Advanced pharmaceutical bulletin. 13(2):328-338.
doi: 10.34172/apb.2023.032
Research Article
Cefazolin-Loaded Double-Shelled Hollow Mesoporous Silica Nanoparticles/Polycaprolactone Nanofiber Composites: A Delivery Vehicle for Regenerative Purposes
Negar Karimi 1  , Mohsen Khorashadizadeh 2, Mohammad Yahya Hanafi-Bojd 3, Esmat Alemzadeh 4, *
, Mohsen Khorashadizadeh 2, Mohammad Yahya Hanafi-Bojd 3, Esmat Alemzadeh 4, * 
Author information:
1Department of Medical Biotechnology, School of Medicine, Birjand University of Medical Sciences, Birjand, Iran.
2Cellular and Molecular Research Center, Department of Medical Biotechnology, School of Medicine, Birjand University of Medical Sciences, Birjand, Iran.
3Cellular and Molecular Research Center, Department of Pharmaceutics and Pharmaceutical Nanotechnology, School of Pharmacy, Birjand University of Medical Sciences, Birjand, Iran.
4Infectious Diseases Research Center, Department of Medical Biotechnology, School of Medicine, Birjand University of Medical Sciences, Birjand, Iran.
Abstract
Purpose:
As important challenges in burn injuries, infections often lead to delayed and incomplete healing. Wound infections with antimicrobial-resistant bacteria are other challenges in the management of wounds. Hence, it can be critical to synthesize scaffolds that are highly potential for loading and delivering antibiotics over long periods.
Methods:
Double-shelled hollow mesoporous silica nanoparticles (DSH-MSNs) were synthesized and loaded with cefazolin. Cefazolin-loaded DSH-MSNs (Cef*DSH-MSNs) were incorporated into polycaprolactone (PCL) to prepare a nanofiber-mediated drug release system. Their biological properties were assessed through antibacterial activity, cell viability, and qRT-PCR. The morphology and physicochemical properties of the nanoparticles and nanofibers were also characterized.
Results:
The double-shelled hollow structure of DSH-MSNs demonstrated a high loading capacity of cefazolin (51%). According to in vitro findings, the Cef*DSH-MSNs embedded in polycaprolactone nanofibers (Cef*DSH-MSNs/PCL) provided a slow release for cefazolin. The release of cefazolin from Cef*DSH-MSNs/PCL nanofibers inhibited the growth of Staphylococcus aureus. The high viability rate of human adipose-derived stem cells (hADSCs) in contact with PCL and DSH-MSNs/PCL was indicative of the biocompatibility of nanofibers. Moreover, gene expression results confirmed changes in keratinocyte-related differentiation genes in hADSCs cultured on the DSH-MSNs/PCL nanofibers with the up-regulation of involucrin.
Conclusion:
The high drug-loading capacity of DSH-MSNs presents these nanoparticles as suitable vehicles for drug delivery. In addition, the use of Cef*DSH-MSNs/PCL can be an effective strategy for regenerative purposes.
Keywords: Adipose-derived stem cells, Double-shelled hollow mesoporous silica nanopartic, Electrospun nanofibers, Keratinocyte differentiation, Sustained drug release, Tissue engineering
Copyright and License Information
©2023 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Today, chronic wounds are considered an emerging epidemic and have created serious clinical challenges for patients. A significant problem with chronic wounds is microbial biofilm formation, which delays wound healing.1-3 Although the use of antibiotics has significantly reduced the risk of infections in patients, the widespread use of these drugs has increased the number of drug-resistant bacteria.4 Hence, it would be a top priority to discover novel methods for inhibiting bacteria.1
Nanotechnology can help develop therapeutic strategies by designing drug delivery systems.5-7 Advantages of drug delivery systems include improved hydrophobic drug solubility, increased drug half-life, prolonged systemic turnover, a slow release, reduced drug dosage, and targeted delivery of drug compounds. These characteristics overcome the limitations of traditional therapeutic approaches.8,9 Among various delivery vehicles, mesoporous silica nanoparticles (MSNs) have gained much popularity in recent decades. Features such as high biocompatibility, high surface area, optimized mesoporous structure, and the ability to target and control drug release have proposed MSNs as promising materials for drug delivery.10,11 Electrospinning is another promising technique that plays a vital role in drug delivery.12,13 High encapsulation capacity, tunable porosity, and cost-effectiveness are some of the advantages of electrospun nanofibers in drug delivery.1,14-17
As an attempt to assess the advantages of these two classes of nanomaterials, this study developed a double-shelled hollow MSNs-embedded nanofiber (DSH-MSNs/PCL) system to achieve a sustained antibiotic release. For this purpose, the study explored the cefazolin (Cef) release profile and antibacterial activity of nanofibers against Staphylococcus aureus. The potential of nanofiber mats for human adipose-derived stem cells (hADSCs) differentiation into keratinocyte cells was also evaluated after 21 days.
Materials and Methods
Materials
Cetyltrimethylammonium bromide (CTAB), polycaprolactone (PCL), tetraethyl orthosilicate (TEOS), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide (MTT), hydrochloric acid, 3-aminopropyltriethoxysilane (APTES), and acetic acid were provided from Sigma-Aldrich.5 Ammonia solution (32%), sodium carbonate (Na2CO3), ethanol (96%), and methanol (99.9%) were purchased from Merck (Germany). cDNA synthesis and RNA extraction kits were obtained from Parstous Co. (Mashhad, Iran). SYBR Green Real-Time-PCR Master Mix was provided from Ampliqon (Denmark). Fetal bovine serum (FBS), trypsin, penicillin, and streptomycin (P/S) were purchased from GIBCO (Grand Island, NY, USA). Dimethyl sulfoxide (DMSO) was supplied from Amresco (Solon, OH, USA). Lastly, the DMEM medium was purchased from Bio-Idea (Tehran, Iran).
Synthesis of DSH-MSNs
DSH-MSNs were synthesized via previously established methods.18 First, TEOS was hydrolyzed using the Stöber method to prepare monodisperse solid silica spheres. Briefly, a mixed solution containing 10 mL of water, 3.15 mL of ammonia solution (25%), and 74 mL of ethanol was prepared to which TEOS (6 mL) was added. After 1 h, the white suspension was centrifuged at 12 000 rpm for 2 minutes and thoroughly rinsed with ethanol three times. The precipitation was dried at 60°C for 6 h.
Later, 300 mg of the sample was dispersed in 60 mL of water and sonicated for 30 minutes. Subsequently, CTAB (450 mg), water (90 mL), ethanol (90 mL), and ammonia solution (1.65 mL) were added to the suspension and stirred for 30 minutes. 35 μL APTES and 750 μL TEOS were added and stirred for 6 hours. Then, the sample was centrifuged and washed with water and ethanol. The sediment was dried at 60°C for 6 hours. Afterward, the sample was dispersed in water (60 mL), and 1.2 g of anhydrous Na2CO3 was added to the solution. The mixture was stirred at 50°C for 10 hours and subsequently centrifuged and washed. The sedimentation was dispersed in ethanol again. Lastly, 0.1 mL of HCl was added to the sample and stirred at 60°C for 5 hours to eliminate CTAB. DSH-MSNs were obtained after centrifuging, washing, and drying.
Drug loading
For drug loading into DSH-MSNs, different amounts of cefazolin (6, 9, 12, and 15 mg) were suspended in 1 mL of DSH-MSNs (3 mg/mL) aqueous suspension and stirred for 18-24 hours at 25°C. The Cef-loaded DSH-MSNs (Cef*DSH-MSNs) were then centrifuged for 2 minutes at 12 000 rpm and rinsed with water to remove free drug molecules. The sediment was dried in a desiccator.19 The loading content of Cef was measured using the standard curve of the Cef solution. The following formulae were used to determine the drug loading content and loading efficiency19-21:
Drug loading content = (weight of the loaded drug in DSH-MSNs/weight of drug and DSH-MSNs) × 100% (Eq. 1)
Drug loading efficiency = (weight of the loaded drug in DSH-MSNs/weight of feeding drug) × 100% (Eq. 2)
The amount of Cef in the supernatant was measured by spectrophotometry at 270 nm.
Synthesis of nanofibers
PCL solution (20%, viscosity: 27.8 cP) was prepared by dissolving PCL in acetic acid (90%) and stirring for 18-24 hours at 25°C. Subsequently, nanoparticles (DSH-MSNs and Cef*DSH-MSNs) and Cef were added to the PCL solution separately. In the next step, the prepared solutions were poured into a syringe (5 mL) with a needle tip (18G), and the fibers were collected on a grounded collector covered with aluminum foil. The optimal electrospinning parameters included a voltage of 14 kV, a 15 cm needle to collector distance, a flow rate of 1 mL/h, and a rotational speed of 200 rpm.
Physicochemical characterization
The structure of Cef*DSH-MSNs, DSH-MSNs, Cef, Cef*DSH-MSNs/PCL, Cef*PCL, and PCL were evaluated with an FT-IR spectrometer (IR prestige-21, Shimadzu Co., Japan) in the spectral range of 400-4000 cm-1 using a potassium bromide disk (resolution of 4 cm-1). Next, 5-6 mg of samples were mixed, triturated with 100 mg potassium bromide, and placed in a sample holder for the potassium bromide disk. The surface morphologies of the DSH-MSNs and nanofibers were observed by SEM (FEI Quanta 450 Field Emission Scanning Electron Microscope, USA). The particle size of DSH-MSNs was characterized by a Transmission Electron Microscope Test Instrument (Carl Zeiss-EM10C-100 kV, Germany).
The particle size distribution of DSH-MSNs dispersed in water was determined by DLS (Brookhaven, USA). A Zeta sizer Nano apparatus (Brookhaven, USA) was applied to measure the surface charge of the DSH-MSNs and Cef.
The water contact angle (WCA) of nanofibers was measured by a WCA analyzer (Veho discovery VMS-004 Deluxe, England). Moreover, water droplets (1 μL) were pipetted onto the surface of PCL nanofibers before and after loading of DSH-MSNs.
Degradation study
Hydrolytic degradation of the nanofibers was conducted in PBS solution (pH = 7.4) for up to 4 weeks. The membranes were taken out from the PBS and characterized with SEM at week 4.
Mechanical properties and tensile strength of nanofibers
Electrospun nanofiber mats’ mechanical properties and tensile strength play an essential role in tissue engineering. The mechanical properties of PCL nanofibers were evaluated before and after loading DSH-MSNs using a mechanical tensile testing device (Santam, STM-1, Iran). All nanofibers were cut in dimensions of 15 mm × 40 mm. The length of the nanofibers in the machine was 25 mm, and the tensile speed was 10 mm/min.
The cefazolin release profile of nanofiber mats
As a measure to determine the cefazolin release profile, 20 mg (three replicates) of the electrospun nanofiber mats (Cef*DSH-MSNs/PCL and Cef*PCL) were weighted and sterilized with UV light for 20 minutes. Twenty mg of nanofibers were immersed in 3 mL PBS at pH 7.4 in a bain-marie at 37°C.22 The released medium was replaced with a fresh medium (3 mL) after the defined time intervals. Eventually, the concentrations of Cef were measured by UV/V spectroscopy at 270 nm.
Antibacterial assessment
Standard broth dilution method
As a model for antibiotic drug, S. aureus (ATCC® 16538TM) was used to evaluate the antibacterial effects of Cef*DSH-MSNs/PCL, Cef*PCL, PCL, and DSH-MSNs/PCL. For quantitative analysis, 20 mg of the nanofiber mats were incubated in 5 mL of Luria broth (LB) medium containing S. aureus (OD: 0.3) at 37°C for 18-24 hours. Subsequently, 100 μL solution of each tube was diluted and cultured on an agar medium. The number of colonies was counted after incubating plates at 37°C for 17 hours. The bacterial suspension constituted the control group. All experiments were conducted in triplicate.
Disc diffusion method
Disc diffusion test was performed to determine the growth-suppressing action of Cef*DSH-MSNs/PCL, Cef*PCL, PCL, and DSH-MSNs/PCL. In this method, 0.5 McFarland sterile normal saline suspension was prepared from freshly growing S. aureus (ATCC® 16538TM) culture. The punched electrospun nanofiber discs (6 mm) were placed on LB agar medium with the bacteria suspension (0.5 McFarland standard) and incubated at 37°C. A ruler was utilized to measure the inhibition zone in millimeters (mm). Three replicates were conducted under similar conditions for each sample.
Cell culture
Cell viability assay
The toxicity of PCL and DSH-MSNs/PCL was determined using MTT assay on hADSCs. Human adipose-derived stem cells were kindly gifted by Dr. Mohsen Khorashadizadeh from Birjand University of Medical Science.23 For the MTT assay, 3 × 104 cells/well were seeded in 24-well plates, treated with sterile nanofiber mats after 24 hours and incubated at 37°C with 5% CO2 for 72 hours. The medium was then substituted with 100 μL of MTT solution (1 mL fresh medium) and incubated at 37°C for 4 hours. For the formazan crystals formed by live cells to dissolve, the medium was removed entirely, and 500 μL of DMSO was added to each well plate. After 5 minutes, the absorbance was read on an ELISA plate reader at 570 nm.
The cell viability (%) was calculated as follow:
Viable cells (%) = abssample – absblank/abscontrol – absblank × 100 (Eq. 3).
Cell seeding and differentiation of hADSCs into keratinocytes
PCL and DSH-MSNs/PCL nanofibers were cut (circular disks in 15 mm diameter) and placed in a laminar flow hood to sterilize using UV radiation so as to prepare nanofiber mats for cell seeding. Afterward, the nanofiber mats were washed to remove any residual solvent. The nanofibers were immersed in DMEM overnight to facilitate cell attachment on the nanofiber surfaces. Next, 200 μL of cell suspension (cell density: 2 × 104 cells/mL) were poured onto the surface of scaffolds and placed in an incubator for 4 hours (5% CO2 at 37°C). After the cells were attached to the nanofiber mats, the additional medium was added, and incubation was resumed. The fresh medium was replaced every two days during the incubation period, and this process was continued for 21 days.
Quantitative analysis of gene expression
After 21 days, qRT-PCR was performed to assess the expressions of specific genes. Total RNA was extracted from cells using the Total RNA Extraction Kit (Parstous, Mashhad, Iran) as per the manufacturer’s protocol. Complementary DNA was prepared using the RevertAid First-Strand cDNA Synthesis Kit (Parstous, Mashhad, Iran) following the manufacturer’s instructions. For qRT-PCR, the amplification of the involucrin (IVL) and cytokeratin 18 (CK18) was performed using SYBR Green qRT-PCR Master Mix (A325402, Ampliqon, Denmark). Thermal cycling was carried out for 35 cycles of 30 seconds at 95°C, 1 minute at 60°C for IVL and 58°C for CK18, and 30 seconds at 72°C. Primer sequences were as follows: IVL, forward: 5′ CAGCACTCCACCAAAGCCTC 3′ and reverse: 3′ GCTCCTGATGGGTATTGACTG 5′; CK18, forward: 5′ TCGCAAATACTGTGGACAATGC 3′ and reverse: 3′ GCAGTCGTGTGATATTGGTGT; and GAPDH, forward: 5′ TGGACTCCACGACGTACTCAG 3′ and reverse: 3′ CGGGAAGCTTGTCATCAATGGAA 5′. The qRT-PCR data were analyzed by the 2-ΔΔct method and normalized to glyceraldehyde-3-phosphate dehydrogenase.
Statistical analysis
All data were summarized as mean ± SD, and statistical analysis was performed by t test (two-tailed) using GraphPad Prism (version 8.3.0) and REST (2009). A P value of less than 0.05 was considered to be statistically significant.
Results and Discussion
Characterization of DSH-MSNs
DSH-MSNs were successfully synthesized by the Stöber method (Figure 1). DSH-MSNs had a round shape, an average particle size of approximately 400 nm, and a shell thickness of ~10 nm, as shown in SEM and TEM images (Figure 2a-f). The TEM image depicted a double-shelled structure, highly uniform size, and the shell thickness of nanoparticles (Figure 2f). The nanoparticles’ zeta potential (pH = 7.4) showed that DSH-MSNs were charged negatively (-17.31). The negative zeta potential of nanoparticles also revealed that the CTAB was removed from the structure.
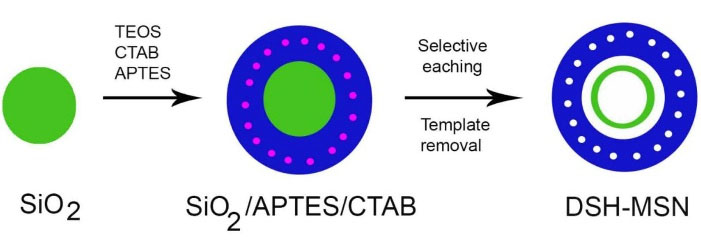
Figure 1.
Schematic illustration of the preparation of DSH-MSNs.
.
Schematic illustration of the preparation of DSH-MSNs.

Figure 2.
SEM (a, b, c) (HV: 30.00 KV, spot: 3.5, WD: 10.3 mm) and TEM (d, e, f) images of DSH-MSNs. Scale bars: (a) 5.0 μm; (b) 2.0 μm; (c) 1.0 μm; (d) 500 nm; (e) 200 nm; (f) 60 nm. FT-IR spectra of DSH-MSNs, Cef, and Cef*DSH-MSNs (g-i).
.
SEM (a, b, c) (HV: 30.00 KV, spot: 3.5, WD: 10.3 mm) and TEM (d, e, f) images of DSH-MSNs. Scale bars: (a) 5.0 μm; (b) 2.0 μm; (c) 1.0 μm; (d) 500 nm; (e) 200 nm; (f) 60 nm. FT-IR spectra of DSH-MSNs, Cef, and Cef*DSH-MSNs (g-i).
In this study, the adsorption method was used for loading drugs into the pores of MSNs. Accordingly, DSH-MSNs were immersed in the concentrated cefazolin solution. After 24 hours, the cefazolin molecules were absorbed on the pore walls. Drug-carrier interactions such as covalent bonding, electrostatic binding, hydrogen bonding, and van der Waals interactions facilitate the absorption of drug molecules in MSNs.24,25 Moreover, Cef loading into DSH-MSNs was achieved by hydrogen bonding interactions between the OH of DSH-MSNs (silica) and Cef. The FT-IR spectrum of DSH-MSNs (Figure 2g) showed sharp bands at 3415, 2927, 2856, 1635, 1097, 962, 800, and 472 cm-1.18 The FT-IR spectrum of Cef (Figure 2h) displayed sharp bands at 3415, 3284, 3143, 3059, 2960, 2862, 1761, 1674, 1600, 1550, 1489, 1282, 1240,1184, 1101, and 1064 cm-1.26 The sharp peaks at 3415, 3284, and 1600 cm-1 were related to the vibration of N-H stretching and the bending vibration of Cef.
The FT-IR spectrum of the Cef*DSH-MSNs (Figure 2i) indicated sharp bands at 3419, 3304, 3005, 2926, 2858, 1761, 1649, 1625, 1544, 1490, 1103, and 958 cm-1 and broadband at 3600-2500 cm-1. The peaks at 3419, 3304, and 1597 cm-1 were related to N-H stretching and the bending vibration of Cef and DSH-MSNs’ second amine at the Cef*DSH-MSNs, respectively. Besides, the peaks at 1103 and 958 cm-1 were connected with the C-O stretching vibration of the Cef and DSH-MSNs, respectively. The FT-IR spectrum of the prepared Cef*DSH-MSNs indicated that the peak at 3005 cm-1 was characteristic of C-H stretching vibration, and the peaks at 1625, 1544, and 1490 cm-1 were related to C = C vinyl and aromatic stretching vibration of Cef. The peaks at 1761 and 1647 cm-1 were also correlated with C = O from Cef. The changes in the peak positions were observed from 3415 to 3419, 1674 to 1649, and 1600 to 1544 cm-1. These results confirm the presence of Cef and DSH-MSNs in the spectrum of Cef*DSH-MSNs. In addition, the results confirm the intermolecular hydrogen-bonding reaction between the OH of DSH-MSNs and Cef.
In this study, the Cef*DSH-MSNs were mixed with the PCL solution to synthesize the nanofibers. It should be noted that the acid pH of the solution has no negative impact on the stability of silica nanoparticles.27 In this regard, Hadipour Moghaddam et al demonstrated that the degradation rate of MSNs increased in alkaline conditions.28 Similarly, Ahmed and Day evaluated the stability of cefazolin in different pH levels, indicating a higher degradation of cefazolin in the alkaline pH.29 The FT-IR spectrum of PCL nanofibers was employed to investigate PCL, Cef*PCL, and Cef*DSH-MSN/PCL structures and the intermolecular hydrogen-bonding reaction between PCL and Cef. The FT-IR spectrum of Cef*DSH-MSNs/PCL (Figure 3o) indicated sharp bands at 3342, 3040, 2927, 2860, 1728, 1670, 1587, 1456, 1292, 1238, 1182, 1045, 960, and 731 cm-1. The broad peak at 2500-3500 cm-1 region also corresponded to OH stretching vibration group of carboxylic acid from Cef. The sharp peaks at 3342 and 1587 cm-1 were characteristic of N-H stretching vibration and the bending vibration of Cef at the Cef*DSH-MSNs/PCL.
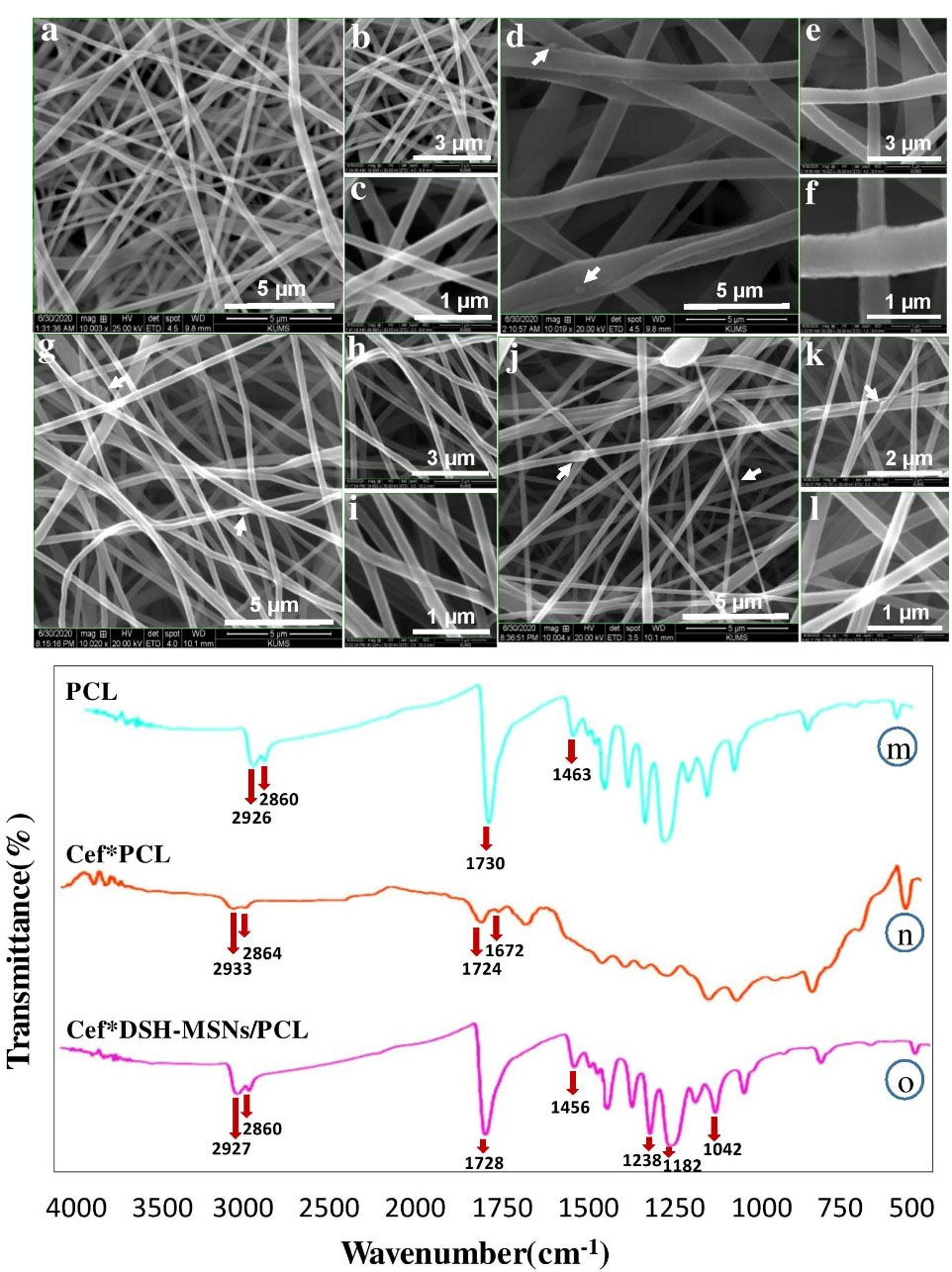
Figure 3.
SEM images of PCL nanofibers. PCL (a, b, c), Cef*PCL (d, e, f), DSH-MSNs/PCL (g, h, i), and Cef*DSH-MSNs/PCL (j, k, l). Scale bars: (a, d, g, j) 5.0 μm; (b, e, h) 3.0 μm; (k) 2.0 μm; (c, f, i, l) 1.0 μm. FT-IR spectra of Cef*DSH-MSNs/PCL, Cef*PCL, and PCL (m-o).
.
SEM images of PCL nanofibers. PCL (a, b, c), Cef*PCL (d, e, f), DSH-MSNs/PCL (g, h, i), and Cef*DSH-MSNs/PCL (j, k, l). Scale bars: (a, d, g, j) 5.0 μm; (b, e, h) 3.0 μm; (k) 2.0 μm; (c, f, i, l) 1.0 μm. FT-IR spectra of Cef*DSH-MSNs/PCL, Cef*PCL, and PCL (m-o).
The presence of the peak at 1728 cm-1 was correlated with C = O from Cef and PCL, and the peaks at 3342 and 1587 cm-1 were a feature of N-H stretching vibration and bending vibration of Cef, which can confirm the synthesis of Cef*DSH-MSNs/PCL nanofiber mats. Also, the FT-IR spectrum of Cef*DSH-MSNs/PCL showed prominent shifting of the peaks compared with those of PCL and Cef. The peaks at 1105, 880, 451 cm-1 were linked with Si–O–Si and Si–O stretching vibrations of DSH-MSNs, confirming the synthesis of Cef*DSH-MSNs. The peak positions shifted from 3400 to 3342, 1730 and 1724 to 1728, and 1591 to 1587 cm-1. These findings confirm the presence of PCL and Cef in the spectrum of Cef*DSH-MSNs/PCL and the synthesis of Cef*DSH-MSNs/PCL nanofiber mats.
WCA was calculated for PCL and DSH-MSNs/PCL nanofiber mats (Figure 4a-c). The hydrophobicity of pure PCL and DSH-MSNs/PCL nanofiber mats were 105 and 133 degrees, respectively. Figure 4c showed that the hydrophobicity of PCL nanofiber mats significantly increased after the addition of DSH-MSNs. Previous research also indicates that MSNs-embedded electrospun nanofibers can increase the hydrophobicity of nanofibers.30
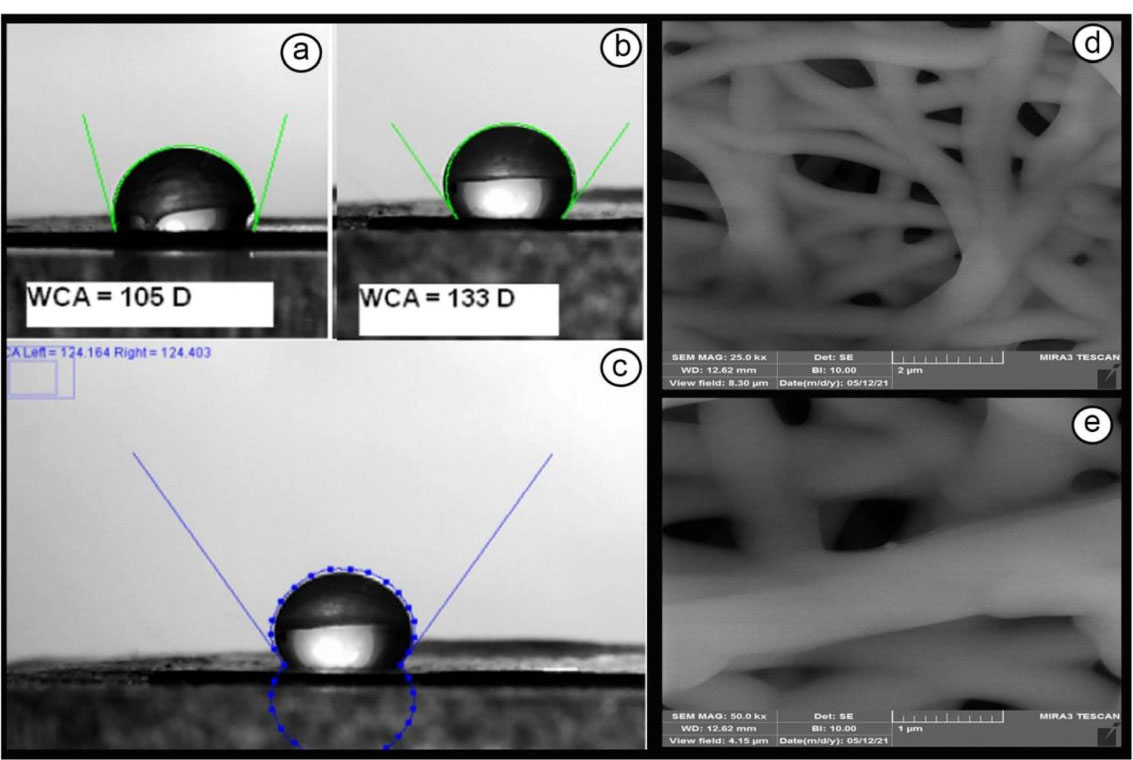
Figure 4.
Water contact angle (a) Water drop on PCL nanofiber mats with 105 degrees (b) Water drop on DSH-MSNs/PCL nanofiber mats with 133 degrees (c) A water drop on the DSH-MSNs/PCL nanofiber mat surface showing contact angles of reflection below. (Note: water contact angle larger than 90 degrees, is considered hydrophobic). (d, e) FE-SEM images of in vitro degradation of electrospun PCL in PBS (pH = 7.4). Scale bars: (d) 2 μm; (e) 1 μm.
.
Water contact angle (a) Water drop on PCL nanofiber mats with 105 degrees (b) Water drop on DSH-MSNs/PCL nanofiber mats with 133 degrees (c) A water drop on the DSH-MSNs/PCL nanofiber mat surface showing contact angles of reflection below. (Note: water contact angle larger than 90 degrees, is considered hydrophobic). (d, e) FE-SEM images of in vitro degradation of electrospun PCL in PBS (pH = 7.4). Scale bars: (d) 2 μm; (e) 1 μm.
The degradability of PCL was evaluated after four weeks using FE-SEM (Figure 4d and 4e). After in vitro incubation in PBS, the diameter of PCL nanofibers increased, indicating that the swelling of nanofibers occurred during incubation.
Mechanical properties and tensile strength of nanofibers
Electrospun nanofiber mats’ tensile strength and mechanical properties play an essential role in tissue engineering. The mechanical properties of PCL nanofibers were evaluated before and after loading DSH-MSNs using a mechanical tensile testing device (Santam, STM-1, Iran). All nanofibers were cut in dimensions of 15 mm × 40 mm. The length of the nanofibers in the machine was 25 mm, and the tensile speed was 10 mm/min.
Figure 5a and Table 1 present the typical stress-strain curves of the PCL and DSH-MSNs/PCL nanofiber mats. DSH-MSNs/PCL nanofibers have significantly higher mechanical properties than PCL ones. Indeed, when DSH-MSNs were added to PCL nanofibers, the nanofibers’ thickness and size uniformity increased, leading to higher adhesion forces (cohesion), which is ultimately associated with enhanced mechanical properties. Furthermore, the high affinity between DSH-MSNs and PCL matrix resulted in better dispersion of DSH-MSNs in nanofibers, which may explain such reinforcement stress transitions.31
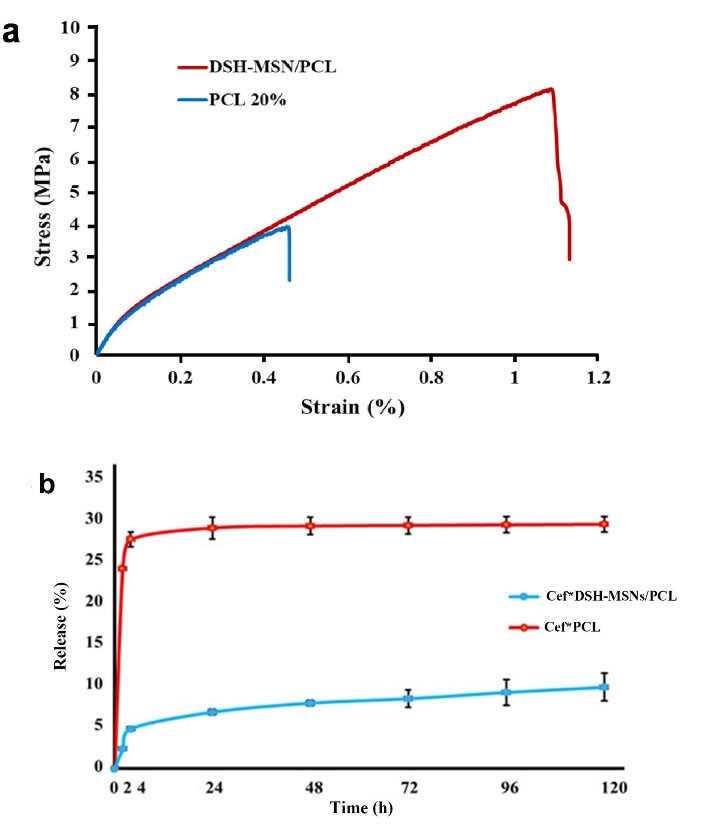
Figure 5.
(a) Stress-strain curves of the PCL and DSH-MSNs/PCL nanofiber mats. (b) Release kinetics of cefazolin from Cef*DSH-MSNs/PCL and Cef*PCL nanofiber mats in phosphate buffer solution (PBS, pH = 7.4) (mean ± SD, n = 3).
.
(a) Stress-strain curves of the PCL and DSH-MSNs/PCL nanofiber mats. (b) Release kinetics of cefazolin from Cef*DSH-MSNs/PCL and Cef*PCL nanofiber mats in phosphate buffer solution (PBS, pH = 7.4) (mean ± SD, n = 3).
Table 1.
Tensile strength, young’s modulus, and elongation at break of the PCL and DSH-MSNs/PCL nanofiber mats
|
|
Young’s modulus (MPa)
|
Ultimate strength (MPa)
|
Elongation at break
|
| PCL |
19.35 ± 2.74 |
3.89 ± 0.28 |
1.09 ± 0.12 |
| DSH-MSNs/PCL |
20.26 ± 1.69 |
8.02 ± 0.41 |
0.45 ± 0.7 |
Gounani et al and Ganesh et al have demonstrated the increase in young’s modulus of PCL mats after silica nanoparticles are added.32,33 Similar to the case with PCL nanofibers, the MSNs embedded into PLGA nanofibers revealed an increase in young’s modulus and tensile strength.34 The similarity of the mechanical properties of scaffolds to those of the host tissue is one of the crucial goals of tissue engineering. Based on the values reported for tensile properties of different body tissues, the PCL and DSH-MSNs/PCL mats can serve as scaffolds in skin tissue engineering.35
The drug loading properties of Cef *DSH-MSNs/PCL and Cef *PCL
As Table 2 indicates, the loading content and loading efficiency of DSH-MSNs were 10.27–51.79% and 5.72–26.85%, respectively. DSH-MSNs and Cef were used in the ratio of 3:12 to synthesize nanofiber mats. Some attractive properties such as large surface area and high pore volume introduce MSNs as excellent materials for loading drugs. Furthermore, a perfect mesoporous structure and the tunable pore size of MSNs help better control drug loading and release kinetics.36 Although MSNs have been broadly evaluated as drug delivery vehicles, the low drug-loading capacity remains the biggest challenge for these materials.37 As a strategy to solve this problem, hollow MSNs with a large cavity inside each original MSN have been developed to enhance the loading capacity of drugs.37 The high loading capacity of DSH-MSNs (51%) in this study indicates the practical application of DSH-MSNs as a drug delivery vehicle.
Table 2.
Cef-loading content and loading efficiency of different concentrations
|
DSH-MSNs
|
Cef
|
Drug loading content (w/w %)
|
Drug loading efficiency (%)
|
| 3 mg |
6 mg |
10.27 |
5.72 |
| 3 mg |
9 mg |
27.41 |
12.58 |
| 3 mg |
12 mg |
51.79 |
26.85 |
| 3 mg |
15 mg |
31.99 |
9.41 |
The drug release properties of Cef *DSH-MSNs/PCL and Cef *PCL nanofiber mats
For the Cef release profile, the drug-loaded nanofibers were soaked in PBS (pH = 7.4). Figure 5b illustrates the findings of the drug release profiles of Cef*DSH-MSNs/PCL and Cef*PCL nanofiber mats. The drug release curve of the samples showed that the release of Cef encapsulated in DSH-MSNs was slower than the Cef incorporated in PCL nanofibers. The drug release rate increased slowly after a few hours in the Cef*DSH-MSNs/PCL nanofiber mats, while the drug in the Cef*PCL nanofibers had a burst release. Multi-shelled hollow MSNs with high penetrating mesostructured shells sustain the drug release to a certain degree.38 However, an initial burst release is typically observed in these drug delivery systems.18 The incorporation of Cef*DSH-MSNs into PCL nanofibers revealed a more controlled release behavior. Drug release from pore DSH-MSNs and subsequently from nanofibers provide compelling evidence for the significantly slow release of Cef from Cef*DSH-MSNs/PCL nanofibers.39 This mechanism can prevent the initial burst release of the drug and increase the local concentration of the drug over time.40 Similarly, antibacterial tests confirmed a slow and controlled release from Cef*DSH-MSNs/PCL nanofiber mats. These preliminary results demonstrate the feasibility of using DSH-MSNs/PCL nanofibers for wound healing applications.
Antibacterial activity of Cef *DSH-MSNs/PCL and Cef *PCL nanofiber mats in broth microdilution under standard in vitro conditions
The antibacterial effects of PCL, DSH-MSNs/PCL, Cef*DSH-MSN/PCL, and Cef*PCL groups were determined via the standard broth dilution method. As Figure 6 depicts, Cef*DSH-MSN/PCL and Cef*PCL significantly decreased bacterial growth after 24 hours. Cef*PCL showed more lethality than Cef*DSH-MSNs/PCL, consistent with experimental data from the drug release profile. However, both groups demonstrated a significant difference from the control group (P < 0.05) (Figure 6a and 6b). The potential antibacterial activities of PCL, DSH-MSNs/PCL, Cef*DSH-MSNs/PCL, and Cef*PCL nanofiber mats were also evaluated against S. aureus (ATCC® 16538TM) through the disc diffusion method. As shown in Figure 6c, Cef*DSH-MSNs/PCL and Cef*PCL nanofiber mats inhibited the growth of S. aureus with inhibition zones of 9 mm for Cef*DSH-MSNs/PCL and 38 mm for Cef*PCL. For PCL and DSH-MSNs/PCL, there was no significant difference in inhibition zones. Infection, a significant problem in burn wounds, can effectively delay healing.41 Moreover, antibacterial resistance is one of the critical challenges for treating burn wounds. Although antibiotics significantly inhibit the growth of bacteria, high-dose antibiotic therapy may lead to toxic side effects in clinical practice.42 On the other hand, bacterial adhesion to the bandage surface and, eventually, biofilm formation are challenges that limit the applicability of some bandages.43
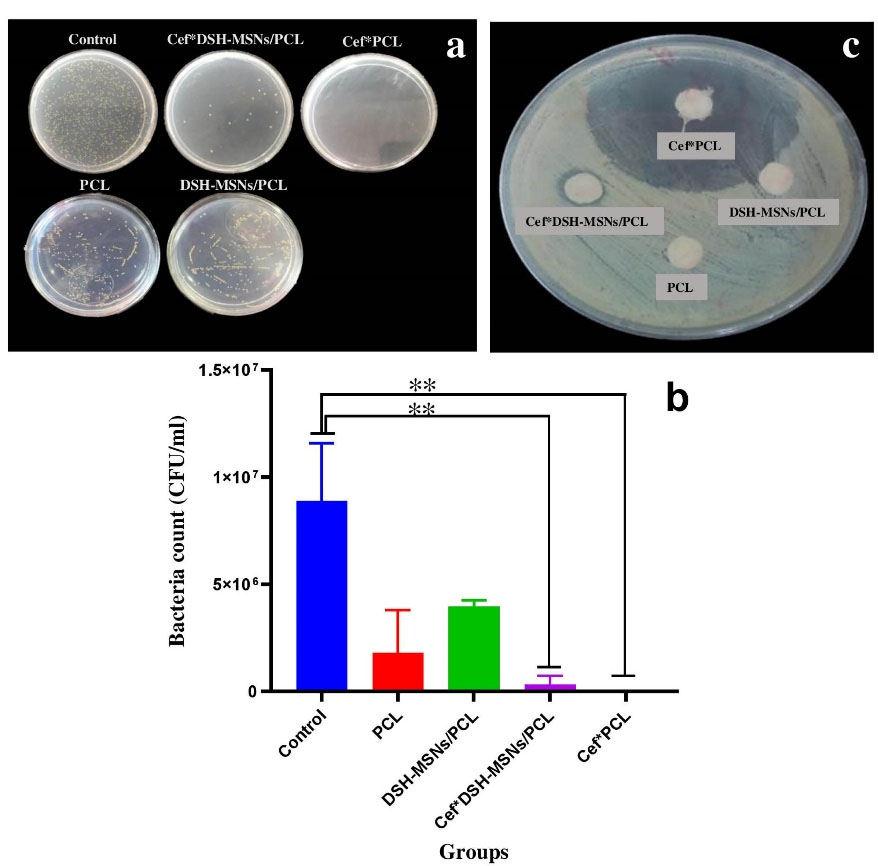
Figure 6.
(a, b) Efficacy of Cef*DSH-MSNs/PCL, Cef*PCL against S. aureus (mean ± SD, n = 3). (Note: * indicates Cef*DSH-MSNs/PCL, Cef*PCL significantly different to the other groups. (* P < 0.05, ** P < 0.01). (c) In vitro antibacterial activity of Cef*DSH-MSNs/PCL, Cef*PCL against S. aureus (ATCC®16538TM). For PCL and DSH-MSNs/PCL there was no significant difference in the zone of inhibition.
.
(a, b) Efficacy of Cef*DSH-MSNs/PCL, Cef*PCL against S. aureus (mean ± SD, n = 3). (Note: * indicates Cef*DSH-MSNs/PCL, Cef*PCL significantly different to the other groups. (* P < 0.05, ** P < 0.01). (c) In vitro antibacterial activity of Cef*DSH-MSNs/PCL, Cef*PCL against S. aureus (ATCC®16538TM). For PCL and DSH-MSNs/PCL there was no significant difference in the zone of inhibition.
Cytotoxicity
The hADSCs viability upon 72 hours exposure to DSH-MSNs and PCL nanofibers was quantified using MTT assay to evaluate possible cytotoxicity of the synthesized nanoparticles and nanofibers (Figure 7a). The nanoengineered membranes showed no toxicity to hADSCs, and cells were well grown on the surface of nanofibers without changes in morphology (P > 0.05). In agreement with our findings, numerous experiments investigating the cytotoxicity of silica nanoparticles in different cell lines have reported that silica nanoparticles have no toxic effects.44-48
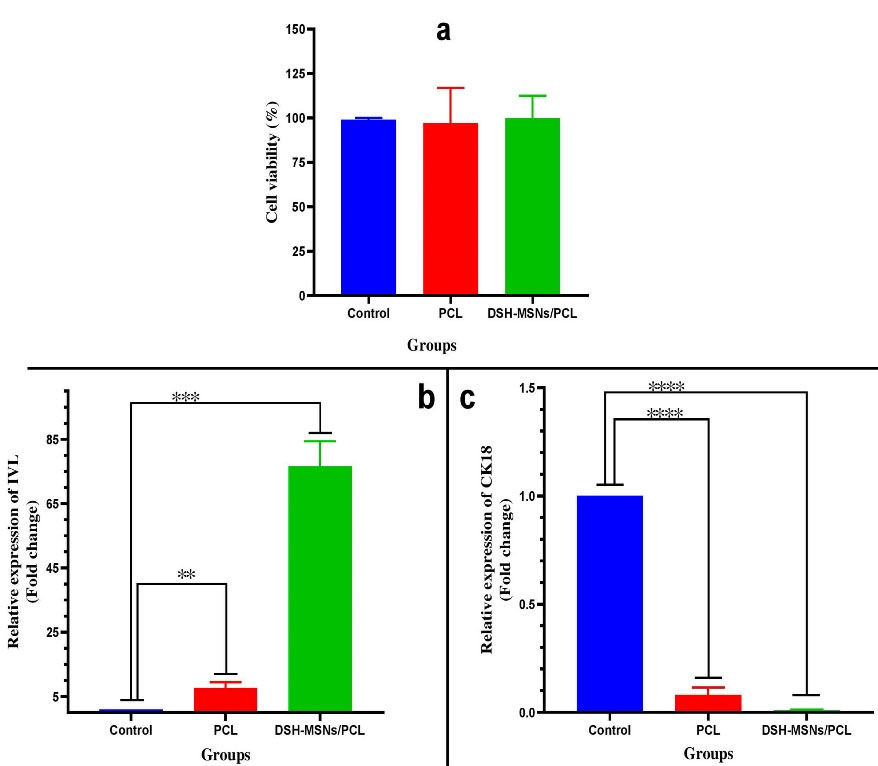
Figure 7.
(a) Cell viability of hADSCs grown on PCL and DSH-MSNs/PCL (P < 0.05). Values are expressed as mean ± SD Modulation of IVL and CK18 profile by PCL and DSH-MSNs/PCL nanofiber mats: The mRNA levels of (b) Involucrin, (c) Cytokeratin18 at 21 days post-culturing, as determined by quantitative real-time PCR (Note: * indicates DSH-MSNs/PCL, PCL significantly different) (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001).
.
(a) Cell viability of hADSCs grown on PCL and DSH-MSNs/PCL (P < 0.05). Values are expressed as mean ± SD Modulation of IVL and CK18 profile by PCL and DSH-MSNs/PCL nanofiber mats: The mRNA levels of (b) Involucrin, (c) Cytokeratin18 at 21 days post-culturing, as determined by quantitative real-time PCR (Note: * indicates DSH-MSNs/PCL, PCL significantly different) (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001).
Gene expression analysis
To determine whether PCL and DSH-MSNs/PCL can stimulate the differentiation of hADSCs toward keratinocytes, we evaluated the expression of IVL and CK18 by qRT-PCR. As shown in Figure 7b, there was a significant increase in the mRNA expression level of IVL in the PCL and DSH-MSNs/PCL nanofiber mats compared with the control group after 21 days (P < 0.05). As against the PCL group, the IVL expression was significantly high in the DSH-MSNs/PCL group (P< 0.05). However, the qRT-PCR data in Figure 7c exhibited a significant reduction in the mRNA expression level of CK18 in the PCL and DSH-MSNs/PCL groups after 21 days (P > 0.05). In this regard, Yang et al also demonstrated that the expression of CK18 in the absence of an induction medium was negative.44,49
Epithelialization is a vital stage in wound healing, and keratinocytes play a crucial role in promoting re-epithelialization.50 Poor quality and insufficient quantity of keratinocytes in unfavorable conditions such as severe burns may impair wound healing.51 Differentiating hASCs toward keratinocytes can be a good solution for promoting burn wound healing. Few researchers have demonstrated the capacity of hADSCs in epidermal differentiation using nanoparticles and scaffolds.52,53 According to previous studies, cells often need an induction medium for differentiation.54 In our study, the differentiation effect of synthesized 3D scaffolds on hADSCs into keratinocytes was assessed without using an induction medium.
The results of qRT-PCR revealed that the IVL expression level in the presence of scaffolds (DSH*MSNs/PCL, PCL) was significantly increased. Notably, the DSH-MSNs/PCL demonstrated a substantially influential role in IVL modulation compared to PCL nanofiber mats. In this regard, several studies have shown that nanoparticles can easily insert into cell membranes, locate in the cytoplasm, affect cellular signaling pathways, and induce differentiation by virtue of their small size.54 Based on Wei and colleagues’ findings, silica nanoparticles’ unique biological and mechanical properties are another possible contributor to stem cell differentiation.54 Nanofiber scaffolds also facilitate the differentiation of ASCs into epidermal cells by mimicking the structure and function of ECM.52 Alongside this, Ganesh et al demonstrated, for the first time, that electrospun MSNs/PCL nanofiber mats could induce differentiation of hMSCs to osteogenic cells.32 It should be noted that three-dimensional culture provides the biochemical aspects of cell-cell communication, signaling mechanisms, plasticity, cell proliferation, and migration to induce differentiation.55,56 Besides, scaffolds provide conditions for cell growth and differentiation into keratinocytes by mimicking the extracellular matrix and creating a 3D environment.57
The large cavity of DSH-MSNs not only increases the loading capacity but also prolongs the release profile of the drug. The differentiation effect of DSH-MSNs and PCL nanofibers on hADSCs into keratinocytes is another reason that considers DSH-MSNs as a good strategy for promoting wound healing. Our findings are promising and should be explored with other tests. Future work should focus on loading the two drugs, evaluating the long-term toxicity of DSH-MSNs, and assessing their impact on wound healing in animal models.
Conclusion
This study has developed a composite nanofibrous material for controlled and sustained drug release. The incorporation of Cef*DSH-MSNs into PCL nanofibers significantly reduced the burst release of the cefazolin and led to a sustained drug release. The drug-loaded nanofiber mats were effective against S. aureus and significantly inhibited their growth. The DSH-MSNs/PCL also significantly stimulated the differentiation of human adipose stem cells to keratinocytes. Hence, the Cef*DSH-MSNs/PCL nanofiber mats demonstrated their potential in drug delivery and regenerative medicine.
Acknowledgments
The office of Vice-Chancellor for Research and Technology, Birjand University of Medical Sciences, supported his research project (grant number: Ir.bums.456090).
Competing Interests
There is no conflict of interest to declare.
Ethical Approval
Ethical approval was granted by Ethics Committee of Birjand University of Medical Sciences (Ethics No. IR.BUMS.REC.1398.408).
References
- Alemzadeh E, Oryan A. Effectiveness of a Crocus sativus extract on burn wounds in rats. Planta Med 2018; 84(16):1191-200. doi: 10.1055/a-0631-3620 [Crossref] [ Google Scholar]
- Alemzadeh E, Oryan A, Mohammadi AA. Hyaluronic acid hydrogel loaded by adipose stem cells enhances wound healing by modulating IL-1β, TGF-β1, and bFGF in burn wound model in rat. J Biomed Mater Res B Appl Biomater 2020; 108(2):555-67. doi: 10.1002/jbm.b.34411 [Crossref] [ Google Scholar]
- Percival SL, McCarty SM, Lipsky B. Biofilms and wounds: an overview of the evidence. Adv Wound Care (New Rochelle) 2015; 4(7):373-81. doi: 10.1089/wound.2014.0557 [Crossref] [ Google Scholar]
- Li B, Webster TJ. Bacteria antibiotic resistance: new challenges and opportunities for implant-associated orthopedic infections. J Orthop Res 2018; 36(1):22-32. doi: 10.1002/jor.23656 [Crossref] [ Google Scholar]
- Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology 2018; 16(1):71. doi: 10.1186/s12951-018-0392-8 [Crossref] [ Google Scholar]
- Safari J, Zarnegar Z. Advanced drug delivery systems: nanotechnology of health design: a review. J Saudi Chem Soc 2014; 18(2):85-99. doi: 10.1016/j.jscs.2012.12.009 [Crossref] [ Google Scholar]
- Suri SS, Fenniri H, Singh B. Nanotechnology-based drug delivery systems. J Occup Med Toxicol 2007; 2:16. doi: 10.1186/1745-6673-2-16 [Crossref] [ Google Scholar]
- Sharma M, Sharma R, Jain DK. Nanotechnology based approaches for enhancing oral bioavailability of poorly water soluble antihypertensive drugs. Scientifica (Cairo) 2016; 2016:8525679. doi: 10.1155/2016/8525679 [Crossref] [ Google Scholar]
- Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P. Drug delivery systems: an updated review. Int J Pharm Investig 2012; 2(1):2-11. doi: 10.4103/2230-973x.96920 [Crossref] [ Google Scholar]
- Popat A, Hartono SB, Stahr F, Liu J, Qiao SZ, Qing Max Lu G. Mesoporous silica nanoparticles for bioadsorption, enzyme immobilisation, and delivery carriers. Nanoscale 2011; 3(7):2801-18. doi: 10.1039/c1nr10224a [Crossref] [ Google Scholar]
- Vallet-Regí M, Colilla M, Izquierdo-Barba I, Manzano M. Mesoporous silica nanoparticles for drug delivery: current insights. Molecules 2017; 23(1):47. doi: 10.3390/molecules23010047 [Crossref] [ Google Scholar]
- Jafari A, Amirsadeghi A, Hassanajili S, Azarpira N. Bioactive antibacterial bilayer PCL/gelatin nanofibrous scaffold promotes full-thickness wound healing. Int J Pharm 2020; 583:119413. doi: 10.1016/j.ijpharm.2020.119413 [Crossref] [ Google Scholar]
- Saveh-Shemshaki N, L SN, Laurencin CT. Nanofiber-based matrices for rotator cuff regenerative engineering. Acta Biomater 2019; 94:64-81. doi: 10.1016/j.actbio.2019.05.041 [Crossref] [ Google Scholar]
- Qiu K, He C, Feng W, Wang W, Zhou X, Yin Z. Doxorubicin-loaded electrospun poly(l-lactic acid)/mesoporous silica nanoparticles composite nanofibers for potential postsurgical cancer treatment. J Mater Chem B 2013; 1(36):4601-11. doi: 10.1039/c3tb20636j [Crossref] [ Google Scholar]
- Shahriar SMS, Mondal J, Hasan MN, Revuri V, Lee DY, Lee YK. Electrospinning nanofibers for therapeutics delivery. Nanomaterials (Basel) 2019; 9(4):532. doi: 10.3390/nano9040532 [Crossref] [ Google Scholar]
- Torres-Martinez EJ, Cornejo Bravo JM, Serrano Medina A, Pérez González GL, Villarreal Gómez LJ. A summary of electrospun nanofibers as drug delivery system: drugs loaded and biopolymers used as matrices. Curr Drug Deliv 2018; 15(10):1360-74. doi: 10.2174/1567201815666180723114326 [Crossref] [ Google Scholar]
- Zhang Y, Chang M, Bao F, Xing M, Wang E, Xu Q. Multifunctional Zn doped hollow mesoporous silica/polycaprolactone electrospun membranes with enhanced hair follicle regeneration and antibacterial activity for wound healing. Nanoscale 2019; 11(13):6315-33. doi: 10.1039/c8nr09818b [Crossref] [ Google Scholar]
- Cao L, Zhang H, Zhou Z, Xu C, Shan Y, Lin Y. Fluorophore-free luminescent double-shelled hollow mesoporous silica nanoparticles as pesticide delivery vehicles. Nanoscale 2018; 10(43):20354-65. doi: 10.1039/c8nr04626c [Crossref] [ Google Scholar]
- Hanafi-Bojd MY, Jaafari MR, Ramezanian N, Xue M, Amin M, Shahtahmassebi N. Surface functionalized mesoporous silica nanoparticles as an effective carrier for epirubicin delivery to cancer cells. Eur J Pharm Biopharm 2015; 89:248-58. doi: 10.1016/j.ejpb.2014.12.009 [Crossref] [ Google Scholar]
- Gao F, Li L, Zhang H, Yang W, Chen H, Zhou J. Deoxycholic acid modified-carboxymethyl curdlan conjugate as a novel carrier of epirubicin: in vitro and in vivo studies. Int J Pharm 2010; 392(1-2):254-60. doi: 10.1016/j.ijpharm.2010.03.044 [Crossref] [ Google Scholar]
- Hanafi-Bojd MY, Ansari L, Mosaffa F, Malaekeh-Nikouei B. The effect of mesoporous silica nanoparticles loaded with epirubicin on drug-resistant cancer cells. Nanomed J 2017; 4(3):135-41. doi: 10.22038/nmj.2017.8954 [Crossref] [ Google Scholar]
- Tort S, Demiröz FT, Yıldız S, Acartürk F. Effects of UV exposure time on nanofiber wound dressing properties during sterilization. J Pharm Innov 2020; 15(3):325-32. doi: 10.1007/s12247-019-09383-7 [Crossref] [ Google Scholar]
- Khorashadizadeh M, Soleimani M, Khanahmad H, Fallah A, Naderi M, Khorramizadeh M. Bypassing the need for pre-sensitization of cancer cells for anticancer TRAIL therapy with secretion of novel cell penetrable form of Smac from hA-MSCs as cellular delivery vehicle. Tumour Biol 2015; 36(6):4213-21. doi: 10.1007/s13277-015-3058-2 [Crossref] [ Google Scholar]
- Li Z, Zhang Y, Feng N. Mesoporous silica nanoparticles: synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert Opin Drug Deliv 2019; 16(3):219-37. doi: 10.1080/17425247.2019.1575806 [Crossref] [ Google Scholar]
- Trzeciak K, Chotera-Ouda A, Bak S, II II, Potrzebowski MJ. Mesoporous silica particles as drug delivery systems-the state of the art in loading methods and the recent progress in analytical techniques for monitoring these processes. Pharmaceutics 2021; 13(7):950. doi: 10.3390/pharmaceutics13070950 [Crossref] [ Google Scholar]
- Rechelo BS, Kogawa AC, Salgado HRN. Quantitative analysis of cefazolin sodium in lyophilized powder by infrared spectrophotometry: green, low cost, fast and effective. Spectrochim Acta A Mol BiomolSpectrosc 2019; 208:157-61. doi: 10.1016/j.saa.2018.09.058 [Crossref] [ Google Scholar]
- Rastegari E, Hsiao YJ, Lai WY, Lai YH, Yang TC, Chen SJ. An update on mesoporous silica nanoparticle applications in nanomedicine. Pharmaceutics 2021; 13(7):1067. doi: 10.3390/pharmaceutics13071067 [Crossref] [ Google Scholar]
- Hadipour Moghaddam SP, Mohammadpour R, Ghandehari H. In vitro and in vivo evaluation of degradation, toxicity, biodistribution, and clearance of silica nanoparticles as a function of size, porosity, density, and composition. J Control Release 2019; 311-312:1-15. doi: 10.1016/j.jconrel.2019.08.028 [Crossref] [ Google Scholar]
- Ahmed I, Day P. Stability of cefazolin sodium in various artificial tear solutions and aqueous vehicles. Am J Hosp Pharm 1987; 44(10):2287-90. [ Google Scholar]
- Contreras-Cáceres R, Cabeza L, Perazzoli G, Díaz A, López-Romero JM, Melguizo C. Electrospun nanofibers: recent applications in drug delivery and cancer therapy. Nanomaterials (Basel) 2019; 9(4):656. doi: 10.3390/nano9040656 [Crossref] [ Google Scholar]
- Eivazzadeh-Keihan R, Khanmohammadi Chenab K, Taheri-Ledari R, Mosafer J, Hashemi SM, Mokhtarzadeh A. Recent advances in the application of mesoporous silica-based nanomaterials for bone tissue engineering. Mater Sci Eng C Mater Biol Appl 2020; 107:110267. doi: 10.1016/j.msec.2019.110267 [Crossref] [ Google Scholar]
- Ganesh N, Jayakumar R, Koyakutty M, Mony U, Nair SV. Embedded silica nanoparticles in poly(caprolactone) nanofibrous scaffolds enhanced osteogenic potential for bone tissue engineering. Tissue Eng Part A 2012; 18(17-18):1867-81. doi: 10.1089/ten.TEA.2012.0167 [Crossref] [ Google Scholar]
- Gounani Z, Pourianejad S, Asadollahi MA, Meyer RL, Rosenholm JM, Arpanaei A. Polycaprolactone-gelatin nanofibers incorporated with dual antibiotic-loaded carboxyl-modified silica nanoparticles. J Mater Sci 2020; 55(36):17134-50. doi: 10.1007/s10853-020-05253-7 [Crossref] [ Google Scholar]
- Mehrasa M, Asadollahi MA, Nasri-Nasrabadi B, Ghaedi K, Salehi H, Dolatshahi-Pirouz A. Incorporation of mesoporous silica nanoparticles into random electrospun PLGA and PLGA/gelatin nanofibrous scaffolds enhances mechanical and cell proliferation properties. Mater Sci Eng C Mater Biol Appl 2016; 66:25-32. doi: 10.1016/j.msec.2016.04.031 [Crossref] [ Google Scholar]
- Moazzami Goudarzi Z, Behzad T, Ghasemi-Mobarakeh L, Kharaziha M, Enayati MS. Structural and mechanical properties of fibrous poly (caprolactone)/gelatin nanocomposite incorporated with cellulose nanofibers. Polym Bull 2020; 77(2):717-40. doi: 10.1007/s00289-019-02756-5 [Crossref] [ Google Scholar]
- Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine 2015; 11(2):313-27. doi: 10.1016/j.nano.2014.09.014 [Crossref] [ Google Scholar]
- Bharti C, Nagaich U, Pal AK, Gulati N. Mesoporous silica nanoparticles in target drug delivery system: a review. Int J Pharm Investig 2015; 5(3):124-33. doi: 10.4103/2230-973x.160844 [Crossref] [ Google Scholar]
- Li Y, Shi J. Hollow-structured mesoporous materials: chemical synthesis, functionalization and applications. Adv Mater 2014; 26(20):3176-205. doi: 10.1002/adma.201305319 [Crossref] [ Google Scholar]
- Xu C, Yu M, Noonan O, Zhang J, Song H, Zhang H. Core-cone structured monodispersed mesoporous silica nanoparticles with ultra-large cavity for protein delivery. Small 2015; 11(44):5949-55. doi: 10.1002/smll.201501449 [Crossref] [ Google Scholar]
- Chen K, Nikam SP, Zander ZK, Hsu YH, Dreger NZ, Cakmak M. Continuous fabrication of antimicrobial nanofiber mats using post-electrospinning functionalization for roll-to-roll scale-up. ACS Appl Polym Mater 2020; 2(2):304-16. doi: 10.1021/acsapm.9b00798 [Crossref] [ Google Scholar]
- Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev 2006; 19(2):403-34. doi: 10.1128/cmr.19.2.403-434.2006 [Crossref] [ Google Scholar]
- Rios AC, Moutinho CG, Pinto FC, Del Fiol FS, Jozala A, Chaud MV. Alternatives to overcoming bacterial resistances: State-of-the-art. Microbiol Res 2016; 191:51-80. doi: 10.1016/j.micres.2016.04.008 [Crossref] [ Google Scholar]
- Li X, Wu B, Chen H, Nan K, Jin Y, Sun L. Recent developments in smart antibacterial surfaces to inhibit biofilm formation and bacterial infections. J Mater Chem B 2018; 6(26):4274-92. doi: 10.1039/c8tb01245h [Crossref] [ Google Scholar]
- Ema M, Kobayashi N, Naya M, Hanai S, Nakanishi J. Reproductive and developmental toxicity studies of manufactured nanomaterials. ReprodToxicol 2010; 30(3):343-52. doi: 10.1016/j.reprotox.2010.06.002 [Crossref] [ Google Scholar]
- Eom HJ, Choi J. Oxidative stress of silica nanoparticles in human bronchial epithelial cell, Beas-2B. Toxicol In Vitro 2009; 23(7):1326-32. doi: 10.1016/j.tiv.2009.07.010 [Crossref] [ Google Scholar]
- Ha SW, Sikorski JA, Weitzmann MN, Beck GR Jr. Bio-active engineered 50 nm silica nanoparticles with bone anabolic activity: therapeutic index, effective concentration, and cytotoxicity profile in vitro. Toxicol In Vitro 2014; 28(3):354-64. doi: 10.1016/j.tiv.2013.12.001 [Crossref] [ Google Scholar]
- Kim IY, Joachim E, Choi H, Kim K. Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomedicine 2015; 11(6):1407-16. doi: 10.1016/j.nano.2015.03.004 [Crossref] [ Google Scholar]
- Marquis BJ, Love SA, Braun KL, Haynes CL. Analytical methods to assess nanoparticle toxicity. Analyst 2009; 134(3):425-39. doi: 10.1039/b818082b [Crossref] [ Google Scholar]
- Yang F, Zhang WL, Chen SQ, Sun HJ, Lu J, Xiao XF. Differentiation of human adipose-derived stem cells into endometrial epithelial cells. Reprod Dev Med 2020; 4(3):137-45. doi: 10.4103/2096-924.296547 [Crossref] [ Google Scholar]
- Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A. Epithelialization in wound healing: a comprehensive review. Adv Wound Care (New Rochelle) 2014; 3(7):445-64. doi: 10.1089/wound.2013.0473 [Crossref] [ Google Scholar]
- Mishra PJ, Mishra PJ, Banerjee D. Keratinocyte induced differentiation of mesenchymal stem cells into dermal myofibroblasts: a role in effective wound healing. Int J Transl Sci 2016; 2016(1):5-32. doi: 10.13052/ijts2246-8765.2016.002 [Crossref] [ Google Scholar]
- Li M, Ma J, Gao Y, Dong M, Zheng Z, Li Y. Epithelial differentiation of human adipose-derived stem cells (hASCs) undergoing three-dimensional (3D) cultivation with collagen sponge scaffold (CSS) via an indirect co-culture strategy. Stem Cell Res Ther 2020; 11(1):141. doi: 10.1186/s13287-020-01645-3 [Crossref] [ Google Scholar]
- Lotfi M, Naderi-Meshkin H, Mahdipour E, Mafinezhad A, Bagherzadeh R, Sadeghnia HR, et al. Adipose tissue-derived mesenchymal stem cells and keratinocytes co-culture on gelatin/chitosan/β-glycerol phosphate nanoscaffold in skin regeneration. Cell Biol Int 2019. 10.1002/cbin.11119.
- Wei M, Li S, Le W. Nanomaterials modulate stem cell differentiation: biological interaction and underlying mechanisms. J Nanobiotechnology 2017; 15(1):75. doi: 10.1186/s12951-017-0310-5 [Crossref] [ Google Scholar]
- Badekila AK, Kini S, Jaiswal AK. Fabrication techniques of biomimetic scaffolds in three-dimensional cell culture: a review. J Cell Physiol 2021; 236(2):741-62. doi: 10.1002/jcp.29935 [Crossref] [ Google Scholar]
- Patil S, Paul S. A comprehensive review on the role of various materials in the osteogenic differentiation of mesenchymal stem cells with a special focus on the association of heat shock proteins and nanoparticles. Cells Tissues Organs 2014; 199(2-3):81-102. doi: 10.1159/000362226 [Crossref] [ Google Scholar]
- Shokrgozar MA, Fattahi M, Bonakdar S, Ragerdi Kashani I, Majidi M, Haghighipour N. Healing potential of mesenchymal stem cells cultured on a collagen-based scaffold for skin regeneration. Iran Biomed J 2012; 16(2):68-76. doi: 10.6091/ibj.1053.2012 [Crossref] [ Google Scholar]