Advanced pharmaceutical bulletin. 13(4):701-711.
doi: 10.34172/apb.2023.079
Review Article
Lutetium-177-Labeled Prostate-Specific Membrane Antigen-617 for Molecular Imaging and Targeted Radioligand Therapy of Prostate Cancer
Rien Ritawidya Conceptualization, Formal analysis, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing, 1, 2 
Hendris Wongso Conceptualization, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing, 1, 2 
Nurmaya Effendi Conceptualization, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing, 3 
Anung Pujiyanto Investigation, Software, Writing – original draft, Writing – review & editing, 1
Wening Lestari Conceptualization, Investigation, Project administration, Visualization, Writing – original draft, Writing – review & editing, 1 
Herlan Setiawan Investigation, Software, Writing – original draft, Writing – review & editing, 1 
Titis Sekar Humani Conceptualization, Formal analysis, Project administration, Validation, Writing – original draft, Writing – review & editing, 1, * 
Author information:
1Research Center for Radioisotope, Radiopharmaceutical, and Biodosimetry Technology, National Research and Innovation Agency (BRIN), Kawasan Puspiptek, Setu, Tangerang Selatan, 15314 Indonesia.
2Research Collaboration Center for Theranostic Radiopharmaceuticals, National Research and Innovation Agency, Jl. Raya Bandung-Sumedang KM 21, Sumedang, 45363, Indonesia.
3Faculty of Pharmacy, University of Muslim Indonesia, Kampus II UMI, Jl. Urip Sumoharjo No.225, Panaikang, Panakkukang, Kota, Makassar, Sulawesi Selatan 90231.
Abstract
Prostate-specific membrane antigen (PSMA) represents a promising target for PSMA-overexpressing diseases, especially prostate cancer-a common type of cancer among men worldwide. In response to the challenges in tackling prostate cancers, several promising PSMA inhibitors from a variety of molecular scaffolds (e.g., phosphorous-, thiol-, and urea-based molecules) have been developed. In addition, PSMA inhibitors bearing macrocyclic chelators have attracted interest due to their favorable pharmacokinetic properties. Recently, conjugating a small PSMA molecule inhibitor-bearing 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) chelator, as exemplified by [177Lu]Lu-PSMA-617 could serve as a molecular imaging probe and targeted radioligand therapy (TRT) of metastatic castration resistant prostate cancer (mCRPC). Hence, studies related to mCRPC have drawn global attention. In this review, the recent development of PSMA ligand-617-labeled with 177Lu for the management of mCRPC is presented. Its molecular mechanism of action, safety, efficacy, and future direction are also described.
Keywords: Prostate cancer, Metastatic-castration resistant prostate cancer, Lutetium-177, PSMA-617, Radioligand
Copyright and License Information
©2023 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Prostate cancer is the second most frequent type of cancer among men in the world.1 The incidence rate of this type of malignancy varies worldwide and it is considered the leading cause of mortality in men. According to the Global Cancer Observatory: Cancer Today (GLOBOCAN), in 2020, there were estimated 1 414 259 (7.3%) incidences occurred across countries, with a number of mortalities estimated 375 304 (3.8%).2 This situation reflects how prostate cancer has become a major health problem on a global scale.
Treatment options available for prostate cancer in the early stages of the disease progression mainly rely on surgery, external beam radiation therapy, and brachytherapy,3 while other treatments such as hormone therapy, chemotherapy, and radiation therapy administered alone or in combination, are usually considered for the treatment of malignant metastases or as additional therapies in the early stages of prostate cancer.3,4 Androgen-deprivation therapy is emerging as the first-line treatment for advanced prostate cancer.5-7 However, in most cases, there can be clinical and biochemical progression of this cancer and this condition is termed metastatic castration-resistant prostate cancer (mCRPC).8,9 The most common treatment options at this stage include docetaxel, sipuleucel-T, abiraterone and radium-223 (XofigoTM).9,10 However, this approach is known to lead to suboptimal results.11 Recently, the poly(ADP-ribose) polymerase inhibitors, such as olaparib and rucaparib have been evaluated in phase 2 clinical trials as novel therapy for mCRPC with tumors lacking homologous recombinant repair.12 Olaparib and rucaparib have been approved and shown to be effective in mCRPC patients with BCA1/2 abnormalities.12 Despite the progress and emergence of various therapeutic methods, an effective treatment approach with minimal side effects for mCRPC is still needed.
The serum prostate-specific antigen (PSA) screening test and the digital rectal examination are widely used methods to detect the pathology of prostate cancer.11 PSA level cut-off of 4.0 ng/mL has been used to decide the need for prostate biopsies.13 While transrectal ultrasound (TRUS)-guided multiple systematic transrectal biopsies are typically performed for the diagnosis purposes by obtaining the tissue sample from the gland for histopathological or cytological examination.4 Several imaging techniques, such as magnetic resonance imaging (MRI) and positron emission tomography (PET) play a pivotal role in the management of prostate cancer, especially for early detection and localization, (re-)staging, whole-gland and focal therapy, active surveillance, and detection of recurrence.14,15 In addition to PET, the single-photon emission computed tomography (SPECT) modality enables nuclear diagnostic imaging in prostate cancer. Consequently, the advancement of PET and SPECT modalities led to the necessity of efficient imaging agents or radiopharmaceuticals probes that would enable the detection of prostate cancer.
Prostate-specific membrane antigen (PSMA) is a type II transmembrane glycoprotein (~100 kDa) highly expressed in prostate cancer16 and upregulated in poorly differentiated, metastatic, and hormone-refractory carcinoma, castration-resistant prostate cancer.17 In addition, organ-minimally expressing PSMA can be found in various organs, including the brain, kidney, salivary gland, and intestine.18 PSMA is known to possess neurocarboxypeptidase activities that degrade alpha-linked glutamates from N-acetylaspartylglutamate19 in addition to its prominent role as folate hydrolase I.20 PSMA also plays an important role in angiogenesis.21 Accordingly, PSMA has recently gained growing interest as a promising target for diagnostic imaging and therapy of prostate cancer.1,22
Targeted radioligand therapy (TRT) is a selective or specific administration of a high dose of radiotoxicity to cancer cells without destroying the surrounding healthy cells.23,24 It typically employs targeting vectors such as proteins, peptides, carbohydrates, vitamins, antibodies, and aptamers.25 Metal-based small-molecule PSMA radioligands have shown a growing interest in TRT prostate cancer.26 A common strategy to develop PSMA-specific based radiometal ligands is shown in Figure 1.27,28
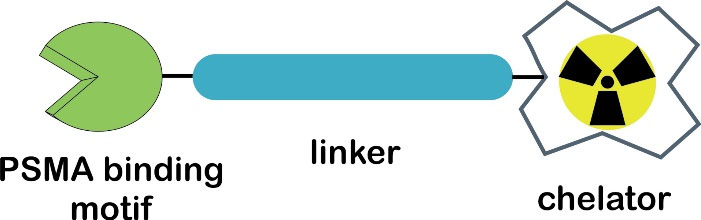
Figure 1.
General design of PSMA-targeted radioligand.27,28
.
General design of PSMA-targeted radioligand.27,28
A macrocyclic chelator 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) is widely used in the field of radiopharmaceuticals, particularly for the complexation of trivalent (3 + ) ions such as the diagnostic PET radionuclide 68Ga and therapeutic radionuclides (177Lu and 90Y).26,28,29 The presence of linkers can connect two different moieties: a chelating agent and a pharmacophore.30 Complexation of DOTA and a trivalent radiometal resulted in a thermodynamically and kinetically stable binding.28 Furthermore, this approach allows that the theranostic concept in nuclear medicine, which defines ideal radiopharmaceuticals should be able to assemble the application for both diagnostic and therapeutic purposes when radiolabeled with a diagnostic and a therapeutic radionuclide, respectively.26,31
PSMA
PSMA has emerged as a promising protein target for prostate cancer for both diagnosis and therapeutic purposes (e.g., radionuclide-based therapy or other therapeutic strategies including immunotoxins, immune cells retargeting, prodrug activation, PSMA vaccines, plasmid DNA, and adenoviral immunizations.30-32 This mechanism leads to the internalization of radionuclides into the cancer cells and eventually causes cell death33 as shown in Figure 2. The unique characteristics of PSMA make it an excellent marker for prostate cancer, mainly due to several characteristics including: 1) expressed in the prostate, 2) upregulated in all stages of the disease, 3) overexpressed in disease progression or in metastases, 4) intact on the cell surface as membrane glycoproteins, present and not released into the circulation, 5) internalized after ligand binding (receptor-mediated endocytosis), 6) associated with enzymatic activity.3,18,23
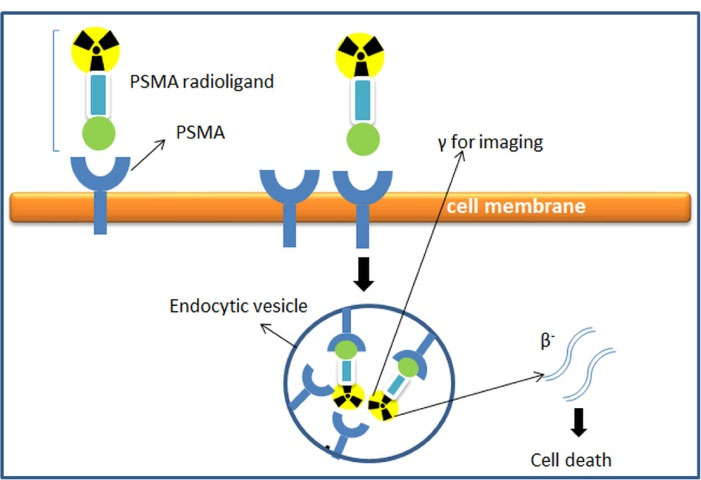
Figure 2.
Mechanism of receptor-mediated endocytosis upon radioligand-based PSMA binding to PSMA.27
.
Mechanism of receptor-mediated endocytosis upon radioligand-based PSMA binding to PSMA.27
PSMA shares sequence similarities to a certain extent (~54%) with transferrin receptors,18,34 and therefore, like transferrin, PSMA undergoes receptor and ligand functions.18 Immunofluorescence analysis or immunoelectron microscopy shows that after ligand binding, the PSMA-antibody complex is internalized through clathrin-coated pits and enters the lysosomes.34
Radiolabeled PSMA
A radiolabeled monoclonal antibody ProstaScintTM (Capromab Pendetide) is a murine IgG1 7E11-C5.3 which is linked to a linker-chelator glycyl-tyrosyl-(N’- diethylenetriaminepentaacetic acid)-lysine hydrochloride35 and it was developed to accurately diagnose, stage, and detect the new and recurrent prostate cancer.36 ProstaScintTM targets PSMA by binding to the intracellular domain (amino-terminus) of PSMA35 and areas of tumor necrosis.18 Accordingly, this radiotracer found limited use in nuclear medicine to diagnose prostate cancer.26 The development of monoclonal antibodies J591 that bind to the extracellular domain of PSMA has been reported in the literature. The J591 monoclonal antibody demonstrated high and specific binding against cell-adherent PSMA.37 In addition, J591 was the first PSMA-based humanized monoclonal antibody used in the clinical application.38,39 Several SPECT and PET tracer-based J591,40-42 as well as radioimmunotherapeutic agents have been developed.43 Some of the PSMA-specific radioligands studied so far are shown in Figure 3.
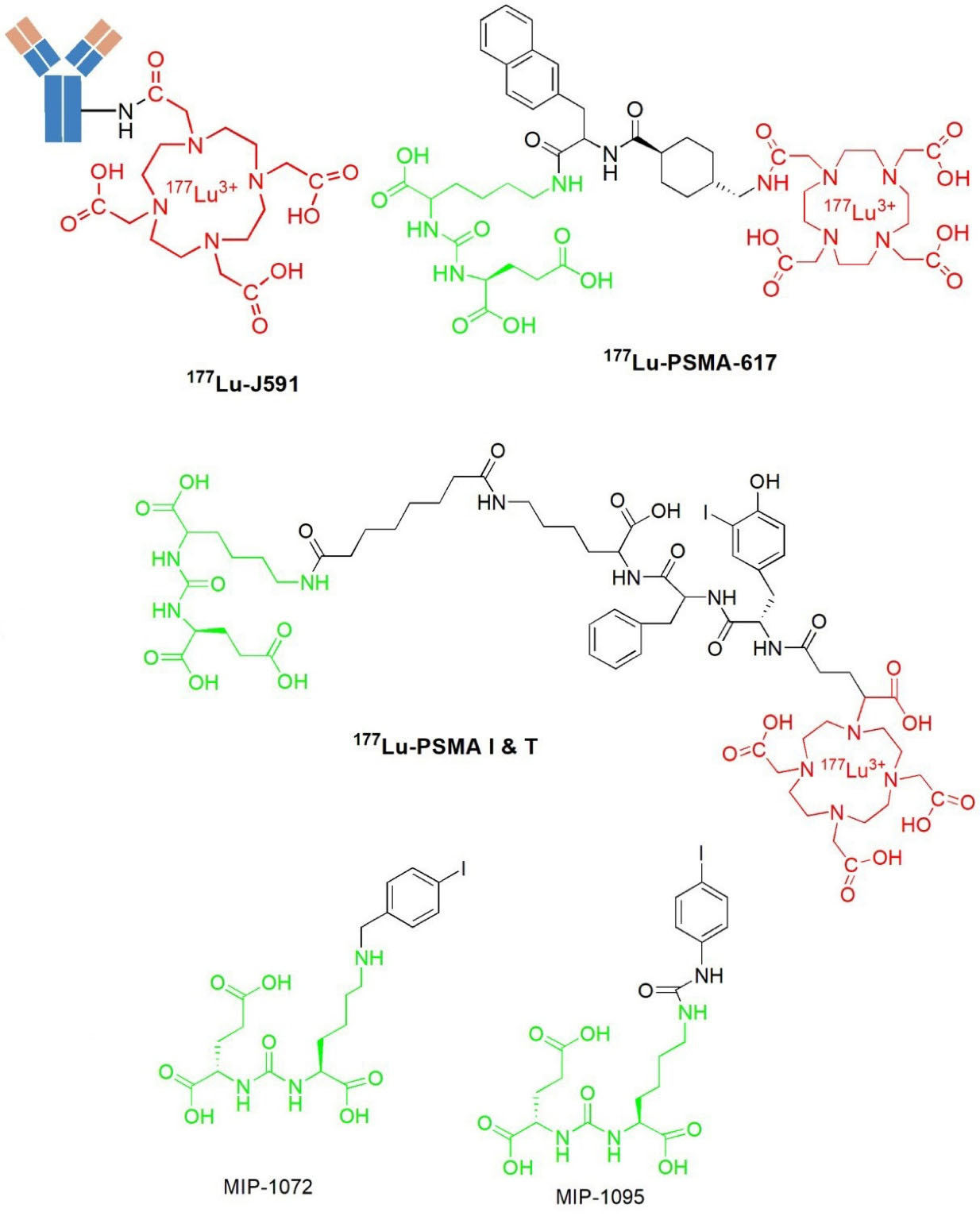
Figure 3.
Some radiolabeled PSMA ligands
.
Some radiolabeled PSMA ligands
However, the nature of the monoclonal antibody, including slow clearance and low uptake, underlines the need for imaging to be performed several days after its administration to patients.39 Therefore, the waiting time between post-administration and the imaging time seems to hinder the potential application of this PSMA-targeted J591 monoclonal antibody.39,44
Continued efforts to discover several specific-PSMA inhibitors with a higher affinity and specificity for PSMA led to various small molecule inhibitors. Small molecule PSMA inhibitors are typically zinc-binding compounds incorporated into glutamate or glutamate isostere and are divided into three classes: 1) phosphonate, phosphate, and phosphoramide compounds; 2) thiols; and 3) ureas (Figure 4).45-47 The phosphorus-based ligands seem to be the gold standard that provide binding to binuclear zinc ions positioned in the active PSMA domain. However, the development of these ligands is limited by their high polarity properties. PSMA ligands bearing thiol functionality, on the other hand, could undergo disulfide bond formation, resulting in low metabolic stability. Thus, some urea-based PSMA ligands have been developed. These molecules display favorable binding affinity and stability with very efficient internalization into the cells.46-48
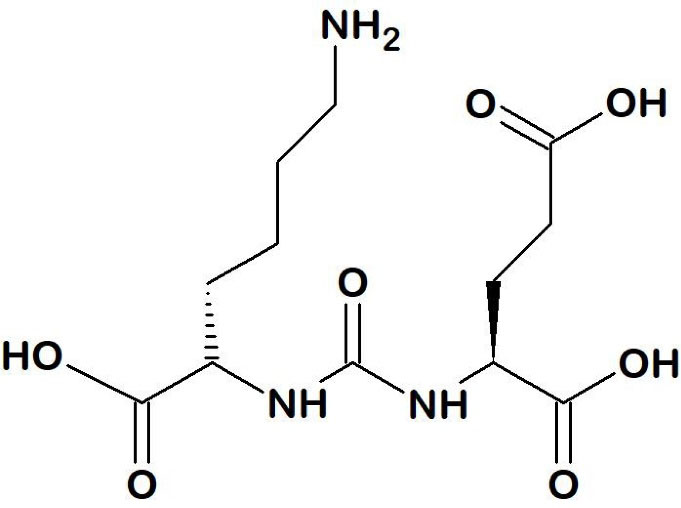
Figure 4.
Lys-urea-Glu (K-u-E) binding motif
.
Lys-urea-Glu (K-u-E) binding motif
The first urea-based compound to target PSMA in the brain was designed by Kozikowski et al.49 To date, urea-based PSMA radiopharmaceuticals are the most sophisticated class which is commonly consisting of three parts, a binding motif (glutamate-urea-lysine [Glu-urea-Lys]), a linker, and a radiolabeled moiety (usually a chelator or prosthetic groups) depending on the radionuclide.23
Liu et al evaluated the dependence of linker length on inhibitory potency, mode of inhibition, and in vitro imaging of three different fluorescent inhibitors.50 They found that choosing the right linker, along with its length, are such crucial considerations in the development of PSMA detection probes and therapy tracers that specifically target PSMA-overexpressing cells.50
The discovery of new developed radioiodinated, 123I-MIP-1072 and 123I-MIP-1095 (Figure 3) PSMA ligands based on urea scaffold have been reported in the literature.25,51-53 Despite the encouraging earlier clinical results, it appears that further attempts to optimize the efficacy and reduce the side effects of these radioiodinated ligands are warranted.30 As a result, the development of 123I-MIP-1072 and 123I-MIP-1095 has initiated the development of other PSMA-based urea binding motif radiopharmaceuticals eligible for prostate cancer.23
The radiometal-based PSMA binding motif [Glu-Urea-Lys] has shown a growing interest in the endoradiotherapy of prostate cancer.26 Due to its favorable coordination chemistry properties, the DOTA chelator can be used to conjugate several radiometals, including 177Lu and 68Ga, whereas the linker can connect two different moieties: chelator and pharmacophore.30 In 2014, a research group in Munich reported the development of the metabolically resistant 1,4,7,10-tetraazacyclododecane,1-(glutaric acid)-4,7,10-triacetic acid (DOTAGA) chelator moiety based on their previously advanced affinity PSMA ligand [68Ga]Ga-DOTAFfK(Sub-KuE)).54 In 2015, a research group in Heidelberg developed a DOTA-containing PSMA inhibitor, PSMA-617.30 This PSMA-617 contains three molecule entities, which are the pharmacophore (binding motif), glutamate-urea-lysine; the chelating agent DOTA, and a linker connecting these two moieties.30 The presence of a linker in peptide-based radiopharmaceuticals can improve metabolic stability and modulate the biodistribution.55 In addition, the linker plays an important role in bridging between a chelator and a pharmacophore; thereby maintaining peptide affinity for the receptor and avoiding the steric hindrance.56 The linker can trigger multiple effects by modulating the size, shape, solubility, stability, and molecular weight of the chemical structure, which positively aids the overall radiopharmaceutical behaviours.57 Benesová et al investigated the influence of chemically modified linkers on PSMA targeting and the pharmacokinetic profile, including PSMA inhibitory activity, cellular internalization, and biodistribution properties of a series of DOTA-PSMA small molecules.58 The study approach led to a more accurate and rational structure-activity relationships design of a new specific PSMA-based glutamate-urea motif, resulting in a promising DOTA-PSMA conjugate that can potentially be radiolabeled for theranostic application of prostate cancer.58
Numerous attempts have been made by the scientific community to develop various PSMA radionuclides based on PSMA ligands. Of several radiolabeled ligands reported in the literature, the radiopharmaceutical 177Lu-PSMA-617 has been one of the most extensively studied PSMA radioligands for both prostate cancer imaging and therapy. Phase III clinical trials of radioligand VISION (177Lu-PSMA-617, NCT03511664) is currently being conducted.59 Accordingly, the presence of extensive knowledge, experience, and information related to this radiopharmaceutical leads us to develop an “in-house” PSMA-617-based-radioligand devoted to the management of metastatic prostate cancer in Indonesia. In this review, the recent development of PSMA ligand-617-labeled with 177Lu for the management of mCRPC is presented. Its molecular mechanism of action, safety, efficacy, and future direction are also described.
Recently, 177Lu-PSMA-617 (Figure 5) was a novel promising radiopharmaceutical for nuclear imaging and TRT that is reported to be safe and can prolong overall survival in mCRPC patients.60-64 The development of this urea-based small PSMA inhibitor labeled with a beta particle-emitting radionuclide (Lu-177) was initially performed by a research group from the German Cancer Research Center (Deutsche Krebforschungszentrum, DKFZ) and its collaborating partner, the University Hospital of Heidelberg Germany in 2015.30
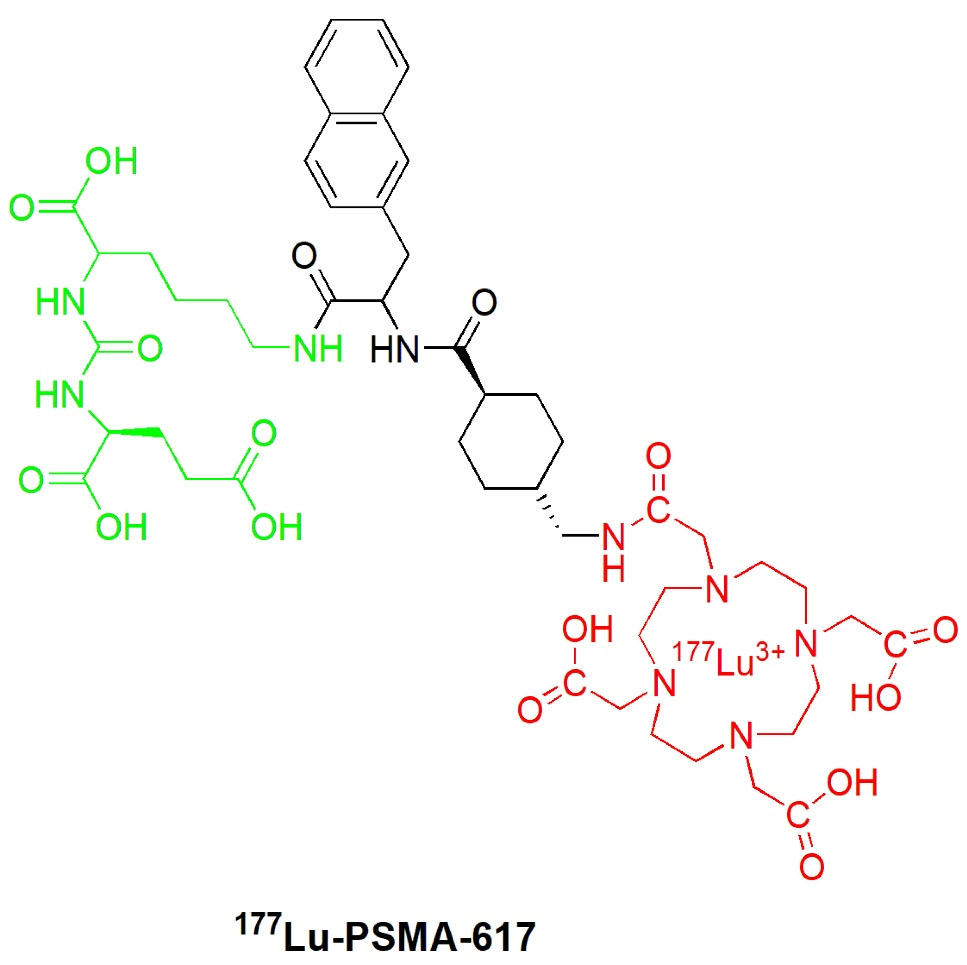
Figure 5.
Structure of 177Lu-PSMA-617 radioligand.
.
Structure of 177Lu-PSMA-617 radioligand.
The PSMA-617 ligand was synthesized by the solid phase peptide method as described in the previous literature.65 Small peptides represent several advantages over monoclonal antibodies, including high penetration, better pharmacokinetics, high affinity and specificity for the target site.66,67 These features often resulted in a higher target-to-non-target ratio, which is important for both imaging and the successful therapeutic application of absorbed dose.68
This custom-designed DOTA containing the small PSMA inhibitor PSMA-617 was reported to be successfully radiolabeled with 177Lu in a small amount (0.5 mg, 0.5 nmol) in sodium acetate buffer, pH 5 with an excellent radiochemical yield ( > 99%).30 The preparation of 177Lu-PSMA-617 is also described in the literature.69 The 177Lu-PSMA-617 prepared “in-house” by our group resulted in a comparable radiochemical yield of more than 99% (data not reported), which is consistent with that reported in the literature.
177Lutetium radionuclide
Therapeutic radionuclides fall into three classification groups, namely beta particles (β-), alpha emitter (α), and Auger electron.70 Of these therapeutic radionuclides for targeted therapy, the beta particles emitter 177Lu has gained remarkable applications in recent years.70
177Lu can be routinely produced in high activity levels with a high specific activity in a nuclear reactor available worldwide.70 Although 177Lu can be crafted in a particle-accelerating machine or cyclotron,71 nuclear reactor production via neutron activation is preferred. Two methods for 177Lu production via a nuclear reactor are available, including a direct method and an indirect method.72 The direct method production or carrier-added approach employs enriched 176Lu as the irradiation target. While the latter one uses an enriched ytterbium (176Yb) target for irradiation.72,73 High specific activity of 177Lu is of great importance for the application of TRT, especially for the production of various therapeutic radiopharmaceuticals based on peptides and antibodies.72 The generator-based production of 177Lu from its long-live isomer 177mLu was reported.70,74 In addition to the generator radionuclide approach, the separation method of 177Lu from chemically and physically similar 177mLu based on the nuclear after-effect of nuclear decay was described.75
177Lu emits β- particles for therapeutic disease purposes and its γ emission is useful for SPECT imaging. The cross-fire effect of 177Lu has pointed this radionuclide as a suitable radionuclide for targeted therapy of various malignant disorders.63,76 The physical and chemical properties of 177Lu (t1/2 = 6.73 days, Eβmax = 497 keV, Eγ = 113 keV (6.4%) and 208 keV (11%)) makes it a favorable radionuclide for the development of therapeutic radiopharmaceuticals. Its β- particle energy (0.5 MeV maximum energy β-emission) allows the delivery of radiotoxicity specifically towards the tumors rather than the healthy tissue.77 The range of 177Lu penetration towards the tissue is appropriate for small tumors ( < 2 mm) and metastases compared to the longer penetration of yttrium-90 (12 mm), and may result in minimal kidney radiation exposure.77,78 Its cross-fire effect has become the important mechanism of the therapeutic outcome of radioligand therapy by destroying the surrounding cells of tracer-accumulating cells.79 Additionally, its lower gamma emission is sufficient for SPECT imaging allowing in vivo biodistribution imaging and pharmacokinetic studies as well as dosimetry measurements.72
Considerable interest in 177Lu applications has been growing since an established application 177Lu-DOTA-TATE (Lutathera®) as a peptide receptor radionuclide therapy (PRRT) radiopharmaceutical for the treatment of somatostatin receptor-positive cancers, such as neuroendocrine tumors.80 Lutathera® is the first PRRT radiopharmaceutical and was approved by The European Medicines Agency (EMA) in 2017 and by The Food and Drug Administration (FDA) in 2018.80 Preparation of several radiopharmaceuticals based on 177Lu has been reported in previous studies.81–86 Recently, the potential application of 177Lu for therapy of another target receptor, such as the gastrin-releasing peptide receptor (GRPR) has been described.1,87,88 GRPR is overexpressed in a variety of cancers such as prostate cancer.24,89 Rousseau et al described the development of the GRPR-targeted radiopharmaceutical, 177Lu-NeoBOMB1, as a promising radiopharmaceutical for prostate cancer.87 The preclinical studies investigating the use of the antagonist GRPR NeoBOMB1 for theranostic usage with 68Ga and 177Lu were investigated.90 The findings showed that 177Lu-NeoBOMB1 and 68Ga-NeoBOMB1 exhibited significant tumor uptake and favorable pharmacokinetic properties, and therefore can be potentially used as promising radiotracers for imaging and treatment of GRPR-positive cancers.90 Kurth et al reported the first human studies of another selective antagonist peptide towards GRPR, RM2-labeled with therapeutic 177Lu radionuclide.88 177Lu-RM2 has been found effective for treating mCRPC for patients with an insufficient amount of PSMA. Four patients who showed high GRPR expression on 68Ga-RM2 PET/CT imaging received 177Lu-RM2. The results showed that 177Lu-RM2 therapy was considered a safe treatment in terms of radiation safety for both patients and caregivers.88 A promising therapeutic application of 177Lu-DOTA-trastuzumab for the treatment HER-2-breast cancers was reported.91 The planar and SPECT/CT imaging results showed uptake at both the primary as well as the metastatic sites. In addition, the lack of localization of 177Lu-DOTA-trastuzumab in negative HER-2 breast cancer patients indicates the specificity of this radiopharmaceutical for treatment of HER-2-positive breast cancer in the future.91
Preclinical and clinical investigations of 177Lu
177Lu-PSMA-617is characterized by its high radiolytic stability for at least 72 hours, a high inhibitory potency ([Ki] = 2.34 ± 2.94 nM on LNCaP, Ki = 0.37 ± 0.21 nM enzymatically determined), and high internalization into LNCaP cells. In addition, the dynamic small PET imaging demonstrated high tumor-to-background contrast 1 hour p.i. The radiolabeled PSMA-617 also demonstrated rapid renal clearance and favorable pharmacokinetic properties, resulting in very high tumor-to-blood and tumor-to-muscle ratios of 1058 and 529, respectively.30
Clinical studies were conducted to evaluate the potential of this novel radioligand as a radioendotherapeutic agent for prostate cancer. Several multicenters around the world have demonstrated the high response rate as well as the low toxicity achieved after therapy with this 177Lu-labeled PSMA-617.60-62,69,70,92-96
In general, the clinical studies investigating the efficacy and safety of 177Lu-PSMA-617 are based on retrospective studies in patients with metastatic castration-resistant prostate cancer who have failed three in-line therapies, including chemotherapy, second generation anti-androgen and radium-223.64 Table 1 summarizes retrospective clinical trials with 177Lu-PSMA-617 in different multicenter.
Table 1.
Clinical studies of 177Lu-PSMA-617 in various multicenter
|
Toxicities
|
PSA evaluation
|
Response
PSA decline (≥50%) (%)
|
Activity range
per cycle (GBq)
|
n
|
References
|
| Grade 3–4: anemia (10%); thrombocytopenia (4%); and leukopenia (3%) |
2-4 wk after |
45/99 (45%) |
2-8 |
99 |
61
|
| Grade 1 dry mouth (87%); grade 3 or 4 thrombocytopenia (13%) |
3-4 wk after |
17/30 (57%) |
4.4-8.7 |
30 |
96
|
| Grade 3-4 haemotoxicity, leucopenia grade 2 |
2 months |
5/10 (50%) |
4.1-6.1 |
10 |
62
|
| Moderate acute haemotoxicities, grade 1 leucopenia (20%); grade 2 leucopenia (7%) |
every 4 wk |
13/30 (57%) |
3.7-4.0 |
30 |
69
|
| Mild nausea |
every 8 wk |
cycle 1 10/24 (41.6%);
cycle 2 (59%) |
4.1-7.1 |
24 |
60
|
| Hemoglobin toxicity: grade 2(1) and grade 3(1) |
2 wk, 4 wk, and 3 months |
Biochemical response: complete
2/31 (6%), partial 20/31 (64.5%) |
Mean activity
5.069 ± 1.845 |
31 |
95
|
| Grade 3 or 4 hematologic toxicity (4) (3.4%) |
after one course of PRLT |
46/80 (57.5%) |
2.0-9.7 |
119 |
94
|
| Grade 3 leukocytopenia (2) and grade 3 (1) anemia |
4 wk after
3rd treatment |
31/54 (58%) |
7.4 |
54 |
92
|
| Grade 3 leukocytopenia (2) |
every 2 wk |
5/14 (36%) |
6.0-8.0 |
14 |
93
|
An early report on side effects and the efficacy of this 177Lu-PSMA-617 radiotherapeutic agent was published by Ahmadzadehfar et al.62 A total number of ten patients involved in this trial received only this radiolabeled agent as a single treatment. The PSA biochemical response was an indication of efficacy and was measured two months after treatment. The tolerability of the therapy was evaluated with regard to the occurrence of post-therapeutic symptoms and toxicities. Notably, seven patients had reduced PSA levels, with 50% of them experiencing a decreased PSA level ( ≥ 50%). No patients showed serious side effects during and after hospitalization. Following this promising initial result, a larger cohort of 24 patients was selected to undergo up to two cycles of 177Lu-PSMA-617 radioligand therapy ranging from 4.1-7.1 GBq (mean of 6.0 GBq).60 Similar to the previous study, no patient showed side effects immediately after administration of 177Lu-PSMA-617. Of 24 patients evaluated 2 months after the first cycle of 177Lu-PSMA-617, 19 patients (79.1%) showed decrease PSA level; 13/24 patients (PSA decline by more than 30%) and 41.6% experienced a PSA reduction more than 50%, while 5 patients demonstrated disease progression. Twenty-two of the 24 patients were recruited to undergo a second cycle, and 15 patients (68.2%) experienced a fall in PSA level, with 59% showing more than 50% PSA decline. The most common side effect in the first 2 days after injection was mild nausea (in 3 patients). In the same year, Kratochwil et al conducted retrospective studies in 30 patients.69 Each patient received 1-3 cycles of 177Lu-PSMA-617. Most patients experienced mild to moderate toxicity.69
PSMA labeled with alpha emitter for targeted alpha therapy (TAT)
Alpha-labeled-PSMA-617 display a great potential for the treatment of metastatic prostate cancer. Therapeutic alpha-emitting radionuclides such as Ac-225, Tb-149, At-211, Bi-212 (lead-212), Bi-213, Ra-223, and Th-227 have higher energy compared to beta particle-emitting radionuclides and a short penetration path length.97–99 Therefore, they present a higher linear energy transfer. A high linear energy transfer of the alpha emitter can lead to the DNA double-strand break when interacting with nuclei. Consequently, compared to the beta emitter, TAT results in a more cytotoxic dose to cancer cells while keeping the dose to the surrounding healthy cells minimal.59,100 Kratochwil reported the first human studies of 225Ac-PSMA-617 in two patients who showed positive PSMA expression with PET/CT imaging of 68Ga-PSMA-11.101 After 225Ac-PSMA-617 therapy, the patients showed significantly lower PSA levels and complete imaging responses. Despite the remarkable results of 225Ac-PSMA-617 therapy, availability, isolation and separation chemistry for 225Ac, and stable targeting systems accompanied by a high labeling yield are still considered challenging issues.102 Therefore, the application of 177Lu-PSMA-617 to treat mCRPC is of great interest. Despite the promising results of 225Ac-PSMA-617, only a limited number of clinical studies have been reported. The success of TAT-PSMA therapy also depends on the chelating agents, improved tumor uptake of linkers and targeting vectors, and reduced toxicity and progeny redistribution.59 Because PSMA-TAT can potentially lead to xerostomia,101 tandem beta (β-) emitting 177Lu-labeled PSMA may help reduce the occurrence of dose-limiting toxicity, including xerostomia.103 In addition, it can lower the 225Ac-PSMA-617 and improve the effectiveness of 177Lu-PSMA-617.103 Recently, Yadav et al studied the efficacy and toxicity of 225Ac-PSMA-617.104 They reported the promising salvage therapy accompanied by minimal toxicity, indicating the great benefit possibility for mCRPC patients who have failed standard care, including 177Lu-PSMA-617.104
Conclusion
177Lu-PSMA-617 is a promising radiopharmaceutical for diagnostic imaging and therapeutic of mCRPC. Due to its mild toxicities and suitable in vitro and in vivo properties, this radioligand possesses greater biomedical applications. Therefore, 177Lu-PSMA-617 could become the modality of choice for the management of prostate cancer in clinical settings, including oncology and nuclear medicine.
Acknowledgments
The authors would like to acknowledge funding from The National Research and Innovation Agency (BRIN) under the Research and Innovation for Advanced Indonesia 2022 scheme (decree number 82/II.7/HK/2022) and The Indonesian Endowment Funds for Education (LPDP). The authors would also like to thank Dr. Tita Puspitasari and Dr. Rohadi Awaludin for their valuable comments on this manuscript.
Competing Interests
The authors declare no conflict of interests.
Ethical Approval
Not applicable.
References
- EANM’16. Eur J Nucl Med Mol Imaging 2016;43(Suppl 1):1-734. 10.1007/s00259-016-3484-4.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71(3):209-49. doi: 10.3322/caac.21660 [Crossref] [ Google Scholar]
- Bouchelouche K, Choyke PL, Capala J. Prostate specific membrane antigen- a target for imaging and therapy with radionuclides. Discov Med 2010; 9(44):55-61. [ Google Scholar]
- Damber JE, Aus G. Prostate cancer. Lancet 2008; 371(9625):1710-21. doi: 10.1016/s0140-6736(08)60729-1 [Crossref] [ Google Scholar]
- Crawford ED, Heidenreich A, Lawrentschuk N, Tombal B, Pompeo ACL, Mendoza-Valdes A. Androgen-targeted therapy in men with prostate cancer: evolving practice and future considerations. Prostate Cancer Prostatic Dis 2019; 22(1):24-38. doi: 10.1038/s41391-018-0079-0 [Crossref] [ Google Scholar]
- Ahmadi H, Daneshmand S. Androgen deprivation therapy for prostate cancer: long-term safety and patient outcomes. Patient Relat Outcome Meas 2014; 5:63-70. doi: 10.2147/prom.s52788 [Crossref] [ Google Scholar]
- He L, Fang H, Chen C, Wu Y, Wang Y, Ge H. Metastatic castration-resistant prostate cancer: Academic insights and perspectives through bibliometric analysis. Medicine (Baltimore) 2020; 99(15):e19760. doi: 10.1097/md.0000000000019760 [Crossref] [ Google Scholar]
- Marinova M, Alamdar R, Ahmadzadehfar H, Essler M, Attenberger U, Mücke M. Improving quality of life in patients with metastatic prostate cancer following one cycle of 177Lu-PSMA-617 radioligand therapy: a pilot study. Nuklearmedizin 2020; 59(6):409-14. doi: 10.1055/a-1234-5891 [Crossref] [ Google Scholar]
- Sun M, Niaz MO, Nelson A, Skafida M, Niaz MJ. Review of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Cureus 2020; 12(6):e8921. doi: 10.7759/cureus.8921 [Crossref] [ Google Scholar]
- Crawford ED, Higano CS, Shore ND, Hussain M, Petrylak DP. Treating patients with metastatic castration resistant prostate cancer: a comprehensive review of available therapies. J Urol 2015; 194(6):1537-47. doi: 10.1016/j.juro.2015.06.106 [Crossref] [ Google Scholar]
- Mochtar CA, Atmoko W, Umbas R, Hamid A. Prostate cancer detection rate in Indonesian men. Asian J Surg 2018; 41(2):163-9. doi: 10.1016/j.asjsur.2017.01.001 [Crossref] [ Google Scholar]
- Brönimann S, Lemberger U, Bruchbacher A, Shariat SF, Hassler MR. Poly(ADP-ribose) polymerase inhibitors in prostate and urothelial cancer. Curr Opin Urol 2020; 30(4):519-26. doi: 10.1097/mou.0000000000000776 [Crossref] [ Google Scholar]
- Catalona WJ, Smith DS, Wolfert RL, Wang TJ, Rittenhouse HG, Ratliff TL. Evaluation of percentage of free serum prostate-specific antigen to improve specificity of prostate cancer screening. JAMA 1995; 274(15):1214-20. [ Google Scholar]
- Turkbey B, Pinto PA, Choyke PL. Imaging techniques for prostate cancer: implications for focal therapy. Nat Rev Urol 2009; 6(4):191-203. doi: 10.1038/nrurol.2009.27 [Crossref] [ Google Scholar]
- Sarkar S, Das S. A review of imaging methods for prostate cancer detection. Biomed Eng Comput Biol 2016; 7(Suppl 1):1-15. doi: 10.4137/becb.s34255 [Crossref] [ Google Scholar]
- Yadav MP, Ballal S, Sahoo RK, Dwivedi SN, Bal C. Radioligand therapy with 177Lu-PSMA for metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. AJR Am J Roentgenol 2019; 213(2):275-85. doi: 10.2214/ajr.18.20845 [Crossref] [ Google Scholar]
- Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 1997; 3(1):81-5. [ Google Scholar]
- Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem 2004; 91(3):528-39. doi: 10.1002/jcb.10661 [Crossref] [ Google Scholar]
- Carter RE, Feldman AR, Coyle JT. Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc Natl Acad Sci U S A 1996; 93(2):749-53. doi: 10.1073/pnas.93.2.749 [Crossref] [ Google Scholar]
- Pinto JT, Suffoletto BP, Berzin TM, Qiao CH, Lin S, Tong WP. Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clin Cancer Res 1996; 2(9):1445-51. [ Google Scholar]
- Conway RE, Petrovic N, Li Z, Heston W, Wu D, Shapiro LH. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Mol Cell Biol 2006; 26(14):5310-24. doi: 10.1128/mcb.00084-06 [Crossref] [ Google Scholar]
- Hillier SM, Maresca KP, Femia FJ, Marquis JC, Foss CA, Nguyen N. Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res 2009; 69(17):6932-40. doi: 10.1158/0008-5472.can-09-1682 [Crossref] [ Google Scholar]
- Czerwińska M, Bilewicz A, Kruszewski M, Wegierek-Ciuk A, Lankoff A. Targeted radionuclide therapy of prostate cancer-from basic research to clinical perspectives. Molecules 2020; 25(7):1743. doi: 10.3390/molecules25071743 [Crossref] [ Google Scholar]
- Wei W, Rosenkrans ZT, Liu J, Huang G, Luo QY, Cai W. ImmunoPET: concept, design, and applications. Chem Rev 2020; 120(8):3787-851. doi: 10.1021/acs.chemrev.9b00738 [Crossref] [ Google Scholar]
- Yeole MP, Dhole SN, Kulkarni NS. Peptide nanomedicine in cancer treatment. Asian J Pharm Clin Res 2013; 6(Suppl 2):28-32. [ Google Scholar]
- Gourni E, Henriksen G. Metal-based PSMA radioligands. Molecules 2017; 22(4):523. doi: 10.3390/molecules22040523 [Crossref] [ Google Scholar]
- Ruigrok EAM, van Weerden WM, Nonnekens J, de Jong M. The future of PSMA-targeted radionuclide therapy: an overview of recent preclinical research. Pharmaceutics 2019; 11(11):560. doi: 10.3390/pharmaceutics11110560 [Crossref] [ Google Scholar]
- Baranyai Z, Tircsó G, Rösch F. The use of the macrocyclic chelator DOTA in radiochemical separations. Eur J Inorg Chem 2020; 2020(1):36-56. doi: 10.1002/ejic.201900706 [Crossref] [ Google Scholar]
- Karczmarczyk U, Wojdowska W, Mikołajczak R, Maurin M, Laszuk E, Garnuszek P. Influence of DOTA chelators on radiochemical purity and biodistribution of 177Lu- and 90Y-Rituximab in xenografted Mice. Iran J Pharm Res 2018; 17(4):1201-8. doi: 10.22037/ijpr.2018.2298 [Crossref] [ Google Scholar]
- Benešová M, Schäfer M, Bauder-Wüst U, Afshar-Oromieh A, Kratochwil C, Mier W. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med 2015; 56(6):914-20. doi: 10.2967/jnumed.114.147413 [Crossref] [ Google Scholar]
- Elsässer-Beile U, Bühler P, Wolf P. Targeted therapies for prostate cancer against the prostate specific membrane antigen. Curr Drug Targets 2009; 10(2):118-25. doi: 10.2174/138945009787354601 [Crossref] [ Google Scholar]
- Potemkin R, Strauch B, Kuwert T, Prante O, Maschauer S. Development of 18F-fluoroglycosylated PSMA-ligands with improved renal clearance behavior. Mol Pharm 2020; 17(3):933-43. doi: 10.1021/acs.molpharmaceut.9b01179 [Crossref] [ Google Scholar]
- Srinivasarao M, Galliford CV, Low PS. Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat Rev Drug Discov 2015; 14(3):203-19. doi: 10.1038/nrd4519 [Crossref] [ Google Scholar]
- Liu H, Rajasekaran AK, Moy P, Xia Y, Kim S, Navarro V. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res 1998; 58(18):4055-60. [ Google Scholar]
- Mohammed AA, Shergill IS, Vandal MT, Gujral SS. ProstaScintTM and its role in the diagnosis of prostate cancer. Expert Rev Mol Diagn 2007; 7(4):345-9. doi: 10.1586/14737159.7.4.345 [Crossref] [ Google Scholar]
- Han M, Partin AW. Current clinical applications of the 111In-capromab pendetide scan (ProstaScint® Scan, Cyt-356). Rev Urol 2001; 3(4):165-71. [ Google Scholar]
- Liu H, Moy P, Kim S, Xia Y, Rajasekaran A, Navarro V. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res 1997; 57(17):3629-34. [ Google Scholar]
- Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol 2005; 23(21):4591-601. doi: 10.1200/jco.2005.05.160 [Crossref] [ Google Scholar]
- Bander NH, Trabulsi EJ, Kostakoglu L, Yao D, Vallabhajosula S, Smith-Jones P. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J Urol 2003; 170(5):1717-21. doi: 10.1097/01.ju.0000091655.77601.0c [Crossref] [ Google Scholar]
- Kampmeier F, Williams JD, Maher J, Mullen GE, Blower PJ. Design and preclinical evaluation of a 99mTc-labelled diabody of mAb J591 for SPECT imaging of prostate-specific membrane antigen (PSMA). EJNMMI Res 2014; 4(1):13. doi: 10.1186/2191-219x-4-13 [Crossref] [ Google Scholar]
- Pandit-Taskar N, O’Donoghue JA, Beylergil V, Lyashchenko S, Ruan S, Solomon SB. ⁸⁹Zr-huJ591 immuno-PET imaging in patients with advanced metastatic prostate cancer. Eur J Nucl Med Mol Imaging 2014; 41(11):2093-105. doi: 10.1007/s00259-014-2830-7 [Crossref] [ Google Scholar]
- Tagawa ST, Akhtar NH, Nikolopoulou A, Kaur G, Robinson B, Kahn R. Bone marrow recovery and subsequent chemotherapy following radiolabeled anti-prostate-specific membrane antigen monoclonal antibody J591 in men with metastatic castration-resistant prostate cancer. Front Oncol 2013; 3:214. doi: 10.3389/fonc.2013.00214 [Crossref] [ Google Scholar]
- Niaz MO, Sun M, Ramirez-Fort MK, Niaz MJ. Review of lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for the treatment of metastatic castration-resistant prostate cancer. Cureus 2020; 12(2):e7107. doi: 10.7759/cureus.7107 [Crossref] [ Google Scholar]
- Abou D, Benabdallah N, Jiang W, Peng L, Zhang H, Villmer A. Prostate cancer theranostics - an overview. Front Oncol 2020; 10:884. doi: 10.3389/fonc.2020.00884 [Crossref] [ Google Scholar]
- Kiess AP, Minn I, Chen Y, Hobbs R, Sgouros G, Mease RC. Auger radiopharmaceutical therapy targeting prostate-specific membrane antigen. J Nucl Med 2015; 56(9):1401-7. doi: 10.2967/jnumed.115.155929 [Crossref] [ Google Scholar]
- uz Zaman M, Fatima N, Zaman A, Sajid M, Zaman U, Zaman S. Diagnostic challenges in prostate cancer and 68Ga-PSMA PET imaging: a game changer?. Asian Pac J Cancer Prev 2017; 18(10):2625-8. doi: 10.22034/apjcp.2017.18.10.2625 [Crossref] [ Google Scholar]
- Debnath S, Zhou N, McLaughlin M, Rice S, Pillai AK, Hao G. PSMA-targeting imaging and theranostic agents-current status and future perspective. Int J Mol Sci 2022; 23(3):1158. doi: 10.3390/ijms23031158 [Crossref] [ Google Scholar]
- Tateishi U. Prostate-specific membrane antigen (PSMA)-ligand positron emission tomography and radioligand therapy (RLT) of prostate cancer. Jpn J Clin Oncol 2020; 50(4):349-56. doi: 10.1093/jjco/hyaa004 [Crossref] [ Google Scholar]
- Kozikowski AP, Nan F, Conti P, Zhang J, Ramadan E, Bzdega T. Design of remarkably simple, yet potent urea-based inhibitors of glutamate carboxypeptidase II (NAALADase). J Med Chem 2001; 44(3):298-301. doi: 10.1021/jm000406m [Crossref] [ Google Scholar]
- Liu T, Nedrow-Byers JR, Hopkins MR, Berkman CE. Spacer length effects on in vitro imaging and surface accessibility of fluorescent inhibitors of prostate specific membrane antigen. Bioorg Med Chem Lett 2011; 21(23):7013-6. doi: 10.1016/j.bmcl.2011.09.115 [Crossref] [ Google Scholar]
- Maresca KP, Hillier SM, Femia FJ, Keith D, Barone C, Joyal JL. A series of halogenated heterodimeric inhibitors of prostate specific membrane antigen (PSMA) as radiolabeled probes for targeting prostate cancer. J Med Chem 2009; 52(2):347-57. doi: 10.1021/jm800994j [Crossref] [ Google Scholar]
- Barrett JA, Coleman RE, Goldsmith SJ, Vallabhajosula S, Petry NA, Cho S. First-in-man evaluation of 2 high-affinity PSMA-avid small molecules for imaging prostate cancer. J Nucl Med 2013; 54(3):380-7. doi: 10.2967/jnumed.112.111203 [Crossref] [ Google Scholar]
- Wang Z, Tian R, Niu G, Ma Y, Lang L, Szajek LP. Single low-dose injection of Evans blue modified PSMA-617 radioligand therapy eliminates prostate-specific membrane antigen positive tumors. Bioconjug Chem 2018; 29(9):3213-21. doi: 10.1021/acs.bioconjchem.8b00556 [Crossref] [ Google Scholar]
- Weineisen M, Simecek J, Schottelius M, Schwaiger M, Wester HJ. Synthesis and preclinical evaluation of DOTAGA-conjugated PSMA ligands for functional imaging and endoradiotherapy of prostate cancer. EJNMMI Res 2014; 4(1):63. doi: 10.1186/s13550-014-0063-1 [Crossref] [ Google Scholar]
- Evans BJ, King AT, Katsifis A, Matesic L, Jamie JF. Methods to enhance the metabolic stability of peptide-based PET radiopharmaceuticals. Molecules 2020; 25(10):2314. doi: 10.3390/molecules25102314 [Crossref] [ Google Scholar]
- Brandt M, Cardinale J, Aulsebrook ML, Gasser G, Mindt TL. An overview of PET radiochemistry, part 2: radiometals. J Nucl Med 2018; 59(10):1500-6. doi: 10.2967/jnumed.117.190801 [Crossref] [ Google Scholar]
- Li X, Cai H, Wu X, Li L, Wu H, Tian R. New frontiers in molecular imaging using peptide-based radiopharmaceuticals for prostate cancer. Front Chem 2020; 8:583309. doi: 10.3389/fchem.2020.583309 [Crossref] [ Google Scholar]
- Benešová M, Bauder-Wüst U, Schäfer M, Klika KD, Mier W, Haberkorn U. Linker modification strategies to control the prostate-specific membrane antigen (PSMA)-targeting and pharmacokinetic properties of DOTA-conjugated PSMA inhibitors. J Med Chem 2016; 59(5):1761-75. doi: 10.1021/acs.jmedchem.5b01210 [Crossref] [ Google Scholar]
- Juzeniene A, Stenberg VY, Bruland Ø S, Larsen RH. Preclinical and clinical status of PSMA-targeted alpha therapy for metastatic castration-resistant prostate cancer. Cancers (Basel) 2021; 13(4):779. doi: 10.3390/cancers13040779 [Crossref] [ Google Scholar]
- Ahmadzadehfar H, Eppard E, Kürpig S, Fimmers R, Yordanova A, Schlenkhoff CD. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget 2016; 7(11):12477-88. doi: 10.18632/oncotarget.7245 [Crossref] [ Google Scholar]
- Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med 2017; 58(1):85-90. doi: 10.2967/jnumed.116.183194 [Crossref] [ Google Scholar]
- Ahmadzadehfar H, Rahbar K, Kürpig S, Bögemann M, Claesener M, Eppard E. Early side effects and first results of radioligand therapy with 177Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res 2015; 5(1):114. doi: 10.1186/s13550-015-0114-2 [Crossref] [ Google Scholar]
- International Atomic Energy Agency (IAEA). Comparative Evaluation of Therapeutic Radiopharmaceuticals. Technical Reports Series No. 458. Vienna: IAEA; 2007.
- Ferdinandus J, Violet J, Sandhu S, Hofman MS. Prostate-specific membrane antigen theranostics: therapy with lutetium-177. Curr Opin Urol 2018; 28(2):197-204. doi: 10.1097/mou.0000000000000486 [Crossref] [ Google Scholar]
- Eder M, Schäfer M, Bauder-Wüst U, Hull WE, Wängler C, Mier W. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem 2012; 23(4):688-97. doi: 10.1021/bc200279b [Crossref] [ Google Scholar]
- Okarvi SM. Recent developments of prostate-specific membrane antigen (PSMA)-specific radiopharmaceuticals for precise imaging and therapy of prostate cancer: an overview. Clin Transl Imaging 2019; 7(3):189-208. doi: 10.1007/s40336-019-00326-3 [Crossref] [ Google Scholar]
- Okarvi SM. Peptide-based radiopharmaceuticals and cytotoxic conjugates: potential tools against cancer. Cancer Treat Rev 2008; 34(1):13-26. doi: 10.1016/j.ctrv.2007.07.017 [Crossref] [ Google Scholar]
- Zechmann CM, Afshar-Oromieh A, Armor T, Stubbs JB, Mier W, Hadaschik B. Radiation dosimetry and first therapy results with a 124I/ 131I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging 2014; 41(7):1280-92. doi: 10.1007/s00259-014-2713-y [Crossref] [ Google Scholar]
- Kratochwil C, Giesel FL, Stefanova M, Benešová M, Bronzel M, Afshar-Oromieh A. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med 2016; 57(8):1170-6. doi: 10.2967/jnumed.115.171397 [Crossref] [ Google Scholar]
- Banerjee S, Pillai MR, Knapp FF. Lutetium-177 therapeutic radiopharmaceuticals: linking chemistry, radiochemistry, and practical applications. Chem Rev 2015; 115(8):2934-74. doi: 10.1021/cr500171e [Crossref] [ Google Scholar]
- Kambali I. Production of Lu-177 radionuclide using deuteron beams: comparison between (d,n) and (d,p) nuclear reactions. J Phys Conf Ser 2018; 1120(1):012011. doi: 10.1088/1742-6596/1120/1/012011 [Crossref] [ Google Scholar]
- Dash A, Pillai MR, Knapp FF Jr. Production of 177Lu for targeted radionuclide therapy: available options. Nucl Med Mol Imaging 2015; 49(2):85-107. doi: 10.1007/s13139-014-0315-z [Crossref] [ Google Scholar]
- Vogel WV, van der Marck SC, Versleijen MWJ. Challenges and future options for the production of lutetium-177. Eur J Nucl Med Mol Imaging 2021; 48(8):2329-35. doi: 10.1007/s00259-021-05392-2 [Crossref] [ Google Scholar]
- Bhardwaj R, Wolterbeek HT, Denkova AG, Serra-Crespo P. Radionuclide generator-based production of therapeutic 177Lu from its long-lived isomer 177mLu. EJNMMI Radiopharm Chem 2019; 4(1):13. doi: 10.1186/s41181-019-0064-5 [Crossref] [ Google Scholar]
- Bhardwaj R, van der Meer A, Das SK, de Bruin M, Gascon J, Wolterbeek HT. Separation of nuclear isomers for cancer therapeutic radionuclides based on nuclear decay after-effects. Sci Rep 2017; 7:44242. doi: 10.1038/srep44242 [Crossref] [ Google Scholar]
- Yousefnia H, Radfar E, Jalilian AR, Bahrami-Samani A, Shirvani-Arani S, Arbabi A. Development of 177Lu-DOTA-anti-CD20 for radioimmunotherapy. J Radioanal Nucl Chem 2011; 287(1):199-209. doi: 10.1007/s10967-010-0676-4 [Crossref] [ Google Scholar]
- Nitipir C, Niculae D, Orlov C, Barbu MA, Popescu B, Popa AM. Update on radionuclide therapy in oncology. Oncol Lett 2017; 14(6):7011-5. doi: 10.3892/ol.2017.7141 [Crossref] [ Google Scholar]
- Baur B, Solbach C, Andreolli E, Winter G, Machulla HJ, Reske SN. Synthesis, radiolabelling and in vitro characterization of the gallium-68-, yttrium-90- and lutetium-177-labelled PSMA ligand, CHX-A’’-DTPA-DUPA-Pep. Pharmaceuticals (Basel) 2014; 7(5):517-29. doi: 10.3390/ph7050517 [Crossref] [ Google Scholar]
- Haberkorn U, Giesel F, Morgenstern A, Kratochwil C. The future of radioligand therapy: α, β, or both?. J Nucl Med 2017; 58(7):1017-8. doi: 10.2967/jnumed.117.190124 [Crossref] [ Google Scholar]
- Hennrich U, Kopka K. Lutathera®: the first FDA- and EMA-approved radiopharmaceutical for peptide receptor radionuclide therapy. Pharmaceuticals (Basel) 2019; 12(3):114. doi: 10.3390/ph12030114 [Crossref] [ Google Scholar]
- Humani TS, Sutari Sutari, Triningsih Triningsih, Ramli M, Ritawidya R, Haryuni RD. Preparation of (177Lu-DOTA)n-PAMAM-[nimotuzumab-F(ab’)2] as a therapeutic radioimmunoconjugate for EGFR overexpressed cancer treatment. J Math Fundam Sci 2017; 49(3):258-68. doi: 10.5614/j.math.fund.sci.2017.49.3.4 [Crossref] [ Google Scholar]
- Ramli M, Hidayat B, Aguswarini S, Karyadi K, Ardiyatno CN, Subur H. Preclinical study of 177Lu-DOTA-trastuzumab: a potential radiopharmaceutical for therapy of breast cancer positive HER-2. Jurnal Ilmu Kefarmasian Indonesia 2013; 11(2):116-22. [ Google Scholar]
- Hermanto S, Haryuni RD, Ramli M, Mutalib A, Hudiyono S. Synthesis and stability test of radioimmunoconjugate 177Lu-DOTA-F(ab′)2-trastuzumab for theranostic agent of HER2 positive breast cancer. J Radiat Res Appl Sci 2016; 9(4):441-8. doi: 10.1016/j.jrras.2016.07.001 [Crossref] [ Google Scholar]
- Ramli M, Hidayat B, Rustendi CT, Subur M, Ardiyatno CN, Karyadi K. In vitro and in vivo testing of 177Lu-DOTA-nimotuzumab, a potential radioimmunotherapeutical agent of cancers. ITB J Sci 2012; 44(4):333-45. doi: 10.5614/itbj.sci.2012.44.4.4 [Crossref] [ Google Scholar]
- Vyas M. Lutetium-177: a flexible radionuclide therapeutic options. J Nucl Med 2021; 62(Suppl 1):3039. [ Google Scholar]
- Wang T, Peng Y, Li X, Li D, Zuo C. Preliminary study of 177Lu-labeled Herceptin as theranostic agent of HER2-positive human lung adenocarcinoma xenografts in mice. J Nucl Med 2019; 60(Suppl 1):1055. [ Google Scholar]
- Rousseau E, Lau J, Zhang Z, Zhang C, Kwon D, Uribe CF. Comparison of biological properties of [177Lu]Lu-ProBOMB1 and [177Lu]Lu-NeoBOMB1 for GRPR targeting. J Labelled Comp Radiopharm 2020; 63(2):56-64. doi: 10.1002/jlcr.3815 [Crossref] [ Google Scholar]
- Kurth J, Krause BJ, Schwarzenböck SM, Bergner C, Hakenberg OW, Heuschkel M. First-in-human dosimetry of gastrin-releasing peptide receptor antagonist [177Lu]Lu-RM2: a radiopharmaceutical for the treatment of metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 2020; 47(1):123-35. doi: 10.1007/s00259-019-04504-3 [Crossref] [ Google Scholar]
- Baratto L, Duan H, Mäcke H, Iagaru A. Imaging the distribution of gastrin-releasing peptide receptors in cancer. J Nucl Med 2020; 61(6):792-8. doi: 10.2967/jnumed.119.234971 [Crossref] [ Google Scholar]
- Dalm SU, Bakker IL, de Blois E, Doeswijk GN, Konijnenberg MW, Orlandi F. 68Ga/177Lu-NeoBOMB1, a novel radiolabeled GRPR antagonist for theranostic use in oncology. J Nucl Med 2017; 58(2):293-9. doi: 10.2967/jnumed.116.176636 [Crossref] [ Google Scholar]
- Bhusari P, Vatsa R, Singh G, Parmar M, Bal A, Dhawan DK. Development of Lu-177-trastuzumab for radioimmunotherapy of HER2 expressing breast cancer and its feasibility assessment in breast cancer patients. Int J Cancer 2017; 140(4):938-47. doi: 10.1002/ijc.30500 [Crossref] [ Google Scholar]
- Rasul S, Hacker M, Kretschmer-Chott E, Leisser A, Grubmüller B, Kramer G. Clinical outcome of standardized 177Lu-PSMA-617 therapy in metastatic prostate cancer patients receiving 7400 MBq every 4 weeks. Eur J Nucl Med Mol Imaging 2020; 47(3):713-20. doi: 10.1007/s00259-019-04584-1 [Crossref] [ Google Scholar]
- Emmett L, Crumbaker M, Ho B, Willowson K, Eu P, Ratnayake L. Results of a prospective phase 2 pilot trial of 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer including imaging predictors of treatment response and patterns of progression. Clin Genitourin Cancer 2019; 17(1):15-22. doi: 10.1016/j.clgc.2018.09.014 [Crossref] [ Google Scholar]
- Kulkarni HR, Singh A, Schuchardt C, Niepsch K, Sayeg M, Leshch Y. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: the Bad Berka experience since 2013. J Nucl Med 2016; 57(Suppl 3):97S-104S. doi: 10.2967/jnumed.115.170167 [Crossref] [ Google Scholar]
- Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, Seth A. 177Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging 2017; 44(1):81-91. doi: 10.1007/s00259-016-3481-7 [Crossref] [ Google Scholar]
- Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol 2018; 19(6):825-33. doi: 10.1016/s1470-2045(18)30198-0 [Crossref] [ Google Scholar]
- Kostelnik TI, Orvig C. Radioactive main group and rare earth metals for imaging and therapy. Chem Rev 2019; 119(2):902-56. doi: 10.1021/acs.chemrev.8b00294 [Crossref] [ Google Scholar]
- Martin S, Tönnesmann R, Hierlmeier I, Maus S, Rosar F, Ruf J. Identification, characterization, and suppression of side products formed during the synthesis of [177Lu]Lu-PSMA-617. J Med Chem 2021; 64(8):4960-71. doi: 10.1021/acs.jmedchem.1c00045 [Crossref] [ Google Scholar]
- Seo Y. Quantitative imaging of alpha-emitting therapeutic radiopharmaceuticals. Nucl Med Mol Imaging 2019; 53(3):182-8. doi: 10.1007/s13139-019-00589-8 [Crossref] [ Google Scholar]
- Morgenstern A, Apostolidis C, Bruchertseifer F, Capote R, Gouder T, Simonelli F. Cross-sections of the reaction 232Th(p,3n)230Pa for production of 230U for targeted alpha therapy. Appl Radiat Isot 2008; 66(10):1275-80. doi: 10.1016/j.apradiso.2008.02.066 [Crossref] [ Google Scholar]
- Kratochwil C, Bruchertseifer F, Giesel FL, Weis M, Verburg FA, Mottaghy F. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med 2016; 57(12):1941-4. doi: 10.2967/jnumed.116.178673 [Crossref] [ Google Scholar]
- Robertson AKH, Ramogida CF, Schaffer P, Radchenko V. Development of 225Ac radiopharmaceuticals: TRIUMF perspectives and experiences. Curr Radiopharm 2018; 11(3):156-72. doi: 10.2174/1874471011666180416161908 [Crossref] [ Google Scholar]
- Kulkarni H, Zhang J, Langbein T, Schuchardt C, Singh A, Mueller D. Radioligand therapy using combination of Ac-225 and Lu-177 labelled PSMA ligands for progressive end-stage metastatic prostate cancer: effective trade-off between response and toxicity. J Nucl Med 2019; 60(Suppl 1):464. [ Google Scholar]
- Yadav MP, Ballal S, Sahoo RK, Tripathi M, Seth A, Bal C. Efficacy and safety of 225Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant prostate cancer patients. Theranostics 2020; 10(20):9364-77. doi: 10.7150/thno.48107 [Crossref] [ Google Scholar]