Advanced pharmaceutical bulletin. 14(2):266-277.
doi: 10.34172/apb.2024.043
Review Article
Review on Hyaluronic Acid Functionalized Sulfur and Nitrogen Co-Doped Graphene Quantum Dots Nano Conjugates for Targeting of Specific Type of Cancer
Vinit Sudhakar Patil Data curation, Writing – original draft, Writing – review & editing, 1
Kedar Rupa Bavaskar Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, 2, * 
Dilip Omprakash Morani Conceptualization, Resources, Visualization, 3
Ashish Suresh Jain Formal analysis, Software, 4
Author information:
1Shri D.D. Vispute College of Pharmacy and Research Center, Devad-Vichumbe, New Panvel, India-410206.
2Department of Pharmaceutics, Shri D.D. Vispute College of Pharmacy and Research Center, Devad-Vichumbe, New Panvel, India-410206.
3Department of Pharmaceutics, Bombay Institute of Pharmacy and Research, Dombivali India-421204.
4Department of Pharmacognosy, Shri D.D. Vispute College of Pharmacy and Research Center, Devad-Vichumbe, New Panvel, India-410206.
Abstract
Many people lose their lives to cancer each year. The prevalence of illnesses, metabolic disorders, high-risk infections, and other conditions has been greatly slowed down by expanding scientific research. Chemotherapy and radiation are still the initial lines of treatment for cancer patients, along with surgical removal of tumors. Modifications have been made in chemotherapy since medicines frequently have substantial systemic toxicity and poor pharmacokinetics and still do not reach the tumor site at effective concentrations. Chemotherapy may now be administered more safely and effectively thanks to nanotechnology. Nanotechnology-based graphene quantum dots (GQDs) are very applicable in breast cancer detection, as a drug delivery system, and in the treatment of breast cancer because of their physical and chemical properties, lower toxicity, small size, fluorescence, and effective drug delivery. This paper analyzes the GQDs as cutting-edge platforms for biotechnology and nanomedicine also its application in drug delivery in cancer. It shows that GQDs can be effectively conjugated with hyaluronic acid (HA) to achieve efficient and target-specific delivery.
Keywords: Nanotechnology, Hyaluronic acid, Graphene quantum dots, Cancer, Nanoparticles, Fluorescence, Tumor, Drug delivery
Copyright and License Information
©2024 The Authors.
This is an open access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Funding Statement
The preparation of this article was not supported by external findings.
Introduction
Each year, millions of people die from cancer, among the most deadly illnesses.1 Because cancer is an aggressive disease, tumor cells frequently spread from primary tumors to faraway organs and tissues. The advancement of metastatic disease indicates a poor prognosis because metastatic illness is typically the ultimate and deadly stage of cancer development.2 Despite being the cause of as many as 88% of cancer-related deaths, metastasis is the aspect of cancer pathogenesis that is least understood. During metastatic spread, cancerous cells from a primary tumor follow the processes below: It locally enters the surrounding tissue, move through the lymphatic and vascular systems, survive and largely translocate via the circulatory system for microvessels for distant tissues, leave the bloodstream, endure in the microenvironment of distant tissues, and then ultimately adjusts into the external microenvironment that surrounds these tissues in ways that support the proliferation of cells and the development of a macroscopic secondary tumor.3 Breast cancer is the most prevalent malignancy in women and the leading cause of death. Managing breast cancer is becoming more difficult due to metastasis and tumor recurrence.4 According to GLOBOCAN statistics, in 2018, there were 18.2 million fresh cases of breast cancer worldwide. Breast cancer is the second major cause of cancer-related death in women, this accounts for 13% of all new cases of cancer and 24% among all cancers in women.5 When cells from the tumor mass travel to other areas of the body or infiltrate nearby healthy cells, the tumor mass progresses and turns malignant.6,7 Sometimes chemotherapy is started after a mastectomy to entirely eradicate the patient’s metastatic cancer cells.8 While chemotherapy successfully extends patients’ lives, anticancer medications have several restrictions on how they can be given to patients. Due to the components’ lipophilicity, oral distribution is severely constrained; therefore, parenteral administration at high concentrations is required to sustain the effective concentration for a prolonged period. There are significant cytotoxic effects brought on by this increased drug concentration in the systemic circulation, including non-specific pharmacokinetics.9 Despite the dangers involved, cytotoxic cancer chemotherapy drugs like taxanes and anthracyclines have been an integral treatment component for more than 50 years.10 The fusion of engineering and biology is an emerging concept to enhance the delivery of medications directly to tumors and decrease damage to healthy cells and tissue.11 To accomplish this, a range of tools and methods have been used, including polymers, lipids, hydrogels, and inorganic carriers.12 Moreover, it has been demonstrated that medication resistances have an impact on both the medicines themselves and the bioengineering techniques employed to enhance therapy response.13 Hence, utilizing research on human cancer and drug resistance to build tailored nanotherapeutics rationally may help to overcome a lot of these difficulties.14 With the help of nanotechnology, it may be possible to increase the solubility and stability of pharmaceuticals as well as their plasma half-lives, reduce side effects that aren’t intended, and concentrate medications where they’re needed.15 The selection of viable treatments is based on the features of the tumor, including biomarkers, tumor size, metastatic illness, ligands, antigens, or the expression of endocrine receptors. Chemotherapy and radiation going to be the primary line of therapy for cancer patients, along with surgical resection.16 Chemotherapies have been improved since medicines frequently have substantial systemic toxicities and poor pharmacokinetics and still do not reach the tumor site at effective concentrations. Chemotherapy may now be administered more safely and effectively thanks to nanotechnology.17 Additionally, nanoparticles are being actively developed for targeted medication delivery, cancer biomarker biomolecular profiling, and in vivo tumor imaging. These nanotechnology-based methods can be used extensively in the treatment of numerous malignant disorders.18
Nanotechnology in cancer targeting
The administration of medications using nanotechnology can change the path that diseases including cancer, diabetes, infections, neurological disorders, blood-related disease, and orthopedic issues are treated.19 For more specific drug targeting, these strategies should ideally improve therapeutic concentration, medication absorption, and stability. An further characteristic of nano-based drug delivery systems is their continuous release of drug within the targeted tissue.20 The formation of nano-platforms with certain shapes, surfaces, and size qualities as a consequence of the rational design of nanotherapeutics is essential for biological interactions and the subsequent therapeutic effects.21 Formulations that depend on nanotechnology have chemical and physiological properties that are used to treat several illnesses.22 The healthcare industry benefits greatly from the current reports on nanoformulations. Most nano-based therapeutic items on the market are intended for intravenous administration, with a few exceptions intended for oral administration.23 Numerous preclinical and clinical trials have led to the development of new nanotherapeutics that are not administered through parenteral routes, such as pulmonary, ocular, nasal, vaginal, and cutaneous. For the delivery of drugs, the route of delivery and the associated barriers to be overcome are of particular interest. Many nanoparticle-based formulations have been created over time to enhance the medication delivery mechanism.24 Selective cancer targeting has undergone a major transformation thanks to nanotechnology. Nanoparticles may be modified in a variety of ways, including modifying their size, shape, chemical and physical characteristics, and so on, to train them to target certain cells. They can either actively or passively target the cancerous cells. In the case of active targeting, chemotherapeutic agent-containing nano-sized particles are created in a fashion that they interact with the damaged cells directly. Molecular recognition underpins active targeting. As a result, the surface of the nanoparticles is altered to target malignant cells. Targeting agents are often added to the surface of nanoparticles for molecular recognition. Nanoparticles are designed to target malignant cells via ligand-receptor interaction or antibody-antigen recognition.25-27
The three major components of a nanotechnology-based targeted delivery system are
-
An apoptosis-inducing agent (anticancer medication).
-
A stimulant for targeted moiety penetration.
-
A nanoparticle is composed of several different substances.
Ceramics, polymers, lipids, and metals are all commonly utilized materials. Cancer can also be targeted by nanoparticles via passive targeting. After apoptosis pauses in cancerous cells, they continue sucking nutritional substances abnormally through the blood vessels, resulting in the formation of broad and leaky blood capillaries around the cells generated by angiogenesis. Basement membrane anomalies and a reduction in the number of pericytes lining rapidly proliferating endothelial cells cause the formation of leaky blood vessels.28 Nanocarriers can be classified into three categories based on the materials that they are made from. Different kinds of nanocarriers used for drug delivery are shown in Figure 1.
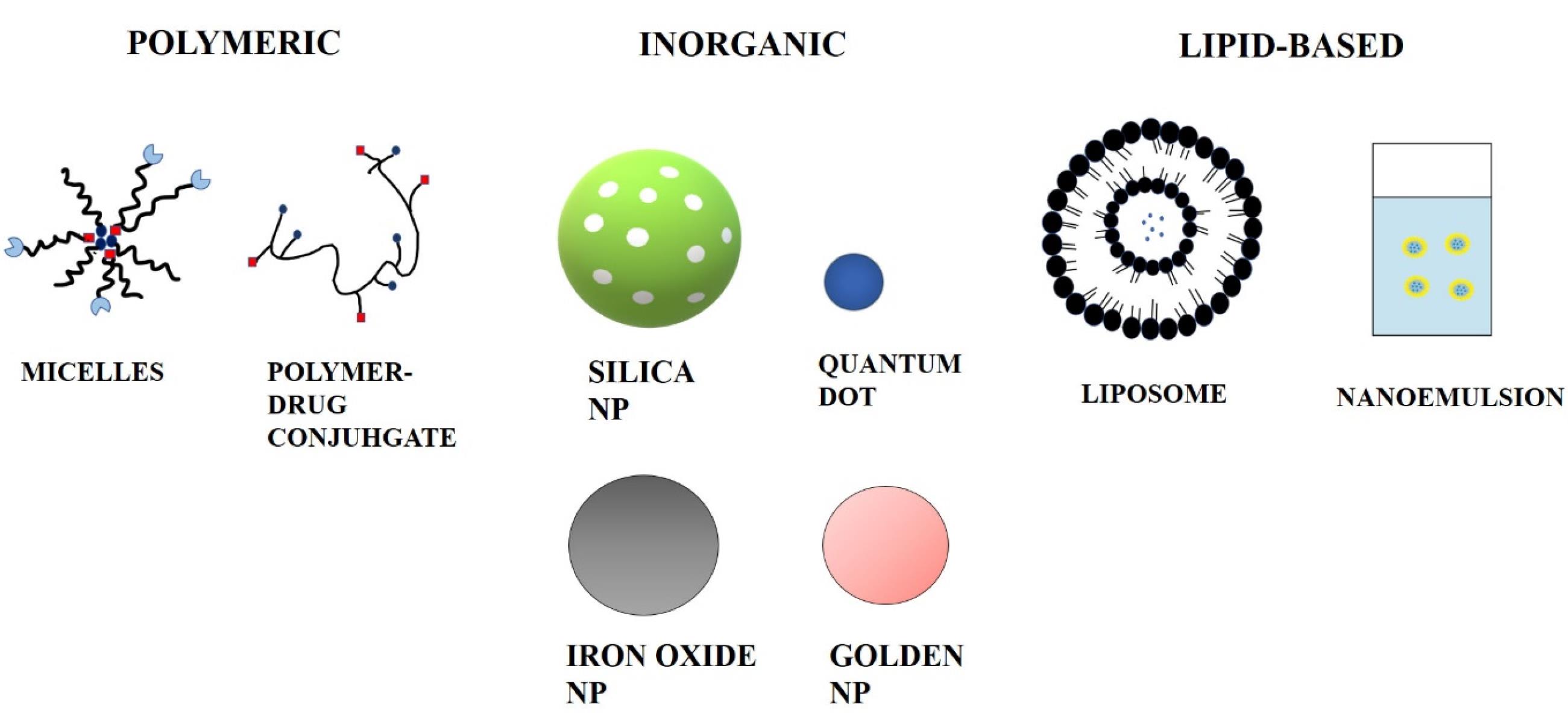
Figure 1.
Different Kinds of nanocarriers used for drug delivery
.
Different Kinds of nanocarriers used for drug delivery
Lipid-based nanoparticles
Most of the lipid-based NPs are spherical platforms with one or more lipid bilayers circulating at the interior aqueous compartment. Lipid-containing NPs comprise several component configurations. Lipid-based NPs have many benefits as a delivery system, such as straightforward formulation, self-assembly, biocompatibility, high bioavailability, capacity for carrying large payloads, and various physicochemical properties that can be controlled to modulate their biological characteristics. These factors make lipid-based NPs the most relevant type of nanomedicines that have received FDA approval.29,30
Polymeric nanoparticles
Systems of polymeric nanoparticles are created using biodegradable and biocompatible polymers. Dendrimers, polymer-drug conjugates, and micelles are examples of polymeric nanocarriers. Poly lactic-co-glycolic acid (PLGA), poly lactic acid (PLA), and polyethylene glycol (PEG) are just a few examples of the many biodegradable polymers that have been employed to create polymeric nanoparticles.31 These nanostructures have also been encapsulated using polysaccharides including pectin, chitosan, and alginate. Block copolymers with variable hydrophilicity and made up of two or more polymer chains are used in the self-assembly method used to create these nanoparticles.32 Drugs that are both hydrophilic and hydrophobic can be enclosed in polymeric nanoparticles. This approach permits regulated pH-dependent controlled release and surface changes.33
Inorganic nanoparticles
Inorganic nanoparticles, which can include iron oxide, silica, gold, and graphene quantum dots (GQDs) among other substances, are one type of nanocarrier that is being created for the detection and treatment of cancer.34 These inorganic NPs may be made to have a broad range of sizes, topologies, and geometries since they are carefully formed. The most extensively researched NPs, gold (AuNPs), are employed in a variety of structures, including nanospheres, nanorods, nanostars, nanoshells, and nanocages. Additionally, because of the characteristics of the base material itself, inorganic NPs have special physical, electrical, magnetic, and optical capabilities.35 Due to their potential for diagnosis and treatment in anticancer systems, these significant nanoparticles have attracted interest in preclinical investigations. They have a number of applications, including tumour imaging, medication delivery, and advancements in radiotherapy. Recent developments in nanotechnology highlight the significance of inorganic nanoparticles since they can be made of various materials, such as gold, oxide iron, and graphene, and because cells can internalize them through the endocytosis process.36
Types of targeting tumor
There are two types of tumors that can be targeted: (1) active and (2) passive. In active tumour targeting, biological markers such a natural ligand, an antibody, an aptamer, and a carbohydrate are used to decorate the surfaces of the nanocarriers. Then, through ligand-receptor interaction, the active targeted nano-size drug-carriers (ligand conjugated nanocarriers) will identify and bind to the tumour, and bound nanocarriers are internalized inside the cells.37 The enhanced permeability and retention (EPR) effect and defective lymphatic drainage in the tumor’s location, in contrast, are distinct physiological parameters that are dependent on passive tumour targeting. Additionally, passive targeting might help to modify drug distribution following direct tissue delivery.38
Passive targeting
The purpose of passive targeting is to take advantage of the differences between tumour and normal tissue. Drugs are successfully transported to the target site by passive targeting in order to perform a therapeutic function. Large pores in the vascular wall cause neovascularization, which worsens the permselectivity of tumour vessels relative to healthy vessels.39 High cancer cell proliferation also promotes neovascularization. Macromolecules, such as NPs, can leak from blood vessels supplying the tumour and accumulate within tumour tissue as a result of the fast and deficient angiogenesis.40 The retention of NPs is increased in cancer due to poor lymphatic drainage, which enables the nanocarriers to transfer their contents to tumour cells. One of the driving forces for passive targeting, the EPR effect, is brought on by these processes. The tumour microenvironment, in addition to the EPR effect, plays a significant role in the passive distribution of nanomedicines.41
Active targeting
In order to extend circulation time and achieve passive targeting coupling of a particular ligand on the surface that will be recognized by the cells present at the illness site, active targeting to the disease site relies on addition to PEG modification of nanocarriers.42 The therapeutic drug must be conjugated to a ligand that is specific to a tissue or cell in order to accomplish active targeting.43 To transport drugs to the target region, various types of nanoparticles have been produced. These ligands are unique in that they have the ability to identify and attach to complimentary molecules, or receptors, that are present on the surface of tumour cells. A greater amount of the anticancer medicine locates and enters the tumour cell when such targeting molecules are introduced to drug delivery nanoparticles, boosting treatment effectiveness and lowering harmful effects on surrounding healthy tissue.44
Hyaluronic acid
Hyaluronic acid (HA) commonly known as hyaluronan is an anionic and nonsulfated glycosaminoglycan that is found widely in connective and epithelial tissues Mucopolysaccharide, is the most crucial part of the extracellular matrix.45 helps significantly in cell proliferation and migration and therefore is responsible for the growth of several malignant tumors.46 HA, a nonsulfated linear glycosaminoglycan found mostly in the extracellular matrix, regulates tissue hydration through its high hydrophilicity and water-holding capacity.47 Because of its superior biocompatibility, selective targeting, and high drug-loading capacity, it is a promising biopolymer for bioconjugation.48
HA can be used as a tumor site-specific drug delivery method due to its high binding affinity for the CD44 receptor, a member of the cell adhesion protein family, which is overexpressed along the outermost layers of numerous carcinoma cells, including breast cancer cells.49 CD44, on the other hand, has been demonstrated to be expressed at very low levels on normal As a result, HA-modified nanoparticles or micelles appear to be viable carriers for CD44-targeted chemotherapy agents.50 HA has multiple functional groups that are employed in various conjugations and modifications. Because of these qualities, HA is an important component of multipurpose NPs used to administer synergistic cancer therapy.51 To make use of HA’s targeting characteristics, many ways of generating HA NP formulations have evolved.52
Quantum dots
One of the terms for quantum dots (QDs) is ‘artificial atoms,’ because they have distinct energy levels and their bandgap may be accurately adjusted by adjusting their size.53 They may be a great source of light ranging from UV to IR based on their size and composition. A QDs crystal core has around 100-100 000 atoms.54 QDs are semiconductor crystals with nano-sized scales made up of elements from groups II to VI or III to V, and they are described as particles having physical dimensions less than the exciton Bohr radius.55 A quantum dot generally has a diameter of 2 to 10 nm. The diameter of the QD depends on the chemicals used in synthesis.56
QDs have distinct luminescence or electronic features, like broad and sustained narrow emission, absorption spectra, and good photostability.57 These absorb white light and, based on the band gap of the material, release an identifiable color a few nanoseconds later.58,59 QDs made with different legends or anticancer agents/genes to concurrently image tumor cells, and malignant growth treatment via particular authority to receptors overexpressed on tumor cells and tissue surface may substantially improve fluorescent bioimaging and target delivery proficiency.60
Graphene quantum dots
The scientific community has been interested in carbon nanoparticles because of their optical, thermal, electrical, and mechanical properties. They have a range of functionalization chemistry and are safer and more secure than metal-based nanoparticles for usage in cancer theranostics.61,62 GQDs have garnered a lot of interest since carbon is among the most prevalent compound on the planet and because they may sometimes take the place of semiconducting QDs in certain applications.63
Scientists have recently paid a lot of attention to GQDs, which show exciton confinement and the quantum-size effect and are composed of very thin (usually 3-20 nm) graphene sheets. The scientific community has been interested in carbon nanomaterials because of their distinctive electrical, optical, thermal, and mechanical capabilities. For use in cancer theranostics, they have a variety of functionalization chemistry and are safer and more secure than metal-based nanoparticles64,65 Since carbon is one of the most common elements on Earth and because they might occasionally replace semiconducting QDs in some applications, GQDs have attracted a lot of attention.66
Recently, scientists have focused a lot of interest on GQDs, which are made of incredibly thin (about 3–20 nm) graphene sheets and exhibit confinement and the quantum-size effect. GQDs also exhibit tunable luminescence, photobleach resistance, constant Photoluminescence (PL), and high solubility in a variety of solvents. Colloidal inorganic semi-conductive QDs, which are poisonous due to the leakage of heavy metals including cadmium, and zinc from their core and have gained a lot of interest in coming years for their electrical and optical properties.67 GQDs effectively take the place of coating. GQDs are separate from carbon nanodots (C-dots), even though they are classed as the same thing. C-dots are NPs with PL properties that are quasi-spherical and have a diameter of less than 10 nm. GQDs, on the other hand, are graphene nanosheets containing functional groups (carboxyl, carbonyl, hydroxyl, and epoxide) commonly located at their edges. They normally have a thickness of less than 10 nm and a lateral dimension of 100 nm. By changing their electron density, these groups can operate as reaction sites and affect PL emission from the QD.68
Synthesis of GQDs
The known approaches for the synthesis of GQDs may be broadly classified as top-down and bottom-up procedures. As with bottom-up approaches, the synthesis of GQDs needs complicated reaction pathways and particular organic ingredients, making optimization challenging. Therefore, it is preferable to employ the top-down strategy, which entails fragmenting big blocks of carbon material.69 There are several top-down strategies for the synthesis of GQDs, including chemical oxidation methods,70-72 hydrothermal methods,73,74 ultrasonic aided methods,75 electrochemical oxidation methods,76 chemical vapor deposition methods,77-79 and pulsed laser ablation (PLA) techniques,80-83 GQDs feature a hexagonal crystalline structure of carbon atoms grouped in rings of six atoms, which is a graphene lattice by definition, even if the structure of GQDs depends on the synthesis circumstances.84
Different methods of synthesis of QDs
Different methods of synthesis of GQDs are given in following Table 1.85-87
Table 1.
Different methods of synthesis of graphene quantum dots
|
Type
|
Method
|
Advantages
|
Disadvantages
|
| Top down |
Oxidation method |
Most used method for large-scale production since it is easy to use and efficient. |
Strong oxidizers that must be utilized may burn or explode |
|
|
Hydrothermal/ solvothermal method |
It is an easy and quick procedure.
Hydrothermal technology is also ecologically beneficial. |
Long reaction time, a reaction also involves high pressure and temperature. |
|
|
Electrochemical oxidation |
high levels of stability and a homogeneous size distribution |
Product yield is poor. |
|
|
Microwave-assisted/ ultrasonic-assisted process |
It can increase the manufacturing yield in addition to reducing response time. |
Costly ultrasonic reactor. |
| Bottom up |
Controllable method |
GQDs gets homogeneous shape and size |
Multistep complex process and low quantum yield |
|
|
Carbonization |
It is an easy and ecologically beneficial technique. |
tough to properly control |
Structure of GQDs
Each carbon atom is covalently linked to three more carbon atoms in this way, resulting in the sp2 hybridized property to the sheet’s plane. These characteristics are responsible for their exceptional electrical and optical qualities.88,89 GQDs have two sorts of edges: armchair and zigzag. Triple carbon bonds may be seen at the armchair edges, and the edge has a carbene-like structure. Carbene-like edges are defined by the presence of two unshared valence electrons on each carbon atom at the zigzag edges.90-92 The edge types influence the form of GQDs and, as a result, their optical and electrical characteristics.93 Structure of GQD is shown in Figure 2.
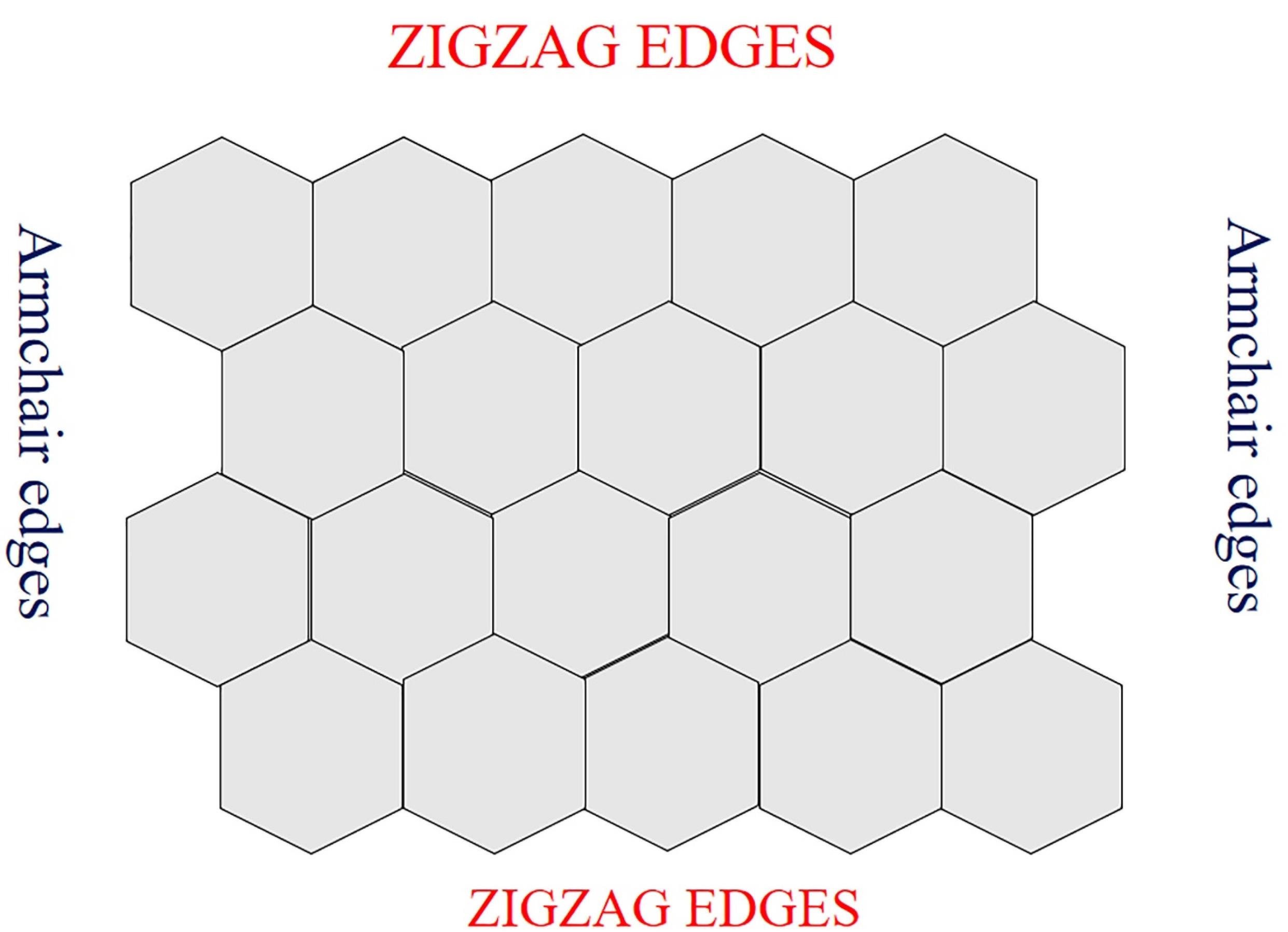
Figure 2.
Structure of GQD
.
Structure of GQD
Photoluminescence mechanism of GQDs
GQDs are the most basic Carbon dots, having a single-layer carbon core and chemical groups attached on the surface or edges. Therefore, GQDs are an excellent model for studying the PL methodology of CDs. The PL behavior of chemically produced GO is provided first to explain the PL mechanism of GQDs since GO is an essential raw material for GQD synthesis and consequently GO and GQDs have comparable chemical structures. Oxygen-based functional groups are found in GO, around the edges, or on the basal plane. As a consequence, a linearly aligned epoxy and hydroxyl-boned sp3 C-O matrix cover the 2-3 nm aromatic sp2 domains.94,95 Fluorescence can be facilitated by radiation-induced recombination of electron-hole (e-h) pairs in such sp2 clusters.96 The band gaps of various sp2 size distributions encompass a wide range due to the large size distribution of sp2 domains in GO, resulting in a broad PL emission spectrum from visible to near-infrared. Many researchers have looked at the fluorescence of GO and decreased GO.97 GQDs are more porous, have more surface functional groups, and have more surface flaws than GO. The GO fluorescence measurements can be utilized to help explain these emissions in GQDs. Graphene excitants have an unlimited Bohr diameter. As a result, any size graphene fragment will exhibit quantum confinement effects. GQDs have a non-zero band gap and PL on excitation as a result. By altering the GQDs’ size and surface chemistry, the band gap may be changed. In the recent five years, there has been a significant advancement in the production of GQDs, and researchers have identified plausible PL mechanisms: conjugated surface/edge state π-domains.98
Bioimaging with GQDs
Nowadays, non-toxic diagnostic imaging for cells has been created. When compared to other materials, GQDs show a substantial advantage in cell imaging.99,100 With the use of different wavelengths of the electromagnetic spectrum, bioimaging is a significant technology that is employed in both research and clinical contexts. It enables the monitoring of biological processes such as targeted delivery, cellular uptake, and biodistribution of medicines in a precise, isolated manner.101-103 Imaging is important in cancer diagnosis because sensitive imaging allows quick cancer diagnosis in addition to the recognition of metastatic or the recurrence of the disease.104 Based on modified GQDs, targeted tumor cell imaging can be achieved. This is possible when GQDs are changed or connected with certain bioactive species. Sun et al. employed chemically abridged GQDs and photo-reduced GQDs as fluorescent probes to image A549 cells in a typical investigation.105
Functionalization of graphene quantum dots
Doped graphene quantum dots
Together with transition metals and metal atoms like Si and Mn, the bulk of the atoms doped with GQDs include N, C, P, B, and S. Metals and transition metal ion ions can be hazardous, restricting their usage in fluorescence sensing applications where toxicity is a concern. Also, due to the larger radiuses of metal and transition metal atoms than those of carbon, doping with GQDs frequently ends in dispersed and inefficient integration. As a result, doping with nonmetallic atoms offers clear advantages. GQDs doped with nonmetallic atoms are more biocompatible for use in human medicine. Additionally, because non-metal ions and carbon atoms have comparable sizes, they may be included uniformly and modify the electronic structure of GQDs, causing physical flaws that enhance the QY and particular binding properties of GQDs.106 Figure 3 shows the advantages of Functionalization of GQDs.
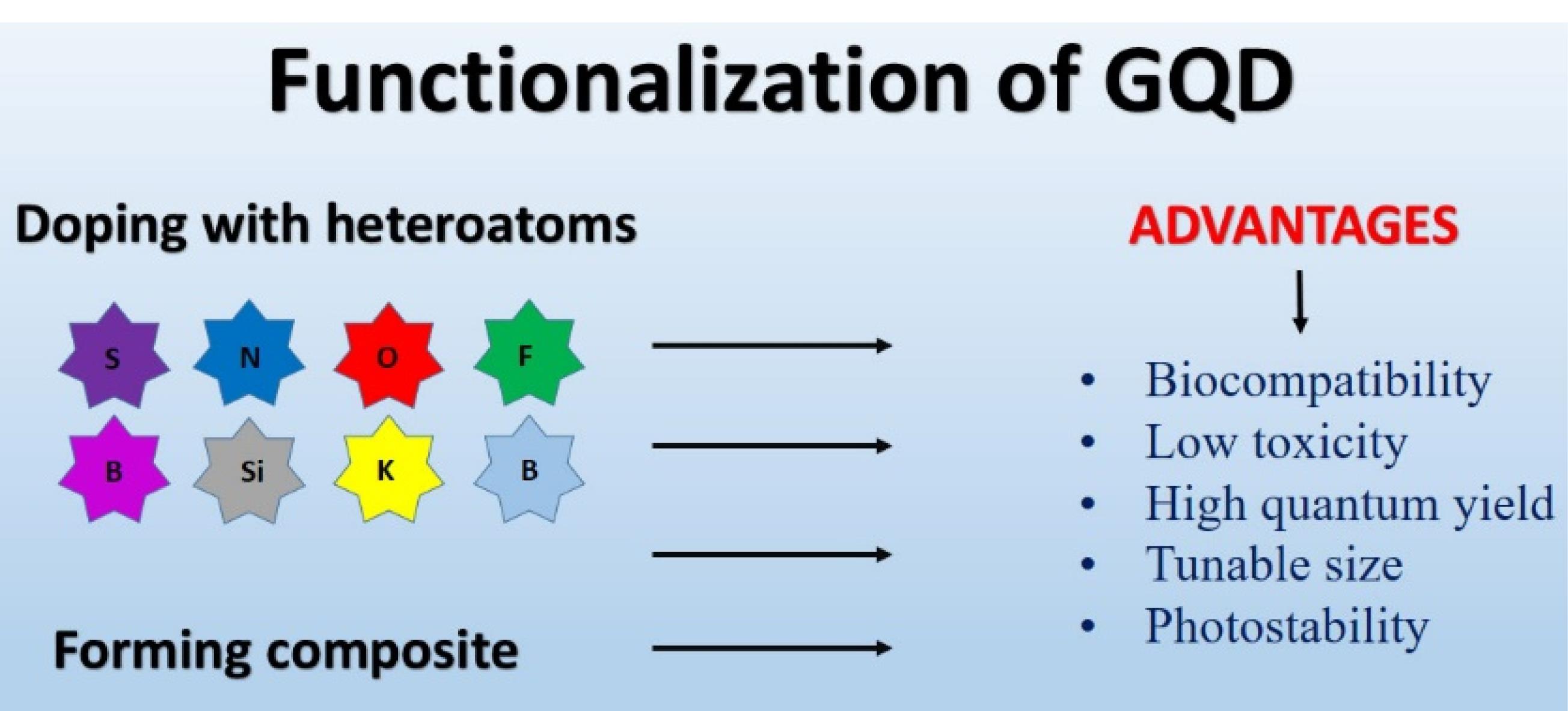
Figure 3.
Advantages of functionalization of GQDs
.
Advantages of functionalization of GQDs
Nitrogen doping (N-GQDs)
It is feasible to create nitrogen doped GQDs via the constant inclusion of nitrogen since nitrogen has a comparable size to carbon and may establish strong covalent connections with it.107 The electrical characteristics and surface state defects of GQDs are enhanced by N-doping, and the amount of reactive groups enhances the fluorescence quantum yield of GQDs. Because of the recent interest in N-GQDs, a range of methods for producing N-GQDs have been developed. The preparation begins with the combination of a carbon and nitrogen source, followed by heating in a poly tetrafluoroethylene-lined tank, followed by separation, and then purification. Transmission electron microscopy (TEM), atomic force microscopy (AFM), and X-ray diffraction are regularly used to clarify structure, whereas X-ray photoelectron spectroscopy (XPS), and infrared spectroscopy (IR spectroscopy) are usually used to establish chemistry (X-ray diffraction (XRD)). Citric acid is a popular source of carbon that has been coupled with ethylenediamine, glutamic acid, urea, ammonia, or glycine as a nitrogen source. The use of glycine in the manufacture of N-GQDs can result in consistent size distributions (e.g. 2.2 1.5 nm), with fluorescence excitation and emission wavelengths of 353 nm and 450 nm, respectively.108 N-GQDs were synthesized by Du et al using GO and ammonia. TEM yielded an average diameter of 5 nm. The resulting N-GQDs glow bright blue when exposed to UV light.109
Sulfur doping and Co-doping
Sulfur may also be utilized to dope GQDs, resulting in different characteristics. The size of the s atom exceeds that of the carbon atom. Because the outermost orbitals of two atoms differ, In GQDs, the combination of S leads to an unequal spin density distribution.110 The electronegativity distinction between S and C is quite modest, indicating that there is little driving force for electron transport between the two materials.111 All of these traits make it challenging to integrate S doping into a GQD architecture. Despite these obstacles, many techniques for synthesizing S-doped GQDs (S-GQDs) have recently been published. Functionally, bottom-up process steps were similar to the techniques discussed in the previous section for manufacturing N-GQDs and require a carbon and a sulfur source.112 Citric acid, 3-mercapto-succinic acid, and NaOH were employed as synthetic ingredients to create S-GQDs in DMF. Bian et al. used 1,3,6-nitropyrene as the carbon source, with either Na2S or 3-mercaptopropionic acid (MPA) as the sulfur atom source. In AFM, the synthesized S-GQDs exhibit a homogeneous particle size distribution when utilizing MPA, with an average diameter of 2.5 nm and an average height of 0.8 nm. XPS indicates the presence of carbon, sulfur, and oxygen on the surface of S-GQDs, as well as carboxyl groups. A solution of S-GQDs looks yellow and generates intense blue fluorescence when exposed to 365 nm ultraviolet light. The fluorescence intensity rises from 310 nm to 360 nm, then decreases when the excitation wavelength goes higher from 375 nm to 390 nm. These S-GQDs have maximal excitation and emission wavelengths of 360 nm and 450 nm, respectively.113 After synthesizing S-GQDS with citric acid and MPA as raw materials, Kadian et al used FT-IR and XPS to demonstrate the C-S bond. The peak excitation and emission wavelengths of S-GQDS were measured to be 340 nm and 440 nm, respectively. It indicates that the sulfur atom was effectively doped into GQDs.114
Doping with other heteroatoms
There have been reports of doping of GQDs with additional atoms, including K, Si, B, Cl, S, and P, in addition to the individual and combined doping of N and S. Doping changes the properties of GQDs. The BC bond is 0.7% longer than the CC bond, and electron loss causes energy state defects in GQDs, resulting in surface defect discharge. Boron-GQDs (B-GQDs) can thereby alter the optical characteristics of GQDs by generating a high number of active sites.115 Ge et al synthesized B-GQDs using a NaOH solution utilizing 1,3, 6-nitropyrene as a source of carbon as well as borax as a source of boron. B-GQDs produce a brilliant yellow solution. The B-GQDs showed good crystallinity as evidenced by TEM; AFM demonstrated graphene having one or two layers of thickness; and XPS analysis of the components revealed that the B-GQDs have abundant groups that contain oxygen on their surfaces. XPS also demonstrated that B atoms were successfully integrated within the GQD lattice. In visible light, the solution that forms of B-GQDs is pale yellow, while in ultraviolet light, it is green.116
GQDs based drug delivery systems
Prior to the advent of nanotechnology, organic fluorescent dyes were thought to be useful tools for bioimaging applications. The previously held idea, however, has been altered by recently released luminous nanomaterial kinds.117,118 In order to achieve targeted and visible medication administration, QD, which are important in nanomedicine, enable the integration of medicines, affinity ligands, and imaging moieties within a single nanostructure. These semiconductor nanoparticles help transport various kinds of efficient anti-cancer medications for gene therapy and immunotherapy in addition to improving the pharmacologic features of currently used treatments. In overall, nanoparticle-based drug delivery methods improve circulation times, minimise drug toxicity, increase bioavailability, and regulate drug release and targeting. Because of this, drug delivery that utilizes nanocarriers has a number of benefits over traditional drug delivery systems.119
Recently used quantum dots to target tumors
Different types of QD have been used recently for targeting cancer cells or tumors, such as nitrogen-doped GQDs, graphitic carbon nitride QDs, black phosphorus QDs, etc.120-126 A list of these QD and their activities is given in Table 2.
Table 2.
List of quantum dots used for tumor targeting
|
Quantum dots
|
Activity
|
Reference
|
| Nitrogen-doped GQDs |
Spread of cancer cells |
121
|
| MnO2 QD stabilized by cysteine |
Dopamine detection |
122
|
| pH-responsive black phosphorus QDs |
Photodynamic treatment for tumors |
123
|
| Graphitic carbon nitride QDs |
Combination chemo-photodynamic treatment for tumors |
124
|
| Black phosphorus QDs |
Synergistic chemo-phototherapy and dual-modality cancer imaging |
125
|
| CQDs - quinic acid |
Gemcitabine to breast cancer cells |
126
|
| Duplex metal co-doped CQDs |
Synergistic cancer treatment |
127
|
GQDS-HA release remedy
As demonstrated by XPS, dopamine hydrochloride conjugated to HA has been attached to a GQD that takes the fascinating adhesive properties of the catechol molecule. Transmission electron microscopy confirmed the particle size as being 20 nm, and the spectrum of fluorescence revealed significant fluorescence power despite HA attachment.
Al-Nahain et al investigated how to use hyaluronic acid (GQD-HA) as a targeting agent to deliver a GQD efficiently and precisely to the intended target. A GQD that adopts the intriguing binding capabilities of dopamine which is the catechol moiety, has been used to attach HA. In vivo, biodistribution analysis showed that the tumor tissue emitted more intense fluorescence when the produced GQD-HA was administered to female Balb/c mice with tumor-bearing CD44 receptors. Using a confocal laser scanning microscope, in vitro cellular imaging revealed that CD44 overexpressed A549 cells fluoresced brightly. Results from both in vitro and in vivo experiments demonstrated the value of utilizing HA as a targeting moiety. The hydrophobic drug doxorubicin’s loading and release kinetics from a GQD in moderately acidic circumstances demonstrated that a GQD may be thought of as a new drug transporter, and the MTT assay’s nontoxic results support the claim that GQD-HA is a biocompatible substance.101 HA-GQD coupling and targeting to cancer cell is shown in Figure 4.
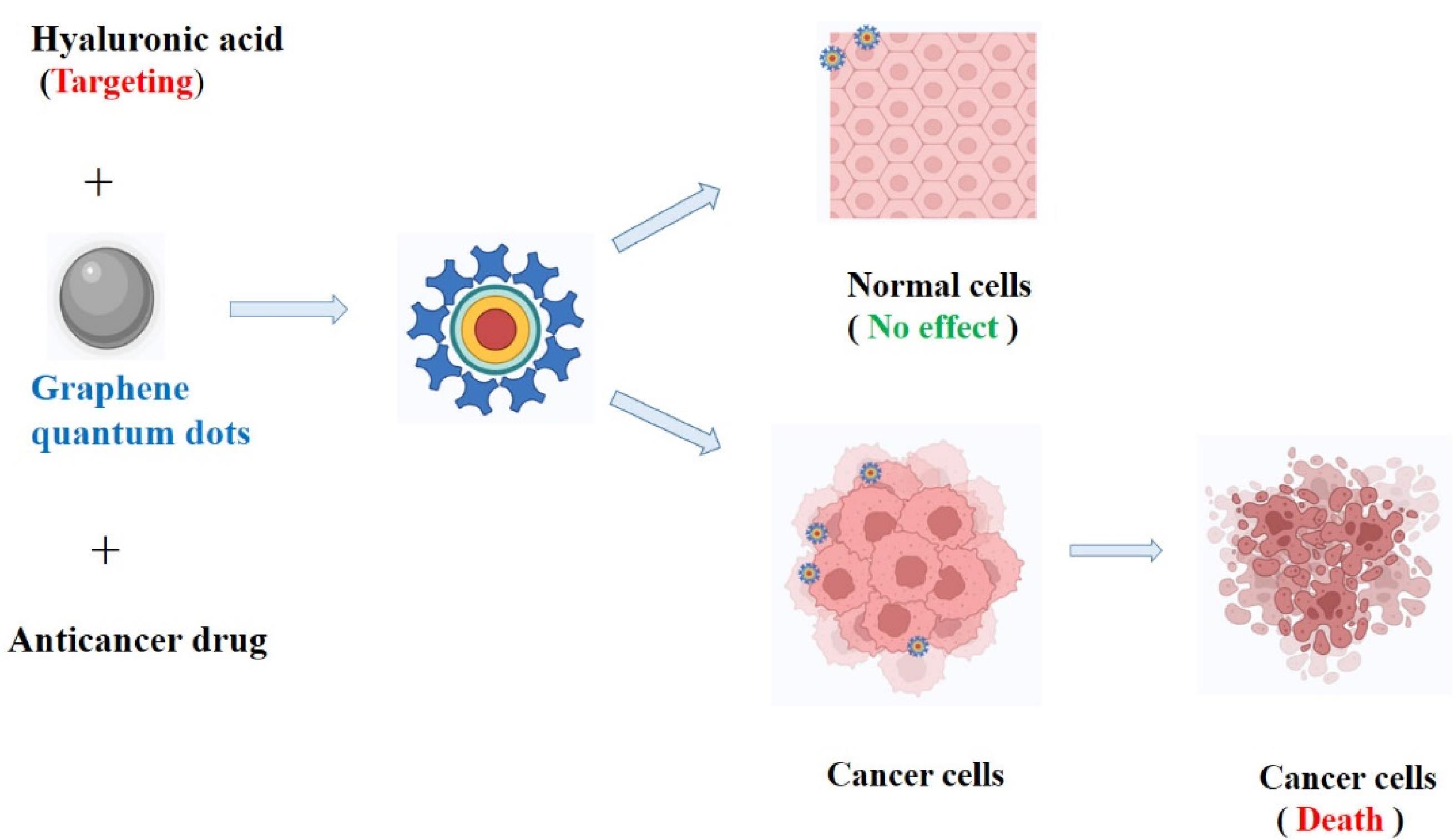
Figure 4.
HA-GQD coupling and targeting to cancer cell
.
HA-GQD coupling and targeting to cancer cell
Wu et al studied the HA conjugation to graphene oxide for targeted drug delivery. HA binds to nanoscale graphene oxide through the creation of amide bonds after adipic acid dihydrazide functionalizes GO to add amine groups. The outcomes of the toxicological tests conducted in vivo and in vitro demonstrate that the resultant GO-HA displays minimal cytotoxicity, great blood compatibility, and no overt toxic effect on mice. Experiments on cellular uptake show that the GO-HA can specifically carry anticancer medicines into the cells through receptor-mediated endocytosis. The anticancer medication DOX has a high loading capacity for the GO-HA, and the resultant GO-HA/DOX demonstrates a high level of cytotoxicity to HeLa cells.127
Zheng et al investigated the detection of human tumor cells using HA-linked nitrogen-doped GQDs (N-GQDs). Blue luminous N-GQDs were created utilizing a hydrothermal method approach that was speedy and easy to prevent by-products, highlighted the binding sites to ensure correct arrangement, and were simple to utilize. In an outcome of the nitrogen component doping, an amide II bond is adequately established, and several binding sites for HA crosslinking were provided. MCF-7 cells fluoresced strongly when CD44, which was overexpressed on the surface of the cells, was combined with HA-conjugated N-GQDs (HA-N-GQDs). HA-N-GQDs’ excellent fluorescence, low toxicity, and high cytocompatibility suggested that they may be used in fluorescence imaging enabling accurate detection of cancer cells.128
Conclusion
Recent advances in GQD research have demonstrated GQDs’ potential as novel platforms in biotechnology and nanomedicine. Novel experimental techniques were developed to enhance the physicochemical characteristics of GQDs to ensure they adhere to the requirements of a specific application. Thus, incorporating the receptor-binding molecule into the GQD can improve cell efficiency. Because of its biocompatible, biodegradable, and nontoxic properties, HA is widely used as a primary receptor, it also has a high affinity for the CD44 receptor. Catechol, the side chain of the rare amino acid 3,4-dihydroxy-L-phenylalanine, L-DOPA, often known as DN, is a common component of marine mussel adhesion substances is notable as a bonding agent for surface modification and biological systems. As a result, HA-DN nanotherapeutics are an excellent cancer treatment alternative.
Acknowledgments
All individuals listed as authors have contributed substantially to the work and are required to indicate their specification contribution.
Competing Interests
Authors declare no conflict of interest.
Ethical Approval
Not applicable.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69(1):7-34. doi: 10.3322/caac.21551 [Crossref] [ Google Scholar]
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011; 331(6024):1559-64. doi: 10.1126/science.1203543 [Crossref] [ Google Scholar]
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 2003; 3(6):453-8. doi: 10.1038/nrc1098 [Crossref] [ Google Scholar]
- Tagde P, Kulkarni GT, Mishra DK, Kesharwani P. Recent advances in folic acid engineered nanocarriers for treatment of breast cancer. J Drug Deliv Sci Technol 2020; 56(Pt A):101613. doi: 10.1016/j.jddst.2020.101613 [Crossref] [ Google Scholar]
- Sparano JA, Gray R, Oktay MH, Entenberg D, Rohan T, Xue X. A metastasis biomarker (MetaSite BreastTM Score) is associated with distant recurrence in hormone receptor-positive, HER2-negative early-stage breast cancer. NPJ Breast Cancer 2017; 3:42. doi: 10.1038/s41523-017-0043-5 [Crossref] [ Google Scholar]
- Devi L, Gupta R, Jain SK, Singh S, Kesharwani P. Synthesis, characterization and in vitro assessment of colloidal gold nanoparticles of gemcitabine with natural polysaccharides for treatment of breast cancer. J Drug Deliv Sci Technol 2020; 56(Pt A):101565. doi: 10.1016/j.jddst.2020.101565 [Crossref] [ Google Scholar]
- de Rinaldis E, Tutt A, Dontu G. Breast cancer. In: Breast Pathology. Elsevier; 2011. p. 352-9. 10.1016/b978-1-4377-1757-0.00028-7.
- Straver ME, Glas AM, Hannemann J, Wesseling J, van de Vijver MJ, Rutgers EJ. The 70-gene signature as a response predictor for neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat 2010; 119(3):551-8. doi: 10.1007/s10549-009-0333-1 [Crossref] [ Google Scholar]
- Choudhury H, Gorain B, Tekade RK, Pandey M, Karmakar S, Pal TK. Safety against nephrotoxicity in paclitaxel treatment: oral nanocarrier as an effective tool in preclinical evaluation with marked in vivo antitumor activity. RegulToxicolPharmacol 2017; 91:179-89. doi: 10.1016/j.yrtph.2017.10.023 [Crossref] [ Google Scholar]
- Schirrmacher V. From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment. Int J Oncol 2019; 54(2):407-19. doi: 10.3892/ijo.2018.4661 [Crossref] [ Google Scholar]
- Alexander-Bryant AA, Vanden Berg-Foels WS, Wen X. Bioengineering strategies for designing targeted cancer therapies. Adv Cancer Res 2013; 118:1-59. doi: 10.1016/b978-0-12-407173-5.00002-9 [Crossref] [ Google Scholar]
- Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 2021; 20(2):101-24. doi: 10.1038/s41573-020-0090-8 [Crossref] [ Google Scholar]
- Craig M, Jenner AL, Namgung B, Lee LP, Goldman A. Engineering in medicine to address the challenge of cancer drug resistance: from micro- and nanotechnologies to computational and mathematical modeling. Chem Rev 2021; 121(6):3352-89. doi: 10.1021/acs.chemrev.0c00356 [Crossref] [ Google Scholar]
- Saha T, Mondal J, Khiste S, Lusic H, Hu ZW, Jayabalan R. Nanotherapeutic approaches to overcome distinct drug resistance barriers in models of breast cancer. Nanophotonics 2021; 10(12):3063-73. doi: 10.1515/nanoph-2021-0142 [Crossref] [ Google Scholar]
- Ledford H. ‘Master protocol’ aims to revamp cancer trials. Nature 2013; 498(7453):146-7. doi: 10.1038/498146a [Crossref] [ Google Scholar]
- Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015; 26 Suppl 5:v8-30. doi: 10.1093/annonc/mdv298 [Crossref] [ Google Scholar]
- Rocha M, Chaves N, Báo S. Nanobiotechnology for breast cancer treatment. In: Van Pham P, ed. Breast Cancer - From Biology to Medicine. Rijeka: IntechOpen; 2017. 10.5772/66989.
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 2005; 5(3):161-71. doi: 10.1038/nrc1566 [Crossref] [ Google Scholar]
- Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 2010; 9(8):615-27. doi: 10.1038/nrd2591 [Crossref] [ Google Scholar]
- Shrestha M. Nanotechnology to revolutionize medicine. J Drug DelivTher 2012; 2(5):156-61. doi: 10.22270/jddt.v2i5.302 [Crossref] [ Google Scholar]
- Duncan R, Gaspar R. Nanomedicine(s) under the microscope. Mol Pharm 2011; 8(6):2101-41. doi: 10.1021/mp200394t [Crossref] [ Google Scholar]
- Bhaskar S, Tian F, Stoeger T, Kreyling W, de la Fuente JM, Grazú V. Multifunctional nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: perspectives on tracking and neuroimaging. Part FibreToxicol 2010; 7:3. doi: 10.1186/1743-8977-7-3 [Crossref] [ Google Scholar]
- Hafner A, Lovrić J, Lakoš GP, Pepić I. Nanotherapeutics in the EU: an overview on current state and future directions. Int J Nanomedicine 2014; 9:1005-23. doi: 10.2147/ijn.s55359 [Crossref] [ Google Scholar]
- Prasad M, Lambe UP, Brar B, Shah I, Manimegalai J, Ranjan K. Nanotherapeutics: an insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed Pharmacother 2018; 97:1521-37. doi: 10.1016/j.biopha.2017.11.026 [Crossref] [ Google Scholar]
- Chen W, Li H, Liu Z, Yuan W. Lipopolyplex for therapeutic gene delivery and its application for the treatment of Parkinson’s disease. Front Aging Neurosci 2016; 8:68. doi: 10.3389/fnagi.2016.00068 [Crossref] [ Google Scholar]
- Jin C, Wang K, Oppong-Gyebi A, Hu J. Application of nanotechnology in cancer diagnosis and therapy - a mini-review. Int J Med Sci 2020; 17(18):2964-73. doi: 10.7150/ijms.49801 [Crossref] [ Google Scholar]
- Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res 2008; 14(5):1310-6. doi: 10.1158/1078-0432.ccr-07-1441 [Crossref] [ Google Scholar]
- Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur J Pharm Sci 2013; 48(3):416-27. doi: 10.1016/j.ejps.2012.12.006 [Crossref] [ Google Scholar]
- Fonseca-Santos B, Gremião MP, Chorilli M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int J Nanomedicine 2015; 10:4981-5003. doi: 10.2147/ijn.s87148 [Crossref] [ Google Scholar]
- Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol 2015; 6:286. doi: 10.3389/fphar.2015.00286 [Crossref] [ Google Scholar]
- Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces 2010; 75(1):1-18. doi: 10.1016/j.colsurfb.2009.09.001 [Crossref] [ Google Scholar]
- Gu F, Zhang L, Teply BA, Mann N, Wang A, Radovic-Moreno AF. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc Natl Acad Sci U S A 2008; 105(7):2586-91. doi: 10.1073/pnas.0711714105 [Crossref] [ Google Scholar]
- Lo YL, Sung KH, Chiu CC, Wang LF. Chemically conjugating polyethylenimine with chondroitin sulfate to promote CD44-mediated endocytosis for gene delivery. Mol Pharm 2013; 10(2):664-76. doi: 10.1021/mp300432s [Crossref] [ Google Scholar]
- Izci M, Maksoudian C, Manshian BB, Soenen SJ. The use of alternative strategies for enhanced nanoparticle delivery to solid tumors. Chem Rev 2021; 121(3):1746-803. doi: 10.1021/acs.chemrev.0c00779 [Crossref] [ Google Scholar]
- Yang W, Liang H, Ma S, Wang D, Huang J. Gold nanoparticle based photothermal therapy: development and application for effective cancer treatment. Sustain Mater Technol 2019; 22:e00109. doi: 10.1016/j.susmat.2019.e00109 [Crossref] [ Google Scholar]
- Dziewięcka M, Pawlyta M, Majchrzycki Ł, Balin K, Barteczko S, Czerkawska M. The structure-properties-cytotoxicity interplay: a crucial pathway to determining graphene oxide biocompatibility. Int J Mol Sci 2021; 22(10):5401. doi: 10.3390/ijms22105401 [Crossref] [ Google Scholar]
- Rajitha B, Malla RR, Vadde R, Kasa P, Prasad GLV, Farran B. Horizons of nanotechnology applications in female specific cancers. Semin Cancer Biol 2021; 69:376-90. doi: 10.1016/j.semcancer.2019.07.005 [Crossref] [ Google Scholar]
- Sharker SM, Lee JE, Kim SH, Jeong JH, In I, Lee H. pH triggered in vivo photothermal therapy and fluorescence nanoplatform of cancer based on responsive polymer-indocyanine green integrated reduced graphene oxide. Biomaterials 2015; 61:229-38. doi: 10.1016/j.biomaterials.2015.05.040 [Crossref] [ Google Scholar]
- Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci 2020; 77(9):1745-70. doi: 10.1007/s00018-019-03351-7 [Crossref] [ Google Scholar]
- Wu J. The enhanced permeability and retention (EPR) effect: the significance of the concept and methods to enhance its application. J Pers Med 2021; 11(8):771. doi: 10.3390/jpm11080771 [Crossref] [ Google Scholar]
- Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol 2019; 71(8):1185-98. doi: 10.1111/jphp.13098 [Crossref] [ Google Scholar]
- Wagner E. Programmed drug delivery: nanosystems for tumor targeting. Expert Opin Biol Ther 2007; 7(5):587-93. doi: 10.1517/14712598.7.5.587 [Crossref] [ Google Scholar]
- Montaseri H, Kruger CA, Abrahamse H. Review: organic nanoparticle based active targeting for photodynamic therapy treatment of breast cancer cells. Oncotarget 2020; 11(22):2120-36. doi: 10.18632/oncotarget.27596 [Crossref] [ Google Scholar]
- Shi P, Cheng Z, Zhao K, Chen Y, Zhang A, Gan W. Active targeting schemes for nano-drug delivery systems in osteosarcoma therapeutics. J Nanobiotechnology 2023; 21(1):103. doi: 10.1186/s12951-023-01826-1 [Crossref] [ Google Scholar]
- Alberts B, Bray D, Hopkin K, Johnson AD, Lewis J, Raff M, et al. Essential Cell Biology. New York: W.W. Norton & Company; 2013. 10.1201/9781315815015.
- Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol 2004; 83(7):317-25. doi: 10.1078/0171-9335-00392 [Crossref] [ Google Scholar]
- Luo Y, Prestwich GD. Hyaluronic acid-N-hydroxysuccinimide: a useful intermediate for bioconjugation. Bioconjug Chem 2001; 12(6):1085-8. doi: 10.1021/bc015513p [Crossref] [ Google Scholar]
- Cyphert JM, Trempus CS, Garantziotis S. Size matters: molecular weight specificity of hyaluronan effects in cell biology. Int J Cell Biol 2015; 2015:563818. doi: 10.1155/2015/563818 [Crossref] [ Google Scholar]
- Louhichi T, Ziadi S, Saad H, Dhiab MB, Mestiri S, Trimeche M. Clinicopathological significance of cancer stem cell markers CD44 and ALDH1 expression in breast cancer. Breast Cancer 2018; 25(6):698-705. doi: 10.1007/s12282-018-0875-3 [Crossref] [ Google Scholar]
- Cortes-Dericks L, Schmid RA. CD44 and its ligand hyaluronan as potential biomarkers in malignant pleural mesothelioma: evidence and perspectives. Respir Res 2017; 18(1):58. doi: 10.1186/s12931-017-0546-5 [Crossref] [ Google Scholar]
- Lokeshwar VB, Mirza S, Jordan A. Targeting hyaluronic acid family for cancer chemoprevention and therapy. Adv Cancer Res 2014; 123:35-65. doi: 10.1016/b978-0-12-800092-2.00002-2 [Crossref] [ Google Scholar]
- Kim JH, Moon MJ, Kim DY, Heo SH, Jeong YY. Hyaluronic acid-based nanomaterials for cancer therapy. Polymers (Basel) 2018; 10(10):1133. doi: 10.3390/polym10101133 [Crossref] [ Google Scholar]
- Klimov VI. Spectral and dynamical properties of multiexcitons in semiconductor nanocrystals. Annu Rev Phys Chem 2007; 58:635-73. doi: 10.1146/annurev.physchem.58.032806.104537 [Crossref] [ Google Scholar]
- Chen L, Liang J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater Sci Eng C Mater Biol Appl 2020; 112:110924. doi: 10.1016/j.msec.2020.110924 [Crossref] [ Google Scholar]
- Chan WC, Maxwell DJ, Gao X, Bailey RE, Han M, Nie S. Luminescent quantum dots for multiplexed biological detection and imaging. CurrOpinBiotechnol 2002; 13(1):40-6. doi: 10.1016/s0958-1669(02)00282-3 [Crossref] [ Google Scholar]
- Das P, Maruthapandi M, Saravanan A, Natan M, Jacobi G, Banin E. Carbon dots for heavy-metal sensing, pH-sensitive cargo delivery, and antibacterial applications. ACS Appl Nano Mater 2020; 3(12):11777-90. doi: 10.1021/acsanm.0c02305 [Crossref] [ Google Scholar]
- Bruchez M Jr, Moronne M, Gin P, Weiss S, Alivisatos AP. Semiconductor nanocrystals as fluorescent biological labels. Science 1998; 281(5385):2013-6. doi: 10.1126/science.281.5385.2013 [Crossref] [ Google Scholar]
- Dabbousi BO, Rodriguez-Viejo J, Mikulec FV, Heine JR, Mattoussi H, Ober R. (CdSe)ZnS core−shell quantum dots: synthesis and characterization of a size series of highly luminescent nanocrystallites. J Phys Chem B 1997; 101(46):9463-75. doi: 10.1021/jp971091y [Crossref] [ Google Scholar]
- Bakalova R, Ohba H, Zhelev Z, Ishikawa M, Baba Y. Quantum dots as photosensitizers?. Nat Biotechnol 2004; 22(11):1360-1. doi: 10.1038/nbt1104-1360 [Crossref] [ Google Scholar]
- Savla R, Taratula O, Garbuzenko O, Minko T. Tumor targeted quantum dot-mucin 1 aptamer-doxorubicin conjugate for imaging and treatment of cancer. J Control Release 2011; 153(1):16-22. doi: 10.1016/j.jconrel.2011.02.015 [Crossref] [ Google Scholar]
- Saleem J, Wang L, Chen C. Carbon-based nanomaterials for cancer therapy via targeting tumor microenvironment. Adv Healthc Mater 2018; 7(20):e1800525. doi: 10.1002/adhm.201800525 [Crossref] [ Google Scholar]
- Fadeel B, Bussy C, Merino S, Vázquez E, Flahaut E, Mouchet F. Safety assessment of graphene-based materials: focus on human health and the environment. ACS Nano 2018; 12(11):10582-620. doi: 10.1021/acsnano.8b04758 [Crossref] [ Google Scholar]
- Tian P, Tang L, Teng KS, Lau SP. Graphene quantum dots from chemistry to applications. Mater Today Chem 2018; 10:221-58. doi: 10.1016/j.mtchem.2018.09.007 [Crossref] [ Google Scholar]
- Yan X, Cui X, Li LS. Synthesis of large, stable colloidal graphene quantum dots with tunable size. J Am Chem Soc 2010; 132(17):5944-5. doi: 10.1021/ja1009376 [Crossref] [ Google Scholar]
- Kim S, Hwang SW, Kim MK, Shin DY, Shin DH, Kim CO. Anomalous behaviors of visible luminescence from graphene quantum dots: interplay between size and shape. ACS Nano 2012; 6(9):8203-8. doi: 10.1021/nn302878r [Crossref] [ Google Scholar]
- Xia X, Zheng Y. Comment on “one-step and high yield simultaneous preparation of single- and multi-layer graphene quantum dots from CX-72 carbon black”. J Mater Chem 2012; 22(40):21776. doi: 10.1039/c2jm32560h [Crossref] [ Google Scholar]
- Frigerio C, Ribeiro DS, Rodrigues SS, Abreu VL, Barbosa JA, Prior JA. Application of quantum dots as analytical tools in automated chemical analysis: a review. Anal Chim Acta 2012; 735:9-22. doi: 10.1016/j.aca.2012.04.042 [Crossref] [ Google Scholar]
- Jin SH, Kim DH, Jun GH, Hong SH, Jeon S. Tuning the photoluminescence of graphene quantum dots through the charge transfer effect of functional groups. ACS Nano 2013; 7(2):1239-45. doi: 10.1021/nn304675g [Crossref] [ Google Scholar]
- Sweetman MJ, Hickey SM, Brooks DA, Hayball JD, Plush SE. A practical guide to prepare and synthetically modify graphene quantum dots. Adv Funct Mater 2019; 29(14):1808740. doi: 10.1002/adfm.201808740 [Crossref] [ Google Scholar]
- Zhao C, Song X, Liu Y, Fu Y, Ye L, Wang N. Synthesis of graphene quantum dots and their applications in drug delivery. J Nanobiotechnol 2020; 18(1):142. doi: 10.1186/s12951-020-00698-z [Crossref] [ Google Scholar]
- Shang W, Cai T, Zhang Y, Liu D, Liu S. Facile one pot pyrolysis synthesis of carbon quantum dots and graphene oxide nanomaterials: all carbon hybrids as eco-environmental lubricants for low friction and remarkable wear-resistance. Tribol Int 2018; 118:373-80. doi: 10.1016/j.triboint.2017.09.029 [Crossref] [ Google Scholar]
- Wang S, Shen J, Wang Q, Fan Y, Li L, Zhang K. High-performance layer-by-layer self-assembly PANI/GQD-rGO/CFC electrodes for a flexible solid-state supercapacitor by a facile spraying technique. ACS Appl Energy Mater 2019; 2(2):1077-85. doi: 10.1021/acsaem.8b01631 [Crossref] [ Google Scholar]
- Ying Y, He P, Ding G, Peng X. Ultrafast adsorption and selective desorption of aqueous aromatic dyes by graphene sheets modified by graphene quantum dots. Nanotechnology 2016; 27(24):245703. doi: 10.1088/0957-4484/27/24/245703 [Crossref] [ Google Scholar]
- Luo P, Guan X, Yu Y, Li X, Yan F. Hydrothermal synthesis of graphene quantum dots supported on three-dimensional graphene for supercapacitors. Nanomaterials (Basel) 2019; 9(2):201. doi: 10.3390/nano9020201 [Crossref] [ Google Scholar]
- Kumar K, Srivastav S, Sharanagat VS. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: a review. UltrasonSonochem 2021; 70:105325. doi: 10.1016/j.ultsonch.2020.105325 [Crossref] [ Google Scholar]
- He J, Li Z, Zhao R, Lu Y, Shi L, Liu J. Aqueous synthesis of amphiphilic graphene quantum dots and their application as surfactants for preparing of fluorescent polymer microspheres. Colloids Surf A PhysicochemEng Asp 2019; 563:77-83. doi: 10.1016/j.colsurfa.2018.11.064 [Crossref] [ Google Scholar]
- Li Y, Li S, Wang Y, Wang J, Liu H, Liu X. Electrochemical synthesis of phosphorus-doped graphene quantum dots for free radical scavenging. Phys Chem Chem Phys 2017; 19(18):11631-8. doi: 10.1039/c6cp06377b [Crossref] [ Google Scholar]
- Deka MJ, Chowdhury D. CVD assisted hydrophobic graphene quantum dots: fluorescence sensor for aromatic amino acids. ChemistrySelect 2017; 2(5):1999-2005. doi: 10.1002/slct.201601737 [Crossref] [ Google Scholar]
- Huang K, Lu W, Yu X, Jin C, Yang D. Highly pure and luminescent graphene quantum dots on silicon directly grown by chemical vapor deposition. Particle & Particle Systems Characterization 2016; 33(1):8-14. doi: 10.1002/ppsc.201500132 [Crossref] [ Google Scholar]
- Lee J, Kim K, Park WI, Kim BH, Park JH, Kim TH. Uniform graphene quantum dots patterned from self-assembled silica nanodots. Nano Lett 2012; 12(12):6078-83. doi: 10.1021/nl302520m [Crossref] [ Google Scholar]
- Kang SH, Mhin S, Han H, Kim KM, Jones JL, Ryu JH. Ultrafast method for selective design of graphene quantum dots with highly efficient blue emission. Sci Rep 2016; 6:38423. doi: 10.1038/srep38423 [Crossref] [ Google Scholar]
- Russo P, Liang R, Jabari E, Marzbanrad E, Toyserkani E, Zhou YN. Single-step synthesis of graphene quantum dots by femtosecond laser ablation of graphene oxide dispersions. Nanoscale 2016; 8(16):8863-77. doi: 10.1039/c6nr01148a [Crossref] [ Google Scholar]
- Santiago SR, Lin TN, Yuan CT, Shen JL, Huang HY, Lin CA. Origin of tunable photoluminescence from graphene quantum dots synthesized via pulsed laser ablation. Phys Chem Chem Phys 2016; 18(32):22599-605. doi: 10.1039/c6cp03159e [Crossref] [ Google Scholar]
- Calabro RL, Yang DS, Kim DY. Liquid-phase laser ablation synthesis of graphene quantum dots from carbon nano-onions: comparison with chemical oxidation. J Colloid Interface Sci 2018; 527:132-40. doi: 10.1016/j.jcis.2018.04.113 [Crossref] [ Google Scholar]
- Chen W, Lv G, Hu W, Li D, Chen S, Dai Z. Synthesis and applications of graphene quantum dots: a review. Nanotechnol Rev 2018; 7(2):157-85. doi: 10.1515/ntrev-2017-0199 [Crossref] [ Google Scholar]
- Khayal A, Dawane V, Amin MA, Tirth V, Yadav VK, Algahtani A. Advances in the methods for the synthesis of carbon dots and their emerging applications. Polymers (Basel) 2021; 13(18):3190. doi: 10.3390/polym13183190 [Crossref] [ Google Scholar]
- Jung H, Sapner VS, Adhikari A, Sathe BR, Patel R. Recent progress on carbon quantum dots based photocatalysis. Front Chem 2022; 10:881495. doi: 10.3389/fchem.2022.881495 [Crossref] [ Google Scholar]
- Kundu S, Pillai VK. Synthesis and characterization of graphene quantum dots. Phys Sci Rev 2020; 5(4):20190013. doi: 10.1515/psr-2019-0013 [Crossref] [ Google Scholar]
- Ohta T, Bostwick A, Seyller T, Horn K, Rotenberg E. Controlling the electronic structure of bilayer graphene. Science 2006; 313(5789):951-4. doi: 10.1126/science.1130681 [Crossref] [ Google Scholar]
- Valencia AM, Caldas MJ. Single vacancy defect in graphene: insights into its magnetic properties from theoretical modeling. Phys Rev B 2017; 96(12):125431. doi: 10.1103/PhysRevB.96.125431 [Crossref] [ Google Scholar]
- Li L, Wu G, Yang G, Peng J, Zhao J, Zhu JJ. Focusing on luminescent graphene quantum dots: current status and future perspectives. Nanoscale 2013; 5(10):4015-39. doi: 10.1039/c3nr33849e [Crossref] [ Google Scholar]
- Lee MW, Kim J, Suh JS. Characteristics of graphene quantum dots determined by edge structures: three kinds of dots fabricated using thermal plasma jet. RSC Adv 2015; 5(83):67669-75. doi: 10.1039/c5ra12223f [Crossref] [ Google Scholar]
- Zhu S, Song Y, Zhao X, Shao J, Zhang J, Yang B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res 2015; 8(2):355-81. doi: 10.1007/s12274-014-0644-3 [Crossref] [ Google Scholar]
- Eda G, Lin YY, Mattevi C, Yamaguchi H, Chen HA, Chen IS. Blue photoluminescence from chemically derived graphene oxide. Adv Mater 2010; 22(4):505-9. doi: 10.1002/adma.200901996 [Crossref] [ Google Scholar]
- Qian J, Wang D, Cai FH, Xi W, Peng L, Zhu ZF. Observation of multiphoton-induced fluorescence from graphene oxide nanoparticles and applications in in vivo functional bioimaging. Angew Chem Int Ed Engl 2012; 51(42):10570-5. doi: 10.1002/anie.201206107 [Crossref] [ Google Scholar]
- Chien CT, Li SS, Lai WJ, Yeh YC, Chen HA, Chen IS. Tunable photoluminescence from graphene oxide. Angew Chem Int Ed Engl 2012; 51(27):6662-6. doi: 10.1002/anie.201200474 [Crossref] [ Google Scholar]
- Luo Z, Vora PM, Mele EJ, Johnson ATC, Kikkawa JM. Photoluminescence and band gap modulation in graphene oxide. Appl Phys Lett 2009; 94(11):111909. doi: 10.1063/1.3098358 [Crossref] [ Google Scholar]
- Zhu S, Tang S, Zhang J, Yang B. Control the size and surface chemistry of graphene for the rising fluorescent materials. Chem Commun (Camb) 2012; 48(38):4527-39. doi: 10.1039/c2cc31201h [Crossref] [ Google Scholar]
- Song SH, Jang MH, Jeong JM, Yoon H, Cho YH, Jeong WI. Primary hepatocyte imaging by multiphoton luminescent graphene quantum dots. Chem Commun (Camb) 2015; 51(38):8041-3. doi: 10.1039/c5cc01801c [Crossref] [ Google Scholar]
- Shao T, Wang G, An X, Zhuo S, Xia Y, Zhu C. A reformative oxidation strategy using high concentration nitric acid for enhancing the emission performance of graphene quantum dots. RSC Adv 2014; 4(89):47977-81. doi: 10.1039/c4ra06935h [Crossref] [ Google Scholar]
- Abdullah-Al-Nahain Abdullah-Al-Nahain, Lee JE, In I, Lee H, Lee KD, Jeong JH. Target delivery and cell imaging using hyaluronic acid-functionalized graphene quantum dots. Mol Pharm 2013; 10(10):3736-44. doi: 10.1021/mp400219u [Crossref] [ Google Scholar]
- Nurunnabi M, Khatun Z, Huh KM, Park SY, Lee DY, Cho KJ. In vivo biodistribution and toxicology of carboxylated graphene quantum dots. ACS Nano 2013; 7(8):6858-67. doi: 10.1021/nn402043c [Crossref] [ Google Scholar]
- Fasbender S, Allani S, Wimmenauer C, Cadeddu RP, Raba K, Fischer JC. Uptake dynamics of graphene quantum dots into primary human blood cells following in vitro exposure. RSC Adv 2017; 7(20):12208-16. doi: 10.1039/c6ra27829a [Crossref] [ Google Scholar]
- Chung S, Revia RA, Zhang M. Graphene quantum dots and their applications in bioimaging, biosensing, and therapy. Adv Mater 2021; 33(22):e1904362. doi: 10.1002/adma.201904362 [Crossref] [ Google Scholar]
- Sun H, Wu L, Gao N, Ren J, Qu X. Improvement of photoluminescence of graphene quantum dots with a biocompatible photochemical reduction pathway and its bioimaging application. ACS Appl Mater Interfaces 2013; 5(3):1174-9. doi: 10.1021/am3030849 [Crossref] [ Google Scholar]
- Li B, Wang Y, Huang L, Qu H, Han Z, Wang Y. Review of performance improvement strategies for doped graphene quantum dots for fluorescence-based sensing. Synth Met 2021; 276:116758. doi: 10.1016/j.synthmet.2021.116758 [Crossref] [ Google Scholar]
- Wang Y, Shao Y, Matson DW, Li J, Lin Y. Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano 2010; 4(4):1790-8. doi: 10.1021/nn100315s [Crossref] [ Google Scholar]
- Sohal N, Maity B, Basu S. Recent advances in heteroatom-doped graphene quantum dots for sensing applications. RSC Adv 2021; 11(41):25586-615. doi: 10.1039/d1ra04248c [Crossref] [ Google Scholar]
- Du F, Sun L, Zen Q, Tan W, Cheng Z, Ruan G. A highly sensitive and selective “on-off-on” fluorescent sensor based on nitrogen doped graphene quantum dots for the detection of Hg2 + and paraquat. Sens Actuators B Chem 2019; 288:96-103. doi: 10.1016/j.snb.2019.02.109 [Crossref] [ Google Scholar]
- Bian S, Shen C, Hua H, Zhou L, Zhu H, Xi F. One-pot synthesis of sulfur-doped graphene quantum dots as a novel fluorescent probe for highly selective and sensitive detection of lead(ii). RSC Adv 2016; 6(74):69977-83. doi: 10.1039/c6ra10836a [Crossref] [ Google Scholar]
- CRC Handbook of Chemistry and Physics: A Ready-Reference of Chemical and Physical Data, 85th ed Edited by David R. Lide (National Institute of Standards and Technology). CRC Press LLC: Boca Raton, FL. 2004. J Am Chem Soc 2005;127(12):4542. 10.1021/ja041017a
- Gao H, Liu Z, Song L, Guo W, Gao W, Ci L. Synthesis of S-doped graphene by liquid precursor. Nanotechnology 2012; 23(27):275605. doi: 10.1088/0957-4484/23/27/275605 [Crossref] [ Google Scholar]
- Gliniak J, Lin JH, Chen YT, Li CR, Jokar E, Chang CH. Sulfur-doped graphene oxide quantum dots as photocatalysts for hydrogen generation in the aqueous phase. ChemSusChem 2017; 10(16):3260-7. doi: 10.1002/cssc.201700910 [Crossref] [ Google Scholar]
- Kadian S, Manik G. Sulfur doped graphene quantum dots as a potential sensitive fluorescent probe for the detection of quercetin. Food Chem 2020; 317:126457. doi: 10.1016/j.foodchem.2020.126457 [Crossref] [ Google Scholar]
- Zhou J, Zhou H, Tang J, Deng S, Yan F, Li W. Carbon dots doped with heteroatoms for fluorescent bioimaging: a review. Microchim Acta 2017; 184(2):343-68. doi: 10.1007/s00604-016-2043-9 [Crossref] [ Google Scholar]
- Ge S, He J, Ma C, Liu J, Xi F, Dong X. One-step synthesis of boron-doped graphene quantum dots for fluorescent sensors and biosensor. Talanta 2019; 199:581-9. doi: 10.1016/j.talanta.2019.02.098 [Crossref] [ Google Scholar]
- Conte C, Moret F, Esposito D, Dal Poggetto G, Avitabile C, Ungaro F. Biodegradable nanoparticles exposing a short anti-FLT1 peptide as antiangiogenic platform to complement docetaxel anticancer activity. Mater Sci Eng C Mater Biol Appl 2019; 102:876-86. doi: 10.1016/j.msec.2019.04.054 [Crossref] [ Google Scholar]
- Patra JK, Das G, Fraceto LF, Ramos Campos EV, Rodriguez-Torres MD, Acosta-Torres LS. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology 2018; 16(1):71. doi: 10.1186/s12951-018-0392-8 [Crossref] [ Google Scholar]
- Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci 2020; 7:193. doi: 10.3389/fmolb.2020.00193 [Crossref] [ Google Scholar]
- Frieler M, Pho C, Lee BH, Dobrovolny H, Akkaraju GR, Naumov AV. Effects of doxorubicin delivery by nitrogen-doped graphene quantum dots on cancer cell growth: experimental study and mathematical modeling. Nanomaterials (Basel) 2021; 11(1):140. doi: 10.3390/nano11010140 [Crossref] [ Google Scholar]
- Ma Z, Xu Y, Li P, Cheng D, Zhu X, Liu M. Self-catalyzed surface reaction-induced fluorescence resonance energy transfer on cysteine-stabilized MnO2 quantum dots for selective detection of dopamine. Anal Chem 2021; 93(7):3586-93. doi: 10.1021/acs.analchem.0c05102 [Crossref] [ Google Scholar]
- Liu YY, Yu NY, Fang WD, Tan QG, Ji R, Yang LY. Photodegradation of carbon dots cause cytotoxicity. Nat Commun 2021; 12(1):812. doi: 10.1038/s41467-021-21080-z [Crossref] [ Google Scholar]
- Zhang W, Dang G, Dong J, Li Y, Jiao P, Yang M. A multifunctional nanoplatform based on graphitic carbon nitride quantum dots for imaging-guided and tumor-targeted chemo-photodynamic combination therapy. Colloids Surf B Biointerfaces 2021; 199:111549. doi: 10.1016/j.colsurfb.2020.111549 [Crossref] [ Google Scholar]
- Yang X, Shang C, Zhou S, Zhao J. MBenes: emerging 2D materials as efficient electrocatalysts for the nitrogen reduction reaction. Nanoscale Horiz 2020; 5(7):1106-15. doi: 10.1039/d0nh00242a [Crossref] [ Google Scholar]
- Samimi S, Shafiee Ardestani M, Abedin Dorkoosh F. Preparation of carbon quantum dots- quinic acid for drug delivery of gemcitabine to breast cancer cells. J Drug Deliv Sci Technol 2021; 61:102287. doi: 10.1016/j.jddst.2020.102287 [Crossref] [ Google Scholar]
- Han M, Gao X, Su JZ, Nie S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat Biotechnol 2001; 19(7):631-5. doi: 10.1038/90228 [Crossref] [ Google Scholar]
- Wu H, Shi H, Wang Y, Jia X, Tang C, Zhang J. Hyaluronic acid conjugated graphene oxide for targeted drug delivery. Carbon 2014; 69:379-89. doi: 10.1016/j.carbon.2013.12.039 [Crossref] [ Google Scholar]
- Tao J, Feng S, Liu B, Pan J, Li C, Zheng Y. Hyaluronic acid conjugated nitrogen-doped graphene quantum dots for identification of human breast cancer cells. Biomed Mater 2021; 16(5):055001. doi: 10.1088/1748-605X/ac0d93 [Crossref] [ Google Scholar]