Advanced pharmaceutical bulletin. 10(1):39-45.
doi: 10.15171/apb.2020.005
Research Article
Development and Characterization of Nanoliposomal Hydroxyurea Against BT-474 Breast Cancer Cells
Azam Akbari 1, 2  , Azim Akbarzadeh 2, *
, Azim Akbarzadeh 2, *  , Morteza Rafiee Tehrani 1, Reza Ahangari Cohan 2
, Morteza Rafiee Tehrani 1, Reza Ahangari Cohan 2  , Mohsen Chiani 2, Mohammad Reza Mehrabi 2
, Mohsen Chiani 2, Mohammad Reza Mehrabi 2
Author information:
1Department of Pharmaceutics, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.
2Department of Nanobiotechnology, Pasteur Institute of Iran, Tehran, Iran.
Abstract
Purpose:
Hydroxyurea (HU) is a well-known chemotherapy drug with several side effects which limit its clinical application. This study was conducted to improve its therapeutic efficiency against breast cancer using liposomes as FDA-approved drug carriers.
Methods:
PEGylated nanoliposomes-containing HU (NL-HU) were made via a thin-film hydration method, and assessed in terms of zeta potential, size, morphology, release, stability, cellular uptake, and cytotoxicity. The particle size and zeta potential of NL-HU were specified by zeta-sizer. The drug release from liposomes was assessed by dialysis diffusion method. Cellular uptake was evaluated by flow cytometry. The cytotoxicity was designated by methyl thiazolyl diphenyl-tetrazolium bromide (MTT) test.
Results:
The size and zeta value of NL-HU were gotten as 85 nm and -27 mV, respectively. NL-HU were spherical.NL-HU vesicles were detected to be stable for two months. The slow drug release and Weibull kinetic model were obtained. Liposomes considerably enhanced the uptake of HU into BT-474 human breast cancer cells. The cytotoxicity of NL-HU on BT-474 cells was found to be significantly more than that of free HU.
Conclusion:
The results confirmed these PEGylated nanoliposomes containing drug are potentially suitable against in vitro model of breast cancer.
Keywords: Breast neoplasms, Drug carriers, Hydroxyurea, Liposomes
Copyright and License Information
© 2020 The Author (s)
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Breast cancer is one of the most prevalent diseases, with a high death rate among women.
1,2
Current methods for breast cancer therapy are chemotherapy, radiotherapy, and surgery, but all three types of treatment cause high toxicity for patients by killing healthy cells beside cancer cells and leading to severe side effects.
3
Furthermore, only a little quantity of the drug reaches the target tumor, and most of the drug enters healthy tissues or is rapidly removed.
4
Among the chemotherapy agents, hydroxyurea (HU) is a well-known, low cost, effective, and safe drug that is extensively applied in the treatment of human cancers.
5,6
Moreover, this drug has several defects, such as rapid clearance from circulation, low bioavailability, and side effects in patients.
7
Liposomes are widely used as carriers for delivering anticancer drugs, with multiple approved products for use by patients.
8-12
In recent years, some researchers have focused on the designing nanosize liposomes with a prolonged circulation time in the blood flow that is believed to increase drug delivery to the tumor. Where nanosize liposomes would pass the tumors vasculature and accumulate in large quantities.-
16
Several efforts have been made in this field. In a study, Alavi et al prepared and evaluated liposomal hydroxyurea on MCF7 cells.
17
O’Shaughnessy et al also showed the successful use of PEGylated liposomes containing doxorubicin for breast cancer treatment.
18
In the present investigation, HU-loaded PEGylated nanoliposomes were prepared and, first, assessed on BT-474 breast cancer cell.
Materials and Methods
Materials and cells
Dipalmitoyl phosphatidylcholine (DPPC) and methoxy-polyethylene glycol-derivatized di- stearoyl phosphatidylethanolamine (DSPE-mPEG2000) were obtained from Lipoid GmbH (Ludwigshafen, Germany). Fluorescein isothiocyanate (FITC), HU and cholesterol (Chol) were provided from Sigma-Aldrich (St. Louis, MO, USA), Sucrose, Chloroform, and methanol were prepared from Merck (Darmstadt, Germany). Polycarbonate screens were supplied from Northern Lipids (Vancouver, Canada). Streptomycin-penicillin-glutamine (S/P/G), SephadexG-50 were taken from Invitrogen (Carlsbad, CA, USA). All other chemicals were of analytical grade. As well, the BT-474 cell line was bought from the American Type Culture Collection.
Preparation of liposomes containing drug
Liposomes were manufactured via the thin-film hydration technique. Briefly, DPPC: Chol: DSPE-mPEG2000 at 7:4:0.18 molar proportions solubilized in a chloroform-methanol mix at a ratio (2:1).
After evaporation of the solvents, the film was dried and suspended in phosphate buffer saline (PBS, pH = 7.4) containing HU (molar ratio drug/lipid = 0.2/1), stirred and warmed up to 50℃ in the water bath. The liposomes were mixed using a homogenizer (Ultra Turrax, NJ, USA) for 4 min at 16 000 rpm and extruded through polycarbonate screens 100 nm for ten circles by an Extruder (Avestin Inc., Ottawa, Canada). The NL-HU were segregated from free HU on a Sephadex G50 column. To determine the encapsulation efficiency (EE), one milliliter of purified NL-HU was disrupted by isopropanol (1:1 ratio) to release the drug.
The value of drug encapsulated in liposomes was computed by a spectrophotometer (UV-1601PC, SHIMADZU, Japan) at 214 nm via plot standard curve and the following formula
19,20
:
Phospholipid quantification
The Stewart assay,
21
was executed to measure the lipid content in the nanoliposomal formulations. Lipids were solubilized in chloroform, followed by removing the solvent by a rotary motion. The thin film was hydrated in DW, 1 mL chloroform added and stored overnight in an oven at 90°C. Then was dissolved by 70 mL chloroform and immediately after centrifugation of the dispersals, we calculated the optical density (OD) of the standard and tests at 485 nm and detected the concentration by the standard curve.
Size, polydispersity index, and zeta potential
The size, zeta index, and polydispersity value of the NL-HU were examined by a zeta-sizer instrument (Nano ZS3600, Malvern Panalytical Ltd, Malvern, UK) at 25°C in triplicate.
Morphology of NL-HU
Morphology of NL-HU was assessed by a scanning electron microscopy (SEM, Carl Zeiss EVO LS15, NY, USA) and the transmission electron microscopy (TEM, Zeiss EM 900, Jena, Germany). For SEM analysis, a small quantity of liposomal suspension was scattered on a stub and parched at environment temperature. NL-HU were layered with gold by sputter coater (Nano-Structured Coatings Co. DSR1, Tehran, Iran) that is equipped with a rotary pump to attain vacuum less than 50 militorr, the proper vacuum range for noble metals, and sample coating with an additional thin layer (~10 nm) of the gold conductive material to gather high-quality information from SEM. Finally, were examined by SEM at 26 kV. For TEM study, one droplet of well-dispersed formulations was settled on the carbon-layered copper grid, wiped at environment temperature, and photographed using TEM at 150 kV.
Drug release, kinetic models and mechanism
To specify the percentage of drug release, 1 mL of NL-HU and free HU were put into the dialysis bags (cut off: 14 000), immersed in 50 mL PBS (pH 7.4) and left on a stirrer for 36 hours at 37℃. At specific time intervals, 1 mL of reservoir buffer was taken and superseded by the same content of the fresh buffer. The amounts of HU released in the PBS were determined by spectrophotometer at 214 nm and the standard curve. The below formula computed the percentage of drug released (Er):
MHU illustrates the content of HU in the liposomes, V0 declares the whole volume of the release media and Ci and Cn manifest the concentration of HU in ith and nth aliquot removed, respectively.
To designate the release mechanism from kinetic models including first order, zero order, Weibull, Higuchi, and Korsmeyer Peppas, the release data were analyzed using Excel 2017 (Microsoft Corporation, Redmond, WA, USA) Add-In DD Solver program. The coefficient of determination (R2), release rate constants (kx), diffusion exponent (n), shape factor (b) were calculated. Well-fit kinetic equation with result data was considered as the best model.
Stability assessment
Stability of NL-HU was evaluated in buffer PBS at specified intervals within 2 months of storage at 4°C. The zeta potential and size of the specimens were considered as the stability performance.
Cellular uptake of formulations by flow cytometry
Internalization of the NL-HU and free HU into BT-474 cells was investigated by flow cytometry. Free HU and NL-HU were labeled by FITC. Briefly, 130 μL of PBS (pH = 7.4) and 65 μL of FTIC (2 mg in 1 cc DMSO) were poured into the free HU solution and NL-HU suspension and mixed gently. Unbounded FITC was separated by Sephadex G-50 column. Cells (300 × 103/well) were placed into six-well plates and incubated with the labeled free HU and NL-HU in the incubator at 37°C for 4 hours. The untreated cells were as the control. Finally, the cells were rinsed with cold PBS and appraised using the flow cytometry (Becton Dickinson FACSCalibur, Franklin Lakes, NJ, USA). WinMDI software was used to analyze the results.
Cytotoxicity assessment
The cytotoxicity of NL-HU against BT-474 cells was designated by MTT assay. Briefly, the cells were kept in RPMI medium containing S/P/G and 10% FBS in a 5% CO2 incubator at 37°C. Then, they were sub-cultured in 96-well plate (50 000 cells/well) in 100 µL of media at 37°C. After 24 hours of incubation, the cells were pasted to the wells. The free HU (as positive control) and NL-HU at the drug concentrations of 200, 400 and 600 µM were poured in the wells and incubated for 48 hours. Then the PBS-containing MTT (5 mg/mL) was added to the treatments and incubated for 3 hours; afterward, the formazan crystals were dissolved in 100 µL of DMSO. The OD was monitored using ELISA reader at 570 nm. Cell viability percentage and IC50 (50% inhibitory concentration of cell growth in comparison to untreated control cells as negative control),
22,23
were determined. All tests were done in triplicate.
Statistical analysis
Data were shown as mean ± standard deviation from three tests. The results were analyzed in SPSS software (version 20, IBM, Armonk, NY) with the student t test and one-way ANOVA followed by Tukey’s HSD post hoc test. Statistical significance was set at P< 0.05.
Results and Discussion
Preparation and characterization of NL-HU
The size of NL-HU was approximately 85 ± 2.2 nm, which was lower than the previous report (Figure 1).
17
The polydispersity index was equal to 0.12±0.030 for nanoliposomal HU; also, the mean zeta potential of the NL-HU was equal to -27 ± 0.51 mV (Figure 1). NL-HU were spherical, and rather homogenous, as is shown in Figure 2. EE% was equivalent to 88%, which was more than the prior study.
17
Lipid content of liposomes was equal to 89 ± 3.6%. Particle size and polydispersity index are fundamental factors for the preparation of proper nano-drug delivery systems; they contribute to the toxicity and delivery ability of these systems. For this purpose, the right size should be between 10 and 200 nm, where, the value of 85 nm was in this range due to the suitable choice of constituents, their proportions, and preparation method.
24-28
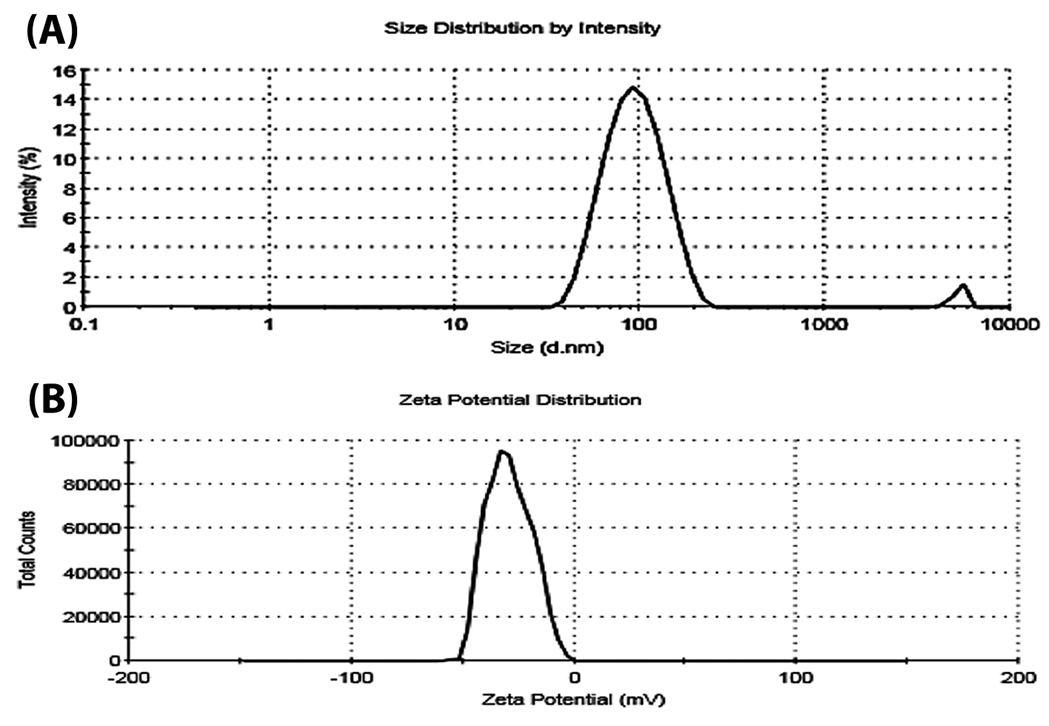
Figure 1.
Hydrodynamic size (A) and zeta potentials images (B) of PEGylated liposomes containing Hydroxyurea (NL-HU) by zeta-sizer instrument.
.
Hydrodynamic size (A) and zeta potentials images (B) of PEGylated liposomes containing Hydroxyurea (NL-HU) by zeta-sizer instrument.
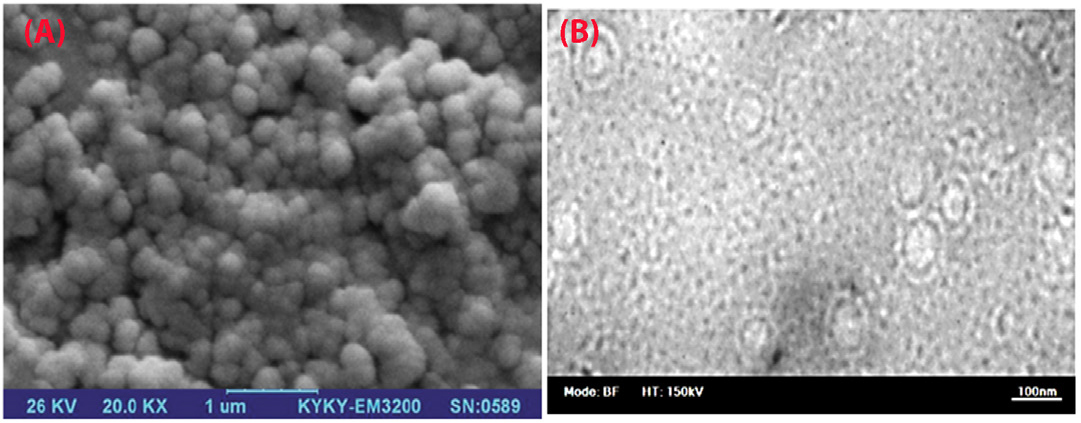
Figure 2.
Scanning electron microscopy (A) and Transmission electron microscopy (B) photographs of PEGylated liposomes containing hydroxyurea (NL-HU).
.
Scanning electron microscopy (A) and Transmission electron microscopy (B) photographs of PEGylated liposomes containing hydroxyurea (NL-HU).
Accordingly, the PDI was calculated based on the square of the ratio of standard deviation over diameter; the PDI <0.2 in this study indicates the high homogeneity of the liposomes.
29
Zeta potential is a significant criterion in the stability of liposome formulations and in blocking their aggregation, as well.
30
Zeta potential of nanoliposomes was negative because of the existence of carboxylic groups of lipids, drug–to–lipid ratio, pH, and type of lipids used in the liposomes. The obtained zeta potential was enough to keep the stability of liposomal formulations. Based on SEM and TEM images, the NL-HU were found to be spherical due to the appropriate method of liposome preparation. The observed dimensions of liposomes using SEM and TEM were slightly smaller than those of the zeta-sizer because of zeta-sizer displays the hydrodynamic diameter of liposomes, but SEM and TEM depict the dried form of the liposomes.
31
The EE% of HU was high because of the suitable HU-loading method and drug to lipid ratio.
30,32
Profile, kinetic, and mechanism of drug release
The pattern of drug release for free HU and NL-HU at pH = 7.4 was presented in Figure 3. Within ten h of drug release at 37°C in the buffer, 67 ± 4.2% of free HU and 11 ± 0.41% of NL-HU were released, respectively. After, the drug release was continued for up to 36 hours that 83 ± 4.8 and 14 ± 1.2% yield release for free HU and NL-HU were obtained, respectively. It is in consistency with the study by Alavi et al.
17
Drug release from NL-HU was significantly less than free HU because drugs are surrounded by liposomes that prevent them from being released. The liposomes exhibited fast drug release during the first ten hours. The reason for that is HU adsorbed on the liposome or the release of the drug-loaded close to the liposome surface. It was followed by a slow release due to liposome components, the liposome erosion, and HU diffusion mechanisms.
33
The sustained release pattern from liposomes could diminish prescription times. The mathematical modeling of the release data for NL-HU was done as well, and the kinetic parameters listed in Table 1. Based on our results, the release data of HU from NL-HU within the first 10 hours and up to 36 were found to be in harmony with the Weibull formula. The Weibull equation describes drug dissolution and releases from dosage forms. The factor β in this model is an index of the mechanism of drug transport through the polymer matrix, and Fickian diffusion is the typical release mechanism where β < 0.75. Accordingly, the calculated values of β = 0.72 for first 10 hours and β = 0.51 for up to 36 hours release represented the diffusion mechanism for drug release from nanoliposomes.
34-37
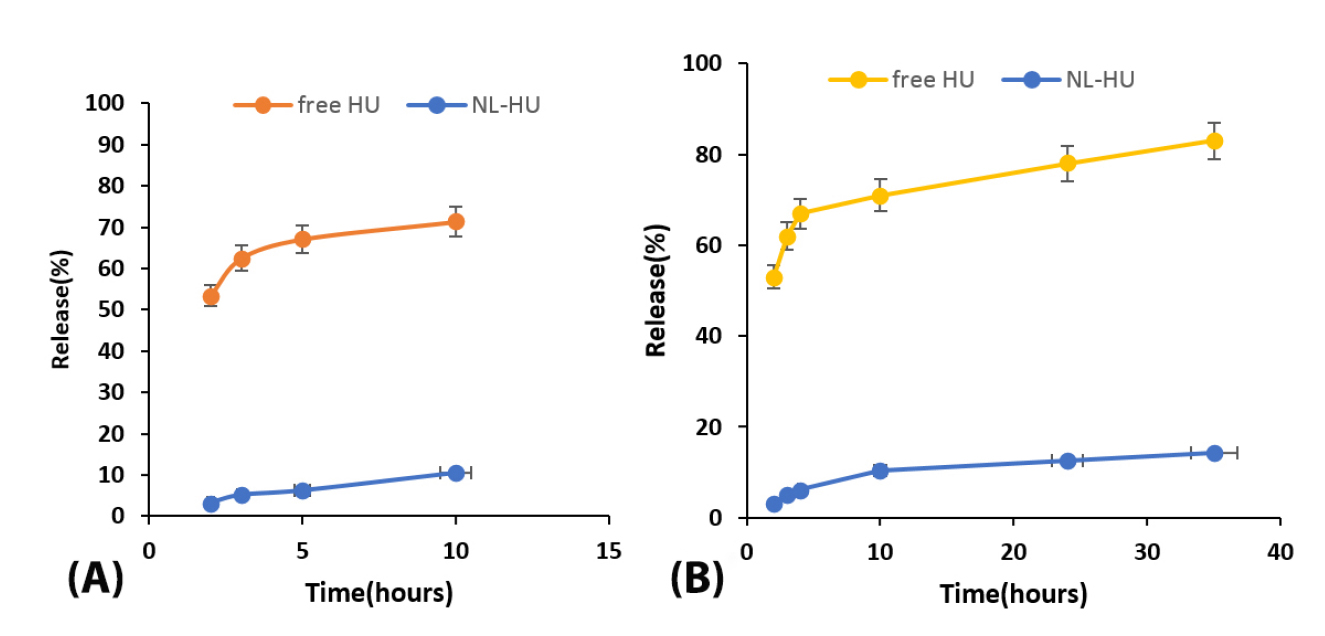
Figure 3.
In vitro release of PEGylated liposomes containing Hydroxyurea (NL-HU) and free Hydroxyurea (free HU) in PBS buffer (pH=7.4) at 37°C within first 10 h (A) and 36 h (B).
.
In vitro release of PEGylated liposomes containing Hydroxyurea (NL-HU) and free Hydroxyurea (free HU) in PBS buffer (pH=7.4) at 37°C within first 10 h (A) and 36 h (B).
Table 1.
Kinetic models parameters of release data
|
Sample
|
Model
|
Zero order
F
=
k
0
t
|
First order
Ln
(1 −
F
) = −
k
f
t
|
Higuchi
F
=
k
H
√ t
|
Weibull
ln[-ln(1-F
)]=
ln k
w
+
β
lnt
|
Peppas–Korsmeyer
F
=
k
kp
t
n
|
|
R
2
|
k
|
R
2
|
k
|
R
2
|
k
|
R
2
|
k
|
b
|
R
2
|
k
|
| NL-HU |
0.85 |
0.0031 |
0.86 |
0.0034 |
0.93 |
0.023 |
0.94 |
0.0090 |
0.51 |
0.94 |
0.027 |
| NL-HU (first 10 h) |
0.97 |
0.0086 |
0.98 |
0.0093 |
0.98 |
0.040 |
0.98 |
0.048 |
0.72 |
0.97 |
0.021 |
Abbreviation: NL-HU: PEGylated nanoliposomes containing Hydroxyurea.
Note: R2 is determination coefficient; F is the released drug fractions; k0, kf, kH, kw, and kkp, are constants of the kinetic models; n is the diffusion exponent of the Peppas–Korsmeyer model; β is the shape factor in Weibull model.
Stability analysis
As is seen in Figure 4, stability analysis confirmed no considerable changes in the mean zeta potential and particle size for the NL-HU within two months in PBS buffer at 4°C.
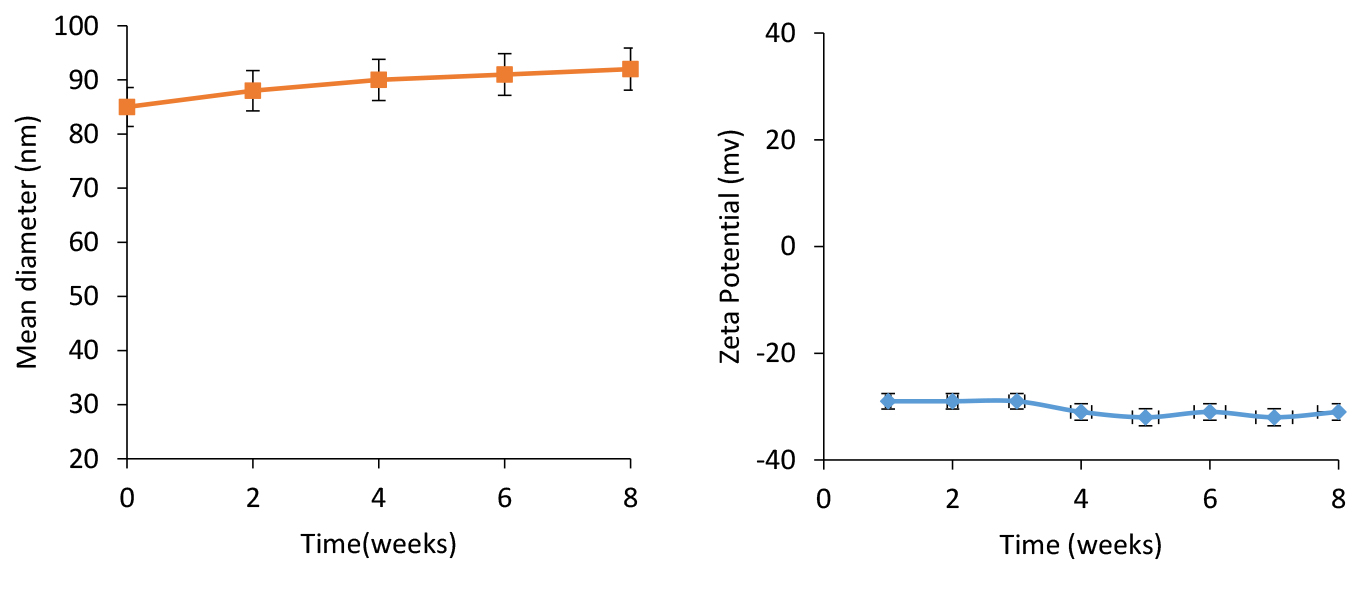
Figure 4.
Stability of PEGylated liposomes containing Hydroxyurea (NL-HU) based on mean diameter (A) and zeta potential(B) at 4°C within two months.
.
Stability of PEGylated liposomes containing Hydroxyurea (NL-HU) based on mean diameter (A) and zeta potential(B) at 4°C within two months.
These findings were related to the suitability of the applied method, components, and their ratios of liposomes.
38
Cellular uptake studies
The results of quantitative cellular internalization analysis by flow cytometry in Figure 5 showed the fluorescence intensity of the NL-HU was remarkably better than that of the free HU in BT-474 cells, which means more uptake of NL-HU compared to free HU in BT-474 cells. It is due to the suitable size of liposomal formulations, type, size of cells, incubation time, and a high tendency between liposome and the cell.22,
39-42
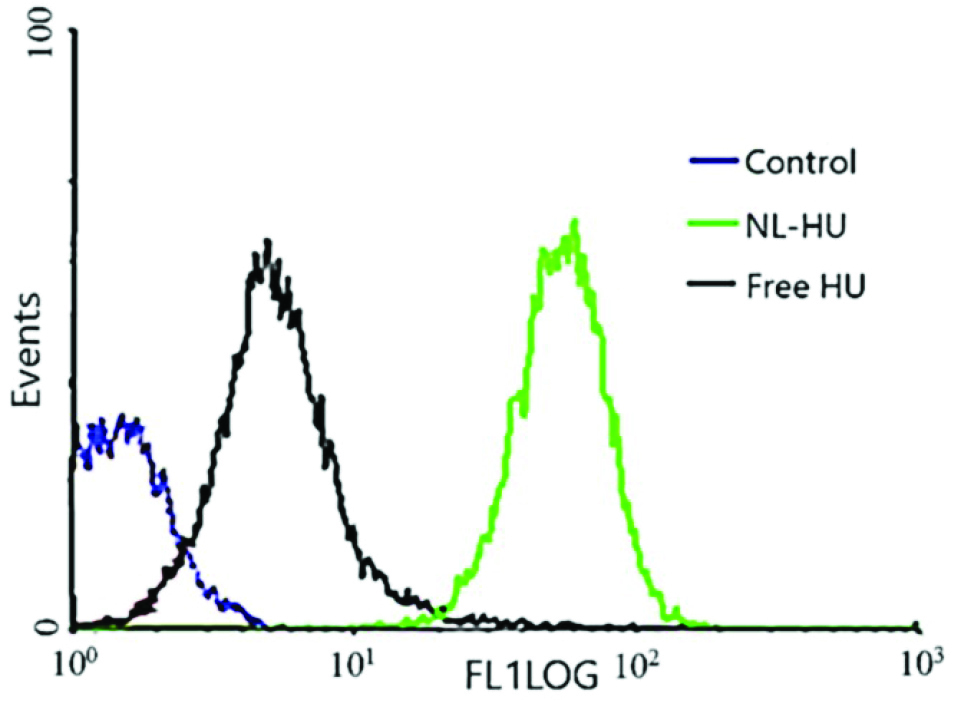
Figure 5.
Uptake of PEGylated nanoliposomes containing Hydroxyurea (NL-HU), Hydroxyurea (free HU) and untreated cells control after four h incubation at 37°C with BT-474 cells by flow cytometry.
.
Uptake of PEGylated nanoliposomes containing Hydroxyurea (NL-HU), Hydroxyurea (free HU) and untreated cells control after four h incubation at 37°C with BT-474 cells by flow cytometry.
Cell cytotoxicity
As is viewed in Figure 6, NL-HU were more toxic to BT-474 cells than free HU, which emphasizes the more anticancer potential of NL-HU against breast cancer than free HU at different concentrations. Cytotoxicity analysis verified less viability and lower IC50 (superior toxicity) of NL-HU (419.29 µM) versus free HU (601.14 µM) at all the concentrations on BT-474 cells; which is attributed to the cell type, exposure time, and liposome charge.
25,43-45
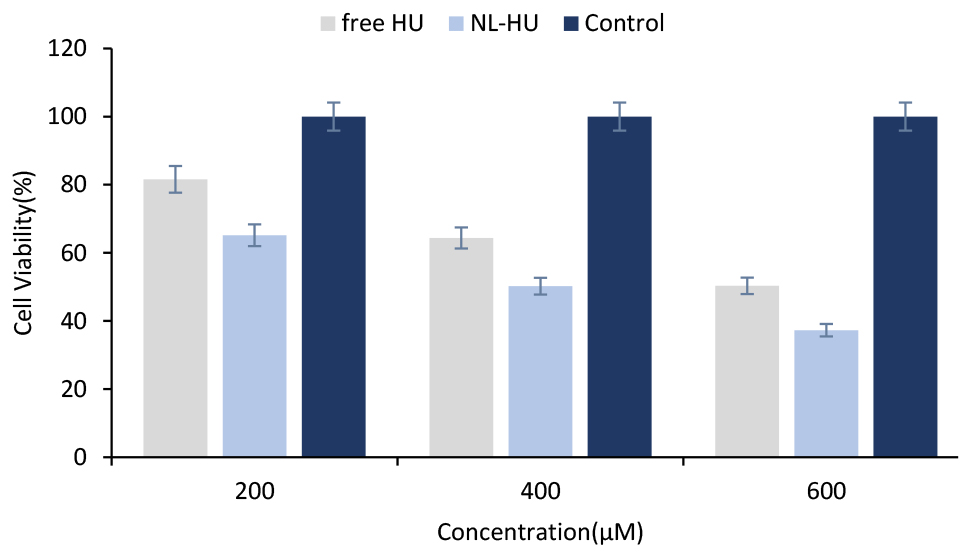
Figure 6.
Viability of BT-474 cells with free Hydroxyurea (free HU), PEGylated liposomes containing Hydroxyurea (NL-HU) and negative control at various concentrations after 48 hours.
.
Viability of BT-474 cells with free Hydroxyurea (free HU), PEGylated liposomes containing Hydroxyurea (NL-HU) and negative control at various concentrations after 48 hours.
Conclusion
We designed and evaluated HU-loaded PEGylated nanoliposomes on an in vitro model of breast cancer. The results demonstrated this liposomal drug system could be potentially useful for delivery of the hydrophilic anticancer drug. Nevertheless, further investigations are needed to assess this formulation on other breast cancer cells, and in vivo.
Ethical Issues
Not applicable.
Conflict of Interest
Authors declare no conflict of interest in this study.
References
- Rivera-Franco MM, Leon-Rodriguez E. Delays in breast cancer detection and treatment in developing countries. Breast Cancer (Auckl) 2018; 12:1178223417752677. doi: 10.1177/1178223417752677 [Crossref] [ Google Scholar]
- Oakman C, Santarpia L, Di Leo A. Breast cancer assessment tools and optimizing adjuvant therapy. Nat Rev Clin Oncol 2010; 7(12):725-32. doi: 10.1038/nrclinonc.2010.170 [Crossref] [ Google Scholar]
- Arruebo M, Vilaboa N, Sáez-Gutierrez B, Lambea J, Tres A, Valladares M. Assessment of the evolution of cancer treatment therapies. Cancers (Basel) 2011; 3(3):3279-330. doi: 10.3390/cancers3033279 [Crossref] [ Google Scholar]
- Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release 2011; 153(3):198-205. doi: 10.1016/j.jconrel.2011.06.001 [Crossref] [ Google Scholar]
- Madaan K, Kaushik D, Verma T. Hydroxyurea: a key player in cancer chemotherapy. Expert Rev Anticancer Ther 2012; 12(1):19-29. doi: 10.1586/era.11.175 [Crossref] [ Google Scholar]
- Heeney MM, Whorton MR, Howard TA, Johnson CA, Ware RE. Chemical and functional analysis of hydroxyurea oral solutions. J Pediatr Hematol Oncol 2004; 26(3):179-84. doi: 10.1097/00043426-200403000-00007 [Crossref] [ Google Scholar]
- Gwilt PR, Tracewell WG. Pharmacokinetics and pharmacodynamics of hydroxyurea. Clin Pharmacokinet 1998; 34(5):347-58. doi: 10.2165/00003088-199834050-00002 [Crossref] [ Google Scholar]
- Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine 2015; 10:975-99. doi: 10.2147/ijn.s68861 [Crossref] [ Google Scholar]
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 2005; 4(2):145-60. doi: 10.1038/nrd1632 [Crossref] [ Google Scholar]
- Alavi M, Karimi N, Safaei M. Application of various types of liposomes in drug delivery systems. Adv Pharm Bull 2017; 7(1):3-9. doi: 10.15171/apb.2017.002 [Crossref] [ Google Scholar]
- Ezzati Nazhad Dolatabadi J, Valizadeh H, Hamishehkar H. Solid lipid nanoparticles as efficient drug and gene delivery systems: recent breakthroughs. Adv Pharm Bull 2015; 5(2):151-9. doi: 10.15171/apb.2015.022 [Crossref] [ Google Scholar]
- García-Pinel B, Porras-Alcalá C, Ortega-Rodríguez A, Sarabia F, Prados J, Melguizo C. Lipid-based nanoparticles: application and recent advances in cancer treatment. Nanomaterials (Basel) 2019; 9(4). doi: 10.3390/nano9040638 [Crossref]
- Tran S, DeGiovanni PJ, Piel B, Rai P. Cancer nanomedicine: a review of recent success in drug delivery. Clin Transl Med 2017; 6(1):44. doi: 10.1186/s40169-017-0175-0 [Crossref] [ Google Scholar]
- Hussain Z, Khan JA, Murtaza S. Nanotechnology: An Emerging Therapeutic Option for Breast Cancer. Crit Rev Eukaryot Gene Expr 2018; 28(2):163-75. doi: 10.1615/CritRevEukaryotGeneExpr.2018022771 [Crossref] [ Google Scholar]
- Fung VW, Chiu GN, Mayer LD. Application of purging biotinylated liposomes from plasma to elucidate influx and efflux processes associated with accumulation of liposomes in solid tumors. Biochim Biophys Acta 2003; 1611(1-2):63-9. doi: 10.1016/s0005-2736(02)00704-6 [Crossref] [ Google Scholar]
- Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev 2016; 99(Pt A):28-51. doi: 10.1016/j.addr.2015.09.012 [Crossref] [ Google Scholar]
- Alavi SE, Esfahani MK, Ghassemi S, Akbarzadeh A, Hassanshahi G. In vitro evaluation of the efficacy of liposomal and pegylated liposomal hydroxyurea. Indian J Clin Biochem 2014; 29(1):84-8. doi: 10.1007/s12291-013-0315-2 [Crossref] [ Google Scholar]
- O’Shaughnessy JA. Pegylated liposomal doxorubicin in the treatment of breast cancer. Clin Breast Cancer 2003; 4(5):318-28. doi: 10.3816/CBC.2003.n.037 [Crossref] [ Google Scholar]
- Poy D, Akbarzadeh A, Ebrahimi Shahmabadi H, Ebrahimifar M, Farhangi A, Farahnak Zarabi M. Preparation, characterization, and cytotoxic effects of liposomal nanoparticles containing cisplatin: an in vitro study. Chem Biol Drug Des 2016; 88(4):568-73. doi: 10.1111/cbdd.12786 [Crossref] [ Google Scholar]
- Zare Kazemabadi F, Heydarinasab A, Akbarzadeh A, Ardjmand M. Preparation, characterization and in vitro evaluation of PEGylated nanoliposomal containing etoposide on lung cancer. Artif Cells Nanomed Biotechnol 2019; 47(1):3222-30. doi: 10.1080/21691401.2019.1646265 [Crossref] [ Google Scholar]
- Khan I, Yousaf S, Subramanian S, Korale O, Alhnan MA, Ahmed W. Proliposome powders prepared using a slurry method for the generation of beclometasone dipropionate liposomes. Int J Pharm 2015; 496(2):342-50. doi: 10.1016/j.ijpharm.2015.10.002 [Crossref] [ Google Scholar]
- Li S, Hu J, Zhang L, Zhang L, Sun Y, Xie Y. In-vitro and in-vivo evaluation of austocystin D liposomes. J Pharm Pharmacol 2013; 65(3):355-62. doi: 10.1111/j.2042-7158.2012.01606.x [Crossref] [ Google Scholar]
- Alavi SE, Muflih Al Harthi S, Ebrahimi Shahmabadi H, Akbarzadeh A. Cisplatin-Loaded Polybutylcyanoacrylate Nanoparticles with Improved Properties as an Anticancer Agent. Int J Mol Sci 2019; 20(7). doi: 10.3390/ijms20071531 [Crossref]
- Cheng WW, Allen TM. Targeted delivery of anti-CD19 liposomal doxorubicin in B-cell lymphoma: a comparison of whole monoclonal antibody, Fab’ fragments and single chain Fv. J Control Release 2008; 126(1):50-8. doi: 10.1016/j.jconrel.2007.11.005 [Crossref] [ Google Scholar]
- Moghimipour E, Rezaei M, Kouchak M, Ramezani Z, Amini M, Ahmadi Angali K. A mechanistic study of the effect of transferrin conjugation on cytotoxicity of targeted liposomes. J Microencapsul 2018; 35(6):548-58. doi: 10.1080/02652048.2018.1547325 [Crossref] [ Google Scholar]
- Haghighi M, Yarmand MS, Emam-Djomeh Z, McClements DJ, Saboury AA, Rafiee-Tehrani M. Design and fabrication of pectin-coated nanoliposomal delivery systems for a bioactive polyphenolic: Phloridzin. Int J Biol Macromol 2018; 112:626-37. doi: 10.1016/j.ijbiomac.2018.01.108 [Crossref] [ Google Scholar]
- Chen JH, Ling R, Yao Q, Li Y, Chen T, Wang Z. Effect of small-sized liposomal Adriamycin administered by various routes on a metastatic breast cancer model. Endocr Relat Cancer 2005; 12(1):93-100. doi: 10.1677/erc.1.00871 [Crossref] [ Google Scholar]
- Afzal E, Zakeri S, Keyhanvar P, Bagheri M, Mahjoubi P, Asadian M. Nanolipodendrosome-loaded glatiramer acetate and myogenic differentiation 1 as augmentation therapeutic strategy approaches in muscular dystrophy. Int J Nanomedicine 2013; 8:2943-60. doi: 10.2147/ijn.s43219 [Crossref] [ Google Scholar]
- Clayton KN, Salameh JW, Wereley ST, Kinzer-Ursem TL. Physical characterization of nanoparticle size and surface modification using particle scattering diffusometry. Biomicrofluidics 2016; 10(5):054107. doi: 10.1063/1.4962992 [Crossref] [ Google Scholar]
- Chiani M, Shokrgozar MA, Azadmanesh K, Norouzian D, Mehrabi MR, Najmafshar A. Preparation, characterization, and in vitro evaluation of bleomycin-containing nanoliposomes. Chem Biol Drug Des 2017; 89(4):492-7. doi: 10.1111/cbdd.12869 [Crossref] [ Google Scholar]
- Ruozi B, Belletti D, Tombesi A, Tosi G, Bondioli L, Forni F. AFM, ESEM, TEM, and CLSM in liposomal characterization: a comparative study. Int J Nanomedicine 2011; 6:557-63. doi: 10.2147/ijn.s14615 [Crossref] [ Google Scholar]
- Thomas AM, Kapanen AI, Hare JI, Ramsay E, Edwards K, Karlsson G. Development of a liposomal nanoparticle formulation of 5-fluorouracil for parenteral administration: formulation design, pharmacokinetics and efficacy. J Control Release 2011; 150(2):212-9. doi: 10.1016/j.jconrel.2010.11.018 [Crossref] [ Google Scholar]
- Huang CY, Chen CM, Lee YD. Synthesis of high loading and encapsulation efficient paclitaxel-loaded poly (n-butyl cyanoacrylate) nanoparticles via miniemulsion. Int J Pharm 2007; 338(1-2):267-75. doi: 10.1016/j.ijpharm.2007.01.052 [Crossref] [ Google Scholar]
- Vandghanooni S, Eskandani M, Barar J, Omidi Y. AS1411 aptamer-decorated cisplatin-loaded poly (lactic-co-glycolic acid) nanoparticles for targeted therapy of miR-21-inhibited ovarian cancer cells. Nanomedicine (Lond) 2018; 13(21):2729-58. doi: 10.2217/nnm-2018-0205 [Crossref] [ Google Scholar]
- Ragab DM, Rohani S, Consta S. Controlled release of 5-fluorouracil and progesterone from magnetic nanoaggregates. Int J Nanomedicine 2012; 7:3167-89. doi: 10.2147/ijn.s30190 [Crossref] [ Google Scholar]
- Barzegar-Jalali M, Adibkia K, Valizadeh H, Shadbad MR, Nokhodchi A, Omidi Y. Kinetic analysis of drug release from nanoparticles. J Pharm Pharm Sci 2008; 11(1):167-77. [ Google Scholar]
- Adibkia K, Siahi Shadbad MR, Nokhodchi A, Javadzedeh A, Barzegar-Jalali M, Barar J. Piroxicam nanoparticles for ocular delivery: physicochemical characterization and implementation in endotoxin-induced uveitis. J Drug Target 2007; 15(6):407-16. doi: 10.1080/10611860701453125 [Crossref] [ Google Scholar]
- Yang T, Cui FD, Choi MK, Cho JW, Chung SJ, Shim CK. Enhanced solubility and stability of PEGylated liposomal paclitaxel: in vitro and in vivo evaluation. Int J Pharm 2007; 338(1-2):317-26. doi: 10.1016/j.ijpharm.2007.02.011 [Crossref] [ Google Scholar]
- Ravichandiran V, Masilamani K, Senthilnathan B, Maheshwaran A, Wong TW, Roy P. Quercetin-decorated curcumin liposome design for cancer therapy: in-vitro and in-vivo studies. Curr Drug Deliv 2017; 14(8):1053-9. doi: 10.2174/1567201813666160829100453 [Crossref] [ Google Scholar]
- Lundberg BB, Griffiths G, Hansen HJ. Cellular association and cytotoxicity of doxorubicin-loaded immunoliposomes targeted via Fab’ fragments of an anti-CD74 antibody. Drug Deliv 2007; 14(3):171-5. doi: 10.1080/10717540601036831 [Crossref] [ Google Scholar]
- Papadimitriou E, Antimisiaris SG. Interactions of PC/Chol and PS/Chol liposomes with human cells in vitro. J Drug Target 2000; 8(5):335-51. doi: 10.3109/10611860008997910 [Crossref] [ Google Scholar]
- Zakeri-Milani P, Mussa Farkhani S, Shirani A, Mohammadi S, Shahbazi Mojarrad J, Akbari J. Cellular uptake and anti-tumor activity of gemcitabine conjugated with new amphiphilic cell penetrating peptides. EXCLI J 2017; 16:650-62. doi: 10.17179/excli2017-249 [Crossref] [ Google Scholar]
- AlQahtani SA, Harisa GI, Badran MM, AlGhamdi KM, Kumar A, Salem-Bekhit MM. Nano-erythrocyte membrane-chaperoned 5-fluorouracil liposomes as biomimetic delivery platforms to target hepatocellular carcinoma cell lines. Artif Cells Nanomed Biotechnol 2019; 47(1):989-96. doi: 10.1080/21691401.2019.1577887 [Crossref] [ Google Scholar]
- Rodallec A, Brunel JM, Giacometti S, Maccario H, Correard F, Mas E. Docetaxel-trastuzumab stealth immunoliposome: development and in vitro proof of concept studies in breast cancer. Int J Nanomedicine 2018; 13:3451-65. doi: 10.2147/ijn.s162454 [Crossref] [ Google Scholar]
- Hatziantoniou S, Dimas K, Georgopoulos A, Sotiriadou N, Demetzos C. Cytotoxic and antitumor activity of liposome-incorporated sclareol against cancer cell lines and human colon cancer xenografts. Pharmacol Res 2006; 53(1):80-7. doi: 10.1016/j.phrs.2005.09.008 [Crossref] [ Google Scholar]