Advanced pharmaceutical bulletin. 12(2):237-247.
doi: 10.34172/apb.2022.025
Review Article
Exendin-4 as a Versatile Therapeutic Agent for the Amelioration of Diabetic Changes
Hadi Rajabi 1  , Mahdi Ahmadi 2, Somayeh Aslani 3, Shirin Saberianpour 4, Reza Rahbarghazi 5, 6, *
, Mahdi Ahmadi 2, Somayeh Aslani 3, Shirin Saberianpour 4, Reza Rahbarghazi 5, 6, * 
Author information:
1Koc University Research Center for Translational Medicine (KUTTAM), Koc University School of Medicine, Istanbul, Turkey.
2Tuberculosis and Lung Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
3Department of Biochemistry and Clinical Laboratories, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran.
4Vascular and Endovascular Surgery Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
5Stem Cell Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
6Department of Applied Cell Sciences, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Type 2 diabetes mellitus (T2DM) is a chronic metabolic abnormality leading to microvascular and macrovascular complications. Non-insulin Incretin mimic synthetic peptide exendin-4 was introduced as an anti-diabetic drug which helped diabetic patients with triggering insulin secretion; further researches have revealed an effective role of exendin-4 in treatment of T2DM related diseases. Exendin-4 is approximately similar to Glucagon-like peptide, thus it can bind to the glucagon-like peptide-1 receptor (GLP-1R) and activated different signaling pathways that are involved in various bioactivities such as apoptosis, insulin secretion and inactivation of microglial. In this review, we investigated the interesting role of exendin-4 in various kinds of T2DM related disorders through the activation of different signaling pathways.
Keywords: Diabetes complications, Exendin-4, Signaling pathways
Copyright and License Information
©2022 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Type 2 diabetes mellitus (T2DM) is a progressive metabolic disorder with a high rate of prevalence worldwide contributing to profound socio-economic implications.
1
T2DM is mainly identified by a hyperglycemic condition that is caused by the combination of cell resistance to insulin and/or insufficiency of pancreatic β cell to synthesize and release insulin to the systemic circulation in response to high levels of glucose. Irrespective of diabetic conditions, the imbalance of insulin could promote microvascular and macrovascular pathologies.
2
The use of traditional approaches such as metformin administration and insulin therapy are the most common and prominent options for patients with T2DM (Table 1).
3,4
However, numerous side effects have been reported in subjects with T2DM (Figure 1). In the case of pharmacological approaches, exendin-4 belongs to the incretin family is a glucagon-like peptide-1 receptor (GLP-1R) agonist with an ability to control hyperglycemic conditions in patients with T2DM and approved by food and drug administration and conceived as anti-diabetic agent.
5,6
In the pharmaceutical industry, Exenatide is the synthetic form of exendin-4 with a 39 amino acid similar to GLP-1commonly seen in the saliva of the Gila monster (Figure 2).
7,8
Table 1.
Various medications which normally used for treating T2DM
| Medications |
Mechanism of action |
Side effects |
| Metformin |
Enhances insulin sensitivity in liver and peripheral tissues by activation of AMP activated protein kinase |
|
| Sulfonylureas |
Activates sulfonylurea receptor on beta cell to stimulate endogenous insulin secretion |
Increased risk of cancer-related mortality compared with metformin |
| Insulin |
activates insulin receptors to regulate metabolism of carbohydrate, fat and protein |
|
Thiazolidinedione Pioglitazone (Actos)
Rosiglitazone (Avandia)
|
Enhances insulin sensitivity in peripheral tissues and liver by activation of peroxisome proliferator-activated receptor-gamma receptors |
|
| Dipeptidyl peptidase 4 inhibitor |
improves incretin pathway activation via inhibition of enzymatic breakdown of endogenous GLP-1 and gastric inhibitory peptide |
|
| Meglitinides |
Increase insulin secretion during the early phase of insulin release |
|
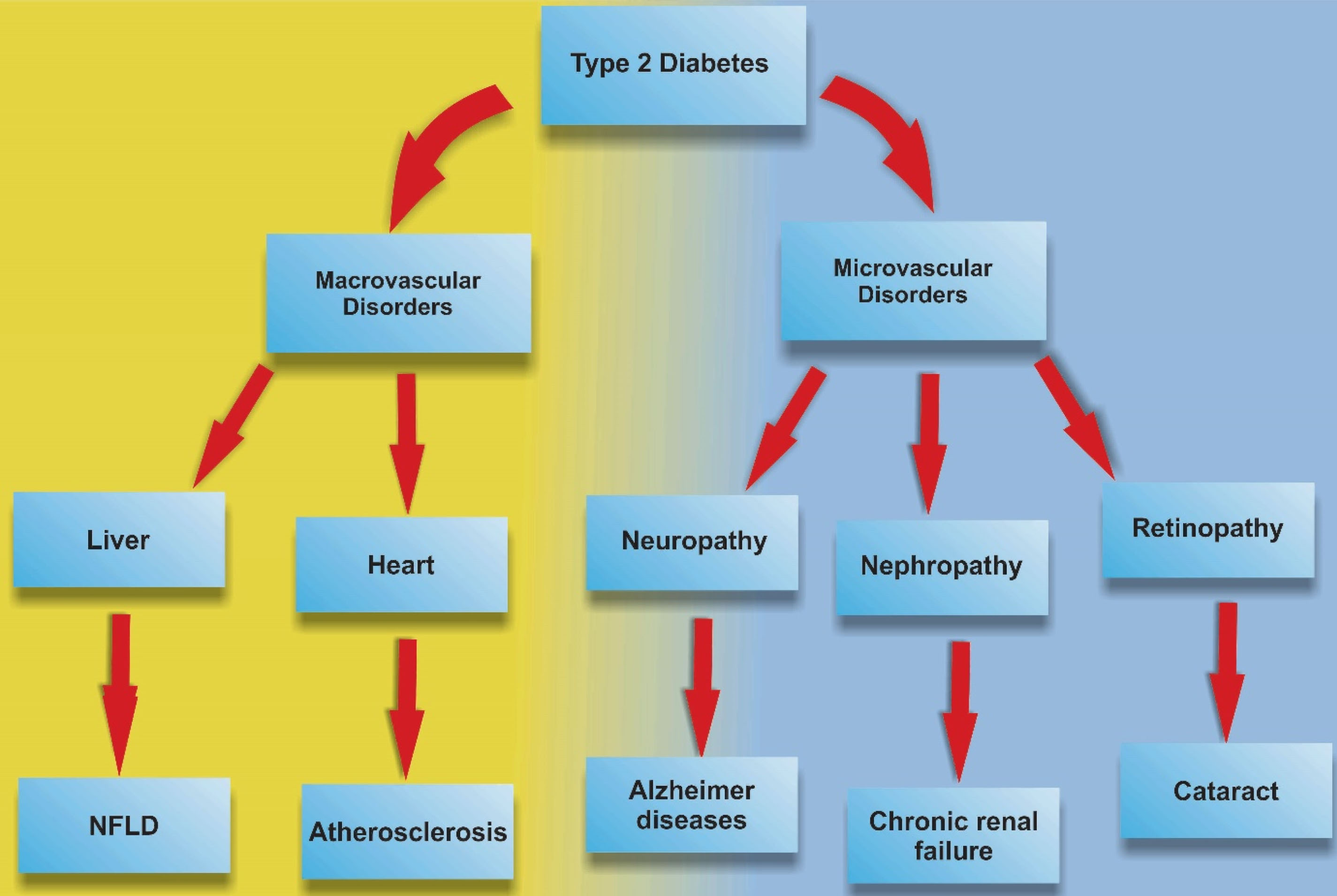
Figure 1.
Type 2 Diabetes and related disorders.
.
Type 2 Diabetes and related disorders.
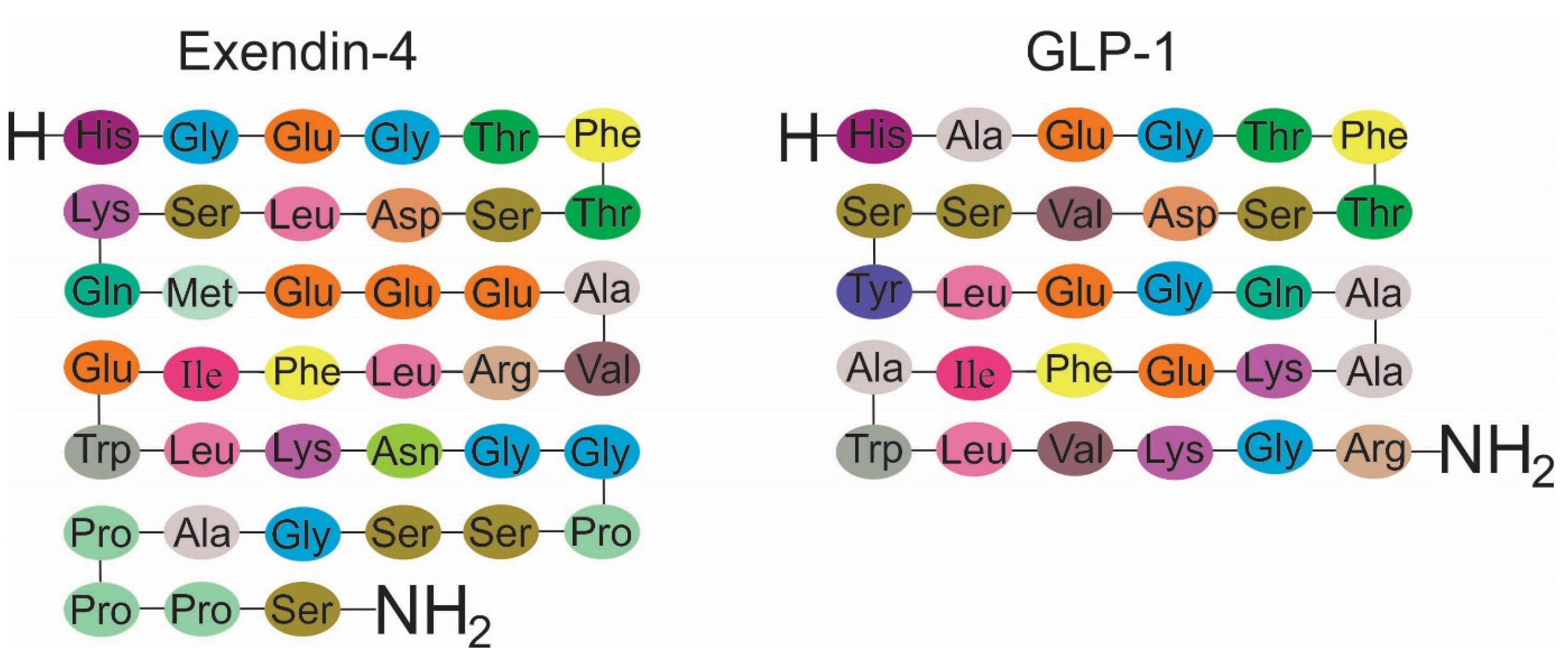
Figure 2.
Exendin-4 shares 53% similarity with Glucagon-like peptide-1.
.
Exendin-4 shares 53% similarity with Glucagon-like peptide-1.
GLP-1 is formed by the post-translational stage of pro-glucagon hormone in the L-cells of the small intestine in response to the ingestion of food.
9
GLP-1 has the potential to reduce systemic glucose levels by inducing insulin secretion. This factor also delays gastric emptying, inhibits glucagon secretion and reduces food intake rate.
10
GLP-1 exerts its effects through GLP-1R.
8
GLP-1R is a G protein-coupled receptor from glucagon receptor family by located on chromosome 6.
11,12
Several studies have confirmed that GLP-1R is not exclusive for pancreatic tissue but also seen in the peripheral tissues such as lungs, stomach, intestines, kidneys, and heart in addition to the most areas of the brain.
13
This receptor contains two major domains ECD (extracellular domain) and TM (transmembrane domain).
14
As previously documented in different experiments, it demonstrated that EX-4 regulates glucose level in diabetic subjects via triggering insulin secretion, reducing glucagon secretion and food intake.
15-17
Compared to GLP-1, EX-4 is resistant to dipeptidyl peptidase-IV activity, thereby possess a much longer plasma half-life and activity.
18
In spite of the similarity between EX-4 and GLP-1 function and structure, there are some differences in the binding capacity to GLP-1R. The isolated GLP-1R ECD binds EX-4 with high affinity rather than GLP-1. Even, GLP-1 could attach with high affinity to TM part of GLP-1R with site-directed mutagenesis as compared with EX-4. In the case with GLP-1 N-terminal truncation, EX-4 loses affinity to attach this receptor but the interaction of GLP-1 with GLP-1 remains unaffected.
19
However, there are some controversies related to EX-4 and GLP-1 interaction with GLP-1R. For instance, de Maturana et al showed the 400-fold binding affinity of Ex-4 (Kd = 6 nM) to the N-terminal domain of GLP-1R compared to GLP-1-GLP-1R attachment.
20
EX-4 related signaling pathway (s)
The critical activity of EX-4 correlates with the induction of multiple downstream effectors from different signaling pathways.
21
As previously mentioned, different biological processes such as cell proliferation and apoptosis modulation are initiated through activation of phosphatidylinositol 3-kinase/Akt (PI3K/AKT) adenylyl cyclase/protein kinase A signaling pathways.
22
In PI3K/AKT axis, the activation of PI3K phosphorylates Akt factor which in turn affects the dynamic of different factors.
23
For example, it was mentioned that activated Akt could increase the level of nitric oxide (NO) by phosphorylation of endothelial nitric oxide (eNOS) and contributes to the modulation of nitrosative stress.
24
Of note, EX-4 has the potential to regulate the function of neurons by the suppression of glycogen synthase kinase-3β (GSK-3β). The decrease of GSK-3β function declines Tau protein phosphorylation thereby prohibits the aggregation of α-synuclein. Therefore, EX-4 is able to modulate the function of neurons and endothelial cells (ECs) during pathological conditions. It is noteworthy to mention that the application of EX-4 could decrease the cytopathic effects of Parkinson’s and Alzheimer’s diseases. In addition, it was well-established that the activation of microglial nuclear factor-ƙB (NF-ƙB) after administration of EX-4 provokes pro-inflammatory responses. One of the possible therapeutic effects of EX-4 on the injured cells could be related to the modulation of apoptosis. It was determined that the activation of Akt by EX-4 inhibits Caspase cascade such as caspase-9 and -3. In addition, the inhibition of pro-apoptotic factors such as Bad and Forkhead box O (FOXO) transcription factors decreased apoptotic changes.
25
The above-mentioned factors are thought to participate in different cell activities (Figure 3). For instance, the critical role of EX-4 was determined in the regulation of β-cell proliferation via PI3K/Akt and mTORC1/S6K1-dependent signaling pathway.
26,27
In contrast to the induction of cell proliferation, and increased proliferation rate could be correlated with the abortion of lipotoxicity-induced apoptosis after induction of protein kinase B and inhibition of mitochondrial-derived Caspase 3 while the transcription of Bcl-2 was found to up-regulate under these conditions.
28,29
In addition to the therapeutic effects of EX-4 on insulin-producing cells, exposure of human endothelial cell lines to EX-4 pre-treated with apoptosis inducer tunicamycin decreased the possibility of atherosclerosis by reducing phosphorylated inositol requiring enzyme-1α/inositol requiring enzyme-1α ratio. Phosphorylation of c-JNK is also diminished in EX-4-treated human endothelial cell lines.
30
Considering the detrimental effects of accumulated free acids in the promotion of endothelial apoptotic changes, initiation of endothelial active fatty acids metabolic reaction is thought to be an efficient strategy to reduce diabetic micro- and macro-vascular pathologies.
31
However, cautions must be considered for the administration of Ex-4 in diabetic candidates. For example, prolonged and continuous subcutaneous use of EX-4 possibly causes nausea, vomiting and a negligible increase in the heart rate. In addition, systemic intravenous injection of this compound was found to yield severe tachycardia and arrhythmias in non-diabetic and diabetes cases in the swine model.
32
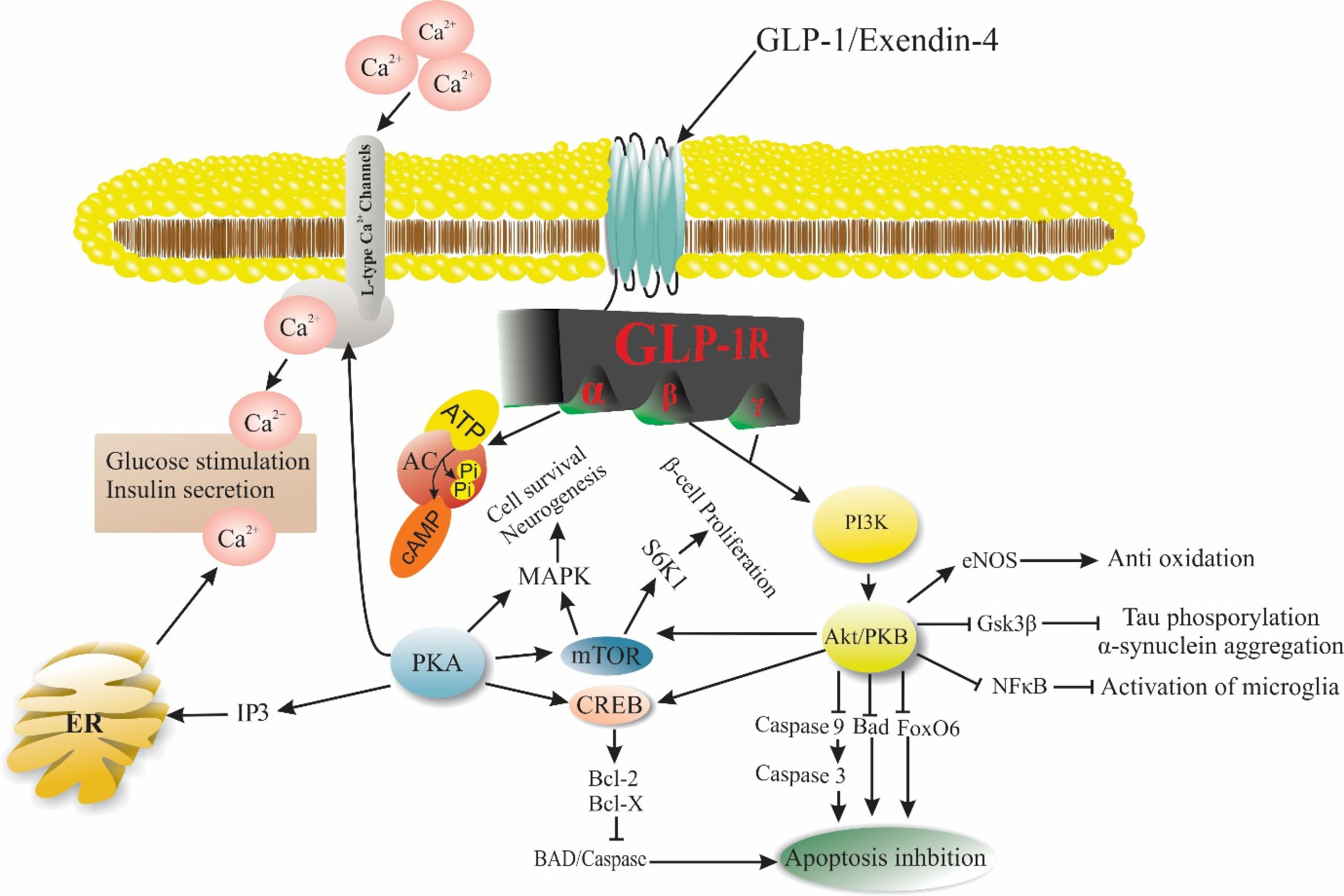
Figure 3.
Exendin-4, a GLP-1R agonist plays a vibrant role in various biological activities such as apoptosis, Beta-cells proliferation, and glucose through different signaling pathways. Abbreviations: GLP-1: Glucagon-like peptide-1, GLP-1R: Glucagon-like peptide-1 receptor, AC: Adenylyl cyclase, PKA: Protein kinase A, IP3: Inositol 3 phosphate, ER: Endoplasmic reticulum, PI3K: Phosphoinositide 3-kinase, PKB/Akt: Protein kinase B, eNOS: Endothelial nitric oxide, Gsk3β: Glycogen synthase kinase 3 beta, NFκB: nuclear factor kappa-light-chain-enhancer of activated B cells, FoxO6: Forkhead Box O6, Bad: Bcl-2-associated death promoter, CREB: cAMP response element-binding, mTOR: mechanistic target of rapamycin, MAPK: mitogen-activated protein kinase, S6K1: Ribosomal protein S6 kinase beta-1.
.
Exendin-4, a GLP-1R agonist plays a vibrant role in various biological activities such as apoptosis, Beta-cells proliferation, and glucose through different signaling pathways. Abbreviations: GLP-1: Glucagon-like peptide-1, GLP-1R: Glucagon-like peptide-1 receptor, AC: Adenylyl cyclase, PKA: Protein kinase A, IP3: Inositol 3 phosphate, ER: Endoplasmic reticulum, PI3K: Phosphoinositide 3-kinase, PKB/Akt: Protein kinase B, eNOS: Endothelial nitric oxide, Gsk3β: Glycogen synthase kinase 3 beta, NFκB: nuclear factor kappa-light-chain-enhancer of activated B cells, FoxO6: Forkhead Box O6, Bad: Bcl-2-associated death promoter, CREB: cAMP response element-binding, mTOR: mechanistic target of rapamycin, MAPK: mitogen-activated protein kinase, S6K1: Ribosomal protein S6 kinase beta-1.
Effect of EX-4 on epitheliogenesis and cutaneous wounds
The accumulation of pro-oxidants and byproducts in squamous and non-squamous epithelial layers leads to cutaneous and gastric wounds in diabetic subjects.
33,34
Lower-extremity amputation may be the only choice by the progression of diabetic foot ulcers after the onset of peripheral neuropathy, peripheral arterial disease, and immunosuppression.
34,35
Epithelialization and wound repair provoke the recruitment of immune cells, fibroblasts, and keratinocytes and endothelial lineage. The successful cutaneous regeneration needs reciprocal cell-to-cell crosstalk through juxtacrine and paracrine interactions. Multiple growth factors such as vasculature endothelial growth factor, transforming growth factor-β (TGF-β), IL-1 and -6, tumor necrosis factor-α (TNF-α)) and other cytokines participate actively in epithelium repair.
36
Systemic and topical administration of Ex-4 was found to decrease superoxide anions content and serum level of IL-6. Ex-4 has also angiogenesis potential in diabetic subjects. For example, it was found that the CD34+/KDR+ positive endothelial progenitor cells in the rat model of diabetes. Based on the data, the tubulogenesis rate increased in Ex-4-treated cells. In line with these finding, protein levels of VEGFR-2, matrix metalloproteinase-2 (MMP-2), p-eNOS, and TGF-β in these cells showing an increased angiogenesis rate. In addition to angiogenesis potential, keratinocyte proliferation rate was also induced in Ex-4 treated rats.
36
In gastro-intestinal tract, the frequency of gastric ulcer is also diminished after administration of Ex-4. The suppression of inflammation rate and oxidative stress is prohibited in the periphery of ulcers. These features are found to confine the progression of ulcers and accelerate the healing.
33
Anti-cancer effect of Ex-4
T2DM has been shown to predispose the risk of many malignancies, such as colon and breast and other cancer types.
37,38
Previous data demonstrated the anti-cancer properties of Ex-4 on cancer cell types such as breast cancer cells.
39
As previously described, Ex-4 has the potential to inhibit breast cancer cell proliferation and metastasis and promote apoptosis by the modulation of effectors such as Caspase-9, Akt, and MMP-2. In addition, the clonogenic property of cancer cells by up-regulation of tissue inhibitor of metalloproteinases-1 and -2 expressions. It was elucidated that the exposure of tumor cells to Ex-4 has the potential to confine tumor mass via engaging GLP-1R signaling pathway. Moreover, Ex-4 has the potential to attenuate ovarian cancer cell proliferation with the induction of GLP-1R mRNA expression.
40
The promotion of pro-inflammatory status governed by NF-κB seems to be a critical tumoricidal mechanism.
41
In other conditions such as pancreas and prostate cancers, the size of tumors decreases after exposure to Ex-4. Ex-4 provokes CD8+ cytotoxic T cells and modulates the function of Foxp3+ regulatory T cells. These changes limit the expansion and progression of cancers.
42
Based on data, the modulatory effect of Ex-4 is different regarding cancer type. For example, the cytotoxic effect of Ex-4 on prostate cancer is mediated by modulation of ERK-MAPK via the cAMP-PKA without affecting apoptotic signaling pathways. Therefore, cautions must be considered in cancer inhibition based on cancer phenotype.
43,44
Insulin resistance is touted as one of the most metabolic disorders during the onset of cancers that lead to cachectic status.
45
Previous studies noted that hyperinsulinemia in response to insulin resistance may lead to tumor expansion by increasing the hepatic IGF-1 level while protein content of insulin-like growth factor binding proteins 1 and 2 decreases.
46
Irrespective of the reasons for induction of insulin production, high levels of insulin provokes estrogen bioactivity which in turn increases the risk of cancer formation in breast.
47
It is suggested to apply a combined therapy for the better tumoricidal effect of Ex-4 in cancer candidates. Simultaneous application of metformin and Ex-4 was found to yield better therapeutic effects.
48
Interestingly; Ex-4 sensitizes cancer cells to ionizing radiation through adenosine monophosphate activated protein kinase (AMPK) activation, contributing to the reduction of colony number and density via reduction of phosphorylated mTOR.
49
The application of Ex-4 in endometrial cancer Ishikawa xenografts in nude mice yielded promising outcomes.
50,51
Honors and Kinzig found the therapeutic effects of Ex-4 in preventing cancer-associated cachexia in male rats with Yoshida sarcoma.
52
However, it seems that the Ex-4 modulatory effect was more prominent in cases with small-sized tumor masses compared to subjects with large sized cancers. Administration of Ex-4 is able to return insulin to basal levels in tumor-bearing candidates. These data stand for a fact that Ex-4 could be used as anti-cancer agent in diabetic subjects susceptible to incidence of different cancer types.
Ex-4 on neurodegenerative disorders
The existence of receptor GLP-1R has been previously described in CNS.
53
In addition to neuroprotective potential of Ex-4 on CNS by the inhibition of apoptosis, this factor is also able to pass across the blood-brain barrier and binds to GLP-1R followed by the activation of adenylyl cyclase, PKC and mitogen-activated protein signaling pathways could regulate brain bioactivity and memory function.
54
The administration of Ex-4 was shown to promote SERCA expression through activation of PKA/cAMP signaling pathways and subsequently leads to inhibition apoptosis after the onset of spinal cord injury.
55
The levels of pro-apoptotic effectors such as Bax, Caspase-3 and cytochrome C are decreased while the expression of Bcl-2.
56
Therefore, it seems that the application of Ex-4 could reverse the detrimental effects of Ex-4 on acute CNS pathologies. Based on data, the onset of diabetic changes could predispose the possibility of degenerative diseases such as Alzheimer’s disease or Parkinson’s disease. Under the emergence of degenerative diseases, hyperinsulinemia and down-regulated insulin signaling per se are seen routinely in the context of CNS.
57
The promotion of degenerative diseases contributes to the unusual aggregation of peptides and/or proteins in the specific regions of the brain. For instance, the deposition of Aβ forms plaques in the extraneuronal area as well as Tau deposited in the form of filaments inside neurons.
58
The introduction of insulin causes to activation of PI3K/AKT and GSK-3β, as two main downstream players in the context of the brain. Ex-4 has the potential to decrease the phosphorylation of GSK-3β and thereby inhibits the bioactivity of tau kinase. Along with these changes, the aggregation of Tau protein is dramatically diminished followed by extracellular protein accumulation removal.
59
Most notably, the administration of Ex-4 could directly increase insulin signaling pathways via phosphorylation of insulin receptor substrate 1 (IRS-1).
60
The phosphorylation of IRS-1 phosphorylation per se increased phosphorylated levels of AKT and GSK-3β contributing to the dephosphorylation of protein tau.
61
The application of Ex-4 in experimentally induced Parkinson’s disease by using 1-methyl-4- phenyl-1, 2, 3, 6-tetrahydropyridine in the model of a mouse, decreased the degeneration of dopaminergic neurons and secretion of MMP-3 released from microglia.
62
The occurrence of ischemic changes and stroke has been reported in diabetic candidates after the endothelial cells’ injury. It seems that the application of Ex-4 in diabetic changes could inhibit endothelial injury by engaging the PI3K/p-Akt/Bcl-xl/Bcl-2 axis.
63
Modulatory effect of Ex-4 cardiovascular disease
It is mighty that diabetic patients are susceptible to cardiovascular disorders.
44
Hyperglycemia has detrimental effects on cardiac structure and function thereby contributing to pathological cardiomyopathy, cardiac hypertrophy,
64
interstitial fibrosis,
65
as well as increased apoptosis and oxidative stress also reported after the onset of insulin resistance.
66
The participation of distinct signaling pathways was reported in diabetic hearts. For instance, it has recently documented that the modulation of AMPK in the pathophysiology of cardiomyocytes metabolism.
67
Therefore, pharmacological activation/inhibition of effector AMPK brings inevitable impacts on the status and intensity of cardiac injury exposed to various metabolic situations. In downstream pathways, AMPK affects the activity of the mTOR signaling pathway that acts as a cross-talk linker between different cell bioactivities.
68
Similar to some mTOR blockers such as rapamycin, activation of GLP-1/GLP-1R axis phosphorylates AMPK and reduces hypertrophic stress.
69
During the occurrence of myocardial infarction (MI), the reduction of Akt-1 and MAPK kinase-3 phosphorylation is seen in the infarcted zones. Ex-4 treatment was found to increase the phosphorylation of these factors and reduce the extent of aberrant remodeling and the number of apoptotic cells juxtaposed to infarct regions.
70
Furthermore, Ex-4 has the potential to promote angiogenesis and neovascularization by the proliferation of vascular endothelial cells and α-smooth muscle actin positive cells toward injured cardiac tissue.
70
In addition, it was shown that Ex-4 exerts a prophylactic role against myocardial injury via the activation of the Sirt1/PGC1α signaling pathway. The activation of this axis could promote mitochondrial function coincided with the suppression of oxidative stress insults.
71
APPL1 plays versatile roles to inhibit cardiomyocytes apoptosis via the interaction with systemic adipokines, adiponectin. This interaction per se contributes to the promotion of AMPK and proliferator-activated receptor-α (PPAR-α). Under the occurrence if MI, Ex-4 decreases cardiac tissue apoptosis by amplifying the systemic adiponectin concentration and APPL1 activation and phosphorylation of AMPK.
72
Other cardiovascular abnormalities mainly heart failure happens due to the disruption in calcium recycling. Ex-4 ameliorates Ca2+ homeostasis in HF subjects by promoting eNOS/cGMP/PKG axis and of restoration of SERCA2a activity. These changes reduce cytoplasmic Ca2+ content and activity of CaMKII.
24
The uncontrolled proliferation and migration of vascular smooth muscle cells (VSMCs) are touted as one of the risk factors resulting in atherosclerosis. During the development of atherosclerotic plaques, VSMCs undergo phenotype shifting with an enhanced proliferation rate.
73
Along with these changes, the dynamic of multiple factors mainly angiotensins are changed. Of note, angiotensin-II is the main peptide that part take in the progression of atherosclerotic pathologies by the induction of VSMCs proliferation and migration via controlling the enzymes catalyze the phosphorylation of extracellular signal-regulated kinase 1/2 and JNK.
74
Scientific literature added a notion that Ex-4 administration inhibits Ang II-induced phosphorylation of ERK1/2 and JNK, improving hypertension and atherosclerosis in the candidate population.
75
In addition to critical role of ERK1/2 and JNK in the dynamic growth of VSMCs, some studies have revealed that nuclear receptor superfamily like neuron derived orphan receptor 1 (NOR1), one of the key regulators of VSMCs proliferation during the occurrence of atherosclerosis, activity removal may lead to the control of neo-intima formation in injured vascular context. The incubation of VSMCs with Ex-4 precisely regulates the proliferation of these cells through inhibiting NOR1 promoter activity.
76
In patients with pulmonary arterial hypertension (PAH), a serious disorder which affects near to 30% of patients suffering from congenital heart disease is originated from extreme pulmonary blood flow due to excessive drug use, a genetic mutation in different factors like bone morphogenetic protein receptor type II.
77,78
By applying Ex-4, the improvement of PAH is initiated via the reduction of pro-inflammatory cytokines interleukin-1α and 1β in PAH rats. After incubation of PAH rats with Ex-4, the function of right ventricular function was restored the number of the amount of Smooth muscle myosin heavy chain class II and α-SMA.
79
Dynamic of Ex-4 in renal tissue
Diabetic nephropathy is the consequent of T2DM. The elevation of excessive glucose contents injures the renal filtration system, thereby contributing to the occurrence of proteinuria or macroalbuminuria.
80
The promotion of diabetic nephropathy causes to chronic kidney disease by time, leading to renal insufficiency. The possibility of infectious nephritis is also reported in diabetic conditions.
81
Commensurate with these data, lowering systemic glucose contents seems a priority in the control of nephropathy. The application of Ex-4 in diabetic nephropathy has shown satisfactory results rat models by the increase of fasting insulin levels.
82
The serum levels of creatinine and blood urea nitrogen were also reduced after EX-4 administration. It seems that Ex-4 has the ability to alleviate oxidative stress via decreasing malondialdehyde production and decrease of lipid peroxidation rate while the bioactivity of superoxide dismutase and glutathione peroxidase were also induced.
83
One of the most therapeutic aspects related to Ex-4 therapeutic effects correlates with the anti-inflammatory property via the control of TNF-α, IL-6, hypersensitive C-reactive protein and CCL5 as commonly described in end-stage renal failure.
84,85
Scientific literature highlighted the potent role of Ex-4 in alleviating renal proximal tubular cell injury in diabetic mice. It was established that the levels of IL-1β, TNF-α and ROS content and malondialdehyde (MDA) levels were increased in renal tissue with the progression of disease. The development of diabetic condition predisposes the initiation of tubulointerstitial fibrosis that hastens renal failure.
86
During T2DM hyperglycemic condition, the accumulation of extracellular matrix is extensively promoted in spaces between renal tubular epithelial cells. The occupation of connective tissue with the extracellular matrix can make multiple resident renal cells to release extracellular vesicles containing proteins, mRNA, and miRNA, contributing to the progression of fibrotic changes.
87
The application of Ex-4 decreases diabetic-associated fibrosis by decreasing TGF-β. Along with these changes, the proliferation of fibroblasts and collagen deposition are decreased in renal niche.
85
Another modulatory effect of Ex-4 in reducing diabetes associated fibrosis could be related to changes in extracellular vesicle cargoes. It seems that the type and intensity of specific miRNAs and factors are modulated that subsequently decreases diabetic-fibrosis.
88
In addition to diabetes-related nephropathies, cardio-renal syndrome (CRS) is defined as a common disorder between heart and kidneys in which any abnormalities in one of these organs may impact other tissue and vice versa. CRS is classified into five subtypes (Table 2).
89
As above-mentioned, T2DM is a major risk factor that affects both organs via engaging different mechanisms such as hypertension, oxidative stress or insulin resistance. For instance, T2DM-derived heart failure or left cardiac ventricular dysfunction causes renal abnormalities.
90
Studies have confirmed that combined therapy of Ex-4 and melatonin can be effective in preserving renal and cardiac functionality in CRS subjected. Using Ex-4 and melatonin in a rat model of CRS, significant favor effects were observed in renal function via decreasing pro-inflammatory factors (TNF-α, NF-κB, MMP-9, RANTES, and iNOS) and oxidative stress (NOX-1, -2 and -4) markers. Besides, the synthesis of apoptosis (Bax, cleaved Caspase 3 and cleaved PARP), fibrosis (Samd3 and TGF-β) markers and DNA damage (γ-H2AX) is inhibited after Ex-4 and melatonin treatment. Pathological features were decreased after using Ex-4 and melatonin in CRS patients which indicated the effective impact of this method on kidney function.
91
Table 2.
Cardiorenal syndrome subtypes and explanations
|
CRS type
|
Definition
|
| Type 1: Acute cardio-renal |
Acute heart dysfunction leads to kidney injury or dysfunction |
| Type 2: Chronic cardio-renal |
Chronic heart dysfunction leads to kidney injury or dysfunction |
| Type 3: Acute reno-cardiac |
Acute kidney dysfunction leads to heart injury or dysfunction |
| Type 4: Chronic reno-cardiac |
Chronic kidney dysfunction leads to heart injury or dysfunction |
| Type 5: Secondary CRS |
Systemic conditions leading to simultaneous injury and/or dysfunction of heart and kidney |
Furthermore, Ex-4 along with melatonin improved mitochondrial function and suppressed βMHC mRNA/protein expression, resulting in the alleviation of cardiac hypertrophy, is decreased. These findings indicated the compensatory role of Ex-4 and melatonin treatment on cardiac function.
92
Effect of Ex-4 in the function of hepatic tissue
Due to the high-rate metabolic activity of hepatic tissue, this tissue is eligible for different metabolic disorders and diseases. Non-alcoholic fatty liver diseases (NAFLDs) are clinically determined with the accumulation of byproduct three acyl glycerol (TAG) in the hepatic tissue that coincided with the development of intracytoplasmic lipid droplets affecting over 5% of total hepatocytes. Beside, an excessive lipid accumulation causes to hepatic cells lipotoxicity and oxidative stress via increased lipid peroxidation, mitochondrial insufficiency, and high content of ROS production.
93
A close relation between T2DM and NAFLD has been previously well-established in which insulin-resistance and compensatory hyperinsulinemia are commonly seen by defective lipid metabolism and accumulation of triglyceride in hepatic tissue in patients with NAFLD or T2DM associate β-cell dysfunction.
94
Along with these changes, NAFLD can lead to hepatic steatosis, NASH, hepatic cirrhosis, and hepatocellular carcinoma.
95
In patients with T2DM, both glucose and lipid metabolisms are deteriorated, leading to the increase of plasma levels of low-density lipoprotein, and adversely reduction of high-density lipoprotein.
96
In the case of NAFLD, the content of different serum transaminases is slightly raised with NAFLD and the administration of GLP-1R could decrease systemic transaminase levels via the promotion of hepatic lipid metabolism. One possible mechanism is that hepatic lipid metabolism is activated via triggering GLP-1R signaling cascade and the promotion of PPAR-α. These changes diminish the synthesis of apolipoprotein C which accelerates the degradation of fatty acid in plasma, after the use of Ex-4 and stimulation of GLP-1R signaling pathway.
97
Experiments have shown that incubation of ob/ob mice with Ex-4 exerted anti-hepatic steatosis and -fibrosis capacities.
98
The modulatory effect of Ex-4 on hepatic steatosis is done by reducing lipid droplets. The deposition of collagens along the hepatic sinusoids in the fibrotic liver is reduced after Ex-4 treatment. It was found that Ex-4 increased the reduced PPAR-α and glucose transporter 4 expressions in ob/ob mice.
98
The activity of FTO correlates with energy homeostasis and the control of energy consumption. Tha is gene is commonly upregulated in NFLD candidates, contributing to oxidative stress indicated by an increased MDA production and decrease of superoxide dismutase activity. Meanwhile, the rate of lipogenesis is increased inside hepatocytes. Under these conditions, the basal dynamic of effectors such as PI3K and Akt is prohibited.
In NFLD animal models, the use of Ex-4 leads to alleviation of NFLD related complications via suppression of FTO expression and MDA production and restoration Superoxide dismutase activity.
99
In addition, the expression of genes participating in fatty acid oxidation promotes hepatocyte functional behavior after phosphorylation of Akt.
99
As above-mentioned, steatosis is routinely reported in the NFLD patients correlated with an increased lipogenesis rate and TAGs accumulation inside hepatocytes.
100
It is thought that the promotion of Wnt/β-catenin signaling could prohibit adipogenesis by suppressing CCAAT/enhancer-binding proteins-induced PPARγ expression in which a decrease in the expression of β-catenin was unveiled in in vitro model of steatosis. Ex-4 has the ability to activate β-catenin which in turn stops lipogenesis by suppression of PPARγ and SREBP-1.
101
The use of Ex-4 in steatoric patients remove excessive fatty acids via regulating lipid metabolism and reducing VLDL synthesis after suppression of genes such as peroxisome Pgc1β, transcription factor Srebp-1c and Fasn, Dgat1, and Apob. Fasn participates in de novo lipogenesis and Dgat1 promotes the last steps in hepatic three glyceride synthesis.
102
It was found that Sirt-1 expression is decreased in high-fat-diet animal models and Ex-4 could reverse the Sirt-1 function either in in vivo and in vitro modelscoincided with nicotinamide phosphoribosyl transferase (Nampt) activity. The Nampt/visfatin enzyme could catalyze the conversion of NAM toward NAD+ and is required for Sirt-1 activity.
103
As previously mentioned, endoplasmic reticulum (ER) plays a critical role in lipid and protein biosynthesis.
104
Disruption of ER homeostasis contributes to the accumulation of misfolded/unfolded proteins seen in obesity. Dysfunction of insulin function and T2DM also has a pivotal role in fatty liver disease development.
74
Progressive ER stress contributes to the development of NFLD and nonalcoholic steatohepatitis by different molecular mechanisms, including lipogenesis, inflammation, apoptosis, and autophagy.
105
Documents showed that Ex-4 can alleviate hepatic steatosis and ER stress via controlling lipin-1β/α ratio and lipid hemostasis through Sirt1 and AMPK signaling pathways.
106
Hepatic glycoproteins like SEPP1 and fetuin-A are known as novel biomarkers who participated in insulin resistance and NFLD. Overactivity of these glycoproteins in palmitic acid-treated hepatocytes contributes to ER stress after Ex-4 treatment via activating Sirt-1 and AMPK expression.
107
Conclusion
According to versatile functions of Ex-4 in different tissues and similar activities to GLP-1, it seems that the use of Ex-4 is possibly helpful in the modulation of pathologies correlated with insulin dysfunction and the promotion of GLP-1R signaling pathway.
Ethical Issue
Not applicable
Conflict of Interest
The authors have no conflict of interest to declare.
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37 Suppl 1:S81-90. doi: 10.2337/dc14-S081 [Crossref] [ Google Scholar]
- Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 2008; 26(2):77-82. doi: 10.2337/diaclin.26.2.77 [Crossref] [ Google Scholar]
- Roussel R, Travert F, Pasquet B, Wilson PW, Smith SC Jr, Goto S. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med 2010; 170(21):1892-9. doi: 10.1001/archinternmed.2010.409 [Crossref] [ Google Scholar]
- Raskin P, Allen E, Hollander P, Lewin A, Gabbay RA, Hu P. Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care 2005; 28(2):260-5. doi: 10.2337/diacare.28.2.260 [Crossref] [ Google Scholar]
- Cirincione B, Mager DE. Population pharmacokinetics of exenatide. Br J Clin Pharmacol 2017; 83(3):517-26. doi: 10.1111/bcp.13135 [Crossref] [ Google Scholar]
- Simonsen L, Holst JJ, Deacon CF. Exendin-4, but not glucagon-like peptide-1, is cleared exclusively by glomerular filtration in anaesthetised pigs. Diabetologia 2006; 49(4):706-12. doi: 10.1007/s00125-005-0128-9 [Crossref] [ Google Scholar]
- Mann RJ, Nasr NE, Sinfield JK, Paci E, Donnelly D. The major determinant of exendin-4/glucagon-like peptide 1 differential affinity at the rat glucagon-like peptide 1 receptor N-terminal domain is a hydrogen bond from SER-32 of exendin-4. Br J Pharmacol 2010; 160(8):1973-84. doi: 10.1111/j.1476-5381.2010.00834.x [Crossref] [ Google Scholar]
- Cho YM, Wideman RD, Kieffer TJ. Clinical application of glucagon-like peptide 1 receptor agonists for the treatment of type 2 diabetes mellitus. Endocrinol Metab (Seoul) 2013; 28(4):262-74. doi: 10.3803/EnM.2013.28.4.262 [Crossref] [ Google Scholar]
- Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem 1986; 261(25):11880-9. [ Google Scholar]
- Vilsbøll T. The effects of glucagon-like peptide-1 on the beta cell. Diabetes Obes Metab 2009; 11 Suppl 3:11-8. doi: 10.1111/j.1463-1326.2009.01073.x [Crossref] [ Google Scholar]
- Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A 1992; 89(18):8641-5. doi: 10.1073/pnas.89.18.8641 [Crossref] [ Google Scholar]
- Brubaker PL, Drucker DJ. Structure-function of the glucagon receptor family of G protein-coupled receptors: the glucagon, GIP, GLP-1, and GLP-2 receptors. Recept Channels 2002; 8(3-4):179-88. doi: 10.3109/10606820213687 [Crossref] [ Google Scholar]
- Kim DS, Choi HI, Wang Y, Luo Y, Hoffer BJ, Greig NH. A new treatment strategy for Parkinson’s disease through the gut-brain axis: the glucagon-like peptide-1 receptor pathway. Cell Transplant 2017; 26(9):1560-71. doi: 10.1177/0963689717721234 [Crossref] [ Google Scholar]
- Donnelly D. The structure and function of the glucagon-like peptide-1 receptor and its ligands. Br J Pharmacol 2012; 166(1):27-41. doi: 10.1111/j.1476-5381.2011.01687.x [Crossref] [ Google Scholar]
-
Eng J. Exendin-3 and Exendin-4 Polypeptides, and Pharmaceutical Compositions Comprising Same. Google Patents; 1995.
- Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2003; 88(7):3082-9. doi: 10.1210/jc.2002-021545 [Crossref] [ Google Scholar]
- Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 2012; 62(5-6):1916-27. doi: 10.1016/j.neuropharm.2011.12.022 [Crossref] [ Google Scholar]
- Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 1995; 136(8):3585-96. doi: 10.1210/endo.136.8.7628397 [Crossref] [ Google Scholar]
- Underwood CR, Garibay P, Knudsen LB, Hastrup S, Peters GH, Rudolph R. Crystal structure of glucagon-like peptide-1 in complex with the extracellular domain of the glucagon-like peptide-1 receptor. J Biol Chem 2010; 285(1):723-30. doi: 10.1074/jbc.M109.033829 [Crossref] [ Google Scholar]
- de Maturana RL, Willshaw A, Kuntzsch A, Rudolph R, Donnelly D. The isolated N-terminal domain of the glucagon-like peptide-1 (GLP-1) receptor binds exendin peptides with much higher affinity than GLP-1. J Biol Chem 2003; 278(12):10195-200. doi: 10.1074/jbc.M212147200 [Crossref] [ Google Scholar]
- Erdogdu O, Nathanson D, Sjöholm A, Nyström T, Zhang Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol 2010; 325(1-2):26-35. doi: 10.1016/j.mce.2010.04.022 [Crossref] [ Google Scholar]
- Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene 2003; 22(56):8983-98. doi: 10.1038/sj.onc.1207115 [Crossref] [ Google Scholar]
- Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev 2004; 30(2):193-204. doi: 10.1016/j.ctrv.2003.07.007 [Crossref] [ Google Scholar]
- Chen J, Wang D, Wang F, Shi S, Chen Y, Yang B. Exendin-4 inhibits structural remodeling and improves Ca2+ homeostasis in rats with heart failure via the GLP-1 receptor through the eNOS/cGMP/PKG pathway. Peptides 2017; 90:69-77. doi: 10.1016/j.peptides.2017.02.008 [Crossref] [ Google Scholar]
- King D, Yeomanson D, Bryant HE. PI3King the lock: targeting the PI3K/Akt/mTOR pathway as a novel therapeutic strategy in neuroblastoma. J Pediatr Hematol Oncol 2015; 37(4):245-51. doi: 10.1097/mph.0000000000000329 [Crossref] [ Google Scholar]
- Wang C, Chen X, Ding X, He Y, Gu C, Zhou L. Exendin-4 promotes beta cell proliferation via PI3k/Akt signalling pathway. Cell Physiol Biochem 2015; 35(6):2223-32. doi: 10.1159/000374027 [Crossref] [ Google Scholar]
- Xie J, El Sayed NM, Qi C, Zhao X, Moore CE, Herbert TP. Exendin-4 stimulates islet cell replication via the IGF1 receptor activation of mTORC1/S6K1. J Mol Endocrinol 2014; 53(1):105-15. doi: 10.1530/jme-13-0200 [Crossref] [ Google Scholar]
- Wei Q, Sun YQ, Zhang J. Exendin-4, a glucagon-like peptide-1 receptor agonist, inhibits cell apoptosis induced by lipotoxicity in pancreatic β-cell line. Peptides 2012; 37(1):18-24. doi: 10.1016/j.peptides.2012.06.018 [Crossref] [ Google Scholar]
- Liu L, Wang Y, Wang L, Lin Y, Liu X, Liu X. Exendin-4 protects murine pancreatic β-cells from free fatty acid-induced apoptosis through PI-3K signaling. Endocr Res 2013; 38(1):40-7. doi: 10.3109/07435800.2012.713423 [Crossref] [ Google Scholar]
- Wu L, Liu X, Wang L, Wang Y, Wang L, Guan B. Exendin-4 protects HUVECs from tunicamycin-induced apoptosis via inhibiting the IRE1a/JNK/caspase-3 pathway. Endocrine 2017; 55(3):764-72. doi: 10.1007/s12020-016-1190-4 [Crossref] [ Google Scholar]
- Zhang C, Shao M, Yang H, Chen L, Yu L, Cong W. Attenuation of hyperlipidemia- and diabetes-induced early-stage apoptosis and late-stage renal dysfunction via administration of fibroblast growth factor-21 is associated with suppression of renal inflammation. PLoS One 2013; 8(12):e82275. doi: 10.1371/journal.pone.0082275 [Crossref] [ Google Scholar]
- Rydén A, Nyman G, Nalin L, Andreasson S, Velikyan I, Korsgren O. Cardiovascular side-effects and insulin secretion after intravenous administration of radiolabeled exendin-4 in pigs. Nucl Med Biol 2016; 43(7):397-402. doi: 10.1016/j.nucmedbio.2016.04.002 [Crossref] [ Google Scholar]
- Chen YC, Ho CC, Yi CH, Liu XZ, Cheng TT, Lam CF. Exendin-4, a glucagon-like peptide-1 analogue accelerates healing of chronic gastric ulcer in diabetic rats. PLoS One 2017; 12(11):e0187434. doi: 10.1371/journal.pone.0187434 [Crossref] [ Google Scholar]
- Kasznicki J, Kosmalski M, Sliwinska A, Mrowicka M, Stanczyk M, Majsterek I. Evaluation of oxidative stress markers in pathogenesis of diabetic neuropathy. Mol Biol Rep 2012; 39(9):8669-78. doi: 10.1007/s11033-012-1722-9 [Crossref] [ Google Scholar]
- Aumiller WD, Dollahite HA. Pathogenesis and management of diabetic foot ulcers. JAAPA 2015; 28(5):28-34. doi: 10.1097/01.JAA.0000464276.44117.b1 [Crossref] [ Google Scholar]
- Roan JN, Cheng HN, Young CC, Lee CJ, Yeh ML, Luo CY. Exendin-4, a glucagon-like peptide-1 analogue, accelerates diabetic wound healing. J Surg Res 2017; 208:93-103. doi: 10.1016/j.jss.2016.09.024 [Crossref] [ Google Scholar]
- Yuhara H, Steinmaus C, Cohen SE, Corley DA, Tei Y, Buffler PA. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer?. Am J Gastroenterol 2011; 106(11):1911-21. doi: 10.1038/ajg.2011.301 [Crossref] [ Google Scholar]
- Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am J Clin Nutr 2007; 86(3):s823-35. doi: 10.1093/ajcn/86.3.823S [Crossref] [ Google Scholar]
- Fidan-Yaylalı G, Dodurga Y, Seçme M, Elmas L. Antidiabetic exendin-4 activates apoptotic pathway and inhibits growth of breast cancer cells. Tumour Biol 2016; 37(2):2647-53. doi: 10.1007/s13277-015-4104-9 [Crossref] [ Google Scholar]
- He W, Yu S, Wang L, He M, Cao X, Li Y. Exendin-4 inhibits growth and augments apoptosis of ovarian cancer cells. Mol Cell Endocrinol 2016; 436:240-9. doi: 10.1016/j.mce.2016.07.032 [Crossref] [ Google Scholar]
- Iwaya C, Nomiyama T, Komatsu S, Kawanami T, Tsutsumi Y, Hamaguchi Y. Exendin-4, a glucagonlike peptide-1 receptor agonist, attenuates breast cancer growth by inhibiting NF-κB activation. Endocrinology 2017; 158(12):4218-32. doi: 10.1210/en.2017-00461 [Crossref] [ Google Scholar]
- He L, Law PTY, Wong CK, Chan JCN, Chan PKS. Exendin-4 exhibits enhanced anti-tumor effects in diabetic mice. Sci Rep 2017; 7(1):1791. doi: 10.1038/s41598-017-01952-5 [Crossref] [ Google Scholar]
- Rodríguez-Berriguete G, Fraile B, Martínez-Onsurbe P, Olmedilla G, Paniagua R, Royuela M. MAP kinases and prostate cancer. J Signal Transduct 2012; 2012:169170. doi: 10.1155/2012/169170 [Crossref] [ Google Scholar]
- Nomiyama T, Kawanami T, Irie S, Hamaguchi Y, Terawaki Y, Murase K. Exendin-4, a GLP-1 receptor agonist, attenuates prostate cancer growth. Diabetes 2014; 63(11):3891-905. doi: 10.2337/db13-1169 [Crossref] [ Google Scholar]
- Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D. Cachexia: a new definition. Clin Nutr 2008; 27(6):793-9. doi: 10.1016/j.clnu.2008.06.013 [Crossref] [ Google Scholar]
- Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer 2009; 16(4):1103-23. doi: 10.1677/erc-09-0087 [Crossref] [ Google Scholar]
- Wairagu PM, Phan AN, Kim MK, Han J, Kim HW, Choi JW. Insulin priming effect on estradiol-induced breast cancer metabolism and growth. Cancer Biol Ther 2015; 16(3):484-92. doi: 10.1080/15384047.2015.1016660 [Crossref] [ Google Scholar]
- Tsutsumi Y, Nomiyama T, Kawanami T, Hamaguchi Y, Terawaki Y, Tanaka T. Combined treatment with exendin-4 and metformin attenuates prostate cancer growth. PLoS One 2015; 10(10):e0139709. doi: 10.1371/journal.pone.0139709 [Crossref] [ Google Scholar]
- Zhang Y, Xu F, Liang H, Cai M, Wen X, Li X. Exenatide inhibits the growth of endometrial cancer Ishikawa xenografts in nude mice. Oncol Rep 2016; 35(3):1340-8. doi: 10.3892/or.2015.4476 [Crossref] [ Google Scholar]
- He W, Li J. Exendin-4 enhances radiation response of prostate cancer. Prostate 2018; 78(15):1125-33. doi: 10.1002/pros.23687 [Crossref] [ Google Scholar]
- Fader AN, Arriba LN, Frasure HE, von Gruenigen VE. Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol 2009; 114(1):121-7. doi: 10.1016/j.ygyno.2009.03.039 [Crossref] [ Google Scholar]
- Honors MA, Kinzig KP. Chronic exendin-4 treatment prevents the development of cancer cachexia symptoms in male rats bearing the Yoshida sarcoma. Horm Cancer 2014; 5(1):33-41. doi: 10.1007/s12672-013-0163-9 [Crossref] [ Google Scholar]
- Hamilton A, Hölscher C. Receptors for the incretin glucagon-like peptide-1 are expressed on neurons in the central nervous system. Neuroreport 2009; 20(13):1161-6. doi: 10.1097/WNR.0b013e32832fbf14 [Crossref] [ Google Scholar]
- Yamamoto K, Amako M, Yamamoto Y, Tsuchihara T, Nukada H, Yoshihara Y. Therapeutic effect of exendin-4, a long-acting analogue of glucagon-like peptide-1 receptor agonist, on nerve regeneration after the crush nerve injury. Biomed Res Int 2013; 2013:315848. doi: 10.1155/2013/315848 [Crossref] [ Google Scholar]
- Sun Z, Liu Y, Kong X, Wang R, Xu Y, Shang C. Exendin-4 plays a protective role in a rat model of spinal cord injury through SERCA2. Cell Physiol Biochem 2018; 47(2):617-29. doi: 10.1159/000490017 [Crossref] [ Google Scholar]
- Li H, Jia Z, Li G, Zhao X, Sun P, Wang J. Neuroprotective effects of exendin-4 in rat model of spinal cord injury via inhibiting mitochondrial apoptotic pathway. Int J Clin Exp Pathol 2015; 8(5):4837-43. [ Google Scholar]
- Yang Y, Hu S, Zhang J, Zhang M, Gong C. Alzheimer-like hyperphosphorylation of tau in brains of rats with obesity and type 2 diabetes. Prog Biochem Biophys 2006; 33(5):458-64. [ Google Scholar]
- Ahmad K, Baig MH, Mushtaq G, Kamal MA, Greig NH, Choi I. Commonalities in biological pathways, genetics, and cellular mechanism between Alzheimer disease and other neurodegenerative diseases: an in silico-updated overview. Curr Alzheimer Res 2017; 14(11):1190-7. doi: 10.2174/1567205014666170203141151 [Crossref] [ Google Scholar]
- Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol 2011; 225(1):54-62. doi: 10.1002/path.2912 [Crossref] [ Google Scholar]
- Yang Y, Ma D, Xu W, Chen F, Du T, Yue W. Exendin-4 reduces tau hyperphosphorylation in type 2 diabetic rats via increasing brain insulin level. Mol Cell Neurosci 2016; 70:68-75. doi: 10.1016/j.mcn.2015.10.005 [Crossref] [ Google Scholar]
- Zhu H, Zhang W, Zhao Y, Shu X, Wang W, Wang D. GSK3β-mediated tau hyperphosphorylation triggers diabetic retinal neurodegeneration by disrupting synaptic and mitochondrial functions. Mol Neurodegener 2018; 13(1):62. doi: 10.1186/s13024-018-0295-z [Crossref] [ Google Scholar]
- Kim S, Moon M, Park S. Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson’s disease. J Endocrinol 2009; 202(3):431-9. doi: 10.1677/joe-09-0132 [Crossref] [ Google Scholar]
- Xie Z, Enkhjargal B, Wu L, Zhou K, Sun C, Hu X. Exendin-4 attenuates neuronal death via GLP-1R/PI3K/Akt pathway in early brain injury after subarachnoid hemorrhage in rats. Neuropharmacology 2018; 128:142-51. doi: 10.1016/j.neuropharm.2017.09.040 [Crossref] [ Google Scholar]
- Han SS, Wang G, Jin Y, Ma ZL, Jia WJ, Wu X. Investigating the mechanism of hyperglycemia-induced fetal cardiac hypertrophy. PLoS One 2015; 10(9):e0139141. doi: 10.1371/journal.pone.0139141 [Crossref] [ Google Scholar]
- Liu C, Fu H, Li J, Yang W, Cheng L, Liu T. Hyperglycemia aggravates atrial interstitial fibrosis, ionic remodeling and vulnerability to atrial fibrillation in diabetic rabbits. Anadolu Kardiyol Derg 2012; 12(7):543-50. doi: 10.5152/akd.2012.188 [Crossref] [ Google Scholar]
- Singh VP, Le B, Khode R, Baker KM, Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes 2008; 57(12):3297-306. doi: 10.2337/db08-0805 [Crossref] [ Google Scholar]
- Wong AK, Howie J, Petrie JR, Lang CC. AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin Sci (Lond) 2009; 116(8):607-20. doi: 10.1042/cs20080066 [Crossref] [ Google Scholar]
- Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K, Hara K. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells 2003; 8(1):65-79. doi: 10.1046/j.1365-2443.2003.00615.x [Crossref] [ Google Scholar]
- Zhou Y, He X, Chen Y, Huang Y, Wu L, He J. Exendin-4 attenuates cardiac hypertrophy via AMPK/mTOR signaling pathway activation. Biochem Biophys Res Commun 2015; 468(1-2):394-9. doi: 10.1016/j.bbrc.2015.09.179 [Crossref] [ Google Scholar]
- Du J, Zhang L, Wang Z, Yano N, Zhao YT, Wei L. Exendin-4 induces myocardial protection through MKK3 and Akt-1 in infarcted hearts. Am J Physiol Cell Physiol 2016; 310(4):C270-83. doi: 10.1152/ajpcell.00194.2015 [Crossref] [ Google Scholar]
- Cai YY, Zou SZ, Fan CX, Wu CY, Fang S, Li P. [Exendin-4 alleviates diabetic cardiomyopathy in mice by regulating Sirt1/PGC1α]. Nan Fang Yi Ke Da Xue Xue Bao 2018; 38(5):520-6. doi: 10.3969/j.issn.1673-4254.2018.05.03 [Crossref] [ Google Scholar]
- XiaoTian L, QiNan W, XiaGuang G, WuQuan D, Bing C, ZiWen L. Exenatide activates the APPL1-AMPK-PPARα axis to prevent diabetic cardiomyocyte apoptosis. J Diabetes Res 2016; 2016:4219735. doi: 10.1155/2016/4219735 [Crossref] [ Google Scholar]
- Tabas I, García-Cardeña G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol 2015; 209(1):13-22. doi: 10.1083/jcb.201412052 [Crossref] [ Google Scholar]
- Özcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Özdelen E. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004; 306(5695):457-61. doi: 10.1126/science.1103160 [Crossref] [ Google Scholar]
- Nagayama K, Kyotani Y, Zhao J, Ito S, Ozawa K, Bolstad FA. Exendin-4 prevents vascular smooth muscle cell proliferation and migration by angiotensin II via the inhibition of ERK1/2 and JNK signaling pathways. PLoS One 2015; 10(9):e0137960. doi: 10.1371/journal.pone.0137960 [Crossref] [ Google Scholar]
- Takahashi H, Nomiyama T, Terawaki Y, Kawanami T, Hamaguchi Y, Tanaka T. GLP-1 receptor agonist exendin-4 attenuates NR4A orphan nuclear receptor NOR1 expression in vascular smooth muscle cells. J Atheroscler Thromb 2019; 26(2):183-97. doi: 10.5551/jat.43414 [Crossref] [ Google Scholar]
- Engelfriet PM, Duffels MG, Möller T, Boersma E, Tijssen JG, Thaulow E. Pulmonary arterial hypertension in adults born with a heart septal defect: the Euro Heart Survey on adult congenital heart disease. Heart 2007; 93(6):682-7. doi: 10.1136/hrt.2006.098848 [Crossref] [ Google Scholar]
- Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009; 54(1 Suppl):S43-S54. doi: 10.1016/j.jacc.2009.04.012 [Crossref] [ Google Scholar]
- Roan JN, Hsu CH, Fang SY, Tsai HW, Luo CY, Huang CC. Exendin-4 improves cardiovascular function and survival in flow-induced pulmonary hypertension. J Thorac Cardiovasc Surg 2018; 155(4):1661-9. doi: 10.1016/j.jtcvs.2017.10.085 [Crossref] [ Google Scholar]
- Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 2005; 28(1):164-76. doi: 10.2337/diacare.28.1.164 [Crossref] [ Google Scholar]
- Chen CH, Cheng BC, Chen KH, Shao PL, Sung PH, Chiang HJ. Combination therapy of exendin-4 and allogenic adipose-derived mesenchymal stem cell preserved renal function in a chronic kidney disease and sepsis syndrome setting in rats. Oncotarget 2017; 8(59):100002-20. doi: 10.18632/oncotarget.21727 [Crossref] [ Google Scholar]
- Gao W, Jusko WJ. Pharmacokinetic and pharmacodynamic modeling of exendin-4 in type 2 diabetic Goto-Kakizaki rats. J Pharmacol Exp Ther 2011; 336(3):881-90. doi: 10.1124/jpet.110.175752 [Crossref] [ Google Scholar]
- Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006; 29(2):254-8. doi: 10.2337/diacare.29.02.06.dc05-1558 [Crossref] [ Google Scholar]
- Wang X, Li Z, Huang X, Li F, Liu J, Li Z. An experimental study of exenatide effects on renal injury in diabetic rats1. Acta Cir Bras 2019; 34(1):e20190010000001. doi: 10.1590/s0102-865020190010000001 [Crossref] [ Google Scholar]
- Sancar-Bas S, Gezginci-Oktayoglu S, Bolkent S. Exendin-4 attenuates renal tubular injury by decreasing oxidative stress and inflammation in streptozotocin-induced diabetic mice. Growth Factors 2015; 33(5-6):419-29. doi: 10.3109/08977194.2015.1125349 [Crossref] [ Google Scholar]
- Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest 2010; 120(11):4040-54. doi: 10.1172/jci43025 [Crossref] [ Google Scholar]
- Borges FT, Melo SA, Özdemir BC, Kato N, Revuelta I, Miller CA. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol 2013; 24(3):385-92. doi: 10.1681/asn.2012101031 [Crossref] [ Google Scholar]
- Jia Y, Zheng Z, Guan M, Zhang Q, Li Y, Wang L. Exendin-4 ameliorates high glucose-induced fibrosis by inhibiting the secretion of miR-192 from injured renal tubular epithelial cells. Exp Mol Med 2018; 50(5):1-13. doi: 10.1038/s12276-018-0084-3 [Crossref] [ Google Scholar]
- Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol 2008; 52(19):1527-39. doi: 10.1016/j.jacc.2008.07.051 [Crossref] [ Google Scholar]
- Sarraf M, Masoumi A, Schrier RW. Cardiorenal syndrome in acute decompensated heart failure. Clin J Am Soc Nephrol 2009; 4(12):2013-26. doi: 10.2215/cjn.03150509 [Crossref] [ Google Scholar]
- Chen KH, Chen CH, Wallace CG, Chen YT, Yang CC, Sung PH. Combined therapy with melatonin and exendin-4 effectively attenuated the deterioration of renal function in rat cardiorenal syndrome. Am J Transl Res 2017; 9(2):214-29. [ Google Scholar]
- Chua S, Lee FY, Chiang HJ, Chen KH, Lu HI, Chen YT. The cardioprotective effect of melatonin and exendin-4 treatment in a rat model of cardiorenal syndrome. J Pineal Res 2016; 61(4):438-56. doi: 10.1111/jpi.12357 [Crossref] [ Google Scholar]
- Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology 2014; 59(2):713-23. doi: 10.1002/hep.26672 [Crossref] [ Google Scholar]
- Forlani G, Giorda C, Manti R, Mazzella N, De Cosmo S, Rossi MC. The burden of NAFLD and its characteristics in a nationwide population with type 2 diabetes. J Diabetes Res 2016; 2016:2931985. doi: 10.1155/2016/2931985 [Crossref] [ Google Scholar]
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 2006; 43(2 Suppl 1):S99-S112. doi: 10.1002/hep.20973 [Crossref] [ Google Scholar]
- Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci 2010; 55(3):560-78. doi: 10.1007/s10620-009-1081-0 [Crossref] [ Google Scholar]
- Wang XC, Gusdon AM, Liu H, Qu S. Effects of glucagon-like peptide-1 receptor agonists on non-alcoholic fatty liver disease and inflammation. World J Gastroenterol 2014; 20(40):14821-30. doi: 10.3748/wjg.v20.i40.14821 [Crossref] [ Google Scholar]
- Kim S, Jung J, Kim H, Heo RW, Yi CO, Lee JE. Exendin-4 improves nonalcoholic fatty liver disease by regulating glucose transporter 4 expression in ob/ob mice. Korean J Physiol Pharmacol 2014; 18(4):333-9. doi: 10.4196/kjpp.2014.18.4.333 [Crossref] [ Google Scholar]
- Li S, Wang X, Zhang J, Li J, Liu X, Ma Y. Exenatide ameliorates hepatic steatosis and attenuates fat mass and FTO gene expression through PI3K signaling pathway in nonalcoholic fatty liver disease. Braz J Med Biol Res 2018; 51(8):e7299. doi: 10.1590/1414-431x20187299 [Crossref] [ Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002; 346(16):1221-31. doi: 10.1056/NEJMra011775 [Crossref] [ Google Scholar]
- Seo MH, Lee J, Hong SW, Rhee EJ, Park SE, Park CY. Exendin-4 inhibits hepatic lipogenesis by increasing β-catenin signaling. PLoS One 2016; 11(12):e0166913. doi: 10.1371/journal.pone.0166913 [Crossref] [ Google Scholar]
- Parlevliet ET, Wang Y, Geerling JJ, Schröder-Van der Elst JP, Picha K, O’Neil K. GLP-1 receptor activation inhibits VLDL production and reverses hepatic steatosis by decreasing hepatic lipogenesis in high-fat-fed APOE*3-Leiden mice. PLoS One 2012; 7(11):e49152. doi: 10.1371/journal.pone.0049152 [Crossref] [ Google Scholar]
- Lee J, Hong SW, Chae SW, Kim DH, Choi JH, Bae JC. Exendin-4 improves steatohepatitis by increasing Sirt1 expression in high-fat diet-induced obese C57BL/6J mice. PLoS One 2012; 7(2):e31394. doi: 10.1371/journal.pone.0031394 [Crossref] [ Google Scholar]
- Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol 2004; 167(1):35-41. doi: 10.1083/jcb.200406136 [Crossref] [ Google Scholar]
- Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol 2011; 54(4):795-809. doi: 10.1016/j.jhep.2010.11.005 [Crossref] [ Google Scholar]
- Lee J, Hong SW, Kwon H, Park SE, Rhee EJ, Park CY. Exendin-4 improves ER stress-induced lipid accumulation and regulates lipin-1 signaling in HepG2 cells. Cell Stress Chaperones 2018; 23(4):629-38. doi: 10.1007/s12192-017-0872-z [Crossref] [ Google Scholar]
- Lee J, Hong SW, Park SE, Rhee EJ, Park CY, Oh KW. Exendin-4 inhibits the expression of SEPP1 and fetuin-A via improvement of palmitic acid-induced endoplasmic reticulum stress by AMPK. Endocrinol Metab (Seoul) 2015; 30(2):177-84. doi: 10.3803/EnM.2015.30.2.177 [Crossref] [ Google Scholar]