Advanced pharmaceutical bulletin. 12(2):206-216.
doi: 10.34172/apb.2022.023
Editorial
Mesenchymal Stem Cells in the Treatment of New Coronavirus Pandemic: A Novel Promising Therapeutic Approach
Sara Razi 1, 2  , Zahra Molavi 1, Seyed Amir Mirmotalebisohi 3, 4, Zahra Niknam 1, Marzieh Sameni 3, 4, Vahid Niazi 5, Amirjafar Adibi 6, Mohsen Yazdani 7, Mohammad Mehdi Ranjbar 8, Hakimeh Zali 1, 5, *
, Zahra Molavi 1, Seyed Amir Mirmotalebisohi 3, 4, Zahra Niknam 1, Marzieh Sameni 3, 4, Vahid Niazi 5, Amirjafar Adibi 6, Mohsen Yazdani 7, Mohammad Mehdi Ranjbar 8, Hakimeh Zali 1, 5, * 
Author information:
1Proteomics Research Center, Shahid Beheshti University of Medical Science, Tehran, Iran.
2Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran.
3Student Research Committee, Department of Biotechnology, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
5Department of Tissue Engineering and Applied Cell Sciences, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
6Departments of Orthopedics, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
7Institute of Biochemistry and Biophysics, Tehran University, Tehran, Iran.
8Razi Vaccine and Serum Research Institute, Agricultural Research, Education and Extension Organization, Karaj, Iran.
Abstract
After severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) outbreaks, coronavirus disease 2019 (COVID-19) is the third coronavirus epidemic that soon turned into a pandemic. This virus causes acute respiratory syndrome in infected people. The mortality rate of SARS-CoV-2 infection will probably rise unless efficient treatments or vaccines are developed. The global funding and medical communities have started performing more than five hundred clinical examinations on a broad spectrum of repurposed drugs to acquire effective treatments. Besides, other novel treatment approaches have also recently emerged, including cellular host-directed therapies. They counteract the unwanted responses of the host immune system that led to the severe pathogenesis of SARS-CoV-2. This brief review focuses on mesenchymal stem cell (MSC) principles in treating the COVID-19. The US clinical trials database and the world health organization database for clinical trials have reported 82 clinical trials (altogether) exploring the effects of MSCs in COVID-19 treatment. MSCs also had better be tried for treating other pathogens worldwide. MSC treatment may have the potential to end the high mortality rate of COVID-19. Besides, it also limits the long-term inability of survivors.
Keywords: COVID-19, SARS-CoV-2, Stem cell therapy, Mesenchymal stem cell, Cytokine storm
Copyright and License Information
©2022 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) is a member of a large family of viruses called Coronaviridae that cause a various range of illnesses from the common cold to the more severe diseases, such as Middle East respiratory syndrome (MERS) and SARS. The infectious disease caused by SARS-CoV-2 has been named coronavirus disease 2019 (COVID-19).
1
Coronaviruses were first discovered and studied in the 1960s.
2
The virus was under vigorous investigations until the mid-1980s.
3
The virus naturally spreads to birds and mammals; however, seven human-transmitted coronaviruses have been discovered. The most recent coronaviruses, the agent of SARS-CoV-2, became epidemic in 2019 in Wuhan, China. The new coronavirus soon spread worldwide and turned into a disastrous pandemic, leading to thousands of deaths.
2
Symptoms of the new virus that cause COVID-19 usually start a few days after being infected. However, in certain people, the symptoms may appear later (from two to fourteen days after infection). According to statistics and research, symptoms include fever, dry cough, respiratory distress, fatigue, muscle aches, and even diarrhea (in 3.8% of cases). The average incubation period was four days. Turbidity, or Ground-glass opacity, was observed in 4.56% of chest scans of patients with COVID-19. Around nine percent of the patients with the non-severe form of the disease did not show any problems in their radiology or scintigraphy outcomes. Lymphocytopenia (decrease in circulating lymphocytes) was observed in a subset of patients at admission.
4
The patients infected with SARS-CoV-2 may show no manifestations of the disease. However, many patients experience mild to severe symptoms that may lead to deadly pneumonia.
5
The immune response is essential for the control and elimination of the SARS-CoV-2. Any defects in the immune system may lead to a severe form of the disease.
6
Currently, little is known about the status of the innate immune system in COVID-19. The innate system is our first line of defense against SARS-CoV-2. Various case studies have shown that in some COVID-19 patients, the number of lymphocytes increases. Besides, neutrophil counts are shown to decrease in some patients with COVID-19. The lymphopenia is shown to be directly associated with the severity of the disease.
7
Initially, the immune system needs to identify the virus. The immune system uses specific immune receptors, called pathogen-associated molecular pattern molecules (PAMPs), to detect the invaders. PAMP is the abbreviation for Pathogen-associated molecular pattern molecules. These receptors can detect the genetic material of various viruses. The genetic material of the SARS-CoV-2 is made of single-strand RNA. Identifying the viral genome leads to the activation of intracellular pathways that eventually lead to the production of interferon type 1 (IFN-I).
8
IFN-I is the most effective intrinsic immune defense against the virus, and its successful increase leads to inhibition of virus replication and elimination in the early stages.
8,9
SARS-CoV-2 suppresses this response by disrupting interferon production or messaging. This strategy is closely related to the severity of the disease. Following the suppression of this defense barrier, immune cells present at the site of entry of the virus produce increased levels of IFN-I to compensate, which infiltrates more macrophages and neutrophils to the location of the inflammation. Finally, the cytokine storm occurs. It is a phenomenon in which uncontrolled large amounts of cytokines are rapidly produced in response to an infectious agent. This unbridled response destroys lung tissue and impairs its function in exchanging respiratory gases.
10,11
Mesenchymal stems cells (MSCs) are a well-known subset of stem cells recommended to treat COVID- 19.
12
The expression and secretion of specific cytokines by MSCs may help improve severe viral infection. They possibly can have immunomodulatory properties and a rapid effect on reducing inflammation and tissue damage.
13
They are promising tools that can suppress the cytokine storm by secreting paracrine or creating a direct interaction with some immune cells.
14,15
In various clinical trials, MSC therapies have already been shown to be effective and safe. It has been used in some inflammatory diseases mediated by the immune system, such as GVHD and SLE.
16,17
The effects of the MSCs on the immune system are further elevated by activating the Toll-like receptors (TLRs) in MSCs. These receptors can be stimulated by pathogen-related molecules such as double-strand RNA of various viruses or lipopolysaccharides.
18,19
Recently some studies have tried to assess the role of MSCs in the modulation of cytokine storms, regulation of Inflammatory response, pulmonary adaptation, preservation of the alveolar microenvironment and endogenous tissue repair. Some studies have proposed the hypothesis of the possible role of MSCs in treating the COVID-19.
20
The role of MSCs in treating COVID-19 needs to be investigated in different aspects. This brief review aims to investigate the possible healing effect of MSC in COVID-19 and describe the molecular mechanisms by which MSC therapy can probably be beneficial for COVID-19 treatment. This novel method needs to be investigated more since it is cheap, easy to obtain, and without side effects.
Pathogenesis of COVID-19
According to the WHO, the new coronavirus called SARS-CoV-2 is the agent of the COVID-19 worldwide. The COVID-19 Common symptoms include fever, shortness of breath, and cough. Some of the COVID-19 symptoms that are less common include sore throat, indigestion, redness of the eyes, pain in muscles, and sputum.
21-23
Most COVID-19 cases show mild symptoms.
24
However, the severe involvement of some organs is also observed. For example, the disease may lead to respiratory failure of the lungs in a subset of patients. Some other patients may experience chest pains.
25
In 56.4% of cases, an opaque glass sign was observed on the patients’ chest scan results. However, approximately three percent of severe patients did not exhibit any signs of a problem in their radiology or scintigraphy outcomes.
26,27
The mortality rate is reported to vary from one to five percent, depending on age and health conditions.
28,29
Tiny respiratory droplets are considered the leading cause of the disease. The disease can be transmitted when people are infected by patients’ coughs or sneezes.
30
The duration of exposure to the virus and the onset of symptoms is between two to fourteen days.
27
The lung is an organ that can be severely affected by COVID-19 due to the abundance of angiotensin-converting enzyme 2 (ACE2) receptors in alveolar type II lung cells. ACE2 is known as the pivotal receptor for virus entry. The virus can enter the cell when a specific type of glycoprotein, called a spike, binds to ACE2.
31-33
The ACE2 concentration in each tissue is associated with disease severity in that tissue, so it can be assumed that decreasing the ACE2 activity may be a protective strategy in drug repurposing.
34,35
The new coronavirus may also lead to respiratory failure by impacting the brainstem since other CoVs have been previously discovered to attack the central nervous system (CNS). The virus has been identified in the autopsy of cerebrospinal fluid; the exact mechanism of its pathogenesis in the CNS remains unknown. It possibly attacks the peripheral nerves since low levels of ACE2 are available in the brain.
36,37
ACE2 is found abundantly in the epithelial and endothelial cells of the gastrointestinal tract organs. The virus also influences the organs of the digestive tract.
38,39
The virus may lead to acute and chronic damage to cardiovascular system.
40
However, the acute seems to be more common in patients.
41
The reason may be ACE2 in myocardial cells that play a role in heart function and is highly expressed in these cells, causing inflammatory responses.
41,42
Besides, blood vessel function and clot formation play a pivotal role in COVID-19 mortality. The formed clots may lead to pulmonary embolism or ischemic events in the brain. Infection with SARS-CoV-2 can lead to a chain of the vaso-constrictor responses in the body. The narrowing of the blood vessels within the respiratory system circulation has been suggested to reduce oxygen delivery.
43
Another major cause of death is acute kidney damage. Mainly in people with chronic diseases, including high blood pressure and diabetes, which in particular causes nephropathy in the long term.
44
The number of lymphocytes, especially natural killer cells, decreases to a lower level in peripheral blood of a subset of COVID-19 patients.
45
Inflammatory parameters, including C-reactive protein and some cytokines, are overexposed, including interleukin (IL)-6, tumor necrosis factor alpha (TNFα), and IL-8.
46,47
The immune system becomes destroyed by atrophy of lymph nodes and spleen, accompanying a decrease in lymphocytes in the lymph nodes. Most immune cells that penetrate the lung lesions are reported to be macrophages and monocytes. However, the minimum penetration of lymphocytes is reported.
48
In SARS-CoV-2, Similar to SARS and MERS viral infections, the Cytokine storm and acute respiratory distress syndrome (ARDS) is observed due to excessive secretion of inflammatory factors.
49,50
Huang et al investigated the inflammatory factors and cytokine levels in patients with COVID-19. Forty-one patients were hospitalized with various cytokines, including IL-1B, IL-1RA, interleukins (IL-7, 8, 9, and 10), fibroblast growth factor, and granulocyte-mucosal factor. Other cytokines are also reported in severe patients, including MCP1, MIP1A, PDGF, TNFα, GM-CSF, IFNG, G-CSF, IP10, VEGF, IL-2.
51-53
Choosing a suitable approach to block the cytokine storm and when to use anti-inflammatory medications to reduce COVID-19 mortality is a critical decision.
54
We have summarized the commonly known pathogenesis of the Coronavirus in Figure 1.
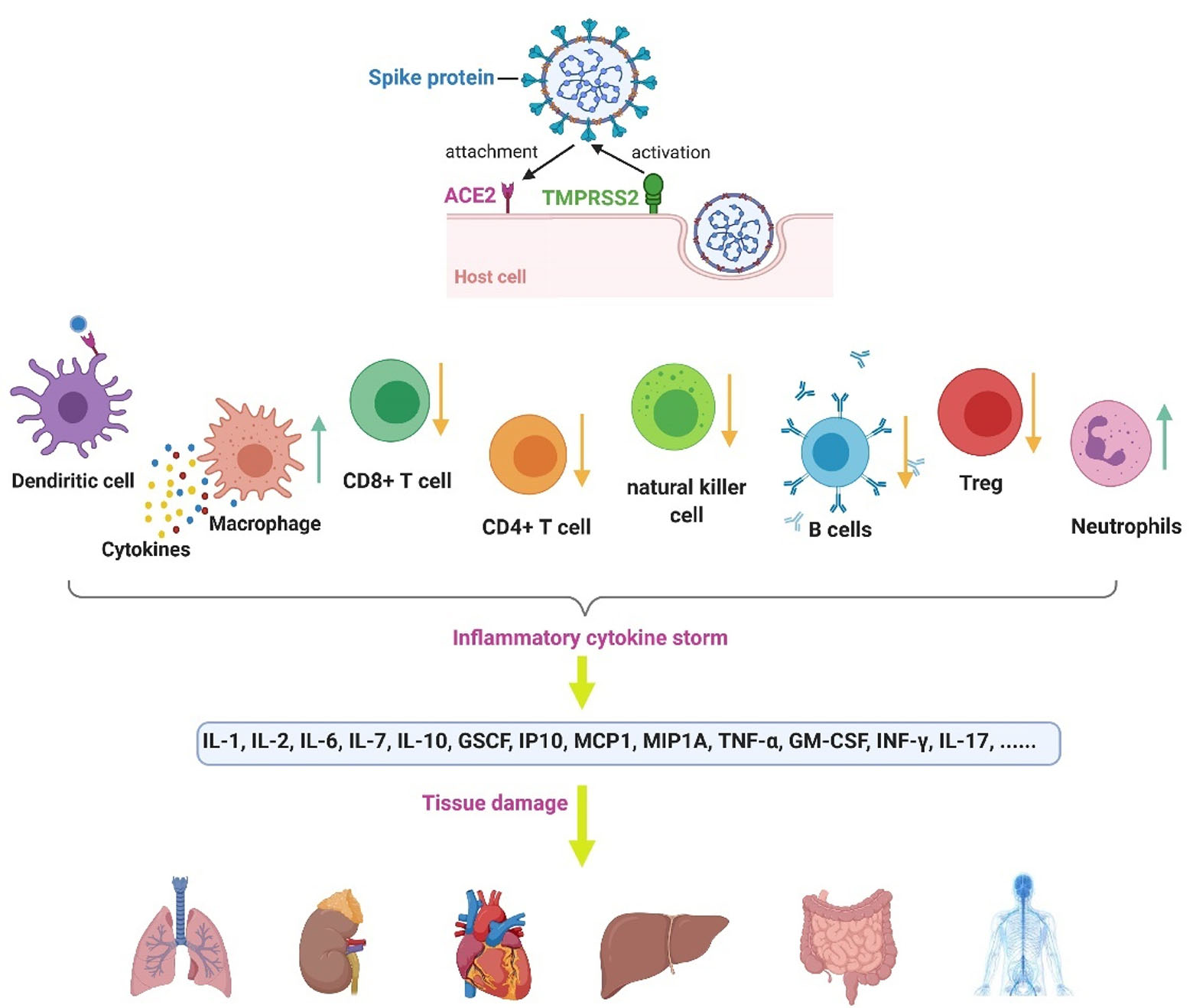
Figure 1.
Coronavirus pathogenesis. The viral spike protein becomes activated by TMPRSS2 (a cell protease). The spike then binds to the ACE2 receptor in the host cell.
55
Infection with COVID-19 causes intracellular components and virus particles and released to the extracellular space, which results in the attraction of immune cells and massive inflammatory responses.
56
The total count of some lymphocytes, including CD4 + T cells, CD + 8 T cells, Treg cells, NK cells, and B cells, decreases. However, the count of neutrophils and leukocytes increases. In COVID-19, the dendritic cells present the antigens.
57
Besides, the macrophages' vast release of cytokines, which can contribute to the cytokine storm and its followed tissue damages.
58
The figure was created by BioRender.com
.
Coronavirus pathogenesis. The viral spike protein becomes activated by TMPRSS2 (a cell protease). The spike then binds to the ACE2 receptor in the host cell.
55
Infection with COVID-19 causes intracellular components and virus particles and released to the extracellular space, which results in the attraction of immune cells and massive inflammatory responses.
56
The total count of some lymphocytes, including CD4 + T cells, CD + 8 T cells, Treg cells, NK cells, and B cells, decreases. However, the count of neutrophils and leukocytes increases. In COVID-19, the dendritic cells present the antigens.
57
Besides, the macrophages' vast release of cytokines, which can contribute to the cytokine storm and its followed tissue damages.
58
The figure was created by BioRender.com
Stem cell therapy
Cell therapy is based on using the regeneration potential of stem cells to treat some diseases. The therapeutic use of stem cells rehabilitates patients after traumatic injuries in some severe illnesses.
55,56
Stem cell therapy is described as the usage of stem cells to heal or limit diseases or conditions.
57
Bone marrow transplantation is the most common treatment for stem cells, but some treatments for umbilical cord blood, adipose stem cells, and embryonic stem cells are also being used.
58
In recent years, there have been several successful stem cell therapies worldwide. Various stem cells have been used in the treatments, including stem cells isolated from adipose tissue, umbilical cord tissue, and other sources.
58,59
Research on various stem cell sources is underway to help treat various diseases, including neurological conditions, diabetes, heart disease, respiratory diseases and especially coronavirus infection.
60
Stem cells have the potential to repair damaged tissue. They have a unique ability to become cells in the blood, liver, myocardium, bone, cartilage or nerve tissue, and thus can repair damaged organs and restore their function.
61
Immunosuppression is the other use of stem cell therapy.
62
Inflammation is one of the essential parts of an efficient immune response. Successful removal of infection without causing inflammation is one of the most challenging.
63
The inflammatory response first starts with the identification of pathogens. Then, the pathogens cause the body’s immune cells to be absorbed into the infected region. The immune cells then destroy the pathogens and eventually lead to homeostasis and tissue repair.
64
However, SARS-CoV-2 increases the long-term response of cytokines/chemokines in infected patients, called cytokine storms. A cytokine storm leads to ARDS or dysfunction of several organs, and ultimately death. Immediate control of cytokine storm in its preceding stages is the key to successfully treating the patients and decreasing the mortality rate.
65
Stem cell therapy using different stem cell sources has shown promising cytokine storm regulation.
59,65
MSC therapy
MSCs therapy can be useful in two ways. Firstly, it affects and modulates the immune system. Secondly, cell differentiation’s ability possibly contributes to regenerate the damaged tissue to some extent since they can differentiate into various cells. MScs are easy to access since they can be prepared from vast resources such as bone marrow, adipose tissue cells, fetal liver, umbilical cord blood, mobilized peripheral blood, fetal lung, placenta, umbilical cord, tooth pulp, synovial membrane, endometrium, and trabecular.
19,60,66
MSCs produce a large variety of cytokines by paracrine secretion. Besides, they have direct immunomodulatory activity by direct interaction with various parts of the immune system. MSCs are stimulated by a pathogenic agent, such as lipid polysaccharide or RNA strands of viruses.
18
The restorative properties of these cells also may enhance endogenous repair.
67
Some studies have investigated MSCs trafficking and homing. A study of liver resection in mice showed that after transplanting the trackable MSCs in mice tail vein, MSCs first became located in the lungs and then gradually moved to the damaged liver and proliferated. The liver histology and function assessment indicated that the inflammation caused by liver resection subsided after transplanting the MSCs. It can be concluded that MSCs have an immune-regulatory and anti-inflammatory effect. They have a multi-grade differentiation potential.
68,69
It has been reported that the lung structures such as bronchi or alveoli surfaces remain intact after MSCs are located and trapped in the lungs. It probably is promising and shows that the migration of MSCs into the lungs in their first movement stages does not probably harm the lungs.
70
When MSCs are injected intravenously, they lead to partial involvement of the lungs. A broad range of mediators mediates the process, including extracellular vesicles, antimicrobial peptides (AMPs), anti-inflammatory cytokines, and secreted angiogenic growth factors.
71,72
The release pattern of the mediators is regulated by activating pathogen-related receptors. The pathogen-related receptors are expressed in MSCs.
73,74
TLRs leads to the activation of MSCs and some cell signaling pathways.
18
For example, TLR3 can be activated by viral RNA. However, TLR9 becomes activated by viral CpF-DNA. MSCs are also reported to impact the repair of alveolar and capillary disorders in the injured lung by secretion of keratinocyte growth factor and Ang-1.
73
In COVID-19 respiratory viral infection, MSC may also offer two distinct antiviral mechanisms. First, it probably mediates antiviral protection by increasing MSC-specific interferon-stimulated gene (ISG) and secondary response to IFN, leading to ISG induction and widespread viral resistance. MSCs also activate innate and acquired immune systems.
73,75
MSCs have an effective immune system regulatory activity (immunomodulatory). Based on these reasons, so far, 82 clinical trials have been registered to evaluate the possible therapeutic effect of MSC therapy in treating SARS-CoV-2 infection (available in Table 1)
Table 1.
List of the registered MSC therapy clinical trials for treating SARS-CoV-2 infection
|
No.
|
MSC type
|
Participants number
|
Locations
|
Registration code
|
| 1 |
Allogenic human cord tissue mesenchymal stromal cells (hCT-MSC) |
No.: 30 participants |
Duke Hospital, Durham, North Carolina, United States |
NCT04399889 |
| 2 |
WJ-MSCs |
No.: 5 participants |
Stem Cells Arabia, Amman, Jordan |
NCT04313322 |
| 3 |
Mesenchymal stem (stromal) cells (MSCs) |
No.: 20 participants |
Beijing Hospital of China |
NCT04252118 |
| 4 |
UC-MSCs |
No.: 30 participants |
Puren Hospital, Wuhan, Hubei, China |
NCT04339660 |
| 5 |
MSCs |
No.: 40 participants |
University Hospital Tuebingen, Tuebingen, Germany |
NCT04377334 |
| 6 |
UC-MSCs |
No.: 48 participants |
Union Hospital, Wuhan, China |
NCT04273646 |
| 7 |
BM-MSCs |
No.: 20 participants |
Guangzhou Institute of Respiratory Health, China |
NCT04346368 |
| 8 |
UC-MSCs |
No.: 100 participants |
Maternal and Child Hospital of Hubei, China |
NCT04288102 |
| 9 |
UC-MSCs |
No.: 0 participants
(possibly not recruited yet)
|
Puren Hospital Wuhan, Hubei, China |
NCT04293692 |
| 10 |
Remestemcel-L |
No.: 50 adult participants |
Mount Sinai Hospital, New York, US |
NCT04366830 |
| 11 |
Umbilical cord mesenchymal stem cells (UC-MSC) |
No.: 24 participants |
Diabetes Research Institute, Miami, Florida, US |
NCT04355728 |
| 12 |
ACT-20-MSC -umbilical derived mesenchymal stem cells in conditioned media) |
No.: 70 participants |
Aspire Health Science, United States |
NCT04398303 |
| 13 |
Remestemcel-L |
No.: 300 participants |
-The University of Southern California,
-Lutheran Hospital, Fort Wayne, US
-Emory University, Atlanta, US, and 12 more.
|
NCT04371393 |
| 14 |
XCEL-UMC-BETA |
No.: 30 participants |
-Hospital de Bellvitge, Spain
-Mútua de Terrassa, Spain
-Hospital del Mar, Spain
-and two more
|
NCT04390139 |
| 15 |
MSC |
No.: 60 participants |
Royan Institute, Tehran, Iran |
NCT04366063 |
| 16 |
Autologous adipose MSC's |
No.: 20 participants |
Regeneris Medical, United States |
NCT04352803 |
| 17 |
MSCs |
No.: 30 participants |
Houston Methodist Hospital, Houston, Texas, United States |
NCT04345601 |
| 18 |
UC-MSCs |
No.: 10 participants |
Zhongnan Hospital of Wuhan University, China |
NCT04269525 |
| 19 |
MSCs |
No.: 9 participants |
The Ottawa Hospital, Ottawa, Ontario, Canada |
NCT04400032 |
| 20 |
Umbilical cord-derived MSCs |
No.: 75 participants |
Northern Ireland, United Kingdom |
NCT03042143 |
| 21 |
MSC treatment |
No.: 30 participants |
Istinye University, Istanbul, Turkey;
SBÜ Dr. Sadi Konuk Eğitim ve Araştırma Hastanesi, Istanbul, Turkey.
|
NCT04392778 |
| 22 |
Bone marrow harvest |
No.: 10 participants |
Cambridge University Hospitals NHS |
NCT04397471 |
| 23 |
Umbilical cord Wharton's jelly-derived human |
No.: 40 participants |
Hôpital Pitié-Salpêtrière – APHP, Paris, France; Hôpital Européen Georges Pompidou – APHP, Paris, France |
NCT04333368 |
| 24 |
Wharton's jelly derived MSCs + standard therapy |
No.: 40 participants |
BioXcellerator, Medellin, Antioquia-CO, Colombia; Clinical Somer, Rionegro, Antioquia, Colombia |
NCT04390152 |
| 25 |
BM-Allo.MSC |
No.: 45 participants |
St. Francis Medical Center, Lynwood, California, United States |
NCT04397796 |
| 26 |
MSCs-derived exosomes |
No.: 30 participants |
Ruijin Hospital Shanghai Jiao Tong University School of Medicine, Shanghai, Shanghai, China |
NCT04276987 |
| 27 |
NK cells and MSCs |
No.: 20 participants |
Hospital of Nanchang University, China |
ChiCTR2000030944 |
| 28 |
HUMSCs and exosomes treat |
No.: 90 participants |
Shiyan Taihe hospital, China |
ChiCTR2000030484 |
| 29 |
MSCs |
No.: 20 participants |
Hospital of Xinxiang Medical University, China |
ChiCTR2000030835 |
| 30 |
UC-MSC |
No.: 30 participants
Control group:30
|
Chinese PLA General Hospital, China |
ChiCTR2000030138 |
| 31 |
Human umbilical cord MSCs |
No.: 30 participants |
The First Hospital of Changsha, China |
ChiCTR2000030866 |
| 32 |
Human umbilical cord MSCs |
No.: 100 participants
Control group:100
|
The Fifth Medical Center of PLA General Hospital, China |
ChiCTR2000031430 |
| 33 |
HB-adMSCs |
No.: 100 participants |
Hope Biosciences Stem Cell Research Foundation, Sugar Land, Texas, United States |
NCT04348435 |
| 34 |
HB-adMSCs |
No.: 56 participants |
Hope Biosciences Stem Cell Research Foundation, Sugar Land, Texas, United States |
NCT04349631 |
| 35 |
MSCs |
No.: 60 participants |
Royan Institute, Iran |
IRCT20200217046526N2 |
| 36 |
Placental MSCs |
No.: 20 participants |
Tarbiat Modares University, Iran |
IRCT20200413047063N1 |
| 37 |
HB-adMSC |
No.: 100 participants |
River Oaks Hospital and Clinics, Houston, Texas, United States; United Memorial Medical Center, Houston, Texas, United States |
NCT04362189 |
| 38 |
Mesenchymoangioblast-derived MSCs |
No.: 24 participants |
Cynata Therapeutics Limited, Australia |
ACTRN12620000612910 |
| 39 |
MSCs |
No.: 6 participants |
Royan Institute, Iran |
IRCT20200217046526N1 |
| 40 |
Allogenic mesenchymal stem cell-derived umbilical cord transplantation |
No.: 20 participants |
Mashhad University of Medical Sciences, Iran |
IRCT20160809029275N1 |
| 41 |
MSCT |
No.: 150 participants |
Assiut university, Assiut, Egypt |
NCT04492865 |
| 42 |
P-MMSCs |
No.: 30 participants |
Institute of Cell Therapy, Kyiv, Ukraine |
NCT04461925 |
| 43 |
MSC |
No.: 20 participants |
Hospital Regional Lic Adolfo Lopez Mateos, Mexico City, Ciudad De Mexico CDMX (Mexico City), Mexico |
NCT04611256 |
| 44 |
LMSCs |
No.: 70 participants |
Miami VA Healthcare System, Miami, Florida, United States; University of Maryland Medical Center, Baltimore, Maryland, United States; Wake Forest Baptist Medical Center, Winston-Salem, North Carolina, United States |
NCT04629105 |
| 45 |
UCMSCs |
No.: 21 participants |
University of Miami, Miami, Florida, United States |
NCT04490486 |
| 46 |
Mesenchymal stromal stem cells - KI-MSC-PL-205 |
No.: 9 participants |
Uppsala University Hospital, Uppsala, Sweden |
NCT04447833 |
| 47 |
MSC |
No.: 10 participants |
Royal Perth Hospital Cell & Tissue Therapies WA, Australia |
ACTRN12620000840987 |
| 48 |
Human umbilical cord MSCs |
No.: 16 participants |
The First Affiliated Hospital of Nanchang University, China |
ChiCTR2000030116 |
| 49 |
hucMSCs |
No.: 9 participants |
Nanjing Second Hospital, China |
ChiCTR2000030300 |
| 50 |
umbilical cord MSCs |
No.: 14 Control: 14 Exp |
Histocell S.L., Spain |
EUCTR2019-002688-89-ES |
| 51 |
Human umbilical cord MSCs |
No.: 0 participants
(possibly not recruited yet)
|
CHU de Liège, Belgium |
EUCTR2020-002102-58-BE |
| 52 |
Remestemcel-L |
No.: 0 participants
(possibly not recruited yet)
|
Hospital Universitario Puerta de Hierro, Spain |
EUCTR2020-002193-27-ES |
| 53 |
MSC |
No.: 50 Control,
50 Exp
|
Fundación Instituto de Investigación Sanitaria Fundación Jiménez Díaz, Spain |
EUCTR2020-001266-11-ES |
| 54 |
Human umbilical cord MSCs |
No.: 15 Control,
15 Exp
|
Banc de Sang i Teixits, Spain |
EUCTR2020-001505-22-ES |
| 55 |
MSC |
No.: 5 participants |
Bagheiat-allah University of Medical Sciences, Iran |
IRCT20200325046860N2 |
| 56 |
MSC |
No.: 30 participants |
Hamedan University of Medical Sciences, Iran |
IRCT20200426047206N2 |
| 57 |
Human umbilical cord MSCs |
No.: 45 Control, 45 Exp |
Shahid Modares hospital, Iran |
IRCT20200421047150N1 |
| 58 |
Adipose-derived MSCs |
No.: 13 control, 13 Exp |
Hospital Universitario de Jerez de la Frontera, Jerez de la Frontera, Cádiz, Spain; Hospital Reina Sofía, Córdoba, Spain; Hospital Universitario Virgen de las Nieves, Granada, Spain
(and 3 more...)
|
NCT04366323 |
| 59 |
Human umbilical cord MSCs |
No.: 15 Control
15 Exp
|
-Fundación Universitaria de Ciencias de la Salud.
-Hospital de San Jose.
-Hospital Infantil Universitario de San Jose, Colombia
|
NCT04429763 |
| 60 |
AdMSCs |
No.: 100 Control, 100 Exp |
Celltex Therapeutics Corporation, United States |
NCT04428801 |
| 61 |
MSCs |
No.: 5 Control, 5 Exp |
Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico |
NCT04416139 |
| 62 |
Pooled olfactory mucosa-derived MScs |
No.: 20 Control,
20 Exp
|
Institute of Biophysics and Cell Engineering of National Academy of Sciences of Belarus, Minsk, Belarus |
NCT04382547 |
| 63 |
Remestemcel-L |
No.: 53 Control,
53 Exp
|
Hospital Universitario de Getafe, Getafe, Madrid, Spain; Hospital Universitario de Cruces, Barakaldo, Spain; Hospital Universitario de La Princesa, Madrid, Spain (and 3 more...) |
NCT04366271 |
| 64 |
Remestemcel-L |
No.: 30 Control,
30 Exp
|
Fuzhou General Hospital,
Fuzhou, Fujian, China
|
NCT04371601 |
| 65 |
Remestemcel-L |
No.: 12 Control,
12 Exp
|
Hospital Universitario Rio Hortega,
Valladolid, Spain
|
NCT04361942 |
| 66 |
Remestemcel-L |
No.: 13 Control,
13 Exp
|
Red Andaluza de Diseño y Traslación de Terapias Avanzadas-Fundación Progreso y Salud, Spain |
EUCTR2020-001364-29-ES |
| 67 |
Human umbilical cord MSCs |
No.: 10 Exp |
Iran University Of Medical Science, Iran |
IRCT20140528017891N8 |
| 68 |
Dental mesenchymal pulp stem cells |
No.: 10 Exp |
Kerman University of Medical Sciences, Iran |
IRCT20140911019125N6 |
| 69 |
Remestemcel-L |
No.: 12 Control,
12 Exp
|
CITOSPIN S.L., Spain |
EUCTR2020-001682-36-ES |
| 70 |
Adipose-derived MSCs |
No.: 50 Control,
50 Exp
|
-Instituto de Investigación Sanitaria de la Fundación Jiménez Díaz.
-Instituto de Investigación Sanitaria y Biomédica de Alicante.
-Hospital General Universitario Gregorio Marañon.
-Clinica Universidad de Navarra, Universidad de Navarra.
-Hospital Universitario de Salamanca.
-Hospital General Universitario de Alicante.
-Hospital Clínico Universitario Virgen de la Arrixaca.
Spain
|
NCT04348461 |
| 71 |
Adipose-derived MScs |
No.: 20 Control,
20 Exp
|
2014 Department of Cardiology, The Heart Centre, University Hospital, Rigshospitalet, Copenhagen, Denmark |
NCT04341610 |
| 72 |
Dental mesenchymal pulp stem cells |
No.: 10 Control,
10 Exp
|
Renmin Hospital of Wuhan University (East Campus), Wuhan, Hubei, China |
NCT04336254 |
| 73 |
Remestemcel-L |
No.: 66 Exp |
Hospital Vera Cruz, Campinas, São Paulo, Brazil; Hospital de Barueri, São Paulo, Brazil; IncCOR, São Paulo, Brazil; UNIFESP, São Paulo, Brazil |
NCT04315987 |
| 74 |
Remestemcel-L |
No.: 18 Control,
18 Exp
|
The Jiangsu Cell Tech Medical Research Institute, China |
ChiCTR2000031494 |
| 75 |
Dental mesenchymal pulp stem cells |
No.: 10 Control,
10 Exp
|
Center for Regenerative Medicine, Renmin Hospital of Wuhan University, China |
ChiCTR2000031319 |
| 76 |
Dental mesenchymal pulp stem cells |
No.: 24 Exp |
CAR-T (Shanghai) Biotechnology Co., Ltd., China |
NCT04302519 |
| 77 |
MSCs (origin not specified) + Ruxolitinib |
No.: 35 Control,
35 Exp
|
Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, China |
ChiCTR2000029580 |
| 78 |
MSC exosomes (origin not specified) |
No.: 13 Control,
13 Exp
|
Wuxi Fifth People's Hospital, China |
ChiCTR2000030261 |
| 79 |
Remestemcel-L |
No.: 20 Exp |
Second Hospital of University of South China, Hengyang, China |
ChiCTR2000030020 |
| 80 |
Human umbilical cord MSCs |
No.: 20 Control,
20 Exp
|
The Sixth Medical Center of PLA General Hospital, China |
ChiCTR2000030088 |
| 81 |
Remestemcel-L |
No.: 60 Control,
60 Exp
|
Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, China |
ChiCTR2000029990 |
| 82 |
Human umbilical cord MSCs |
No.: 30 Control,
30 Exp
|
Hunan yuanpin Cell Biotechnology Co., Ltd, China |
ChiCTR2000030173 |
A study at Beijing’s YouAn hospital examined whether the treatment using MSC improved the outcomes of seven patients admitted with COVID-19 pneumonia. Various clinical results of MSC injection were investigated in seven patients within 14 days. The clinical results included changes in inflammation, immune function, and side effects. They reported that MSCs improved the performance of seven patients without observation of any side effects. Pulmonary function and manifestations of these seven patients improved significantly during two days after MSC treatment. Peripheral lymphocytes were then extracted and studied. The C-reactive protein was reduced. Inactive cytokine-secreting immune cells vanished within three to six days, including CXCR3+ CD8+ T cells, CXCR3+ CD4+ T cells, and CXCR3+ NK cells. Besides, a subset of DC cell populations regulating CD14+ CD11c+ CD11bmid increased significantly. TNF-α levels were decreased significantly. However, IL-10 levels augmented in the MSC therapy group compared with the placebo. The gene expression specifications indicated that MSCs were negative for TMPRSS2 and ACE2, showing that MSCs were probably free of the SARS-CoV-2. Capillary endothelial and alveolar (type II) cells can express the ACE2 and TMPRSS2 receptors. These receptors can help the virus enter into the host cell and contribute to its spreading.
73,76
In sum, they reported that intravenous transplantation of MSCs was useful and safe in the treatment of a subset of patients with COVID-19.
76
We have summarized the crucial molecules and cells intermediating in MSC therapy in COVID-19 patients in Figure 2.
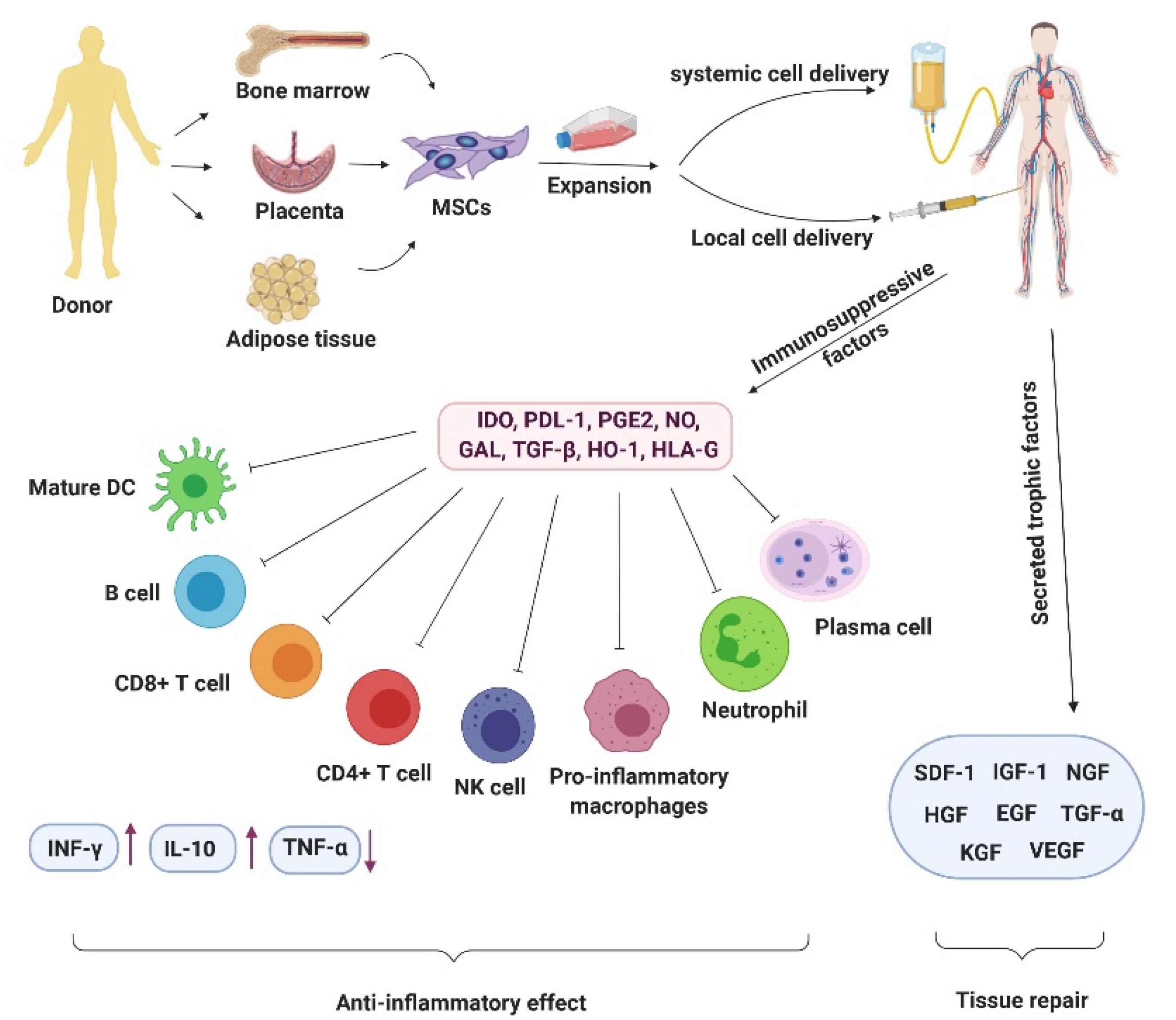
Figure 2.
The picture describes the crucial molecules and cells intermediating in MSC therapy in COVID-19 patients. The figure was created by BioRender.com
.
The picture describes the crucial molecules and cells intermediating in MSC therapy in COVID-19 patients. The figure was created by BioRender.com
Secretome of MSCs in COVID-19 therapy
At the beginning of the viral disease, pneumocytes type II are infected; therefore, the respiratory system becomes injured.
77
Previous studies have shown that the secretome of MSCs can be applied to treat injuries in the respiratory system. The MSC secretome has therapeutic ingredients, including exosomes, microvesicles, various proteins, cytokines, miRNAs, and lipids.
77
The exosomes derived from the MSCs of the bone marrow improve lung oxygenation. The exosomes are shown to modulate lymphocyte counts and reduce cytokine storms. In sum, it could improve the health of hospitalized COVID-19 patients.
78
Bari et al have shown that the secretome could be a useful therapeutic in the pneumonia of COVID-19 patients.
78
The secretome modulates the immune system and has an anti-inflammatory function. It also has an essential role in pro-angiogenic and anti-fibrotic processes.
79
MSCs have potential antimicrobial impacts and can directly or indirectly stimulate the immune system since they can produce some AMPs when pathogens infect the organism.
80
They can probably be considered a first-line defense that protects the organism against various infections.
81
AMPs may have extracellular or intracellular molecular targets; therefore, they can disrupt membrane integrity or inhibit proteins or DNA/RNA synthesis. MSCs produce various AMP types, such as the human cysteine-rich protein called β-defensin (hBD). The hBD contributes to the human body in defending against microbes. MSCs and some other cells can secrete different β-Defensin family members such as hBD-1, hBD-2 and hBD-3.
82-84
Defensin can inhibit some viral cycle mechanisms. For example, it can inhibit the viral entry and traffic against the respiratory syncytial virus, influenza A virus, and some coronaviruses (SARS). This therapeutic effect of defensin can boost the host immune system and lead to dysfunction of the infectious pathogens. It also can contribute to employ various immune cells such as dendrites, T cells, and macrophages into some tissues.
85
Some AMPs can facilitate bacterial clearance during MSC therapy and even contribute to ARDS inhibition.
86,87
Finally, it has been hypothesized that the use of MSCs’ secretome can be an alternative method in treating COVID-19 patients in their severe condition.
Conclusion
The safety and anti-inflammatory properties of MSC therapy in the treatment of COVID-19 have been confirmed and documented by 40 clinical studies (https://clinicaltrials.gov) (https://www.who.int/ictrp/en/). MSC therapy seems to be promising in patients with COVID-19. The essential sources of MSCs include the umbilical cord, umbilical cord blood, Wharton gel, menstrual blood, and bone marrow. The therapeutic results are expected to be observed in a short period. We suggest a combination of therapeutic approaches for patients with COVID-19. However, we emphasize that MSC therapy should be more paid attention to and investigated in this regard. Further scientific investigations probably will ensure the effectiveness and safety of this type of treatment soon.
Acknowledgments
We thank the Proteomics Research Centre of Shahid Beheshti University of Medical Sciences for their support in this study.
Ethical Issues
The ethics committee of Shahid Beheshti University of Medical Sciences approved this study.
Conflict of Interest
The authors declare that they have no conflict of interests.
References
-
Cascella M, Rajnik M, Aleem A, Dulebohn S, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2020.
- Kothai R, Arul B. 2019 novel coronavirus: a mysterious threat from Wuhan, China–a current review. Int J Res Pharm Sci 2020; 11(1 Suppl):7-15. doi: 10.26452/ijrps.v11iSPL1.1975 [Crossref] [ Google Scholar]
- Pyrc K, Berkhout B, van der Hoek L. The novel human coronaviruses NL63 and HKU1. J Virol 2007; 81(7):3051-7. doi: 10.1128/jvi.01466-06 [Crossref] [ Google Scholar]
- Yi Y, Lagniton PNP, Ye S, Li E, Xu RH. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci 2020; 16(10):1753-66. doi: 10.7150/ijbs.45134 [Crossref] [ Google Scholar]
- Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses 2020; 12(4):372. doi: 10.3390/v12040372 [Crossref] [ Google Scholar]
- Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P. Coronavirus infections and immune responses. J Med Virol 2020; 92(4):424-32. doi: 10.1002/jmv.25685 [Crossref] [ Google Scholar]
- Verdugo-Paiva F, Izcovich A, Ragusa M, Rada G. Lopinavir-ritonavir for COVID-19: a living systematic review. Medwave 2020; 20(6):e7967. doi: 10.5867/medwave.2020.06.7966 [Crossref] [ Google Scholar]
- Nan Y, Nan G, Zhang YJ. Interferon induction by RNA viruses and antagonism by viral pathogens. Viruses 2014; 6(12):4999-5027. doi: 10.3390/v6124999 [Crossref] [ Google Scholar]
- Teijaro JR. Type I interferons in viral control and immune regulation. Curr Opin Virol 2016; 16:31-40. doi: 10.1016/j.coviro.2016.01.001 [Crossref] [ Google Scholar]
- Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res 2020; 178:104791. doi: 10.1016/j.antiviral.2020.104791 [Crossref] [ Google Scholar]
-
Lokugamage KG, Hage A, de Vries M, Valero-Jimenez AM, Schindewolf C, Dittmann M, et al. Type I interferon susceptibility distinguishes SARS-CoV-2 from SARS-CoV. bioRxiv 2020. 10.1101/2020.03.07.982264
- Xu X, Chen P, Wang J, Feng J, Zhou H, Li X. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020; 63(3):457-60. doi: 10.1007/s11427-020-1637-5 [Crossref] [ Google Scholar]
- Zayed M, Iohara K. Immunomodulation and regeneration properties of dental pulp stem cells: a potential therapy to treat coronavirus disease 2019. Cell Transplant 2020; 29:963689720952089. doi: 10.1177/0963689720952089 [Crossref] [ Google Scholar]
- Majolo F, da Silva GL, Vieira L, Timmers L, Laufer S, Goettert MI. Review of trials currently testing stem cells for treatment of respiratory diseases: facts known to date and possible applications to COVID-19. Stem Cell Rev Rep 2021; 17(1):44-55. doi: 10.1007/s12015-020-10033-6 [Crossref] [ Google Scholar]
- Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 2013; 13(4):392-402. doi: 10.1016/j.stem.2013.09.006 [Crossref] [ Google Scholar]
- Wang LT, Ting CH, Yen ML, Liu KJ, Sytwu HK, Wu KK. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J Biomed Sci 2016; 23(1):76. doi: 10.1186/s12929-016-0289-5 [Crossref] [ Google Scholar]
- Kamen DL, Nietert PJ, Wang H, Duke T, Cloud C, Robinson A. CT-04 Safety and efficacy of allogeneic umbilical cord-derived mesenchymal stem cells (MSCs) in patients with systemic lupus erythematosus: results of an open-label phase I study. Lupus Sci Med 2018; 5(Suppl 2):A46-A7. doi: 10.1136/lupus-2018-lsm.76 [Crossref] [ Google Scholar]
- Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One 2010; 5(4):e10088. doi: 10.1371/journal.pone.0010088 [Crossref] [ Google Scholar]
- Li W, Ren G, Huang Y, Su J, Han Y, Li J. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ 2012; 19(9):1505-13. doi: 10.1038/cdd.2012.26 [Crossref] [ Google Scholar]
- Lin F, Ichim TE, Pingle S, Jones LD, Kesari S, Ashili S. Mesenchymal stem cells as living anti-inflammatory therapy for COVID-19 related acute respiratory distress syndrome. World J Stem Cells 2020; 12(10):1067-79. doi: 10.4252/wjsc.v12.i10.1067 [Crossref] [ Google Scholar]
-
Alipio MM. Chest radiographic findings of patients infected with 2019-nCOV. Chest 2020.
- Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020; 11(7):995-8. doi: 10.1021/acschemneuro.0c00122 [Crossref] [ Google Scholar]
-
Vance D, Shah P, Sataloff RT. COVID-19: Impact on the Musician and Returning to Singing; A Literature Review [published online ahead of print, 2021 Jan 14]. J Voice 2021;S0892-1997(21)00003-5. 10.1016/j.jvoice.2020.12.042
-
Dorigatti I, Okell L, Cori A, Imai N, Baguelin M, Bhatia S, et al. Report 4: Severity of 2019-Novel Coronavirus (nCoV). London: Imperial College London; 2020. 10.25561/77154
- Ghelichi-Ghojogh M, Allah Kalteh E, Fararooei M. Coronavirus disease 2019; epidemiology and recommendations. J Prev Epidemiol 2020; 5(1):e01. doi: 10.34172/jpe.2020.01 [Crossref] [ Google Scholar]
- Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology 2020; 158(6):1518-9. doi: 10.1053/j.gastro.2020.02.054 [Crossref] [ Google Scholar]
- Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol 2020; 5(4):562-9. doi: 10.1038/s41564-020-0688-y [Crossref] [ Google Scholar]
- Gundlapally P, Pingili S, Doragolla B. A novel coronavirus disease (COVID-19) outbreak as a pandemic crisis. Int J Res Rev 2020; 7(4):495-51. [ Google Scholar]
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus A first step in understanding SARS pathogenesis. J Pathol 2004; 203(2):631-7. doi: 10.1002/path.1570 [Crossref] [ Google Scholar]
- Hui DS, E IA, Madani TA, Ntoumi F, Kock R, Dar O. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 2020; 91:264-6. doi: 10.1016/j.ijid.2020.01.009 [Crossref] [ Google Scholar]
- Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal 2020; 10(2):102-8. doi: 10.1016/j.jpha.2020.03.001 [Crossref] [ Google Scholar]
- Koyuncu Irmak D, Darıcı H, Karaöz E. Stem cell based therapy option in COVID-19: is it really promising?. Aging Dis 2020; 11(5):1174-91. doi: 10.14336/ad.2020.0608 [Crossref] [ Google Scholar]
- Robson B. Bioinformatics studies on a function of the SARS-CoV-2 spike glycoprotein as the binding of host sialic acid glycans. Comput Biol Med 2020; 122:103849. doi: 10.1016/j.compbiomed.2020.103849 [Crossref] [ Google Scholar]
- Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 2020; 92(6):552-5. doi: 10.1002/jmv.25728 [Crossref] [ Google Scholar]
- Sohrabi C, Alsafi Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg 2020; 76:71-6. doi: 10.1016/j.ijsu.2020.02.034 [Crossref] [ Google Scholar]
- Tavakoli A, Vahdat K, Keshavarz M. Novel coronavirus disease 2019 (COVID-19): an emerging infectious disease in the 21st century. Iran South Med J 2020; 22(6):432-50. doi: 10.29252/ismj.22.6.432 [Crossref] [ Google Scholar]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323(11):1061-9. doi: 10.1001/jama.2020.1585 [Crossref] [ Google Scholar]
- Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 2020; 12(1):8. doi: 10.1038/s41368-020-0074-x [Crossref] [ Google Scholar]
- Yoon SH, Lee KH, Kim JY, Lee YK, Ko H, Kim KH. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): analysis of nine patients treated in Korea. Korean J Radiol 2020; 21(4):494-500. doi: 10.3348/kjr.2020.0132 [Crossref] [ Google Scholar]
- Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 2020; 46(4):586-90. doi: 10.1007/s00134-020-05985-9 [Crossref] [ Google Scholar]
- Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020; 17(5):259-60. doi: 10.1038/s41569-020-0360-5 [Crossref] [ Google Scholar]
- Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci 2004; 25(6):291-4. doi: 10.1016/j.tips.2004.04.001 [Crossref] [ Google Scholar]
-
Wadman M, Couzin-Frankel J, Kaiser J, Matacic C. How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes. 2020. Available from: https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/covidwho-71625.
- Diao B, Wang C, Wang R, Feng Z, Zhang J, Yang H. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun 2021; 12(1):2506. doi: 10.1038/s41467-021-22781-1 [Crossref] [ Google Scholar]
- Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis 2020; 221(11):1762-9. doi: 10.1093/infdis/jiaa150 [Crossref] [ Google Scholar]
- Rafe T, Shawon PA, Salem L, Chowdhury NI, Kabir F, Bin Zahur SM. Preventive role of resveratrol against inflammatory cytokines and related diseases. Curr Pharm Des 2019; 25(12):1345-71. doi: 10.2174/1381612825666190410153307 [Crossref] [ Google Scholar]
- Darabi R, Li Y. Stem cell therapies for COVID-19: Strategy and application. J Cell Biochem 2020. doi: 10.1002/jcb.29816 [Crossref]
- Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol 2020; 214:108393. doi: 10.1016/j.clim.2020.108393 [Crossref] [ Google Scholar]
- Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest 2020; 130(5):2202-5. doi: 10.1172/jci137647 [Crossref] [ Google Scholar]
- Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol 2017; 39(5):517-28. doi: 10.1007/s00281-017-0639-8 [Crossref] [ Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395(10223):497-506. doi: 10.1016/s0140-6736(20)30183-5 [Crossref] [ Google Scholar]
- Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents 2020; 34(2):327-31. doi: 10.23812/conti-e [Crossref] [ Google Scholar]
- Bamba C, Singh SP, Choudhury S. Can mesenchymal stem cell therapy be the interim management of COVID-19?. Drug Discov Ther 2020; 14(3):139-42. doi: 10.5582/ddt.2020.03032 [Crossref] [ Google Scholar]
- Verdiner RE, Choukalas CG, Siddiqui S, Stahl DL, Galvagno SM Jr, Jabaley CS. COVID-activated emergency scaling of anesthesiology responsibilities intensive care unit. Anesth Analg 2020; 131(2):365-77. doi: 10.1213/ane.0000000000004957 [Crossref] [ Google Scholar]
- Kumar A, Narayanan K, Chaudhary RK, Mishra S, Kumar S, Vinoth KJ. Current perspective of stem cell therapy in neurodegenerative and metabolic diseases. Mol Neurobiol 2017; 54(9):7276-96. doi: 10.1007/s12035-016-0217-4 [Crossref] [ Google Scholar]
- Pulendran B, S Arunachalam P, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov 2021; 20(6):454-475. doi: 10.1038/s41573-021-00163-y [Crossref] [ Google Scholar]
- Mahla RS. Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol 2016; 2016:6940283. doi: 10.1155/2016/6940283 [Crossref] [ Google Scholar]
- Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther 2019; 10(1):68. doi: 10.1186/s13287-019-1165-5 [Crossref] [ Google Scholar]
- Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Rev Rep 2020; 16(3):427-33. doi: 10.1007/s12015-020-09973-w [Crossref] [ Google Scholar]
- Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells-current trends and future prospective. Biosci Rep 2015; 35(2):e00191. doi: 10.1042/bsr20150025 [Crossref] [ Google Scholar]
- Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cells 2019; 8(8):886. doi: 10.3390/cells8080886 [Crossref] [ Google Scholar]
- Wang M, Yuan Q, Xie L. Mesenchymal stem cell-based immunomodulation: properties and clinical application. Stem Cells Int 2018; 2018:3057624. doi: 10.1155/2018/3057624 [Crossref] [ Google Scholar]
- Nicholson LB. The immune system. Essays Biochem 2016; 60(3):275-301. doi: 10.1042/ebc20160017 [Crossref] [ Google Scholar]
- Hirayama D, Iida T, Nakase H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int J Mol Sci 2017; 19(1):92. doi: 10.3390/ijms19010092 [Crossref] [ Google Scholar]
- Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect 2020; 80(6):607-13. doi: 10.1016/j.jinf.2020.03.037 [Crossref] [ Google Scholar]
- Freitag J, Wickham J, Shah K, Tenen A. Mesenchymal stem cell use in acute respiratory distress syndrome: a potential therapeutic application. Future Sci OA 2020; 6(6):FSO584. doi: 10.2144/fsoa-2020-0048 [Crossref] [ Google Scholar]
- Glenn JD, Whartenby KA. Mesenchymal stem cells: emerging mechanisms of immunomodulation and therapy. World J Stem Cells 2014; 6(5):526-39. doi: 10.4252/wjsc.v6.i5.526 [Crossref] [ Google Scholar]
- Yang X, Meng Y, Han Z, Ye F, Wei L, Zong C. Mesenchymal stem cell therapy for liver disease: full of chances and challenges. Cell Biosci 2020; 10:123. doi: 10.1186/s13578-020-00480-6 [Crossref] [ Google Scholar]
- Chen D, Li Q, Meng Z, Guo L, Tang Y, Liu Z. Bright polymer dots tracking stem cell engraftment and migration to injured mouse liver. Theranostics 2017; 7(7):1820. doi: 10.7150/thno.18614 [Crossref] [ Google Scholar]
- Schmelzer E, Miceli V, Chinnici CM, Bertani A, Gerlach JC. Effects of mesenchymal stem cell coculture on human lung small airway epithelial cells. Biomed Res Int 2020; 2020:9847579. doi: 10.1155/2020/9847579 [Crossref] [ Google Scholar]
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009; 5(1):54-63. doi: 10.1016/j.stem.2009.05.003 [Crossref] [ Google Scholar]
- Hu S, Park J, Liu A, Lee J, Zhang X, Hao Q. Mesenchymal stem cell microvesicles restore protein permeability across primary cultures of injured human lung microvascular endothelial cells. Stem Cells Transl Med 2018; 7(8):615-24. doi: 10.1002/sctm.17-0278 [Crossref] [ Google Scholar]
- Rajarshi K, Chatterjee A, Ray S. Combating COVID-19 with mesenchymal stem cell therapy. Biotechnol Rep (Amst) 2020; 26:e00467. doi: 10.1016/j.btre.2020.e00467 [Crossref] [ Google Scholar]
- Liotta F, Angeli R, Cosmi L, Filì L, Manuelli C, Frosali F. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells 2008; 26(1):279-89. doi: 10.1634/stemcells.2007-0454 [Crossref] [ Google Scholar]
- Khoury M, Cuenca J, Cruz FF, Figueroa FE, Rocco PRM, Weiss DJ. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J 2020; 55(6):200085. doi: 10.1183/13993003.00858-2020 [Crossref] [ Google Scholar]
- Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis 2020; 11(2):216-28. doi: 10.14336/ad.2020.0228 [Crossref] [ Google Scholar]
- Deffune E, Prudenciatti A, Moroz A. Mesenchymal stem cell (MSc) secretome: a possible therapeutic strategy for intensive-care COVID-19 patients. Med Hypotheses 2020; 142:109769. doi: 10.1016/j.mehy.2020.109769 [Crossref] [ Google Scholar]
- Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev 2020; 29(12):747-54. doi: 10.1089/scd.2020.0080 [Crossref] [ Google Scholar]
- Bari E, Ferrarotti I, Saracino L, Perteghella S, Torre ML, Corsico AG. Mesenchymal stromal cell secretome for severe COVID-19 infections: premises for the therapeutic use. Cells 2020; 9(4):924. doi: 10.3390/cells9040924 [Crossref] [ Google Scholar]
- Sutton MT, Fletcher D, Ghosh SK, Weinberg A, van Heeckeren R, Kaur S. Antimicrobial properties of mesenchymal stem cells: therapeutic potential for cystic fibrosis infection, and treatment. Stem Cells Int 2016; 2016:5303048. doi: 10.1155/2016/5303048 [Crossref] [ Google Scholar]
- Izadpanah A, Gallo RL. Antimicrobial peptides. J Am Acad Dermatol 2005; 52(3):381-90. doi: 10.1016/j.jaad.2004.08.026 [Crossref] [ Google Scholar]
- Sau S, Sun J, Lee CY. Molecular characterization and transcriptional analysis of type 8 capsule genes in Staphylococcus aureus. J Bacteriol 1997; 179(5):1614-21. doi: 10.1128/jb.179.5.1614-1621.1997 [Crossref] [ Google Scholar]
- Kim JM. Antimicrobial proteins in intestine and inflammatory bowel diseases. Intest Res 2014; 12(1):20-33. doi: 10.5217/ir.2014.12.1.20 [Crossref] [ Google Scholar]
- De Smet K, Contreras R. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett 2005; 27(18):1337-47. doi: 10.1007/s10529-005-0936-5 [Crossref] [ Google Scholar]
- Verma YK, Verma R, Tyagi N, Behl A, Kumar S, Gangenahalli GU. COVID-19 and its therapeutics: special emphasis on mesenchymal stem cells based therapy. Stem Cell Rev Rep 2021; 17(1):113-31. doi: 10.1007/s12015-020-10037-2 [Crossref] [ Google Scholar]
- Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med 2013; 187(7):751-60. doi: 10.1164/rccm.201206-0990OC [Crossref] [ Google Scholar]
- Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 2012; 67(6):533-9. doi: 10.1136/thoraxjnl-2011-201176 [Crossref] [ Google Scholar]