Advanced pharmaceutical bulletin. 13(4):792-798.
doi: 10.34172/apb.2023.073
Original Article
Modulatory Effect of Vitamin C on Hypoxia Induced Breast Cancer Stem Cells
Masoumeh Kazemi Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft, 1, 2 
Soheila Montazersaheb Data curation, Methodology, Resources, Software, Validation, Writing – original draft, 3 
Mina Noroozpour Formal analysis, Resources, Visualization, Writing – review & editing, 4 
Safar Farajnia Funding acquisition, 1 
Hojjatollah Nozad Charoudeh Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing, 1, * 
Author information:
1Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
2Department of Medical Genetics, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3Molecular Medicine Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
4Faculty of Materials Science and Engineering, Sahand University of Technology, Tabriz, Iran.
Abstract
Purpose:
Eliminating cancer stem cells (CSCs) is a challenge because of their enhanced resistance to anti-cancer drugs. Vitamin C, which is insufficient in patients with higher stages of cancer, has been gaining attention as a potential treatment for human malignancies. Hence this study aimed to analyze the effect of high-dose vitamin C treatment on the gene expression level of HIF-1α, NF-κB1, BAX, and DNMT1 in the MCF7 cells undergoing hypoxia, as an inducer of CSCs characteristics. As a result, vitamin C could be possibly used as a promising therapeutic adjuvant.
Methods:
Here we first analyzed the breast CSC population alteration in MCF7 cells following hypoxia induction. Then, we evaluated the impact of vitamin C treatment on the gene expression level of four stemness-related genes in hypoxic MCF7 cells.
Results:
Our results indicate that vitamin C could reduce proliferation and stemness states in CSCs possibly by induction of apoptotic markers such as BAX, along with attenuating stemness markers, including NF-κB1, and DNMT1 gene expressions.
Conclusion:
According to our findings, vitamin C administration would become a new approach to avoiding the stimulation of CSCs during cancer therapies.
Keywords: Vitamin C, Hypoxia, MCF7, Cancer Stem Cell
Copyright and License Information
©2023 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Cancer stem cells (CSCs) are a smaller group of tumor cells that are responsible for cancer development, progress, recurrence, and metastasis. This can be attributed to several features, including self-renewal, unlimited proliferation potency, and invasion and migration potency.1,2 CSCs are relatively resistant to conventional chemotherapy, thereby there is a need to develop novel approaches to eradicate CSCs from tumor niche.3 It is possible for cancer to relapse if CSCs are not cleared. Therefore, targeting the CSCs is in demand for developing a successful cancer therapeutic regimen.
Based on clinical findings, one of the indications related to poor prognosis and metastasis is tumor hypoxia,4 which can promote resistance to chemo and radiotherapeutic agents.5,6 Moreover, recent researches indicate that in many human cancers, hypoxic niche displays a crucial impact in the evolution of CSCs, becoming an imperative focus for studying tumor malignancy.7 In this regard, previous studies provided evidence that hypoxia increases breast cancer stem cells (bCSCs) population in a hypoxia-inducible factor-1 (HIF-1) dependent manner.8-10 In other similar studies, it is proposed that hypoxia provokes bCSCs enrichment by m6A-demethylation of NANOG mRNA via RNA demethylase ALKBH511 or adenosine receptor 2B expression (A2BR) following HIF-1 induction.12
A number of evidence declare the beneficial effect of antioxidants in cancer therapy. Vitamin C is an antioxidant that prevents oxidative damage to cells by inhibiting free radicals production. Consistent with this notion, a high dose of vitamin C has been considered as a therapeutic potential in malignancies.13,14 The anti-cancer activity of vitamin C is possibly mediated by redox mechanism, co-factor activity,15 and apoptosis induction.16 Furthermore, it has been revealed that a high dose of vitamin C triggers DNA damage in CSCs and upregulates epigenetic demethylases that eventually reverse CSCs phenotypes.13,15,17
This research is thus compiled to analyze the impact of the higher doses of vitamin C on the expression level of HIF-1α, NF-κB1, BAX, and DNMT1 genes in MCF7 cells undergoing hypoxia, as an inducer of CSCs characteristics; investigating the probability that high dosages of vitamin C can inhibit tumor recurrence by eradicating CSCs.
Materials and Methods
Cell culture
MCF7 cell line (purchased from Pasteur Institute; NCBI code: C135) was cultured in RPMI1640 medium (Sigma), supplemented with 10% Fetal Bovine Serum (Gibco), and 1% Pen-Strep solution. Cultured cells were kept in a humidified incubator that provided 95% O2 and 5% CO2 at 37 °C. Cells were regularly passaged every three days and all assays were carried out when cells were sub-confluent. To perform hypoxic exposure, hypoxia was imitated using a humidified gas mixture of 94% N2, 5% CO2, and 1% O2 at 37 °C.
Vitamin C preparation
A stock solution containing 0.2 M (0.035 g/mL) of vitamin C (Sigma) was prepared by dissolving it in dimethyl sulfoxide (DMSO, Merck). Then a different concentration of the working solution was prepared by dilution of the stock solution with RPMI-1640 immediately before use.
Cell viability assay
To study how vitamin C affects cell viability, an MTT assay was conducted.18 In brief, 1 × 104 cells/well were seeded in a 96-well plate and cultured for 24 hours in 10% FBS RPMI1640 medium. After 24 hours, variable concentrations of vitamin C (2.5-50 mM) were added to each well and cell plates were incubated in the same medium containing 2% FBS for 24 hours, 48 hours, and 72 hours. Following this, 20 mL of MTT solution (5 mg/mL) was loaded into each well for 4 hours (Sigma). After removing the media containing MTT, 200 μL of DMSO solution was added to solubilize the formazan crystals. The absorbance rate was then determined at 570 nm with a microplate reader (BioTek). The experiments were done in triplicate and the relative viability was calculated relative to the control cells (percentage of control).
Magnetic-activated cell sorting assay
To verify whether hypoxic conditions increase the number of CSCs, we isolated and enriched CD44+ and CD24- cells from hypoxic MCF7 cells using magnetic-activated cell sorting (MACS). After dissociating adherent cells with 0.25% trypsin-EDTA (Gibco), the cells were washed and resuspended in PBS. Next, 1 × 107 cells were incubated with 20 μL anti-CD44 MACS microbeads at 4 °C for 15 minutes. After resuspending the cells in PBS and Miltenyi buffer (500 μL), the cells passed through LS positive selection column in the presence of a magnetic field, so that the CD44+ cells remained in the column. Then CD44+ cells were obtained after the magnetic separator was removed from the column and the cell culture was washed with Miltenyi buffer. A subsequent experiment involved resuspending 1 × 107 CD44+ cells in 40 μL of buffer and adding 10 μL of monoclonal CD24 antibody conjugated to biotin and incubating at 4 °C for 15 minutes. Following washing and centrifugation, the cells were resuspended in Miltenyi buffer and 20 μL Anti biotin-CD24 microbeads for 15 minutes at 4 °C. Following the washing of cells in PBS and resuspending in Miltenyi buffer, CD24- cells were passed through the column and collected. Obtained cells were CD44+/CD24- cells.
Flow cytometric analysis
Briefly, three cultured groups of MCF7 cells (normoxic, hypoxic, and vitamin C-treated hypoxic cells) were detached and washed two times with PBS. The cells (106 in 1% bovine serum albumin in PBS) were incubated with 10 μL of FITC-CD24 and PE-CD44 antibodies (Miltenyi Biotec) at 1/100 dilution at 4 °C for 30 minutes in the dark. The CSCs populations were then isolated based on the CD44+/CD24- markers, applying FACSCalibur flow cytometer (BD Bioscience). The results were evaluated using FlowJo Software. Nonspecific results were discovered by proper isotype-matched antibodies.19,20
RNA isolation, reverse–transcription, and real-time PCR
In brief, 2 × 106 cells were treated and collected from each group to test the impacts of vitamin C administration on the expression of cancer stemness-associated genes. Using an RNA extraction kit (Yekta Tajhiz Azma, Iran), RNA was extracted and reverse transcripted (0.5 μg RNA) to cDNA. The real-time PCR was conducted by applying QuantiTect SYRB Green dye (TakaRa) and a Corbett Rotor-GeneTM 6000 HRM system. The target genes were normalized to the reference gene GAPDH, and the data were presented as the relative fold difference between the cDNA of the study and the calibrator samples using the ΔΔCT method. All experiments were carried out in triplicate. Real-time PCR primers were specifically designed to span an exon-exon junction or be separated by at least one intron on the corresponding genomic DNA to only amplify the mRNA sequences (Table 1). In addition, to verify primer amplification, PCR products were visualized on 2% agarose gel (Cinnagen).21
Table 1.
Sequences of primers used for real-time PCR analysis
|
Genes
|
Primer sequences
|
Product size (bp)
|
|
GAPDH
|
F: 5'-TTGACCTCAACTACATGGTTTACA-3'
R: 5'-GCTCCTGGAAGATGGTGATG-3' |
126 |
|
HIF-1 α
|
F: 5'-TAGCCGAGGAAGAACTATGAAC-3'
R: 5'-ACTGAGGTTGGTTACTGTTGG-3' |
101 |
|
NF- κ B1
|
F: 5'-CAATCATCCACCTTCATTCTCAAC-3'
R: 5'-CCACCACATCTTCCTGCTTAG-3' |
147 |
|
BAX
|
F: 5'-TCAGGATGCGTCCACCAAGAAG-3'
R: 5'-TGTGTCCACGGCGGCAATCATC-3' |
103 |
|
DNMT1
|
F: 5'-GCGGCTCAAAGATTTGGAAAGA-3'
R: 5'-CAGGTAGCCCTCCTCGGAT-3' |
160 |
Statistical analyses
The data of independent tests were shown as mean ± standard deviation (SD). To find out whether the results are significant, data analysis was performed by ANOVA and Tukey’s post hoc test in GraphPad Prism version 7.0 (GraphPad Software Inc.). P value < 0.05 was considered statistically significant.
Results and Discussion
Effect of vitamin C on the viability of MCF7s
MCF7 cells were incubated with different concentrations of vitamin C ranging from 2.5 to 50 mM. The cells exhibited different growth rates in various concentrations of vitamin C (Figure 1). As shown in Figure 1, as the concentration of vitamin C increased, the viability of MCF7s decreased. In addition, these results indicated that incubation of MCF7s with concentrations of 25 and 50 mM of vitamin C displayed a promising cytotoxic effect. Accordingly, vitamin C at a concentration of 2.5, 5, and 10 mM was selected for subsequent analyses, for 24 hours.
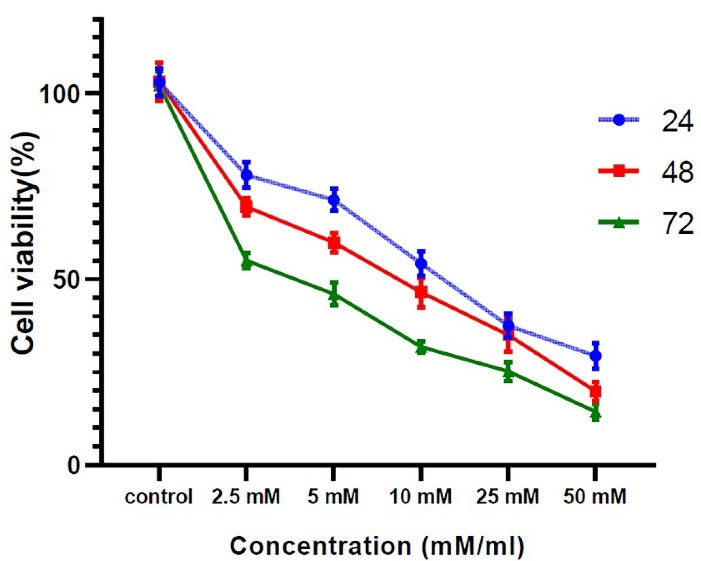
Figure 1.
The effect of vitamin C on MCF7s survival rate. MCF7 cells showed the highest survival rate after being exposed to 2.5 mM vitamin C for 24 hours. Contrary, treatment of cells with the dose of 25 and 50 mM leads to cell viability below 50 percent. So, three concentrations of 2.5, 5, and 10 mM were used for the following processes. All experiments were performed in triplicate and data were expressed as mean ± SD
.
The effect of vitamin C on MCF7s survival rate. MCF7 cells showed the highest survival rate after being exposed to 2.5 mM vitamin C for 24 hours. Contrary, treatment of cells with the dose of 25 and 50 mM leads to cell viability below 50 percent. So, three concentrations of 2.5, 5, and 10 mM were used for the following processes. All experiments were performed in triplicate and data were expressed as mean ± SD
Effect of hypoxia on breast cancer stem cell population in MCF7 cells
To induce CSCs enrichment in MCF7 cell culture, the cells were kept in a hypoxic condition. Characterization of CSCs in MCF7 cells was verified according to the expression of both CD24 and CD44 markers. Immunofluorescence staining on MACS-isolated cells from normoxic and hypoxic MCF7 cells revealed that the percentage of the positive cells for the CD44 marker and negative cells for the CD24 marker was increased in cells cultured under the hypoxic condition when compared to MCF7 cells without hypoxia pre-treatment (32.9% versus 18.6%) (data are not shown).
Effect of vitamin C on breast cancer stem cells during hypoxia induction
Flow cytometric data revealed that vitamin C could reduce the number of bCSCs (Figure 2). Compared with untreated hypoxic cells, a significant reduction was found in the level of CD24-/CD44+ cells in the group receiving vitamin C (Figure 3). These results imply that vitamin C can potentially reduce the rate of bCSCs in human MCF7s during hypoxia induction.
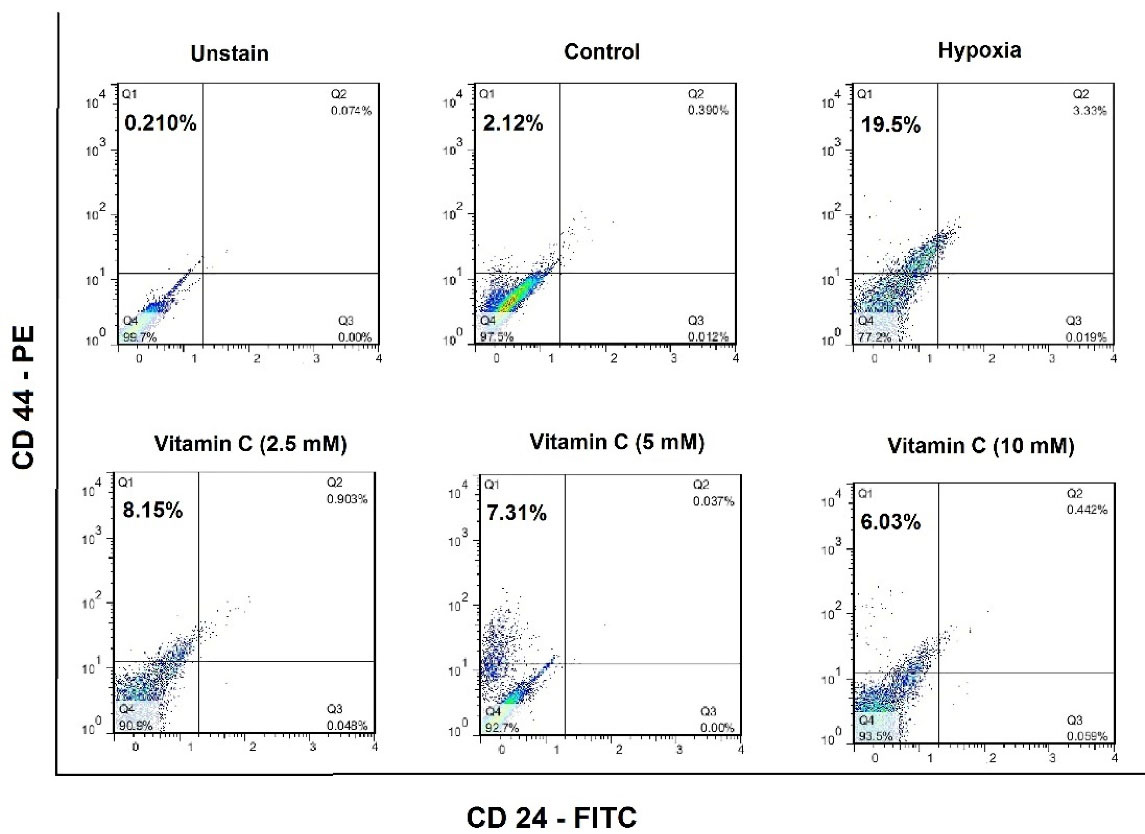
Figure 2.
Cancer stem cells (CSCs) population in the normoxic, hypoxic, and vitamin C-treated MCF7 cell line. MCF7 cells were labeled with FITC-conjugated anti-CD24 and PE-conjugated anti-CD44 antibodies, and then the cell populations for each group of control, hypoxia and Vitamin C-treated ones, were characterized according to the surface markers using a FACSCalibur flow cytometer (BD Bioscience, USA). The data were collected and analyzed using FlowJo Software.
.
Cancer stem cells (CSCs) population in the normoxic, hypoxic, and vitamin C-treated MCF7 cell line. MCF7 cells were labeled with FITC-conjugated anti-CD24 and PE-conjugated anti-CD44 antibodies, and then the cell populations for each group of control, hypoxia and Vitamin C-treated ones, were characterized according to the surface markers using a FACSCalibur flow cytometer (BD Bioscience, USA). The data were collected and analyzed using FlowJo Software.
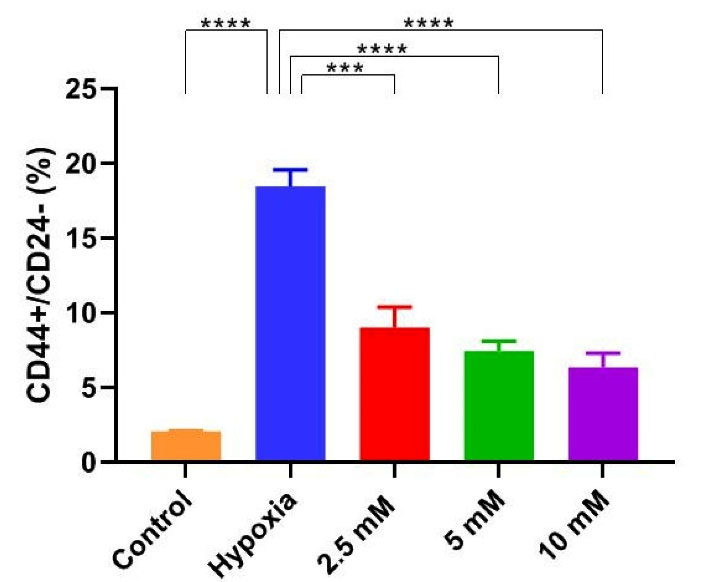
Figure 3.
Vitamin C reduced the breast Cancer stem cells (bCSCs) population during hypoxia induction. CD24-/CD44+ cells as an indicator of bCSCs were stained using FITC-conjugated anti-CD24 and PE-conjugated anti-CD44 antibodies, and analyzed by Flow cytometry in hypoxic MCF7 cells, that were
incubated for 24 hours in medium containing three concentrations of 2.5, 5 and 10 mM vitamin C. Data are expressed as mean ± SD of three independent experiments. Statistically significant differences are indicated as *** P < 0.0002 and ****P < 0.0001.
.
Vitamin C reduced the breast Cancer stem cells (bCSCs) population during hypoxia induction. CD24-/CD44+ cells as an indicator of bCSCs were stained using FITC-conjugated anti-CD24 and PE-conjugated anti-CD44 antibodies, and analyzed by Flow cytometry in hypoxic MCF7 cells, that were
incubated for 24 hours in medium containing three concentrations of 2.5, 5 and 10 mM vitamin C. Data are expressed as mean ± SD of three independent experiments. Statistically significant differences are indicated as *** P < 0.0002 and ****P < 0.0001.
Effect of vitamin C on the gene expression level of NF-κB1, BAX, HIF-1α, and DNMT1 genes
In order to assess the impact of vitamin C on gene expression of NF-κB1, BAX, HIF-1α, and DNMT1, real-time RT-PCR was carried out. NF-κB1 mRNA showed an almost 12-fold higher expression level in hypoxia-induced bCSC cells as compared to normoxic MCF7 cells, reaching the lowest level at 24 hours incubation with vitamin C (10 mM) (Figure 4a).
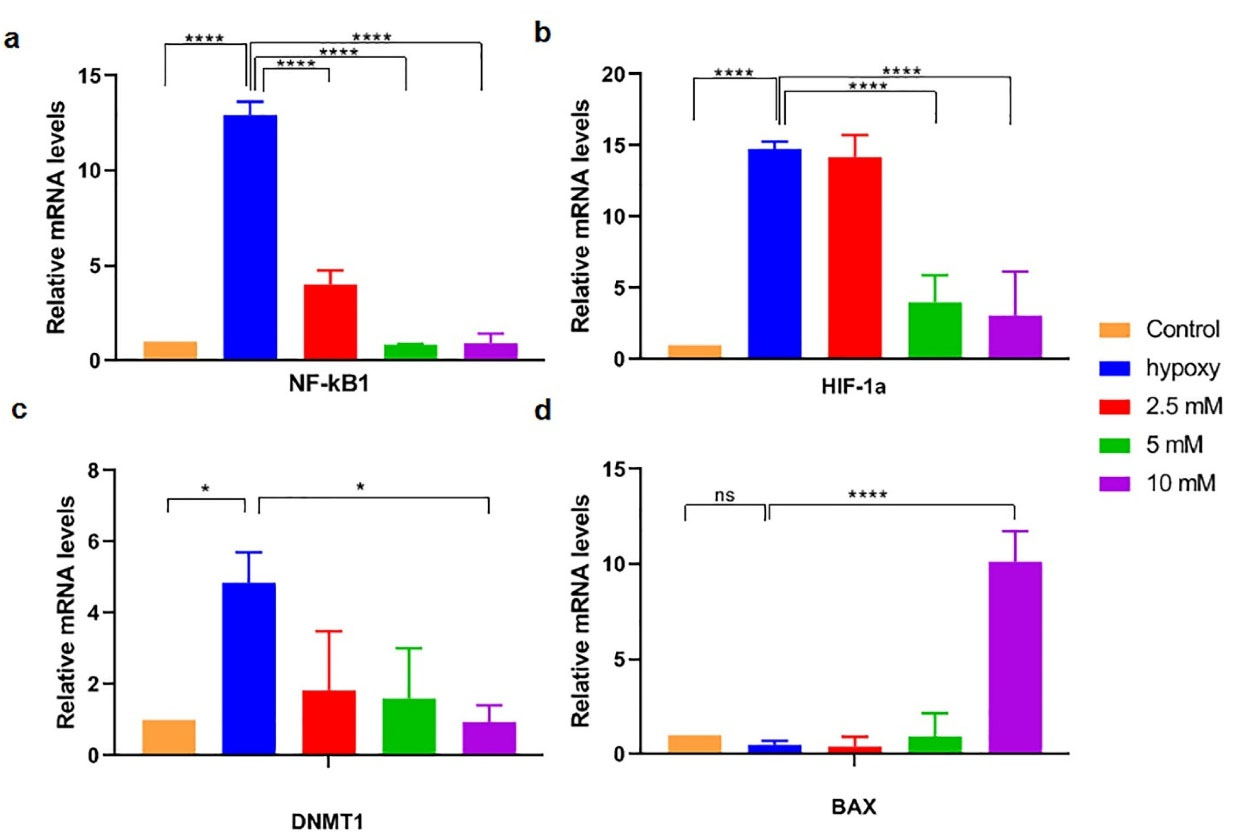
Figure 4.
mRNA expression of NF-κB1 (a), HIF-1α (b), DNMT1 (c), and BAX (d), in hypoxic MCF7 cells was carried out by real-time RT-PCR and compared with that expression in normoxic MCF7cells after incubation of the cells with vitamin C (2.5, 5, and 10 mM) for 24 hours. Results are presented as mean ± SD of three independent experiments. Statistically significant differences are indicated as * P < 0.05 and **** P < 0.00001.
.
mRNA expression of NF-κB1 (a), HIF-1α (b), DNMT1 (c), and BAX (d), in hypoxic MCF7 cells was carried out by real-time RT-PCR and compared with that expression in normoxic MCF7cells after incubation of the cells with vitamin C (2.5, 5, and 10 mM) for 24 hours. Results are presented as mean ± SD of three independent experiments. Statistically significant differences are indicated as * P < 0.05 and **** P < 0.00001.
HIF-1α and DNMT1 mRNA expression levels in hypoxic bCSC were also significantly increased in CSCs than that level in normoxic MCF7 cells (respectively 4.8 and 14.7 fold changes; P < 0.05) and decreased the following incubation with vitamin C (respectively reached to 0.95 and 3 fold changes; P < 0.05) (Figure 4b, c).
Regarding mRNA expression of BAX, vitamin C-treated cells with a dose of 10 mM could significantly increase the gene expression level up to 10-fold, as compared to the control and hypoxic MCF7 cells (Figure 4d).
As evidenced, CSCs have the intrinsic capacity for self-renewal and differentiation. These cells are known to be the origin of most cancer cells and are responsible for tumor resistance and relapse. It has been reported that CSCs possess elevated protection levels against oxidative stress which was induced by reactive oxygen species compared with non-stem-like cancer cells.22,23
Evidence indicates that hypoxia, the major feature of solid tumors, enhances the proportion of CSCs in a HIF-1 dependent way.9,12,24
Vitamin C, a prominent antioxidant, has a binary function in CSCs dynamics by scavenging free radicals and protecting cells from oxidative damage.25,26 Beyond the protective effect, vitamin C has also cytotoxic effects on cancer cells that are mediated by increasing the ROS levels and impeding the homeostasis of energy at high concentrations.27 Contrary to low vitamin C concentrations (5–25 μM), which leads to CSCs proliferation,27 higher concentrations (10 g/d) are conceivably toxic to cancerous cells compared to healthy cells.15,28
In this study, we considered the impact s of vitamins C at three concentrations of 2.5, 5, and 10 mM on the genes expression level of NF-κB1, HIF-1α, BAX, and DNMT1 in the MCF7 cells undergoing hypoxia, as an inducer of CSCs characteristics.
The molecular pathways needed for CSCs preservation are explained in several studies. Among them, NF- κB is the known transcription factor with pivotal effects on cell survival, immunity, and inflammation. New findings show that mammalian NF- κB controls the self-renewal of bCSCs in a Her2-dependent manner.29 Deregulation of NF- κB activity leads to the constant nuclear localization of p65, p52, p50, RelB, and cRel, resulting in the up-regulation of anti-apoptotic factors and the interference with cell proliferation and death balance in the following.30,31 It is also revealed that activation of NF- κB by inflammatory cytokines or epigenetic dysregulation could stimulate NOTCH signaling pathway in CSCs, increasing CSC populations. Therefore, NF- κB activation plays a critical role in regulating of CSCs populations.32 In this way, it is reported that small molecules like parthenolide, pyrrolidine dithiocarbamate, and its analog diethyldithiocarbamate could target bCSCs.29 It is also evident from in vitro studies that curcumin and epigallocatechin gallate diminished the stemness characteristics of the breast cancer cells by adjusting STAT3–NF- κB signaling pathways.33
In our study, the NF-κB1 mRNA content in hypoxic MCF7 was significantly higher than that level in normoxic MCF7 cells which was significantly decreased following incubation with vitamin C for 24 hours. Our finding is in line with the prior research indicating that vitamin C can inhibit the NF- κB activation in a dose-dependent way in addition to inhibited TNFα-induced degradation of IkBcα.34
In some cancers, growing data indicates that hypoxia plays an important function in CSCs expansion,23 and oxygen-dependent transcription activators such as hypoxia-inducible factors (HIFs), are the major mediators of cell response to low oxygen status.35,36 These factors mediate tumor adaption with stressful situations, leading to various gene transcriptions which take part in glycolysis, angiogenesis, metastasis and resistance to radio and chemotherapy.37,38 Enhanced activity of HIF in hypoxic situations is correlated with an enhanced level of antioxidant production which is necessary for maintaining redox homeostasis and promoting the appearance of stem cell properties in breast cancer.37,39 These events lead to poor outcomes in a range of cancers. As a result, now HIFs are thought to be a key goal for cancer treatment. It is worth noting that vitamin C, as a cofactor is required for hydroxylation reactions which can control the activity and stability of HIFs α subunits.40 Accordingly, supplementation of cancer cells with increased vitamin C concentration could promote the hydroxylation and then reduce the activity of the HIFs, thus attenuating tumorigenesis.41,42 In the present research in order to evaluate the effect of vitamin C in the process of the bCSC-like cells, the relative mRNA expression of HIF-1α was analyzed. The finding showed that the expression level of the HIF-1α gene was decreased significantly in hypoxic-treated cells in relation to non-treated hypoxic cells. This finding is inconsistent with earlier studies showing that vitamin C decreased HIF-1α levels in a dose-dependent pattern.40,43
To clarify the impact of high-dose vitamin C in apoptotic events, we evaluated BAX pro-apoptotic gene expression. Our data showed that vitamin C at 10 mM concentration could significantly enhance the expression level of BAX. In this context, it is noteworthy to highlight that Bax activity is counteracted by Bcl-2, an apoptosis-promoting protein. It was demonstrated that produced ROS by a high dose of vitamin C induces programmed cell death in bCSCs. These results imply that a higher concentration of vitamin C, 10 and 20 mM, results in several events in bCSCs as follows: cell damage by enhancing the ROS level, mitochondrial damage by induction of oxidative stress, and intrinsic apoptosis pathway.28
In addition, the role of vitamin C in the epigenetic control of gene expression has received growing notice in many backgrounds, from the normal performance of cells to cancer therapy.26,44,45 It was determined that vitamin C acts as a cofactor for methylcytosine dioxygenases which acts as DNA demethylase and some JmjC domain-containing histone demethylases.46 Here, we examined the effect of vitamin C on the gene expression level of DNMT1. Previous data support a crucial role for DNMT1 in the tumorigenic phenotype of CSCs and show that suppression of DNMT activity reverses the atypical self-renewal characteristics of CSCs.47 Based on our results vitamin C treatment with the dose of 10 mM significantly reduces the DNMT1 gene expression level compared to non-treated hypoxic MCF7 cells.
Overall, our results indicate that vitamin C can reduce proliferation and stemness states in CSCs possibly through the induction of apoptotic markers such as BAX, along with attenuating stemness markers, including NF-κB1 and DNMT1 gene expressions.
Conclusion
To conclude, a high dose of vitamin C could potentially inhibit CSCs, an aspect of the new medical trend of targeting CSCs. Despite the limitation of the study, our findings in concordance with the recent studies28,48 revealed that high doses of vitamin C drive cytotoxicity and gene expression modifications related to the genes that are involved in cancer stemness phenotypes. Therefore, the pharmacological dose of vitamin C (~ > 10 mM) could be possibly applied as a hopeful future therapeutic adjuvant, particularly in higher stages of breast cancer, and needs confirmation pre-clinically.
Acknowledgments
The authors greatly appreciate the Drug Applied Research Center of the Tabriz University of Medical Science for supporting this project. (Grant number: 5/104/672).
Competing Interests
The authors declared no conflicts of interest.
Ethical Approval
Not applicable.
References
- Sottoriva A, Verhoeff JJ, Borovski T, McWeeney SK, Naumov L, Medema JP. Cancer stem cell tumor model reveals invasive morphology and increased phenotypical heterogeneity. Cancer Res 2010; 70(1):46-56. doi: 10.1158/0008-5472.can-09-3663 [Crossref] [ Google Scholar]
- Dalerba P, Clarke MF. Cancer stem cells and tumor metastasis: first steps into uncharted territory. Cell Stem Cell 2007; 1(3):241-2. doi: 10.1016/j.stem.2007.08.012 [Crossref] [ Google Scholar]
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008; 8(10):755-68. doi: 10.1038/nrc2499 [Crossref] [ Google Scholar]
- Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res 2010; 16(24):5928-35. doi: 10.1158/1078-0432.ccr-10-1360 [Crossref] [ Google Scholar]
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011; 11(6):393-410. doi: 10.1038/nrc3064 [Crossref] [ Google Scholar]
- Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 2004; 4(6):437-47. doi: 10.1038/nrc1367 [Crossref] [ Google Scholar]
- Bar EE, Lin A, Mahairaki V, Matsui W, Eberhart CG. Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am J Pathol 2010; 177(3):1491-502. doi: 10.2353/ajpath.2010.091021 [Crossref] [ Google Scholar]
- Xiang L, Gilkes DM, Hu H, Takano N, Luo W, Lu H. Hypoxia-inducible factor 1 mediates TAZ expression and nuclear localization to induce the breast cancer stem cell phenotype. Oncotarget 2014; 5(24):12509-27. doi: 10.18632/oncotarget.2997 [Crossref] [ Google Scholar]
- Brooks DL, Schwab LP, Krutilina R, Parke DN, Sethuraman A, Hoogewijs D. ITGA6 is directly regulated by hypoxia-inducible factors and enriches for cancer stem cell activity and invasion in metastatic breast cancer models. Mol Cancer 2016; 15:26. doi: 10.1186/s12943-016-0510-x [Crossref] [ Google Scholar]
- Semenza GL. Regulation of the breast cancer stem cell phenotype by hypoxia-inducible factors. Clin Sci (Lond) 2015; 129(12):1037-45. doi: 10.1042/cs20150451 [Crossref] [ Google Scholar]
- Zhang C, Samanta D, Lu H, Bullen JW, Zhang H, Chen I. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m⁶A-demethylation of NANOG mRNA. Proc Natl Acad Sci U S A 2016; 113(14):E2047-56. doi: 10.1073/pnas.1602883113 [Crossref] [ Google Scholar]
- Lan J, Lu H, Samanta D, Salman S, Lu Y, Semenza GL. Hypoxia-inducible factor 1-dependent expression of adenosine receptor 2B promotes breast cancer stem cell enrichment. Proc Natl Acad Sci U S A 2018; 115(41):E9640-e8. doi: 10.1073/pnas.1809695115 [Crossref] [ Google Scholar]
- Kim TJ, Byun JS, Kwon HS, Kim DY. Cellular toxicity driven by high-dose vitamin C on normal and cancer stem cells. BiochemBiophys Res Commun 2018; 497(1):347-53. doi: 10.1016/j.bbrc.2018.02.083 [Crossref] [ Google Scholar]
- Xia J, Xu H, Zhang X, Allamargot C, Coleman KL, Nessler R. Multiple myeloma tumor cells are selectively killed by pharmacologically-dosed ascorbic acid. EBioMedicine 2017; 18:41-9. doi: 10.1016/j.ebiom.2017.02.011 [Crossref] [ Google Scholar]
- Vissers MCM, Das AB. Potential mechanisms of action for vitamin C in cancer: reviewing the evidence. Front Physiol 2018; 9:809. doi: 10.3389/fphys.2018.00809 [Crossref] [ Google Scholar]
- Hong SW, Jin DH, Hahm ES, Yim SH, Lim JS, Kim KI. Ascorbate (vitamin C) induces cell death through the apoptosis-inducing factor in human breast cancer cells. Oncol Rep 2007; 18(4):811-5. [ Google Scholar]
- Satheesh NJ, Samuel SM, Büsselberg D. Combination therapy with vitamin C could eradicate cancer stem cells. Biomolecules 2020; 10(1):79. doi: 10.3390/biom10010079 [Crossref] [ Google Scholar]
- Montazersaheb S, Kazemi M, Nabat E, Nielsen PE, Hejazi MS. Downregulation of TdT expression through splicing modulation by antisense peptide nucleic acid (PNA). Curr Pharm Biotechnol 2019; 20(2):168-78. doi: 10.2174/1389201020666190206202650 [Crossref] [ Google Scholar]
- Montazersaheb S, Kabiri F, Saliani N, Nourazarian A, Biray Avcı Ç, Rahbarghazi R. Prolonged incubation with Metformin decreased angiogenic potential in human bone marrow mesenchymal stem cells. Biomed Pharmacother 2018; 108:1328-37. doi: 10.1016/j.biopha.2018.09.135 [Crossref] [ Google Scholar]
- Valipour B, Abedelahi A, Naderali E, Velaei K, Movassaghpour A, Talebi M. Cord blood stem cell derived CD16( + ) NK cells eradicated acute lymphoblastic leukemia cells using with anti-CD47 antibody. Life Sci 2020; 242:117223. doi: 10.1016/j.lfs.2019.117223 [Crossref] [ Google Scholar]
- Montazersaheb S, Biray Avcı Ç, Goker Bagca B, Ozates Ay NP, Tarhriz V, Nielsen PE. Targeting TdT gene expression in Molt-4 cells by PNA-octaarginine conjugates. Int J Biol Macromol 2020; 164:4583-90. doi: 10.1016/j.ijbiomac.2020.09.081 [Crossref] [ Google Scholar]
- Sun X, Lv X, Yan Y, Zhao Y, Ma R, He M. Hypoxia-mediated cancer stem cell resistance and targeted therapy. Biomed Pharmacother 2020; 130:110623. doi: 10.1016/j.biopha.2020.110623 [Crossref] [ Google Scholar]
- Najafi M, Farhood B, Mortezaee K, Kharazinejad E, Majidpoor J, Ahadi R. Hypoxia in solid tumors: a key promoter of cancer stem cell (CSC) resistance. J Cancer Res Clin Oncol 2020; 146(1):19-31. doi: 10.1007/s00432-019-03080-1 [Crossref] [ Google Scholar]
- Xiang L, Semenza GL. Hypoxia-inducible factors promote breast cancer stem cell specification and maintenance in response to hypoxia or cytotoxic chemotherapy. Adv Cancer Res 2019; 141:175-212. doi: 10.1016/bs.acr.2018.11.001 [Crossref] [ Google Scholar]
- Campbell EJ, Vissers MCM, Wohlrab C, Hicks KO, Strother RM, Bozonet SM. Pharmacokinetic and anti-cancer properties of high dose ascorbate in solid tumours of ascorbate-dependent mice. Free Radic Biol Med 2016; 99:451-62. doi: 10.1016/j.freeradbiomed.2016.08.027 [Crossref] [ Google Scholar]
- Mastrangelo D, Pelosi E, Castelli G, Lo-Coco F, Testa U. Mechanisms of anti-cancer effects of ascorbate: cytotoxic activity and epigenetic modulation. Blood Cells Mol Dis 2018; 69:57-64. doi: 10.1016/j.bcmd.2017.09.005 [Crossref] [ Google Scholar]
- Pires AS, Marques CR, Encarnação JC, Abrantes AM, Mamede AC, Laranjo M. Ascorbic acid and colon cancer: an oxidative stimulus to cell death depending on cell profile. Eur J Cell Biol 2016; 95(6-7):208-18. doi: 10.1016/j.ejcb.2016.04.001 [Crossref] [ Google Scholar]
- Sen U, Chaudhury D, Shenoy PS, Bose B. Differential sensitivities of triple-negative breast cancer stem cell towards various doses of vitamin C: an insight into the internal antioxidant systems. J Cell Biochem 2021; 122(3-4):349-66. doi: 10.1002/jcb.29863 [Crossref] [ Google Scholar]
- Shostak K, Chariot A. NF-κB, stem cells and breast cancer: the links get stronger. Breast Cancer Res 2011; 13(4):214. doi: 10.1186/bcr2886 [Crossref] [ Google Scholar]
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol 2002; 3(3):221-7. doi: 10.1038/ni0302-221 [Crossref] [ Google Scholar]
- Lv N, Shan Z, Gao Y, Guan H, Fan C, Wang H. Twist1 regulates the epithelial-mesenchymal transition via the NF-κB pathway in papillary thyroid carcinoma. Endocrine 2016; 51(3):469-77. doi: 10.1007/s12020-015-0714-7 [Crossref] [ Google Scholar]
- Yamamoto M, Taguchi Y, Ito-Kureha T, Semba K, Yamaguchi N, Inoue J. NF-κB non-cell-autonomously regulates cancer stem cell populations in the basal-like breast cancer subtype. Nat Commun 2013; 4:2299. doi: 10.1038/ncomms3299 [Crossref] [ Google Scholar]
- Chung SS, Vadgama JV. Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via down-regulation of STAT3-NFκB signaling. Anticancer Res 2015; 35(1):39-46. [ Google Scholar]
- Son EW, Mo SJ, Rhee DK, Pyo S. Vitamin C blocks TNF-alpha-induced NF-kappaB activation and ICAM-1 expression in human neuroblastoma cells. Arch Pharm Res 2004; 27(10):1073-9. doi: 10.1007/bf02975434 [Crossref] [ Google Scholar]
- Eales KL, Hollinshead KE, Tennant DA. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 2016; 5(1):e190. doi: 10.1038/oncsis.2015.50 [Crossref] [ Google Scholar]
- Samanta D, Semenza GL. Metabolic adaptation of cancer and immune cells mediated by hypoxia-inducible factors. BiochimBiophys Acta Rev Cancer 2018; 1870(1):15-22. doi: 10.1016/j.bbcan.2018.07.002 [Crossref] [ Google Scholar]
- Semenza GL. Hypoxia-inducible factors: coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. EMBO J 2017; 36(3):252-9. doi: 10.15252/embj.201695204 [Crossref] [ Google Scholar]
- Semenza GL. The hypoxic tumor microenvironment: a driving force for breast cancer progression. BiochimBiophys Acta 2016; 1863(3):382-91. doi: 10.1016/j.bbamcr.2015.05.036 [Crossref] [ Google Scholar]
- De Francesco EM, Bonuccelli G, Maggiolini M, Sotgia F, Lisanti MP. Vitamin C and doxycycline: a synthetic lethal combination therapy targeting metabolic flexibility in cancer stem cells (CSCs). Oncotarget 2017; 8(40):67269-86. doi: 10.18632/oncotarget.18428 [Crossref] [ Google Scholar]
- Jóźwiak P, Ciesielski P, Zaczek A, Lipińska A, Pomorski L, Wieczorek M. Expression of hypoxia inducible factor 1α and 2α and its association with vitamin C level in thyroid lesions. J Biomed Sci 2017; 24(1):83. doi: 10.1186/s12929-017-0388-y [Crossref] [ Google Scholar]
- Kuiper C, Vissers MC. Ascorbate as a co-factor for Fe- and 2-oxoglutarate dependent dioxygenases: physiological activity in tumor growth and progression. Front Oncol 2014; 4:359. doi: 10.3389/fonc.2014.00359 [Crossref] [ Google Scholar]
- Wohlrab C, Kuiper C, Vissers MC, Phillips E, Robinson BA, Dachs GU. Ascorbate modulates the hypoxic pathway by increasing intracellular activity of the HIF hydroxylases in renal cell carcinoma cells. Hypoxia (Auckl) 2019; 7:17-31. doi: 10.2147/hp.s201643 [Crossref] [ Google Scholar]
- Knowles HJ, Raval RR, Harris AL, Ratcliffe PJ. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res 2003; 63(8):1764-8. [ Google Scholar]
- Young JI, Züchner S, Wang G. Regulation of the epigenome by vitamin C. Annu Rev Nutr 2015; 35:545-64. doi: 10.1146/annurev-nutr-071714-034228 [Crossref] [ Google Scholar]
- Gillberg L, Ørskov AD, Liu M, Harsløf LBS, Jones PA, Grønbæk K. Vitamin C - a new player in regulation of the cancer epigenome. Semin Cancer Biol 2018; 51:59-67. doi: 10.1016/j.semcancer.2017.11.001 [Crossref] [ Google Scholar]
- Cimmino L, Neel BG, Aifantis I. Vitamin C in stem cell reprogramming and cancer. Trends Cell Biol 2018; 28(9):698-708. doi: 10.1016/j.tcb.2018.04.001 [Crossref] [ Google Scholar]
- Pathania R, Ramachandran S, Elangovan S, Padia R, Yang P, Cinghu S. DNMT1 is essential for mammary and cancer stem cell maintenance and tumorigenesis. Nat Commun 2015; 6:6910. doi: 10.1038/ncomms7910 [Crossref] [ Google Scholar]
- Ghanbari-Movahed M, Shiri Varnamkhasti B, Shourian M. Inhibiting Notch activity in breast cancer stem cells by functionalized gold nanoparticles with gamma-secretase inhibitor DAPT and vitamin C. Chem Zvesti 2022; 76(2):1157-70. doi: 10.1007/s11696-021-01936-w [Crossref] [ Google Scholar]