Advanced pharmaceutical bulletin. 13(3):469-482.
doi: 10.34172/apb.2023.046
Review Article
Virus-Specific T Cells: Promising Adoptive T Cell Therapy Against Infectious Diseases Following Hematopoietic Stem Cell Transplantation
Arsalan Jalili Conceptualization, Writing – original draft, 1, 2 
Abbas Hajifathali Methodology, Writing – original draft, 3
Mozhdeh Mohammadian Methodology, Writing – original draft, Writing – review & editing, 3, 4
Ghazaleh Sankanian Validation, Writing – original draft, 3
Maryam Sayahinouri Validation, Writing – original draft, 5, 6
Mahmoud Dehghani Ghorbi Visualization, Writing – original draft, 7
Elham Roshandel Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Supervision, 3, * 
Nasser Aghdami Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Supervision, 2, 8, * 
Author information:
1Department of Applied Cell Sciences, Faculty of Basic Sciences and Advanced Medical Technologies, Royan Institute, ACECR, Tehran, Iran.
2Department of Stem Cells and Developmental Biology at Cell Science Research Center, Royan Institute for Stem Cell Biology and Technology, ACECR, Tehran.
3Hematopoietic Stem Cell Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4Department of Hematology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
5Department of Immunology, Afzalipour Faculty of Medicine, Kerman University of Medical Sciences, Kerman, Iran.
6Parvaz Research Ideas Supporter institute, Tehran, Iran.
7Department of Internal Medicine, Imam Hossein Hospital, School of Medicine Shahid Beheshti University of Medical Science, Tehran, Iran.
8Department of Regenerative Medicine, Cell Science Research Center, Royan Institute for Stem Cell Biology and Technology, ACECR, Tehran, Iran.
Abstract
Hematopoietic stem cell transplantation (HSCT) is a life-saving therapy for various hematologic disorders. Due to the bone marrow suppression and its long recovery period, secondary infections, like cytomegalovirus (CMV), Epstein-Bar virus (EBV), and adenovirus (AdV), are the leading causes of morbidity and mortality in HSCT cases. Drug resistance to the antiviral pharmacotherapies makes researchers develop adoptive T cell therapies like virus-specific T cell therapy. These studies have faced major challenges such as finding the most effective T cell expansion methods, isolating the expected subtype, defining the functionality of the end-cell population, product quality control, and clinical complications after the injection. This review discusses the viral infections after HSCT, T cells characteristics during chronic viral infection, application of virus-specific T cells (VSTs) for refractory infections, standard methods for producing VSTs and their limitation, clinical experiences on VSTs, focusing on outcomes and side effects that can be helpful in decision-making for patients and further researches.
Keywords: Post-hematopoietic stem cell transplantation infec, Adoptive T cell therapy, Virus-specific T cells
Copyright and License Information
©2023 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Hematopoietic stem cell transplantation
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is applied for treating various hematologic diseases such as aplastic anemia, paroxysmal nocturnal hemoglobinuria, acute myeloid leukemia, acute lymphoblastic leukemia, and chronic myeloid leukemia.1 This therapeutic approach is accompanied by dramatic damage to the immune system, causing severe immune deficiencies in HSCT recipients. The symptoms occur during the first trimester following HSCT, and the immune system recovery takes about three to six months.2 Due to the long recovery, secondary infections are one of the major reasons for death in HSCT cases.3
Viral infections following HSCT
About one-third of mortality-related deaths following HSCT are due to viruses like cytomegalovirus (CMV), Epstein-Bar virus (EBV), and adenovirus (AdV).4 Generally, CMV is not associated with specific symptoms in adults; however, it causes various complications in pregnant women and immunocompromised patients.5 EBV causes infectious mononucleosis associated with either non-malignant or premalignant and malignant lymphoproliferative diseases. This latent virus is usually reactivated after allo-HSCT and generates a post-transplant proliferative disease (PTLD).6 AdV is a highly prevalent virus that affects many children before entering school, associated with various respiratory and gastrointestinal symptoms. Similarly, this virus is quite common in patients undergoing HSCT. Although the local infections can be easily managed, the mortality rate increases in the presence of systemic infections, leading to a decrease in acquired immune response mediated by T cells and subsequent death.7
Anti-EBV, anti-AdV, and anti-CMV drugs show significant effective results.8 However, some complications like toxicity, immune reactions, and lack of response have been observed in immunocompromised patients.9 Controlling resistant viral infections is mainly impressed via the capacity of the immune system restoration. Adoptive T cell therapy is a pretty effective way to restore the immune system.10 This immune support has been identified by assessing specific T cell responses in peripheral blood; unfortunately, there is little information about other predictive markers.
T cell adoptive therapy
Using T cells as biological anti-infection tools is a promising therapeutic choice because suppressing the immune system impairs T cells’ antiviral function. The leading limitations of the conventional HSCT, including the type of transplantation, human leukocyte antigen (HLA) compatibility, and patients’ background diseases, do not influence the adoptive T cell transplantation outcomes much. In this therapeutic approach, T cells must be derived from seropositive donors,11,12 which is easily accessible due to the abundance of CMV seropositive individuals in the community. The transplant can even be derived from third-party donors.12 However, T cells derived from the same donors for HSCT are more efficient than those derived from third-party donors. So far, adoptive T cell therapy has not been successful under high doses of steroids; this is one of the complications of combinational therapy that physicians should consider.13
Despite the progress, lethal infections are still one of the leading reasons for morbidity and mortality following allo-HSCT. Due to the drug resistance to the current viral pharmacotherapies and the high expense of antibody therapy, researchers focus on adoptive immunotherapy with virus-specific T cells (VSTs). Clinical trials on CMV- and EBV-specific T cells confirmed their effectiveness and safety in preventing and curing these viral diseases. Moreover, multi-virus-specific T cells (MVSTs) target the most prevalent viruses following HSCT, including AdV, BK virus, and herpesvirus. VSTs can help fast reconstruction of antiviral immunity in the immunosuppressed allo-HSCT recipient. VSTs can be derived from transplant donors or third-party donors.14
This study discusses T cell changes during viral infection, conventional methods for producing VSTs, different stimulatory factors for T cell expansion, using VSTs in post HSCT recurrent infections, and clinical experiences in this field.
T cells in chronic viral infection
Several factors can result in T cell exhaustion, like permanent antigen exposure or reduced cross-presentation to CD4 + T cells.15 Continuous exposure to antigens affects cell function, and antigen over-exposure or increased viral load causes more severe T cell exhaustion. Antigen exposure must last at least two weeks to one month to cause T cell exhaustion. Some other factors can also stimulate the cell exhaustion, including inhibitory receptors such as programmed cell death protein 1 (PD1), cytotoxic T-lymphocyte-associated protein 4 (CTLA4), T cell immunoglobulin and mucin domain 3 (TIM3), lymphocyte activation gene 3 protein (LAG3),16 and soluble molecules such as interleukin (IL)-10 (released by tumor cells) and transforming growth factor ꞵ (TGF-ꞵ).17 Regulatory T cells, an important source for IL-10 and TGF-ꞵ, also serve a key role in T cell exhaustion.18 Therefore, T cell exhaustion can be eliminated by reducing regulatory T-cells and blocking inhibitory receptors. T-cell exhaustion can also be caused by infections, especially sepsis, resulting in cytokine storms.19
Although cell exhaustion in cancer cases can be relieved by blocking the inhibitory receptors (such as PD-1 and CTLA4) and chemotherapy, this method cannot be considered effective for patients with recurrent CMV infections; since the cause of infection cannot be eliminated, and it is not possible to use blocking agents for a lifetime. Thus, it can be concluded that adoptive T cell therapy can be considered a promising approach to control recurrent viral infections in patients who underwent allo-HSCT (Figure 1).
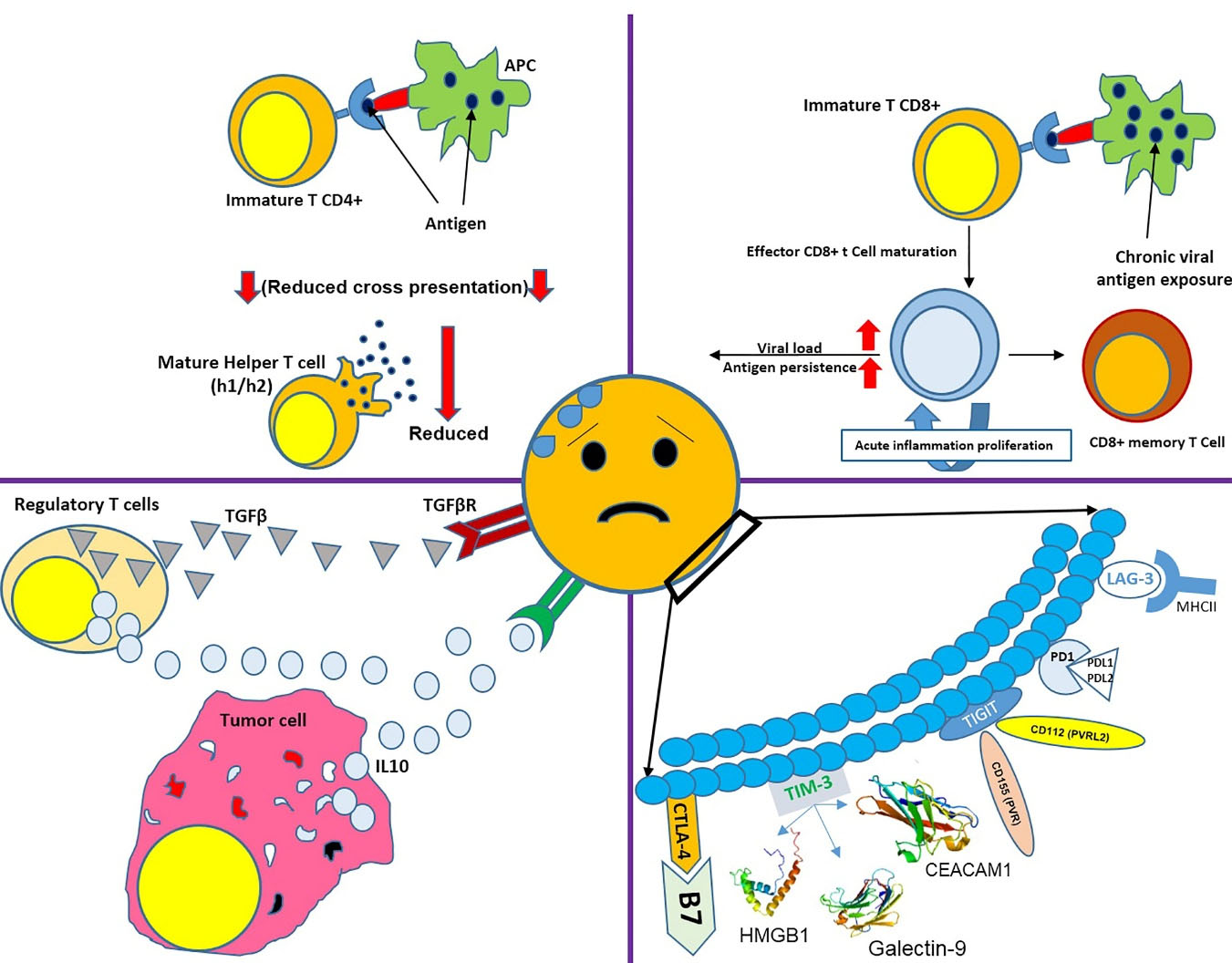
Figure 1.
There are different factors causing cell exhaustion. This figure shows the effect of chronic viral exposure, inhibitory receptors, and their ligands, reduced cross-presentation with T CD4 + , and high levels of IL10 and TGFβ in T cells’ environment.
.
There are different factors causing cell exhaustion. This figure shows the effect of chronic viral exposure, inhibitory receptors, and their ligands, reduced cross-presentation with T CD4 + , and high levels of IL10 and TGFβ in T cells’ environment.
Common methods for producing VSTs
Over the past 20 years, using VSTs has been significantly increased to reduce the uncontrolled proliferation of viruses in HSCT patients or other immune-suppressed patients. Numerous in vitro studies have been performed to reach an optimal condition and method for inducing T cell proliferation and selecting VSTs for clinical use.20
In the first immunotherapy studies using VSTs, T cells were cultured with CMV lysates and CMV-infected fibroblasts, requiring cleanroom, quality control, and quality assurance to provide good manufacturing practice (GMP) grade production. Nowadays, one of the standard methods applied for VSTs production is using tetramers to select and isolate specific T cells from the patient’s entire T population.21 The main advantage of this method is the simplicity of the cell selection process. Besides, it does not need antigen-presenting cells (APCs), exogenous cytokines, or ex vivo manipulations. Additionally, since this process is performed using closed-system devices, there is no need for cleanroom and GMP equipment. However, this method only selects the T cells possessing a specific epitope for one type of HLA. Thus, it is only applicable to HLA-matched donors. Sometimes focusing on viral reactions with a particular epitope can lead to antigen escape, as observed in EBV cases.22
Another method for VST isolation is based on immunomagnetic cell sorting, which is specified for isolating interferon-gamma (IFN-γ) secreting T cells created via culturing T cells with viral peptides.23 In addition to the fast proliferation, which is the most significant advantage, this method does not require much manipulation. This method is superior to previous ones because it can cover all viruses and antigens based on the stimulation.20
Stimulating peripheral blood mononuclear cells (PBMCs) by APCs is another technique to produce GMP-grade VSTs. In 1990, this method was developed to produce EBV-specific T cells. It is initiated via stimulating CD8 + T cells with EBV24 and followed by coculture with dendritic cells (DCs) transduced by AdV vectors specific for CMV and EBV.25 Cytotoxic T cells (CTLs) generated by this method can enable T cells to detect all three viruses, including CMV (engineered from adenovirus vectors), EBV (from lymphoblastoid cell lines (LCLs)), and adenovirus (from adenovirus vectors). All the processes can be performed in a culture medium with a low blood amount (50-60 mL). This method is very time-consuming and takes long, even three months. Besides, it requires very expensive clinical viral vectors. In order to replace the viral vectors, nucleofected DCs with DNA plasmids of different viruses were used to create multi-specific VSTs. Thus, the CTLs were ready to be used after a single stimulation.26
Despite the mentioned improvements, none of these methods can generate VSTs from seropositive donors, which is considered a major limitation. Because one of the greatest risks in viral infections is the absence of memory T cells in transplanted cells (like cells derived from the umbilical cord blood (UCB) sources or seropositive donors), leading to recipient infection infected with the pathogens.27 In this regard, numerous studies have tried to isolate and proliferate naive T cells from the UCB to solve this problem.28 Proliferating UCB-derived T cells using the G-Rex gas permeable device29 to sufficient amounts for clinical applications indicates the possibility of using a system that has not experienced viral conditions to produce VSTs.30 Clinical use of T cells to treat or prevent post-HSCT viral infections is limited by factors such as the time-consuming and complicated nature of the cell production process. Therefore, few immunotherapy centers can provide such services. Many different groups have conducted studies to overcome these limitations. Tetramer-based isolation is an up-and-coming method. However, it is costly for routine clinical applications31 (Figure 2).
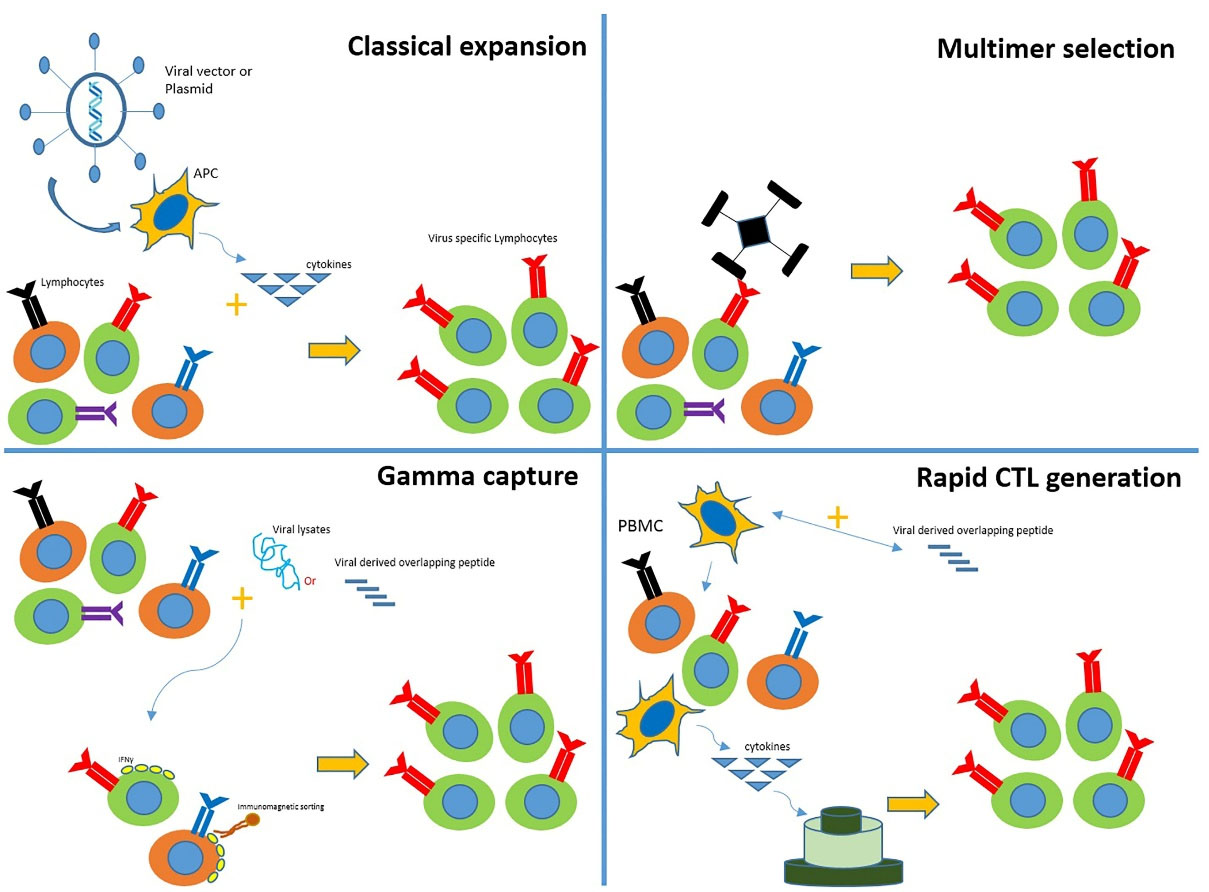
Figure 2.
Conventional methods for producing VSTs (classical expansion, multimer selection, gamma capture, and rapid CTL generation) are illustrated in this figure.
.
Conventional methods for producing VSTs (classical expansion, multimer selection, gamma capture, and rapid CTL generation) are illustrated in this figure.
Different stimulation ways for T cell expansion
The main strategy to proliferate T cells performs using magnetic beads with immobilized monoclonal antibodies to CD332 or CD3 and CD28,33 along with cytokines. T cells in different maturation states and memory T cells show different proliferation and activation abilities; for instance, memory T cells have more proliferation potential than naive T cells. In addition, the proliferative potential of memory T cell subsets differs from central memory T cells, which show the highest proliferative rate. While T cell stimulation with the CD3 receptor (T-cell receptor) serves an important part in the differentiation fate of T cells,34 hemostatic cytokines are involved in proliferation, differentiation, and viability of T cells in vivo35 and in vitro.36
IL-2 is an essential growth factor in T cell proliferation, used at concentrations of 20-1800 IU/mL.37 IL-7 plays a crucial part in the T cell viability and antigen-dependent proliferation of Naïve T cells.38 IL-15 is involved in the proliferation rate of CD8 + 35 and CD4 + memory cells in the absence of IL-7.39 IL-15 shares many biological features with IL-2,40 like stimulating the differentiation and proliferation of central memory T cells to effector memory T cells.34 A variety of studies that used IL-2 as a proliferation agent showed favorable results. Thus, IL-2 is considered the gold standard for stimulating T cell proliferation in most clinical trials (Table 1).
Table 1.
Clinical trials using virus-specific T cells in post-HSCT viral infections
|
Type of cell
|
Source
|
Dose
|
Expansion
|
Targeted virus
|
Transplantation
|
Infusion
|
No. of cases
|
Results and side effects
|
Ref.
|
CMV- specific
CD8 + clones |
BM |
3.3 × 107–109 cells/m2 |
IL-2 |
CMV |
Allogeneic |
- IV
- Each week for four consecutive weeks |
3 |
- 3/3: Prevention of viremia and pneumonia
- No changes in vital signs, oximetry, chest x-ray
- No GVHD |
41
|
CMV- specific
CD8 + clones
(Phase I) |
PBMC |
3.3 × 107–109 cells/m2 |
IL-2 |
CMV |
Allogeneic |
- IV
- 4 escalating doses (33, 100, and 330 million, and 1 billion cells/m2),
- Each dose given one week apart
- Over a 30-minute period
- Through a Hickman catheter |
11 |
- 11/11: CMV prevention
- 4/11: No GVHD
- No significant changes in vital signs, oxygen saturation, blood-chemistry, chest radiographs, and cell blood counts
- 1/11: Transient fever |
42
|
EBV-specific
CD8 + T cells |
PB |
0.2–1.2 × 108 cells/m2 |
IL-2 |
EBV |
Allogeneic |
- IV
- 60 to 185 days post BMT |
10 |
- 7/10: No evidence of EBV reactivation
- 3/10: Evidence of uncontrolled EBV replication |
24
|
EBV-specific
CD8 + T cells |
PB |
0.2–1.0 × 108 cells/m2 |
IL-2 |
EBV |
Allogeneic/Mismatched family member or closely matched unrelated
Donor |
- IV
- At a median of 88 days post-transplantation |
39 |
- 39/39: 2-4 log decreases in viral DNA levels within 2-3 weeks after infusion
- No lymphoma development
- No toxic effects
- Full response in 2 additional patients who did not received prophylaxis and developed overt immunoblastic lymphoma |
43
|
| CMV- specific polyclonal CD8 + and CD4 + T cells |
PBMC |
107 cells/m2 |
IL-2 |
CMV |
Allogeneic |
- IV
- In the presence of virus |
8 |
- 5/7: Successful anti-CMV cellular therapy
- 2/7: Only transient reductions in virus load
- No pulmonary toxicity or other acute side effects |
44
|
| EBV-specific polyclonal CD8 + and CD4 + T cells (Phase I/II) |
PBM |
106 cells/kg |
IL-2 |
EBV |
Allogeneic |
- IV
- Every 2 weeks, until complete remission either had (complete regression of tumor) or no response (Tumor enlargment). |
5 |
- 3/5: Complete remission
- 2/5: No clinical response
- No GVHD or allo-specific antibodies
- 3/5: Graft function improvement
- Tumor responses in those with early, localized, polyclonal disease
- 5/5: Decreased EBV load in PB to undetectable levels |
45
|
CMV-specific
polyclonal CD8 + and
CD4 + T cells |
PBMC |
105 cells/kg |
IL-2
In-vivo expansion |
CMV |
Allogeneic |
IV |
16 |
- 14/16: No virus reactivation
- 11/16: No GVHD
- 2/16: No evaluable GVHD - 3/19: Grade I GVHD |
46
|
CMV-specific
CD4 + clones |
PBMC |
3 × 105-106 cells/kg |
IL-2
(only from day 14 onward to
ensure specificity) |
CMV |
Haploidentical |
- IV
- Between days 13 and 37 post transplantation |
25 |
- 7/25: CMV-reactivation
- No acute or chronic GvHD
- No infusion-related toxicity
- 5/25: Developed CMV-disease (3 eliminated infection) |
47
|
| CMV-specific CD8 + T cells using MHC-I-tetramers |
PBMC |
1.2–33 × 103 cells/kg |
In vivo expansion |
CMV |
Allogeneic |
IV |
9 |
- 8/9: Eliminated infection
- 2/9: Mild GvHD (grades 1 and 2) within 2 week (both had shown grade 1 GvHD before infusion) |
21
|
AdV-specific polyclonal
CD8 + and CD4 + T cells |
PBMC |
1.2–50 × 103 cells/kg |
IL-2 |
AdV |
Allogeneic |
IV |
9 |
- No acute clinical side effects
- 2/9: Died due to preexisting clinical problems |
48
|
| CMV-, EBV-, and AdV-specific CD8 + T cells |
PBMC |
5 × 106–1 × 108 cells/m2 |
Expand in response to viral challenge after injection |
AdV/
CMV/
EBV |
Allogeneic (related or un-related donor) |
IV |
11 |
- 11/11: elimination of the viral pathogen
- No GvHD over 3 months
- No toxicities over 3 months |
25
|
EBV-specific
polyclonal CD8 + and CD4 + T cells
(Phase II) |
PBMC |
2 × 106 cells/kg |
Weekly stimulation with autologous EBV immortalized LCLs |
EBV |
Allogeneic |
- IV
- For 4 weeks |
33 |
- No adverse effects
- 14/33: Complete remission, - 3/33 partial response
- 6/33: No response at 6 months |
45
|
| EBV- and AdV-specific CD8 + T cells |
PBMC |
0.5–13.5 × 107 cells/m2 |
IL-2 |
EBV/
CMV |
Allogeneic (unrelated and haploidentical) |
IV |
13 |
- 13/13: AdV clearance
- 13/13 no PTLD
- No de novo GVHD |
49
|
EBV-specific
CD8 + T cells |
PBMC |
1–5 × 107 cells/m2 |
Stimulation with LCLCs and IL-2 |
EBV |
Autologous |
- IV
- In different doses |
114 |
- No immediate adverse reaction
- No de novo GVHD after CTL infusion
- 51/114: GVHD (36: grade I; 13: grade II; 2: grade III)
- 8/51: recurrent GVHD
(6: grade I; 2: grade II) |
50
|
| CMV-specific polyclonal CD8 + andCD4 + T cells |
PBMC |
1.2–166 × 103 cells/kg |
In vivo expansion/
Short-term in vitro expansion for 2 patients (IL-2) |
CMV |
Transplant donor (15)
Third party donor (2) |
- IV
- Directly after isolation, without in vitro expansion
- In vitro expansion was performed for 2 cases |
18 |
- 15/18: Responded
- No acute side effects |
51
|
EBV-specific CD8 +
T cells using MHC-I-pentamers |
PBMC |
1.1 × 104 cells/kg and
2 × 104 cells/kg |
In vivo expansion |
EBV |
Haplo-identical (mother) |
IV |
1 |
- 1/1: Complete response
- No GVHD |
52
|
EBV-specific polyclonal CD8 +
and CD4 + T cells |
PBMC |
0.4–9.7 × 104 cells/kg |
In vivo expansion |
EBV |
Allogeneic |
IV |
6 |
- 3/6: Responded |
23
|
CMV-specific polyclonal CD8 + and CD4 + T cells
(Phase I/II) |
Leukapheresis |
3.5 × 104 cells/kg |
In vivo expansion |
CMV |
Allogeneic related donors |
- IV
- At 21 days after HSCT |
18 |
- No infusion-related toxicity
- 2/18: No need for antiviral treatment |
20
|
| CMV-specific CD8 + T cells using MHC-I-streptamers |
PBMC |
0.37-2.2 × 105 cells/kg |
In vivo expansion |
CMV |
Allogeneic |
IV |
2 |
- 2/2: controlled CMV virema |
53
|
CMV-specific polyclonal CD8 + and CD4 + T cells
(Phase I/II) |
PBMC |
0.9 × 104-3.1 × 105 cells/kg |
IL-2 |
CMV |
Autologous and Allogeneic |
IV |
6 |
- No acute adverse events
- No GVHD
- CMV load was disappeared |
54
|
| CMV-and AdV-specific CD8 + T cells using MHC-I-pentamers |
PBMC |
0.8–24.6 × 104 cells/kg (CMV)/
3.1 × 104 and 1.7 × 104 cells/kg (AdV) |
In vivo expansion |
CMV/
AdV |
Allogeneic/Haploidentical (frozen donor material and third-party donors) |
IV |
CMV:5
AdV:8 |
- CMV specific T cells: 4/5 responded
- AdV specific T cells: 5/6 responded
- No CTL-associated GVHD |
55
|
EBV-specific
CD8 + T cells |
PBMC |
106 cells/kg |
Stimulation with autologous
EBV transformed B cells
+ IL2 on day 16 |
EBV |
Allogeneic |
- IV
- 1 × 106 EBV-specific CTLs/kg
IV weekly for 3 weeks
- One-time 0.2-1 × 106 unselected
CD3 + T cells/kg |
19 |
- 13/19: Complete response
- No immediate adverse reactions due to cell therapy
- No de novo acute or chronic GVHD or a flare of preexisting GVHD |
56
|
CMV-specific polyclonal
CD8 + and CD4 + T cells
(Phase II) |
PBMC (T cells)/
Stem cell harvest product |
2 × 107 cells/m2 |
In vitro expansion
IL-2 |
CMV |
Allogeneic |
- IV
- On or after day 28 post-transplantation
- Treatment was delayed in case of active GVHD, organ dysfunction or active infection |
50 |
- Median overall survival: 76 months (95% CI 56 to 96)
- 13/50: relapsed over the follow-up period
- Progression free survival (PFS) at one year was 89% and at 5 years was 66%
- 1/50: Transplant associated microangiopathy (TAM)
-1/50: Graft failure
- 2/50: Lung adenocarcinoma
- 1/50: Pulmonary hypertension
- 6/50: Bacterial infection
- 6/50: Yeast and fungal non-
- 1/50: Fatal intracerebral hemorrhage |
57
|
| EBV-specific polyclonal CD8 + and CD4 + T cells |
Whole blood or unstimulated apheresis (PBMC) |
0.15-53.8 × 103 cells/kg |
LG2 cells + phythemagglutinine-L + IL-2 |
EBV |
Allogeneic |
IV |
10 |
- 7/10: Responded
- No acute adverse reaction
- 1/7: Transient grade I-II acute skin GVHD (15 days after the first infusion/ responded well to treatment and resolved within 3-4 weeks) |
58
|
AdV-specific polyclonal
CD8 + and CD4 + T cells |
PBMC |
104 cells/kg |
In vivo expansion |
AdV |
Allogeneic Haploidentical |
IV |
5 |
- 3/5: Responded
- No acute infusion-related toxicities
- No GVHD |
59
|
CMV-,
EBV-, and AdV-specific
CD8 + T cells
(Phase I/II) |
PBMC |
5 × 106 – 2 × 107 cells/m2 |
In vitro with G-Rex10 |
CMV/
EBV/
AdV |
Allogeneic.
5 Haplo, 3 MUD, 1MMUD, and 1MRD) |
IV |
Total: 10
CMV: 3
AdV: 1
EBV: 2 EBV + / AdV +: 2
CMV + / AdV +: 2 |
- 8/10: Complete responses
- 1/10: Mild and localized skin rash and intercurrent BK infection who showed similar rash during an earlier episode of BK reactivation, prior to CTL therapy
- 9/10: No infusion-related toxicity |
55
|
AdV-specific
polyclonal CD8 + and
CD4 + T cells |
PBMC |
104 cells/kg |
IL-15 |
AdV |
Allogeneic |
IV |
2 |
- 1/2: Complete response
- 1/2: Partial response
- 1/2: Developed severe GvHD |
60
|
EBV-specific polyclonal CD8 + and CD4 + T cells
(Phase I/II) |
PBMC |
5 × 106 cells/kg |
T: BLCL/
Stimulation with IL-2 |
EBV |
Allogeneic |
IV |
10 |
- 6/10: No response
- 1/10: Partial response
- 3/10: Complete response |
61
|
AdV-specific polyclonal
CD8 + and CD4 + T cells |
PBMC |
0.3–24 × 103 cells/kg |
In vivo expansion |
AdV |
Allogeneic |
IV |
30 |
- 21/30: Responded
- No acute side effects |
62
|
CMV-specific
(Phase I) |
PBMC |
0.66–15.41 × 107 CD8 + and
0.68–9.25 × 105 CD4 + |
IL-2
IL-15
Anti-CD3 antibody
IFNγ |
CMV |
Allogeneic |
- IV |
32 |
- 27/32: Responded
- 3/32: Died (not due to T cell therapy)
- No infusion-related side effects |
63
|
CMV-specific CD8 +
T cells using MHC-I-streptamers (Phase I/IIa) |
PBMC (third-party donor) |
6.3 × 106 cells
1.4 × 107 cells |
In vivo expansion |
CMV |
Allogeneic
(Third party donor) |
- IV
- Within 24 hours after selection or 72 hours after apheresis. |
16 |
- No transfusion-related reaction
- 2/16: Acute and chronic GVHD |
13
|
CMV-, EBV, AdV, and
varicella-zoster virus-specific CD8 + and CD4 + T cells
(Phase I) |
PBMC |
2.0 × 107 cells/m2 |
IL-2 |
CMV/
EBV/
AdV/
multivirus |
Allogeneic
(Third party donor) |
- IV
- At a median of 75 days post HSCT |
CMV:27
EBV: 1
AdV:1
Multi-virus: 1 |
- CMV specific T cells: 26/27 responded
- EBV specific T cells: 0/1 responded
- Adv specific T cells: 1/1 responded
- Multi specific T cells: 1/1 responded |
64,65
|
| CMV-, EBV-, AdV-specific polyclonal CD8 + and CD4 + T cells/Multi: CMV- and EBV-specific or CMV- and AdV-specific CD8 + and CD4 + T cells |
Leukapheresis |
CMV: 7.5–16.2 × 104 cells/kg
EBV: 1.8–2.3 × 104 cells/kg
AdV: 2.7 × 104 cells/kg
Multi: 3.2–4.8 × 104 cells/kg |
In vivo expansion |
CMV/
EBV/
AdV |
7 matched unrelated, 1 sibling, and 1 haploidentical donor |
- IV
- Following a premedication by antihistamines and acetaminophen |
CMV: 3
EBV: 2
AdV: 1
CMVa+ AdV +: 2
CMV + EBV +: 1 |
- Complete response in all cases (except 1 CMV)
- Treatment failure in a newly acquired viral illness.
- No developing or worsening |
66
|
Multivirus-Specific Cytotoxic T cells
(Cohort) |
PBMC |
5 × 106 mCTLs/m2 |
Not exactly mentioned |
EBV/
CMV/
AdV/ HHV6/ BK |
Allogeneic |
IV |
4 |
4/4: Responded |
67
|
| 1 × 107 mCTLs/m2 |
Not exactly mentioned |
EBV/
CMV/
AdV/ HHV6/
BK |
Allogeneic |
IV |
4 |
- 1/4: General disorders
- 1/4: Respiratory, thoracic and mediastinal disorders |
| 2 × 107 mCTLs/m2 |
Not exactly mentioned |
EBV/
CMV/
AdV/ HHV6/
BK |
Allogeneic |
IV |
13 |
- 1/13: Gastrointestinal disorders
- 1/13: General disorders
- 1/13: Infections
- 1/13: Renal and urinary disorders
- 1/13: Reproductive system and breast disorders |
| CMV-specific CTLs |
PBMC |
1 × 106 cells/kg |
Not exactly mentioned |
CMV |
Allogeneic |
- Bolus IV
- Once a week for 3 weeks |
58 |
- 8/58: Complete response
- 50/58: No complete response
- 11/58: Dead
- 1/58: Hepatobiliary disorders
- 2/58: Catheter related infection
- 1/58: Alanine aminotransferase increased
- 1/58: Aspartate aminotransferase increased
- 1/58: Neutrophil count decreased
- 1/58: Acidosis
- 1/58: Dehydration
- 1/58: Renal and urinary disorders
- 7/58: Hypoxia
- 1/58: Thromboembolic event |
68
|
| Multivirus-specific Cytotoxic T cells |
PBMC |
2 × 107 cells/m2 |
Not exactly mentioned |
AdV/
CMV/ EBV/
BKV/ HHV6 |
Allogeneic |
IV |
58 |
- 6/58: All-cause mortality
- 1/58: Blood and lymphatic system disorders
- 4/58: Gastrointestinal disorders:3/58
- 2/58: General disorders
- 3/58: Multi-organ failure
- 1/58: Infections
- 1/58: Nervous system disorders
- 1/58: Vascular disorders |
69
|
Abbreviations: CMV, Cytomegalovirus; BM, Bone marrow; IV, Intravenous; GVHD, Graft versus host disease; PBMC, Peripheral blood mononuclear cells; EBV, Epstein–Barr virus; PB, Peripheral blood; DNA, Deoxyribonucleic acid; AdV, Adenovirus; LCLs, Lymphoblastoid cell lines; PTLD, Post-transplant lymphoproliferative disorder; MHC-I, Major histocompatibility complex-I; CTL, Cytotoxic T lymphocytes; BLCL, B lymphoblastoid cell lines; MUD, Matched unrelated donor; MMUD, Mismatched unrelated donor; MRD, Minimal residual disease; BKV, BK virus; HHV6, Human herpesvirus 6.
Using VSTs in post-HSCT recurrent viral infections
HSCT can be considered one of the best treatments for malignant blood disorders. In selecting an appropriate donor, reducing the number of cytotoxic T cells should be considered, as it can reduce the risk of graft versus host disease (GVHD). GVHD prevention strategy introduces other hematopoietic stem cell sources, like haploidentical donors or UCBCs, which still result in infection, one of the most important reasons for transplant-related mortality.70 CMV, EBV, and AdV are the most prevalent virus pathogens in HSCT patients.71
Despite the routine use of antiviral drugs, their prescription is accompanied by limitations. First, antiviral medications may suppress the immune system72 and cause complications in chemotherapy and radiotherapy patients. Second, although clinical trials revealed the ability of the anti-CMV and anti-EBV drugs, the efficacy of anti-AdV drugs has not been reported in trials.73 Antiviral drugs, especially those applied for CMV, can cause late-onset CMV infection. As soon as antiviral therapies cease, late-onset CMV may initiate, more severe than normal, with delayed immune recovery.74 As a result, HSC transplanted patients with viral infections may need several courses of antiviral treatments that are very costly and may lead to drug resistance. Approximately 94% of CMV species resist ganciclovir due to their mutations in the UL97 gene.75 In addition, Nichols et al. reported that about one-third of allo-HSCT recipients experienced an increased viral load after antiviral therapy.76 Many patients show drug resistance to antiviral drugs due to their continuous use, leading to severe complications like liver encephalopathy.77 Therefore, adoptive immunotherapy with VSTs can be applied as one of the most attractive and creative substitutes for pharmacological antivirals.
Clinical experience with VSTs
CMV-specific T cells
The first clinical trials were implemented in the early 1990s, in which CMV-specific T cells were obtained from donors, cultured, and injected into the recipients. T cell clones derived from donors were injected into fourteen HSCT patients; no recipient showed CMV infection.42 Parallel to efforts to produce CMV-specific T cells, many attempts were made to reduce the ex vivo expansion time. Peggs et al generated VSTs by targeting IFNγ-secreting cells and managed 18 patients by pre-emptive therapy. Their findings showed that this treatment significantly benefited patients on prophylaxis regimens, as six out of the seven patients did not experience reactive CMV. However, this method appears very effective in primary infections because nine of the eleven patients who received antiviral prophylaxis needed additional antiviral drugs later. In addition, many patients showed GVHD symptoms due to the active T cells.20
Using T-cells isolated by tetramers was first performed by Cobbold and colleagues. They treated nine transplant patients with CMV reactivity. Following CMV-specific T cells prescription, eight patients were treated, and two developed GVHD.21 These findings were supported by other clinical trials and confirmed that VST is a safe therapeutic strategy and can solve many limitations of antiviral drugs.78 Qasim et al treated adenoviremia in HSCT pediatric patients by isolating IFN-γ-secreting T cells. However, a third-party donor was required for two out of five patients.51
AdV -specific T cells
Feuchtinger et al first reported using AdV-specific T cells for HSCT patients with resistant infection. AdV-specific T cells were generated based on isolating IFN-γ -secreting T cells and proliferating by IL-2 and feeder cell stimulation. According to their results, AdV disappeared in five cases, and GVHD was seen in one case.79
EBV-specific T cells
Due to APCs and LCLs delivering viral antigens to T cells, the conditions in which VSTs are obtained by LCLs and APCs can be critical. EBV-specific T cells were firstly established in 1996 and 1998.48 In a multi-institutional study in 2009, 114 HSCT patients were candidates for adoptive EBV-specific T cell therapy. A total of 101 patients received EBV-specific CTLs, and none of them (either prophylaxis or preemptive therapy) showed recurrent PTLD or de novo GVHD symptoms.80 MSKCC group treated 47 HSCT patients with EBV-specific CTLs derived from an HSCT donor or a third-party donor. They reported an overall response of 68% and no GVHD symptoms.56 Other studies with fewer patients were performed and admitted the potency and safety of the EBV-specific T cells obtained by LCLs.81,82
Multi-virus-specific T cells
MVSTs target the most common post-HSCT viruses. In order to enhance the specificity of CTLs against viruses and target more infections, different studies were conducted to target specific viruses, like targeting EBV and AdV,80 CMV and AdV,37 and CMV, AdV, and EBV.25
HSCT recipients can also be infected with other viruses, including BK, human herpesvirus 6 (HHV6), influenza, parainfluenza, coronavirus, and respiratory syncytial virus, causing morbidity and mortality. In order to expand VSTs for other viruses, a group of scientists developed a way to create polyclonal T cells (CD4 +, CD8 + ) for different viruses, including Elizabethkingia, CMV, Adv, BK, HHV6, respiratory syncytial virus, and influenza, to face against other post-HSCT viral risk factors.83
MVSTs were also used in the clinic. The most critical inclusion criterium in all clinical trials is donor seropositivity. Clinical experiments in T-cell immunotherapy with seropositive donors are minimal, but recently UCB-derived CTLs have been assessed in phase I clinical trials. Among the nine patients who used MVSTs against CMV, EBV, and Adv, only three represented active viral reactivation. One patient showed both active CMV and AdV infection, represented increased CMV and AdV-specific T cells and decreased CMV and AdV viruses following VSTs injections, and was successfully treated without antiviral pharmacotherapy. Two patients who experienced EBV reactivation or infection before or immediately after VSTs injection were treated without using antiviral drugs, and VSTs were detectable in their peripheral blood.84
Third party donor’s T cells
Although the frequency of refractory infections is relatively low in HSCT recipients, which makes it unreasonable to prepare VSTs for all at-risk patients, the aggressive character of the viral pathogens demands immediate availability to VSTs in antiviral therapy non-responder patients. Waiting 8- to 10-week to generate VSTs is too long for patients who developed an infection. This obstacle can be dominated by off-the-shelf third-party T cells. Production and storage of HLA-matched VSTs from third-party donors (bio-banking) could be a promising approach for adoptive VSTs therapy for patients with no HLA-matched donor.
Previous studies in this regard are summarised in Table 1. First, Haque et al. applied EBV-specific T cells to 33 HSCT or solid tumors surgery patients to treat their PTLD. They reported a total response of 64% and 52% in five weeks and six months, respectively. No evidence of GVHD or rejection was observed. Best results were obtained when patients received HLA-matched transplants.45 In 2011, Qasim et al. conducted a clinical trial on AdV-infected HSCT patients and applied VSTs obtained from third-party donors using isolating IFN-γ secretory cell method. They reported an increased risk of alloreactive T cells and GVHD.85 The Memorial Sloan-Kettering group evaluated the efficacy and safety of this method by comparing EBV-specific T cells and donor lymphocyte infusion (DLIs) in PTLD patients. Although the responsiveness was the same in both groups, GVHD incidence was higher in the DLI group recipients.86
Finally, a multi-institutional study used previously-stored VSTs from a third-party donor and showed that the procedure was safe. According to their study, HLA incompatibility can lead to complications; in other words, the shared allele must identify the specific epitope of the virus. A professional team is required to isolate, store, and inject the virus-specific T cell products.87
Conclusion
Trials using VSTs (mono-specific or multi-virus) demonstrate their ability to cure recurrent and pharmacotherapy refractory viral infections. No side effects such as GVHD were reported, even with alternative donors. Third-party donors offer a great opportunity to use the off-the-shelf product for many post-HSCT infections. It can be a promising therapeutic approach for many post-HSCT infections. Despite the limitations in generating VSTs, they have shown promising outcomes in clinical trials.
Competing Interests
None.
Ethical Approval
Not applicable.
References
- Giralt S, Bishop MR. Principles and overview of allogeneic hematopoietic stem cell transplantation. Cancer Treat Res 2009; 144:1-21. doi: 10.1007/978-0-387-78580-6_1 [Crossref] [ Google Scholar]
- Styczyński J, Tridello G, Koster L, Iacobelli S, van Biezen A, van der Werf S. Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Bone Marrow Transplant 2020; 55(1):126-36. doi: 10.1038/s41409-019-0624-z [Crossref] [ Google Scholar]
- Sachdev V, Hsieh M, Jeffries N, Noreuil A, Li W, Sidenko S. Reversal of a rheologic cardiomyopathy following hematopoietic stem cell transplantation for sickle cell disease. Blood Adv 2019; 3(19):2816-24. doi: 10.1182/bloodadvances.2019000387 [Crossref] [ Google Scholar]
- Atilla E, Atilla PA, Bozdağ SC, Demirer T. A review of infectious complications after haploidentical hematopoietic stem cell transplantations. Infection 2017; 45(4):403-11. doi: 10.1007/s15010-017-1016-1 [Crossref] [ Google Scholar]
- Giménez E, Torres I, Albert E, Piñana JL, Hernández-Boluda JC, Solano C. Cytomegalovirus (CMV) infection and risk of mortality in allogeneic hematopoietic stem cell transplantation (Allo-HSCT): a systematic review, meta-analysis, and meta-regression analysis. Am J Transplant 2019; 19(9):2479-94. doi: 10.1111/ajt.15515 [Crossref] [ Google Scholar]
- Martinez OM. Biomarkers for PTLD diagnosis and therapies. Pediatr Nephrol 2020; 35(7):1173-81. doi: 10.1007/s00467-019-04284-w [Crossref] [ Google Scholar]
- Takamatsu A, Tagashira Y, Hasegawa S, Honda H. Disseminated adenovirus infection in a patient with a hematologic malignancy: a case report and literature review. Future Sci OA 2019; 5(8):FSO412. doi: 10.2144/fsoa-2019-0072 [Crossref] [ Google Scholar]
- Cho SY, Lee DG, Kim HJ. Cytomegalovirus infections after hematopoietic stem cell transplantation: current status and future immunotherapy. Int J Mol Sci 2019; 20(11):2666. doi: 10.3390/ijms20112666 [Crossref] [ Google Scholar]
- Meesing A, Razonable RR. New developments in the management of cytomegalovirus infection after transplantation. Drugs 2018; 78(11):1085-103. doi: 10.1007/s40265-018-0943-1 [Crossref] [ Google Scholar]
- Ottaviano G, Chiesa R, Feuchtinger T, Vickers MA, Dickinson A, Gennery AR. Adoptive T cell therapy strategies for viral infections in patients receiving haematopoietic stem cell transplantation. Cells 2019; 8(1):47. doi: 10.3390/cells8010047 [Crossref] [ Google Scholar]
- Full F, Lehner M, Thonn V, Goetz G, Scholz B, Kaufmann KB. T cells engineered with a cytomegalovirus-specific chimeric immunoreceptor. J Virol 2010; 84(8):4083-8. doi: 10.1128/jvi.02117-09 [Crossref] [ Google Scholar]
- Bodey GP. Infection in cancer patients A continuing association. Am J Med 1986; 81(1A):11-26. doi: 10.1016/0002-9343(86)90510-3 [Crossref] [ Google Scholar]
- Neuenhahn M, Albrecht J, Odendahl M, Schlott F, Dössinger G, Schiemann M. Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after allo-HSCT. Leukemia 2017; 31(10):2161-71. doi: 10.1038/leu.2017.16 [Crossref] [ Google Scholar]
- Barrett AJ, Prockop S, Bollard CM. Reprint of: virus-specific T cells: broadening applicability. Biol Blood Marrow Transplant 2018; 24(3S):S1-S6. doi: 10.1016/j.bbmt.2017.12.787 [Crossref] [ Google Scholar]
- Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E. Defining ‘T cell exhaustion’. Nat Rev Immunol 2019; 19(11):665-74. doi: 10.1038/s41577-019-0221-9 [Crossref] [ Google Scholar]
- Tu L, Guan R, Yang H, Zhou Y, Hong W, Ma L. Assessment of the expression of the immune checkpoint molecules PD-1, CTLA4, TIM-3 and LAG-3 across different cancers in relation to treatment response, tumor-infiltrating immune cells and survival. Int J Cancer 2020; 147(2):423-39. doi: 10.1002/ijc.32785 [Crossref] [ Google Scholar]
- Sawant DV, Yano H, Chikina M, Zhang Q, Liao M, Liu C. Adaptive plasticity of IL-10 + and IL-35 + T(reg) cells cooperatively promotes tumor T cell exhaustion. Nat Immunol 2019; 20(6):724-35. doi: 10.1038/s41590-019-0346-9 [Crossref] [ Google Scholar]
- Shi L, Feng M, Du S, Wei X, Song H, Yixin X. Adenosine generated by regulatory T cells induces CD8 + T cell exhaustion in gastric cancer through A2aR pathway. Biomed Res Int 2019; 2019:4093214. doi: 10.1155/2019/4093214 [Crossref] [ Google Scholar]
- Shankar-Hari M, Fish M, Azoulay E. Should we consider blocking the inhibitory immune checkpoint molecules for treating T cell exhaustion in sepsis?. Intensive Care Med 2020; 46(1):119-21. doi: 10.1007/s00134-019-05814-8 [Crossref] [ Google Scholar]
- Peggs KS, Thomson K, Samuel E, Dyer G, Armoogum J, Chakraverty R. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis 2011; 52(1):49-57. doi: 10.1093/cid/ciq042 [Crossref] [ Google Scholar]
- Cobbold M, Khan N, Pourgheysari B, Tauro S, McDonald D, Osman H. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med 2005; 202(3):379-86. doi: 10.1084/jem.20040613 [Crossref] [ Google Scholar]
- Gottschalk S, Ng CY, Perez M, Smith CA, Sample C, Brenner MK. An Epstein-Barr virus deletion mutant associated with fatal lymphoproliferative disease unresponsive to therapy with virus-specific CTLs. Blood 2001; 97(4):835-43. doi: 10.1182/blood.v97.4.835 [Crossref] [ Google Scholar]
- Moosmann A, Bigalke I, Tischer J, Schirrmann L, Kasten J, Tippmer S. Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood 2010; 115(14):2960-70. doi: 10.1182/blood-2009-08-236356 [Crossref] [ Google Scholar]
- Rooney CM, Smith CA, Ng CY, Loftin S, Li C, Krance RA. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet 1995; 345(8941):9-13. doi: 10.1016/s0140-6736(95)91150-2 [Crossref] [ Google Scholar]
- Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med 2006; 12(10):1160-6. doi: 10.1038/nm1475 [Crossref] [ Google Scholar]
- Gerdemann U, Christin AS, Vera JF, Ramos CA, Fujita Y, Liu H. Nucleofection of DCs to generate multivirus-specific T cells for prevention or treatment of viral infections in the immunocompromised host. Mol Ther 2009; 17(9):1616-25. doi: 10.1038/mt.2009.140 [Crossref] [ Google Scholar]
- Ugarte-Torres A, Hoegh-Petersen M, Liu Y, Zhou F, Williamson TS, Quinlan D. Donor serostatus has an impact on cytomegalovirus-specific immunity, cytomegaloviral disease incidence, and survival in seropositive hematopoietic cell transplant recipients. Biol Blood Marrow Transplant 2011; 17(4):574-85. doi: 10.1016/j.bbmt.2010.07.020 [Crossref] [ Google Scholar]
- Safdar A, Decker WK, Li S, Xing D, Robinson SN, Yang H. De novo T-lymphocyte responses against baculovirus-derived recombinant influenzavirus hemagglutinin generated by a naive umbilical cord blood model of dendritic cell vaccination. Vaccine 2009; 27(10):1479-84. doi: 10.1016/j.vaccine.2009.01.017 [Crossref] [ Google Scholar]
- Vera JF, Brenner LJ, Gerdemann U, Ngo MC, Sili U, Liu H. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex). J Immunother 2010; 33(3):305-15. doi: 10.1097/CJI.0b013e3181c0c3cb [Crossref] [ Google Scholar]
- Hanley PJ, Cruz CR, Savoldo B, Leen AM, Stanojevic M, Khalil M. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood 2009; 114(9):1958-67. doi: 10.1182/blood-2009-03-213256 [Crossref] [ Google Scholar]
- Luo XH, Huang XJ, Liu KY, Xu LP, Liu DH. Protective immunity transferred by infusion of cytomegalovirus-specific CD8 + T cells within donor grafts: its associations with cytomegalovirus reactivation following unmanipulated allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2010; 16(7):994-1004. doi: 10.1016/j.bbmt.2010.02.007 [Crossref] [ Google Scholar]
- Yang OO, Kalams SA, Rosenzweig M, Trocha A, Jones N, Koziel M. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J Virol 1996; 70(9):5799-806. doi: 10.1128/jvi.70.9.5799-5806.1996 [Crossref] [ Google Scholar]
- Herndon TM, Pirone DM, Tsokos GC, Chen CS. T cell-to-T cell clustering enhances NF-kappaB activity by a PI3K signal mediated by Cbl-b and Rho. BiochemBiophys Res Commun 2005; 332(4):1133-9. doi: 10.1016/j.bbrc.2005.05.064 [Crossref] [ Google Scholar]
- Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naïve, central memory and effector memory CD4 + T cells. Pathol Biol (Paris) 2003; 51(2):64-6. doi: 10.1016/s0369-8114(03)00098-1 [Crossref] [ Google Scholar]
- Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol 2009; 9(7):480-90. doi: 10.1038/nri2580 [Crossref] [ Google Scholar]
- Onlamoon N, Hudson K, Bryan P, Mayne AE, Bonyhadi M, Berenson R. Optimization of in vitro expansion of macaque CD4 T cells using anti-CD3 and co-stimulation for autotransfusion therapy. J Med Primatol 2006; 35(4-5):178-93. doi: 10.1111/j.1600-0684.2006.00182.x [Crossref] [ Google Scholar]
- Winstone N, Guimarães-Walker A, Roberts J, Brown D, Loach V, Goonetilleke N. Increased detection of proliferating, polyfunctional, HIV-1-specific T cells in DNA-modified vaccinia virus Ankara-vaccinated human volunteers by cultured IFN-gamma ELISPOT assay. Eur J Immunol 2009; 39(4):975-85. doi: 10.1002/eji.200839167 [Crossref] [ Google Scholar]
- Soares MV, Borthwick NJ, Maini MK, Janossy G, Salmon M, Akbar AN. IL-7-dependent extrathymic expansion of CD45RA + T cells enables preservation of a naive repertoire. J Immunol 1998; 161(11):5909-17. [ Google Scholar]
- Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4 + memory T cells are IL-15 dependent. J Exp Med 2007; 204(4):951-61. doi: 10.1084/jem.20061805 [Crossref] [ Google Scholar]
- Picker LJ, Reed-Inderbitzin EF, Hagen SI, Edgar JB, Hansen SG, Legasse A. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest 2006; 116(6):1514-24. doi: 10.1172/jci27564 [Crossref] [ Google Scholar]
- Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science 1992; 257(5067):238-41. doi: 10.1126/science.1352912 [Crossref] [ Google Scholar]
- Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med 1995; 333(16):1038-44. doi: 10.1056/nejm199510193331603 [Crossref] [ Google Scholar]
- Krishna AB, Manikyam HK, Sharma VK, Sharma N. Plant cardenolides in therapeutics. Int J Indigenous Med Plants 2015; 48(2):1871-96. [ Google Scholar]
- Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Löffler J. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 2002; 99(11):3916-22. doi: 10.1182/blood.v99.11.3916 [Crossref] [ Google Scholar]
- Haque T, Wilkie GM, Jones MM, Higgins CD, Urquhart G, Wingate P. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood 2007; 110(4):1123-31. doi: 10.1182/blood-2006-12-063008 [Crossref] [ Google Scholar]
- Peggs KS, Verfuerth S, Pizzey A, Khan N, Guiver M, Moss PA. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet 2003; 362(9393):1375-7. doi: 10.1016/s0140-6736(03)14634-x [Crossref] [ Google Scholar]
- Perruccio K, Tosti A, Burchielli E, Topini F, Ruggeri L, Carotti A. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood 2005; 106(13):4397-406. doi: 10.1182/blood-2005-05-1775 [Crossref] [ Google Scholar]
- Heslop HE, Ng CY, Li C, Smith CA, Loftin SK, Krance RA. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med 1996; 2(5):551-5. doi: 10.1038/nm0596-551 [Crossref] [ Google Scholar]
- Schaiquevich P, Fabius AW, Francis JH, Chantada GL, Abramson DH. Ocular pharmacology of chemotherapy for retinoblastoma. Retina 2017; 37(1):1-10. doi: 10.1097/iae.0000000000001275 [Crossref] [ Google Scholar]
- Micklethwaite KP, Clancy L, Sandher U, Hansen AM, Blyth E, Antonenas V. Prophylactic infusion of cytomegalovirus-specific cytotoxic T lymphocytes stimulated with Ad5f35pp65 gene-modified dendritic cells after allogeneic hemopoietic stem cell transplantation. Blood 2008; 112(10):3974-81. doi: 10.1182/blood-2008-06-161695 [Crossref] [ Google Scholar]
- Feuchtinger T, Opherk K, Bethge WA, Topp MS, Schuster FR, Weissinger EM. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood 2010; 116(20):4360-7. doi: 10.1182/blood-2010-01-262089 [Crossref] [ Google Scholar]
- Uhlin M, Okas M, Gertow J, Uzunel M, Brismar TB, Mattsson J. A novel haplo-identical adoptive CTL therapy as a treatment for EBV-associated lymphoma after stem cell transplantation. Cancer Immunol Immunother 2010; 59(3):473-7. doi: 10.1007/s00262-009-0789-1 [Crossref] [ Google Scholar]
- Schmitt A, Tonn T, Busch DH, Grigoleit GU, Einsele H, Odendahl M. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8 + T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion 2011; 51(3):591-9. doi: 10.1111/j.1537-2995.2010.02940.x [Crossref] [ Google Scholar]
- Meij P, Jedema I, Zandvliet ML, van der Heiden PL, van de Meent M, van Egmond HM. Effective treatment of refractory CMV reactivation after allogeneic stem cell transplantation with in vitro-generated CMV pp65-specific CD8 + T-cell lines. J Immunother 2012; 35(8):621-8. doi: 10.1097/CJI.0b013e31826e35f6 [Crossref] [ Google Scholar]
- Uhlin M, Gertow J, Uzunel M, Okas M, Berglund S, Watz E. Rapid salvage treatment with virus-specific T cells for therapy-resistant disease. Clin Infect Dis 2012; 55(8):1064-73. doi: 10.1093/cid/cis625 [Crossref] [ Google Scholar]
- Doubrovina E, Oflaz-Sozmen B, Prockop SE, Kernan NA, Abramson S, Teruya-Feldstein J. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV + lymphomas after allogeneic hematopoietic cell transplantation. Blood 2012; 119(11):2644-56. doi: 10.1182/blood-2011-08-371971 [Crossref] [ Google Scholar]
- Blyth E, Clancy L, Simms R, Ma CK, Burgess J, Deo S. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood 2013; 121(18):3745-58. doi: 10.1182/blood-2012-08-448977 [Crossref] [ Google Scholar]
- Icheva V, Kayser S, Wolff D, Tuve S, Kyzirakos C, Bethge W. Adoptive transfer of Epstein-Barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol 2013; 31(1):39-48. doi: 10.1200/jco.2011.39.8495 [Crossref] [ Google Scholar]
- Qasim W, Gilmour K, Zhan H, Derniame S, McNicol AM, Ip W. Interferon-γ capture T cell therapy for persistent adenoviraemia following allogeneic haematopoietic stem cell transplantation. Br J Haematol 2013; 161(3):449-52. doi: 10.1111/bjh.12251 [Crossref] [ Google Scholar]
- Geyeregger R, Freimüller C, Stemberger J, Artwohl M, Witt V, Lion T. First-in-man clinical results with good manufacturing practice (GMP)-compliant polypeptide-expanded adenovirus-specific T cells after haploidentical hematopoietic stem cell transplantation. J Immunother 2014; 37(4):245-9. doi: 10.1097/cji.0000000000000034 [Crossref] [ Google Scholar]
- Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang Z. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Biosci Trends 2015; 9(1):16-34. doi: 10.5582/bst.2015.01019 [Crossref] [ Google Scholar]
- Feucht J, Opherk K, Lang P, Kayser S, Hartl L, Bethge W. Adoptive T-cell therapy with hexon-specific Th1 cells as a treatment of refractory adenovirus infection after HSCT. Blood 2015; 125(12):1986-94. doi: 10.1182/blood-2014-06-573725 [Crossref] [ Google Scholar]
- Pei XY, Zhao XY, Chang YJ, Liu J, Xu LP, Wang Y. Cytomegalovirus-specific T-cell transfer for refractory cytomegalovirus infection after haploidentical stem cell transplantation: the quantitative and qualitative immune recovery for cytomegalovirus. J Infect Dis 2017; 216(8):945-56. doi: 10.1093/infdis/jix357 [Crossref] [ Google Scholar]
- Withers B, Blyth E, Clancy LE, Yong A, Fraser C, Burgess J. Long-term control of recurrent or refractory viral infections after allogeneic HSCT with third-party virus-specific T cells. Blood Adv 2017; 1(24):2193-205. doi: 10.1182/bloodadvances.2017010223 [Crossref] [ Google Scholar]
- Withers B, Clancy L, Burgess J, Simms R, Brown R, Micklethwaite K. Establishment and operation of a third-party virus-specific T cell bank within an allogeneic stem cell transplant program. Biol Blood Marrow Transplant 2018; 24(12):2433-42. doi: 10.1016/j.bbmt.2018.08.024 [Crossref] [ Google Scholar]
- Kállay K, Kassa C, Réti M, Karászi É, Sinkó J, Goda V. Early experience with CliniMACS prodigy CCS (IFN-gamma) system in selection of virus-specific T cells from third-party donors for pediatric patients with severe viral infections after hematopoietic stem cell transplantation. J Immunother 2018; 41(3):158-63. doi: 10.1097/cji.0000000000000197 [Crossref] [ Google Scholar]
- ClinicalTrails.gov. ARMS - Rapidly Generated Multivirus-Specific CTLs for Prophylaxis & Treatment of EBV, CMV, Adenovirus, HHV6 & BK Virus (ARMS). 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT01570283.
- ClinicalTrails.gov. Primary Transplant Donor Derived CMVpp65 Specific T-cells for The Treatment of CMV Infection or Persistent CMV Viremia After Allogeneic Hematopoietic Stem Cell Transplantation. 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT01646645.
- ClinicalTrails.gov. Multivirus-specific T Cells for the Treatment of Virus Infections After Stem Cell Transplant (CHARMS). 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT02108522.
- Kennedy-Nasser AA, Bollard CM, Myers GD, Leung KS, Gottschalk S, Zhang Y. Comparable outcome of alternative donor and matched sibling donor hematopoietic stem cell transplant for children with acute lymphoblastic leukemia in first or second remission using alemtuzumab in a myeloablative conditioning regimen. Biol Blood Marrow Transplant 2008; 14(11):1245-52. doi: 10.1016/j.bbmt.2008.08.010 [Crossref] [ Google Scholar]
- Brunstein CG, Weisdorf DJ, DeFor T, Barker JN, Tolar J, van Burik JA. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood 2006; 108(8):2874-80. doi: 10.1182/blood-2006-03-011791 [Crossref] [ Google Scholar]
- Salzberger B, Bowden RA, Hackman RC, Davis C, Boeckh M. Neutropenia in allogeneic marrow transplant recipients receiving ganciclovir for prevention of cytomegalovirus disease: risk factors and outcome. Blood 1997; 90(6):2502-8. doi: 10.1182/blood.V90.6.2502 [Crossref] [ Google Scholar]
- Matthes-Martin S, Feuchtinger T, Shaw PJ, Engelhard D, Hirsch HH, Cordonnier C. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL-4 (2011). Transpl Infect Dis 2012; 14(6):555-63. doi: 10.1111/tid.12022 [Crossref] [ Google Scholar]
- Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood 2003; 101(2):407-14. doi: 10.1182/blood-2002-03-0993 [Crossref] [ Google Scholar]
- Lurain NS, Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev 2010; 23(4):689-712. doi: 10.1128/cmr.00009-10 [Crossref] [ Google Scholar]
- Nichols WG, Corey L, Gooley T, Drew WL, Miner R, Huang M. Rising pp65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood 2001; 97(4):867-74. doi: 10.1182/blood.v97.4.867 [Crossref] [ Google Scholar]
- Kogiso T, Sagawa T, Kodama K, Taniai M, Katagiri S, Egawa H. Hepatocellular carcinoma after direct-acting antiviral drug treatment in patients with hepatitis C virus. JGH Open 2019; 3(1):52-60. doi: 10.1002/jgh3.12105 [Crossref] [ Google Scholar]
- Mackinnon S, Thomson K, Verfuerth S, Peggs K, Lowdell M. Adoptive cellular therapy for cytomegalovirus infection following allogeneic stem cell transplantation using virus-specific T cells. Blood Cells Mol Dis 2008; 40(1):63-7. doi: 10.1016/j.bcmd.2007.07.003 [Crossref] [ Google Scholar]
- Feuchtinger T, Matthes-Martin S, Richard C, Lion T, Fuhrer M, Hamprecht K. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br J Haematol 2006; 134(1):64-76. doi: 10.1111/j.1365-2141.2006.06108.x [Crossref] [ Google Scholar]
- Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood 2009; 114(19):4283-92. doi: 10.1182/blood-2009-07-232454 [Crossref] [ Google Scholar]
- Comoli P, Basso S, Zecca M, Pagliara D, Baldanti F, Bernardo ME. Preemptive therapy of EBV-related lymphoproliferative disease after pediatric haploidentical stem cell transplantation. Am J Transplant 2007; 7(6):1648-55. doi: 10.1111/j.1600-6143.2007.01823.x [Crossref] [ Google Scholar]
- Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 2010; 115(5):925-35. doi: 10.1182/blood-2009-08-239186 [Crossref] [ Google Scholar]
- Schönberger S, Meisel R, Adams O, Pufal Y, Laws HJ, Enczmann J. Prospective, comprehensive, and effective viral monitoring in children undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2010; 16(10):1428-35. doi: 10.1016/j.bbmt.2010.04.008 [Crossref] [ Google Scholar]
- Sokos DR, Berger M, Lazarus HM. Intravenous immunoglobulin: appropriate indications and uses in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2002; 8(3):117-30. doi: 10.1053/bbmt.2002.v8.pm11939601 [Crossref] [ Google Scholar]
- Qasim W, Derniame S, Gilmour K, Chiesa R, Weber M, Adams S. Third-party virus-specific T cells eradicate adenoviraemia but trigger bystander graft-versus-host disease. Br J Haematol 2011; 154(1):150-3. doi: 10.1111/j.1365-2141.2011.08579.x [Crossref] [ Google Scholar]
- Barker JN, Doubrovina E, Sauter C, Jaroscak JJ, Perales MA, Doubrovin M. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood 2010; 116(23):5045-9. doi: 10.1182/blood-2010-04-281873 [Crossref] [ Google Scholar]
- Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 2013; 121(26):5113-23. doi: 10.1182/blood-2013-02-486324 [Crossref] [ Google Scholar]