affected by this process. Genetic factors, withdrawal of neurotropic factors, exposure to reactive oxygen species etc. are the main causative agents expected to be involved in RGC degeneration.
18,51,52
Some of the compounds having neuroprotective property and thus improves RGCs survival are mentioned in Table 2.
53
Table 2.
Different compounds which provide neuroprotection in glaucoma
|
Compound
|
Mode of action
|
| CoQ10 |
Effect on mitochondrial dysfunction |
| Ginkgo biloba |
Antioxidant |
| Melatonin |
Antioxidant |
| Minocycline |
Anti-inflammatory |
| Resveratrol |
Antioxidant |
| Citicoline |
Neuroprotective |
| Brimonidine tartrate |
Neuroprotective |
Brimonidine
Brimonidine acts as a successful α2 adrenergic agonist which is in use for the treatment of glaucoma. Research works illustrate that 0.2% brimonidine tartrate solution is capable to activate the α2 receptors present in the retina.
54
WoldeMussieet al studied the neuroprotective effect of brimonidine on RGCs in rats treated with laser- induced chronic ocular model of hypertension. The investigation is able to conclude that the brimonidine is able to decrease ganglion cell death by 26±1% and 15±2% at doses of 0.5 and 1 mg/kg respectively.
55
Ferencz et al studied the neuroprotective effect of topically applied brimonidine in patients undergone laser treatment for choroidal neovascularization and reported the significant improvement in vision.
56
Citicoline
Citicoline or cytidine 5’-diphosphocholine is an endogenous compound made up of ribose, pyrophosphate, cytosine and choline. It is a nontoxic moiety which increases the brain neurotransmitters such as dopamine, noradrenaline, serotonin etc.
57
It also acts as an intermediate in the synthesis of CNS phospholipids and also the drug is believed to improve the synthesis of phosphatidylcholine.
58
Oshitari et al reported the neuroprotective effect of citicoline in 2002. TUNEL staining (terminal deoxynucleotidyl transferase dUTP nick end labelling) is done to explore the consequence of the administration of citicoline to mouse retinal explants. An obvious expansion in the number of neurons or regeneration is observed after the administration of citicoline.
59
Ottobelli et alevaluated the consequence of oral use of citicoline on visual field rates in 41 patients with progressing glaucoma and reported the decreased progression of the disease in these patients, indicates its neuroprotective effects.
60
Gingko biloba
Gingko biloba extracts is typically composed of flavonoids and terpenoids. EGb761 and LI1370 are reported to be isolated from the extracts possessing antioxidant properties.
61
Moreover, the extracts are reported to cause vasodilation, thereby improve the blood flow and safeguard mitochondrial metabolic process.
62
Quaranta et al performed a double-masked randomized crossover trial with 27 patients to identify the effect of Gingko biloba extract on pre-existing visual field damage in normal-tension glaucoma and reported the improved visual field damage.
63
Ubiquinone/Coenzyme Q 10 (CoQ10)
Ubiquinone acts as a cofactor in the mitochondrial electron transport chain, which is responsible for the transport of electrons from complex I &II to complex III.
64,65
Mitochondrial dysfunction is reported to be involved in apoptosis of neurons and ubiquinone can counteract these effects.
53
A study was conducted by Lee et al to investigate the neuroprotective effect of ubiquinone on RGCs in glutamate excitotoxicity and oxidative stress model in DBA/2J mice. CoQ10 is found to boost the growth of neurons in the ONH by 29%, as well as decreases the apoptotic cell death by suppressing the expression of proapoptotic Bax (Bcl-2-associated X protein) or by enhancing the expression of antiapoptotic Bad protein.
66
Another study conducted by M. Cordeiro et al reported the decreased RGC death on topical application of CoQ10 in an experimental model of glaucoma, suggested it as a promising candidate for use in glaucoma.
67
Resveratrol
Resveratrol is a plant polyphenol commonly found in grapes. Chemically it is a stilbenoid and is considered as a phytoalexin generated by various plants in response to injury or when attacked by pathogens.
68
Luna et al carried out a study to reveal its effect on the expression of glaucoma markers induced by chronic oxidative stress and is reported that resveratrol limited the expression of inflammatory markers like IL-1α, IL-6, IL-8, etc., along with a decrease in senescence marker lipofuscin manifested in glaucomatous condition. Resveratrol is effective in repairing the abnormalities associated with trabecular meshwork cells in primary open-angle glaucoma.
69
It is also reported that the resveratrol can induce a delay in RGC death in hyaluronic acid (HA) model of glaucoma.
70
Novel ocular drug delivery approaches
Glaucoma is said to affect around 111.8 million people by the year 2040.
71
Therefore, the requirement of a novel therapeutic strategy is the need of the hour. A variety of factors are there to influence the manufacturing of drug delivery systems and novel delivery approaches. These factors need to be considered much to develop ideal delivery systems for the treatment of glaucoma.
Mucoadhesive polymers
Use of the right polymer or a mixture of polymers is essential to improve the ocular residence period as well as the bioavailability.
72
Polymers used in the preparation of formulations play a key role in stabilizing the carrier systems.
73
These polymers are categorized into biodegradable and non- biodegradable polymers. Biodegradable polymers have a short duration of action compared to non-biodegradable forms.
74,75
These polymers should possess good viscosity, adherence and a reduction in the drug drainage rate. Attachment of drug carrier with the mucin coat covering the conjunctiva & cornea elevates the drug residence time, establishes a contact between the drug and the tissue, which absorbs it. This results in a higher drug concentration at the target.
76
Hyaluronic acid (HA) is a natural glycosaminoglycan remains as the first among these polymers. This mucoadhesive biological polymer has the advantage of having a highwater binding capacity, low irritancy, better viscosity and pseudoplastic behaviour.
77,78
Other mucoadhesive polymers include carboxymethylcellulose, polyacrylic acid derivatives, xanthan gum, carrageenan etc.
78,79
Chitosan is a polysaccharide obtained from chitin by deacetylation and has several features that make it favourable for the construction of a controlled drug delivery devices to the eye.
80,81
It is a hydrophilic mucoadhesive polymer which is biocompatible and biodegradable with good eye tolerability. Moreover, chitosan exhibits antimicrobial and wound healing properties.
82
It is reported that the positively charged amino groups of chitosan associates with the negatively charged sialic acid residues of mucin, thereby prolonging the contact time of the drug. It is a widely utilized polymer for application in various drug targeting methodologies and also acts as an excipient in vaccine delivery, gene therapy etc. Chitosan offers sufficient muco-adhesiveness, ocular permeability, biocompatibility and corneal wound healing along with antibacterial and antifungal effects.
83
It can easily turn to gels by physico-chemical methods or through co-ordination linkages without the need of any additives. Chitosan in situ gelling systems are used to deliver bioactive compounds by instillation into the eye, which upon exposure to the ocular media changes to gel.
84
These are liquid at room temperature and transform into gel after application to the ocular surface due to changes in temperature, pH or ionic strength. Chitosan may interact with water-soluble macromolecules such as anionic polysaccharides, dextran sulphate, collagen or anionic polymers such as polyacrylic acid.
85
Carbopol is a polyacrylic acid derivative which initiates sol-gel transition in aqueous solution when the pH of the medium rises above 5.5. It is non-toxic and non-irritating to humans following topical application. However, the concentration required to form gel resulted in acidic solutions that cannot be rapidly neutralized by lacrimal fluid buffers.
86
Chitosan can be complexed with Carbopol through the electrostatic interactions between the amino groups of chitosan and the carboxyl groups of carbopol. Cross-linking of chitosan is imperative to ameliorate its properties like stability and durability for drug delivery.
87
Various other polymers like collagen, pluronic F-127, polyvinyl alcohol etc are utilized widely in manufacturing novel drug delivery systems. Collagen is a naturally occurring polymer reported to have optimum strength and low toxicity.
88
Pluronic F127 is also called as Poloxamer 407; contains both polar and non-polar substituent in it and is amphiphilic in nature. It possesses good gelation property but it is difficult to maintain its stability.
89
Contact lenses
One of the major problems associated with glaucoma is low medication adherence. The topical medications need to be instilled two or three times a day. This issue can also be managed by the usage of contact lenses as they can provide a controlled or sustained release of medication.
90
Thus the problems of low ocular availability and less residence time of the drug carrier system can be tackled by using medicated contact lenses. Both conventional and silicone hydrogel contact lenses release ophthalmic drugs in a short period.
91
But nanoparticle laden lenses, biomimetic and imprinted contact lenses with layered structures may permit an extended drug delivery along with improved ocular availability and reduced side effects. The usage of contact lenses in glaucomatous patients is reported to achieve the same efficacy which is comparable with other therapeutical regimens.
92,93
Peng et al state that contact lenses with only 20% of the drug loading are able to achieve the same efficacy as that achieved with eye drops. A significant lowering of IOP is achieved by the continuous usage of it.
94
Hsu et al formulated contact lenses loaded with both dorzolamide and timolol and the system is reported to prolong the release of the loaded moieties for 2 days with sufficient decrease in IOP when compared to eye drops. Also, the system in combination with Vitamin E is proved to be more beneficial due to the antioxidant potential of vitamin E.
95
Ophthalmic inserts
These are solid gadgets available to place into the conjunctiva of eye designed to induce the release of drug for an extended time frame at a constant rate to improve patient compliance.
96,97
Ophthalmic inserts also promote non-corneal diffusion. But the systems are reported to cause discomfort among its users as these are not dissolving in the conjunctival sac.
98
This factor encourages the development of non-irritant, non-toxic, more convenient and soluble the ophthalmic inserts by using polymers like polyacrylamide, polyvinyl pyrrolidineetc.
99
Iontophoresis
Ocular drug delivery can be achieved through non-invasive techniques like iontophoresis. In this technique a weak current supply the charged species through the ocular tissue and another ground electrode is placed elsewhere on the body to complete the circuit.
100
Medications are retained in the iontophoretic device by two methods, either as a solution or as saturated in a gel. Ocular iontophoresis is achieved by trans corneal or transscleral iontophoresis. Trans corneally the drug is delivered onto the anterior segment and is suggested to be beneficial in diseases like glaucoma, inflammations, keratitis etc.
101,102
Transscleral drug delivery helps in the administration of high concentrations of the drug into the inner or posterior segments of the eye. Current density and iontophoretic exposure intervals influence the drug delivery to the various regions of the eye. The procedure emphasizes minimal discomfort for the patient; however, it possesses several disadvantages such as epithelial oedema, burns, damage to the site of application etc.
103
The technique is also limited to drugs having a small size; ionic nature and lower molecular weight.
104
Nanoformulations
Recently different nanoformulations are reported to have favourable properties for ideal use for ophthalmic applications. The size of different nanoformulations is usually below 1000 nm. These are fabricated through chemical processes to control the release of therapeutic agents and to enhance the ocular penetration. The size of the complex drug particles in an ophthalmic preparation should be less than 10 µm to avoid ocular irritation. The major advantages of utilizing nanocarriers in the treatment of ocular diseases are to enhance the bioavailability of topical administration, to achieve a controlled release, permit targeted drug delivery and ultimately to achieve improved therapeutic efficacy.
105,106
Nanocarriers also offers higher drug retention time, lower dosage requirements, decreased dosing frequency and higher patient compliance (Figure 3). Cornea and conjunctiva possess negative surface charges and enhances the retention time of positively charged nanoparticles more efficiently than the anionic carriers, providing an increased opportunity for the drug to enter into the eye.
107,108
Nanoparticles are becoming a resourceful therapeutic agent in eradicating various disorders associated with the posterior and anterior regions of the eye (Table 3).
109
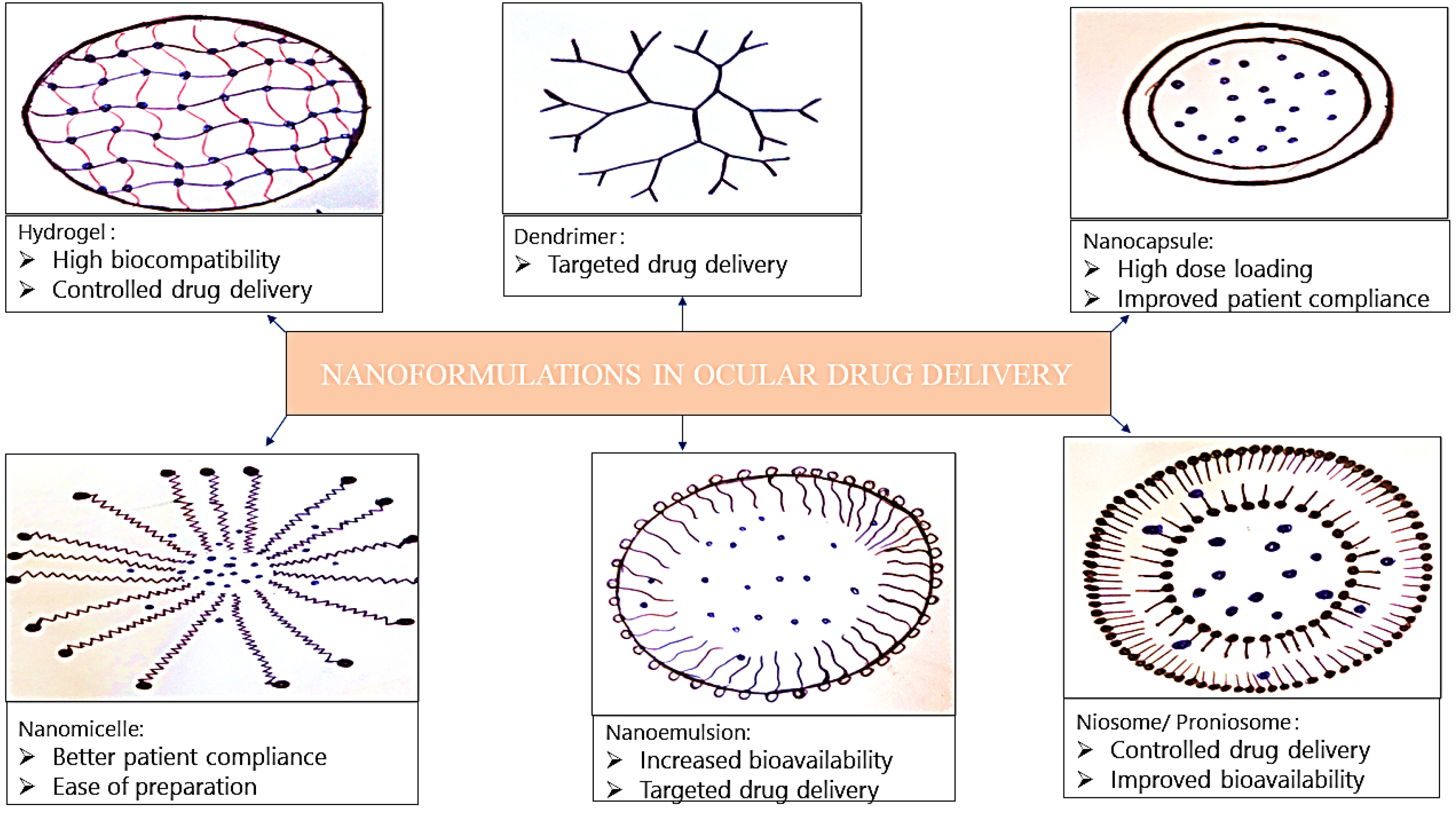
Figure 3.
Nanoformulations used in ocular drug delivery.
.
Nanoformulations used in ocular drug delivery.
Table 3.
Nanoformulations reported for ocular drug delivery
|
Formulation
|
Drug loaded
|
Polymers used
|
Method of preparation
|
| Nano micelle |
a) Methazolamide
b) Rapamycin
c) Cyclosporin A
|
a) Methoxy-poly (ethylene glycol)-b-poly(ε-caprolactone)
b) Vitamin E tocopherol polyethylene glycol succinate&octoxynol-40
c)Polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol & Pluronic F127
|
a) Thin film hydration
b) Solvent evaporation
c)Solvent evaporation/ Film hydration
|
| Nano emulsion |
a) Dorzolamide
b) Moxifloxacin
c) Acetazolamide
|
a) Poloxamer 407
b) Soluphor® P
c) Peanut oil
|
|
| Nano capsule |
a) Pilocarpine
b) Prednisolone
c) Pilocarpine
|
a) Poly(ε-caprolactone) & Pluronic F68
b) Poly(ε-caprolactone) or Eudragit RS100
c) Poly isobutyl cyanoacrylate
|
a) Double emulsion
b) Interfacial deposition
c) Interfacial polymerization
|
| Dendrimer |
a) Brimonidine & Timolol
b) Fluocinolone
c) Timolol analogue
|
a) Polyethylene glycol
b) Hydroxyl-terminated poly amido amine
c)Heterobifunctional amine polyethylene glycol acetic acid
|
|
| Hydrogel |
a) Timolol
b) Cannabigerolic acid
c) Bevacizumab
|
a) Propoxylated glyceryl triacrylate
b) Hyaluronic acid & methyl cellulose
c) Glycol chitosan & oxidized alginate
|
a) Thermal polymerization |
| Proniosome/Niosome |
a) Lomefloxacin
b) brimonidine
c) Dorzolamide
|
a) Cholesterol
b) L-α-phosphatidylcholine & Cholesterol
c) L-α-lecithin & Cholesterol
|
a) Coacervation phase separation
b) Coacervation phase separation
c) Coacervation phase separation
|
Nanomicelles
Agents that are amphiphilic and capable of self-assembling above the critical micellar concentrations are termed as nanomicelles; measuring about 10–100 nm in size.
110,111
Nanomicelles consists of a hydrophobic core where the drug is confined and the hydrophilic tail protrudes to the outside environment.
112
Nanomicelles are advantageous as they maintain an ideal concentration of the medicament in the respected targets, better patient compliance, easy to prepare, small in size and improves the availability of medications in visual tissues.
113
The utility of micellar scale preparations are limited as it may experience rapid tear dilution, untimely drug release & cost.
114
Elmowafy et al reported the possibility of a better evolved therapy in glaucoma using nanomicelles. The authors converted methazolamide, a carbonic anhydrase inhibitor into a polymeric micelle which is found to improve the drug entrapment efficiency and ocular tolerability. The formulation showed an initial burst followed by a sustained release.
115
Rapamycin loaded nanomicellar formulation for the delivery to the posterior segment of the eye is reported by Cholkar et al. The formulation is reported to have a size of approximately 10 nm and could transport the rapamycin into the retina and choroid following topical application. The in vitrocytotoxicity analysis demonstrated very little cytotoxic effects.
116
Guo et al fabricated cyclosporine A loaded nanomicellar formulation using PVCL-PVA-PEG copolymer to achieve efficient drug delivery to treat immune-mediated corneal disease. This nanoformulation is found to possess good tolerance, stability on storage, no cytotoxicity and adequate ocular tolerance. Multiple and even single instillations of the formulation delivered promising levels of cyclosporine A into theeye.
117
Nanoemulsions
Nanoemulsions are thermodynamically stable drug carriers within the size range of 10-1000 nm with an interfacial surfactant layer separating the oil and water environments.
118
These drug carriers are identified as oil in water, water in oil and bicontinuous nanoemulsions. Emulsions and nanoemulsions are easily identified from their appearance as the former tend to be cloudy in nature but the latter is translucent.
119
Compared to other possible drug carrier systems, nanoemulsions offer increased drug bioavailability, improved drug absorption, rapid drug penetration with the protection of medicament from chemical reactions like hydrolysis and oxidation.
120
Nanoemulsions allow the entrapment of hydrophilic and lipophilic drug particles. Besides, it is easy to adjust the dose of the drugs without altering the efficacy of the system.
121
Manufacturing of nanoemulsions can often be allotted as an intricate process as precise temperature and pH conditions have to be maintained to ensure the stability of the preparation. Along with that, the excipients used especially the surfactant concentrations need to be adjusted so as to be non-toxic to the human tissues.
122
Dorzolamide hydrochloride loaded in situ gel nanoemulsion is developed by Ammar et al. Nanoemulsion form of the drug is found to have longer ocular residence time and therapeutic potential when compared with the free drug solution. This formulation offers a lesser frequency of instillation of dorzolamide and intensified the action of the medication.
123
Shah et al formulated nanoemulsion loaded with moxifloxacin. In vivo studies are conducted on rabbit models to evaluate the pharmacokinetic properties. The formulation showed good tolerance to toxicity, improved antibacterial efficacy and prolonged ocular availability. The emulsion system exhibited appropriate viscosity and improved stability.
124
Nanoemulsion based electrolyte triggered in situ gel for ocular delivery of acetazolamide is reported by Morsi et al. In the work acetazolamide loaded nanoemulsions is prepared using peanut oil, tween 8- and or cremophor EL along with transcutol P or propylene glycol as co-surfactant. This is incorporated into an in-situ gelling system using gellan/xanthan. Formulation exhibited better stability and improved IOP lowering effect when compared with the marketed formulation of brinzolamide and oral acetazolamide tablets.
125
Nanocapsules
Nanocapsules are a drug delivery system in which the drug is enclosed inside a polymeric membrane or protective matrix with size starting from 10 nm. The drug is entrapped, adsorbed or dissolved into the matrix system.
126
It is one of the efficient means of drug delivery system and is considered more advantageous than the free drug counterparts with improved bioavailability, sustained release, decreased toxicity and greater reproducibility. Nanocapsules markedly reduce the drug content required to acquire maximum therapeutic efficacy. Delivery of the drugs to the targeted sites at a decreased dose avoids many of the major side effects without compromising the intended therapeutic effectiveness.
127
Lee et alused poly (ɛ-caprolactone) nanocapsule carriers to develop a sustained release nanoformulation of pilocarpine (PILO PCL NC). The release time of the drug from the capsular barrier is said to be over a period of 42 days. In comparison with pilocarpine loaded poly ɛ-caprolactone nanospheres, PILO PCL NC is observed to be almost 3 times higher in pilocarpine loading efficiency with a sustained pattern of drug release when tested in vivo. It showed a long-term effect in suppressing the pressure-related ocular damage in the retinal and corneal regions of the eye.
128
Katzer et al developed prednisolone loaded nanocapsules for the treatment of ocular inflammatory disorders. The formulation has an encapsulation efficacy of 50% with the particle size ranging from 100-300 nm. The study results concluded that the nano model can regulate the release of prednisolone in a controlled manner for 5 hours without causing any significant irritation to the eye.
129
Desai et alformulated pluronic F127 based ocular delivery system with biodegradable poly isobutyl cyanoacrylate nanocapsules for pilocarpine by interfacial polymerization technique. The formulation can deliver the drug for longer periods with increased effectiveness. The gel-like property of formulation provides sufficient adhesiveness and thereby improving the contact time with the cornea and hence bioavailability of pilocarpine.
130
Dendrimers
These are symmetrically shaped nanosized particles having a particle size between 1-100 nm, with reactive end group which develops into an internal cavity encapsulating the drug molecules.
131,132
Drug delivery using dendrimers can be carried out by either enclosing a lipophilic moiety within a hydrophobic cavity or by attaching drugs onto the dendrimer surface.
133,134
The dendrimeric structure consists of three parts: (a) Initiator core (b) Interior layers (c) Exterior functionality.
135,136
Dendrimers are advantageous as it possesses a lower polydispersity index and the hollow core is available inside the dendrimeric structure which can be utilized for entrapping drug moieties. It also helps to target the drug delivery.
137-139
Holden et al prepared poly amido amine dendrimer hydrogel for enhanced delivery of antiglaucoma drugs brimonidine and timolol maleate. Mucoadhesiveness and non-toxic nature of the dendrimer hydrogel makes it a suitable nanoformulation for ocular application. A sustained release of drug is observed for 56-72 hours. Overall dendrimer hydrogel formulations are found to increase the efficacy of the selected anti-glaucoma drugs.
140
Dendrimer based targeted intravitreal therapy for attenuation of neuroinflammation and retinal degeneration using polyamidoamine drug conjugate nanodevice is developed by Iezzi et al. The drug (fluocinolone) is released for a period of 90 days from the nanodevice and is observed to be better than the free drug solution.
141
DenTimol as a dendrimeric timolol analogue for glaucoma therapy is developed by Lancina et al. A significant decrease in the IOP (approximately 30% reduction from baseline) is observed 30 minutes after the instillation of the drug topically to Brown Norway male rats, without exhibiting any toxicity.
142
Hydrogel
These are highly absorbable natural or synthetic polymers formed as a three-dimensional network. The flexibility of the polymer matrix is achieved by providing sufficient water.
143
Hydrogels swell when exposed to an aqueous environment. The three-dimensional crosslinks render these structures insoluble in water because of anionic interactions and hydrogen bonds.
136,144
Hydrogels that releases drugs or undergo a change in their phase in response to a stimulus are widely studied.
145
Porosity of the gel allows the timely release of the drug from the matrix structure. When used in topical ocular formulations, the hydrogels are capable to maintain muco-adhesiveness and improved ocular residence time to permit adequate action.
146
Jung et al investigated the timolol nanoparticle loaded silicone hydrogel contact lenses for prolonged drug delivery. In the formulation, the ester bond of timolol-propoxylated glyceryl triacylate (PGT) particle is hydrolysed slowly resulting in an extended-release. The in vivo studies confirmed the extended-release of timolol with improved efficacy and safety.
147
Kabiri et al investigated an in situ forming; Cannabigerolic acid (CBGA) loaded nanoparticle - laden hydrogel for ocular drug delivery in a controlled manner. CBGA is synthesized by genetic engineering methods from E. coli. A 300% increase in trans corneal penetration is achieved with the use of approximately 0.015% of the CBGA. The results suggested that the formulation would coat the corneal surface by blinking.
148
Age related macular degeneration and proliferative diabetic retinopathy is treated by Avastin® (bevacizumab). Xu et alstudied the sustained release of avastin from polysaccharides cross-linked hydrogels for ocular drug delivery. To reduce the multiple application of the drug into the eye, it is modified into a hydrogel using glycol chitosan and oxidized alginate. In vitro studies revealed a sustained release profile with an initial burst release for 4 hours followed by a decrease in release rate.
149
Maulvi et aldeveloped a novel implantation hydrogel contact lenses for controlled drug delivery of timolol maleate. In vitro release studies revealed that the implant contact lens can maintain a sustained release of drug within the therapeutic window. The implant contact lenses need to be stored at a dry state and need to be hydrated before its use to preserve the integrity of the formulation. In vivo experiments revealed the sustained drug release in tear fluid for a period of more than 192 h with IOP reduction without any ocular toxicity and irritancy.
150
Hsiao et aldeveloped a depot injectable formulation to sustain the release of latanoprost for the management of glaucoma. When tested with the rabbit glaucoma model, the formulation is found to be able to lower IOP at a stable rate for a period of 40 days. The results obtained from the study prove the suitability of the system to undergo further clinical trials.
151
Niosomes and proniosomes
These are prepared by self-assembling the hydrated non-ionic surfactant molecules to form bilayers. The medication is encapsulated inside a vesicle surrounded by the bilayer.
152,153
The structure of the niosomes are composed of non-ionic surfactant, cholesterol and a charge inducing molecule.
154,155
Proniosomes are free-flowing, dry formulations of surfactant coated carriers which on hydration provides the multilamellar niosomes and provides better stability on storage, handling, transportation etc compared to niosomes.
156,157
Proniosomes avoids the stability related problems like aggregation, fusion, and hydrolysis of the drug which is entrapped within it and improve the shelf life of the loaded moiety.
158,159
Proniosomal gel-derived niosomes to sustain and improve the ocular delivery of brimonidine tartrate is formulated by Emad Eldeeb et al. A higher entrapment of brimonidine tartrate with sustained release profile over 24 hours is observed. The in vivopharmacodynamic study of the formulation demonstrated the improved bioavailability and sustained drug release without irritation on rabbit.
160
Research conducted by Fouda et al revealed a sustained delivery of Dorzolamide –HCl via proniosomal gel formulation. The formulation exhibited a significant reduction in IOP and increased ocular availability of dorzolamide in comparison with the marketed eye drops.
161
Proniosomes are employed for the delivery of Lomefloxacin hydrochloride for the treatment of bacterial conjunctivitis. The proniosomal gel preparations of the drug increased the therapeutic efficacy, the penetration properties and retention time of the loaded moiety. The entrapment efficiency of the formulation is found to be greater than 80%, a controlled drug release rate for 12 hours is observed and is found to be stable for three months when tested in vitro.
162
Conclusion
Glaucoma is a chronic ailment that poses a great threat to vision, if not identified and treated immediately. Early diagnosis of the disease and maintaining strict medication adherence is necessary to prevent the advancement of the disease. In this review, a brief outline is given regarding glaucoma, with its pathology and treatment methods available. The review focuses on newer delivery approaches available for glaucoma with their advantages. The current treatment strategy for glaucoma is based on the sole reduction in the IOP by topically applying agents like prostaglandins, alpha agonists, carbonic anhydrase inhibitors etc. Certain new and emerging technologies involving the use of nanomedicine are being developed which may prove beneficial to the diseased society.
Ethical Issues
Not applicable
Conflict of Interest
The authors have no affiliation or financial conflict with the subject of materials discussed in the manuscript with any of the organization or entity.
References
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet 2004; 363(9422):1711-20. doi: 10.1016/s0140-6736(04)16257-0 [Crossref] [ Google Scholar]
- Nair RV, Nair SC, Anoop KR. Current trends in ocular drug delivery systems and its applications. Res J Pharm Technol 2015; 8(5):629-36. doi: 10.5958/0974-360x.2015.00101.8 [Crossref] [ Google Scholar]
-
Tortora GJ, Derrickson BH. Principles of Anatomy and Physiology. John Wiley & Sons; 2018.
-
Howland RD, Mycek MJ, Harvey RA, Champe PC. Lippincott’s Illustrated Reviews: Pharmacology. Philadelphia: Lippincott Williams & Wilkins; 2006.
- Willoughby CE, Ponzin D, Ferrari S, Lobo A, Landau K, Omidi Y. Anatomy and physiology of the human eye: effects of mucopolysaccharidoses disease on structure and function–a review. Clin Exp Ophthalmol 2010; 38(Suppl 1):2-11. doi: 10.1111/j.1442-9071.2010.02363.x [Crossref] [ Google Scholar]
-
Kolb H. Gross anatomy of the eye. In: Kolb H, Fernandez E, Nelson R, eds. Webvision: The Organization of the Retina and Visual System. Salt Lake City, UT: University of Utah Health Sciences Center; 1995.
- Malhotra A, Minja FJ, Crum A, Burrowes D. Ocular anatomy and cross-sectional imaging of the eye. Semin Ultrasound CT MR 2011; 32(1):2-13. doi: 10.1053/j.sult.2010.10.009 [Crossref] [ Google Scholar]
-
Rehman I, Hazhirkarzar B, Patel BC. Anatomy, head and neck, eye. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019.
- Kels BD, Grzybowski A, Grant-Kels JM. Human ocular anatomy. Clin Dermatol 2015; 33(2):140-6. doi: 10.1016/j.clindermatol.2014.10.006 [Crossref] [ Google Scholar]
- Hughes MO. A pictorial anatomy of the human eye/anophthalmic socket: a review for ocularists. Eye 2007; 4(5):51-63. [ Google Scholar]
- Gupta N, Yücel YH. Glaucoma as a neurodegenerative disease. Curr Opin Ophthalmol 2007; 18(2):110-4. doi: 10.1097/ICU.0b013e3280895aea [Crossref] [ Google Scholar]
- Nair RV, S S, Suresh A, K RA, Nair SC. Sustained release timolol maleate loaded ocusert based on biopolymer composite. Int J Biol Macromol 2018; 110:308-17. doi: 10.1016/j.ijbiomac.2018.01.029 [Crossref] [ Google Scholar]
- Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med 2009; 360(11):1113-24. doi: 10.1056/NEJMra0804630 [Crossref] [ Google Scholar]
-
Types of Glaucoma. Glaucoma Research Foundation; 2019. Available from: https://www.glaucoma.org/glaucoma/types-of-glaucoma.php. Accessed 24 October 2019.
-
Types of Glaucoma. Glaucoma UK. Available from: https://www.glaucoma-association.com/about-glaucoma/types-of-glaucoma/. Accessed 27 October 2019.
- Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA 2014; 311(18):1901-11. doi: 10.1001/jama.2014.3192 [Crossref] [ Google Scholar]
- Vohra R, Tsai JC, Kolko M. The role of inflammation in the pathogenesis of glaucoma. Surv Ophthalmol 2013; 58(4):311-20. doi: 10.1016/j.survophthal.2012.08.010 [Crossref] [ Google Scholar]
- Levin LA. Retinal ganglion cells and neuroprotection for glaucoma. Surv Ophthalmol 2003; 48 Suppl 1:S21-4. doi: 10.1016/s0039-6257(03)00007-9 [Crossref] [ Google Scholar]
- Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retin Eye Res 2012; 31(6):702-19. doi: 10.1016/j.preteyeres.2012.07.001 [Crossref] [ Google Scholar]
- Izzotti A, Bagnis A, Saccà SC. The role of oxidative stress in glaucoma. Mutat Res 2006; 612(2):105-14. doi: 10.1016/j.mrrev.2005.11.001 [Crossref] [ Google Scholar]
- Chrysostomou V, Rezania F, Trounce IA, Crowston JG. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr Opin Pharmacol 2013; 13(1):12-5. doi: 10.1016/j.coph.2012.09.008 [Crossref] [ Google Scholar]
- Saccà SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res 2007; 84(3):389-99. doi: 10.1016/j.exer.2006.10.008 [Crossref] [ Google Scholar]
- Wang N, Chintala SK, Fini ME, Schuman JS. Ultrasound activates the TM ELAM-1/IL-1/NF-kappaB response: a potential mechanism for intraocular pressure reduction after phacoemulsification. Invest Ophthalmol Vis Sci 2003; 44(5):1977-81. doi: 10.1167/iovs.02-0631 [Crossref] [ Google Scholar]
- Tezel G. Immune regulation toward immunomodulation for neuroprotection in glaucoma. Curr Opin Pharmacol 2013; 13(1):23-31. doi: 10.1016/j.coph.2012.09.013 [Crossref] [ Google Scholar]
- Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res 2005; 24(5):612-37. doi: 10.1016/j.preteyeres.2004.10.003 [Crossref] [ Google Scholar]
- Allingham RR, Liu Y, Rhee DJ. The genetics of primary open-angle glaucoma: a review. Exp Eye Res 2009; 88(4):837-44. doi: 10.1016/j.exer.2008.11.003 [Crossref] [ Google Scholar]
- Vajaranant TS, Nayak S, Wilensky JT, Joslin CE. Gender and glaucoma: what we know and what we need to know. Curr Opin Ophthalmol 2010; 21(2):91-9. doi: 10.1097/ICU.0b013e3283360b7e [Crossref] [ Google Scholar]
- Lee DA, Higginbotham EJ. Glaucoma and its treatment: a review. Am J Health Syst Pharm 2005; 62(7):691-9. doi: 10.1093/ajhp/62.7.691 [Crossref] [ Google Scholar]
- Cohen LP, Pasquale LR. Clinical characteristics and current treatment of glaucoma. Cold Spring Harb Perspect Med 2014; 4(6). doi: 10.1101/cshperspect.a017236 [Crossref]
- McKinnon SJ, Goldberg LD, Peeples P, Walt JG, Bramley TJ. Current management of glaucoma and the need for complete therapy. Am J Manag Care 2008; 14(1 Suppl):S20-7. [ Google Scholar]
- Kumarasamy NA, Lam FS, Wang AL, Theoharides TC. Glaucoma: current and developing concepts for inflammation, pathogenesis and treatment. Eur J Inflamm 2006; 4(3):129-37. doi: 10.1177/1721727x0600400301 [Crossref] [ Google Scholar]
- Schwartz K, Budenz D. Current management of glaucoma. Curr Opin Ophthalmol 2004; 15(2):119-26. doi: 10.1097/00055735-200404000-00011 [Crossref] [ Google Scholar]
- Bagnis A, Papadia M, Scotto R, Traverso CE. Current and emerging medical therapies in the treatment of glaucoma. Expert Opin Emerg Drugs 2011; 16(2):293-307. doi: 10.1517/14728214.2011.563733 [Crossref] [ Google Scholar]
- Beidoe G, Mousa SA. Current primary open-angle glaucoma treatments and future directions. Clin Ophthalmol 2012; 6:1699-707. doi: 10.2147/opth.s32933 [Crossref] [ Google Scholar]
- Conlon R, Saheb H, Ahmed II. Glaucoma treatment trends: a review. Can J Ophthalmol 2017; 52(1):114-24. doi: 10.1016/j.jcjo.2016.07.013 [Crossref] [ Google Scholar]
- Mendrinos E, Mermoud A, Shaarawy T. Nonpenetrating glaucoma surgery. Surv Ophthalmol 2008; 53(6):592-630. doi: 10.1016/j.survophthal.2008.08.023 [Crossref] [ Google Scholar]
- Pajic B, Pajic-Eggspuehler B, Haefliger I, Hafezi F. The high-frequency deep sclerotomy glaucoma procedure. European Ophthalmic Review 2012; 6(1):20. doi: 10.17925/eor.2012.06.01.20 [Crossref] [ Google Scholar]
- Tsai JC, Kanner EM. Current and emerging medical therapies for glaucoma. Expert Opin Emerg Drugs 2005; 10(1):109-18. doi: 10.1517/14728214.10.1.109 [Crossref] [ Google Scholar]
- Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev 2006; 58(11):1131-5. doi: 10.1016/j.addr.2006.07.027 [Crossref] [ Google Scholar]
- Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J 2010; 12(3):348-60. doi: 10.1208/s12248-010-9183-3 [Crossref] [ Google Scholar]
- Barar J, Javadzadeh AR, Omidi Y. Ocular novel drug delivery: impacts of membranes and barriers. Expert Opin Drug Deliv 2008; 5(5):567-81. doi: 10.1517/17425247.5.5.567 [Crossref] [ Google Scholar]
- Kuno N, Fujii S. Recent advances in ocular drug delivery systems. Polymers 2011; 3(1):193-221. doi: 10.3390/polym3010193 [Crossref] [ Google Scholar]
- Ghate D, Edelhauser HF. Barriers to glaucoma drug delivery. J Glaucoma 2008; 17(2):147-56. doi: 10.1097/IJG.0b013e31814b990d [Crossref] [ Google Scholar]
- Kwatra D, Mitra AK. Drug delivery in ocular diseases: barriers and strategies. World J Pharmacol 2013; 2(4):78-83. doi: 10.5497/wjp.v2.i4.78 [Crossref] [ Google Scholar]
- Davies NM. Biopharmaceutical considerations in topical ocular drug delivery. Clin Exp Pharmacol Physiol 2000; 27(7):558-62. doi: 10.1046/j.1440-1681.2000.03288.x [Crossref] [ Google Scholar]
- Liu S, Jones L, Gu FX. Nanomaterials for ocular drug delivery. Macromol Biosci 2012; 12(5):608-20. doi: 10.1002/mabi.201100419 [Crossref] [ Google Scholar]
- Kaufman PL, Rasmussen CA. Advances in glaucoma treatment and management: outflow drugs. Invest Ophthalmol Vis Sci 2012; 53(5):2495-500. doi: 10.1167/iovs.12-9483m [Crossref] [ Google Scholar]
- Clark AF, Pang IH. Advances in glaucoma therapeutics. Expert Opin Emerg Drugs 2002; 7(1):141-63. doi: 10.1517/14728214.7.1.141 [Crossref] [ Google Scholar]
- Gupta SK, Niranjan DG, Agrawal SS, Srivastava S, Saxena R. Recent advances in pharmacotherapy of glaucoma. Indian J Pharmacol 2008; 40(5):197-208. doi: 10.4103/0253-7613.44151 [Crossref] [ Google Scholar]
- Kaushik S, Pandav SS, Ram J. Neuroprotection in glaucoma. J Postgrad Med 2003; 49(1):90-5. doi: 10.4103/0022-3859.917 [Crossref] [ Google Scholar]
- Baltmr A, Duggan J, Nizari S, Salt TE, Cordeiro MF. Neuroprotection in glaucoma–is there a future role?. Exp Eye Res 2010; 91(5):554-66. doi: 10.1016/j.exer.2010.08.009 [Crossref] [ Google Scholar]
- Vasudevan SK, Gupta V, Crowston JG. Neuroprotection in glaucoma. Indian J Ophthalmol 2011; 59(Suppl 1):S102-13. doi: 10.4103/0301-4738.73700 [Crossref] [ Google Scholar]
-
Bagli E, Kitsos G. Neuroprotective agents in glaucoma. In: Kubena T, ed. The Mystery of Glaucoma. Rijeka: InTech; 2011. p. 115-44. 10.5772/19067
- Rahman MQ, Ramaesh K, Montgomery DM. Brimonidine for glaucoma. Expert Opin Drug Saf 2010; 9(3):483-91. doi: 10.1517/14740331003709736 [Crossref] [ Google Scholar]
- WoldeMussie E, Ruiz G, Wijono M, Wheeler LA. Neuroprotection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension. Invest Ophthalmol Vis Sci 2001; 42(12):2849-55. [ Google Scholar]
- Ferencz JR, Gilady G, Harel O, Belkin M, Assia EI. Topical brimonidine reduces collateral damage caused by laser photocoagulation for choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 2005; 243(9):877-80. doi: 10.1007/s00417-005-1160-7 [Crossref] [ Google Scholar]
- Roberti G, Tanga L, Michelessi M, Quaranta L, Parisi V, Manni G. Cytidine 5’-diphosphocholine (citicoline) in glaucoma: rationale of its use, current evidence and future perspectives. Int J Mol Sci 2015; 16(12):28401-17. doi: 10.3390/ijms161226099 [Crossref] [ Google Scholar]
- Nucci C, Martucci A, Giannini C, Morrone LA, Bagetta G, Mancino R. Neuroprotective agents in the management of glaucoma. Eye 2018; 32(5):938-45. doi: 10.1038/s41433-018-0050-2 [Crossref] [ Google Scholar]
- Oshitari T, Fujimoto N, Adachi-Usami E. Citicoline has a protective effect on damaged retinal ganglion cells in mouse culture retina. Neuroreport 2002; 13(16):2109-11. doi: 10.1097/00001756-200211150-00023 [Crossref] [ Google Scholar]
- Ottobelli L, Manni GL, Centofanti M, Iester M, Allevena F, Rossetti L. Citicoline oral solution in glaucoma: is there a role in slowing disease progression?. Ophthalmologica 2013; 229(4):219-26. doi: 10.1159/000350496 [Crossref] [ Google Scholar]
- Cybulska-Heinrich AK, Mozaffarieh M, Flammer J. Ginkgo biloba: an adjuvant therapy for progressive normal and high tension glaucoma. Mol Vis 2012; 18:390-402. [ Google Scholar]
- Rhee DJ, Katz LJ, Spaeth GL, Myers JS. Complementary and alternative medicine for glaucoma. Surv Ophthalmol 2001; 46(1):43-55. doi: 10.1016/s0039-6257(01)00233-8 [Crossref] [ Google Scholar]
- Quaranta L, Riva I, Floriani I. Ginkgo biloba extract improves visual field damage in some patients affected by normal-tension glaucoma. Invest Ophthalmol Vis Sci 2014; 55(4):2417. doi: 10.1167/iovs.14-13942 [Crossref] [ Google Scholar]
- Mozaffarieh M, Grieshaber MC, Orgül S, Flammer J. The potential value of natural antioxidative treatment in glaucoma. Surv Ophthalmol 2008; 53(5):479-505. doi: 10.1016/j.survophthal.2008.06.006 [Crossref] [ Google Scholar]
- Mozaffarieh M, Flammer J. A novel perspective on natural therapeutic approaches in glaucoma therapy. Expert Opin Emerg Drugs 2007; 12(2):195-8. doi: 10.1517/14728214.12.2.195 [Crossref] [ Google Scholar]
- Lee D, Shim MS, Kim KY, Noh YH, Kim H, Kim SY. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Invest Ophthalmol Vis Sci 2014; 55(2):993-1005. doi: 10.1167/iovs.13-12564 [Crossref] [ Google Scholar]
- Cordeiro M, Guo L, Cheung W, Wood N, Salt TE. Topical CoQ10 is neuroprotective in experimental glaucoma. Invest Ophthalmol Vis Sci 2007; 48(13):4369. [ Google Scholar]
- Abu-Amero KK, Kondkar AA, Chalam KV. Resveratrol and ophthalmic diseases. Nutrients 2016; 8(4):200. doi: 10.3390/nu8040200 [Crossref] [ Google Scholar]
- Luna C, Li G, Liton PB, Qiu J, Epstein DL, Challa P. Resveratrol prevents the expression of glaucoma markers induced by chronic oxidative stress in trabecular meshwork cells. Food Chem Toxicol 2009; 47(1):198-204. doi: 10.1016/j.fct.2008.10.029 [Crossref] [ Google Scholar]
- Pirhan D, Yüksel N, Emre E, Cengiz A, Kürşat Yıldız D. Riluzole- and resveratrol-induced delay of retinal ganglion cell death in an experimental model of glaucoma. Curr Eye Res 2016; 41(1):59-69. doi: 10.3109/02713683.2015.1004719 [Crossref] [ Google Scholar]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014; 121(11):2081-90. doi: 10.1016/j.ophtha.2014.05.013 [Crossref] [ Google Scholar]
- Wagh VD, Inamdar B, Samanta MK. Polymers used in ocular dosage form and drug delivery systems. Asian J Pharm 2014; 2(1):12-7. [ Google Scholar]
- Bucolo C, Drago F, Salomone S. Ocular drug delivery: a clue from nanotechnology. Front Pharmacol 2012; 3:188. doi: 10.3389/fphar.2012.00188 [Crossref] [ Google Scholar]
- Alhalafi AM. Applications of polymers in intraocular drug delivery systems. Oman J Ophthalmol 2017; 10(1):3-8. doi: 10.4103/0974-620x.200692 [Crossref] [ Google Scholar]
- Tsai CH, Wang PY, Lin IC, Huang H, Liu GS, Tseng CL. Ocular drug delivery: role of degradable polymeric nanocarriers for ophthalmic application. Int J Mol Sci 2018; 19(9):2830. doi: 10.3390/ijms19092830 [Crossref] [ Google Scholar]
- Rajasekaran A, Kumaran K, Preetha JP, Karthika K. A comparative review on conventional and advanced ocular drug delivery formulations. Int J Pharmtech Res 2010; 2(1):668-74. [ Google Scholar]
- Jin YJ, Ubonvan T, Kim DD. Hyaluronic acid in drug delivery systems. J Pharm Investig 2010; 40:33-43. doi: 10.4333/kps.2010.40.s.033 [Crossref] [ Google Scholar]
- Yadav AK, Mishra P, Agrawal GP. An insight on hyaluronic acid in drug targeting and drug delivery. J Drug Target 2008; 16(2):91-107. doi: 10.1080/10611860802095494 [Crossref] [ Google Scholar]
- Sultana Y, Jain R, Aqil M, Ali A. Review of ocular drug delivery. Curr Drug Deliv 2006; 3(2):207-17. doi: 10.2174/156720106776359186 [Crossref] [ Google Scholar]
- Alonso MJ, Sánchez A. The potential of chitosan in ocular drug delivery. J Pharm Pharmacol 2003; 55(11):1451-63. doi: 10.1211/0022357022476 [Crossref] [ Google Scholar]
- Panonnummal R, Jayakumar R, Sabitha M. Comparative anti-psoriatic efficacy studies of clobetasol loaded chitin nanogel and marketed cream. Eur J Pharm Sci 2017; 96:193-206. doi: 10.1016/j.ejps.2016.09.007 [Crossref] [ Google Scholar]
- de la Fuente M, Raviña M, Paolicelli P, Sanchez A, Seijo B, Alonso MJ. Chitosan-based nanostructures: a delivery platform for ocular therapeutics. Adv Drug Deliv Rev 2010; 62(1):100-17. doi: 10.1016/j.addr.2009.11.026 [Crossref] [ Google Scholar]
- Bernkop-Schnürch A, Dünnhaupt S. Chitosan-based drug delivery systems. Eur J Pharm Biopharm 2012; 81(3):463-9. doi: 10.1016/j.ejpb.2012.04.007 [Crossref] [ Google Scholar]
- Paolicelli P, de la Fuente M, Sánchez A, Seijo B, Alonso MJ. Chitosan nanoparticles for drug delivery to the eye. Expert Opin Drug Deliv 2009; 6(3):239-53. doi: 10.1517/17425240902762818 [Crossref] [ Google Scholar]
- Nagpal K, Singh SK, Mishra DN. Chitosan nanoparticles: a promising system in novel drug delivery. Chem Pharm Bull (Tokyo) 2010; 58(11):1423-30. doi: 10.1248/cpb.58.1423 [Crossref] [ Google Scholar]
- Goyal G, Garg T, Rath G, Goyal AK. Current nanotechnological strategies for treating glaucoma. Crit Rev Ther Drug Carrier Syst 2014; 31(5):365-405. doi: 10.1615/critrevtherdrugcarriersyst.2014010123 [Crossref] [ Google Scholar]
- Wadhwa S, Paliwal R, Paliwal SR, Vyas SP. Chitosan and its role in ocular therapeutics. Mini Rev Med Chem 2009; 9(14):1639-47. doi: 10.2174/138955709791012292 [Crossref] [ Google Scholar]
- Lee CH, Singla A, Lee Y. Biomedical applications of collagen. Int J Pharm 2001; 221(1-2):1-22. doi: 10.1016/s0378-5173(01)00691-3 [Crossref] [ Google Scholar]
- Almeida H, Amaral MH, Lobão P, Sousa Lobo JM. Applications of poloxamers in ophthalmic pharmaceutical formulations: an overview. Expert Opin Drug Deliv 2013; 10(9):1223-37. doi: 10.1517/17425247.2013.796360 [Crossref] [ Google Scholar]
- Hsu KH, Gause S, Chauhan A. Review of ophthalmic drug delivery by contact lenses. J Drug Deliv Sci Technol 2014; 24(2):123-35. doi: 10.1016/s1773-2247(14)50021-4 [Crossref] [ Google Scholar]
- Maulvi FA, Soni TG, Shah DO. A review on therapeutic contact lenses for ocular drug delivery. Drug Deliv 2016; 23(8):3017-26. doi: 10.3109/10717544.2016.1138342 [Crossref] [ Google Scholar]
- Guzman-Aranguez A, Colligris B, Pintor J. Contact lenses: promising devices for ocular drug delivery. J Ocul Pharmacol Ther 2013; 29(2):189-99. doi: 10.1089/jop.2012.0212 [Crossref] [ Google Scholar]
- Lavik E, Kuehn MH, Kwon YH. Novel drug delivery systems for glaucoma. Eye (Lond) 2011; 25(5):578-86. doi: 10.1038/eye.2011.82 [Crossref] [ Google Scholar]
- Peng CC, Burke MT, Carbia BE, Plummer C, Chauhan A. Extended drug delivery by contact lenses for glaucoma therapy. J Control Release 2012; 162(1):152-8. doi: 10.1016/j.jconrel.2012.06.017 [Crossref] [ Google Scholar]
- Hsu KH, Carbia BE, Plummer C, Chauhan A. Dual drug delivery from vitamin E loaded contact lenses for glaucoma therapy. Eur J Pharm Biopharm 2015; 94:312-21. doi: 10.1016/j.ejpb.2015.06.001 [Crossref] [ Google Scholar]
- Kaur IP, Kanwar M. Ocular preparations: the formulation approach. Drug Dev Ind Pharm 2002; 28(5):473-93. doi: 10.1081/ddc-120003445 [Crossref] [ Google Scholar]
- Sikandar M, Sharma PK, Visht S. Ocular drug delivery system: an overview. Int J Pharm Sci Res 2011; 2(5):1168-75. [ Google Scholar]
- Nisha S, Deepak K. An insight to ophthalmic drug delivery system. Int J Pharm Sci Res 2012; 3(2):9-13. [ Google Scholar]
- Rathore KS, Nema RK. Review on ocular inserts. Int J Pharmtech Res 2009; 1(2):164-9. [ Google Scholar]
- Eljarrat-Binstock E, Domb AJ. Iontophoresis: a non-invasive ocular drug delivery. J Control Release 2006; 110(3):479-89. doi: 10.1016/j.jconrel.2005.09.049 [Crossref] [ Google Scholar]
- Semalty A, Semalty M, Singh R, Saraf SK, Saraf S. Iontophoretic drug delivery system: a review. Technol Health Care 2007; 15(4):237-45. doi: 10.3233/thc-2007-15402 [Crossref] [ Google Scholar]
- Shoeibi N, Mahdizadeh M, Shafiee M. Iontophoresis in ophthalmology: a review of the literature. Rev Clin Med 2014; 1(4):183-8. doi: 10.17463/rcm.2014.04.003 [Crossref] [ Google Scholar]
- Sharma S, Parvez N, Sharma PK. Iontophoresis--models and applications: a review. Afr J Basic Appl Sci 2015; 7(1):1-7. doi: 10.5829/idosi.ajbas.2015.7.1.9236 [Crossref] [ Google Scholar]
- Patane MA, Cohen AE, Sheppard JD, Nguyen QD. Ocular iontophoresis for drug delivery. Retina Today 2011; 6:64-6. [ Google Scholar]
- Mudgil M, Gupta N, Nagpal M, Pawar P. Nanotechnology: a new approach for ocular drug delivery system. Int J Pharm Pharm Sci 2012; 4(2):105-12. [ Google Scholar]
- Aldrich DS, Bach CM, Brown W, Chambers W, Fleitman J, Hunt D. Ophthalmic preparations. US Pharmacopeia 2013; 39(5):1-21. [ Google Scholar]
- Vandervoort J, Ludwig A. Ocular drug delivery: nanomedicine applications. Nanomedicine (Lond) 2007; 2(1):11-21. doi: 10.2217/17435889.2.1.11 [Crossref] [ Google Scholar]
- Raju HB, Goldberg JL. Nanotechnology for ocular therapeutics and tissue repair. Expert Rev Ophthalmol 2008; 3(4):431-6. doi: 10.1586/17469899.3.4.431 [Crossref] [ Google Scholar]
- Araújo J, Gonzalez E, Egea MA, Garcia ML, Souto EB. Nanomedicines for ocular NSAIDs: safety on drug delivery. Nanomedicine 2009; 5(4):394-401. doi: 10.1016/j.nano.2009.02.003 [Crossref] [ Google Scholar]
- Patel A, Cholkar K, Agrahari V, Mitra AK. Ocular drug delivery systems: an overview. World J Pharmacol 2013; 2(2):47-64. doi: 10.5497/wjp.v2.i2.47 [Crossref] [ Google Scholar]
- Trivedi R, Kompella UB. Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomedicine (Lond) 2010; 5(3):485-505. doi: 10.2217/nnm.10.10 [Crossref] [ Google Scholar]
- Vaishya RD, Khurana V, Patel S, Mitra AK. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2014; 6(5):422-37. doi: 10.1002/wnan.1272 [Crossref] [ Google Scholar]
- Singh P, Verma N. A review on impact of nanomicelle for ocular drug delivery system. Int J Pharm Sci Res 2018; 9(4):1397-404. doi: 10.13040/ijpsr.0975-8232.9(4).1397-04 [Crossref] [ Google Scholar]
- Grimaudo MA, Pescina S, Padula C, Santi P, Concheiro A, Alvarez-Lorenzo C. Topical application of polymeric nanomicelles in ophthalmology: a review on research efforts for the noninvasive delivery of ocular therapeutics. Expert Opin Drug Deliv 2019; 16(4):397-413. doi: 10.1080/17425247.2019.1597848 [Crossref] [ Google Scholar]
- Elmowafy E, Gad H, Biondo F, Casettari L, Soliman ME. Exploring optimized methoxy poly(ethylene glycol)-block-poly(ε-caprolactone) crystalline cored micelles in anti-glaucoma pharmacotherapy. Int J Pharm 2019; 566:573-84. doi: 10.1016/j.ijpharm.2019.06.011 [Crossref] [ Google Scholar]
- Cholkar K, Gunda S, Earla R, Pal D, Mitra AK. Nanomicellar topical aqueous drop formulation of rapamycin for back-of-the-eye delivery. AAPS PharmSciTech 2015; 16(3):610-22. doi: 10.1208/s12249-014-0244-2 [Crossref] [ Google Scholar]
- Guo C, Zhang Y, Yang Z, Li M, Li F, Cui F. Nanomicelle formulation for topical delivery of cyclosporine A into the cornea: in vitro mechanism and in vivo permeation evaluation. Sci Rep 2015; 5(1):12968. doi: 10.1038/srep12968 [Crossref] [ Google Scholar]
- Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ. Nano-emulsions. Curr Opin Colloid Interface Sci 2005; 10(3-4):102-10. doi: 10.1016/j.cocis.2005.06.004 [Crossref] [ Google Scholar]
- Mason TG, Wilking JN, Meleson K, Chang CB, Graves SM. Nanoemulsions: formation, structure, and physical properties. J Phys Condens Matter 2007; 19(7):079001. doi: 10.1088/0953-8984/19/7/079001 [Crossref] [ Google Scholar]
- Cholkar K, Patel SP, Vadlapudi AD, Mitra AK. Novel strategies for anterior segment ocular drug delivery. J Ocul Pharmacol Ther 2013; 29(2):106-23. doi: 10.1089/jop.2012.0200 [Crossref] [ Google Scholar]
- Reimondez-Troitiño S, Csaba N, Alonso MJ, de la Fuente M. Nanotherapies for the treatment of ocular diseases. Eur J Pharm Biopharm 2015; 95(Pt B):279-93. doi: 10.1016/j.ejpb.2015.02.019 [Crossref] [ Google Scholar]
- Thiagarajan P. Nanoemulsions for drug delivery through different routes. Res Biotechnol 2011; 2(3):1-13. [ Google Scholar]
- Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride. AAPS PharmSciTech 2009; 10(3):808-19. doi: 10.1208/s12249-009-9268-4 [Crossref] [ Google Scholar]
- Shah J, Nair AB, Jacob S, Patel RK, Shah H, Shehata TM. Nanoemulsion based vehicle for effective ocular delivery of moxifloxacin using experimental design and pharmacokinetic study in rabbits. Pharmaceutics 2019; 11(5):230. doi: 10.3390/pharmaceutics11050230 [Crossref] [ Google Scholar]
- Morsi N, Ibrahim M, Refai H, El Sorogy H. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of acetazolamide. Eur J Pharm Sci 2017; 104:302-14. doi: 10.1016/j.ejps.2017.04.013 [Crossref] [ Google Scholar]
-
Couvreur P, Gref R. Nanocapsules: preparation, characterization and therapeutic applications. In: Torchilin VP, ed. Nanoparticulates as Drug Carriers. World Scientific; 2006. p. 255-77.
- Yellepeddi VK, Palakurthi S. Recent advances in topical ocular drug delivery. J Ocul Pharmacol Ther 2016; 32(2):67-82. doi: 10.1089/jop.2015.0047 [Crossref] [ Google Scholar]
- Lee CH, Li YJ, Huang CC, Lai JY. Poly(ε-caprolactone) nanocapsule carriers with sustained drug release: single dose for long-term glaucoma treatment. Nanoscale 2017; 9(32):11754-64. doi: 10.1039/c7nr03221h [Crossref] [ Google Scholar]
- Katzer T, Chaves P, Bernardi A, Pohlmann A, Guterres SS, Ruver Beck RC. Prednisolone-loaded nanocapsules as ocular drug delivery system: development, in vitro drug release and eye toxicity. J Microencapsul 2014; 31(6):519-28. doi: 10.3109/02652048.2013.879930 [Crossref] [ Google Scholar]
- Desai SD, Blanchard J. Pluronic F127-based ocular delivery system containing biodegradable polyisobutylcyanoacrylate nanocapsules of pilocarpine. Drug Deliv 2000; 7(4):201-7. doi: 10.1080/107175400455128 [Crossref] [ Google Scholar]
- Wang Y, Xu X, Gu Y, Cheng Y, Cao F. Recent advance of nanoparticle-based topical drug delivery to the posterior segment of the eye. Expert Opin Drug Deliv 2018; 15(7):687-701. doi: 10.1080/17425247.2018.1496080 [Crossref] [ Google Scholar]
- Jain A, Dubey S, Kaushik A, Tyagi A. Dendrimer: a complete drug carrier. Int J Pharm Sci Res 2010; 1(4):38-52. doi: 10.13040/ijpsr.0975-8232.1(4).38-52 [Crossref] [ Google Scholar]
- Bharti JP, Prajapati SK, Jaiswal MK, Yadav RD. Dendrimer multifunctional nano-device: a review. Int J Pharm Sci Res 2011; 2(8):1947-60. doi: 10.13040/ijpsr.0975-8232.2(8).1947-60 [Crossref] [ Google Scholar]
- Kandekar U, Chaudhari PD, Tambe VS, Vichare VS, Dhole SN. Dendrimers: novel drug nanocarriers. Int J Pharm Sci Res 2011; 2(5):1086-98. doi: 10.13040/ijpsr.0975-8232.(5).1086-98 [Crossref] [ Google Scholar]
- Toraskar MP, Pande VG, Kadam VJ. Dendrimer: a new approach in pharmacy. Int J Res Pharm Chem 2011; 1(4):1100-7. [ Google Scholar]
- Yadav KS, Rajpurohit R, Sharma S. Glaucoma: Current treatment and impact of advanced drug delivery systems. Life Sci 2019; 221:362-76. doi: 10.1016/j.lfs.2019.02.029 [Crossref] [ Google Scholar]
- Gondkar SB, Rasal SP, Saudagar RB. Dendrimer: a review. Asian J Pharm Res 2016; 6(3):186-90. doi: 10.5958/2231-5691.2016.00027.7 [Crossref] [ Google Scholar]
- Shinde MS, Bhalerao MB, Thakre S, Franklin J, Jain A. Dendrimers-an excellent polymer for drug delivery system. Asian J Pharm Res Dev 2014; 2(2):24-34. [ Google Scholar]
- Akbarzadeh A, Khalilov R, Mostafavi E, Annabi N, Abasi E, Kafshdooz T. Role of dendrimers in advanced drug delivery and biomedical applications: a review. Exp Oncol 2018; 40(3):178-83. [ Google Scholar]
- Holden CA, Tyagi P, Thakur A, Kadam R, Jadhav G, Kompella UB. Polyamidoamine dendrimer hydrogel for enhanced delivery of antiglaucoma drugs. Nanomedicine 2012; 8(5):776-83. doi: 10.1016/j.nano.2011.08.018 [Crossref] [ Google Scholar]
- Iezzi R, Guru BR, Glybina IV, Mishra MK, Kennedy A, Kannan RM. Dendrimer-based targeted intravitreal therapy for sustained attenuation of neuroinflammation in retinal degeneration. Biomaterials 2012; 33(3):979-88. doi: 10.1016/j.biomaterials.2011.10.010 [Crossref] [ Google Scholar]
- Lancina MG 3rd, Wang J, Williamson GS, Yang H. DenTimol as a dendrimeric timolol analogue for glaucoma therapy: synthesis and preliminary efficacy and safety assessment. Mol Pharm 2018; 15(7):2883-9. doi: 10.1021/acs.molpharmaceut.8b00401 [Crossref] [ Google Scholar]
- Achouri D, Alhanout K, Piccerelle P, Andrieu V. Recent advances in ocular drug delivery. Drug Dev Ind Pharm 2013; 39(11):1599-617. doi: 10.3109/03639045.2012.736515 [Crossref] [ Google Scholar]
- Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev 2012; 64 Suppl:18-23. doi: 10.1016/j.addr.2012.09.010 [Crossref] [ Google Scholar]
- Ramteke KH, Chavanke MS, Chavanke PS. Stimuli sensitive hydrogels in drug delivery systems. Int J Pharm Sci Res 2012; 3(12):4604-16. doi: 10.13040/ijpsr.0975-8232.3(12).4604-16 [Crossref] [ Google Scholar]
- Kirchhof S, Goepferich AM, Brandl FP. Hydrogels in ophthalmic applications. Eur J Pharm Biopharm 2015; 95(Pt B):227-38. doi: 10.1016/j.ejpb.2015.05.016 [Crossref] [ Google Scholar]
- Jung HJ, Abou-Jaoude M, Carbia BE, Plummer C, Chauhan A. Glaucoma therapy by extended release of timolol from nanoparticle loaded silicone-hydrogel contact lenses. J Control Release 2013; 165(1):82-9. doi: 10.1016/j.jconrel.2012.10.010 [Crossref] [ Google Scholar]
- Kabiri M, Kamal SH, Pawar SV, Roy PR, Derakhshandeh M, Kumar U. A stimulus-responsive, in situ-forming, nanoparticle-laden hydrogel for ocular drug delivery. Drug Deliv Transl Res 2018; 8(3):484-95. doi: 10.1007/s13346-018-0504-x [Crossref] [ Google Scholar]
- Xu X, Weng Y, Xu L, Chen H. Sustained release of Avastin® from polysaccharides cross-linked hydrogels for ocular drug delivery. Int J Biol Macromol 2013; 60:272-6. doi: 10.1016/j.ijbiomac.2013.05.034 [Crossref] [ Google Scholar]
- Maulvi FA, Soni TG, Shah DO. Effect of timolol maleate concentration on uptake and release from hydrogel contact lenses using soaking method. J Pharm Appl Sci 2014; 1(1):17-23. [ Google Scholar]
- Hsiao MH, Chiou SH, Larsson M, Hung KH, Wang YL, Liu CJ. A temperature-induced and shear-reversible assembly of latanoprost-loaded amphiphilic chitosan colloids: characterization and in vivo glaucoma treatment. Acta Biomater 2014; 10(7):3188-96. doi: 10.1016/j.actbio.2014.03.016 [Crossref] [ Google Scholar]
- Yadav JD, Kulkarni PR, Vaidya KA, Shelke GT. Niosomes: a review. J Pharm Res 2011; 4(3):632-6. [ Google Scholar]
- Rajitha P, Biswas R, Sabitha M, Jayakumar R. Methotrexate in the treatment of psoriasis and rheumatoid arthritis: mechanistic insights, current issues and novel delivery approaches. Curr Pharm Des 2017; 23(24):3550-66. doi: 10.2174/1381612823666170601105439 [Crossref] [ Google Scholar]
- Debnath A, Kumar A. Structural and functional significance of niosome and proniosome in drug delivery system. Int J Pharm Eng 2015; 3(3):621-37. [ Google Scholar]
- Hu C, Rhodes DG. Proniosomes: a novel drug carrier preparation. Int J Pharm 1999; 185(1):23-35. doi: 10.1016/s0378-5173(99)00122-2 [Crossref] [ Google Scholar]
- Yasam VR, Jakki SL, Natarajan J, Kuppusamy G. A review on novel vesicular drug delivery: proniosomes. Drug Deliv 2014; 21(4):243-9. doi: 10.3109/10717544.2013.841783 [Crossref] [ Google Scholar]
- Radha GV, Rani TS, Sarvani B. A review on proniosomal drug delivery system for targeted drug action. J Basic Clin Pharm 2013; 4(2):42-8. doi: 10.4103/0976-0105.113609 [Crossref] [ Google Scholar]
- Khatoon M, Shah KU, Din FU, Shah SU, Rehman AU, Dilawar N. Proniosomes derived niosomes: recent advancements in drug delivery and targeting. Drug Deliv 2017; 24(Suppl 1):56-69. doi: 10.1080/10717544.2017.1384520 [Crossref] [ Google Scholar]
- Jadhav KR, Pawar AY, Bachhav AA, Ahire SA. Proniosome: a novel non-ionic provesicules as potential drug carrier. Asian J Pharm 2016; 10(3):S210-22. doi: 10.22377/ajp.v10i03.757 [Crossref] [ Google Scholar]
- Emad Eldeeb A, Salah S, Ghorab M. Proniosomal gel-derived niosomes: an approach to sustain and improve the ocular delivery of brimonidine tartrate; formulation, in-vitro characterization, and in-vivo pharmacodynamic study. Drug Deliv 2019; 26(1):509-21. doi: 10.1080/10717544.2019.1609622 [Crossref] [ Google Scholar]
- Fouda NH, Abdelrehim RT, Hegazy DA, Habib BA. Sustained ocular delivery of Dorzolamide-HCl via proniosomal gel formulation: in-vitro characterization, statistical optimization, and in-vivo pharmacodynamic evaluation in rabbits. Drug Deliv 2018; 25(1):1340-9. doi: 10.1080/10717544.2018.1477861 [Crossref] [ Google Scholar]
- Khalil RM, Abdelbary GA, Basha M, Awad GE, El-Hashemy HA. Design and evaluation of proniosomes as a carrier for ocular delivery of lomefloxacin HCl. J Liposome Res 2017; 27(2):118-29. doi: 10.3109/08982104.2016.1167737 [Crossref] [ Google Scholar]