Advanced pharmaceutical bulletin. 15(1):7-10.
doi: 10.34172/apb.42806
Editorial
c-Abl Inhibitors in Parkinson’s: Exploring Hypotheses on Alpha-Synuclein Modulation
Jyutia Nargish Conceptualization, Writing – original draft, 
Hirok Jyoti Baishya Writing – original draft, 
Piyong Sola Supervision, Writing – review & editing, * 
Author information:
Department of Pharmacology, NETES Institute of Pharmaceutical Science, NEMCARE Group of Institutions, Shantipur, Mirza, Kamrup, Assam, India.
Abstract
Parkinson’s disease (PD) stands as the second most prevalent neurodegenerative disorder, impacting a global population estimated between 6 to 10 million individuals. The condition primarily arises from a dopamine deficiency and the presence of α-synuclein, forming Lewy bodies in the substantia nigra pars compacta (SNcp). Despite the ongoing quest to unravel the precise pathophysiological mechanisms underlying PD, recent literature reviews posit that heightened activation of the Abelson non-receptor tyrosine kinase(c-Abl), in brain tissues plays a pivotal role in neurodegeneration observed in PD patients. Notably, these studies put forth compelling evidence suggesting that c-Abl inhibitors’ interventions exhibit notable therapeutic potential. The potential benefits encompass enhancements in motor function, prevention of dopamine neuron loss, and the meticulous regulation of α-synuclein phosphorylation and clearance. These findings collectively advocate for the exploration of c-Abl as a prospective therapeutic target, thereby presenting inhibitors of this kinase as promising candidates for intervention in the management of PD.
Keywords: Parkinson’s disease, Neurodegeneration, c-Abl, α-synuclein, Phosphorylation
Copyright and License Information
© 2025 The Author (s).
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Funding Statement
Not applicable.
Introduction
Parkinson disease (PD) is a chronic, progressive condition that affects 1% of people over 60.1 Primarily PD characterized by the early and pronounced degeneration of dopaminergic cells in the substantia nigra pars compacta (SNpc), PD manifests as a neurological disorder, leading to a deficit of dopamine in the basal ganglia and resulting in a movement disorder akin to parkinsonism.2 The process of neurodegeneration in PD is concomitant with the occurrence of cytoplasmic inclusions, known as Lewy bodies (LBs), and neuritic inclusions termed Lewy neurites (LNs). These inclusions manifest in surviving dopaminergic neurons and other affected regions within the central nervous system (CNS).3 The precise mechanisms underlying the degeneration of dopaminergic neurons in the SNpc in PD remain elusive. Multiple factors, including protein misfolding and aggregation, compromised protein clearance pathways, cell-autonomous processes, and the phenomenon of “prion-like protein infection,” are implicated in the pathogenesis of PD. Additionally, potential contributors encompass mitochondrial damage, energy insufficiency, oxidative stress, and excitotoxicity, thereby emphasizing the multifaceted nature of processes that may contribute to the development of this neurological disorder.4
The manifestations of this condition can be categorized into two primary domains: motor features and non-motor symptoms. Motor features encompass a spectrum of clinical presentations, notably characterized by bradykinesia, gait disturbance, tremor, rigidity, and speech deficits. On the other hand, non-motor symptoms encompass a diverse array of manifestations, including but not limited to depression, hyposmia, cognitive impairment, sleep disorders, and constipation. This comprehensive classification facilitates a nuanced understanding of the multifaceted nature of the observed clinical presentations in individuals affected by the condition.5
c- Abl and tyrosine kinase
Kinases have emerged as a promising class of drug targets for PD therapy. Studies have highlighted the significant role of disrupted kinase activity and altered phosphorylation pathways in the development and progression of PD.6 The only other member of the Abl family of non-receptor tyrosine kinases is Arg (ABL2; Abl-related gene), which includes c-Abl (ABL1; Abelson tyrosine kinase). The c-Abl protein, with a molecular weight of approximately 120 kDa, is a member of the cytoplasmic tyrosine kinase family.7 Structurally c-Abl consist of N-terminal Src-homology domain 2 (SH2) and Src-homology domain (SH3).8 The c-Abl protein exhibits a high degree of evolutionary conservation across metazoan organisms and is characterized by ubiquitous expression within various subcellular compartments, including the cytoplasm, nucleus, mitochondria, and endoplasmic reticulum.7 The c-Abl protein demonstrates a broad spectrum of functional interactions, engaging with an array of cellular components such as cell signaling adaptors, kinases, and phosphatases.7,9 It has been demonstrated recently that SNCA induced neurodegeneration is mostly caused by dysregulated activation of c-Abl.10 Numerous stimuli, such as growth hormones, chemokines, oxidative stress, DNA damage, adhesion receptors, and microbial pathogens, closely control endogenous Abl kinases.11,12 Upon activation, Abl kinases control signaling pathways involved in cytoskeletal remodeling, which play a crucial role in cellular protrusion formation, cell migration, morphogenesis, adhesion, endocytosis, and phagocytosis.13 Through a number of pathways, such as the activation of parkin, the aggregation of α-synuclein (SNCA), the activation of microglia, and the activation of protein kinase C delta, the c-Abl tyrosine kinase contributes significantly to the pathophysiology of PD. As a result, the neuroprotective potential of c-Abl inhibitors in treating PD has been investigated in a number of preclinical investigations.14 Table 1 presents an overview of the procedures and results from these investigations.
Table 1.
Overview of the researches that looked into how c-Abl inhibition might protect animal models against PD
|
Animals
|
Intervention
|
Conclusion
|
References
|
Adult mice of the wild type with conditional neural knockout of c-Abl.
Age-matched mice of the wild type. |
At two-hour intervals, mice were given four intraperitoneal injections of either saline or MPTP (20 mg/kg). Seven days later, the c-Abl activity was measured use an antibody against p-tyrosine 245 c-Abl. Additionally, the levels of FBP1 and AIMP2 were measured by using certain antibodies to target them. |
One crucial posttranslational alteration that inhibits parkin's activity is its phosphorylation by c-Abl, which may be a factor in the causes of sporadic PD. Furthermore, PD may be treated by inhibiting c-Abl, which may have a neuroprotective effect. |
Ko et al, 201015 |
| Adult male wild type mice |
Alpha-synuclein or lentiviral c-Abl were stereotaxically injected into the substantia nigra of mice on both sides. Following three weeks of lentiviral injection c-Abl, half of the rats received dimethyl sulfoxide alone for an additional three weeks, while the other half received a daily dose of 10 mg/kg of nilotinib dissolved in dimethyl sulfoxide. Following animal sacrifice, brain tissues were prepared for neurochemical analysis. |
Alpha-synuclein particles are autophagically cleared by nilotinib in several brain regions, including the striatum, hippocampus, and the brain. |
Hebron et al, 201316 |
| Male C57Bl/6 mice aged 7-8 weeks. |
At two-hour intervals, mice were given four intraperitoneal injections of either saline or MPTP. They received either a vehicle or a nilotinib injection three days later. Mice were then tested for motor coordination and behavior. They subsequently got an intraperitoneal a lethal dosage of pentobarbital, and samples of their brains were prepared for neurochemical analysis. |
Nilotinib increases the effectiveness of dopaminergic signalling by preventing cyclin-dependent kinase 5 (Cdk5) from becoming phosphorylated.
Additionally, it works in concert with D1 agonists to boost the expression of the c-fos gene. When administered systemically, it normalizes aberrant motor activities brought on by MPTP. |
Tanabe et al, 201417 |
| Adult mice of the wild type with conditional neural knockout of c-Abl |
Mice were given four intraperitoneal injections at one-day intervals of 30 mg/kg imatinib, 5 mg/kg SB203580 (a particular inhibitor of the p38-mitogen activated protein kinase (MAPK) pathway), or saline (12 hours prior to and following the MPTP injection). After that, mice received four intraperitoneal injections of MPTP at a dose of 20 mg/kg. The animals were killed seven days following the final MPTP injection, and the striatum was ready for western blot examination. |
Cell death caused by oxidative stress is mediated by C-Abl and its substrate p38α.
Imatinib-induced c-Abl knockout reduced MPTP-induced dopaminergic loss of neurons. |
Wu et al, 201518 |
Hypotheses
c-Abl protein is involved in various biological processes, including the coordination of cellular growth and survival, modulation of integrin signaling, facilitation of actin polymerization, and the regulation of cell migration. From several literature reviews we found that c-Abl level is adjusted in postmortem striatum and autophosphorylation of c-Abl at y412 enhanced the catalytic activity in the substantia nigra and striatum of the PD patient. Phosphorylation of c-Abl protein increases in the SNCA overexpression and result in SNCA aggregation. Therefore, the inhibition of the c-Abl protein may prevent SNCA accumulation and can be useful in the management of PD.
Justification for the hypotheses
Pathologically aggregated conformations of SNCA constitute a prominent causative factor in the degeneration of dopamine neurons within the substantia nigra, contributing significantly to the observed pathological changes.19-21 Elevated c-Abl activity, quantified by the ratio of phosphorylated Y245 c-Abl to total c-Abl, is discernible in the postmortem brains of individuals afflicted with PD, particularly within the substantia nigra and striatum. In the human brain, SNCA is typically phosphorylated at Ser129 only to a limited extent under normal conditions.22,23 A significant buildup of pS129 SNCA is observed in transgenic animal models and the brains of individuals with PD. These results suggest that pS129 may represent the pathological or toxic form of the protein.22,24,25 The discovery that c-Abl mediates alternate phosphorylation sites on SNCA, namely at Tyr39 (pY39) and Tyr125 (pY125), raises the possibility that c-Abl is involved in the development of neurodegenerative disorders.10,26 These brain regions are known to be pathologically implicated in PD.10,15,27 The activation of c-Abl, as gauged by phosphorylation at the (Ser129) pS129 site, is likewise evident in postmortem brains of individuals diagnosed with PD.16,28-30 These observations suggest a discernible correlation between the activation of c-Abl and the pathological manifestations associated with PD.10 The kinase c-Abl operates within a rigorously regulated framework, with activation occurring in response to either oxidative or genotoxic stress. Moreover, the functional efficacy of this protein is contingent upon its precise subcellular localization.31-33 In a recent study by Hebron et al, compelling evidence was presented elucidating a bidirectional interplay between SNCA and c-Abl in vivo. Research indicates that c-Abl activation may be a critical factor in driving neurodegeneration, as c-Abl knockout or chemical inhibition has shown neuroprotective effects in animal models of PD. The findings delineate a reciprocal relationship, wherein elevated SNCA expression prompts the phosphorylation and subsequent activation of c-Abl. Conversely, an augmentation in c-Abl expression and activity leads to the accumulation of SNCA. These observations suggest that the inhibition of c-Abl may represent a promising therapeutic strategy for mitigating SNCA accumulation and safeguarding against SNCA-induced toxicity in the context of PD34 (Figure 1).
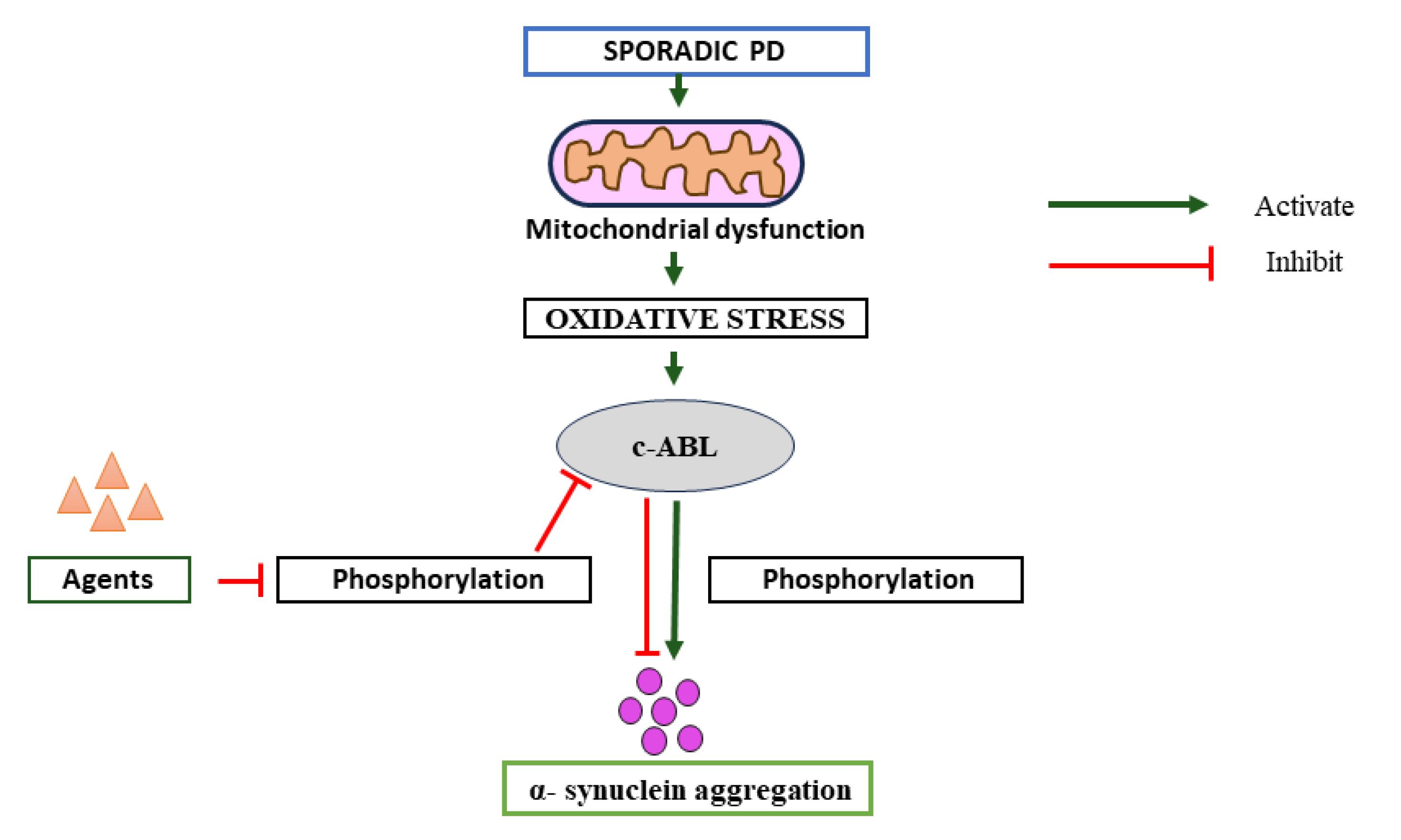
Figure 1.
Pathway of Parkinson’s disease; significance of c-ABL inhibitor on progression of disease
.
Pathway of Parkinson’s disease; significance of c-ABL inhibitor on progression of disease
Conclusion
c-Abl, a central orchestrator of critical cellular functions, undergoes meticulous regulation to maintain its basal activity. Interestingly, the aberrant activation of c-Abl is observed in postmortem brain tissue from PD patients, specifically within regions marked by pathological neurodegeneration. c-Abl activation, triggered by reactive oxygen species, links to cell death in PD, revealing its dual role in neurodegeneration and oxidative stress regulation. Evidence suggests c-Abl activation, not just SNCA accumulation, initiates neurodegeneration. Our hypothesis proposes intracellular pathogenic SNCA transformation via c-Abl activation triggers neurodegenerative cascade. Inhibiting c-Abl activation emerges as a potential disease-modifying strategy for PD.
Competing Interests
All the authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare that they have no conflict of interest.
Consent for Publication
Not applicable.
Ethical Approval
Not applicable.
Acknowledgements
I want to acknowledge the Department of Pharmacology, NETES Institute of Pharmaceutical Science, NEMCARE Group of Institutions, for providing the opportunities and facilities to carry out the work in the institution.
References
- Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA 2014; 311(16):1670-83. doi: 10.1001/jama.2014.3654 [Crossref] [ Google Scholar]
- Kalia LV, Lang AE. Parkinson’s disease. Lancet 2015; 386(9996):896-912. doi: 10.1016/s0140-6736(14)61393-3 [Crossref] [ Google Scholar]
- Recchia A, Debetto P, Negro A, Guidolin D, Skaper SD, Giusti P. Alpha-synuclein and Parkinson’s disease. Faseb j 2004; 18(6):617-26. doi: 10.1096/fj.03-0338rev [Crossref] [ Google Scholar]
- Maiti P, Manna J, Dunbar GL. Current understanding of the molecular mechanisms in Parkinson’s disease: targets for potential treatments. TranslNeurodegener 2017; 6:28. doi: 10.1186/s40035-017-0099-z [Crossref] [ Google Scholar]
- Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. TranslNeurodegener 2015; 4:19. doi: 10.1186/s40035-015-0042-0 [Crossref] [ Google Scholar]
- Mehdi SJ, Rosas-Hernandez H, Cuevas E, Lantz SM, Barger SW, Sarkar S. Protein kinases and Parkinson’s disease. Int J Mol Sci 2016; 17(9):1585. doi: 10.3390/ijms17091585 [Crossref] [ Google Scholar]
- Hantschel O, Superti-Furga G. Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat Rev Mol Cell Biol 2004; 5(1):33-44. doi: 10.1038/nrm1280 [Crossref] [ Google Scholar]
- Brahmachari S, Karuppagounder SS, Ge P, Lee S, Dawson VL, Dawson TM. c-Abl and Parkinson’s disease: mechanisms and therapeutic potential. J Parkinsons Dis 2017; 7(4):589-601. doi: 10.3233/jpd-171191 [Crossref] [ Google Scholar]
- Moresco EM, Donaldson S, Williamson A, Koleske AJ. Integrin-mediated dendrite branch maintenance requires Abelson (Abl) family kinases. J Neurosci 2005; 25(26):6105-18. doi: 10.1523/jneurosci.1432-05.2005 [Crossref] [ Google Scholar]
- Brahmachari S, Ge P, Lee SH, Kim D, Karuppagounder SS, Kumar M. Activation of tyrosine kinase c-Abl contributes to α-synuclein-induced neurodegeneration. J Clin Invest 2016; 126(8):2970-88. doi: 10.1172/jci85456 [Crossref] [ Google Scholar]
- Colicelli J. ABL tyrosine kinases: evolution of function, regulation, and specificity. Sci Signal 2010; 3(139):re6. doi: 10.1126/scisignal.3139re6 [Crossref] [ Google Scholar]
- Greuber EK, Smith-Pearson P, Wang J, Pendergast AM. Role of ABL family kinases in cancer: from leukaemia to solid tumours. Nat Rev Cancer 2013; 13(8):559-71. doi: 10.1038/nrc3563 [Crossref] [ Google Scholar]
- Bradley WD, Koleske AJ. Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. J Cell Sci 2009; 122(Pt 19):3441-54. doi: 10.1242/jcs.039859 [Crossref] [ Google Scholar]
- Abushouk AI, Negida A, Elshenawy RA, Zein H, Hammad AM, Menshawy A. c-Abl inhibition; a novel therapeutic target for Parkinson’s disease. CNS Neurol Disord Drug Targets 2018; 17(1):14-21. doi: 10.2174/1871527316666170602101538 [Crossref] [ Google Scholar]
- Ko HS, Lee Y, Shin JH, Karuppagounder SS, Gadad BS, Koleske AJ. Phosphorylation by the c-Abl protein tyrosine kinase inhibits parkin’s ubiquitination and protective function. Proc Natl Acad Sci U S A 2010; 107(38):16691-6. doi: 10.1073/pnas.1006083107 [Crossref] [ Google Scholar]
-
Correction to: nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of α-synuclein in Parkinson’s disease models. Hum Mol Genet 2023;32(1):172-6. doi: 10.1093/hmg/ddac274.
- Filipov NM, Seegal RF, Lawrence DA. Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B-dependent mechanism. Toxicol Sci 2005; 84(1):139-48. doi: 10.1093/toxsci/kfi055 [Crossref] [ Google Scholar]
- Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M. Comparison of kindreds with parkinsonism and α-synuclein genomic multiplications. Ann Neurol 2004; 55(2):174-9. doi: 10.1002/ana.10846 [Crossref] [ Google Scholar]
- Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol 2013; 9(1):13-24. doi: 10.1038/nrneurol.2012.242 [Crossref] [ Google Scholar]
- Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson’s disease. Neuron 2010; 66(5):646-61. doi: 10.1016/j.neuron.2010.04.034 [Crossref] [ Google Scholar]
- Maries E, Dass B, Collier TJ, Kordower JH, Steece-Collier K. The role of α-synuclein in Parkinson’s disease: insights from animal models. Nat Rev Neurosci 2003; 4(9):727-38. doi: 10.1038/nrn1199 [Crossref] [ Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS. Alpha-synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 2002; 4(2):160-4. doi: 10.1038/ncb748 [Crossref] [ Google Scholar]
- Muntané G, Ferrer I, Martinez-Vicente M. Alpha-synuclein phosphorylation and truncation are normal events in the adult human brain. Neuroscience 2012; 200:106-19. doi: 10.1016/j.neuroscience.2011.10.042 [Crossref] [ Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Haass C. Physiology and pathophysiology of α-synuclein Cell culture and transgenic animal models based on a Parkinson’s disease-associated protein. Ann N Y Acad Sci 2000; 920:33-41. doi: 10.1111/j.1749-6632.2000.tb06902.x [Crossref] [ Google Scholar]
- Okochi M, Walter J, Koyama A, Nakajo S, Baba M, Iwatsubo T. Constitutive phosphorylation of the Parkinson’s disease associated α-synuclein. J Biol Chem 2000; 275(1):390-7. doi: 10.1074/jbc.275.1.390 [Crossref] [ Google Scholar]
- Mahul-Mellier AL, Fauvet B, Gysbers A, Dikiy I, Oueslati A, Georgeon S. c-Abl phosphorylates α-synuclein and regulates its degradation: implication for α-synuclein clearance and contribution to the pathogenesis of Parkinson’s disease. Hum Mol Genet 2014; 23(11):2858-79. doi: 10.1093/hmg/ddt674 [Crossref] [ Google Scholar]
- Imam SZ, Zhou Q, Yamamoto A, Valente AJ, Ali SF, Bains M. Novel regulation of parkin function through c-Abl-mediated tyrosine phosphorylation: implications for Parkinson’s disease. J Neurosci 2011; 31(1):157-63. doi: 10.1523/jneurosci.1833-10.2011 [Crossref] [ Google Scholar]
- Lee S, Kim S, Park YJ, Yun SP, Kwon SH, Kim D. The c-Abl inhibitor, radotinib HCl, is neuroprotective in a preclinical Parkinson’s disease mouse model. Hum Mol Genet 2018; 27(13):2344-56. doi: 10.1093/hmg/ddy143 [Crossref] [ Google Scholar]
- Karuppagounder SS, Brahmachari S, Lee Y, Dawson VL, Dawson TM, Ko HS. The c-Abl inhibitor, nilotinib, protects dopaminergic neurons in a preclinical animal model of Parkinson’s disease. Sci Rep 2014; 4:4874. doi: 10.1038/srep04874 [Crossref] [ Google Scholar]
- Imam SZ, Trickler W, Kimura S, Binienda ZK, Paule MG, Slikker W Jr. Neuroprotective efficacy of a new brain-penetrating c-Abl inhibitor in a murine Parkinson’s disease model. PLoS One 2013; 8(5):e65129. doi: 10.1371/journal.pone.0065129 [Crossref] [ Google Scholar]
- Sun X, Wu F, Datta R, Kharbanda S, Kufe D. Interaction between protein kinase C delta and the c-Abl tyrosine kinase in the cellular response to oxidative stress. J Biol Chem 2000; 275(11):7470-3. doi: 10.1074/jbc.275.11.7470 [Crossref] [ Google Scholar]
- Stuart JR, Kawai H, Tsai KK, Chuang EY, Yuan ZM. c-Abl regulates early growth response protein (EGR1) in response to oxidative stress. Oncogene 2005; 24(55):8085-92. doi: 10.1038/sj.onc.1208953 [Crossref] [ Google Scholar]
- Yoshida K. Regulation for nuclear targeting of the Abl tyrosine kinase in response to DNA damage. Adv Exp Med Biol 2007; 604:155-65. doi: 10.1007/978-0-387-69116-9_15 [Crossref] [ Google Scholar]
- Hebron ML, Lonskaya I, Moussa CE. Tyrosine kinase inhibition facilitates autophagic SNCA/α-synuclein clearance. Autophagy 2013; 9(8):1249-50. doi: 10.4161/auto.25368 [Crossref] [ Google Scholar]