Advanced pharmaceutical bulletin. 11(4):712-718.
doi: 10.34172/apb.2021.080
Research Article
Combination of Estradiol with Leukemia Inhibitory Factor Stimulates Granulosa Cells Differentiation into Oocyte-Like Cells
Soudabe Yousefi 1, 2, 3  , Maryam Akbarzadeh 4
, Maryam Akbarzadeh 4  , Jafar Soleimanirad 1, Kobra Hamdi 5, Laya Farzadi 5, Aalie Ghasemzadeh 5, Mahdi Mahdipour 1, Reza Rahbarghazi 1, 5, , * #
, Jafar Soleimanirad 1, Kobra Hamdi 5, Laya Farzadi 5, Aalie Ghasemzadeh 5, Mahdi Mahdipour 1, Reza Rahbarghazi 1, 5, , * #  , Mohammad Nouri 1, 5, , * #
, Mohammad Nouri 1, 5, , * # 
Author information:
1Stem Cell Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
2Department of Biochemistry and Clinical Laboratories, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran.
3Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran.
4Stem Cell and Regenerative Medicine Institute, Tabriz University of Medical Sciences, Tabriz, Iran.
5Department of Applied Cell Sciences, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
6Women’s Reproductive Health Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
#These authors contributed equally to this work.
Abstract
Purpose:
Previous studies have documented that cumulus granulosa cells (GCs) can trans-differentiation into different non-ovarian cells, showing their multipotentiality to repopulate the injured cells in ovarian tissue. The current experiment is aimed to assess the differentiation capacity of human cumulus GCs toward the oocyte-like phenotype in vitro.
Methods:
GCs were isolated from healthy female volunteers subjected to in vitro fertilization or intra-cytoplasmic sperm injection (IVF-ICSI). The effect of different media supplemented with leukemia inhibitory factors (LIFs), 5 ng/mL estradiol, and 0.005 IU/mL follicle-stimulating hormone (FSH) were investigated to the differentiation of GCs toward oocyte-like phenotype via monitoring the expression of Oct3/4 and GATA-4 using flow cytometry analysis. The expression of genes such as FIGLA, NOBOX, and SYCP3 was measured by real-time polymerase chain reaction (PCR) assay. We also assess morphological adaptation by using bright-field microscopic imaging.
Results:
Exposure of GCs to LIFs increased the number of cells expressing stemness factor Oct3/4 coincided with the suppression of GATA-4 after 7 days (P < 0.05). We found that the transcript level of all genes FIGLA, Nobox, and SYCP-3 decreased in cells after treatment with a FSH (P < 0.05). According to our data, the incubation of GCs with estradiol increased the expression of genes related to the oocyte-like phenotype.
Conclusion:
Our finding revealed that the combination of LIFs and estradiol could induce the GCs’ oogenesis capacity and thereby is possibly suggested as a therapeutic strategy during the occurrence of gynecological disorders.
Keywords: Granulosa cells, Leukemia inhibitory factors, Follicle-stimulating hormone, Estradiol, Oocyte-like cells
Copyright and License Information
© 2021 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Infertility is a multifactorial disorder and induced by numerous environmental factors such as changes in lifestyle and nutritional habits.
1
Some conventional techniques such as in vitro fertilization/intra-cytoplasmic sperm injection (IVF-ICSI) are commonly used to circumvent the complications after the onset of infertilities. Unfortunately, conventional approaches do not always contribute to reliable results. Therefore, alternative approaches with the potential to improve IVF-ICSI efficiency are extensively under investigation.
2
Female infertility is originated from a variety of endogenous reasons such as hypothalamic dysfunction, premature ovarian failure, polycystic ovarian syndrome or early menopause, and tube fallopian insufficiency. Nevertheless, the absence or reduction of follicles within ovaries and oogenesis suppression is thought of as the most leading cause of infertility. Recently, ovarian progenitor cells and their differentiation toward oocyte-like cells have been of great interest to restore the ovarian tissue competence.
3-5
In mammals, oocytes are surrounded by a large number of granulosa cells (GCs) layers during folliculogenesis. After entering the prenatal stage, theca cells envelop the follicle containing oocytes. Theca cells in collaboration with GCs produce estrogen to provide structural integrity and maintain the synthesis of androgen substrates.
6
The existence of stem cells in follicular theca and ovarian epithelium was previously reported. For instance, Hubner et al revealed an inherent capacity of embryonic stem cells trans-differentiation into oogonia with normal meiosis. These cells were also able to recruit neighboring cells to generate follicle-like unites and blastocysts.
7
In another study done by Bukovsky and colleagues, the differentiation capacity of ovarian tunica mesenchymal cells toward epithelial-like cells was found. These cells could pass through ovarian blood vessels, and play as ovarian germ cells. In the next steps, these cells participate in the reformation of follicles in adult women post-puberty.
8
Further studies on a variety of pluripotent cells showed that GCs are capable to express stem cell markers.
9
In 2012, Yamanaka proposed four factors OCT4, SOX2, KIF4, and c-MYC as pluripotent stem cells markers.
10
Later, the expression of these factors in GCs was shown in various studies, indicating the suitability of these cells for reprogramming.
11,12
Considering the stemness feature of GCs and ease of extraction during surgery and biopsy procedure, it is noteworthy to mention that a large GCs number could be achieved for in vitro culture systems. Here, we aimed to investigate the oocyte-like differentiation of GCs after exposure to leukemia inhibitory factors (LIFs), follicle-stimulating hormone (FSH), and estradiol (E2).
Materials and Methods
Granulosa cells expansion
In this study, follicles were sampled by transvaginal ultrasound-guided aspiration from the women patients who referred to the infertility clinic for ICSI procedure. All candidates signed the informed consent form. Inclusion criteria were regular monthly ovulation, the mature oocytes, and normal values of thyroid and sex hormones. Patients with the history of polycystic ovaries, ovulation disorders, dysfunction in sex hormones, and HIV also CMV diseases were excluded from the study. Follicular fluid (FF) was aspirated by an expert embryologist, and then the GCs surrounding oocytes were separated and transferred into a sterile falcon tube containing an ISM1 medium. After a quick spin, the cells were washed twice with sterile phosphate-buffered saline (PBS) and centrifuged at 1200 rpm for 10 minutes to remove FF. Then, cells were cultured as described in the next section. The aspirated FF was also centrifuged at 3000 rpm for 10 minutes, inactivated at 56˚C for 45 minutes and filtered. The filtered liquid was stored at -20°C until used.
Cell culture
GCs were expanded in DMEM/F12 medium (Gibco) containing 10% FF, 2% fetal bovine serum (FBS; Gibco) and %1 penicillin-streptomycin (Sigma) solution. 3×105 cells/well were placed in each well of 6 well-plates (TPP) and maintained at 37˚C in a humidified atmosphere and 5% CO2. Culture media were replenished every 48 hours. In the second week, GCs were incubated with 1000 IU LIF (Peprotec) to induce a stem cell-like phenotype. From the beginning of the 3rd week, cells were incubated in DMEM/F12 medium supplemented with 10% FF, 2% FBS, 10 ng/mL basic fibroblast growth factor (bFGF; Sigma), 10 µL/mL non-essential amino acids (Invitrogen), 0.1 µL/mL retinoic acid (Sigma), 10 ng/mL epidermal growth factor (EGF; Sigma) and 1 mM 2-Mercaptoethanol. In this study, cells were then divided into five main groups as follows (Figure 1); (I) Control, (II) cells received 0.0025 and 0.005 IU/mL FSH and III: cells were incubated with 5 and 10 ng/mL E2. The medium was replaced every 24 hours and the cells were passaged over 5 weeks.
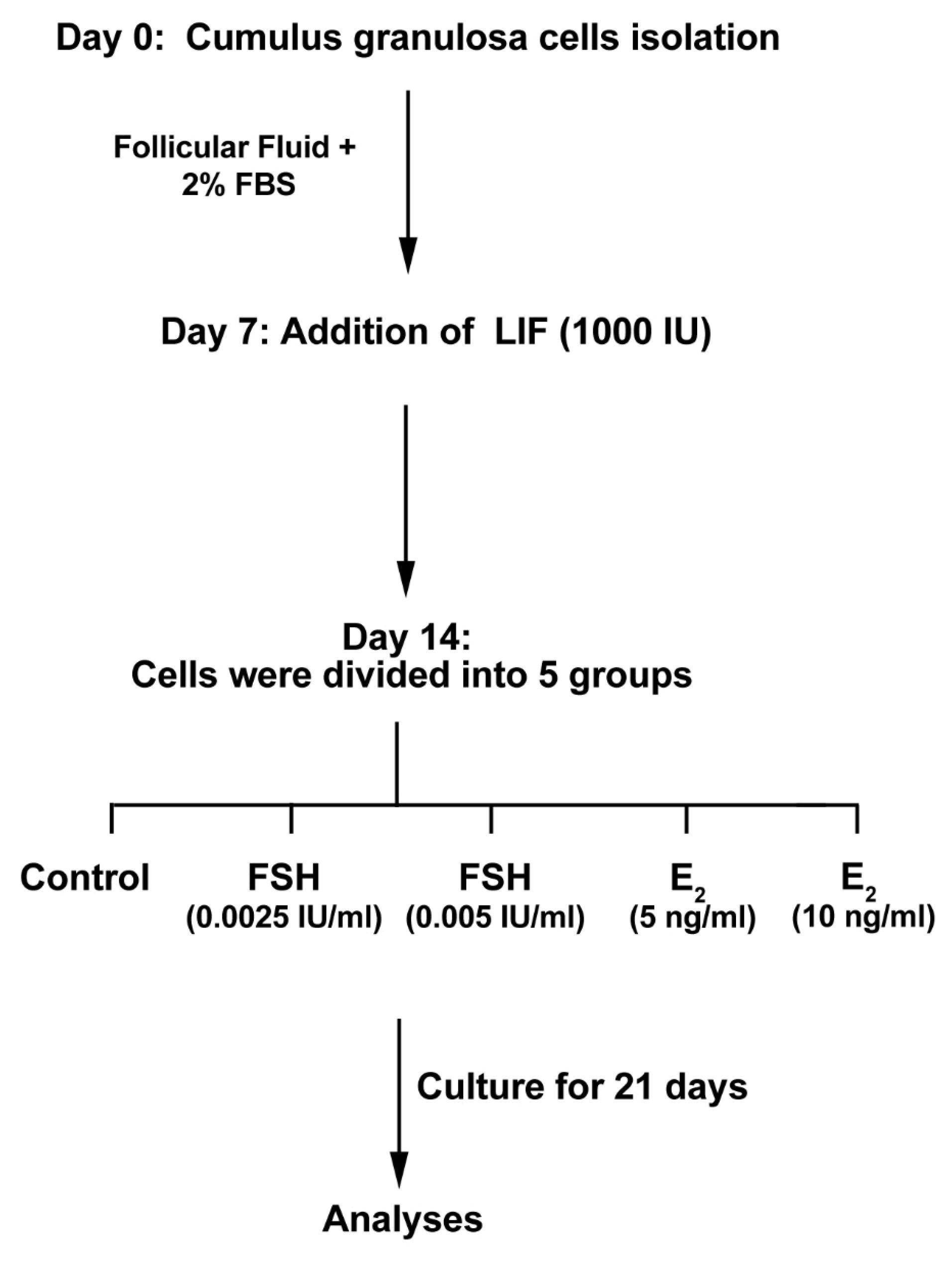
Figure 1.
Diagram of the experimental procedure of cumulus granulosa cells exposed to leukemia inhibitory factor (LIF) and estradiol (E2), and follicle-stimulating hormone (FSH) throughout 35 days to assess differentiation potential toward oocyte-like cells.
.
Diagram of the experimental procedure of cumulus granulosa cells exposed to leukemia inhibitory factor (LIF) and estradiol (E2), and follicle-stimulating hormone (FSH) throughout 35 days to assess differentiation potential toward oocyte-like cells.
Detecting E2 receptor (ER) by immunofluorescence assay
For this purpose, 104 GCs were placed in each well of 8-well slide champers. After 24 hours, GCs were fixed by using a pre-cooled paraformaldehyde solution (4% w/v). Then, cells were incubated in 1% Triton-X100 solution (Sigma-Aldrich) for 5 minutes for permeabilization. To detect ER, rabbit anti-human ER (dilution: 1:500; Abcam) and Texas Red-conjugated antibody (Dilution: 1:2000, Abcam) were used. For nuclear staining 200 µL, DAPI (1 µg/mL) was poured onto wells and the slides were imaged using fluorescence microscopy.
Flow cytometry analysis
The flow cytometry analysis was done to confirm the existence of stem cell-specific marker Oct3/4 (BD) and granulosa cell-specific marker GATA4 (BD). To this end, 7 and 14 days after treatment with different factors, cells were detached from the plates using 0.25% Trypsin-EDTA solution (Gibco). Permeabilization was performed by using the TritonX-100 solution (0.1% w/w). A panel of primary antibodies including Oct3/4 and GATA4 was used. Appropriate fluorescent secondary antibodies were applied for cell staining. The BD FACSCaliburTM system and FlowJo software (ver.7.6.1) were used to perform flow cytometry analysis. This experiment was used in triplicate.
Real-time PCR assay
Expression of FIGLA, NOBOX, and SYCP3 was evaluated using real-time polymerase chain reaction (PCR) assay. On day 14, the whole RNA was extracted by using an RNX PLUS Kit (Cinnagen, Iran). The content of RNA was measured by a NanoDrop (2000c spectrophotometer; Thermo Fisher). We used the cDNA synthesis kit (Bioneer) to synthesize cDNA. The real-time PCR analysis was performed by Corbett Rotor-Gene 6000 machine (Corbett Life Science) in a final volume of 14 µL reaction system containing 0.8 µL of each primer (Table 1), 7 µL of SYBR green reagent (Takara Bio, Japan), 0.8 µL of cDNA template, and nuclease-free water.
Table 1.
Primer list
|
Gene
|
Primer sequence
|
Accession number
|
Annealing (°C)
|
|
GAPDH
|
F: 5´AAGCTCATTTCCTGGTATGACAACG-3´ |
NM_002046.3 |
58 |
| R: 5´TCTTCCTCTTGTGCTCTTGCTGG-3´ |
|
FIGL A
|
F: 5´-CCAAGGAGCGTGAGCGGATAA-3´ |
NM_001004311.3 |
60 |
| R: 5´-TACTATAGCTCTGCTCATCTGG-3´ |
|
NOBOX
|
F: 5´-CTGATGGATGTTGCTGGCAGTGA-3´ |
NM_001080413.3 |
59 |
| R: 5´-AAGGGGAAAGTGGGGAGGTAGGG-3´ |
|
SYCP
3
|
F: 5´-CTCAGAAGCGTCGCGGAGAAG-3´ |
NM_001177948.1 |
61 |
| R: 5´-CTTCCGCAATGGCCGAGGACCAG-3´ |
Statistical analysis
All experiments were done in three independent replicates. Results were reported as mean ± SD. Statistical analyses were performed using GraphPad Prism (version7.0). Significant differences were calculated using a one-way analysis of variance (ANOVA) and a Student ttest. The mean difference between the data was significant at the level of *P < 0.05, **P < 0.01 and *** P < 0.001.
Results and Discussion
Immunofluorescence staining showed typical markers in cultured granulosa cells
We examined the existence of ER in GCs using IF imaging (Figure 2A). As presented in Figure 1A, the cellular distribution of ER was identified in GCs obtained from human samples. Here, we showed a dim expression of ER in cultured GCs. These results showed that GCs had the potential to express ER, indicating an inherent cell ability to respond to E2.
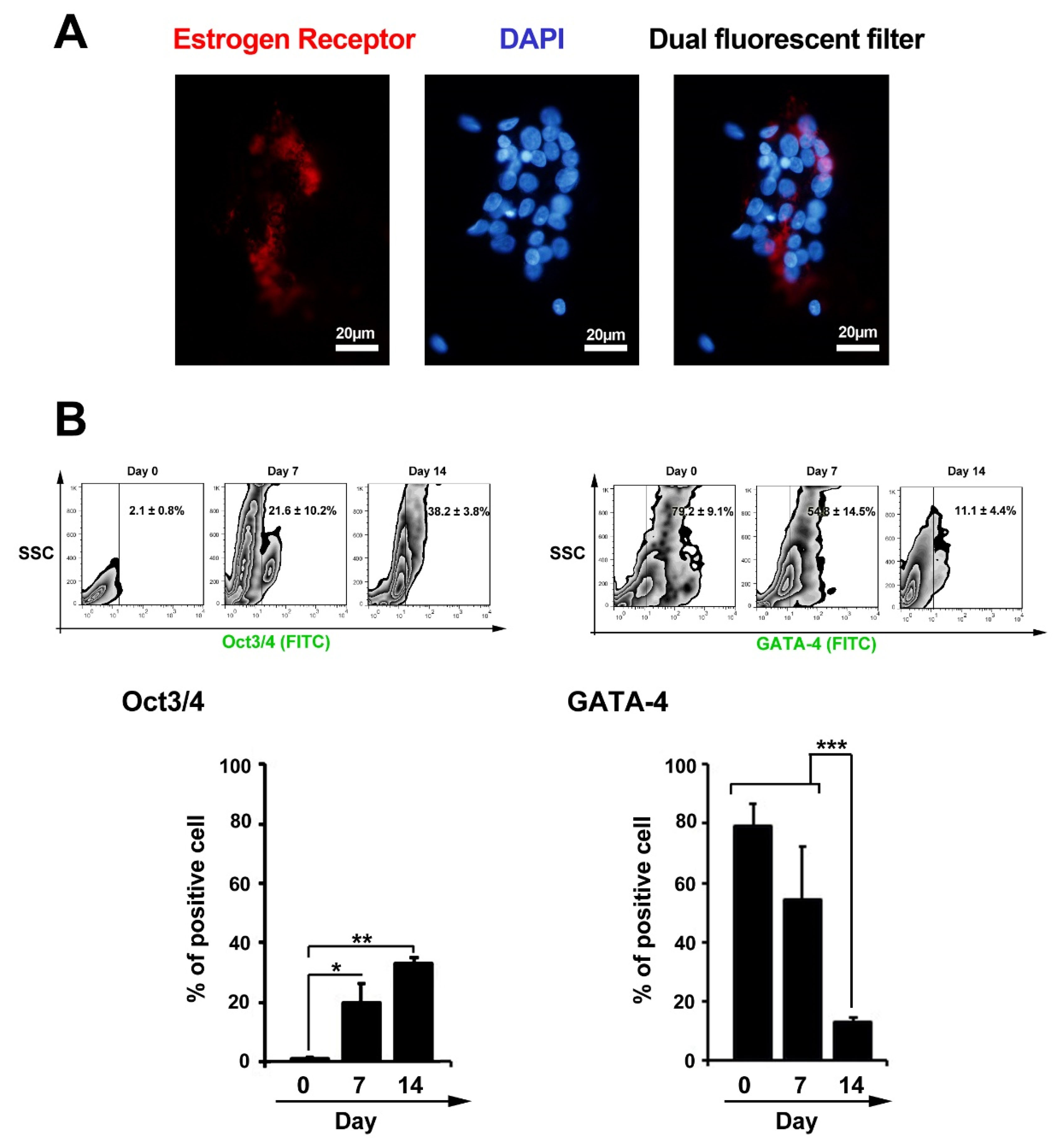
Figure 2.
Measuring estrogen receptors in cultured granulosa cells using immunofluorescence staining (A). Data showed a dim expression of estrogen receptors in granulosa cells at passage three. Flow cytometric analysis of Oct3/4 and GATA-4 in granulosa cells 7 days after treatment with leukemia inhibitory factor (B). Data showed statistically significant induction and reduction of Oct3/4 and GATA-4 in granulosa cells, respectively (n=3); One-way ANOVA with Tukey post hoc test *P < 0.05; **P < 0.01 and ***P < 0.001).
.
Measuring estrogen receptors in cultured granulosa cells using immunofluorescence staining (A). Data showed a dim expression of estrogen receptors in granulosa cells at passage three. Flow cytometric analysis of Oct3/4 and GATA-4 in granulosa cells 7 days after treatment with leukemia inhibitory factor (B). Data showed statistically significant induction and reduction of Oct3/4 and GATA-4 in granulosa cells, respectively (n=3); One-way ANOVA with Tukey post hoc test *P < 0.05; **P < 0.01 and ***P < 0.001).
Changes in the levels of Oct3/4 and GATA-4 in granulosa cells treated with leukemia inhibitory factor
Flow cytometry analysis showed that GCs could express Oct3/4 14 days after incubation with LIF (Figure 2B). Compared to non-treated cells at initial seeding time, the percent of Oct3/4 positive GCs were reached 38.2 ± 3.8% on day 14 (PDay 14 versus Day 0 < 0.01; PDay 7 versus Day 0 < 0.05). These data confirm stemness-like features in GCs after exposure to the LIF. Besides, the level of GATA-4 reached to minimum levels, from 79.2 ± 9.1% to 11.1 ± 4.4%, at the end of incubation time (PDay 14 versus Day 0 < 0.001; Figure 2B). We also found a significant drop in the level of GATA-4 coincided with the induction of Oct3/4 in GCs. These data showed the potential of LIF to induce multipotentiality in the GCs. Parte et al first reported the expression of the pluripotent genes, including STAT-3, NANOG, Oct-4, TERT, and Sox-2, in the ovarian epithelium.
13
Virant-Klun et al identified adult stem cells in the human ovaries with the ability to trans-differentiate into oocyte-like cells and form parthenote-like complexes.
14
Studies pointed multipotent stem cells could commit to germ cell lineages and functional gametes by applying numerous differentiation methodologies, including the addition of LIF or FF to the culture medium with ovarian GCs.
15-18
LIF, as one of the most studied pro-pluripotency factors, promotes the self-renewal by activating various signaling pathways such as STAT3 and BMP4 and MAP kinase pathways.
19
This factor is secreted from the outside of the fetus, as well as many other types of mature cells such as endometrial cells, fibroblasts, bone cells, monocytes, macrophages, and T lymphocytes. LIF initiates intracellular signaling pathways after binding to receptors LIFR and gp130.
18,20,21
The cell distribution of the CD29, POU5F1, CD90, CD44, CD166, CD105, and CD117 factors was reported on the surface of GCs by Kossowska-Tomaszczuk et al.
22
Real-time PCR analysis showed up-regulation of oocyte-related genes
Real-time PCR analysis showed the up-regulation of FIGLA-a, Nobox, and SYCP-3 in GCs treated with 5 ng/mL E2 and these effects were less in groups received 10 ng/mL E2. According to these data, it seems that E2 enhanced oocyte-like stemness in GCs in a certain dose (Figure 3). In contrast, data showed that FSH treatment, at doses 0.0025 IU and 0.005 IU per ml, suppressed the activity of FIGLA-a, Nobox, and SYCP-3 compared to the control cells (Figure 3). In vitro studies have further demonstrated that GCs are capable of differentiation into lineages of neurons, osteoblasts, and chondrocytes which are not observed in normal ovarian follicles.
22
Varras et al showed that DAZL mRNA, a typical germ cell marker, was not detectable in GCs, suggesting that GCs are not originated from primordial germ cells
12
. The maintenance of GCs stemness is one of the most important and considerable issues in the trans-differentiation of the GCs into another cell lineage.
23
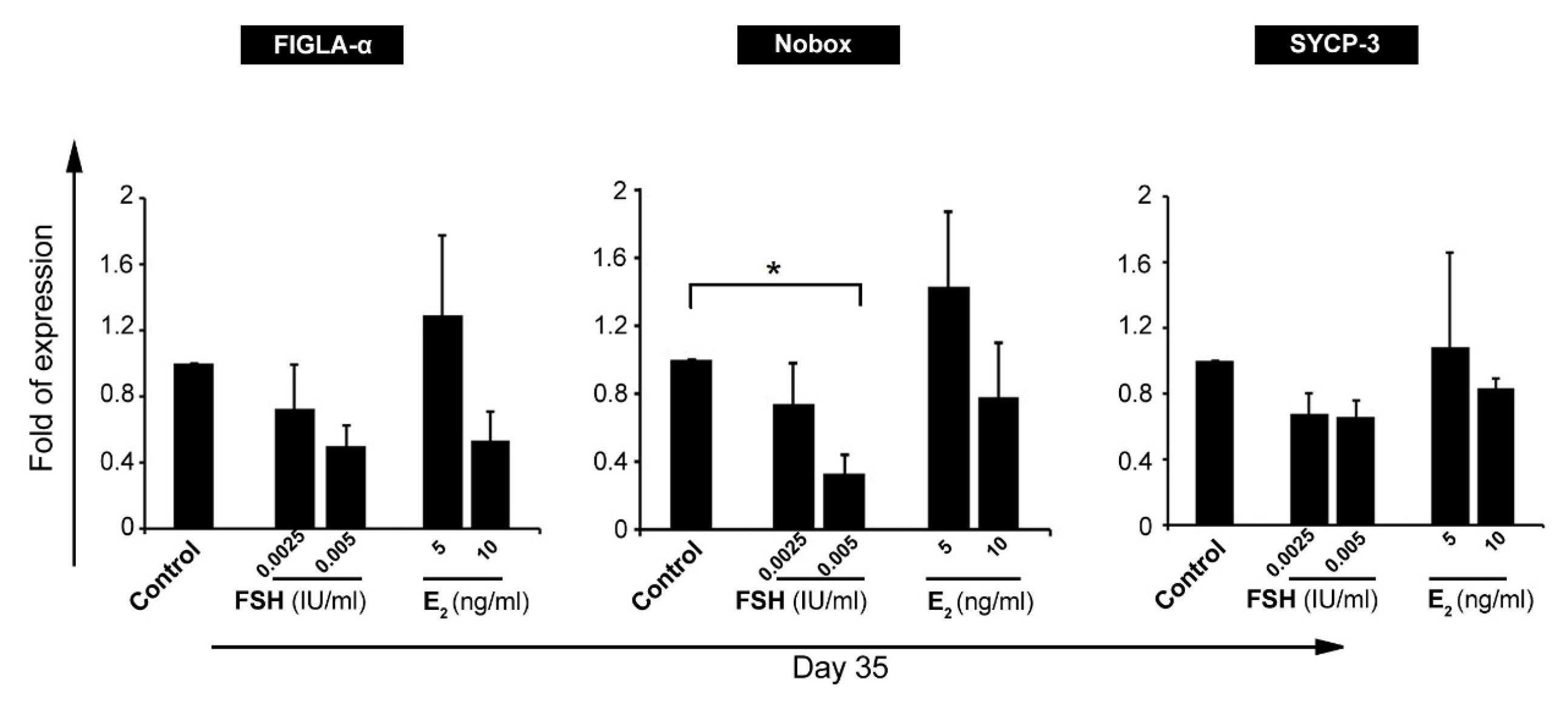
Figure 3.
Real-time PCR analysis of oocyte-related genes, FIGLA-α, Nobox, and SYCP-3, in granulosa cells after exposure to follicle-stimulating hormone (FSH) and estradiol (E2) (n=3). Gene expression analysis confirmed the significant reduction of Nobox gene in granulosa cells after treatment with FSH. These data showed the potency of FSH in inhibition granulosa cell differentiation toward oocyte-like cells.
.
Real-time PCR analysis of oocyte-related genes, FIGLA-α, Nobox, and SYCP-3, in granulosa cells after exposure to follicle-stimulating hormone (FSH) and estradiol (E2) (n=3). Gene expression analysis confirmed the significant reduction of Nobox gene in granulosa cells after treatment with FSH. These data showed the potency of FSH in inhibition granulosa cell differentiation toward oocyte-like cells.
Morphological adaptation was shown in granulosa cells after exposure to estradiol and follicle-stimulating hormone
Photomicrographs showed the ability of freshly isolated GCs to attach the bottom of the culture. GCs exhibited an epithelial-like appearance after 7 days post-seeding (Figure 4A). The addition of LIF to culture medium generated micro-aggregates and colonies. Using FSH and E2, colonies became more compact and large gaps were evident between the colonies (Figure 4A). On day 35, single oocyte-like cells were observed with a round shape (Figure 4B). It has been elucidated that FF contains various factors secreted from GCs, theca cells and oocytes, and plasma, namely GDF9b, GDF9, stem cell factor (SCF), bFGF, and E2.
24,25
These factors are involved in the regulation of follicular development.
24
In a study conducted by Virant-Klun et al, the culture of epithelial cells in a medium with FF generated round-shaped-cell clusters with alkaline phosphatase activity and primitive oocyte phenotype and up-regulation of SOX-2 and SSEA-4.
26
We, here, showed that the exposure of GCs to E2 and FF contributed to the formation of colonies and oocyte-like cells. These cells can synthesize factors OCT4, SOX-2, etc., which are known pluripotency markers of stem cells.
17
Dyce et al tested several culture systems to identify conditions in which porcine skin-derived sphere cells could differentiate into germ-cells. They documented that FF advocates the induction of markers expression coincided with germ-cell differentiation.
17
These data support the notion that the combination of FF with the appropriate factor levels could promote GCs orientation to different lineage, especially oocyte-like cells. In line with this statement, some previous studies documented that FSH and E2 have beneficial effects on the antrum-like reorganization, proliferation, differentiation as well as endocrine function of the GCs.
27-29
Unlike these results, we did not find any changes in the level of oocyte-associated markers after cell exposure to FSH. Robker and Richards showed that E2 and FSH can directly and independently regulate the process of the cell cycle in GCs by increasing levels of cyclin D2.
29
However, there is not enough evidence about the effects of the FSH and E2 on the trans-differentiation capacity of GCs into oocyte-like cells.
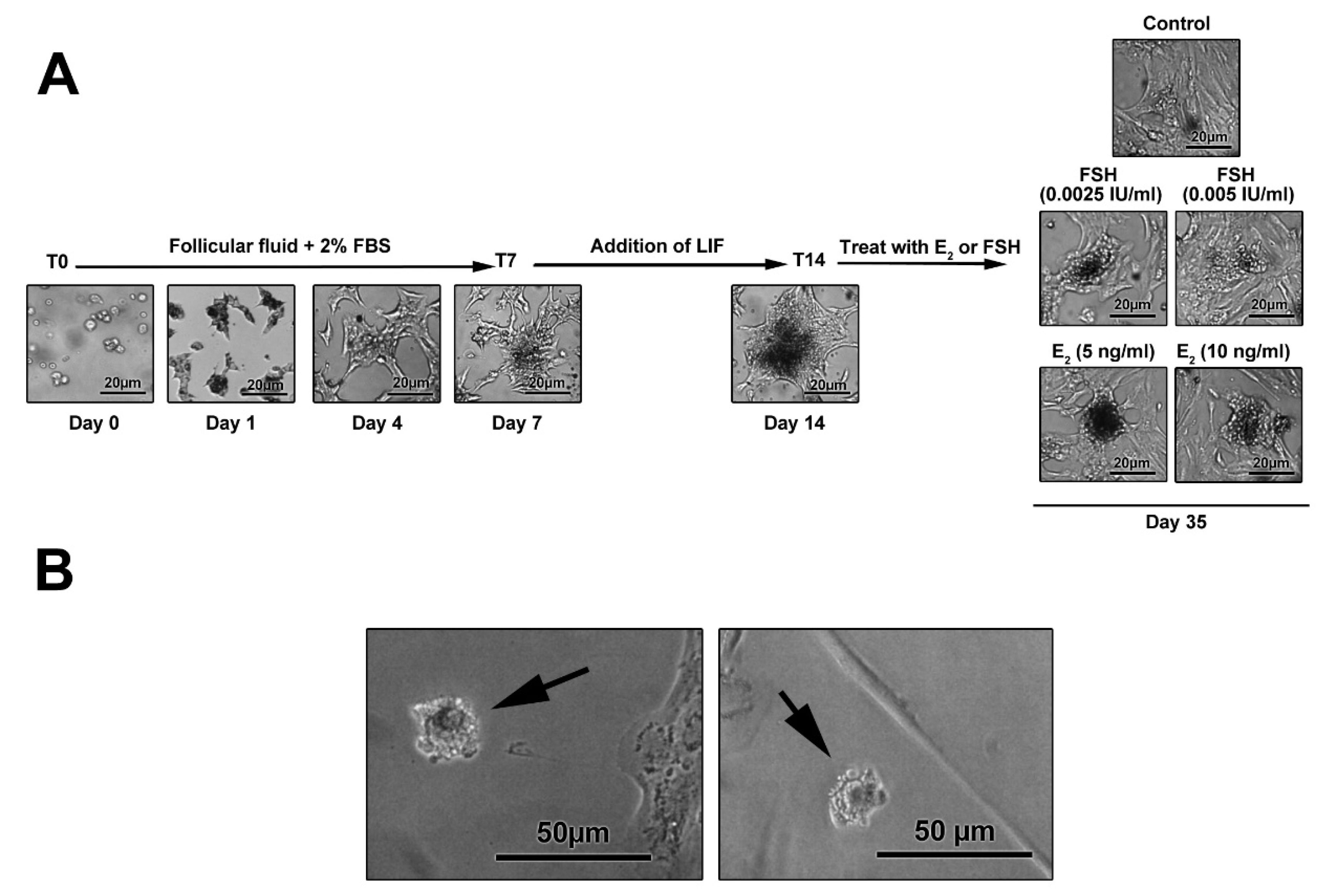
Figure 4.
The morphological adaptation of granulosa cells cultured in different media supplemented with leukemia inhibitory factor (LIF), estradiol (E2), and follicle-stimulating hormone (FSH) after 35 days. Cell morphology in E2-treated cells (B). After the completion of the experimental protocol, cells lose epithelial-like appearance and acquire a round shape. The cell spreading and flattening are confined and the extent of projection decreased following treatment with LIF and E2. It seems that the up-regulation of oocyte-like genes, such as Nobox, in granulosa cells coincides with prominent morphological adaptation.
.
The morphological adaptation of granulosa cells cultured in different media supplemented with leukemia inhibitory factor (LIF), estradiol (E2), and follicle-stimulating hormone (FSH) after 35 days. Cell morphology in E2-treated cells (B). After the completion of the experimental protocol, cells lose epithelial-like appearance and acquire a round shape. The cell spreading and flattening are confined and the extent of projection decreased following treatment with LIF and E2. It seems that the up-regulation of oocyte-like genes, such as Nobox, in granulosa cells coincides with prominent morphological adaptation.
Discussion
Nowadays, a lot of research is done to address the molecular mechanisms causing the low quality of oocytes.
13,30
Identification of a subpopulation of GCs to exhibit a pluripotent and self-renewing potential opens new horizons in augmenting new therapeutic strategies for patients suffering from ovarian insufficiencies.
22,26
We showed that the culture of GCs with LIF increased the expression of OCT3/4 while down-regulated GATA-4 in GCs. Additionally, the treatment of GSs with FSH diminished the expression of oocyte-related genes. E2 can promote the expression of FIGLA-a, Nobox, and SYCP-3. These data support the notion that E2 could efficiently preserve the stemness characteristics of the GCs as compared to FSH-treated cells.
Conclusion
In conclusion, our study showed that the exposure of the GCs to the LIF and E2 efficiently preserves the GCs multipotentiality by inducing the expression of oocyte-like cell genes. This approach offers a novel strategy in the medication of infertility and ovary restoration in the treatment of gynecological disorders.
Ethical Issues
All experiments and procedures were conducted in compliance with the ethical principles of Tabriz University of Medical Science, Tabriz, Iran (TBZMED.REC.1394.100). This study was supported by a grant from Tabriz University of Medical Sciences.
Conflict of Interest
None declared.
Acknowledgments
The authors kindly thank Dr. Shaneband to design primers.
References
- Källén B. Maternal morbidity and mortality in in-vitro fertilization. Best Pract Res Clin Obstet Gynaecol 2008; 22(3):549-58. doi: 10.1016/j.bpobgyn.2008.02.001 [Crossref] [ Google Scholar]
- Youssry M, Orief Y, Ozmen B, Al-Hasani S, Zohni K. Human sperm DNA damage in the context of assisted reproductive techniques. Iran J Reprod Med 2007; 5(4):137-50. [ Google Scholar]
- Letourneau JM, Ebbel EE, Katz PP, Oktay KH, McCulloch CE, Ai WZ. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer 2012; 118(7):1933-9. doi: 10.1002/cncr.26403 [Crossref] [ Google Scholar]
- Bukovsky A, Caudle MR. Immunoregulation of follicular renewal, selection, POF, and menopause in vivo, vs neo-oogenesis in vitro, POF and ovarian infertility treatment, and a clinical trial. Reprod Biol Endocrinol 2012; 10:97. doi: 10.1186/1477-7827-10-97 [Crossref] [ Google Scholar]
- Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod 2008; 23(3):462-77. doi: 10.1093/humrep/dem426 [Crossref] [ Google Scholar]
- Latham KE, Bautista FD, Hirao Y, O’Brien MJ, Eppig JJ. Comparison of protein synthesis patterns in mouse cumulus cells and mural granulosa cells: effects of follicle-stimulating hormone and insulin on granulosa cell differentiation in vitro. Biol Reprod 1999; 61(2):482-92. doi: 10.1095/biolreprod61.2.482 [Crossref] [ Google Scholar]
- Hübner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R. Derivation of oocytes from mouse embryonic stem cells. Science 2003; 300(5623):1251-6. doi: 10.1126/science.1083452 [Crossref] [ Google Scholar]
- Bukovsky A, Svetlikova M, Caudle MR. Oogenesis in cultures derived from adult human ovaries. Reprod Biol Endocrinol 2005; 3:17. doi: 10.1186/1477-7827-3-17 [Crossref] [ Google Scholar]
- Chronowska E. Stem cell characteristics of ovarian granulosa cells-review. Ann Anim Sci 2012; 12(2):151-7. doi: 10.2478/v10220-012-0012-8 [Crossref] [ Google Scholar]
- Liu X, Huang J, Chen T, Wang Y, Xin S, Li J. Yamanaka factors critically regulate the developmental signaling network in mouse embryonic stem cells. Cell Res 2008; 18(12):1177-89. doi: 10.1038/cr.2008.309 [Crossref] [ Google Scholar]
- Mao J, Zhang Q, Ye X, Liu K, Liu L. Efficient induction of pluripotent stem cells from granulosa cells by Oct4 and Sox2. Stem Cells Dev 2014; 23(7):779-89. doi: 10.1089/scd.2013.0325 [Crossref] [ Google Scholar]
- Varras M, Griva T, Kalles V, Akrivis C, Paparisteidis N. Markers of stem cells in human ovarian granulosa cells: is there a clinical significance in ART?. J Ovarian Res 2012; 5(1):36. doi: 10.1186/1757-2215-5-36 [Crossref] [ Google Scholar]
- Parte S, Bhartiya D, Telang J, Daithankar V, Salvi V, Zaveri K. Detection, characterization, and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovary. Stem Cells Dev 2011; 20(8):1451-64. doi: 10.1089/scd.2010.0461 [Crossref] [ Google Scholar]
- Virant-Klun I, Rozman P, Cvjeticanin B, Vrtacnik-Bokal E, Novakovic S, Rülicke T. Parthenogenetic embryo-like structures in the human ovarian surface epithelium cell culture in postmenopausal women with no naturally present follicles and oocytes. Stem Cells Dev 2009; 18(1):137-49. doi: 10.1089/scd.2007.0238 [Crossref] [ Google Scholar]
- Hirai H, Firpo M, Kikyo N. Establishment of leukemia inhibitory factor (LIF)-independent iPS cells with potentiated Oct4. Stem Cell Res 2015; 15(3):469-80. doi: 10.1016/j.scr.2015.09.002 [Crossref] [ Google Scholar]
- Park S, Park HR, Lee WD, Hur CY, Lee YJ. Establishment of a xeno-free culture system that preserves the characteristics of placenta mesenchymal stem cells. Cytotechnology 2015; 67(5):851-60. doi: 10.1007/s10616-014-9725-0 [Crossref] [ Google Scholar]
- Dyce PW, Wen L, Li J. In vitro germline potential of stem cells derived from fetal porcine skin. Nat Cell Biol 2006; 8(4):384-90. doi: 10.1038/ncb1388 [Crossref] [ Google Scholar]
- Qing T, Shi Y, Qin H, Ye X, Wei W, Liu H. Induction of oocyte-like cells from mouse embryonic stem cells by co-culture with ovarian granulosa cells. Differentiation 2007; 75(10):902-11. doi: 10.1111/j.1432-0436.2007.00181.x [Crossref] [ Google Scholar]
- Hao J, Li TG, Qi X, Zhao DF, Zhao GQ. WNT/beta-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev Biol 2006; 290(1):81-91. doi: 10.1016/j.ydbio.2005.11.011 [Crossref] [ Google Scholar]
- Mathieu ME, Saucourt C, Mournetas V, Gauthereau X, Thézé N, Praloran V. LIF-dependent signaling: new pieces in the Lego. Stem Cell Rev Rep 2012; 8(1):1-15. doi: 10.1007/s12015-011-9261-7 [Crossref] [ Google Scholar]
- Hirai H, Karian P, Kikyo N. Regulation of embryonic stem cell self-renewal and pluripotency by leukaemia inhibitory factor. Biochem J 2011; 438(1):11-23. doi: 10.1042/bj20102152 [Crossref] [ Google Scholar]
- Kossowska-Tomaszczuk K, De Geyter C, De Geyter M, Martin I, Holzgreve W, Scherberich A. The multipotency of luteinizing granulosa cells collected from mature ovarian follicles. Stem Cells 2009; 27(1):210-9. doi: 10.1634/stemcells.2008-0233 [Crossref] [ Google Scholar]
- Dzafic E, Stimpfel M, Virant-Klun I. Plasticity of granulosa cells: on the crossroad of stemness and transdifferentiation potential. J Assist Reprod Genet 2013; 30(10):1255-61. doi: 10.1007/s10815-013-0068-0 [Crossref] [ Google Scholar]
- McNatty KP, Moore LG, Hudson NL, Quirke LD, Lawrence SB, Reader K. The oocyte and its role in regulating ovulation rate: a new paradigm in reproductive biology. Reproduction 2004; 128(4):379-86. doi: 10.1530/rep.1.00280 [Crossref] [ Google Scholar]
- Paulini F, Melo EO. The role of oocyte-secreted factors GDF9 and BMP15 in follicular development and oogenesis. Reprod Domest Anim 2011; 46(2):354-61. doi: 10.1111/j.1439-0531.2010.01739.x [Crossref] [ Google Scholar]
- Virant-Klun I, Skutella T, Stimpfel M, Sinkovec J. Ovarian surface epithelium in patients with severe ovarian infertility: a potential source of cells expressing markers of pluripotent/multipotent stem cells. J Biomed Biotechnol 2011; 2011:381928. doi: 10.1155/2011/381928 [Crossref] [ Google Scholar]
- Gore-Langton RE, Daniel SA. Follicle-stimulating hormone and estradiol regulate antrum-like reorganization of granulosa cells in rat preantral follicle cultures. Biol Reprod 1990; 43(1):65-72. doi: 10.1095/biolreprod43.1.65 [Crossref] [ Google Scholar]
- Wandji SA, Eppig JJ, Fortune JE. FSH and growth factors affect the growth and endocrine function in vitro of granulosa cells of bovine preantral follicles. Theriogenology 1996; 45(4):817-32. doi: 10.1016/0093-691x(96)00011-8 [Crossref] [ Google Scholar]
- Robker RL, Richards JS. Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol 1998; 12(7):924-40. doi: 10.1210/mend.12.7.0138 [Crossref] [ Google Scholar]
- Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update 2008; 14(2):159-77. doi: 10.1093/humupd/dmm040 [Crossref] [ Google Scholar]