Advanced pharmaceutical bulletin. 15(1):217-222.
doi: 10.34172/apb.43661
Short Communication
Preparation and Anti-cancer Evaluation of Methotrexate-loaded Inositol-6 Phosphate Cross-linked Chitosan Nanoparticles on Breast Cancer
Masoud Farshbaf Data curation, Writing – original draft, 1, 2 
Nasrin Gobakhlou Investigation, Writing – original draft, 1, 2
Muhammad Sarfraz Project administration, 3
Javid Shahbazi-Mojarrad Methodology, 4
Mohammad Feyzizadeh Software, Writing – review & editing, 2, 5, * 
Hamed Hamishehkar Formal analysis, 4, 6
Parvin Zakeri-Milani Validation, Visualization, 7 
Hadi Valizadeh Conceptualization, Project administration, Supervision, 4 
Author information:
1Department of Medical Nanotechnology, Faculty of Advanced Medical Science, Tabriz University of Medical Science, Tabriz, Iran.
2Student Research Committee, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
3College of Pharmacy, Al Ain University, Al Ain 64141, United Arab Emirates.
4Drug Applied Research Center, Faculty of Pharmacy, Tabriz University of Medical Science, Tabriz, Iran.
5Department of Pharmaceutics, Faculty of Pharmacy, Tabriz University of Medical Science, Tabriz, Iran.
6Research Center of New Material and Green Chemistry, Khazar University, 41 Mehseti Street, AZ1096, Baku, Azerbaijan
7Liver and Gastrointestinal Diseases Research Center, Faculty of Pharmacy, Tabriz University of Medical Science, Tabriz, Iran.
Abstract
Purpose:
Chitosan nanoparticles (CNs) have directed considerable research efforts towards developing biocompatible, biodegradable, inexpensive and efficient particulate drug delivery systems.
Methods:
In the present investigation, we utilized green and safe inositol hexaphosphate (InsP6) as a physical cross-linker to obtain CNs (InsP6CNs) and compared their size, zeta potential and cell uptake ability with the CNs cross-linked with tripolyphosphate (TPP) as a commonly used cross-linker (TPPCNs). Methotrexate (MTX) as the model drug was physically incorporated within the both types of CNs (InsP6CNsMTX and TPPCNsMTX) and their time-dependent anti-cancer behavior was evaluated on MCF-7 cell line.
Results:
Compared to TPPCNs, InsP6CNs were bigger in hydrodynamic diameter and showed far different zeta potential value. The MTX encapsulation efficiency was much higher for InsP6CNsMTX than that of TPPCNsMTX. InsP6CNs and TPPCNs showed similar in vitro cell uptake behavior, examined on MCF-7 cell line. Furthermore, after 24 h, InsP6CNsMTX had the most in vitro antitumor effect on the MCF-7 cells, compared to free MTX and TPPCNsMTX.
Conclusion:
Consequently, InsP6 can be presented as an accessible and cost-effective member of physical cross-linkers to prepare efficient CNs as drug delivery systems.
Keywords: Chitosan, Inositol hexaphosphate, Methotrexate, Breast cancer
Copyright and License Information
© 2025 The Author (s).
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Funding Statement
This study was written based on a dataset of Master of Science thesis, registered in Tabriz University of Medical Sciences and financially supported by Drug Applied Research Centre of Tabriz University of Medical Sciences [Grant No. 93/2-8/6].
Introduction
Chitin is one the most abundant polysaccharides in nature consisting β-1,4-linked glucosamine as its sugar backbone with a high degree of N-acetylation.1 Chitosan, the main derivative of chitin, is a natural biodegradable cationic polymer with a random distribution of N-acetyl glucosamine and D-glucosamine in its backbone and commonly obtained by alkaline deacetylation.2 Due to its unique biological properties including nontoxicity, biocompatibility, biodegradability and mucoadhesive behavior, chitosan has attracted much of attention upon using in different fields of biomedical applications such as drug and gene delivery systems and biomedical engineering.3,4 Having the pKa around 6.5, chitosan is considered as a pH-sensitive polymer, by which at the pH values beneath this pKa, the amine groups of chitosan get protonated, forming polyelectrolyte with high charge density. On the other hand, at pH values near neutral (7-7.4), those amine groups become deprotonated and decrease the charge density of polymer. This behavior also influences the soluble-insoluble transition of chitosan. Among the many of techniques to obtain chitosan nanoparticles (CNs), emulsion cross-linking has been commonly employed in recent years.5 Cross-linking agents possess at least two reactive sites by which they can form bridges between the polymer chains. There are two different types of cross-linkers including chemical and physical. Through intra- and intermolecular covalent bindings, the reactive site of chemical cross-linkers like genipin, cinnamaldehyde and glutaraldehyde react with the amine groups of chitosan and form a complex, irreversible and unresponsive net of polymer and consequently rigid CNs.6,7 On the other hand, physical cross-linkers are mostly negative charged ions which form electrostatic interactions with the cationic amine groups of chitosan. This method is facile and the obtained nanoparticles have massive hydrophilic capacity within, presenting pH-responsive ability and are more biocompatible than the previous ones.8 Tripolyphosphate (TPP) is the most employed physical cross-linker for the CNs preparation,9 though it is a chemical agent, and besides being expensive to provide, it may cause cytotoxic effects upon using in the body. Thus, it is worth introducing a safer, cheaper and more accessible alternative. Inositol hexaphosphate (InsP6) is a vitamin-like naturally occurring polyphosphorylated carbohydrate mostly found in oil seeds, nuts, soybean and animals. InsP6 has been proved with anti-cancer effect on prostate,10 colorectal11 and breast cancers.12 Considering the chemical structure of InsP6, as a polyphosphate species, it is capable of forming ionic interactions with chitosan and acting as a green and accessible physical cross-linker with therapeutic action (Figure 1).13 In this study, we use InsP6 as a green cross-linker to obtain CNs (InsP6CNs) loaded with methotrexate (MTX) (InsP6CNsMTX) as an anti-cancer model drug. For comparison, the CNs cross-linked with TPP (TPPCNs) loaded with the same drug (TPPCNsMTX) was also prepared.
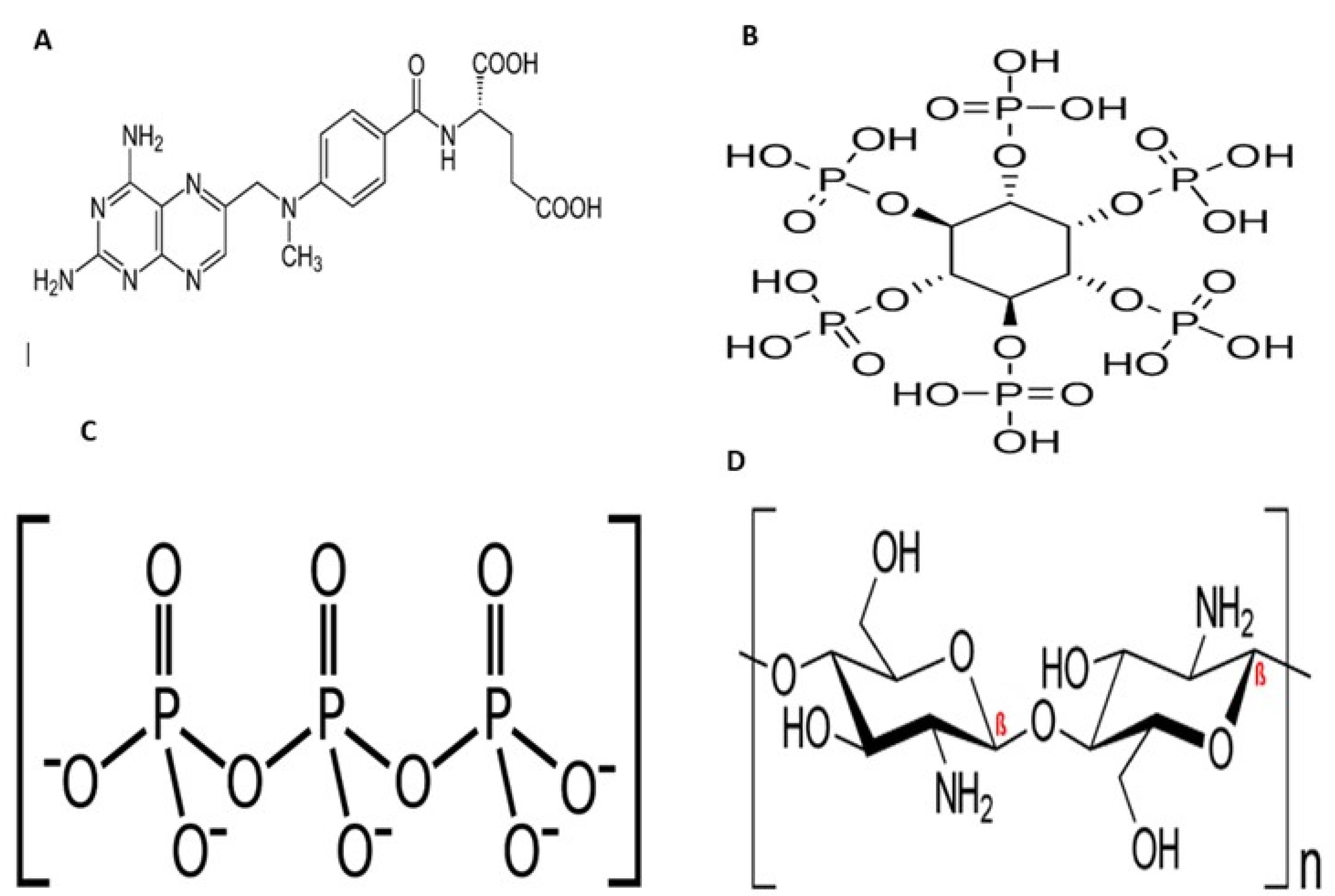
Figure 1.
Chemical structures of MTX (A), InsP6 (B), TPP (C), and Chitosan (D)
.
Chemical structures of MTX (A), InsP6 (B), TPP (C), and Chitosan (D)
Materials and Methods
Materials
Chitosan (medium molecular weight), MTX, fluorescein isothiocyanate (FITC), TPP and InsP6 were purchased from Sigma-Aldrich (St. Louis, MO, USA). RPMI1640 medium and fetal bovine serum (FBS) were provided by Gibco® (Grand Island, NY, USA). 3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyltetrazolium bromide (MTT) was purchased from CARL ROTH (Roth, Karlsruhe, Germany). MCF-7 Cell Line was obtained from Pasteur Institute (Tehran, Iran). All the other organic reagents were of analytical grade and purchased from Merck (Darmstadt, Germany).
Synthesis of InsP6CNs
InsP6CNs were synthesized by a facile method using InsP6 as a green and safe cross-linker for chitosan at an acidic environment. Briefly, 105 mg of chitosan was added to 35 mL of acetic acid (1%) in a round-bottom flask. After fine dispersion of chitosan in the solution, 14 mL of a mixture with concentration of 1 mg mL-1 of InsP6 in deionized (DI) water was added drop-wise to the above solution under stirring (600 rpm) at 65 °C for 30 minutes. Once the solution became almost opaque, the stirring speed was decreased to 400 rpm for 1 min and the nanoparticles were settled down. Finally, the supernatant was collected and the obtained InsP6CNs were washed using DI water and then re-dispersed in fresh DI water using the Ultrasonic Cleaner (Otto, Italy).
Synthesis of TPPCNs
The procedure for preparation of TPPCNs was similar to that of InsP6CNs using the same concentrations and with only difference in the type of cross-linker and reaction temperature which was carried out by using TPP and at ambient temperature, respectively.
Synthesis of InsP6CNsMTX and TPPCNsMTX
In order to physically encapsulate the MTX within the InsP6CNs and TPPCNs, a 2.2 mg mL-1 solution of MTX was obtained by dissolving it in the cross-linker solution (InsP6 or TPP) prior to addition to the chitosan solution. The rest of the protocol was same as for the synthesis of blank InsP6CNs or TPPCNs. After synthesis of InsP6CNsMTX and TPPCNsMTX, the unloaded drug was separated by centrifugation (Hettich, Germany) for 10 min at 5000 rpm. In order to calculate the encapsulation efficiency (EE) of MTX in both InsP6CNsMTX and TPPCNsMTX, the collected supernatants were analyzed using ultraviolet-visible (UV-Vis) spectrophotometer (Shimadzu, Japan) at λmax = 306 nm and the calculations were carried out according to the equation 1:
Eq. 1
Synthesis of fluorescence CNs
In order to prepare FITC-tagged CNs (InsP6CNsFITC and TPPCNsFITC), 10 mg FITC (0.71 mg mL-1) was added to the cross-linker solution (InsP6 or TPP) prior to addition to the chitosan solution. The rest of the protocol was same as for the synthesis of blank InsP6CNs or TPPCNs, and carried out in a dark room to prevent photo-leaching.
Characterization techniques
The mean hydrodynamic diameter, polydispersity index (PDI), and zeta potential of InsP6CNs and TPPCNs were assessed using Nano S zetasizer (Malvern Instruments, Malvern, UK). The samples were diluted with DI water and the mean hydrodynamic diameter and zeta potential were measured in three replicates at pH = 7.4 and 25 °C.
Cell culture and in vitro cell viability assay
Breast cancer MCF-7 cell line was cultured according to the ref14 and used to investigate the in vitro antitumor effect of InsP6CNsMTX and TPPCNsMTX and cellular uptake of InsP6CNsFITC and TPPCNsFITC. Briefly, cells were cultured in Gibco® RPMI1640 containing 10% (v/v) FBS, 100 U mL-1 penicillin G, and 100 µg mL-1 streptomycin and incubated at 37 °C in an atmosphere of 5% CO2 and 95% airfor 24 h. After reaching to proper population and their detachment with 0.25% trypsin in phosphate buffer saline (PBS), cells were seeded in 96-well plates with cell density of 1.0 × 104 cells/well and allowed to grow for 24 h with the same temperature and atmosphere. Then, in order to perform time-dependent viability study, cells were treated with equivalent concentrations of chitosan, TPP, InsP6, MTX,TPPCNs,InsP6CNs, InsP6CNsMTX and TPPCNsMTX. Equivalent volume of fresh mediumwasadded to control group. After incubation for 24 h the MTT assay was carried out in triplicates. To this end, the old media was removed and 100 µL of fresh medium with 10 µL of a 5 mg mL-1 MTT in PBS was added to each well and after covering with aluminum foil, incubated for 4 h. The old medium was replaced with 100 µL DMSO in order to dissolve the formazan crystals. After shaking for 10 min, an ELISA plate reader(Bio-Tek Instruments, USA) was used to measure the absorbance of each well at 570 nm with a background correction at 630 nm.
Study of in vitro cellular uptake
The MCF-7 cells with density of 5 × 105 cells/well were seeded into 6-well plate and incubated for 20 h. Then, the culture medium was replaced with 100 µl fresh medium (with no antibiotic) containing proper amount of InsP6CNsFITC or TPPCNsFITC (2 wells for each sample) and incubation was continued at 37 °C. After 90 min, the fluorescent intensity and quantitative analysis were evaluated by flow cytometry (FACSCalibur, Becton Dickinson, San Jose, CA, USA).
Statistical analysis
Data analysis was conducted using GraphPad Prism version 6. Data were represented as mean ± standard deviation (SD). One way analysis of variance (ANOVA) was used to perform comparisons between groups. P value < 0.05 was considered statistically significant.
Results and Discussion
Synthesis of nanoparticles
In this study, we used a facile method in which InsP6 acted as a naturally occurring and non-harmful physical cross-linker to prepare CNs in an acidic medium. In order to compare the results, we also synthesized CNs with the commonly used synthetic cross-linker, TPP. When the cross-linker solution (InsP6 or TPP) was being added dropwise, the formation of either InsP6CNs or TPPCNs was obvious by gradually turning the color of solution to opaque. FITC was used as a fluorescence dye to tag the InsP6CNs and TPPCNs and to evaluate their in vitro cell uptake behavior. FITC was physically encapsulated within the nanoparticles via the electrostatic interaction of its hydroxyl groups with the amine groups of CNs resulting in a mild colorful suspension. Similarly, MTX was trapped into the InsP6CNs and TPPCNs with proposed interactions of its pteridine ring by negative phosphate groups. EE of MTX in InsP6CNsMTX and TPPCNsMTX was calculated to be 32.5% and 8.2%, indicating the superior ability of InsP6CNs to encapsulate the MTX. This could be due to the fact that, the encapsulation of MTX was basically dependent on the electrostatic interactions of its cationic end groups with the anionic parts of cross-linker and chitosan. As InsP6 has higher negative net charge than its counterpart, TPP, the higher MTX EE for InsP6CNsMTX was expected.15
Size and charge of InsP6CNs and TPPCNs
The hydrodynamic diameter of InsP6CNs and TPPCNs were measured in DI water using dynamic light scattering (DLS) instrument and based on the light scattering profile of nanoparticles. The mean size of InsP6CNs and TPPCNs were measured to be 918.5 ± 103.6 nm and 463.3 ± 21 nm, respectively. CNs cross-linked with InsP6 were bigger in size than those of with TPP, which could be possibly due to the different molecular structure and reactive site of InsP6 and its higher negative net charge compared to TPP. Furthermore, TPPCNs were relatively mono-disperse with narrow range of size distribution (Figure 2A) compared to poly-disperse behavior of InsP6CNs having wide range of size distribution (Figure 2B). Thus, it is better to use TPP as cross-linker, if smaller CNs are required for a specific purpose. Of course, smaller InsP6CNs may also be obtained by applying diverse changes in synthesis procedure like using different concentrations of chitosan and InsP6 and different solvents, and also benefiting from some of the top-down techniques such as high pressure homogenizer (HPH).16 The zeta potential values for InsP6CNs and TPPCNs were -6.97 ± 2.36 mV and + 43.9 ± 12.51 mV, respectively (Figure 2C, 2D), which could be due to the higher negative charge density of InsP6 compared to that of TPP. The higher cationic surface charge of TPPCNs resulted in electrostatic repulsion between the nanoparticles and kept them dispersed in DI water, while there was relatively lower stability shown with InsP6CNs against agglomeration. However, in drug delivery systems, the immune responses are insignificant to negatively charged nanoparticles and they are reported to be less toxic than highly destructive cationic ones.17
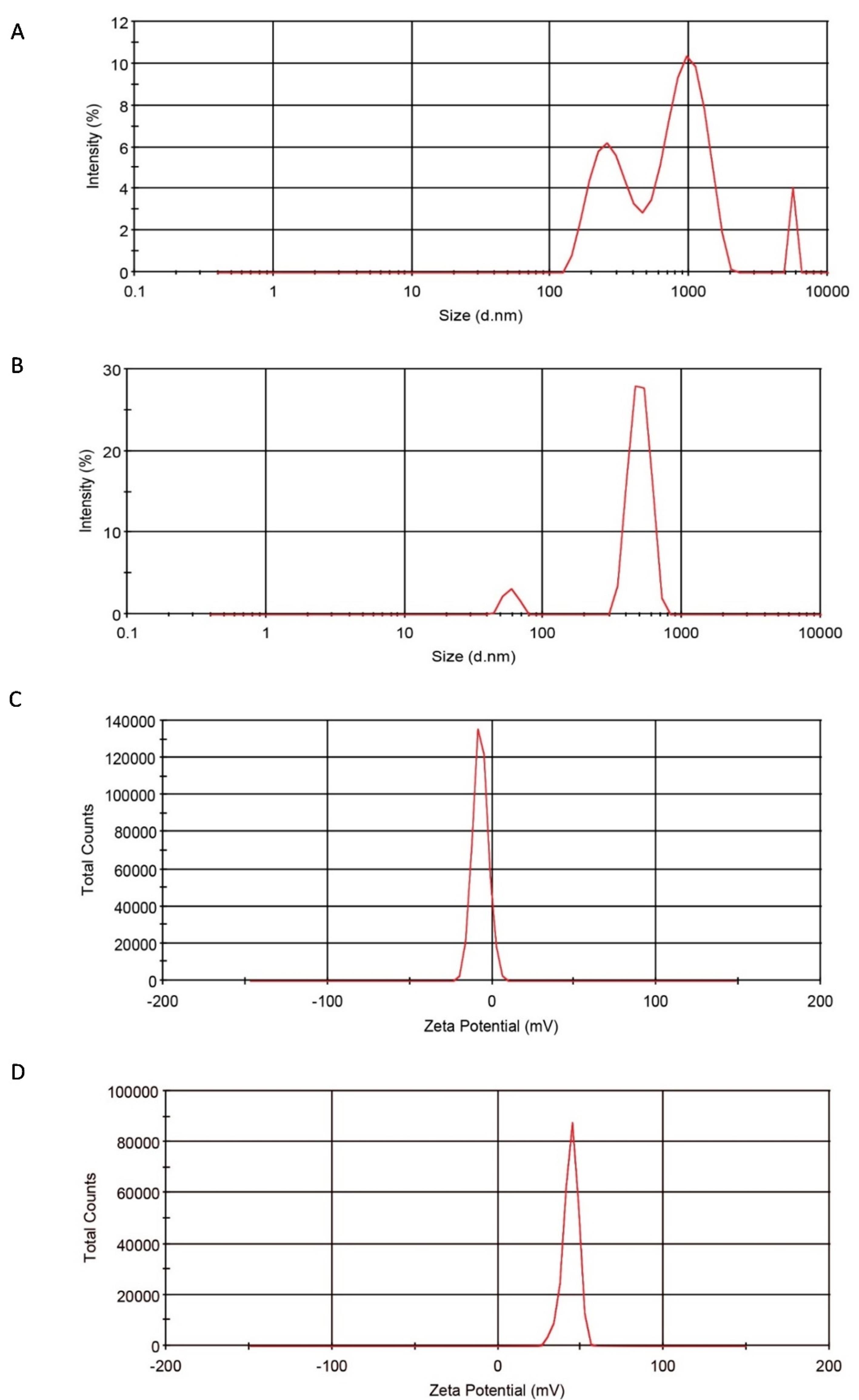
Figure 2.
Size distribution of InsP6CNs and TPPCNs (A, B), and zeta potential of InsP6CNs and TPPCNs (C, D)
.
Size distribution of InsP6CNs and TPPCNs (A, B), and zeta potential of InsP6CNs and TPPCNs (C, D)
In vitro cell viability assay
The therapeutic efficiency of InsP6CNsMTX and TPPCNsMTXwere studied compared to each other along with TPP, InsP6, MTX, chitosan, InsP6CNs, and TPPCNs using MTT assay on MCF-7 cell line after 24 h of incubation. As shown in Figure 3, after 24 hours, InsP6CNsMTX exhibited highest time-dependent cell cytotoxicity compared to other groups especially to those which were treated with TPPCNsMTX and free MTX, that may be due to the combination of higher MTX loading of InsP6CNsMTX and anti-cancer behavior of InsP6, which was shown in cells treated with free InsP6. Both of the blankInsP6CNs and TPPCNs showed cytotoxic behavior, mainly because of above-mentioned anti-cancer behavior of InsP6 involved within theInsP6CNs and high cationic charge of TPPCNs which disrupted the cell membrane and resulted in cell death. Interestingly, free chitosan enhanced the cell proliferation, indicating the fact that the cells might use chitosan as a power supply.
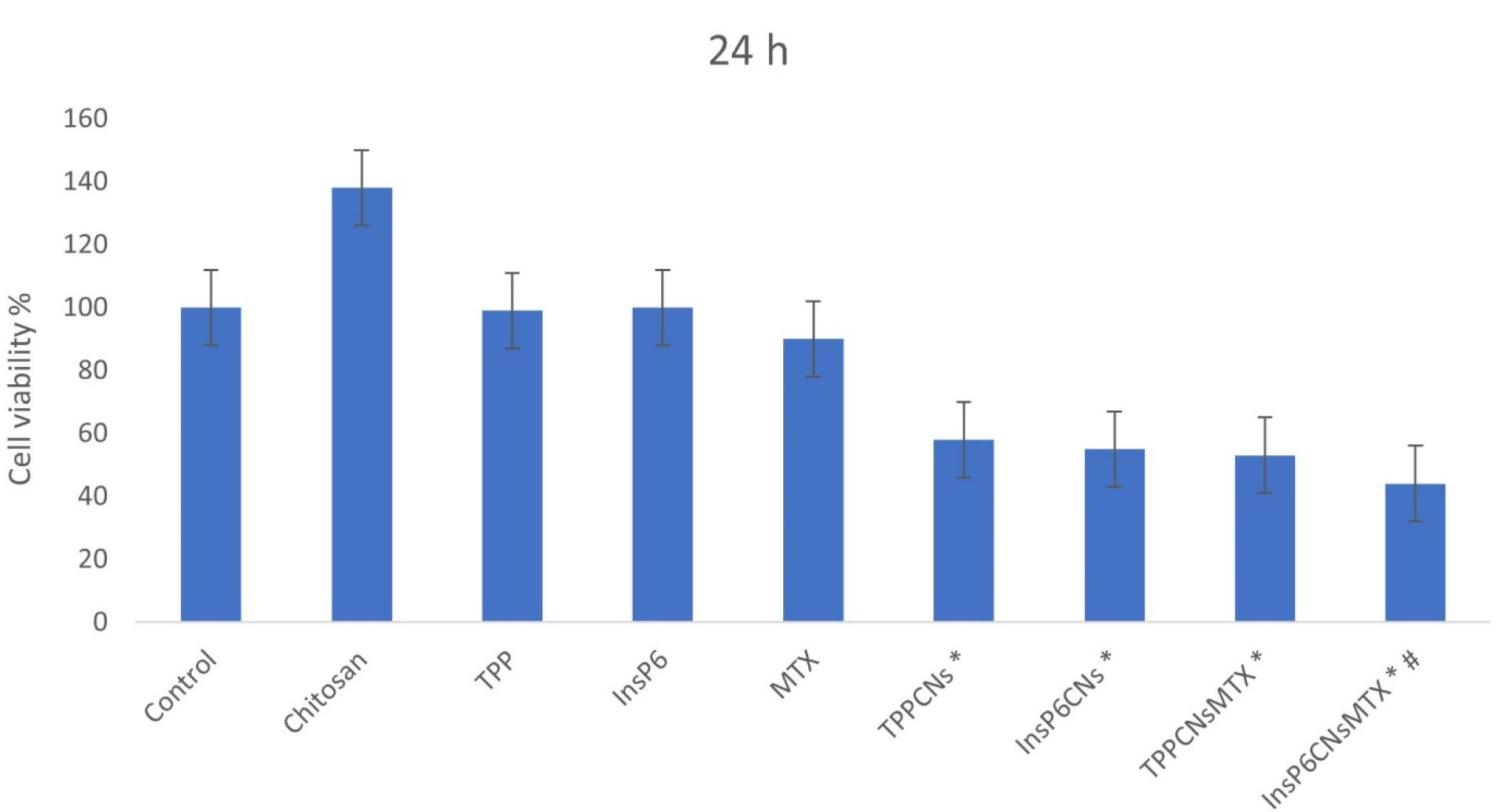
Figure 3.
Anti-proliferation effect of different formulations after 24 h against MCF-7 cells; n = 3; * p < 0.01
compared to control group, # P < 0.05 compared to MTX group
.
Anti-proliferation effect of different formulations after 24 h against MCF-7 cells; n = 3; * p < 0.01
compared to control group, # P < 0.05 compared to MTX group
In vitro cellular uptake
The in vitro cell uptake ability of InsP6CNsFITC and TPPCNsFITCon MCF-7 cells was evaluated quantitatively after 30 and 90 minutes using flow cytometry. Based on florescence intensity, both samples showed similar cell uptake behavior (Figure 4) and as expected, by increasing the incubation time to 90 min, the cell uptake was enhanced for both InsP6CNsFITC and TPPCNsFITC. Moreover, as MTX has similar structure to folic acid, it can act as a potential ligand for the overexpressed folate receptors onto the cancer cells allowing the InsP6CNsMTX and TPPCNsMTX into the cells through receptor-mediated endocytosis.14
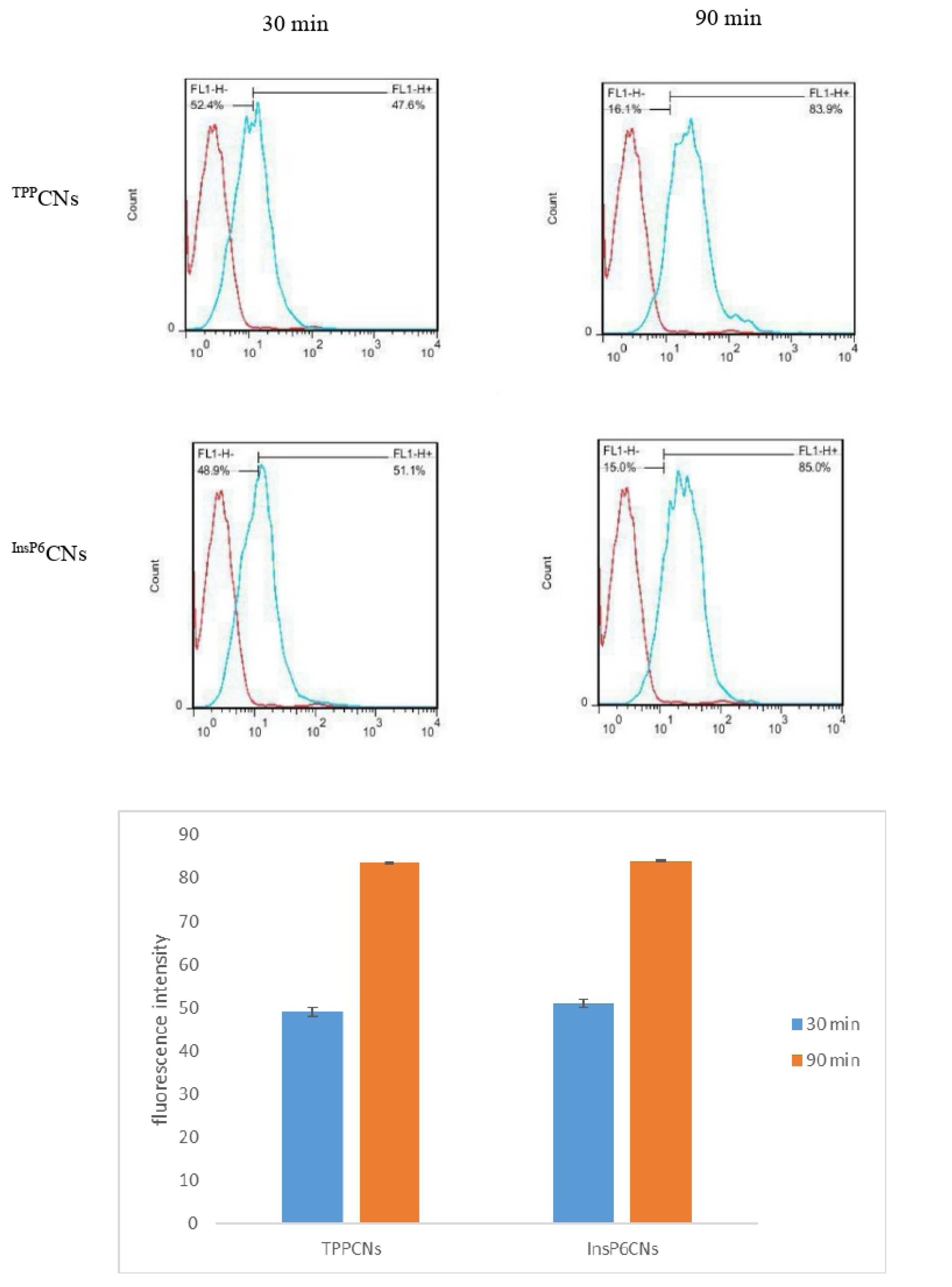
Figure 4.
Cellular uptake of FITC-tagged InsP6CNs and TPPCNs on MCF-7 cells after 30 and 90 min
.
Cellular uptake of FITC-tagged InsP6CNs and TPPCNs on MCF-7 cells after 30 and 90 min
Conclusion
In the current study, we presented InsP6 as a member of physical cross-linkers for CNs preparation. The synthesis procedure was quite similar for both of InsP6CNs and TPPCNs. Though, InsP6CNs were bigger in size, but they possessed negative zeta potential compared to highly cationic TPPCNs, which can reduce the following charge-related cytotoxicity. That is why the MTT assay emphasized on the cytotoxic behavior of blank TPPCNs on MCF-7 cells. Due to enhanced electrostatic interactions and better physical entrapment of MTX within the carrier, InsP6CNsMTX showed higher encapsulation efficiency than TPPCNsMTX. InsP6CNsMTX showed excellent time-dependent in vitro antitumor behavior compared to TPPCNsMTX and free MTX. Moreover, both InsP6CNs and TPPCNs presented similar in vitro cellular uptake capability towards MCF-7 cells. In conclusion, InsP6 can be employed as a promising physical cross-linker to attain efficient CNs for drug delivery applications.
Competing Interests
The authors declare that they have no competing interests. The authors alone are responsible for the content and writing of this article.
Ethical Approval
This study was approved by research ethical committee of Tabriz university of medical sciences.
References
- Felt O, Buri P, Gurny R. Chitosan: a unique polysaccharide for drug delivery. Drug Dev Ind Pharm 1998; 24(11):979-93. doi: 10.3109/03639049809089942 [Crossref] [ Google Scholar]
- Farshbaf M, Davaran S, Zarebkohan A, Annabi N, Akbarzadeh A, Salehi R. Significant role of cationic polymers in drug delivery systems. Artif Cells NanomedBiotechnol 2018; 46(8):1872-91. doi: 10.1080/21691401.2017.1395344 [Crossref] [ Google Scholar]
- Nag M, Gajbhiye V, Kesharwani P, Jain NK. Transferrin functionalized chitosan-PEG nanoparticles for targeted delivery of paclitaxel to cancer cells. Colloids Surf B Biointerfaces 2016; 148:363-70. doi: 10.1016/j.colsurfb.2016.08.059 [Crossref] [ Google Scholar]
- Al-Kassas R, Wen J, Cheng AE, Kim AM, Liu SSM, Yu J. Transdermal delivery of propranolol hydrochloride through chitosan nanoparticles dispersed in mucoadhesive gel. CarbohydrPolym 2016; 153:176-86. doi: 10.1016/j.carbpol.2016.06.096 [Crossref] [ Google Scholar]
- Birch NP, Schiffman JD. Characterization of self-assembled polyelectrolyte complex nanoparticles formed from chitosan and pectin. Langmuir 2014; 30(12):3441-7. doi: 10.1021/la500491c [Crossref] [ Google Scholar]
- Lai JY. Biocompatibility of genipin and glutaraldehyde cross-linked chitosan materials in the anterior chamber of the eye. Int J Mol Sci 2012; 13(9):10970-85. doi: 10.3390/ijms130910970 [Crossref] [ Google Scholar]
- Gadkari RR, Suwalka S, Yogi MR, Ali W, Das A, Alagirusamy R. Green synthesis of chitosan-cinnamaldehyde cross-linked nanoparticles: characterization and antibacterial activity. CarbohydrPolym 2019; 226:115298. doi: 10.1016/j.carbpol.2019.115298 [Crossref] [ Google Scholar]
- Fan W, Yan W, Xu Z, Ni H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf B Biointerfaces 2012; 90:21-7. doi: 10.1016/j.colsurfb.2011.09.042 [Crossref] [ Google Scholar]
- Thamaket P, Raviyan P. Preparation and physical properties of carotenoids encapsulated in chitosan cross-linked tripolyphosphate nanoparticles. Food Appl Biosci J 2015; 3(1):69-84. doi: 10.14456/fabj.2015.8 [Crossref] [ Google Scholar]
- Raina K, Kandhari K, Jain AK, Ravichandran K, Maroni P, Agarwal C. Stage-specific effect of inositol hexaphosphate on cancer stem cell pool during growth and progression of prostate tumorigenesis in TRAMP model. Cancers (Basel) 2022; 14(17):4204. doi: 10.3390/cancers14174204 [Crossref] [ Google Scholar]
- Liu G, Song Y, Cui L, Wen Z, Lu X. Inositol hexaphosphate suppresses growth and induces apoptosis in HT-29 colorectal cancer cells in culture: PI3K/Akt pathway as a potential target. Int J Clin Exp Pathol 2015; 8(2):1402-10. [ Google Scholar]
- Bizzarri M, Dinicola S, Bevilacqua A, Cucina A. Broad spectrum anticancer activity of myo-inositol and inositol hexakisphosphate. Int J Endocrinol 2016; 2016(1):5616807. doi: 10.1155/2016/5616807 [Crossref] [ Google Scholar]
- Sang Z, Qian J, Han J, Deng X, Shen J, Li G. Comparison of three water-soluble polyphosphate tripolyphosphate, phytic acid, and sodium hexametaphosphate as crosslinking agents in chitosan nanoparticle formulation. CarbohydrPolym 2020; 230:115577. doi: 10.1016/j.carbpol.2019.115577 [Crossref] [ Google Scholar]
- Farshbaf M, Salehi R, Annabi N, Khalilov R, Akbarzadeh A, Davaran S. pH- and thermo-sensitive MTX-loaded magnetic nanocomposites: synthesis, characterization, and in vitro studies on A549 lung cancer cell and MR imaging. Drug Dev Ind Pharm 2018; 44(3):452-62. doi: 10.1080/03639045.2017.1397686 [Crossref] [ Google Scholar]
- Marques Gonçalves M, Florencio Maluf D, Pontarolo R, Ketzer Saul C, Almouazen E, Chevalier Y. Negatively charged chitosan nanoparticles prepared by ionotropic gelation for encapsulation of positively charged proteins. Int J Pharm 2023; 642:123164. doi: 10.1016/j.ijpharm.2023.123164 [Crossref] [ Google Scholar]
- Wijesena RN, Tissera N, Kannangara YY, Lin Y, Amaratunga GA, de Silva KM. A method for top down preparation of chitosan nanoparticles and nanofibers. CarbohydrPolym 2015; 117:731-8. doi: 10.1016/j.carbpol.2014.10.055 [Crossref] [ Google Scholar]
- Clogston JD, Patri AK. Zeta potential measurement. Methods Mol Biol 2011; 697:63-70. doi: 10.1007/978-1-60327-198-1_6 [Crossref] [ Google Scholar]