Advanced pharmaceutical bulletin. 13(4):712-722.
doi: 10.34172/apb.2023.087
Review Article
Current Advances in Nanotechnology-Mediated Delivery of Herbal and Plant-Derived Medicines
Amir Jalili Writing – original draft, Writing – review & editing, 1 
Rafieh Bagherifar Software, Writing – review & editing, 2, 3 
Ali Nokhodchi Supervision, Validation, 4, 5
Barbara Conway Supervision, Validation, 6, 7, * 
Yousef Javadzadeh Conceptualization, Project administration, Supervision, Validation, 8, * 
Author information:
1Department of Pharmaceutical Technology, Faculty of Pharmacy, Eastern Mediterranean University, Famagusta, North Cyprus.
2Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran.
3Department of Pharmaceutics, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran.
4Pharmaceutics Research Laboratory, School of Life Sciences, University of Sussex, Arundel Building, Brighton BNI 9QJ, UK.
5Lupin Research Center, Coral Springs, Florida, USA.
6Department of Pharmacy, School of Applied Sciences, University of Huddersfield, Huddersfield, UK.
7Institute of Skin Integrity and Infection Prevention, University of Huddersfield, Huddersfield, UK.
8Biotechnology Research Center, and Faculty of Pharmacy, Tabriz University of Medical Science, Tabriz, Iran.
Abstract
Phytomedicine has been used by humans since ancient times to treat a variety of diseases. However, herbal medicines face significant challenges, including poor water and lipid solubility and instability, which lead to low bioavailability and insufficient therapeutic efficacy. Recently, it has been shown that nanotechnology-based drug delivery systems are appropriate to overcome the above-mentioned limitations. The present review study first discusses herbal medicines and the challenges involved in the formulation of these drugs. The different types of nano-based drug delivery systems used in herbal delivery and their potential to improve therapeutic efficacy are summarized, and common techniques for preparing nanocarriers used in herbal drug delivery are also discussed. Finally, a list of nanophyto medicines that have entered clinical trials since 2010, as well as those that the FDA has approved, is presented.
Keywords: Phytomedicine, Herbal drug, Nanotechnology, Drug delivery systems, Nanophytomedicine
Copyright and License Information
©2023 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Introduction
Phytomedicines also called herbal medicines, are mixtures of plant metabolites containing pharmacologically active compounds with some healing and therapeutic properties. due to the benefits such as fewer adverse effects and low cost, herbal medicines have been used since ancient times as therapeutic agents in various diseases. In addition, over one-third of all FDA-approved new molecular entities are natural products and their derivatives.1,2 The first plant-derived drug was painkiller morphine, with a mechanism of inhibiting the discharge of neurotransmitters from presynaptic neurons and was authorized for utilization in 1827.3 Later, many other products were developed, including paclitaxel, which is used today as an anticancer agent in ovarian, breast, lung, and other cancers and extracted from the pacific yew plant (Taxus brevifolia).4,5
The significant steps to obtain herbal extracts or oils from plant materials generally include harvesting (to suppress plant metabolism at the right time), drying (to protect the active substance by inhibiting enzymes), size reduction (to increase the surface area and thus the improvement of solvent extraction) and extraction (in order to obtain therapeutic portion and omission of inert parts). Finally, the resulting extract can be traditionally formulated in various dosage forms such as solid, liquid, and semi-solid, or encapsulated in novel drug delivery systems such as liposomes, pyrosomes, polymeric NPs, etc.6-8
Despite the prominent pharmacological actions of herbal drugs in various diseases, several challenges, including pharmacokinetic drawbacks such as low bioavailability and limited absorption and physicochemical challenges like poor water and lipid solubility, large molecular size, and instability, can reduce their efficacy, primarily upon oral administration.9,10 An effective drug delivery system is needed to overcome the abovementioned barriers, reduce repeated administration, and increase patient compliance.11
In recent decades, nanotechnology-based delivery systems have received much attention in phytomedicine. The encapsulation of herbal drugs in nanocarriers and overcoming the above-mentioned limitations provides benefits such as improved solubility, protection from degradation, reduction of side effects, controlled release, and consequently optimal bioavailability and therapeutic efficacy.12-14
This review outlines the challenges of phyto/herbal medicines, including physicochemical and pharmacokinetic drawbacks. Different types of nanocarriers are also discussed as novel and efficient strategies in herbal drug delivery with the potential to overcome the above-mentioned challenges. Some of the common techniques used for the formulation of nanoparticles (NPs) have been reviewed. Therefore, an overview of FDA-approved nanophytomedicines as well as those being used in clinical trials since 2010, has been provided.
Herbal medicines: Challenges
Herbal medicines are a mixture of various ingredients with different physicochemical properties.15 In addition, poor gastrointestinal (GI) absorption and consequent low oral bioavailability of herbal drugs are due to various factors, including high molecular weight, poor solubility in GI fluids, limited permeability through cell membranes, degradation in the GI tract, hepatic presystemic metabolism, and P-glycoprotein (P-GP/MDR1/ABCB1)]-mediated gut efflux.16,17 Therefore, the development and preparation of herbal formulations face various challenges.
Nanotechnology-based techniques have been developed to overcome the above-mentioned limitations and increase the bioavailability of herbal medicines.
Nanotechnology for herbal drug delivery
The importance of nanotechnology
Nanotechnology can be used to develop products with novel and improved actions and physicochemical properties particularly in the medical field.18 Nanocarriers protect their payload from degradation, improve bioavailability, reduce the therapeutic dose and side effects, and provide targeted therapy and controlled release of phytomedicine.19-21 Different classes of nanocarriers, including lipid-based NPs, polymer-based NPs, and inorganic NPs, have been used for drug delivery in phytomedicine, which will be discussed in detail below. A schematic of common nanocarriers is shown in Figure 1.
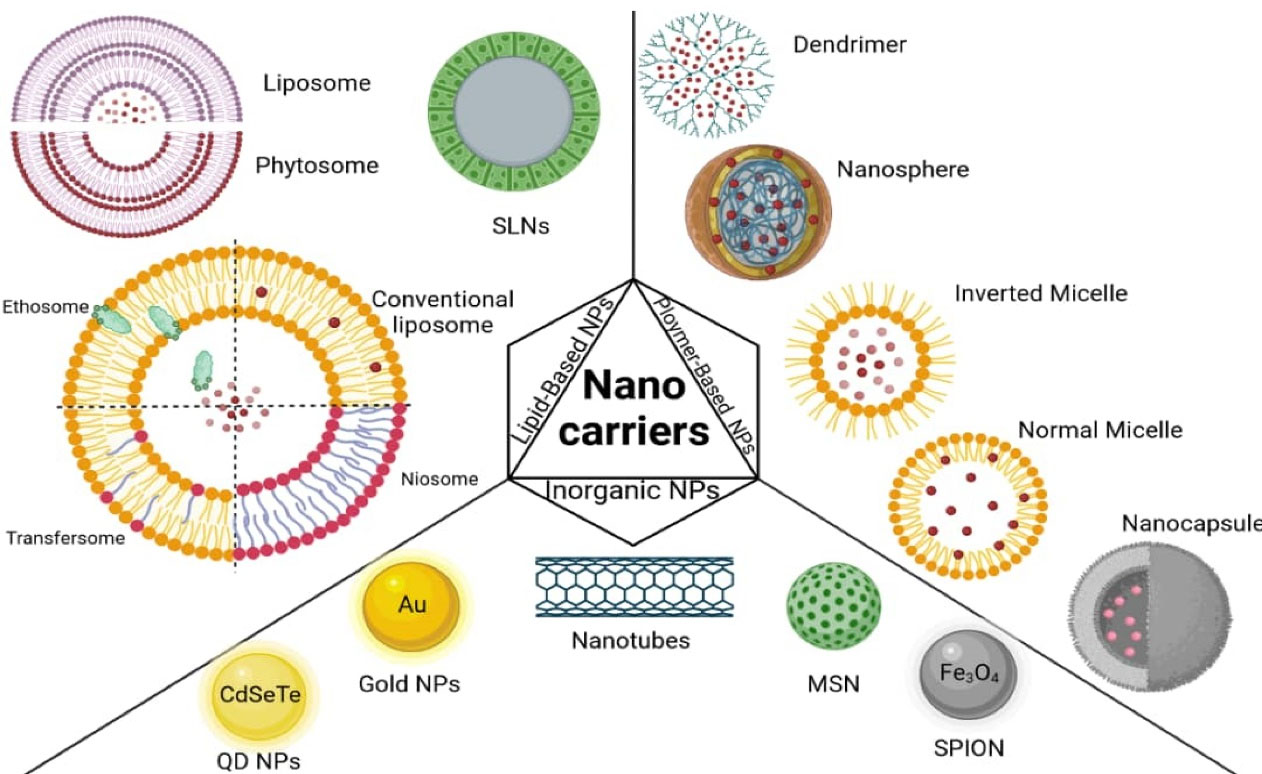
Figure 1.
Schematic representation of common nanocarriers for herbal drug delivery
.
Schematic representation of common nanocarriers for herbal drug delivery
Lipid-based nanocarriers for herbal drug delivery
In addition to the benefits mentioned in the previous section, lipid-based NPs such as solid lipid nanoparticles (SLNs), liposomes, and phytosomes also have the advantages of biocompatibility and the ability to improve the aqueous solubility of poorly soluble herbal drugs.22 Lipid-based nanocarriers are prepared using various materials and methods depending on their target. Challenges like scale-up and physical instability such as aggregation must be considered in the choice of preparation method.23 Following the preparation of NPs, parameters such as size, morphology, and surface properties should be determined because they play an essential role in the cellular uptake and pharmacological effects of NPs.24
Liposomes are vesicular NPs which consist of concentric lipid bilayers made of amphipathic phospholipid molecules that assemble to create spherical structures in aqueous media and surround part of the solvent.25 In addition to increasing the solubility of the loaded drug, the liposome has been considered as a suitable carrier in herbal delivery in terms of its ability to load both hydrophilic and lipophilic drugs besides improving bioavailability and therapeutic efficacy.26,27
In 1989, an Italian pharmaceutical and nutraceutical company, Indena, successfully generated complexes of phospholipids (phosphatidylcholine) and plant actives called Phytosome® and then patented the innovation.28 Phytosomes (refer to Figure 1), also called phytolipid delivery systems, are more stable than liposomes. Because, unlike liposomes, they have a chemical bond in their structure. Phytosomes increase the bioavailability of poorly soluble herbal medicines by increasing their absorption in GI. Some of the phytosomes comprising various phytoconstituents such as grape seed, hawthorn, Ginkgo biloba, milk thistle, ginseng, and green tea are commercialized in the USA.29,30
In 1990, SLNs as colloidal NPs which containing lipids that are in solid state at room and body temperature were developed. SLNs have advantages such as excellent physicochemical stability and higher protection compared to other NPs such as liposomes and polymeric NPs. In addition, due to biocompatibility and small size (50 to 1000 nm), it is possible to use SLN herbal formulations in various routes of administration.31,32 Table 1 summarizes the studies performed on the most common herbal medicines loaded in lipid-based NPs in the last 5 years.
Table 1.
A summary of lipid-based herbal nanoformulations
|
Nanocarrier type
|
Active ingredients/product
|
Therapeutic activity/disease
|
Results (benefits of nanotechnology)
|
Ref.
|
| Liposome |
Triptolide |
Anticancer activity |
Significant antitumor ability on breast cancer |
33
|
| Curcumin |
Anti-inflammatory activity |
Improved antioxidant and behavioral responses in inflamed mice |
34
|
| Anticancer activity |
Higher therapeutic efficiency |
35
|
| Significant cytotoxic effect on MCF-7 cells |
36
|
| Prolonged release of curcumin Improved antitumor effect |
37
|
| Anti-inflammatory activity |
Prolonged release of curcumin Reduced inflammatory markers |
38
|
| Capsaicin |
Anticancer activity |
Enhanced anticancer activity Improved pharmacokinetics properties |
39
|
| Usnic acid |
Antimicrobial activity |
Increased antimicrobial activity |
40
|
| Antimycobacterial activity |
Effective antimycobacterial activity against infected macrophages |
41
|
| Catechins |
Anticancer activity |
Significantly higher inhibition activity |
42
|
| Antioxidant activity |
Higher stability and antioxidant and antibacterial effects |
43
|
| Phytosome |
Quercetin |
Anticancer activity |
Significantly increased apoptosis |
44
|
| Naringenin |
Acute lung injury |
Sustained release of Naringenin Enhanced pulmonary bioavailability of Naringenin |
45
|
| Silybin |
Hepatoprotection activity |
Higher hepatoprotection efficacy Higher drug bioavailability |
46
|
| Epigallocatechin-3-gallate |
Anti-Inflammatory activity |
Significant anti-inflammatory activity of epigallocatechin-3-gallate |
47
|
| Curcumin |
Inflammation and anxiety |
Reduction of adverse effects of stress on anxiety and inflammation parameters |
48
|
| Ginsenosides |
Antioxidant activity |
Improved efficacy and bioavailability of the ginsenosides |
49
|
| SLN |
Triptolide |
Rheumatoid arthritis |
Remarkable inhibition of inflammation and reduction of knee edema |
50
|
| Antige + n-induced arthritis |
Better therapeutic effect |
51
|
| Berberine |
Anticancer activity |
Prolonged release of berberine |
52
|
| Wogonin |
Enhanced cytotoxicity Sustained and controlled release |
53
|
| Epigallocatechin gallate |
Antioxidant and anticancer activities |
Enhanced stability |
54
|
| Curcumin |
Anticancer activity |
Stronger cytotoxicity Higher uptake efficiency |
55
|
| Pgp inhibitor |
Effective reduction of the sensitivity to doxorubicin against drug-resistant TNBC tumors |
56
|
| CNS diseases |
Increased brain accumulation |
57
|
| Anticancer activity |
Increased bioavailability |
58
|
| Hodgkin's lymphoma |
Enhanced growth inhibitory effect |
59
|
| Antioxidant activity |
Improved stability |
60
|
| Hibiscus rosa sinensis extract |
Antidepressant activity |
Greater antidepressant activity |
61
|
| Myricetin |
Anticancer activity |
Significant increase in necrosis percentage |
62
|
| Silybin |
Type 2 diabetes |
Enhanced absorption of silybin after oral administration |
63
|
| Linalool |
Anticancer activity |
Higher tumor inhibitory effects |
64
|
Polymeric nanocarriers for herbal drug delivery
Recently, polymeric NPs have attracted more attention as a drug delivery system in phytomedicine. These NPs have a particle size of 10 to 1000 nm and are divided into two categories of nanospheres and nanocapsules based on structure. Nanospheres are polymeric matrices in which the active substance is uniformly dispersed, while nanocapsules have a core-shell structure with a polymeric shell, and the active ingredient is encapsulated in the core or is adsorbed on the polymeric membrane. Biodegradable and biocompatible synthetic or natural polymers are used to prepare polymeric NPs. These particles allow the controlled release of the drug and target it to a specific site in the body.65-67
Dendrimers have been extensively studied in herbal delivery among polymers due to their unique polyvalency, monodispersity, and controllable structure.68 Dendrimers consist of three parts: the central core, the generations, and the terminal groups. The drug can be attached to the terminal group either covalently or non-covalently and it can be encapsulated in the hydrophobic core. Polyamidoamine (PAMAM) is the first commercialized dendrimer, which is also used to increase the absorption of poorly water-soluble drugs.69,70
Polymeric micelles with a core-shell structure (10-100 nm) are another polymeric NPs that are formed by self-assembly of block copolymers consisting of both a hydrophilic block and a hydrophobic block in an aqueous medium. The hydrophobic core provides benefits such as increased solubility and protection against degradation and intracellular accumulation of the drug. The outer hydrophilic layer can achieve improved biocompatibility and active targeting. In general, the stability of polymeric micelles is higher than that of surfactant micelles.71-73 The studies conducted on the delivery of most common herbal medicines using different polymeric NPs during the last 5 years are summarized in Table 2.
Table 2.
Polymer-based herbal nanoformulations
|
Nanocarrier type
|
Active ingredients/product
|
Therapeutic activity/disease
|
Results (benefits of nanotechnology)
|
Ref.
|
| Nanospheres |
Curcumin |
Anticancer activity |
Higher anticancer activity and apoptosis in HepG2 cells |
74
|
| Increased growth inhibition and apoptosis in breast cancer cells |
75
|
| Improved serum stability Enhanced apoptotic effects on tumor cells |
76
|
| Skin wound healing process |
Enhanced potential in cutaneous wound repair |
77
|
| Berberine |
Anticancer activity |
Increased dissolution rate and bioavailability |
78
|
| Artemether |
Antimalarial activity |
Sustained release of artemether |
79
|
| Nanocapsules |
Berberine |
Anticancer activity |
Improved efficiency and controlled release of berberine |
80
|
| Curcumin |
Neuroprotective activity |
Improvement in the blockade of apomorphine-induced behavioral changes |
81
|
| Antimalarial activity |
Controlled release of curcumin |
82
|
| Dendrimer |
Quercetin |
Antibacterial efficacy |
Sustained drug release Enhanced therapeutic potential of quercetin |
83
|
| Silybin |
Antioxidant activity |
Extended-release time and improved solubility and stability |
84
|
| Curcumin |
Anticancer activity |
Reduction of the viability of glioblastoma cell lines |
85
|
| Improved antitumor effect |
86
|
| Polymeric micelles |
Berberine |
Anticancer activity |
Enhanced cellular uptake and improved solubility and delivery |
87
|
| Higher cellular uptake Enhanced cytotoxic effect against HCT116 cells |
80
|
| 10-Hydroxycamptothecin |
Improved liver targeting and absorption |
88
|
| Curcumin |
Antibacterial activity |
Enhanced penetration into the biofilms and antibacterial activity |
89
|
Inorganic nanoparticles
Recently, various types of inorganic NPs, such as metal NPs, mesoporous silica nanoparticles (MSNs), carbon nanotubes (CNTs), and magnetic NPs, have been used for applications in drug delivery.
Metal NPs, the most important of which are quantum dots (QDs), gold, silver, platinum, iron (II, III) oxide, titanium dioxide, and zinc oxide, were discovered by Faraday in 1908. Recently, metal NPs have attracted attention in herbal drug delivery due to their unique properties, like the high surface area to volume ratio, many low coordination sites, the transition between metallic and molecular states, and high surface energies.90-92
MSNs are capable of carrying large amounts of cargo due to their large surface area and porosity. In addition, they are widely used in both oral and parenteral drug delivery due to because of unique properties such as excellent chemical stability and biocompatibility.93,94
CNTs are relatively more compatible than other inorganic NPs. These NPs, which have a tubular structure, are obtained by curling up graphite sheets and are divided into two categories: single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs). SWCNTs can increase the solubility and bioavailability of herbal medicines. In addition, due to their hollow structure and the possibility of surface functionalization, they play an essential role in improving the physical and chemical properties of herbal drugs.95,96
Magnetic NPs are another group of inorganic NPs, among which Fe2O3 in the form of superparamagnetic NPs is not sensitive to oxidation compared to other magnetic NPs such as nickel and cobalt, so it has the potential application in biomedicine, mainly targeted drug delivery. In fact, the possibility of accumulation of magnetic NPs in the target tissue by applying an external magnetic field leads to target therapy.97
The studies performed during the last 5 years on the delivery of most common herbal medicines using different types of an inorganic nanocarriers are summarized in Table 3.
Table 3.
Inorganic NPs used in herbal nanoformulations
|
Inorganic nanocarrier
|
Nanocarrier type
|
Active ingredients/product
|
Therapeutic activity/disease
|
Results (benefits of nanotechnology)
|
Ref.
|
| Metal NP |
Gold |
Berberine |
Anticancer activity |
Remarkable reduction of tumor weight |
98
|
| Spinal cord injury |
Higher anti-apoptotic and anti-inflammatory effects |
99
|
| Curcumin |
Anticancer activity |
Higher inhibition of tumor cell growth |
100
|
| Silver |
Curcumin |
Antibacterial activity |
Improved curcumin photostability and antibacterial activity |
101
|
| Carbon tetrachloride induced hepatic injury |
Significant antioxidant activity |
102
|
| Anticancer activity |
Promoted cytotoxic effect on the tumor cells |
103
|
| QD |
Curcumin |
Anticancer activity |
Better inhibitory effect on tumor cells |
104
|
| MSN |
folic acid–conjugated MSN |
Curcumin |
Antioxidant, Anticancer activity |
Enhanced cellular uptake and sustained release Induction of apoptosis in vitro. Enhanced in vitro antioxidant activity |
105
|
| PEGylated lipid bilayer-coated MSN |
Paclitaxel and curcumin |
Improved stability, solubility, and sustained release in vitro
Enabled iv administration of hydrophobic drugs
Promoted in vitro cytotoxic activity against breast cancer cells |
106
|
| Magnetic NP |
Fe2O3/chitosan/montmorillonite |
Quercetin |
Anticancer activity |
Decreased toxicity Controlled and targeted release of the quercetin |
107
|
| α-Fe2O3 |
Sida cordifolia plant extract |
Antibacterial activity |
Enhanced antimicrobial activity through targeted delivery |
108
|
| Fe3O4 |
Gallic acid |
Anticancer activity |
Higher anticancer activity |
109
|
| Quercetin |
Improved anticancer activity |
110
|
| Fe3O4–β-cyclodextrin |
Epilepsy disorder |
Improved therapeutic efficacy |
111
|
| Fe3O4 |
Silymarin |
Anticancer activity |
Higher antioxidant activity |
112
|
| CNT |
MWCNT |
Curcumin, Glycyrrhizin and Rutin |
Anticancer activity |
Increased stability of suspension of CNTs in aqueous media
Decreased toxicity of delivery system |
113
|
| Curcumin |
Prolonged-release property High adsorption capacity for curcumin |
114
|
| SWCNT |
Curcumin |
Increase in population of necrotic cells |
115
|
| Improved inhibition of cancer cell proliferation |
116
|
| Cancer cell membrane-modified SWCNT |
Berberine |
Increased accumulation in liver cancer tissue
Prolonged circulation time |
117
|
Techniques used for the formulation of nanophytomedicines
High-pressure homogenization method
In the high-pressure homogenization method, lipid particles are converted into nanoscale particles using high pressure and high shear stress. This method, divided into hot and cold homogenization, is widely used to produce lipid-based NPs, including emulsions, liposomes, and SLNs at large scales. In both cases, the first step involves dissolving of the drug in the molten lipid. In hot homogenization, homogenization is applied to the pre-emulsion at a higher temperature than the melting point of lipid. In contrast, in cold homogenization, homogenization of suspension is performed at room temperature.118,119
Solvent emulsification–diffusion method
In this method, the polymer or lipid is dissolved in an organic solvent and then emulsified into an aqueous phase containing an emulsifier. Finally, the solvent is evaporated under a vacuum to form polymeric or lipid-based NPs. The advantage of this method over the homogenization method is the lack of high temperature, so it is a suitable method for formulating temperature-sensitive drugs. However, organic solvents may cause toxicological problems.120,121
Co-precipitation method
Co-precipitation is the most used method for the preparation of metal oxide and core-shell NPs. It is a cost-effective, fast, straightforward, and easily transposable on a larger scale method for industrial applications. This method gives nanomaterials via high purity and doesn’t require high pressure or temperature and hazardous organic solvents.122
Phase coacervation
Coacervation is one of the common methods of microencapsulation and is divided into two categories: simple and complex. In simple coacervation, a colloidal solute such as ethyl cellulose or chitosan is used, while in the case of complex coacervation, a polymer solution is prepared by the interaction between two oppositely charged agents such as gelatin and chitosan. Generally, this method involves the phase-separation of two separate liquid phases to form a polymer-rich phase (coacervate) and a polymer-depleted phase (equilibrium solution).123,124
Salting out method
Both the drug and polymer are first dissolved in a solvent in this method. Then, the solubility of the polymer is reduced by adding an electrolyte, and as a result, it precipitates and encapsulates the drug. This technique is primarily used for the preparation of nanospheres.125,126
Supercritical fluid-based methods
The supercritical fluid technique with the potential to produce NPs with a narrow size distribution without solvent residues in the final product is considered an essential tool for preparing a wide range of biomedical nanomaterials. Carbon dioxide and water are most commonly used supercritical solvents in this method.127 The basis of this method is the dissolution of the drug and carrier materials (e.g., polymer) in the supercritical solvent at critical temperature and pressure and then its expansion by spraying in the expansion chamber at lower pressures, which leads to the deposition of materials and the formation of NPs.128
Nanoprecipitation technique
Nanoprecipitation techniques, also called solvent displacement methods, were developed by Fessi et al.129 Usually, in this method, the polymer and drug are dissolved in a water-miscible solvent and then added to a non-solvent. The solubility of the polymer decreases as soon as it enters the nonsolvent and the polymer precipitates encapsulate the drug. The presence of an emulsifier or stabilizer, such as poloxamers is vital to avoid the aggregation of NPs during the nanoprecipitation process.130
Self-assembly methods
Self-assembly is the spontaneous arrangement of individual units to create well-defined structures, which is more suitable for preparing two-dimensional nanostructures such as nanosheets. Self-assembly can occur under the influence or in the absence of external intervention, which is called dynamic and static processes, respectively.131,132
Clinical trials and FDA-approved herbal drug delivery nanoformulations
Cosmetochem Company specialized in the production of a range of botanical extracts in a liposomal powder named Liposome Herbasec®. Similarly, a line of Phytosome® technology-based products has been developed and commercialized by the Indena Company. Both liposomal and phytosomal NPs are very efficient penetration enhancers, so they are used as drug carriers for skin with the ability to increase the bioavailability of plant extracts.15,133
In addition, different companies have offered various nanoformulations of anticancer phytomedicines. A summary of anticancer nanophytomedicines, which have entered clinical trials and have also been approved by the FDA, is given in Table 4.
Table 4.
Clinical trials and FDA-approved anticancer nanophytomedicines
|
Phytomedicine
|
Brand name
|
Nanocarrier
|
FDA approved
|
Clinical trials (phase)
|
Govt. clinical trials
|
| Docetaxel |
DoceAqualip |
Lipid nanosuspension |
Approved in India |
I/II/ III |
NCT01957995 NCT03671044 |
| SYP-0709 |
Polymeric NPs |
- |
I |
NCT02274610 NCT01103791 |
| LE-DT/ ATI-1123 |
Liposome |
- |
I/II |
NCT01151384 |
| CriPec® docetaxel/ CPC634 |
CriPec NPs |
- |
I/II |
NCT02442531 NCT03742713 NCT03712423 |
| Docetaxel-PM/ SYP-0704A/ NANOXEL- M |
Polymeric micelle |
- |
II/III |
NCT02639858 NCT02982395 NCT03585673 |
| Irinotecan |
Onivyde® |
Liposome |
Yes |
- |
NCT00702182 NCT01494506 ChiCTR-IPR- 15005856 |
| Vincristine |
Marqibo® |
Liposome |
Yes |
- |
- |
| Vinorelbine tartrate |
Navelbine/ NanoVNB® |
Liposome |
Yes |
- |
NCT03518606 NCT02925000 |
| Curcumin |
IMX-110 |
Curcumin/doxorubicin- encapsulating nanoparticle |
Yes |
I/II |
NCT03382340 |
| LipocurcTM |
Liposome |
- |
I/II |
NCT02138955 |
| Camptothecin |
CRLX101/ NLG207 |
Polymeric nanoparticle |
- |
I/II |
NCT02010567 NCT01380769 NCT01612546 |
| Paclitaxel |
NK105 |
Micellar nanoparticle |
- |
III |
NCT01644890 |
| Genexol-PM/ IG-001/ Cynviloq |
Polymeric micelle |
- |
I/II/ III/IV |
NCT03618758 |
| Lipusu® |
Liposome |
- |
I/II/ III/IV |
NCT02142790 NCT02996214 |
| Abraxane® |
Albumin-stabilized nanoparticle |
Yes |
- |
NCT02555696 NCT02151149 |
Conclusion
Despite the potential use of plant-derived drugs in the treatment of various diseases, they have considerable limitations due to their high molecular weight, high required dose, poor solubility, and high toxicity. Novel nanotechnology-based drug delivery systems, including polymeric, lipid, and inorganic nanocarriers are beneficial in overcoming these limitations. Nanocarriers containing herbal medicines provide benefits such as increased therapeutic efficacy and bioavailability. Today, many herbal and plant-derived nanoformulations have been approved by the FDA, and many clinical studies are underway in this field.
Acknowledgments
The figures were created with Biorender.com.
Competing Interests
All authors declare that they have no conflicts of interest.
Ethical Approval
Not applicable.
References
- Hafez DA, Elkhodairy KA, Teleb M, Elzoghby AO. Nanomedicine-based approaches for improved delivery of phyto-therapeutics for cancer therapy. Expert Opin Drug Deliv 2020; 17(3):279-85. doi: 10.1080/17425247.2020.1723542 [Crossref] [ Google Scholar]
- Sharma R, Hazra J, Prajapati PK. Nanophytomedicines: a novel approach to improve drug delivery and pharmacokinetics of herbal medicine. Bio Bull 2017; 3(1):132-5. [ Google Scholar]
- Patridge E, Gareiss P, Kinch MS, Hoyer D. An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov Today 2016; 21(2):204-7. doi: 10.1016/j.drudis.2015.01.009 [Crossref] [ Google Scholar]
- Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents VI The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 1971; 93(9):2325-7. doi: 10.1021/ja00738a045 [Crossref] [ Google Scholar]
- Foa R, Norton L, Seidman AD. Taxol (paclitaxel): a novel anti-microtubule agent with remarkable anti-neoplastic activity. Int J Clin Lab Res 1994; 24(1):6-14. doi: 10.1007/bf02592403 [Crossref] [ Google Scholar]
- Bart HJ, Pilz S. Industrial Scale Natural Products Extraction. John Wiley & Sons; 2011.
- Vlietinck A, Pieters L, Apers S. Legal requirements for the quality of herbal substances and herbal preparations for the manufacturing of herbal medicinal products in the European Union. Planta Med 2009; 75(7):683-8. doi: 10.1055/s-0029-1185307 [Crossref] [ Google Scholar]
- Singh J. Maceration, percolation and infusion techniques for the extraction of medicinal and aromatic plants. Extraction Technologies for Medicinal and Aromatic Plants 2008; 67:32-5. [ Google Scholar]
- Kumar K, Rai AK. Miraculous therapeutic effects of herbal drugs using novel drug delivery systems. Int Res J Pharm 2012; 3(2):27-30. [ Google Scholar]
- Gunasekaran T, Haile T, Nigusse T, Dhanaraju MD. Nanotechnology: an effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac J Trop Biomed 2014; 4(Suppl 1):S1-7. doi: 10.12980/apjtb.4.2014c980 [Crossref] [ Google Scholar]
- Sandhiya V, Ubaidulla U. A review on herbal drug loaded into pharmaceutical carrier techniques and its evaluation process. Futur J Pharm Sci 2020; 6(1):51. doi: 10.1186/s43094-020-00050-0 [Crossref] [ Google Scholar]
- Rahman HS, Othman HH, Hammadi NI, Yeap SK, Amin KM, Abdul Samad N. Novel drug delivery systems for loading of natural plant extracts and their biomedical applications. Int J Nanomedicine 2020; 15:2439-83. doi: 10.2147/ijn.s227805 [Crossref] [ Google Scholar]
- Aqil F, Munagala R, Jeyabalan J, Vadhanam MV. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett 2013; 334(1):133-41. doi: 10.1016/j.canlet.2013.02.032 [Crossref] [ Google Scholar]
- Thakur L, Ghodasra U, Patel N, Dabhi M. Novel approaches for stability improvement in natural medicines. Pharmacogn Rev 2011; 5(9):48-54. doi: 10.4103/0973-7847.79099 [Crossref] [ Google Scholar]
- Ajazuddin Ajazuddin, Saraf S. Applications of novel drug delivery system for herbal formulations. Fitoterapia 2010; 81(7):680-9. doi: 10.1016/j.fitote.2010.05.001 [Crossref] [ Google Scholar]
- Zhang W, Yang S, He H, Liu C, Chen W, Tang X. Technology for improving the bioavailability of small molecules extracted from traditional Chinese medicines. Expert Opin Drug Deliv 2009; 6(11):1247-59. doi: 10.1517/17425240903206963 [Crossref] [ Google Scholar]
- He SM, Chan E, Zhou SF. ADME properties of herbal medicines in humans: evidence, challenges and strategies. Curr Pharm Des 2011; 17(4):357-407. doi: 10.2174/138161211795164194 [Crossref] [ Google Scholar]
- Bagherifar R, Kiaie SH, Hatami Z, Ahmadi A, Sadeghnejad A, Baradaran B. Nanoparticle-mediated synergistic chemoimmunotherapy for tailoring cancer therapy: recent advances and perspectives. J Nanobiotechnology 2021; 19(1):110. doi: 10.1186/s12951-021-00861-0 [Crossref] [ Google Scholar]
- Alexander A, Ajazuddin Ajazuddin, Patel RJ, Saraf S, Saraf S. Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives. J Control Release 2016; 241:110-24. doi: 10.1016/j.jconrel.2016.09.017 [Crossref] [ Google Scholar]
- Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine 2013; 9(1):1-14. doi: 10.1016/j.nano.2012.05.013 [Crossref] [ Google Scholar]
- Istrati D, Lacatusu I, Bordei N, Badea G, Oprea O, Stefan LM. Phyto-mediated nanostructured carriers based on dual vegetable actives involved in the prevention of cellular damage. Mater Sci Eng C Mater Biol Appl 2016; 64:249-59. doi: 10.1016/j.msec.2016.03.087 [Crossref] [ Google Scholar]
- Chen ML. Lipid excipients and delivery systems for pharmaceutical development: a regulatory perspective. Adv Drug Deliv Rev 2008; 60(6):768-77. doi: 10.1016/j.addr.2007.09.010 [Crossref] [ Google Scholar]
- Devi VK, Jain N, Valli KS. Importance of novel drug delivery systems in herbal medicines. Pharmacogn Rev 2010; 4(7):27-31. doi: 10.4103/0973-7847.65322 [Crossref] [ Google Scholar]
- Shi F, Zhao JH, Liu Y, Wang Z, Zhang YT, Feng NP. Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int J Nanomedicine 2012; 7:2033-43. doi: 10.2147/ijn.s30085 [Crossref] [ Google Scholar]
- Kiaie SH, Mojarad-Jabali S, Khaleseh F, Allahyari S, Taheri E, Zakeri-Milani P. Axial pharmaceutical properties of liposome in cancer therapy: recent advances and perspectives. Int J Pharm 2020; 581:119269. doi: 10.1016/j.ijpharm.2020.119269 [Crossref] [ Google Scholar]
- Xi J, Guo R. Studies on molecular interactions between puerarin and PC liposomes. Chin Sci Bull 2007; 52(19):2612-7. doi: 10.1007/s11434-007-0395-6 [Crossref] [ Google Scholar]
- Sarangi MK, Padhi S. Novel herbal drug delivery system: an overview. Arch Med Health Sci 2018; 6(1):171-9. doi: 10.4103/amhs.amhs_88_17 [Crossref] [ Google Scholar]
- Gnananath K, Sri Nataraj K, Ganga Rao B. Phospholipid complex technique for superior bioavailability of phytoconstituents. Adv Pharm Bull 2017; 7(1):35-42. doi: 10.15171/apb.2017.005 [Crossref] [ Google Scholar]
- Awasthi R, Kulkarni G, Pawar V. Phytosomes: an approach to increase the bioavailability of plant extracts. Int J Pharm Pharm Sci 2011; 3(2):1-3. [ Google Scholar]
- Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans I Review of 97 bioavailability studies. Am J Clin Nutr 2005; 81(1 Suppl):230S-42S. doi: 10.1093/ajcn/81.1.230S [Crossref] [ Google Scholar]
- Martins S, Costa-Lima S, Carneiro T, Cordeiro-da-Silva A, Souto EB, Ferreira DC. Solid lipid nanoparticles as intracellular drug transporters: an investigation of the uptake mechanism and pathway. Int J Pharm 2012; 430(1-2):216-27. doi: 10.1016/j.ijpharm.2012.03.032 [Crossref] [ Google Scholar]
- Pople PV, Singh KK. Development and evaluation of topical formulation containing solid lipid nanoparticles of vitamin A. AAPS PharmSciTech 2006; 7(4):91. doi: 10.1208/pt070491 [Crossref] [ Google Scholar]
- Zheng W, Wang C, Ding R, Huang Y, Li Y, Lu Y. Triptolide-loaded nanoparticles targeting breast cancer in vivo with reduced toxicity. Int J Pharm 2019; 572:118721. doi: 10.1016/j.ijpharm.2019.118721 [Crossref] [ Google Scholar]
- Baradaran S, Hajizadeh Moghaddam A, Khanjani Jelodar S, Moradi-Kor N. Protective effects of curcumin and its nano-phytosome on carrageenan-induced inflammation in mice model: behavioral and biochemical responses. J Inflamm Res 2020; 13:45-51. doi: 10.2147/jir.s232462 [Crossref] [ Google Scholar]
- Zhang T, Chen Y, Ge Y, Hu Y, Li M, Jin Y. Inhalation treatment of primary lung cancer using liposomal curcumin dry powder inhalers. Acta Pharm Sin B 2018; 8(3):440-8. doi: 10.1016/j.apsb.2018.03.004 [Crossref] [ Google Scholar]
- Mahmoudi R, Mirahmadi-Babaheidri SA, Delaviz H, Fouani MH, Alipour M, Jafari Barmak M. RGD peptide-mediated liposomal curcumin targeted delivery to breast cancer cells. J Biomater Appl 2021; 35(7):743-53. doi: 10.1177/0885328220949367 [Crossref] [ Google Scholar]
- Wang WY, Cao YX, Zhou X, Wei B. Delivery of folic acid-modified liposomal curcumin for targeted cervical carcinoma therapy. Drug Des DevelTher 2019; 13:2205-13. doi: 10.2147/DDDT.S205787 [Crossref] [ Google Scholar]
- Ng ZY, Wong JY, Panneerselvam J, Madheswaran T, Kumar P, Pillay V. Assessing the potential of liposomes loaded with curcumin as a therapeutic intervention in asthma. Colloids Surf B Biointerfaces 2018; 172:51-9. doi: 10.1016/j.colsurfb.2018.08.027 [Crossref] [ Google Scholar]
- Al-Samydai A, Alshaer W, Al-Dujaili EAS, Azzam H, Aburjai T. Preparation, characterization, and anticancer effects of capsaicin-loaded nanoliposomes. Nutrients 2021; 13(11):3995. doi: 10.3390/nu13113995 [Crossref] [ Google Scholar]
- Francolini I, Giansanti L, Piozzi A, Altieri B, Mauceri A, Mancini G. Glucosylated liposomes as drug delivery systems of usnic acid to address bacterial infections. Colloids Surf B Biointerfaces 2019; 181:632-8. doi: 10.1016/j.colsurfb.2019.05.056 [Crossref] [ Google Scholar]
- Lima Salviano T, Dos Santos Macedo DC, de Siqueira Ferraz Carvalho R, Pereira MA, de Arruda Barbosa VS, Dos Santos Aguiar J. Fucoidan-coated liposomes: a target system to deliver the antimicrobial drug usnic acid to macrophages infected with Mycobacterium tuberculosis. J Biomed Nanotechnol 2021; 17(8):1699-710. doi: 10.1166/jbn.2021.3139 [Crossref] [ Google Scholar]
- Hong SC, Park KM, Hong CR, Kim JC, Yang SH, Yu HS. Microfluidic assembly of liposomes dual-loaded with catechin and curcumin for enhancing bioavailability. Colloids Surf A PhysicochemEng Asp 2020; 594:124670. doi: 10.1016/j.colsurfa.2020.124670 [Crossref] [ Google Scholar]
- Wu J, Guan R, Cao G, Liu Z, Wang Z, Shen H. Antioxidant and antimicrobial effects of catechin liposomes on Chinese dried pork. J Food Prot 2018; 81(5):827-34. doi: 10.4315/0362-028x.jfp-17-452 [Crossref] [ Google Scholar]
- Alhakamy NA, Fahmy UA, Eldin SMB, Ahmed OAA, Aldawsari HM, Okbazghi SZ. Scorpion venom-functionalized quercetin phytosomes for breast cancer management: in vitro response surface optimization and anticancer activity against MCF-7 cells. Polymers (Basel) 2021; 14(1):93. doi: 10.3390/polym14010093 [Crossref] [ Google Scholar]
- Yu Z, Liu X, Chen H, Zhu L. Naringenin-loaded dipalmitoylphosphatidylcholine phytosome dry powders for inhaled treatment of acute lung injury. J Aerosol Med Pulm Drug Deliv 2020; 33(4):194-204. doi: 10.1089/jamp.2019.1569 [Crossref] [ Google Scholar]
- Chi C, Zhang C, Liu Y, Nie H, Zhou J, Ding Y. Phytosome-nanosuspensions for silybin-phospholipid complex with increased bioavailability and hepatoprotection efficacy. Eur J Pharm Sci 2020; 144:105212. doi: 10.1016/j.ejps.2020.105212 [Crossref] [ Google Scholar]
- Shariare MH, Afnan K, Iqbal F, Altamimi MA, Ahamad SR, Aldughaim MS. Development and optimization of epigallocatechin-3-gallate (EGCG) nano phytosome using design of experiment (DoE) and their in vivo anti-inflammatory studies. Molecules 2020; 25(22):5453. doi: 10.3390/molecules25225453 [Crossref] [ Google Scholar]
- Nemati Karimooy F, Vaez A, Asadi I, Fereidouni A, Saadat M. Therapeutic effects of nano-phytosome of curcumin on anxiety-like behaviors, neuroinflammation and biochemical parameters in rats exposed to stress. Chemical Methodologies 2021; 5(3):219-26. doi: 10.22034/chemm.2021.126582 [Crossref] [ Google Scholar]
- Merchant NA, Kavya T, Srinivasa R, Rao P, Narayanan P, Bhat S. Ginsenoside Rg1 nanophytosome synthesis and their characterization: an initiative towards the treatment of amyotrophic lateral sclerosis. In: 2021 IEEE 21st International Conference on Nanotechnology (NANO). Montreal, QC: IEEE; 2021. 10.1109/nano51122.2021.9514358.
- Gu Y, Tang X, Yang M, Yang D, Liu J. Transdermal drug delivery of triptolide-loaded nanostructured lipid carriers: preparation, pharmacokinetic, and evaluation for rheumatoid arthritis. Int J Pharm 2019; 554:235-44. doi: 10.1016/j.ijpharm.2018.11.024 [Crossref] [ Google Scholar]
- Li S, Su L, Lv G, Luo W, Kang Y. Ultrasound guided intra-articular injection of triptolide-loaded solid lipid nanoparticle for treatment of antigen-induced arthritis in rabbits. Front Pharmacol 2022; 13:824015. doi: 10.3389/fphar.2022.824015 [Crossref] [ Google Scholar]
- Kabary DM, Helmy MW, Elkhodairy KA, Fang JY, Elzoghby AO. Hyaluronate/lactoferrin layer-by-layer-coated lipid nanocarriers for targeted co-delivery of rapamycin and berberine to lung carcinoma. Colloids Surf B Biointerfaces 2018; 169:183-94. doi: 10.1016/j.colsurfb.2018.05.008 [Crossref] [ Google Scholar]
- Baek JS, Na YG, Cho CW. Sustained cytotoxicity of wogonin on breast cancer cells by encapsulation in solid lipid nanoparticles. Nanomaterials (Basel) 2018; 8(3):159. doi: 10.3390/nano8030159 [Crossref] [ Google Scholar]
- Shtay R, Keppler JK, Schrader K, Schwarz K. Encapsulation of (─)-epigallocatechin-3-gallate (EGCG) in solid lipid nanoparticles for food applications. J Food Eng 2019; 244:91-100. doi: 10.1016/j.jfoodeng.2018.09.008 [Crossref] [ Google Scholar]
- Wang W, Chen T, Xu H, Ren B, Cheng X, Qi R. Curcumin-loaded solid lipid nanoparticles enhanced anticancer efficiency in breast cancer. Molecules 2018; 23(7):1578. doi: 10.3390/molecules23071578 [Crossref] [ Google Scholar]
- Fathy Abd-Ellatef GE, Gazzano E, Chirio D, Hamed AR, Belisario DC, Zuddas C. Curcumin-loaded solid lipid nanoparticles bypass P-glycoprotein mediated doxorubicin resistance in triple negative breast cancer cells. Pharmaceutics 2020; 12(2):96. doi: 10.3390/pharmaceutics12020096 [Crossref] [ Google Scholar]
- Sadegh Malvajerd S, Azadi A, Izadi Z, Kurd M, Dara T, Dibaei M. Brain delivery of curcumin using solid lipid nanoparticles and nanostructured lipid carriers: preparation, optimization, and pharmacokinetic evaluation. ACS Chem Neurosci 2019; 10(1):728-39. doi: 10.1021/acschemneuro.8b00510 [Crossref] [ Google Scholar]
- Minafra L, Porcino N, Bravatà V, Gaglio D, Bonanomi M, Amore E. Radiosensitizing effect of curcumin-loaded lipid nanoparticles in breast cancer cells. Sci Rep 2019; 9(1):11134. doi: 10.1038/s41598-019-47553-2 [Crossref] [ Google Scholar]
- Guorgui J, Wang R, Mattheolabakis G, Mackenzie GG. Curcumin formulated in solid lipid nanoparticles has enhanced efficacy in Hodgkin’s lymphoma in mice. Arch BiochemBiophys 2018; 648:12-9. doi: 10.1016/j.abb.2018.04.012 [Crossref] [ Google Scholar]
- Santonocito D, Sarpietro MG, Carbone C, Panico A, Campisi A, Siciliano EA. Curcumin containing PEGylated solid lipid nanoparticles for systemic administration: a preliminary study. Molecules 2020; 25(13):2991. doi: 10.3390/molecules25132991 [Crossref] [ Google Scholar]
- Vijayanand P, Jyothi V, Aditya N, Mounika A. Development and characterization of solid lipid nanoparticles containing herbal extract: in vivo antidepressant activity. J Drug Deliv 2018; 2018:2908626. doi: 10.1155/2018/2908626 [Crossref] [ Google Scholar]
- Khorsandi L, Mansouri E, Rashno M, Karami MA, Ashtari A. Myricetin loaded solid lipid nanoparticles upregulate MLKL and RIPK3 in human lung adenocarcinoma. Int J Pept Res Ther 2020; 26(2):899-910. doi: 10.1007/s10989-019-09895-3 [Crossref] [ Google Scholar]
- Piazzini V, Cinci L, D’Ambrosio M, Luceri C, Bilia AR, Bergonzi MC. Solid lipid nanoparticles and chitosan-coated solid lipid nanoparticles as promising tool for silybin delivery: formulation, characterization, and in vitro evaluation. Curr Drug Deliv 2019; 16(2):142-52. doi: 10.2174/1567201815666181008153602 [Crossref] [ Google Scholar]
- Rodenak-Kladniew B, Islan GA, de Bravo MG, Durán N, Castro GR. Design, characterization and in vitro evaluation of linalool-loaded solid lipid nanoparticles as potent tool in cancer therapy. Colloids Surf B Biointerfaces 2017; 154:123-32. doi: 10.1016/j.colsurfb.2017.03.021 [Crossref] [ Google Scholar]
- Khuda-Bukhsh AR, Bhattacharyya SS, Paul S, Boujedaini N. Polymeric nanoparticle encapsulation of a naturally occurring plant scopoletin and its effects on human melanoma cell A375. Zhong Xi Yi Jie He Xue Bao 2010; 8(9):853-62. doi: 10.3736/jcim20100909 [Crossref] [ Google Scholar]
- Mainardes RM, Gremião MP, Evangelista RC. Thermoanalytical study of praziquantel-loaded PLGA nanoparticles. Rev Bras Cienc Farm 2006; 42(4):523-30. doi: 10.1590/s1516-93322006000400007 [Crossref] [ Google Scholar]
- Sureshkumar R, Munikumar M, Ganesh GN, Jawahar N, Nagasamyvenkatesh D, Senthil V. Formulation and evaluation of pectin-hydroxypropyl methylcellulose coated curcumin pellets for colon delivery. Asian J Pharm 2009; 3(2):138-42. doi: 10.22377/ajp.v3i2.255 [Crossref] [ Google Scholar]
- Tolia G, Choi H. The role of dendrimers in topical drug delivery. Pharm Technol 2008; 32(11):88-98. [ Google Scholar]
- Klajnert B, Bryszewska M. Dendrimers: properties and applications. Acta Biochim Pol 2001; 48(1):199-208. [ Google Scholar]
- D’Emanuele A, Attwood D. Dendrimer-drug interactions. Adv Drug Deliv Rev 2005; 57(15):2147-62. doi: 10.1016/j.addr.2005.09.012 [Crossref] [ Google Scholar]
- Biswas S, Kumari P, Lakhani PM, Ghosh B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur J Pharm Sci 2016; 83:184-202. doi: 10.1016/j.ejps.2015.12.031 [Crossref] [ Google Scholar]
- Zou F, Wei K, Peng X. Thermodynamics of micellization and sustained release of folate targeted capecitabine loaded nanomicelles. J NanosciNanotechnol 2016; 16(8):8519-27. doi: 10.1166/jnn.2016.12710 [Crossref] [ Google Scholar]
- Wang Z, Yu Y, Ma J, Zhang H, Zhang H, Wang X. LyP-1 modification to enhance delivery of artemisinin or fluorescent probe loaded polymeric micelles to highly metastatic tumor and its lymphatics. Mol Pharm 2012; 9(9):2646-57. doi: 10.1021/mp3002107 [Crossref] [ Google Scholar]
- Rajasekar A, Devasena T, Suresh S, Senthil B, Sivaramakrishnan R, Pugazhendhi A. Curcumin nanospheres and nanorods: synthesis, characterization and anticancer activity. Process Biochem 2022; 112:248-53. doi: 10.1016/j.procbio.2021.12.007 [Crossref] [ Google Scholar]
- Afzali E, Eslaminejad T, Yazdi Rouholamini SE, Shahrokhi-Farjah M, Ansari M. Cytotoxicity effects of curcumin loaded on chitosan alginate nanospheres on the KMBC-10 spheroids cell line. Int J Nanomedicine 2021; 16:579-89. doi: 10.2147/ijn.s251056 [Crossref] [ Google Scholar]
- Duse L, Agel MR, Pinnapireddy SR, Schäfer J, Selo MA, Ehrhardt C. Photodynamic therapy of ovarian carcinoma cells with curcumin-loaded biodegradable polymeric nanoparticles. Pharmaceutics 2019; 11(6):282. doi: 10.3390/pharmaceutics11060282 [Crossref] [ Google Scholar]
- Kim DW, Choi CH, Park JP, Lee SJ. Nanospheres loaded with curcumin improve the bioactivity of umbilical cord blood-mesenchymal stem cells via c-Src activation during the skin wound healing process. Cells 2020; 9(6):1467. doi: 10.3390/cells9061467 [Crossref] [ Google Scholar]
- Jia J, Zhang K, Zhou X, Zhou D, Ge F. Precise dissolution control and bioavailability evaluation for insoluble drug berberine via a polymeric particle prepared using supercritical CO₂. Polymers (Basel) 2018; 10(11):1198. doi: 10.3390/polym10111198 [Crossref] [ Google Scholar]
- Bhide AR, Jindal AB. Fabrication and evaluation of artemether loaded polymeric nanorods obtained by mechanical stretching of nanospheres. Int J Pharm 2021; 605:120820. doi: 10.1016/j.ijpharm.2021.120820 [Crossref] [ Google Scholar]
- Ghaffarzadegan R, Khoee S, Rezazadeh S. Fabrication, characterization and optimization of berberine-loaded PLA nanoparticles using coaxial electrospray for sustained drug release. Daru 2020; 28(1):237-52. doi: 10.1007/s40199-020-00335-y [Crossref] [ Google Scholar]
- de Oliveira Pacheco C, de Gomes MG, da Silva Neto MR, Parisotto AJM, Dos Santos RB, Maciel TR. Surface-functionalized curcumin-loaded polymeric nanocapsules could block apomorphine-induced behavioral changes in rats. Pharmacol Rep 2022; 74(1):135-47. doi: 10.1007/s43440-021-00331-2 [Crossref] [ Google Scholar]
- Dos Santos RB, Nakama KA, Pacheco CO, de Gomes MG, de Souza JF, de Souza Pinto AC. Curcumin-loaded nanocapsules: influence of surface characteristics on technological parameters and potential antimalarial activity. Mater Sci Eng C Mater Biol Appl 2021; 118:111356. doi: 10.1016/j.msec.2020.111356 [Crossref] [ Google Scholar]
- Rehman K, Ali I, El-Haj BM, Kanwal T, Maharjan R, Saifullah S. Synthesis of novel biocompatible resorcinarene based nanosized dendrimer-vesicles for enhanced anti-bacterial potential of quercetin. J Mol Liq 2021; 341:116921. doi: 10.1016/j.molliq.2021.116921 [Crossref] [ Google Scholar]
- Diaz C, Guzmán J, Jiménez VA, Alderete JB. Partially PEGylated PAMAM dendrimers as solubility enhancers of Silybin. Pharm Dev Technol 2018; 23(7):689-96. doi: 10.1080/10837450.2017.1315134 [Crossref] [ Google Scholar]
- Gallien J, Srinageshwar B, Gallo K, Holtgrefe G, Koneru S, Otero PS. Curcumin loaded dendrimers specifically reduce viability of glioblastoma cell lines. Molecules 2021; 26(19):6050. doi: 10.3390/molecules26196050 [Crossref] [ Google Scholar]
- Mohammadpour K, Salahvarzi S, Dadgar Z. Connection of poly (propylene imine) dendrimer to curcumin and investigation into anti-cancer effects of its products. Asian J Nanosci Mater 1999; 3(4):340-50. doi: 10.26655/ajnanomat.2020.4.8 [Crossref] [ Google Scholar]
- Abdelmoneem MA, Mahmoud M, Zaky A, Helmy MW, Sallam M, Fang JY. Dual-targeted casein micelles as green nanomedicine for synergistic phytotherapy of hepatocellular carcinoma. J Control Release 2018; 287:78-93. doi: 10.1016/j.jconrel.2018.08.026 [Crossref] [ Google Scholar]
- Wu H, Yu T, Tian Y, Wang Y, Zhao R, Mao S. Enhanced liver-targeting via coadministration of 10-Hydroxycamptothecin polymeric micelles with vinegar baked Radix Bupleuri. Phytomedicine 2018; 44:1-8. doi: 10.1016/j.phymed.2018.04.022 [Crossref] [ Google Scholar]
- Barros CHN, Hiebner DW, Fulaz S, Vitale S, Quinn L, Casey E. Synthesis and self-assembly of curcumin-modified amphiphilic polymeric micelles with antibacterial activity. J Nanobiotechnology 2021; 19(1):104. doi: 10.1186/s12951-021-00851-2 [Crossref] [ Google Scholar]
- Jain S, Saxena N, Sharma MK, Chatterjee S. Metal nanoparticles and medicinal plants: present status and future prospects in cancer therapy. Mater Today Proc 2020; 31(Pt 4):662-73. doi: 10.1016/j.matpr.2020.06.602 [Crossref] [ Google Scholar]
- Singla R, Guliani A, Kumari A, Yadav SK. Metallic nanoparticles, toxicity issues and applications in medicine. In: Yadav SK, ed. Nanoscale Materials in Targeted Drug Delivery, Theragnosis and Tissue Regeneration. Singapore: Springer; 2016. p. 41-80. 10.1007/978-981-10-0818-4_3.
- Kiaie S, Karami C, Khodadadian A, Taher M, Soltanian S. A facile method for detection of N-acetylcysteine and L-cysteine with silver nanoparticle in aqueous environments. Journal of Bioequivalence & Bioavailability 2016; 8(5):197-203. doi: 10.4172/jbb.1000294 [Crossref] [ Google Scholar]
- Zhang Y, Wang J, Bai X, Jiang T, Zhang Q, Wang S. Mesoporous silica nanoparticles for increasing the oral bioavailability and permeation of poorly water soluble drugs. Mol Pharm 2012; 9(3):505-13. doi: 10.1021/mp200287c [Crossref] [ Google Scholar]
- Hao N, Jayawardana KW, Chen X, Yan M. One-step synthesis of amine-functionalized hollow mesoporous silica nanoparticles as efficient antibacterial and anticancer materials. ACS Appl Mater Interfaces 2015; 7(2):1040-5. doi: 10.1021/am508219g [Crossref] [ Google Scholar]
- Li YL, Li J, Yan CY, Lai ZF, Hu GJ. Chinese medicine single-walled carbon nanotube targeting compound for antitumor therapy: a feasible way?. Chin J Integr Med 2014; 20(1):63-7. doi: 10.1007/s11655-012-1080-4 [Crossref] [ Google Scholar]
- Jogi H, Maheshwari R, Raval N, Kuche K, Tambe V, Mak KK. Carbon nanotubes in the delivery of anticancer herbal drugs. Nanomedicine (Lond) 2018; 13(10):1187-220. doi: 10.2217/nnm-2017-0397 [Crossref] [ Google Scholar]
- Varadan VK, Chen L, Xie J. Nanomedicine: Design and Applications of Magnetic Nanomaterials, Nanosensors and Nanosystems. John Wiley & Sons; 2008.
- Chiu CF, Fu RH, Hsu SH, Yu YA, Yang SF, Tsao TC. Delivery capacity and anticancer ability of the berberine-loaded gold nanoparticles to promote the apoptosis effect in breast cancer. Cancers (Basel) 2021; 13(21):5317. doi: 10.3390/cancers13215317 [Crossref] [ Google Scholar]
- Zhou Z, Li D, Fan X, Yuan Y, Wang H, Wang D. Gold nanoclusters conjugated berberine reduce inflammation and alleviate neuronal apoptosis by mediating M2 polarization for spinal cord injury repair. Regen Biomater 2022; 9:rbab072. doi: 10.1093/rb/rbab072 [Crossref] [ Google Scholar]
- Fu C, Ding C, Sun X, Fu A. Curcumin nanocapsules stabilized by bovine serum albumin-capped gold nanoclusters (BSA-AuNCs) for drug delivery and theranosis. Mater Sci Eng C Mater Biol Appl 2018; 87:149-54. doi: 10.1016/j.msec.2017.12.028 [Crossref] [ Google Scholar]
- Azeez L, Lateef A, Adebisi SA. Silver nanoparticles (AgNPs) biosynthesized using pod extract of Cola nitida enhances antioxidant activity and phytochemical composition of Amaranthus caudatus Linn. Appl Nanosci 2017; 7(1):59-66. doi: 10.1007/s13204-017-0546-2 [Crossref] [ Google Scholar]
- Ebaid H, Habila M, Hassan I, Al-Tamimi J, Omar MS, Rady A. Curcumin-containing silver nanoparticles prevent carbon tetrachloride-induced hepatotoxicity in mice. Comb Chem High Throughput Screen 2021; 24(10):1609-17. doi: 10.2174/1386207323666201211100830 [Crossref] [ Google Scholar]
- Garg S, Garg A. Encapsulation of curcumin in silver nanoparticle for enhancement of anticancer drug delivery. Int J Pharm Sci Res 2018; 9(3):1160-6. doi: 10.13040/ijpsr.0975-8232.9(3).1160-66 [Crossref] [ Google Scholar]
- Khan FA, Lammari N, Muhammad Siar AS, Alkhater KM, Asiri S, Akhtar S. Quantum dots encapsulated with curcumin inhibit the growth of colon cancer, breast cancer and bacterial cells. Nanomedicine (Lond) 2020; 15(10):969-80. doi: 10.2217/nnm-2019-0429 [Crossref] [ Google Scholar]
- AbouAitah K, Swiderska-Sroda A, Farghali AA, Wojnarowicz J, Stefanek A, Gierlotka S. Folic acid-conjugated mesoporous silica particles as nanocarriers of natural prodrugs for cancer targeting and antioxidant action. Oncotarget 2018; 9(41):26466-90. doi: 10.18632/oncotarget.25470 [Crossref] [ Google Scholar]
- Lin J, Cai Q, Tang Y, Xu Y, Wang Q, Li T. PEGylated Lipid bilayer coated mesoporous silica nanoparticles for co-delivery of paclitaxel and curcumin: design, characterization and its cytotoxic effect. Int J Pharm 2018; 536(1):272-82. doi: 10.1016/j.ijpharm.2017.10.043 [Crossref] [ Google Scholar]
- Ahmadi M, Pourmadadi M, Ghorbanian SA, Yazdian F, Rashedi H. Ultra pH-sensitive nanocarrier based on Fe2O3/chitosan/montmorillonite for quercetin delivery. Int J Biol Macromol 2021; 191:738-45. doi: 10.1016/j.ijbiomac.2021.09.023 [Crossref] [ Google Scholar]
- Pallela P, Ummey S, Ruddaraju LK, Gadi S, Cherukuri CS, Barla S. Antibacterial efficacy of green synthesized α-Fe2O3 nanoparticles using Sida cordifolia plant extract. Heliyon 2019; 5(11):e02765. doi: 10.1016/j.heliyon.2019.e02765 [Crossref] [ Google Scholar]
- Rosman R, Saifullah B, Maniam S, Dorniani D, Hussein MZ, Fakurazi S. Improved anticancer effect of magnetite nanocomposite formulation of gallic acid (Fe₃O₄-PEG-GA) against lung, breast and colon cancer cells. Nanomaterials (Basel) 2018; 8(2):83. doi: 10.3390/nano8020083 [Crossref] [ Google Scholar]
- Ghafelehbashi R, Tavakkoli Yaraki M, Heidarpoor Saremi L, Lajevardi A, Haratian M, Astinchap B. A pH-responsive citric-acid/α-cyclodextrin-functionalized Fe3O4 nanoparticles as a nanocarrier for quercetin: an experimental and DFT study. Mater Sci Eng C Mater Biol Appl 2020; 109:110597. doi: 10.1016/j.msec.2019.110597 [Crossref] [ Google Scholar]
- Hashemian M, Ghasemi-Kasman M, Ghasemi S, Akbari A, Moalem-Banhangi M, Zare L. Fabrication and evaluation of novel quercetin-conjugated Fe3O4-β-cyclodextrin nanoparticles for potential use in epilepsy disorder. Int J Nanomedicine 2019; 14:6481-95. doi: 10.2147/ijn.s218317 [Crossref] [ Google Scholar]
- Zare M, Sarkati MN. Chitosan‐functionalized Fe3O4 nanoparticles as an excellent biocompatible nanocarrier for silymarin delivery. Polym Adv Technol 2021; 32(10):4094-100. doi: 10.1002/pat.5416 [Crossref] [ Google Scholar]
- Ohadi M, Rezaei P, Mehrabani M, Behnam B, Ansari M. Synthesis, characterization and toxicity assessment of the novel non covalent functionalized multi-walled carbon nanotubes with glycyrrhizin, curcumin and rutin. J Clust Sci 2022; 33(3):975-84. doi: 10.1007/s10876-021-02026-3 [Crossref] [ Google Scholar]
- Koupaei Malek S, Gabris MA, Hadi Jume B, Baradaran R, Aziz M, Abd Karim K. Adsorption and in vitro release study of curcumin form polyethyleneglycol functionalized multi walled carbon nanotube: kinetic and isotherm study. Daru 2019; 27(1):9-20. doi: 10.1007/s40199-018-0232-2 [Crossref] [ Google Scholar]
- Tiwari J, Garg A, Jain AP. Synthesis and characterization of single walled carbon nanotubes (SWCNTs) anchored curcumin for breast cancer targeting. Int J Adv Sci Technol 2020; 29(5):13708-19. [ Google Scholar]
- Singh N, Sachdev A, Gopinath P. Polysaccharide functionalized single walled carbon nanotubes as nanocarriers for delivery of curcumin in lung cancer cells. J NanosciNanotechnol 2018; 18(3):1534-41. doi: 10.1166/jnn.2018.14222 [Crossref] [ Google Scholar]
- Yue J, Wang Z, Shao D, Chang Z, Hu R, Li L. Cancer cell membrane-modified biodegradable mesoporous silica nanocarriers for berberine therapy of liver cancer. RSC Adv 2018; 8(70):40288-97. doi: 10.1039/c8ra07574c [Crossref] [ Google Scholar]
- Sahni JK, Baboota S, Ali J. Promising role of nanopharmaceuticals in drug delivery. Pharma Times 2011; 43(10):16-8. [ Google Scholar]
- Ansari SH, Islam F, Sameem M. Influence of nanotechnology on herbal drugs: a review. J Adv Pharm Technol Res 2012; 3(3):142-6. doi: 10.4103/2231-4040.101006 [Crossref] [ Google Scholar]
- Trotta M, Debernardi F, Caputo O. Preparation of solid lipid nanoparticles by a solvent emulsification-diffusion technique. Int J Pharm 2003; 257(1-2):153-60. doi: 10.1016/s0378-5173(03)00135-2 [Crossref] [ Google Scholar]
- Siekmann B, Westesen K. Melt-homogenized solid lipid nanoparticles stabilized by the nonionic surfactant tyloxapol I Preparation and particle size determination. Pharm Pharmacol Lett 1994; 3(5):194-7. [ Google Scholar]
- Cruz IF, Freire C, Araújo JP, Pereira C, Pereira AM. Multifunctional ferrite nanoparticles: from current trends toward the future. In: El-Gendy AA, Barandiarán JM, Hadimani RL, eds. Magnetic Nanostructured Materials. Elsevier; 2018. p. 59-116. 10.1016/b978-0-12-813904-2.00003-6.
- Chadha S. Recent advances in nano-encapsulation technologies for controlled release of biostimulants and antimicrobial agents. In: Jogaiah S, Singh HB, Fraceto LF, de Lima R, eds. Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture. Woodhead Publishing; 2021. p. 29-55. 10.1016/b978-0-12-820092-6.00002-1.
- Salaün F. Microencapsulation technology for smart textile coatings. In: Hu J, ed. Active Coatings for Smart Textiles. Elsevier; 2016. p. 179-220. 10.1016/b978-0-08-100263-6.00009-5.
- Yoo HS, Oh JE, Lee KH, Park TG. Biodegradable nanoparticles containing doxorubicin-PLGA conjugate for sustained release. Pharm Res 1999; 16(7):1114-8. doi: 10.1023/a:1018908421434 [Crossref] [ Google Scholar]
- Kumari B. A review on nanoparticles: their preparation method and applications. Indian Res J Pharm Sci 2018; 5(2):1420-6. doi: 10.21276/irjps.2018.5.2.3 [Crossref] [ Google Scholar]
- Pathak K. Effective formulation strategies for poorly water soluble drugs. In: Nayak AK, Pal K, Banerjee I, Maji S, Nanda U, eds. Advances and Challenges in Pharmaceutical Technology. Elsevier; 2021. p. 181-228. 10.1016/b978-0-12-820043-8.00004-9.
- Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev 2007; 59(6):454-77. doi: 10.1016/j.addr.2007.04.011 [Crossref] [ Google Scholar]
- Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm 1989; 55(1):R1-R4. doi: 10.1016/0378-5173(89)90281-0 [Crossref] [ Google Scholar]
- Quintanar-Guerrero D, Allémann E, Fessi H, Doelker E. Preparation techniques and mechanisms of formation of biodegradable nanoparticles from preformed polymers. Drug Dev Ind Pharm 1998; 24(12):1113-28. doi: 10.3109/03639049809108571 [Crossref] [ Google Scholar]
- Ghalia MA, Dahman Y. Advanced nanobiomaterials in tissue engineering: synthesis, properties, and applications. In: Grumezescu AM, ed. Nanobiomaterials in Soft Tissue Engineering. Elsevier; 2016. p. 141-72. 10.1016/b978-0-323-42865-1.00006-4.
- Yadav S, Sharma AK, Kumar P. Nanoscale self-assembly for therapeutic delivery. Front BioengBiotechnol 2020; 8:127. doi: 10.3389/fbioe.2020.00127 [Crossref] [ Google Scholar]
- Semalty A, Semalty M, Rawat MS, Franceschi F. Supramolecular phospholipids-polyphenolics interactions: the PHYTOSOME strategy to improve the bioavailability of phytochemicals. Fitoterapia 2010; 81(5):306-14. doi: 10.1016/j.fitote.2009.11.001 [Crossref] [ Google Scholar]