Advanced pharmaceutical bulletin. 10(1):146-149.
doi: 10.15171/apb.2020.020
Short Communication
Isolation of Indigenous Selenium Tolerant Yeast and Investigation of the Relationship Between Growth and Selenium Biotransformation
Maryam Hosseindokht Khujin 1  , Hamed Zare 2, 3, *
, Hamed Zare 2, 3, * 
Author information:
1Department of Genetics and Molecular Biology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
2Cellular and Molecular Research Center, Birjand University of Medical Sciences, Birjand, Iran.
3Pharmaceutical Biotechnology Department, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Abstract
Purpose:
Organic selenium compound such as selenomethionine plays a significant function in the response to oxidative stress. Saccharomyces cerevisiae have the ability to accumulate selenium and selenium biotransformation. Selection of indigenous selenium tolerant yeast is our goals. The relationship between cell growth and selenium biotransformation was also investigated.
Methods:
The screening of the yeast cell was carried out at two steps in order to select yeast with high capacity for resistance and accumulation of selenium. The isolates were selected according to produced high biomass at different concentrations of selenium. Secondly, best yeast strains from previous step were grown in presence of 25 mg/L of sodium selenite and organic selenium content was measured.
Results:
The S17 isolate showed had maximum organic selenium accumulation (2515 ppm) and biomass production (2.73 g/L) compared to the other isolates. The biomass production and organic selenium accumulation of the S17 during 120 hours was shown a direct relationship between growth and biotransformation.
Conclusion:
This increase in organic selenium content was achieved with yeast screening. It is interesting to know that organic selenium has high bioavailability and low toxicity compared with inorganic selenium. Therefore, finding yeast strains which are resistant to selenium can be very helpful in cancer prevention.
Keywords: Yeast, Selenium, Biotransformation, Screening, Saccharomyces cerevisiae
Copyright and License Information
© 2020 The Author (s)
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Selenium has been shown to be a crucial micronutrient of human diets.
1,2
This element was discovered in 1817.
3,4
selenium is located in glutathione peroxidase.
5,6
This enzyme along with catalase and superoxide dismutase protects cells from the oxidation damage.
4,7
It has been shown that selenium has important roles in prevention of several cancers such as prostate cancer.
6,8-10
Deficiency of selenium is linked to the incidence of several diseases including heart disease, infertility, cancer and reversible cardiomyopathy that known as Keshan disease.
4,11,12
A lot of people in many countries consume a small amount of selenium.
1
Addition of inorganic salts of selenium in food products is one of the ways to overcome insufficiency of selenium, but this element has a narrow border for essential and toxic concentrations.
9,13
Interestingly, organic selenium to be the most bioavailable for human consumption.
6,9
Saccharomyces cerevisiae strains have the ability to absorb inorganic selenium and also they can transform inorganic selenium into organic selenium ingredients.
13,14
The advantage of Seleno-yeast is easy ingestion, low cost and high content of selenomethionine.
1,10,15
In this study, the ability of the yeast strains was measured in organic selenium production. In the following, the relationship between growth and organic selenium production was investigated.
Materials and Methods
Materials
Culture medium and salts were supplied by Merck (Germany). Master Mix PCR was obtained from Genaxxon. Standard yeast S. cerevisiae (PTCC 5157) was obtained from Iranian Biological Resource Center.
Yeast isolates
The yeast isolates from our previous study
15
were identified according to the criteria of Kurtzman and Fell.
16,17
Molecular identification was done with PCR reaction.
18
Lastly, the best yeast isolates with high resistance against selenium were confirmed by sequencing of the LSU rRNA gene D1/D2 domain of 26S rDNA and ITS region including 5.8S rRNA gene.
19-21
Screening for biomass production in the presence of selenium
The isolated strains were grown in SD (Sabouraud Dextrose) broth containing different concentrations of sodium selenite, i.e., 5 mg/L, 15 mg/L and 25 mg/L, and incubated at 30°C for 24 hours. The dry cell weight (DCW) was measured and strains with highest biomass were chosen for more screening.
Screening for selenium biotransformation
Selected strains were grown in SD broth containing 25 mg/L of sodium selenite, and incubated at 30°C for 24 hours. The yeast cultures were centrifuged at 8000 g for 10 min, washed 3 times for elimination of selenium from the cell surface. Cell sediment was dried at 85°C and weighed. Total and inorganic selenium was determined according to the atomic absorption spectroscopy method.
22
Calculation of organic selenium yield was obtained from the difference between the total and inorganic selenium.
22
Relationship between growth and selenium biotransformation
Two isolates with the high selenium tolerance and maximum organic selenium accumulation (S17 and S34) were chosen for more investigations. S17 isolate was grown in 5 ml SD broth and incubated at 30°C for 24 hours. One milliliters of this culture was added to 5 × 100 mL SD broth containing 25 mg/L of sodium selenite and was incubated at 30°C for 120 hours. Biomass production and organic selenium accumulation were measured at 24, 48 h, 72, 96 and 120 hours.
Results and Discussion
Screening for biomass production in the presence of selenium
The 40 isolates from the previous study were cultured in SD broth medium containing different concentrations of sodium selenite (i.e., 5 mg/L, 15 mg/L and 25 mg/L) to separate species with high yeast biomass production. Biomass concentration of yeast isolates decreased; while selenium concentration increased from 5 mg/L to 25 mg/L (Figure 1). This decrease is due to selenium toxicity. One-way ANOVA used in order to find any significant variation between yeast biomass production in 3 groups (The mean difference is significant at the 0.05 level). After incubation, only nine yeast cells (S8, S13, S17, S23, S30, S31, S34, S36 and S38) were gained to acceptable biomass (above 2.5 g/L DCW in 25 mg/L selenium) (Figure 2). Yeast cells can elevate resistance to toxic metals and therefore the yeast cells are able to obtain new metabolic capacities while exposed to the selection pressure.
23
Although the yeast cells can grow in medium with selenium, their ability to selenium biotransformation must be examined.
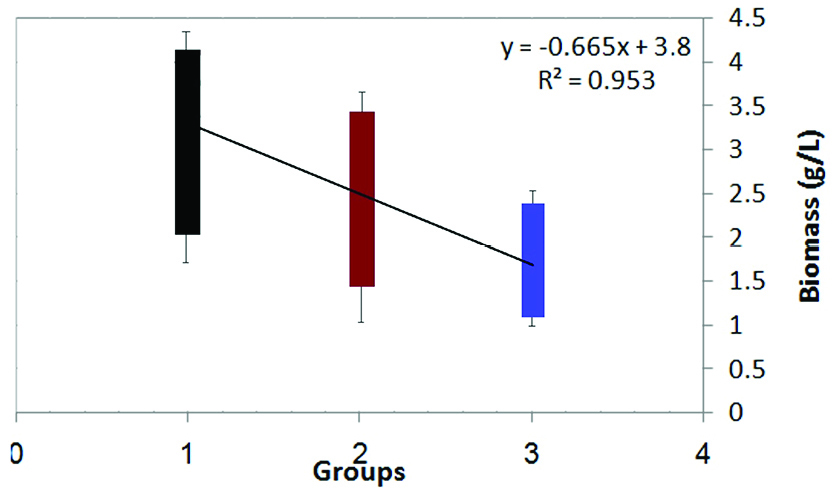
Figure 1.
Biomass production in Saccharomyces cerevisiae isolates under different concentrations of selenium. Selenium concentration in group 1, group 2 and group 3 is 5, 15 and 25 mg/L respectively.
.
Biomass production in Saccharomyces cerevisiae isolates under different concentrations of selenium. Selenium concentration in group 1, group 2 and group 3 is 5, 15 and 25 mg/L respectively.
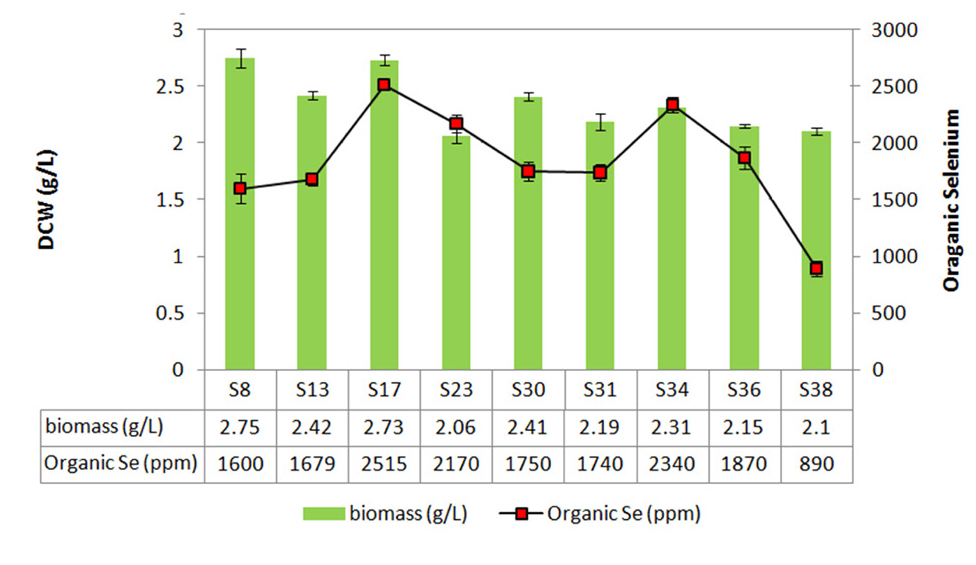
Figure 2.
Screening of selenium tolerant yeast isolate for high yeast biomass and organic selenium production.
.
Screening of selenium tolerant yeast isolate for high yeast biomass and organic selenium production.
Screening for selenium biotransformation
The best selected isolates from previous step were grown in SD broth containing 25 mg/L of sodium selenite. After incubation organic selenium content was measured. Organic selenium accumulation of the S8, S13, S17, S23, S30, S31, S34, S36 and S38 isolates were 1600, 1679, 2515, 2170, 1750, 1740, 2340, 1870 and 890 ppm respectively. The S17 isolate demonstrated greatest organic selenium (2515 ppm) and biomass creation (2.73 g/L) in comparison to other isolates (Figure 2). S17 and S34 isolates have given high biomass and best organic selenium content. The S8 isolate with the high biomass production, had low organic selenium content. On the other hand, the S23 isolate had high organic selenium content with low biomass production. Two isolates with the high resistant to selenium and best selenium biotransformation (S17 and S34) were selected for further characterization. The results of sequencing the ITS region and the LSU rRNA gene D1/D2 domains (data not shown) were indicated S17 and S34 isolates must be strains of S. cerevisiae.
The relationship between growth and biotransformation
Finally, S17 isolate was grown in presence of selenium. Biomass and selenium biotransformation measurement at different times showed a direct relationship between biomass growth and selenium biotransformation in the first 72 hours (Figure 3).
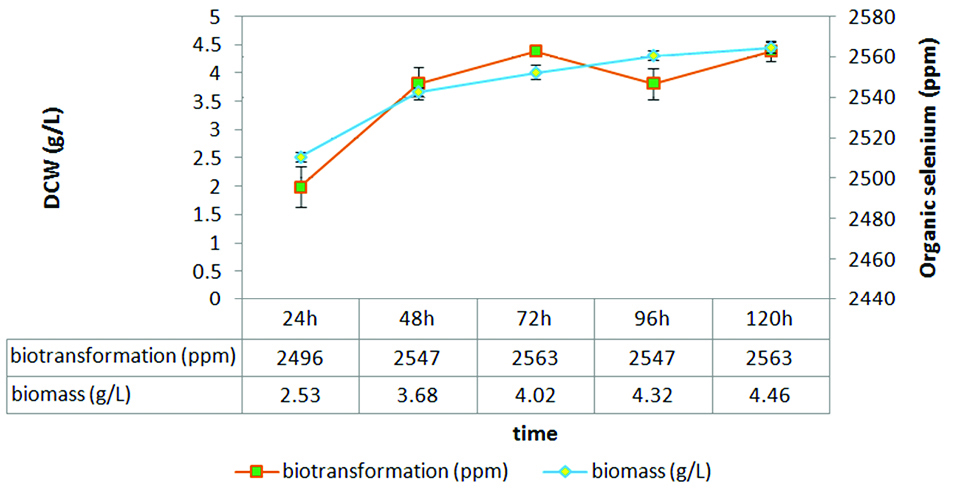
Figure 3.
The relationship between biomass and selenium biotransformation in S17 isolate.
.
The relationship between biomass and selenium biotransformation in S17 isolate.
Previous studies had shown the selenium amount that could be entered into yeast cells is between 500 to 3000 ppm.
4
Marinescu and Stoicescu showed organic selenium production in the range of 300 to 2200 ppm in yeast cells by supplementation of the medium with 30 to180 μg/mL sodium selenite.
6
Maximum biotransformation of selenium (2718 ppm) was acquired with synthetic medium by Rajashree and Muthukumar.
4
Suhajda et al reported Seleno-yeast with 1200 to 1400 µg selenium per gram dried yeast.
9
Conclusion
Even though selenium species are formed by Lactobacillus and Saccharomyces; but selenomethionine (the major selenium-containing amino acid) is synthesized in S. cerevisiae yeast.
23
On the other hand S. cerevisiae is one of the most known probiotics in the food industry and human nutrition.
24
It is interesting to know that organic selenium has high bioavailability and low toxicity compared with inorganic selenium.
4
Therefore, finding yeast strains resistant to selenium can be very helpful. In this study, the results showed strain (S17) capable of producing 2515 ppm organic selenium in presence of 25 mg/L of selenium, which is higher than the other isolates used in this work. This selenium biotransformation was performed only with screening, without manipulating the media.
Ethical Issues
Not applicable.
Conflicts of Interest
The authors have declared that no conflict of interest exists.
Acknowledgments
This study is proposed and approved by the school of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- Bierla K, Szpunar J, Yiannikouris A, Lobinski R. Comprehensive speciation of selenium in selenium-rich yeast. Trends Anal Chem 2012; 41:122-32. doi: 10.1016/j.trac.2012.08.006 [Crossref] [ Google Scholar]
- Kieliszek M, Blazejak S. Selenium: significance and outlook for supplementation. Nutrition 2013; 29:713-8. doi: 10.1016/j.nut.2012.11.012 [Crossref] [ Google Scholar]
- Kokarnig S, Tsirigotaki A, Wiesenhofer T, Lackner V, Francesconi KA, Pergantis SA. Concurrent quantitative HPLC-ICP mass spectrometry profiling of small selenium species in human serum and urine after ingestion of selenium supplements. J Trace Elem Med Biol 2015; 29:83-90. doi: 10.1016/j.jtemb.2014.06.012 [Crossref] [ Google Scholar]
- Rajashree K, Muthukumar T. Selection of Culture medium and conditions for the production of selenium enriched Saccharomyces cerevisiae. Afr J Biotechnol 2013; 12:2972-7. doi: 10.5897/AJB2012.11207 [Crossref] [ Google Scholar]
- Oraby MM, Allababidy T, Ramadan EM. The bioavailability of selenium in Saccharomyces cerevisiae. Ann Agric Sci 2015; 60:307-15. doi: 10.1016/j.aoas.2015.10.006 [Crossref] [ Google Scholar]
- Marinescu G, Stoicescu AG, Teodorof L. Industrial nutrient medium use for yeast selenium preparation. Food Technol 2011; 35:45-53. [ Google Scholar]
- Rajashree K, Muthukumar T. Preparation of selenium tolerant yeast Saccharomyces cerevisiae. J Microbiol Biotechnol Res 2013; 3:46-53. [ Google Scholar]
- El-Bayoumy K, Das A, Russell S, Wolfe S, Jordan R, Renganathan K. The effect of selenium enrichment on baker’s yeast proteome. J Proteomics 2012; 75:1018-30. doi: 10.1016/j.jprot.2011.10.013 [Crossref] [ Google Scholar]
- Suhajda A, Hegoczki J, Janzso B, Pais I, Vereczkey G. Preparation of selenium yeasts I Preparation of selenium-enriched Saccharomyces cerevisiae. J Trace Elem Med Biol 2000; 14:43-7. doi: 10.1016/S0946-672X(00)80022-X [Crossref] [ Google Scholar]
- Yin H, Chen Z, Gu Z, Han Y. Optimization of natural fermentative medium for selenium-enriched yeast by D-optimal mixture design. LWT Food Sci Technol 2009; 42:327-31. doi: 10.1016/j.lwt.2008.04.002 [Crossref] [ Google Scholar]
- Yang B, Wang D, Wei G, Liu Z, Ge X. Selenium-enriched Candida utilis: Efficient preparation with l-methionine and antioxidant capacity in rats. J Trace Elem Med Biol 2013; 27:7-11. doi: 10.1016/j.jtemb.2012.06.001 [Crossref] [ Google Scholar]
- Zare H, Rajabibazl M, Rasooli I, Ebrahimizadeh W, Bakherad H, Ardakani LS. Production of nanobodies against prostate-specific membrane antigen (PSMA) recognizing LnCaP cells. Int J Biol Markers 2014; 29:169-79. doi: 10.5301/jbm.5000063 [Crossref] [ Google Scholar]
- Stabnikova O, Ivanov V, Larionova I, Stabnikov V, Bryszewska MA, Lewis J. Ukrainian dietary bakery product with selenium-enriched yeast. LWT Food Sci Technol 2008; 41:890-5. doi: 10.1016/j.lwt.2007.05.021 [Crossref] [ Google Scholar]
- Sanchez MM, da Silva EG, Perez-Corona T, Camara C, Ferreira SL, Madrid Y. Selenite biotransformation during brewing Evaluation by HPLC-ICP-MS. Talanta 2012; 88:272-6. doi: 10.1016/j.talanta.2011.10.041 [Crossref] [ Google Scholar]
- Zare H, Vahidi H, Owlia P, Hosseindokht Khujin M, Khamisabadi A. Yeast enriched with selenium: a promising source of selenomethionine and seleno-proteins. Trends in Peptide and Protein Sciences 2017; 1:130-34. doi: 10.22037/tpps.v1i3.16778 [Crossref] [ Google Scholar]
- Hampsey M. A review of phenotypes in Saccharomyces cerevisiae. Yeast 1997; 13:1099-133. doi: 10.1002/(SICI)1097-0061 [Crossref] [ Google Scholar]
-
Kurtzman C, Fell JW, Boekhout T. The Yeasts: A Taxonomic Study. Amsterdam: Elsevier; 2011.
- Josepa SN, Guillamon JNM, Cano JN. PCR differentiation of Saccharomyces cerevisiae from Saccharomyces bayanus/Saccharomyces pastorianus using specific primers. FEMS Microbiol Lett 2000; 193:255-9. doi: 10.1111/j.1574-6968.2000.tb09433.x [Crossref] [ Google Scholar]
- Alimadadi N, Soudi MR, Wang SA, Wang QM, Talebpour Z, Bai FY. Starmerella orientalis fa, sp nov, an ascomycetous yeast species isolated from flowers. Int J Syst Evol Microbiol 2016; 66:1476-81. doi: 10.1099/ijsem.0.000905 [Crossref] [ Google Scholar]
-
White TJ, Bruns T, Lee SJ, Taylor JW. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. Amsterdam: Elsevier; 1990.
- Kurtzman CP, Robnett CJ. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Anton Leeuw J 1998; 73:331-71. [ Google Scholar]
- Esmaeili S, Khosravi-Darani K, Pourahmad R, Komeili R. An experimentaldesign for production of selenium-enriched yeast. Worl Appl Sci J 2012; 19:31-7. doi: 10.5829/idosi.wasj.2012.19.01.2634 [Crossref] [ Google Scholar]
- Yang L, Sturgeon RE, McSheehy S, Mester ZE. Comparison of extraction methods for quantitation of methionine and selenomethionine in yeast by species specific isotope dilution gas chromatography-mass spectrometry. J chromatogr A 2004; 1055:177-84. doi: 10.1016/j.chroma.2004.09.018 [Crossref] [ Google Scholar]
- Tahmasebi T, Nosrati R, Zare H, Saderi H, Moradi R, Owlia P. Isolation of indigenous glutathione producifng Saccharomyces cerevisiae strains. Iran J Pathol 2016; 11:354-62. [ Google Scholar]