Advanced pharmaceutical bulletin. 10(1):125-129.
doi: 10.15171/apb.2020.016
Research Article
Lactobacillus casei UT1 Isolated from Northwest of Iran Traditional Curd Exerts Anti-proliferative and Apoptosis Inducing Effects in Human Colorectal Tumor HCT 116 Cells
Mitra Rabiei , Gholamreza Zarrini *  , Majid Mahdavi
, Majid Mahdavi
Author information:
Department of Biology, Faculty of Natural Science, University of Tabriz, Tabriz, Iran.
*
Corresponding Author: Gholamreza Zarrini, Tel:+984133392707, Fax: +984133356027, Email:
zarrini@tabrizu.ac.ir
Abstract
Purpose:
The present study was mainly designed to assess anti-cancer effects of lactobacilli isolated from traditional dairy products, on HCT116 colorectal cancer cell lines.
Methods:
Traditional dairy products samples were collected from the region of Azarbayjan and the suspensions were cultured in MRS agar medium. The isolates were identified by biochemical and molecular methods. Isolated bacteria were cultured in MRS broth. Supernatants of the isolates cultures were collected and their cytotoxicity was evaluated on HCT116 cancer cells. Morphological changes of the treated cells by supernatant were observed using an inverted microscope. Cell metabolic activity was assessed by MTT assay. The morphology of apoptotic cells was examined using a fluorescent microscope. In cell cycle analysis, content measurement of DNA was performed by flow cytometry.
Results:
Out of 30 lactobacilli were isolated from dairy products samples, six isolates belong to curd samples. Cell-based assays showed that culture supernatant of one isolate (UT1) had a significant anticancer effect on colorectal HCT116 cell lines (P<0.05). The 16S rRNA sequence analysis revealed that the isolate UT1 was 99% compatible with Lactobacillus casei.
Conclusion:
It is noteworthy that the supernatant of L. casei UT1 can be candidate for studies on compounds having anti-cancer effect.
Keywords: Apoptosis, HCT116 cells, Lactobacillus, Probiotic
Copyright and License Information
© 2020 The Author (s)
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
One of the most important emerging fields in the food industry is the concept of “food function” or “functional foods”. For individual’s health promotion and/or disease prevention, a word “food function” can be often used with the meaning of pharmacological effect of food-stuffs as well as their ingredients.
1
Today, many of the dietary agents and natural health products have attracted the attention of scientists. One of them is probiotics.
2
Within the functional foods, the use of probiotics is rapidly expanding.
3
Probiotics are defined as “live microorganisms” that, when administered in adequate amounts, confer health benefits on the host.
4-8
Probiotics can be bacteria, moulds, yeast. But most probiotics are bacteria. Among bacteria, lactic acid bacteria (LAB) are more popular, and most probiotics belong to this group.
3
Probiotics, in the form of dairy foods containing LAB, have been consumed for centuries by humans.
9
During the past decade, the importance of LAB in the maintenance of gut health has increasingly been recognized. Accordingly, the possible health-related benefits associated with consumption of LAB as a dietary supplement are well-documented. In particular, the potentiality of dietary LAB to prevent chronic diseases such as cancer is so promising. Researches indicate that fermented milk products have been shown to have strong chemopreventive activity towards carcinogenesis.
10
A number of clinical studies have been performed on the ability of probiotic in the prevention, control and treatment of various cancers, especially the gastrointestinal tract. Due to the large quantities of probiotic bacteria in the gut, probiotics seem to be one of the most interesting candidates for the treatment of colorectal cancer.
11
HCT116 cell line as a type of carcinoma, expresses transforming growth factor beta 1 and beta 2. In addition, HCT116 has a mutation in codon 13 of Ras proto-oncogene.
12
In terms of useful properties, especially anti-cancer effects, the most important and common genera LAB in fermented foods, particularly dairy products, are lactobacilli.
13
Some studies show that increase in lactobacilli in the gut, by competing with pathogenic bacteria, reduce the production of some mutagenic compounds.
9
Some strains of lactobacilli and their fermented products could reduce the risks of certain types of cancer and inhibit the growth of certain tumors in vitro assays, animal studies, human studies, epidemiological and intervention studies.
14
During this project, due to the major role of dairy products probiotics to control intestinal health, anti-cancer effects of lactobacilli isolated from traditional dairy products, on HCT116 colorectal cancer cell lines are examined.
Materials and Methods
Sampling and isolation
Traditional dairy products samples were taken aseptically and transferred to microbiology lab. Different dilutions of the samples were made in physiological saline and inoculated on MRS (De Man, Rogosa and Sharpe) agar (HiMedia, India). Inoculated plates were incubated anaerobically for 48 hours at 37˚C. Appeared colonies were examined by gram staining and catalase test and lactobacilli were isolated.
15
Strains identification
Morphological and biochemical identification
The isolates were identified following morphological and biochemical characterization according to Bergey’s Manual of Systematic Bacteriology, and Voges-Proskauer (VP), carbohydrate fermentation (glucose, galactose, lactose, arabinose, maltose, mannitol, sorbitol, sucrose, xylose, melibiose, raffinose, trehalose), arginine hydrolysis and growth at different temperatures (15˚C and 45˚C) tests were performed.
16-18
Molecular identification
Molecular methods are important for bacterial identification.
19
For molecular characterization, PCR analysis of 16S rRNA gene was performed using the universal primers and followed by agarose gel electrophoresis. Sequence (performed by Bioneer, Germany) homologies were examined by comparing the obtained sequence with those in the DNA databases (http//www.ncbi.nim.nih.gov/BLAST)
16,20-22
and dendrogram of the isolates were depicted by Mega 5 software.
Preparation of cell-free supernatant
All the isolated bacteria were cultured in MRS broth (Biolife, Italy) and were incubated for 24 hours at 37˚C. Optical density (OD) of cultures was measured at 600 nm and the number of bacteria was calculated (CFU [colony-forming unit]= OD × 8 × 108). Supernatants were isolated by centrifugation (3000 rpm, 30 min) and were sterilized using a 0.22 µm filter.
23
Cell analysis
Cell culture
The HCT116 cell line were purchased from Pasteur institute of Iran and were cultured in RPMI (Gibco, England) medium with 10% FBS (Sigma, Germany).
24
Cell viability assay
The cells were cultured in 96 wells plate. 0.5, 1, 1.5, 2 and 5 × 107CFU/mL concentrations of the supernatants were added to the wells, respectively and cultures were incubated for 24, 48 and 72 hours at 37˚C and 5% CO2. Morphological changes of treated cells by supernatant were observedusing a microscope. After 72 h incubation, 20 μL of MTT solution (5 mg/ml) was added to each well and incubated for another 3 h. The medium was then removed and the blue formazan crystals were solubilized with 200 μL of Dimethyl sulfoxide (DMSO). MTT converted to formazan by metabolically viable cells and its absorbance was measured using an ELISA reader at 570 nm.
11,24,25
Fluorescent staining
After cell culture and treatment for 72 h, 20 μL of trypsin was added into each well. When cells had sloughed off, suspensions were transferred to micro-tubes. Dual fluorescent staining solutions (1 μL) containing 100 μg/mL acridine orange (AO) and 100 μg/mL ethidium bromide (EB) were added to each suspension and then covered with a coverslip.
26
The morphology of apoptotic cells was examined using a fluorescent microscope.
Cell cycle assay
Primary steps were performed according to the fluorescence test. After transferring of cell suspensions to micro-tubes, the cells were washed twice with phosphate buffered solutions (PBS) and were centrifuged. The pellet was dissolved in 50 μL of cold PBS and 450 μL of cold ethanol for 1 hour at 4˚C. The cells were centrifuged again for 5 minutes and the pellet was washed with PBS and cells were centrifuged. Then, the pellet was dissolved in PBS containing RNAase enzyme (20 μg/mL) and the cells were incubated for 30 minutes in 37˚C. Then, the cells were incubated with propidium iodide (50 μg/mL) for 1 hour. After these steps, the result was examined by flow cytometry.
27
Statistical analysis
All values are expressed as mean ± SD from three independent experiments. Statistical analysis was done using GraphPad Prism with two-way analysis of variance (ANOVA) and Tukey multiple comparison tests. P< 0.05 was considered statistically significant
Results and Discussion
Typically isolated colonies of Lactobacillus appeared on MRS agar were circular, convex, opaque, smooth and white to creamy.
21,28
During this study, 30 lactobacilli were isolated from dairy products samples and were designated as CT1 until CT3 (from “Lighvan” traditional cheese), JT1 until JT14 (from “jug” traditional cheese), ST1 until ST3 (from “Shoor” traditional dairy product), UT1 until UT6 (from traditional curd) and YT1 until YT2 (from traditional yogurt) based on their morphological characteristics. All isolates were Gram positive and catalase negative. Microscopic analyze of the isolates supernatants for primary screening identification of the highest strain in terms of cytotoxic activity was investigated (The results not shown) and the strain with the most cytotoxic effects (UT1) on tumor cells was selected for more evaluations.
The biochemical characteristics of Lactobacillus sp. UT1 is shown in Figure 1. Agarose gel electrophoresis confirmed sequence amplification in the PCR reaction (Figure 2). According to the results, Sequence comparison using BLAST nucleotide database from the National Center for Biotechnology Information (NCBI) confirmed that isolated strain UT1 belonged to species of Lactobacillus was identified as Lactobacillus casei (Figure 3). The sequence of strain UT1 was 99% similar to that in the Gene bank and was registered with accession number MF506844 in NCBI.
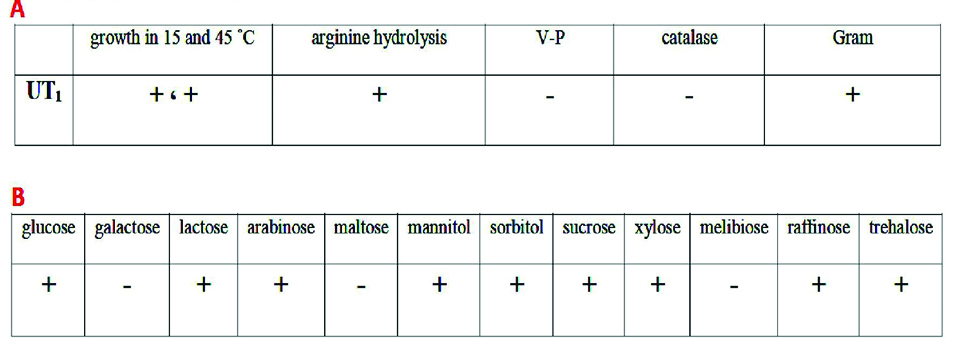
Figure 1.
A: Biochemical characteristics of Lactobacillus sp. UT1. B: carbohydrate fermentation results of Lactobacillus sp. UT1. (+: growth, -: no growth)
.
A: Biochemical characteristics of Lactobacillus sp. UT1. B: carbohydrate fermentation results of Lactobacillus sp. UT1. (+: growth, -: no growth)

Figure 2.
Agarose gel electrophoresis of PCR reaction product (A: DNA ladder: 50 bp, B: bound of UT1 sample, about 1500 bp).
.
Agarose gel electrophoresis of PCR reaction product (A: DNA ladder: 50 bp, B: bound of UT1 sample, about 1500 bp).
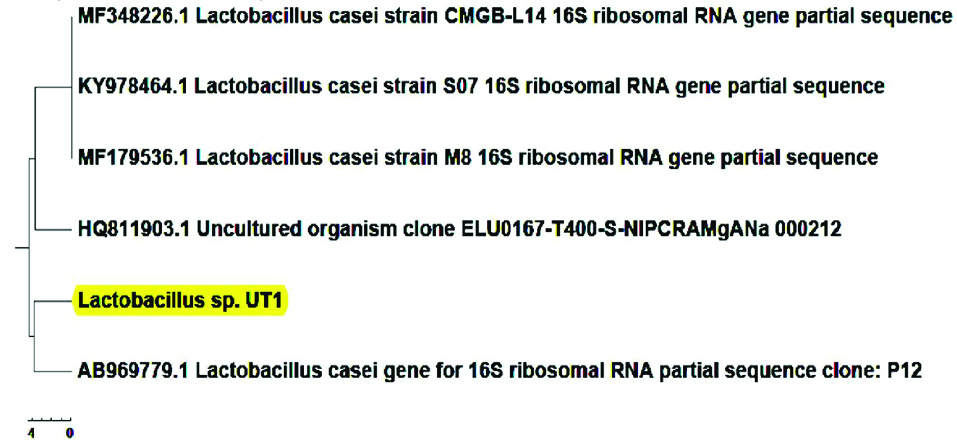
Figure 3.
Dendogram of Lactobacillus sp. UT1. The distance between the reference strains of similar lactobacilli and isolated strain have been shown.
.
Dendogram of Lactobacillus sp. UT1. The distance between the reference strains of similar lactobacilli and isolated strain have been shown.
Cell viability analysis using MTT assay provides evidence that traditional crude from UT1 has a cytotoxic activity (Figure 4). HCT 116 cells were incubated with MTT solution after treatment with 0.5-5×107 CFU/mL of the bacterial supernatant. As shown in Figure 4, the inhibition of cancer cells proliferation increased in a dose and time-dependent manner. Following 72 h exposure, the IC50 value of the bacterial supernatant was calculated 1×107 CFU/mL (Figure 4). As shown in inverted microscopy images (Figure 5), the untreated cells, apparently got wrinkled after treatment with an IC50 value of the bacterial supernatant. Cell rupturing and fragmentation observed as time increased. According to the result of fluorescent staining, after 24, 48 and 72 hours of the treatment, the incidence of apoptosis was observed (Figure 6). Apoptosis occurrence was also confirmed by flow cytometry in the cells. As shown in Figure 7, during treatment, the amount of cells in the sub G1 phase has increased compared to control cells. Increasing the presence of cells in the sub G1 phase indicated that the cells have transferred to the apoptotic stage. Therefore, evaluated metabolite in the present study can induce apoptosis in the HCT116 cells.

Figure 4.
Effect of lactobacilli supernatant on HCT116 cells proliferation for 24, 48 and 72 hours. The inhibition of cancer cells proliferation increased in a dose and time dependent manner (* P < 0.05).
.
Effect of lactobacilli supernatant on HCT116 cells proliferation for 24, 48 and 72 hours. The inhibition of cancer cells proliferation increased in a dose and time dependent manner (* P < 0.05).
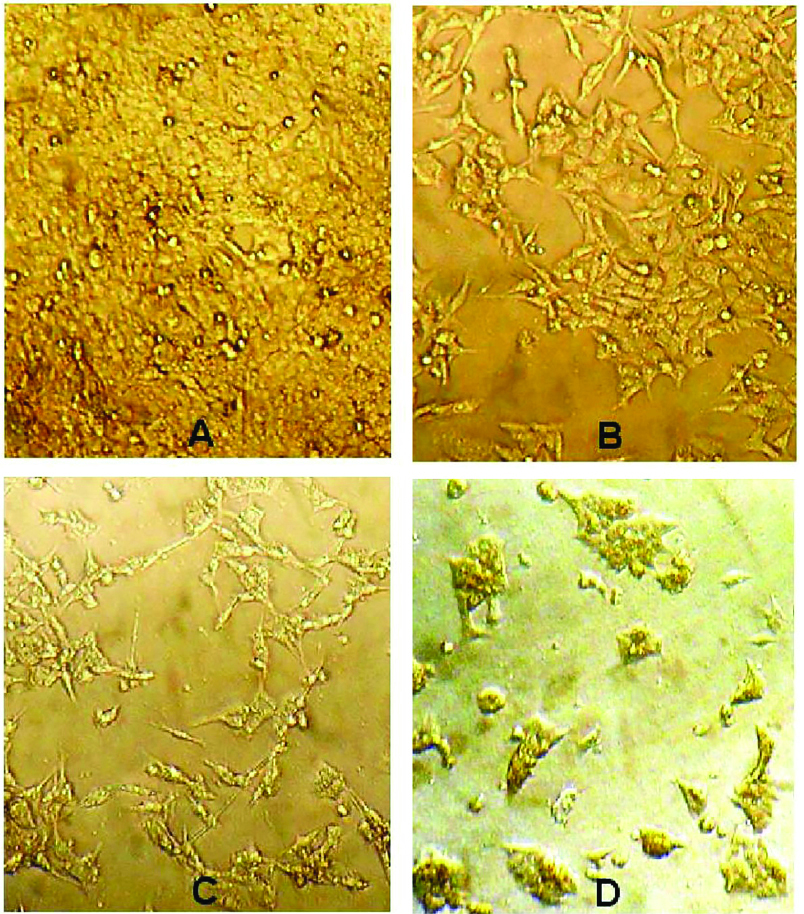
Figure 5.
Morphological changes of HCT 116 cells affected by the IC50 value (1×107 CFU/mL) of the bacterial supernatant (A: control cells, B: treated cells after 24 h, C: treated cells after 48 h, D: treated cells after 72 h).
.
Morphological changes of HCT 116 cells affected by the IC50 value (1×107 CFU/mL) of the bacterial supernatant (A: control cells, B: treated cells after 24 h, C: treated cells after 48 h, D: treated cells after 72 h).
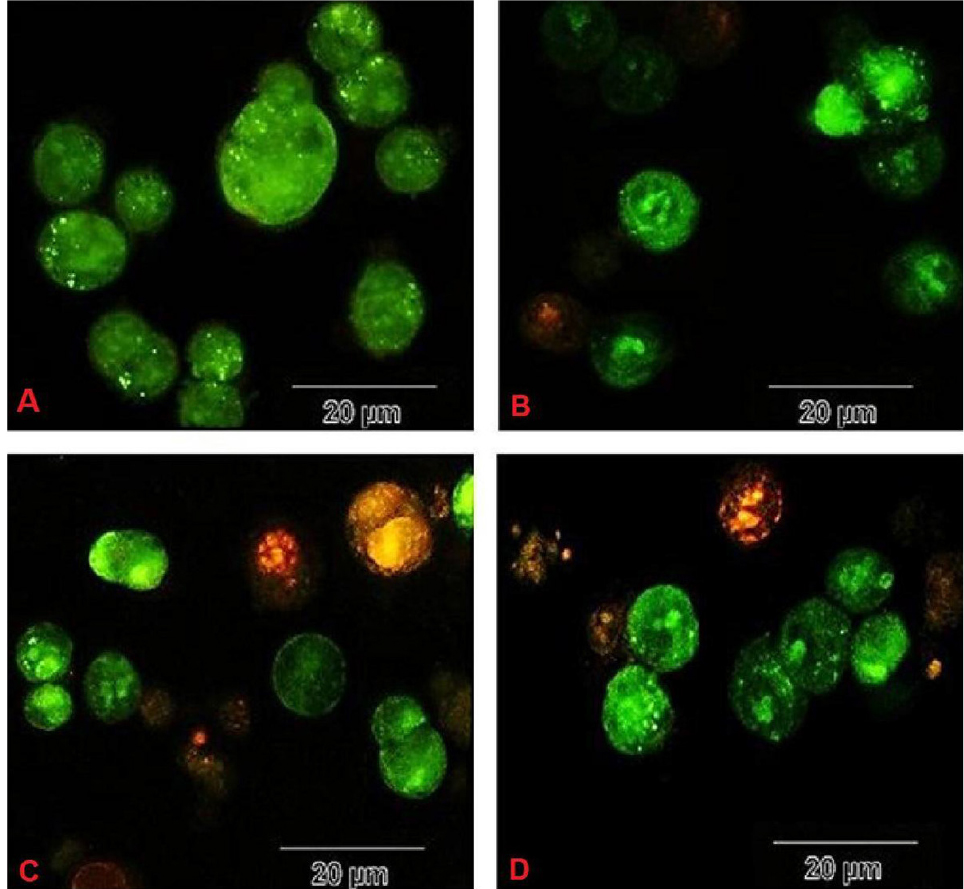
Figure 6.
The incidence of apoptosis in HCT116 cells during treatment with metabolites from the strain UT1 (A: negative control cells, B: treated cells in 24h, C: treated cells in 48h, D: treated cells in 72h). The circular nucleus uniformly distributed in the center of the cell in untreated (control) cells (A). Nucleus showed yellow-green fluorescence by acridine orange (AO) staining and concentrated into a crescent or granular that located in 1 side of cells in early apoptotic cells (treated cells). After 48-72 h treatment, late apoptosis (orange spots) are seen (C and D).
.
The incidence of apoptosis in HCT116 cells during treatment with metabolites from the strain UT1 (A: negative control cells, B: treated cells in 24h, C: treated cells in 48h, D: treated cells in 72h). The circular nucleus uniformly distributed in the center of the cell in untreated (control) cells (A). Nucleus showed yellow-green fluorescence by acridine orange (AO) staining and concentrated into a crescent or granular that located in 1 side of cells in early apoptotic cells (treated cells). After 48-72 h treatment, late apoptosis (orange spots) are seen (C and D).
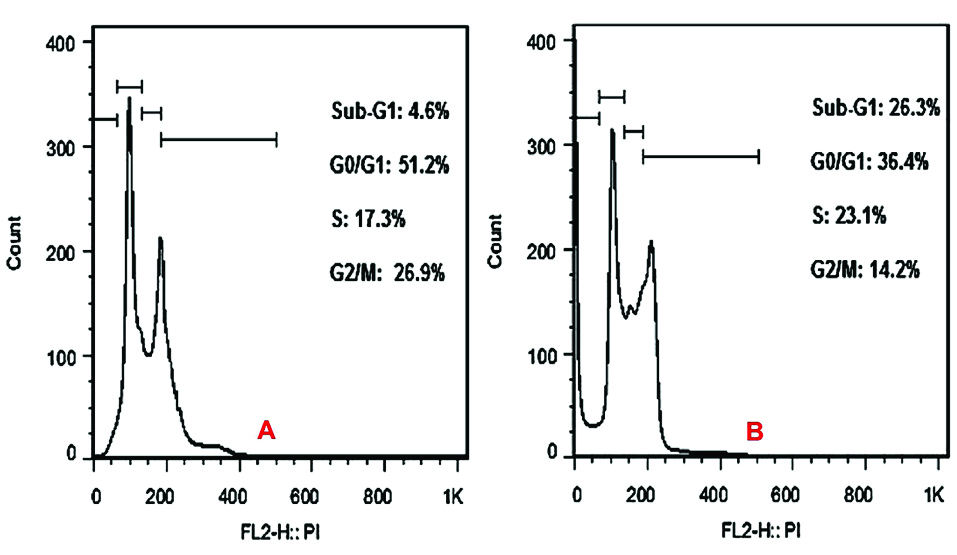
Figure 7.
The results of cell cycle assay. Increasing the amount of cells in the sub G1 phase compared to control cells is seen. According to the results, the presence of cells in the sub G1 phase has increased from 4.6% in control (A) to 26.3% in the treatment for 72h (B).
.
The results of cell cycle assay. Increasing the amount of cells in the sub G1 phase compared to control cells is seen. According to the results, the presence of cells in the sub G1 phase has increased from 4.6% in control (A) to 26.3% in the treatment for 72h (B).
The results indicated that L.casei UT1 shows the inhibitory effect of cancer cells proliferation, as well as apoptosis induction activity. We are currently attempting to identify which components in the supernatant are responsible for the observed effects. The supernatant of strain seems to contain compounds with anti-proliferative effects. The inhibition of cancer cell growth by Lactobacillus has also been reported in other studies. Choi et al in their study in 2005 showed that Lactobacillus acidophilus extract decreases cell survival compared to controls 21%-28%. These results compared to the values obtained in the present study are much lower inhibitory effect. Soltan Dallal et al in 2015 examined the anticancer effect of supernatant of L. acidophilus on the Caco-2 cells at a concentration similar to this project. It was observed that cytotoxic effects were no more than 38%.
11
Kahouli et al in 2015 evaluated cytotoxic effect of Lactobacillus fermentum supernatant on colorectal cancer cells.
25
The results were almost identical to the results of this project. Er et al in 2015 examined anti-cancer effect of the large number lactobacilli on the Caco-2 cells.
30
The results were not significant. Proper method and time for the release of metabolites can vindicate the significant cytotoxic effect of metabolites obtained from isolates in this project compared to other studies. In addition, the following resources to isolation of bacteria that are traditional dairy products, it is noteworthy that these compounds can significantly candidate for studies on compounds having anti-cancer effect.
Conclusion
Results achieved in this study suggest that the use of lactobacilli probiotics can serve as a promising tool to prevent the incidence of colorectal cancer. The use of probiotics to reduce intestinal inflammatory responses that predispose to colorectal cancer reduces the risk of developing this disease. Laboratory studies suggest that probiotic bacteria prevent forming and proliferation of tumors. The new findings suggest that the use of lactobacilli can be a new method in the prevention of colorectal cancer.
11
LAB pose as an avenue which can be exploited with both health and economic benefits.
31
Further research is currently underway to investigate the anticancer properties and anticancer mechanism of lactobacilli.
14,24,32
As well as, the identified isolate can be considered as starter cultures for their commercial uses.
Ethical Issues
The current article does not contain any studies with human or animal subjects.
Conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgements
The authors appreciate the support of this investigation by the research council of University of Tabriz, Tabriz, Iran.
References
- Yasuda S, Igoshi K. Anticancer Effect in HL-60 Human Leukemia Cells and Other Helath-Beneficial Functions of Cheese. Open J Blood Dis 2013; 3(3):7-10. doi: 10.4236/ojbd.2013.33A002 [Crossref] [ Google Scholar]
- Daniluk U. Probiotics, the New Approach for Cancer Prevention and/or Potentialization of Anti-Cancer Treatment?. J Clin Exp Oncol 2012; 1(2):1-2. doi: 10.4172/2324-9110.1000e105 [Crossref] [ Google Scholar]
- Suvarna VC, Boby VU. Probiotics in human health: a current assessment. Curr Sci 2005; 88(11):1744-8. [ Google Scholar]
- Kazemi Darsanaki R, Hassani Kolavani M, Mohammad Doost Chakoosari M, Ebadi Shalkeh S, Tajehmiri A. Biological control of aflatoxin B1 by probiotic bacteria. Trends Life Sci 2014; 1(1):8-11. [ Google Scholar]
- Bernardeau M, Guguen M, Vernoux JP. Beneficial lactobacilli in food and feed: long-term use, biodiversity and proposals for specific and realistic safety assessments. FEMS Microbiol Rev 2006; 30(4):487-513. doi: 10.1111/j.1574-6976.2006.00020.x [Crossref] [ Google Scholar]
- Pessi T, Sutas Y, Saxelin M, Kallioinen H, Isolauri E. Antiproliferative effects of homogenates derived from five strains of candidate probiotic bacteria. Appl Environ Microbiol 1999; 65(11):4725-8. [ Google Scholar]
- Niazi Amraii H, Abtahi H, Jafari P, Mohajerani HR, Fakhroleslam MR, Akbari N. In vitro study of potentially probiotic lactic acid bacteria strains isolated from traditional dairy products. Jundishapur J Microbiol 2014; 7(6):e10168. doi: 10.5812/jjm.10168 [Crossref] [ Google Scholar]
- Subhashini S, Lavanya J, Meignanalakshmi S. In Vitro Studies on Adhesion and the Effect of Cytotoxicity of Bifidobacterium spp Using Cell Lines. Eur Sci J 2013; 9(18):311-26. [ Google Scholar]
- Rafter JJ. The role of lactic acid bacteria in colon cancer prevention. Scand J Gastroenterol 1995; 30(6):497-502. doi: 10.3109/00365529509089779 [Crossref] [ Google Scholar]
- Kim JY, Woo HJ, Kim KH, Kim ER, Jung HK, Juhn HN. Antitumor Activity of Lactobacillus plantarum Cytoplasm on Teratocarcinoma-Bearing Mice. J Microbiol Biotechnol 2002; 12(6):998-1001. [ Google Scholar]
- Soltan Dallal MM, Mojarrad M, Baghbani F, Raoofian R, Mardaneh J, Salehipour Z. Effects of probiotic Lactobacillus acidophilus and Lactobacillus casei on colorectal tumor cells activity (CaCo-2). Arch Iran Med 2015; 18(3):167-72. [ Google Scholar]
- Brattain MG, Fine WD, Khaled FM, Thompson J, Brattain DE. Heterogeneity of malignant cells from a human colonic carcinoma. Cancer Res 1981; 41(5):1751-6. [ Google Scholar]
- Nami Y, Abdullah N, Haghshenas B, Radiah D, Rosli R, Khosroushahi AY. Assessment of probiotic potential and anticancer activity of newly isolated vaginal bacterium Lactobacillus plantarum 5BL. Microbiol Immunol 2014; 58(9):492-502. doi: 10.1111/1348-0421.12175 [Crossref] [ Google Scholar]
-
Wang S, Zhang L, Gu W. Effects of lactobacillus strains on colon cancer cell proliferation and cell cycle blockage. In International Conference on Biomedical Engineering and Biotechnology IEEE; 2012. p. 1015-8. 10.1109/iCBEB.2012.183.
- Kermanshahi RK, Peymanfar S. Isolation and identification of lactobacilli from cheese, yoghurt and silage by 16S rDNA gene and study of bacteriocin and biosurfactant production. Jundishapur J Microbiol 2012; 5(4):528-32. doi: 10.5812/jjm.3444 [Crossref] [ Google Scholar]
- Bassyouni RH, Abdel-all WS, Fadl MG, Abdel-all S, kamel Z. Characterization of lactic acid bacteria isolated from dairy products in Egypt as a probiotic. Life Sci J 2012; 9(4):2924-33. [ Google Scholar]
- Dodamani S, Kaliwal B. Identification and characterization of Lactococcus garvieae and antimicrobial activity of its bacteriocin isolated from cow’s milk. Asian J Pharm Clin Res 2013; 6 Suppl 3:104-8. [ Google Scholar]
- Havaz E. Isolation and identification of probiotic lactic acid bacteria from curd and in vitro evaluation of its growth inhibition activities against pathogenic bacteria. Afr J Microbiol Res 2014; 8(13):1419-25. doi: 10.5897/AJMR2014.6639 [Crossref] [ Google Scholar]
-
Beasley S. Isolation, identification and exploitation of lactic acid bacteria from human and animal microbiota. University of Helsinki; 2004.
- Chang CK, Wang SC, Chiu CK, Chen SY, Chen ZT, Duh PD. Effect of lactic acid bacteria isolated from fermented mustard on immunopotentiating activity. Asian Pac J Trop Biomed 2015; 5(4):281-6. doi: 10.1016/S2221-1691(15)30346-4 [Crossref] [ Google Scholar]
- Jose NM, Bunt CR, Hussain MA. Comparison of microbiological and probiotic characteristics of lactobacilli isolates from dairy food products and animal rumen contents. Microorganisms 2015; 3(2):198-212. doi: 10.3390/microorganisms3020198 [Crossref] [ Google Scholar]
- Plengvidhya V, Breidt F, Jr Jr, Lu Z, Fleming HP. DNA fingerprinting of lactic acid bacteria in sauerkraut fermentations. Appl Environ Microbiol 2007; 73(23):7697-702. doi: 10.1128/aem.01342-07 [Crossref] [ Google Scholar]
- Hu P, Song W, Shan Y, Du M, Huang M, Song C. Lactobacillus paracasei subsp paracasei M5L induces cell cycle arrest and calreticulin translocation via the generation of reactive oxygen species in HT-29 cell apoptosis. Food Funct 2015; 6(7):2257-65. doi: 10.1039/c5fo00248f [Crossref] [ Google Scholar]
- Sadeghi-Aliabadi H, Mohammadi F, Fazeli H, Mirlohi M. Effects of Lactobacillus plantarum A7 with probiotic potential on colon cancer and normal cells proliferation in comparison with a commercial strain. Iran J Basic Med Sci 2014; 17(10):815-9. doi: 10.22038/IJBMS.2014.3459 [Crossref] [ Google Scholar]
- Kahouli I, Malhotra M, Alaoui-Jamali M, Prakash S. In-Vitro Characterization of the Anti-Cancer Activity of the Probiotic Bacterium Lactobacillus Fermentum NCIMB 5221 and Potential against Colorectal Cancer. J Cancer Sci Ther 2015; 7(7):224-35. doi: 10.4172/1948-5956.1000354 [Crossref] [ Google Scholar]
- Liu K, Liu PC, Liu R, Wu X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med Sci Monit Basic Res 2015; 21:15-20. doi: 10.12659/msmbr.893327 [Crossref] [ Google Scholar]
- Yun JM, Afaq F, Khan N, Mukhtar H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, induces apoptosis and cell cycle arrest in human colon cancer HCT116 cells. Mol Carcinog 2009; 48(3):260-70. doi: 10.1002/mc.20477 [Crossref] [ Google Scholar]
- Zahid M, Ashraf M, Arshad M, Muhammad G, Yasmin A, Hameed HM. Antimicrobial Activity of Bacteriocins Isolated from Lactic Acid Bacteria Against Resistant Pathogenic Strains. Int J Nutr Food Sci 2015; 4(3):326-31. doi: 10.11648/j.ijnfs.20150403.20 [Crossref] [ Google Scholar]
- Choi SS, Kim Y, Han KS, You S, Oh S, Kim SH. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett Appl Microbiol 2006; 42(5):452-8. doi: 10.1111/j.1472-765X.2006.01913.x [Crossref] [ Google Scholar]
- Er S, Koparal AT, Kivanç M. Cytotoxic effects of various lactic acid bacteria on Caco-2 cells. Turk J Biol 2015; 39(1):23-30. doi: 10.3906/biy-1402-62 [Crossref] [ Google Scholar]
- Nandhini B, Palaniswamy M. Anticancer effect of goat milk fermented by Lactobacillus plantarum and Lactobacillus paracasei. Int J Pharm Pharm Sci 2013; 5(3):898-901. [ Google Scholar]
- Azadnia P, Khan Nazer AH. Identification of lactic acid bacteria isolated from traditional drinking yoghurt in tribes of Fars province. Iran J Vet Res 2009; 10(3):235-40. doi: 10.22099/ijvr.2009.1698 [Crossref] [ Google Scholar]