Advanced pharmaceutical bulletin. 14(3):504-512.
doi: 10.34172/apb.2024.023
Editorial
Ready to Eat Food: A Reason for Enhancement in Multidrug Resistance in Humans
Sheetal Negi Writing – original draft, Writing – review & editing, 1 
Sarika Sharma Conceptualization, Data curation, Formal analysis, Supervision, Validation, Visualization, 2, * 
Author information:
1Department of Microbiology, Lovely Professional University Phagwara (Punjab), India.
2Department of Sponsored Research, Division of Research & Development, Lovely Professional University Phagwara (Punjab), India.
Abstract
The increasing trend of consuming ready-to-eat (RTE) food has become a global phenomenon, and this has raised concerns about the potential negative impacts on human health. Recent studies have shown a correlation between the consumption of RTE foods and the expansion of multidrug resistance (MDR) in humans. MDR is a significant challenge in the effective theory of infectious diseases, as it limits the effectiveness of antibiotics and other drugs used in therapy. Consumption of RTE food contribute to the development of MDR in humans. Additionally, there are potential risks of consuming RTE food contaminated with antibiotic-resistant bacteria, which can cause severe health consequences. The article highlights the need for awareness campaigns on the potential hazard related to the ingestion of RTE food and the importance of responsible and safe food production practices. It also recommends the need for regulatory bodies to establish strict guidelines for the production and distribution of RTE food to ensure that they are free from harmful contaminants and that their consumption does not lead to the development of MDR in humans. Overall, this article provides a comprehensive analysis of the potential negative impacts of RTE food consumption on human health and emphasizes the need for a more cautious approach to food consumption to protect public health.
Keywords: Ready-to-eat (RTE), Drug resistance, Food, Pathogens, Multi-drug resistance
Copyright and License Information
©2024 The Author (s).
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Funding Statement
There is no funding.
Introduction
Ready-to-eat (RTE) food has become increasingly popular in recent years and become an integral part of our busy and fast-paced lifestyles. These foods are readily available and require little to no preparation before consumption.1 Examples of RTE foods include sandwiches, salads, pre-cooked meals, and packaged snacks. These foods are often pre-cooked or prepared and require minimal preparation before consumption.2 While RTE foods may be convenient, they have also been associated with the beginning and spreading of multi-drug resistant (MDR) bacteria in humans. Consumption of RTE foods has been known as an important hazard cause for the transmission of MDR bacteria to humans.3
MDR bacteria are bacteria that have developed resistance to multiple types of antibiotics. These bacteria pose a significant public health threat, as they are difficult to treat and can cause intense and sometimes serious infections. The emergence and spread of MDR bacteria are due to various factors, including the large-scale use of antibiotics in agriculture and food production, the global movement of people and food products, and the overuse and misuse of antibiotics in human medicine.4
RTE foods can be a source of MDR bacteria in several ways. First, the processing and handling of RTE foods can lead to the contamination of these foods with MDR bacteria.5 Secondly, the utilization of antibacterial drug in agriculture and food production can contribute to the development of MDR bacteria, which can then contaminate RTE foods. Finally, the consumption of RTE foods that have been contaminated with MDR bacteria can lead to the transportation of these bacteria to humans.
The utilization of antibiotics in agriculture and food production has been linked to the beginning of MDR bacteria in food products (Figure 1), including RTE foods.6 The more use of antibiotics in animal husbandry and agriculture has led to the development of antibiotic-resistant bacteria, which can contaminate food products and cause infections in humans. Additionally, the processing and handling of RTE foods can also lead to the transmission of MDR bacteria.7
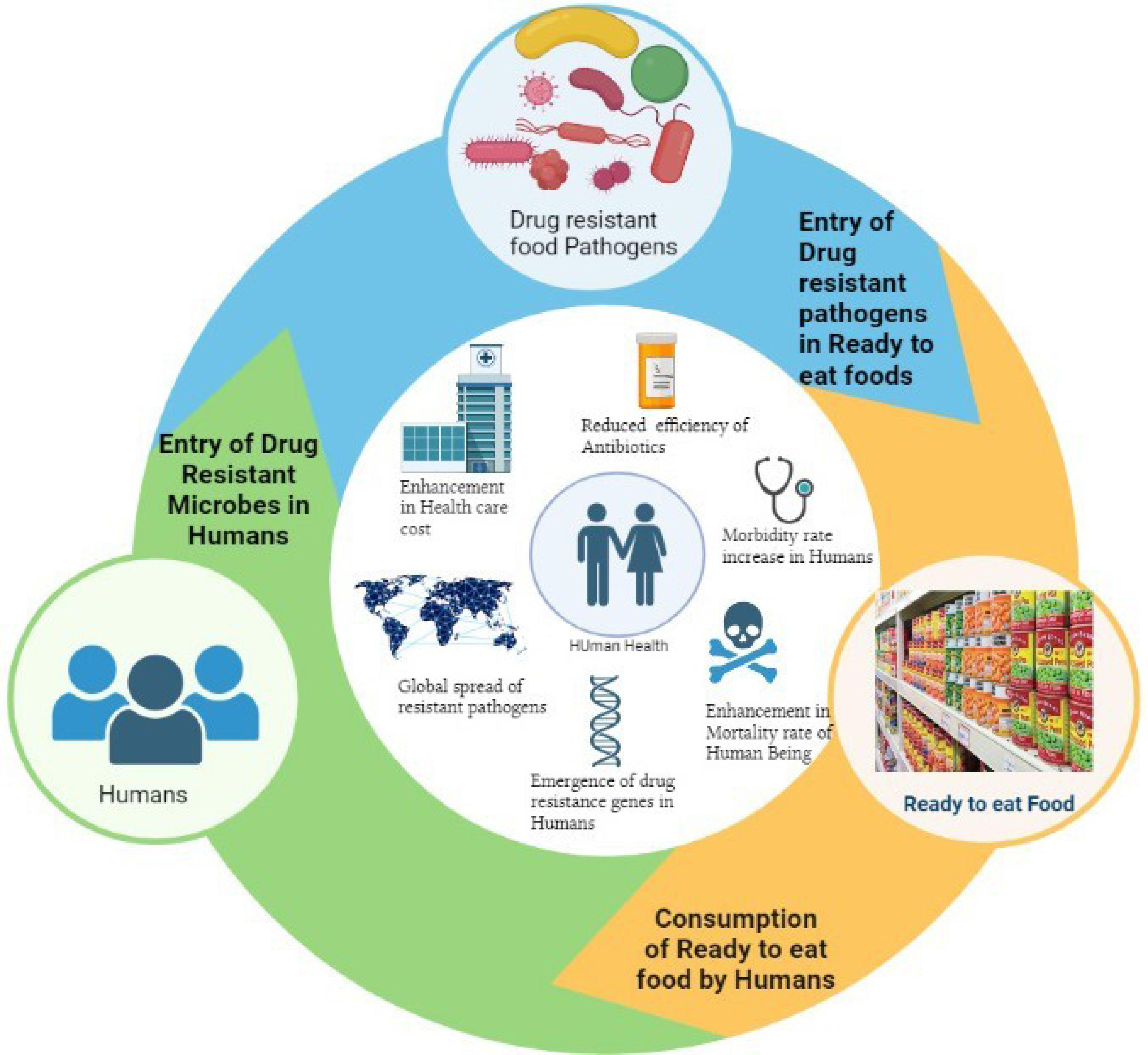
Figure 1.
Entry of drug resistance food pathogens from food to human and its effects on human health
.
Entry of drug resistance food pathogens from food to human and its effects on human health
Several types of MDR bacteria have been associated with RTE foods, including methicillin-resistant Staphylococcus aureus (MRSA), Salmonella, and Escherichia coli. MRSA is a type of bacteria that is resistant to many types of antibiotics and can lead to soft tissue infections, skin infections, pneumonia, and bloodstream infections.4 Salmonella is a type of bacteria that can cause food poisoning, with symptoms including diarrhea, fever, and sepsis in humans.8 E. coli is a type of bacteria that can cause severe gastrointestinal illness, with symptoms ranging from mild to severe diarrhea, abdominal pain, and fever.9
The link between RTE foods and MDR bacteria is a significant public health concern. The emergence and dissemination of MDR bacteria are challenging to control, and the evolution of new antibiotics is slow and costly. Therefore, it is crucial to take steps to reduce the consumption of RTE foods and to implement measures to prevent the transmission of MDR bacteria in food products.4
Ready to eat food
RTE foods are convenient and popular due to their minimal preparation requirements and short cooking times. They cater to busy lifestyles and are especially favored during the COVID-19 pandemic.10 RTE foods include items like frozen snacks and bakery products.11 RTE foods, such as RTE cereal, are known for their nutritional adequacy and are particularly beneficial for children and adolescents. Studies show that consuming RTE cereal is associated with better nutrient intake.12 Shelf life is a key factor contributing to the popularity of RTE foods, especially in regions like Asia, Europe, and America. Brown rice, rich in dietary fibers and nutrients, has a shorter shelf life and longer cooking time compared to white rice. However, the development of RTE brown rice products overcomes these limitations.13 RTE fish is preserved in chilling and freezing conditions, maintaining its quality for extended periods.14 In metros and urban areas, RTE food is a trend, catering to individuals with hectic schedules who lack the time to prepare meals. RTE cereals save time and are a healthy breakfast option.15 RTE frozen fruits and vegetables retain their nutritional value and can be easily transported.16 Additionally, RTE foods are cost-effective. Despite their convenience, RTE foods have drawbacks. They often contain added ingredients like preservatives, artificial colors, sugar, and fats, leading to health issues such as obesity, type 2 diabetes, and heart disease.17 High trans-fat content in ultra-processed foods can increase the risk of cardiovascular diseases.18 RTE vegetables can absorb harmful chemicals, posing risks like bladder cancer and reproductive problems.19 Excessive fluoride in RTE infant foods can lead to dental fluorosis.20
RTE raw fish, like sushi and sashimi, is consumed for its nutritional quality but carries biological and chemical hazards. Contaminants in fish tissue can impact human health.21 RTE salad dishes are susceptible to contamination from handling, dirty water, and cross-contamination between cooked and raw ingredients.22 Ingesting RTE salads and sprouts can lead to foodborne illnesses, including non-tuberculosis mycobacteria (NTM) infections in immunocompromised individuals. NTM can be present in soil, water, and fertilizers and are transmitted to fresh fruits and vegetables.23 RTE foods can also be a reservoir of antibiotic-resistant bacteria, which can transfer antibiotic-resistant genes to the bacteria in the human gut. This poses health risks as these antibiotic-resistant genes can affect human health.24
Pathogens associated with RTE food
RTE foods become most appropriate medium for the growth of pathogenic microorganisms which causes threat to public health. According to World Health Organization (WHO) there are more than 200 diseases spread by foods.25 Some major food pathogens are:
Listeria monocytogenes
A Gram-positive pathogenic bacteria called Listeria monocytogenes causes listeriosis which is a food borne illness. This microbe is prevalent in RTE meat because of the poor hygienic conditions, exposure of the meat during sale and storage conditions of meat.26 Listeriosis mainly affect the people whose immunity is weak i.e., immunocompromised patient, old people, pregnant women and neonates. Listeria mostly affect the pregnant women. During the infection pregnant women suffer from mild febrile illness. Maternal listeriosis can passed onto the fetus where it causes neonatal listeriosis which has a rate of high mortality of 25%-30% depending upon the gestational age.27
H. pylori
Helicobacter pylori is a foodborne pathogen present in RTE food like hamburger, minced beef, RTE tuna meat etc. Various samples contained H. pylori like hamburger (1.42%) and in minced beef (12.5%).28 TheDNA of H. pylori was found in 36% raw chicken and 44% in RTE raw tuna meat samples.29 Furthermore 550 samples of RTE foods, out of 74% of samples detecting H. pylori are; restaurant salad (30%), olive salad (36%), soup (22%) and fruit salad (28%) these samples were contaminated with H. pylori.30 H. pylori was also detected in 60 out of 300 RTE food samples like hamburger (8.33%), meat sandwiches (10%), RTE fish (15%), vegetable sandwiches (18%), minced meat (32%) and chicken sandwiches (5%).31 There are more prevalence of H. pylori in RTE foods due to the contamination in hygiene and also during the handling of RTE food, preparing and packing of RTE food so, the H. pylori when transmitted to humans can cause disease in humans such as chronic disorders of upper gastrointestinal tract, gastric ulcer disease, gastric adenocarcinoma, low grade B cell MALtoma ( Mucosa associated lymphoid tissue lymphoma of the stomach) etc. it can also cause iron deficiency anemia, autoimmune thyroid disease, rosacea, thrombocytopenic purpura, idiopathic urticarial and extra-gastric disorders like coronary heart diseases. 80% of population in developing countries including young children are H. pylori positive whereas in industrialized countries the presence of H. pylori remains 40% and present in high numbers in adults and old people rather than young children and adolescents.32
Salmonella enterica
Salmonella enterica is a food borne pathogen and found in RTE meats. 300 samples of RTE meat was tested that includes beef, chicken, mutton, chevon, pork, guinea fowl. The street RTE meat are contaminated in many ways by poor handling of meat or not washing hands, do’ not drink, smoke or eat while selling the meat it increases the risk of contamination.33 Salmonella enterica strains are a common reason for concern in the field of food safety as they are the primary culprit in outbreaks of bacterial food poisoning around the world.34 Sushi and sashimi, two well-known RTE foods, have been linked to salmonellosis epidemics.35 The importance of RTE salad vegetables in the human diet is rising. Inadequate cleaning during processing, however, can result in some foodborne infections, such salmonellosis, because they are eaten raw. In the study on foodborne disease outbreaks, Salmonella spp. were the pathogens most commonly reported (41.0%) which was published by the Public Health Laboratory Service (PHLS) Communicable Disease Surveillance Centre (CDSC) during 1992-2000. 83 (5.5%) of the 1518 foodborne outbreaks of infectious intestinal illness were related to the eating of fruit or salad vegetables.36
Klebsiella pneumonia
Klebsiella pneumonia is a food borne pathogen present in RTE meat [Luncheon-meat] and it causes diarrhea, septicemia in humans and the infection caused by K. pneumonia in humans is known as nosocomial infections.37 Klebsiella also responsible for pneumonia, lower biliary, urinary tract disease, blood stream infections, pyrogenic liver abscess, meningitis and intra-abdominal infections in humans.38 350 meat samples were collected Mansoura city, Egypt from which 44 (12.6%) K. pneumonia species were isolated from RTE meat samples.37
Aeromonas spp
Aeromonas sppis found in RTE seafood products and seafood products are at the highest risk for foodborne outbreaks and it is because people are more interested towards RTE seafood in industrialized countries. Aeromonas bacteria is an aquatic bacterium and is a human pathogen due to presence in all types of food but especially in seafood as it causes spoilage. Some of the Aeromonas spp can grow unrepressed in food during the course of refrigeration under modified packaging atmosphere, NaCl and pH concentration. Most clinical strains come from a subset of four species that are more frequently linked to infections in humans. Aeromonas caviae, Aeromonas dhakensis, Aeromonas veronii biovar sobria, and Aeromonas hydrophila causes acute gastroenteritis, potentially fatal infections like septicemia, and meningitis.39
Escherichia coli
Escherichia coli belongs to a family of Enterobacteriaceaea foodborne pathogen in RTE meat and food that are sold in street causes food borne illnesses in humans by producing Shiga toxins and causing illness and deaths. Presence of E. coli in RTE meats include eight samples of chicken (16%), three samples of pork (6%), ten samples of chevon (20%), four samples of beef (8%), nine sample of guinea fowl (18%) and four samples of mutton (8%) were contaminated by E. coli.40 A study conducted in Egypt showed the presence of E. coli in total 90 samples of RTE sandwiches of meat and chicken in which 15 of each samples were taken from both meat and chicken, from meat shawarma (33.3%), kofta (40%) and hawawshi (46.7%) and from chicken shiesh tawook (26.7%), panee (33.3%, shawerma (33.3%).41 A study was conducted in Himachal Pradesh in which 265 RTE milk and milk-related products altogether were gathered to check the presence of E. coli. 65 samples of burfi collected from which four (6.15%) were positive for E. coli, 54 samples of paneer from which four (7.40%) were positive, 42 samples of cream roll and 12 of curd from which no E. coli was detected, 31 samples of milk tea out of which three were positive, 47 samples of ice cream out of which one was positive and 14 samples of pasteurized milk out of which one was positive for E. coli strain. Several strains of E. coli can lead to hemolytic uremic syndrome and diarrhea.42
Bacillus cereus
Bacillus cereus has been screened from RTE foods. B. cereus can result in food poisoning at relatively low concentrations, if a food contains higher amount of B. cereus than 103 than it is considered unsafe for consumption.43 860 RTE food samples were taken from China which include rice/noodle, cooked meat and cold vegetables dishes in sauce. From all these samples 302 samples were positive for Bacillus, including 59 of 119 the rice/noodles samples (50%), 19 out of the 85 cold vegetable dishes in sauce samples (22%) and 224 out of the 656 cooked meat samples (34%) so, this indicate that RTE foods are harmful for consumption as it causes severe food safety problems.44
Staphylococcus aureus
Staphylococcus aureus is found in RTE items such as sushi and sashimi which causes food poisoning and Salmonellosis outbreak to the people who consume it. These RTE food are prepared with bare hands so level of contamination with foodborne pathogens is high in these foods. In a study, 200 sample of sushi and 51 of sashimi was taken out of which Staphylococcus was detected in 26%.35
Drug resistance in RTE food pathogens
Drug resistance in RTE food pathogens is a growing concern in the field of food safety. RTE foods are those that do not require cooking before consumption, such as deli meats, salads, and fresh fruits. Pathogens such as Salmonella, Listeria, and E. coli can contaminate these foods during production, processing, and packaging. Utilizing antibiotics in agriculture and animal husbandry has been identified as a major contributor to the emergence of drug-resistant forms of these diseases. When these drug-resistant pathogens infect humans, they are challenging to treat with conventional antibiotics, raising the danger of serious illness and death. Drug resistance limit the use of drugs and hinder the treatment process for the patients. Drug resistance is mainly associated with the changes in the gene like gene amplification, site mutations and deletions. Resistance against any drug involve decreasing the effectiveness of the drug as the drug was administered frequently so the pathogen developed resistance against that drug and hence the effectiveness of the drug is decreased.45 The mechanism of drug resistance includes drug target modification, active drug efflux, drug uptake restriction, and drug inactivation.46 The antibiotic resistant pathogen can also transfer their resistance genes to other microbes and humans via food chain. RTE products are potential vehicle for spreading antibiotic-resistant pathogenic bacteria. There are more chances of survival and growth of microorganisms in RTE foods because of handling and processing of food in unhygienic conditions and no requirement of.47 It has been reported globally that antibiotic resistant bacteria cause death of 700 000 people and the mortality rate increases to 10 million by the year 2050.48 Antibiotic resistance in Listeria monocytogenes also found in RTE Foods in Turkey. Salmonella one of the food-borne diseases that carries a significant danger to human health and has a remarkable global reach are resistant to multiple drugs.49 S. aureus species are frequent human and animal infections that were first identified as penicillin-resistant in 1948. These resistant microorganisms are crucial for dairy products.50 Birds and mammals’ intestinal tracts frequently harbor enterococcispp., which are recognized as sign of enteric contamination in food. These pathogens can withstand unfavorable conditions like saline waters, low or high pH, and temperature, which shows that resistant enterococcican play a significant part in the expansion of diseases in the community. Additionally, resistantenterococcimay indirectly cause harm by passing on their resistance genes to strains that have been adapted to live in humans.51
Table 1 summarizes the list of major drug resistant food pathogens from various ready to eat food items.
Table 1.
List of food pathogens resistant to drugs
|
Name of the pathogen
|
Drug resistance
|
References
|
|
Salmonella enterica
|
Nalidixic acid, neomycin, sulfonamides, tetracycline (TET) |
36
|
|
Listeria monocytogenes
|
TET, trimethoprim-sulfamethoxazole, ampicillin (AMP), erythromycin, penicillin G (PEN), TET |
52,53
|
| Staphylococcus aureus |
TET, cefoxitin, gentamicin, amikacin, vancomycin, erythromycin, ciprofloxacin, trimethoprim-sulfamethoxazole, chloramphenicol, cefoperazone, penicillin, erythromycin, clindamycin, azithromycin, trimethoprim/sulfamethoxazole |
54,55
|
|
Yersinia enterocolitica
|
AMC, cephalothin, amoxycillin, TET, imipenem, gentamycin, piperacillin, amikacin, aztreonam, ciprofloxacin |
56
|
|
Escherichia coli
|
AMC, ciprofloxacin, chloramphenicol, cotrimoxazole, imipenem, ertapenem, cefepime, cefoperazone sulbactam, gentamicin, amikacin, ciprofloxacin |
57,58
|
|
Bacillus cereus
|
Cefixime (CFM), PEN, amoxicillin/clavulanic acid (AMC), ceftazidime (CAZ), AMP, ceftriaxone (CTR), cefotaxime, clindamycin |
59
|
|
Klebsiella pneumoniae
|
AMP, cefepime, cefuroxime, cefotaxime, meropenem, ciprofloxacin, gentamicin |
58
|
|
Helicobacter pylori
|
Amoxicillin, AMP, metronidazole, TET |
30
|
|
Aeromonas hydrophila
|
Erythromycin, AMP, AMC, azithromycin, gentamicin, streptomycin, sulfisoxazole, TET trimethoprim/sulfamethoxazole |
60
|
Genes associated with drug resistance in RTE food pathogens
Genes associated with drug resistance in RTE food pathogens are of great concern due to the potential for serious health consequences. These genes encode proteins that confer resistance to antibiotics and other drugs, making infections caused by these pathogens more difficult to treat. Commonly identified genes linked to drug resistance in RTE food pathogens include those for tetracycline, fluoroquinolones, and aminoglycosides. These genes can be present in the bacterial genome or acquired through horizontal gene transfer from other bacteria. The spread of drug-resistant RTE food microorganisms is a significant public health issue, emphasizing the importance of food safety measures and antibiotic stewardship in both human and animal populations. In one study, antibiotic resistance genes were found in bacterial strains from RTE foods, including chloramphenicol resistance genes in S. maltophilia and Pseudomonas spp., gentamycin resistance genes in Acinetobacter spp. and Pseudomonas spp., and tetracycline resistance genes in Enterobacteriaceae spp. Additionally, various other resistance genes like ESBLs, OXA, carbapenemases, and plasmid-mediated AmpC genes were detected.61
In 244 RTE foods, 267 S. aureus strains were isolated from various sources, with different antimicrobial resistance genes detected, including beta-lactamase gene blaZ, iblaI, methicillin resistance gene mecA, vancomycin resistance gene vanB, macrolide resistance gene msr (A), tetracycline resistance genes tet (K) and tet (M), chloramphenicol resistance gene cat, macrolide/clindamycin resistance gene remA /C, tobramycin resistance gene aaaD, and metallothiol transferase resistance gene fosB. These genes were detected using DNA microarray systems.62
Salmonella contamination was found in 81 RTE salads and vegetables, with resistance to sulfonamide antibiotics due to sul1, sul2, and sul3 resistance genes, detected via polymerase chain reaction.36
Listeria monocytogenes was isolated from various RTE foods, and the antibiotic resistance genes fosX, norB, mprF, and lin were found, conferring resistance to antibiotics such as Fosfomycin, quinolones, and lincosamides. tetA and tetC genes provided resistance to tetracycline antibiotics, and the resistance genes had different mechanisms.63 In RTE meat products, including hamburger and raw kebab, E. coli, S. aureus, L. monocytogenes, and Salmonella spp. were detected, with resistance genes like blaSHV, blaZ, blaTEM, and mecA identified using PCR.47
Dairy products made from raw or bovine milk contained pathogens like S. aureus, L. monocytogenes, Salmonella spp., and E. coli, with antibiotic resistance genes like blaTEM, blaSHV, blaZ, and mecA identified in foodborne bacteria isolated from bovine milk.64 The prevalence of these genes varied in different pathogens. Resistance gene subtypes were also mentioned, with different genes identified in RTE meat, vegetables, and fruit. For example, catA1 was the most abundant ARG in RTE meat, followed by bacA, tetM and tetA were also present in RTE meat. In RTE vegetables, genes like mexF, mexB, mexW, acrB, acrA, emrD, and tolC were found, and in RTE fruit, genes like acrB, acrA, emrD, tolC, mdtH, mdfA, bacA, and Bcr were identified.65
Effects of drug resistance food pathogen in RTE food
Drug resistance occurs when various microorganisms like bacteria, fungi, viruses etc. adapt themselves in the presence of any drug or antibiotics and thus causes serious threats to public health. The effect of drug resistance in human health includes increase mortality rate among humans, increase morbidity rate, increased risk of transmission and dissemination, as well as higher healthcare costs, reduced efficacy of related antibiotics used in humans.66
The resistant bacteria against drugs or antibiotics will increases the serious health issues and increase the chances of death among humans. According to a global status because of the drug resistant infections each year 700 000 people lose their lives.48 In an CDC (Center for Disease Control and Prevention) study mortality rate data among two countries i.e., United States and Europe were compared from which the number of affected people from drug resistance were 2 million approx. in both the countries and the number of mortality rate in the United States was 23 000 and in Europe. If no effective measure will be taken against drug resistance, it was estimated that about 10 million people will die by 2050 globally.66 According to a European study, because of drug resistance, more than 33 000 people die in Europe each year. Drug resistant infections also increases the morbidity rate among humans and prolonged stays in hospital which are infectious and spread their infections to other people.67 It also increases the healthcare costs among humans as the patients need isolated beds, prolonged hospitalization, ICUs, additional antibiotics, more diagnostic test to prevent the spread of infection. In the United States for treating the person with drug resistant infection, the hospital bill adds about $1400 and it goes upto more than $2 billion every year this was according to CDC data.66 This healthcare cost increases globally by 2050 from $300 billion to $1 trillion annually. Apart from serious health issue caused by drug resistant infection in humans, it also has global economic impact. Due to global economic impact, it was estimated that by 2050, 7% of GDP will be lost and because of this it is more difficult for developing countries to overcome this situation. And if actions have not taken there is an adverse impact on economy worldwide.68 According to CDC, in US the cost of drug resistance infection is $55 billion from which $20 billion for healthcare and $35 billion for lost productivity. A study showed that global GDP decrease to 1% and by 2050 the GDP decreased to 5-7%. Among pathogenic bacteria there is a antibiotic resistance is growing which was a major risk to the public’s health.66
Methods to regulate drug resistance in RTE food
In the context of RTE foods, their convenience makes them high-risk for foodborne outbreaks if mishandled or stored at improper temperatures, promoting bacterial growth. To ensure the safety of RTE foods, it is essential to regulate bacteria concentrations.69 Drug resistance in RTE foods can be mitigated through Good Manufacturing Practices (GMPs), which encompass proper handling, storage, cleaning, disinfection, and hygiene practices in food production facilities.70
Additionally, preventing contamination of raw materials, especially those originating from animals or prone to cross-contamination by animal feces, requires various preventive controls.71 These include ensuring suppliers adhere to appropriate agricultural practices, utilizing kill-step methods like irradiation and pasteurization, and conducting environmental sampling to eliminate pathogens in the post-processing environment.72
Furthermore, implementing Good Agricultural Practices (GAP) in animal and plant production, as well as adopting Hazard Analysis and Critical Control Points (HACCP), can play vital roles in ensuring food safety throughout the production, distribution, and consumption of RTE foods.72 Regulations limiting antibiotic use in animal agriculture, particularly in RTE foods, can also reduce the emergence of antibiotic-resistant bacteria.
Consumers’ preference for antibiotic-free meat, along with other measures like improved animal housing, immunization, and hygiene, can help decrease antibiotic usage in animals. The direct or indirect transmission of antibiotic-resistant illnesses to humans through food consumption emphasizes the need to prohibit the use of antibiotics for growth promotion and enforce rules for their therapeutic administration in animals. Employing immunizations, herbal medicines, enzyme preparations, and other techniques can reduce antibiotic use while enhancing animal health.73
To control drug resistance in RTE foods, it is crucial to identify potential sources of contamination, implement remedial actions, and conduct testing for antibiotic-resistant bacteria. Educational programs targeting both consumers and food workers can promote proper food safety procedures.74 Moreover, alternatives to antibiotics, such as natural antimicrobials and probiotics, can be employed to limit bacterial growth and reduce the risk of drug resistance in RTE foods.51
Conclusion
In conclusion, the uptake of RTE food has become a component of our daily routine, especially for those who have busy lifestyles. However, the convenience of such food comes at a cost. Studies have shown that the regular consumption of these foods is contributing to the increase in multidrug resistance in humans. The use of antibiotics in the production of these foods, along with poor hygiene practices during their preparation, has led to the appearance of resistant bacteria, which is a serious public health concern. It is imperative that we take measures to reduce the consumption of RTE food and promote healthy eating habits. This can be achieved by increasing awareness about the risks associated with the consumption of such food and encouraging people to adopt healthy food choices. Additionally, proper regulation of the use of antibiotics in food production, along with good hygiene practices during food preparation, can go a long way in reducing the spread of antibiotic-resistant bacteria. Overall, the issue of multidrug resistance in humans because of the uptake of RTE food is a complex problem that requires a multifaceted solution. The public, the food industry, and the government all have a role to play in addressing this issue and ensuring that we can continue to enjoy convenient food options while safeguarding our health.
Future prospects
The demand for frozen food is increasing as the RTE food market expands because it takes less time to prepare, requires little to no heat, can be consumed at any time, such as during breakfast, lunch, or dinner, and has a longer shelf life, making it simple to store for future use. This is because there are more people working in developed countries and because their lives are busier and more hectic. When kept at room temperature in a sealed container, frozen RTE food is shelf-stable and is prepared for consumption. Because it is more convenient for people to shop for groceries online these days, customers bought RTE food online because it is simple to choose and there is no need to go outside to get food. The RTE market will expand more in the future as a result of expanding urbanization and people’s growing knowledge of the advantages of frozen meals. Since artificial preservatives can hurt our bodies, consumers want RTE foods that are lightly processed and have a longer shelf life without losing quality food is in such high demand that the market’s growth curve will incline upward. More quality RTE food items, such meals inspired by different cuisines, are already available on the market as the population’s preference for RTE food grows.
Competing Interests
None.
Ethical Approval
Not applicable.
References
- Patel D, Rathod R. Ready to eat food perception, food preferences and food choice: a theoretical discussion. World Wide J Multidiscip Res Dev 2017; 3(8):198-205. [ Google Scholar]
- Kokkinakis MN, Fragkiadakis GA, Lapidakis NE, Kokkinaki AN. Assessing microbiological quality of ready-to-eat prepacked sandwiches, in Crete, Greece. J Food Sci Technol 2020; 57(11):4220-7. doi: 10.1007/s13197-020-04460-z [Crossref] [ Google Scholar]
- Rodriguez MB, Junior CA, Carneiro CS, Franco RM, Mano SB. The effect of carbon dioxide on the shelf life of ready-to-eat shredded chicken breast stored under refrigeration. Poult Sci 2014; 93(1):194-9. doi: 10.3382/ps.2013-03045 [Crossref] [ Google Scholar]
- van Duin D, Paterson DL. Multidrug-resistant bacteria in the community: trends and lessons learned. Infect Dis Clin North Am 2016; 30(2):377-90. doi: 10.1016/j.idc.2016.02.004 [Crossref] [ Google Scholar]
- Makinde OM, Adetunji MC, Ezeokoli OT, Odumosu BT, Ngoma L, Mwanza M. Bacterial contaminants and their antibiotic susceptibility patterns in ready-to-eat foods vended in Ogun state, Nigeria. Lett Appl Microbiol 2021; 72(2):187-95. doi: 10.1111/lam.13407 [Crossref] [ Google Scholar]
- Manyi-Loh C, Mamphweli S, Meyer E, Okoh A. Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules 2018; 23(4):795. doi: 10.3390/molecules23040795 [Crossref] [ Google Scholar]
- Economou V, Gousia P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect Drug Resist 2015; 8:49-61. doi: 10.2147/idr.s55778 [Crossref] [ Google Scholar]
- Zha L, Garrett S, Sun J. Salmonella infection in chronic inflammation and gastrointestinal cancer. Diseases 2019; 7(1):28. doi: 10.3390/diseases7010028 [Crossref] [ Google Scholar]
- Yang SC, Lin CH, Aljuffali IA, Fang JY. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch Microbiol 2017; 199(6):811-25. doi: 10.1007/s00203-017-1393-y [Crossref] [ Google Scholar]
- Rahman N, Ishitsuka K, Piedvache A, Tanaka H, Murayama N, Morisaki N. Convenience food options and adequacy of nutrient intake among school children during the COVID-19 pandemic. Nutrients 2022; 14(3):630. doi: 10.3390/nu14030630 [Crossref] [ Google Scholar]
- Temgire S, Borah A, Kumthekar S, Idate A. Recent trends in ready to eat/cook food products. Pharma Innov J 2021; 10(5):211-7. doi: 10.22271/tpi.2021.v10.i5c.6207 [Crossref] [ Google Scholar]
- Zhu Y, Jain N, Normington J, Holschuh N, Sanders LM. Ready-to-eat cereal is an affordable breakfast option associated with better nutrient intake and diet quality in the US population. Front Nutr 2022; 9:1088080. doi: 10.3389/fnut.2022.1088080 [Crossref] [ Google Scholar]
- Federici E, Gentilucci V, Bernini V, Vittadini E, Pellegrini N. Ready to eat shelf-stable brown rice in pouches: effect of moisture content on product’s quality and stability. Eur Food Res Technol 2021; 247(11):2677-85. doi: 10.1007/s00217-021-03790-2 [Crossref] [ Google Scholar]
- Tavares J, Martins A, Fidalgo LG, Lima V, Amaral RA, Pinto CA. Fresh fish degradation and advances in preservation using physical emerging technologies. Foods 2021; 10(4):780. doi: 10.3390/foods10040780 [Crossref] [ Google Scholar]
- Navale SA, Swami SB, Thakor NJ. Extrusion cooking technology for foods: a review. J Ready Eat Food 2015; 2(3):66-80. [ Google Scholar]
- Storey M, Anderson P. Total fruit and vegetable consumption increases among consumers of frozen fruit and vegetables. Nutrition 2018; 46:115-21. doi: 10.1016/j.nut.2017.08.013 [Crossref] [ Google Scholar]
- Dziedzinska R, Makovcova J, Kaevska M, Slany M, Babak V, Moravkova M. Nontuberculous mycobacteria on ready-to-eat, raw and frozen fruits and vegetables. J Food Prot 2016; 79(8):1452-6. doi: 10.4315/0362-028x.jfp-16-030 [Crossref] [ Google Scholar]
- Juul F, Vaidean G, Parekh N. Ultra-processed foods and cardiovascular diseases: potential mechanisms of action. Adv Nutr 2021; 12(5):1673-80. doi: 10.1093/advances/nmab049 [Crossref] [ Google Scholar]
- Coroneo V, Carraro V, Marras B, Marrucci A, Succa S, Meloni B. Presence of trihalomethanes in ready-to-eat vegetables disinfected with chlorine. Food AdditContam Part A Chem Anal Control Expo Risk Assess 2017; 34(12):2111-7. doi: 10.1080/19440049.2017.1382723 [Crossref] [ Google Scholar]
- Chandio N, John JR, Floyd S, Gibson E, Wong DK, Levy SM. Fluoride content of ready-to-eat infant foods and drinks in Australia. Int J Environ Res Public Health 2022; 19(21):14087. doi: 10.3390/ijerph192114087 [Crossref] [ Google Scholar]
- Lehel J, Yaucat-Guendi R, Darnay L, Palotás P, Laczay P. Possible food safety hazards of ready-to-eat raw fish containing product (sushi, sashimi). Crit Rev Food Sci Nutr 2021; 61(5):867-88. doi: 10.1080/10408398.2020.1749024 [Crossref] [ Google Scholar]
- Chau ML, Aung KT, Hapuarachchi HC, Lee PS, Lim PY, Kang JS. Microbial survey of ready-to-eat salad ingredients sold at retail reveals the occurrence and the persistence of Listeria monocytogenes sequence types 2 and 87 in pre-packed smoked salmon. BMC Microbiol 2017; 17(1):46. doi: 10.1186/s12866-017-0956-z [Crossref] [ Google Scholar]
- Cerna-Cortes JF, Leon-Montes N, Cortes-Cueto AL, Salas-Rangel LP, Helguera-Repetto AC, Lopez-Hernandez D. Microbiological quality of ready-to-eat vegetables collected in Mexico City: occurrence of aerobic-mesophilic bacteria, fecal coliforms, and potentially pathogenic nontuberculous mycobacteria. Biomed Res Int 2015; 2015:789508. doi: 10.1155/2015/789508 [Crossref] [ Google Scholar]
- Habboush Y, Guzman N. Antibiotic resistance. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024.
- Bintsis T. Foodborne pathogens. AIMS Microbiol 2017; 3(3):529-63. doi: 10.3934/microbiol.2017.3.529 [Crossref] [ Google Scholar]
- Henriques AR, Cristino JM, Fraqueza MJ. Genetic characterization of Listeria monocytogenes isolates from industrial and retail ready-to-eat meat-based foods and their relationship with clinical strains from human listeriosis in Portugal. J Food Prot 2017; 80(4):551-60. doi: 10.4315/0362-028x.jfp-16-310 [Crossref] [ Google Scholar]
- Madjunkov M, Chaudhry S, Ito S. Listeriosis during pregnancy. Arch GynecolObstet 2017; 296(2):143-52. doi: 10.1007/s00404-017-4401-1 [Crossref] [ Google Scholar]
- Gilani A, Razavilar V, Rokni N, Rahimi E. VacA and cagA genotypes status and antimicrobial resistance properties of Helicobacter pylori strains isolated from meat products in Isfahan province, Iran. Iran J Vet Res 2017; 18(2):97-102. [ Google Scholar]
- Meng X, Zhang H, Law J, Tsang R, Tsang T. Detection of Helicobacter pylori from food sources by a novel multiplex PCR assay. J Food Saf 2008; 28(4):609-19. doi: 10.1111/j.1745-4565.2008.00135.x [Crossref] [ Google Scholar]
- Hemmatinezhad B, Momtaz H, Rahimi E. VacA, cagA, iceA and oipA genotypes status and antimicrobial resistance properties of Helicobacter pylori isolated from various types of ready to eat foods. Ann Clin Microbiol Antimicrob 2016; 15:2. doi: 10.1186/s12941-015-0115-z [Crossref] [ Google Scholar]
- Ghorbani F, Gheisari E, Dehkordi FS. Genotyping of vacA alleles of Helicobacter pylori strains recovered from some Iranian food items. Trop J Pharm Res 2016; 15(8):1631-6. doi: 10.4314/tjpr.v15i8.5 [Crossref] [ Google Scholar]
- Quaglia NC, Dambrosio A. Helicobacter pylori: a foodborne pathogen?. World J Gastroenterol 2018; 24(31):3472-87. doi: 10.3748/wjg.v24.i31.3472 [Crossref] [ Google Scholar]
- Aduah M, Adzitey F, Amoako DG, Abia AL, Ekli R, Teye GA. Not all street food is bad: low prevalence of antibiotic-resistant Salmonella enterica in ready-to-eat (RTE) meats in Ghana is associated with good vendors’ knowledge of meat safety. Foods 2021; 10(5):1011. doi: 10.3390/foods10051011 [Crossref] [ Google Scholar]
- Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B. Correction: World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med 2015; 12(12):e1001940. doi: 10.1371/journal.pmed.1001940 [Crossref] [ Google Scholar]
- Suat MP, Kek HC, Jin Ai MA. Prevalence of Staphylococcus aureus and Salmonella enterica in ready-to-eat sushi and sashimi. Trop Biomed 2017; 34(1):45-51. [ Google Scholar]
- Taban BM, Aytac SA, Akkoc N, Akcelik M. Characterization of antibiotic resistance in Salmonella enterica isolates determined from ready-to-eat (RTE) salad vegetables. Braz J Microbiol 2013; 44(2):385-91. doi: 10.1590/s1517-83822013005000047 [Crossref] [ Google Scholar]
- Abdel-Rhman SH. Characterization of β-lactam resistance in K pneumoniae associated with ready-to-eat processed meat in Egypt. PLoS One 2020; 15(9):e0238747. doi: 10.1371/journal.pone.0238747 [Crossref] [ Google Scholar]
- Zhang S, Yang G, Ye Q, Wu Q, Zhang J, Huang Y. Phenotypic and genotypic characterization of Klebsiella pneumoniae isolated from retail foods in China. Front Microbiol 2018; 9:289. doi: 10.3389/fmicb.2018.00289 [Crossref] [ Google Scholar]
- Hoel S, Vadstein O, Jakobsen AN. The significance of mesophilic Aeromonas spp in minimally processed ready-to-eat seafood. Microorganisms 2019; 7(3):91. doi: 10.3390/microorganisms7030091 [Crossref] [ Google Scholar]
- Abass A, Adzitey F, Huda N. Escherichia coli of ready-to-eat (RTE) meats origin showed resistance to antibiotics used by farmers. Antibiotics (Basel) 2020; 9(12):869. doi: 10.3390/antibiotics9120869 [Crossref] [ Google Scholar]
- Hassanin FS, Reham AA, Shawky NA, Gomaa WM. Incidence of Escherichia coli and Salmonella in ready to eat foods. Benha Vet Med J 2014; 27(1):84-91. [ Google Scholar]
- Ahmadi SA, Panda AK, Shalmali Shalmali, Kumar Y, Brahmne HG. Prevalence of Escherichia coli and Salmonella spp in ready-to-eat meat and meat products in Himachal Pradesh. J Commun Dis 2012; 44(2):71-7. [ Google Scholar]
- Granum PE, Lund T. Bacillus cereus and its food poisoning toxins. FEMS Microbiol Lett 1997; 157(2):223-8. doi: 10.1111/j.1574-6968.1997.tb12776.x [Crossref] [ Google Scholar]
- Yu S, Yu P, Wang J, Li C, Guo H, Liu C. A study on prevalence and characterization of Bacillus cereus in ready-to-eat foods in China. Front Microbiol 2019; 10:3043. doi: 10.3389/fmicb.2019.03043 [Crossref] [ Google Scholar]
- Leidner F, Kurt Yilmaz N, Schiffer CA. Deciphering complex mechanisms of resistance and loss of potency through coupled molecular dynamics and machine learning. J Chem Theory Comput 2021; 17(4):2054-64. doi: 10.1021/acs.jctc.0c01244 [Crossref] [ Google Scholar]
- Reygaert WC. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol 2018; 4(3):482-501. doi: 10.3934/microbiol.2018.3.482 [Crossref] [ Google Scholar]
- Rajaei M, Moosavy MH, Nouri Gharajalar S, Khatibi SA. Antibiotic resistance in the pathogenic foodborne bacteria isolated from raw kebab and hamburger: phenotypic and genotypic study. BMC Microbiol 2021; 21(1):272. doi: 10.1186/s12866-021-02326-8 [Crossref] [ Google Scholar]
- Taneja N, Sharma M. Antimicrobial resistance in the environment: the Indian scenario. Indian J Med Res 2019; 149(2):119-28. doi: 10.4103/ijmr.IJMR_331_18 [Crossref] [ Google Scholar]
- Olsen SJ, Ying M, Davis MF, Deasy M, Holland B, Iampietro L. Multidrug-resistant Salmonella typhimurium infection from milk contaminated after pasteurization. Emerg Infect Dis 2004; 10(5):932-5. doi: 10.3201/eid1005.030484 [Crossref] [ Google Scholar]
- Huttner A, Harbarth S, Carlet J, Cosgrove S, Goossens H, Holmes A. Antimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob Resist Infect Control 2013; 2:31. doi: 10.1186/2047-2994-2-31 [Crossref] [ Google Scholar]
- Hashempour-Baltork F, Hosseini H, Shojaee-Aliabadi S, Torbati M, Alizadeh AM, Alizadeh M. Drug resistance and the prevention strategies in food borne bacteria: an update review. Adv Pharm Bull 2019; 9(3):335-47. doi: 10.15171/apb.2019.041 [Crossref] [ Google Scholar]
- Yan SF, Wang W, Bai L, Hu YJ, Dong YP, Xu J. Antimicrobial resistance, virulence profile, and molecular characterization of Listeria monocytogenes isolated from ready-to-eat food in China, 2013-2014. Biomed Environ Sci 2016; 29(6):448-52. doi: 10.3967/bes2016.058 [Crossref] [ Google Scholar]
- de Vasconcelos Byrne V, Hofer E, Vallim DC, de Castro Almeida RC. Occurrence and antimicrobial resistance patterns of Listeria monocytogenes isolated from vegetables. Braz J Microbiol 2016; 47(2):438-43. doi: 10.1016/j.bjm.2015.11.033 [Crossref] [ Google Scholar]
- Puah SM, Chua KH, Tan JA. Virulence factors and antibiotic susceptibility of Staphylococcus aureus isolates in ready-to-eat foods: detection of S aureus contamination and a high prevalence of virulence genes. Int J Environ Res Public Health 2016; 13(2):199. doi: 10.3390/ijerph13020199 [Crossref] [ Google Scholar]
- Luo K, Shao F, Kamara KN, Chen S, Zhang R, Duan G. Molecular characteristics of antimicrobial resistance and virulence determinants of Staphylococcus aureus isolates derived from clinical infection and food. J Clin Lab Anal 2018; 32(7):e22456. doi: 10.1002/jcla.22456 [Crossref] [ Google Scholar]
- Seakamela EM, Diseko L, Malatji D, Makhado L, Motau M, Jambwa K. Characterisation and antibiotic resistance of Yersinia enterocolitica from various meat categories, South Africa. Onderstepoort J Vet Res 2022; 89(1):e1-e11. doi: 10.4102/ojvr.v89i1.2006 [Crossref] [ Google Scholar]
- Sapkota S, Adhikari S, Pandey A, Khadka S, Adhikari M, Kandel H. Multi-drug resistant extended-spectrum beta-lactamase producing E coli and Salmonella on raw vegetable salads served at hotels and restaurants in Bharatpur, Nepal. BMC Res Notes 2019; 12(1):516. doi: 10.1186/s13104-019-4557-9 [Crossref] [ Google Scholar]
- Giri S, Kudva V, Shetty K, Shetty V. Prevalence and characterization of extended-spectrum β-lactamase-producing antibiotic-resistant Escherichia coli and Klebsiella pneumoniae in ready-to-eat street foods. Antibiotics (Basel) 2021; 10(7):850. doi: 10.3390/antibiotics10070850 [Crossref] [ Google Scholar]
- Rana N, Panda AK, Pathak N, Gupta T, Thakur SD. Bacillus cereus: public health burden associated with ready-to-eat foods in Himachal Pradesh, India. J Food Sci Technol 2020; 57(6):2293-302. doi: 10.1007/s13197-020-04267-y [Crossref] [ Google Scholar]
- Poole TL, Schlosser WD, Anderson RC, Norman KN, Beier RC, Nisbet DJ. Whole-genome sequence of Aeromonas hydrophila CVM861 isolated from diarrhetic neonatal swine. Microorganisms 2020; 8(11):1648. doi: 10.3390/microorganisms8111648 [Crossref] [ Google Scholar]
- Lin L, Wang SF, Yang TY, Hung WC, Chan MY, Tseng SP. Antimicrobial resistance and genetic diversity in ceftazidime non-susceptible bacterial pathogens from ready-to-eat street foods in three Taiwanese cities. Sci Rep 2017; 7(1):15515. doi: 10.1038/s41598-017-15627-8 [Crossref] [ Google Scholar]
- Baumgartner A, Niederhauser I, Johler S. Virulence and resistance gene profiles of Staphylococcus aureus strains isolated from ready-to-eat foods. J Food Prot 2014; 77(7):1232-6. doi: 10.4315/0362-028x.jfp-14-027 [Crossref] [ Google Scholar]
- Parra-Flores J, Holý O, Bustamante F, Lepuschitz S, Pietzka A, Contreras-Fernández A. Virulence and antibiotic resistance genes in Listeria monocytogenes strains isolated from ready-to-eat foods in Chile. Front Microbiol 2021; 12:796040. doi: 10.3389/fmicb.2021.796040 [Crossref] [ Google Scholar]
- Hassani S, Moosavy MH, Nouri Gharajalar S, Khatibi SA, Hajibemani A, Barabadi Z. High prevalence of antibiotic resistance in pathogenic foodborne bacteria isolated from bovine milk. Sci Rep 2022; 12(1):3878. doi: 10.1038/s41598-022-07845-6 [Crossref] [ Google Scholar]
- Li Y, Cao W, Liang S, Yamasaki S, Chen X, Shi L. Metagenomic characterization of bacterial community and antibiotic resistance genes in representative ready-to-eat food in southern China. Sci Rep 2020; 10(1):15175. doi: 10.1038/s41598-020-72620-4 [Crossref] [ Google Scholar]
- Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist 2019; 12:3903-10. doi: 10.2147/idr.s234610 [Crossref] [ Google Scholar]
- Laxminarayan R, Bhutta Z, Duse A, Jenkins P, O’Brien T, Okeke IN, et al. Drug resistance. In: Disease Control Priorities in Developing Countries. 2nd ed. New York: Oxford University Press; 2006.
- Ansari MM, Kuche K, Ghadi R, Date T, Chaudhari D, Khan R, et al. Socioeconomic impact of antimicrobial resistance and their integrated mitigation by one health approach. In: Akhtar N, Singh KS, Prerna, Goyal D, eds. Emerging Modalities in Mitigation of Antimicrobial Resistance. Cham; 2022. p. 135-56. 10.1007/978-3-030-84126-3_7.
- Beshiru A, Okoh AI, Igbinosa EO. Processed ready-to-eat (RTE) foods sold in Yenagoa Nigeria were colonized by diarrheagenic Escherichia coli which constitute a probable hazard to human health. PLoS One 2022; 17(4):e0266059. doi: 10.1371/journal.pone.0266059 [Crossref] [ Google Scholar]
- Meghwal M. Good manufacturing practices for food processing industries: purposes, principles and practical applications. In: Meghwal M, Goyal MR, Kaneria MJ, eds. Food Technology: Applied Research and Production Techniques. USA: CRC Press, Apple Academic Press; 2016.
- Penakalapati G, Swarthout J, Delahoy MJ, McAliley L, Wodnik B, Levy K. Exposure to animal feces and human health: a systematic review and proposed research priorities. Environ Sci Technol 2017; 51(20):11537-52. doi: 10.1021/acs.est.7b02811 [Crossref] [ Google Scholar]
- Burange PS, Wargantiwar RK, Gonde AD, Rajendran TP. Good agricultural practices-relevance for crop health management. Ecol Environ Conserv 2015; 21(1):155-64. [ Google Scholar]
- Ma F, Xu S, Tang Z, Li Z, Zhang L. Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosaf Health 2021; 3(1):32-8. doi: 10.1016/j.bsheal.2020.09.004 [Crossref] [ Google Scholar]
- Fung F, Wang HS, Menon S. Food safety in the 21st century. Biomed J 2018; 41(2):88-95. doi: 10.1016/j.bj.2018.03.003 [Crossref] [ Google Scholar]