Advanced pharmaceutical bulletin. 10(4):566-576.
doi: 10.34172/apb.2020.067
Review Article
Eco-Friendly Greener Synthesis of Nanoparticles
Brahamdutt Bhardwaj *  , Pritam Singh , Arun Kumar , Sandeep Kumar , Vikas Budhwar
, Pritam Singh , Arun Kumar , Sandeep Kumar , Vikas Budhwar 
Author information:
Department of Pharmaceutical Sciences, Maharshi Dayanand University, Rohtak-124001, India.
Abstract
The exploitation of naturally obtained resources like biopolymers, plant-based extracts, microorganisms etc., offers numerous advantages of environment-friendliness and biocompatibility for various medicinal and pharmaceutical applications, whereas hazardous chemicals are not utilized for production protocol. Plant extracts based synthetic procedures have drawn consideration over conventional methods like physical and chemical procedures to synthesize nanomaterials. Greener synthesis of nanomaterials has become an area of interest because of numerous advantages such as non-hazardous, economical, and feasible methods with variety of applications in biomedicine, nanotechnology and nano-optoelectronics, etc.
Keywords: Nanoparticles, Eco-friendly methods, Polysaccharides, Nano-biotechnology, Antimicrobial activities
Copyright and License Information
© 2020 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
In the current scenario of drug delivery, nanosystems like nanoparticles (NPs), liposomes, dendrimers, solid lipid NPs and others are being employed for a controlled, sustained and targeted delivery of active pharmaceutical entities. All of these nanomaterials have various advantages and patient-friendly because of reduction in dose frequency and much better retention time of drugs within the targeted site compared to conventional dosage forms. The primary aim of these nanosystems is to sustain the therapeutic amount of drug within the bloodstream for a longer time period. But still, there are some important factors that affect the delivery of drugs as the drug carrier, targeted site for delivery of drugs, drug administration route and the tactic considered to boost therapeutic efficiency of medication. These factors reduce the undesirable effects of the active pharmaceutical entity and improved the therapeutic performance of drugs.
1
Although UV irradiation, aerosol technologies, lithography, laser ablation, ultrasonic fields, and photochemical reduction techniques have been used successfully to produce NPs, they remain expensive and involve the use of hazardous chemicals, which leads to major attention toward the expansion of eco-friendly and sustainable greener synthesis of NPs.
2
Nano-biotechnology is a newer term formed through merging of three different fields i.e. nanotechnology, microbiology and biotechnology as microbes are being used for synthesis of nanomaterials through biotechnological methods. Bioremediation and bioleaching bio-mineralization have been performed through metal–microbe interactions, but nano-biotechnology is at its early stage period. In spite of their potent outcomes, it carries an encouraging application in drug delivery through nano-methods. This review article highlights the green synthesis of NPs from various sources such as plants, polysaccharides and microbes with their applications in different areas.
3
Why green methods for synthesis of nanoparticles?
Currently, there are numerous chemical and physical methods available in the literature for production of nanomaterials, which deliver a higher rate of production and well-controlled size and shape of nanomaterials but these approaches are discouraging due to higher loss of energy and capital, use of noxious chemicals, and production of large amount of bio-waste. These key factors influence the commercial level scale-up process of nanomaterials economically as well as environmentally. Additionally, the clinical use of nanomaterials prepared through chemical methods has been limited due to issues of biocompatibility, toxicity and stability. These components elevates requirement of eco-friendly, cheaper and biocompatible methods for production of nanomaterials. In comparison to conventional physical and chemical methods, greener route for NPs synthesis offers economical, environment-friendly and nontoxic approaches (Figure 1).
3
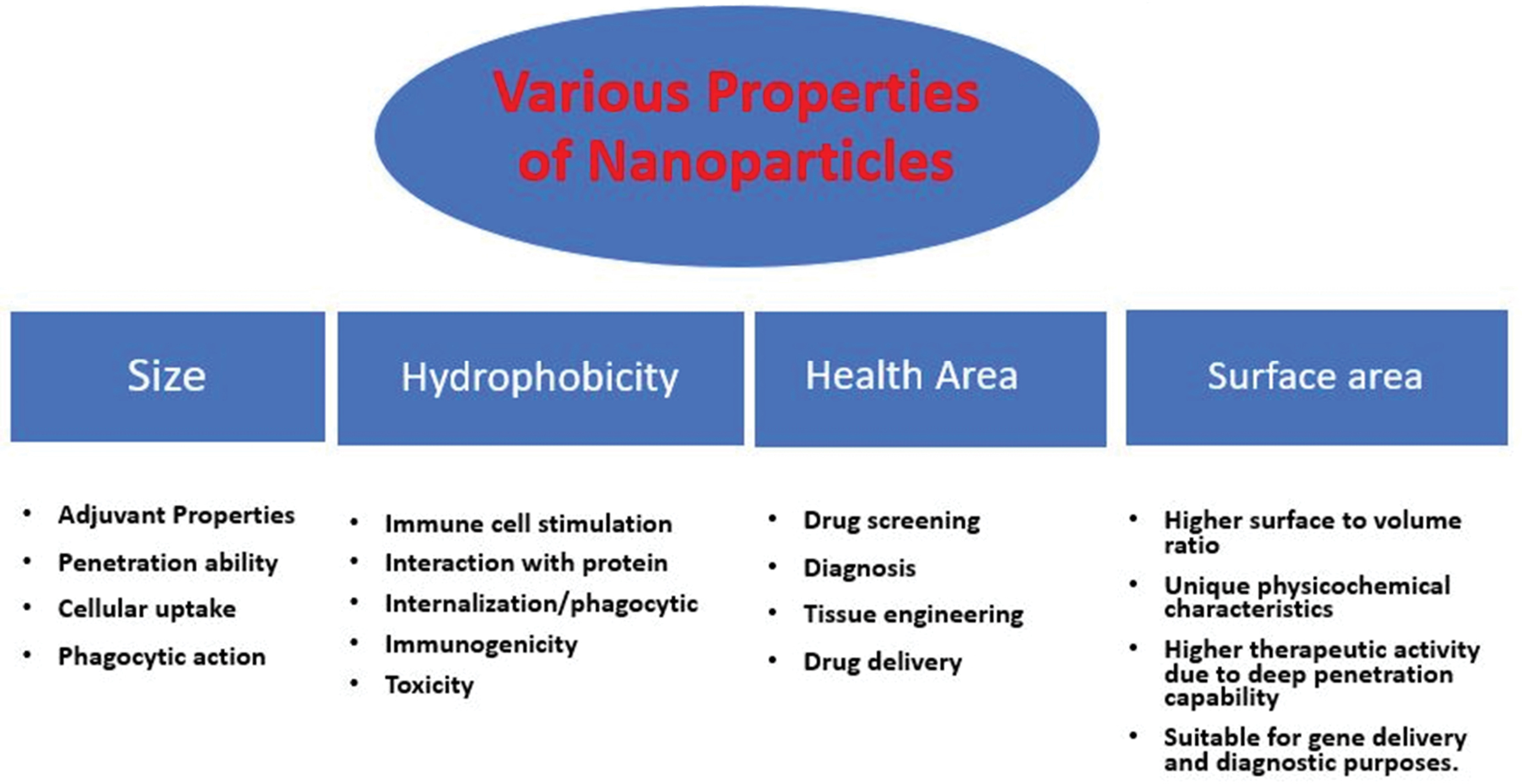
Figure 1.
Different properties of nanoparticles.
.
Different properties of nanoparticles.
Nanoparticles synthesis from plants and their extracts
Due to environment friendly behavior, lower toxicity, cheap, more biocompatibility and better size controlling aspects offered a higher prominence for the production of nanomaterials through greener ways over physical and chemical methods. The primary goal of nanotechnology is to develop a reliable and better production method, which regulates the chemical composition, morphology and better monodispersing systems in large scale production of nanomaterials. Numerous eco-friendly methods for synthesis of NPs systems from plants, bacteria, and fungi have been recommended in literature because of their economical, low toxicity profile and biocompatible in nature. Jayprakash et al, prepared silver NPs (AgNPs) with Tamarindus indica natural fruit extract through microwave-assisted greener synthesis. The plant-based extract was acted as a reducing as well as capping mediator for AgNPs synthesis. Morphological characterization of NPs was performed using different techniques such as X-ray diffraction (XRD), high-resolution scanning electron microscopy and transmission electron microscopy. The average particle size of prepared NPs was found to be 6-8 nm and XRD studies revealed the face-centered cubic silver presence. Good antibacterial action was exhibited by the prepared silver NPs through a simplistic, economical and greener method. AgNPs production methods using plant extracts are reported in literature like Mangifera indica leaf, Murraya koenigii leaf, Jatropha curcas , Mangosteen leaf, Cinnamomum zeylanicum leaf, Aloe vera , Camellia sinensis , honey and mushroom. Fruit extracts were also being utilized for NPs preparation such as lemon, pear, papaya, goose berry and tansy etc. NPs prepared through consuming fruit or plant extracts offer an advantage of non-aggregation of NPs over long term storage conditions.
4
Aloe vera plant extract was used for the synthesis of spinal shaped polycrystalline nanopowders of NixCu0.25Zn0.75–xFe2O4 (where x = 0.25, 0.35, 0.5) having an average particle size of 15-40 nm via simple solution method consuming metallic nitrates and Aloe vera plant extract mixture. Ferromagnetic activities were exhibited from obtained nanomaterials.
5
Coffee and tea extracts had been exploited for synthesis of stable NPs of noble metals (i.e. Pd and Ag) in the size range of 20-60 nm. These stated approaches might be employed for NPs production of other noble metals like Pt and Au.
6
Oxides of various metals had been utilized for nanoparticle production like titanium oxide. Nyctanthes leaf extract and titanium isopropoxide solution were used for obtaining titanium (IV) oxide nanoparticle having average size (100–150 nm).
7
Aqueous extracts of the manna of Hedysarum plant and the soap-root (Acanthophyllum bracteatum ) plant were exploited to prepare the NPs and an average diameter of the prepared NPs in solution was about 29–68 nm (Table 1).
8
Table 1.
Nanoparticles prepared from plants and their extracts
8-25
|
Source
|
Plant part used
|
Nanomaterial
|
Application
|
|
Acanthophyllum bracteatum plant
|
Aqueous extracts of the manna of Hedysarum plant and the soap-root
|
Silver Nanoparticles |
Antibacterial activity |
|
Aloe barbadensis Miller
|
Leaf extract |
Zinc oxide NPs |
Antimicrobial, and dermatologic application |
|
Plectranthus amboinicus
|
Leaf extract |
Zinc oxide NPs |
Photocatalytic activity |
|
Garcinia xanthochymus
|
|
Zinc oxide nanoparticle |
Antioxidant activity, photocatalytic activity |
| Honey |
|
Zinc oxide nanopowder |
Cytotoxicity effects |
|
Lycopersicon esculentum Mill.
|
Leaf extract |
Silver and gold NPs |
Antimicrobial activity |
|
Artocarpus heterophyllus Lam.
|
Fruit extract |
Gold NPs |
Antimicrobial activity |
|
Trianthema decandra
|
|
Silver and gold NPs |
Antimicrobial activity |
|
Helianthus annuus
|
Flower |
Gold NPs |
Antimicrobial activity |
|
Saraca indica
|
Bark extract |
Gold NPs |
Catalytic reducing agent |
|
Phyllanthus amarus Schum and Thon
|
Aqueous leaf extract |
Silver and gold NPs |
|
|
Azadirachta indica
|
Leaves |
Silver NPs |
Biolarvicidal |
|
Catharanthus roseus
|
Leaves |
Palladium NPs |
Catalytic activity in dye degradation |
| Banana |
Peel |
Cadmium sulphide |
|
| Red ginseng |
Root |
Silver NPs |
Antibacterial |
|
Cocos nucifera
|
Leaves |
Lead NPs |
Antibacterial and photo- catalytic activity |
|
Citrus medica
|
Fruits |
Copper NPs |
Antimicrobial |
|
Abutilon indicum
|
Leaves |
Silver NPs |
Antibacterial |
Nanoparticles synthesis from natural polysaccharides
In spite of plant parts extracts polysaccharides are also being employed for nanomaterials preparation as an eco-friendly approach. Sulfated polysaccharides obtained from marine red algae (Porphyra vietnamensis ) were utilized for silver NPs synthesis. The particle size of prepared nanoparticle was found to be about 13 ± 3 nm and surface plasmon resonance centred at 404 nm. The spectroscopic study revealed the connection of reduction of silver nitrate by sulfate moiety of obtained polysaccharide.
26
The Greener method for preparation of silver NPs was employed by dissolving silver (III) ion-containing rice wine and soda over-temperature raging (25-55°C) at pH 6.5 without using extra protective material. In this technique, rice wine played dual role as solvent and reducing agent while soda was utilized as base catalyst and protective agent. The obtained mixture exhibited higher stability and negligible precipitation even after long term storage for months.
27
In another study, Chen et al proposed deformable liposome of flurbiprofen coated with chitosan for ocular drug delivery to improve the transcorneal absorption and enhanced the pre-corneal drug residence time. These liposomes were formulated through the modified ethanol injection technique and then chitosan was coated over them. Gamma scintigraphy technique was employed to check the pre-corneal retention period and draining out dynamics of drug in-vivo . The deformable liposome of flurbiprofen coated with chitosan prolonged the area under the remaining activity-time up to 2.84 and 1.53-fold compare to flurbiprofen solution and deformable liposome of flurbiprofen respectively. No ocular injury or irritation was reported with use of deformable liposome of flurbiprofen coated with chitosan in-vivo .
28
Curcumin, N,O-carboxymethyl chitosan and oxidized alginate-based in situ injectable nanocomposite hydrogel formulation showed a novel dermal wound dressing application. The development of nanocomposite of curcumin involved incorporation of methoxy poly(ethylene glycol)-β-poly(-caprolactone) copolymer into N,O-carboxymethyl chitosan and oxidized alginate hydrogels system. Prepared hydrogels were injected on rat dorsal injuries to study the healing process. The study revealed the considerable improvement in epidermal re-epithelialization and deposition of collagen within the tissue of wound.
29
In 2012, Tian et al, prepared glycyrrhetinic acid and modified sulfated chitosan-based drug carrier system for anticancer activity. The prepared drug-carrying nanosystem was found to be spherical in shape and around 200 nm in size, showing a significant anticancer activity.
30
Among the various biological NPs, those produced by medicinal plants have been found to be the most pharmacologically active, possibly due to the attachment of several pharmacologically active residues (Table 2).
Table 2.
Nanoparticles prepared from natural polysaccharides
31-44
|
Material used
|
Drug
|
Nanomaterial
|
Uses
|
| Alginate |
Isoniazid and pyrazinamide |
NPs |
Anti-tubercular activity |
| Alginate–oligochitosan–Eudragit L100-55 |
Naproxen |
Microparticles |
Non-steroidal anti-inflammatory activity |
| Sodium Alginate |
Isoniazid |
Microspheres |
Anti-tubercular activity |
| Chitosan |
Zinc sulphide and mannose |
Nanoprobes |
Targeted cancer imaging |
| Galactosylated chitosan |
Doxorubicin |
Microbubbles |
Anticancer activity |
| Chitosan |
Prednisolone |
NPs |
Renal targeting drug delivery |
| Lauryl succinyl chitosan |
Human insulin |
Micro/nano-particles |
Oral peptide delivery system |
| Hyaluronic acid |
Tacrolimus |
Niosomes |
Ocular drug delivery |
| Alginate |
Cisplatin and doxorubicin |
Liposome |
Anticancer drug delivery |
| Chitosan |
Artemisinin |
Magnetic NPs |
Drug delivery in breast cancer cell |
| Chitosan |
IR820- iron oxide |
Magnetic nanosystem |
Imaging agent against melanoma |
| Gelatine |
Dextran sulphate |
NPs |
Expression of MUC5AC in ocular surface epithelial cells |
| Gum cordia |
Fluconazole |
NPs |
- |
| Cationized gelatine |
Dextran sulphate and chondroitin sulphate |
NPs
|
Ophthalmic drug delivery |
Nanoparticles synthesis from microbial origin
Plant-based extracts and microbial cultures have been used for the greener or eco-friendly synthesis of NPs all over the world. Due to quick growth rate, low-cost cultivation and capability of survival in ambient environmental conditions like temperature, pressure and pH make microbes a favorable candidate for NPs synthesis. These have inherent potential to prepare NPs of inorganic materials via reduction mechanism through intracellular and extracellular routes because of their survival capability in the metallic noxious surroundings. Metallic ions present in the environment are trapped by microbes and with the help of enzymatic activity and microbes convert these ions into their elemental forms.
3
Fungi based greener synthesis of nanomaterials is attaining much popularity worldwide.
45
In comparison to bacteria, higher yield of NPs is obtained using fungal strains, because of larger biomass. NPs with different shapes and sizes were prepared by using numerous fungal species such as Fusarium oxysporum , Verticillium luteoalbum , Trichothecium sp., Colletotrichum sp., Alternata alternate, Aspergillus oryzae , Trichoderma viride, etc.
46
Largely, the use of toxic or hazardous chemicals can be eliminated for production of biologically and pharmaceutically important materials by the use of eco-friendly greener chemicals and microorganisms. Numerous reports have been published for greener synthesis of metal oxide NPs (like manganese oxide, copper oxide, iron oxide, titania) with the use of microorganism’s cultures like Lactobacillus sp., Yeast cells, Fusarium oxysporum, Shewanella oneidensis, Saccharomyces cerevisiae and Bacillus sp. cells etc.
45
Metallic ions felt great reduction effect over them due to bacteria leads to synthesize NPs. Research studies revealed the bacterial based reduction mechanism over metallic ions leads to precipitation of metals to nanometres scale. Fungal species had different enzymes (intracellular and extracellular) which could produce a well-defined size and shaped mono-dispersed NPs.
46
In a study, Malarkodi et al, biosynthesized NPs of titanium dioxide using Planomicrobium sp. and their anti-microbial activities were estimated against K. planticola , Bacillus Subtilis and Asper niger .
47
NPs of iron were prepared using Fusarium oxysporum presenting antimicrobial activity against Escherichia coli, Staphylococcus sp. and Bacillus . The respiration mechanism of microbes depends on concentration of substrates, was restricted by these iron NPs via limiting the oxygen supply.
48
The concentration of substrates, pH and temperature of the incubated medium influenced the growth, mono-dispersion and dimensions of the formulated NPs.
49
In a similar study, Sharma et al revealed that the capping agent and incubation time period directly influenced the stability and size of formulated NPs, respectively.
50
Synergistic action of different antibiotics viz. nitrofurantoin, ciprofloxacin and carbenicillin with silver NPs prepared via eco-friendly method from R. stolonifer were exhibited against ESBL-strains of Enterobacteriaceae . Both ciprofloxacin and Carbenicillin exhibited increment of 30.53% and 33.56% respectively, while around 50% of increment was reported with nitrofurantoin.
51
In the similar fashion, combination of silver NPs prepared from Brevibacterium frigoritolerans with various antibiotics (like penicillin G, novobiocin, oleandomycin, vancomycin, rifampicin) improved the antimicrobial effect of these antibiotics especially against pathogenic strains of Bacillus cereus, Escherichia coli, Salmonella enterica, Vibrio parahaemolyticus, Candida albicans and Bacillus anthracis .
52
Breast cancer malevolence is one of the major causes of death among women. According to the reports described in literature, these microbes based metallic NPs are offering significant anticancer activity. Platinum NPs biosynthesized from Saccharomyces boulardii tested against A-431 and MCF-7 cell lines exhibiting anticancer activity.
53
Silver NPs prepared using Cryptococcus laurentii present a better anticancer effect against cancerous cell line especially breast cancer cell lines. The stimulation of apoptosis, sustainability and endocytic action of tumor cell lines were affected by greener synthesized silver NPs. The endocytic activity of tumour cell was found to be equivalent to efficiency of silver NPs.
54
Selenium is trace element with anticancer activities and Streptomyces bikiniensis was utilized to biological preparation of selenium nanorods exhibiting antitumor activity against MCF-7 and Hep-G2 cancer cells. Deployment of copper bound to chromatin trailed by pro-oxidant effect leads to decrease Hep-G2 and MCF-7 cells and this was the mechanism of action that followed by these nanorods.
55
In vitro anticancer activity against breast cancer and human liver cells viz. MCF-7 and HEPG-2, respectively, were conducted with gold NPs synthesized from Streptomyces cyaneus revealing stimulation of mitochondrial apoptosis and cytokinesis detention lead to DNA impairment.
56
Gold NPs synthesized with Candida albicans were estimated to analyze the cancer cells of liver through attachment of NPs with surface-specific antibodies of liver cancer cell. These NPs bounded antibody attached clearly with superficial antigen of affected cell and could recognizably differentiate cancer cell from normal cells.
57
The use of microbially synthesized nanomaterial in diagnostics is at its initial stages and further research in this area would provide more feasible perspective for future.
Fungal species Fusarium oxysporum released a bioactive material via silver nitrate reduction extracellularly. An admirable anti-inflammatory and antibacterial activity are unveiled by silver NPs helps in improvement of wounds healing process. The fungal culture released protein which help in stabilization of silver NPs and nitrate dependent reductase enzyme and quinine shuttle reduce the metallic ions. The antibacterial action of silver NPs prepared by the above-discussed method was evaluated on silk and cotton cloths against S. aureus .
58
Similarly, algae released their protein which not only reduce the silver ions, but also the NPs and thus stabilized the silver NPs. The protein released by Chlorella vulgaris played a double role through reduction of silver ions as well as controlling the synthesis and morphology of NPs. The -OH and -COOH groups present in tyrosine and Asp/Glu residues helped in reduction process of silver ions. The metabolites of marine algae-like Chaetoceros calcitrans, Chlorella salina, Isochrysis galbana and Tetraselmis gracilis reduced the silver ions and thereby synthesized the Silver NPs (Table 3).
59
Table 3.
Nanoparticles Prepared from Microbial Sources
60-92
|
Microbial culture used
|
Type of nanoparticles
|
Size of nanoparticles
|
Morphology
|
|
Aspergillus flavus
|
Silver |
100 nm |
Spherical |
|
Aspergillus fumigatus
|
Silver |
10-25 nm |
Spherical |
|
Brevibacterium casei
|
Silver |
10-50 nm |
Spherical |
|
Fusarium oxysporum
|
Silver |
15-50 nm |
Spherical |
|
Cladosporium cladosporioides
|
Silver |
<100 nm |
Spherical |
|
Brevibacterium casei
|
Gold |
<50 nm |
Spherical |
|
Trichoderma viride
|
Silver |
3-5 nm |
Irregular |
|
Verticillium sp.
|
Silver |
<50 nm |
Spherical |
|
Plectonema boryanum
|
Gold |
<25 nm |
Cubic |
|
Plectonema boryanum
|
Gold |
<6 µm |
Octahedral |
|
Pseudomonas aeruginosa
|
Gold |
30 nm |
Irregular |
|
Rhodococcus sp.
|
Gold |
<15 nm |
Spherical |
|
Shewanella Algae
|
Platinum |
5 nm |
Irregular |
|
Enterobacter sp.
|
Hg |
<5 nm |
Spherical |
|
Fusarium oxysporum
|
Alloy of silver and gold |
<15 nm |
Spherical |
|
Desulfovibrio desulfuricans
|
Palladium |
<50 nm |
Spherical |
|
Yarrowia lipolytica
|
Gold |
<15 nm |
Triangle |
|
V. luteoalbum
|
Gold |
<100 nm |
Irregular |
|
Ureibacillus thermosphaericus
|
Gold |
<100 nm |
Irregular |
|
Escherichia coli
|
CdTe |
2-4 nm |
Spherical |
|
Lactobacillus sp.
|
BaTiO3
|
<100 nm |
Tetragonal |
| HSMV-1 |
Fe3O4
|
<100 nm |
Bullet shaped |
|
Shewanella oneidensis
|
Fe3O4
|
<50 nm |
Rectangular |
|
Fusarium oxysporum
|
BaTiO3
|
<5 nm |
Spherical |
| Yeast |
FePO4
|
<100 nm |
Nanopowder |
|
Fusarium oxysporum
|
TiO2
|
<15 nm |
Spherical |
|
Lactobacillus sp.
|
TiO2
|
<35 nm |
Spherical |
|
Aeromonas hydrophila
|
ZnO |
55-75 nm |
Spherical |
|
Fusarium
|
ZrO2
|
3-11 nm |
Spherical |
|
Fusarium oxysporum
|
CdS |
<20 nm |
Spherical |
|
Rhodobacter sphaeroides
|
CdS |
<10 nm |
Hexagonal |
|
Rhodopseudomonas palustris
|
CdS |
<10 nm |
Cubic |
|
Rhodobacter sphaeroides
|
PbS |
<10 nm |
Spherical |
|
Desulfobacteraceae
|
ZnS |
<5 nm |
Bio-Film |
| Prokaryotes |
Fe3S4
|
<100 nm |
Irregular |
Enzyme-mediated and protein-mediated synthesis of nanoparticles
Biological systems could be used for greener synthesis of NPs in terms of their unique shapes and sizes in a controlled manner. Rangnekar et al prepared gold NPs by using pure α-amylase. In the similar fashion, EcoRI, an endonuclease having free cysteine, reduces the gold ions, while other enzymes were unable to reduce the chloroauric acid to gold NPs without free cysteine exposure.
93
In another study, Roy et al investigated the capacity of cysteine as a reducing agent in spite of the role of cysteine as a capping material on gold NPs. Various analytical techniques were utilized to investigate the linkage of cysteine with gold NPs like ultra-violet visible spectrophotometry, Fourier transform infrared spectroscopy, XRD and Raman spectroscopy.
94
Sharma et al carried out a study of gold and gold: platinum NPs synthesis by using urease enzyme as reducing agent. They investigated the role of cysteine in NPs formation. They modified the cysteine in urease by its reaction with 5,5′–dithiobis in non-denaturation conditions. Due to this modification, there was no NPs formation occurred. Patela et al prepared Glycine max’ (soybean) leaf extract mediated palladium NPs. In this study, the protein present in leaf extract acts as reducing agent for formation of palladium NPs. The possible reaction of tyrosine with palladium ions leads to the donation of electron and conversion of palladium to palladium NPs.
95
Similarly, the glucose oxidase interaction with palladium leads to the formation of palladium NPs. Selenium NPs were produced by using α-amylase from Bacillus methylotrophicus but unfortunately, no mechanism behind the study was discussed (Table 4).
96
Table 4.
Nanoparticles prepared from various natural sources having antioxidant properties
97-108
|
Species Name
|
Part of species used for extraction
|
Medium used for extraction
|
Antioxidant Properties
|
|
Cassia occidentalis
|
Seeds and Leaves |
Methanol |
Ferric reducing antioxidant activity |
|
Terminalia chebula
|
Leaves |
Ethanol |
Increased free radical scavenging potential |
|
Schotia latifolia
|
Stem bark |
Aqueous |
Free radical scavenging activities |
|
Pistacia integerrima
|
Leaf gall extracts |
Ethanol |
Higher content of total phenolics and flavonoids found in the ethanolic extract was directly associated with higher antioxidant activity |
| Poly(acrylonitrile-butadiene-styrene) |
|
Chloroform |
Scavenge free radical |
|
Xanthomonas campestris produce Xanthan polymer
|
Bacteria |
|
Antioxidant properties |
|
Acetobacter xylinum produce Cellulose polymer
|
Bacteria |
|
Reducing power |
|
Sinorhizobium meliloti produce Curdlan polymer
|
Bacteria |
|
Antioxidant properties |
|
Leuconostoc mesenteroides produce Dextran polymer
|
Bacteria |
|
Antioxidant properties |
|
Cystoseira barbata
|
Seaweed |
Aqueous |
Cystoseira barbata based alginate polymer exerted moderate antioxidant activity
|
| Bacterial nanocellulose |
Cellulose based membrane loaded with caffeic, ellagic and gallic acids |
Aqueous |
Higher antioxidant properties |
|
Ficus glomerata
|
Leaf gall extracts |
Aqueous and methanol |
Enhanced antioxidant properties of methanolic extract comparative to Aqueous extraction. |
Shortcomings in green synthesis of nanoparticles
Though microbes offer a safe, eco-friendly and economically viable approach for synthesis of NPs as compared to their chemical alternates, lack of monodispersing system, uncontrolled size, and time-consuming production process and these disadvantages have limited their use on commercial scale. Owing to nontoxicity of biosynthesized NPs, they showed propitious potential in nanomedicine yet their use in drug delivery and diagnostics is at its infancy.
3
The toxicity of natural polysaccharides could be assessed by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2Htetrazolium (MTS) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays, which are currently widely applied since they allow to assess the effect of chitosan NPs onto the cell metabolic activity.
109
De Campos et al assessed the toxicity profile for chitosan via simple colorimetric assay with tryptan blue dye. The study revealed that up to 2 mg/mL of chitosan concentration exhibited no toxicity. The higher concentration of chitosan may be hazardous for survival of cells but, some scientists claimed that acetate buffer solution (pH 6.0) might contributes to the toxicity for cell survival.
110
On the basis of MTT assays, some of the reports considered cytotoxic behavior of chitosan NPs were greater in macrophages than in fibroblasts. The higher concentration of particles caused cells death by modifying the metabolism process of cell via nanoparticle internalization, but not due to membrane degradation.
111
In the case of plants, the charge present over various phytochemicals got changed due to change in pH, which caused the changes in their capability of binding and metallic ions reduction mechanism during synthesis of NPs, affecting the production and morphological characters of NPs. The gold NPs of Avena sativa were prepared in large quantity at pH 3.0-4.0 while a bunch of NPs was observed on pH 2.0. The process of aggregation dominated the reduction mechanism of metallic ions, in case of low pH range.
112
Fungal cultures are extensively being used for the eco-friendly production of nanomaterials. Due to greater quantity of bioactive material secreted by fungi, these were much preferred for large scale production of NPs.
58
But there are some drawbacks regarding fungi-based NPs production as laborious, time-consuming and costly intensive down flowing process, so for commercial-scale production, cheaper and economical method will be needed. While in the case of bacterial based synthesis methods on large scale, the requirements of hazardous chemicals are low but process of bacterial culturing is laborious and control on the nanoparticle’s morphological parameters is less.
46
Organized and meaningful studies are required for understanding some of the mechanisms involving in various reactions to find a more well-defined outcome. There were numerous concepts regarding reduction of Ag+ to Ag0 and the bacteriostatic activity of silver NPs.
2
Finding of study
The study reports found green NPs synthesis as far as physical and chemical methods are concerned to be considered much more effective and environmentally friendly. Due to its diverse characteristics, flexibility, various benefits and applications for humans, NPs are one of the most essential and versatile materials. Green sources are a stabilizing and reducing agent for the synthesis of controlled-size and shape NPs. The application of NPs to crops in general increases agricultural growth and yield. As a constant increase in demand for food, there is a low yield for a staple crop. It is therefore important for sustainable agriculture to market metal oxide NPs. During various processes, such as bioimaging, drug delivery, biosensors and gene delivery, the biomedical applications in this field are being stepped up daily. NPs can serve as intelligent weapons against multiple drug-resistant microorganisms and can replace antibiotics in terms of their toxicity properties. This study is intended to further streamline research in this area on novel analytical and clinical associations.
Conclusion
In summary, here we have discussed various biological or eco-friendly green synthesis of nanomaterials and their biomedical applications. Though, the physical and chemical methods for production of nanomaterials are available currently biological methods are preferred because of their non-hazardous nature as compared to chemical methods. Some of the key factors (like expensive chemicals, higher energy consumption and toxicity) cause the chemically produced nanomaterials unfavorable for use. Thus, a need for biocompatible, greener and economical approaches arises for production of NPs. Plants based extracts, naturally obtained polysaccharides and microbes are the targeted materials for fulfilling the desire of suitable methods for biological production of NPs. But still some numerous concepts are required to be probed in more details like methods for large scale production with cheaper cost and controlled behavior. Detailed investigations regarding controlled morphology, biocompatibility and pharmacokinetic studies are also desirable. So, more research work should be focussed on understanding the concepts and mechanisms involved in biological and economical production of nanosystems using plant sources and microorganisms.
Ethical Issues
Not applicable.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
The authors thank the Department of Pharmaceutical Sciences, M.D. University for providing the necessary facilities.
References
- Budhwar V, Brahamdutt Brahamdutt, Choudhary M. Nanotechnology: applications in pharmaceutical drug delivery systems. J Chem Pharm Res 2016; 8(8):259-65. [ Google Scholar]
- Kharissova OV, Dias HV, Kharisov BI, Pérez BO, Pérez VM. The greener synthesis of nanoparticles. Trends Biotechnol 2013; 31(4):240-8. doi: 10.1016/j.tibtech.2013.01.003 [Crossref] [ Google Scholar]
- Fariq A, Khan T, Yasmin A. Microbial synthesis of nanoparticles and their potential applications in biomedicine. J Appl Biomed 2017; 15(4):241-8. doi: 10.1016/j.jab.2017.03.004 [Crossref] [ Google Scholar]
- Jayaprakash N, Vijaya JJ, Kaviyarasu K, Kombaiah K, Kennedy LJ, Ramalingam RJ. Green synthesis of Ag nanoparticles using Tamarind fruit extract for the antibacterial studies. J Photochem Photobiol B 2017; 169:178-85. doi: 10.1016/j.jphotobiol.2017.03.013 [Crossref] [ Google Scholar]
- Laokul P, Maensiri S. Aloe vera solution synthesis and magnetic properties of Ni-Cu-Zn ferrite nanopowders. J Optoelectron Adv M 2009; 11(6):857-62. [ Google Scholar]
- Nadagouda MN, Varma RS. Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem 2008; 10(8):859-62. doi: 10.1039/B804703K [Crossref] [ Google Scholar]
- Sundrarajan M, Gowri S. Green synthesis of titanium dioxide nanoparticles by Nyctanthes arbor-tristis leaves extract. Chalcogenide Lett 2011; 8(8):447-51. [ Google Scholar]
- Forough M, Farhadi K. Biological and green synthesis of silver nanoparticles. Turkish J Eng Environ Sci 2010; 34(4):281-7. doi: 10.3906/muh-1005-30 [Crossref] [ Google Scholar]
- Sangeetha G, Rajeshwari S, Venckatesh R. Green synthesis of zinc oxide nanoparticles by Aloe barbadensis Miller leaf extract: structure and optical properties. Mater Res Bull 2011; 46(12):2560-6. doi: 10.1016/j.materresbull.2011.07.046 [Crossref] [ Google Scholar]
- Fu L, Fu Z. Plectranthus amboinicus leaf extract–assisted biosynthesis of ZnO nanoparticles and their photocatalytic activity. Ceram Int 2015; 41(2 Pt A):2492-6. doi: 10.1016/j.ceramint.2014.10.069 [Crossref] [ Google Scholar]
- Nethravathi PC, Shruthi GS, Suresh D, Udayabhanu Udayabhanu, Nagabhushana H, Sharma SC. Garcinia xanthochymus mediated green synthesis of ZnO nanoparticles: photoluminescence, photocatalytic and antioxidant activity studies. Ceram Int 2015; 41(7):8680-7. doi: 10.1016/j.ceramint.2015.03.084 [Crossref] [ Google Scholar]
- Hoseini SJ, Darroudi M, Kazemi Oskuee R, Gholami L, Khorsand Zak A. Honey-based synthesis of ZnO nanopowders and their cytotoxicity effects. Adv Powder Technol 2015; 26(3):991-6. doi: 10.1016/j.apt.2015.04.003 [Crossref] [ Google Scholar]
- Asmathunisha N, Kathiresan K. Rapid biosynthesis of antimicrobial silver and gold nanoparticles by in vitro callus and leaf extracts from Lycopersicon esculentum Mill. Int J Pharma Bio Sci 2013; 4(1):334-44. [ Google Scholar]
- Basavegowda N, Dhanya Kumar G, Tyliszczak B, Wzorek Z, Sobczak-Kupiec A. One-step synthesis of highly-biocompatible spherical gold nanoparticles using Artocarpus heterophyllus Lam (jackfruit) fruit extract and its effect on pathogens. Ann Agric Environ Med 2015; 22(1):84-9. doi: 10.5604/12321966.1141374 [Crossref] [ Google Scholar]
- Geethalakshmi R, Sarada DV. Gold and silver nanoparticles from Trianthema decandra: synthesis, characterization, and antimicrobial properties. Int J Nanomedicine 2012; 7:5375-84. doi: 10.2147/ijn.s36516 [Crossref] [ Google Scholar]
- Liny P, Divya TK, Barasa M, Malakar B, Nagaraj B, Krishnamurthy N, Dinesh R. Preparation of gold nanoparticles from Helianthus annuus (sun flower) flowers and evaluation of their antimicrobial activities. Int J Pharma Bio Sci 2012; 3(1):P439-46. [ Google Scholar]
- Dash SS, Majumdar R, Sikder AK, Bag BG, Patra BK. Saraca indica bark extract mediated green synthesis of polyshaped gold nanoparticles and its application in catalytic reduction. Appl Nanosci 2014; 4(4):485-90. doi: 10.1007/s13204-013-0223-z [Crossref] [ Google Scholar]
- Annamalai A, Babu ST, Jose NA, Sudha D, Lyza CV. Biosynthesis and characterization of silver and gold nanoparticles using Aqueous leaf extraction of Phyllanthus amarus Schum & Thonn. World Appl Sci J 2011; 13(8):1833-40. [ Google Scholar]
- Poopathi S, De Britto LJ, Praba VL, Mani C, Praveen M. Synthesis of silver nanoparticles from Azadirachta indica--a most effective method for mosquito control. Environ Sci Pollut Res Int 2015; 22(4):2956-63. doi: 10.1007/s11356-014-3560-x [Crossref] [ Google Scholar]
- Kalaiselvi A, Roopan SM, Madhumitha G, Ramalingam C, Elango G. Synthesis and characterization of palladium nanoparticles using Catharanthus roseus leaf extract and its application in the photo-catalytic degradation. Spectrochim Acta A Mol Biomol Spectrosc 2015; 135:116-9. doi: 10.1016/j.saa.2014.07.010 [Crossref] [ Google Scholar]
- Zhou GJ, Li SH, Zhang YC, Fu YZ. Biosynthesis of CdS nanoparticles in banana peel extract. J Nanosci Nanotechnol 2014; 14(6):4437-42. doi: 10.1166/jnn.2014.8259 [Crossref] [ Google Scholar]
- Singh P, Kim YJ, Wang C, Mathiyalagan R, El-Agamy Farh M, Yang DC. Biogenic silver and gold nanoparticles synthesized using red ginseng root extract, and their applications. Artif Cells Nanomed Biotechnol 2016; 44(3):811-6. doi: 10.3109/21691401.2015.1008514 [Crossref] [ Google Scholar]
- Elango G, Roopan SM. Green synthesis, spectroscopic investigation and photocatalytic activity of lead nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 2015; 139:367-73. doi: 10.1016/j.saa.2014.12.066 [Crossref] [ Google Scholar]
- Shende S, Ingle AP, Gade A, Rai M. Green synthesis of copper nanoparticles by Citrus medica Linn (Idilimbu) juice and its antimicrobial activity. World J Microbiol Biotechnol 2015; 31(6):865-73. doi: 10.1007/s11274-015-1840-3 [Crossref] [ Google Scholar]
- Ashokkumar S, Ravi S, Kathiravan V, Velmurugan S. Synthesis of silver nanoparticles using A indicum leaf extract and their antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc 2015; 134:34-9. doi: 10.1016/j.saa.2014.05.076 [Crossref] [ Google Scholar]
- Venkatpurwar V, Pokharkar V. Green synthesis of silver nanoparticles using marine polysaccharide: study of in-vitro antibacterial activity. Mater Lett 2011; 65(6):999-1002. doi: 10.1016/j.matlet.2010.12.057 [Crossref] [ Google Scholar]
- Wu CC, Chen DH. A facile and completely green route for synthesizing gold nanoparticles by the use of drink additives. Gold Bull 2007; 40(3):206-12. doi: 10.1007/BF03215582 [Crossref] [ Google Scholar]
- Chen H, Pan H, Li P, Wang H, Wang X, Pan W. The potential use of novel chitosan-coated deformable liposomes in an ocular drug delivery system. Colloids Surf B Biointerfaces 2016; 143:455-62. doi: 10.1016/j.colsurfb.2016.03.061 [Crossref] [ Google Scholar]
- Li X, Chen S, Zhang B, Li M, Diao K, Zhang Z. In situ injectable nano-composite hydrogel composed of curcumin, N, O-carboxymethyl chitosan and oxidized alginate for wound healing application. Int J Pharm 2012; 437(1-2):110-9. doi: 10.1016/j.ijpharm.2012.08.001 [Crossref] [ Google Scholar]
- Tian Q, Wang XH, Wang W, Zhang CN, Wang P, Yuan Z. Self-assembly and liver targeting of sulfated chitosan nanoparticles functionalized with glycyrrhetinic acid. Nanomedicine 2012; 8(6):870-9. doi: 10.1016/j.nano.2011.11.002 [Crossref] [ Google Scholar]
- Ahmad Z, Sharma S, Khuller GK. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int J Antimicrob Agents 2005; 26(4):298-303. doi: 10.1016/j.ijantimicag.2005.07.012 [Crossref] [ Google Scholar]
- Calija B, Cekić N, Savić S, Daniels R, Marković B, Milić J. pH-sensitive microparticles for oral drug delivery based on alginate/oligochitosan/Eudragit(®) L100-55 “sandwich” polyelectrolyte complex. Colloids Surf B Biointerfaces 2013; 110:395-402. doi: 10.1016/j.colsurfb.2013.05.016 [Crossref] [ Google Scholar]
- Rastogi R, Sultana Y, Aqil M, Ali A, Kumar S, Chuttani K. Alginate microspheres of isoniazid for oral sustained drug delivery. Int J Pharm 2007; 334(1-2):71-7. doi: 10.1016/j.ijpharm.2006.10.024 [Crossref] [ Google Scholar]
- Jayasree A, Sasidharan S, Koyakutty M, Nair S, Menon D. Mannosylated chitosan-zinc sulphide nanocrystals as fluorescent bioprobes for targeted cancer imaging. Carbohydr Polym 2011; 85(1):37-43. doi: 10.1016/j.carbpol.2011.01.034 [Crossref] [ Google Scholar]
- Villa R, Cerroni B, Viganò L, Margheritelli S, Abolafio G, Oddo L. Targeted doxorubicin delivery by chitosan-galactosylated modified polymer microbubbles to hepatocarcinoma cells. Colloids Surf B Biointerfaces 2013; 110:434-42. doi: 10.1016/j.colsurfb.2013.04.022 [Crossref] [ Google Scholar]
- Yuan ZX, Sun X, Gong T, Ding H, Fu Y, Zhang ZR. Randomly 50% N-acetylated low molecular weight chitosan as a novel renal targeting carrier. J Drug Target 2007; 15(4):269-78. doi: 10.1080/10611860701289875 [Crossref] [ Google Scholar]
- Rekha MR, Sharma CP. Synthesis and evaluation of lauryl succinyl chitosan particles towards oral insulin delivery and absorption. J Control Release 2009; 135(2):144-51. doi: 10.1016/j.jconrel.2009.01.011 [Crossref] [ Google Scholar]
- Zeng W, Li Q, Wan T, Liu C, Pan W, Wu Z. Hyaluronic acid-coated niosomes facilitate tacrolimus ocular delivery: Mucoadhesion, precorneal retention, aqueous humor pharmacokinetics, and transcorneal permeability. Colloids Surf B Biointerfaces 2016; 141:28-35. doi: 10.1016/j.colsurfb.2016.01.014 [Crossref] [ Google Scholar]
- Ruttala HB, Ramasamy T, Gupta B, Choi HG, Yong CS, Kim JO. Multiple polysaccharide-drug complex-loaded liposomes: a unique strategy in drug loading and cancer targeting. Carbohydr Polym 2017; 173:57-66. doi: 10.1016/j.carbpol.2017.05.062 [Crossref] [ Google Scholar]
- Natesan S, Ponnusamy C, Sugumaran A, Chelladurai S, Shanmugam Palaniappan S, Palanichamy R. Artemisinin loaded chitosan magnetic nanoparticles for the efficient targeting to the breast cancer. Int J Biol Macromol 2017; 104(Pt B):1853-9. doi: 10.1016/j.ijbiomac.2017.03.137 [Crossref] [ Google Scholar]
- Hou X, Zhou H, Wang L, Tang J, Chen C, Jiang G. Multifunctional near-infrared dye-magnetic nanoparticles for bioimaging and cancer therapy. Cancer Lett 2017; 390:168-75. doi: 10.1016/j.canlet.2016.12.026 [Crossref] [ Google Scholar]
- Zorzi GK, Contreras-Ruiz L, Párraga JE, López-García A, Romero Bello R, Diebold Y. Expression of MUC5AC in ocular surface epithelial cells using cationized gelatin nanoparticles. Mol Pharm 2011; 8(5):1783-8. doi: 10.1021/mp200155t [Crossref] [ Google Scholar]
- Yadav M, Ahuja M. Preparation and evaluation of nanoparticles of gum cordia, an anionic polysaccharide for ophthalmic delivery. Carbohydr Polym 2010; 81(4):871-7. doi: 10.1016/j.carbpol.2010.03.065 [Crossref] [ Google Scholar]
- Zorzi GK, Párraga JE, Seijo B, Sánchez A. Hybrid nanoparticle design based on cationized gelatin and the polyanions dextran sulfate and chondroitin sulfate for ocular gene therapy. Macromol Biosci 2011; 11(7):905-13. doi: 10.1002/mabi.201100005 [Crossref] [ Google Scholar]
- Shamaila S, Sajjad AKL, Ryma N, Farooqi SA, Jabeen N, Majeed S. Advancements in nanoparticle fabrication by hazard free eco-friendly green routes. Appl Mater Today 2016; 5:150-99. doi: 10.1016/j.apmt.2016.09.009 [Crossref] [ Google Scholar]
- Jeevanandam J, Chan YS, Danquah MK. Biosynthesis of metal and metal oxide nanoparticles. ChemBioEng Rev 2016; 3(2):55-67. doi: 10.1002/cben.201500018 [Crossref] [ Google Scholar]
- Malarkodi C, Chitra K, Rajeshkumar S, Gnanajobitha G, Paulkumar K, Vanaja M, Annadurai G. Novel eco-friendly synthesis of titanium oxide nanoparticles by using Planomicrobium sp and its antimicrobial evaluation. Der Pharmacia Sinica 2013; 4(3):59-66. [ Google Scholar]
- Abdeen S, Praseetha PK. Diagnostics and treatment of metastatic cancers with magnetic nanoparticles. J Nanomedine Biotherapeutic Discov 2013; 3(2):115. doi: 10.4172/2155-983x.1000115 [Crossref] [ Google Scholar]
- Barabadi H, Honary S, Ebrahimi P, Mohammadi MA, Alizadeh A, Naghibi F. Microbial mediated preparation, characterization and optimization of gold nanoparticles. Braz J Microbiol 2014; 45(4):1493-501. doi: 10.1590/s1517-83822014000400046 [Crossref] [ Google Scholar]
- Sharma N, Pinnaka AK, Raje M, Fnu A, Bhattacharyya MS, Choudhury AR. Exploitation of marine bacteria for production of gold nanoparticles. Microb Cell Fact 2012; 11:86. doi: 10.1186/1475-2859-11-86 [Crossref] [ Google Scholar]
- Banu A, Rathod V, Ranganath E. Silver nanoparticle production by Rhizopus stolonifer and its antibacterial activity against extended spectrum β-lactamase producing (ESBL) strains of Enterobacteriaceae. Mater Res Bull 2011; 46(9):1417-23. doi: 10.1016/j.materresbull.2011.05.008 [Crossref] [ Google Scholar]
- Singh P, Kim YJ, Singh H, Wang C, Hwang KH, Farh Mel A. Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles. Int J Nanomedicine 2015; 10:2567-77. doi: 10.2147/ijn.s72313 [Crossref] [ Google Scholar]
- Borse V, Kaler A, Banerjee UC. Microbial synthesis of platinum nanoparticles and evaluation of their anticancer activity. Int J Emerg Trends Elect Electron 2015; 11(2):26-31. doi: 10.13140/RG.2.1.3132.5283 [Crossref] [ Google Scholar]
- Ortega FG, Fernández-Baldo MA, Fernández JG, Serrano MJ, Sanz MI, Diaz-Mochón JJ. Study of antitumor activity in breast cell lines using silver nanoparticles produced by yeast. Int J Nanomedicine 2015; 10:2021-31. doi: 10.2147/ijn.s75835 [Crossref] [ Google Scholar]
- Ahmad MS, Yasser MM, Sholkamy EN, Ali AM, Mehanni MM. Anticancer activity of biostabilized selenium nanorods synthesized by Streptomyces bikiniensis strain Ess_amA-1. Int J Nanomedicine 2015; 10:3389-401. doi: 10.2147/ijn.s82707 [Crossref] [ Google Scholar]
- El-Batal AI, Al Tamie MS. Biosynthesis of gold nanoparticles using marine Streptomyces cyaneus and their antimicrobial, antioxidant and antitumor (in vitro) activities. J Chem Pharm Res 2015; 7(7):1020-36. [ Google Scholar]
- Chauhan A, Zubair S, Tufail S, Sherwani A, Sajid M, Raman SC. Fungus-mediated biological synthesis of gold nanoparticles: potential in detection of liver cancer. Int J Nanomedicine 2011; 6:2305-19. doi: 10.2147/ijn.s23195 [Crossref] [ Google Scholar]
- Mouxing FU, Qingbiao LI, Daohua SU, Yinghua LU, Ning HE, Xu DE. Rapid preparation process of silver nanoparticles by bioreduction and their characterizations. Chin J Chem Eng 2006; 14(1):114-7. doi: 10.1016/s1004-9541(06)60046-3 [Crossref] [ Google Scholar]
- Govindaraju K, Basha SK, Kumar VG, Singaravelu G. Silver, gold and bimetallic nanoparticles production using single-cell protein (Spirulina platensis) Geitler. J Mater Sci 2008; 43(15):5115-22. doi: 10.1007/s10853-008-2745-4 [Crossref] [ Google Scholar]
- Vigneshwaran N, Ashtaputre NM, Varadarajan PV, Nachane RP, Paralikar KM, Balasubramanya RH. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater Lett 2007; 61(6):1413-8. doi: 10.1016/j.matlet.2006.07.042 [Crossref] [ Google Scholar]
- Bhainsa KC, D’Souza SF. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf B Biointerfaces 2006; 47(2):160-4. doi: 10.1016/j.colsurfb.2005.11.026 [Crossref] [ Google Scholar]
- Kalishwaralal K, Deepak V, Ram Kumar Pandian S, Kottaisamy M, BarathmaniKanth S, Kartikeyan B. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf B Biointerfaces 2010; 77(2):257-62. doi: 10.1016/j.colsurfb.2010.02.007 [Crossref] [ Google Scholar]
- Senapati S, Mandal D, Ahmad A, Khan MI, Sastry M, Kumar R. Fungus mediated synthesis of silver nanoparticles: a novel biological approach. Indian J Phys 2004; 78:101-5. [ Google Scholar]
- Balaji DS, Basavaraja S, Deshpande R, Mahesh DB, Prabhakar BK, Venkataraman A. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf B Biointerfaces 2009; 68(1):88-92. doi: 10.1016/j.colsurfb.2008.09.022 [Crossref] [ Google Scholar]
- Mohammed Fayaz A, Balaji K, Kalaichelvan PT, Venkatesan R. Fungal based synthesis of silver nanoparticles--an effect of temperature on the size of particles. Colloids Surf B Biointerfaces 2009; 74(1):123-6. doi: 10.1016/j.colsurfb.2009.07.002 [Crossref] [ Google Scholar]
- Lengke MF, Fleet ME, Southam G. Morphology of gold nanoparticles synthesized by filamentous cyanobacteria from gold(I)-thiosulfate and gold(III)--chloride complexes. Langmuir 2006; 22(6):2780-7. doi: 10.1021/la052652c [Crossref] [ Google Scholar]
- Lengke MF, Ravel B, Fleet ME, Wanger G, Gordon RA, Southam G. Mechanisms of gold bioaccumulation by filamentous cyanobacteria from gold(III)-chloride complex. Environ Sci Technol 2006; 40(20):6304-9. doi: 10.1021/es061040r [Crossref] [ Google Scholar]
- Husseiny MI, El-Aziz MA, Badr Y, Mahmoud MA. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim Acta A Mol Biomol Spectrosc 2007; 67(3-4):1003-6. doi: 10.1016/j.saa.2006.09.028 [Crossref] [ Google Scholar]
- Ahmad A, Senapati S, Khan MI, Kumar R, Ramani R, Srinivas V. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 2003; 14(7):824-8. doi: 10.1088/0957-4484/14/7/323 [Crossref] [ Google Scholar]
- Konishi Y, Ohno K, Saitoh N, Nomura T, Nagamine S, Hishida H. Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae. J Biotechnol 2007; 128(3):648-53. doi: 10.1016/j.jbiotec.2006.11.014 [Crossref] [ Google Scholar]
- Sinha A, Khare SK. Mercury bioaccumulation and simultaneous nanoparticle synthesis by Enterobacter sp cells. Bioresour Technol 2011; 102(5):4281-4. doi: 10.1016/j.biortech.2010.12.040 [Crossref] [ Google Scholar]
- Senapati S, Ahmad A, Khan MI, Sastry M, Kumar R. Extracellular biosynthesis of bimetallic Au-Ag alloy nanoparticles. Small 2005; 1(5):517-20. doi: 10.1002/smll.200400053 [Crossref] [ Google Scholar]
- Lloyd JR, Yong P, Macaskie LE. Enzymatic recovery of elemental palladium by using sulfate-reducing bacteria. Appl Environ Microbiol 1998; 64(11):4607-9. [ Google Scholar]
- Agnihotri M, Joshi S, Kumar AR, Zinjarde S, Kulkarni S. Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Mater Lett 2009; 63(15):1231-4. doi: 10.1016/j.matlet.2009.02.042 [Crossref] [ Google Scholar]
- Gericke M, Pinches A. Biological synthesis of metal nanoparticles. Hydrometallurgy 2006; 83(1-4):132-40. doi: 10.1016/j.hydromet.2006.03.019 [Crossref] [ Google Scholar]
- Juibari MM, Abbasalizadeh S, Jouzani GS, Noruzi M. Intensified biosynthesis of silver nanoparticles using a native extremophilic Ureibacillus thermosphaericus strain. Mater Lett 2011; 65(6):1014-7. doi: 10.1016/j.matlet.2010.12.056 [Crossref] [ Google Scholar]
- Bao H, Lu Z, Cui X, Qiao Y, Guo J, Anderson JM. Extracellular microbial synthesis of biocompatible CdTe quantum dots. Acta Biomater 2010; 6(9):3534-41. doi: 10.1016/j.actbio.2010.03.030 [Crossref] [ Google Scholar]
- Jha AK, Prasad K. Ferroelectric BaTiO3 nanoparticles: biosynthesis and characterization. Colloids Surf B Biointerfaces 2010; 75(1):330-4. doi: 10.1016/j.colsurfb.2009.09.005 [Crossref] [ Google Scholar]
- Lefèvre CT, Abreu F, Schmidt ML, Lins U, Frankel RB, Hedlund BP. Moderately thermophilic magnetotactic bacteria from hot springs in Nevada. Appl Environ Microbiol 2010; 76(11):3740-3. doi: 10.1128/aem.03018-09 [Crossref] [ Google Scholar]
- Perez-Gonzalez T, Jimenez-Lopez C, Neal AL, Rull-Perez F, Rodriguez-Navarro A, Fernandez-Vivas A. Magnetite biomineralization induced by Shewanella oneidensis. Geochim Cosmochim Acta 2010; 74(3):967-79. doi: 10.1016/j.gca.2009.10.035 [Crossref] [ Google Scholar]
- Bansal V, Poddar P, Ahmad A, Sastry M. Room-temperature biosynthesis of ferroelectric barium titanate nanoparticles. J Am Chem Soc 2006; 128(36):11958-63. doi: 10.1021/ja063011m [Crossref] [ Google Scholar]
- Zhou W, He W, Zhang X, Yan S, Sun X, Tian X. Biosynthesis of iron phosphate nanopowders. Powder Technol 2009; 194(1-2):106-8. doi: 10.1016/j.powtec.2009.03.034 [Crossref] [ Google Scholar]
- Bansal V, Rautaray D, Bharde A, Ahire K, Sanyal A, Ahmad A. Fungus-mediated biosynthesis of silica and titania particles. J Mater Chem 2005; 15(26):2583-9. doi: 10.1039/b503008k [Crossref] [ Google Scholar]
- Jha AK, Prasad K, Kulkarni AR. Synthesis of TiO2 nanoparticles using microorganisms. Colloids Surf B Biointerfaces 2009; 71(2):226-9. doi: 10.1016/j.colsurfb.2009.02.007 [Crossref] [ Google Scholar]
- Jayaseelan C, Rahuman AA, Kirthi AV, Marimuthu S, Santhoshkumar T, Bagavan A. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim Acta A Mol Biomol Spectrosc 2012; 90:78-84. doi: 10.1016/j.saa.2012.01.006 [Crossref] [ Google Scholar]
- Bansal V, Rautaray D, Ahmad A, Sastry M. Biosynthesis of zirconia nanoparticles using the fungus Fusarium oxysporum. J Mater Chem 2004; 14(22):3303-5. doi: 10.1039/b407904c [Crossref] [ Google Scholar]
- Ahmad A, Mukherjee P, Mandal D, Senapati S, Khan MI, Kumar R. Enzyme mediated extracellular synthesis of CdS nanoparticles by the fungus, Fusarium oxysporum. J Am Chem Soc 2002; 124(41):12108-9. doi: 10.1021/ja027296o [Crossref] [ Google Scholar]
- Bai HJ, Zhang ZM, Gong J. Biological synthesis of semiconductor zinc sulfide nanoparticles by immobilized Rhodobacter sphaeroides. Biotechnol Lett 2006; 28(14):1135-9. doi: 10.1007/s10529-006-9063-1 [Crossref] [ Google Scholar]
- Bai HJ, Zhang ZM, Guo Y, Yang GE. Biosynthesis of cadmium sulfide nanoparticles by photosynthetic bacteria Rhodopseudomonas palustris. Colloids Surf B Biointerfaces 2009; 70(1):142-6. doi: 10.1016/j.colsurfb.2008.12.025 [Crossref] [ Google Scholar]
- Bai HJ, Zhang ZM. Microbial synthesis of semiconductor lead sulfide nanoparticles using immobilized Rhodobacter sphaeroides. Mater Lett 2009; 63(9-10):764-6. doi: 10.1016/j.matlet.2008.12.050 [Crossref] [ Google Scholar]
- Labrenz M, Druschel GK, Thomsen-Ebert T, Gilbert B, Welch SA, Kemner KM. Formation of sphalerite (ZnS) deposits in natural biofilms of sulfate-reducing bacteria. Science 2000; 290(5497):1744-7. doi: 10.1126/science.290.5497.1744 [Crossref] [ Google Scholar]
- Lefèvre CT, Abreu F, Lins U, Bazylinski DA. Nonmagnetotactic multicellular prokaryotes from low-saline, nonmarine aquatic environments and their unusual negative phototactic behavior. Appl Environ Microbiol 2010; 76(10):3220-7. doi: 10.1128/aem.00408-10 [Crossref] [ Google Scholar]
- Rangnekar A, Sarma TK, Singh AK, Deka J, Ramesh A, Chattopadhyay A. Retention of enzymatic activity of α-amylase in the reductive synthesis of gold nanoparticles. Langmuir 2007; 23(10):5700-6. doi: 10.1021/la062749e [Crossref] [ Google Scholar]
- Roy M, Mukherjee P, Mandal BP, Sharma RK, Tyagi AK, Kale SP. Biomimetic synthesis of nanocrystalline silver sol using cysteine: stability aspects and antibacterial activities. RSC Adv 2012; 2(16):6496-503. doi: 10.1039/c2ra00785a [Crossref] [ Google Scholar]
- Sharma B, Mandani S, Sarma TK. Biogenic growth of alloys and core-shell nanostructures using urease as a nanoreactor at ambient conditions. Sci Rep 2013; 3:2601. doi: 10.1038/srep02601 [Crossref] [ Google Scholar]
- Petla RK, Vivekanandhan S, Misra M, Mohanty AK, Satyanarayana N. Soybean (Glycine max) leaf extract based green synthesis of palladium nanoparticles. J Biomater Nanobiotechnol 2012; 3(1):16695. doi: 10.4236/jbnb.2012.31003 [Crossref] [ Google Scholar]
-
Rostami R, Gharib-Bibalan F, Mollania N. Biological method for selenium nanoparticles synthesis assisted by α-amylase enzyme from Bacillus methylotrophicus. Tabriz, Iran: 1st Tabriz International Life Science Conference & 12th Iran Biophysical Chemistry Conference; 2013.
- Kolar FR, Gogi CL, Khudavand MM, Choudhari MS, Patil SB. Phytochemical and antioxidant properties of some Cassia species. Nat Prod Res 2018; 32(11):1324-8. doi: 10.1080/14786419.2017.1342085 [Crossref] [ Google Scholar]
- Eshwarappa RS, Ramachandra YL, Subaramaihha SR, Subbaiah SG, Austin RS, Dhananjaya BL. Antioxidant activities of leaf galls extracts of Terminalia chebula (Gaertn) Retz (Combretaceae). Acta Sci Pol Technol Aliment 2015; 14(2). doi: 10.17306/j.afs.2015.2.11 [Crossref]
- Mbaebie BO, Edeoga HO, Afolayan AJ. Phytochemical analysis and antioxidants activities of aqueous stem bark extract of Schotia latifolia Jacq. Asian Pac J Trop Biomed 2012; 2(2):118-24. doi: 10.1016/s2221-1691(11)60204-9 [Crossref] [ Google Scholar]
- Rigoussen A, Verge P, Raquez JM, Dubois P. Natural Phenolic Antioxidants As a Source of Biocompatibilizers for Immiscible Polymer Blends. ACS Sustain Chem Eng 2018; 6(10):13349-57. doi: 10.1021/acssuschemeng.8b02999 [Crossref] [ Google Scholar]
- Sellimi S, Younes I, Ayed HB, Maalej H, Montero V, Rinaudo M. Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed. Int J Biol Macromol 2015; 72:1358-67. doi: 10.1016/j.ijbiomac.2014.10.016 [Crossref] [ Google Scholar]
- Eshwarappa RS, Iyer S, Subaramaihha SR, Richard SA, Dhananjaya BL. Antioxidant activities of Ficus glomerata (moraceae) leaf gall extracts. Pharmacognosy Res 2015; 7(1):114-20. doi: 10.4103/0974-8490.147225 [Crossref] [ Google Scholar]
- Shenton W, Douglas T, Young M, Stubbs G, Mann S. Inorganic–organic nanotube composites from template mineralization of tobacco mosaic virus. Adv Mater 1999; 11(3):253-6. doi: 10.1002/(sici)1521-4095(199903)11:3<253::aid-adma253>3.0.co;2-7 [Crossref] [ Google Scholar]
- Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine 2007; 3(2):168-71. doi: 10.1016/j.nano.2007.02.001 [Crossref] [ Google Scholar]
- Marshall MJ, Beliaev AS, Dohnalkova AC, Kennedy DW, Shi L, Wang Z. c-Type cytochrome-dependent formation of U(IV) nanoparticles by Shewanella oneidensis. PLoS Biol 2006; 4(9):e268. doi: 10.1371/journal.pbio.0040268 [Crossref] [ Google Scholar]
- Dameron CT, Reese RN, Mehra RK, Kortan AR, Carroll PJ, Steigerwald ML. Biosynthesis of cadmium sulphide quantum semiconductor crystallites. Nature 1989; 338(6216):596-7. doi: 10.1038/338596a0 [Crossref] [ Google Scholar]
- Vigneshwaran N, Kathe AA, Varadarajan PV, Nachane RP, Balasubramanya RH. Biomimetics of silver nanoparticles by white rot fungus, Phaenerochaete chrysosporium. Colloids Surf B Biointerfaces 2006; 53(1):55-9. doi: 10.1016/j.colsurfb.2006.07.014 [Crossref] [ Google Scholar]
- Bugnicourt L, Ladavière C. Interests of chitosan nanoparticles ionically cross-linked with tripolyphosphate for biomedical applications. Prog Polym Sci 2016; 60:1-17. doi: 10.1016/j.progpolymsci.2016.06.002 [Crossref] [ Google Scholar]
- de Campos AM, Diebold Y, Carvalho ELS, Sánchez A, Alonso MJ. Chitosan nanoparticles as new ocular drug delivery systems: in vitro stability, in vivo fate, and cellular toxicity. Pharm Res 2004; 21(5):803-10. doi: 10.1023/b:pham.0000026432.75781.cb [Crossref] [ Google Scholar]
- Nasti A, Zaki NM, de Leonardis P, Ungphaiboon S, Sansongsak P, Rimoli MG. Chitosan/TPP and chitosan/TPP-hyaluronic acid nanoparticles: systematic optimisation of the preparative process and preliminary biological evaluation. Pharm Res 2009; 26(8):1918-30. doi: 10.1007/s11095-009-9908-0 [Crossref] [ Google Scholar]
- Singh P, Kim YJ, Zhang D, Yang DC. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol 2016; 34(7):588-99. doi: 10.1016/j.tibtech.2016.02.006 [Crossref] [ Google Scholar]