Advanced pharmaceutical bulletin. 13(4):723-735.
doi: 10.34172/apb.2023.081
Systematic Review
ChAdOx1 nCoV-19 Vaccine and Thrombosis with Thrombocytopenia Syndrome among Adults: A Systematic Review
Homa Faghihi Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing – original draft, Writing – review & editing, 1 
Negar Mottaghi-Dastjerdi Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing – original draft, Writing – review & editing, 2, * 
Mohammad Sharifzadeh Investigation, Resources, Supervision, Validation, Visualization, Writing – review & editing, 3
Nader Rahimi Kakavandi Formal analysis, Project administration, Validation, Writing – review & editing, 4, 5 
Author information:
1Department of Pharmaceutics and Pharmaceutical Nanotechnology, School of Pharmacy Iran University of Medical Sciences, Tehran, Iran.
2Department of Pharmacognosy and Pharmaceutical Biotechnology, School of Pharmacy, Iran University of Medical Sciences, Tehran, Iran.
3Department of Pharmacology and Toxicology, Faculty of Pharmacy, Toxicology and Poisoning Research Centre, Tehran University of Medical Sciences, Tehran, Iran.
4Department of Toxicology & Pharmacology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.
5Health and Environment Research Center, Ilam University of Medical Sciences, Ilam, Iran.
Abstract
Several vaccine-induced thrombotic thrombocytopenia syndrome (VITTS) cases have been reported after the ChAdOx1 nCov-19 vaccination. The current study systematically reviewed the reported post-ChAdOx1 nCoV-19 vaccination thrombotic thrombocytopenia cases. Their laboratory and clinical features, as well as the diagnostic and therapeutic measures, were investigated. Online databases were searched until 25 August 2021. Studies reporting post-ChAdOx1 nCov-19 vaccination thrombotic thrombocytopenia syndrome (TTS) were included. Overall, 167 cases (21-77 years old) from 53 publications were included showing a female dominance of 1.75 times. About 85% of the cases exhibited the primary symptoms within the first two weeks post-vaccination. Headache was the most common initial symptom (>44.2%), and hemorrhage/thrombotic problems (22.46%), as well as discoordination/weakness/numbness/ hemiparesis/cyanotic toes (19.6%), were the most prevalent uncommon initial symptoms. Prothrombin time (PT), D-dimers, and C-reactive protein were the most remarkable increased laboratory parameters in 50.6%, 99.1%, and 55.6% of cases, respectively. In comparison, platelet and fibrinogen were the most remarkable decreased laboratory parameters in 92.7% and 50.5% of cases, respectively. Most VITT cases presented with cerebral venous thrombosis/cerebral venous sinus thrombosis, supraventricular tachycardia, transverse sinus/cerebral thrombosis, pulmonary embolism, and cerebral hemorrhage. Anti-PF4 antibody measurement through immunoassays and functional assays were positive in 86.2% and 73% of cases, respectively. About 31% of the cases died. Early diagnosis and proper therapeutic measures are important in ChAdOx1 nCov-19 vaccine-induced VITTS patients. Therefore, experts are recommended to know the corresponding clinical and laboratory features, as well as diagnostic methods. Elucidation of the pathophysiologic mechanism of ChAdOx1 nCov-19 vaccine-induced TTS deserves further investigation.
Keywords: Cerebral venous sinus thrombosis, Cerebral venous thrombosis, ChAdOx1 nCov-19 vaccine, Oxford AstraZeneca COVID-19 vaccine, Thrombotic thrombocytopenia syndrome
Copyright and License Information
©2023 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Vaccine-induced thrombotic thrombocytopenia (VITT) is a severe adverse event upon vaccination associated with extraordinary thrombosis and a concurrent decrease in platelet counts. VITT is likewise known as vaccine-induced prothrombotic immune thrombocytopenia and/or thrombosis with thrombocytopenia syndrome (TTS).1 ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and Janssen COVID-19 vaccines, as adenoviral vector-based vaccines, have been implicated in creating VITT. The probable explanation for such phenomenon is that the free existing DNA in these vaccines might bind to anti-platelet factor 4 (PF4) antibodies.2 VITT is mainly attributed to PF4 antibodies partially like heparin-induced thrombocytopenia (HIT) regarding clinical and biochemical aspects. These immunoglobulin G class antibodies activate platelets via FcɣRIIa receptors, causing them to clump together, leading to clot formation and thrombocytopenia.3
About a hundred cases of thrombosis at atypical sites such as cerebral sinus, splanchnic veins, and the right ventricle with variable degrees of thrombocytopenia have been reported 5 to 30 days upon vaccination with Oxford/AstraZeneca and Janssen COVID-19 vaccines. Microvascular events in the brain, the lungs, and the kidneys have been additionally observed.4 The precise incidence of VITT after vaccination against COVID-19 remains ambiguous due to insufficient clinical experiences, complicated diagnostic methods, several feasibly-involved mechanisms, and lack of well-defined periods for follow-up.5,6 Based on data latest updated in November 2021 offered by Uptodate.com, the highest incidence rates following Oxford/AstraZeneca and Janssen vaccine were 1 in 26 000 and 1 in 533 333, respectively. While crucial risk factors for VITT have not been comprehensively known, young females are proposed as the most vulnerable groups to such an adverse event. Unfortunately, patients with VITT often exhibit intravascular coagulation combined with thrombocytopenia without noticeable clinical symptoms until the immediate onset of thrombosis.7
Infection with SARS-CoV-2 can cause the systemic release of viral RNA leading to activation of the innate immune coagulation pathway associated with systemic and pulmonary immunothrombosis. Recently, COVID-19 viral vectored vaccines such as the ChAdOx1 nCoV-19 vaccine are associated with thrombotic thrombocytopenia after vaccination called VITT.3 One of the main mechanisms clarified by the Greifswald Working Group with Andreas Greinacher leadership was antibody formation against platelet antigens (anti-PF4) due to the stimulation of the immune system and inflammatory reactions. These antibodies can finally lead to an extensive activation of the platelets via the Fc receptor, which resembles HIT.8 After intramuscular administration of an adenoviral-vectored vaccine, a cascade of events occurs, including microvascular damage, microbleeding and activation of the platelets with the release of PF4 and disperse of the adenovirus cargo with the engagement of DNA-PF4 can interrupt the immune tolerance causing rare autoimmunity directed by PF4.3 According to the reported deaths associated with ChAdOx1 nCoV-19 post-vaccination VITT, early diagnosis and fast therapeutic measures could benefit the outcome of the patients.
In this study, we systematically reviewed the reported cases of post-vaccination thrombotic thrombocytopenia contributed to the ChAdOx1 nCoV-19 vaccine and investigated their laboratory and clinical features and the diagnostic and therapeutic measures applied in these cases.
Methods
This study was performed based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) protocol for reporting systematic reviews and meta-analyses.
Search strategy
We performed a comprehensive literature search in the online databases of PubMed, Scopus, and Google Scholar up to August 25th, 2021. In the investigation, we purposed to identify case reports investigating the effects of ChAdOx1 nCoV-19 vaccination on vaccine-induced immune thrombotic thrombocytopenia in adults. The following keywords were used in the search strategy: (Thrombosis OR Thromboses OR Thrombus OR “Blood Clot” OR “Blood Clots” OR “Clot, Blood” OR “Clots, Blood” OR Thrombocytopenia OR “cerebral venous sinus thrombosis (CVST )” OR “Vaccine-induced immune thrombotic thrombocytopenia (VITT)” OR “vaccine-induced prothrombotic immune thrombocytopenia (VIPIT)” OR Platelets OR “Blood Platelet” OR “Platelet, Blood” OR “Platelets, Blood” OR Thrombocytes OR thrombocyte OR platelets OR platelet OR “low-platelet syndrome” OR Thrombocytopenias OR thrombopenia OR thrombopenias) AND (AstraZeneca OR “ChAdOx1 SARS2 vaccine” OR “ChAdOx1 nCoV-19 “ OR Covishield OR AZD1222 OR “Oxford AstraZeneca COVID-19 vaccine” OR “ChAdOx1 COVID-19 vaccine” OR “COVID-19 vaccine” OR “SARS-CoV-2 vaccine”). No time restriction was applied. References of the relevant publications were manually screened to avoid missing any eligible studies. Unpublished researches were not included. Two independent investigators conducted a literature search.
Inclusion criteria
We included eligible studies that met the following criteria: 1) case reports, 2) studies that administered ChAdOx1 nCoV-19, 3) case reports and case series with thrombocytopenia and thrombosis after ChAdOx1 nCoV-19 administration. The complete one was included if > 1 article was published for one dataset.
Exclusion criteria
In the current systematic review, we excluded experimental studies, those with a cohort, cross-sectional, and case-control design, clinical trials, and review articles. We also excluded studies with reports on thrombosis without thrombocytopenia or thrombocytopenia without thrombosis after ChAdOx1 nCoV-19 vaccine administration.
Data extraction
Two independent investigators performed data extraction from each eligible case report and case series. The following information was extracted: name of the first author, publication year, individuals’ characteristics (age, sex, ethnicity, smoker/non-smoker), place of measure, number of cases in each study, first dose of ChAdOx1 nCoV-19 vaccine, medical history, previous exposure to heparin, medication on admission, chief complaint/initial symptom, onset of symptom (days) post vaccination (initial symptoms/thrombocytopenia/thrombotic complications), therapeutic measures, medical examination/imaging method/site of thrombosis/hemorrhages, outcome, laboratory parameters including hemoglobin, platelets/white/red blood cells count, prothrombin time (PT)/ international normalized ratio (INR), activated partial thromboplastin time (ratio)/aPTT/PTT, thrombin time, fibrinogen, D-dimers, antithrombin, C-reactive protein, haptoglobin, neutrophil, lymphocyte, monocyte, eosinophil, aminotransferase, Gamma glutamyl transferase, sodium, potassium, calcium, glucose, nitrogen urea, lactate dehydrogenase, lactate, total bilirubin, amylase, coagulation factor, folic acid, vitamin B12, pro-calcitonin, creatinine, total protein, albumin, paroxysmal nocturnal haemoglobinuria, ferritin, interleukin, cholesterol/ high-density lipoprotein ratio, urea, EXTEM CT, EXTEM A10, FIBTEM A10, INTEM CT, fibrin degradation product, protein C activity, protein S (free antigen), VWF, thrombophilia mutations (factor V Leiden, prothrombin G20210A, MTHFR, Janus kinase 2 (JAK2)), antiphospholipid antibodies, anti-thyroid peroxidase, myeloperoxidase, anti-neutrophil cytoplasmic anti-bodies, rheumatoid factor, antinuclear antibodies, extractable nuclear antigen, dsDNA, anti-globulin test, neutrophil DNA extracellular traps, schistocytes on peripheral blood smear, blood film, immunoglobulins IgA/IgM/IgG, complement, cryoglobulins, ADAMTS13, antiplatelet antibodies, homocysteine, septic screen of blood/urine/respiratory cultures, acute kidney injury, SARS-CoV-2 screening, test for other possible causes of thrombocytopenia including hepatitis B virus, hepatitis C virus, HIV, Epstein–Barr virus, cytomegalovirus, hantaviruses, and Helicobacter pylori infections, as well as anti-PF4 antibodies screening (immunoassays and functional assays). If data on laboratory parameters were reported in different units, we converted them to the most frequently used unit.
Results and Discussion
Many regulatory agencies approve the Oxford-AstraZeneca COVID-19 vaccine with the viral vector platform for the prevention of COVID-19. Also called Vaxzevria, Covishield, AZD1222, ChAdOx1 SARS2 vaccine, and ChAdOx1 nCov-19, this vaccine has an efficacy of 66.7% two weeks after the second dose.9 Common side effects of the ChAdOx1 nCoV-19 vaccine include site reaction (tenderness, pain, warmth, itching or bruising), chills or feverish, headache, nausea, fatigue, unwell feeling, and joint pain, or muscle ache; all be resolved within a few days.10 However, there were few reports on rare cases of thrombosis at unusual sites associated with thrombocytopenia shortly after ChAdOx1 nCov-19 vaccine administration.2,11 Accordingly, in this systematic review, we investigated different features of the studies reporting the cases with thrombotic thrombocytopenia syndrome (TTS) after the ChAdOx1 nCov-19 vaccine administration.
Totally, 4924 publications were identified in our initial search. After the screening, 4856 unrelated articles were excluded based on duplication (n = 108), title, and abstract assessment. Then, 62 publications remained for further evaluation of a full text. Out of these 62 eligible publications, five studies were also excluded due to the absence of thrombosis,12-16 and four studies were excluded due to the lack of thrombocytopenia.17-20 The flow diagram of study selection is outlined in Figure 1.
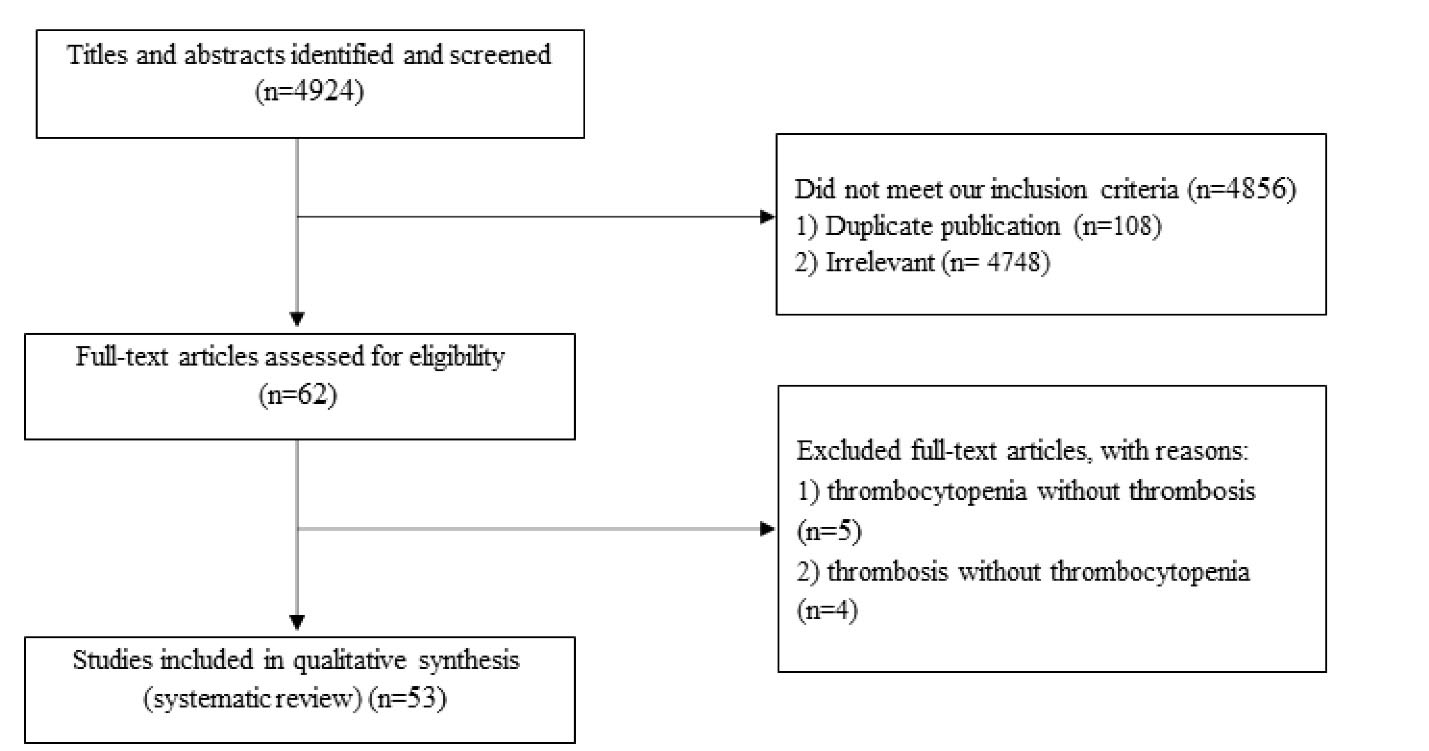
Figure 1.
Flow diagram of study selection
.
Flow diagram of study selection
Characteristics of the included studies
The characteristics of 53 case reports included in the current systematic review are illustrated in Table 1.One hundred and sixty-seven cases are included in the 53 studies in this systematic review (63.6% females and 36.4% males). These cases were between 21 to 77 years old (19.6% (20-29 years old), 20.9% (30-39 years old), 21.5% (40-49 years old), 15.3% (50-59 y/o), 16.6% (60-69 years old), and 6.1% (70-77 years old)). The primary symptoms after ChAdOx1 nCov-19 vaccination initiated from the first day ranged from 0-26 days after vaccination. However, these cases’ thrombocytopenia/thrombotic complications started from day 2 to 35 days after vaccination with ChAdOx1 nCov-19.
Table 1.
Summary of case reports on VITT after ChAdOx1 nCov-19 vaccination
|
First Author
|
Year
|
No. of cases
|
Onset of primary side effects post vaccination, days
|
Onset of symptoms (thrombocytopenia /thrombotic
complications) post vaccination, days
|
Age (y)
|
Sex
|
Place of measure
|
Ethnicity
|
Ref.
|
| Greinacher |
2021 |
9 |
4-16 |
4-16 |
22-49 |
8 F, 1 M |
Germany |
NA |
11
|
| Al-Mayhani |
2021 |
3 |
6-21 |
11-21 |
35-43 |
2 F, 1 M |
UK |
2 Asian
1 White |
21
|
| Scully |
2021 |
23 |
NA |
6-24 (22 out of 23) |
21-77 |
14 F, 9 M |
UK |
NA |
22
|
| Schultz |
2021 |
5 |
7-10 |
7-10 |
32-54 |
4 F, 1 M |
Norway |
NA |
23
|
| Greinacher |
2021 |
11 |
NA |
5-16 |
22-49 |
9 F, 2 M |
Germany and Austria |
NA |
2
|
| Bayas |
2021 |
1 |
0 |
8 |
55 |
1 F |
Germany |
NA |
24
|
| Blauenfeldt |
2021 |
1 |
< 7 |
7 |
60 |
1 F |
Denmark |
Danish |
25
|
| Mehta |
2021 |
2 |
6-9 |
6-9 |
25-32 |
2 M |
UK |
White |
26
|
| Castelli |
2021 |
1 |
7 |
7 |
50 |
1 M |
Italy |
Caucasian |
27
|
| D’Agostino |
2021 |
1 |
12 |
12 |
54 |
1 F |
Italy |
NA |
28
|
| Abu Esba |
2021 |
2 |
14 |
> 14-16 |
40-61 |
1 F, 1 M |
Saudi Arabia |
NA |
29
|
| Franchini |
2021 |
1 |
7 |
11 |
50 |
1 M |
Italy |
White |
30
|
| Garnier |
2021 |
1 |
0 |
8 |
26 |
1 F |
France |
NA |
31
|
| Geeraerts |
2021 |
2 |
NA |
NA |
NA |
NA |
France |
NA |
32
|
| Gras-Champel |
2021 |
27 |
NA |
2-35 |
21-74 |
13 F, 14 M |
France |
NA |
33
|
| Jones |
2021 |
1 |
20 |
25 |
63 |
1 M |
UK |
NA |
34
|
| Ramdeny |
2021 |
1 |
14 |
21 |
54 |
1 M |
UK |
NA |
35
|
| Wolf |
2021 |
3 |
0-8 |
7-17 |
22-46 |
3 F |
Germany |
NA |
36
|
| Xie |
2021 |
1 |
< 7 |
7 |
23 |
1 F |
UK |
NA |
37
|
| Muster |
2021 |
1 |
8 |
8 |
51 |
1 F |
Austria |
NA |
38
|
| Aladdin |
2021 |
1 |
14 |
14 |
36 |
1 F |
Saudi Arabia |
NA |
39
|
| Althaus |
2021 |
8 |
6-20 |
6-20 |
41.5 (24-53) |
5 F, 3 M |
Germany |
NA |
40
|
| Bano |
2021 |
3 |
8-13 |
10-16 |
53-61 |
2 F
1 M |
UK |
NA |
41
|
| Bonato |
2021 |
1 |
14 |
14 |
26 |
1 F |
Italy |
Italian |
42
|
| Bourguignon |
2021 |
3 |
7-18 |
15-24 |
63-72 |
1 F, 2 M |
Canada |
NA |
43
|
| Choi |
2021 |
1 |
0 |
12 |
33 |
1 M |
Korea |
Korean |
44
|
| Cliff-Patel |
2021 |
3 |
8-21 |
8-28 |
28-61 |
3 M |
UK |
NA |
45
|
| Gangi |
2021 |
6 |
3-26 |
9-31 |
38 (26-48) |
4 M, 2 F |
UK |
NA |
46
|
| Gattringer |
2021 |
2 |
6-8 |
8-12 |
24-39 |
2 F |
Austria |
NA |
47
|
| Gessler |
2021 |
2 |
7-12 |
7-12 |
47-50 |
2 F |
Germany |
NA |
48
|
| Graf |
2021 |
1 |
9 |
14 |
29 |
1 M |
Germany |
NA |
49
|
| Guan |
2021 |
1 |
5 |
10 |
52 |
1 M |
Taiwan |
East Asian |
50
|
| Huang |
2021 |
1 |
0 |
10 |
34 |
1 M |
Taiwan |
Asian |
51
|
| Ikenberg |
2021 |
1 |
0 |
7 |
early 30s |
1 F |
Germany |
NA |
52
|
| Jacob |
2021 |
1 |
0 |
7 |
39 |
1 F |
UK |
NA |
53
|
| Khuhapinant |
2021 |
1 |
3 |
8 |
26 |
1 F |
Thailand |
Asian |
54
|
| Mauriello |
2021 |
1 |
1 |
18 |
48 |
1 F |
Italy |
Caucasian |
55
|
| De Michele |
2021 |
2 |
7-9 |
9-10 |
55-57 |
2 F |
Italy |
NA |
56
|
| Panovska-Stavridis |
2021 |
1 |
9 |
10 |
29 |
1 F |
Macedonia |
Caucasian |
57
|
| Patriquin |
2021 |
3 |
10-16 |
10-16 |
45-48 |
3 F |
Canada |
NA |
58
|
| Paul |
2021 |
1 |
5 |
5 |
middle aged |
1 M |
India |
NA |
59
|
| Ramessur |
2021 |
1 |
0 |
14 |
73 |
1 M |
UK |
NA |
60
|
| Soleimani |
2021 |
3 |
4-11 |
10-14 |
34- 59 |
1 M, 2 F |
UK |
NA |
61
|
| Tølbøll Sørensen |
2021 |
1 |
8 |
11 |
30 |
1 F |
Denmark |
NA |
62
|
| Suresh |
2021 |
1 |
2 |
12 |
27 |
1 M |
UK |
NA |
63
|
| Tejpal |
2021 |
1 |
20 |
20 |
61 |
1 M |
Canada |
NA |
64
|
| Tiede |
2021 |
5 |
5-11 |
6-15 |
41-67 |
5 F |
Germany |
NA |
65
|
| Turi |
2021 |
1 |
2 |
6 |
57 |
1 F |
Italy |
NA |
66
|
| Varona |
2021 |
1 |
10 |
11 |
47 |
1 M |
Spain |
NA |
67
|
| Vayne |
2021 |
9 |
9-18 |
9-18 |
44 (21-69) |
2 M, 7 F |
Singapore |
NA |
68
|
| Wang |
2021 |
1 |
0 |
7 |
41 |
1 F |
Taiwan |
NA |
69
|
| Wiedmann |
2021 |
1 |
7 |
10 |
34 |
1 F |
Norway |
NA |
70
|
| Zanferrari |
2021 |
1 |
0 |
7 |
40 |
1 F |
Italy |
NA |
71
|
Findings from the systematic review
Patient characteristics, site of thrombosis, and outcome of case reports on VITT after ChAdOx1 nCov-19 vaccination
Our results showed that TTS occurs 1.75 times more in women. Most cases (62%) aged between 20-49 years old. Among the cases with TTS in this systematic review, about 60% had no remarkable PMH of any personal or familial disease/risk factors, and about 24% had pre-existing conditions. In addition, less than 30% of the cases had several regular medications with contraceptives as the most used medication.22,23,33,40,42,46,62,70 However, the number of cases for each condition or medication is not enough to find a relationship between the pre-existing condition or regular medication and TTS risk post-ChAdOx1 nCoV-19 vaccination.
In addition to the common symptoms associated with administration of ChAdOx1 nCoV-19 vaccine with headache as the most common initial symptom ( > 44.2%),2,11,21-23,25-27,29-31,35,36,39,41-43,45-55,57,58,60-63,65,66,69-71 cases in this systematic review reported having some uncommon initial symptoms, including hemorrhage/thrombotic problems (22.46%)11,22,40,58,67,68 and discoordination/weakness/numbness/hemiparesis/cyanotic toes (19.6%)21,23,25,26,34,36,39,41-45,53,58,61,65,70 as the most prevalent uncommon initial symptoms which could be a signal alert to be aware of vaccine-induced thrombotic thrombocytopenia syndrome (VITTS). However, further study is needed to figure out a relationship between these initial symptoms and the risk of VITTS.
The initial symptoms were appeared within the first, second, third, fourth, and fifth week post vaccination with ChAdOx1 nCov-19 vaccine in 32.1%,2,11,21-27,30,31,33,36,37,40,43,44,46-48,50-56,59-61,63,65,66,69-71 53.3%,2,11,21-23,26,28,29,33,35,36,38-43,45-49,56-58,61,62,65,67,68 11.5%,2,11,22,33,34,40,43,45,58,64,68 1.8%,22,33,46 and 1.2%33 of the cases, respectively. These results showed that the initial symptoms after ChAdOx1 nCov-19 vaccination might start from the first day ranging from 0-26 days after vaccination. However, the thrombocytopenia/thrombotic complications in these cases started from day 2 to 35 days after vaccination with ChAdOx1 nCov-19. Our results confirmed that more than 85% of the cases showed the initial symptoms within the first- and second-week post-vaccination.
This systematic review also investigated the clinical features in a cohort of cases presenting with acute atypical thrombosis, mainly involving the cerebral veins and concurrent thrombocytopenia. Most cases involved in this systematic review presented with cerebral venous thrombosis/cerebral venous sinus thrombosis 2,11,21-23,29,30,32,33,35,40,41,44,46,50,52,61,62,65,67-69, supraventricular tachycardia (thrombosis of the portal, mesenteric, splenic, ileal, or hepatic veins),2,11,21-23,26,28-33,35,37,39-41,43,44,46,49,50,52,56,58,61-63,65-67,69 transverse sinus/cerebral thrombosis21,23,27-31,36,39,41-44,46,47,49,50,52,55,58,61,65,70 and pulmonary embolism,2,11,21-23,31,34,37,38,40,41,43,45,46,56,58,61,64,66-69 as well as cerebral hemorrhage (occipital, temporo-occipital, frontal, juxtacortical, cerebellar, intraparenchymal/hemispheric/parenchymal hemorrhage/subarachnoid hemorrhage).2,22,23,26-28,30,36,41,42,44,46,47,49,52,55,61,63,70,71 However, further study is needed to make a reliable conclusion on the relationship between the outcome of the cases and ChAdOx1 nCov-19 vaccine-induced TTS.
About 31.4% of the reported cases died2,11,21-23,25-28,30,32,33,39-41,44,48,55,56,63,70 and about 68.6% of the cases fully recovered.2,11,21-24,29,31,33-38,40-43,45-47,49-54,56-62,64,65,67,69,71 Details on patient characteristics, therapeutic measures, site of thrombosis, and outcome of case reports on VITT after ChAdOx1 nCov-19 vaccination are illustrated in Table S1a and summarized in Table S1b (see Supplementary fie 1).
Laboratory tests
Common laboratory parameters including hemoglobin, WBC, platelet, PT/INR, aPTT, fibrinogen, thrombin, D-dimers, C-reactive protein, and antithrombin were also assessed in the included cases with ChAdOx1 nCov-19 vaccine-induced TTS in our systematic review. Summary of these laboratory blood tests are illustrated in Table 2 and the details are illustrated in Table S2. Our results showed normal levels of antithrombin in all cases, high levels of WBC, PT/INR, aPTT, fibrinogen, thrombin, D-dimers, C-reactive protein in 33.3%, 50.6%, 20.5%, 4.4%, 14.3%, 99.1%, and 55.6% of cases, respectively as well as lower than normal levels of hemoglobin, WBC, platelet, PT, aPTT and fibrinogen in 22.2%, 13.3%, 92.7%, 1.3%, 11.5%, and 50.5% of cases, respectively. PT, D-dimers, and C-reactive protein were the most remarkable increased laboratory parameters in 50.6%, 99.1%, and 55.6% of cases, respectively. In comparison, platelet and fibrinogen were the most remarkable decreased laboratory parameters in 92.7% and 50.5% of cases, respectively. Accordingly, these laboratory parameters should be considered most. In addition, abnormally increased levels of CRP are correlated with worse prospects and more rates of mortality in patients with COVID-19.72 However, further studies are needed to find a reliable relationship between these common laboratory parameters and ChAdOx1 nCov-19 vaccine-induced TTS.
Table 2.
Summary of common laboratory blood tests and SARS-CoV-2 screening of case reports on VITT after ChAdOx1 nCov-19 vaccination
|
Hemoglobin (n=27)
|
White cell (n=15)
|
Platelets
(n=164)
|
PT/INR
(n=79)
|
Activated partial thromboplastin time (ratio)/aPTT/PTT
(n=78)
|
Thrombin time
(n=8)
|
Fibrinogen
(n=91)
|
D-dimers
(n=111)
|
Antithrombin
(n=12)
|
C-reactive protein
(n=27)
|
SARS-CoV-2 screening
(n=80)
|
| Normal: 77.8% |
Normal: 53.3% |
Normal: 7.3% |
Normal: 48.1% |
Normal: 67.9% |
Normal: 75% |
Normal: 45.1% |
Normal: 0.9% |
Normal: 100% |
Normal: 44.4% |
Negative: 98.75% |
| Low: 22.2% |
High: 33.3% |
Thrombocytopenia: 92.7% |
High: 50.6% |
High: 20.5% |
High: 25% |
High: 4.4% |
High: 99.1% |
|
High: 55.6% |
Positive: 1.25% |
|
|
Low: 13.3% |
|
Low: 1.3% |
Low: 11.5% |
|
Low: 50.5% |
|
|
|
|
Less common laboratory tests of case reports on VITT after ChAdOx1 nCov-19 vaccination are summarized in Table 3, and the details are illustrated in Table S3.
Table 3.
Summary of less common laboratory tests of case reports on VITT after ChAdOx1 nCov-19 vaccination
|
Laboratory parameters
|
Results
|
| Haptoglobin (n = 3) |
Normal: 100% |
| RBC (n = 5) |
Normal: 80%; Low: 20% |
| Neutrophil (n = 8) |
Normal: 37.5%; High: 62.5% |
| Lymphocyte (n = 5) |
Normal: 60%; Low: 40% |
| Monocyte (n = 5) |
Normal: 80%; Low: 20% |
| Eosinophil (n = 5) |
Normal: 100% |
| Basophil (n = 2) |
Normal: 100% |
| Aspartate aminotransferase (n = 6) |
AST |
| Alanine aminotransferase (n = 8) |
Normal: 100% |
| ALT |
| Normal: 37.5%; High: 62.5% |
| Gamma glutamyl transferase (n = 4) |
Normal: 25%; High: 75% |
| Alkaline phosphatase (n = 3) |
Normal: 100% |
| Sodium; Potassium (n = 2) |
Normal: 100% |
| Nitrogen urea (n = 2) |
Normal: 100% |
| Lactate dehydrogenase (n = 12) |
Normal: 41%; High: 59% |
| Lactate (n = 3) |
Normal: 100% |
| Total bilirubin (n = 10) |
Normal: 70%; High: 30% |
| Clotting factors (n = 9) |
Normal: 55%; Low:33%; High: 11% |
| Folic acid (n = 2) |
Low:100% |
| Vitamin B12 (n = 1) |
Normal: 100% |
| Procalcitonin (n = 1) |
Normal: 100% |
| Creatinine (n = 13) |
Normal: 85%; High: 15% |
| Total protein; Albumin (n = 1) |
Normal: 100% |
| Paroxysmal nocturnal hemoglobinuria (n = 3) |
Normal: 100% |
| Septic screening of blood, urine, and respiratory cultures (n = 1) |
Normal: 100% |
| Acute kidney injury (n = 2) |
Normal: 50%; High: 50% |
| Ferritin (n = 2) |
Normal: 100% |
| Cholesterol high-density lipoprotein ratio (n = 2) |
Normal: 50%; High: 50% |
| Urea (n = 1) |
Normal: 100% |
Summary of thrombophilia marker assays in case reports on VITT after ChAdOx1 nCov-19 vaccination is illustrated in Table 4. Antiphospholipid antibodies (lupus anticoagulant, beta-2-glycoprotein 1, and anticardiolipin antibodies) as the most commonly evaluated thrombophilia markers were evaluated in a couple of cases as follows2,11,22-26,30,32,34,36,37,41,42,47,53-57,61,62,65,66,71: Lupus anticoagulant measured in 20.3% of cases (positive in 8 cases, negative in 26 cases), beta-2-glycoprotein was evaluated in 20.3% of cases (positive in 2 cases, negative in 32 cases), and anticardiolipin antibodies were measured in 21.5% of cases (positive in 4 cases, negative in 32 cases). Details of thrombophilia marker assays in case reports on VITT after ChAdOx1 nCov-19 vaccination is illustrated in Table S4.Coagulopathy has been found in association with the detection of antiphospholipid antibodies, but the latter’s relation with VITT remains controversial. Although the levels of these markers have been measured in about 20% of all-studied cases, the outcome did not provide significant evidence of antiphospholipid antibody linkages to the occurrence of VITT post-vaccination. It should be noted that there were no complete details of such screening in all cases, and therefore, the pathogenesis of these antibodies in VITT deserves further attention. Von Willebrand factor (VWF) was measured in 8 cases which were positive in 73%. In a previously performed research work, VWF and P-selectin were introduced as key factors which were involved in the formation of platelet–leukocyte complex, leading to platelet activation and enhanced thrombocytopenia upon adenovirus exposure which highlights the further assessments of this marker to elucidate its possible role in the pathogenesis of VITT. 73
Table 4.
Summary of thrombophilia marker assays in case reports on VITT after ChAdOx1 nCov-19 vaccination
|
Thrombophilia marker assays
|
Results
|
| EXTEM CTa (n = 1) |
High: 100% |
| EXTEM A10b (n = 1) |
High: 100% |
| FIBTEM A10c (n = 1) |
Normal: 100% |
| INTEM CTd (n = 1) |
High: 100% |
| Fibrin degradation product (n = 1) |
High: 100% |
| Protein C activity (n = 19) |
High: 5.26%; Normal: 94.74% |
| Protein S, free antigen (n = 19) |
Normal: 100% |
| VWF (n = 8) |
Normal: 12.5%; High: 87.5% |
| Thrombophilia mutations (Factor V Leiden, prothrombin G20210A, MTHFR, JAK2) |
- Generally mentioned as Thrombophilia (n = 32) |
| Normal: 100% |
| - Factor V Leiden (n = 12) |
| Normal: 33%; High: 67% |
| - Prothrombin (G20210A) (n = 7) |
| Normal: 43%; High: 57% |
| - MTHFR (n = 2) |
| High: 100% |
| - JAK2 (n = 4) |
| Normal: 100% |
| Antiphospholipid antibodies |
- Lupus anticoagulant (n = 34) |
| Normal: 76.5%; High: 23.5% |
| - Beta-2-glycoprotein (n = 34) |
| Normal: 94%; High: 6% |
| - Anticardiolipin antibodies (n = 36) |
| Normal: 88.8%; High: 11.2% |
| Anti-thyroid peroxidase, myeloperoxidase, anti-neutrophil cytoplasmic anti-bodies, rheumatoid factor, and antinuclear antibodies, extractable nuclear antigen, dsDNA, Anti-globulin test |
- Antinuclear antibodies (n = 31) |
| Normal: 100% |
| - Extractable nuclear antigen (n = 26) |
| Normal: 96.16%; High: 3.84% |
| - Myeloperoxidase (n = 2) |
| Normal: 50%; High: 50% |
| - Proteinase 3 antineutrophil cytoplasmic antibody (n = 1) |
| High: 100% |
| - Anti–double-stranded DNA antibody (n = 2) |
| Normal: 50%; High: 50% |
| - Antineutrophilic antibodies (n = 2) |
| Normal: 100% |
| - Anti-globulin (n = 1) |
| Normal: 100% |
| Neutrophil DNA extracellular traps (n = 1) |
High: 100% |
| Schistocytes (n = 11) |
Normal: 18%; High: 82% |
| Blood film (n = 16) |
Normal: 20% |
| - Thrombocytopenia without cell fragments: 20% |
| - Leukocytosis of neutrophils with polychromasia, anisocytosis, and moderate thrombocytopenia: 6.66% |
| - No hemolysis or fragments of red cell: 40% |
| - Giant platelets: 6.66% |
| - Platelet anisocytosis: 6.66% |
| Immunoglobulins (n = 3) |
Normal: 67%; High: 33% |
| Complement (n = 11) |
Normal: 72.7%; High: 9.1%; Low: 18.2% |
| Cryoglobulins (n = 1) |
Normal: 100% |
| ADAMTS13 (n = 12) |
Normal: 91.7%; High: 8.3% |
| Antiplatelet antibodies (n = 8) |
Normal: 50%; High: 50% |
| Homocysteine (n = 8) |
Normal: 50%; High: 50% |
a EXTEM: extrinsic pathway (tissue factor) clotting time, b EXTEM A10: amplitude of formed clot 10 min after formation, c FIBTEM A10: fibrin-dependent clot formation, amplitude of the formed clot at 10 min, d INTEM CT: intrinsic pathway clotting time.
Anti-PF4 antibody measurement through immunoassays and functional assays were positive in 86.2%2,11,22,23,25,26,30-33,41-46,50-54,56-58,61-63,65,67-71 and 73% of cases, 22,23,31,32,34,41-43,56,58,64,68,70 respectively. Details on anti-PF4 antibody assays of case reports on VITT after ChAdOx1 nCov-19 vaccination is illustrated in Table S5.
Binding of viral protein and free DNA particles in the vaccine to PF4 and generation of specific antigens are assumed to stimulate the secretion of antibodies against PF4 further. The immune complex of the PF4-IgG antibody and heparin activates platelets via interaction with cellular FcγRIIa receptors on the platelet surface. Subsequent clotting will be developed in combination with thrombocytopenia known as VITT.39 VITT resembles HIT in terms of clinical symptoms and pathobiology. Autoimmune or spontaneous HIT syndrome provides anti-PF4 antibodies despite the absence of previous exposure to heparin and includes persisting HIT, spontaneous HIT syndrome, flush heparin HIT, and fondaparinux-associated HIT.62 The serological and clinical features of autoimmune HIT are like those seen in VITT. In contrast to HIT, platelet activation in VITT occurs in the presence of PF4 rather than low heparin concentrations. The specific detection of antibodies contributed to ChAdOx1 nCov-19 vaccination should be regarded as a crucial task in confirming suspected VITT. The GTH expert committee highly recommends screening anti-PF4 antibodies if any thromboembolic events or thrombocytopenia observed during 2 weeks upon vaccination.8 There are two main approaches to verify the existence of VITT-related antibodies as is demonstrated in Figure 2. The preliminary screening phase is employment of immunoassays to confirm the presence and quantify the level of anti-PF4 antibodies. The further confirmatory step is via utilizing functional assays to detect the presence of platelet –activating antibodies independently of heparin.
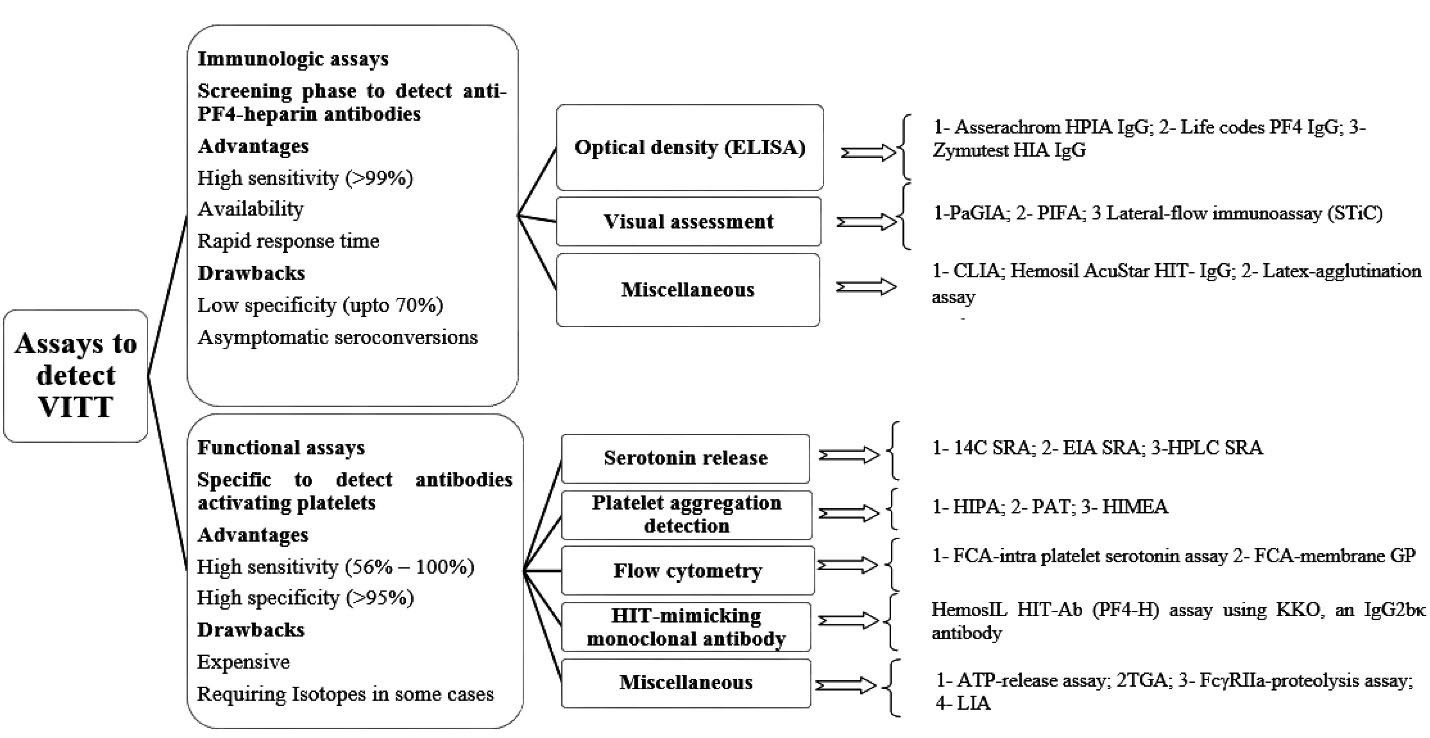
Figure 2.
Functional and immunologic assays for detection of anti-PF4 antibodies. Abbreviations:ATP, adenosine triphosphate; 14C, carbon 14; EIA, enzyme immunoassay; FCA, flow-cytometry assay; GP, glycoprotein; HPLC, high-pressure liquid chromatography; HIT, heparin-induced thrombocytopenia; HIMEA, heparin-induced multielectrode aggregometry; HIPA, heparin-induced platelet activation; KKO, murine HIT–like monoclonal antibody; LIA, latex immunoturbidimetric assay; PF4, platelet factor 4; PAT, platelet-aggregation assay; SRA, serotonin-release assay; TGA, thrombin-generation assay; PaGIA, particle-gel immunoassay; PIFA, Particle-immunofiltration assay
.
Functional and immunologic assays for detection of anti-PF4 antibodies. Abbreviations:ATP, adenosine triphosphate; 14C, carbon 14; EIA, enzyme immunoassay; FCA, flow-cytometry assay; GP, glycoprotein; HPLC, high-pressure liquid chromatography; HIT, heparin-induced thrombocytopenia; HIMEA, heparin-induced multielectrode aggregometry; HIPA, heparin-induced platelet activation; KKO, murine HIT–like monoclonal antibody; LIA, latex immunoturbidimetric assay; PF4, platelet factor 4; PAT, platelet-aggregation assay; SRA, serotonin-release assay; TGA, thrombin-generation assay; PaGIA, particle-gel immunoassay; PIFA, Particle-immunofiltration assay
As it has been reported in the results, the immunoassays were used to detect anti-PF4 antibodies in more than 82% of all-evaluated cases and resulted in 85% positive outcomes. Generally considering, immunoassays are categorized in 3 main subgroups namely optical density-based assessments, visual-based assessments as well as miscellaneous (such as CLIA and Latex-agglutination assay). Although screening for anti PF4 antibodies via a particle gel immune assay (ID-PaGIA) was positive in a few cases,57 lack of precise sensitivity to recognize anti-PF4 antibodies were observed by PaGIA, and CLIA. While the results of a couple of studies exhibited the acceptable sensitivity of immunoassays such as Immucor® (which employs polyvinyl sulfonate and PF4) to recognize antibody-inducing VITT with up to 100% accuracy, employment of PaGIA or CLIA was not sufficiently precise. A prominent conclusion is indicative of the fact that establishing a diagnosis of VITT may require a combination of diverse tests. CLIA was previously reported to be well sensitive for HIT antibodies while VITT antibodies were not desirably responsive to CLIA which is possibly due to differences between epitopes in VITT and HIT antibodies.4
It is additionally worth noting that, among diverse IgG-specific ELISAs, the sensitivity and specificity has been reported to significantly vary in HIT from that of VITT. For instance, Asserachrom HPIA IgG has demonstrated 91.1 and 100% sensitivity and specificity for diagnosis of VITT while 72 and 93.8% sensitivity and specificity for diagnosis of HIT. Lifecodes PF4 IgG and Zymutest HIA IgG assays provide 94.1 and 77.8 % sensitivity and specificity to detect VITT while supplying more than 99 and 85% respective sensitivity and specificity to quantify HIT. It is worth considering that HemosIL AcuStar HIT-IgG and STic Expert, as frequently applicable rapid tests, with almost complete specificity for detection of both VITT and HIT are poorly sensitive to VITT-related antibodies, up to 5.9 and 4.2%, respectively. The sensitivity of both mentioned assays to detect HIT antibodies is more than 98%.74 In conclusion, the immunoassays, including HYPHEN BioMed ZYMUTEST and the Immucor, could be introduced as acceptably sensitive for VITT-relevant antibodies. The results of other comprehensive investigations concluded that for detection of VITT, the ELISA assays from Stago, Hyphen, and LIFECODES were acceptable. The assays from Acustar HIT assay, LIA, Stago STIC, were not reliably sensitive.75
Immunoassays to detect antibodies against the PF4/heparin complex exhibit positive results should be further evaluated via functional tests since all existing tests for HIT diagnosis are not necessarily validated to detect antibodies involved in pathogenesis of VITT. Functional assays are technically complicated procedures to perform routinely. They are classified as serotonin release- based assay, platelet aggregation detection, flow cytometry, HIT-mimicking monoclonal antibody- based assay and miscellaneous (such as Latex immunoturbidimetric assay). In the current review, 63 cases were evaluated through functional assays which platelet-activating antibodies were detected in 73% of the totally assessed cases.
It is recommended that upon positive immunoassays of PF4-heparin antibodies, a heparin-induced platelet activation (HIPA) assay or serotonin-release assay (SRA) be performed to confirm the diagnosis further. The advantage of HIPA over SRA is visual confirmation of platelet aggregation, unlike SRA, which makes use of radioactive agents.76 Simpler technologies are flow cytometry, turbidometry, aggregometry, and assessment of luciferase activity. For functional assays, measured end points, employed technology, accurate selection of platelet-donor, varied types of heparin and between laboratory differences should be regarded as critical variables which have influence on interpretation of achieved results.77
Functional assays can detect platelet-activating antibodies in both typical and autoimmune HIT. If conventional HIPA or SRA tests do not represent autoimmune HIT, a modified HIPA test is suggested which indicates the vaccine-induced prothrombotic immune thrombocytopenia with a different reaction pattern.8 Similarly, it has been exhibited that in VITT, the SRA could investigate anti-PF4 IgG in the absence of heparin.78
Recommended algorithm for diagnosis and treatments of patients with suspected ChAdOx1 nCoV-19 vaccine-induced TTS, 2 to 35 days after ChAdOx1 nCoV-19 vaccination is outlined in Figure 3. Based on the GTH guideline, in the screening test for PF4/heparin antibodies with a positive result, functional confirmatory tests should be performed. These assays detect antibodies able to activate platelets dependent on (typical HIT) or independent of exogenous heparin (autoimmune HIT).8 About 43% of the cases in this systematic review had no previous exposure to heparin and therefore, positive test results in theses cases can establish the diagnosis of other mechanisms including autoimmune HIT.
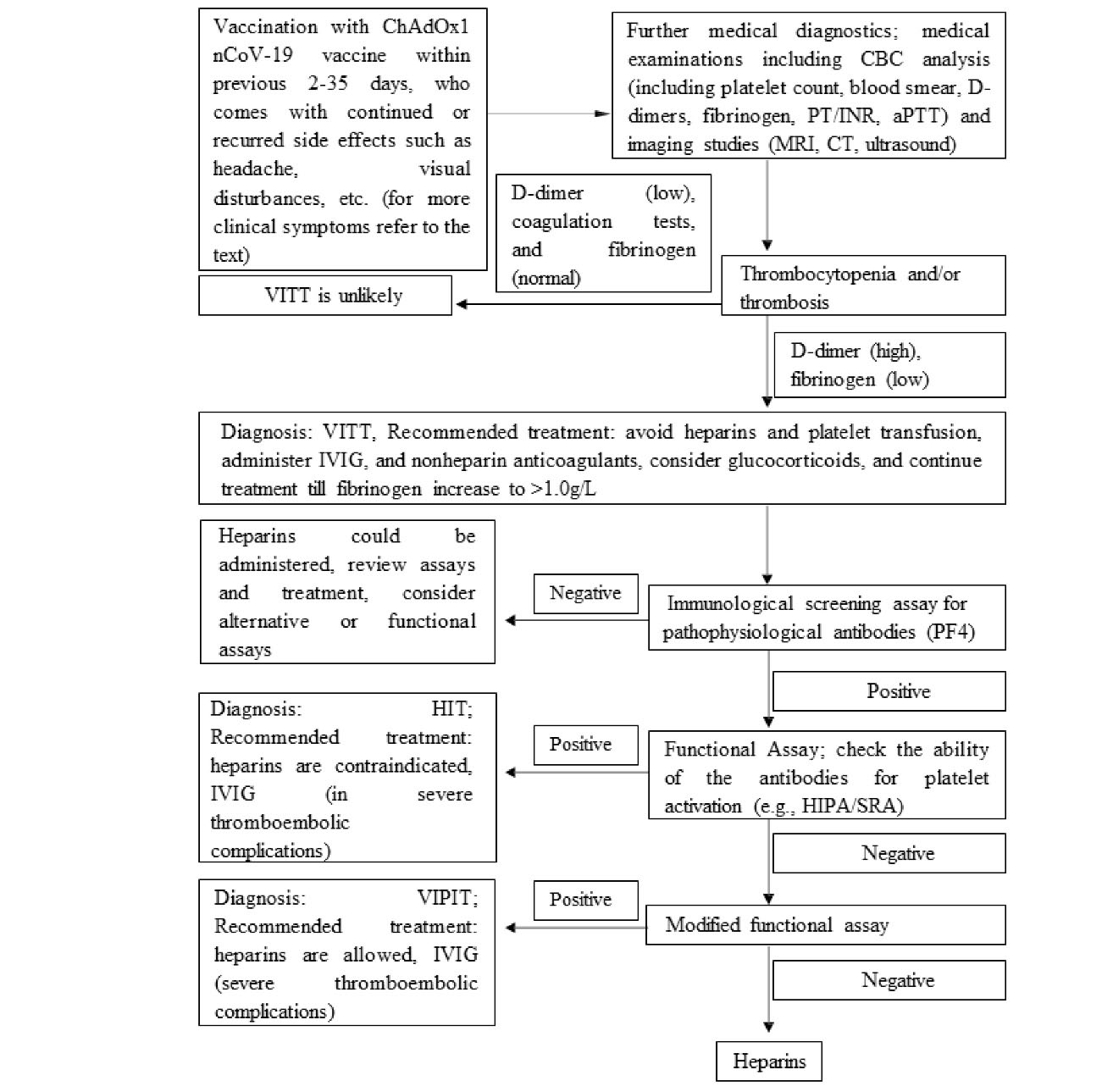
Figure 3.
Recommended algorithms for diagnosis and treatments of patients with suspected ChAdOx1 nCoV-19 vaccine-induced TTS, 2 to 35 days after ChAdOx1 nCoV-19 vaccination
.
Recommended algorithms for diagnosis and treatments of patients with suspected ChAdOx1 nCoV-19 vaccine-induced TTS, 2 to 35 days after ChAdOx1 nCoV-19 vaccination
As the unknown mechanism and pathophysiological basis of VITTS after the administration of ChAdOx1 nCoV-19 vaccine, careful consideration should be prompted for the treatment. Platelet transfusion can provide further substrate for coagulopathy through antibody-mediated platelet activation mechanism.22 A comprehensive understanding of the exact mechanism involved in ChAdOx1 nCov-19 vaccine associated TTS may allow for more targeted and efficient therapeutic measures.
There was no evidence yet suggesting the exacerbation of the patient condition after heparin administration, however, anticoagulation using a non-heparin anticoagulant including danaparoid, fondaparinux, or Argatroban, or DOACs is recommended. IVIG administration has been shown to be useful in patients with autoimmune HIT as the closest clinical manifestation to VITTS syndrome. Furthermore, plasma exchange with plasma instead of albumin can cause a temporary reduction in the levels of pathologic antibodies leading to amelioration of the coagulopathy in terms of hypofibrinogenemia.22
Gundry and his colleagues used the PLUS cardiac test (GD Biosciences, Inc, Irvine, CA) as a clinically validated examination to measure different protein-based markers leading to the generation of a 5-year risk predicting the score of acute coronary syndrome. Their results showed that the COVID-19 mRNA vaccines could substantially raise endothelium inflammation and T cell infiltration in the cardiac muscle. This might justify the high rate of cardiomyopathy, thrombosis, and different vascular problems observed after mRNA-based vaccination. Accordingly, it is recommended to do the same study with ChAdOx1 nCov-19 induced TTS patients, which can help elucidate the possible TTS mechanisms in these patients.
The most practical implication of these findings is that a low threshold should be considered to request immunoassays and confirmatory functional assays in cases with unexpected VITT clinical symptoms upon ChAdOx1 nCov-19 vaccination. No individual immunoassay test can detect all feasibly existing VITT cases, and if a single test becomes negative, a further ELISA or a functional assay should be ordered in case of strong clinical features of VITT. It is additionally recommended that upon positive ELISA results, functional assays perform as well.
Conclusion
ChAdOx1 nCov-19 vaccine-induced VITTS is an unusual and potentially fatal disorder that necessitates early diagnosis and appropriate therapeutic measures. Accordingly, familiarity with the clinical and laboratory features as well as diagnostic methods of this clotting complication is of great importance for health care providers to reduce the mortality rate in VITT patients. Further investigations on the pathophysiologic and molecular mechanisms of ChAdOx1 nCov-19 vaccine-induced VITTS may improve the diagnostic and therapeutic measures.
Acknowledgments
We appreciate the support from the Iran University of Medical Sciences.
Competing Interests
The authors declare no conflict of interest.
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and/or its supplementary material].
Ethical Approval
Not applicable.
Supplementary Files
Supplementary file 1 contains Table S1a, Table S1b and Tables S2-S5.
(pdf)
References
- Chen PW, Tsai ZY, Chao TH, Li YH, Hou CJ, Liu PY. Addressing vaccine-induced immune thrombotic thrombocytopenia (VITT) following COVID-19 vaccination: a mini-review of practical strategies. Acta Cardiol Sin 2021; 37(4):355-64. doi: 10.6515/acs.202107_37(4).20210628a [Crossref] [ Google Scholar]
- Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021; 384(22):2092-101. doi: 10.1056/NEJMoa2104840 [Crossref] [ Google Scholar]
- McGonagle D, De Marco G, Bridgewood C. Mechanisms of immunothrombosis in vaccine-induced thrombotic thrombocytopenia (VITT) compared to natural SARS-CoV-2 infection. J Autoimmun 2021; 121:102662. doi: 10.1016/j.jaut.2021.102662 [Crossref] [ Google Scholar]
- Sachs UJ, Cooper N, Czwalinna A, Müller J, Pötzsch B, Tiede A. PF4-dependent immunoassays in patients with vaccine-induced immune thrombotic thrombocytopenia: results of an interlaboratory comparison. ThrombHaemost 2021; 121(12):1622-7. doi: 10.1055/a-1535-9002 [Crossref] [ Google Scholar]
- Smadja DM, Yue QY, Chocron R, Sanchez O, Lillo-Le Louet A. Vaccination against COVID-19: insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur Respir J 2021; 58(1):2100956. doi: 10.1183/13993003.00956-2021 [Crossref] [ Google Scholar]
- von Hundelshausen P, Lorenz R, Siess W, Weber C. Vaccine-induced immune thrombotic thrombocytopenia (VITT): targeting pathomechanisms with Bruton tyrosine kinase inhibitors. ThrombHaemost 2021; 121(11):1395-9. doi: 10.1055/a-1481-3039 [Crossref] [ Google Scholar]
- Greinacher A, Selleng K, Mayerle J, Palankar R, Wesche J, Reiche S. Anti-platelet factor 4 antibodies causing VITT do not cross-react with SARS-CoV-2 spike protein. Blood 2021; 138(14):1269-77. doi: 10.1182/blood.2021012938 [Crossref] [ Google Scholar]
- Oldenburg J, Klamroth R, Langer F, Albisetti M, von Auer C, Ay C. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseologie 2021; 41(3):184-9. doi: 10.1055/a-1469-7481 [Crossref] [ Google Scholar]
- Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 2021; 397(10277):881-91. doi: 10.1016/s0140-6736(21)00432-3 [Crossref] [ Google Scholar]
- Hernández AF, Calina D, Poulas K, Docea AO, Tsatsakis AM. Safety of COVID-19 vaccines administered in the EU: should we be concerned?. Toxicol Rep 2021; 8:871-9. doi: 10.1016/j.toxrep.2021.04.003 [Crossref] [ Google Scholar]
- Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle P, Eichinger S. A prothrombotic thrombocytopenic disorder resembling heparin-induced thrombocytopenia following coronavirus-19 vaccination. Res Sq [Preprint]. April 7, 2021. 10.21203/rs.3.rs-362354/v2.
- Candelli M, Rossi E, Valletta F, De Stefano V, Franceschi F. Immune thrombocytopenic purpura after SARS-CoV-2 vaccine. Br J Haematol 2021; 194(3):547-9. doi: 10.1111/bjh.17508 [Crossref] [ Google Scholar]
- Ryan E, Benjamin D, McDonald I, Barrett A, McHugh J, Ryan K. AZD1222 vaccine-related coagulopathy and thrombocytopenia without thrombosis in a young female. Br J Haematol 2021; 194(3):553-6. doi: 10.1111/bjh.17530 [Crossref] [ Google Scholar]
- Paulsen FO, Schaefers C, Langer F, Frenzel C, Wenzel U, Hengel FE. Immune thrombocytopenic purpura after vaccination with COVID-19 vaccine (ChAdOx1 nCov-19). Blood 2021; 138(11):996-9. doi: 10.1182/blood.2021012790 [Crossref] [ Google Scholar]
- Koch M, Fuld S, Middeke JM, Fantana J, von Bonin S, Beyer-Westendorf J. Secondary immune thrombocytopenia (ITP) associated with ChAdOx1 COVID-19 vaccination - a case report. TH Open 2021; 5(3):e315-e8. doi: 10.1055/s-0041-1731774 [Crossref] [ Google Scholar]
- Lwin ZT, Naing TKP. A case report: COVID vaccine-induced immune thrombocytopenia. Clin Surg 2021; 5(12):1-3. [ Google Scholar]
- Miller L, Binder W. A 38-year-old woman with cerebral venous sinus thrombosis. R I Med J (2013) 2021; 104(5):34-7. [ Google Scholar]
- Bandapaati S, Bobba H, Navinan MR. Coeliac artery and splenic artery thrombosis complicated with splenic infarction 7 days following the first dose of Oxford vaccination, causal relationship or coincidence?. BMJ Case Rep 2021; 14(7):e243799. doi: 10.1136/bcr-2021-243799 [Crossref] [ Google Scholar]
- Bjørnstad-Tuveng TH, Rudjord A, Anker P. Fatal cerebral haemorrhage after COVID-19 vaccine. Tidsskr Nor Laegeforen 2021;141. 10.4045/tidsskr.21.0312.
- Haakonsen HB, Nystedt A. Deep vein thrombosis more than two weeks after vaccination against COVID-19. Tidsskr Nor Laegeforen 2021;141. 10.4045/tidsskr.21.0274.
- Al-Mayhani T, Saber S, Stubbs MJ, Losseff NA, Perry RJ, Simister RJ. Ischaemic stroke as a presenting feature of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. J Neurol Neurosurg Psychiatry 2021; 92(11):1247-8. doi: 10.1136/jnnp-2021-326984 [Crossref] [ Google Scholar]
- Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021; 384(23):2202-11. doi: 10.1056/NEJMoa2105385 [Crossref] [ Google Scholar]
- Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021; 384(22):2124-30. doi: 10.1056/NEJMoa2104882 [Crossref] [ Google Scholar]
- Bayas A, Menacher M, Christ M, Behrens L, Rank A, Naumann M. Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Lancet 2021; 397(10285):e11. doi: 10.1016/s0140-6736(21)00872-2 [Crossref] [ Google Scholar]
- Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas AM. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J ThrombHaemost 2021; 19(7):1771-5. doi: 10.1111/jth.15347 [Crossref] [ Google Scholar]
- Mehta PR, Apap Mangion S, Benger M, Stanton BR, Czuprynska J, Arya R. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination - a report of two UK cases. Brain BehavImmun 2021; 95:514-7. doi: 10.1016/j.bbi.2021.04.006 [Crossref] [ Google Scholar]
- Castelli GP, Pognani C, Sozzi C, Franchini M, Vivona L. Cerebral venous sinus thrombosis associated with thrombocytopenia post-vaccination for COVID-19. Crit Care 2021; 25(1):137. doi: 10.1186/s13054-021-03572-y [Crossref] [ Google Scholar]
- D’Agostino V, Caranci F, Negro A, Piscitelli V, Tuccillo B, Fasano F. A rare case of and disseminated intravascular coagulation temporally associated to the COVID-19 vaccine administration. J Pers Med 2021; 11(4):285. doi: 10.3390/jpm11040285 [Crossref] [ Google Scholar]
- Abu Esba LC, Al Jeraisy M. Reported adverse effects following COVID-19 vaccination at a tertiary care hospital, focus on cerebral venous sinus thrombosis (CVST). Expert Rev Vaccines 2021; 20(8):1037-42. doi: 10.1080/14760584.2021.1940145 [Crossref] [ Google Scholar]
- Franchini M, Testa S, Pezzo M, Glingani C, Caruso B, Terenziani I. Cerebral venous thrombosis and thrombocytopenia post-COVID-19 vaccination. Thromb Res 2021; 202:182-3. doi: 10.1016/j.thromres.2021.04.001 [Crossref] [ Google Scholar]
- Garnier M, Curado A, Billoir P, Barbay V, Demeyere M, Dacher JN. Imaging of Oxford/AstraZeneca® COVID-19 vaccine-induced immune thrombotic thrombocytopenia. Diagn Interv Imaging 2021; 102(10):649-50. doi: 10.1016/j.diii.2021.04.005 [Crossref] [ Google Scholar]
- Geeraerts T, Montastruc F, Bonneville F, Mémier V, Raposo N. Oxford-AstraZeneca COVID-19 vaccine-induced cerebral venous thrombosis and thrombocytopaenia: a missed opportunity for a rapid return of experience. Anaesth Crit Care Pain Med 2021; 40(4):100889. doi: 10.1016/j.accpm.2021.100889 [Crossref] [ Google Scholar]
- Gras-Champel V, Liabeuf S, Baud M, Albucher JF, Benkebil M, Boulay C. Atypical thrombosis associated with VaxZevria® (AstraZeneca) vaccine: data from the French network of regional pharmacovigilance centres. Therapie 2021; 76(4):369-73. doi: 10.1016/j.therap.2021.05.007 [Crossref] [ Google Scholar]
- Jones M, Boisvert A, Landry J, Petrasek PF. Limb ischemia and pulmonary artery thrombosis after the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine: a case of vaccine-induced immune thrombotic thrombocytopenia. CMAJ 2021; 193(24):E906-E10. doi: 10.1503/cmaj.210795 [Crossref] [ Google Scholar]
- Ramdeny S, Lang A, Al-Izzi S, Hung A, Anwar I, Kumar P. Management of a patient with a rare congenital limb malformation syndrome after SARS-CoV-2 vaccine-induced thrombosis and thrombocytopenia (VITT). Br J Haematol 2021; 195(3):299. doi: 10.1111/bjh.17619 [Crossref] [ Google Scholar]
- Wolf ME, Luz B, Niehaus L, Bhogal P, Bäzner H, Henkes H. Thrombocytopenia and intracranial venous sinus thrombosis after “COVID-19 vaccine AstraZeneca” exposure. J Clin Med 2021; 10(8):1599. doi: 10.3390/jcm10081599 [Crossref] [ Google Scholar]
- Xie C, Vincent L, Chadwick A, Peschl H. COVID-19 vaccine induced prothrombotic immune thrombocytopenia. Eur Heart J 2021; 42(33):3206. doi: 10.1093/eurheartj/ehab237 [Crossref] [ Google Scholar]
- Muster V, Gary T, Raggam RB, Wölfler A, Brodmann M. Pulmonary embolism and thrombocytopenia following ChAdOx1 vaccination. Lancet 2021; 397(10287):1842. doi: 10.1016/s0140-6736(21)00871-0 [Crossref] [ Google Scholar]
- Aladdin Y, Algahtani H, Shirah B. Vaccine-induced immune thrombotic thrombocytopenia with disseminated intravascular coagulation and death following the ChAdOx1 nCoV-19 vaccine. J Stroke Cerebrovasc Dis 2021; 30(9):105938. doi: 10.1016/j.jstrokecerebrovasdis.2021.105938 [Crossref] [ Google Scholar]
- Althaus K, Möller P, Uzun G, Singh A, Beck A, Bettag M. Antibody-mediated procoagulant platelets in SARS-CoV-2-vaccination associated immune thrombotic thrombocytopenia. Haematologica 2021; 106(8):2170-9. doi: 10.3324/haematol.2021.279000 [Crossref] [ Google Scholar]
- Bano F, Badugama B, Chandra D. Thrombosis and thrombocytopaenia after ChAdOx1 nCoV-19 vaccination: a single UK centre experience. BMJ Case Rep 2021; 14(7):e243894. doi: 10.1136/bcr-2021-243894 [Crossref] [ Google Scholar]
- Bonato S, Artoni A, Lecchi A, Schwarz G, La Marca S, Padovan L. Massive cerebral venous thrombosis due to vaccine-induced immune thrombotic thrombocytopenia. Haematologica 2021; 106(11):3021-4. doi: 10.3324/haematol.2021.279246 [Crossref] [ Google Scholar]
- Bourguignon A, Arnold DM, Warkentin TE, Smith JW, Pannu T, Shrum JM. Adjunct immune globulin for vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med 2021; 385(8):720-8. doi: 10.1056/NEJMoa2107051 [Crossref] [ Google Scholar]
- Choi JK, Kim S, Kim SR, Jin JY, Choi SW, Kim H. Intracerebral hemorrhage due to thrombosis with thrombocytopenia syndrome after vaccination against COVID-19: the first fatal case in Korea. J Korean Med Sci 2021; 36(31):e223. doi: 10.3346/jkms.2021.36.e223 [Crossref] [ Google Scholar]
- Cliff-Patel N, Moncrieff L, Ziauddin V. Renal vein thrombosis and pulmonary embolism secondary to vaccine-induced thrombotic thrombocytopenia (VITT). Eur J Case Rep Intern Med 2021; 8(7):002692. doi: 10.12890/2021_002692 [Crossref] [ Google Scholar]
- Gangi A, Mobashwera B, Ganczakowski M, Ayto R. Imaging and hematologic findings in thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 (AstraZeneca) vaccination. Radiology 2022; 302(2):319-25. doi: 10.1148/radiol.2021211546 [Crossref] [ Google Scholar]
- Gattringer T, Gressenberger P, Gary T, Wölfler A, Kneihsl M, Raggam RB. Successful management of vaccine-induced immune thrombotic thrombocytopenia-related cerebral sinus venous thrombosis after ChAdOx1 nCov-19 vaccination. Stroke Vasc Neurol 2022; 7(1):86-8. doi: 10.1136/svn-2021-001142 [Crossref] [ Google Scholar]
- Gessler F, Schmitz AK, Dubinski D, Bernstock JD, Lehmann F, Won SY. Neurosurgical considerations regarding decompressive craniectomy for intracerebral hemorrhage after SARS-CoV-2-vaccination in vaccine induced thrombotic thrombocytopenia-VITT. J Clin Med 2021; 10(13):2777. doi: 10.3390/jcm10132777 [Crossref] [ Google Scholar]
- Graf T, Thiele T, Klingebiel R, Greinacher A, Schäbitz WR, Greeve I. Immediate high-dose intravenous immunoglobulins followed by direct thrombin-inhibitor treatment is crucial for survival in SARS-COVID-19-adenoviral vector vaccine-induced immune thrombotic thrombocytopenia VITT with cerebral sinus venous and portal vein thrombosis. J Neurol 2021; 268(12):4483-5. doi: 10.1007/s00415-021-10599-2 [Crossref] [ Google Scholar]
- Guan CY, Tsai SH, Fan JS, Lin YK, Kao CC. Middle-age Asian male with cerebral venous thrombosis after COVID-19 AstraZeneca vaccination. Am J Emerg Med 2022;51:427.e3-427.e4. 10.1016/j.ajem.2021.07.011.
- Huang CT, Hsu SY, Wang CH, Tseng WJ, Yang CY, Ng CJ. Double high-dose immunoglobulin for ChAdOx1 nCov-19 vaccine-induced immune thrombotic thrombocytopenia. Thromb Res 2021; 206:14-7. doi: 10.1016/j.thromres.2021.07.017 [Crossref] [ Google Scholar]
- Ikenberg B, Demleitner AF, Thiele T, Wiestler B, Götze K, Mößmer G. Cerebral venous sinus thrombosis after ChAdOx1 nCov-19 vaccination with a misleading first cerebral MRI scan. Stroke Vasc Neurol 2021; 6(4):668-70. doi: 10.1136/svn-2021-001095 [Crossref] [ Google Scholar]
- Jacob C, Rani KA, Holton PJ, Boyce SR, Weir NU, Griffith CR. Malignant middle cerebral artery syndrome with thrombotic thrombocytopenia following vaccination against SARS-CoV-2. J Intensive Care Soc 2022; 23(4):479-84. doi: 10.1177/17511437211027496 [Crossref] [ Google Scholar]
- Khuhapinant A, Rungjirajittranon T, Suwanawiboon B, Chinthammitr Y, Ruchutrakool T. Successful venous thromboprophylaxis in a patient with vaccine-induced immune thrombotic thrombocytopenia (VITT): a case report of the first reported case in Thailand. Thromb J 2021; 19(1):65. doi: 10.1186/s12959-021-00317-3 [Crossref] [ Google Scholar]
- Mauriello A, Scimeca M, Amelio I, Massoud R, Novelli A, Di Lorenzo F. Thromboembolism after COVID-19 vaccine in patients with preexisting thrombocytopenia. Cell Death Dis 2021; 12(8):762. doi: 10.1038/s41419-021-04058-z [Crossref] [ Google Scholar]
- De Michele M, Iacobucci M, Chistolini A, Nicolini E, Pulcinelli F, Cerbelli B. Malignant cerebral infarction after ChAdOx1 nCov-19 vaccination: a catastrophic variant of vaccine-induced immune thrombotic thrombocytopenia. Nat Commun 2021; 12(1):4663. doi: 10.1038/s41467-021-25010-x [Crossref] [ Google Scholar]
- Panovska-Stavridis I, Pivkova-Veljanovska A, Trajkova S, Lazarevska M, Grozdanova A, Filipche V. A rare case of superior ophthalmic vein thrombosis and thrombocytopenia following ChAdOx1 nCoV-19 vaccine against SARS-CoV-2. Mediterr J Hematol Infect Dis 2021; 13(1):e2021048. doi: 10.4084/mjhid.2021.048 [Crossref] [ Google Scholar]
- Patriquin CJ, Laroche V, Selby R, Pendergrast J, Barth D, Côté B. Therapeutic plasma exchange in vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med 2021; 385(9):857-9. doi: 10.1056/NEJMc2109465 [Crossref] [ Google Scholar]
- Paul M, Abraham L, Sophy M, Varghese D, Thomas J, Varghese J. Idiopathic thrombotic microangiopathy with ChAdOx1 nCov-19 vaccination in a middle aged male: a clinical challenge in the COVID era. J HematolThrombo Dis 2021; 9(7):431. doi: 10.24105/2329-8790.2021.9.441 [Crossref] [ Google Scholar]
- Ramessur R, Saffar N, Czako B, Agarwal A, Batta K. Cutaneous thrombosis associated with skin necrosis following Oxford-AstraZeneca COVID-19 vaccination. Clin Exp Dermatol 2021; 46(8):1610-2. doi: 10.1111/ced.14819 [Crossref] [ Google Scholar]
- Soleimani B, Turaga S, Khan D, Davies C, Duodu Y, Botcherby E, et al. Syndrome of cerebral venous sinus thrombosis and thrombocytopenia after vaccination for COVID-19. Res Sq [Preprint]. April 19, 2021. 10.21203/rs.3.rs-439289/v1.
- Tølbøll Sørensen AL, Rolland M, Hartmann J, Harboe ZB, Roed C, Jensen T. A case of thrombocytopenia and multiple thromboses after vaccination with ChAdOx1 nCoV-19 against SARS-CoV-2. Blood Adv 2021; 5(12):2569-74. doi: 10.1182/bloodadvances.2021004904 [Crossref] [ Google Scholar]
- Suresh P, Petchey W. ChAdOx1 nCOV-19 vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis (CVST). BMJ Case Rep 2021; 14(6):e243931. doi: 10.1136/bcr-2021-243931 [Crossref] [ Google Scholar]
- Tejpal A, Economopoulos P, Andreou R, Stevenson J. Vaccine-induced immune thrombotic thrombocytopenia after receiving the ChAdOx1 nCoV-19 vaccine. Can J Gen Intern Med 2021; 16(2):34-7. doi: 10.22374/cjgim.v16i2.559 [Crossref] [ Google Scholar]
- Tiede A, Sachs UJ, Czwalinna A, Werwitzke S, Bikker R, Krauss JK. Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood 2021; 138(4):350-3. doi: 10.1182/blood.2021011958 [Crossref] [ Google Scholar]
- Turi MC, Spitaleri F, Gori AM, Parruti G, Rogolino AA, Albani A. A case of vaccine-induced immune thrombotic thrombocytopenia with massive artero-venous thrombosis. Blood Transfus 2021; 19(4):343-6. doi: 10.2450/2021.0131-21 [Crossref] [ Google Scholar]
- Varona JF, García-Isidro M, Moeinvaziri M, Ramos-López M, Fernández-Domínguez M. Primary adrenal insufficiency associated with Oxford-AstraZeneca ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia (VITT). Eur J Intern Med 2021; 91:90-2. doi: 10.1016/j.ejim.2021.06.025 [Crossref] [ Google Scholar]
- Vayne C, Rollin J, Gruel Y, Pouplard C, Galinat H, Huet O. PF4 immunoassays in vaccine-induced thrombotic thrombocytopenia. N Engl J Med 2021; 385(4):376-8. doi: 10.1056/NEJMc2106383 [Crossref] [ Google Scholar]
- Wang RL, Chiang WF, Shyu HY, Chen MH, Lin CI, Wu KA. COVID-19 vaccine-associated acute cerebral venous thrombosis and pulmonary artery embolism. QJM 2021; 114(7):506-7. doi: 10.1093/qjmed/hcab185 [Crossref] [ Google Scholar]
- Wiedmann M, Skattør T, Stray-Pedersen A, Romundstad L, Antal EA, Marthinsen PB. Vaccine induced immune thrombotic thrombocytopenia causing a severe form of cerebral venous thrombosis with high fatality rate: a case series. Front Neurol 2021; 12:721146. doi: 10.3389/fneur.2021.721146 [Crossref] [ Google Scholar]
- Zanferrari C, Fanucchi S, Liberato NL, Lauria G, Persico A, Cavallini A. Excellent response to high-dose intravenous immunoglobulin in anti-PF4 positive cerebral thrombosis following Oxford-AstraZeneca AZD1222 vaccine. Res Sq [Preprint]. April 12, 2021. 10.21203/rs.3.rs-399801/v1.
- Saberi-Movahed F, Mohammadifard M, Mehrpooya A, Rezaei-Ravari M, Berahmand K, Rostami M, et al. Decoding clinical biomarker space of COVID-19: exploring matrix factorization-based feature selection methods. medRxiv [Preprint]. July 9, 2021. Available from: https://www.medrxiv.org/content/10.1101/2021.07.07.21259699v1.
- Othman M, Labelle A, Mazzetti I, Elbatarny HS, Lillicrap D. Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood 2007; 109(7):2832-9. doi: 10.1182/blood-2006-06-032524 [Crossref] [ Google Scholar]
- Platton S, Bartlett A, MacCallum P, Makris M, McDonald V, Singh D. Evaluation of laboratory assays for anti-platelet factor 4 antibodies after ChAdOx1 nCOV-19 vaccination. J ThrombHaemost 2021; 19(8):2007-13. doi: 10.1111/jth.15362 [Crossref] [ Google Scholar]
- Reilly-Stitt C, Kitchen S, Jennings I, Horner K, Jones R, Makris M. Anti-PF4 testing for vaccine-induced immune thrombocytopenia and thrombosis and heparin induced thrombocytopenia: results from a UK National External Quality Assessment Scheme exercise April 2021. J ThrombHaemost 2021; 19(9):2263-7. doi: 10.1111/jth.15423 [Crossref] [ Google Scholar]
- Minet V, Dogné JM, Mullier F. Functional assays in the diagnosis of heparin-induced thrombocytopenia: a review. Molecules 2017; 22(4):617. doi: 10.3390/molecules22040617 [Crossref] [ Google Scholar]
- Pouplard C, Amiral J, Borg JY, Laporte-Simitsidis S, Delahousse B, Gruel Y. Decision analysis for use of platelet aggregation test, carbon 14-serotonin release assay, and heparin-platelet factor 4 enzyme-linked immunosorbent assay for diagnosis of heparin-induced thrombocytopenia. Am J Clin Pathol 1999; 111(5):700-6. doi: 10.1093/ajcp/111.5.700 [Crossref] [ Google Scholar]
- Marchandot B, Curtiaud A, Trimaille A, Sattler L, Grunebaum L, Morel O. Vaccine-induced immune thrombotic thrombocytopenia: current evidence, potential mechanisms, clinical implications, and future directions. Eur Heart J Open 2021; 1(2):oeab014. doi: 10.1093/ehjopen/oeab014 [Crossref] [ Google Scholar]