Advanced pharmaceutical bulletin. 13(4):772-783.
doi: 10.34172/apb.2023.086
Original Article
Screening of Cyclodextrins in the Processing of Buserelin Dry Powders for Inhalation Prepared by Spray Freeze-Drying
Mostafa Rostamnezhad Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Writing – original draft, Writing – review & editing, 1 
Katayoon Mireskandari Data curation, Investigation, Visualization, 1
Mohammad Reza Rouini Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, 1
Samira Ansari Supervision, 2, 3
Majid Darabi Software, 1
Alireza Vatanara Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing, 1, * 
Author information:
1Department of Pharmaceutics, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.
2CinnaGen Medical Biotechnology Research Center, Alborz University of Medical Sciences, Karaj, Iran.
3CinnaGen Research and Production Co., Alborz, Iran.
Abstract
Purpose:
In this study, we prepared inhalable buserelin microparticles using the spray freeze-drying (SFD) method for pulmonary drug delivery. Raffinose as a cryoprotectant carrier was combined with two levels of five different cyclodextrins (CDs) and then processed by SFD.
Methods:
Dry powder diameters were evaluated by laser light scattering and morphology was determined by scanning electron microscopy (SEM). Differential scanning calorimetry (DSC) and X-ray diffraction (XRD) analysis were utilized for the determination of crystalline structures. The aerodynamic properties of the spray freeze-dried powders were evaluated by twin stage impinger (TSI) and the stability of prepared samples was assessed under normal and accelerated conditions.
Results:
The prepared powders were mostly porous spheres and the size of microparticles ranged from 9.08 to 13.53 μm, which are suitable as spray-freeze dried particles. All formulations showed amorphous structure confirmed by DSC and XRD. The aerosolization performance of the formulation containing buserelin, raffinose and 5% beta-cyclodextrin (β-CD), was the highest and its fine particle fraction (FPF) was 69.38%. The more circular and separated structures were observed in higher concentrations of CDs, which were compatible with FPFs. The highest stability was obtained in the formulation containing hydroxypropyl beta-cyclodextrin (HP-β-16. CD) 5%. On the contrary, sulfobutylether beta-cyclodextrin (SBE-β-CD) 5% bearing particles showed the least stability.
Conclusion:
By adjusting the type and ratio of CDs in the presence of raffinose, the prepared formulations could effectively enhance the aerosolization and stability of buserelin. Therefore, they can be proposed as a suitable career for lung drug delivery.
Keywords: Spray freeze-drying, Buserelin, Raffinose, Cyclodextrins, Microparticles, Aerodynamic behavior
Copyright and License Information
©2023 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Pulmonary delivery of medicines is a promising manner due to its non-invasive characteristic for both systemic and local effects in the treatment of a wide range of disorders such as cystic fibrosis, chronic obstructive pulmonary disease, asthma, diabetes, etc.1,2 High surface area and lack of the first-pass metabolism are outstanding properties that contribute to selection of the respiratory tract as a suitable site for drug delivery.3,4
Extensive studies in the last decade have shown that for some reasons such as lower proteolytic enzymatic degradation, high absorption of macromolecules in the lungs, rapid onset of action, etc., delivery of peptides and proteins through the lungs can be very effective. But the acquisition of inhalable peptides and proteins with suitable aerodynamic properties is associated with challenges.5
Buserelin is a peptide analog of hypothalamic gonadotropin-releasing hormone (GnRH) used to treat premature puberty, prostate cancer, uterine leiomyoma, endometriosis and ovulation stimulation.6,7 Comprehensive application of GnRH analogs has been a reason for developing various dosage forms, including injectable solutions, slow-release systems and nasal spray.8 Due to the invasive nature of injection and the variation in the bioavailability of the drug through the nasal route (2.5% to 6%), it seems to be useful to obtain a dosage form that solves the problems of available buserelin products in the market.9
Dried forms of protein and peptide drugs provide more stable formulations than liquid ones.10 A wide range of dry powder inhalers (DPIs) have been proposed for respiratory drug delivery. DPIs should provide appropriate mass median aerodynamic diameter and reduce particle adhesion, leading to an increase in drug deposition in the lungs.11
There are a wide variety of particle engineering techniques that can be exploited for DPIs production, including micronization and blending, supercritical fluid processing, freeze drying, spray drying (SD) and spray freeze-drying (SFD).12,13 SFD is a relatively novel particle engineering technique, suitable for production of porous, fine and uniform particles. Therefore, it is an attractive method among different particle engineering methods.14-16 The general principle of this method is atomization of feed solution into liquid nitrogen and then lyophilization of frozen droplets to allow the solvent to be sublimated at low temperature and vacuum conditions (Figure 1).7,8 Spraying and rapid freezing of the droplets and sublimation of ice particles in this method create porous spherical particles. As a result, SFD method creates particles with suitable aerodynamic properties, size, density17 and leading to higher stability in the lungs and nasal mucosa than other conventional drying methods.18 This in turn improves performance of engineered drug delivery system. Also, the low applied temperature exploited in this method preserves the nature of drugs (especially protein and peptide drugs).18,19
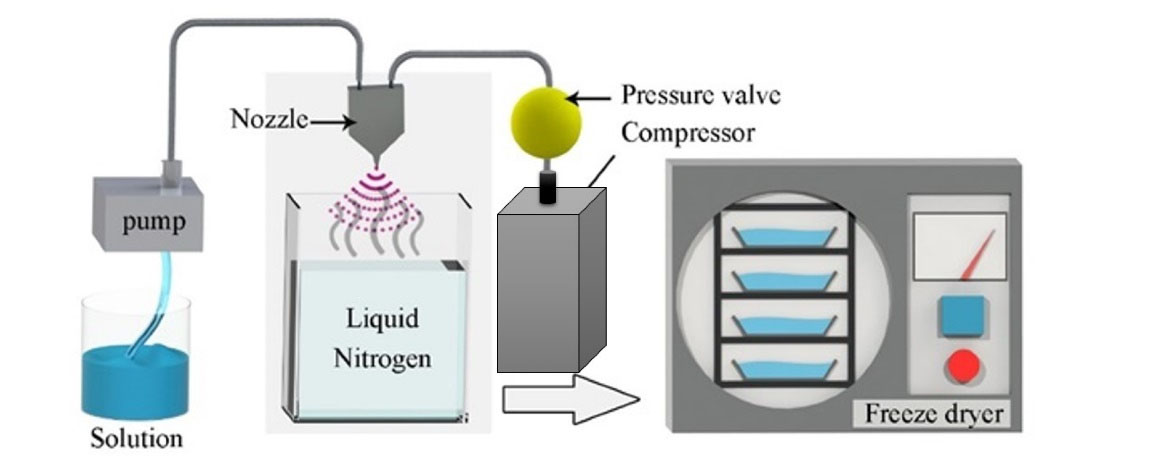
Figure 1.
Schematic of SFD process
.
Schematic of SFD process
In contrast, there are some drawbacks to the SFD method. Several stresses are applied on the peptide and proteins during the SFD process can cause some irreparable damages on the molecule structures, causing aggregation and structural perturbations. Mechanical stress of spraying from the nozzle, the air-water interface formation and cold denaturation are some sources of damage in this process. To overcome this, different stabilizing agents are employed to prevent process instability20-22 and numerous studies have shown the protective effects of various sugars and polyhydroxy compounds on the stability of peptides and proteins during dehydration, freezing, and storage.23-25 As these excipients interact with applied drugs in different ways, further studies need to be implemented to introduce specific combinations for an individual drug and the aerodynamic behavior of each drug in the presence of different sugars is unique; So, studying various aspects of excipients can be helpful.
The most common stabilizing agent utilized in dried formulations are sugars like lactose, mannitol, trehalose, raffinose and sucrose. Currently, raffinose has been used in some studies due to its desirable properties, more specifically, the formation of microparticles containing raffinose and a protein model, with proper aerodynamic and micrometric properties for pulmonary delivery.26,27 It provides several advantages over other common sugars such as trehalose and sucrose.28 Raffinose, is a non-reducing oligosaccharide preventing Millard’s reaction in proteins26 represents its merits via two mechanisms, water replacement and vitrification relating to the kinetic and thermodynamic aspects of stabilization, respectively.29,30 Two main parameters affecting the physical and chemical stability of biomolecules are glass transition temperature (Tg) and collapse temperature (Tc).31 Through water replacement mechanism, raffinose makes more effective direct hydrogen bonds with biomolecules than trehalose and sucrose.23,32 Finally, it is a suitable stabilizer for the long-term storage of dried formulations.33-35
Cyclodextrins (CDs) as cyclic oligosaccharides are also appropriate excipients that can be employed to inhibit protein and peptide aggregation.36-38 While CDs at concentrations greater than 1% (w/v) act as lyoprotectant and stabilize protein by both water replacement and vitrification mechanisms, at concentrations of less than 1% (w/v) play as a non-ionic surfactant agent, protecting the protein and peptide from the air-water interface.39 It was found that CD-raffinose binary carriers can be exploited as an ideal candidate for inhaled drug delivery27 due to the improvement of anti-hygroscopicity and aerodynamic properties of dry powders prepared by SD method.
Despite the fact that broad studies have been focused on CDs employment for particle engineering purposes, due to the structural diversity and different mechanisms of CDs to protect biologics at different concentrations, further investigations can provide valuable information about the impact of different CDs on physicochemical characteristics of fabricated particles. In this study, we aimed to evaluate the effect of combination of raffinose and five different CDs on the aerodynamic properties and stability of DPIs prepared by SFD process.
Materials and Methods
Materials
Buserelin was kindly donated by CinnaGen Company, Iran. Highly branch cyclic dextrin (HBCD) was obtained from Glico Nutrition Co. Ltd., Japan. Gamma-cyclodextrin (Gamma-CD) and sulfobutylether-β-cyclodextrin (SBE-β-CD), were purchased from CYCLOLAB 106. Ltd., Hungary. Beta-cyclodextrin (β-CD), Hydroxypropyl beta-cyclodextrin (HP-β-CD), raffinose, analytical grade of acetonitrile and phosphoric acid 85%, were purchased from Sigma, Germany. Size 2 hydroxypropyl methylcellulose (HPMC) capsules were purchased from Cipla, India.
Methods
Preparation of microparticles processed by SFD
Firstly, aqueous solutions containing buserelin (4 mg), raffinose (400 mg) and five different CDs (HBCD, β-CD, HP-β-CD, Gamma-CD, and SBE-β-CD) with two ratios of CD: raffinose (0.05 and 5 % w/v) were prepared according to Table 1. In each experiment, 0.4 L of liquid nitrogen was poured into a 2 L glass container as cryogenic vapour. The feed solution was atomized into vapour above liquid nitrogen by a laboratory scale atomizer equipped with a pneumatic nozzle at a flow rate of 0.6 mL/min. After forming the frozen droplets at the bottom of the glass container and nitrogen evaporation, the lyophilization step was performed by a freezer dryer (Dorsatech, Iran). While primary drying was done at –50 °C and 0.005 mbar within 24 hours, the secondary drying took place in 24 hours and the temperature gradually reached to -20 °C. The lyophilized powders were gathered in glass vials for further evaluation.
Table 1.
Types of feed solutions and particle size distribution for spray freeze-dried formulations
|
Formulation
|
CD (%)
|
d
10%
(μm)
|
d
50%
(μm)
|
d
90%
(μm)
|
Span
|
| F1 |
HBCD (0.05) |
2.69 |
9.26 |
30.73 |
3.02 |
| F2 |
HBCD (5) |
2.61 |
9.08 |
29.74 |
2.98 |
| F3 |
β-CD (0.05) |
3.27 |
9.99 |
28.04 |
2.47 |
| F4 |
β-CD (5) |
2.38 |
11.65 |
29.81 |
2.35 |
| F5 |
HP-β-CD (0.05) |
3.43 |
10.75 |
30.38 |
2.50 |
| F6 |
HP-β-CD (5) |
2.91 |
13.53 |
30.91 |
2.06 |
| F7 |
Gamma-CD (0.05) |
1.79 |
11.76 |
30.38 |
2.43 |
| F8 |
Gamma-CD (5) |
1.74 |
11.22 |
28.96 |
2.42 |
| F9 |
SBE-β-CD (0.05) |
1.48 |
9.38 |
26.63 |
2.68 |
| F10 |
SBE-β-CD (5) |
2.01 |
11.04 |
27.93 |
2.34 |
HPLC analysis
A reverse-phase high-performance liquid chromatography system (Knauer, Germany) equipped with a C18 column (4.6 × 150 mm, 5 µm particle size, Tosoh Bioscience, Japan) was used for buserelin assay. Analysis was performed under isocratic elution with mobile phase containing 75:25 (v/v) phosphate buffer/acetonitrile, pH 2.5. The detection wavelength, flow rate and injection volume were 220 nm, 1 mL/min and 50 µl, respectively. The LOD and LOQ of this method are 30 and 100 ng/mL, respectively. HPLC analysis is based on the buserelin monograph in the European Pharmacopoeia.40
Laser light scattering for particle size analysis
For particle size analysis by laser light scattering instrument, (Sympatec GmbH,Germany), 10 mg of each spray freeze-dried formulation was dispersed in 5 mL of acetonitrile as a dispersion medium before they were sonicated in a sonicator bath (Starsonic, Italy) for 5 minutes. In three measurements, the average particle size was obtained at obscuration between 15% to 20%. The span, a volume-based size distribution parameter, was obtained by Eq. 1:
Eq. (1)
Scanning electron microscopy (SEM)
The morphological characteristics of spray freeze-dried particles were examined by SEM at an accelerated voltage of 25 kV. (XL30, the Netherlands). The processed powders were poured on aluminum stub covered with carbon tape, then sputter-coated with gold at room temperature under vacuum (BAL-TEC, Switzerland).
Differential scanning calorimetry (DSC)
Thermal behaviour and crystallinity of SFD processed powders were assessed by DSC apparatus (Mettler Toledo, Switzerland). Approximately, 10 mg of each sample was placed in the aluminum pan, sealed and then heated from -20 to 200 °C. Scan speed was 10 °C per min.
X-ray diffraction (XRD) analysis
X-ray diffractometer (SmartLab, Rigaku, Japan) was applied to affirm the DSC results on the crystallinity of the powders. Samples were evaluated with Cu Κα at 40 kV with 30 mA and were scanned from 5 to 100 °C. Scan speed was 5 °C per min.
Particle density
The bulk density of the selected formulations was measured by determining the volume of a known mass of the sample that has been poured into a 10 mL measuring cylinder. The true density was also measured using a helium pycnometer (Multipycnometer, Quantachrome Instruments, USA) after calibration of the instrument using standard stainless steel spheres provided by the manufacturer. Each formulation was analyzed three times.
In vitro aerodynamic behavior
To compare the in vitro aerodynamic behavior of all samples, the twin stage impinger (TSI) was used. Then, for a more detailed investigation, three selected formulations were evaluated by Andersen cascade impactor (ACI).
Approximately, 5 mg of each powder which contains 50 µg of buserelin was poured into an HPMC capsule and placed in a Cyclohaler® device. The aerodynamic properties of the SFD processed powders were evaluated by TSI at vacuum strength of 60 L/min for 5 seconds calibrated with a flowmeter (Erweka, Germany) according to the British Pharmacopoeia 2022.41 The capsule, device and various parts of the TSI were washed by mobile phase. The drug contents of each part were determined by RP-HPLC and then, the emitted dose (ED), fine particle dose (FPD) and fine particle fraction (FPF) for each formulation were calculated. ED is the amount of drug taken out of the capsule and device into TSI, the FPD is the amount of drug collected in stage 2 and the FPF is the FPD divided by ED multiplied by 100.
For further clarification, the aerodynamic properties of the selected powders were evaluated by ACI (Copley Scientific, Nottingham, UK) at vacuum strength of 60 L/min for 4 seconds calibrated with a flowmeter (Erweka, Germany) at a pressure drop of 4 kPa. Approximately, 10 mL mobile phase was applied to the preseparator (conforming to USP apparatus 3) before analysis to avoid particle bouncing. The capsule, device and various parts of the ACI were washed by mobile phase. The drug contents of each part were determined by RP-HPLC and then, the ED and FPF for three selected formulations, were calculated. ED is the amount of drug taken out of the capsule and device into ACI, the FPF is defined as the percent fraction of ED with particle aerodynamic diameter < 3.2 mm; i.e. ED deposited on stage 2 to F.42
Stability studies
For stability studies, the SFD processed samples were placed in sealed glass vials with parafilmand stored at 25 167. °C, 40% and 45 °C, 60% relative humidity. Drug recovery for each formulation was evaluated by RP-HPLC at 1 month and 3 months storage.
Results
The ultra-fast freezing step, different rheological and energy-mass transfer phenomena in SFD method lead to obtaining products with different properties from those of spray drying (SD) or freeze drying.43 The quality and characteristics of spray freeze-dried particles have shown a significant correlation with the type and ratio of formulation components.44
Particle size distribution evaluation
The particle size distribution parameters of the prepared particles are presented in Table 1. Mean particle size of the formulations was between 9.08 to 13.53 μm. Due to the formation of porous particles in the SFD process, this particle size range can be acceptable for inhalation. Formulation F6, containing HP-β-CD 5%, showed the largest mean particle size with the lowest span (2.06) and formulation F1, containing HBCD 0.05%, had the highest Span (3.02).
Scanning electron microscopy
The morphologies of microparticles fabricated by SFD technique were evaluated by SEM. According to micrographs (Figure 2), all formulations were formed in relatively spherical shape and pores within and on the surface. This fine porous structure is one of the most vital points of the SFD method compared to the conventional methods for particle engineering.45 Of course, the particles are different in terms of adhesion and porosity and there is a morphology difference as the concentration of components is changed. Except in formulations containing SBE-β-CD, in which the particles tend to be more aggregated and showed lower FPF, in other formulations, aggregation between particles decreased as the CD content increased from 0.05 to 5%. Also broken particles were found in the formulation containing SBE-β-CD 0.05%.

Figure 2.
SEM images of spray freeze-dried formulations (a:F1; b:F2; c:F3; d:F4; e:F5; f:F6; g:F7; h:F8; i:F9; j:F10)
.
SEM images of spray freeze-dried formulations (a:F1; b:F2; c:F3; d:F4; e:F5; f:F6; g:F7; h:F8; i:F9; j:F10)
Differential scanning calorimetry
Thermal behavior of processed powders was assessed by DSC experiments. Figure 3 indicates the DSC thermograms of pure buserelin, pure excipients and processed powders with the combinations of raffinose and different cryoprotectants, including HBCD, β-CD, HP-β-CD, Gamma-CD and SBE-β-CD. Buserelin showed an endothermic peak around 180-190 °C, which was referred to its melting. This peak sharpness was seen in most formulations which could mean the buserelin remained crystalline after SFD. Moreover, there was a broad peak in some CDs between 100-140 °C, corresponding to water loss. Similarly, a sharp peak between 80-90 °C was seen for pentahydrated raffinose. This peak was not seen in the formulations since the raffinose became amorphous.
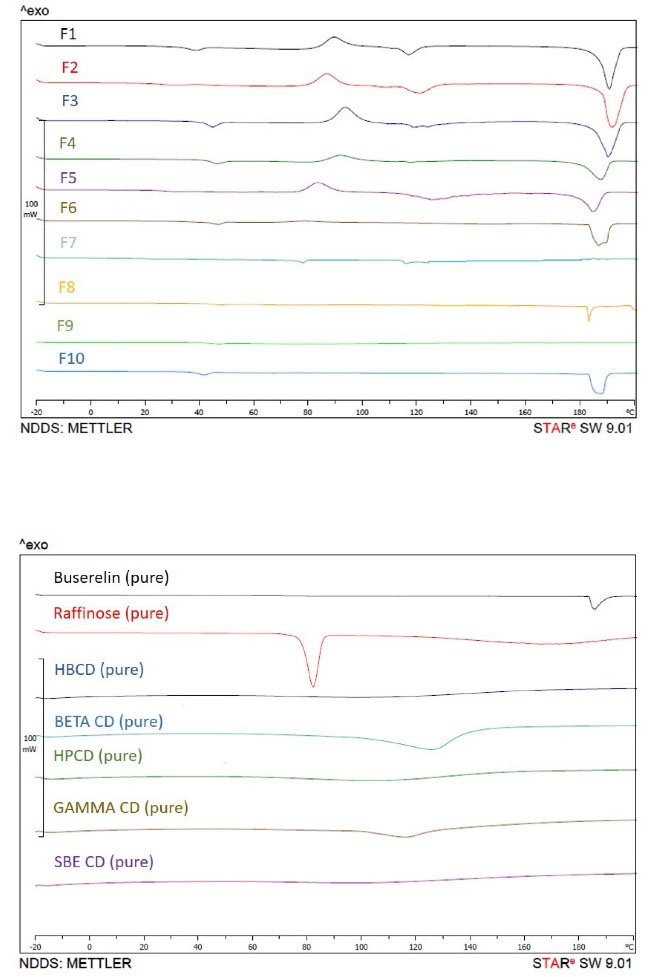
Figure 3.
DSC thermograms of the spray freeze-dried formulations (a), and excipients (b)
.
DSC thermograms of the spray freeze-dried formulations (a), and excipients (b)
X-ray diffraction analysis
For confirmation of the obtained data from DSC, analysis by XRD was performed. As shown in Figure 4a, all spray freeze-dried formulations showed an amorphous structure; Whereas, pure excipients, raffinose and β-CD were crystalline (Figure 4b). Due to the rapid freezing of atomized droplets in contact with liquid nitrogen and rapid formation of particles, there is not enough time for crystallization and the amorphous habit of the particles is reasonable. Particle aggregation observed in SEM images is consistent with the amorphous nature of the particles.
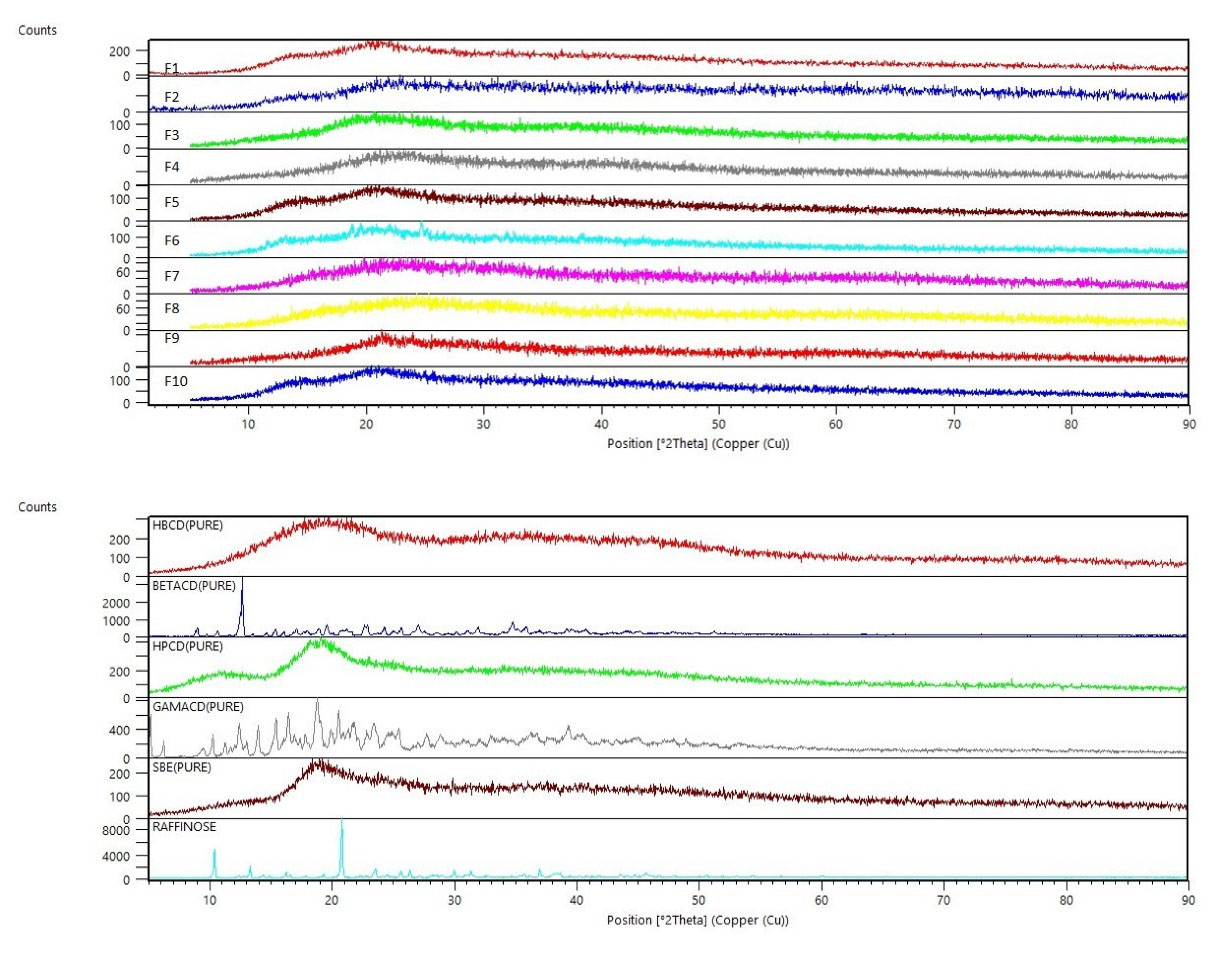
Figure 4.
XRD patterns of spray freeze-dried formulations (a), and excipients (b)
.
XRD patterns of spray freeze-dried formulations (a), and excipients (b)
Particle density
The bulk density, true density, and tapped density values for selected samples were ranging from 0.081 to 0.095 g/cm3, 0.52 to 0.76 g/cm3, and 0.17 to 0.2 g/cm3 respectively (Table 2). Formulation F4 showed the lowest density values which was consistent with its highest FPF.
Table 2.
Bulk density, true density and tapped density values of the spray freeze-dried samples.
|
Formulation
|
Bulk density
(g/cm3)
|
True density
(g/cm3)
|
Tapped density
(g/cm3)
|
| F4 |
0.081 ± 0.011 |
0.52 ± 0.002 |
0.17 ± 0.022 |
| F6 |
0.092 ± 0.003 |
0.64 ± 0.021 |
0.18 ± 0.03 |
| F8 |
0.095 ± 0.015 |
0.76 ± 0.017 |
0.2 ± 0.026 |
Aerodynamic performance of prepared powders
The in vitro aerosol performance of spray freeze-dried microparticles is illustrated in Figure 5. This study showed that the aerosolization of microparticles containing HBCD 0.05% had the lowest ED and FPF, 88.53% and 23.79%, respectively, while the FPF increased to 2-times for HBCD 5% (FPF = 49.32%). In contrast, β-CD 5% had the highest aerosolization property by far, with ED 92.53% and FPF 69.38%. The FPF value for gamma-CD 0.05% approximately halved (36.9%) and decreased to 1.2-times for gamma-CD 5% (57.52%). Although employing higher concentrations (5%) increased FPF amounts 1.3 and 1.08-fold for HP-β-CD and β-CD than 0.05%, respectively, it fell 12% for SBE-β-CD 5% compared to lower concentrations.
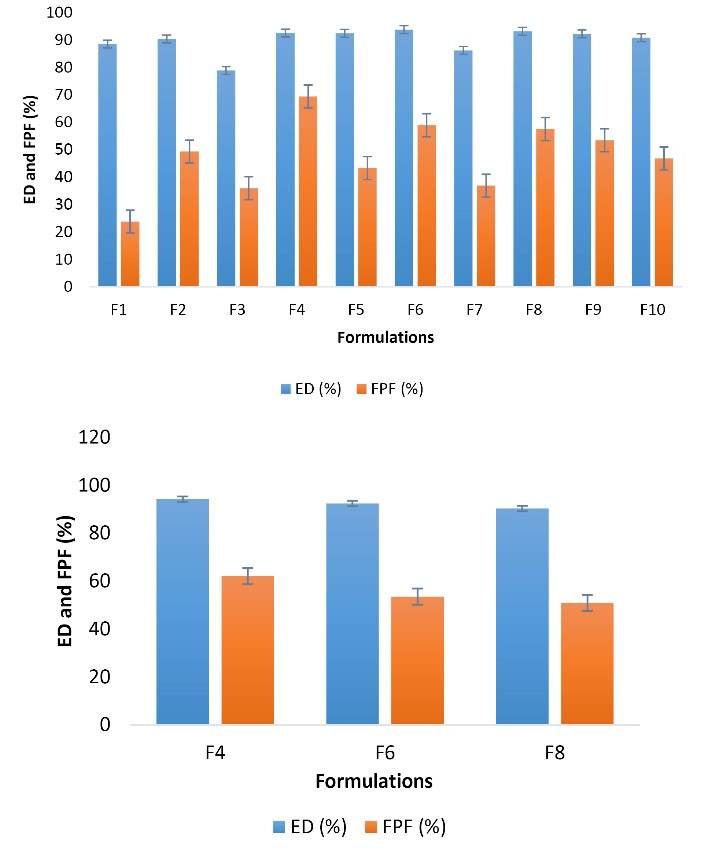
Figure 5.
In vitro drug deposition results for spray freeze-dried formulations by TSI (a), and ACI (b)
.
In vitro drug deposition results for spray freeze-dried formulations by TSI (a), and ACI (b)
The in vitro aerosolization behavior of selected formulations was determined using an ACI. ED% and FPF % data obtained for buserelin after aerosolization of spray freeze-dried formulations by using a Cyclohaler® are shown in Figure 6. The results obtained by ACI were comparable with the results obtained by TSI. ED % of all three samples measured by this method was higher than 90%. The highest FPF value was related to β-CD 5% containing formulation (FPF = 62.1%). On the other hand, the β-CD 5% containing particles dispersed well, with most powders depositing at the lower stages of the ACI and having higher FPF compared to the two formulations containing HP-β-CD 5% and Gamma-CD 5%.
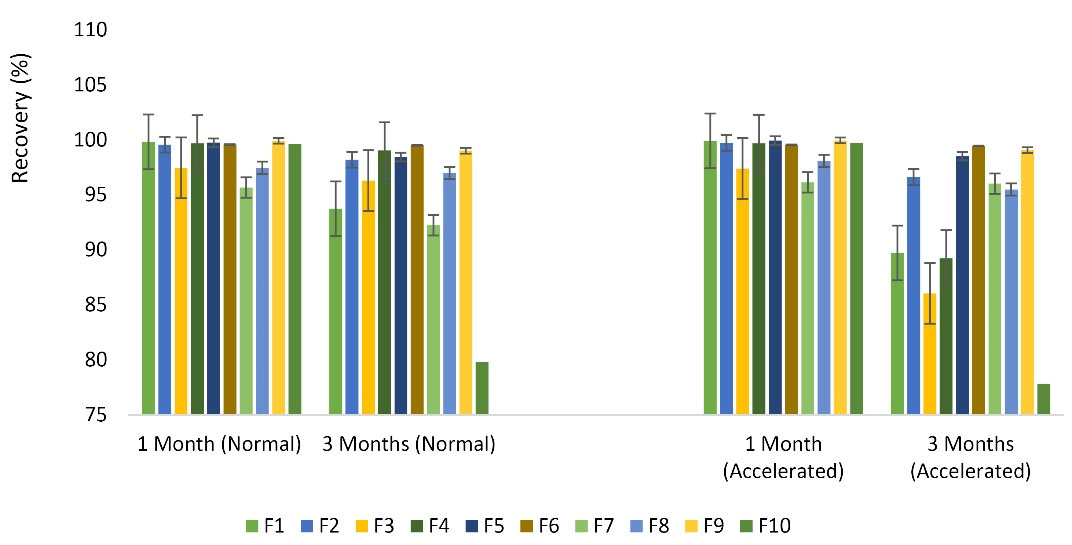
Figure 6.
The recovery percent of spray freeze-dried formulations after 1 month, and 3 months of storage at the normal, and accelerated conditions (n = 3, Mean ± SD)
.
The recovery percent of spray freeze-dried formulations after 1 month, and 3 months of storage at the normal, and accelerated conditions (n = 3, Mean ± SD)
Stability evaluation
The recovery percent of buserelin in the processed microparticles was assessed in normal and accelerated conditions for 1 and 3 months. The slightest changes were obtained for HP-β-CD 5% that reached to 99.58 and 99.50% for normal groups, 99.55 and 99.45% for accelerated samples in the first and third months. The most remarkable changes were related to SBE-β-237. CD 5%, reaching to 79.82 and 70% for normal and accelerated samples in the third month, respectively (Figure 6).
Discussion
Today, peptide and protein drugs are an important part of the pharmaceutical market. A large number of peptides and proteins are undergoing pre-clinical and clinical phases to enter the pharmaceutical market. However, most of these drugs are injectable, reducing the acceptance of these drugs, especially in the case of chronic diseases requiring long-term usage of drugs. To address this issue, inhalation drugs can be considered a desirable alternative to the injection route of administration due to the special characteristics of the respiratory system like very high surface area of the lung, low thickness of the alveolar membrane, and extensive blood supply to the lung tissue.46
However, there can be found some challenges for inhalation drug delivery, including maintaining the chemical and structural stability of peptides and proteins during formulation and storage, preparing particles with desirable physicochemical properties, providing a deep lung penetration, and passing macromolecules through the epithelial membrane and entering the bloodstream.47
The possibility of preparing particles for inhalation employing the SFD process was demonstrated in 1998 by Maa et al. Despite the unique ability of this method to prepare DPIs containing peptides and proteins (due to lack of exposure to high temperatures), few studies have been conducted in this field.48
As the interaction of each drug with excipients (e.g., sugars and CDs) is unique, their effects on the aerodynamic properties and stability of the drugs will be different. Therefore, it is worthwhile to study different aspects of these excipients in the SFD process. This study is focused on the processing of formulations containing buserelin, fabricated by the SFD method for pulmonary drug delivery applications. To stabilize the denaturation of biomolecules through the SFD process, some stabilizing compounds have been used. The notable ability of sugars in protecting peptides and proteins has been proven in several studies.13,49 CDs are. other additives that can be effective in stabilizing peptides and proteins. We hypothesize that the combination of these two stabilizing agents (raffinose and CD) can provide a more stable formulation for buserelin with improved aerodynamic behavior against stresses applied to proteins and peptides during the SFD process.27
In this study, five types of CDs (β-CD, HP-β-CD, HBCD, Gamma-CD, SBE-β-CD) and raffinose are employed to improve and optimize the aerodynamic properties and stability of microparticles containing buserelin fabricated by the SFD method.
According to our results, as the CD concentration increased the FPF, ED and aerodynamic performance improved (except SBE-β-CD). These results can be referred to as the presence of more CDs on the surface of the frozen sprayed droplets and lower moisture absorption. The effects of CDs on the properties of proteins and peptides are variable due to different structures and the presence of other hydrophobic amino acids on their surface, so the effects of CDs on various proteins and peptides should be assessed.
In all formulations, the FPF values were above 23%. According to the results, it can be concluded that all prepared formulations had relatively acceptable inhalation properties. The best formulation in terms of inhalation properties was related to the F4 formulation, containing β-CD 5%, with FPF = 69.38% (by TSI). The results obtained by ACI had the same trend as the results obtained by TSI. The β-CD 5% containing formulation with the most powders depositing at the lower stages of the ACI had higher FPF compared to the formulation containing HP-β-CD 5%. Several studies have shown that high concentrations of HP-β-CD reduce the melting point of proteins and may impair their aerodynamic properties.50,51 Also, HP-β-CD molecules have hydrophobic chains, deposited on the surface of particles. This can lead to powder adhesion and reduce FPF in the presence of hydrophobic interactions. The adhesion of microparticles of the F6 formulation can be seen in their SEM images and the increase in HP-β-CD resulted in a decrease in FPF compared to the F4 formulation (our optimal sample).
The average ED in all selected formulations is above 78%. The obtained high amount of ED indicates a low level of electrostatic attraction and the efficiency of the SFD method in reducing this force. Moreover, this high ED is a sign of the low moisture content of the powder. As moisture is absorbed, the capillary forces prevent the powder particles from opening and leaving the capsule correctly.52
The used combination of CD and raffinose can be effective in preventing the absorption of moisture. The combination of these two excipients could create a core-shell complex in which the raffinose is placed in the core part of this structure. In other words, a micelle-like structure can be created in which raffinose molecules are enclosed by CD molecules. According to this theory, CDs that are more hydrophobic than raffinose are more prone to move to the outer surfaces of atomized droplets and form the core-shell structure. Finally, with the drying of atomized droplets, CDs accumulate on the surface of the dried particles, resulting in limited molecular mobility and lower moisture access to raffinose in this micelle-like structure. That is an effective way to reduce moisture absorption and improve the aerodynamic behavior of microparticles.27
Equation 2 shows the calculation of aerodynamic diameter with respect to geometric diameter.
where dae, dp, ρp, and ρ0 are aerodynamic diameter, geometric diameter, particle density, and reference density (water density ~ 1000 mg/cm3), respectively. The shape factor, χ, depends on the sphericity of the particles. This factor is equal to 1 for spherical particles and higher than 1 for non-spherical particles.53
SEM images show that the prepared particles are very porous and therefore ρp is very small. As a result, the aerodynamic diameter is significantly smaller than the geometric diameter. It has been proven that if the particles are spherical, depending on the porosity and density, the aerodynamic diameter, is about 20%-40% of the geometric diameter.44 So, the inhalation properties of DPIs are not only dependent on the particle size but also the shape and porosity play an important role. According to the mentioned cases, despite the relatively large apparent diameter (9 μm to 13 μm), the fabricated particles showed low aerodynamic diameter and suitable inhalation properties.
In a study on terbutaline and salbutamol, it was shown that despite the geometric diameter of 41 μm of SFD-prepared particles, a significant number of particles had proper aerodynamic diameters to penetrate into the lungs. In fact, the lower surface-to-volume ratio of particles in the SFD method results in a less adhesion force between the particles and thus facilitates particle dispersion.54
Maa et al presented a comparison between the aerodynamic properties of rhDNase and anti-IgE particles prepared by SD and SFD methods. Under the same atomization conditions, it was shown that the FPF values of spray freeze-dried particles (10 μm, porous particles) was significantly higher than that of spray dried particles (3 μm, dense particles). The FPF values reached 46% in spray dried particles to about 70% in spray freeze-dried particles. These observations can prove the theory that porous particles have a lower aerodynamic size and therefore exhibit more suitable inhalation properties.55 During SD, water leaks out of the droplets, causing the particles to shrink, reducing the original geometric diameter. In contrast, the particle diameter increases slowly during freezing in the SFD method. As a result, according to equation 1, it is evident that if particles with the same solid content are processed by both SD and SFD processes, the particles prepared by SFD have a lower aerodynamic diameter.44
Figure 2 shows the morphology of particles containing buserelin. According to the intact structure of the particles and lack of damage during the collection and preparation of samples, it can be said that these particles have good structural strength. There are many studies on the surface and internal structures of spray dried powders. In contrast, fewer studies have been performed on the microscopic structure of spray freeze-dried particles as it is a newer manner of particles fabrication. As shown in Figure 2, almost all samples have a very porous structure. Despite the large size of the particles, their highly porous structure with low densities makes them ideal for inhalation. Furthermore, the large size of these particles helps them escape from the alveolar macrophage system.56 During SFD, the dispersed droplets are frozen rapidly, resulting in the formation of ice crystals. Sublimation of these ice crystals leads to the formation of porous particles. In SEM images, some formulations represent adhesion or fusion of particles due to the amorphous structure of the processed microparticles. The existence of these amorphous structures has been confirmed by DSC and XRD analysis.
The amorphous phase of microparticles can be suitable for maintaining the structure of peptides and proteins.57 According to the thermal evaluation of SFD-derived microparticles, an amorphous structure is obtained in the presence of CDs. The exotherm at about 100 °C can be due to the crystallization or rearrangement of raffinose molecules, and the subsequent endotherm may be due to their melting (in DSC thermograms). Also, the ultra-fast solidification step in the SFD method reduces the possibility of crystalline structure, leading to the expansion of amorphous glass.38 In our study, this was confirmed by XRD analysis of spray freeze-dried powders.
Chemical and physical degradation of peptides and proteins and their instability are major challenges in formulation, production, and storage. To maintain the biological activity of peptides and proteins, saving their chemical and structural stability is essential.47 Peptides and proteins are subjected to various stresses during drying and engineering processes that may lead to changes in their structure.58 Structural changes may lead to complete or partial destruction of the biological activity of the peptides or proteins. As a result, a lower dose of the drug reaches the patient. Also, degradation of peptides may lead to toxic or immunogenic compounds.59 During SFD, the peptide faces stresses such as exposure to the liquid-air surface, atomization, etc., which may affect its stability; Therefore, the use of stabilizers in the formulation seems necessary.60
Sugars have been shown to have a significant effect on the stability of peptides and proteins during the lyophilization process. In fact, preserving the structure of peptides and proteins in aqueous solutions is the result of their interaction with water molecules through the formation of hydrogen bonds. In general, sugars can form hydrogen bonds with peptides and proteins and prevent their degradation during the drying process.61 CDs are other additives that can be effective in stabilizing peptides and proteins. Also, they are more likely to improve the bioavailability of drugs,62 leading to improve pulmonary drug delivery efficiency. Biocompatibility of CDs has been proven in many studies in which there was no significant tissue damage.63
Raffinose is more effective in forming hydrogen bonds with biomolecules than compounds such as trehalose and sucrose. The collapse temperature (Tc) of formulations containing raffinose is higher than sugars such as maltose, lactose, trehalose, and sucrose. Also, it increases the Tg in the remaining amorphous matrix. Due to the thermophysical properties of raffinose, this material is preferred as an excipient and stabilizer in particle engineering processes.26
Moreover, the concentration of HP-β-CD has a significant effect on the stability of buserelin. The recovery percentage of buserelin from processed powders increases with increasing HP-β-CD ratio in the formulation. Numerous studies have been conducted on the stabilizing effects of various types of CDs on peptides or proteins during SD and lyophilization.
CDs in lyophilized formulations have been used in very different concentrations from 0.0001% (w/v) to 10% (w/v). Based on different concentrations of CDs employed in lyophilized formulations, they can be a cryoprotectant or a surfactants. At high concentrations, CDs play the role of lyoprotectant. Sugars are stabilizing agents through the mechanism of “water molecule replacement”. If the purpose of using CDs is their lyoprotectant role, they should be used at least in a weight ratio equal to peptide or protein.28 Serno et al showed that at low concentrations (less than 1%) the surfactant effect of CDs is more pronounced.64
The surfactant effect is used to maintain the structure of peptides and proteins during atomization and drying. Surfactants reduce the contact of proteins and peptides with hydrophobic surfaces (e.g, the surface between water-air or water-ice) and prevent their structural disruption and accumulation.65 The pattern obtained in the stabilization of peptides and proteins seems to be true during the lyophilization process in SFD. In this regard, similar studies have been performed by this team on the effect of CDs on the stability of IgG, calcitonin, and parathormone during SFD. The results represented the significant effects of CDs on improving the stability of these drugs compared to CD-free formulations.22,49,66
Determining the exact ratio of CD: peptide or protein depends on the type of peptide or protein as well as other components of the formulations. Among different types of CDs, β-CD, HP-β-CD, Gamma-CD, SBE-β-CD, have shown the highest level of safety in injectable compounds.67 Studies have shown that the ability of CDs to stabilize peptides and proteins is varying. β-CD derivatives are more efficient at stabilizing peptides and proteins than alpha and gamma CDs, which may be due to the greater compatibility of amino acid benzene groups with the internal cavity of β-CD derivatives.38
The addition of SBE-β-CD to the formulations (formulations F9 and F10) resulted in less stability than other formulations. It has been shown that the ionic strength of CDs has a significant effect on the stability of peptides and proteins. Due to the higher negative charge in SBE-β-CD, the migration of these CDs to the surface of microparticles in the core-shell structure decreases, which can be lead to lower stability and poor aerodynamic performance of microparticles.68
The best stability results of this study are related to the F6 formulation. The protection of the peptide can be due to the hydroxyl groups in HP-β-CD and the formation of hydrogen bonds. HP-β-CD with a concentration of 5% with higher properties of “replacement of water molecules”, offers better stability compared to the formula F5. In our previous study, a combination of trehalose and HP-β-CD was used to prepare DPIs containing calcitonin by the SFD technique. HP-β-CD significantly improved the stability of calcitonin, which may be due to the surfactant effect of this CD.50 The formation of protein or peptide complexes by HP-β-CD is greater than β-CD because more hydrophobic segments are formed within the complex and provide deeper cavities due to the hydroxypropyl groups. Also, the surfactant property of HP-β-CD is higher than β-CD, so it has more competition for placement at air-water interfaces and provides better protection.69 Also, in a study on the stabilizing effect of various CDs on lactate dehydrogenase during the lyophilization process, it was found that HP-β-CD had the greatest stabilizing effect.70 The results show that branched CDs such as HP-β-CDs can stabilize peptides or proteins better than β-CD. Comparing the results of this study with other studies, it was found that the effect of CDs on aerodynamic properties and stability of microparticles varies according to the type of peptide. Due to the mechanism of action, CDs were used in two ratios of 0.05 % and 5% with raffinose and at higher CD percentages, the aerodynamic properties and stability of the formulations were improved.
Conclusion
In this study, inhalable microparticle containing buserelin, raffinose and five different CDs (HBCD, β-CD, HP- β-CD, Gamma-CD, SBE- β-CD) were fabricated via SFD method (F1-F10). The morphology of prepared microparticles was semi-spherical with a rough, porous surface. The highest stability of microparticles was obtained for HP-β-CD 5% in normal and accelerated conditions after one and three months. The highest aerosolization property was showed for β-CD 5%. Overall, the type and concentration of utilized CDs effectively impacted FPF and ED of produced microparticles. Generally, employing a higher concentration of CDs improved the FPF of microparticles. But the increase in HP-β-CD portions led to a decrease in FPF than sample containing β-CD 5% due to high concentrations of HP-β-CD may impair aerodynamic properties. As shown in previous studies, the amorphous matrix showed better protection for the proteins and peptides structures. The amorphous structure of all fabricated microparticles was confirmed by DSC and XRD evaluation. In conclusion, the employed SFD process and co-cryoprotectants can be utilized to prepare microparticles containing peptides like buserelin for lung delivery applications.
Acknowledgments
We wish to acknowledge Tehran University of Medical Sciences (TUMS).
Competing Interests
The authors report no declarations of interest.
References
- Dalby R, Suman J. Inhalation therapy: technological milestones in asthma treatment. Adv Drug Deliv Rev 2003; 55(7):779-91. doi: 10.1016/s0169-409x(03)00077-2 [Crossref] [ Google Scholar]
- Adi H, Young PM, Chan HK, Agus H, Traini D. Co-spray-dried mannitol-ciprofloxacin dry powder inhaler formulation for cystic fibrosis and chronic obstructive pulmonary disease. Eur J Pharm Sci 2010; 40(3):239-47. doi: 10.1016/j.ejps.2010.03.020 [Crossref] [ Google Scholar]
- Liang Z, Ni R, Zhou J, Mao S. Recent advances in controlled pulmonary drug delivery. Drug Discov Today 2015; 20(3):380-9. doi: 10.1016/j.drudis.2014.09.020 [Crossref] [ Google Scholar]
- Patil JS, Sarasija S. Pulmonary drug delivery strategies: a concise, systematic review. Lung India 2012; 29(1):44-9. doi: 10.4103/0970-2113.92361 [Crossref] [ Google Scholar]
- Liang W, Pan HW, Vllasaliu D, Lam JKW. Pulmonary delivery of biological drugs. Pharmaceutics 2020; 12(11):1025. doi: 10.3390/pharmaceutics12111025 [Crossref] [ Google Scholar]
- Costantino HR, Firouzabadian L, Wu C, Carrasquillo KG, Griebenow K, Zale SE. Protein spray freeze drying 2 Effect of formulation variables on particle size and stability. J Pharm Sci 2002; 91(2):388-95. doi: 10.1002/jps.10059 [Crossref] [ Google Scholar]
- Luy B, Stamato H. Spray freeze drying. In: Ohtake S, Izutsu KI, Lechuga-Ballesteros D, eds. Drying Technologies for Biotechnology and Pharmaceutical Applications. John Wiley & Sons; 2020. p. 217-37. 10.1002/9783527802104.ch8.
- Hu J, Rogers TL, Brown J, Young T, Johnston KP, Williams RO 3rd. Improvement of dissolution rates of poorly water soluble APIs using novel spray freezing into liquid technology. Pharm Res 2002; 19(9):1278-84. doi: 10.1023/a:1020390422785 [Crossref] [ Google Scholar]
- Holland FJ, Fishman L, Costigan DC, Luna L, Leeder S. Pharmacokinetic characteristics of the gonadotropin-releasing hormone analog D-Ser(TBU)-6EA-10luteinizing hormone-releasing hormone (buserelin) after subcutaneous and intranasal administration in children with central precocious puberty. J Clin Endocrinol Metab 1986; 63(5):1065-70. doi: 10.1210/jcem-63-5-1065 [Crossref] [ Google Scholar]
- Horn J, Mahler HC, Friess W. Drying for stabilization of protein formulations. In: Ohtake S, Izutsu KI, Lechuga-Ballesteros D, eds. Drying Technologies for Biotechnology and Pharmaceutical Applications. John Wiley & Sons; 2020. p. 91-119. 10.1002/9783527802104.ch4.
- Otake H, Okuda T, Okamoto H. Development of spray-freeze-dried powders for inhalation with high inhalation performance and antihygroscopic property. Chem Pharm Bull (Tokyo) 2016; 64(3):239-45. doi: 10.1248/cpb.c15-00824 [Crossref] [ Google Scholar]
- Hojjati M, Vatanara A, Yamini Y, Moradi M, Rouholamini Najafabadi A. Supercritical CO2 and highly selective aromatase inhibitors: experimental solubility and empirical data correlation. J Supercrit Fluids 2009; 50(3):203-9. doi: 10.1016/j.supflu.2009.06.015 [Crossref] [ Google Scholar]
- Daneshmand B, Faghihi H, Amini Pouya M, Aghababaie S, Darabi M, Vatanara A. Application of disaccharides alone and in combination, for the improvement of stability and particle properties of spray-freeze dried IgG. Pharm Dev Technol 2019; 24(4):439-47. doi: 10.1080/10837450.2018.1507039 [Crossref] [ Google Scholar]
- Rostamnezhad M, Jafari H, Moradikhah F, Bahrainian S, Faghihi H, Khalvati R. Spray freeze-drying for inhalation application: process and formulation variables. Pharm Dev Technol 2022; 27(3):251-67. doi: 10.1080/10837450.2021.2021941 [Crossref] [ Google Scholar]
- Keyhan Shokouh M, Faghihi H, Darabi M, Mirmoeini M, Vatanara A. Formulation and evaluation of inhalable microparticles of rizatriptan benzoate processed by spray freeze-drying. J Drug Deliv Sci Technol 2021; 62:102356. doi: 10.1016/j.jddst.2021.102356 [Crossref] [ Google Scholar]
- Emami F, Vatanara A, Vakhshiteh F, Kim Y, Kim TW, Na DH. Amino acid-based stable adalimumab formulation in spray freeze-dried microparticles for pulmonary delivery. J Drug Deliv Sci Technol 2019; 54:101249. doi: 10.1016/j.jddst.2019.101249 [Crossref] [ Google Scholar]
- Ishwarya SP, Anandharamakrishnan C, Stapley AGF. Spray-freeze-drying: a novel process for the drying of foods and bioproducts. Trends Food Sci Technol 2015; 41(2):161-81. doi: 10.1016/j.tifs.2014.10.008 [Crossref] [ Google Scholar]
- Vishali DA, Monisha J, Sivakamasundari SK, Moses JA, Anandharamakrishnan C. Spray freeze drying: emerging applications in drug delivery. J Control Release 2019; 300:93-101. doi: 10.1016/j.jconrel.2019.02.044 [Crossref] [ Google Scholar]
- Dutta S, Moses JA, Anandharamakrishnan C. Modern frontiers and applications of spray-freeze-drying in design of food and biological supplements. J Food Process Eng 2018; 41(8):e12881. doi: 10.1111/jfpe.12881 [Crossref] [ Google Scholar]
- Junping W, Maitani Y, Takayama K, Nagai T. In vivo evaluation of doxorubicin carried with long circulating and remote loading proliposome. Int J Pharm 2000; 203(1-2):61-9. doi: 10.1016/s0378-5173(00)00410-5 [Crossref] [ Google Scholar]
- Bhatnagar BS, Bogner RH, Pikal MJ. Protein stability during freezing: separation of stresses and mechanisms of protein stabilization. Pharm Dev Technol 2007; 12(5):505-23. doi: 10.1080/10837450701481157 [Crossref] [ Google Scholar]
- Milani S, Faghihi H, Roulholamini Najafabadi A, Amini M, Montazeri H, Vatanara A. Hydroxypropyl beta cyclodextrin: a water-replacement agent or a surfactant upon spray freeze-drying of IgG with enhanced stability and aerosolization. Drug Dev Ind Pharm 2020; 46(3):403-11. doi: 10.1080/03639045.2020.1724131 [Crossref] [ Google Scholar]
- Carpenter JF, Crowe JH. An infrared spectroscopic study of the interactions of carbohydrates with dried proteins. Biochemistry 1989; 28(9):3916-22. doi: 10.1021/bi00435a044 [Crossref] [ Google Scholar]
- Franks F. Long-term stabilization of biologicals. Biotechnology (N Y) 1994; 12(3):253-6. doi: 10.1038/nbt0394-253 [Crossref] [ Google Scholar]
- Franks F. Thermomechanical properties of amorphous saccharides: their role in enhancing pharmaceutical product stability. Biotechnol Genet Eng Rev 1999; 16:281-92. doi: 10.1080/02648725.1999.10647979 [Crossref] [ Google Scholar]
- Ogáin ON, Li J, Tajber L, Corrigan OI, Healy AM. Particle engineering of materials for oral inhalation by dry powder inhalers I-Particles of sugar excipients (trehalose and raffinose) for protein delivery. Int J Pharm 2011; 405(1-2):23-35. doi: 10.1016/j.ijpharm.2010.11.039 [Crossref] [ Google Scholar]
- Zhao Z, Huang Z, Zhang X, Huang Y, Cui Y, Ma C. Low density, good flowability cyclodextrin-raffinose binary carrier for dry powder inhaler: anti-hygroscopicity and aerosolization performance enhancement. Expert Opin Drug Deliv 2018; 15(5):443-57. doi: 10.1080/17425247.2018.1450865 [Crossref] [ Google Scholar]
- Davidson P, Sun WQ. Effect of sucrose/raffinose mass ratios on the stability of co-lyophilized protein during storage above the Tg. Pharm Res 2001; 18(4):474-9. doi: 10.1023/a:1011002326825 [Crossref] [ Google Scholar]
- Grasmeijer N, Stankovic M, de Waard H, Frijlink HW, Hinrichs WL. Unraveling protein stabilization mechanisms: vitrification and water replacement in a glass transition temperature controlled system. BiochimBiophys Acta 2013; 1834(4):763-9. doi: 10.1016/j.bbapap.2013.01.020 [Crossref] [ Google Scholar]
- Serno T, Geidobler R, Winter G. Protein stabilization by cyclodextrins in the liquid and dried state. Adv Drug Deliv Rev 2011; 63(13):1086-106. doi: 10.1016/j.addr.2011.08.003 [Crossref] [ Google Scholar]
- Alhajj N, O’Reilly NJ, Cathcart H. Designing enhanced spray dried particles for inhalation: a review of the impact of excipients and processing parameters on particle properties. Powder Technol 2021; 384:313-31. doi: 10.1016/j.powtec.2021.02.031 [Crossref] [ Google Scholar]
- Mayolo-Deloisa K, Martínez LM, Rito-Palomares M. [Chromatographic techniques and their application to studies of conformational changes, stability and refolding of proteins]. Rev Mex Ing Quim 2012; 11(3):415-29. [ Google Scholar]
- Bellows RJ, King CJ. Product collapse during freeze drying of liquid foods. AICHE Symp Ser 1973; 69(132):33-41. [ Google Scholar]
- Kajiwara K, Franks F. Crystalline and amorphous phases in the binary system water–raffinose. J Chem Soc Faraday Trans 1997; 93(9):1779-83. doi: 10.1039/a608572e [Crossref] [ Google Scholar]
- Saleki-Gerhardt A, Stowell JG, Byrn SR, Zografi G. Hydration and dehydration of crystalline and amorphous forms of raffinose. J Pharm Sci 1995; 84(3):318-23. doi: 10.1002/jps.2600840311 [Crossref] [ Google Scholar]
- Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov 2004; 3(12):1023-35. doi: 10.1038/nrd1576 [Crossref] [ Google Scholar]
- Cooper A, Lovatt M, Nutley MA. Energetics of protein-cyclodextrin interactions. J Incl Phenom Macrocycl Chem 1996; 25(1):85-8. doi: 10.1007/bf01041542 [Crossref] [ Google Scholar]
- Tavornvipas S, Tajiri S, Hirayama F, Arima H, Uekama K. Effects of hydrophilic cyclodextrins on aggregation of recombinant human growth hormone. Pharm Res 2004; 21(12):2369-76. doi: 10.1007/s11095-004-7691-5 [Crossref] [ Google Scholar]
- Carpenter JF, Pikal MJ, Chang BS, Randolph TW. Rational design of stable lyophilized protein formulations: some practical advice. Pharm Res 1997; 14(8):969-75. doi: 10.1023/a:1012180707283 [Crossref] [ Google Scholar]
- European pharmacopoeia. European Directorate for the Quality of Medicine & Health Care of the Council of Europe (EDQM); 2017.
- British pharmacopoeia. 2022. Available from: https://www.pharmacopoeia.com/.
- Telko MJ, Hickey AJ. Dry powder inhaler formulation. Respir Care 2005; 50(9):1209-27. [ Google Scholar]
- Claussen IC, Ustad TS, Strømmen I, Walde PM. Atmospheric freeze drying—a review. Dry Technol 2007; 25(6):947-57. doi: 10.1080/07373930701394845 [Crossref] [ Google Scholar]
- Gala RP, Popescu C, Knipp GT, McCain RR, Ubale RV, Addo R. Physicochemical and preclinical evaluation of a novel buccal measles vaccine. AAPS PharmSciTech 2017; 18(2):283-92. doi: 10.1208/s12249-016-0566-3 [Crossref] [ Google Scholar]
- Tewa-Tagne P, Briançon S, Fessi H. Preparation of redispersible dry nanocapsules by means of spray-drying: development and characterisation. Eur J Pharm Sci 2007; 30(2):124-35. doi: 10.1016/j.ejps.2006.10.006 [Crossref] [ Google Scholar]
- Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet 2011; 377(9770):1032-45. doi: 10.1016/s0140-6736(10)60926-9 [Crossref] [ Google Scholar]
- Malik DK, Baboota S, Ahuja A, Hasan S, Ali J. Recent advances in protein and peptide drug delivery systems. Curr Drug Deliv 2007; 4(2):141-51. doi: 10.2174/156720107780362339 [Crossref] [ Google Scholar]
- Sonner C, Maa YF, Lee G. Spray-freeze-drying for protein powder preparation: particle characterization and a case study with trypsinogen stability. J Pharm Sci 2002; 91(10):2122-39. doi: 10.1002/jps.10204 [Crossref] [ Google Scholar]
- Poursina N, Vatanara A, Rouini MR, Gilani K, Rouholamini Najafabadi A. The effect of excipients on the stability and aerosol performance of salmon calcitonin dry powder inhalers prepared via the spray freeze drying process. Acta Pharm 2016; 66(2):207-18. doi: 10.1515/acph-2016-0012 [Crossref] [ Google Scholar]
- Härtl E, Winter G, Besheer A. Influence of hydroxypropyl-beta-cyclodextrin on the stability of dilute and highly concentrated immunoglobulin G formulations. J Pharm Sci 2013; 102(11):4121-31. doi: 10.1002/jps.23729 [Crossref] [ Google Scholar]
- Tavornvipas S, Hirayama F, Takeda S, Arima H, Uekama K. Effects of cyclodextrins on chemically and thermally induced unfolding and aggregation of lysozyme and basic fibroblast growth factor. J Pharm Sci 2006; 95(12):2722-9. doi: 10.1002/jps.20715 [Crossref] [ Google Scholar]
- Müller RH, Runge S, Ravelli V, Mehnert W, Thünemann AF, Souto EB. Oral bioavailability of cyclosporine: solid lipid nanoparticles (SLN) versus drug nanocrystals. Int J Pharm 2006; 317(1):82-9. doi: 10.1016/j.ijpharm.2006.02.045 [Crossref] [ Google Scholar]
- DeCarlo PF, Slowik JG, Worsnop DR, Davidovits P, Jimenez JL. Particle morphology and density characterization by combined mobility and aerodynamic diameter measurements Part 1: theory. Aerosol Sci Technol 2004; 38(12):1185-205. doi: 10.1080/027868290903907 [Crossref] [ Google Scholar]
- Sharma G, Mueannoom W, Buanz AB, Taylor KM, Gaisford S. In vitro characterisation of terbutaline sulphate particles prepared by thermal ink-jet spray freeze drying. Int J Pharm 2013; 447(1-2):165-70. doi: 10.1016/j.ijpharm.2013.02.045 [Crossref] [ Google Scholar]
- Maa YF, Zhao L, Payne LG, Chen D. Stabilization of alum-adjuvanted vaccine dry powder formulations: mechanism and application. J Pharm Sci 2003; 92(2):319-32. doi: 10.1002/jps.10294 [Crossref] [ Google Scholar]
- Crowder TM, Rosati JA, Schroeter JD, Hickey AJ, Martonen TB. Fundamental effects of particle morphology on lung delivery: predictions of Stokes’ law and the particular relevance to dry powder inhaler formulation and development. Pharm Res 2002; 19(3):239-45. doi: 10.1023/a:1014426530935 [Crossref] [ Google Scholar]
- Tewes F, Gobbo OL, Ehrhardt C, Healy AM. Amorphous calcium carbonate based-microparticles for peptide pulmonary delivery. ACS Appl Mater Interfaces 2016; 8(2):1164-75. doi: 10.1021/acsami.5b09023 [Crossref] [ Google Scholar]
- Cleland JL, Powell MF, Shire SJ. The development of stable protein formulations: a close look at protein aggregation, deamidation, and oxidation. Crit Rev Ther Drug Carrier Syst 1993; 10(4):307-77. [ Google Scholar]
- Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A 2009; 106(10):3794-9. doi: 10.1073/pnas.0804543106 [Crossref] [ Google Scholar]
- Costantino HR, Firouzabadian L, Hogeland K, Wu C, Beganski C, Carrasquillo KG. Protein spray-freeze drying Effect of atomization conditions on particle size and stability. Pharm Res 2000; 17(11):1374-83. doi: 10.1023/a:1007570030368 [Crossref] [ Google Scholar]
- Selva C, Malferrari M, Ballardini R, Ventola A, Francia F, Venturoli G. Trehalose preserves the integrity of lyophilized phycoerythrin-antihuman CD8 antibody conjugates and enhances their thermal stability in flow cytometric assays. J Pharm Sci 2013; 102(2):649-59. doi: 10.1002/jps.23398 [Crossref] [ Google Scholar]
- Patton JS, Fishburn CS, Weers JG. The lungs as a portal of entry for systemic drug delivery. Proc Am Thorac Soc 2004; 1(4):338-44. doi: 10.1513/pats.200409-049TA [Crossref] [ Google Scholar]
- Asai K, Morishita M, Katsuta H, Hosoda S, Shinomiya K, Noro M. The effects of water-soluble cyclodextrins on the histological integrity of the rat nasal mucosa. Int J Pharm 2002; 246(1-2):25-35. doi: 10.1016/s0378-5173(02)00345-9 [Crossref] [ Google Scholar]
- Serno T. Inhibition of Therapeutic Protein Aggregation by Cyclodextrins. Cuvillier Verlag; 2011.
- Randolph TW, Jones LS. Surfactant-protein interactions. In: Carpenter JF, Manning MC, eds. Rational Design of Stable Protein Formulations: Theory and Practice. New York: Kluwert Academic, Plenum Publishers; 2002.
- Poursina N, Vatanara A, Rouini MR, Gilani K, Rouholamini Najafabadi A. Systemic delivery of parathyroid hormone (1-34) using spray freeze-dried inhalable particles. Pharm Dev Technol 2017; 22(6):733-9. doi: 10.3109/10837450.2015.1125924 [Crossref] [ Google Scholar]
- Munro IC, Newberne PM, Young VR, Bär A. Safety assessment of gamma-cyclodextrin. RegulToxicolPharmacol 2004; 39 Suppl 1:S3-13. doi: 10.1016/j.yrtph.2004.05.008 [Crossref] [ Google Scholar]
- Mauritz JM, Morrisby RS, Hutton RS, Legge CH, Kaminski CF. Imaging pharmaceutical tablets with optical coherence tomography. J Pharm Sci 2010; 99(1):385-91. doi: 10.1002/jps.21844 [Crossref] [ Google Scholar]
- He J, Zheng ZP, Zhu Q, Guo F, Chen J. Encapsulation mechanism of oxyresveratrol by β-cyclodextrin and hydroxypropyl-β-cyclodextrin and computational analysis. Molecules 2017; 22(11):1801. doi: 10.3390/molecules22111801 [Crossref] [ Google Scholar]
- Iwai J, Ogawa N, Nagase H, Endo T, Loftsson T, Ueda H. Effects of various cyclodextrins on the stability of freeze-dried lactate dehydrogenase. J Pharm Sci 2007; 96(11):3140-3. doi: 10.1002/jps.20847 [Crossref] [ Google Scholar]