Advanced pharmaceutical bulletin. 10(2):307-314.
doi: 10.34172/apb.2020.037
Research Article
Interleukin-6, -8, and TGF-β Secreted from Mesenchymal Stem Cells Show Functional Role in Reduction of Telomerase Activity of Leukemia Cell Via Wnt5a/β-Catenin and P53 Pathways
Ezzatollah Fathi 1  , Behnaz Valipour 2
, Behnaz Valipour 2  , Zohreh Sanaat 3
, Zohreh Sanaat 3  , Hojjatollah Nozad Charoudeh 4
, Hojjatollah Nozad Charoudeh 4  , Raheleh Farahzadi 3, *
, Raheleh Farahzadi 3, * 
Author information:
1Department of Clinical Sciences, Faculty of Veterinary Medicine, University of Tabriz, Tabriz, Iran.
2Stem Cell Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
3Hematology and Oncology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
4Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
*
Corresponding Author: Raheleh Farahzadi, Tel: +98-41-33343626, Fax: +98-41-33343844, E-mail:
farahzadir@tbzmed.ac.ir
Abstract
Purpose: The effect of mesenchymal stem cells (MSCs) on the immortality features of malignant cells, such as hematologic cancerous cells, are controversial, and the associated mechanisms are yet to be well understood. The aim of the present study was to investigate the in vitro effect of bone marrow-derived MSCs (BMSCs) on the chronic myeloid leukemia cell line K562 through telomere length measurements, telomerase activity assessments, and hTERT gene expression. The possible signaling pathways involved in this process, including Wnt-5a/β-catenin and P53, were also evaluated.
Methods: Two cell populations (BMSCs and K562 cell line) were co-cultured on transwell plates for 7 days. Next, K562 cells were collected and subjected to quantitative real-time PCR, PCR-ELISA TRAP assay, and the ELISA sandwich technique for telomere length, hTERT gene expression, telomerase activity assay, and cytokine measurement, respectively. Also, the involvement of the mentioned signaling pathways in this process was reported by real-time PCR and Western blotting through gene and protein expression, respectively.
Results: The results showed that BMSCs caused significant decreases in telomere length, telomerase activity, and the mRNA level of hTERT as a regulator of telomerase activity. The significant presence of interleukin (IL)-6, IL-8, and transforming growth factor beta (TGF-β) was obvious in the co-cultured media. Also, BMSCs significantly decreased and increased the gene and protein expression of β-catenin and P53, respectively.
Conclusion: It was concluded that the mentioned effects of IL-6, IL-8, and TGF-β cytokines secreted from MSCs on K562 cells as therapeutic agents were applied by Wnt-5a/β-catenin and P53 pathways
Keywords: Mesenchymal stem cells, Telomere length, Telomerase activity, Cytokines, Wnt5a/β-catenin, P53 signaling pathways
Copyright and License Information
© 2020 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Chronic myeloid leukemia (CML) is a hematopoietic stem cell disorder caused by a reciprocal translocation between chromosomes 9 and 22, resulting in the BCR-ABL fusion gene.
The BCR-ABL active tyrosine kinase promotes proliferation and cell survival through several intracellular pathways, eventually leading to malignant transformation.1 There are different therapy options for different kinds of leukemia, and the transplantation of stem cells is one of the most important options.2,3 Mesenchymal stem cells (MSCs) are multipotent cells that can be differentiated into adipocytes, osteocytes, and chondrocytes and have been harvested from adult tissues including bone marrow, adipose, heart, brain, amniotic fluid, etc.4 According to previous research, MSCs derived from various tissues have the same characteristics, such as the same morphology, self-renewal, and multi-lineage differentiation potential, and the ability to express different cell surface markers.5 Given the characteristics of MSCs, they have numerous applications in cell therapy as well as regenerative medicine.6 Because of their ability to migrate, secrete cytokines, and kill tumors, they have received more attention in the last decade.7 All these properties have led to the application of MSCs as clinical agents in therapeutic strategies.8,9 In addition, these cells have been genetically engineered and used as strong therapeutic agents in in vitro experimental models.10
Despite some reports that MSCs inhibit tumor growth and proliferation, others suggest that MSCs accelerate tumor progression. For example, Sun et al reported that bone marrow-derived MSCs (BMSCs) promote tumor growth and improve microanatomy sites of melanoma cells.11 Zhang and Zhang, however, showed that BMSCs inhibited the cell proliferation of CML cells.12 Telomeres with TTAGGG repeats at the end of eukaryotic chromosomes are structures that protect chromosomes from genome instability and degradation.13 These nucleoprotein sequences are maintained by the ribonucleoprotein enzyme telomerase reverse transcriptase. In most somatic and stem cells, due to the end-replication problem, telomeres gradually shortened. In some cancers, telomerase is activated to maintain telomere length; in some others telomere length is elongated under the mechanisms called alternative lengthening of telomeres.14 Therefore, reducing telomerase activity and telomere length can be used as therapeutic approaches to overcome cancer. Previous studies have shown dramatically reduced telomere lengths of leukemic cells as opposed to non-leukemic T-cells in peripheral blood cells of CML patients. Furthermore, a correlation of age-adapted telomere length with disease stage and response to treatment has also been revealed.15,16 With all the studies that have been done about the antitumor properties of BMSCs, the precise cellular and molecular mechanisms involved in their impact on tumor progression through the study of telomere length and telomerase activity is yet to be reported. Thus, the current study reports the in vitro effects of BMSCs on the mortality of the CML cell line by investigating the telomere length, telomerase activity, and gene expression of telomerase components. The possible signaling pathways involved in this process including Wnt-5a/β-catenin and P53 were also evaluated
Materials and Methods
Isolation of rat BMSCs
BMSCs was isolated as described previously by Blanc et al and Fathi et al.17,18 In brief, after giving ethical consent, 5 (5- to 8-week-old) rats were euthanized with an overdose of ketamine/xylazine and bone marrow contains was flushed with phosphate-buffered saline (PBS) supplemented with 5% fetal bovine serum (FBS) (washing buffer). Bone marrow contents was centrifuged and the cell pellet was re-suspended and was layered over same volume of Ficoll-Paque (Innotrain, Germany) and centrifuged at 850×g for 25 minutes at 4°C. In the following, mononuclear cell layer was collected and was re-suspended in Dulbecco’s modified Eagle’s medium (DMEM) culture medium containing 10% FBS. Cell cultures were incubated in a 37°C incubator and passaged with 0.25% trypsin/ethylene diamine tetra acetic acid (EDTA).19 A general overview of methods steps was described as Figure 1.
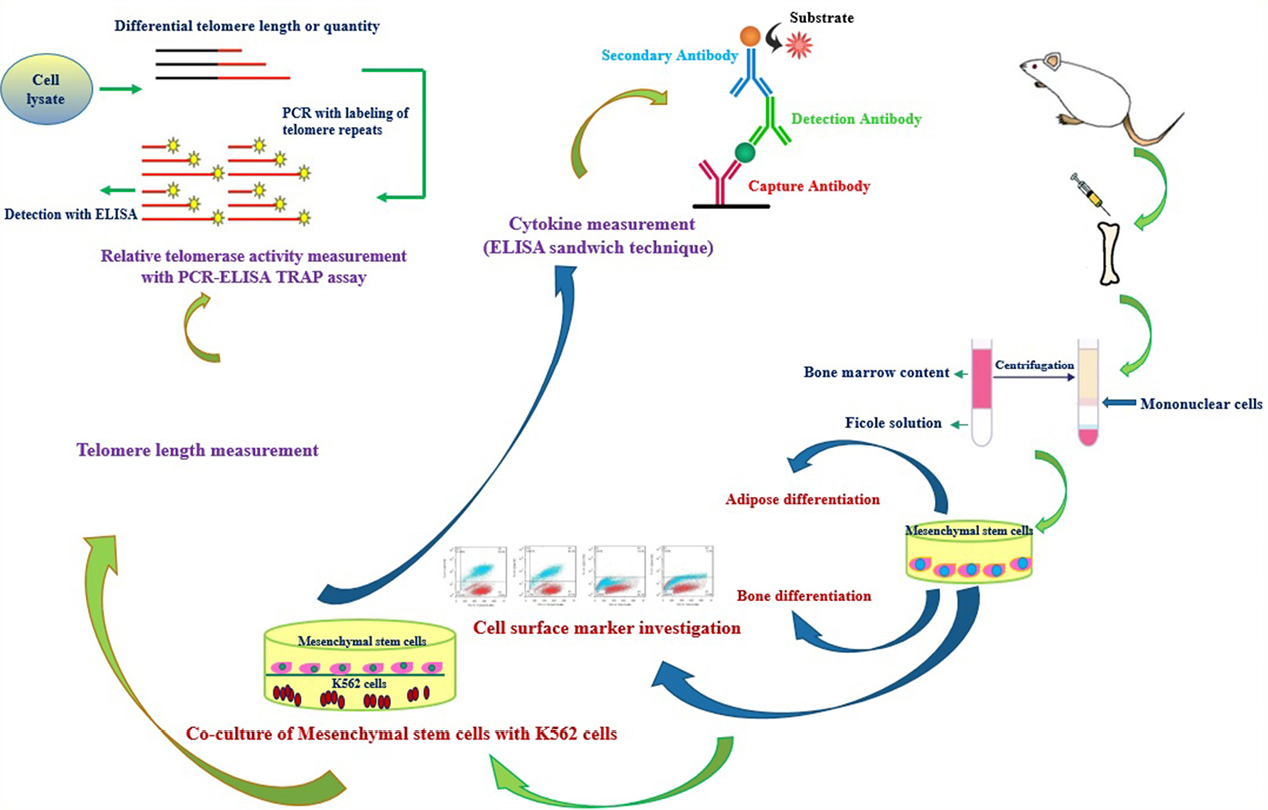
Figure 1.
An overview of the experimental procedures that have been done in this paper.
.
An overview of the experimental procedures that have been done in this paper.
Characterization of BMSCs by cell surface markers detection and multi-lineage differentiation
Flow cytometry was used for immune-characterization of BMSCs as previously described by Fathi et al.18 Briefly, 10×104 BMSCs were trypsinized and incubated with 5 µL of fluorescein isothiocyanate-conjugated antibody CD31 and CD34 and phycoerythrin–conjugated CD73 and CD44 (BD Pharmingen, USA) for 40 minutes on ice. At the end of incubating time, FACS instrument (Becton, USA) was used to quantify the fluorescence intensity of cells. In addition to flow-cytometry, the multipotency of BMSCs was also assessed. For this purpose, BMSCs were cultured in one of the adipogenic, osteogenic and chondrogenic induction medium as previously reported by Fathi and Farahzadi.20 At the end of 21th day, the BMSCs were fixed with 4% paraformaldehyde and were subjected to Sudan III (1% in 96% ethanol), Alizarin red (2% in distilled water) and toluidine blue (0.1% in distilled water) for adipogenesis, osteogenesis and chondrogenesis, respectively.21
Culture of myeloid leukemia cell line K562
K562 (CML cell line) was purchased from Institute Pasteur (Iran) and was cultured in Roswell Park Memorial Institute (RPMI)-1640 supplemented with 10% (v/v) FBS.
Co-culture of BMSCs and CML-cell line (K562)
BMSCs was plated into 6-well trans-well platesat 10×104 cells/well in DMEM complete culture medium solution. After 24 h, 10×105 K562 cells/well was added respectively into two groups; control group (culture of K562 alone) and experimental group (co-cultured K562 and BMSCs). After end of treatment time (7 days), two groups of cells were subjected to DNA isolation for measuring telomere length and protein extraction for western blotting and PCR-ELISA TRAP assay. Also, at the end of 7th day, culture media of two groups were collected for cytokine measuring by ELISA.
Real-time PCR assessment
At the end of co-culture period, 10×105 K562 cells/well from control group as well as experimental group were collected and total RNA from the cells was isolated and cDNA was synthesized using 2 µg RNA with YTA kit (Yekta Tajhiz Azma, Iran) as manufacturer’s instruction, respectively.22 The mRNA expressions of target genes in this experiment included hTERT, P53, Wnt5a, β-catenin and β-actin. Fluorescence data was analyzed to get CT values. The CT values were calculated in relation to β-actin CT values by the 2-ΔΔCT method. The primer sequences are listed in Table 1.
Table 1.
Primer sequences used for the real-time PCR assays
|
No.
|
Gene
|
Primer pair sequence (5'-3')
|
Product length (bp)
|
| NM_001193376.1 |
hTERT
|
CAGCAAGTTTGGAAGAACCC
GACATCCCTGCGTTCTTGG |
98 |
| NM_199173.5 |
Wnt-5a
|
GGCAGCGAGGTAGTGAAGA
TCAGCCAACTCGTCACAGTC |
131 |
| NM_001330729.1 |
β-catenin
|
CATCTGACCAGCCGACACC
CGAATCAATCCAACAGTAGCC |
137 |
| NM_001126118.1 |
P53
|
TCAGTCTACCTCCCGCCATAA
AGTGGGGAACAAGAAGTGGAG |
86 |
| NM_001101.4 |
β-actin
|
AAACTGGAACGGTGAAGGTG
TATAGAGAAGTGGGGTGGCT |
174 |
Telomere and single copy gene (SCG) standard curve
A standard curve is created by dilution of known quantities of TTAGGG repeated 14 times. Also as discussed and shown by Farahzadi et al.23 36B4, was used as a control for determining genome copies per sample. A serial dilutions of TEL STD A (10-1 [1.18 ×108] through to 10-6 [1.18 × 103] dilution) as well as SCG STD A (10-1 through to 10-6 dilution) was performed for generating a standard curve.
DNA isolation and telomere length measurement
Genomic DNA was isolated from two groups of cells as explained above and samples were dissolved in 30 μL final volume, 2 μL of these samples were used for DNA concentration measurement by NanoDrop Spectrophotometer. In the following, absolute telomere length measurement was done by real-time PCR as previously reported by Farahzadi et al.23 Data obtained from real-time PCR technique to measure absolute telomere length were analyzed as kb/reaction and genome copies/reaction for telomere and SCG, respectively, as described in detail previously by O’Callaghan & Fenech24 and Farahzadi et al.23
Telomerase activity assay
PCR-ELISA TRAP assay was performed to determine the telomerase activity. For this purpose, total protein was extracted from control and experimental groups and the relative telomerase activity was assessed using the Telomerase PCR-ELISA kit (Roche Life Science, Germany). In brief, cell extracts were incubated with biotin-labeled primers at 25°C, and the telomeric repeats added onto the ends of the primers were amplified by PCR. The PCR products were added to digoxigenin-labeled detection specific probes and following were allowed to bind to a streptavidin-coated 96-well plate. Finally, the optical density of the blue color was measured at 450 nm by ELISA reader (Dynatech, USA).
Western blotting analysis for Wnt-5a, β-catenin and P53 protein expression
To investigate the Wnt-5a/β-catenin and P53 signaling pathways involved in BMSCs effect on K562 cells, these protein expression was assessed by western blotting. For this stage, K562 cells protein in both groups (control and experimental) were extracted and 50 μg of each cell protein sample was electrophoresed on 12% polyacrylamide slab gels and transferred to poly vinylidene difluoride membrane. In the following, the membrane was incubated with primary antibodies of Wnt-5a, β-catenin and P53 (1:500, Santa Cruz Biotechnology, CA) and was incubated with goat anti-mouse secondary antibody (1:5000 Santa Cruz) for 60 minutes at 25°C. Also, β-actin was used as the internal control to normalize. Next, the protein bands detected with X-ray film. Protein bands intensity were measured and then calculated the ratio of target protein/β-actin and the obtained values were graphed.
Cytokine measuring by ELISA
Culture media was collected from each group including, K562 cell line, BMSCs and co-culture of K562 cell line with BMSCs. ELISA was performed according to the manufacturer’s instructions (ExCell Biology, Shanghai, China; CINC-1 from R&D Systems). Briefly, a 96-well plate was coated with detection Reagent A and stored overnight at 4°C. Then, 50 μL of cell culture media from each groups and standard solution were added into the 96-well plate, which had been coated with human interleukin (IL)-6, IL-8 and transforming growth factor beta (TGF-β) antibodies, and detected via the ELISA sandwich technique. After terminating the reaction, the optical density at a wavelength of 450 nm in each well was determined using a spectrophotometer.
Statistical analysis
The data were analyzed by one-way and two-way ANOVA followed by Dunnett’s post hoc test. Values were measured statistically significant at P < 0.05 by GraphPad Prism version 6.01. A comprehensive overview of methods that have been used in this paper was described as Figure 1.
Results
Culturing and characterization of BMSCs
Bone marrow derived-MSCs like another MSCs, morphologically appear as spindle-shaped cells resembling fibroblasts (Figure 2A and B). After isolation and culturing of the BMSCs, adipogenesis and osteogenesis was done. Sudan III, Alizarin red and toluidine blue were used for staining the lipid droplets, mineralized compartments and aggrecan aggregates, respectively (Figure 2C-E). Immunophenotypic characterization of BMSCs was done by flow cytometry. Figure 2F shows that mesenchymal markers CD73 (95.5%) and CD44 (90.2%) are expressed. However, the hematopoietic markers CD31 (3.02%) and CD34 (6.3%) are not expressed in BMSCs.
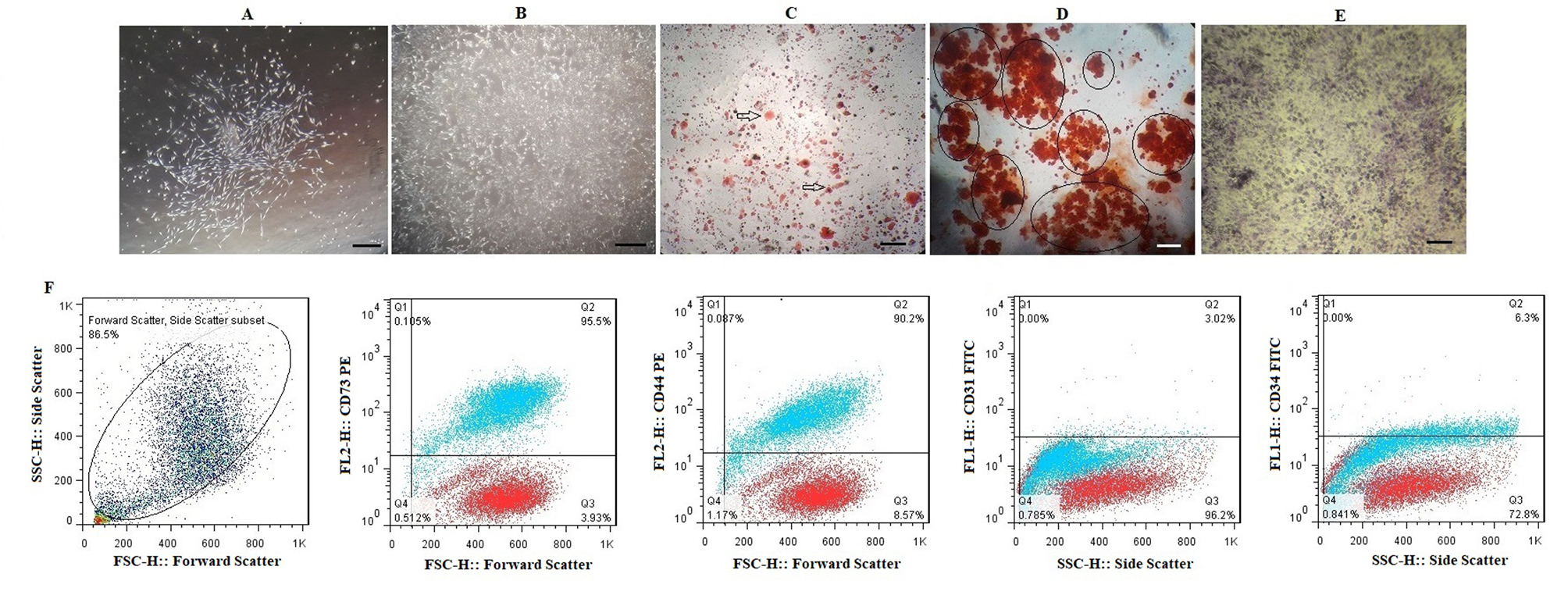

Figure 2.
Morphological features of bone marrow derived-MSCs; (A) Spindle-shaped morphology of bone marrow cells at day 7, (B) More confluent bone marrow derived-MSCs at 21 days of culturing cells. Multi-lineage differentiation of bone marrow derived-MSCs;(C)Generation of lipid vacuoles after adipogenesis and staining by Sudan III; (D) Osteogenic differentiation and Alizarin red staining of mineralized cell aggregates; (E) Chondrogenic differentiation and Toluidine blue staining. (F) The expression of the cell surface markers of bone marrow derived-MSCs that analyzed by flow cytometry. Each antibody was tested individually and the isotopes controls were used as the negative control in this experiment; (A) a total population of cells, the bone marrow derived-MSCs were positive for CD73 (95.5%) and CD44 (90.2%) and negative for CD31 (3.02%) and CD34 (6.3 %). For CD31 and CD34, the isotype control was mouse IgG1 and for CD73 and CD44, the isotype control was mouse IgG2b. Also, isotype control is seen with red dots.
.
Morphological features of bone marrow derived-MSCs; (A) Spindle-shaped morphology of bone marrow cells at day 7, (B) More confluent bone marrow derived-MSCs at 21 days of culturing cells. Multi-lineage differentiation of bone marrow derived-MSCs;(C)Generation of lipid vacuoles after adipogenesis and staining by Sudan III; (D) Osteogenic differentiation and Alizarin red staining of mineralized cell aggregates; (E) Chondrogenic differentiation and Toluidine blue staining. (F) The expression of the cell surface markers of bone marrow derived-MSCs that analyzed by flow cytometry. Each antibody was tested individually and the isotopes controls were used as the negative control in this experiment; (A) a total population of cells, the bone marrow derived-MSCs were positive for CD73 (95.5%) and CD44 (90.2%) and negative for CD31 (3.02%) and CD34 (6.3 %). For CD31 and CD34, the isotype control was mouse IgG1 and for CD73 and CD44, the isotype control was mouse IgG2b. Also, isotype control is seen with red dots.
Investigation of telomere length, telomerase activity and hTERT gene expression
Absolute telomere length measurement was evaluated at the end of 7th day co-culture period. As shown in Figure 3A, telomere length significantly decreased (19.13 kbp) compared to the control group (74 kbp) (***P < 0.001). Also, the telomerase activity has decreased by 50% in the experimental group compared to the control group (Figure 3B) (**P < 0.01). In addition, it was shown that the expression of hTERT gene was 0.4-fold decreased in the experimental group in compared with control group (Figure 3C) (*P < 0.05).
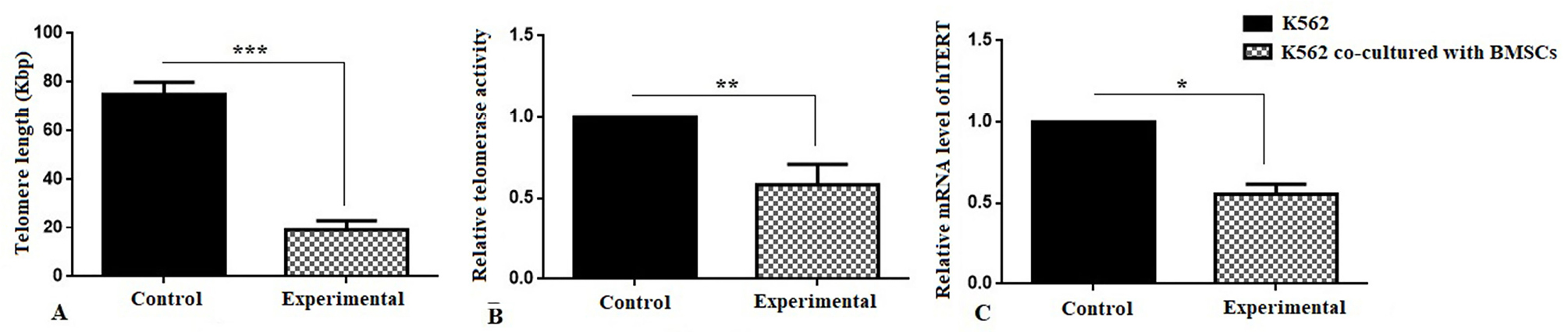
Figure 3.
(A) Absolute telomere length measurement;BMSCs were significantly decreased the telomere length (***P <0.001) of K562 cell line compared with the control group. (B) Relative telomerase activity measurement and (C) RelativehTERT gene expression levels of K562 cell line at the end of 7th day co-culture period with BMSCs.BMSCs were significantly decreased the telomerase activity (**P <0.01) and hTERT gene expression of K562 cell line (*P <0.05) compared with the control group.
.
(A) Absolute telomere length measurement;BMSCs were significantly decreased the telomere length (***P <0.001) of K562 cell line compared with the control group. (B) Relative telomerase activity measurement and (C) RelativehTERT gene expression levels of K562 cell line at the end of 7th day co-culture period with BMSCs.BMSCs were significantly decreased the telomerase activity (**P <0.01) and hTERT gene expression of K562 cell line (*P <0.05) compared with the control group.
BMSCs cause to change the gene and protein expression of Wnt-5a, β-catenin and P53 in K562 cell line
For evaluating the signaling pathways involved in the effect of factors and agents secreted from BMSCs on K562 cell, the protein and mRNA expression was examined by western blotting and real-time PCR, respectively. In this panel, the gene and protein expression of Wnt-5a, β-catenin and P53was investigated. As shown in Figures 4A and B, the protein expression levels of β-catenin and P53 were significantly decreased and increased, respectively (**P < 0.01). A significant decrease and increase in mRNA expression levels of β-catenin and P53, respectively, was also seen (Figure 4C) (**P < 0.01).
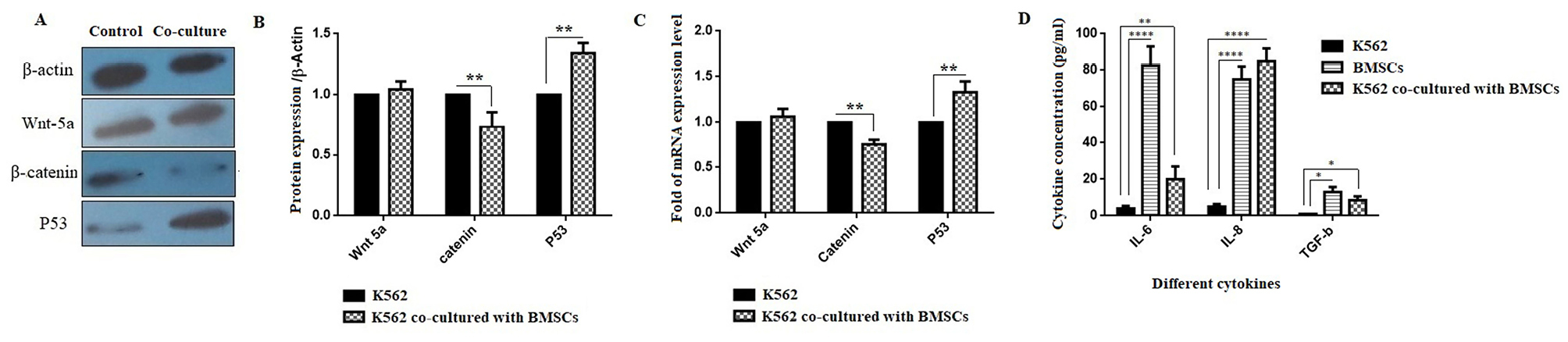
Figure 4.
Effect of bone marrow derived-MSCs on gene and protein expression of Wnt5a,β-catenin and P53 in K562 cell line. (A and B) The protein and (C) the mRNA expression levels of Wnt5a, β-catenin and P53. (D) The secretion levels of cytokines TNF-α, IL-6, IL-8 and TGF-β (compared with control group) (*P < 0.05; **P < 0.01 and ***P < 0.001).
.
Effect of bone marrow derived-MSCs on gene and protein expression of Wnt5a,β-catenin and P53 in K562 cell line. (A and B) The protein and (C) the mRNA expression levels of Wnt5a, β-catenin and P53. (D) The secretion levels of cytokines TNF-α, IL-6, IL-8 and TGF-β (compared with control group) (*P < 0.05; **P < 0.01 and ***P < 0.001).
Measuring cytokine secretion of BMSCs by ELISA
ELISA results revealed that the secretion of IL-6, IL-8, and TGF-β was significantly increased in both BMSCs and the co-culture of the K562 cell line with BMSCs groups compared to the K562 group (Figure 4D) (**P < 0.01 and ****P < 0.0001). In addition to IL-6 and IL-8, as shown in Figure 4D, the TGF-β level was markedly increased in both the BMSCs group and the co-cultured group compared with the K562 group (*P < 0.05).
Discussion
This study demonstrated the effect of BMSCs on the K562 cell as a CML cell line by investigating telomere length, telomerase activity, and hTERT gene expression through P53, Wnt5a and β-catenin signaling pathways. To investigate the hypothesis of this research, K562 cells were co-cultured with BMSCs on trans-well plates for 7 days. To explore the mechanism of the effect of BMSCs on the CML cell line K562, at the end of co-culture period, K562 cells were collected and subjected to PCR-ELISA TRAP assay, quantitative real-time PCR, and western blotting to assay telomerase activity, telomere length, and protein expression, respectively. Also, the culture media was collected for measuring IL-6, IL-8, and TGF-β cytokines.
As seen from the results, hTERT gene expression, telomerase activity, and telomere length were significantly decreased in the experimental group (co-cultured K562 and BMSCs) by more than 3.9-, 0.5-, and 1.7-fold, respectively, when compared with the control group (culture of K562 alone).
Beside the studies on cancer, the potential role of telomerase upregulation in cancer and its inhibition in the cancer treatment were also of great interest and there are still many studies on this field. But the molecular mechanisms and signaling pathways involved are yet to be completely known and it seems at least some of the effects may be due to the same properties.25
Keller et al have previously shown that telomere length measured in peripheral blood of patients with CML correlates with disease stage, clinical prognostic scores, and response to treatment.26 Also, Samassekou et al reported that in the early stage of CML ontogenesis, long telomeres on key chromosomes may contribute to a cell proliferation advantage.27 In another study, Braig et al demonstrated that telomerase-targeting strategy could alleviate the tumor promoting effect of BCR-ABL via induce senescence in CML-like cells.28
The current results showed a significant relationship among hTERT gene expression, telomerase activity, and telomere length in K562 cells co-cultured with BMSCs. Since telomerase activity is regulated by the expression of the hTERT gene,29 a correlation between the telomerase activity and the expression of hTERT can be expressed.
Given the several involved mechanisms in telomerase activity, the regulation of telomere length seems to be complex. Based on the results of previous studies, it was hypothesized that the reductions in telomere length, telomerase activity and hTERT gene expression were heavily governed by cytokines.30 Among these factors, IL-6, IL-8, and TGF-β are possible candidates. In this study, cytokines were measured using ELISA.Results from ELISA demonstrated that the secretion of IL-6, IL-8, and TGF-β was significantly increased in both the control and the experimental groups compared with the K562 group. Liu and Hwang had measured cytokine expression by UCB-MSCs found that IL-6 and IL-8 were numerous proteins expressed, which agrees with our data.31
Two prominent cytokines (IL-6 and IL-8) detected in the co-culture were most probably derived from BMSCs, as the BMSCs culture alone indicated a high secretion of these cytokines, but they were absent in the K562 cell culture (no data obtained). Compared with the K562 cell culture, BMSCs secreted a great amount of both IL-6 and IL-8.
As previously shown by Klassen et al, IL-6 has wide range of performance for the differentiation, development, regeneration and tissue remodeling.32,33 The findings further showed that BMSCs co-cultured with K562 cells expressed higher amounts of IL-8 compared with the BMSCs cultured alone, consistent with previous reports.34,35
IL-8 as a pro-inflammatory cytokine has mitogenic and angiogenic potential. In the other words, IL-8 has been attracted as a main cytokine in the tumor progression in the modulation of angiogenesis.36 The reason for high expression of IL-6 and IL-8 in BMSCs cultured media remains to be investigated. In addition, TGF-β as an anti-inflammatory cytokine has apoptotic function to restrain cell proliferation and the loss of these effects leads to hyper proliferative disorders that are the hallmarks of tumors.37
Previous results of cytokine measurement showed detectable levels of TGF-β in the BMSCs and co-cultured media. It can be concluded that the presence of TGF-β is one reason for the decreasing telomere length and telomerase activity. These results confirm the results obtained in the current study.
As previously reported, MSCs are capable to inhibit the proliferation of cancer cells. In one study, Tyndall et al reported that MSCs being able to suppress immune reactions in an MHC-independent manner via their secretive soluble factors.30 In another study by Fonseka et al, it was demonstrated that MSCs derived from umbilical cord blood (UCB-MSCs) cause to significantly inhibited the proliferation of leukemic cells.35 Considering that the inhibition of tumor cells growth is dose-dependent, suggesting that the UCB-MSCs as well as other sources of MSCs such as bone marrow, adipose tissue etc can be used as anti-tumor agent.38 As indicated in vivo and in vitro study by Ahn et al, it was shown that the growth of tumor in mice was inhibited by adipose tissue-derived MSCs. Also, it was administered that homing of MSCs to tumor is well-established. It was shown that homing potential of MSCs can be mediated by cytokines secreted by tumors or/and their related stroma.4 These results confirm the results obtained by our study.
In addition to the mentioned items, many signaling pathways involved in the pathogenesis of carcinomas have been characterized; among these, the Wnt-5a/β-catenin and P53 pathways are important. During the activation process of the Wnt-5a/β-catenin signaling pathway and after entering β-catenin into the nucleus, the transcription of target genes including c-myc and cyclin D1 are activated. Improper activation of this pathway contributes to carcinogenesis and malignant behaviors.
In addition, the Wnt-5a/β-catenin pathway has been extensively investigated as a key target in cancer treatment and in fine-tuning the regulation of hematopoiesis.39,40 Previous studies were indicated that aberrantly expression and high expression of β-catenin cause to AML and poor prognosis in AML patients, respectively.41,42 Also it was reported that the Wnt/β-catenin pathway activation was detected in samples from patients with CML in blastic crisis. Additionally, it can be said that this study was confirmed the results from our study.
As mentioned above, the results of real-time PCR and western blotting showed that BMSCs could decrease the gene and protein expression of β-catenin. With all these interpretations, the molecular link between Wnt/β-catenin signaling and the expression of telomerase expression and telomere length was also reported by Hoffmeyer et al.43 It was shown that β-catenin-deficient mouse embryonic stem cells have short telomeres; conversely, stem cells expressing an activated form of β-catenin have long telomeres.
The direct relationship between the reduction in telomere length, telomerase activity and β-catenin expression was shown in this study. In addition to the Wnt/β-catenin pathway, the P53 pathway also plays an important role in cancer treatment targeting.44 In the present study, the mRNA and protein expression of p53 in K562 cells was significantly upregulated.
In one study, Basu and Haldar indicated that overexpression of P53 in some cancer cells via reducing the bcl-2 protein expression leading to in apoptotic cell death.45 In another study, Brassat et al reported that telomerase inhibition in CML patient is highly dependent on the presence of functional p53.46 These results are consistent with the results of the current study that overexpression of P53 is associated with decreasing telomeric length and telomerase activity. It can be concluded that the reduction in telomere length, telomerase activity and hTERT gene expression was governed by the P53, Wnt5a, and β-catenin signaling pathways.
Conclusion
In conclusion, we provided evidence showing that BMSCs has an effect on reducing the hTERT gene expression, telomerase activity and telomere length of K562 cell, which is brought about via P53, Wnt5a and β-catenin signaling pathways. Without any ethical concerns, MSCs are easily obtained and cell therapeutic strategy using these cells seems to be a better choice for cancers, however, further researches are needed to use MSCs as clinical application.
Ethical Issues
This study was approved by ethical committee of Tabriz University of Medical Sciences, Tabriz, Iran (Ethical No: IR.TBZMED.REC.1396.849).
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This study was supported by a grant of Research Vice-Chancellor of Tabriz University of Medical Sciences, Tabriz, Iran.
References
- Bouillon AS, Ventura Ferreira MS, Awad SA, Richter J, Hochhaus A, Kunzmann V. Telomere shortening correlates with leukemic stem cell burden at diagnosis of chronic myeloid leukemia. Blood Adv 2018; 2(13):1572-9. doi: 10.1182/bloodadvances.2018017772 [Crossref] [ Google Scholar]
- Choupani J, Nariman-Saleh-Fam Z, Saadatian Z, Ouladsahebmadarek E, Masotti A, Bastami M. Association of mir-196a-2 rs11614913 and mir-149 rs2292832 polymorphisms with risk of cancer: an updated meta-analysis. Front Genet 2019; 10:186. doi: 10.3389/fgene.2019.00186 [Crossref] [ Google Scholar]
- Nariman-Saleh-Fam Z, Bastami M, Somi MH, Samadi N, Abbaszadegan MR, Behjati F. In silico dissection of miRNA targetome polymorphisms and their role in regulating miRNA-mediated gene expression in esophageal cancer. Cell Biochem Biophys 2016; 74(4):483-97. doi: 10.1007/s12013-016-0754-5 [Crossref] [ Google Scholar]
- Ahn JO, Coh YR, Lee HW, Shin IS, Kang SK, Youn HY. Human adipose tissue-derived mesenchymal stem cells inhibit melanoma growth in vitro and in vivo. Anticancer Res 2015; 35(1):159-68. [ Google Scholar]
- Orbay H, Tobita M, Mizuno H. Mesenchymal stem cells isolated from adipose and other tissues: basic biological properties and clinical applications. Stem Cells Int 2012; 2012:461718. doi: 10.1155/2012/461718 [Crossref] [ Google Scholar]
- Montazersaheb S, Kabiri F, Saliani N, Nourazarian A, Avci CB, Rahbarghazi R. Prolonged incubation with Metformin decreased angiogenic potential in human bone marrow mesenchymal stem cells. Biomed Pharmacother 2018; 108:1328-37. doi: 10.1016/j.biopha.2018.09.135 [Crossref] [ Google Scholar]
- Secchiero P, Zorzet S, Tripodo C, Corallini F, Melloni E, Caruso L. Human bone marrow mesenchymal stem cells display anti-cancer activity in SCID mice bearing disseminated non-Hodgkin’s lymphoma xenografts. PLoS One 2010; 5(6):e11140. doi: 10.1371/journal.pone.0011140 [Crossref] [ Google Scholar]
- Tang XJ, Lu JT, Tu HJ, Huang KM, Fu R, Cao G. TRAIL-engineered bone marrow-derived mesenchymal stem cells: TRAIL expression and cytotoxic effects on C6 glioma cells. Anticancer Res 2014; 34(2):729-34. [ Google Scholar]
- Park CW, Kim KS, Bae S, Son HK, Myung PK, Hong HJ. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int J Stem Cells 2009; 2(1):59-68. doi: 10.15283/ijsc.2009.2.1.59 [Crossref] [ Google Scholar]
- Chen Q, Cheng P, Song N, Yin T, He H, Yang L. Antitumor activity of placenta-derived mesenchymal stem cells producing pigment epithelium-derived factor in a mouse melanoma model. Oncol Lett 2012; 4(3):413-8. doi: 10.3892/ol.2012.772 [Crossref] [ Google Scholar]
- Sun T, Sun BC, Ni CS, Zhao XL, Wang XH, Qie S. Pilot study on the interaction between B16 melanoma cell-line and bone-marrow derived mesenchymal stem cells. Cancer Lett 2008; 263(1):35-43. doi: 10.1016/j.canlet.2007.12.015 [Crossref] [ Google Scholar]
- Zhang HM, Zhang LS. Influence of human bone marrow mesenchymal stem cells on proliferation of chronic myeloid leukemia cells. Ai Zheng 2009; 28(1):29-32. [ Google Scholar]
- Fathi E, Charoudeh HN, Sanaat Z, Farahzadi R. Telomere shortening as a hallmark of stem cell senescence. Stem Cell Investig 2019; 6:7. doi: 10.21037/sci.2019.02.04 [Crossref] [ Google Scholar]
- Bryan TM, Englezou A, Dalla-Pozza L, Dunham MA, Reddel RR. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med 1997; 3(11):1271-4. doi: 10.1038/nm1197-1271 [Crossref] [ Google Scholar]
- Wenn K, Tomala L, Wilop S, Vankann L, Hasenbank C, Frank O. Telomere length at diagnosis of chronic phase chronic myeloid leukemia (CML-CP) identifies a subgroup with favourable prognostic parameters and molecular response according to the ELN criteria after 12 months of treatment with nilotinib. Leukemia 2015; 29(12):2402-4. doi: 10.1038/leu.2015.245 [Crossref] [ Google Scholar]
- Brümmendorf TH, Balabanov S. Telomere length dynamics in normal hematopoiesis and in disease states characterized by increased stem cell turnover. Leukemia 2006; 20(10):1706-16. doi: 10.1038/sj.leu.2404339 [Crossref] [ Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004; 363(9419):1439-41. doi: 10.1016/s0140-6736(04)16104-7 [Crossref] [ Google Scholar]
- Fathi E, Farahzadi R, Valipour B, Sanaat Z. Cytokines secreted from bone marrow derived mesenchymal stem cells promote apoptosis and change cell cycle distribution of K562 cell line as clinical agent in cell transplantation. PLoS One 2019; 14(4):e0215678. doi: 10.1371/journal.pone.0215678 [Crossref] [ Google Scholar]
- Farahzadi R, Fathi E, Mesbah-Namin SA, Zarghami N. Anti-aging protective effect of L-carnitine as clinical agent in regenerative medicine through increasing telomerase activity and change in the hTERT promoter CpG island methylation status of adipose tissue-derived mesenchymal stem cells. Tissue Cell 2018; 54:105-13. doi: 10.1016/j.tice.2018.08.012 [Crossref] [ Google Scholar]
- Fathi E, Farahzadi R. Zinc sulphate mediates the stimulation of cell proliferation of rat adipose tissue-derived mesenchymal stem cells under high intensity of EMF exposure. Biol Trace Elem Res 2018; 184(2):529-35. doi: 10.1007/s12011-017-1199-4 [Crossref] [ Google Scholar]
- Fathi E, Farahzadi R, Sheikhzadeh N. Immunophenotypic characterization, multi-lineage differentiation and aging of zebrafish heart and liver tissue-derived mesenchymal stem cells as a novel approach in stem cell-based therapy. Tissue Cell 2019; 57:15-21. doi: 10.1016/j.tice.2019.01.006 [Crossref] [ Google Scholar]
- Mehdizadeh A, Somi MH, Darabi M, Farajnia S, Akbarzadeh A, Montazersaheb S. Liposome-mediated RNA interference delivery against Erk1 and Erk2 does not equally promote chemosensitivity in human hepatocellular carcinoma cell line HepG2. Artif Cells Nanomed Biotechnol 2017; 45(8):1612-9. doi: 10.1080/21691401.2016.1269117 [Crossref] [ Google Scholar]
- Farahzadi R, Fathi E, Mesbah-Namin SA, Zarghami N. Zinc sulfate contributes to promote telomere length extension via increasing telomerase gene expression, telomerase activity and change in the TERT gene promoter CpG island methylation status of human adipose-derived mesenchymal stem cells. PLoS One 2017; 12(11):e0188052. doi: 10.1371/journal.pone.0188052 [Crossref] [ Google Scholar]
- O’Callaghan NJ, Fenech M. A quantitative PCR method for measuring absolute telomere length. Biol Proced Online 2011; 13:3. doi: 10.1186/1480-9222-13-3 [Crossref] [ Google Scholar]
- Montazersaheb S, Kazemi M, Nabat E, Nielsen PE, Hejazi MS. Downregulation of TdT expression through splicing modulation by antisense peptide nucleic acid (PNA). Curr Pharm Biotechnol 2019; 20(2):168-78. doi: 10.2174/1389201020666190206202650 [Crossref] [ Google Scholar]
- Keller G, Brassat U, Braig M, Heim D, Wege H, Brümmendorf TH. Telomeres and telomerase in chronic myeloid leukaemia: impact for pathogenesis, disease progression and targeted therapy. Hematol Oncol 2009; 27(3):123-9. doi: 10.1002/hon.901 [Crossref] [ Google Scholar]
- Samassekou O, Li H, Hébert J, Ntwari A, Wang H, Cliché CG. Chromosome arm-specific long telomeres: a new clonal event in primary chronic myelogenous leukemia cells. Neoplasia 2011; 13(6):550-60. doi: 10.1593/neo.11358 [Crossref] [ Google Scholar]
- Braig M, Pällmann N, Preukschas M, Steinemann D, Hofmann W, Gompf A. A ‘telomere-associated secretory phenotype’ cooperates with BCR-ABL to drive malignant proliferation of leukemic cells. Leukemia 2014; 28(10):2028-39. doi: 10.1038/leu.2014.95 [Crossref] [ Google Scholar]
- Guilleret I, Benhattar J. Unusual distribution of DNA methylation within the hTERT CpG island in tissues and cell lines. Biochem Biophys Res Commun 2004; 325(3):1037-43. doi: 10.1016/j.bbrc.2004.10.137 [Crossref] [ Google Scholar]
- Tyndall A, Walker UA, Cope A, Dazzi F, De Bari C, Fibbe W. Immunomodulatory properties of mesenchymal stem cells: a review based on an interdisciplinary meeting held at the Kennedy Institute of Rheumatology Division, London, UK, 31 October 2005. Arthritis Res Ther 2007; 9(1):301. doi: 10.1186/ar2103 [Crossref] [ Google Scholar]
- Liu CH, Hwang SM. Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine 2005; 32(6):270-9. [ Google Scholar]
- Klassen HJ, Imfeld KL, Kirov Kirov, II II, Tai L, Gage FH, Young MJ. Expression of cytokines by multipotent neural progenitor cells. Cytokine 2003; 22(3-4):101-6. doi: 10.1016/s1043-4666(03)00120-0 [Crossref] [ Google Scholar]
- Gadient RA, Otten UH. Interleukin-6 (IL-6)--a molecule with both beneficial and destructive potentials. Prog Neurobiol 1997; 52(5):379-90. doi: 10.1016/s0301-0082(97)00021-x [Crossref] [ Google Scholar]
- Wang LX, Lu H, Liu P, Fei XM, Wu YJ, Wang CY. [Effect of co-culture of bone marrow mesenchymal stem cells and K562 cells on expression of interleukin 8]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2008; 16(1):156-8. [ Google Scholar]
- Fonseka M, Ramasamy R, Tan BC, Seow HF. Human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSC) inhibit the proliferation of K562 (human erythromyeloblastoid leukaemic cell line). Cell Biol Int 2012; 36(9):793-801. doi: 10.1042/cbi20110595 [Crossref] [ Google Scholar]
- Miller LJ, Kurtzman SH, Wang Y, Anderson KH, Lindquist RR, Kreutzer DL. Expression of interleukin-8 receptors on tumor cells and vascular endothelial cells in human breast cancer tissue. Anticancer Res 1998; 18(1A):77-81. [ Google Scholar]
- Derynck R, Jarrett JA, Chen EY, Eaton DH, Bell JR, Assoian RK. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature 1985; 316(6030):701-5. doi: 10.1038/316701a0 [Crossref] [ Google Scholar]
- Ding Y, Lu H, Lu SF, Lu RN, Liu P, Wu YJ. [Effects of human bone marrow mesenchymal stem cells on proliferation and apoptosis of K562 cells]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2009; 17(1):137-40. [ Google Scholar]
- Ashihara E, Takada T, Maekawa T. Targeting the canonical Wnt/beta-catenin pathway in hematological malignancies. Cancer Sci 2015; 106(6):665-71. doi: 10.1111/cas.12655 [Crossref] [ Google Scholar]
- Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol 2006; 7(10):1048-56. doi: 10.1038/ni1381 [Crossref] [ Google Scholar]
- Ysebaert L, Chicanne G, Demur C, De Toni F, Prade-Houdellier N, Ruidavets JB. Expression of beta-catenin by acute myeloid leukemia cells predicts enhanced clonogenic capacities and poor prognosis. Leukemia 2006; 20(7):1211-6. doi: 10.1038/sj.leu.2404239 [Crossref] [ Google Scholar]
- Serinsöz E, Neusch M, Büsche G, Wasielewski R, Kreipe H, Bock O. Aberrant expression of beta-catenin discriminates acute myeloid leukaemia from acute lymphoblastic leukaemia. Br J Haematol 2004; 126(3):313-9. doi: 10.1111/j.1365-2141.2004.05049.x [Crossref] [ Google Scholar]
- Hoffmeyer K, Raggioli A, Rudloff S, Anton R, Hierholzer A, Del Valle I. Wnt/beta-catenin signaling regulates telomerase in stem cells and cancer cells. Science 2012; 336(6088):1549-54. doi: 10.1126/science.1218370 [Crossref] [ Google Scholar]
- Valipour B, Mohammadi SM, Abedelahi A, Faramarzian Azimi Maragheh B, Naderali E, Dehnad A. Culture filtrate ether extracted metabolites from Streptomyces levis ABRIINW111 increased apoptosis and reduced proliferation in acute lymphoblastic leukemia. Biomed Pharmacother 2018; 108:216-23. doi: 10.1016/j.biopha.2018.09.050 [Crossref] [ Google Scholar]
- Basu A, Haldar S. The relationship between BcI2, Bax and p53: consequences for cell cycle progression and cell death. Mol Hum Reprod 1998; 4(12):1099-109. doi: 10.1093/molehr/4.12.1099 [Crossref] [ Google Scholar]
- Brassat U, Balabanov S, Bali D, Dierlamm J, Braig M, Hartmann U. Functional p53 is required for effective execution of telomerase inhibition in BCR-ABL-positive CML cells. Exp Hematol 2011; 39(1):66-76 e1. doi: 10.1016/j.exphem.2010.10.001 [Crossref] [ Google Scholar]