Advanced pharmaceutical bulletin. 10(3):458-463.
doi: 10.34172/apb.2020.056
Research Article
Activation of PPARγ Inhibits TLR4 Signal Transduction Pathway in Melanoma Cancer In Vitro
Nasim Dana 1  , Golnaz Vaseghi 2, 1
, Golnaz Vaseghi 2, 1  , Shaghayegh Haghjooy Javanmard 1, *
, Shaghayegh Haghjooy Javanmard 1, * 
Author information:
1Applied Physiology Research Center, Cardiovascular Research Institute, Isfahan University of Medical sciences, Isfahan, Iran.
2Isfahan Cardiovascular Research Center, Cardiovascular Research Institute, Isfahan University of Medical sciences, Isfahan, Iran.
Abstract
Purpose:
Although peroxisome proliferator-activated receptor γ (PPARγ) is known as a regulator of fatty acid storage, fat cell differentiation, glucose and lipid metabolism, recent studies show that PPARγ has anticancer effects. The mechanisms of PPARγ activation in melanoma cancer remain unclarified. Recently, increased TLR4 expression has been associated with the melanoma cancer progression. We investigated whether the anti-cancer effect of PPARγ is through regulating TLR4 signaling pathway.
Methods: Mouse melanoma cells (B16F10) were treated in different groups: control, pioglitazone (1, 10, 100, 300 µmol/L), lipopolysaccharide (LPS) (5 µg/mL) and LPS + pioglitazone. In another experiment, they were treated with CLI-095 (1 μM), and after 1 hour pioglitazone was added and subsequently stimulated with LPS. MTT assay was performed to measure the cell viability in vitro. The expression of Tlr4, Myd88, Nf-κb genes were evaluated by quantitative reverse transcription PCR (qRT-PCR) in different groups. The concentration of tumor necrosis factor alpha and Interleukin 1 beta in the cell culture medium were measured by enzyme-linked immunosorbent assay (ELISA) kits.
Results: We show that activation of PPARγ by its agonist, pioglitazone, reduces cell proliferation, Tlr-4, Myd-88, Nf-kb mRNA expression, and tumor necrosis factor-alpha (TNF-α) production but not interleukin-1 β (IL-1β) in B16F10 LPS–stimulated cells in vitro. Moreover, treatment of B16F10 cells with TLR4 inhibitor prior treatment with pioglitazone indicate that the anticancer effects of pioglitazone on melanoma cells was dependent on TLR4.
Conclusion: The results indicate that pioglitazone has a beneficial protective effect against melanoma by affecting the TLR4 signaling pathway.
Keywords: Peroxisome proliferatoractivated receptor, Toll-like receptor 4, Melanoma, Pioglitazone
Copyright and License Information
© 2020 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Melanoma is a type of skin cancer that begins in the melanocytes after malignant transformation such as genetic mutations and tumor microenvironmental alterations in these cells.1 Some of these changes are mediated by dysregulation of the NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells). NF-κB is a major anti-apoptotic factor and has a fundamental role in melanoma progression.2,3
Peroxisome proliferator-activated receptor gamma (PPARγ) is a transcription factor that belongs to the superfamily of nuclear receptors.4 PPARγ can regulate gene expression of proinflammatory genes such as NF-κB. Pioglitazone is known to be a ligand for PPARγ.5 It has been shown that activation of PPARγ can inhibit the proliferation of melanoma cells but its mechanism is not clear.
Melanoma tumors by using NF-κB can achieve to survival, proliferation and resistance to apoptosis.6
One of the reasons for the increase in NF-κB levels in melanoma cells can be over-expression of toll like receptor 4 by melanoma cells.7 Toll-like receptors (TLRs), like other pattern recognition receptors, are responsible for recognizing different molecular patterns for example molecular patterns of pathogens.8 It has been shown that TLR4 is overexpressed in different cancers. It plays different roles in cancer development and progression.9
TLR4 signaling in response to lipopolysaccharide (LPS) stimulation, increase migration, invasion and adhesive properties of tumor cells such as breast, esophageal and colorectal cancer cell lines.10-13
Recent evidence suggests that PPARs and TLRs signaling pathways have crosstalk in different diseases.14 However, there is a little knowledge about the interaction between TLR4 pathway and the anti-inflammatory effect of PPARγ in cancer. So the aim of this study was to investigate the probable relation between PPARγ and TLR4 in melanoma cancer in vitro.
Materials and Methods
Cell and reagents
The mouse melanoma cell line (B16F10) purchased from the National Cell Bank of Iran (affiliated to Pasteur Institute, Tehran, Iran). Culture media and supplements were obtained from Gibco BRL (Carlsbad, CA, USA. Ultrapure LPS-EB from E. coli 0111: B4 and CLI-095 [resatorvid, ethyl (6R )-6-[N -(2-chloro-4-fluorophenyl)sulfamoyl] cyclohex-1-ene-1-carboxylate] were provided by Invivogen (San Diego, CA). Pioglitazone and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) were produced by Sigma (St. Louis, MO, USA). ELISA kits were prepared from eBioscience (San Diego, CA, USA).
Cell culture and treatment
The B16-F10 cells were grown in complete media under standard conditions. Cells were seeded into the wells of the plate at densities of 10 000 cell/well (in 96 well) and 200 000 cell/well (in 24 well) for different assays and incubated for 24 hours.
After the incubation period, cells were divided into control, pioglitazone (1, 10, 100, 300 µmol/L), LPS (5 µg/mL) and LPS + pioglitazone (corresponding LPS group plus (1, 10, 100, 300 µmol/L) pioglitazone) groups. There were two different control groups in this study, one control group didn’t receive any treatment and all groups that have not treated with LPS were compared with this control. The second control group was the cells that were treated with LPS and all LPS treated groups were compared with this control. For the experiments initially, the cells were treated with different dose of pioglitazone for one hour. Following stimulation, the cells were treated with LPS subsequently. After 24 hours, MTT assay was done, mRNA levels of Tlr-4, Myd-88 and Nf-kb were measured by real-time quantitative reverse transcription PCR (qRT-PCR). For measurable amounts of tumor necrosis factor-alpha (TNF-α) and interleukin-1 β (IL-1β) in cell culture supernatant.
MTT assay
B16-F10 cells were treated with or without pioglitazone and LPS as described above. Then MTT assay was done followed by 4 hours incubation of cells with MTT solution. After incubation, the medium/MTT mixtures were removed, and the formazan crystals were dissolved in DMSO. Optical densities at 570 nm were measured with a microplate reader (BioTek Instruments, Epoch, USA). The percentage cell viability was calculated by the formula according to our previous study.15
RNA extraction
After treatment of B16F10 cells as described above, total RNA was extracted by using a GeneJET RNA purification kit (Thermo Scientific, (EU) Lithuania). At first RNA concentration and purity of each sample was verified and then it was converted to cDNA using a RevertAid First Strand cDNA Synthesis Kit (Fermentas, Vilnius, Lithuania).
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
We used real time qRT-PCR to quantify mRNA expression levels of Tlr - 4, Myd-88 andNf-kb. The primer sequences for Tlr-4, Myd-88, Nf-kb, and beta-actin Primer sequences were as follows: (Tlr-4, 5′-AGTGGCTGGATTTATCCAGGTGTG-3′(fwd) and 5′-TTGAGAGGTGGTGTAAGCCATGCC-3′(rev); Myd-88, 5′-AAGTCTAGGAAGGCCCCAAA-3′ (fwd) and 5′-CTGGGGAGAAAACAGCTGAG-3′ (rev);Nf-kb, 5′- ACACGAGGCTACAACTCTGC-3′(fwd) and 5′- GGTACCCCCAGAGACCTCAT-3′(rev); β-actin, 5′- GCTGTATTCCCCTCCATCGTG -3′(fwd) and 5′- CACGGT TGGCCT TAGGGTTCAG -3′(rev)).
Real-time RT-PCR was performed by the Maxima SYBR Green Rox qPCR master mix kit (Fermentas, Vilnius, Lithuania). Real-time PCR reactions were performed using Corbett machine, Rotorgene 6000 (Australia). The other conditions and procedures were as described previously.16
The expression level of each target gene normalized with respect to the expression of housekeeping β-actin gene was calculated as 2-ΔΔCt.
All treatments were performed in triplicate wells for each condition and repeated at least twice.
Enzyme-linked immunosorbent assay (ELISA)
B16F10 cells at a concentration of 200000 cells/well, were treated with pioglitazone (1, 10, 100, 300 µmol/L) with or without LPS (5 µg/mL). In another experiment, at first the cells were treated with CLI-095(1 µM) for one hour then pioglitazone (300 µmol/L) was added. Subsequently cells were stimulated with LPS (5 µg/mL) for 24 hours. Culture medium was collected from each well, centrifuged and stored at -80°C. The concentration of TNF-α and IL-1β was determined by ELISA kits (Mouse TNF-α Instant ELISA and mouse IL-1β Instant ELISA).
Statistical analysis
For comparison of the difference between groups unpaired Student’s t test or one-way ANOVA followed by post hoc Dunn’s multiple comparison tests was used. P values <0.05 were considered as statistically significant. All statistical analysis was performed using SPSS 20.
Results
Pioglitazone activation suppresses proliferation of B16F10 cells
The effect of pioglitazone and LPS separately on B16F10 cells proliferation was assessed. As shown in Figure 1A, treatment of B16F10 cells with LPS alone compare with control group significantly increased (P < 0.05) the cell viability. Cell treatment with different concentrations of pioglitazone illustrated that only the highest concentration of pioglitazone (300 μmol/L) inhibited significantly proliferation of B16F10 cells (P < 0.001). Cell stimulation with both LPS and pioglitazone indicated that pioglitazone treatment in comparison with the LPS-stimulated control group significantly inhibited the effect of LPS on cell proliferation by all of the pioglitazone doses (P < 0.001; Figure 1B).
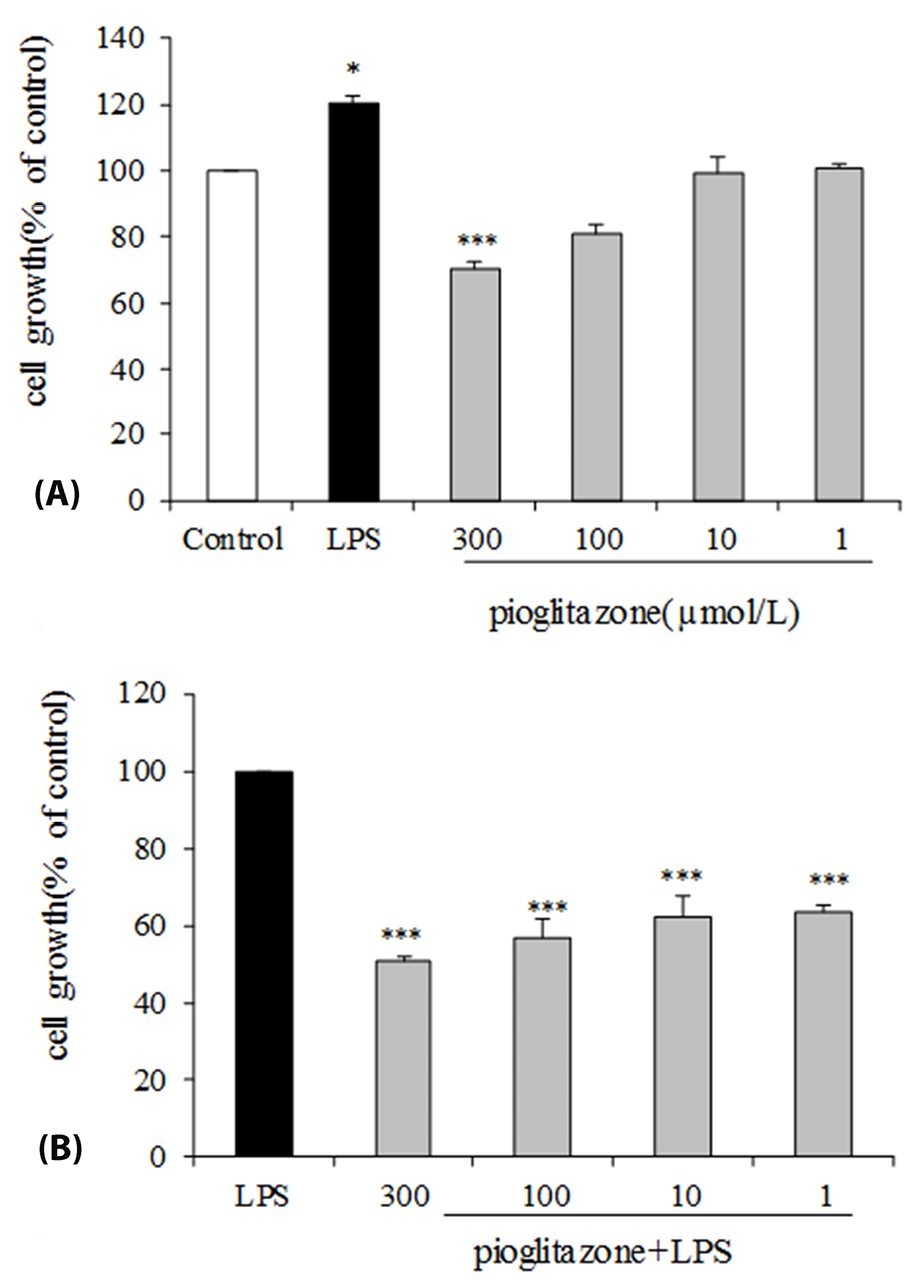
Figure 1.
Effects of LPS-EB Ultrapure and pioglitazone treatment on B16F10 cell line viability. (A) B16F10 cells were incubated with pioglitazone (1, 10, 100, 300 µM) or LPS (5 µg/mL) for 24 h. (B) B16F10 cells were pre-incubated for 1 h with our without pioglitazone (1, 10, 100, 300 µM) then continuing the cells incubation with LPS (5 µg/mL) for 24 h. Cell viability was determined by MTT assay. * P <0.05; *** P <0.001 compared with negative control. The data are representative of three independent experiments and are expressed as mean± SEM.
.
Effects of LPS-EB Ultrapure and pioglitazone treatment on B16F10 cell line viability. (A) B16F10 cells were incubated with pioglitazone (1, 10, 100, 300 µM) or LPS (5 µg/mL) for 24 h. (B) B16F10 cells were pre-incubated for 1 h with our without pioglitazone (1, 10, 100, 300 µM) then continuing the cells incubation with LPS (5 µg/mL) for 24 h. Cell viability was determined by MTT assay. * P <0.05; *** P <0.001 compared with negative control. The data are representative of three independent experiments and are expressed as mean± SEM.
Pioglitazone reduces expression of Tlr4, Myd-88, and Nf-kb mRNA
As shown in Figure 2A, pioglitazone treatment for 24 hours decreased the Tlr-4,Myd-88 and Nf-kB mRNA expression in B16F10 cells by all doses. Incubation of B16F10 cells with different concentration of pioglitazone (1, 10, 100, 300 µmol/L) resulted in a dose-dependent inhibition of mRNA expression levels of Tlr - 4 (1 (P < 0.01), 10, 100, 300 µmol/L (P < 0.001)).
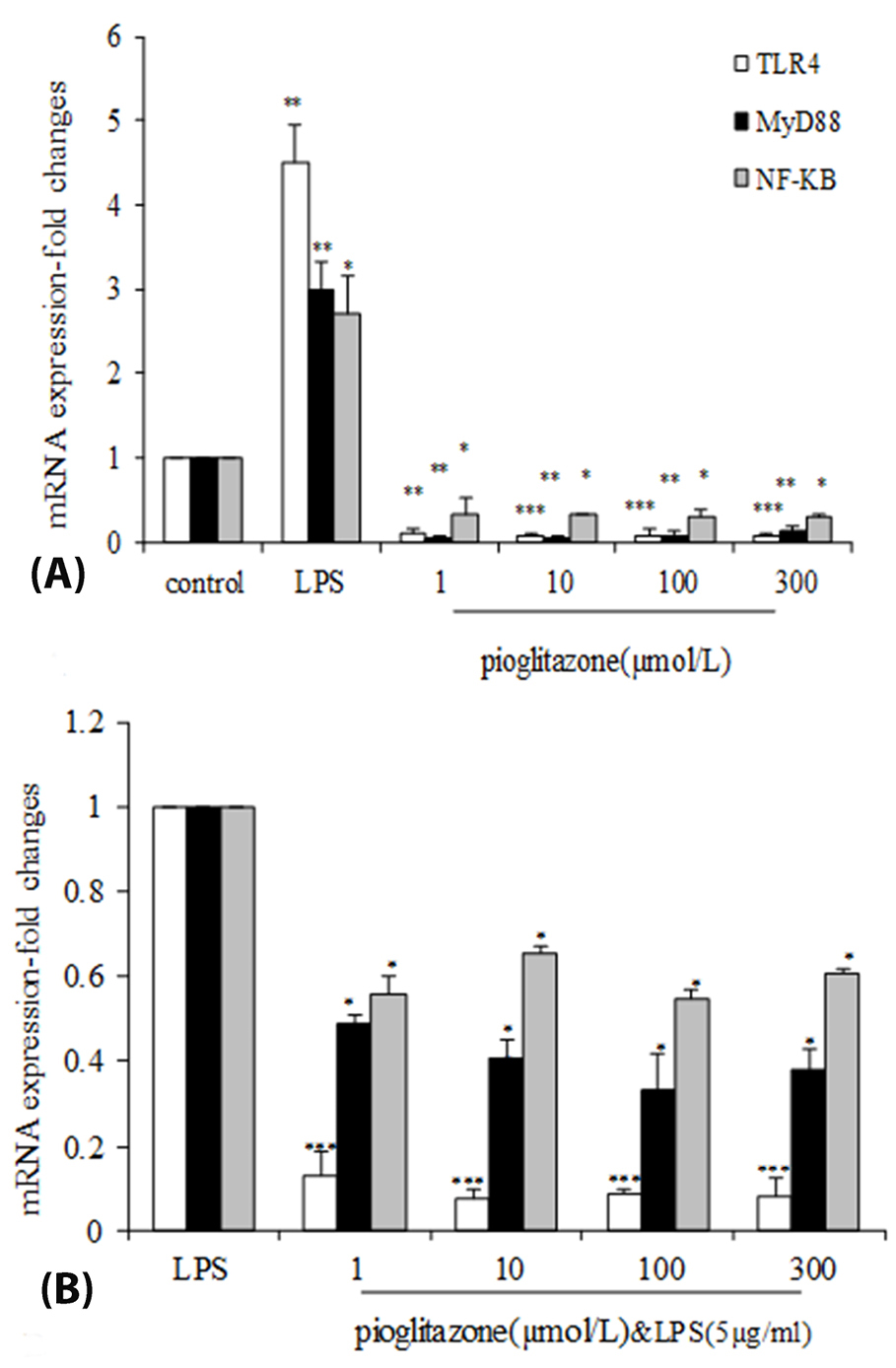
Figure 2.
Effects of LPS-EB Ultrapure and pioglitazone treatment on expression of Tlr4, Myd88 and Nf- κ b. (A) B16F10 cells were incubated with pioglitazone (1, 10, 100, 300 µM) or LPS (5 µg/mL)for 24 h. (B) B16F10 cells were pre-incubated for 1 h with our without pioglitazone (1, 10, 100, 300 µM) then continuing the cells incubation with LPS (5 µg/mL) for 24 h. mRNA expression was measured using quantitative real-time PCR. * P <0.05; **P <0.01; *** P <0.001 compared with negative control One representative experiment of three is depicted. Each graph has been represented as Mean ± SEM.
.
Effects of LPS-EB Ultrapure and pioglitazone treatment on expression of Tlr4, Myd88 and Nf- κ b. (A) B16F10 cells were incubated with pioglitazone (1, 10, 100, 300 µM) or LPS (5 µg/mL)for 24 h. (B) B16F10 cells were pre-incubated for 1 h with our without pioglitazone (1, 10, 100, 300 µM) then continuing the cells incubation with LPS (5 µg/mL) for 24 h. mRNA expression was measured using quantitative real-time PCR. * P <0.05; **P <0.01; *** P <0.001 compared with negative control One representative experiment of three is depicted. Each graph has been represented as Mean ± SEM.
Treatment with all doses of pioglitazone significantly reduced the expression of Myd-88 (P < 0.01),Nf-kb mRNA (P < 0.05) compared with the control group.
Pretreatment with pioglitazone (1, 10, 100, 300 µmol/L) before LPS(5 µg/mL) (PIO+LPS) significantly decreased the inducedTlr4 mRNA level by LPS (P < 0.001) (Figure 2B). As shown in Figure 2B, after stimulation of Myd-88 mRNA expression by LPS, pioglitazone significantly inhibited its expression (P < 0.01). We also observed decreased Nf-kb mRNA expression in pioglitazone treated cells (P < 0.05).
Pioglitazone decreases TNF-α in B16F10 cell culture supernatant
To explore whether the effect of pioglitazone on TNF-α and IL-1β protein are mediated through TLR4, we further observed the change of the effects of pioglitazone in different concentration with or without LPS pretreatment of B16F10 cells.
As shown in Figure 3A the results have shown that treatment of cells with pioglitazone alone decrease the levels of TNF-α in 100, 300 µmol/L doses compare to control group (untreated cells) (P < 0.05). Moreover, TNF-α levels after treatment with pioglitazone in the combination of LPS were significantly lower than treatment with LPS alone in these doses (P < 0.001). We observed no statistical changes in the IL-1β levels in pioglitazone or LPS groups after treatment (Figure 3B).
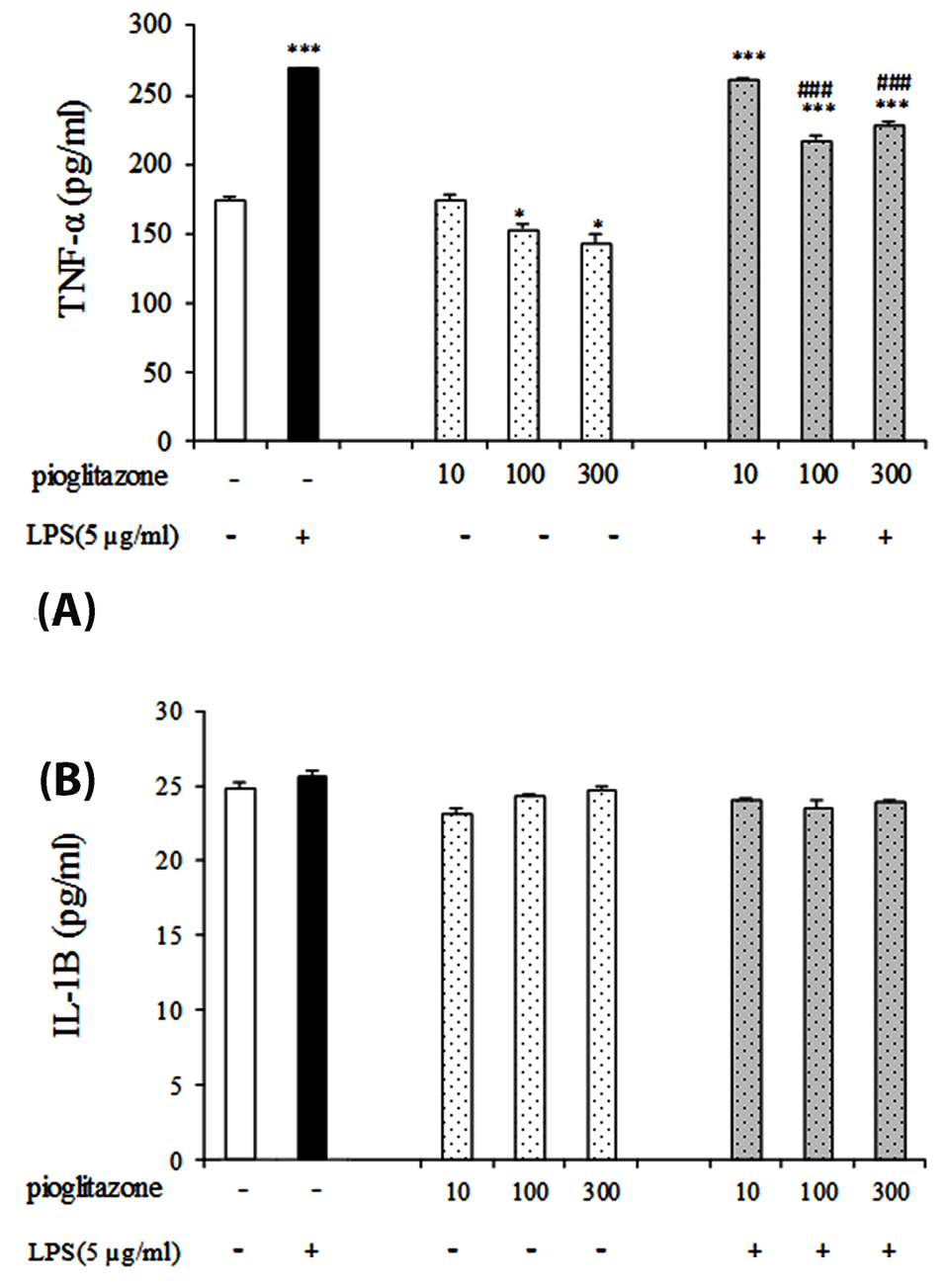
Figure 3.
Effect of pioglitazone with or without LPS on TNF-α and IL-1β production in B16F10 cell supernatant. *P < 0.05; *** P < 0.001, in compare with control group and ###P < 0.001 compare LPS group. Each graph has been represented as mean ± SEM.
.
Effect of pioglitazone with or without LPS on TNF-α and IL-1β production in B16F10 cell supernatant. *P < 0.05; *** P < 0.001, in compare with control group and ###P < 0.001 compare LPS group. Each graph has been represented as mean ± SEM.
Effect of TLR4 inhibitor on anti-inflammatory potential of pioglitazone
As mentioned above, pioglitazone can decrease TNF-α production, and downregulate Tlr4 expression. As shown in Figure 4, compared with the control, treatment of the cells with LPS led to TNF-α elevation, whereas the CLI-095 and pioglitazone each one alone inverted the LPS-induced effect on TNF-α in B16F10 cells. Moreover, treatment of the cells with both CLI-095 and pioglitazone synergistically reversed the effects induced by LPS in comparison with the treatment of the CLI-095 or pioglitazone alone. Considering that the TLR4 inhibitor antagonizes effects of LPS on TNF-α and pioglitazone also downregulates TLR4 expression in B16F10 cells, the modulatory effects of pioglitazone on TNF-α production in these cells is related to TLR4.
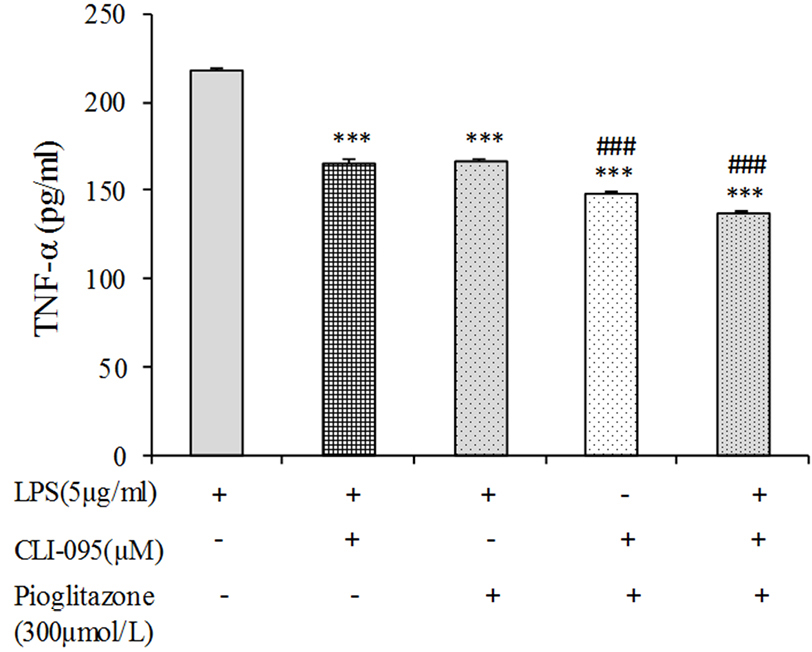
Figure 4.
Effects of the TLR4 inhibitor, CLI-095, on the production of TNF-α induced by LPS in B16F10 cells. The cells were pretreated with pioglitazone alone or in combination with CLI-095 before LPS treatment. Following 24 h treatment, the amounts of TNF-α production was measured in the supernatants. Data are means ± SEM of three independent experiments (***P < 0.001 compare LPS-treated cells; ###P < 0.001 compare LPS plus pioglitazone treated cells).
.
Effects of the TLR4 inhibitor, CLI-095, on the production of TNF-α induced by LPS in B16F10 cells. The cells were pretreated with pioglitazone alone or in combination with CLI-095 before LPS treatment. Following 24 h treatment, the amounts of TNF-α production was measured in the supernatants. Data are means ± SEM of three independent experiments (***P < 0.001 compare LPS-treated cells; ###P < 0.001 compare LPS plus pioglitazone treated cells).
Discussion
We have shown for the first time that pioglitazone reduced melanoma progression by suppressing TLR4 signaling pathway and inflammatory cytokines.
We observed that pioglitazone treatment alone has an anti-proliferative effect on B16F10 cells only in 300 μmol/L concentration. PPARs Play a critical role in melanoma cell proliferation and progression.17-20 Some studies have demonstrated the effect of PPARγ function in skin cancer and the mechanisms by which these receptors affect skin carcinogenesis, such as differentiation, proliferation, apoptosis, inflammation and angiogenesis.21
Several studies have shown the contradictory effects of PPAR agonists on the proliferation of melanoma cells and the mechanism underlying these growth inhibitory effects.Conflicting reports are based on the cell model and concentration of PPAR agonists.22 In the other cancers a PPARγ agonist was shown to inhibit hepatocellular carcinoma,23 gastric24 and prostate cancer cell growth.25 PPAR agonists exert this regulatory effect by regulation of gene expression and blocks the proto-oncogene proteins.26 Also They modulate NF-kB-dependent inflammatory response in innate immunity initiated by activation of TLRs.27
On the other hand, we observed that pioglitazone reversed the proliferative effect of LPS on B16F10 cells in all concentrations. The effect of TLR4 on cancer progression has been shown in prostate,28 breast,29 ovarian30 and lung cancer31 and it is upregulated in different cancer cells. Activation of TLR4 can inhibit melanoma cell death while TLR4 inhibition led to inhibition of their survival.32
Our data demonstrated that LPS treatment of B16F10 cells led to significantly increase the levels of Tlr4, Myd-88 and Nf-κb expression in this cell, while pioglitazone reversed this effect. So we concluded that pioglitazone suppressed B16F10 cell progression by blocking the TLR4 signaling. Cross talk between different toll like receptors and PPARs have been shown in different inflammatory diseases.14 Previous study demonstrated that pioglitazone exert its effect against AngII-induced inflammatory effect on cardiac fibroblast cells with interferring by TLR4 signaling pathway.33 The other PPARγ agonist, rosiglitazone can inhibit Tlr4 mRNA and protein expression in alveolar macrophages, which are consistent with our study.34 A recent study highlighted that PPARγ agonist, rosiglitazone, resisted the effect of LPS on Tlr4, Myd-88 expression in esophageal cancer cells.26 Also, it has been shown that PPARγ 15d-PGJ2 regulates Tlr4 mRNA and protein expression in HT-29 cells.35
Finally, we examine the effect of pioglitazone on TNF-α and IL-1β concentration by B16F10 cells. We observed that pioglitazone decrease the amount of TNF-α in both LPS treated and untreated cells. But it has not any effect on IL-1β. PPARγ agonists, glitazones, have established the ability to reduce inflammatory cytokine production such as TNF-α. Several studies show that thiazolidinedione have anti-tumor activity
For an assessment of this propose that pioglitazone may counteract LPS-stimulated inflammation via the blockade of TLR4, we assessed the effects of TLR4 inhibitor with or without pioglitazone on the concentration of TNF-α in B16F10. We observed that simultaneous treatment of B16F10 cells with pioglitazone and TLR4 inhibitor synergistically suppress LPS-induced levels of TNF-α. Our result has shown that the combined treatment decreased the amount of TNF-α more in comparison with the pioglitazone or TLR4 inhibitor alone treated cells. Inhibition of TLR4 binding with LPS by TLR4 inhibitor can indicate the antagonistic effect of pioglitazone against TLR4. Moreover, the negative interactions between TLR4 and PPAR-γ caused by LPS were retarded by the inhibition of the TLR4/MyD-88/NF-kB signaling pathway.
In conclusion, to our knowledge, this is the first study providing experimental evidence on effects of PPARγ activator, pioglitazone, to counter-regulate melanoma cancer by affecting on TLR4 signaling (TLR4/MyD-88/NF-kB) pathway. Further research in in vivo models of melanoma is needed to better understand of interaction between PPARγ and TLR4 signaling in this cancer.
Conflict of Interest
None.
Ethical Issues
Not applicable.
Acknowledgments
This article is driven from a PhD thesis promoted at Isfahan University of Medical Sciences with a grant number of No. 394617. This work was financially supported by Iran National Science Foundation (INSF) [grant No. 95844116].
References
- Leonardi GC, Falzone L, Salemi R, Zanghi A, Spandidos DA, McCubrey JA. Cutaneous melanoma: from pathogenesis to therapy (Review). Int J Oncol 2018; 52(4):1071-80. doi: 10.3892/ijo.2018.4287 [Crossref] [ Google Scholar]
- Lee KR, Lee JS, Kim YR, Song IG, Hong EK. Polysaccharide from Inonotus obliquus inhibits migration and invasion in B16-F10 cells by suppressing MMP-2 and MMP-9 via downregulation of NF-kappaB signaling pathway. Oncol Rep 2014; 31(5):2447-53. doi: 10.3892/or.2014.3103 [Crossref] [ Google Scholar]
- Guarneri C, Bevelacqua V, Polesel J, Falzone L, Cannavò PS, Spandidos DA. NFkappaB inhibition is associated with OPN/MMP9 downregulation in cutaneous melanoma. Oncol Rep 2017; 37(2):737-46. doi: 10.3892/or.2017.5362 [Crossref] [ Google Scholar]
- Youssef J, Badr M. Peroxisome proliferator-activated receptors features, functions, and future. Nucl Receptor Res 2015; 2:1-30. doi: 10.11131/2015/101188 [Crossref] [ Google Scholar]
- Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta 2007; 1771(8):926-35. doi: 10.1016/j.bbalip.2007.02.013 [Crossref] [ Google Scholar]
- Madonna G, Ullman CD, Gentilcore G, Palmieri G, Ascierto PA. NF-kappaB as potential target in the treatment of melanoma. J Transl Med 2012; 10:53. doi: 10.1186/1479-5876-10-53 [Crossref] [ Google Scholar]
- Mittal D, Saccheri F, Vénéreau E, Pusterla T, Bianchi ME, Rescigno M. TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J 2010; 29(13):2242-52. doi: 10.1038/emboj.2010.94 [Crossref] [ Google Scholar]
- Suresh R, Mosser DM. Pattern recognition receptors in innate immunity, host defense, and immunopathology. Adv Physiol Educ 2013; 37(4):284-91. doi: 10.1152/advan.00058.2013 [Crossref] [ Google Scholar]
- Liu WT, Jing YY, Yu GF, Han ZP, Yu DD, Fan QM. Toll like receptor 4 facilitates invasion and migration as a cancer stem cell marker in hepatocellular carcinoma. Cancer Lett 2015; 358(2):136-43. doi: 10.1016/j.canlet.2014.12.019 [Crossref] [ Google Scholar]
- Yang H, Wang B, Wang T, Xu L, He C, Wen H. Toll-like receptor 4 prompts human breast cancer cells invasiveness via lipopolysaccharide stimulation and is overexpressed in patients with lymph node metastasis. PLoS One 2014; 9(10):e109980. doi: 10.1371/journal.pone.0109980 [Crossref] [ Google Scholar]
- Hsu RY, Chan CH, Spicer JD, Rousseau MC, Giannias B, Rousseau S. LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer Res 2011; 71(5):1989-98. doi: 10.1158/0008-5472.can-10-2833 [Crossref] [ Google Scholar]
- Gonçalves M, Cappellari ÁR, dos Santos Junior AA, de Marchi FO, Macchi FS, Antunes KH. Effect of LPS on the viability and proliferation of human oral and esophageal cancer cell lines. Braz Arch Biol Technol 2016; 59:e16150485. doi: 10.1590/1678-4324-2016150485 [Crossref] [ Google Scholar]
- Rousseau MC, Hsu RY, Spicer JD, McDonald B, Chan CH, Perera RM. Lipopolysaccharide-induced toll-like receptor 4 signaling enhances the migratory ability of human esophageal cancer cells in a selectin-dependent manner. Surgery 2013; 154(1):69-77. doi: 10.1016/j.surg.2013.03.006 [Crossref] [ Google Scholar]
- Dana N, Vaseghi G, Haghjooy Javanmard S. Crosstalk between peroxisome proliferator-activated receptors and toll-like receptors: a systematic review. Adv Pharm Bull 2019; 9(1):12-21. doi: 10.15171/apb.2019.003 [Crossref] [ Google Scholar]
- Dana N, Javanmard Sh H, Rafiee L. Antiangiogenic and antiproliferative effects of black pomegranate peel extract on melanoma cell line. Res Pharm Sci 2015; 10(2):117-24. [ Google Scholar]
- Dana N, Haghjooy Javanmard S, Vaseghi G. The effect of fenofibrate, a PPARalpha activator on toll-like receptor-4 signal transduction in melanoma both in vitro and in vivo. Clin Transl Oncol 2020; 22(4):486-94. doi: 10.1007/s12094-019-02150-7 [Crossref] [ Google Scholar]
- Eastham LL, Mills CN, Niles RM. PPARalpha/gamma expression and activity in mouse and human melanocytes and melanoma cells. Pharm Res 2008; 25(6):1327-33. doi: 10.1007/s11095-007-9524-9 [Crossref] [ Google Scholar]
- Freudlsperger C, Moll I, Schumacher U, Thies A. Anti-proliferative effect of peroxisome proliferator-activated receptor gamma agonists on human malignant melanoma cells in vitro. Anticancer Drugs 2006; 17(3):325-32. doi: 10.1097/00001813-200603000-00011 [Crossref] [ Google Scholar]
- Placha W, Gil D, Dembińska-Kieć A, Laidler P. The effect of PPARgamma ligands on the proliferation and apoptosis of human melanoma cells. Melanoma Res 2003; 13(5):447-56. doi: 10.1097/00008390-200310000-00003 [Crossref] [ Google Scholar]
- Núñez NP, Liu H, Meadows GG. PPAR-gamma ligands and amino acid deprivation promote apoptosis of melanoma, prostate, and breast cancer cells. Cancer Lett 2006; 236(1):133-41. doi: 10.1016/j.canlet.2005.05.009 [Crossref] [ Google Scholar]
- Sertznig P, Reichrath J. Peroxisome proliferator-activated receptors (PPARs) in dermatology: challenge and promise. Dermatoendocrinol 2011; 3(3):130-5. doi: 10.4161/derm.3.3.15025 [Crossref] [ Google Scholar]
- Freudlsperger C, Schumacher U, Reinert S, Hoffmann J. The critical role of PPARgamma in human malignant melanoma. PPAR Res 2008; 2008:503797. doi: 10.1155/2008/503797 [Crossref] [ Google Scholar]
- Cao LQ, Shao ZL, Liang HH, Zhang DW, Yang XW, Jiang XF. Activation of peroxisome proliferator-activated receptor-gamma (PPARgamma) inhibits hepatoma cell growth via downregulation of SEPT2 expression. Cancer Lett 2015; 359(1):127-35. doi: 10.1016/j.canlet.2015.01.004 [Crossref] [ Google Scholar]
- Cho SJ, Kook MC, Lee JH, Shin JY, Park J, Bae YK. Peroxisome proliferator-activated receptor gamma upregulates galectin-9 and predicts prognosis in intestinal-type gastric cancer. Int J Cancer 2015; 136(4):810-20. doi: 10.1002/ijc.29056 [Crossref] [ Google Scholar]
- Bolden A, Bernard L, Jones D, Akinyeke T, Stewart LV. The PPAR gamma agonist troglitazone regulates Erk 1/2 phosphorylation via a PPARgamma-independent, MEK-dependent pathway in human prostate cancer cells. PPAR Res 2012; 2012:929052. doi: 10.1155/2012/929052 [Crossref] [ Google Scholar]
- Wu K, Yang Y, Liu D, Qi Y, Zhang C, Zhao J. Activation of PPARgamma suppresses proliferation and induces apoptosis of esophageal cancer cells by inhibiting TLR4-dependent MAPK pathway. Oncotarget 2016; 7(28):44572-82. doi: 10.18632/oncotarget.10067 [Crossref] [ Google Scholar]
- Scirpo R, Fiorotto R, Villani A, Amenduni M, Spirli C, Strazzabosco M. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-gamma limits NF-kappaB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology 2015; 62(5):1551-62. doi: 10.1002/hep.28000 [Crossref] [ Google Scholar]
- Ou T, Lilly M, Jiang W. The pathologic role of toll-like receptor 4 in prostate cancer. Front Immunol 2018; 9:1188. doi: 10.3389/fimmu.2018.01188 [Crossref] [ Google Scholar]
- Hao B, Chen Z, Bi B, Yu M, Yao S, Feng Y. Role of TLR4 as a prognostic factor for survival in various cancers: a meta-analysis. Oncotarget 2018; 9(16):13088-99. doi: 10.18632/oncotarget.24178 [Crossref] [ Google Scholar]
- Li Z, Block MS, Vierkant RA, Fogarty ZC, Winham SJ, Visscher DW. The inflammatory microenvironment in epithelial ovarian cancer: a role for TLR4 and MyD88 and related proteins. Tumour Biol 2016; 37(10):13279-86. doi: 10.1007/s13277-016-5163-2 [Crossref] [ Google Scholar]
- Wang K, Wang J, Wei F, Zhao N, Yang F, Ren X. Expression of TLR4 in non-small cell lung cancer is associated with PD-L1 and poor prognosis in patients receiving pulmonectomy. Front Immunol 2017; 8:456. doi: 10.3389/fimmu.2017.00456 [Crossref] [ Google Scholar]
- Chen X, Chang L, Qu Y, Liang J, Jin W, Xia X. Tea polyphenols inhibit the proliferation, migration, and invasion of melanoma cells through the down-regulation of TLR4. Int J Immunopathol Pharmacol 2018; 32:394632017739531. doi: 10.1177/0394632017739531 [Crossref] [ Google Scholar]
- Dengfeng G, Meng Z, Liu Z, Dong X, Dengfeng G. GW25-e5248 PPARgamma agonist pioglitazone suppresses angii-induced inflammation in cardiac fibroblast cells via TLR4-depended signaling pathway. J Am Coll Cardiol 2014; 64(16 Supplement):C13-C4. doi: 10.1016/j.jacc.2014.06.071 [Crossref] [ Google Scholar]
- Yin Y, Hou G, Li E, Wang Q, Kang J. PPARgamma agonists regulate tobacco smoke-induced toll-like receptor 4 expression in alveolar macrophages. Respir Res 2014; 15:28. doi: 10.1186/1465-9921-15-28 [Crossref] [ Google Scholar]
- Eun CS, Han DS, Lee SH, Paik CH, Chung YW, Lee J. Attenuation of colonic inflammation by PPARgamma in intestinal epithelial cells: effect on toll-like receptor pathway. Dig Dis Sci 2006; 51(4):693-7. doi: 10.1007/s10620-006-3193-0 [Crossref] [ Google Scholar]