Advanced pharmaceutical bulletin. 14(3):513-523.
doi: 10.34172/apb.2024.061
Review Article
Nanomedicine Strategies Utilizing Lipid-Based Nanoparticles for Liver Cancer Therapy: Exploring Signaling Pathways and Therapeutic Modalities
Fereshteh Asgharzadeh Conceptualization, Investigation, Methodology, Resources, Writing – original draft, 1 
Maryam Moradi Binabaj Conceptualization, Investigation, Resources, Writing – original draft, 2
Sahar Fanoudi Conceptualization, Investigation, Writing – original draft, 3
William C. Cho Resources, Validation, 4
Yu-jeong Yang Visualization, 5
Maryam Azarian Formal analysis, Software, Writing – original draft, 6
Mehdi Shafiee Ardestani Validation, 7
Nasim Nasiri Visualization, 8
Marzieh Ramezani Farani Data curation, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing, 5, , # * 
Yun Suk Huh Funding acquisition, Supervision, Writing – review & editing, 5, , # * 
Author information:
1Metabolic Syndrome Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
2Department of Nutrition, Food Sciences and Clinical Biochemistry, School of Medicine, Social Determinants of Health Research Center, Gonabad University of Medical Science, Gonabad, Iran.
3Department of Basic Medical Sciences, Neyshabur University of Medical Sciences, Neyshabur, Iran.
4Department of Clinical Oncology, Queen Elizabeth Hospital, Kowloon, Hong Kong.
5NanoBio High-Tech Materials Research Center, Department of Biological Sciences and Bioengineering, Inha University, Incheon 22212, Republic of Korea.
6Department of Radiology, Charité - Universitätsmedizin Berlin, Berlin, Germany.
7Department of Radiopharmacy, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.
8Department of Cell and Molecular Sciences, Faculty of Biological Sciences, Kharazmi University, Tehran, Iran.
#These authors have equal contributions.
Abstract
Liver cancer, specifically hepatocellular carcinoma (HCC), is the second leading cause of cancer-related deaths, following pancreatic cancer. The 5-year overall survival rate for HCC remains relatively low. Currently, there are multiple treatment options available for HCC, including systemic drugs, minimally invasive local therapies such as radiofrequency ablation, transarterial chemoembolization (TACE), and arterial radioembolization (TARE), as well as surgical interventions like liver resection or transplantation. However, the effectiveness of drug delivery to the cancerous liver is hindered by pathophysiological changes in the organ. In order to address this challenge, lipid-based nanoparticles (LNPs) have emerged as promising platforms for delivering a diverse range of therapeutic drugs. LNPs offer various structural configurations that enhance their physical stability and enable them to accommodate different types of cargo with varying mechanical properties and degrees of hydrophobicity. In this article, we provide a comprehensive review of the current applications of LNPs in the development of anti-HCC therapies. By examining the existing research, we aim to shed light on the potential future directions and advancements in this field.
Keywords: Liver cancer, Hepatocellular carcinoma, Signaling pathways, Therapeutic approaches, Lipid-based nanoparticles, Nanomedicine
Copyright and License Information
©2024 The Author (s).
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Funding Statement
This work was supported by the South Korean Ministry of Science, ICT & Future Planning through the National Research Foundation (2022H1D3A2A02085952, 2022M3J7A1062940, and RS-2023-00240052).
Introduction
Liver cancer is projected to cause over one million deaths by 2030, according to the World Health Organization (WHO).1 The United States has observed a significant 43% increase in liver cancer mortality between 2000 and 2016.2 Hepatocellular carcinoma (HCC), the second leading cause of fatal malignancy after pancreatic cancer, has an overall 5-year survival rate of only 18%.3 In 2020, primary liver cancers (PLCs) were estimated to account for 830,180 cancer deaths and 905,677 new cases worldwide, ranking them as the third most common diagnosed diseases. The highest incidence rates were reported in Eastern and South-Eastern Asia, as well as Northern and Western Africa.4 HCC is the most prevalent type of PLC globally, accounting for nearly 90% of all liver cancer cases,5 while cholangiocarcinoma (CCA) is the second most common form. CCA is a highly lethal malignancy with a 5-year survival rate of less than 10%. It is classified into intrahepatic (iCCA), peripheral (pCCA), and distal (dCCA) subtypes based on anatomic location. iCCA is more commonly associated with hepatitis B virus (HBV)-related cirrhosis, while pCCA is closely related to primary sclerosing cholangitis presenting as chronic inflammation.6 Other rare liver cancers include juvenile hepatoblastoma, fibrolamellar HCC, and hepatocellular mixed cholangiocarcinoma (HCC-CCA).7,8
HCC and liver cancer rank as the third deadliest disease and the sixth most common diseases worldwide, according to the WHO.9,10 Liver cancer primarily affects men, with the highest incidence rates observed in East Asia, North Africa, and Micronesia. Mortality rates are particularly alarming in East Asia, North Africa, and Southeast Asia. More than 80% of HCC cases are attributable to PLCs.11 Inflammatory liver disorders such as alcoholism, nonalcoholic fatty liver disease, and viral hepatitis are common causes of HCC.12,13 Epigenetic changes and mutations play a role in the development of chronic liver disorders, including HCC, by activating molecular signaling cascades related to cell proliferation and inhibiting apoptosis.14 However, delivering drugs to the cancerous liver is challenging due to pathophysiological changes. While normal liver tissue relies on hepatic artery perfusion for blood supply, portal vein perfusion accounts for 80% of the liver’s oxygen supply.15 Systemic administration of drugs results in minimal drug penetration due to insufficient portal vein perfusion. HCC exhibits fibrosis-like behavior, characterized by reduced sinus fenestrae, the need for drugs to cross endothelial barriers, the extracellular matrix, and the tumor stromal barrier to reach the cells. This leads to increased angiogenesis, microvascular density, and permeability in the HCC tumor microenvironment.16
The enhanced permeability and retention (EPR) effect, coupled with impaired lymphatic drainage, can result in the selective accumulation of macromolecules and nanoparticles in HCC.17-19 Several cell proliferation-related receptors and proteins expressed on the surface of HCC cells can be targeted, including ASGPR,20 glypican-3,21 transferrin receptor,22 somatostatin receptor,23 glycyrrhetinic acid receptor,22 cluster of differentiation 44,24 and AF-20 antigen.25 Additionally, drugs can be targeted to increase tumor metabolic rate and production of lactic acid and glutathione (GSH).26 HCC is characterized by a low pH (pH 6.5) and a strong reducing potential (GSH: 2–10 mM) at the tumor site compared to the extracellular environment and normal tissues (pH 7.4). The pH or redox differential can be utilized to trigger the release of tumor-specific cargoes.27 Treatment options for HCC are determined based on staging after diagnosis (Figure 1).
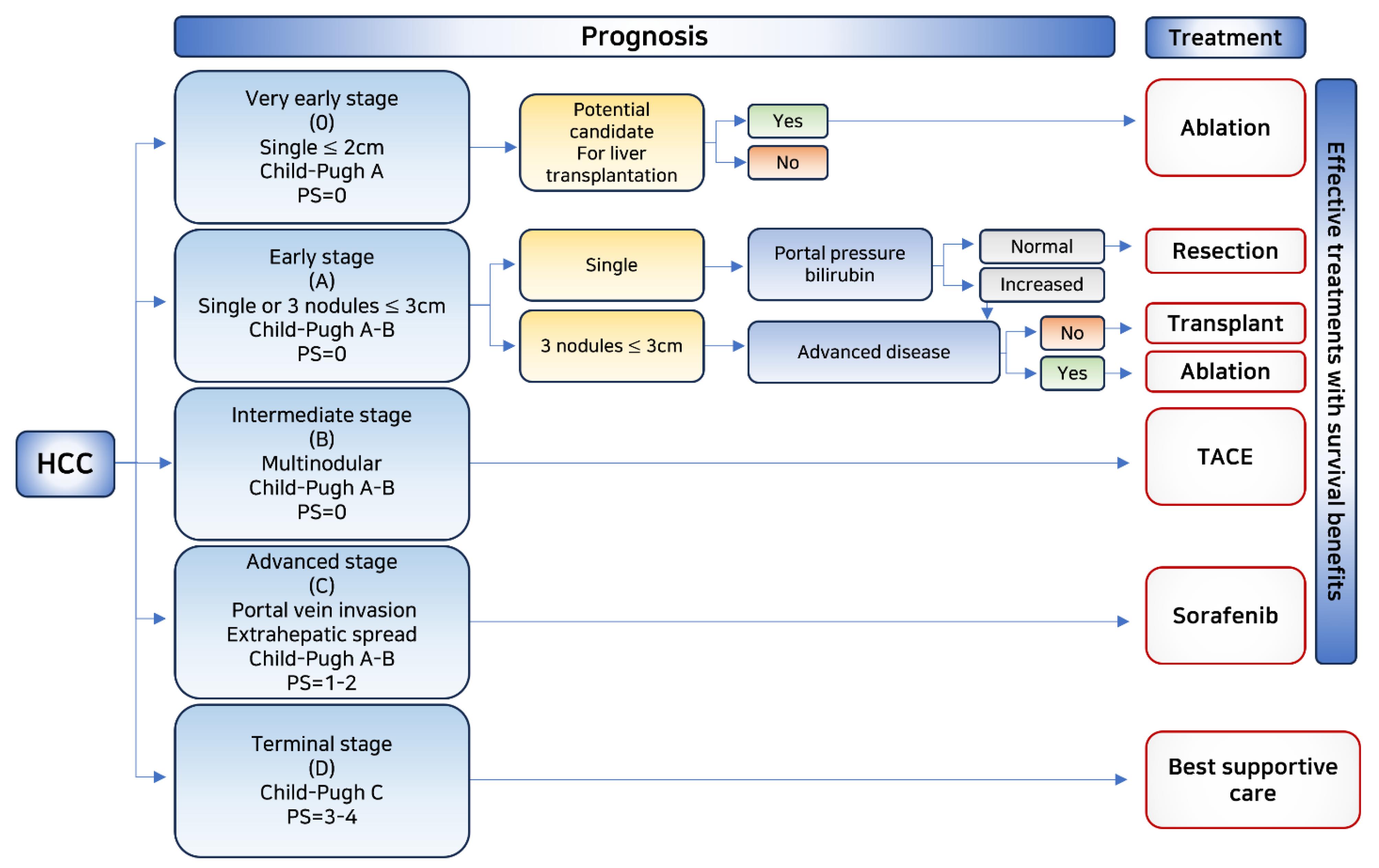
Figure 1.
Flowchart depicting prognosis and effective treatment options with survival benefits in HCC
.
Flowchart depicting prognosis and effective treatment options with survival benefits in HCC
The initial phases of the illness primarily focus on its management. Hence, primary treatment choices consist of hepatectomy and liver transplantation, if required. In situations where surgical removal is not practicable, radiofrequency (RF) ablation and microwave ablation are suggested. For intermediate stages, the most efficient treatments are chemoembolism, radioembolism, and simple embolism. Despite continuous advancements, systemic therapy for liver disease has been a matter of debate until recently. The development of a targeted multikinase inhibitor, sorafenib, which exhibited a 2.8-month survival advantage over a placebo in 2008, marked its initial development as a treatment option. In 2017, regorafenib, a tyrosine kinase inhibitor, was discovered and replaced despite being an expensive and highly toxic treatment.28,29 Lenvatinib is another tyrosine kinase inhibitor recently approved for unresectable HCCs.30 Due to the serious side effects of sorafenib and regorafenib, immunotherapy is the chosen first-line therapeutic strategy. Second-line approved therapies include immune checkpoint inhibitors (ICIs) such as nivolumab and pembrolizumab. Previously, phase III studies with immunotherapy treatments failed to show a statistically significant association between progression-free and overall survival rates.
HCC immunotherapy encounters a significant obstacle as there is currently no delivery technology capable of precisely targeting therapeutic agents to their intended destinations. To address this challenge, lipid-based nanoparticles (LBNPs), for example, liposomes and lipid nanoparticles (LNPs), have been created as potential platforms for delivering various therapeutic drugs.31,32 Compared to other delivery methods, LBNPs are superior because they have less systemic toxicity and are clinically more effective than polymeric and inorganic nanoparticles since they are readily soluble in water.33
Lipid-based nanoparticles are the most prevalent type of nanomedicine approved by the US Food and Drug Administration (FDA). These nanoparticles come in a variety of structural configurations that improve their physical stability and their ability to accommodate cargo with varying mechanical properties and degrees of hydrophobicity. The original liposome formulation, created in the 1960s and 1970s for delivering active pharmaceutical ingredients, consisted of a blue inner core surrounded by lipid bilayers. Subsequently, LNPs adopted a micelle-like shape like liposomes but with different lipid structures. It has been demonstrated that the more complex internal lipid architecture of LNPs makes them better suited for encapsulating genetic cargo.34 These droplets are composed of nanodroplets that mix the phases of oil and water. Unlike water-in-oil nanoemulsions (NEs), oil-in-water NEs, as an alternative to liposomes and LNPs, have micelle-like structures but are coated with oil droplets.35 NEs are less thermodynamically stable than liposomes and LNPs, causing them to undergo phase separation during long-term storage. To improve the kinetic stability of NEs and ensure their efficacy with hydrophobic drugs, a surfactant and lipid adjuvant can be used.36 Throughout this article, we reviewed the current applications of LNPs in the development of anti-HCC therapies, which may shed light on how the field may develop in the future.
Development (signaling pathways)
The development of liver cancer stem cells (LCSCs) and the preservation of their characteristics during the liver cancer process involve several signaling pathways, including Wnt/Catenin, Hippo, IL-6/STAT3, MAPK, and Notch pathways. The most prominent signaling systems involved in maintaining the stemness of HCC cells are the Wnt/Catenin and IL-6/STAT3 pathways. Somatic mutations in Wnt-related pathway genes are commonly observed in HCC. Additionally, activation of STAT3 frequently occurs in HCC and can transactivate certain genes expressions, such as NANOG, OCT4, and SOX2, which are transcription factors that stimulate the growth of LCSCs.37 Interleukin (IL)-6, which is produced by immune cells and hepatocytes, is an upstream regulator of STAT3.38,39 The function of STAT3 in different human cancers has been highlighted, and it is an oncogenic factor with a versatile function in accelerating tumorigenesis and the development of drug resistance.40-43
While chronic liver inflammation is frequently linked to the development of HCC, it is worth noting that hepatocytes producing IL-6 may lead to the autocrine activation of STAT3 in hepatocytes. This effect can trigger the reprogramming of liver cells into stem cells, ultimately leading to the development of liver cancer. Furthermore, the tumor suppressor pathway hypo-signaling limits the proliferation of mature stem cells as well as progenitor cells.44 When Hippo signaling is activated, the transcriptional coactivators Yes-associated protein 1 (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) are phosphorylated, leading them to stay in the cytoplasm. This event stops TEA family nuclear transcription factors (TEAD) from interacting with YAP and TAZ, hence suppressing gene expression. Studies have shown that suppression of TAZ expression slows the development of HCC cells. It can also result in the compensatory upregulation of YAP, enhance tumor cell chemoresistance, and increase the expression of CD90, a unique cancer stem cell (CSC) marker for HCC. As YAP and TAZ have different functional roles, simultaneous inhibition of both transcriptional activators is crucial when aiming for the Hippo signaling pathway.45 A dysgene and metalloprotease must cleave NOTCH for cancer progression in CSCs (ADAM). NOTCH (NICD) signaling is initiated by inducible nitric oxide synthase (iNOS) via ADAM17/transarterial chemoembolization (TACE) activation, resulting in an active NOTCH intracellular domain.46 Interestingly, when three members of the retinoblastoma (RB) family—RB, p107, and p130—were not expressed in the liver, the NOTCH pathway was activated, leading to reduced HCC formation. This finding shows the complex role of NOTCH in the development of liver cancer.47
Liver cirrhosis, which is a leading cause of HCC, involves the crucial role of liver stellate cells or activated fibroblasts. These cells release hepatocyte growth factor (HGF), which can activate the transcription factor FRA1, inducing HEY1 expression in hepatocytes by binding to their c-MET receptor. HEY1 is a downstream target of the NOTCH signaling pathway, and its increased expression may result in an increase in the number of LCSCs and the chemoresistance of hepatocytes, thereby promoting HCC development.48 The protein arginine methyltransferase 6 (PRMT6) is methylated and linked to arginine-100 (R100) of c-Raf to inhibit MAPK signaling. When PRMT6 is inhibited, MAPK signaling is stimulated, improving the stemness of CD133+ LCSCs.49 About 80 to 90% of HCC patients have cirrhosis due to long-term inflammation of the liver.50 The primary initiator of inflammation associated with liver cancer is the death of epithelial cells. Tumor necrosis factor (TNF)-α, signal transducer and activator of transcription 3 (STAT3), IL-6, C-Jun N-terminal kinase (JNK), nuclear factor kappa B (NF-κB), innate and adaptive immune signaling, and immunity are examples of pathways that contribute to inflammation-mediated hepatocarcinogenesis.51
Risk factors
Males have a higher incidence of HCC than females, with a two-fold increased risk. The incidence of liver cancer increases with cirrhosis from any cause, ranging from 2 to 4 percent per year. The risk varies depending on the underlying cause, region, age, gender, and the extent of liver damage.52 Worldwide, HCC is mainly caused by HBV infection. Although vaccination against HBV has reduced the incidence of liver cancer,53 many unvaccinated individuals are still at risk of developing liver cancer and HBV infection (257 million in 2015), primarily in Asia and sub-Saharan Africa.54 Moreover, individuals with HBV infection who have a specific mutation in TP53 at position 249 (RS) are more prone to developing HCC. Conversely, in Western countries and Japan, HCV infection is the primary cause of liver cancer. HBV infection has a direct carcinogenic impact regardless of the severity of underlying liver fibrosis,55 whereas liver cancer is uncommon in HCV-infected individuals without extensive fibrosis. Non-alcoholic fatty liver disease (NAFLD) is increasing the prevalence of HCC worldwide. In the United States, this rate is expected to rise by 122% between 2016 and 2030.56 Liver cancer is commonly caused by alcoholic cirrhosis. Additionally, HCC can arise due to concurrent smoking and human immunodeficiency virus (HIV) infection. While antiviral medications significantly reduce the likelihood of developing HCC in these individuals, the risk cannot be completely eliminated.57 The use of direct-acting antiviral drugs reduces the risk of liver cancer in HCV-infected patients.58
Currently, the primary global risk factors for HCC are HBV and HCV. However, the prevalence of these conditions is declining due to improved care and vaccination efforts. In contrast, the incidence of HCC due to NAFLD/non-alcoholic steatohepatitis (NASH) is rising and could soon surpass viral causes as the leading risk factor. Other significant risk factors include excessive alcohol consumption, exposure to AFB1, obesity, and diabetes. Therefore, greater efforts should be made to combat obesity and diabetes to reduce the incidence of NAFLD. Additionally, more effective strategies to reduce alcohol consumption and control mycotoxin contamination are necessary.59
Staging systems
HCC should be staged because proper staging leads to treatment recommendations and an accurate prognosis. There are some new HCC staging systems that have emerged in different countries, but none of them are widely recognized or allow for the comparison of different treatment methods. Some clinical staging systems for HCC include the CLIP (Cancer of the Liver Italian Programme) score, MESIAH (Model to Estimate Survival in Ambulatory HCC Patients) score, ITA.LI.CA (Italian Liver Cancer) score, BCLC (Barcelona Clinic Liver Cancer) staging, HKLC (Hong Kong Liver Cancer) staging, and the Alberta algorithm.60 Since most patients with HCC also have concurrent liver disease, the benefits of tumor therapy must be evaluated against the risks of medical therapy in individuals with cirrhosis.
The comprehensive management of liver cancer involves various disciplines such as hepatology, hepatobiliary surgery, pathology, oncology, radiology (both diagnostic and interventional), and specialized nursing. To determine the prognosis of patients, a staging system must consider the extent of liver dysfunction, tumor burden, and performance status. The BCLC staging system, introduced in 1999,61 is the most widely used.62 This grading approach is recognized as a standard for the development of clinical trials in HCC and is supported by clinical practice guidelines.63,64 The algorithm categorizes patients into five stages—very early, early, intermediate, advanced, or late stage—and provides stage-based treatment recommendations (Figure 2). Other staging methods, such as the CLIP,65 and the HKLC staging system,66 are available but are limited to certain geographic areas. Cross-sectional imaging is used to assess tumor burden, considering factors such as size, number of nodules, and the presence of macrovascular tumor invasion or extrahepatic spread.
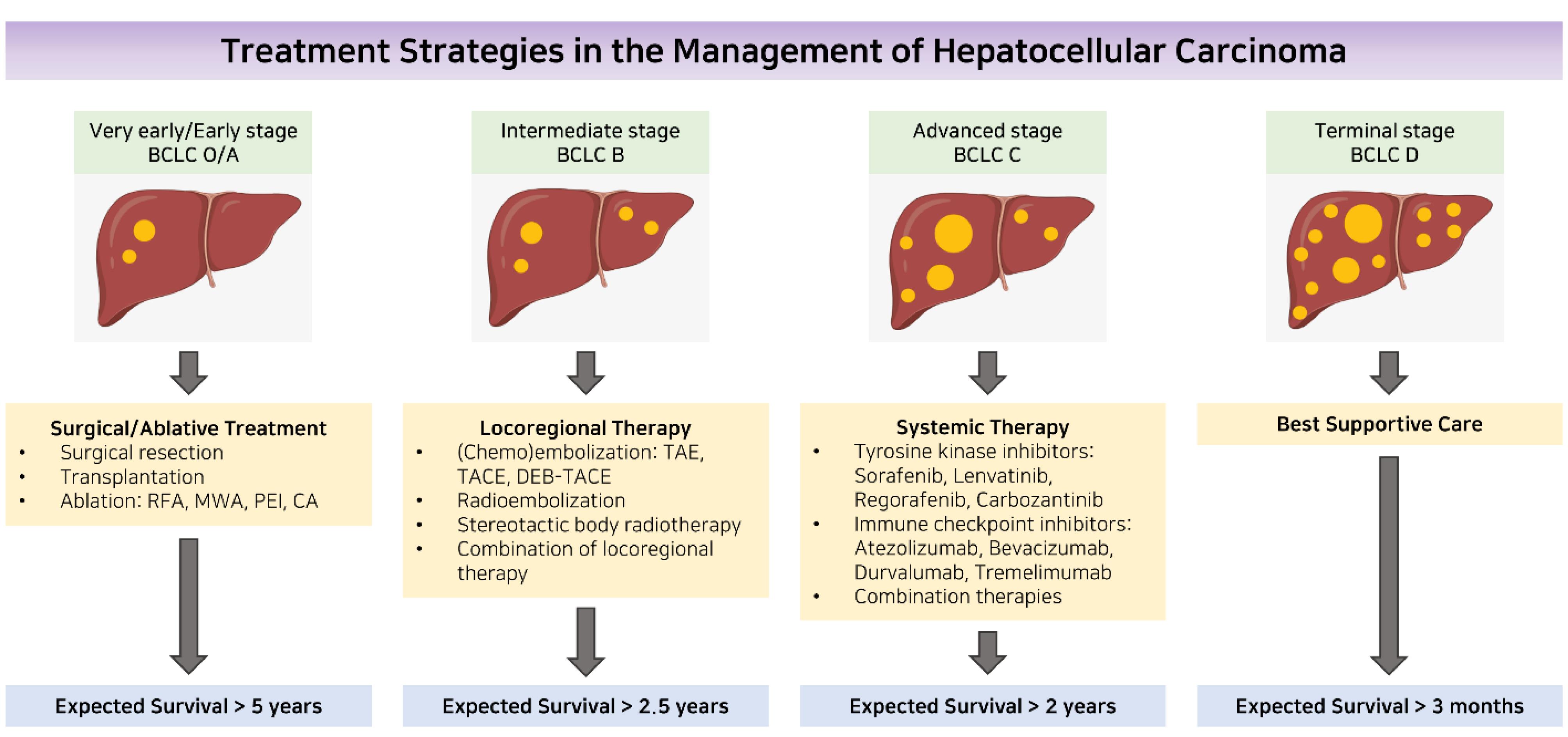
Figure 2.
Managing hepatocellular carcinoma through a treatment strategy. Abbreviations: BCLC stands for Barcelona Clinic Liver Cancer; CA stands for cryoablation; DEB-TACE stands for drug-eluting beads transarterial chemoembolization; MWA stands for microwave ablation; PEI stands for percutaneous ethanol injection; RFA stands for radiofrequency ablation; TACE stands for transarterial chemoembolization; and TAE stands for transarterial embolization.67 Reprinted with permission from Kung and Ng67 (Copyright 2022, OAE Publishing Inc)
.
Managing hepatocellular carcinoma through a treatment strategy. Abbreviations: BCLC stands for Barcelona Clinic Liver Cancer; CA stands for cryoablation; DEB-TACE stands for drug-eluting beads transarterial chemoembolization; MWA stands for microwave ablation; PEI stands for percutaneous ethanol injection; RFA stands for radiofrequency ablation; TACE stands for transarterial chemoembolization; and TAE stands for transarterial embolization.67 Reprinted with permission from Kung and Ng67 (Copyright 2022, OAE Publishing Inc)
Diagnosis methods
Imaging techniques can be used in cirrhotic patients to identify HCC. This is because malignant nodules are typically supplied by the hepatic arteries, while benign lesions like regenerative and dysplastic nodules are supplied by branches of the portal system.68 Contrast-enhanced CT or magnetic resonance imaging (MRI) scans can reveal the characteristic changes caused by HCC. These changes are seen as hypervascularity during the arterial phase of the scan, followed by hypoperfusion during the portal or delayed phases. This pattern has a sensitivity ranging from 66% to 82% and a specificity of over 90% in detecting HCC in individuals with cirrhosis and nodule diameters larger than 1 cm.69 In specialized centers, particularly in Europe,63 and Asia,70 contrast-enhanced ultrasound is also used to characterize hepatic nodules, although its precise diagnostic performance is still under investigation. For individuals without cirrhosis or with indeterminate patterns on imaging, a biopsy should be relied upon for diagnosis. Histological diagnosis can be challenging in patients with small nodules; however, a panel of immunostaining markers, such as heat shock protein 70, glypican 3, and glutamine synthetase, improves the accuracy of diagnosis.71 Patients with cirrhosis and nodules smaller than 1 cm should undergo ultrasound monitoring every 3–4 months, and if the nodule size remains stable after 12 months, routine surveillance may be an option.63
Therapeutic approaches
Currently, there are several treatment options available for HCC, including systemic drugs, minimally invasive local therapies like radiofrequency ablation, TACE, and arterial radioembolization (TARE), as well as surgical options like liver resection or transplantation. The decision for liver transplantation is based on the Milan criteria.72 However, it has been suggested that broader selection criteria than Milan should be considered in some cases.73,74 Radiofrequency ablation (RFA), cryoablation, and microwave ablation deliver heat directly to the tumor using needle electrodes, effectively killing tumor cells.75 Transarterial injection with a combination of lipiodol, chemotherapeutic medicines, gel foam, or microspheres (traditional TACE), drug-eluting beads (DEB-TACE), or yttrium-99 (TARE) promotes tumor necrosis by reaching the tumor site, as HCC is sustained by blood from the hepatic artery.76 Selective internal radiation therapy (SIRT) eliminates tumor cells by generating beta rays.77 For patients with advanced-stage HCC, sorafenib, an oral tyrosine kinase inhibitor that targets the fibrosarcoma (Raf)/mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway, vascular endothelial growth factor receptor (VEGFR), and platelet-derived growth factor receptor (PDGFR), is recommended and demonstrated.78,79
Between 2017 and 2019, new first- and second-line systemic therapies targeting tyrosine kinase receptors were developed: regorafenib (a multikinase inhibitor with a mechanism similar to sorafenib),28 lenvatinib (a multikinase inhibitor that targets VEGFR 1-3, fibroblast growth factor receptors (FGFR) 1-4, PDGFR α, RET, and KIT),80 cabozantinib (a tyrosine kinase inhibitor that targets MET, VEGFR2, and RET),81 and ramucirumab (a VEGFR2 antagonist).82,83 Nivolumab, another programmed cell death protein-1 (PD-1) inhibitor, was authorized in 2017 as second-line therapy for advanced HCC.84 Although new antiviral agents for HBV replication, such as tenofovir and entecavir, inhibit HBV replication, they do not completely eliminate the risk of HCC in people with cirrhosis.85 On the other hand, antiviral drugs for HCV, such as sofosbuvir, daclatasvir, ledipasvir, and ribavirin, have shown efficacy in preventing irreversible hepatic decompensation and reducing tumor recurrence after curative therapy.86
The increasing incidence of HCC caused by NASH, particularly in the Indian subcontinent,87 requires careful monitoring of HCC progression in patients with NAFLD, as well as the promotion of lipid-lowering medications such as statins and lifestyle adjustments to manage metabolic syndrome. New medications, such as elafibranor, obeticholic acid, cenicriviroc, liraglutide, and aramacular, have the potential to treat NASH and limit the development of HCC. However, their efficacy needs to be studied in future clinical research.88,89
Nanotechnology has the potential to enhance the activity of drugs and destroying cancer cells.90,91 Its application can facilitate the development or improvement of treatments that result in better outcomes for neoplastic diseases. This could involve optimizing medication levels and dosages or using tissue-specific delivery systems to target specific locations, reducing the risk of systemic toxicity and unwanted effects.92 The use of nanotechnology may revolutionize current approaches to combination therapy by enhancing pharmacokinetic, permeation, and retention profiles, resulting in reduced side effects.93,94 Nanoparticle approaches in medicine pave the way for a promising future through therapeutic regimens that combine multiple substances to enhance the effects of drugs.95,96 The use of nanotechnology has been explored to enhance drug delivery and efficacy in liver cancer treatment. One potential application is the development of different drug delivery systems using nanocarriers, which can reduce the required drug doses, increase the therapeutic index, minimize the risk of systemic toxicity, enable sustained drug release over days after a single dose, and enhance the selective targeting of liver cancer cells (Table 1).
Table 1.
Advantage and disadvantage of lipid nanoparticles
|
LBNPs
|
Advantages
|
Disadvantage
|
References
|
|
|
|
|
97,98
|
|
|
|
|
99,100
|
|
|
|
-
Production costs are high
-
Leakage of drug substances
-
Insufficient half-life
-
Use of phospholipids may result in oxidation and hydrolysis
|
100,101
|
|
|
-
Synthesis in a hurry
-
Dispersibility is simple
-
Economies of scale
|
-
Controlling particle size is difficult
-
Scaling up can be challenging
-
Stability of the product over a short period
|
102
|
|
|
|
|
103,104
|
|
|
|
|
105,106
|
|
|
|
|
107,108
|
Abbreviations: SLNs, solid lipid nanoparticles; NLCs, nanostructured lipid carriers; LCNs, lipid-coated nanoparticles
Evaluating and summarizing different studies on the application of LBNPs in HCC
LBNP technology is currently being explored as a potential treatment for HCC. Table 2 presents published reports on studies investigating the use of LBNPs for HCC treatment. However, the physicochemical properties of drugs often limit the effectiveness of HCC treatments. Chemotherapy and targeted drugs like sorafenib have minimal impact on patient survival, while radiotherapy is frequently ineffective. To improve treatment efficacy and patient survival, some nanoplatforms have been combined with drugs. For instance, in liver cancer treatment, cubosomes and NLCs loaded with 5-FU and PTX have been utilized. This treatment increases the accumulation of 5-FU in the liver by preventing its rapid degradation by enzymes. Additionally, PTX-loaded NLCs enhance the plasma persistence and aggregation of Intaxelfi, the commercial formulation.109,110 Furthemore, a dual treatment system consisting of sorafenib and superparamagnetic iron oxide nanoparticles (SPIONs) loaded into SLNs has been used to treat HepG2 cells.111 The antitumor strategies for liver cancer are further enhanced by the development and testing of new nanoformulations. Two new MEs have been developed by Lin et al112 and Qu et al113: (1) an ME made from soybeans loaded with curcumin (CUR); and (2) an ME made from Coix seed ingredients (C-ME) modified to target the overexpressed asialoglycoprotein receptor.
Table 2.
Clinical trials for lipid-based nanoparticles (LBNPs) for HCC treatment.
|
Stages of the clinical trial
|
Lipid-based NPs
|
Drugs that have been loaded
|
LBNPs target
|
Reference
|
| Phase 3 |
Liposomes that respond to temperature |
Dox |
Release in response to stimulus |
NCT00617981 |
| Phase 3 |
Liposomes that respond to temperature |
Dox |
Release in response to stimulus |
NCT02112656 |
| Phase 1b/2 |
Nanoparticles containing lipids |
Small interfering RNA oligonucleotide |
The development of gene therapies targeted at the liver |
NCT02314052 |
| Phase 1/2 |
The combination of glycyrrhizin and gemcitabine (Gem) |
Gem |
Targeting with an active approach |
NCT02449109 |
| Phase 1 |
Liposome |
Mitoxantrone hydrochloride |
An approach that is passive |
NCT04331743 |
| Phase 3 |
Liposomes that respond to temperature |
Dox |
Release in response to stimulus |
NCT02112656 |
| Phase 1 |
Liposomes that respond to temperature |
Dox |
Release in response to stimulus |
NCT00441376 |
| Phase 1 |
Liposome |
RNA with double strands |
The development of gene therapies targeted at the liver |
NCT02716012 |
| Phase 1/2 |
Liposome |
Aroplatin |
An approach that is passive |
NCT00057395 |
| Phase 2 |
Liposomes with pegylation |
Dox |
An approach that is passive |
NCT00003296 |
| Phase 2 |
Liposome |
Irinotecan |
An approach that is passive |
NCT03044587 |
| Phase 1 |
Liposomal mimic |
miR-34a |
The development of gene therapies targeted at the liver |
NCT01829971 |
At concentrations of 15 mM, toxicity was mainly observed in HepG2 cells, but at higher concentrations, animals with HepG2 tumor xenografts showed increased internalization and toxicity. Additionally, Hu et al114 developed a chemotherapeutic and photothermal polyethylene with doxorubicin (Dox) and indocyanine green (ICG). Combining this approach with near-infrared (NIR) laser irradiation completely inhibited H22 tumor models.
Concluding and future perspectives
Current cancer treatments are limited to surgery, radiation therapy, and chemotherapy. However, these procedures carry the risk of damaging normal tissues or incomplete eradication of cancer. Nanotechnology provides tools to directly and selectively target chemotherapy to cancer cells and tumors, guide surgical tumor removal, and enhance the effectiveness of radiotherapy and other existing treatments. These advancements can reduce risks for patients and improve their chances of survival. Based on the findings in this review, lipid-based nanostructures hold promise as candidates in liver cancer therapy. Several reasons contribute to the popularity of lipidic nanoparticles, such as their stability, biocompatibility, and decreased undesirable side effects. The cytotoxic activity of anti-neoplastic agents is significantly enhanced by the use of lipidic nanoparticles. A variety of targeting techniques can further enhance lipid-based nanoparticle efficiency. Antineoplastic agents are more likely to reach tumors through targeted approaches, which minimizes their effect on normal tissues. Increasing the effectiveness of anti-neoplastic agents by using lipid-based nanoparticles holds great promise. However, there is still room for improvement in order to increase the number of clinically approved medications. A particular emphasis should be placed on reducing the potential risk of toxicity. It is important to find alternatives to cationic lipids, which may have toxic effects, and to develop solutions that can achieve the positive effects of lipidic nanoparticles without the associated risk of toxicity. This will help to enhance the safety profile of the formulations. Additionally, the methods of preparation used should be a focus for further advancement. The use of novel, environmentally friendly methods that do not rely on organic solvents will not only improve scalability and reduce production costs, but also eliminate the risk of toxic residuals.
Conclusion
LBNPs have gained significant traction in both preclinical and clinical settings for drug delivery applications, demonstrating superior advantages compared to other delivery methods. The approval of numerous LBNPs for clinical use underscores their efficacy. The innovation in lipid design and advanced formulations of LBNPs holds the potential for expanding their application in drug delivery. However, a key challenge remains in achieving drug specificity. Despite efforts to develop targeted delivery approaches, practical implementation in clinical settings lags. Various strategies have been investigated to target the effective delivery of LBNPs. The choice of lipid composition influences the biodistribution of medications, with DSPC-based LBNPs showing a predilection for the spleen, while DOPE-based LBNPs tend to accumulate in the liver. Future enhancements in LBNP design for drug delivery will benefit from a comprehensive understanding of structure-function relationships and lipid chemistry. The integration of cutting-edge technologies such as machine learning and meta-data analysis in research publications is anticipated to provide valuable insights for optimizing LBNP design in the future.
Acknowledgments
This research was supported by the Brain Pool program funded by the Ministry of Science and ICT through the National Research Foundation of Korea (Grant Number: 1244 2022H1D3A2A02085952). This research was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2022M3J7A1062940 and RS-2023-00240052).
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
Not applicable.
References
- Bahrami A, Hasanzadeh M, Shahidsales S, Farazestanian M, Hassanian SM, Moetamani Ahmadi M. Genetic susceptibility in cervical cancer: from bench to bedside. J Cell Physiol 2018; 233(3):1929-39. doi: 10.1002/jcp.26019 [Crossref] [ Google Scholar]
- Bahrami A, Khazaei M, Hassanian SM, Shahidsales S, Joudi-Mashhad M, Maftouh M. Targeting the tumor microenvironment as a potential therapeutic approach in colorectal cancer: Rational and progress. J Cell Physiol 2018; 233(4):2928-36. doi: 10.1002/jcp.26041 [Crossref] [ Google Scholar]
- Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson AB. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst 2017; 109(9):djx030. doi: 10.1093/jnci/djx030 [Crossref] [ Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71(3):209-49. doi: 10.3322/caac.21660 [Crossref] [ Google Scholar]
- Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 2006; 6(9):674-87. doi: 10.1038/nrc1934 [Crossref] [ Google Scholar]
- Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014; 383(9935):2168-79. doi: 10.1016/s0140-6736(13)61903-0 [Crossref] [ Google Scholar]
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380(9859):2095-128. doi: 10.1016/s0140-6736(12)61728-0 [Crossref] [ Google Scholar]
- Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. World Health Organization; 2010.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6):394-424. doi: 10.3322/caac.21492 [Crossref] [ Google Scholar]
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer; 2020.
- Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019; 16(10):589-604. doi: 10.1038/s41575-019-0186-y [Crossref] [ Google Scholar]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132(7):2557-76. doi: 10.1053/j.gastro.2007.04.061 [Crossref] [ Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55(2):74-108. doi: 10.3322/canjclin.55.2.74 [Crossref] [ Google Scholar]
- Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 2008; 48(4):1312-27. doi: 10.1002/hep.22506 [Crossref] [ Google Scholar]
- Cabrera R, Nelson DR. Review article: the management of hepatocellular carcinoma. Aliment PharmacolTher 2010; 31(4):461-76. doi: 10.1111/j.1365-2036.2009.04200.x [Crossref] [ Google Scholar]
- Zhu AX. Systemic therapy of advanced hepatocellular carcinoma: how hopeful should we be?. Oncologist 2006; 11(7):790-800. doi: 10.1634/theoncologist.11-7-790 [Crossref] [ Google Scholar]
- Yang S, Cai C, Wang H, Ma X, Shao A, Sheng J. Drug delivery strategy in hepatocellular carcinoma therapy. Cell Commun Signal 2022; 20(1):26. doi: 10.1186/s12964-021-00796-x [Crossref] [ Google Scholar]
- Kalyane D, Raval N, Maheshwari R, Tambe V, Kalia K, Tekade RK. Employment of enhanced permeability and retention effect (EPR): nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater Sci Eng C Mater Biol Appl 2019; 98:1252-76. doi: 10.1016/j.msec.2019.01.066 [Crossref] [ Google Scholar]
- Huang D, Sun L, Huang L, Chen Y. Nanodrug delivery systems modulate tumor vessels to increase the enhanced permeability and retention effect. J Pers Med 2021; 11(2):124. doi: 10.3390/jpm11020124 [Crossref] [ Google Scholar]
- Shi B, Abrams M, Sepp-Lorenzino L. Expression of asialoglycoprotein receptor 1 in human hepatocellular carcinoma. J HistochemCytochem 2013; 61(12):901-9. doi: 10.1369/0022155413503662 [Crossref] [ Google Scholar]
- Hanaoka H, Nakajima T, Sato K, Watanabe R, Phung Y, Gao W. Photoimmunotherapy of hepatocellular carcinoma-targeting glypican-3 combined with nanosized albumin-bound paclitaxel. Nanomedicine (Lond) 2015; 10(7):1139-47. doi: 10.2217/nnm.14.194 [Crossref] [ Google Scholar]
- Tros de Ilarduya C, Düzgüneş N. Delivery of therapeutic nucleic acids via transferrin and transferrin receptors: lipoplexes and other carriers. Expert Opin Drug Deliv 2013; 10(11):1583-91. doi: 10.1517/17425247.2013.837447 [Crossref] [ Google Scholar]
- Abdellatif AA, Zayed G, El-Bakry A, Zaky A, Saleem IY, Tawfeek HM. Novel gold nanoparticles coated with somatostatin as a potential delivery system for targeting somatostatin receptors. Drug Dev Ind Pharm 2016; 42(11):1782-91. doi: 10.3109/03639045.2016.1173052 [Crossref] [ Google Scholar]
- Mattheolabakis G, Milane L, Singh A, Amiji MM. Hyaluronic acid targeting of CD44 for cancer therapy: from receptor biology to nanomedicine. J Drug Target 2015; 23(7-8):605-18. doi: 10.3109/1061186x.2015.1052072 [Crossref] [ Google Scholar]
- Takahashi H, Ozturk M, Wilson B, Maki A, Ozawa K, Koizumi M. In vivo expression of two novel tumor-associated antigens and their use in immunolocalization of human hepatocellular carcinoma. Hepatology 1989; 9(4):625-34. doi: 10.1002/hep.1840090419 [Crossref] [ Google Scholar]
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 1989; 49(23):6449-65. [ Google Scholar]
- Chen F, Zhang J, Wang L, Wang Y, Chen M. Tumor pH(e)-triggered charge-reversal and redox-responsive nanoparticles for docetaxel delivery in hepatocellular carcinoma treatment. Nanoscale 2015; 7(38):15763-79. doi: 10.1039/c5nr04612b [Crossref] [ Google Scholar]
- Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 389(10064):56-66. doi: 10.1016/s0140-6736(16)32453-9 [Crossref] [ Google Scholar]
- Jackson R, Psarelli EE, Berhane S, Khan H, Johnson P. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol 2017; 35(6):622-8. doi: 10.1200/jco.2016.69.5197 [Crossref] [ Google Scholar]
- Bouattour M, Mehta N, He AR, Cohen EI, Nault JC. Systemic treatment for advanced hepatocellular carcinoma. Liver Cancer 2019; 8(5):341-58. doi: 10.1159/000496439 [Crossref] [ Google Scholar]
- Xu Y, Wang C, Shen F, Dong Z, Hao Y, Chen Y. Lipid-coated CaCO3 nanoparticles as a versatile pH-responsive drug delivery platform to enable combined chemotherapy of breast cancer. ACS Appl Bio Mater 2022; 5(3):1194-201. doi: 10.1021/acsabm.1c01234 [Crossref] [ Google Scholar]
- Zhuang X, Qi Y, Wang M, Yu N, Nan F, Zhang H. mRNA vaccines encoding the HA protein of influenza A H1N1 virus delivered by cationic lipid nanoparticles induce protective immune responses in mice. Vaccines (Basel) 2020; 8(1):123. doi: 10.3390/vaccines8010123 [Crossref] [ Google Scholar]
- Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 2021; 20(2):101-24. doi: 10.1038/s41573-020-0090-8 [Crossref] [ Google Scholar]
- Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater 2021; 6(12):1078-94. doi: 10.1038/s41578-021-00358-0 [Crossref] [ Google Scholar]
- McClements DJ. Edible nanoemulsions: fabrication, properties, and functional performance. Soft Matter 2011; 7(6):2297-316. doi: 10.1039/c0sm00549e [Crossref] [ Google Scholar]
- Sutradhar KB, Amin ML. Nanoemulsions: increasing possibilities in drug delivery. Eur J Nanomed 2013; 5(2):97-110. doi: 10.1515/ejnm-2013-0001 [Crossref] [ Google Scholar]
- Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer 2014; 14(11):736-46. doi: 10.1038/nrc3818 [Crossref] [ Google Scholar]
- Kumagai N, Tsuchimoto K, Tsunematsu S, Toda K, Takeuchi O, Saito H. Inhibition of growth of human hepatoma cells by dual-function antisense IL-6 oligonucleotides. Hepatol Res 2002; 22(2):119-26. doi: 10.1016/s1386-6346(01)00128-0 [Crossref] [ Google Scholar]
- He G, Dhar D, Nakagawa H, Font-Burgada J, Ogata H, Jiang Y. Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling. Cell 2013; 155(2):384-96. doi: 10.1016/j.cell.2013.09.031 [Crossref] [ Google Scholar]
- Ashrafizadeh M, Zarrabi A, Orouei S, Zarrin V, Rahmani Moghadam E, Zabolian A. STAT3 pathway in gastric cancer: signaling, therapeutic targeting and future prospects. Biology (Basel) 2020; 9(6):126. doi: 10.3390/biology9060126 [Crossref] [ Google Scholar]
- Ashrafizadeh M, Gholami MH, Mirzaei S, Zabolian A, Haddadi A, Vasheghani Farahani M. Dual relationship between long non-coding RNAs and STAT3 signaling in different cancers: new insight to proliferation and metastasis. Life Sci 2021; 270:119006. doi: 10.1016/j.lfs.2020.119006 [Crossref] [ Google Scholar]
- Mirzaei S, Gholami MH, Khaksary Mahabady M, Nabavi N, Zabolian A, Banihashemi SM. Pre-clinical investigation of STAT3 pathway in bladder cancer: paving the way for clinical translation. Biomed Pharmacother 2021; 133:111077. doi: 10.1016/j.biopha.2020.111077 [Crossref] [ Google Scholar]
- Ashrafizadeh M, Mohan CD, Rangappa S, Zarrabi A, Hushmandi K, Kumar AP. Noncoding RNAs as regulators of STAT3 pathway in gastrointestinal cancers: roles in cancer progression and therapeutic response. Med Res Rev 2023; 43(5):1263-321. doi: 10.1002/med.21950 [Crossref] [ Google Scholar]
- Wang Y, Rattray JB, Thomas SA, Gurney J, Brown SP. Author correction: in silico bacteria evolve robust cooperation via complex quorum-sensing strategies. Sci Rep 2020; 10(1):11979. doi: 10.1038/s41598-020-68366-8 [Crossref] [ Google Scholar]
- Hayashi H, Higashi T, Yokoyama N, Kaida T, Sakamoto K, Fukushima Y. An imbalance in TAZ and YAP expression in hepatocellular carcinoma confers cancer stem cell-like behaviors contributing to disease progression. Cancer Res 2015; 75(22):4985-97. doi: 10.1158/0008-5472.can-15-0291 [Crossref] [ Google Scholar]
- Wang R, Li Y, Tsung A, Huang H, Du Q, Yang M. iNOS promotes CD24( + )CD133( + ) liver cancer stem cell phenotype through a TACE/ADAM17-dependent Notch signaling pathway. Proc Natl Acad Sci U S A 2018; 115(43):E10127-36. doi: 10.1073/pnas.1722100115 [Crossref] [ Google Scholar]
- Viatour P, Ehmer U, Saddic LA, Dorrell C, Andersen JB, Lin C. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway. J Exp Med 2011; 208(10):1963-76. doi: 10.1084/jem.20110198 [Crossref] [ Google Scholar]
- Lau EY, Lo J, Cheng BY, Ma MK, Lee JM, Ng JK. Cancer-associated fibroblasts regulate tumor-initiating cell plasticity in hepatocellular carcinoma through c-Met/FRA1/HEY1 signaling. Cell Rep 2016; 15(6):1175-89. doi: 10.1016/j.celrep.2016.04.019 [Crossref] [ Google Scholar]
- Chan LH, Zhou L, Ng KY, Wong TL, Lee TK, Sharma R, et al. PRMT6 regulates RAS/RAF binding and MEK/ERK-mediated cancer stemness activities in hepatocellular carcinoma through CRAF methylation. Cell Rep 2018;25(3):690-701.e8. 10.1016/j.celrep.2018.09.053.
- Ringelhan M, Pfister D, O’Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol 2018; 19(3):222-32. doi: 10.1038/s41590-018-0044-z [Crossref] [ Google Scholar]
- Yang YM, Kim SY, Seki E. Inflammation and liver cancer: molecular mechanisms and therapeutic targets. Semin Liver Dis 2019; 39(1):26-42. doi: 10.1055/s-0038-1676806 [Crossref] [ Google Scholar]
- Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology 2009; 136(1):138-48. doi: 10.1053/j.gastro.2008.09.014 [Crossref] [ Google Scholar]
- Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst 2009; 101(19):1348-55. doi: 10.1093/jnci/djp288 [Crossref] [ Google Scholar]
- Yuen MF, Chen DS, Dusheiko GM, Janssen HL, Lau DT, Locarnini SA. Hepatitis B virus infection. Nat Rev Dis Primers 2018; 4:18035. doi: 10.1038/nrdp.2018.35 [Crossref] [ Google Scholar]
- Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol 2016; 64(1 Suppl):S84-101. doi: 10.1016/j.jhep.2016.02.021 [Crossref] [ Google Scholar]
- Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol 2018; 69(4):896-904. doi: 10.1016/j.jhep.2018.05.036 [Crossref] [ Google Scholar]
- Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004; 351(15):1521-31. doi: 10.1056/NEJMoa033364 [Crossref] [ Google Scholar]
- Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 2017;153(4):996-1005.e1. 10.1053/j.gastro.2017.06.012.
- McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology 2021; 73(Suppl 1):4-13. doi: 10.1002/hep.31288 [Crossref] [ Google Scholar]
- Tellapuri S, Sutphin PD, Beg MS, Singal AG, Kalva SP. Staging systems of hepatocellular carcinoma: a review. Indian J Gastroenterol 2018; 37(6):481-91. doi: 10.1007/s12664-018-0915-0 [Crossref] [ Google Scholar]
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999; 19(3):329-38. doi: 10.1055/s-2007-1007122 [Crossref] [ Google Scholar]
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018; 391(10127):1301-14. doi: 10.1016/s0140-6736(18)30010-2 [Crossref] [ Google Scholar]
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69(1):182-236. 10.1016/j.jhep.2018.03.019
- Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018; 68(2):723-50. doi: 10.1002/hep.29913 [Crossref] [ Google Scholar]
- A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998;28(3):751-5. 10.1002/hep.510280322.
- Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014;146(7):1691-700.e3. 10.1053/j.gastro.2014.02.032.
- Kung JW, Ng KK. Role of locoregional therapies in the management of patients with hepatocellular carcinoma. Hepatoma Res 2022; 8:17. doi: 10.20517/2394-5079.2021.138 [Crossref] [ Google Scholar]
- Matsui O, Kobayashi S, Sanada J, Kouda W, Ryu Y, Kozaka K. Hepatocelluar nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom Imaging 2011; 36(3):264-72. doi: 10.1007/s00261-011-9685-1 [Crossref] [ Google Scholar]
- Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK. Imaging for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatology 2018; 67(1):401-21. doi: 10.1002/hep.29487 [Crossref] [ Google Scholar]
- Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017; 11(4):317-70. doi: 10.1007/s12072-017-9799-9 [Crossref] [ Google Scholar]
- Tremosini S, Forner A, Boix L, Vilana R, Bianchi L, Reig M. Prospective validation of an immunohistochemical panel (glypican 3, heat shock protein 70 and glutamine synthetase) in liver biopsies for diagnosis of very early hepatocellular carcinoma. Gut 2012; 61(10):1481-7. doi: 10.1136/gutjnl-2011-301862 [Crossref] [ Google Scholar]
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334(11):693-9. doi: 10.1056/nejm199603143341104 [Crossref] [ Google Scholar]
- Chan SC, Fan ST. Selection of patients of hepatocellular carcinoma beyond the Milan criteria for liver transplantation. Hepatobiliary Surg Nutr 2013; 2(2):84-8. doi: 10.3978/j.issn.2304-3881.2012.12.04 [Crossref] [ Google Scholar]
- Shah SR. Living-related transplantation for hepatocellular carcinoma: how far do we travel beyond Milan?. Indian J Gastroenterol 2008; 27(4):139-41. [ Google Scholar]
- Gervais DA, Arellano RS. Percutaneous tumor ablation for hepatocellular carcinoma. AJR Am J Roentgenol 2011; 197(4):789-94. doi: 10.2214/ajr.11.7656 [Crossref] [ Google Scholar]
- Daher S, Massarwa M, Benson AA, Khoury T. Current and future treatment of hepatocellular carcinoma: an updated comprehensive review. J Clin Transl Hepatol 2018; 6(1):69-78. doi: 10.14218/jcth.2017.00031 [Crossref] [ Google Scholar]
- Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol 2012; 56(2):464-73. doi: 10.1016/j.jhep.2011.07.012 [Crossref] [ Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359(4):378-90. doi: 10.1056/NEJMoa0708857 [Crossref] [ Google Scholar]
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10(1):25-34. doi: 10.1016/s1470-2045(08)70285-7 [Crossref] [ Google Scholar]
- Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018; 391(10126):1163-73. doi: 10.1016/s0140-6736(18)30207-1 [Crossref] [ Google Scholar]
- Kelley RK, Verslype C, Cohn AL, Yang TS, Su WC, Burris H. Cabozantinib in hepatocellular carcinoma: results of a phase 2 placebo-controlled randomized discontinuation study. Ann Oncol 2017; 28(3):528-34. doi: 10.1093/annonc/mdw651 [Crossref] [ Google Scholar]
- Zhu AX, Galle PR, Kudo M, Finn RS, Qin S, Xu Y. A study of ramucirumab (LY3009806) versus placebo in patients with hepatocellular carcinoma and elevated baseline alpha-fetoprotein (REACH-2). J Clin Oncol 2018; 36(4 Suppl):TPS538. doi: 10.1200/JCO.2018.36.4_suppl.TPS538 [Crossref] [ Google Scholar]
- Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20(2):282-96. doi: 10.1016/s1470-2045(18)30937-9 [Crossref] [ Google Scholar]
- El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389(10088):2492-502. doi: 10.1016/s0140-6736(17)31046-2 [Crossref] [ Google Scholar]
- Papatheodoridis GV, Dalekos GN, Yurdaydin C, Buti M, Goulis J, Arends P. Incidence and predictors of hepatocellular carcinoma in Caucasian chronic hepatitis B patients receiving entecavir or tenofovir. J Hepatol 2015; 62(2):363-70. doi: 10.1016/j.jhep.2014.08.045 [Crossref] [ Google Scholar]
- Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS Jr. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015; 149(3):649-59. doi: 10.1053/j.gastro.2015.05.010 [Crossref] [ Google Scholar]
- David D, Raghavendran A, Goel A, Bharath Kumar C, Kodiatte TA, Burad D. Risk factors for non-alcoholic fatty liver disease are common in patients with non-B non-C hepatocellular carcinoma in India. Indian J Gastroenterol 2017; 36(5):373-9. doi: 10.1007/s12664-017-0785-x [Crossref] [ Google Scholar]
- Eshraghian A. Current and emerging pharmacological therapy for non-alcoholic fatty liver disease. World J Gastroenterol 2017; 23(42):7495-504. doi: 10.3748/wjg.v23.i42.7495 [Crossref] [ Google Scholar]
- Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013; 10(11):686-90. doi: 10.1038/nrgastro.2013.171 [Crossref] [ Google Scholar]
- Zare I, Chevrier DM, Cifuentes-Rius A, Moradi N, Xianyu Y, Ghosh S. Protein-protected metal nanoclusters as diagnostic and therapeutic platforms for biomedical applications. Mater Today 2023; 66:159-93. doi: 10.1016/j.mattod.2020.10.027 [Crossref] [ Google Scholar]
- Nuri Ertas Y, Abedi Dorcheh K, Akbari A, Jabbari E. Nanoparticles for targeted drug delivery to cancer stem cells: a review of recent advances. Nanomaterials (Basel) 2021; 11(7):1755. doi: 10.3390/nano11071755 [Crossref] [ Google Scholar]
- Reddy LH, Couvreur P. Nanotechnology for therapy and imaging of liver diseases. J Hepatol 2011; 55(6):1461-6. doi: 10.1016/j.jhep.2011.05.039 [Crossref] [ Google Scholar]
- Ashrafizadeh M, Zarrabi A, Bigham A, Taheriazam A, Saghari Y, Mirzaei S. (Nano)platforms in breast cancer therapy: drug/gene delivery, advanced nanocarriers and immunotherapy. Med Res Rev 2023; 43(6):2115-76. doi: 10.1002/med.21971 [Crossref] [ Google Scholar]
- Entezari M, Gholamiyan Yousef Abad G, Sedghi B, Ettehadi R, Asadi S, Beiranvand R. Gold nanostructure-mediated delivery of anticancer agents: biomedical applications, reversing drug resistance, and stimuli-responsive nanocarriers. Environ Res 2023; 225:115673. doi: 10.1016/j.envres.2023.115673 [Crossref] [ Google Scholar]
- Singh SK, Lillard JW Jr, Singh R. Reversal of drug resistance by planetary ball milled (PBM) nanoparticle loaded with resveratrol and docetaxel in prostate cancer. Cancer Lett 2018; 427:49-62. doi: 10.1016/j.canlet.2018.04.017 [Crossref] [ Google Scholar]
- Livney YD, Assaraf YG. Rationally designed nanovehicles to overcome cancer chemoresistance. Adv Drug Deliv Rev 2013; 65(13-14):1716-30. doi: 10.1016/j.addr.2013.08.006 [Crossref] [ Google Scholar]
- Duan Y, Dhar A, Patel C, Khimani M, Neogi S, Sharma P. A brief review on solid lipid nanoparticles: part and parcel of contemporary drug delivery systems. RSC Adv 2020; 10(45):26777-91. doi: 10.1039/d0ra03491f [Crossref] [ Google Scholar]
- Mishra V, Bansal KK, Verma A, Yadav N, Thakur S, Sudhakar K. Solid lipid nanoparticles: emerging colloidal nano drug delivery systems. Pharmaceutics 2018; 10(4):191. doi: 10.3390/pharmaceutics10040191 [Crossref] [ Google Scholar]
- Duong VA, Nguyen TT, Maeng HJ. Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method. Molecules 2020; 25(20):4781. doi: 10.3390/molecules25204781 [Crossref] [ Google Scholar]
- Jangde R, Singh D. Preparation and optimization of quercetin-loaded liposomes for wound healing, using response surface methodology. Artif Cells NanomedBiotechnol 2016; 44(2):635-41. doi: 10.3109/21691401.2014.975238 [Crossref] [ Google Scholar]
- Hashemi M, Ghadyani F, Hasani S, Olyaee Y, Raei B, Khodadadi M. Nanoliposomes for doxorubicin delivery: Reversing drug resistance, stimuli-responsive carriers and clinical translation. J Drug Deliv Sci Technol 2023; 80:104112. doi: 10.1016/j.jddst.2022.104112 [Crossref] [ Google Scholar]
- Zendrini A, Paolini L, Busatto S, Radeghieri A, Romano M, Wauben MHM. Augmented COlorimetric NANoplasmonic (CONAN) method for grading purity and determine concentration of EV microliter volume solutions. Front BioengBiotechnol 2019; 7:452. doi: 10.3389/fbioe.2019.00452 [Crossref] [ Google Scholar]
- Jiang L, Lee HW, Loo SCJ. Therapeutic lipid-coated hybrid nanoparticles against bacterial infections. RSC Adv 2020; 10(14):8497-517. doi: 10.1039/c9ra10921h [Crossref] [ Google Scholar]
- Krishnamurthy S, Vaiyapuri R, Zhang L, Chan JM. Lipid-coated polymeric nanoparticles for cancer drug delivery. Biomater Sci 2015; 3(7):923-36. doi: 10.1039/c4bm00427b [Crossref] [ Google Scholar]
- Barani M, Sangiovanni E, Angarano M, Rajizadeh MA, Mehrabani M, Piazza S. Phytosomes as innovative delivery systems for phytochemicals: a comprehensive review of literature. Int J Nanomedicine 2021; 16:6983-7022. doi: 10.2147/ijn.s318416 [Crossref] [ Google Scholar]
- Amith Kumar B, Habbu P, Thimmasetty Thimmasetty, Lakshman Lakshman, Hullatti P, Ravi Kumar S. Phytosomes as novel drug delivery system for herbal medicine? A review. Syst Rev Pharm 2017; 8(1):5-7. doi: 10.5530/srp.2017.1.2 [Crossref] [ Google Scholar]
- Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech 2015; 5(2):123-7. doi: 10.1007/s13205-014-0214-0 [Crossref] [ Google Scholar]
- Muzaffar F, Singh UK, Chauhan L. Review on microemulsion as futuristic drug delivery. Int J Pharm Pharm Sci 2013; 5(3):39-53. [ Google Scholar]
- Harshita Harshita, Barkat MA, Rizwanullah M, Beg S, Pottoo FH, Siddiqui S. Paclitaxel-loaded nanolipidic carriers with improved oral bioavailability and anticancer activity against human liver carcinoma. AAPS PharmSciTech 2019; 20(2):87. doi: 10.1208/s12249-019-1304-4 [Crossref] [ Google Scholar]
- Nasr M, Ghorab MK, Abdelazem A. In vitro and in vivo evaluation of cubosomes containing 5-fluorouracil for liver targeting. Acta Pharm Sin B 2015; 5(1):79-88. doi: 10.1016/j.apsb.2014.12.001 [Crossref] [ Google Scholar]
- Grillone A, Riva ER, Mondini A, Forte C, Calucci L, Innocenti C. Active targeting of sorafenib: preparation, characterization, and in vitro testing of drug-loaded magnetic solid lipid nanoparticles. Adv Healthc Mater 2015; 4(11):1681-90. doi: 10.1002/adhm.201500235 [Crossref] [ Google Scholar]
- Lin CC, Lin HY, Chi MH, Shen CM, Chen HW, Yang WJ. Preparation of curcumin microemulsions with food-grade soybean oil/lecithin and their cytotoxicity on the HepG2 cell line. Food Chem 2014; 154:282-90. doi: 10.1016/j.foodchem.2014.01.012 [Crossref] [ Google Scholar]
- Qu D, Liu M, Huang M, Wang L, Chen Y, Liu C. Octanoyl galactose ester-modified microemulsion system self-assembled by coix seed components to enhance tumor targeting and hepatoma therapy. Int J Nanomedicine 2017; 12:2045-59. doi: 10.2147/ijn.s125293 [Crossref] [ Google Scholar]
- Hu H, Xiao C, Wu H, Li Y, Zhou Q, Tang Y. Nanocolloidosomes with selective drug release for active tumor-targeted imaging-guided photothermal/chemo combination therapy. ACS Appl Mater Interfaces 2017; 9(48):42225-38. doi: 10.1021/acsami.7b14796 [Crossref] [ Google Scholar]