Advanced pharmaceutical bulletin. 12(4):757-762.
doi: 10.34172/apb.2022.077
Mini Review
Inorganic Nanoparticles: Toxic Effects, Mechanisms of Cytotoxicity and Phytochemical Interactions
Rashid Bhatti 1, *  , Hadia Shakeel 1, Kausar Malik 1, Muhammad Qasim 2, Mohsin Ahmad Khan 3, Nadeem Ahmed 3, Shajia Jabeen 1
, Hadia Shakeel 1, Kausar Malik 1, Muhammad Qasim 2, Mohsin Ahmad Khan 3, Nadeem Ahmed 3, Shajia Jabeen 1
Author information:
1Molecular Medicine Lab, Centre of Excellence in Molecular Biology, University of the Punjab, Lahore, Pakistan.
2Department of Bioinformatics and Biotechnology, Government College University, Faisalabad, Pakistan.
3Development of Recombinant Biopharmaceuticals Lab, Centre of Excellence in Molecular Biology, University of the Punjab, Lahore, Pakistan.
Abstract
During the last few decades, nanotechnology has gained many applications in almost all fields of life because of the unique properties of nanoparticles (NPs). Nanotechnology has specially marked its name in the field of medicine. However, NPs toxicity is detrimental to human health and is a prime concern in applied medicine. They can cause insomnia, vertigo, madarosis, epistaxis, hypokalemia, lymphopenia, Alzheimer’s and Parkinson’s diseases, etc. There is a gap in knowledge regarding the study of the toxicological effects of NPs. Mechanisms that are responsible for this toxicity are not fully understood yet. Phytochemicals have natural therapeutic effects of reducing metal NPs’ toxicity by acting as stabilizers and nontoxic reducing agents. However, the interaction between phytochemicals and NPs is remained to be elucidated. This review will provide in-depth knowledge about the various types of inorganic NPs and their associated toxicities, key parameters determining the toxic behaviour of NPs, and the mechanisms behind their cytotoxicity. It also emphasizes the need for further research to understand the interaction between various phytochemicals and NPs for therapeutic purposes.
Keywords: Medicine, Nanoparticles, Phytochemicals, Toxicity, Therapeutic effects
Copyright and License Information
©2022 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
A lot of progress has been made in the area of nanotechnology over the last few decades. Nanoparticles (NPs) usually have a nano scale size, i.e., a diameter of less than or equal to 100 nm.
1
Because of their unique properties, they have applications in numerous fields including cosmetics, electronics and medicine.
2
Silver, gold, zinc oxide (ZnO), and titanium oxide (TiO2) NPs are used in cosmetics because of their excellent drug delivery system, skin whitening and moisture retention properties. Their use in cosmetics is safe as they do not penetrate the skin. Therefore, they are not as harmful as long as they are used dermally.
3
NPs use in diagnostics and therapeutics is growing day by day. However, safety is needed to be ensured for their effective use in various fields like food, cosmetics, medicine, etc.
4
Plants possess abundant radical scavenging molecules like vitamins, phenolic compounds, terpenoids, etc. These molecules have antioxidant activity, thus enabling them to reduce toxicity caused by NPs.
5
As NPs are toxic to health, workers dealing with them must wear personal protective equipment (PPE) such as respirators, nitrile gloves, lab coats, goggles and closed-toed shoes. Fume hoods, gloves, biosafety cabinets should be employed for handling NPs.
6
This review discusses different types of iNPs, their toxicity, factors affecting toxicity of iNPs, mechanisms behind their toxicity, strategies to avoid toxicity and the interaction between phytochemicals and inorganic NPs.
Types of inorganic nanoparticles and their toxicity
Of all the NPs, inorganic NPs (quantum dots, metallic NPs, etc) are among those that are most abundantly produced and used commercially.
7
They are being used as therapeutic agents because of their anticancer and antimicrobial activities.
8
They can be used to create antimicrobial nanocomposite films. TYiO2-NPs were incorporated into chitosan to produce a biocomposite membrane that reduced the oxidative stress levels and apoptosis in mouse fibroblast cells due to the superior porosity, crystallinity, mechanical strength and structural flexibility.
9
However, their increased exposure may cause inflammation, genotoxicity, and oxidative stress, leading to cancer and metabolic diseases.
10
Different types of inorganic NPs and their associated toxicities are mentioned in Table 1.
Table 1.
Inorganic nanoparticles and their toxicity
|
Type of inorganic nanoparticle
|
Source reducing Agent
|
Particle size
|
Mechanism
|
References
|
| TMAT-AuNP |
Gold |
1.3 nm |
Progression of eye pigmentation |
11
|
| Ag-NP |
Silver |
10 nm |
Oxidative stress |
12
|
| Multi-walled carbon nanotubes |
Carbon nanotubes |
15-50 nm |
Inflammation |
13
|
|
TiO2
|
Titanium |
5-90 nm |
Apoptosis |
14
|
ZnS
CdS
Quantum dots (QD)
|
Cores: Zinc
Cadmium
Shells: Sulphide
|
10 ± 2 nm
8 ± 2 nm
|
Increased lipid peroxidation & catalase activity |
15
|
Gold and silver nanoparticles toxicity
“Nanogold” is a suspension of sub-micrometer-sized gold particles in a fluid, usually water.
16
Because of chemical stability and good optical properties, gold nanoparticles (AuNPs) are being used in chemotherapy and drug delivery. They have shown the cytotoxicity in vitro on BALB/3T3 mouse fibroblasts.
17
Silver nanoparticles (AgNPs), because of their antimicrobial activity, are used in medicine and drug delivery.
18
Oxidation of AgNPs results in the release of silver ions that accounts for cytotoxicity related to the AgNPs.
19
A study showed that reactive oxygen species (ROS) generation was more by AgNPs than bulk silver, due to which AgNPs are more toxic than bulk silver.
20
Actually, the toxicity of AgNPs is related to surface area; as the concentration of AgNPs per unit volume of reaction mixture increases, the surface area increases as well. It causes an increase in ROS production, which ultimately contributes to cell toxicity.
21
Moreover, oxidative damage and subacute toxicity of AgNP-PVP and AgNP-20 on the kidneys, lungs and liver of mice have also been reported.
22
Carbon nanotubes toxicity
Carbon nanotubes (CNTs) are the allotropes of carbon and possess fiber-shaped nanostructures.
23
In cell lines, CNTs can activate ROS-associated intracellular signalling pathways.
24
They have also been reported to trigger the release of cytokines including TNF-α, IL-1β, IL-8 and IL-6 from macrophages and mesothelial cells.
25
A study showed that nanocomposites of chitosan CNTs not only improved antimicrobial activity but also caused DNA damage in hepatic cells of Oreochromis niloticus.
26
Titanium dioxide nanoparticles toxicity
Titanium dioxide nanoparticles (TiO2 NPs) are used in cosmetics, food additives and pharmaceutical products because of their chemical stability and photocatalytic properties. TiO2 NPs can induce cytotoxicity, genotoxicity and oxidative stress.
27
They have induced indirect genotoxicity in two lung cell lines, i.e., A549 and BEAS-2B due to impaired DNA repair processes.
28
Quantum dots nanoparticles toxicity
Quantum dots (QDs), the semiconductor NPs, are fluorescent and possess unique optical properties.
29
Just like other NPs, QDs cytotoxicity depends on their shape, size, concentration, redox activity, mechanical stability, surface coatings and charge.
30
Nitrogen and Sulphur co-doped graphene QDs are less toxic and used as fluorescent nano-sensors in living cells.
31
Factors affecting the toxicity of NPs
Major factors associated with NPs toxicity are given below:
Dose and time of exposure
The toxicity of NPs is associated with their number. Cells with more particles have more toxic effects than cells with fewer particles. Both dose and time play a crucial role in determining the toxicity of NPs.
32
However, NP penetration in the cells depends on their exposure time.
Concentration and aggregation
Increased concentration of NPs favours their aggregation as their size is in micrometers, they do not penetrate the cells and their toxicity is lost.
33
On the other hand, another study suggested that aggregation of NPs affects their stability, making them more toxic.
34
Particle size and shape
The toxicity of NPs also depends on their size.
35
Small-sized NPs are toxic than large-sized NPs, e.g., AgNPs of 10 nm have more significant toxicity than the larger AgNPs (20-100 nm).
36
The shape is another factor that helps to determine the toxicity of NPs, i.e., different aspect ratios possess different toxicity levels.
37
Long asbestos fibers (10 µm) can cause lung cancer, while short fibers (5-10µm) can cause mesothelioma or asbestosis (2 µm).
38
Multi-walled CNTs embedded in pleural membrane activated macrophages that secreted IL-1β, which amplify inflammation in mesothelial cells.
39
Crystal structure and route of exposure
Different crystalline structures of NPs can exhibit toxicity differently. NPs can show different oxidative mechanisms, cellular uptake and subcellular localization based upon their crystalline structure.
33
Route of exposure regulates the initial interaction of NPs and cells.
40
Dermal exposure of NPs activate the immune system while their systemic distribution causes spleen and liver toxicities.
36
Pre-exposure and surface functionalization
Pre-exposure to low nanoparticle concentrations can stimulate phagocytic activity and adapt the human body to these NPs.
41
Whereastheir surface properties have drastic effects on oxidation processes. NPs with cationic surface are more cytotoxic than NPs with anionic surface.
42
Mechanisms of nanoparticles cytotoxicity
Nanoparticle toxicity mechanisms include DNA damage, oxidative stress, ROS production (Figure 1), and alteration of protein structures. Different mechanisms associated with nanoparticle cytotoxicity are mentioned in Table 2.
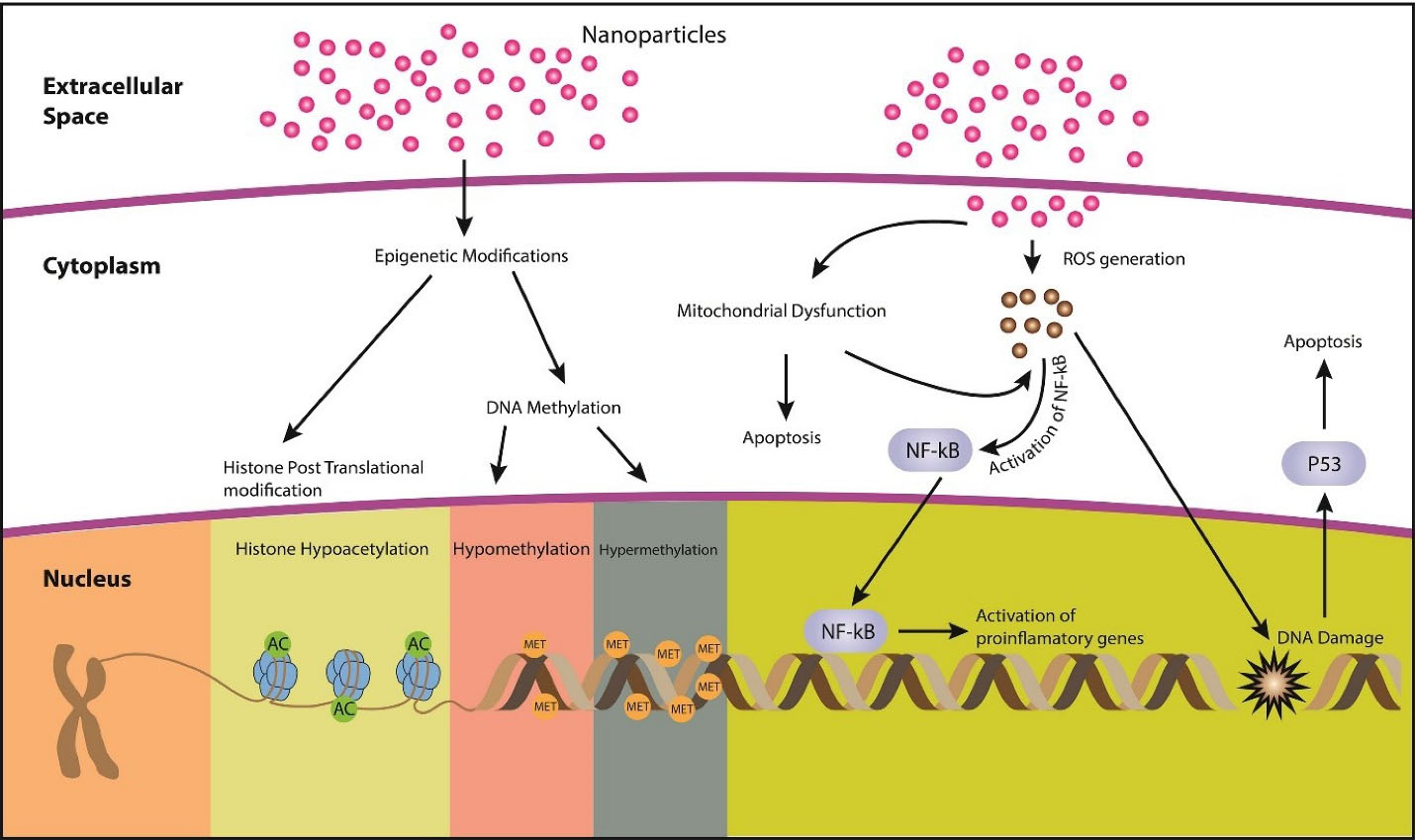
Figure 1.
Different mechanisms associated with nanoparticle toxicity
.
Different mechanisms associated with nanoparticle toxicity
Table 2.
Inorganic nanoparticles and their cytotoxicity
|
Type of inorganic nanoparticle
|
Source reducing agent
|
Particle size
|
Mechanism of cytotoxicity
|
References
|
| Au-NP |
Gold |
25-30 nm |
Oxidative stress |
43
|
| Ag20Pep |
Silver |
20 nm |
ROS formation & calcium dysregulation |
44
|
| Long and short multi-walled carbon nanotubes |
Carbon |
7-26 nm |
Lipid peroxidation and oxidative stress |
45
|
|
TiO2
|
Titanium |
~26.4 ± 1.2 nm |
ROS production and apoptosis |
46
|
| CdTe quantum dots |
Cadmium |
2.3 nm |
ROS generation and apoptosis |
47
|
Two important cytotoxicity mechanisms are discussed below:
Inflammatory response and Oxidative stress
Inflammation is the defense mechanism of the body that involves many cytokines.
48
Macrophages in the macrophage-rich organs, including spleen and liver, usually take up the NPs and release cytokines.
49
ROS induction is the leading cause of nanotoxicity.
50
A large number of nanomaterials have induced toxicity in human erythrocytes and skin fibroblasts through the production of ROS.
51
Moreover, an imbalance in redox state of the cell causes the oxidative stress.
52
Though ROS production is considered normal, but its excessive production is harmful to the cells. ZnO-NPs increase ROS inside cells and activate apoptosis via the caspase cascades in human gingival squamous cell carcinoma.
53
Epigenetic modifications
Epigenetic modifications refer to the heritable changes that are not due to alterations in the nucleotide sequence of DNA. Instead, they are due to the alterations in chromatin structure and DNA accessibility, e.g., histone modification and DNA methylation.
54
Transcriptional machinery of the cell depends upon how tightly DNA is enfolded around histones, while DNA packaging depends upon histone post-translational modifications.
55
Nanoparticle exposure can lead to epigenetic changes. Inorganic nanoparticles (iNPs) can change the gene and chromatin packaging, e.g., Ag-NPs can cross the nuclear membrane and interfere with chromatin remolding enzymes that affect condensation of chromatin and accessibility of DNA, thus altering the expression of genes.
56
Strategies to avoid toxicity caused by inorganic nanoparticles
The main cause of iNPs toxicity is oxidative stress, so their toxicity can be overcome by preventing oxidative stress. Interestingly, it is reported that vitamin C can decrease ROS production in acute myeloid leukaemia cells treated with AgNPs.
57
Another strategy that can be used to avoid oxidative stress is to use methods that slow down the release of metal ions since metal ions play a role in the induction of oxidative stress, e.g., slowing down the release of silver ions produced by AgNPs can reduce AgNP-induced toxicity.
58,59
NPs can be coated with antioxidants or a polymer like polyethylene glycol (PEG) to reduce ROS formation. PEG-coated iron oxide NPs reduce cytotoxicity by blocking the interaction of ROS with Fe2O3-NPs.
60
PVP-Bi2Se3 NPs showed better radiotherapy efficacy in cancer treatment. As selenium can improve immune function by reducing the harmful effects of radiation on normal cells.
61
NPs toxicity can also be minimized by creating metal oxide NPs that are toxic to cancer cells but not to normal cells, e.g., ZnO NPs selectively target cancerous cells leaving normal cells.
62
Interaction between phytochemicals and inorganic nanoparticles
Secondary metabolites derived from harmless microbes and plants are called phytochemicals.
63
These phytochemicals due to their therapeutic effects are used to prepare metal NPs by green synthesis approach. Green synthesis is a biological method for synthesizing NPs based upon oxidation-reduction reaction to reduce metal ions into stable NPs using an organism’s components or its extract.
64
Previous studies showed that phytochemicals with antioxidant properties could possess the ability to protect cells from NPs’ exposure. However, the interaction between phytochemicals, NPs, and their associated toxicities are yet to be understood.
Conclusion
NPs are being used in almost all fields of life today, but nanotoxicity has become a major issue. Oxidative stress is particularly associated with the toxicity of inorganic NPs and reducing this stress may increase the biocompatibility of NPs. Due to low toxicity and high bioactivity, phytochemicals can be coated on NPs to reduce their cytotoxicity efficiently. Research on the toxicity of iNPs is highly dispersed and no definitive conclusions can be drawn from the available literature. So, there is a need for further research to understand the toxicity mechanisms, the interaction between various phytochemicals and inorganic NPs and investigate strategies for synthesizing NPs with optimal properties while minimizing adverse effects on living cells.
Acknowledgments
The authors would like to thank Ms. Zainab Akram and Ms. Khadija Abdul Majid for the help rendered by them.
Ethical Issues
Not applicable.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Aljerf L, AlHamwi B. Carbon nanotubes-synthesis developmental engineering demands will overcome the health challenge of nanotoxicity and its acute mortality for humans. Madridge J Nanotechnol Nanosci 2018; 3(2):118-21. doi: 10.18689/mjnn-1000122 [Crossref] [ Google Scholar]
- Raj S, Jose S, Sumod US, Sabitha M. Nanotechnology in cosmetics: opportunities and challenges. J Pharm Bioallied Sci 2012; 4(3):186-93. doi: 10.4103/0975-7406.99016 [Crossref] [ Google Scholar]
- Dréno B, Alexis A, Chuberre B, Marinovich M. Safety of titanium dioxide nanoparticles in cosmetics. J Eur Acad Dermatol Venereol 2019; 33 Suppl 7:34-46. doi: 10.1111/jdv.15943 [Crossref] [ Google Scholar]
- Singh T, Shukla S, Kumar P, Wahla V, Bajpai VK. Application of nanotechnology in food science: perception and overview. Front Microbiol 2017; 8:1501. doi: 10.3389/fmicb.2017.01501 [Crossref] [ Google Scholar]
- Burlacu E, Tanase C, Coman NA, Berta L. A review of bark-extract-mediated green synthesis of metallic nanoparticles and their applications. Molecules 2019; 24(23):4354. doi: 10.3390/molecules24234354 [Crossref] [ Google Scholar]
- Gupta R, Xie H. Nanoparticles in daily life: applications, toxicity and regulations. J Environ Pathol Toxicol Oncol 2018; 37(3):209-30. doi: 10.1615/JEnvironPatholToxicolOncol.2018026009 [Crossref] [ Google Scholar]
- Alam MW, Qurashi A. Metal chalcogenide quantum dots for hybrid solar cell applications. In: Qurashi A, ed. Metal Chalcogenide Nanostructures for Renewable Energy Applications. Massachusetts: Scrivener Publishing LLC; 2014. p. 233-46.
- Wang H, Zhang F, Wen H, Shi W, Huang Q, Huang Y. Tumor- and mitochondria-targeted nanoparticles eradicate drug resistant lung cancer through mitochondrial pathway of apoptosis. J Nanobiotechnology 2020; 18(1):8. doi: 10.1186/s12951-019-0562-3 [Crossref] [ Google Scholar]
- Wang L, Lin L, Pang J. A novel glucomannan incorporated functionalized carbon nanotube films: synthesis, characterization and antimicrobial activity. Carbohydr Polym 2020; 245:116619. doi: 10.1016/j.carbpol.2020.116619 [Crossref] [ Google Scholar]
- Morsy EA, Hussien AM, Ibrahim MA, Farroh KY, Hassanen EI. Cytotoxicity and genotoxicity of copper oxide nanoparticles in chickens. Biol Trace Elem Res 2021; 199(12):4731-45. doi: 10.1007/s12011-021-02595-4 [Crossref] [ Google Scholar]
- Kim KT, Zaikova T, Hutchison JE, Tanguay RL. Gold nanoparticles disrupt zebrafish eye development and pigmentation. Toxicol Sci 2013; 133(2):275-88. doi: 10.1093/toxsci/kft081 [Crossref] [ Google Scholar]
- Patlolla AK, Hackett D, Tchounwou PB. Silver nanoparticle-induced oxidative stress-dependent toxicity in Sprague-Dawley rats. Mol Cell Biochem 2015; 399(1-2):257-68. doi: 10.1007/s11010-014-2252-7 [Crossref] [ Google Scholar]
- Francis AP, Ganapathy S, Palla VR, Murthy PB, Ramaprabhu S, Devasena T. One time nose-only inhalation of MWCNTs: exploring the mechanism of toxicity by intermittent sacrifice in Wistar rats. Toxicol Rep 2015; 2:111-20. doi: 10.1016/j.toxrep.2015.02.003 [Crossref] [ Google Scholar]
- Jia X, Wang S, Zhou L, Sun L. The potential liver, brain, and embryo toxicity of titanium dioxide nanoparticles on mice. Nanoscale Res Lett 2017; 12(1):478. doi: 10.1186/s11671-017-2242-2 [Crossref] [ Google Scholar]
- Matos B, Martins M, Samamed AC, Sousa D, Ferreira I, Diniz MS. Toxicity evaluation of quantum dots (ZnS and CdS) singly and combined in zebrafish (Danio rerio). Int J Environ Res Public Health 2019; 17(1). doi: 10.3390/ijerph17010232 [Crossref]
- Qiao J, Qi L. Recent progress in plant-gold nanoparticles fabrication methods and bio-applications. Talanta 2021; 223(Pt 2):121396. doi: 10.1016/j.talanta.2020.121396 [Crossref] [ Google Scholar]
- Coradeghini R, Gioria S, García CP, Nativo P, Franchini F, Gilliland D. Size-dependent toxicity and cell interaction mechanisms of gold nanoparticles on mouse fibroblasts. Toxicol Lett 2013; 217(3):205-16. doi: 10.1016/j.toxlet.2012.11.022 [Crossref] [ Google Scholar]
- Gholami N, Ahangari Cohan R, Razavi A, Bigdeli R, Dashbolaghi A, Asgary V. Cytotoxic and apoptotic properties of a novel nano-toxin formulation based on biologically synthesized silver nanoparticle loaded with recombinant truncated pseudomonas exotoxin A. J Cell Physiol 2020; 235(4):3711-20. doi: 10.1002/jcp.29265 [Crossref] [ Google Scholar]
- Zhang T, Wang L, Chen Q, Chen C. Cytotoxic potential of silver nanoparticles. Yonsei Med J 2014; 55(2):283-91. doi: 10.3349/ymj.2014.55.2.283 [Crossref] [ Google Scholar]
- Batchelor-McAuley C, Tschulik K, Neumann CC, Laborda E, Compton RG. Why are silver nanoparticles more toxic than bulk silver? Towards understanding the dissolution and toxicity of silver nanoparticles. Int J Electrochem Sci 2014; 9(3):1132-8. [ Google Scholar]
- Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B 2008; 112(43):13608-19. doi: 10.1021/jp712087m [Crossref] [ Google Scholar]
- Gan J, Sun J, Chang X, Li W, Li J, Niu S. Biodistribution and organ oxidative damage following 28 days oral administration of nanosilver with/without coating in mice. J Appl Toxicol 2020; 40(6):815-31. doi: 10.1002/jat.3946 [Crossref] [ Google Scholar]
- Aljerf L, Nadra R. Developed greener method based on MW implementation in manufacturing CNFs. Int J Nanomanuf 2019; 15(3):269-89. doi: 10.1504/ijnm.2019.100461 [Crossref] [ Google Scholar]
- Garriga R, Herrero-Continente T, Palos M, Cebolla VL, Osada J, Muñoz E. Toxicity of carbon nanomaterials and their potential application as drug delivery systems: in vitro studies in Caco-2 and MCF-7 cell lines. Nanomaterials (Basel) 2020; 10(8):1617. doi: 10.3390/nano10081617 [Crossref] [ Google Scholar]
- Murphy FA, Schinwald A, Poland CA, Donaldson K. The mechanism of pleural inflammation by long carbon nanotubes: interaction of long fibres with macrophages stimulates them to amplify pro-inflammatory responses in mesothelial cells. Part Fibre Toxicol 2012; 9:8. doi: 10.1186/1743-8977-9-8 [Crossref] [ Google Scholar]
- Abu-Elala NM, AbuBakr HO, Khattab MS, Mohamed SH, El-Hady MA, Ghandour RA. Aquatic environmental risk assessment of chitosan/silver, copper and carbon nanotube nanocomposites as antimicrobial agents. Int J Biol Macromol 2018; 113:1105-15. doi: 10.1016/j.ijbiomac.2018.03.047 [Crossref] [ Google Scholar]
- Yang J, Liu J, Wang P, Sun J, Lv X, Diao Y. Toxic effect of titanium dioxide nanoparticles on corneas in vitro and in vivo. Aging (Albany NY) 2021; 13(4):5020-33. doi: 10.18632/aging.202412 [Crossref] [ Google Scholar]
- Biola-Clier M, Beal D, Caillat S, Libert S, Armand L, Herlin-Boime N. Comparison of the DNA damage response in BEAS-2B and A549 cells exposed to titanium dioxide nanoparticles. Mutagenesis 2017; 32(1):161-72. doi: 10.1093/mutage/gew055 [Crossref] [ Google Scholar]
-
Armăşelu A. Quantum dots and fluorescent and magnetic nanocomposites: recent investigations and applications in biology and medicine. In: Stavrou VN, ed. Nonmagnetic and Magnetic Quantum Dots. London: IntechOpen; 2017. 10.5772/intechopen.70614.
- Khalili Fard J, Jafari S, Eghbal MA. A review of molecular mechanisms involved in toxicity of nanoparticles. Adv Pharm Bull 2015; 5(4):447-54. doi: 10.15171/apb.2015.061 [Crossref] [ Google Scholar]
- Qu C, Zhang D, Yang R, Hu J, Qu L. Nitrogen and sulfur co-doped graphene quantum dots for the highly sensitive and selective detection of mercury ion in living cells. Spectrochim Acta A Mol Biomol Spectrosc 2019; 206:588-96. doi: 10.1016/j.saa.2018.07.097 [Crossref] [ Google Scholar]
- Graham UM, Jacobs G, Yokel RA, Davis BH, Dozier AK, Birch ME. From dose to response: in vivo nanoparticle processing and potential toxicity. Adv Exp Med Biol 2017; 947:71-100. doi: 10.1007/978-3-319-47754-1_4 [Crossref] [ Google Scholar]
- Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 2018; 9:1050-74. doi: 10.3762/bjnano.9.98 [Crossref] [ Google Scholar]
- Saifi MA, Khan W, Godugu C. Cytotoxicity of nanomaterials: using nanotoxicology to address the safety concerns of nanoparticles. Pharm Nanotechnol 2018; 6(1):3-16. doi: 10.2174/2211738505666171023152928 [Crossref] [ Google Scholar]
- Sukhanova A, Bozrova S, Sokolov P, Berestovoy M, Karaulov A, Nabiev I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res Lett 2018; 13(1):44. doi: 10.1186/s11671-018-2457-x [Crossref] [ Google Scholar]
- Ivask A, Kurvet I, Kasemets K, Blinova I, Aruoja V, Suppi S. Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS One 2014; 9(7):e102108. doi: 10.1371/journal.pone.0102108 [Crossref] [ Google Scholar]
-
Budama-Kilinc Y, Cakir-Koc R, Zorlu T, Ozdemir B, Karavelioglu Z, Egil AC, et al. Assessment of nano-toxicity and safety profiles of silver nanoparticles. In: Khan M, ed. Silver Nanoparticles: Fabrication, Characterization and Applications. London: IntechOpen; 2018. 10.5772/intechopen.75645.
- Lippmann M. Effects of fiber characteristics on lung deposition, retention, and disease. Environ Health Perspect 1990; 88:311-7. doi: 10.1289/ehp.9088311 [Crossref] [ Google Scholar]
- Xie D, Luo X. Identification of four methylation-driven genes as candidate biomarkers for monitoring single-walled carbon nanotube-induced malignant transformation of the lung. Toxicol Appl Pharmacol 2021; 412:115391. doi: 10.1016/j.taap.2020.115391 [Crossref] [ Google Scholar]
- De Matteis V. Exposure to inorganic nanoparticles: routes of entry, immune response, biodistribution and in vitro/in vivo toxicity evaluation. Toxics 2017; 5(4):29. doi: 10.3390/toxics5040029 [Crossref] [ Google Scholar]
- Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases 2007; 2(4):MR17-71. doi: 10.1116/1.2815690 [Crossref] [ Google Scholar]
- Xia T, Kovochich M, Liong M, Zink JI, Nel AE. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano 2008; 2(1):85-96. doi: 10.1021/nn700256c [Crossref] [ Google Scholar]
- Surapaneni SK, Bashir S, Tikoo K. Gold nanoparticles-induced cytotoxicity in triple negative breast cancer involves different epigenetic alterations depending upon the surface charge. Sci Rep 2018; 8(1):12295. doi: 10.1038/s41598-018-30541-3 [Crossref] [ Google Scholar]
- Haase A, Rott S, Mantion A, Graf P, Plendl J, Thünemann AF. Effects of silver nanoparticles on primary mixed neural cell cultures: uptake, oxidative stress and acute calcium responses. Toxicol Sci 2012; 126(2):457-68. doi: 10.1093/toxsci/kfs003 [Crossref] [ Google Scholar]
- Rezazadeh Azari M, Mohammadian Y. Comparing in vitro cytotoxicity of graphite, short multi-walled carbon nanotubes, and long multi-walled carbon nanotubes. Environ Sci Pollut Res Int 2020; 27(13):15401-6. doi: 10.1007/s11356-020-08036-4 [Crossref] [ Google Scholar]
- Kongseng S, Yoovathaworn K, Wongprasert K, Chunhabundit R, Sukwong P, Pissuwan D. Cytotoxic and inflammatory responses of TiO2 nanoparticles on human peripheral blood mononuclear cells. J Appl Toxicol 2016; 36(10):1364-73. doi: 10.1002/jat.3342 [Crossref] [ Google Scholar]
- Lovrić J, Cho SJ, Winnik FM, Maysinger D. Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chem Biol 2005; 12(11):1227-34. doi: 10.1016/j.chembiol.2005.09.008 [Crossref] [ Google Scholar]
-
Aljerf L, Aljurf M. Improvements in the ecological and nutritional aspects of Down’s syndrome. Res Sq [Preprint]. May 31, 2020. Available from: https://www.preprints.org/manuscript/202005.0512/v1#.
- Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol 2015; 33(9):941-51. doi: 10.1038/nbt.3330 [Crossref] [ Google Scholar]
- Khanna P, Ong C, Bay BH, Baeg GH. Nanotoxicity: an interplay of oxidative stress, inflammation and cell death. Nanomaterials (Basel) 2015; 5(3):1163-80. doi: 10.3390/nano5031163 [Crossref] [ Google Scholar]
- Akhtar MJ, Ahamed M, Kumar S, Siddiqui H, Patil G, Ashquin M. Nanotoxicity of pure silica mediated through oxidant generation rather than glutathione depletion in human lung epithelial cells. Toxicology 2010; 276(2):95-102. doi: 10.1016/j.tox.2010.07.010 [Crossref] [ Google Scholar]
- Aw TY. Molecular and cellular responses to oxidative stress and changes in oxidation-reduction imbalance in the intestine. Am J Clin Nutr 1999; 70(4):557-65. doi: 10.1093/ajcn/70.4.557 [Crossref] [ Google Scholar]
- Wang SW, Lee CH, Lin MS, Chi CW, Chen YJ, Wang GS. ZnO nanoparticles induced caspase-dependent apoptosis in gingival squamous cell carcinoma through mitochondrial dysfunction and p70S6K signaling pathway. Int J Mol Sci 2020; 21(5):1612. doi: 10.3390/ijms21051612 [Crossref] [ Google Scholar]
- Ajong AB, Kenfack B, Ali IM, Yakum MN, Aljerf L, Telefo PB. Hypocalcaemia and calcium intake in pregnancy: a research protocol for critical analysis of risk factors, maternofoetal outcomes and evaluation of diagnostic methods in a third-category health facility, Cameroon. PLoS One 2020; 15(11):e0241812. doi: 10.1371/journal.pone.0241812 [Crossref] [ Google Scholar]
- Zhang H, Kuchroo V. Epigenetic and transcriptional mechanisms for the regulation of IL-10. Semin Immunol 2019; 44:101324. doi: 10.1016/j.smim.2019.101324 [Crossref] [ Google Scholar]
- Qian Y, Zhang J, Hu Q, Xu M, Chen Y, Hu G. Silver nanoparticle-induced hemoglobin decrease involves alteration of histone 3 methylation status. Biomaterials 2015; 70:12-22. doi: 10.1016/j.biomaterials.2015.08.015 [Crossref] [ Google Scholar]
- Aggarwal V, Tuli HS, Varol A, Thakral F, Yerer MB, Sak K. Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Biomolecules 2019; 9(11):735. doi: 10.3390/biom9110735 [Crossref] [ Google Scholar]
- Poljsak B. Strategies for reducing or preventing the generation of oxidative stress. Oxid Med Cell Longev 2011; 2011:194586. doi: 10.1155/2011/194586 [Crossref] [ Google Scholar]
- Ahlberg S, Rancan F, Epple M, Loza K, Höppe D, Lademann J. Comparison of different methods to study effects of silver nanoparticles on the pro- and antioxidant status of human keratinocytes and fibroblasts. Methods 2016; 109:55-63. doi: 10.1016/j.ymeth.2016.05.015 [Crossref] [ Google Scholar]
- Yu M, Huang S, Yu KJ, Clyne AM. Dextran and polymer polyethylene glycol (PEG) coating reduce both 5 and 30 nm iron oxide nanoparticle cytotoxicity in 2D and 3D cell culture. Int J Mol Sci 2012; 13(5):5554-70. doi: 10.3390/ijms13055554 [Crossref] [ Google Scholar]
- Du J, Gu Z, Yan L, Yong Y, Yi X, Zhang X. Poly(vinylpyrollidone)- and selenocysteine-modified Bi2 Se3 nanoparticles enhance radiotherapy efficacy in tumors and promote radioprotection in normal tissues. Adv Mater 2017; 29(34). doi: 10.1002/adma.201701268 [Crossref]
- Rasmussen JW, Martinez E, Louka P, Wingett DG. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin Drug Deliv 2010; 7(9):1063-77. doi: 10.1517/17425247.2010.502560 [Crossref] [ Google Scholar]
- Seukep AJ, Kuete V, Nahar L, Sarker SD, Guo M. Plant-derived secondary metabolites as the main source of efflux pump inhibitors and methods for identification. J Pharm Anal 2020; 10(4):277-90. doi: 10.1016/j.jpha.2019.11.002 [Crossref] [ Google Scholar]
- Singh J, Dutta T, Kim KH, Rawat M, Samddar P, Kumar P. ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnology 2018; 16(1):84. doi: 10.1186/s12951-018-0408-4 [Crossref] [ Google Scholar]