Advanced pharmaceutical bulletin. 10(2):184-202.
doi: 10.34172/apb.2020.023
Review Article
The Role of Natural Antioxidants Against Reactive Oxygen Species Produced by Cadmium Toxicity: A Review
Velid Unsal 1, *  , Tahir Dalkıran 2
, Tahir Dalkıran 2  , Mustafa Çiçek 3
, Mustafa Çiçek 3  , Engin Kölükçü 4
, Engin Kölükçü 4 
Author information:
1Faculty of Health Sciences and Central Research Laboratory, Mardin Artuklu University, Mardin, Turkey.
2Department of Pediatric Intensive Care, Necip Fazıl City Hospital, 46030, Kahramanmaras, Turkey.
3Department of Anatomy, Faculty of Medicine, Kahramanmaraş Sütçü imam University, Kahramanmaras, Turkey.
4Department of Urology, Faculty of Medicine, Gaziosmanpasa University,Tokat, Turkey.
Abstract
Cadmium (Cd) is a significant ecotoxic heavy metal that adversely affects all biological processes
of humans, animals and plants. Exposure to acute and chronic Cd damages many organs in humans and animals (e.g. lung, liver, brain, kidney, and testes). In humans, the Cd concentration at birth is zero, but because the biological half-life is long (about 30 years in humans), the concentration increases with age. The industrial developments of the last century have significantly increased the use of this metal. Especially in developing countries, this consumption is higher. Oxidative stress is the imbalance between antioxidants and oxidants. Cd increases reactive oxygen species (ROS) production and causes oxidative stress. Excess cellular levels of ROS cause damage to proteins, nucleic acids, lipids, membranes and organelles. This damage has been associated with various diseases. These include cancer, hypertension, ischemia/perfusion, cardiovascular diseases, chronic obstructive pulmonary disease, diabetes, insulin resistance, acute respiratory distress syndrome, idiopathic pulmonary fibrosis, asthma, skin diseases, chronic kidney disease, eye diseases, neurodegenerative diseases (amyotrophic lateral sclerosis, Parkinson’s disease, Alzheimer’s disease, and Huntington disease). Natural antioxidants are popular drugs that are used by the majority of people and have few side effects. Natural antioxidants play an important role in reducing free radicals caused by Cd toxicity. Our goal in this review is to establish the relationship between Cd and oxidative stress and to discuss the role of natural antioxidants in reducing Cd toxicity.
Keywords: Cadmium, Oxidative stress, ROS, Natural antioxidants
Copyright and License Information
© 2020 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Cadmium
Cadmium (Cd) is a bright silvery-white soft metal in group II B of the periodic table. Cd has an atomic number of 48, an atomic weight of 112.41 u and a density of 8.64 g/cm3.
1,2
Cd is not pure in nature. However, it was purified for the first time in 1817 and had a commercial designation in the early 1900s.
3
Present in the ground at a rate of 0.15-0.2 mg/kg, Cd is obtained as a by-product during the decomposition of zinc ore.
4
In the United Kingdom and the United States, some black clay deposits contain high levels of Cd, and there is Cd at high concentrations in the ground. The main source of Cd atmospheric release is volcanic activity. It is thought that the amount of Cd released by volcanic activity is between 100-500 tons. Volcanic movements in deep seas are also one of the natural sources of Cd.
5
Cd usage areas
Cd is an easily formable metal due to its physically soft nature. It has a wide range of applications since it has excellent properties against corrosion and has important properties in metal usage such as low melting temperature.
6
Cd is most commonly used in the construction of nickel-Cd batteries.
7,8
Cd is widely used as a coating material in PVC and shipbuilding industry due to its resistance to oxidation. Cd sulfur compounds are also used as color materials in the production of plastic, glass, ceramic, rubber, paint and fireworks.
9,10
In addition, household goods, automobiles and trucks, agricultural tools, aircraft parts, industrial tools, hand tools and fasteners (screw nuts, bolts, screws and nails) are generally covered with Cd. It is also used in tire repair and photography.
11
The effects of Cd
In humans, Cd exposure is due to food, inhalation and predominantly cigarette smoke.
12
Exposure through the skin is less. Cadmium chloride (CaCl2) is the major form of water-soluble Cd in high concentrations and is highly well absorbed from the mouth. Cadmium oxide (CaO) is the most common form exposed through respiration.
13
Many occupational groups are exposed to significant proportions. Among these occupational groups, workers in mining, paint and battery factories are particularly exposed to significant amounts of Cd dust and fumes. Following these groups, smokers are those most exposed to Cd.
14
One cigarette contains 1.5-2 μg of Cd. About 10% of it goes to cigarette smoke, and during smoking, 50% of this cigarette is absorbed by the lungs. Approximately 1 μg of Cd enters the body of a person smoking 20 cigarettes a day.
15
Studies have shown that the Cd levels of smokers are 3-4 times higher than those of non-smokers.
16,17
In non-smokers, Cd exposure is usually linked to food intake. The daily amount of Cd consumed by foodstuffs is about 10-25 μg but may vary with environmental Cd rates. For example, in Japan, the amount of Cd taken by food is 28 μg/day compared to 9.9 μg/day in China and 9-10 μg/day in Germany.
18
In contaminated areas, Cd intake with food can reach several hundred micrograms per day. Drinking water usually contains Cd in low quantities, and the approximate amount is 1 μg/L or less.
19,20
Cd is one of the most effective carcinogenic metals for humans. Cd and its compounds have been described among human carcinogens in 1993 by the International Organization for Cancer Research.
21
Cd affects cellular proliferation, differentiation, apoptosis and other cellular activities.
22
Prolonged exposure to Cd can result in lung, prostate, testicular, and kidney cancer. Because the half-life of Cd is 10 to 30 years, the effect of this metal on it may be carcinogenic.
23
Inactivation of tumor suppressor genes causes the deterioration of cell adhesion mechanism, triggering of apoptosis, suppression of DNA repair, free radical production and affecting the antioxidant system.
24,25
Cd can easily penetrate the body and pass into the cytoplasm via the calcium channels through the cell membrane. It can then bind to intracellular molecules, accumulate in the cell, cause metabolic transformation, or be dislodged. High levels of Cd are bound by Cd by glutathione, sulfhydryl-rich soluble proteins and metallothioneins (MTs) in the cell to try to reduce the intracellular and extracellular fluid levels.
26
Cd causes the release of free oxygen radicals such as superoxide (O2-), hydroxyl (OH-), nitric oxide (NO) and hydrogen peroxide (H2O2) in the organism like other heavy metals.
27
Cd causes peroxidation in membrane lipids, degradation of the antioxidant defense system, the emergence of inflammation, protein structure disorders and the oxidation of nucleic acids, and it negatively affects the DNA repair mechanism. Cd is a very potent toxic metal that indirectly contributes to the production of free oxygen radical species.
28-30
Cd and reproductive system
Exposure to Cd causes a decrease in reproductive performance evaluates such as fertility, abnormal embryonic development, prenatal death and sexual dysfunction.
31
Prolonged exposure may cause structural and functional disturbances in the male and female reproductive system.
32,33
In males, it causes a decrease in sperm motility and spermatogenesis index.
34
Acute Cd-induced damage to the testes manifests itself with hemorrhagic inflammation, organ degeneration and dysfunction and vacuolization of the seminiferous tubules. It has been reported that after high doses of Cd, the seminiferous tubule diameter and the tubular volume density decrease markedly.
32
In one study, 39.98% of the reproductive capacities of rats that received Cd for 52 weeks orally were found to be reduced.
35
In experimental studies on female rats, it has been reported that Cd ovulation is inhibited and that it directly reduces progesterone production by acting directly on granulosa cell morphology and steroid biosynthesis.
36,37
Another in vivo study on female rabbits reported that when Cd chloride was administered at a dose of 1.5 mg/kg, there was a decrease in the number of primary follicles and an increase in the number of atretic follicles in the ovary.
38
Cd and cardiovascular system
Cd can be stored in the heart, like kidney, liver. However, when compared to the kidney and liver, it has been reported that the concentration of Cd in the heart tissue is relatively low. In a study in rats, 50 ppm Cd+2 per day was applied to rats’ diets for 7 weeks. Studies have reported that Cd+2 accumulation occurs in cardiac tissues at ratios of 0.55-1.22 μg.
39
Cd has effects on cardiac tissue. Two important hypotheses have been proposed regarding this topic: the effects of Cd on cardiac tissue structure and integrity and its effects on the cardiac conduction system.
40
The first is based on increased reactive oxygen species (ROS) with increased oxidative stress and necrosis and ultrastructural changes in cells with increased ROS. The second is based on the interaction of Cd with contraction and acceleration systems.
41,42
Cd has been shown to have the potential to serve as a new risk factor on the cardiovascular system, as demonstrated by in vitro animal studies and human studies.
43
Studies show that Cd leads to metabolic and structural disorders in the heart and that it plays a role in the etiology of hypertension even at low concentrations.
44-46
In a recent study, Cd content in serum, hair, and nails of patients with hypertensive and coronary heart disease was compared to those of a healthy control group, and as a result, it was reported that Cd content increased in various tissues of the patient group.
47
Another study found that urinary Cd, the biological indicator of long-term Cd exposure, was associated with increased cardiovascular mortality and increased incidence of cardiovascular disease.
48
These studies support that Cd exposure is a cardiovascular risk factor. However, there are also publications that show the opposite. Vivoli et al reported that urine and hair Cd contents in the hypertensive patient group were similar to those of normotensive individuals, but urinary Cd/copper ratio was significantly lower in hypertensive patients.
49
Cd causes atherosclerosis. It has been reported that the incidence of atherosclerosis is increased in people living in areas with Cd contamination. These findings may shed light on the etiology of atherosclerosis, which is common in smokers.
50
There are various hypotheses on this subject. According to the information available, the dominant hypothesis explaining the effect of Cd on atherosclerosis is the “response to injury hypothesis” proposed by Ross. According to this hypothesis, it is a functional disorder that initiates primary endothelial damage and/or diseases. Cd reduces endothelial barrier function by causing degradation of endothelial cell-cell adhesions, and death of endothelial cells was demonstrated in vivo and in vitro.
51
Cd and placenta
The placenta is a versatile organ that protects the fetus and plays an important role in its development and in placenta functions between mother and fetus during pregnancy. Some lesions developing on the placenta may adversely affect fetal life.
52-54
In addition, it acts as a filter in reducing the passage of harmful substances and protects the embryo and then the fetus without exposure to contaminants.
55
Cd can pass through the mother and to the fetus through the placenta.
56-57
Human placenta is sensitive to toxic Cd activity. Experimental studies have shown the administration of Cd salts in the late period of pregnancy to cause placental damage. Early exposure to Cd in particular is thought to have an impact on baby health, such as neurological, developmental and endocrine disorders.
55
Cd also affects endocrine hormone synthesis (eg, placental progesterone or leptin) and changes trophoblast cell migration.
58
In one study, there was a significant negative correlation between the concentration of Cd in the cord blood and TSH concentration in the neonatal blood.
59
In addition, Cd can damage the fetus by affecting the metabolism of elements such as zinc, copper, iron and selenium.
14
Cd and brain
Cd enters the central nervous system either by smell or by altering the permeability of the blood-brain barrier. Cd causes neurotoxicity with complex pathology-involving behavioral changes, brain biochemical defects, and neurological dysfunction.
60
In the central nervous system, Cd causes oxidative stress and histologically observable membrane disorders due to the decrease of acetylcholinesterase activity, the increase of oxidative stress symptoms and the depletion of glutathione, superoxide dismutase and other antioxidants.
61
Cd and kidney
The kidney is one of the major organs affected by different ways of exposure to Cd. Whether acute or chronic, it is adversely affected in all circumstances. In chronic Cd exposure, approximately 50% of the accumulated dose is stored in the kidneys.
62,63
Acute toxicity occurs when too much Cd is inhaled or taken orally. Acute exposure rarely results in death. The exposure resulting in death is 20-30 mg/kg for humans.
64,65
Exposure to Cd-oxide vapors at doses of 5 mg/m3 for 8 hours has been reported to be lethal to humans.
66
This stored amount represents the amount of Cd not bound to MT. The S-1 segment of the kidney proximal tubules is the main target site for Cd deposition. Cd inhibits reabsorption of protein, amino acid, glucose, bicarbonate and phosphate in the proximal tubules, resulting in tissue damage. It induces apoptosis in tubular cells, causing oxidative stresses in transport proteins and mitochondria.
67,68
Cd disrupts vitamin D metabolism in the kidneys, causing a devastating effect on the bones. This effect causes absorption of calcium from the intestines and impairs collagen metabolism, thus resulting in osteomalacia and osteoporosis. The most important example of this is the Ittai Itai disease in Japan. Renal tubular dysfunction, impaired calcium absorption, anemia and osteomalacia cause severe pain in this disease.
69,70
The earliest sign of kidney damage is the asset of low molecular weight proteins. These are B2 microglobulin (b2M), retinol binding protein (RBP) and enzymes like N-acetyl-β-D-glucosaminidase (NAG). Detection of b2M and RBP in urine provides information about impairment of proximal tubular cell function. Thus, the urinary RBP and b2M are defined as biological markers of proximal tubular dysfunction.
64,71
Cd and endocrine system
Cd has adverse effects on the endocrine system. According to previous research, hormones are affected negatively.
72,73
Endocrine disrupting chemicals are natural or synthetic agents that mimic, enhance or inhibit the effects of endogenous hormones, and recent reports suggest that Cd may be added to endocrine disrupting chemicals as it has the potential to mimic the estrogenic effects of various tissues.
74,75
Jancic and Stosic reported that Cd tends to accumulate not only in the liver, kidneys, or other organs but also in the thyroid gland. They found that the concentration of Cd in the blood is positively correlated with accumulation in the thyroid gland.
76
Chronic exposure to Cd causes many histological and metabolic changes in the thyroid gland.
77
In a study, elevated blood levels of Cd have been associated with suppressed TSH production. However, the increase in Cd to urine ratio has also been correlated with increase in triiodothyronine (T3) and thyroxine (T4) serum levels.
78
In a rat experiment, it was determined that Cd caused calcitonin, synaptophysin, chromogranin A and somatostatin secretion. Ca+2 levels in the serum decreased considerably in these animals. In addition, Cd may impair the metabolism of Ca, as well as other basic metals such as Zn, Se and iodine and the structure and function of thyroid follicular cells in female rats chronically exposed to Cd.
76,79
Cd and liver
The liver plays an important role in maintaining body homeostasis in living organisms. Plasma proteins have functional properties such as construction, regulation of blood composition, detoxification and hormone inactivation.
80,81
The liver is the organ most affected by Cd through all exposure patterns. Cd-induced hepatotoxicity depends on the amount and duration of exposure. As in other tissues and organs, histopathological and metabolic changes occur in the liver, along with some histopathological changes such as loss of normal architecture of the parenchymatous tissue, cytoplasmic vacuolization, cellular degeneration and necrosis, congested blood vessels, destructed mitochondria cristae, fat globules, severe glycogen depletion, lipofuscin pigments, and collagenous fibers.
82,83
These changes may result in both apoptosis and necrosis.
83,84
Mitochondrial-mediated apoptosis may be involved in metal-induced cell deaths. Hepatotoxicity is thought to be caused mainly by the binding of Cd to thiol groups in the mitochondria, leading to mitochondrial dysfunction and related injury.
83
Cd triggers a programmed creation of necrotic cell-killing by the rupture of lysosomes.
41
Increased serum concentrations of amino acids in the urea cycle have been reported in individuals exposed to Cd via diet. This is also an indication of kidney damage.
85
Plasma ALT, AST and GGT enzyme levels are known to be the most important indicators for evaluating the structural and functional status of liver tissue.
86
Cd causes structural and functional impairment in cells by increasing lipid peroxidation. Cd also leads to an increase in blood enzyme levels by transferring these enzymes to the blood and disrupting the membrane permeability of the cells.
87,88
Oxidation of lipids results in degradation of the membrane structure by degradation and crosslinking of polymerization.
89
Cd and bone
Recent studies have established a target for Cd in the bone even at low exposures, as in other tissues and organs.
14,90
Chronic Cd exposure is associated with bone loss, low bone mass, and an increase in fracture incidence. The function of osteoblasts and osteoclasts, and the cells associated with the bones that resemble the bone include development, repair, and skeletal renewal, respectively. Adult skeleton is interestingly dynamic, with the rebuilding of the bone in response to mechanical and metabolic demands. It is not known what effect Cd has on bone loss at the cellular level. Under comparable incubation conditions in organ and cell culture systems, bone resorption appears to be more sensitive than bone formation versus Cd exposure. Cd toxicity reduces phosphate uptake of Fibroblast growth factor 23 (FGF-23) in bones and causes osteomalacia along with phosphaturia.
91
FGF-23 plays an important role in balancing mineral ion homeostasis and bone mineralization.
92
Cd is toxic to an osteoblast variety, MC3T3, by an unknown mechanism and activates osteoclasts and causes osteoporosis.
93-94
In a study on rats, Cd has been reported to reduce serum osteocalcin levels.
95
In children exposed to Cd ‘calciuria’, increased bone resorption and reduced bone mineral concentration have been observed.
96
It has been reported that administration of Cd to rats at a dose of 0.5 mg/kg three times a week increased the risk of osteoporosis by reducing the biomechanical quality of the bone.
97
In another animal study, single-dose oral Cd has been shown to increase calcium excretion in mice. Similar studies have shown that Cd destroys the collagen matrix and causes calcium to be released from bone to blood.
98
Cd and hematopoiesis
Cd toxicity negatively affects hematopoiesis. Anemic hemolysis due to Cd toxicity has been observed. In fact, severe anemia was observed in patients with Itai-itai due to Cd toxicity, which is linked to significant suppression of erythropoietin production.
99
Three mechanisms have been proposed to explain why Cd causes anemia.
-
Hemolysis due to deformities of peripheral red blood cells.
100
-
When iron is absorbed in the duodenum, iron deficiency develops due to Cd competing with iron.
101
-
Renal anemia due to hypo-production of erythropoietin
99
along with these, at high doses, acute Cd has been shown to increase the rate of polychromatic erythrocytes by showing a genotoxic effect in bone marrow.
101
Acute Cd toxicity also causes pial cerebral thrombosis by accelerating platelet aggregation.
89
Cd and immune system
Cd exposure changes the immune system.
102
It has been reported that Cd intoxication in the prenatal period disrupts T lymphocyte production and immunization in the postnatal period and causes abnormal thymocyte development.
103,104
In rats receiving chronic Cd toxicity (40 mg/L/30 days), tumor necrosis factor alpha (TNF-α), interleukin-1β, 6, 10 (IL-1β, 6, 10), peripheral neutrophil levels and interferon gamma (IFN-γ) increased, while lymphocyte counts decreased.
105
In addition to autoimmune formation, lymphocyte proliferation and natural killer cell activity are also suppressed.
106
Cd and lung
The lungs are among the target organs of Cd. Exposure to Cd dust or vapor by respiration irritates the respiratory tissues of humans. The most important source of inhalation Cd poisoning is cigarette smoke. Human lungs reabsorb more than almost half of the Cd (40-60%) in tobacco smoke.
107
Male rats fed orally with CdCl2 for 10 days showed a decrease in lung weight due to the dose. However, the same situation was not observed in female rats. Nonspecific pulmonary lesions were seen in male rats fed with 1.2 mg/kg CdCl2 in water for 200 days.
108
Cd may also contribute to asthma symptoms. Zn and Selenium (Se) can treat the clinical symptoms of asthma such as wheezing, coughing and lung function by competing with and removing Cd in children. Despite the limited number of studies showing that Cd has no association with lung cancer, recent studies have identified an increased risk of lung cancer in the population exposed to Cd.
109,110
It has been reported that Cd levels in 24-hour urine are positively associated with both lung cancer and total cancer risk.
Cd and Cancer
Cd is one of the most effective carcinogenic metals for humans. Chronic exposure to Cd causes lung, prostate, breast, pancreatic and renal cancer.
69,111,112
The cellular and molecular mechanisms of the carcinogenic effect of Cd are given under six forms as below.
110
1) Activation of proto-oncogenes.
2) Inactivation of tumor suppressor genes.
3) Deterioration of cell adhesion mechanism.
4) Induction of apoptosis.
5) Inhibition of DNA repair.
6) Free radical production and affecting the antioxidant system.
Cd promotes the expression of genes (such as c-myc, c-fos, and c-jun) that are members of the activator protein 1 (AP-1) family of proto-oncogenes. In addition, with immediate early response genes, MT increases the expression of stress response genes that encode glutathione and heat shock proteins, suppresses the expression of antioxidant genes such as superoxide dismutase, catalase and glutathione peroxidase. Tumor suppressor proteins such as E-cadherin and VE-cadherin impair cell-cell adhesion.
113-115
Cd and trace elements, metallothioneins
Since Cd has toxicological properties, it can prevent the uptake, transport and use of many other elements.
116
Se is a trace element with antioxidant effects based on the human body that shows oxygen-free radical cleaning, protects organs and tissues in the body from oxidative damage and improves the immune system of the body. Previous in vitro studies have shown Se to be a protective agent against Cd cytotoxicity by blocking ROS production. In addition, Se has been shown to stimulate antioxidant enzymes in the immature kidneys of rats exposed to Cd and to protect against oxidative damage.
117
Zn plays a role in many cellular functions, catalytic functions of many enzymes and structural stability of various cell proteins. Zn also interacts in DNA/RNA binding by the regulation of transcription, chromatin structure, protein-protein interactions and RNA metabolism. It also has an important role in stabilization and protection of biological membranes against oxidative and peroxidative injuries, loss of plasma membrane integrity and change of membrane permeability. Zn supplementation at low concentrations has been shown to reduce Cd-induced oxidative stress.
118
Another study reported Zn supplementation to be beneficial for the system against Cd toxicity.
116
Environmentally essential toxic metal Cd is present as Cd+2 ion in biological systems and is chemically similar to Ca2+ in such cases. Both are bivalent and can enter cells through channels or protein-bound penetration. Cd disrupts calcium homeostasis by inhibiting calcium channels and/or related proteins. Altered calcium homeostasis induced by Cd results in cell apoptosis, autophagy or tumorigenesis.
119,120
Metals, hormones, cytokinins, various chemicals, inflammation, stress and hypoxia, necrosis factors and glucocorticoids induce synthesis of MTs.
121
With their rich thiol groups, MTs are proteins involved in many physiological and pathological events, mainly antioxidant processes. They are involved in many important events, such as the detoxification of heavy metals such as Hg, Cd and lead (Pb), the regulation of essential metals such as Cu and Zn, antioxidant action against oxygen radicals and protection against DNA damage, maintenance of cell viability, angiogenesis, apoptosis and proliferation functions.
122
MTs are thought to play a role in the homeostasis of essential metals such as Zn, Cu and Fe. MTs binds Zn, but Zn can easily be replaced with excess Cu, Fe or Cd.
123
MTs are zinc-containing proteins consisting of 33% cysteine amino acid. MTs protect tissues from the toxic effects of heavy metals that have toxic effects in biological systems such as Cd and Hg. While MT has minimal effects on the absorption of Cd from the intestine, it plays an important role in Cd retention in tissues and reduces the excretion of Cd into bile. Excessive amounts of MT-containing cells are resistant to and are protective against Cd toxicity.
124-126
Oxidative stress
Oxidative stress occurs when the balance between ROS and the antioxidant system is impaired in the oxidant direction. Oxidative stress is a natural process, and there are specialized mechanisms that control this stress. Oxidative damage occurs when these mechanisms are inadequate.
127
The cellular and molecular mechanisms of Cd can be categorized as follows: inactivation of tumor suppressor genes, deterioration of cell adhesion mechanism, triggering of apoptosis, suppression of DNA repair, free radical production and effect on antioxidant systems.
32,33
Most of the damage caused by toxic metals is due to the increase in the free radicals they cause. ROS can lead to oxidative stress within cells by reacting with macromolecules and causing damages. These damages are in the form of increased lipid peroxidation, DNA damage, and oxidation of proteins.
83
ROS
ROS production plays an important role in Cd toxicity, and various suggestions have been put forward in this regard. It has been suggested that acute Cd toxicity mechanisms involve the depletion of glutathione and protein-bound sulfhydryl groups, resulting in increased production of ROS such as superoxide ion, hydrogen peroxide and hydroxyl radicals. Cd-enhanced ROS causes lipid peroxidation and results in DNA damage. Another suggestion is that mitochondria are an important target of Cd toxicity. It has been suggested that Cd initially binds to protein thiols in the mitochondrial membrane, affects mitochondrial permeability transmission, inhibits respiratory chain reaction and then produces ROS. Cancer, diabetes, atherosclerosis, neurodegenerative diseases and many other diseases play a role in the pathogenesis of ROS. For this reason, it is one of the most studied topics in recent years. ROS is a non-stable, highly effective atom or molecule having one or more unpaired electrons. Free radicals are produced according to endogenous or exogenous factors.
127-129
Potential endogenous sources of ROS
Mitochondria, endoplasmic reticulum, cytochrome P-450, peroxisomes, microsomes, and inflammatory cell activation are potential sources of ROS. Mitochondria are the main source of superoxide (O2.–) in the body. For each mg of protein, it produces 2-3 mol O2 per minute while also producing H2O2. When cytochrome P450 is induced, it specifically produces O2- and H2O2. Xanthine oxidase (XO), NADPH oxidase, lipoxygenase and other oxidant enzymes are important ROS sources. The ROS family includes short-lived molecules that result from the reduction of molecular oxygen. The most common ROS are H2O2, O2.– and OH⁻.
130,131
Potential exogenous sources of ROS
-
Antineoplastic agents: Doxorubicin, an anticarcinogenic agent, inhibits DNA replication in the cell. This leads to the formation of H2O2 and O2- and ultimately the initiation of lipid peroxidation.
-
Radiation and environmental agents [air pollution, heavy metals, pesticides, cigarette smoke, solvents, anesthetics, aromatic hydrocarbons, Carbon tetrachloride (CCl4), Iron nitrilotriacetate (Fe-NTA)] cause free radical formation.
132,133
-
Habitual substances: Alcohol and narcotics.
-
Environmental agents: Xenobiotics (air polluting photo chemicals, hyperoxia, pesticides, cigarette smoke, solvents, anesthetic substances, aromatic hydrocarbons).
-
Stress: The level of catecholamine increases in stress. Oxidation of catecholamines is a source of free radicals.
-
Ischemia, trauma.
134
ROS attack protein and enzymes, carbohydrates, and DNA in the cell membrane lipids, causing DNA damage, as well as protein modification
131
(Figure 1).
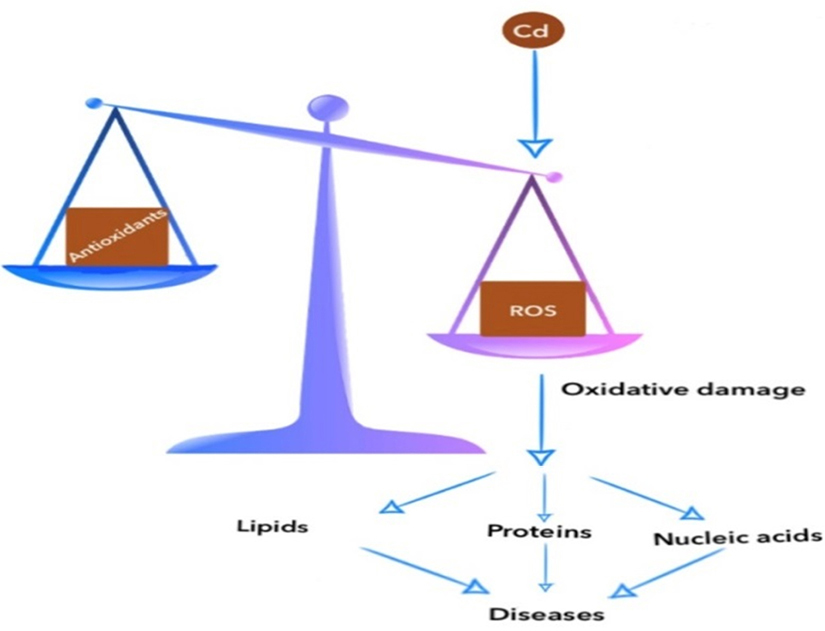
Figure 1.
Demonstration of the relationship between ROS and antioxidants.
Oxidative stress occurs when the balance between ROS and the antioxidant
system is impaired in the oxidant direction. ROS affect all important structures
such as carbohydrates, lipids, Nucleic acids and enzymes. Cd: Cadmium,
ROS: Reactive oxygen species.
.
Demonstration of the relationship between ROS and antioxidants.
Oxidative stress occurs when the balance between ROS and the antioxidant
system is impaired in the oxidant direction. ROS affect all important structures
such as carbohydrates, lipids, Nucleic acids and enzymes. Cd: Cadmium,
ROS: Reactive oxygen species.
Antioxidant defence system
Antioxidants and ROS penetrate the whole of life and constitute the field of redox biology. The antioxidant system prevents radical formation before damage, repairs the oxidative damage, cleans the damaged molecules and prevents mutations.
135,136
Two different mechanisms play an active role against free radical damage. The first is the non-enzymatic nutritional mechanism, and the other is the enzymatic mechanism (Figure 2). In the non-enzymatic feeding mechanism, glutathione (GSH) is present in some trace elements as well as low molecular weight molecules such as vitamin E, vitamin C, beta-carotene and vitamin A. Vitamins act as the carrier or silent receptors of free radicals, whereas trace elements regulate the activities of antioxidant enzymes.
127
Antioxidant defense systems of the human body are complex and are classified in various forms. The final classification involves their functions, their place in the cell, the structure they protect, their solubility and their source.
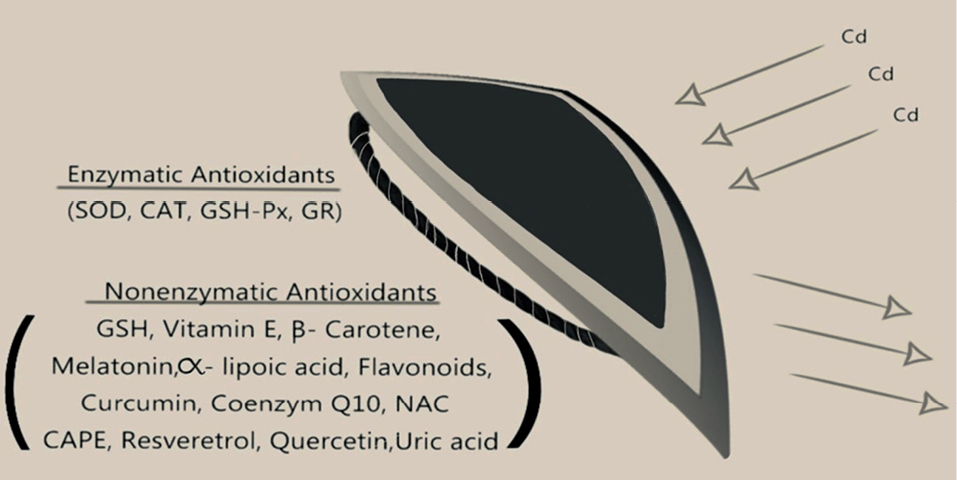
Figure 2.
There are two types of the antioxidant defense systems. The first
is the enzymatic antioxidant system, and the other is the nonenzymatic
antioxidant system. Both serve as shields against the harmful effects of Cd.
SOD: Superoxide dismutase, CAT: Catalase, GSH-Px: Glutathione peroxidase
GR: Glutathione reductase, GSH: Glutathione, NAC: N-acetylcysteine,
CAPE: Caffeic acid phenethyl ester.
.
There are two types of the antioxidant defense systems. The first
is the enzymatic antioxidant system, and the other is the nonenzymatic
antioxidant system. Both serve as shields against the harmful effects of Cd.
SOD: Superoxide dismutase, CAT: Catalase, GSH-Px: Glutathione peroxidase
GR: Glutathione reductase, GSH: Glutathione, NAC: N-acetylcysteine,
CAPE: Caffeic acid phenethyl ester.
Superoxide dismutase (SOD)
SOD is the most important enzyme that protects against O2.– radicals. SOD catalyzes the conversion of superoxide to oxygen and hydrogen peroxides. The SOD enzyme family is named according to the cofactors used to detoxify the excess of O2.– such as Cu/Zn-SOD, Fe-SOD, Ni-SOD and Mn-SOD. It is defined as metalloenzymes because it contains metals. Cu/Zn-SOD is in a dimeric form and is found in cytosol, with Cu and Zn bound to two subunits. SOD is usually found in the mitochondria and is tetrameric.
135,136
The presence of specific SOD isoforms in different subcellular compartments emphasizes the need for tight control of ROS homeostasis.
137-139
The role of SOD has been recognized as an important factor in oxidative stress caused by Cd. It has been shown in in vivo and in vitro studies that Cd reduces SOD activity. SOD with no activity or decreased activity causes an increase of ROS.
139
SOD activities have been reported to decrease in the liver and kidneys of rats that were sacrificed 24 hours after a single dose of 2.5 mg/kg and 5 mg/kg Cd.
140
Catalase (CAT)
CAT is a potent antioxidant enzyme in protein structure. CAT is a hemoprotein with four heme groups in its structure. The molecular weight of hemoprotein CAT is 248 kDa.
141
The most important task of CAT is to remove toxic H2O2 from cells. CAT, an enzyme found in plants, animals and aerobic bacteria, is found mostly in peroxisomes in the cell. A molecule CAT can convert 6 million hydrogen peroxide molecules per minute into water and oxygen. It has high activity in erythrocytes, kidney and liver. In vivo and in vitro studies have shown that CAT activity changes in the negative direction due to interaction with Cd.
142
Glutathione peroxidase (GSH-Px)
The GSH-Px enzyme is key in the living organism antioxidant system under both normal and oxidative stress conditions. GSH-Px has great important defenses against free oxygen radicals, peroxides and carcinogens. GSH-Px also plays an important role in inhibiting tumor formation by altering the lipoxygenase and cyclooxygenase pathways. In tumors, enzyme defense against H2O2 and other peroxides have been reported to cause significant increase in GSH-Px activity, the first enzyme.
131
The GSH-Px enzyme, which catalyzes the reduction of peroxides, converts reduced GSH to oxidized glutathione (GSSG) in the cell. It has been reported that there are 8 GSH-Px in the human organism, and it is called selenoproteins (Se GPxs), which are seen in many different cell types. Only GSH-Px-4, GSH-Px-7 and GSH-Px-8 are monomeric, while the others are tetrameric. GSH-Px can be found in two forms: selenium-bound and not selenium-bound. The selenium-bound group consists of four members that reduce hydrogen peroxide and other organic peroxides. These are GSH-Px1, GSH-Px2, GSH-Px3 and GSHPx4.
143
GSH-Px1 or cellular GSH-Px (cGSH-Px) is a cytosolic enzyme in the tetrameric structure found in all cells. GSH-Px1 is active against organic hydroperoxides and H2O2. This enzyme has been found in all tissues studied, albeit much less in germ cells.
144
GSH-Px 2 or gastrointestinal GSH-Px (GSH-Px-GI) is found in the liver and gastrointestinal tract in humans, but not found in the kidney, heart and lung.
145
GSH-Px 3 or plasma GSH-Px (pGSH-Px) are found in the kidneys and are especially present in the epithelial cells of proximal tubules.
143
GSH-Px4 or phospholipid GSH-Px (PH-GSH-Px) is found in cytosol, mitochondria and cell membrane. The enzyme reduces the phospholipid hydroperoxides to alcohols and protects the membrane against peroxidation in the absence of the most important antioxidant vitamin E.
146
Cd decreases GSH-Px activity in induced cells. The reduced activity of GSH-Px can be explained by the sulfur competition of Cd-metallothionines and GSH-Px containing amino acids.
147,148
Glutathione reductase (GR)
GR is responsible for providing reduced glutathione. It is one of the most abundant thiols in most cells. In its reduced form, glutathione plays an important role in the cellular control of ROS.
146
It is involved in the re-conversion of GSSG into reduced GSH by the reducing power of NADPH.
148
Glutathione S-transferase (GST)
GST is a multifunctional enzyme that provides homeostasis in the detoxification metabolic pathway by catalyzing the first step in the formation of the water-soluble end product mercapturic acid. GST enzymes are a multifunctional polymorphic isoenzyme family that protect the cell from cytotoxic and genotoxic stresses.
149
The most common tissues of the GST are the liver, especially organs such as kidney, small intestine, intestine, lung and breast. The basic biological roles of GSTs include detoxification and protection against oxidative stress. GSTs have four families: cytosolic, mitochondrial, microsomal/membrane-associated and fosfomycin/glyoxalase. There are 17 different cytosolic GSTs divided into 7 classes (alpha, mI, omega, phi, sigma, theta and zeta) based on sequence similarity in humans.
150
Non-enzymatic antioxidants
-
Those in the lipid phase (α-tocopherol, β-carotene)
-
Those found in a liquid phase (cell cytosol or blood plasma) (ascorbic acid, urate, cysteine, ceruloplasmin, transferrin, lactoferrin, myoglobin, hemoglobin, ferritin, albumin, bilirubin, glutathione)
-
Those present in both liquid and lipid phase (melatonin).
Glutathione
GSH is a tripeptide with a nucleophilic structure and strong antioxidant properties. The glutathione system acts as the major redox buffer in most cells. Decreased GSH in tissues can lead to peroxidative tissue damage with the deterioration of the cellular defense mechanism against ROS. In addition, glutathione has a key role in protecting against xenobiotics and heavy metals such as Cd.
151-153
Vitamin E
Vitamin E is a vitamin type that has a high level of antioxidant potency and is soluble in oil. Vitamin E has eight isoforms, α-, β-, γ-, and δ-tocopherol and α-, β-, γ-, and δ-tocotrienol stereoisomers including α, β, γ, δ tocopherol and α, β, γ, δ tocotrienol. Among these stereoisomers, the most biologically active is α-tocopherol.
154,155
The most important property of alpha-tocopherol is its ability to protect against lipid peroxidation and to protect the cell membrane from degenerative effects of free radicals. Vitamin E has been reported to be protective against colon and prostate cancer, some cardiovascular diseases, ischemia, cataract, arthritis and neurological diseases as a result of the studies done.
154
In vivo studies have shown that Vitamin E is protective against Cd toxicity and reduces oxidative damage.
155,156
β–Carotene
Carotene is a chemical terpene with two main types: alpha-carotene (α-carotene) and beta-carotene (β-carotene). There are also gamma, delta and epsilon- (γ, δ and ε-) carotenes.
β-carotene is most commonly available in green fruits and vegetables. β-carotene is particularly abundant in carrots.157 Being a good antioxidant, it has an important effect in eliminating harmful free radicals. In many studies, β-carotene has been shown to reduce oxidative stress damage and to play a protective role against oxidative stress.
157,158
Studies have shown β-carotene to reduce oxidative stress caused by Cd-induced brain and testicular toxicity and to improve the loss of cellular antioxidants in these tissues.
159,160
Melatonin
Melatonin is a neurohormone that is synthesized and secreted by the retina, bone marrow, thymus, ovary and gastrointestinal tract, primarily the pineal gland.
161
In the skin, it is responsible for the protection of deep tissues against the harmful radiation effects of sun rays and the exchange of pigment granules. On the other hand, it plays a role in the protection of oxidative cholesterol derivatives and bile acid to oxidative damage to the bile and mucous membranes.
162
Since melatonin can be dissolved in both liquid and lipid phases, it can easily reach all intracellular components and effectively protect the cell membrane, organelles and nucleus from free radical damage. It also reduces the production of radicals such as O2, H2O2 and OH⁻ that occur in mitochondrial respiration. Its ability to reach the core ensures that DNA is protected against oxidative damage.
163
There are in vitro studies suggesting that melatonin has protective effects on Cd-induced cancer cell proliferation. Furthermore, in vivo studies have shown that melatonin reduces the toxic effects of Cd.
84,164
α-Lipoic acid (ALA)
ALA is a small molecule that contains a thiol group and has antioxidant activity. It is efficient in both lipophilic and hydrophilic environments. Although ALA is found in human diet in sufficient quantities, mitochondria are synthesized de novo by lipoic acid synthase. ALA is found in two forms as oxides and dihydrolipoic acid as in its reduced form. Although both forms are active, it is stated that the most active form is dihydrolipoic acid. However, both forms are antioxidant. In addition to the antioxidant effects that ALA possesses, it strengthens and renews the intracellular levels of other antioxidants (vitamins C, E, GSH, etc). Because an antioxidant neutralizes its free radical, it loses its antioxidant property. Studies have shown that treatment with ALA leads to modulatory effects in Cd toxicity and reduces Cd destructive effects, leading to marked reductions in Cd residues in the liver and kidneys. In conclusion, it is claimed that ALA may be used as a potential therapeutic agent against Cd-induced toxicity.
165-167
Flavonoids
Flavonoids are a large group of polyphenolic compounds found in plant foods consumed regularly (i.e. vegetables and fruits), olive oil and drinks such as tea and wine. Flavonoids are known as phenylpropanoids with important chelating and antioxidant properties and are generally found in plants and cannot be synthesized in the human organism. Flavonoids are classified as flavones (grapes, French bean seeds, rice bran, vetch), flavonols (quercetin, rutin, strawberries, apples, persimmons, onions, cucumbers), flavanones (lemons, rosehips, bitter orange, petit grain, orange, orange juice) flavanonols (vinegar), flavanols or catechins (tea leaves, black tea, oolong tea), anthocyanins (cranberries, blueberries, plums, grapes, cherries, sweet potatoes) and chalcones. It has been found that flavonoids have different properties such as antiviral, antiallergic, antitumor, anticholinesterase, antithrombotic, anti-inflammatory and vasodilating effects besides antioxidant properties. Flavonoids provide protection against damage caused by Cd with these effects.
168,169
The cytoprotective effects of flavonoids against Cd-induced diseases have been explained through specific mechanisms. First, they clean the ROS of flavonoids, reducing lipid peroxide production and increasing the activity of antioxidation enzymes. Second, they chelate Cd, reducing Cd deposition and altering in vivo levels of other basic metal ions. Third, they reduce DNA damage and prevent apoptosis.
170
Curcumin
Curcumin is a hydrophobic polyphenol compound used as a yellow spice for years and is obtained from turmeric. Curcumin has antioxidant, antimicrobial, anti-inflammatory, antiviral, anti-carcinogenic and antiapoptotic properties.
171
It has been shown to be an important agent against Cd-induced hepatotoxicity, immunotoxicity, lung diseases, reproductive toxicity, neurotoxicity, colon toxicity and nephrotoxicity.
172
Antioxidant effect
Curcumin exhibits potent antioxidant activity comparable to vitamins E and C. The antioxidant effect comes from its phenolic structure and β-diketone derivative. Curcumin has been shown to inhibit lipid peroxidation, H2O2 and enhance the activity of antioxidant enzymes (SOD, GST, and GPx).
173,174
Anticancer effect
In vivo and in vitro studies have shown curcumin to have an inhibitory effect in three steps of carcinogenesis: tumor increase, angiogenesis and tumor growth. It has been shown to protect against tumors in many animal studies. This protection has been shown in colon, prostate, duodenum, esophagus, stomach and oral cancers.
175
Anti-inflammatory effect
Various studies have shown that curcumin modulates the production of a variety of inflammatory cytokines and thus exhibits a potent anti-inflammatory activity. In addition, it has an inhibitory effect on proinflammatory cytokines TNF-α and IL-6, which are inflammatory process inhibitors caused by Cd poisoning.
176
Coenzyme Q10 (CoQ10)
CoQ10 is a vitamin-like compound that can dissolve in oil, can exist in all cells and acts as a coenzyme during key enzymatic interaction in forming energy in the cell. The chemical formula of CoQ10 consists of 5-ethyl-2.3-dimethoxy-6-decaprenyl-1.4-benzoquinone. It is synthesized from phenylalanine and mevalonic acid endogenously in the body. The majority of CoQ10 in cell membranes is a reduced form (KoQH2) and is found near the unsaturated lipid chains in membranes. CoQ10 interacts with ROS to prevent protein and lipid peroxidation from initiating and damaging biomolecules. CoQ10 (also known as ubiquinone), an important electron carrier in cellular respiration, exhibits antioxidant properties by removing ROS and protecting oxidative stress cells. The mitochondrial respiratory chain acts as the electron. In addition, the membrane stability of CoQ10 also functions in cell signaling, gene formation, cell growth and apoptosis protection.
177-179
In Cd poisoning study; Cd caused significant changes in hematological and biochemical parameters and increased lipid peroxidation, altered the glutathione circuit and decreased the activity of antioxidant enzymes. CoQ10 provided protection by substantially normalizing these values and reducing lipid peroxidation. The results of this study showed that CoQ10 may be useful in the prevention of Cd-induced hepatotoxicity and can be used as a preventive against acute Cd poisoning of exposed persons.
180
N-Acetyl cysteine (NAC)
NAC is an N-acetylated derivative of a thiol molecule L-Cysteine, a natural amino acid. Its chemical formula is C5H9NO3S, and it has a molecular weight of 163.2 g/mol. Acetylcysteine is a mucolytic that reduces the viscosity of secretions. The drug can be administered orally, intravenously or by the respiratory route. NAC is used intravenously in the treatment of paracetamol (acetaminophen) poisoning, while it is used as the second choice in acrylonitrile and methacrylonitrile poisoning. It has begun to be used in some psychiatric and physiological diseases due to the demonstration of its antioxidant properties. NAC is very effective in neutralizing free radicals in vitro. NAC reacts rapidly with hydroxyl radicals. It is also effective against other ROS such as O2•– and H2O2. It increases SOD activity and GSH levels and inhibits autocatalytic lipid peroxidation. One study showed NAC to improve Cd-induced neurotoxicity and improve memory and learning processes of Cd-poisoned rats.
181,182
Caffeic acid phenethyl ester (CAPE)
CAPE is an active component with a flavonoid-like structure found in propolis produced by honey bees and has anti-inflammatory, neuroprotective, hepatoprotective, immunomodulatory, antiviral, anticancer, antioxidant and apoptosis-regulating properties.
183,184
More than 180 substances were found in the structure of propolis. Among these components, flavonoids, caffeic acid and esters are the most dense and active constituents. CAPE can also be chemically synthesized as a result of esterification of caffeic acid and phenethyl alcohol with acid.
183-187
CAPE consists of two ring structures. In these rings, there are two functional OH groups that carry almost all the chemical properties of the molecule, and they actively play a role in oxidation and reduction. The aromatic and aliphatic structure has a lipophilic character for carrying very long carbon groups. It is a potent inhibitor of enzymes such as ornithine carboxylase, 5-α reductase, protease, cyclooxygenase, lipoxygenase, xanthine oxidase and HIV-1 integrase. In one study, pretreatment with CAPE improved all changes caused by Cd. The results of this study showed CAPE to play a promising role as a mitochondrial-targeted antioxidant against the renal toxicity of Cd.
184,188
Resveratrol
Resveratrol is one of the most widely studied phytochemicals with beneficial effects on human health. In recent years, pharmacological effects of resveratrol in relation to cardiovascular health, diabetes, aging, obesity and cancer have been demonstrated by various biochemical mechanisms in preclinical models.
189
Resveratrol has been reported to play an antiproliferative role on various types of cancer such as breast, lung, prostate, colorectal and stomach cancer, esophageal tumors, pancreatic cancer and leukemia. The anti-cancer activity of resveratrol is mediated through the modulation of various cell-signaling molecules that regulate the cell cycle process, proliferation, apoptosis, metastasis, angiogenesis and invasion of cancer cells.
190,191
There are studies showing that resveratrol inhibits tumor growth in cancerous cells by inhibiting NF-κB activation and decreasing TNF-α production.
192
Resveratrol has different mechanisms of antioxidant effect. Resveratrol shows an antioxidant potential by eliminating free radicals, binding metal ions, reducing the activity of enzymes involved in ROS formation and increasing antioxidant enzyme activities.
193,194
Resveratrol inhibits lipid peroxidation caused by free radicals and prevents DNA damage. It has been reported that resveratrol lipid peroxidation is more effective when compared to vitamins E and C in prevention and that it maintains cell viability and inhibits oxidation by targeting molecules in the cell.
195
Resveratrol has been shown to improve the renal oxidative damage caused by Cd.
196
In addition, in another study, resveratrol has been shown to have a protective role in the amelioration of mitochondrial health through Sirt3-dependent FoxO3a.
197
Quercetin
Quercetin contains three rings and five hydroxyl groups in its structure. Taken at a daily dose of 50-500 mg, quercetin has many important functions for metabolism such as antioxidant, anticancerogenic, antiviral, antithrombotic, anti-ischemic, anti-inflammatory and antiallergic properties. Quercetin is known as the most powerful antioxidant substance in flavonoids. Quercetin protects the cells against endogenous and exogenous ROS tissue damage.
198,199
Quercetin has been reported to have different mechanisms of action on ROS.
-
It exhibits strong antiradical properties against radicals such as hydroxyl radical, peroxide and superoxide anion.
-
It reduces superoxide anion production with xanthine oxidase.
-
Cyclooxygenase and lipoxygenase inhibit enzyme activities.
-
It forms a chelate with metals such as iron and copper.
198
There are quercetin applications for Cd toxicity to remove oxidative stress and degenerative disorders that occur in various tissues and organs of the body. Quercetin has been shown to prevent renal tubular damage and oxidative stress in rats exposed to chronic Cd. In another study, it was observed that Quercetin alleviated liver damage and oxidative stress in Cd-induced liver damage. Unsal et al suggested that Quercetin exhibited a significant protective action against Cd-induced neurotoxicity in rats via inhibiting lipid peroxidation.
199-204
Studies have shown that antioxidants reduce Cd toxicity (Table 1).
Table 1.
Researches in the literature to remove Cd from the body and to reduce oxidative stress
|
Models
|
Study Design
|
Materials
|
Effect
|
Mechanisms and Conclusion
|
Ref.
|
|
CdCl2 (25 mg/kg)
|
Sprague-Dawley rats |
Curcumin (50 mg/kg, oral) |
BUN↓, Creatinine↓, AST↓, ALT↓, Glucose↓ |
Curcumin has protective effect against nephrotoxicity due to Cd. |
205
|
|
CdCl2 (5 mg/kg b.w)
|
Wistar albino rats |
Vitamin E (100 mg/kg b.w) and β-carotene (10 mg/kg b.w) |
GSH↑, TBARS↓, AST↓, ALT↓, Glucose ↓, BUN↓, Creatinine ↓ |
Vitamin E, β-carotene, and/or combinations thereof can alleviate the detrimental effects of CdCl2.
|
206
|
|
CdCl2 (5 mg/kg/d b.w)
|
Wistar rats |
Grape seed extract (400 mg/kg/d b.w) |
GSH-Px↑ CAT↑, GSH -R↑, MDA ↓ |
Grape seed extract has beneficial protective effects against the harmful effects of CdCl2 on testis.
|
207
|
|
CdCl2 (4.4 mg/kg/d b.w)
|
Wistar albino rats |
Grape seed extract (100 mg/kg/d b.w) |
GSH-Px↑ CAT↑, GSH↑, SOD↑, MDA↓, TAC↑ |
Grape seed extract has protective effects on the spleen of rats by alleviating oxidative stress. |
208
|
CdCl2
(5 mg/kg b.w)
|
Wistar albino rats |
Sinapic acid (10 or 20 mg/kg b.w) |
CAT↑, MDA↓, |
Cd protects against the nephrotoxicity caused by sinapic acid. |
209
|
|
CdCl2 (5 mg/kg b.w)
|
Sprague-Dawley rats |
Thymoquinone (40 mg/kg/d) |
GSH↑, SOD↑, MDA↓, |
Thymoquinone prevents the harmful effects of Cd exposure on the kidneys. |
210
|
|
CdCl2 (7 mg/kg b.w)
|
Male mice |
Curcumin (50 mg/kg b.w) Resveratrol (20 mg/kg b.w) Melatonin (12 mg/kg b.w) |
CAT↑, GSH↑, MDA↓ GSH-Px↑ |
Cd-induced toxicity has been shown to protect against lipid peroxidation of curcumin, resveratrol and melatonin treatment and to reduce the negative effect of Cd on antioxidant enzymes. |
211
|
|
CdCl2 (2.5 mg/kg b.w.)
|
Wistar strain albino rats |
Hydroxytyrosol (2-(3,4-dihydroxyphenyl) ethanol, DPE) (9.0 mg/kg b.w.) or MnCl2 (2.0 mg/kg b.w.)
|
SOD↑, CAT↑, GSH-Px↑ |
Manganese and DPE provide a positive contribution to reducing the damage caused by Cd toxicity. In addition, the antioxidant properties exhibited by the DPE in the liver are protective against Cd damage. |
8
|
|
CdCl2 (6.5 mg/kg b.w)
|
Wistar albino rats |
Physalis peruviana L. (200 mg/kg b.w)
|
GSH-Px↑, CAT↑, GSH↑, GR↑, MDA (TBARS)↓ |
Physalis peruviana L. is protective against acute Cd neurotoxicity.
|
212
|
|
CdCl2 (200 µg/mL)
|
Sprague-Dawley rats |
1 % Taurine, 0.02 % Melatonin, 0.5 % NAC and 1 % 4 Taurine, 0.08 % Melatonin, 2 % NAC |
CAT↑, SOD↑, MDA↓, GST↑, MPO↑ |
Taurine, melatonin and N-acetylcysteine have been shown to have some protective effect against Cd accumulation in brain and heart tissues. |
213
|
|
CdCl2 (50 mg/kg b.w)
|
Male Wistar rats |
Rutin, 25, 50 or 100 mg/kg b.w |
GSH-Px↑, CAT↑, GSH↑, SOD↑, MDA↓ |
Rutin has protective effect against oxidative stress by stimulating antioxidant enzymes in Cd-induced testicular damage. |
214
|
|
CdCl2 (20 mg/kg/b.w)
|
Male Wistar rats |
Vit-E (20 mg/b.w) Ca + Zn (2 mg/kg) |
GSH-Px↑, CAT↑, GSH↑, SOD↑, GR↑, LPO (MDA)↓ |
Vit-E supplementation against Cd-induced nephrotoxicity can be an important safeguard. |
215
|
|
CdCl2 2 mg/kg/d b.w)
|
Albino rats |
Sodium selenite (1 mg/kg b.w) |
MDA↓, GST↑, GSH-Px↑ |
Selenium treatment protects kidney tissues against Cd toxicity. |
216
|
|
CdCl2 (1 mg/kg b.w)
|
Wistar rats |
Royal Jelly (100 mg/kg b.w/day) |
SOD↑, GR↑, MDA↓ |
Royal Jelly Cd-induced male infertility can also be a natural preservative. |
217
|
|
CdCl2 (2 mg/kg/day)
|
Male C57 BL/6J mice |
Flavocoxid (20 mg/kg/d) |
GR↑, GSH↑, GSH-Px↑, PC↓ |
Flavocoxid significantly reduces Cd-induced oxidative damage in the kidney. |
218
|
|
CdCl2(2, 4, and 8 mg/kg b.w)
|
Male mice |
Quercetin (75 mg/kg) |
GSH-Px↑, GSH↑, SOD↑, MDA↓ |
Quercetin has a protective effect against Cd by reducing lipid peroxidation with its anti-oxidative property. |
219
|
|
CdCl2 (1.8 mg/kg)
|
Mice |
Extra virgin olive oil (2 mL/kg b.w) |
GSH-Px↑ CAT↑, SOD↑, MDA↓, |
Extra virgin olive oil has been shown to be an important agent in reducing oxidative stress in the prevention of Cd toxicity. |
220
|
|
CdCl2 (5 mg/kg)
|
Male albino rats |
Origan ummajorana L.
1000 mg/kg b.w.
|
CAT↑, GSH↑, SOD↑, TBARS (MDA)↓, |
Origanum majorana L. has protective properties against Cd-induced hepatotoxicity and nephrotoxicity. |
221
|
|
CdCl2 (6.5 mg/kg)
|
Male Wistar rats |
Fragaria ananassa Crude Extract (250 mg/kgb.w)
|
GSH-Px↑, CAT↑, GSH↑, SOD↑, MDA (LPO)↓ |
The crude extract of Fragaria ananassa has been shown to be protective against Cd-induced oxidative stress.
|
147
|
CdCl2: Cadmium chloride, MDA: Malondialdehyde, MPO: Myeloperoxidase, TAC: Total antioxidant capacity, LPO: Lipid peroxidation, TBARS: Thiobarbituric acid reactive substances, SOD: Superoxide dismutase, CAT: Catalase, GSH-Px: Glutathione peroxidase GR: Glutathione reductase, GSH: Glutathione, PC: Protein carbonyl.
Conclusion and Future Perspective
Cd exposure is present in many stages of human life and has become a global problem. Cd affects skeletal, urinal, reproductive, cardiovascular, central and peripheral nerves and respiratory system in human body. If it is almost impossible to escape from Cd in the industrialized world, then we must find ways to remove heavy metals like Cd from the body. The most commonly used agent in Cd poisoning is EDTA. In addition, chelators such as penicillamine, dimercaprol (British anti-Lewisite, BAL) and dithiocarbamates are used. BAL is more toxic than its derivatives, DMPS and DMSA, and is rarely used clinically.
222,223
The antioxidant balance of the body is greatly influenced by the diet. Pathological conditions can occur when the defenses of the body are destroyed due to nutritional deficiencies. Cd increases the ROS level and causes oxidative stress.The increase in ROS and inadequacy of defense systems cause the deterioration of antioxidant balance in the body and the formation of “oxidative stress” conditions. Antioxidants interact with free radicals, rendering them ineffective and preventing damage. They are also known as free radical scavengers. To neutralize free radicals, the body produces some endogenous antioxidants. However, there are also exogenous antioxidants taken with nutrition. Fruits, vegetables and some medical plants are rich sources of antioxidants. It has been reported that the toxic effects of Cd can be protected through antioxidants. Many antioxidant substances have been tested to minimize the oxidative stress that occurs in tissues due to Cd. The reason researchers conduct antioxidant trials is that they appear purer and more reliable. As a result of our research, we found that some antioxidants decreased Cd-induced oxidative stress. However, to elucidate future therapeutic advances, new efforts are needed to remove Cd and reduce oxidative damage, particularly to obtain appropriate doses and more understandable results.
Ethical Issues
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
-
Bradl H. Heavy Metals in the Environment: Origin, Interaction and Remediation. Vol. 6. Academic Press; 2005.
-
Faroon O, Ashizawa A, Wright S, Tucker P, Jenkins K, Ingerman L, et al. Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles. Toxicological Profile for Cadmium. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); 2012.
-
Tsalev DL. Atomic Absorption Spectrometry in Occupational and Environmental Health Practice. Vol. 3. Boca Raton: CRC Press; 1995.
-
Saputra E, Utama P, Muhammad S, Ang HM, Tade M, Wang S. Catalytic Oxidation of Toxic Organics in Aqueous Solution for Wastewater Treatment: a Review. In: Proceedings from TIChE International Conference; Nov 10-11 2011; Hatyai, Songkhla, Thailand, Songkla University.
- Nriagu JO. Global inventory of natural and anthropogenic emissions of trace metals to the atmosphere. Nature 1979; 279(5712):409-11. doi: 10.1038/279409a0 [Crossref] [ Google Scholar]
-
U.S. Geological Survey. Mineral Commodity Summaries 2015. U.S. Geological Survey; 2015. p. 36-8. doi: 10.3133/7014009.
-
U.S. Geological Survey. Mineral Commodity Summaries 2009. U.S. Geological Survey; 2009. p. 38-40. doi: 10.3133/mineral2009.
- Casalino E, Calzaretti G, Sblano C, Landriscina C. Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium. Toxicology 2002; 179(1-2):37-50. doi: 10.1016/s0300-483x(02)00245-7 [Crossref] [ Google Scholar]
-
Scoullos MJ, Vonkeman GH, Thornton I, Makuch Z. Mercury—cadmium—lead handbook for sustainable heavy metals policy and regulation. vol. 31. Dordrecht: Kluwer Academic Publishers; 2001. doi: 10.1007/978-94-010-0403-9.
-
Wang LK, Chen JP, Hung YT, Shammas NK. Heavy Metals in the Environment. 1st ed. Boca Raton: CRC Press; 2009. p. 14-20. doi: 10.1201/9781420073195.
- Kirkham MB. Cadmium in plants on polluted soils: effects of soil factors, hyperaccumulation, and amendments. Geoderma 2006; 137(1-2):19-32. doi: 10.1016/j.geoderma.2006.08.024 [Crossref] [ Google Scholar]
-
Bishop ML, Duben-Engelkirk JL, Fody EP. Clinical Chemistry: Principles, Procedures, Correlations. 4th ed. California, USA: Lippincott Williams & Wilkins; 2000. p. 345-54.
- Cannino G, Ferruggia E, Luparello C, Rinaldi AM. Cadmium and mitochondria. Mitochondrion 2009; 9(6):377-84. doi: 10.1016/j.mito.2009.08.009 [Crossref] [ Google Scholar]
- Järup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure--a review of the literature and a risk estimate. Scand J Work Environ Health 1998; 24 Suppl 1:1-51. [ Google Scholar]
-
World Health Organization (WHO). Cadmium. Environmental Health Criteria. Geneva: WHO; 1992. p. 92-205.
- Mortada WI, Sobh MA, El-Defrawy MM. The exposure to cadmium, lead and mercury from smoking and its impact on renal integrity. Med Sci Monit 2004; 10(3):CR112-6. [ Google Scholar]
- Galazyn-Sidorczuk M, Brzóska MM, Moniuszko-Jakoniuk J. Estimation of Polish cigarettes contamination with cadmium and lead, and exposure to these metals via smoking. Environ Monit Assess 2008; 137(1-3):481-93. doi: 10.1007/s10661-007-9783-2 [Crossref] [ Google Scholar]
- Kikuchi Y, Nomiyama T, Kumagai N, Dekio F, Uemura T, Takebayashi T. Uptake of cadmium in meals from the digestive tract of young non-smoking Japanese female volunteers. J Occup Health 2003; 45(1):43-52. doi: 10.1539/joh.45.43 [Crossref] [ Google Scholar]
- Méranger JC, Subramanian KS, Chalifoux C. Survey for cadmium, cobalt, chromium, copper, nickel, lead, zinc, calcium, and magnesium in Canadian drinking water supplies. J Assoc Off Anal Chem 1981; 64(1):44-53. [ Google Scholar]
- Lim CS, Shaharuddin MS, Sam WY. Risk assessment of exposure to lead in tap water among residents of Seri Kembangan, Selangor state, Malaysia. Glob J Health Sci 2012; 5(2):1-12. doi: 10.5539/gjhs.v5n2p1 [Crossref] [ Google Scholar]
- Verougstraete V, Lison D, Hotz P. Cadmium, lung and prostate cancer: a systematic review of recent epidemiological data. J Toxicol Environ Health B Crit Rev 2003; 6(3):227-55. doi: 10.1080/10937400306465 [Crossref] [ Google Scholar]
- Waisberg M, Joseph P, Hale B, Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 2003; 192(2-3):95-117. doi: 10.1016/s0300-483x(03)00305-6 [Crossref] [ Google Scholar]
- Il’yasova D, Schwartz GG. Cadmium and renal cancer. Toxicol Appl Pharmacol 2005; 207(2):179-86. doi: 10.1016/j.taap.2004.12.005 [Crossref] [ Google Scholar]
- Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta 2016; 1863(12):2977-92. doi: 10.1016/j.bbamcr.2016.09.012 [Crossref] [ Google Scholar]
- Bashir N, Manoharan V, Miltonprabu S. Grape seed proanthocyanidins protects against cadmium induced oxidative pancreatitis in rats by attenuating oxidative stress, inflammation and apoptosis via Nrf-2/HO-1 signaling. J Nutr Biochem 2016; 32:128-41. doi: 10.1016/j.jnutbio.2016.03.001 [Crossref] [ Google Scholar]
- Ivanina AV, Cherkasov AS, Sokolova IM. Effects of cadmium on cellular protein and glutathione synthesis and expression of stress proteins in eastern oysters, Crassostrea virginica Gmelin. J Exp Biol 2008; 211(Pt 4):577-86. doi: 10.1242/jeb.011262 [Crossref] [ Google Scholar]
- Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J 2012; 5(1):9-19. doi: 10.1097/WOX.0b013e3182439613 [Crossref] [ Google Scholar]
- Rani A, Kumar A, Lal A, Pant M. Cellular mechanisms of cadmium-induced toxicity: a review. Int J Environ Health Res 2014; 24(4):378-99. doi: 10.1080/09603123.2013.835032 [Crossref] [ Google Scholar]
- Chaoui A, Mazhoudi S, Ghorbal MH, El Ferjani E. Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L). Plant Sci 1997; 127(2):139-47. doi: 10.1016/S0168-9452(97)00115-5 [Crossref] [ Google Scholar]
- Belkadhi A, De Haro A, Obregon S, Chaibi W, Djebali W. Positive effects of salicylic acid pretreatment on the composition of flax plastidial membrane lipids under cadmium stress. Environ Sci Pollut Res Int 2015; 22(2):1457-67. doi: 10.1007/s11356-014-3475-6 [Crossref] [ Google Scholar]
-
Agency for Toxic Substances and Disease Registry (ATSDR). Cadmium toxicity—Case Studies in Environmental Medicine. Atlanta, GA: ATSDR, U.S. Department of Health and Human Services; 2008.
- Marettová E, Maretta M, Legáth J. Toxic effects of cadmium on testis of birds and mammals: a review. Anim Reprod Sci 2015; 155:1-10. doi: 10.1016/j.anireprosci.2015.01.007 [Crossref] [ Google Scholar]
- Koyuturk M, Yanardag R, Bolkent S, Tunali S. Influence of combined antioxidants against cadmium induced testicular damage. Environ Toxicol Pharmacol 2006; 21(3):235-40. doi: 10.1016/j.etap.2005.08.006 [Crossref] [ Google Scholar]
- Bench G, Corzett MH, Martinelli R, Balhorn R. Cadmium concentrations in the testes, sperm, and spermatids of mice subjected to long-term cadmium chloride exposure. Cytometry 1999; 35(1):30-6. doi: 10.1002/(sici)1097-0320(19990101)35:1<30::aid-cyto5>3.3.co;2-d [Crossref] [ Google Scholar]
- Ono H, Funakoshi T, Shimada H, Kojima S. Comparative effects of disulfiram and diethyldithiocarbamate against testicular toxicity in rats caused by acute exposure to cadmium. J Toxicol Environ Health 1997; 50(4):389-99. doi: 10.1080/009841097160429 [Crossref] [ Google Scholar]
- Peter M, Róbert T, Ferdinand N. Concentrations of cadmium in ovary, oviductus, uterus, testis and tunica albuginea of testis in cattle. J Environ Sci Health A Environ Sci Eng Toxicol 1995; 30(8):1685-92. doi: 10.1080/10934529509376295 [Crossref] [ Google Scholar]
- Massányi P, Uhrín V, Toman R, Pivko J, Lukáč N, Forgács Z. Ultrastructural changes of ovaries in rabbits following cadmium administration. Acta Vet Brno 2005; 74(1):29-35. doi: 10.2754/avb200574010029 [Crossref] [ Google Scholar]
- Massányi P, Lukáč N, Uhrín V, Toman R, Pivko J, Rafay J. Female reproductive toxicology of cadmium. Acta Biol Hung 2007; 58(3):287-99. doi: 10.1556/ABiol.58.2007.3.5 [Crossref] [ Google Scholar]
- Jamall IS, Naik M, Sprowls JJ, Trombetta LD. A comparison of the effects of dietary cadmium on heart and kidney antioxidant enzymes: evidence for the greater vulnerability of the heart to cadmium toxicity. J Appl Toxicol 1989; 9(5):339-45. doi: 10.1002/jat.2550090510 [Crossref] [ Google Scholar]
- Messner B, Bernhard D. Cadmium and cardiovascular diseases: cell biology, pathophysiology, and epidemiological relevance. Biometals 2010; 23(5):811-22. doi: 10.1007/s10534-010-9314-4 [Crossref] [ Google Scholar]
- Ozturk IM, Buyukakilli B, Balli E, Cimen B, Gunes S, Erdogan S. Determination of acute and chronic effects of cadmium on the cardiovascular system of rats. Toxicol Mech Methods 2009; 19(4):308-17. doi: 10.1080/15376510802662751 [Crossref] [ Google Scholar]
- Wang Y, Fang J, Leonard SS, Rao KM. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med 2004; 36(11):1434-43. doi: 10.1016/j.freeradbiomed.2004.03.010 [Crossref] [ Google Scholar]
- Andersen O, Nielsen JB, Svendsen P. Oral cadmium chloride intoxication in mice: effects of dose on tissue damage, intestinal absorption and relative organ distribution. Toxicology 1988; 48(3):225-36. doi: 10.1016/0300-483x(88)90103-5 [Crossref] [ Google Scholar]
- Schroeder HA. Municipal drinking water and cardiovascular death rates. JAMA 1966; 195(2):81-5. doi: 10.1001/jama.1966.03100020069016 [Crossref] [ Google Scholar]
- Giridhar J, Isom GE. Alteration of atrial natriuretic peptide levels by short term cadmium treatment. Toxicology 1991; 70(2):185-94. doi: 10.1016/0300-483x(91)90045-3 [Crossref] [ Google Scholar]
- Jamall IS, Smith JC. Effects of cadmium on glutathione peroxidase, superoxide dismutase, and lipid peroxidation in the rat heart: a possible mechanism of cadmium cardiotoxicity. Toxicol Appl Pharmacol 1985; 80(1):33-42. doi: 10.1016/0041-008x(85)90098-5 [Crossref] [ Google Scholar]
- Tang YR, Zhang SQ, Xiong Y, Zhao Y, Fu H, Zhang HP. Studies of five microelement contents in human serum, hair, and fingernails correlated with aged hypertension and coronary heart disease. Biol Trace Elem Res 2003; 92(2):97-104. doi: 10.1385/bter:92:2:97 [Crossref] [ Google Scholar]
- Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease A prospective cohort study. Ann Intern Med 2013; 159(10):649-59. doi: 10.7326/0003-4819-159-10-201311190-00719 [Crossref] [ Google Scholar]
- Vivoli G, Bergomi M, Borella P, Fantuzzi G, Caselgrandi E. Cadmium in blood, urine and hair related to human hypertension. J Trace Elem Electrolytes Health Dis 1989; 3(3):139-45. [ Google Scholar]
- Houtman JP. Prolonged low-level cadmium intake and atherosclerosis. Sci Total Environ 1993; 138(1-3):31-6. doi: 10.1016/0048-9697(93)90402-r [Crossref] [ Google Scholar]
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med 1999; 340(2):115-26. doi: 10.1056/nejm199901143400207 [Crossref] [ Google Scholar]
- Costa MA. The endocrine function of human placenta: an overview. Reprod Biomed Online 2016; 32(1):14-43. doi: 10.1016/j.rbmo.2015.10.005 [Crossref] [ Google Scholar]
- Al-Saleh I, Shinwari N, Mashhour A, Mohamed Gel D, Rabah A. Heavy metals (lead, cadmium and mercury) in maternal, cord blood and placenta of healthy women. Int J Hyg Environ Health 2011; 214(2):79-101. doi: 10.1016/j.ijheh.2010.10.001 [Crossref] [ Google Scholar]
- Schuler-Maloney D. Placental triage of the singleton placenta. J Midwifery Womens Health 2000; 45(2):104-13. doi: 10.1016/s1526-9523(99)00045-8 [Crossref] [ Google Scholar]
- Caserta D, Graziano A, Lo Monte G, Bordi G, Moscarini M. Heavy metals and placental fetal-maternal barrier: a mini-review on the major concerns. Eur Rev Med Pharmacol Sci 2013; 17(16):2198-206. [ Google Scholar]
- Kuhnert BR, Kuhnert PM, Zarlingo TJ. Associations between placental cadmium and zinc and age and parity in pregnant women who smoke. Obstet Gynecol 1988; 71(1):67-70. [ Google Scholar]
- Schoeters G, Den Hond E, Zuurbier M, Naginiene R, van den Hazel P, Stilianakis N. Cadmium and children: exposure and health effects. Acta Paediatr Suppl 2006; 95(453):50-4. doi: 10.1080/08035320600886232 [Crossref] [ Google Scholar]
- Stasenko S, Bradford EM, Piasek M, Henson MC, Varnai VM, Jurasović J. Metals in human placenta: focus on the effects of cadmium on steroid hormones and leptin. J Appl Toxicol 2010; 30(3):242-53. doi: 10.1002/jat.1490 [Crossref] [ Google Scholar]
- Iijima K, Otake T, Yoshinaga J, Ikegami M, Suzuki E, Naruse H. Cadmium, lead, and selenium in cord blood and thyroid hormone status of newborns. Biol Trace Elem Res 2007; 119(1):10-8. doi: 10.1007/s12011-007-0057-1 [Crossref] [ Google Scholar]
- Jeong EM, Moon CH, Kim CS, Lee SH, Baik EJ, Moon CK. Cadmium stimulates the expression of ICAM-1 via NF-kappaB activation in cerebrovascular endothelial cells. Biochem Biophys Res Commun 2004; 320(3):887-92. doi: 10.1016/j.bbrc.2004.05.218 [Crossref] [ Google Scholar]
- Shagirtha K, Muthumani M, Prabu SM. Melatonin abrogates cadmium induced oxidative stress related neurotoxicity in rats. Eur Rev Med Pharmacol Sci 2011; 15(9):1039-50. [ Google Scholar]
- Wolff NA, Abouhamed M, Verroust PJ, Thévenod F. Megalin-dependent internalization of cadmium-metallothionein and cytotoxicity in cultured renal proximal tubule cells. J Pharmacol Exp Ther 2006; 318(2):782-91. doi: 10.1124/jpet.106.102574 [Crossref] [ Google Scholar]
- Johri N, Jacquillet G, Unwin R. Heavy metal poisoning: the effects of cadmium on the kidney. Biometals 2010; 23(5):783-92. doi: 10.1007/s10534-010-9328-y [Crossref] [ Google Scholar]
-
Friberg L, Piscator M, Nordberg G. Cadmium in the Environment: a Toxicological and Epidemiological Appraisal. Stockholm: United States Environmental Protection Agency; 1971.
- Sinha M, Manna P, Sil PC. Attenuation of cadmium chloride induced cytotoxicity in murine hepatocytes by a protein isolated from the leaves of the herb Cajanus indicus L. Arch Toxicol 2007; 81(6):397-406. doi: 10.1007/s00204-007-0176-7 [Crossref] [ Google Scholar]
-
US Department of Health and Human Services. Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs), (update) PB/95/264370. Atlanta: US Department of Health and Human Services; 1995.
- Thévenod F. Catch me if you can! Novel aspects of cadmium transport in mammalian cells. Biometals 2010; 23(5):857-75. doi: 10.1007/s10534-010-9309-1 [Crossref] [ Google Scholar]
- Uraguchi S, Fujiwara T. Cadmium transport and tolerance in rice: perspectives for reducing grain cadmium accumulation. Rice (N Y) 2012; 5(1):5. doi: 10.1186/1939-8433-5-5 [Crossref] [ Google Scholar]
- Nordberg GF. Historical perspectives on cadmium toxicology. Toxicol Appl Pharmacol 2009; 238(3):192-200. doi: 10.1016/j.taap.2009.03.015 [Crossref] [ Google Scholar]
- Ogawa T, Kobayashi E, Okubo Y, Suwazono Y, Kido T, Nogawa K. Relationship among prevalence of patients with Itai-itai disease, prevalence of abnormal urinary findings, and cadmium concentrations in rice of individual hamlets in the Jinzu River basin, Toyama prefecture of Japan. Int J Environ Health Res 2004; 14(4):243-52. doi: 10.1080/09603120410001725586 [Crossref] [ Google Scholar]
- Wu J, Shao X, Lu K, Zhou J, Ren M, Xie X. Urinary RBP and NGAL levels are associated with nephropathy in patients with type 2 diabetes. Cell Physiol Biochem 2017; 42(2):594-602. doi: 10.1159/000477860 [Crossref] [ Google Scholar]
- Jiménez-Ortega V, Cano Barquilla P, Fernández-Mateos P, Cardinali DP, Esquifino AI. Cadmium as an endocrine disruptor: correlation with anterior pituitary redox and circadian clock mechanisms and prevention by melatonin. Free Radic Biol Med 2012; 53(12):2287-97. doi: 10.1016/j.freeradbiomed.2012.10.533 [Crossref] [ Google Scholar]
- Takiguchi M, Yoshihara S. New aspects of cadmium as endocrine disruptor. Environ Sci 2006; 13(2):107-16. [ Google Scholar]
- Borgeest C, Greenfeld C, Tomic D, Flaws JA. The effects of endocrine disrupting chemicals on the ovary. Front Biosci 2002; 7:d1941-8. doi: 10.2741/borgees [Crossref] [ Google Scholar]
- Garcia-Morales P, Saceda M, Kenney N, Kim N, Salomon DS, Gottardis MM. Effect of cadmium on estrogen receptor levels and estrogen-induced responses in human breast cancer cells. J Biol Chem 1994; 269(24):16896-901. [ Google Scholar]
- Jancic SA, Stosic BZ. Cadmium effects on the thyroid gland. Vitam Horm 2014; 94:391-425. doi: 10.1016/b978-0-12-800095-3.00014-6 [Crossref] [ Google Scholar]
- Piłat-Marcinkiewicz B, Brzóska MM, Sawicki B, Moniuszko-Jakoniuk J. Structure and function of thyroid follicular cells in female rats chronically exposed to cadmium. Bull Vet Inst Pulawy 2003; 47(1):157-63. [ Google Scholar]
- Arbon KS, Christensen CM, Harvey WA, Heggland SJ. Cadmium exposure activates the ERK signaling pathway leading to altered osteoblast gene expression and apoptotic death in Saos-2 cells. Food Chem Toxicol 2012; 50(2):198-205. doi: 10.1016/j.fct.2011.10.031 [Crossref] [ Google Scholar]
- Hammouda F, Messaoudi I, El Hani J, Baati T, Said K, Kerkeni A. Reversal of cadmium-induced thyroid dysfunction by selenium, zinc, or their combination in rat. Biol Trace Elem Res 2008; 126(1-3):194-203. doi: 10.1007/s12011-008-8194-8 [Crossref] [ Google Scholar]
- van den Berghe G. The role of the liver in metabolic homeostasis: implications for inborn errors of metabolism. J Inherit Metab Dis 1991; 14(4):407-20. doi: 10.1007/bf01797914 [Crossref] [ Google Scholar]
- Sawchenko PE, Friedman MI. Sensory functions of the liver--a review. Am J Physiol 1979; 236(1):R5-20. doi: 10.1152/ajpregu.1979.236.1.R5 [Crossref] [ Google Scholar]
- Yamano T, DeCicco LA, Rikans LE. Attenuation of cadmium-induced liver injury in senescent male fischer 344 rats: role of Kupffer cells and inflammatory cytokines. Toxicol Appl Pharmacol 2000; 162(1):68-75. doi: 10.1006/taap.1999.8833 [Crossref] [ Google Scholar]
- Arroyo VS, Flores KM, Ortiz LB, Gómez-Quiroz LE, Gutiérrez-Ruiz MC. Liver and cadmium toxicity. J Drug Metab Toxicol 2012; S5:1-7. doi: 10.4172/2157-7609.S5-001 [Crossref] [ Google Scholar]
- El-Sokkary GH, Nafady AA, Shabash EH. Melatonin administration ameliorates cadmium-induced oxidative stress and morphological changes in the liver of rat. Ecotoxicol Environ Saf 2010; 73(3):456-63. doi: 10.1016/j.ecoenv.2009.09.014 [Crossref] [ Google Scholar]
- Nishino H, Shiroishi K, Kagamimori S, Naruse Y, Watanabe M. Studies on the increase in serum concentrations of urea cycle amino acids among subjects exposed to cadmium. Bull Environ Contam Toxicol 1988; 40(4):553-60. doi: 10.1007/bf01688380 [Crossref] [ Google Scholar]
- Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician 1999; 59(8):2223-30. [ Google Scholar]
- Jurczuk M, Brzóska MM, Moniuszko-Jakoniuk J, Gałazyn-Sidorczuk M, Kulikowska-Karpińska E. Antioxidant enzymes activity and lipid peroxidation in liver and kidney of rats exposed to cadmium and ethanol. Food Chem Toxicol 2004; 42(3):429-38. doi: 10.1016/j.fct.2003.10.005 [Crossref] [ Google Scholar]
- Fahim MA, Nemmar A, Dhanasekaran S, Singh S, Shafiullah M, Yasin J. Acute cadmium exposure causes systemic and thromboembolic events in mice. Physiol Res 2012; 61(1):73-80. [ Google Scholar]
- Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol 2005; 204(3):274-308. doi: 10.1016/j.taap.2004.09.007 [Crossref] [ Google Scholar]
- Jin T, Nordberg G, Ye T, Bo M, Wang H, Zhu G. Osteoporosis and renal dysfunction in a general population exposed to cadmium in China. Environ Res 2004; 96(3):353-9. doi: 10.1016/j.envres.2004.02.012 [Crossref] [ Google Scholar]
- Aranami F, Segawa H, Furutani J, Kuwahara S, Tominaga R, Hanabusa E. Fibroblast growth factor 23 mediates the phosphaturic actions of cadmium. J Med Invest 2010; 57(1-2):95-108. doi: 10.2152/jmi.57.95 [Crossref] [ Google Scholar]
- Guo YC, Yuan Q. Fibroblast growth factor 23 and bone mineralisation. Int J Oral Sci 2015; 7(1):8-13. doi: 10.1038/ijos.2015.1 [Crossref] [ Google Scholar]
- Chen N, He Y, Su Y, Li X, Huang Q, Wang H. The cytotoxicity of cadmium-based quantum dots. Biomaterials 2012; 33(5):1238-44. doi: 10.1016/j.biomaterials.2011.10.070 [Crossref] [ Google Scholar]
- Lizotte J, Abed E, Signor C, Malu DT, Cuevas J, Kevorkova O. Expression of macrophage migration inhibitory factor by osteoblastic cells: protection against cadmium toxicity. Toxicol Lett 2012; 215(3):167-73. doi: 10.1016/j.toxlet.2012.10.006 [Crossref] [ Google Scholar]
- Youness ER, Mohammed NA, Morsy FA. Cadmium impact and osteoporosis: mechanism of action. Toxicol Mech Methods 2012; 22(7):560-7. doi: 10.3109/15376516.2012.702796 [Crossref] [ Google Scholar]
- Sughis M, Penders J, Haufroid V, Nemery B, Nawrot TS. Bone resorption and environmental exposure to cadmium in children: a cross--sectional study. Environ Health 2011; 10:104. doi: 10.1186/1476-069x-10-104 [Crossref] [ Google Scholar]
- Comelekoglu U, Yalin S, Bagis S, Ogenler O, Sahin NO, Yildiz A. Low-exposure cadmium is more toxic on osteoporotic rat femoral bone: mechanical, biochemical, and histopathological evaluation. Ecotoxicol Environ Saf 2007; 66(2):267-71. doi: 10.1016/j.ecoenv.2006.01.006 [Crossref] [ Google Scholar]
- Bhattacharyya MH, Whelton BD, Stern PH, Peterson DP. Cadmium accelerates bone loss in ovariectomized mice and fetal rat limb bones in culture. Proc Natl Acad Sci U S A 1988; 85(22):8761-5. doi: 10.1073/pnas.85.22.8761 [Crossref] [ Google Scholar]
- Horiguchi H, Oguma E, Kayama F. Cadmium induces anemia through interdependent progress of hemolysis, body iron accumulation, and insufficient erythropoietin production in rats. Toxicol Sci 2011; 122(1):198-210. doi: 10.1093/toxsci/kfr100 [Crossref] [ Google Scholar]
- Kunimoto M, Miura T, Kubota K. An apparent acceleration of age-related changes of rat red blood cells by cadmium. Toxicol Appl Pharmacol 1985; 77(3):451-7. doi: 10.1016/0041-008x(85)90185-1 [Crossref] [ Google Scholar]
- Hamilton DL, Valberg LS. Relationship between cadmium and iron absorption. Am J Physiol 1974; 227(5):1033-7. doi: 10.1152/ajplegacy.1974.227.5.1033 [Crossref] [ Google Scholar]
- Müller S, Gillert KE, Krause C, Jautzke G, Gross U, Diamantstein T. Effects of cadmium on the immune system of mice. Experientia 1979; 35(7):909-10. doi: 10.1007/bf01955143 [Crossref] [ Google Scholar]
- Hanson ML, Holásková I, Elliott M, Brundage KM, Schafer R, Barnett JB. Prenatal cadmium exposure alters postnatal immune cell development and function. Toxicol Appl Pharmacol 2012; 261(2):196-203. doi: 10.1016/j.taap.2012.04.002 [Crossref] [ Google Scholar]
- Holásková I, Elliott M, Hanson ML, Schafer R, Barnett JB. Prenatal cadmium exposure produces persistent changes to thymus and spleen cell phenotypic repertoire as well as the acquired immune response. Toxicol Appl Pharmacol 2012; 265(2):181-9. doi: 10.1016/j.taap.2012.10.009 [Crossref] [ Google Scholar]
- El-Boshy ME, Risha EF, Abdelhamid FM, Mubarak MS, Hadda TB. Protective effects of selenium against cadmium induced hematological disturbances, immunosuppressive, oxidative stress and hepatorenal damage in rats. J Trace Elem Med Biol 2015; 29:104-10. doi: 10.1016/j.jtemb.2014.05.009 [Crossref] [ Google Scholar]
- Fortier M, Omara F, Bernier J, Brousseau P, Fournier M. Effects of physiological concentrations of heavy metals both individually and in mixtures on the viability and function of peripheral blood human leukocytes in vitro. J Toxicol Environ Health A 2008; 71(19):1327-37. doi: 10.1080/15287390802240918 [Crossref] [ Google Scholar]
- Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich A. The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol 2006; 1:22. doi: 10.1186/1745-6673-1-22 [Crossref] [ Google Scholar]
- Petering HG, Choudhury H, Stemmer KL. Some effects of oral ingestion of cadmium on zinc, copper, and iron metabolism. Environ Health Perspect 1979; 28:97-106. doi: 10.1289/ehp.792897 [Crossref] [ Google Scholar]
- Demir N, Türksoy VA, Kayaaltı Z, Söylemezoğlu T, Savaş I. The evaluation of arsenic and cadmium levels in biological samples of cases with lung cancer. Tuberk Toraks 2014; 62(3):191-8. doi: 10.5578/tt.7902 [Crossref] [ Google Scholar]
- Waalkes MP. Cadmium carcinogenesis. Mutat Res 2003; 533(1-2):107-20. doi: 10.1016/j.mrfmmm.2003.07.011 [Crossref] [ Google Scholar]
- García-Esquinas E, Pollan M, Tellez-Plaza M, Francesconi KA, Goessler W, Guallar E. Cadmium exposure and cancer mortality in a prospective cohort: the strong heart study. Environ Health Perspect 2014; 122(4):363-70. doi: 10.1289/ehp.1306587 [Crossref] [ Google Scholar]
- Song J, Luo H, Yin X, Huang G, Luo S, Lin du R. Association between cadmium exposure and renal cancer risk: a meta-analysis of observational studies. Sci Rep 2015; 5:17976. doi: 10.1038/srep17976 [Crossref] [ Google Scholar]
- Boiago Gollucke AP, Claudio SR, Yamamura H, Morais DR, Bataglion GA, Eberlin MN. Grape skin extract mitigates tissue degeneration, genotoxicity, and oxidative status in multiple organs of rats exposed to cadmium. Eur J Cancer Prev 2018; 27(1):70-81. doi: 10.1097/cej.0000000000000273 [Crossref] [ Google Scholar]
- Souza V, Escobar Md Mdel C, Gómez-Quiroz L, Bucio L, Hernández E, Cossio EC. Acute cadmium exposure enhances AP-1 DNA binding and induces cytokines expression and heat shock protein 70 in HepG2 cells. Toxicology 2004; 197(3):213-28. doi: 10.1016/j.tox.2004.01.006 [Crossref] [ Google Scholar]
- Salminen WF Jr, Voellmy R, Roberts SM. Induction of hsp 70 in HepG2 cells in response to hepatotoxicants. Toxicol Appl Pharmacol 1996; 141(1):117-23. [ Google Scholar]
- Aravind P, Prasad MNV. Zinc alleviates cadmium-induced oxidative stress in Ceratophyllum demersum L: a free floating freshwater macrophyte. Plant Physiol Biochem 2003; 41(4):391-7. doi: 10.1016/S0981-9428(03)00035-4 [Crossref] [ Google Scholar]
- Wang Y, Wu Y, Luo K, Liu Y, Zhou M, Yan S. The protective effects of selenium on cadmium-induced oxidative stress and apoptosis via mitochondria pathway in mice kidney. Food Chem Toxicol 2013; 58:61-7. doi: 10.1016/j.fct.2013.04.013 [Crossref] [ Google Scholar]
- Tkalec M, Stefanić PP, Cvjetko P, Sikić S, Pavlica M, Balen B. The effects of cadmium-zinc interactions on biochemical responses in tobacco seedlings and adult plants. PLoS One 2014; 9(1):e87582. doi: 10.1371/journal.pone.0087582 [Crossref] [ Google Scholar]
- Choong G, Liu Y, Templeton DM. Interplay of calcium and cadmium in mediating cadmium toxicity. Chem Biol Interact 2014; 211:54-65. doi: 10.1016/j.cbi.2014.01.007 [Crossref] [ Google Scholar]
- Zhou X, Hao W, Shi H, Hou Y, Xu Q. Calcium homeostasis disruption - a bridge connecting cadmium-induced apoptosis, autophagy and tumorigenesis. Oncol Res Treat 2015; 38(6):311-5. doi: 10.1159/000431032 [Crossref] [ Google Scholar]
- Murphy BJ, Andrews GK, Bittel D, Discher DJ, McCue J, Green CJ. Activation of metallothionein gene expression by hypoxia involves metal response elements and metal transcription factor-1. Cancer Res 1999; 59(6):1315-22. [ Google Scholar]
- Thirumoorthy N, Shyam Sunder A, Manisenthil Kumar K, Senthil Kumar M, Ganesh G, Chatterjee M. A review of metallothionein isoforms and their role in pathophysiology. World J Surg Oncol 2011; 9:54. doi: 10.1186/1477-7819-9-54 [Crossref] [ Google Scholar]
- Shaw CF 3rd, Savas MM, Petering DH. Ligand substitution and sulfhydryl reactivity of metallothionein. Methods Enzymol 1991; 205:401-14. doi: 10.1016/0076-6879(91)05122-c [Crossref] [ Google Scholar]
- Klaassen CD, Liu J, Choudhuri S. Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu Rev Pharmacol Toxicol 1999; 39:267-94. doi: 10.1146/annurev.pharmtox.39.1.267 [Crossref] [ Google Scholar]
- Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T. The role of metallothionein in oxidative stress. Int J Mol Sci 2013; 14(3):6044-66. doi: 10.3390/ijms14036044 [Crossref] [ Google Scholar]
- Liu Y, Liu J, Iszard MB, Andrews GK, Palmiter RD, Klaassen CD. Transgenic mice that overexpress metallothionein-I are protected from cadmium lethality and hepatotoxicity. Toxicol Appl Pharmacol 1995; 135(2):222-8. doi: 10.1006/taap.1995.1227 [Crossref] [ Google Scholar]
- Sies H. Oxidative stress: from basic research to clinical application. Am J Med 1991; 91(3c):31s-8s. doi: 10.1016/0002-9343(91)90281-2 [Crossref] [ Google Scholar]
- Liu J, Qu W, Kadiiska MB. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol 2009; 238(3):209-14. doi: 10.1016/j.taap.2009.01.029 [Crossref] [ Google Scholar]
- Nemmiche S. Oxidative signaling response to cadmium exposure. Toxicol Sci 2017; 156(1):4-10. doi: 10.1093/toxsci/kfw222 [Crossref] [ Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39(1):44-84. doi: 10.1016/j.biocel.2006.07.001 [Crossref] [ Google Scholar]
- Unsal V, Belge-Kurutaş E. Experimental hepatic carcinogenesis: oxidative stress and natural antioxidants. Open Access Maced J Med Sci 2017; 5(5):686-91. doi: 10.3889/oamjms.2017.101 [Crossref] [ Google Scholar]
- Umemura T, Sai K, Takagi A, Hasegawa R, Kurokawa Y. Oxidative DNA damage, lipid peroxidation and nephrotoxicity induced in the rat kidney after ferric nitrilotriacetate administration. Cancer Lett 1990; 54(1-2):95-100. doi: 10.1016/0304-3835(90)90097-h [Crossref] [ Google Scholar]
- Myers CE, McGuire WP, Liss RH, Ifrim I, Grotzinger K, Young RC. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science 1977; 197(4299):165-7. doi: 10.1126/science.877547 [Crossref] [ Google Scholar]
- Aruoma OI. Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc 1998; 75(2):199-212. doi: 10.1007/s11746-998-0032-9 [Crossref] [ Google Scholar]
- Halliwell B. Halliwell BReactive species and antioxidantsRedox biology is a fundamental theme of aerobic life. Plant Physiol 2006; 141(2):312-22. doi: 10.1104/pp.106.077073 [Crossref] [ Google Scholar]
- Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans 2007; 35(Pt 5):1147-50. doi: 10.1042/bst0351147 [Crossref] [ Google Scholar]
- Miller AF. Superoxide dismutases: ancient enzymes and new insights. FEBS Lett 2012; 586(5):585-95. doi: 10.1016/j.febslet.2011.10.048 [Crossref] [ Google Scholar]
- Culotta VC, Yang M, O’Halloran TV. Activation of superoxide dismutases: putting the metal to the pedal. Biochim Biophys Acta 2006; 1763(7):747-58. doi: 10.1016/j.bbamcr.2006.05.003 [Crossref] [ Google Scholar]
- Wang Y, Branicky R, Noe A, Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol 2018; 217(6):1915-28. doi: 10.1083/jcb.201708007 [Crossref] [ Google Scholar]
- Yalin S, Comelekoglu U, Bagis S, Sahin NO, Ogenler O, Hatungil R. Acute effect of single-dose cadmium treatment on lipid peroxidation and antioxidant enzymes in ovariectomized rats. Ecotoxicol Environ Saf 2006; 65(1):140-4. doi: 10.1016/j.ecoenv.2005.06.006 [Crossref] [ Google Scholar]
- Banni M, Chouchene L, Said K, Kerkeni A, Messaoudi I. Mechanisms underlying the protective effect of zinc and selenium against cadmium-induced oxidative stress in zebrafish Danio rerio. Biometals 2011; 24(6):981-92. doi: 10.1007/s10534-011-9456-z [Crossref] [ Google Scholar]
- Cherubini A, Ruggiero C, Polidori MC, Mecocci P. Potential markers of oxidative stress in stroke. Free Radic Biol Med 2005; 39(7):841-52. doi: 10.1016/j.freeradbiomed.2005.06.025 [Crossref] [ Google Scholar]
- Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med 2003; 34(2):145-69. doi: 10.1016/s0891-5849(02)01197-8 [Crossref] [ Google Scholar]
-
Lymbury RS. Cardiovascular Disease, Antioxidant Enzyme Systems, and Selenium. Griffith University; 2008.
- Unsal V. Natural phytotherapeutic antioxidants in the treatment of mercury intoxication-a review. Adv Pharm Bull 2018; 8(3):365-76. doi: 10.15171/apb.2018.043 [Crossref] [ Google Scholar]
- Couto N, Wood J, Barber J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic Biol Med 2016; 95:27-42. doi: 10.1016/j.freeradbiomed.2016.02.028 [Crossref] [ Google Scholar]
- Elmallah MIY, Elkhadragy MF, Al-Olayan EM, Abdel Moneim AE. Protective effect of Fragaria ananassa crude extract on cadmium-induced lipid peroxidation, antioxidant enzymes suppression, and apoptosis in rat testes. Int J Mol Sci 2017; 18(5). doi: 10.3390/ijms18050957 [Crossref]
- Olsson U. Selenium deficiency and detoxication functions in the rat: short-term effects of cadmium. Drug Nutr Interact 1986; 4(3):309-19. [ Google Scholar]
- Hayeshi R, Mutingwende I, Mavengere W, Masiyanise V, Mukanganyama S. The inhibition of human glutathione S-transferases activity by plant polyphenolic compounds ellagic acid and curcumin. Food Chem Toxicol 2007; 45(2):286-95. doi: 10.1016/j.fct.2006.07.027 [Crossref] [ Google Scholar]
- Modén O, Mannervik B. Glutathione transferases in the bioactivation of azathioprine. Adv Cancer Res 2014; 122:199-244. doi: 10.1016/b978-0-12-420117-0.00006-2 [Crossref] [ Google Scholar]
- Kundu S, Sengupta S, Chatterjee S, Mitra S, Bhattacharyya A. Cadmium induces lung inflammation independent of lung cell proliferation: a molecular approach. J Inflamm (Lond) 2009; 6:19. doi: 10.1186/1476-9255-6-19 [Crossref] [ Google Scholar]
- Halder S, Kar R, Galav V, Mehta AK, Bhattacharya SK, Mediratta PK. Cadmium exposure during lactation causes learning and memory-impairment in F1 generation mice: amelioration by quercetin. Drug Chem Toxicol 2016; 39(3):272-8. doi: 10.3109/01480545.2015.1092042 [Crossref] [ Google Scholar]
- Delalande O, Desvaux H, Godat E, Valleix A, Junot C, Labarre J. Cadmium-glutathione solution structures provide new insights into heavy metal detoxification. FEBS J 2010; 277(24):5086-96. doi: 10.1111/j.1742-4658.2010.07913.x [Crossref] [ Google Scholar]
- Niki E, Traber MG. A history of vitamin E. Ann Nutr Metab 2012; 61(3):207-12. doi: 10.1159/000343106 [Crossref] [ Google Scholar]
- Nakano M, Onodera A, Saito E, Tanabe M, Yajima K, Takahashi J. Effect of astaxanthin in combination with alpha-tocopherol or ascorbic acid against oxidative damage in diabetic ODS rats. J Nutr Sci Vitaminol (Tokyo) 2008; 54(4):329-34. doi: 10.3177/jnsv.54.329 [Crossref] [ Google Scholar]
- Beytut E, Yuce A, Kamiloglu NN, Aksakal M. Role of dietary vitamin E in cadmium-induced oxidative damage in rabbit’s blood, liver and kidneys. Int J Vitam Nutr Res 2003; 73(5):351-5. doi: 10.1024/0300-9831.73.5.351 [Crossref] [ Google Scholar]
- Karabulut-Bulan O, Bolkent S, Yanardag R, Bilgin-Sokmen B. The role of vitamin C, vitamin E, and selenium on cadmium-induced renal toxicity of rats. Drug Chem Toxicol 2008; 31(4):413-26. doi: 10.1080/01480540802383200 [Crossref] [ Google Scholar]
- Stahl W, Sies H. beta-Carotene and other carotenoids in protection from sunlight. Am J Clin Nutr 2012; 96(5):1179s-84s. doi: 10.3945/ajcn.112.034819 [Crossref] [ Google Scholar]
- Kasperczyk S, Dobrakowski M, Kasperczyk J, Ostalowska A, Zalejska-Fiolka J, Birkner E. Beta-carotene reduces oxidative stress, improves glutathione metabolism and modifies antioxidant defense systems in lead-exposed workers. Toxicol Appl Pharmacol 2014; 280(1):36-41. doi: 10.1016/j.taap.2014.07.006 [Crossref] [ Google Scholar]
- Kini RD, Kumar NA, Noojibail A, Bhagyalakhshmi K, Bhoja SS, Chatterjee PK. Antioxidant Role of Beta Carotene: Protection against Cadmium Induced Testicular Toxicity. Pharmacogn J 2018; 10(6s):s66-s70. doi: 10.5530/pj.2018.6s.13 [Crossref] [ Google Scholar]
- El-Missiry MA, Shalaby F. Role of beta-carotene in ameliorating the cadmium-induced oxidative stress in rat brain and testis. J Biochem Mol Toxicol 2000; 14(5):238-43. doi: 10.1002/1099-0461(2000)14:5<238::aidjbt2>3.0.co;2-x [Crossref] [ Google Scholar]
- Cho EH, Koh PO. Proteomic identification of proteins differentially expressed by melatonin in hepatic ischemia-reperfusion injury. J Pineal Res 2010; 49(4):349-55. doi: 10.1111/j.1600-079X.2010.00799.x [Crossref] [ Google Scholar]
- Pieri C, Marra M, Moroni F, Recchioni R, Marcheselli F. Melatonin: a peroxyl radical scavenger more effective than vitamin E. Life Sci 1994; 55(15):PL271-PL6. doi: 10.1016/0024-3205(94)00666-0 [Crossref] [ Google Scholar]
- Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res 2011; 51(1):1-16. doi: 10.1111/j.1600-079X.2011.00916.x [Crossref] [ Google Scholar]
- Ataei N, Aghaei M, Panjehpour M. The protective role of melatonin in cadmium-induced proliferation of ovarian cancer cells. Res Pharm Sci 2018; 13(2):159-67. doi: 10.4103/1735-5362.223801 [Crossref] [ Google Scholar]
- Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta 2009; 1790(10):1149-60. doi: 10.1016/j.bbagen.2009.07.026 [Crossref] [ Google Scholar]
- Luo T, Liu G, Long M, Yang J, Song R, Wang Y. Treatment of cadmium-induced renal oxidative damage in rats by administration of alpha-lipoic acid. Environ Sci Pollut Res Int 2017; 24(2):1832-44. doi: 10.1007/s11356-016-7953-x [Crossref] [ Google Scholar]
- Xu Y, Zhou X, Shi C, Wang J, Wu Z. alpha-Lipoic acid protects against the oxidative stress and cytotoxicity induced by cadmium in HepG2 cells through regenerating glutathione regulated by glutamate-cysteine ligase. Toxicol Mech Methods 2015; 25(8):596-603. doi: 10.3109/15376516.2015.1044150 [Crossref] [ Google Scholar]
- Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci 2016; 5:e47. doi: 10.1017/jns.2016.41 [Crossref] [ Google Scholar]
- Iranshahi M, Rezaee R, Parhiz H, Roohbakhsh A, Soltani F. Protective effects of flavonoids against microbes and toxins: the cases of hesperidin and hesperetin. Life Sci 2015; 137:125-32. doi: 10.1016/j.lfs.2015.07.014 [Crossref] [ Google Scholar]
- Li X, Jiang X, Sun J, Zhu C, Li X, Tian L. Cytoprotective effects of dietary flavonoids against cadmium-induced toxicity. Ann N Y Acad Sci 2017; 1398(1):5-19. doi: 10.1111/nyas.13344 [Crossref] [ Google Scholar]
- Altenburg JD, Bieberich AA, Terry C, Harvey KA, Vanhorn JF, Xu Z. A synergistic antiproliferation effect of curcumin and docosahexaenoic acid in SK-BR-3 breast cancer cells: unique signaling not explained by the effects of either compound alone. BMC Cancer 2011; 11:149. doi: 10.1186/1471-2407-11-149 [Crossref] [ Google Scholar]
- Mohajeri M, Rezaee M, Sahebkar A. Cadmium-induced toxicity is rescued by curcumin: a review. Biofactors 2017; 43(5):645-61. doi: 10.1002/biof.1376 [Crossref] [ Google Scholar]
- Piper JT, Singhal SS, Salameh MS, Torman RT, Awasthi YC, Awasthi S. Mechanisms of anticarcinogenic properties of curcumin: the effect of curcumin on glutathione linked detoxification enzymes in rat liver. Int J Biochem Cell Biol 1998; 30(4):445-56. doi: 10.1016/s1357-2725(98)00015-6 [Crossref] [ Google Scholar]
- Priyadarsini KI. Free radical reactions of curcumin in membrane models. Free Radic Biol Med 1997; 23(6):838-43. doi: 10.1016/S0891-5849(97)00026-9 [Crossref] [ Google Scholar]
- Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci 2006; 78(18):2081-7. doi: 10.1016/j.lfs.2005.12.007 [Crossref] [ Google Scholar]
- Alghasham A, Salem TA, Meki AR. Effect of cadmium-polluted water on plasma levels of tumor necrosis factor-alpha, interleukin-6 and oxidative status biomarkers in rats: protective effect of curcumin. Food Chem Toxicol 2013; 59:160-4. doi: 10.1016/j.fct.2013.05.059 [Crossref] [ Google Scholar]
-
Dallner G, Roland S. Coenzyme Q10. In: Coates PM, Paul MC, Blackman M, Blackman MR, Cragg GM, Levine M, et al. Encyclopedia of Dietary Supplements. New York: Marcel Dekker; 2005. p. 121-31.
- Chew GT, Watts GF. Coenzyme Q10 and diabetic endotheliopathy: oxidative stress and the ‘recoupling hypothesis’. QJM 2004; 97(8):537-48. doi: 10.1093/qjmed/hch089 [Crossref] [ Google Scholar]
- Yue Y, Zhou H, Liu G, Li Y, Yan Z, Duan M. The advantages of a novel CoQ10 delivery system in skin photo-protection. Int J Pharm 2010; 392(1-2):57-63. doi: 10.1016/j.ijpharm.2010.03.032 [Crossref] [ Google Scholar]
- Paunović MG, Matić MM, Ognjanović BI, Saičić ZS. Antioxidative and haematoprotective activity of coenzyme Q10 and vitamin E against cadmium-induced oxidative stress in Wistar rats. Toxicol Ind Health 2017; 33(10):746-56. doi: 10.1177/0748233717725480 [Crossref] [ Google Scholar]
- Dodd S, Dean O, Copolov DL, Malhi GS, Berk M. N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility. Expert Opin Biol Ther 2008; 8(12):1955-62. doi: 10.1517/14728220802517901 [Crossref] [ Google Scholar]
- Gonçalves JF, Fiorenza AM, Spanevello RM, Mazzanti CM, Bochi GV, Antes FG. N-acetylcysteine prevents memory deficits, the decrease in acetylcholinesterase activity and oxidative stress in rats exposed to cadmium. Chem Biol Interact 2010; 186(1):53-60. doi: 10.1016/j.cbi.2010.04.011 [Crossref] [ Google Scholar]
- Yilmaz HR, Uz E, Yucel N, Altuntas I, Ozcelik N. Protective effect of caffeic acid phenethyl ester (CAPE) on lipid peroxidation and antioxidant enzymes in diabetic rat liver. J Biochem Mol Toxicol 2004; 18(4):234-8. doi: 10.1002/jbt.20028 [Crossref] [ Google Scholar]
- Castaldo S, Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002; 73 Suppl 1:S1-6. doi: 10.1016/s0367-326x(02)00185-5 [Crossref] [ Google Scholar]
- Russo A, Longo R, Vanella A. Antioxidant activity of propolis: role of caffeic acid phenethyl ester and galangin. Fitoterapia 2002; 73 Suppl 1:S21-9. doi: 10.1016/s0367-326x(02)00187-9 [Crossref] [ Google Scholar]
- Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther 2002; 96(2-3):67-202. doi: 10.1016/s0163-7258(02)00298-x [Crossref] [ Google Scholar]
- Kobroob A, Chattipakorn N, Wongmekiat O. Caffeic acid phenethyl ester ameliorates cadmium-induced kidney mitochondrial injury. Chem Biol Interact 2012; 200(1):21-7. doi: 10.1016/j.cbi.2012.08.026 [Crossref] [ Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 2006; 5(6):493-506. doi: 10.1038/nrd2060 [Crossref] [ Google Scholar]
- Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB. Chemosensitization of tumors by resveratrol. Ann N Y Acad Sci 2011; 1215:150-60. doi: 10.1111/j.1749-6632.2010.05852.x [Crossref] [ Google Scholar]
- Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol 2007; 224(3):274-83. doi: 10.1016/j.taap.2006.12.025 [Crossref] [ Google Scholar]
- Martín AR, Villegas I, Sánchez-Hidalgo M, de la Lastra CA. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br J Pharmacol 2006; 147(8):873-85. doi: 10.1038/sj.bjp.0706469 [Crossref] [ Google Scholar]
- Carrizzo A, Forte M, Damato A, Trimarco V, Salzano F, Bartolo M. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem Toxicol 2013; 61:215-26. doi: 10.1016/j.fct.2013.07.021 [Crossref] [ Google Scholar]
- Gambini J, Inglés M, Olaso G, Lopez-Grueso R, Bonet-Costa V, Gimeno-Mallench L. Properties of resveratrol: in vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid Med Cell Longev 2015; 2015:837042. doi: 10.1155/2015/837042 [Crossref] [ Google Scholar]
- Stojanović S, Sprinz H, Brede O. Efficiency and mechanism of the antioxidant action of trans-resveratrol and its analogues in the radical liposome oxidation. Arch Biochem Biophys 2001; 391(1):79-89. doi: 10.1006/abbi.2001.2388 [Crossref] [ Google Scholar]
- Hu J, Zhang B, Du L, Chen J, Lu Q. Resveratrol ameliorates cadmium induced renal oxidative damage and inflammation. Int J Clin Exp Med 2017; 10(5):7563-72. [ Google Scholar]
- Fu B, Zhao J, Peng W, Wu H, Zhang Y. Resveratrol rescues cadmium-induced mitochondrial injury by enhancing transcriptional regulation of PGC-1alpha and SOD2 via the Sirt3/FoxO3a pathway in TCMK-1 cells. Biochem Biophys Res Commun 2017; 486(1):198-204. doi: 10.1016/j.bbrc.2017.03.027 [Crossref] [ Google Scholar]
- Moskaug JØ, Carlsen H, Myhrstad M, Blomhoff R. Molecular imaging of the biological effects of quercetin and quercetin-rich foods. Mech Ageing Dev 2004; 125(4):315-24. doi: 10.1016/j.mad.2004.01.007 [Crossref] [ Google Scholar]
- Aguirre L, Arias N, Macarulla MT, Gracia A, Portillo MP. Beneficial effects of quercetin on obesity and diabetes. Open Nutraceuticals J 2011; 4:189-98. doi: 10.2174/1876396001104010189 [Crossref] [ Google Scholar]
- Grace SC, Logan BA. Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol 1996; 112(4):1631-40. doi: 10.1104/pp.112.4.1631 [Crossref] [ Google Scholar]
- Casagrande R, Georgetti SR, Verri WA, Jabor JR, Santos AC, Fonseca MJ. Evaluation of functional stability of quercetin as a raw material and in different topical formulations by its antilipoperoxidative activity. AAPS PharmSciTech 2006; 7(1):E64-e71. doi: 10.1208/pt070110 [Crossref] [ Google Scholar]
- Morales AI, Vicente-Sánchez C, Sandoval JM, Egido J, Mayoral P, Arévalo MA. Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. Food Chem Toxicol 2006; 44(12):2092-100. doi: 10.1016/j.fct.2006.07.012 [Crossref] [ Google Scholar]
- Renugadevi J, Milton Prabu S. Ameliorative effect of quercetin against cadmium induced toxicity in liver of Wistar rats. J Cell Tissue Res 2009; 9(1):1665-72. [ Google Scholar]
- Unsal C, Kanter M, Aktas C, Erboga M. Role of quercetin in cadmium-induced oxidative stress, neuronal damage, and apoptosis in rats. Toxicol Ind Health 2015; 31(12):1106-15. doi: 10.1177/0748233713486960 [Crossref] [ Google Scholar]
- Kim KS, Lim HJ, Lim JS, Son JY, Lee J, Lee BM. Curcumin ameliorates cadmium-induced nephrotoxicity in Sprague-Dawley rats. Food Chem Toxicol 2018; 114:34-40. doi: 10.1016/j.fct.2018.02.007 [Crossref] [ Google Scholar]
- El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and beta-carotene. Food Chem Toxicol 2004; 42(10):1563-71. doi: 10.1016/j.fct.2004.05.001 [Crossref] [ Google Scholar]
- Alkhedaide A, Alshehri ZS, Sabry A, Abdel-Ghaffar T, Soliman MM, Attia H. Protective effect of grape seed extract against cadmium-induced testicular dysfunction. Mol Med Rep 2016; 13(4):3101-9. doi: 10.3892/mmr.2016.4928 [Crossref] [ Google Scholar]
- Sönmez MF1 Tascioglu S. Protective effects of grape seed extract on cadmium-induced testicular damage, apoptosis, and endothelial nitric oxide synthases expression in rats. Toxicol Ind Health 2016; 32(8):1486-94. doi: 10.1177/0748233714566874 [Crossref] [ Google Scholar]
- Ansari MA, Raish M, Ahmad A, Alkharfy KM, Ahmad SF, Attia SM. Sinapic acid ameliorate cadmium-induced nephrotoxicity: In vivo possible involvement of oxidative stress, apoptosis, and inflammation via NF-kappaB downregulation. Environ Toxicol Pharmacol 2017; 51:100-7. doi: 10.1016/j.etap.2017.02.014 [Crossref] [ Google Scholar]
- Erboga M, Kanter M, Aktas C, Sener U, Fidanol Erboga Z, Bozdemir Donmez Y. Thymoquinone ameliorates cadmium-induced nephrotoxicity, apoptosis, and oxidative stress in rats is based on its anti-apoptotic and anti-oxidant properties. Biol Trace Elem Res 2016; 170(1):165-72. doi: 10.1007/s12011-015-0453-x [Crossref] [ Google Scholar]
- Eybl V, Kotyzova D, Koutensky J. Comparative study of natural antioxidants - curcumin, resveratrol and melatonin - in cadmium-induced oxidative damage in mice. Toxicology 2006; 225(2-3):150-6. doi: 10.1016/j.tox.2006.05.011 [Crossref] [ Google Scholar]
- Abdel Moneim AE, Bauomy AA, Diab MM, Shata MT, Al-Olayan EM, El-Khadragy MF. The protective effect of Physalis peruviana L against cadmium-induced neurotoxicity in rats. Biol Trace Elem Res 2014; 160(3):392-9. doi: 10.1007/s12011-014-0066-9 [Crossref] [ Google Scholar]
- Gülyaşar T, Şener S, Aydoğdu N, Kaymak K, Çakına S, Sipahi T. Trace elements in a rat model of cadmium toxicity: the effects of taurine, melatonin and n-acetylcysteine. Balkan Med J 2010; 27(1):23-7. [ Google Scholar]
- Abarikwu SO, Olufemi PD, Lawrence CJ, Wekere FC, Ochulor AC, Barikuma AM. Rutin, an antioxidant flavonoid, induces glutathione and glutathione peroxidase activities to protect against ethanol effects in cadmium-induced oxidative stress in the testis of adult rats. Andrologia 2017; 49(7). doi: 10.1111/and.12696 [Crossref]
- Adi PJ, Burra SP, Vataparti AR, Matcha B. Calcium, zinc and vitamin E ameliorate cadmium-induced renal oxidative damage in albino Wistar rats. Toxicol Rep 2016; 3:591-7. doi: 10.1016/j.toxrep.2016.07.005 [Crossref] [ Google Scholar]
- El-Sharaky AS, Newairy AA, Badreldeen MM, Eweda SM, Sheweita SA. Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology 2007; 235(3):185-93. doi: 10.1016/j.tox.2007.03.014 [Crossref] [ Google Scholar]
- Ahmed MM, El-Shazly SA, Alkafafy ME, Mohamed AA, Mousa AA. Protective potential of royal jelly against cadmium-induced infertility in male rats. Andrologia 2018; 50(5):e12996. doi: 10.1111/and.12996 [Crossref] [ Google Scholar]
- Micali A, Pallio G, Irrera N, Marini H, Trichilo V, Puzzolo D. Flavocoxid, a natural antioxidant, protects mouse kidney from cadmium-induced toxicity. Oxid Med Cell Longev 2018; 2018:9162946. doi: 10.1155/2018/9162946 [Crossref] [ Google Scholar]
- Bu T, Mi Y, Zeng W, Zhang C. Protective effect of quercetin on cadmium-induced oxidative toxicity on germ cells in male mice. Anat Rec (Hoboken) 2011; 294(3):520-6. doi: 10.1002/ar.21317 [Crossref] [ Google Scholar]
- Wani FA, Ibrahim MAB, Abdel Moneim MM, Almaeen ARHA. Cytoprotectant and Anti-Oxidant Effects of Olive Oil on Cadmium Induced Nephrotoxicity in Mice. Open J Pathol 2017; 8(1):31-46. doi: 10.4236/ojpathology.2018.81004 [Crossref] [ Google Scholar]
- Shati AA. Effects of Origanum majorana L on cadmium induced hepatotoxicity and nephrotoxicity in albino rats. Saudi Med J 2011; 32(8):797-805. [ Google Scholar]
- Bernhoft RA. Cadmium toxicity and treatment. ScientificWorldJournal 2013; 2013:394652. doi: 10.1155/2013/394652 [Crossref] [ Google Scholar]
- Rafati Rahimzadeh M, Rafati Rahimzadeh M, Kazemi S, Moghadamnia AA. Cadmium toxicity and treatment: an update. Caspian J Intern Med 2017; 8(3):135-45. doi: 10.22088/cjim.8.3.135 [Crossref] [ Google Scholar]