Advanced pharmaceutical bulletin. 13(1):202-209.
doi: 10.34172/apb.2023.039
Research Article
Decreased Cardiac NOX4 and SIRT-1 Protein Levels Contribute to Decreased Angiogenesis in the Heart of Diabetic Rats: Rescue Effects of IGF-1 and Exercise
Shiva Roshan Milani 1, 2  , Bagher Pourheydar 3, 2, Saman Daneshfar 4, Leila Chodari 1, 2, *
, Bagher Pourheydar 3, 2, Saman Daneshfar 4, Leila Chodari 1, 2, * 
Author information:
1Department of Physiology, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran.
2Neurophysiology Research Center, Cellular and Molecular Medicine Institute, Urmia University of Medical Sciences, Urmia, Iran.
3Department of Anatomical Sciences, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran.
4Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran.
Abstract
Purpose:
Reduced angiogenesis in the heart tissue is a primary risk factor for heart disease in the diabetes condition. This study was aimed to evaluate the changes of two main angiogenesis mediators, NADPH oxidase 4 (NOX4) and sirtuin 1 (SIRT-1) protein levels in the heart of diabetic rats and the impact of Insulin-like growth factor 1 (IGF-1) and exercise on these proteins.
Methods: Injection of 60 mg/kg of streptozotocin in 40 male Wistar rats led to the induction of type 1 diabetes. Angiogenesis was detected in the hearts by immunostaining for PECAM-1/ CD31 after 30 days of treatment with IGF-1 (2 mg/kg/day) and exercise. ELISA technique was utilized to establish the expression levels of NOX4 and SIRT-1 within the heart.
Results: The results revealed a significant increase in HbA1c and a significant decrease in SIRT1, NOX4 levels and angiogenesis grade in the heart of diabetes group compared to control group. Meanwhile, IGF-1 and exercise alone or in combination completely masked these effects. Additionally, synergistic effect on SIRT-1, HbA1c levels and angiogenesis grade is evident when IGF-1 and exercise are applied simultaneously.
Conclusion: Our findings suggest that reduction in angiogenesis in the heart of diabetic rats may be mediated by down expression of NOX4 and SIRT-1 protein levels. It was also displayed that IGF-1 and exercise as novel therapies increase NOX4 and SIRT-1 protein levels within the hearts of diabetic rats.
Keywords: Diabetes, Heart, Angiogenesis, Exercise, IGF-I, NOX4, SIRT1
Copyright and License Information
©2023 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Diabetes mellitus is a considerable public health problem and has a global impact on human health and economics.1 High blood glucose condition in the diabetes induces macro and microvascular malfunction and lowers angiogenesis in the cardiac tissue that leads to ischemic condition.2 Today, it is well known that decreased angiogenesis is a main reason for cardiac failure in diabetic patient.2 It has been demonstrated that diabetes impairs collateral vessel development in animal models of ischemia and in ischemic human hearts.3
As a result, evaluating the molecular intermediators involved in the angiogenesis signaling pathways in diabetic rats’ heart tissue are critical. Several lines of evidence demonstrated that NOX4 (NADPH oxidase 4) and SIRT-1 (sirtuin 1) have significant impact on angiogenesis signaling pathway.4-6
reactive oxygen species (ROS) scavenging enzymes and ROS producing oxidases cautiously co-regulate angiogenesis. The NOX family, by transferring electrons from NADPH to molecular oxygen produce ROS.7
NOX4 is the key isoform of NADPH oxidases produced in endothelium and largely yields ROS and H2O2 that play crucial role in vascularization.8 It is shown that vascularization in cultured NOX4 -/- endothelial cells was reduced whereas treatment with H2O2 improve formation of new vessels.9
Vasoprotective feature of NOX4 during ischemic also reported, furthermore molecular studies indicate that NOX4 stimulates angiogenesis signaling pathway through production of ROS.10 Although NOXs have been suggested as a therapeutic pathway for a variety of diabetic issues, their role in diabetic cardiomyopathy is still vague.11
Sirtuins (Sirt1–Sirt7) include a family of nicotinamide adenine dinucleotide (NAD + )-dependent deacetylating enzymes.12 Between sirtuins, SIRT1 and SIRT6 are the best considered for their defensive role in opposition to inflammation, vascular and cardiac disease.13 SIRT1 is extremely produced in the vascular bed, where it controls endothelial angiogenic functions during vascular growth.14 In the absence of SIRT1 activity, the sprouting and branching blood vessels reduced.15 It is demonstrated that lowered expression of SIRT1 within the heart of diabetic subjects is enough to cause phenotypes like Diabetic cardiomyopathy.16
Insulin-like growth factor 1 (IGF-1) as an inducer of angiogenesis factor is a peptide hormone that increases cell responsiveness to insulin.17 Numerous literatures have confirmed that in type 1 diabetes, IGF-1 insufficiency is prevalent and therefore sensitivity of cells to insulin decline in the diabetes condition.18 Furthermore, it is shown that IGF-1 therapy refines insulin sensitivity in adults with type 1 diabetes.19 Therefore, IGF-1 can be a useful healing goal in the treatment of heart failure in diabetes condition.
In patients with heart disease, physical exercise is agreed to be one of the mending factor since it decrease myocardial failure, and cardiac risk factors, and enhances cardiac function.20 Regular exercise is known to decrease cardiovascular problems in both type 1 and 2 diabetes.21 Huge studies showed that exercise alleviates diabetes-induced cardiomyopathy by increasing angiogenesis.22
Thus, this research aimed to study the effect of diabetes on cardiac NOX4 and SRIT-1 protein levels as antigenic factors. It also sought to examine the possible protective effects of treatments, alone or together, on angiogenesis in the heart in the diabetes condition and its corresponding molecular mediators (NOX4 and SIRT-1)
Materials and Methods
Animals and study design
Forty male Wistar rats weighing 250 ± 10 purchased from the Urmia University of Medical Science that were casually separated into 5 groups (n = 8). Animals were housed in special cages on a 12L:12D cycle at 24°C room temperature, proportionate moisture of 50% and fed ad libitum on commercial laboratory food pellets. The groupings are as follows: Control (Cont), Diabetes (Dia), Diabetes + Exercise (Dia + Exe), Diabetes + IGF-1 (Dia + IGF-1), Diabetes Exercise + IGF-1 (Dia + Exe + IGF-1) groups. IGF-1 (Sigma Aldrich, USA) was injected daily with 2 mg/kg dose subcutaneously for four weeks.23 It should be noted that rats that didn’t performed exercise were taken to the exercise room and placed on treadmill. Also, rats without IGF-1 intervention received normal saline daily.
Induction of type 1 diabetes
For induction of type 1diabetes, rats were fasted overnight and received streptozotocin (60 mg/kg/d, i.p., dissolved in 0.1 mol/L citrate buffer, pH 4.5,Sigma, St. Louis, Missouri, USA). 3 days after the injection, the rats with fasting plasma glucose above 300 mg/dL (16.67 mmol/L) were classified as diabetic.24
Exercise protocol
The whole training period was 4 weeks. At first, the animals in the Exe group were adjusted to the animal treadmill. The examinations were conducted between 9 and 12 AM. The training program illustrated in the Table 1 that considered as mild training.25
Table 1.
Training program
|
Training program
|
Duration of training (min)
|
Speed (m/min)
|
Inclination of treadmill (degree)
|
| Fist week |
day 1th: 10 min
day 2th: 15 min
day 3th: 20 min
day 4th: 25 min
day 5th: 30 min
day 6th: 30 min
day 7th:30 min |
17 m/min |
0 |
| Second week |
30 m/min |
17 m/min |
0 |
| Third week |
30 m/min |
17 m/min |
0 |
| Fourth week |
30 m/min |
17 m/min |
0 |
Elisa
After treatment for one month, all rats were anesthetized with ketamine (80 mg/kg) and xylazine (5 mg/kg). Heart tissue was removed immediately and the left ventricle was used for measurement of protein levels of SIRT-1 and NOX4 by high-sensitivity ELISA kits (Zelbio, Germany). According to the manufacturer’s instructions, 100 mg samples were homogenized in 1 mL potassium phosphate buffer (pH 7.2 to 7.4) containing anti-protease cocktail for preventing of protein destruction. Then, the homogenate was centrifuged for 20 minutes at 4°C at 1000 ×g. Finally, the resulting supernatant was depleted and target proteins were extracted for ELISA analysis.
Blood glucose and HbA1c assessment
The HbA1c test, is an important blood test that gives a good indication of controlled diabetes condition. For this test, heart-collected blood sample was utilized to measure rat HbA1c levels in HbA1c assay kit (Crystal chem, cat number: 80300).
Immunostaining for PECAM-1/CD31
To evaluate degree of vascularization in the heart, samples of left ventricle were used. So, 4 μm-thick slices were prepared from the samples. In the next step, these sections underwent processing steps such as deparaffinization, dehydration. To suppress endogenous peroxidase activity, incubation with proteinase K and treatment with hydrogen peroxide 0.3% were applied.
The primary antibody CD31 (Santa Cruz, USA) was used as an indicator of angiogenesis to cover the samples. Later on the sections were incubated at 4°C for 12 hours. Then hatched with usual avidin–biotin complex (ABC; Santa Cruz) giving to the manufacturer’s orders. In the next step, light microscope (Olympus BX 40, Japan) was used for evaluating the strength scoring for CD31 staining (at magnification 40×). For this purpose, 3 to 5 1 mm2 areas were selected and staining strength each (at 200×magnification) and amount of positive cells were calculated. As a distinct container of the positive endothelial cell batch of immunoreactivities in interaction alongside with the elected region (1 mm2) was calculated. The granulated tissue was used as a positive control to measure the proportion of immunostaining, and the strength of staining was scored as 0 ( < 10%); 1 (10% to 25%); 2 (25% to 50%); 3 (50% to 75%) or 4 (75% to 100%).26
Statistical analysis
Values are presented as mean ± SEM and were subjected to ANOVA followed by post hoc test (SPSS software version 16). Any P value < 0.05 was considered statistically significant.
Results and Discussion
IGF-1 and exercise decrease HbA1c levels in the blood
According to Figure 1, HbA1C levels were found to be much higher (P < 0.001) in the diabetic subjects compared to the control group. Four weeks exercise and IGF-1 treatment in the Dia + E and Dia + IGF-1 groups significantly decreased HbA1c level (P < 0.001) in comparison to diabetes group. Meanwhile it was found to be noticeably (P < 0.01) higher compared to the control group. Moreover, simultaneous treatment with IGF-1 and exercise considerably decreased HbA1c compared to that in the Dia (P < 0.001), Dia + E (P < 0.05) and Dia + IGF-1 (P < 0.05) groups. According to Figure 1, combination therapy had a synergistic effect on HbA1c levels in the blood. Today, many studies has discovered that vascular complication in diabetes is mediated by hyperglycemia.27 As a result, the use of HBA1C reducing mediators in the treatment of diabetes-related heart failure has received a lot of interest. Many studies have demonstrated IGF-1’s capabilities in lowering blood glucose.28 Thus far, diabetic patients have shown to have lower IGF-1 levels in comparison to non-diabetic patients.28 Additionally, today, the improving role of physical activity in diabetes and its importance in regulating glucose hemostasis has been proven.29
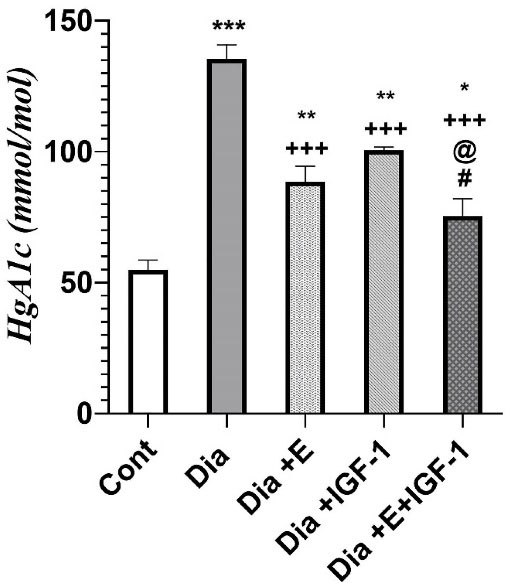
Figure 1.
Effect of exercise and IGF-1 on HbA1c levels in the blood. Values are expressed as Mean ± SD. *P < 0.05,**P < 0.01,*** P < 0.001 vs control group. + + + P < 0.001 vs Dia group. @ P < 0.05 vs Exe group. # P < 0.05 vs IGF-1 group.
.
Effect of exercise and IGF-1 on HbA1c levels in the blood. Values are expressed as Mean ± SD. *P < 0.05,**P < 0.01,*** P < 0.001 vs control group. + + + P < 0.001 vs Dia group. @ P < 0.05 vs Exe group. # P < 0.05 vs IGF-1 group.
IGF-1 and exercise improve NOX4 and SIRT-1 protein levels in the cardiac tissue
The results achieved by ELISA indicated that diabetes in the early stage, remarkably reduced NOX4 (P < 0. 01) and SIRT-1 (P < 0. 001) protein levels in cardiac tissue comparing to that of the control group. As demonstrated in Figure 2 A-B, the four-week treatment of the diabetic rats with exercise remarkably (P < 0.05) grew NOX4 and SIRT-1 protein levels compared to that in the Dia group. Also, Figure 2 shows that IGF-1 therapy noticeably increased NOX4 (P < 0. 01) and SIRT-1 (P < 0. 05) protein levels in comparison to that in the Dia group. Interestingly, exercise and IGF-1 have been able to increase the NOX4 protein level similar to the control group but in regarding to SIRT-1, it was still significantly lower than that of the control group (P < 0.001). Moreover, the simultaneous use of exercise and IGF-1 caused an up rise in cardiac NOX4 level in contrast to Dia (P < 0.01), Exe (P < 0.05) groups. Nevertheless, according to the Figure 2B, in rats with the combination treatment of IGF-1 and exercise did not show any synergistic effect on NOX4 protein level. It seems that the increasing effect of combination therapy on the NOX4 protein levels mediated by IGF-1 and IGF-1 has a more potent effect on NOX4 protein level than exercise. In regarding to SIRT-1, simultaneously treatment with exercise and IGF-1 crucially raised SIRT-1 protein levels compared to Dia (P < 0.01), Dia + E (P < 0.05), Dia + IGF-1 (P < 0.05). It is cleared that combination therapy showed a synergistic effect on SIRT-1 protein levels in the heart tissue. Between a large numbers of molecular intermediaries associated to angiogenesis process, mediators such as NOX4 6 and SIRT-130 also show vital effect in adjust of new vessel development in the diabetes condition. Each of NADPH oxidase (NOX) isoforms are as major sources of ROS in the vessel wall. It is shown thatNOX2 and NOX4 are widely expressed in the heart.31 In this study, we focused on the function of NOX 4 molecule in the myocardium that is less well-understood. Recently, it is widely accepted that NOX4 is a significant source of ROS production in human endothelial cells.32 Also, molecular studies indicate that NOX4 stimulates angiogenesis signaling pathway through production of ROS, activation of tyrosine kinases receptor and the downstream extracellular signal-regulated kinase (ERK) pathway.33 In other hand, it is reported that in some pathological conditions, too much NOX-dependent ROS production, which is related with the over expression of distinct NOX isoforms, disrupt the redox control systems and induce oxidative injury of the cardiovascular cell.34 As, increased expression of NOX4 has been reported in hearts from diabetic model by Maeda et al in 2012.35 While, in this study, our results suggest that NOX4 protein expression declined in the heart of rats with diabetes and exercise and IGF-1 improved this reduction.
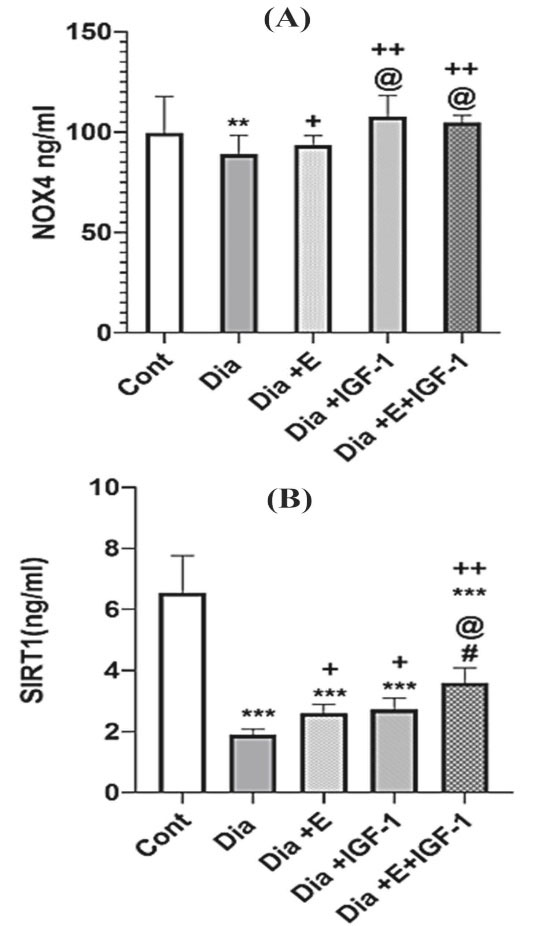
Figure 2.
(A-B) Effect of exercise and IGF-1 on NOX4 and SIRT-1 protein levels in the heart. Values are expressed as mean ± SD. **P < 0.01, *** P < 0.001 vs control group. + P < 0.05, + + P < 0.01, + + + P < 0.001 vs Dia group. @ P < 0.05 vs Exe group. # P < 0.05 vs IGF-1 group.
.
(A-B) Effect of exercise and IGF-1 on NOX4 and SIRT-1 protein levels in the heart. Values are expressed as mean ± SD. **P < 0.01, *** P < 0.001 vs control group. + P < 0.05, + + P < 0.01, + + + P < 0.001 vs Dia group. @ P < 0.05 vs Exe group. # P < 0.05 vs IGF-1 group.
In regards to the role of NOX4 in cardiomyopathy, Guzik et al showed that NOX-derived ROS has concerning the progression of vascular disease in diabetic patients.36 It seems that the role of NOX4 in pathology condition is arguable as specified by studies both reporting helpful37 and harmful effects38 of NOX4 in experimental heart failure. Hansen et al suggested that these discrepancies are due to the severity of the HF applied in the different studies.39 As, NOX4 may moderate advantageous impact via escalated angiogenesis in the development of a less serious heart failure in diabetic models.39 According to the results of the present study and previous research,40 it seems that the expression of NOX4 in the cardiomyocyte decreases in the beginning stage of diabetes but increases in the latter stage of diabetes. For example, Ebrahimian et al40 showed mice that were diabetic for two months had an increasing levels of NOX4, whereas our study showed rats that were diabetic for one month had a decreased levels of NOX4. The current study’s findings are consistent with those of a previous study demonstrating thatrats with diabetes for 4 weeks had increased levels of NOX4 mRNA in the heart but the protein expression of NOX4 did not show any increase.41 Lisa et al in 2011 indicated that subcutaneous injection of IGF1 in diabetic rats with myocardial infarction increased angiogenesis in cardiac tissue.42 The findings of our research also exhibited that IGF-1 injection rose angiogenesis in heart of diabetic rats. Humpert et al in 2008 displayed that insulin increases angiogenesis in endothelial cell through IGF-1 receptors.43 IGF-1 is also thought to promote angiogenesis by engaging with regionally generated factors like VEGF.44 In this study, histopathological assessment confirmed that IGF-1 treated group has a noticeable angiogenic reaction in the heart in compared to untreated rats. Qiao et al showed that IGF-1 increase angiogenesis by activating the AKT/ERK pathway45 and Bakr et al displayed that IGF-1 increased angiogenesis by activating the PI3K/HIF-1a/VEGF-A pathway.46 In completing studies to elucidate the mechanisms used by IGF to induce angiogenesis, we have shown for the first time that IGF-1 exhibits its pro-angiogenesis effects by increasing NOX4, SIRT-1. The current study also displayed those levels of NOX4, SIRT1 and in comparison, to inactive rats, capillary density elevated in the exercised groups. In line with our work, it is reported that treadmill exercise increases angiogenesis in the heart of diabetic subjects. Furthermore, it is showed that exercise increases the expression of SIRT1 in the aorta of rats.47 Exercise has also been shown to induce angiogenesis in the retina of rats by increasing NOX4 expression.48
Furthermore, IGF-1 and exercise had a synergistic effect on HbA1c, cardiac SIRT-1 expression, and on neovascularization. But the combination treatment did not show any synergistic effect on NOX4 protein levels and it seems that the increased levels of NOX4 in the combination therapy group are due to IGF-1.
IGF-1 and exercise increase Angiogenesis in the heart tissue
Platelet endothelial cell adhesion molecule (PECAM-1) also known as cluster of differentiation 31 (CD31) is an extensively utilized marker of angiogenesis. In this study, the expression levels of CD31 were also evaluated by IHC (Figure 3A). The brown-stained tissues show CD-31 immunostained endothelial cells. Diabetes condition caused in a remarkably (P < 0.001) decreased angiogenesis compared to the control group. Each of exercise or IGF-1 had an increasing effect (P < 0.01) on angiogenesis and Dia + E and Dia + IGF-1 do not have any significant difference compared to control group. As, each of these interventions increased angiogenesis similar to that in the control group. Additionally, combination of IGF-1 and exercise in the Dia + E + IGF-1 group led into a noticeable (P < 0.01) rise in immunoreactivity for CD31 in comparison with Dia group. Statistical analysis of the immunohistochemically study showed that there was no notable rise in angiogenesis grade in the combination therapy group in comparison to each of Dia + E and Dia + IGF-1 groups. Actually, simultaneously treatment did not show any synergistic effects on angiogenesis (Figure 3B). Accumulating evidence showed that heart tissue in the diabetes condition displays functional changes that leading to myocardial dysfunction and cardiovascular diseases.28 Growing proof has shown that the progression of diabetes-related heart disorders was linked to a decrease in the development of new arteries.29 Therefore, a comprehensive description of the angiogenesis process and illumination of corresponding signaling pathways and involved molecules might be effective in the reducing heart complications in the diabetes condition. The outcomes of this study revealed that angiogenesis was reduced within the diabetic rat’s heart tissue. In addition, we showed that each of the exercise and IGF-1 interventions alone or in combination can increase the capillary density. In line with molecular results; histopathological comparison amongst the tissue of heart of the rats and the control group revealed a noticeable angiogenic reaction took place in the diabetic rats. We assume decreased angiogenesis in the diabetic rats mediated by decreased expression levels of NOX4. Probably with decrease levels of NOX4, activation of AKT and ERK proteins, which are the most important inducer angiogenesis, are also reduced. The angiogenic activity of endothelial cells is supervised extreme expression of SIRT-1 inside the vasculature throughout blood vessel development.49 Losing of SIRT1 function obstructs sprouting angiogenesis and bifurcation morphogenesis of endothelial cells with subsequent down-regulation of genes involved in blood vessel development and vascular renovation.50 Our results also showed that parallel with the decreased angiogenesis in heart tissue of diabetic rats, there was, a decrease in SIRT-1 expression levels in the heart of this group.50 In accordance to our study, Sulaiman et al51 in 2010 showed that SIRT1 decreased in the hearts of diabetic rats. Also, Huang et al showed that SIRT1 has a defensive impact on heart tissue.50 It has also been shown that SIRT1 increases angiogenesis responses in local ischemia of heart tissue in mice and shows a protective effect by suppressing NF-KB and increasing NO production in blood vessels.52 It has also been reported that sirt1 activates the MAPK/ERK and PI3K-Akt pathways, the two major signaling pathways involved in angiogenesis, and increases neovascularization.13
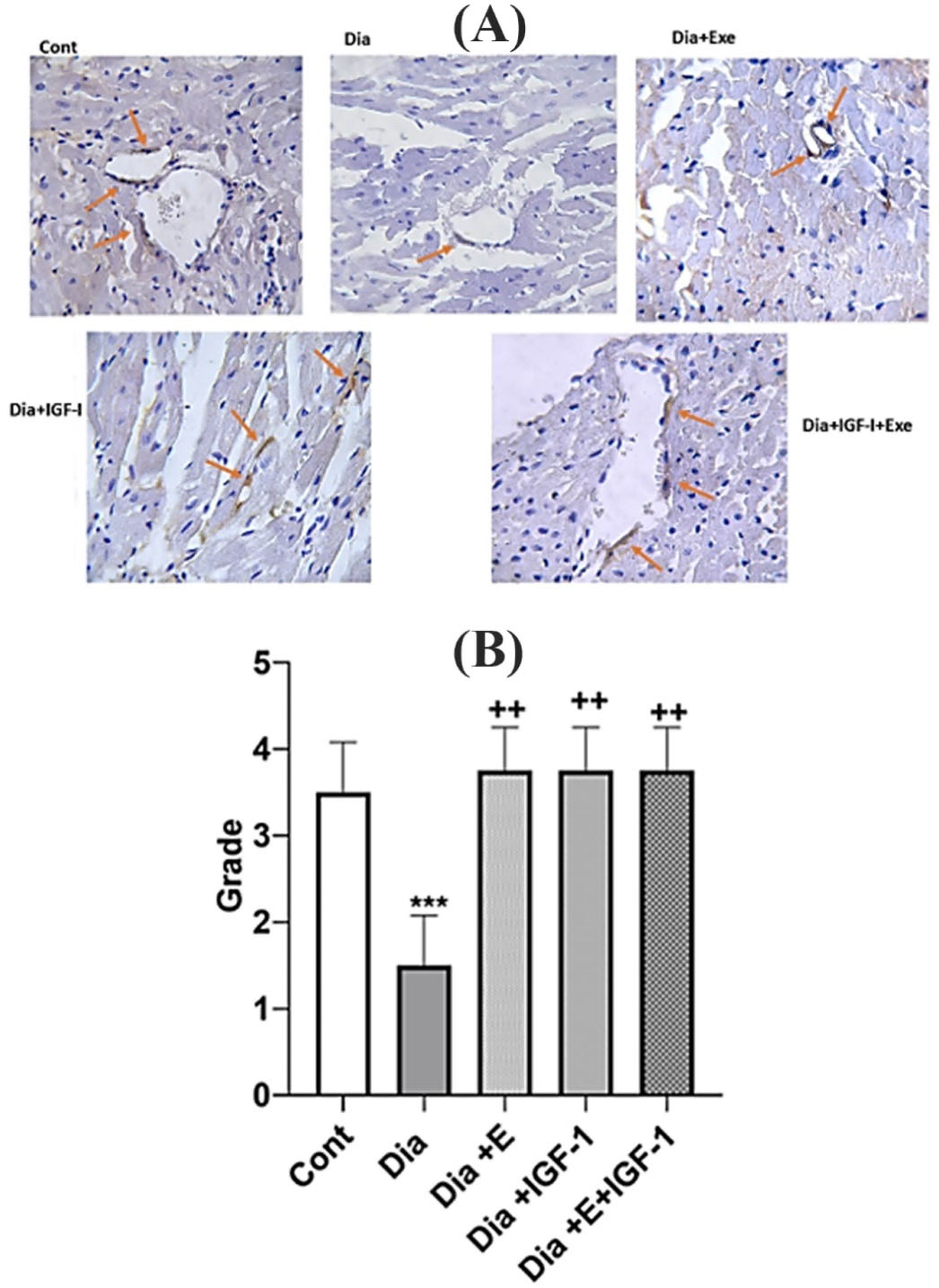
Figure 3.
(A) Immunohistochemical detection of CD31 in heart. CD-31 immunostained endothelial cells were representative brown stained. A: Control; B: Dia; C: Dia + Exe, C:Dia + IGF-1, E: Dia + IGF-1 + Exe. Red arrows indicate the number and intensity of immunostaining for CD31. (B). Effect of exercise and IGF-1 on the angiogenesis in heart. Values are expressed as mean ± SD. *** P < 0.001 vs control group. + +P < 0. 01 vs Dia group.
.
(A) Immunohistochemical detection of CD31 in heart. CD-31 immunostained endothelial cells were representative brown stained. A: Control; B: Dia; C: Dia + Exe, C:Dia + IGF-1, E: Dia + IGF-1 + Exe. Red arrows indicate the number and intensity of immunostaining for CD31. (B). Effect of exercise and IGF-1 on the angiogenesis in heart. Values are expressed as mean ± SD. *** P < 0.001 vs control group. + +P < 0. 01 vs Dia group.
Conclusion
As a result, we showed that angiogenesis, NOX4 and SIRT1 protein levels decrease in the cardiac tissue in the early stage of diabetes. Also, IGF-1 and exercise could raise neovascularization in the diabetic rats maybe by increasing NOX4 and SIRT1expression.Additionally, the combination of IGF-1 and exercise have an improving synergistic impact on HgA1C and SIRT1 levels in the heart tissue of the diabetic rats in the early stage compared with each one alone. As a corollary to these findings, IGF-1 supplementation and exercise training may be considered as new therapeutic strategies for cardiomyopathy in diabetes condition. However, more studies are needed to elucidate the effective role of NOX4 in diabetic cardiomyopathy.
Acknowledgments
Financial support for this investigation by the Faculty of Medicine, Urmia University of Medical Sciences through grant is gratefully acknowledged.
Author Contributions
Conceptualization: Leila Chodari, Shiva Roshan Milani.
Data curation: Saman Daneshfar, Bagher Pourheydar.
Formal Analysis: Leila Chodari, Bagher Pourheydar.
Funding acquisition: Leila Chodari.
Investigation: Shiva Roshan Milani.
Methodology: Leila Chodari,Bagher Pourheydar.
Project administration: Leila Chodari.
Resources: Saman Daneshfar.
Software: Leila Chodari.
Supervision: Leila Chodari.
Validation: Leila Chodari.
Visualization: Bagher Pourheydar.
Writing – original draft: Leila Chodari.
Writing – review & editing: Leila Chodari, Shiva Roshan Milani.
Ethical Issues
The animal care and experimental procedures were carried out according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 2011) and the ethical principles under which the journal operates, and our work complied with this animal ethics checklist. The study was approved by the Animal Ethics Committee of the Urmia University of Medical Sciences, (ethical code: IR.UMSU.REC.1397.150). We have taken all steps to minimize the pain and suffering of the animals
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87(1):4-14. doi: 10.1016/j.diabres.2009.10.007 [Crossref] [ Google Scholar]
- Borghetti G, von Lewinski D, Eaton DM, Sourij H, Houser SR, Wallner M. Diabetic cardiomyopathy: current and future therapies. Beyond glycemic control. Front Physiol 2018; 9:1514. doi: 10.3389/fphys.2018.01514 [Crossref] [ Google Scholar]
- Chodari L, Mohammadi M, Ghorbanzadeh V, Dariushnejad H, Mohaddes G. Testosterone and voluntary exercise promote angiogenesis in hearts of rats with diabetes by enhancing expression of VEGF-A and SDF-1a. Can J Diabetes 2016; 40(5):436-41. doi: 10.1016/j.jcjd.2016.03.004 [Crossref] [ Google Scholar]
- Almeida AJ. Envelhecimento: AspectosMoleculares e SuasImplicaçõesSobre o Sistema Cardiovascular. Brasil: Universidade Federal da Paraíba; 2017.
- Sundaresan NR, Pillai VB, Wolfgeher D, Samant S, Vasudevan P, Parekh V. The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Sci Signal 2011; 4(182):ra46. doi: 10.1126/scisignal.2001465 [Crossref] [ Google Scholar]
- Fukai T, Ushio-Fukai M. Cross-talk between NADPH oxidase and mitochondria: role in ROS signaling and angiogenesis. Cells 2020; 9(8):1849. doi: 10.3390/cells9081849 [Crossref] [ Google Scholar]
- Kim YM, Kim SJ, Tatsunami R, Yamamura H, Fukai T, Ushio-Fukai M. ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis. Am J Physiol Cell Physiol 2017; 312(6):C749-C64. doi: 10.1152/ajpcell.00346.2016 [Crossref] [ Google Scholar]
- Harel S, Mayaki D, Sanchez V, Hussain SNA. NOX2, NOX4, and mitochondrial-derived reactive oxygen species contribute to angiopoietin-1 signaling and angiogenic responses in endothelial cells. VasculPharmacol 2017; 92:22-32. doi: 10.1016/j.vph.2017.03.002 [Crossref] [ Google Scholar]
- Liu B, Ren KD, Peng JJ, Li T, Luo XJ, Fan C. Suppression of NADPH oxidase attenuates hypoxia-induced dysfunctions of endothelial progenitor cells. BiochemBiophys Res Commun 2017; 482(4):1080-7. doi: 10.1016/j.bbrc.2016.11.161 [Crossref] [ Google Scholar]
- Braunersreuther V, Montecucco F, Asrih M, Pelli G, Galan K, Frias M. Role of NADPH oxidase isoforms NOX1, NOX2 and NOX4 in myocardial ischemia/reperfusion injury. J Mol Cell Cardiol 2013; 64:99-107. doi: 10.1016/j.yjmcc.2013.09.007 [Crossref] [ Google Scholar]
- Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A 2009; 106(34):14385-90. doi: 10.1073/pnas.0906805106 [Crossref] [ Google Scholar]
- Pardo PS, Boriek AM. SIRT1 regulation in ageing and obesity. Mech Ageing Dev 2020; 188:111249. doi: 10.1016/j.mad.2020.111249 [Crossref] [ Google Scholar]
- D’Onofrio N, Servillo L, Balestrieri ML. SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antioxid Redox Signal 2018; 28(8):711-32. doi: 10.1089/ars.2017.7178 [Crossref] [ Google Scholar]
- Budbazar E, Rodriguez F, Sanchez JM, Seta F. The role of Sirtuin-1 in the vasculature: focus on aortic aneurysm. Front Physiol 2020; 11:1047. doi: 10.3389/fphys.2020.01047 [Crossref] [ Google Scholar]
- Guarani V, Deflorian G, Franco CA, Krüger M, Phng LK, Bentley K. Acetylation-dependent regulation of endothelial Notch signalling by the SIRT1 deacetylase. Nature 2011; 473(7346):234-8. doi: 10.1038/nature09917 [Crossref] [ Google Scholar]
- Karbasforooshan H, Karimi G. The role of SIRT1 in diabetic cardiomyopathy. Biomed Pharmacother 2017; 90:386-92. doi: 10.1016/j.biopha.2017.03.056 [Crossref] [ Google Scholar]
- Carroll PV, Christ ER, Umpleby AM, Gowrie I, Jackson N, Bowes SB. IGF-1 treatment in adults with type 1 diabetes: effects on glucose and protein metabolism in the fasting state and during a hyperinsulinemic-euglycemic amino acid clamp. Diabetes 2000; 49(5):789-96. doi: 10.2337/diabetes.49.5.789 [Crossref] [ Google Scholar]
- Dunger DB, Cheetham TD, Crowne EC. Insulin-like growth factors (IGFs) and IGF-1 treatment in the adolescent with insulin-dependent diabetes mellitus. Metabolism 1995; 44(10 Suppl 4):119-23. doi: 10.1016/0026-0495(95)90232-5 [Crossref] [ Google Scholar]
- Shih KC, Ho LT. Effects of growth hormone on diurnal insulin sensitivity in normal and type 2 diabetic patients and animal models. J Diabetes Metab 2016; 7(10):709. doi: 10.4172/2155-6156.1000709 [Crossref] [ Google Scholar]
- Gielen S, Laughlin MH, O’Conner C, Duncker DJ. Exercise training in patients with heart disease: review of beneficial effects and clinical recommendations. Prog Cardiovasc Dis 2015; 57(4):347-55. doi: 10.1016/j.pcad.2014.10.001 [Crossref] [ Google Scholar]
- Wu N, Bredin SSD, Guan Y, Dickinson K, Kim DD, Chua Z. Cardiovascular health benefits of exercise training in persons living with type 1 diabetes: a systematic review and meta-analysis. J Clin Med 2019; 8(2):253. doi: 10.3390/jcm8020253 [Crossref] [ Google Scholar]
- Żebrowska A, Hall B, Kochańska-Dziurowicz A, Janikowska G. The effect of high intensity physical exercise and hypoxia on glycemia, angiogenic biomarkers and cardiorespiratory function in patients with type 1 diabetes. Adv Clin Exp Med 2018; 27(2):207-16. doi: 10.17219/acem/66354 [Crossref] [ Google Scholar]
- Saboory E, Gholizadeh-Ghaleh Aziz S, Samadi M, Biabanghard A, Chodari L. Exercise and insulin-like growth factor 1 supplementation improve angiogenesis and angiogenic cytokines in a rat model of diabetes-induced neuropathy. Exp Physiol 2020; 105(5):783-92. doi: 10.1113/ep088069 [Crossref] [ Google Scholar]
- Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights 2016; 11:95-104. doi: 10.4137/bmi.s38440 [Crossref] [ Google Scholar]
- Ghorbanzadeh V, Pourheydar B, Dariushnejad H, Ghalibafsabbaghi A, Chodari L. Curcumin improves angiogenesis in the heart of aged rats: involvement of TSP1/NF-κB/VEGF-A signaling. Microvasc Res 2022; 139:104258. doi: 10.1016/j.mvr.2021.104258 [Crossref] [ Google Scholar]
- Kota SK, Meher LK, Jammula S, Kota SK, Krishna SV, Modi KD. Aberrant angiogenesis: the gateway to diabetic complications. Indian J Endocrinol Metab 2012; 16(6):918-30. doi: 10.4103/2230-8210.102992 [Crossref] [ Google Scholar]
- Nieves-Cintrón M, Flores-Tamez VA, Le T, Baudel MM, Navedo MF. Cellular and molecular effects of hyperglycemia on ion channels in vascular smooth muscle. Cell Mol Life Sci 2021; 78(1):31-61. doi: 10.1007/s00018-020-03582-z [Crossref] [ Google Scholar]
- Teppala S, Shankar A. Association between serum IGF-1 and diabetes among US adults. Diabetes Care 2010; 33(10):2257-9. doi: 10.2337/dc10-0770 [Crossref] [ Google Scholar]
- Gaderpour S, Ghiasi R, Hamidian G, Heydari H, Keyhanmanesh R. Voluntary exercise improves spermatogenesis and testicular apoptosis in type 2 diabetic rats through alteration in oxidative stress and mir-34a/SIRT1/p53 pathway. Iran J Basic Med Sci 2021; 24(1):58-65. doi: 10.22038/ijbms.2020.49498 [Crossref] [ Google Scholar]
- Li X, Wu G, Han F, Wang K, Bai X, Jia Y. SIRT1 activation promotes angiogenesis in diabetic wounds by protecting endothelial cells against oxidative stress. Arch BiochemBiophys 2019; 661:117-24. doi: 10.1016/j.abb.2018.11.016 [Crossref] [ Google Scholar]
- Cheng D, Chen L, Tu W, Wang H, Wang Q, Meng L. Protective effects of valsartan administration on doxorubicin-induced myocardial injury in rats and the role of oxidative stress and NOX2/NOX4 signaling. Mol Med Rep 2020; 22(5):4151-62. doi: 10.3892/mmr.2020.11521 [Crossref] [ Google Scholar]
- Lener B, Kozieł R, Pircher H, Hütter E, Greussing R, Herndler-Brandstetter D. The NADPH oxidase Nox4 restricts the replicative lifespan of human endothelial cells. Biochem J 2009; 423(3):363-74. doi: 10.1042/bj20090666 [Crossref] [ Google Scholar]
- Chen C, Li L, Zhou HJ, Min W. The role of NOX4 and TRX2 in angiogenesis and their potential cross-talk. Antioxidants (Basel) 2017; 6(2):42. doi: 10.3390/antiox6020042 [Crossref] [ Google Scholar]
- Manea A. NADPH oxidase-derived reactive oxygen species: involvement in vascular physiology and pathology. Cell Tissue Res 2010; 342(3):325-39. doi: 10.1007/s00441-010-1060-y [Crossref] [ Google Scholar]
- Maeda Y, Inoguchi T, Takei R, Hendarto H, Ide M, Inoue T. Chymase inhibition prevents myocardial fibrosis through the attenuation of NOX4-associated oxidative stress in diabetic hamsters. J Diabetes Investig 2012; 3(4):354-61. doi: 10.1111/j.2040-1124.2012.00202.x [Crossref] [ Google Scholar]
- Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 2002; 105(14):1656-62. doi: 10.1161/01.cir.0000012748.58444.08 [Crossref] [ Google Scholar]
- Zhang M, Brewer AC, Schröder K, Santos CX, Grieve DJ, Wang M. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A 2010; 107(42):18121-6. doi: 10.1073/pnas.1009700107 [Crossref] [ Google Scholar]
- Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A 2010; 107(35):15565-70. doi: 10.1073/pnas.1002178107 [Crossref] [ Google Scholar]
- Hansen SS, Aasum E, Hafstad AD. The role of NADPH oxidases in diabetic cardiomyopathy. BiochimBiophys Acta Mol Basis Dis 2018; 1864(5 Pt B):1908-13. doi: 10.1016/j.bbadis.2017.07.025 [Crossref] [ Google Scholar]
- Ebrahimian TG, Heymes C, You D, Blanc-Brude O, Mees B, Waeckel L. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am J Pathol 2006; 169(2):719-28. doi: 10.2353/ajpath.2006.060042 [Crossref] [ Google Scholar]
- Novoa U, Arauna D, Moran M, Nuñez M, Zagmutt S, Saldivia S. High-intensity exercise reduces cardiac fibrosis and hypertrophy but does not restore the nitroso-redox imbalance in diabetic cardiomyopathy. Oxid Med Cell Longev 2017; 2017:7921363. doi: 10.1155/2017/7921363 [Crossref] [ Google Scholar]
- Lisa M, Haleagrahara N, Chakravarthi S. Insulin-like growth factor-1 (IGF-1) reduces ischemic changes and increases circulating angiogenic factors in experimentally - induced myocardial infarction in rats. Vasc Cell 2011; 3(1):13. doi: 10.1186/2045-824x-3-13 [Crossref] [ Google Scholar]
- Humpert PM, Djuric Z, Zeuge U, Oikonomou D, Seregin Y, Laine K. Insulin stimulates the clonogenic potential of angiogenic endothelial progenitor cells by IGF-1 receptor-dependent signaling. Mol Med 2008; 14(5-6):301-8. doi: 10.2119/2007-00052.Humpert [Crossref] [ Google Scholar]
- Hellström A, Carlsson B, Niklasson A, Segnestam K, Boguszewski M, de Lacerda L. IGF-1 is critical for normal vascularization of the human retina. J Clin Endocrinol Metab 2002; 87(7):3413-6. doi: 10.1210/jcem.87.7.8629 [Crossref] [ Google Scholar]
- Qiao M, Shapiro P, Kumar R, Passaniti A. Insulin-like growth factor-1 regulates endogenous RUNX2 activity in endothelial cells through a phosphatidylinositol 3-kinase/ERK-dependent and Akt-independent signaling pathway. J Biol Chem 2004; 279(41):42709-18. doi: 10.1074/jbc.M404480200 [Crossref] [ Google Scholar]
- Bakr AG, El-Bahrawy AH, Taha HH, Ali FEM. Diosmin enhances the anti-angiogenic activity of sildenafil and pentoxifylline against hepatopulmonary syndrome via regulation of TNF-α/VEGF, IGF-1/PI3K/AKT, and FGF-1/ANG-2 signaling pathways. Eur J Pharmacol 2020; 873:173008. doi: 10.1016/j.ejphar.2020.173008 [Crossref] [ Google Scholar]
- Machin DR, Auduong Y, Gogulamudi VR, Liu Y, Islam MT, Lesniewski LA. Lifelong SIRT-1 overexpression attenuates large artery stiffening with advancing age. Aging (Albany NY) 2020; 12(12):11314-24. doi: 10.18632/aging.103322 [Crossref] [ Google Scholar]
- Vogel J, Kruse C, Zhang M, Schröder K. Nox4 supports proper capillary growth in exercise and retina neo-vascularization. J Physiol 2015; 593(9):2145-54. doi: 10.1113/jphysiol.2014.284901 [Crossref] [ Google Scholar]
- Alqudah A, Eastwood KA, Jerotic D, Todd N, Hoch D, McNally R, et al. FKBPL and SIRT-1, key angiogenesis proteins, are downregulated by diabetes in pregnancy. medRxiv [Preprint]. October 8, 2020. Available from: https://www.medrxiv.org/content/10.1101/2020.10.06.20208116v1.
- Huang H, Xu Z, Qi Y, Zhang W, Zhang C, Jiang M. Exosomes from SIRT1-overexpressing ADSCs restore cardiac function by improving angiogenic function of EPCs. Mol Ther Nucleic Acids 2020; 21:737-50. doi: 10.1016/j.omtn.2020.07.007 [Crossref] [ Google Scholar]
- Sulaiman M, Matta MJ, Sunderesan NR, Gupta MP, Periasamy M, Gupta M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 2010; 298(3):H833-H843. doi: 10.1152/ajpheart.00418.2009 [Crossref] [ Google Scholar]
- Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res 2008; 80(2):191-9. doi: 10.1093/cvr/cvn224 [Crossref] [ Google Scholar]