Advanced pharmaceutical bulletin. 14(1):34-47.
doi: 10.34172/apb.2024.012
Review Article
A Comprehensive Review on Novel Lipid-Based Nano Drug Delivery
Sonam Suresh Godase Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing, 
Nilesh Shrikant Kulkarni Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, * 
Shashikant Nivrutti Dhole Formal analysis, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing,
Author information:
Department of Pharmaceutics, PES Modern college of Pharmacy (for ladies) Moshi, Pune. Affiliated to Savitribai Phule Pune University, Pune, Maharashtra, India.
Abstract
Novel drug delivery system opens the doors towards nano/micro formulation strategies to overcome the challenges associated with the poorly soluble and permeable drugs. Lipid based nanoparticles are widely accepted that includes liposomes, niosomes and micelles which are FDA approved. Such lipid based drug delivery allows delivery for natural phytoconstituents, biopharmaceutical classification system (BCS) class II and class IV drugs are effectively delivered to improve its solubility, permeability and bioavailability. The article provides the recent advances and application of lipid based dosage form for improvement of therapeutic efficacy.
Keywords: Novel Drug Delivery System, BCS classification, Liposome, Niosomes, Solid lipid nanoparticles, Nanochochleats
Copyright and License Information
©2024 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Novel drug delivery system opens the doors towards Nano/Micro formulation strategies to overcome the challenges associated with the biopharmaceutical classification system (BCS) class II and class IV drugs.1 Such medication or drug delivery targets the drug at required site that too in low concentration and improves therapeutic efficiency. Novel drug delivery system includes microparticles, nanoparticles such as lipid based liposomes, niosomes, phytosomes, micelles, hydrogels, quantum dots, nanotubes, dendrimers etc.2 Nanoparticulate drug delivery system have particle size which ranges between 1 to 100 nm. The drug movement across the barrier will get improved due to development of nanosized particulate system.3 Nanomaterials have wide application in the treatment and diagnostic purpose.4,5
Currently lipid based dosage forms are popular that includes liposomes, niosomes, micelles etc which are FDA approved. Such lipid based drug delivery systems have found to be effective for natural phytoconstituents and inorganic particles like gold.6 The advantages of lipid based novel drug delivery system are associated with the majority of drugs.
Reasons for application of novel drug delivery system for BCS class II and IV drugs.7-11
-
Poor solubility and poor permeability of drug.
-
Decrease in size of particle leads to increase in effective surface area which ultimately improves dissolution rate of poorly soluble drugs.
-
Nanomaterials are being used in many different biological and medical fields because they reframe optical, electrical, chemical and physical properties.
-
Increases mobility of particle that helps to increase bioavailability.
-
Nanomaterials have application in targeted and controlled delivery of biopharmaceuticals.
-
Due to nanosized structure, it can easily cross mucosal membrane whereas Microsystems has capacity to cross epithelial lining.
-
Increased drug therapeutics efficacy and reduced side effects.
-
Protection of drug from first pass metabolism and enzymatic degradation.
Solubility and permeability
Solubility is one of the key parameter that directly affects the activity and bioavailability of drug. The variety of factors that has influence on solubility of the drugs are pKa of drug, pH at gastrointestinal tract (GIT), presence of luminal pH.12,13 Physiological and physicochemical factors have influence on drug solubility.14,15
Solubility depends on chemical, electrical, structural properties of the solute and interaction between solute solvent. The USP 38, European pharmacopoeia categorized solubility in seven different group.16 Biopharmaceutics classification system was developed by Amidon et al in 1995. The BCS classification has application for the development of immediate release oral dosage forms. The drugs will be classified into four classes.17-19 Solubility and permeability improvement for BCS class II and BCS class IV drugs respectively has a major obstacle for the formulation scientist (Table 1). There are various approaches are reported till today to enhance the solubility for such drugs. Permeability study also shows the movement of drug into the circulatory system through GIT.
Table 1.
BCS Classification
|
Class
|
Solubility
|
Permeability
|
Example
|
| Class I |
High |
High |
Metoprolol, diltiazem, verapamil, propranolol etc. |
| Class II |
Low |
High |
Ibuprofen, ketoprofen, carvedilol, ketoconazole, fenofibrate etc. |
| Class III |
High |
Low |
Cimetidine, ranitidine, acyclovir, neomycin B, atenolol, captopril. |
| Class IV |
Low |
Low |
Hydrochlorothiazide, taxol, furosemide. |
The BCS class II drugs will be classified into subclasses considering the acidic and basic strength (Table 2).20-22 Variation in pH environment in GIT has influence on drug solubility for BCS class II drugs.
Table 2.
BCS Sub classification
|
Class II
|
Solubility
|
Example
|
|
Gastric pH solubility
|
Instestinal pH solubility
|
| Class II a (Weakly acidic drugs) |
Low |
Dissolve quickly |
Ibuprofen, ketoprofen, flurbiprofen, naproxen, rifampicin etc |
| Class II b (Weakly basic drugs) |
High |
Precipitate |
Carvedilol, ketoconazole, ibuprofen, ketoprofen etc |
| Class II c (Neutral drugs) |
No dependent on pH change |
Fenofibrate etc. |
BCS classification allows the formulator to correlate the physicochemical properties of drug and its solubility, permeability to make a judgment on bioavailability. It reduces the time, cost of drug delivery and development. It is approved by US Food and Drug Administration (USFDA). The regulatory agencies such as European Medicine Agency (EMEA) and World Health Organization (WHO) for bioavailability/bioequivalence standards for approval of drug product and gives directions for In-Vitro, In-vivo dissolution study.23,24
Basic fundamentals of BCS classification are the three dimensionless numbers as dose number, absorption number and dissolution number which calculates the amount of drug.25
Dose number (high solubility): when the highest clinical dose is dissolved in 250 mL buffer at all pH values within the range 1–7.5.
Permeability: High permeability means the drug product is stable in GIT and drug absorption is greater than 90% of the given dose. Permeability is defined as passage or movement from site of administration (Gastrointestinal track) to the systemic circulation across the biological membranes is called permeability. Permeability depends upon the absorption of drug and absorption is depend upon various properties of drug, receptors, biological membranes, types of transport etc.
Types of lipid based nano drug delivery system
The major obstacle for the drugs to develop into dosage form is associated with poor aqueous solubility, poor permeability, poor absorption, extensive first pass metabolism, systemic metabolism and efflux proteins (P-glycoprotein).26 It is important for further clinical improvements of drugs. Researchers were tried with variety of techniques to overcome these issues which includes Lipid based drug delivery systems, Polymer based drug delivery system, Nanocarriers, Nanocrystals, liquisolid technology, solid dispersions etc (Figure 1). Amongst these techniques lipid based nanoparticulate formulation was found to be beneficial.
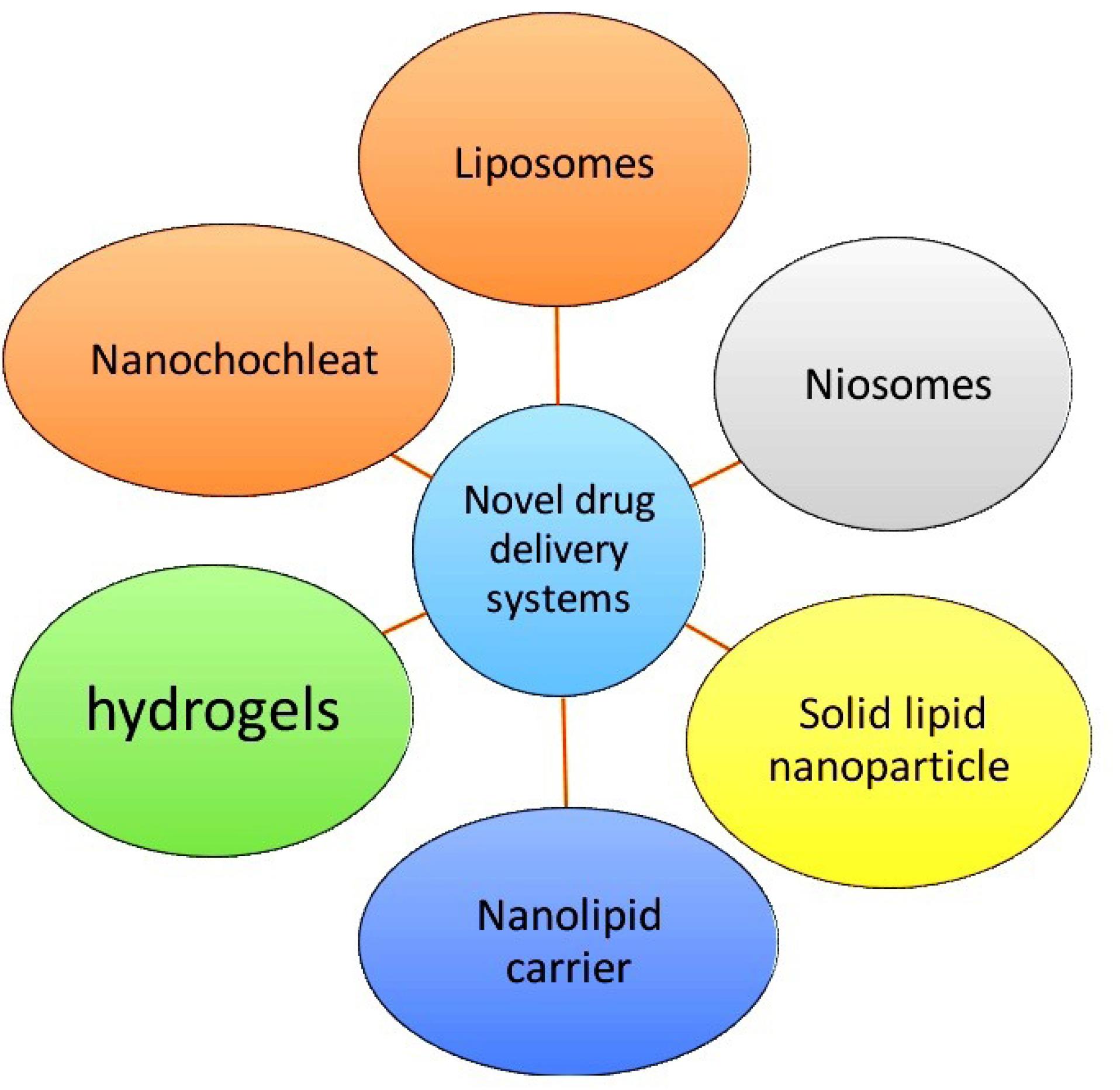
Figure 1.
Types of novel drug delivery system
.
Types of novel drug delivery system
Liposomes
Liposomes are the spherical vesicles made up of amphiphilic phospholipids. Phospholipids has capability to encloses both hydrophilic and hydrophobic drugs and possess property to self assemble.27
Mechanism of liposome formation
The lipids phase is added into the aqueous phase. It forms bilayers by hydrophobic interaction or hydrophilic interaction between lipid–lipid or lipid–water molecules (Figure 2). These formed lipid layers are set as vesicles by external energy such as sonication, homogenization, heating, freezing etc.
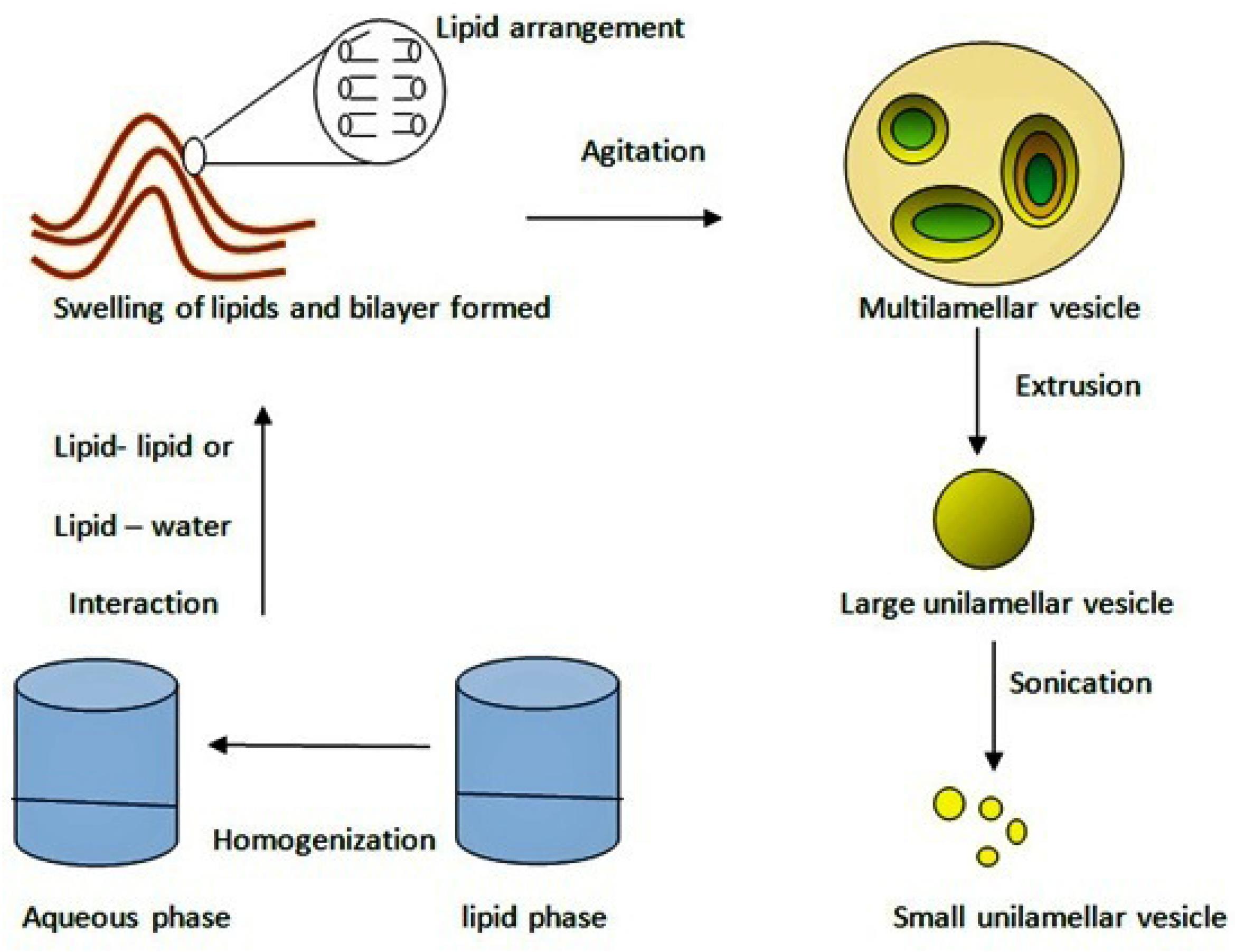
Figure 2.
Mechanism of liposome formation
.
Mechanism of liposome formation
Classification of liposomes
The liposomes will be classified based on material used for the preparation, types of lipid or combination of lipids used, based on method of preparation techniques and depending upon the size of vesicles formed (Figure 3).
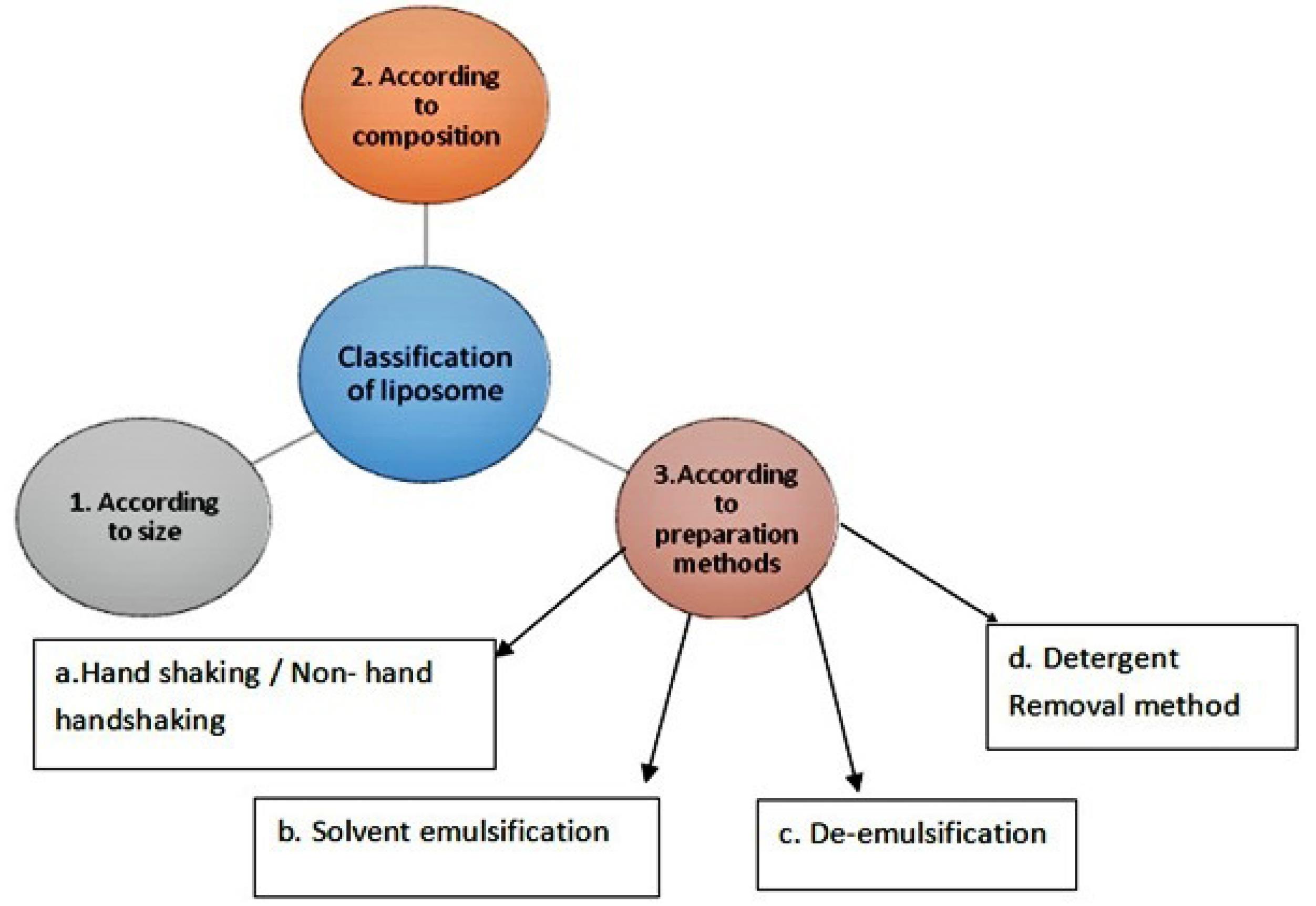
Figure 3.
Classification of liposomes
.
Classification of liposomes
A. According to size and shape of liposome: Liposomes were classified according to the size, number of bilayers formed in particle and according to their pattern. They are classified as multilamellar large vesicle which is greater than 0.5 µm size. Multilamellar liposomes are those which are with a number of lipidic bilayers. Oligolamellar liposomes means vesicles are same as that of multilamellar. Oligolamellar vesicles are made up with 2 to 5 lipid bilayers. More than 5 lipid bilayer considering as multilamellar vesicles. Unilamellar vesicles (ULV), small unilamellar vesicles (SUV) and large unilamellar vesicles (LUV) possess similarity in structure but varies in size (Table 3).
Table 3.
Types of liposomes according to size
|
Type
|
Size
|
| Multilamellar large vesicle |
> 0.5 µm |
| Oligolamellar vesicles (OLV) |
0.1-1.0 µm |
| Unilamellar vesicles (ULV) |
All size range (o.1 nm to 1000 µm) |
| small unilamellar vesicle (SUV) |
20-100 nm |
| Large unilamellar vesicles (LUV) |
> 100 nm |
| Giant unilamellar vesicles (GUV) |
> 1.0 µm |
| Multivariant vesicle |
> 1.0 µm |
B. Based on composition: According to the source of lipids used in preparation of liposome (Table 4).
Table 4.
Types of liposomes based on lipid composition
|
Type of composition
|
Application
|
Examples of lipids
|
| Conventional liposomes |
To improve drug delivery |
Neutral or negatively charged lipids examples phospholipid lecithin, glycerol, fatty acids etc28 |
| pH sensitive liposome |
According to pH intracellular drug delivery |
Neutral to slightly alkaline pH to acidic lipids examples phosphatidyl ethanolamine, dioleoyl phasphatidyl ethanolamine etc29 |
Cationic liposomes
(Positively charged head groups) or lipoplexes |
For delivery of negatively charged macromolecules
(DNA, RNA) |
DOTAP (1,2–dioleoyl-3,3-trimethyl ammonium-propane (chloride salt), DOTMA(1,2,3–dioleoloxy) 3,3 trimethyl ammonpropane etc30 |
| Stealth liposomes or long circulating or PEGylated liposomes |
To avoid immune system and extracellular delivery of drug |
Includes synthetic polymers in liposome composition example Polyethylene glycol31 |
| Immunoliposome |
Cell specific binding with avoiding immune system |
Antibodies attached to conventional liposomes30 |
| Magnetic liposomes |
Use by external vibrating magnetic field at deliberate site for immediate release on site |
Phosphatidylcholine, Cholesterol, linear chain aldehyde and colloidal particles of magnetic iron oxide31 |
| Temperature sensitive or heat sensitive |
Liposomes release drugs at target cell according to temperature or heat change |
Dipalmitoyl phosphatidylcholine28 |
C. Based on method for preparation of liposome: Various methods are reported for the preparation of liposome as mechanical dispersion, solvent dispersion, de-emulsification, detergent removal method (Table 5).
Table 5.
Types of methods of preparation of liposomes
|
Name of method
|
Instruments used
|
Mechanical dispersion:
Process: Co-dissolving lipids in organic solvent, Organic solvent is removed by film deposition under vacuum. |
Hand shaking/Non hand shaking |
| Sonication (bath sonicator or probe sonicator) |
Ultra sonicator32-34 |
| Micro emulsification |
Microfluidizer pump35,36 |
| Extrusion technique |
Extruder37,38 |
Solvent dispersion:
Process: Lipids are dissolved in organic solvent then add into aqueous phase containing drug. |
Ethanol injection (water miscible solvent) |
Fine needle39 |
| Ether injection (water immiscible solvent) |
Fine needle40 |
| Rapid solvent exchange |
Narrow needle41 |
De-emulsification:
Process: breakdown of large emulsion vesicles that have capacity to reform when broken down. |
Reverse phase evaporation technique |
Evaporator42 |
Detergent removal method:
Process: micelles are formed with the help of detergents. |
Dialysis |
Membranes43 |
| Column chromatography |
Columns43 |
Niosomes
Niosomes are the non-ionic surfactants containing liposomes. Surfactants such as fatty alcohol, esters and copolymers are used in the development of niosome formulation. Niosomes formulation contains surfactant.44,45
The main component is surfactant. The surfactants possess both hydrophilic and hydrophobic groups and hence these are widely accepted (Table 6). According to head group properties, surfactants are classified as anionic, cationic, amphoteric and nonionic. Nonionic surfactant is mostly used because they are more stable, less toxic and compatible.46
Table 6.
Examples of niosome prepared by film hydration Technique
|
Technique of preparation
|
Excipients used
|
Compound/ Drug used
|
| Thin film hydration (sonication) |
Tween 80, Tween 20, Phosphate buffer pH 7, Cholesterol |
Curcumin47 |
| Thin film hydration |
Chloroform, Methanol, Span80, Dicetyl phosphate |
Curcumin48 |
| Reverse phase evaporation |
Span 60, DMSO, cholesterol |
Growth factor49 |
| Thin film hydration (evaporator) |
GMS, Cholesterol, Glucose, Sodium chloride, Tween 80, MYRJ 49 |
Ginkgolide50 |
Advantages
-
Designed for drugs which has poor absorption to enhance bioavailability.
-
Solubility/ Permeability is enhanced as niosomeal drug delivery crosses anatomical barriers of GIT via transcytosis of M cells of Peyer’s patches in intestine.
-
Niosomes has capacity to release drugs in the gradual and controlled manner.
-
Niosomes are easily modified due to presence of hydrophilic and lipophilic head groups.
Disadvantages
-
Physical instability (aggregation, fusion)
-
Hydrolysis of entrapped drug.
-
Leaking and leaching of an entrapped.
Solid lipid nanoparticles
The solid lipid nanoparticles (SLNs) are need to be developed to overcome drawbacks associated with traditional colloidal systems such as emulsions, liposomes, polymeric nanoparticles The SLNs are composed of physiological lipids like glycerides of fatty acids which possess biocompatibility and biodegradability. SLNs overcomes the drawbacks associated with traditional colloidal systems as complicated preparation methods, low entrapment efficiency, difficult large scales manufacturing.51
Key ingredients to be used for formulation of SLNs includes:
-
Lipids – triglycerides, partial glycerides
-
fatty acids
-
Steroids
-
Waxes
Different methods of preparation for solid-lipid Nanoparticle are reported (Figure 4).52
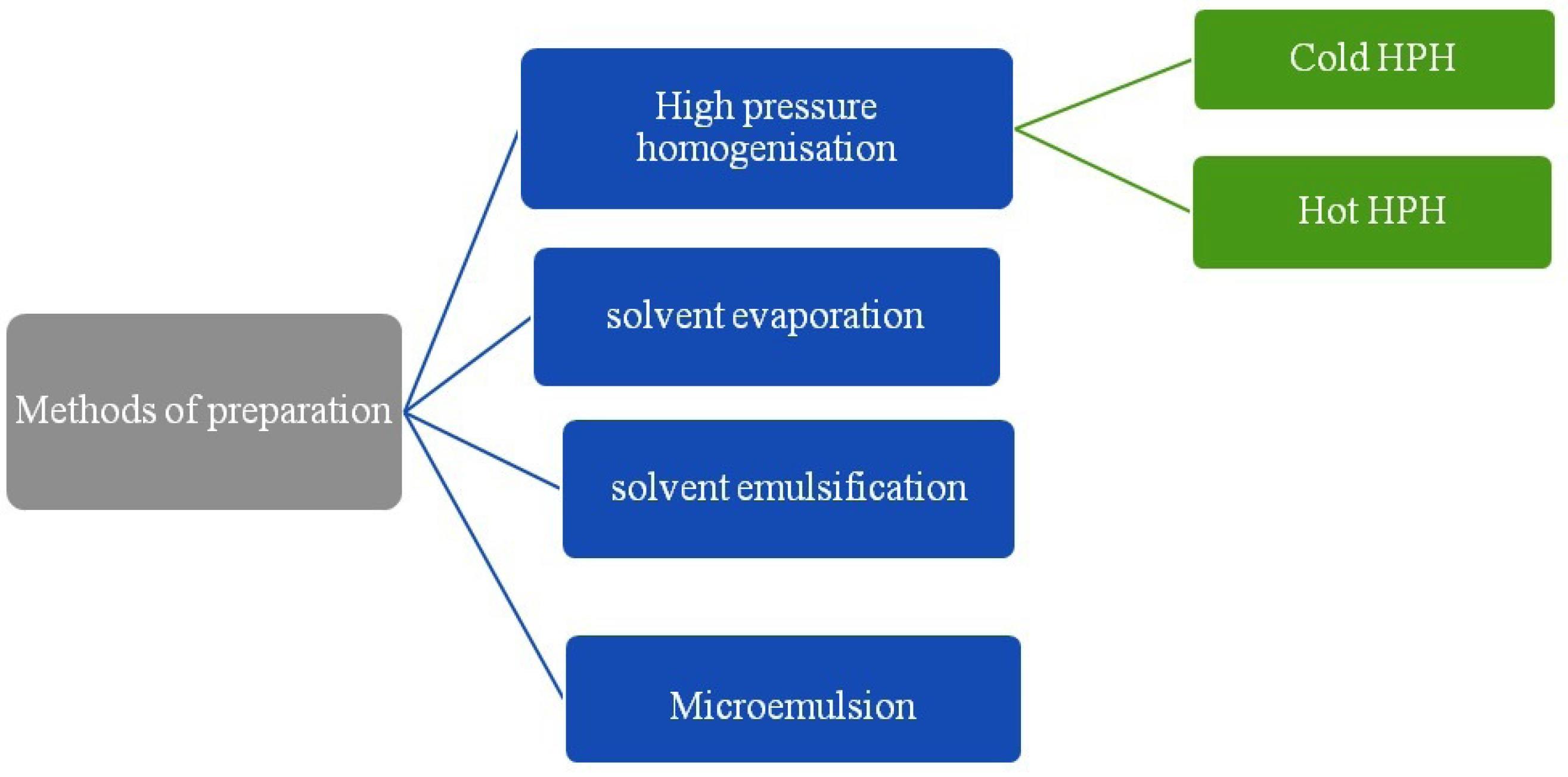
Figure 4.
Methods of preparation of solid lipid nanoparticles
.
Methods of preparation of solid lipid nanoparticles
A. High pressure homogenization (HPH)
HPH is most widely used and accepted technique used in pharmaceutical industries. In the high pressure homogenizer, liquid phase is need to passed with high pressure through narrow orifice of micron or submicron size. This leads to reduction in particle size. HPH process is of two types as hot homogenization and cold homogenization. For both the types drug is to be dissolved in the lipids and dispersion is made. Afterward according to method temperature is need to be maintained.
I. Hot homogenization method
The hot homogenization method includes temperature which is more than the melting point of the lipid. Lipid is allowed to melt and into molten lipid drug is added. This process makes microemulsion also called as pre-emulsion which is maintained at high temperature and mixed with the aqueous phase with surfactants (Figure 5).53
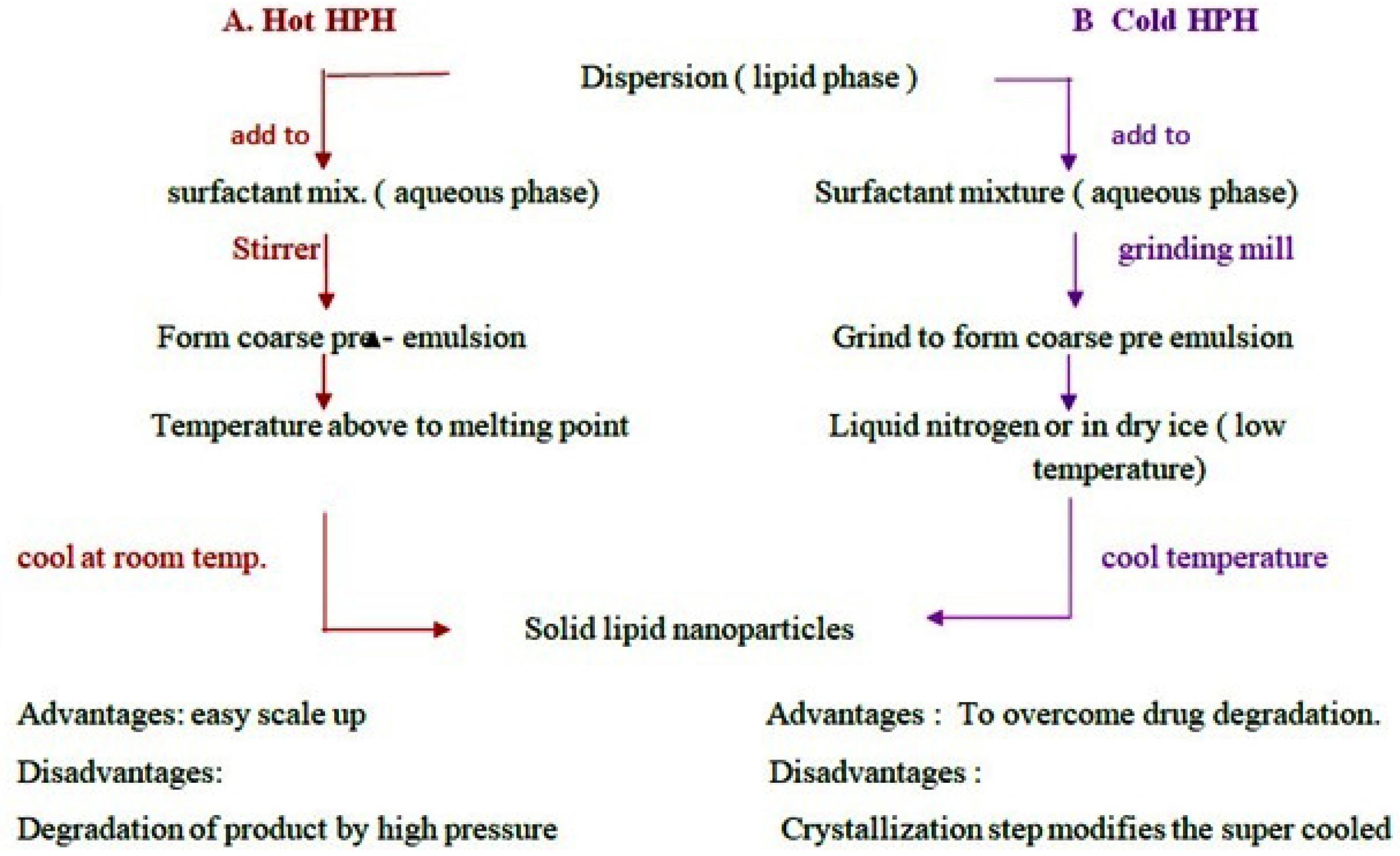
Figure 5.
Types of high pressure homogenization method
.
Types of high pressure homogenization method
II. Cold homogenization method
The heat sensitive drugs undergo degradation at high temperature in hot homogenization method. To improve drug stability cold homogenization method is preferred. The dispersion of drug and lipid is added to liquid nitrogen or dry ice to drop down the temperature of the sample. Afterward sample is allowed to cool at room temperature or lower temperature. The resultant powder product is SLNs (Table 7).53
Table 7.
Examples of Solid lipid nanoparticles prepared by High Pressure Homogenization techniques
|
HPH method
|
Drug
|
Excipient
|
Outcome
|
| Hot HPH |
Eucalyptus
globules oil |
Solid lipid: cocoa butter
Liquid lipids: sesame oil, olive oil, Surfactant: L-α phosphatidyl choline |
Wound healing53 |
| Cold HPH |
Rifampicin,
isoniazid, pyrazinamide |
Poloxamer 188 (pluronic F-68), sodium taurocholate, stearic acid, mannitol, GMS, poloxamer 407, HPMC |
As antitubercular action54 |
| Hot HPH |
Zataria multiflora oil |
Stearic acid, tween 80, span 60, absolute ethanol |
Repellant activity against anopheles stephensi55 |
| Cold HPH |
Streptomycin sulphate |
Soy lecithin, precirol ATO 888, GMS, tween 80, PEG 400, PEG 600, Gelucire 44/14 |
Against mycobacterium for tuberculosis56 |
| Hot HPH |
Curcumin |
Curcumin, Compritol 888 ATO, Soy lecithin (Phospholipon 90 G), |
As wound healing57 |
B. Solvent evaporation/emulsification method
In solvent evaporation/emulsification method lipophilic material is dissolved in an organic solvent and further emulsified in an aqueous phase. It forms a to give an oil in water type of emulsion.58 The prepared emulsion is stirred on mechanical stirrer to allow organic solvent to evaporate. SLNs are formed due to precipitation of lipid phase in water or aqueous phase. In this method polarity of two phases should be of opposite to form o/w emulsion (Table 8).59
Table 8.
Examples of solid lipid nanoparticles containing drug prepared by solvent evaporation method
|
Drug /compound
|
Excipients
|
Outcome
|
| Curcumin |
Poloxamer 188, tween 80, Glyceryl monostearate, PEG-400, Ethyl alcohol |
For treatment of COPD60 |
| Naloxone |
Glyceryl monostearate, Pluronic 127, tween 80 |
To inverse opioid overdose61 |
| Perphenazine |
Tween 80, Soy lecithin, HPLC grade acetonitrile, methanol, glyceryl monostearate |
As an antipsychotic62 |
| Amphotericin- B |
Pluronic F 127, Vitamin B 12, Fluorescein isothiocyanate, Stearic acid, Oxyma sodium bicarbonate, Potassium bromide, phosphotungstic acid, Solutol HS 15, cellulose, Precirol ATO5 |
As am antileshmanial63 |
| Glibenclamide |
Precitrol and Compritol, PEG |
For hypoglycemic effect64 |
| Olmesartan medoxomil |
Glyceryl Monostearate, Soya Phosphatidylcholine and Tween 80 |
As antihypertensive65 |
Limitations
-
Large amount of emulsifiers are needed to get small size particles.
-
Time and energy consuming method.
-
Solvents used if not biocompatible needs further purification is needed.
C. Solvent emulsification diffusion Technique
It consists of preparation of suspension from emulsion by a solvent diffusion technique. This process is also based on water miscibility of solvents (Figure 6). The water miscible solvents such as butyl lactate, benzyl alcohol, methyl acetate, ethyl acetate, isopropyl acetate etc are widely used. Suspensions are prepared from emulsions (with partially water miscible solvents). Process depends upon water miscibility of solvents (Tables 9 and 10).
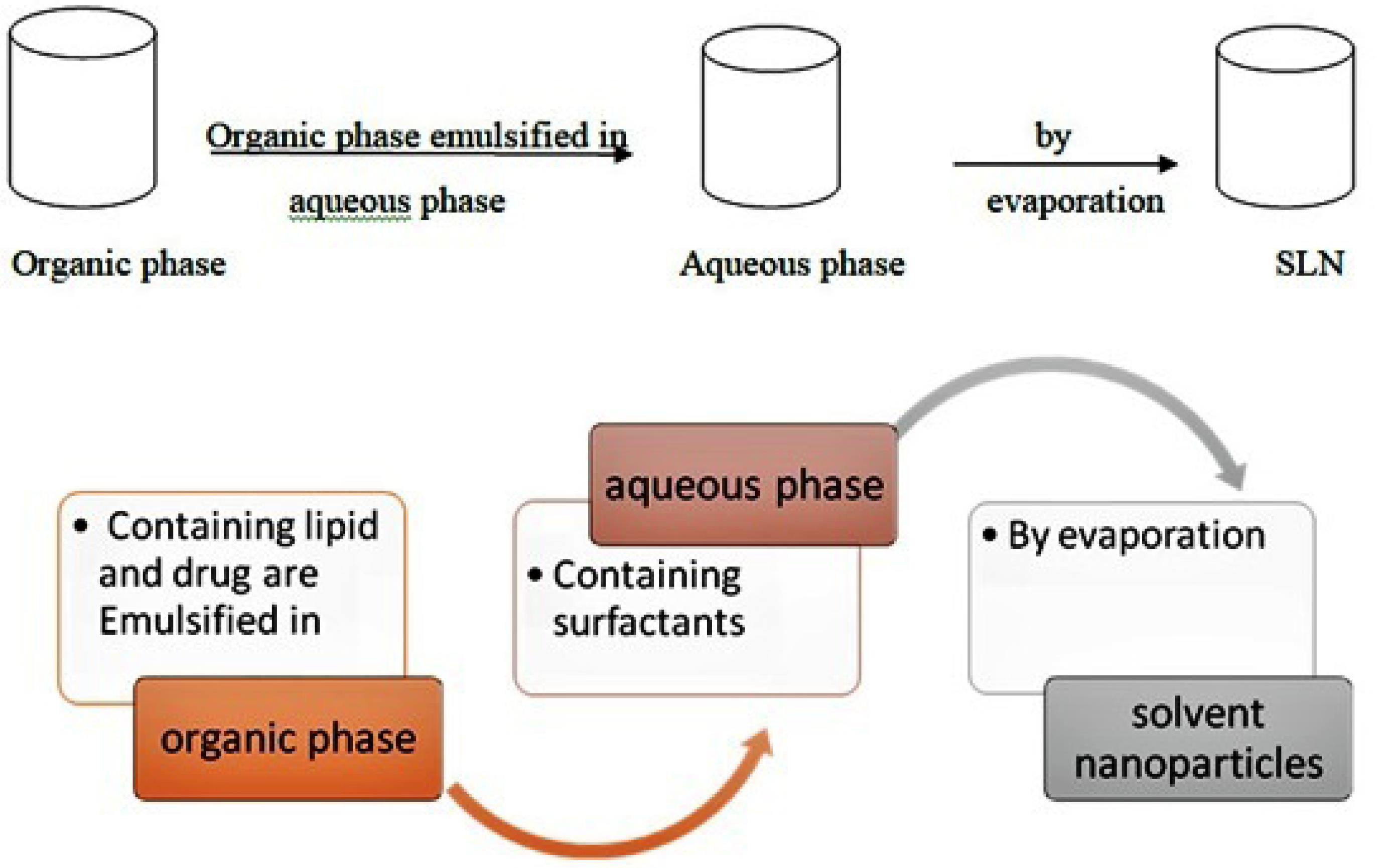
Figure 6.
Process of solvent evaporation or emulsification method
.
Process of solvent evaporation or emulsification method
Table 9.
Examples of solvent emulsification diffusion technique for SLN preparation
|
Drug name
|
Excipients
|
Outcome
|
| Tretinoin gel |
GMS, Compritol 888 ATO, Dnyasan116, Cutina CBS, Epikuron 200, Tween 20, Tween 80 |
To treat acne68 |
| Povidone – iodine gel |
GMS, soyalecithin, Pluronic F 68, carbopol 940, propylene glycol |
As an antiseptic drug for wound healing69 |
| Rutin |
Phospholipon 80 H, Tween 80, Trehalose, Ethanol, Acetate (2:1) |
Use for oxidative stress induced diseases70 |
| Folate conjugated Olaparib nanoparticle |
PEG 4000, stannous octoate, dicyclohexylcarbodiimide (DCC), N- hydroxysuccinimide |
Use as anticancer71 |
Table 10.
Indian Patents published for solid lipid nanoparticles by various methods
|
Application number
|
Year
|
Drug name
|
Title
|
Method
|
Outcome
|
Ingredients
|
| 202111058402 |
2021 |
Lemongrass essential oil |
Formulation of lemongrass essential oil loaded solid lipid nanoparticles |
Hot water technique |
For acne vulgaris |
Oil, tween 80, ethanol, distilled water72 |
| 202142054086 |
2021 |
methotrexate |
Surface modified methotrexate loaded solid lipid nanoparticles to overcome ABCB1 polymorphism |
Microemulsion method |
anticancer |
Stearic acid, soya-lecithin, polyoxyethylene-polyoxypropylene73 |
| 202111052175 |
2021 |
Baicalein |
Baicalein loaded solid- lipid nanoparticles and method of preparation of thereof |
Solvent diffusion method |
Neurodenegrative disorder |
Baicalein stearic acid (lipid), tween80, ethanol, chloroform74 |
| 202141046636 |
2021 |
Clobetasol |
Clobetasol loaded solid lipid nanoparticles and nanostructured lipid carriers for topical treatment of psoriasis |
Melt dispersion |
For psoriasis |
Clobetasol, compritol, oleic acid, tween 8075 |
| 202121046360 |
2021 |
Sertraline hydrochloride |
SLN of sertraline hydrochloride |
Hot homogenization |
Antidepressant and anorectic agent |
Glyceryl monostearate, stearic acid, cetyl palmitate, poloxamer188, triethanolamine, ethanol76 |
| 202121039670 |
2021 |
Aceclofenac |
Development of nanoparticle formulation of aceclofenac |
Solvent evaporation method |
|
Aceclofenac, ethyl cellulose, chitosan, HPMC K100, poly vinyl alcohol, dichloromethane, distilled water77 |
| 202111036625 |
2021 |
Citrus limetta peel |
A novel composition and process for fabrication of SLNs |
Ionotropic gelling agent |
Diabetic neuropathy |
Citrus limetta peel as active pharmaceutical ingredient78 |
Mechanism: It involves addition of organic phase into aqueous phase that leads to formation of o/w emulsion. Emulsion is diluted with water. During agitation provided by mechanical stirrer, dissolved drug in organic solvent gets solidified instantly due to diffusion of the organic solvent from droplets to continuous phase which forms hollow spheres (Figures 7 and 8).
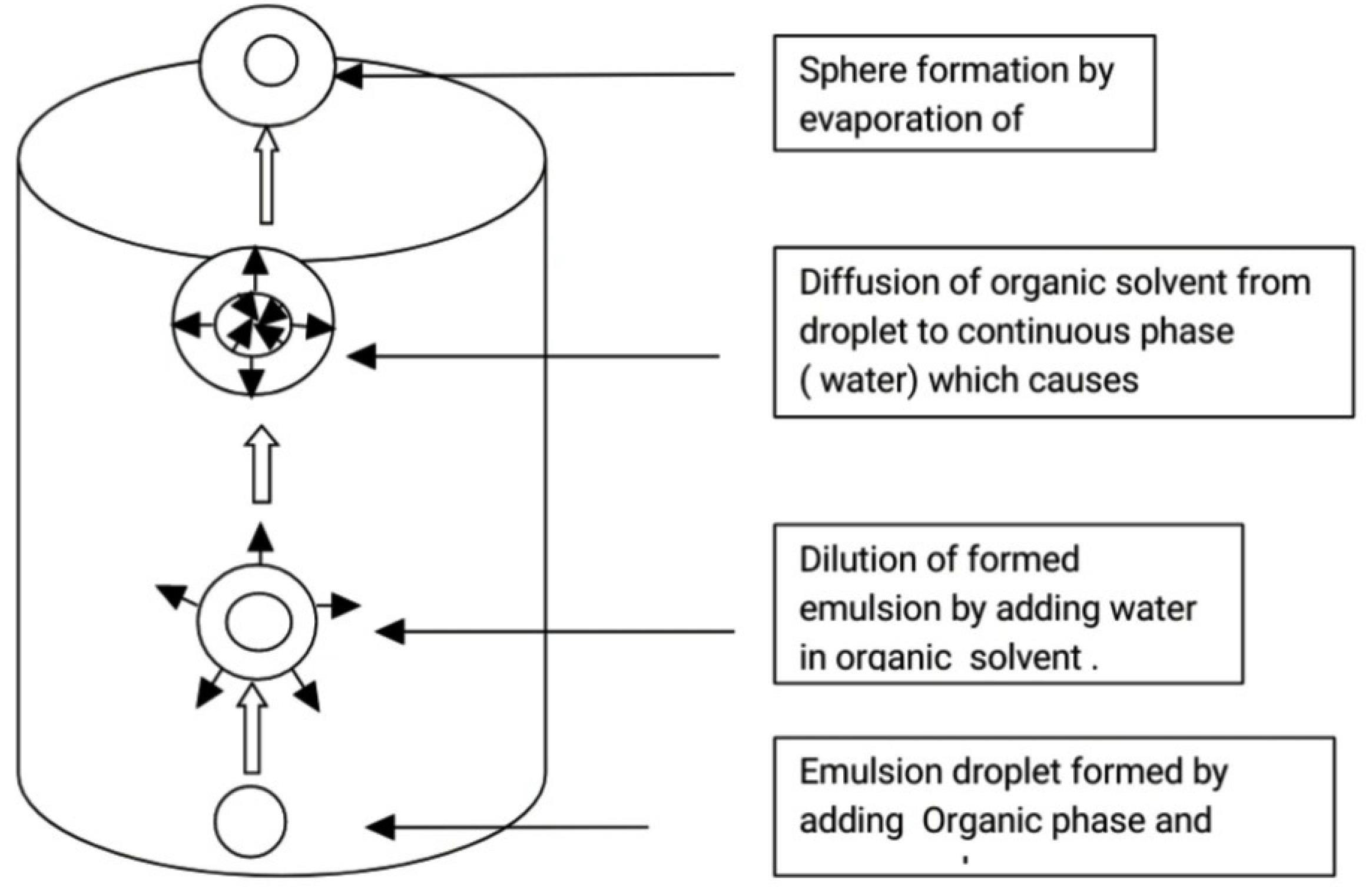
Figure 7.
Mechanism of Sphere particle formation by solvent emulsification diffusion technique
.
Mechanism of Sphere particle formation by solvent emulsification diffusion technique
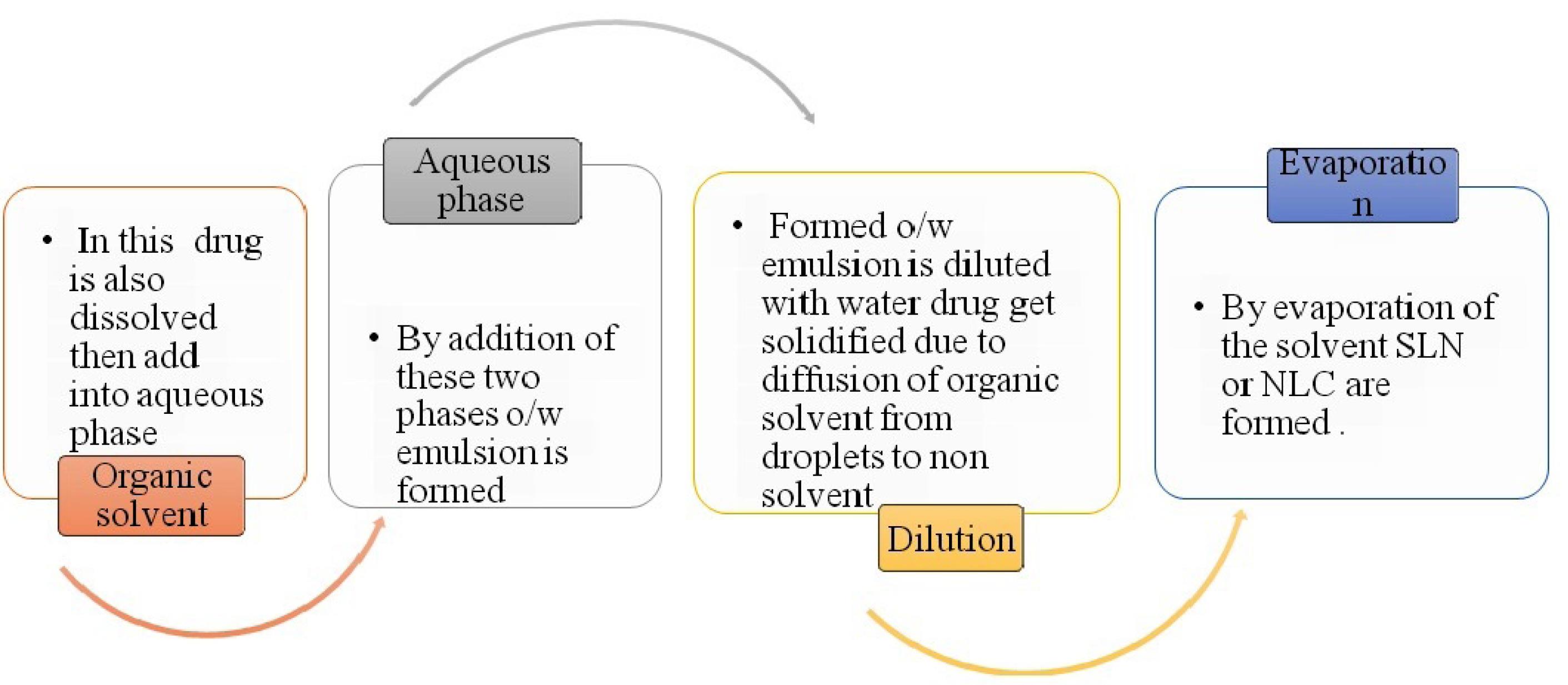
Figure 8.
Process of solvent emulsification diffusion method for SLN or NLC
.
Process of solvent emulsification diffusion method for SLN or NLC
Advantages66
-
The technique is easy to scale-up.
-
Exposure of drug to high temperature and physical stress will be avoided.
-
The technique is suitable for both hydrophilic and hydrophobic drugs.
Disadvantages67
-
The method requires dilution of dispersions
-
Technique requires purification process to remove residual organic solvent.
Nanostructured lipid carriers (NLC)
NLC are prepared by using blend of solid lipid with a liquid lipid which remains solid at body temperature.79-81 The main formulation ingredients include lipids, emulsifiers and water. The preparation methods are similar to that of the SLN. SLN and NLC are similar in characteristic and techniques of preparation (Table 11). In case of SLN preparations, solid lipids are used whereas for NLC, liquid lipids or blend of solid lipid with a liquid lipid are used (Figure 9).
Table 11.
Literature examples for development of Nanostructured lipid carrier formulations
|
Technique of preparation
|
Drug used
|
Excipient used
|
Outcome
|
| Hot HPH |
Rifabutin |
Polysorbate 80, coumarin 9, glyceryldistearate (precirol ATO 5), Epikuron 145 V |
Oral antimycobacterial for Crohn’s disease84 |
| Sonication |
Itraconazole |
Precirol ATO 5, polysorbate 80, oleic acid |
For pulmonary aspergillosis85 |
| HPH |
Tretinoin |
Isopropyl myristate, Cetyl alcohol, Tween 80, Isopropyl alcohol, methyl paraben, propyl paraben. |
For acne vulgaris86 |
| Hot HPH |
Minoxidil |
Stearic acid, GMS, tripolyglycerol monostearate, oleic acid, Isopropyl myristate, Ethyl oleate, Tween 80, span 80. |
For treatment of Alopecia87 |
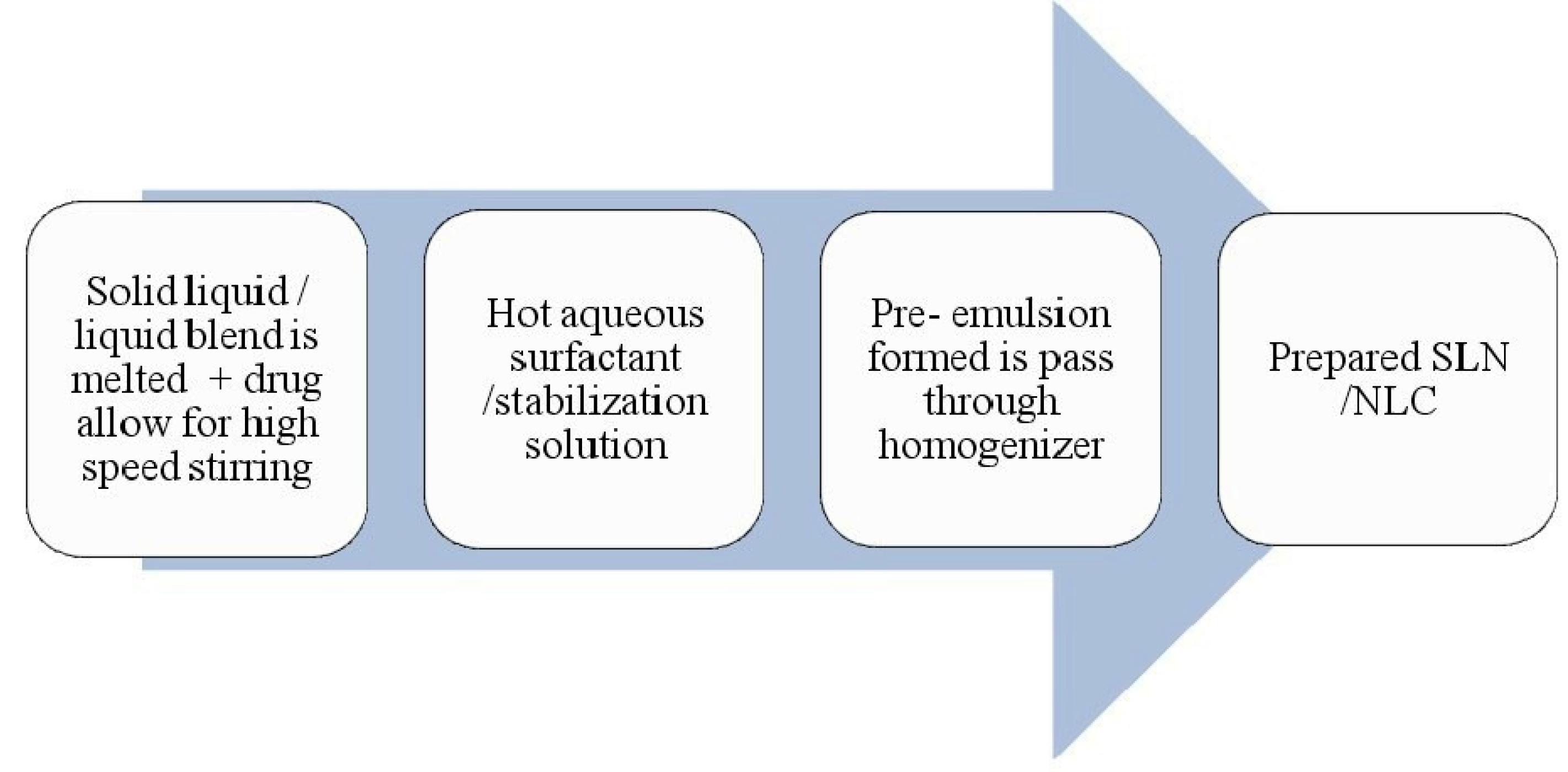
Figure 9.
Process of Microemulsion based method for SLN or NLC
.
Process of Microemulsion based method for SLN or NLC
NLC are of three different types according to their form.82,83
-
Imperfect type
-
Amorphous type
-
Multiple type
a. Imperfect type
Imperfect type of NLC is prepared by different lipids with different structures and it misleads the crystal structure. This misleading can be improved with by changing saturation and number of carbon atoms in lipid. This leads to an increase in the loading capacity for drug.
b. Amorphous type
Amorphous matrix is formed by mixing solid lipids with each other which forms amorphous structure.
c. Multiple type
These are prepared by lipid–solid and solid-water interaction. Multiple type NLC have the advantage of increased drug loading and prolonged release of drugs due to the presence of oil droplets dispersed in solid matrix.
Advantages
-
Increased drug loading capacity as that of SLN.
-
Due to use of liquid mixture, differently structured molecules are formed which makes perfect crystal.
-
Perfectness of NLC system is its imperfectness for crystalline structure because they carry lattice space in between particles
Microemulsion method
These are the transparent system containing two immiscible fluids stabilized by interfacial surfactant or combinations surfactant with cosurfactants film.88 Microemulsions possess ultralow interfacial tension between the immiscible phases which gives thermodynamic solubility, spontaneous formation, simplicity of preparation, solubilize all lipophilic, hydrophilic and amphiphilic solutes, improve solubilisation and bioavailability of hydrophobic drugs and increases permeation (Table 12). Microemulsion method is the oil based two phasic system which contains aqueous phase and oil phase (Figure 10). Diluting microemulsion in a cold aqueous solution result in nanoemulsion then SLN/NLC prepared by lipid precipitation.
Table 12.
Literature examples of lipid based microemulsion formulations
|
Drug /compound
|
Excipients
|
Outcome
|
| Curcumin |
Trilaurine, tristearin, triacetin, myristic acid, GMS, ethyl acetate, benzyl alcohol, polysorbate (20, 40, 80), Pluronic F68, trimyristin |
Use as anticancer88 |
| Anthocyanine |
Palmitic acid, ethanol, isobutanol, Pluronic F127, egg lecithin, Span 85 |
Encapsulation to preserve anthocyanins from degradation89 |
| Artesunate |
GMS, Tween 80, n-butanol, ethanol |
To measure intestinal permeability90 |
| Rifampicin |
Stearic acid, Poloxamer 407, Lipoid s-100 |
Use for infection of brucellosis91 |
| Silymarin |
GMS, Tween 80, stearic acid, cetostearyl alcohol, chloroform, propyl paraben, methyl paraben, methanol, triethanolamine, glycerol |
For photoprotective activity92 |
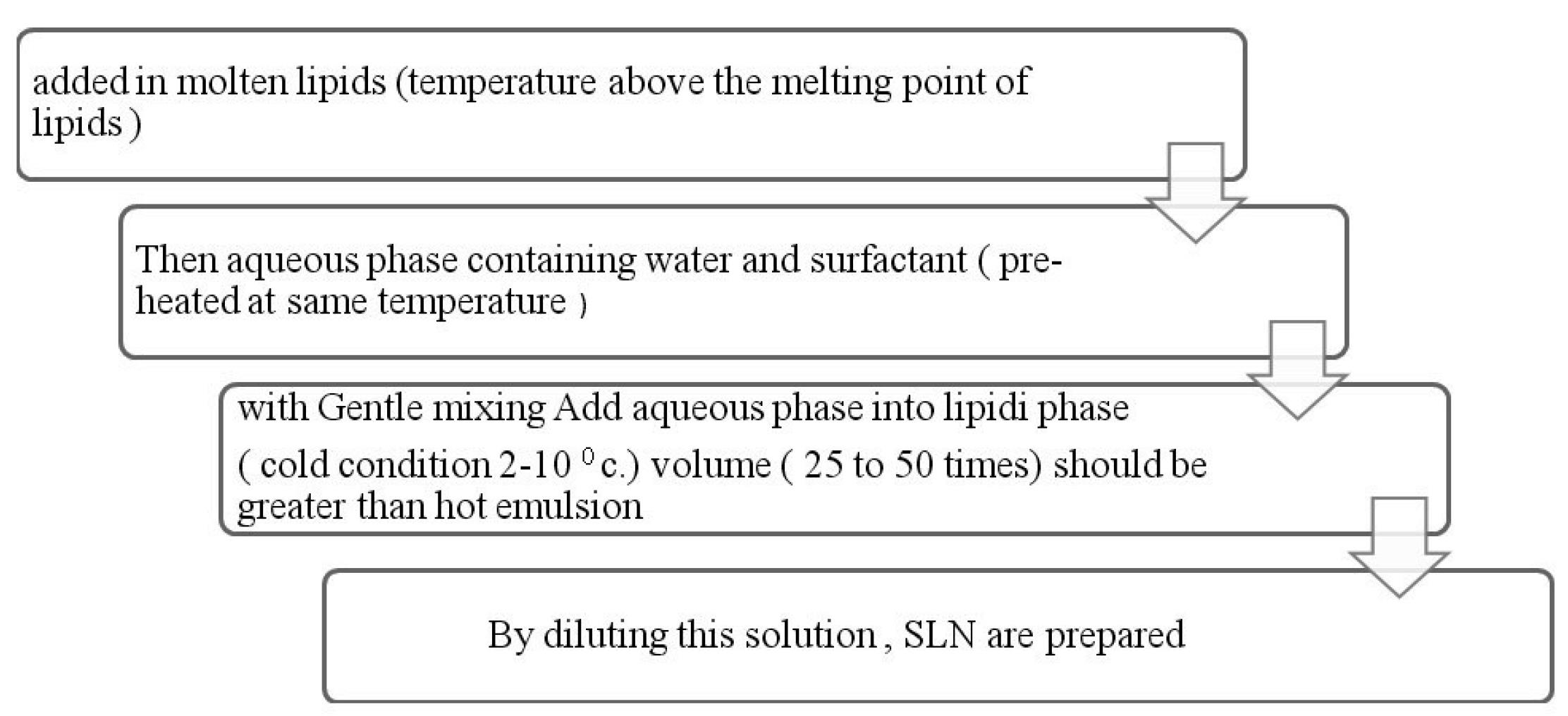
Figure 10.
Process of Microemulsion formulation as SLN or NLCs
.
Process of Microemulsion formulation as SLN or NLCs
Advantages
-
Thermodynamically stable, clear or colorless.
-
large scale manufacturing is possible.
Disadvantages includes requirement of high surfactant concentration.
Hydrogel
Hydrogels are three dimensional structures (formed by chemical or physical cross linking), hydrophilic and polymeric networks (cross linked monomers or chains of co-polymers) with water or biological fluid (Figure 11, Table 13).93 The hydrophilicity of hydrogel is due to the chemical structure of polymer backbone or group such as –OH,-COOH,-CONH,-CONH2,-SO3H and its less solubility is due to covalent bond between polymer chains or hydrophobic force, micellar packing, ionic bonding, crystallizing groups and due to presence of various bonds in the network gels.94,95
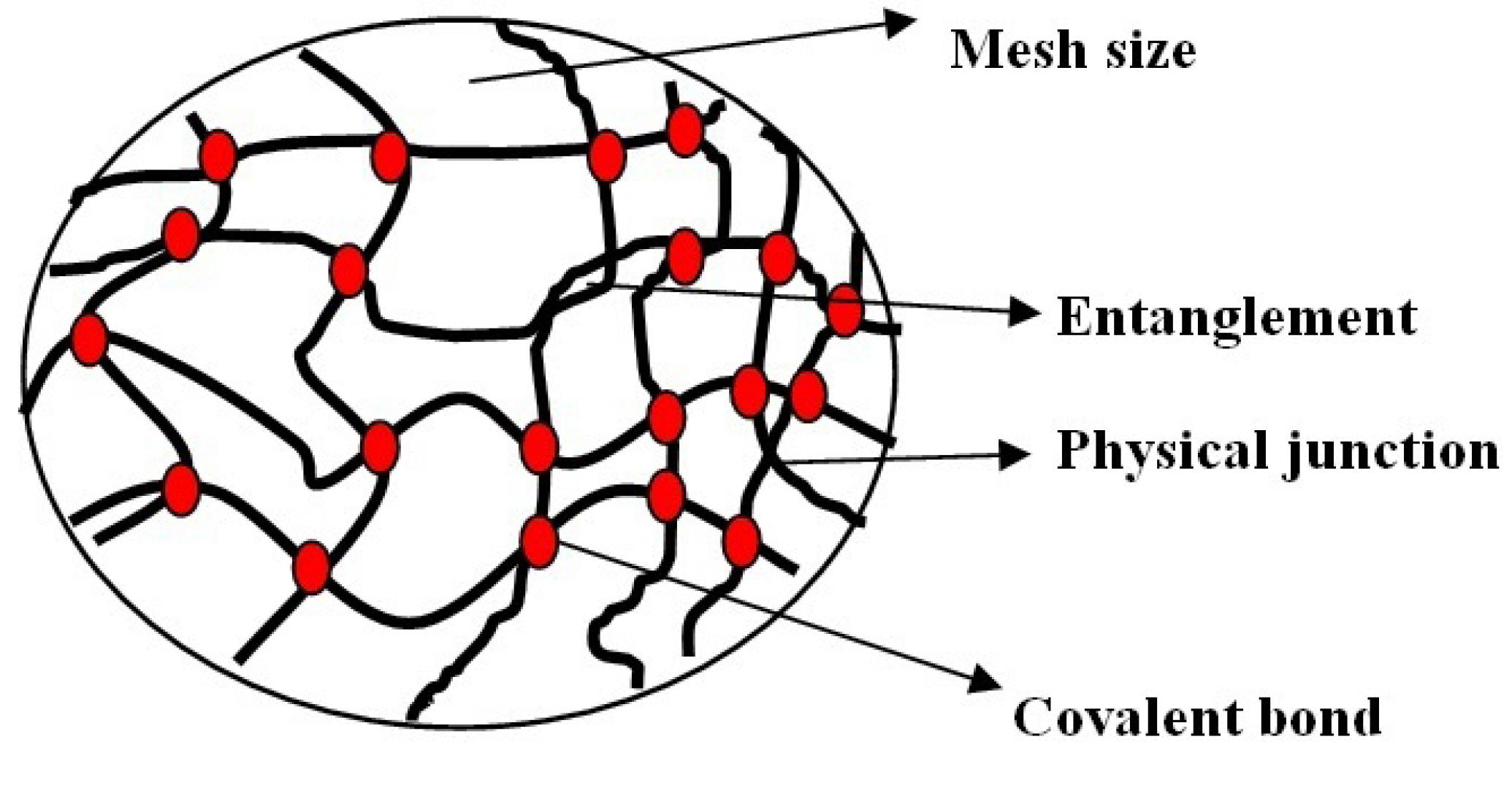
Figure 11.
Structure of hydrogel at molecular level
.
Structure of hydrogel at molecular level
Table 13.
Hydrogel types and mechanism of drug release
|
Type of hydrogel
|
Mechanism of drug release
|
Polymers used
|
Temperature sensitive
Negatively thermosensitive
Positively thermosensitive
Thermoreversible gels. |
Changes in temperature at various site
↓
Changes in the structure of polymer
↓
changes in swelling
↓
drug release |
Poly (N-isopropylacrylamide,
Poly (N,N dimethylacrylamide),
Poly (N-isopropylacrylamide butyl methacrylate)96 |
pH-sensitive
(acidic or basic hydrogels) |
Change in PH of
environment
↓
swelling
↓
drug release |
Polyacrylic acid, polymethacrylic acid, polyethylacrylic acid, polypropylacrylic acid, polyhydroxyethyl methacrylate97 |
| Glucose sensitive hydrogels |
Glucose concentration
increases
↓
swelling of hydrogel
↓
liberate drug |
Hydroxyethyl methacrylate – N,N-dimethylaminoethyl methacrylate, methacrylic acid with polyethylene glycol97 |
| Electric signal sensitive hydrogels |
External electric field
↓
membrane charging (electrophoresis of charged drug)
↓
Swelling and drug release |
Polyelectrolyte such as Polyacrylic acid co-1-vinyl 3butyl imidazole bromide (AAV), Tetraethoxysilane–fluorinated silica nanoparticles98,99 |
| Light sensitive |
UV radiation / visible
light
↓
at fixed temperature swelling of hydrogels
↓
drug release |
a. UV sensitive-triethylene tetramine
b. Visible light sensitive –trisodium salt of copper chlorophyllin to poly (N-isopropyl acrylamide100 |
Hydrogels are classified as (a) Physical hydrogels: By the formation of bonds like ionic, hydrogen or hydrophobic bonds. (b) Chemical hydrogels: Crosslinked networks, cross linking of water soluble polymers. (c) Ionic hydrogels: Polyelectrolyte are combined with multivalent ion of the opposite charge.
Dried hydrogel also called as xero gels are more absorptive than that and called super absorbent.
Nanocochleates
Nanocochleates are cream role like structure which is formed by the lipid bi-layers by interaction of liposomes and cations (Table 14). The sheet of phospholipids carries high tension at their edges which causes nanocochleates binding with tissue membrane (Figure 12).101,102
Table 14.
Literature examples of Phospholipids and cations used for nanocochleates formulation
|
Ingredients
|
Example
|
| Phospholipids |
Phosphotidyl serine (PS) Dioleoyl phosphotidyl serine (DOPS), phosphatidic acid (PA), phosphatidyl ionositol (PI), Phosphatidoyl ethanolamine, Phosphatodoyl Glycerol (PG), Phosphatodyl choline, diolylphosphatidic acid, distearoyl phosphatidylserine, dipalmitoyl phosphatidyl glyceroyl |
| Cations |
Zn+2, Mg+2, Ca+2, Ba+2 |
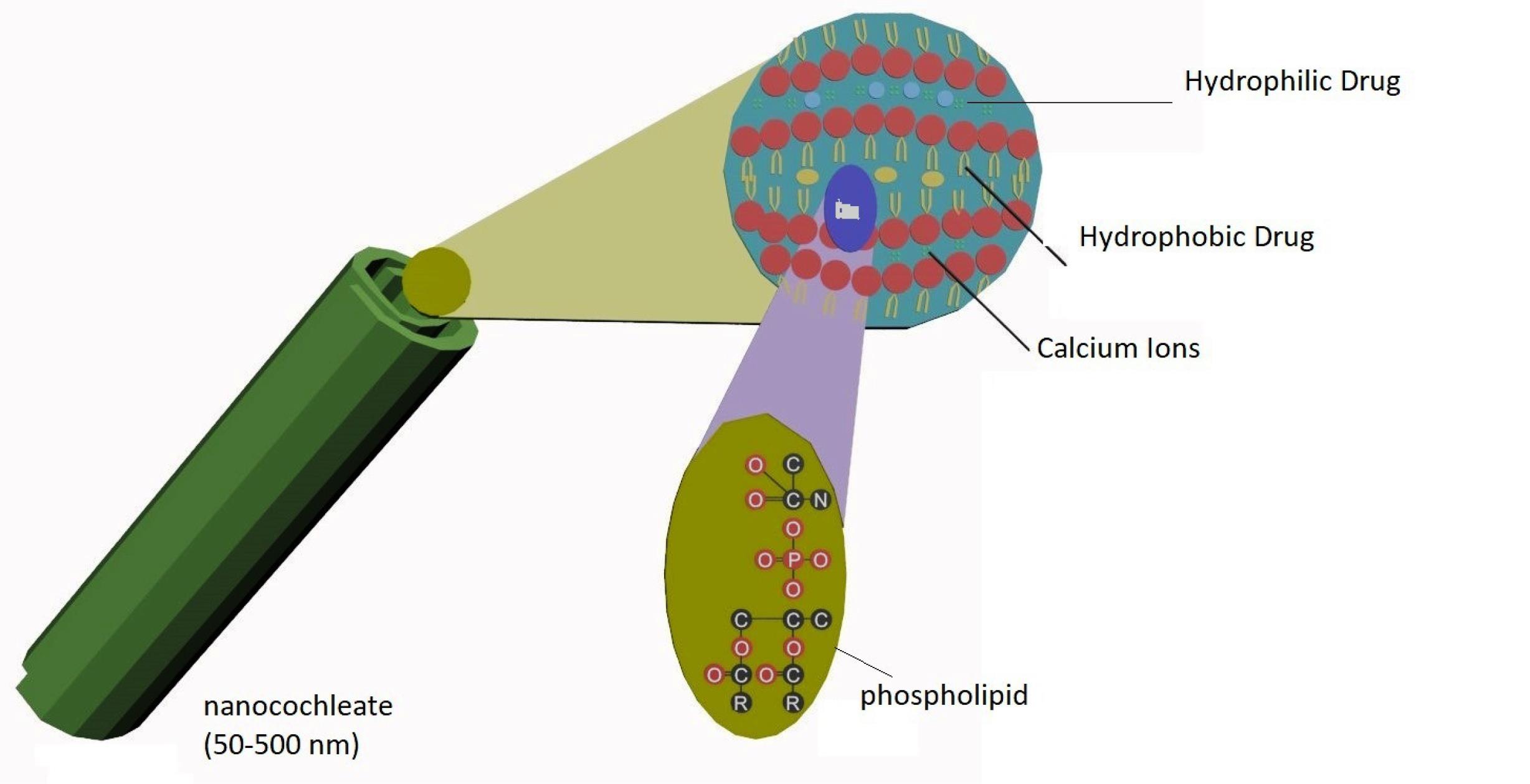
Figure 12.
Structure of nanocochleates
.
Structure of nanocochleates
Conclusion
Novel drug delivery systems had emerged as a promising nanoplatform for efficient drug delivery. Lipid based nanoformulations was found to be beneficial to improve low aqueous solubility/poor solubility of poorly soluble drugs. The lipid based formulations have the advantage of enhancement in bioavailability for the drugs which have extensive drug The various techniques reported till today for formulation and evaluation of dosage forms as liposomes, niosomes, SLNs, nanostructured lipid carriers, nanocholates etc. Novel formulations have advantages in both solubility and permeability enhancement of poorly soluble drugs.
Competing Interests
All authors declare that they have no competing interests.
Ethical Approval
Not applicable.
Funding
None.
References
- Mahomoodally MF, Sadeer N, Edoo M, Venugopala KN. The potential application of novel drug delivery systems for phytopharmaceuticals and natural extracts - current status and future perspectives. Mini Rev Med Chem 2021; 21(18):2731-46. doi: 10.2174/1389557520666200730160911 [Crossref] [ Google Scholar]
- Bandawane A, Saudagar R. A review on novel drug delivery system: a recent trend. J Drug Deliv Ther 2019; 9(3):517-21. doi: 10.22270/jddt.v9i3.2610 [Crossref] [ Google Scholar]
- Rudramurthy GR, Swamy MK, Sinniah UR, Ghasemzadeh A. Nanoparticles: alternatives against drug-resistant pathogenic microbes. Molecules 2016; 21(7):836. doi: 10.3390/molecules21070836 [Crossref] [ Google Scholar]
- Haba Y, Kojima C, Harada A, Ura T, Horinaka H, Kono K. Preparation of poly(ethylene glycol)-modified poly(amido amine) dendrimers encapsulating gold nanoparticles and their heat-generating ability. Langmuir 2007; 23(10):5243-6. doi: 10.1021/la0700826 [Crossref] [ Google Scholar]
- Shi X, Sun K, Baker JR. Spontaneous formation of functionalized dendrimer-stabilized gold nanoparticles. J Phys Chem C Nanomater Interfaces 2009; 112(22):8251-8. doi: 10.1021/jp801293a [Crossref] [ Google Scholar]
- Park SH, Oh SG, Mun JY, Han SS. Loading of gold nanoparticles inside the DPPC bilayers of liposome and their effects on membrane fluidities. Colloids Surf B Biointerfaces 2006; 48(2):112-8. doi: 10.1016/j.colsurfb.2006.01.006 [Crossref] [ Google Scholar]
- Ghadi R, Dand N. BCS class IV drugs: highly notorious candidates for formulation development. J Control Release 2017; 248:71-95. doi: 10.1016/j.jconrel.2017.01.014 [Crossref] [ Google Scholar]
- Emerich DF, Thanos CG. Nanotechnology and medicine. Expert Opin Biol Ther 2003; 3(4):655-63. doi: 10.1517/14712598.3.4.655 [Crossref] [ Google Scholar]
- Desai MP, Labhasetwar V, Amidon GL, Levy RJ. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm Res 1996; 13(12):1838-45. doi: 10.1023/a:1016085108889 [Crossref] [ Google Scholar]
- Vasir JK, Reddy MK, Labhasetwar VD. Nanosystems in drug targeting: opportunities and challenges. Curr Nanosci 2005; 1(1):47-64. doi: 10.2174/1573413052953110 [Crossref] [ Google Scholar]
- Shekhawat PB, Pokharkar VB. Understanding peroral absorption: regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles. Acta Pharm Sin B 2017; 7(3):260-80. doi: 10.1016/j.apsb.2016.09.005 [Crossref] [ Google Scholar]
- Martinez MN, Amidon GL. A mechanistic approach to understanding the factors affecting drug absorption: a review of fundamentals. J Clin Pharmacol 2002; 42(6):620-43. doi: 10.1177/00970002042006005 [Crossref] [ Google Scholar]
- Pavurala N, Achenie LEK. A mechanistic approach for modeling oral drug delivery. Comput Chem Eng 2013; 57:196-206. doi: 10.1016/j.compchemeng.2013.06.002 [Crossref] [ Google Scholar]
- Lennernäs H. Human intestinal permeability. J Pharm Sci 1998; 87(4):403-10. doi: 10.1021/js970332a [Crossref] [ Google Scholar]
- Yu LX, Lipka E, Crison JR, Amidon GL. Transport approaches to the biopharmaceutical design of oral drug delivery systems: prediction of intestinal absorption. Adv Drug Deliv Rev 1996; 19(3):359-76. doi: 10.1016/0169-409x(96)00009-9 [Crossref] [ Google Scholar]
- United States Pharmacopeial Convention. The United States Pharmacopeia: Official from July 1, 1980--20th Revision. Rockville, MD: United States Pharmacopeial Convention; 1979.
- Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 1995; 12(3):413-20. doi: 10.1023/a:1016212804288 [Crossref] [ Google Scholar]
- Dahan A, Miller JM, Amidon GL. Prediction of solubility and permeability class membership: provisional BCS classification of the world’s top oral drugs. AAPS J 2009; 11(4):740-6. doi: 10.1208/s12248-009-9144-x [Crossref] [ Google Scholar]
- Sugano K, Terada K. Rate- and extent-limiting factors of oral drug absorption: theory and applications. J Pharm Sci 2015; 104(9):2777-88. doi: 10.1002/jps.24391 [Crossref] [ Google Scholar]
- Reddy BB, Karunakar A. Biopharmaceutics classification system: a regulatory approach. Dissolution Technol 2011; 18(1):31-7. doi: 10.14227/dt180111p31 [Crossref] [ Google Scholar]
- Tsume Y, Mudie DM, Langguth P, Amidon GE, Amidon GL. The biopharmaceutics classification system: subclasses for in vivo predictive dissolution (IPD) methodology and IVIVC. Eur J Pharm Sci 2014; 57:152-63. doi: 10.1016/j.ejps.2014.01.009 [Crossref] [ Google Scholar]
- Brahmankar DM, Jaiswal SB. Biopharmaceutics and Pharmacokinetics. 2nd ed. Delhi: Vallabh Prakashan; 2009. p. 399-401.
- Cook JA, Bockbrader HN. An industrial implementation of the biopharmaceutics classification system. Dissolution Technol 2002; 9(2):6-9. doi: 10.14227/dt090202p6 [Crossref] [ Google Scholar]
- Cook JA, Davit BM, Polli JE. Impact of biopharmaceutics classification system-based biowaivers. Mol Pharm 2010; 7(5):1539-44. doi: 10.1021/mp1001747 [Crossref] [ Google Scholar]
- Patra JK, Das G, Fraceto LF, Campos EVR, Del Pilar Rodriguez-Torres M, Acosta-Torres LS. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology 2018; 16(1):71. doi: 10.1186/s12951-018-0392-8 [Crossref] [ Google Scholar]
- Singh SP, Patra CN, Swain S, Ansari VA. Liposomes as novel drug delivery vehicle. In: Pharmaceutical Drug Delivery Systems and Vehicles. WPI Publishing; 2018. p. 245-66.
- Daraee H, Etemadi A, Kouhi M, Alimirzalu S, Akbarzadeh A. Application of liposomes in medicine and drug delivery. Artif Cells Nanomed Biotechnol 2016; 44(1):381-91. doi: 10.3109/21691401.2014.953633 [Crossref] [ Google Scholar]
- Balazs DA, Godbey W. Liposomes for use in gene delivery. J Drug Deliv 2011; 2011:326497. doi: 10.1155/2011/326497 [Crossref] [ Google Scholar]
- Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics 2017; 9(2):12. doi: 10.3390/pharmaceutics9020012 [Crossref] [ Google Scholar]
- Sforzi J, Palagi L, Aime S. Liposome-based bioassays. Biology (Basel) 2020; 9(8):202. doi: 10.3390/biology9080202 [Crossref] [ Google Scholar]
- Vlasova KY, Piroyan A, Le-Deygen IM, Vishwasrao HM, Ramsey JD, Klyachko NL. Magnetic liposome design for drug release systems responsive to super-low frequency alternating current magnetic field (AC MF). J Colloid Interface Sci 2019; 552:689-700. doi: 10.1016/j.jcis.2019.05.071 [Crossref] [ Google Scholar]
- de Freitas CF, Calori IR, Tessaro AL, Caetano W, Hioka N. Rapid formation of small unilamellar vesicles (SUV) through low-frequency sonication: an innovative approach. Colloids Surf B Biointerfaces 2019; 181:837-44. doi: 10.1016/j.colsurfb.2019.06.027 [Crossref] [ Google Scholar]
- Inamura K, Komizu Y, Yamakuchi M, Ishida S, Matsumoto Y, Matsushita T. Inhibitory effect of hybrid liposomes on the growth of liver cancer stem cells. Biochem Biophys Res Commun 2019; 509(1):268-74. doi: 10.1016/j.bbrc.2018.12.118 [Crossref] [ Google Scholar]
- Rodriguez EB, Almeda RA, Vidallon MLP, Reyes CT. Enhanced bioactivity and efficient delivery of quercetin through nanoliposomal encapsulation using rice bran phospholipids. J Sci Food Agric 2019; 99(4):1980-9. doi: 10.1002/jsfa.9396 [Crossref] [ Google Scholar]
- Zhang J, Froelich A, Michniak-Kohn B. Topical delivery of meloxicam using liposome and microemulsion formulation approaches. Pharmaceutics 2020; 12(3):282. doi: 10.3390/pharmaceutics12030282 [Crossref] [ Google Scholar]
- Zhang ZJ, Osmałek T, Michniak-Kohn B. Deformable liposomal hydrogel for dermal and transdermal delivery of meloxicam. Int J Nanomedicine 2020; 15:9319-35. doi: 10.2147/ijn.s274954 [Crossref] [ Google Scholar]
- Doskocz J, Dałek P, Przybyło M, Trzebicka B, Foryś A, Kobyliukh A. The elucidation of the molecular mechanism of the extrusion process. Materials (Basel) 2021; 14(15):4278. doi: 10.3390/ma14154278 [Crossref] [ Google Scholar]
- Vakili-Ghartavol R, Rezayat SM, Faridi-Majidi R, Sadri K, Jaafari MR. Optimization of docetaxel loading conditions in liposomes: proposing potential products for metastatic breast carcinoma chemotherapy. Sci Rep 2020; 10(1):5569. doi: 10.1038/s41598-020-62501-1 [Crossref] [ Google Scholar]
- Cai W, Geng C, Jiang L, Sun J, Chen B, Zhou Y. Encapsulation of gemcitabine in RGD-modified nanoliposomes improves breast cancer inhibitory activity. Pharm Dev Technol 2020; 25(5):640-8. doi: 10.1080/10837450.2020.1727920 [Crossref] [ Google Scholar]
- Kanda H, Katsube T, Wahyudiono Wahyudiono, Goto M. Preparation of liposomes from soy lecithin using liquefied dimethyl ether. Foods 2021; 10(8):1789. doi: 10.3390/foods10081789 [Crossref] [ Google Scholar]
- Yao H, Lu H, Zou R, Chen X, Xu H. Preparation of encapsulated resveratrol liposome thermosensitive gel and evaluation of its capability to repair sciatic nerve injury in rats. J Nanomater 2020; 2020:2840162. doi: 10.1155/2020/2840162 [Crossref] [ Google Scholar]
- Wang T, Deng Y, Geng Y, Gao Z, Zou J, Wang Z. Preparation of submicron unilamellar liposomes by freeze-drying double emulsions. Biochim Biophys Acta 2006; 1758(2):222-31. doi: 10.1016/j.bbamem.2006.01.023 [Crossref] [ Google Scholar]
- Schubert R. Liposome preparation by detergent removal. Methods Enzymol 2003; 367:46-70. doi: 10.1016/s0076-6879(03)67005-9 [Crossref] [ Google Scholar]
- Kreuter J. Colloidal Drug Delivery Systems. CRC Press; 2014. p. 203-21.
- Verma AK, Bindal JC. A vital role of niosomes on controlled and novel drug delivery. Indian J Nov Drug Deliv 2011; 3:238-46. [ Google Scholar]
- Zografi G. Interfacial phenomena. In: Gennaro AR. Remington: The Science and Practice of Pharmacy. 19th ed. Easton, Pennsylvania: Mack Publishing Company; 1995. p. 241-51.
- Sahu AK, Mishra J, Mishra AK. Introducing Tween-curcumin niosomes: preparation, characterization and microenvironment study. Soft Matter 2020; 16(7):1779-91. doi: 10.1039/c9sm02416f [Crossref] [ Google Scholar]
- Akbarzadeh I, Shayan M, Bourbour M, Moghtaderi M, Noorbazargan H, Eshrati Yeganeh F. Preparation, optimization and in-vitro evaluation of curcumin-loaded niosome@calcium alginate nanocarrier as a new approach for breast cancer treatment. Biology (Basel) 2021; 10(3):173. doi: 10.3390/biology10030173 [Crossref] [ Google Scholar]
- Moghassemi S, Hadjizadeh A, Hakamivala A, Omidfar K. Growth factor-loaded nano-niosomal gel formulation and characterization. AAPS PharmSciTech 2017; 18(1):34-41. doi: 10.1208/s12249-016-0579-y [Crossref] [ Google Scholar]
- Zhou J, Wu X, Zhao Z, Wang Z, Li S, Chen C. Preparation of a novel ginkgolide B niosomal composite drug. Open Chem 2020; 18(1):1064-74. doi: 10.1515/chem-2020-0089 [Crossref] [ Google Scholar]
- Scioli Montoto S, Muraca G, Ruiz ME. Solid lipid nanoparticles for drug delivery: pharmacological and biopharmaceutical aspects. Front Mol Biosci 2020; 7:587997. doi: 10.3389/fmolb.2020.587997 [Crossref] [ Google Scholar]
- Uchegbu IF. Emulsions and nanosuspensions for the formulation of poorly soluble drugs. Int J Pharm 2001; 212(1):143-4. doi: 10.1016/s0378-5173(00)00604-9 [Crossref] [ Google Scholar]
- Saporito F, Sandri G, Bonferoni MC, Rossi S, Boselli C, Icaro Cornaglia A. Essential oil-loaded lipid nanoparticles for wound healing. Int J Nanomedicine 2018; 13:175-86. doi: 10.2147/ijn.s152529 [Crossref] [ Google Scholar]
- Khatak S, Mehta M, Awasthi R, Paudel KR, Singh SK, Gulati M. Solid lipid nanoparticles containing anti-tubercular drugs attenuate the Mycobacterium marinum infection. Tuberculosis (Edinb) 2020; 125:102008. doi: 10.1016/j.tube.2020.102008 [Crossref] [ Google Scholar]
- Kelidari HR, Momen Bellah-Fard MJ, Morteza-Semnani K, Amoozegar F, Shahriari-Namadi M, Saeedi M. Solid-lipid nanoparticles (SLN)s containing Zataria multiflora essential oil with no-cytotoxicity and potent repellent activity against Anopheles stephensi. J Parasit Dis 2021; 45(1):101-8. doi: 10.1007/s12639-020-01281-x [Crossref] [ Google Scholar]
- Singh M, Schiavone N, Papucci L, Maan P, Kaur J, Singh G. Streptomycin sulphate loaded solid lipid nanoparticles show enhanced uptake in macrophage, lower MIC in Mycobacterium and improved oral bioavailability. Eur J Pharm Biopharm 2021; 160:100-24. doi: 10.1016/j.ejpb.2021.01.009 [Crossref] [ Google Scholar]
- Sandhu SK, Kumar S, Raut J, Singh M, Kaur S, Sharma G. Systematic development and characterization of novel, high drug-loaded, photostable, curcumin solid lipid nanoparticle hydrogel for wound healing. Antioxidants (Basel) 2021; 10(5):725. doi: 10.3390/antiox10050725 [Crossref] [ Google Scholar]
- Sjöström B, Bergenståhl B. Preparation of submicron drug particles in lecithin-stabilized O/W emulsions I Model studies of the precipitation of cholesteryl acetate. Int J Pharm 1992; 88(1-3):53-62. doi: 10.1016/0378-5173(92)90303-J [Crossref] [ Google Scholar]
- Beloqui A, Solinís M, Rodríguez-Gascón A, Almeida AJ, Préat V. Nanostructured lipid carriers: promising drug delivery systems for future clinics. Nanomedicine 2016; 12(1):143-61. doi: 10.1016/j.nano.2015.09.004 [Crossref] [ Google Scholar]
- Li N, Li X, Cheng P, Yang P, Shi P, Kong L. Preparation of curcumin solid lipid nanoparticles loaded with flower-shaped lactose for lung inhalation and preliminary evaluation of cytotoxicity in vitro. Evid Based Complement Alternat Med 2021; 2021:4828169. doi: 10.1155/2021/4828169 [Crossref] [ Google Scholar]
- Hasan N, Imran M, Kesharwani P, Khanna K, Karwasra R, Sharma N. Intranasal delivery of naloxone-loaded solid lipid nanoparticles as a promising simple and non-invasive approach for the management of opioid overdose. Int J Pharm 2021; 599:120428. doi: 10.1016/j.ijpharm.2021.120428 [Crossref] [ Google Scholar]
- Mohammadi P, Mahjub R, Mohammadi M, Derakhshandeh K, Ghaleiha A, Mahboobian MM. Pharmacokinetics and brain distribution studies of perphenazine-loaded solid lipid nanoparticles. Drug Dev Ind Pharm 2021; 47(1):146-52. doi: 10.1080/03639045.2020.1862172 [Crossref] [ Google Scholar]
- Soltani S, Mojiri-Forushani H, Soltani S, Sagha Kahvaz M, Foroutan M. Evaluation of antileishmanial activity employing conventional and solid lipid nanoparticles of amphotericin B on Leishmania major in vitro and in vivo. Infect Disord Drug Targets 2020; 20(6):822-7. doi: 10.2174/1871526519666191015170627 [Crossref] [ Google Scholar]
- Gonçalves LM, Maestrelli F, Di Cesare Mannelli L, Ghelardini C, Almeida AJ, Mura P. Development of solid lipid nanoparticles as carriers for improving oral bioavailability of glibenclamide. Eur J Pharm Biopharm 2016; 102:41-50. doi: 10.1016/j.ejpb.2016.02.012 [Crossref] [ Google Scholar]
- Nooli M, Chella N, Kulhari H, Shastri NR, Sistla R. Solid lipid nanoparticles as vesicles for oral delivery of olmesartan medoxomil: formulation, optimization and in vivo evaluation. Drug Dev Ind Pharm 2017; 43(4):611-7. doi: 10.1080/03639045.2016.1275666 [Crossref] [ Google Scholar]
- Salatin S, Barar J, Barzegar-Jalali M, Adibkia K, Kiafar F, Jelvehgari M. Development of a nanoprecipitation method for the entrapment of a very water soluble drug into Eudragit RL nanoparticles. Res Pharm Sci 2017; 12(1):1-14. doi: 10.4103/1735-5362.199041 [Crossref] [ Google Scholar]
- Kamiya H, Gotoh K, Shimada M, Uchikoshi T, Otani Y, Fuji M, et al. Characteristics and behavior of nanoparticles and its dispersion systems. In: Hosokawa M, Nogi K, Naito M, Yokoyama T, eds. Nanoparticle Technology Handbook. Amsterdam: Elsevier; 2008. p. 113-76. 10.1016/b978-044453122-3.50006-4.
- Patravale VB, Mirani AG. Preparation and characterization of solid lipid nanoparticles-based gel for topical delivery. Methods Mol Biol 2019; 2000:293-302. doi: 10.1007/978-1-4939-9516-5_20 [Crossref] [ Google Scholar]
- Puri D, Mishra A, Singh AP, Gaur PK, Singh M, Yasir M. Formulation development of topical preparation containing nanoparticles of povidone-iodine for wound healing. Assay Drug Dev Technol 2021; 19(2):115-23. doi: 10.1089/adt.2020.1029 [Crossref] [ Google Scholar]
- De Gaetano F, Cristiano MC, Venuti V, Crupi V, Majolino D, Paladini G. Rutin-loaded solid lipid nanoparticles: characterization and in vitro evaluation. Molecules 2021; 26(4):1039. doi: 10.3390/molecules26041039 [Crossref] [ Google Scholar]
- Li D, Hu C, Yang J, Liao Y, Chen Y, Fu SZ. Enhanced anti-cancer effect of folate-conjugated olaparib nanoparticles combined with radiotherapy in cervical carcinoma. Int J Nanomedicine 2020; 15:10045-58. doi: 10.2147/ijn.s272730 [Crossref] [ Google Scholar]
- Ali A. Formulation of Lemongrass Essential Oil Loaded Solid Lipid Nanoparticles, 202111058402. December 2021. Available from: https://iprsearch.ipindia.gov.in/PublicSearch/PublicationSearch/ApplicationStatus.
- Surface Modified Methotrexate Loaded Solid Lipid Nanoparticles to Overcome ABCB1 Polymorphism, 202142054086. 2021. Available from: https://iprsearch.ipindia.gov.in/PublicSearch/PublicationSearch/ApplicationStatus.
- DIT University. Baicalein Loaded Solid-Lipid Nanoparicles and Method of Preparation of Thereof, 202111052175. December 3, 2021. Available from: https://iprsearch.ipindia.gov.in/PublicSearch/PublicationSearch/ApplicationStatus.
- Reddy Kudamala R, Reddy R, Shaik CB, Medarametla KB, Anna B, Challa MC, et al. Clobetasol Loaded Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Topical Treatment of Psoriasis, 202141046636. November 5, 2021. Available from: https://iprsearch.ipindia.gov.in/PublicSearch/PublicationSearch/ApplicationStatus.
- Mohanty PK, Sachin Raosaheb H, Rai JP. Solid Lipid Nanoparticles (SLN) of Sertraline Hydrochloride, 202121046360. October 29, 2021. Available from: https://iprsearch.ipindia.gov.in/PublicSearch/PublicationSearch/ApplicationStatus.
- Development of Nanoparticle Formulation of Aceclofenac, 202121039670. 2021. Available from: https://iprsearch.ipindia.gov.in/PublicSearch/PublicationSearch/ApplicationStatus.
- Goel R, Bhardwaj S, Singh M, Barman M, Mishra R, Sawhey SK. A Novel Composition and Process for Fabrication of Solid Lipid Nanoparticles, 202111036625. January 12, 2022. Available from: https://iprsearch.ipindia.gov.in/PublicSearch/PublicationSearch/ApplicationStatus.
- Dhiman N, Awasthi R, Sharma B, Kharkwal H, Kulkarni GT. Lipid nanoparticles as carriers for bioactive delivery. Front Chem 2021; 9:580118. doi: 10.3389/fchem.2021.580118 [Crossref] [ Google Scholar]
- Bhatt S, Sharma JB, Kamboj R, Kumar M, Saini V, Mandge S. Design and optimization of febuxostat-loaded nano lipid carriers using full factorial design. Turk J Pharm Sci 2021; 18(1):61-7. doi: 10.4274/tjps.galenos.2019.32656 [Crossref] [ Google Scholar]
- Chaudhari VS, Murty US, Banerjee S. Nanostructured lipid carriers as a strategy for encapsulation of active plant constituents: formulation and in vitro physicochemical characterizations. Chem Phys Lipids 2021; 235:105037. doi: 10.1016/j.chemphyslip.2020.105037 [Crossref] [ Google Scholar]
- Khosa A, Reddi S, Saha RN. Nanostructured lipid carriers for site-specific drug delivery. Biomed Pharmacother 2018; 103:598-613. doi: 10.1016/j.biopha.2018.04.055 [Crossref] [ Google Scholar]
- Müller RH, Radtke M, Wissing SA. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm 2002; 242(1-2):121-8. doi: 10.1016/s0378-5173(02)00180-1 [Crossref] [ Google Scholar]
- Rouco H, Diaz-Rodriguez P, Gaspar DP, Gonçalves LMD, Cuerva M, Remuñán-López C. Rifabutin-loaded nanostructured lipid carriers as a tool in oral anti-mycobacterial treatment of Crohn’s disease. Nanomaterials (Basel) 2020; 10(11):2138. doi: 10.3390/nano10112138 [Crossref] [ Google Scholar]
- Shadambikar G, Marathe S, Ji N, Almutairi M, Bandari S, Zhang F. Formulation development of itraconazole PEGylated nano-lipid carriers for pulmonary aspergillosis using hot-melt extrusion technology. Int J Pharm X 2021; 3:100074. doi: 10.1016/j.ijpx.2021.100074 [Crossref] [ Google Scholar]
- Samadi A, Sartipi Z, Ahmad Nasrollahi S, Sheikholeslami B, Nassiri Kashani M, Rouini MR. Efficacy assessments of tretinoin-loaded nano lipid carriers in acne vulgaris: a double blind, split-face randomized clinical study. Arch Dermatol Res 2022; 314(6):553-61. doi: 10.1007/s00403-021-02256-5 [Crossref] [ Google Scholar]
- Wang W, Chen L, Huang X, Shao A. Preparation and characterization of minoxidil loaded nanostructured lipid carriers. AAPS PharmSciTech 2017; 18(2):509-16. doi: 10.1208/s12249-016-0519-x [Crossref] [ Google Scholar]
- Chirio D, Peira E, Dianzani C, Muntoni E, Gigliotti CL, Ferrara B. Development of solid lipid nanoparticles by cold dilution of microemulsions: curcumin loading, preliminary in vitro studies, and biodistribution. Nanomaterials (Basel) 2019; 9(2):230. doi: 10.3390/nano9020230 [Crossref] [ Google Scholar]
- Ravanfar R, Tamaddon AM, Niakousari M, Moein MR. Preservation of anthocyanins in solid lipid nanoparticles: optimization of a microemulsion dilution method using the Placket-Burman and Box-Behnken designs. Food Chem 2016; 199:573-80. doi: 10.1016/j.foodchem.2015.12.061 [Crossref] [ Google Scholar]
- Masiiwa WL, Gadaga LL. Intestinal permeability of artesunate-loaded solid lipid nanoparticles using the everted gut method. J Drug Deliv 2018; 2018:3021738. doi: 10.1155/2018/3021738 [Crossref] [ Google Scholar]
- Ghaderkhani J, Yousefimashouf R, Arabestani M, Roshanaei G, Soleimani Asl S, Abbasalipourkabir R. Improved antibacterial function of Rifampicin-loaded solid lipid nanoparticles on Brucella abortus. Artif Cells Nanomed Biotechnol 2019; 47(1):1181-93. doi: 10.1080/21691401.2019.1593858 [Crossref] [ Google Scholar]
- Netto MG, Jose J. Development, characterization, and evaluation of sunscreen cream containing solid lipid nanoparticles of silymarin. J Cosmet Dermatol 2018; 17(6):1073-83. doi: 10.1111/jocd.12470 [Crossref] [ Google Scholar]
- Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J Adv Res 2015; 6(2):105-21. doi: 10.1016/j.jare.2013.07.006 [Crossref] [ Google Scholar]
- Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev 2008; 60(15):1638-49. doi: 10.1016/j.addr.2008.08.002 [Crossref] [ Google Scholar]
- Kashyap N, Kumar N, Kumar MN. Hydrogels for pharmaceutical and biomedical applications. Crit Rev Ther Drug Carrier Syst 2005; 22(2):107-49. doi: 10.1615/critrevtherdrugcarriersyst.v22.i2.10 [Crossref] [ Google Scholar]
- Seo JW, Shin SR, Lee MY, Cha JM, Min KH, Lee SC. Injectable hydrogel derived from chitosan with tunable mechanical properties via hybrid-crosslinking system. Carbohydr Polym 2021; 251:117036. doi: 10.1016/j.carbpol.2020.117036 [Crossref] [ Google Scholar]
- Chen T, Liu H, Dong C, An Y, Liu J, Li J. Synthesis and characterization of temperature/pH dual sensitive hemicellulose-based hydrogels from eucalyptus APMP waste liquor. Carbohydr Polym 2020; 247:116717. doi: 10.1016/j.carbpol.2020.116717 [Crossref] [ Google Scholar]
- Kim GJ, Kim KO. Novel glucose-responsive of the transparent nanofiber hydrogel patches as a wearable biosensor via electrospinning. Sci Rep 2020; 10(1):18858. doi: 10.1038/s41598-020-75906-9 [Crossref] [ Google Scholar]
- Zhou Y, Fei X, Tian J, Xu L, Li Y. A ionic liquid enhanced conductive hydrogel for strain sensing applications. J Colloid Interface Sci 2022; 606(Pt 1):192-203. doi: 10.1016/j.jcis.2021.07.158 [Crossref] [ Google Scholar]
- Ryplida B, In I, Park SY. Tunable pressure sensor of F-carbon dot-based conductive hydrogel with electrical, mechanical, and shape recovery for monitoring human motion. ACS Appl Mater Interfaces 2020; 12(46):51766-75. doi: 10.1021/acsami.0c16745 [Crossref] [ Google Scholar]
- Panja A, Bairi P, Halder D, Das S, Nandi AK. A robust stimuli responsive Eu3+ - metalo organic hydrogel and xerogel emitting white light. J Colloid Interface Sci 2020; 579:531-40. doi: 10.1016/j.jcis.2020.06.078 [Crossref] [ Google Scholar]
- Tilawat M, Bonde S. Nanocochleates: a potential drug delivery system. J Mol Liq 2021; 334:116115. doi: 10.1016/j.molliq.2021.116115 [Crossref] [ Google Scholar]