Advanced pharmaceutical bulletin. 10(4):502-511.
doi: 10.34172/apb.2020.062
Review Article
PCSK9: A Key Target for the Treatment of Cardiovascular Disease (CVD)
Saeideh Sobati 1, 2, Amir Shakouri 1, Mahdi Edalati 3, 4, Daryoush Mohammadnejad 1, Reza Parvan 5, Javad Masoumi 6, Jalal Abdolalizadeh 7, 4, * 
Author information:
1Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
2Department of Biochemistry, Higher Education Institute of Rab-Rashid, Tabriz, Iran.
3Hematology and Oncology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
4Paramedical Faculty, Tabriz University of Medical Sciences, Tabriz, Iran.
5Department of Biosciences, University of Milan, Via celoria 26, 20133, Milan, Italy.
6Immunology Department, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
7Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Abstract
Proprotein convertase subtilisin/kexin type 9 (PCSK9), as a vital modulator of low-density lipoprotein cholesterol (LDL-C) , is raised in hepatocytes and released into plasma where it binds to LDL receptors (LDLR), leading to their cleavage. PCSK9 adheres to the epidermal growth factor-like repeat A (EGF-A) domain of the LDLR which is confirmed by crystallography. LDLR expression is adjusted at the transcriptional level through sterol regulatory element binding protein 2 (SREBP-2) and at the post translational stages, specifically through PCSK9, and the inducible degrader of the LDLR PCSK9 inhibition is an appealing new method for reducing the concentration of LDL-C. In this review the role of PCSK9 in lipid homeostasis was elucidated, the effect of PCSK9 on atherosclerosis was highlighted, and contemporary therapeutic techniques that focused on PCSK9 were summarized. Several restoration methods to inhibit PCSK9 have been proposed which concentrate on both extracellular and intracellular PCSK9, and they include blockage of PCSK9 production by using gene silencing agents and blockage of it’s binding to LDLR through antibodies and inhibition of PCSK9 autocatalytic processes by tiny molecule inhibitors.
Keywords: Atherosclerosis, Cholesterol, Coronary heart disease, LDL, Monoclonal antibody, PCSK9
Copyright and License Information
© 2020 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
High level of low-density lipoprotein cholesterol (LDL-C) has been continually related to risk of cardiovascular disease, especially coronary heart disease (CHD). The LDL receptors (LDLRs) on hepatic cells collude be eliminate LDL particles from peripheral blood. LDL binds to LDLR and the complicated form, LDL/LDLR, is coopted into clathrin covered vesicles through of endocytosis. After separation of LDL from the receptor, LDLR is recycled for re-use. Simultaneously, LDL is broken down. As a whole, this is a continuous process towards the plasma membrane.
1-4
Proprotein convertase subtilisin/kexin type 9 (PCSK9) encoded via the human PCSK9 gene on chromosome 1.
5
It is universally expressed in a lot of tissues and cells.
6
PCSK9 binds to the receptor of LDL-C within the liver, LDLR eliminates LDL-C from the blood. While PCSK9 binds to LDLR, the receptor is broken down and might not cast-off LDL-C from the blood. If PCSK9 is blocked, extra LDLRs could be existent on the surface of the liver and will do away with greater LDL-C from the blood.
7
Consequently, obstructing PCSK9 can decrease cholesterol levels in plasma.
8-10
The clinical significance of PCSK9 is for acting in cholesterol homeostasis. PCSK9 is blocked by drugs, consequently decreasing LDL-C. The U.S. Food and Drug Administration (FDA) have been approved the primary PCSK9 inhibitors, evolocumab and alirocumab, for decreasing LDL-C in 2015.
The producers did not submit records to reveal that the medicine certainly advanced consequences of cardiovascular sickness, but they assumed that lowering LDLC could deal with cardiovascular disorder.
11,12
The production cost of those new medicines as in 2015 affected their prices.
13,14
History
A biochemistry scientist in the Institute of Montreal in Canada, determined a singular human proprotein convertase gene located on chromosome 1 in 2003 (Nabil Seidah). Also, Catherine Boileau in the Necker-Enfants Malades clinic in Paris investigated groups with familial hypercholesterolaemia at the same time. They recognized a mutation on chromosome 1 in a number of those families; however, they were not able to discover the applicable gene. The labs were given collectively and one year later their work was posted, linking mutations within the gene, now diagnosed as PCSK9, under the given circumstance.
15
They demonstrated that the mutations would probably lead to gene overexpression. Also, investigators at Rockefeller University
16
and University of Texas Southwestern had detected equal protein in mice in the same year. They had demonstrated the new pathway for control of LDL-C related to PCSK9 gene expression.
Temporarily, Abifadel et al at UT-Southwestern analyzed humans with very excessive and low LDL-C and accumulated DNA samples. The approximate function of PCSK9 and its region within the genome, the applicable location of chromosome1 was sequenced in humans with very low cholesterol and nonsense mutations were observed inside the gene, hence this biological target was validated for drug treatment.
17
Finally, the FDA authorized the PCSK9 as a novel therapeutic target in July 2015.
Structure of PCSK9
The PCSK9 is a 72 kDa protein with 692 amino acids and composed of a prodomain with 31–152 residues, signal peptide with 1–30 residues, a C-terminal domain with 153–451 residues and a catalytic domain with 153–451 residues (Figure 1).
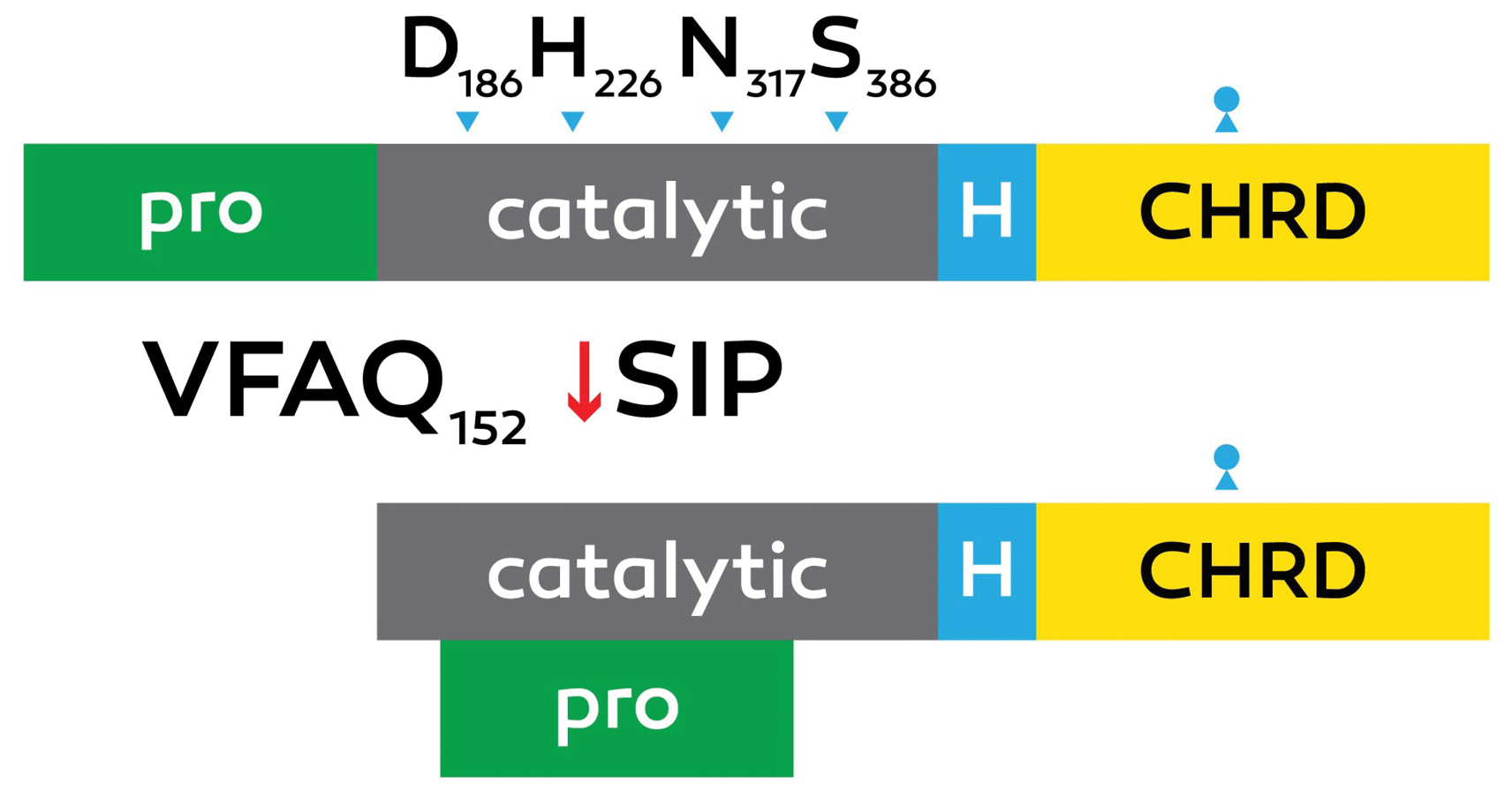
Figure 1.
Schematic design of proprotein convertase subtilisin kexin 9 (PCSK9) processing.
The autocatalytic zymogen processing of pro PCSK9 (75 kDa) at Val-Phe-Ala-Gln152↓Ser-Ile-pro (VFAQ152↓SIP) into the [pro segment (15 k Da) =PCSK9 (62 k Da)] complex is emphasized, collectively with the positions of the active site Asp186, His226, and Ser386 and the oxyanion hole Asn317. The C-terminal hinge area (H) and cys-his–rich domain (CHRD) are revealed.
.
Schematic design of proprotein convertase subtilisin kexin 9 (PCSK9) processing.
The autocatalytic zymogen processing of pro PCSK9 (75 kDa) at Val-Phe-Ala-Gln152↓Ser-Ile-pro (VFAQ152↓SIP) into the [pro segment (15 k Da) =PCSK9 (62 k Da)] complex is emphasized, collectively with the positions of the active site Asp186, His226, and Ser386 and the oxyanion hole Asn317. The C-terminal hinge area (H) and cys-his–rich domain (CHRD) are revealed.
PCSK9 includes 9 members with the modern member of the proprotein convertase circle.
15,18
Prepro PCSK9 undergoes signal peptidase cleavage and this cleavage is needed for the maturation and secretion of PCSK9.
1,19
At some point in rodent improvement, PCSK9 was shown to be transiently expressed in mind centers, including the telencephalon, olfactory bulb, and cerebellum.
20,21
Current in situ hybridization confirmed that PCSK9 mRNA is also ample within the embryonic umbilical artery wall, such as embryonic membranes and presumptive smooth muscle cells. In the adult, PCSK9 remains noticeably expressed in hepatocytes and much less so within the small kidney and intestine.
20,21
Gene and mutations
The human PCSK9 gene is found on chromosome 1 on the band1q32.3; it is made up of 13 exons.
22
This gene generates isoforms through alternatives.
23
Studies concerning splicing mutagenesis have shown that the sequence needed for autocatalytic cleavage is degenerate, which requires additional complex labors to become aware of the ordinary substrate(s) of PCSK9.
24,25
Some of the “loss of function” PCSK9 mutants that will occur undoubtedly are associated with the shape of PCSK9; it is likely that these mutants influence some of PCSK9 features. The shorter versions of PCSK9 is made by three mutations that disrupting right folding and secretion.
26,27
Several extra PCSK9 variants are related to decreased plasma LDL levels and decreased cardiovascular hazard.
28
Transporters of the D374Y-PCSK9 have been determined to possess a five to thirtyfold higher affinity for binding to LDLR in comparison with the wild types and led to the onset of untimely CHD in advance for 10 years.
29
In a recent study by Cohen et al, it was reported that plasma LDL-C is reduced by roughly 1.0 mmol/L by PCSK9 variations, Y142X and C679X; it was also revealed that 88% reduction in CHD prevalence is correlated with PCSK9 variations, Y142X and C679X. Some other variation, R46 L, particularly dealt with in Caucasian subjects, is correlated with an LDL-C decrease of 0.5 mmol/L along with a 47% reduction of CHD risk.
30
Co-regulation of pcsk9 and LDLR expression by SREBP
It is believed that sterol regulatory element-binding protein-2 (SREBP-2), membrane-bonded transcription factor regulating the cholesterol homeostasis in cells
31
is responsible for the simultaneous expression of PCSK9 at the transcriptional level. The main SREBP isoforms expressed in mouse liver have partly overlapping but distinct gene objectives: genes associated with fatty acid synthesis are controlled by SREBP-1c. However, genes associated with synthesis of cholesterol and uptake, including Pcsk9 and LDLR are controlled by SREBP-2. The proteolytic processing of SREBPs resulted in active nuclear forms. This phenomenon occurs in the reaction that decreases cholesterol levels in endoplasmic reticulum (ER) membranes.
32
Each SREBP in mouse liver is impeded by fasting and they can be activated by feeding.
33
In a feeding state, SREBP-2 activity is reduced to the baseline level; but insulin stimulates SREBP-1c at each of the transcriptional and protein processing degrees.
33,34
SREBP-1a, SREBP-1c, and SREBP-2 are three versions of SREBPs. Like SREBP-1a and SREBP-1c, SREBP-2 is synthesized as a pathfinder that joins the triplex blend made up by SREBP cleavage-activating protein (SCAP) and the ER-retention protein insulin-inducible gene accompanied by excessive levels of sterols (largely derived from LDL-related cholesterol); this complex is kept in the ER and remains an inactive pool of SREBP-2. Nevertheless, in low levels of cellular sterol, the SCAP–SREBP-2 complex Shipped to the Golgi system and after proteolytic processing the SREBP-2 is ready to secrete in the cytosol. Then, SREBP-2 transported into the nucleus and acts as a mediating factor for transcriptional activation of genes that involved in cholesterol metabolism like 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) synthase. This enzyme activity induces the transformation of acetyl-CoA to cholesterol, another one is the HMG CoA reductase that is the rate-restricting enzyme that is responsible for converting of HMG-CoA to mevalonate, and the LDLR32, 35, 36. In healthy individuals, fasting reduces plasma PCSK9; but they are extended post-prandially, and possess a diurnal rhythm that replicates markers of hepatic cholesterol synthesis.
29,35
In another study, 12 healthy subjects underwent fasting for 18 hours, but it was shown that a ketogenic diet was not associated with 35% in PCSK9 plasma ranges. The results showed that prolonged fasting decreased PCSK9 sharply (-50%).
36
Further investigations confirmed that 48-hour fasting decreased PCSK9 levels that minimized at 36 hours (58% decrease vs. fed state).
35
It is believed that the Mediterranean diet reduced plasma PCSK9 and LDL-C to -11.7% and -9.9%, respectively, without reducing the weight
37
PCSK9 levels were reduced by diets accompany with oleic acid, which was definitely associated with cholesterol synthesis markers (lathosterol-to-cholesterol ratio.
38
In contrast to a saturated fat food diet, PUFA diet decreased PCSK9 and lathosterol-to-cholesterol ratio. It is believed that PUFA contributes to the development of hepatic LDL cholesterol, which leads to decreased activity of SREBP-2.
39
Functions of PCSK9
Hepatic cholesterol metabolism
The important characteristic of PCSK9 is the degradation of LDLR through a difficult process. Through hepatic uptake, the primary part of LDL-C is eliminated of the plasma. During the endocytosis, transmembrane LDLR internalizes the LDL molecules. Internalized LDL/LDLR will be dissociated then, the LDLR returns to the cytoplasmic membrane for recycling. Every LDLR molecule is able to recycle for 150 times, it demonstrates that moderate fluctuations in LDLR accessibility via PCSK9-prompted destruction may cause significant modifications in the level of LDL-C. The PCSK9 precursor molecule experiences intramolecular autocatalytic cleavage in the N-terminal pro segment domain within the ER
40
(Figures 2 and 3).
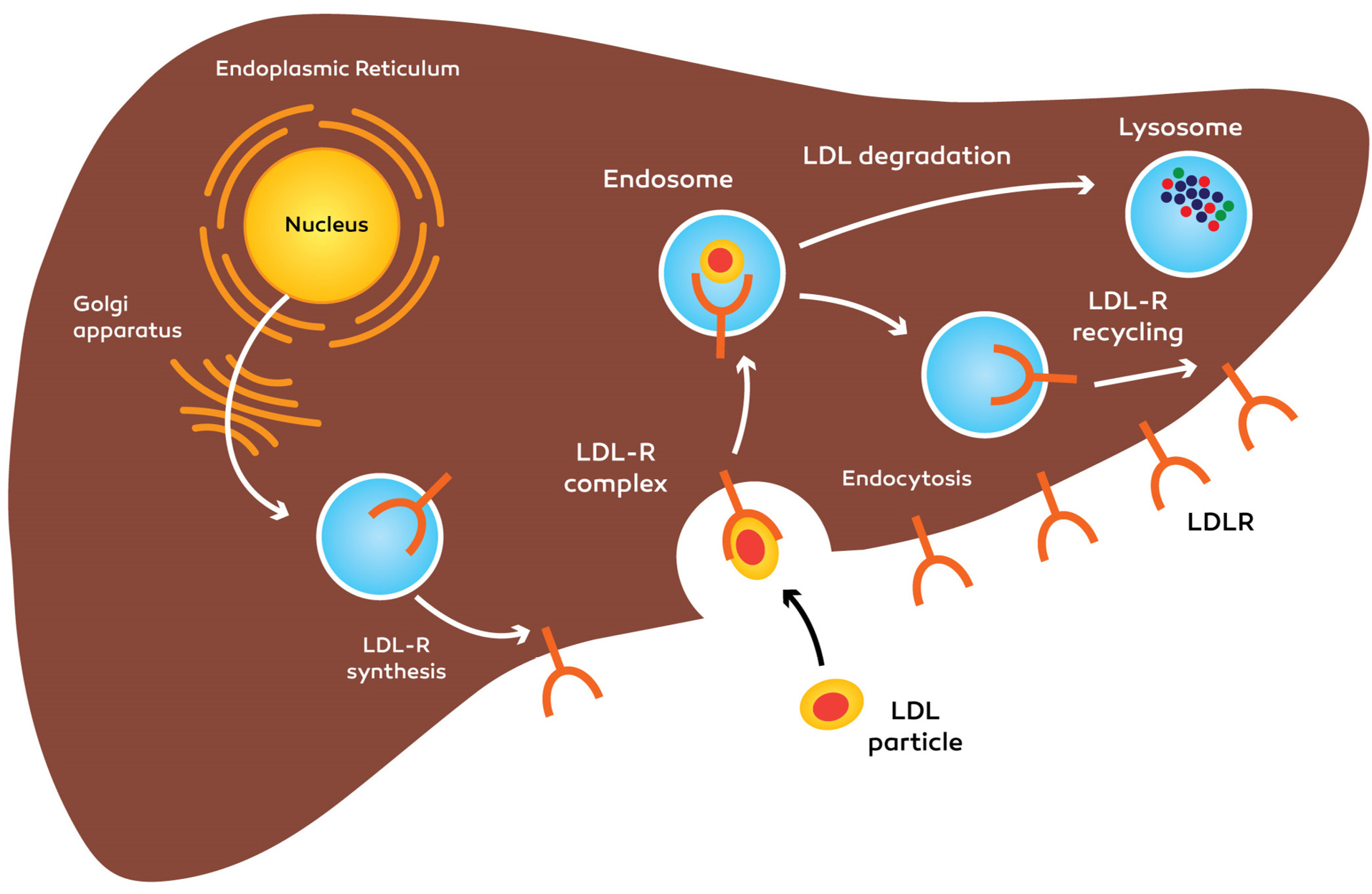
Figure 2.
Function of PCSK9 within the regulation of the hepatocyte LDLR activity: synthesis and recycling of LDLR. LDLR: low-density lipoprotein cholesterol receptor, PCSK9: Proprotein convertase, subtilisin/kexin type 9.
.
Function of PCSK9 within the regulation of the hepatocyte LDLR activity: synthesis and recycling of LDLR. LDLR: low-density lipoprotein cholesterol receptor, PCSK9: Proprotein convertase, subtilisin/kexin type 9.
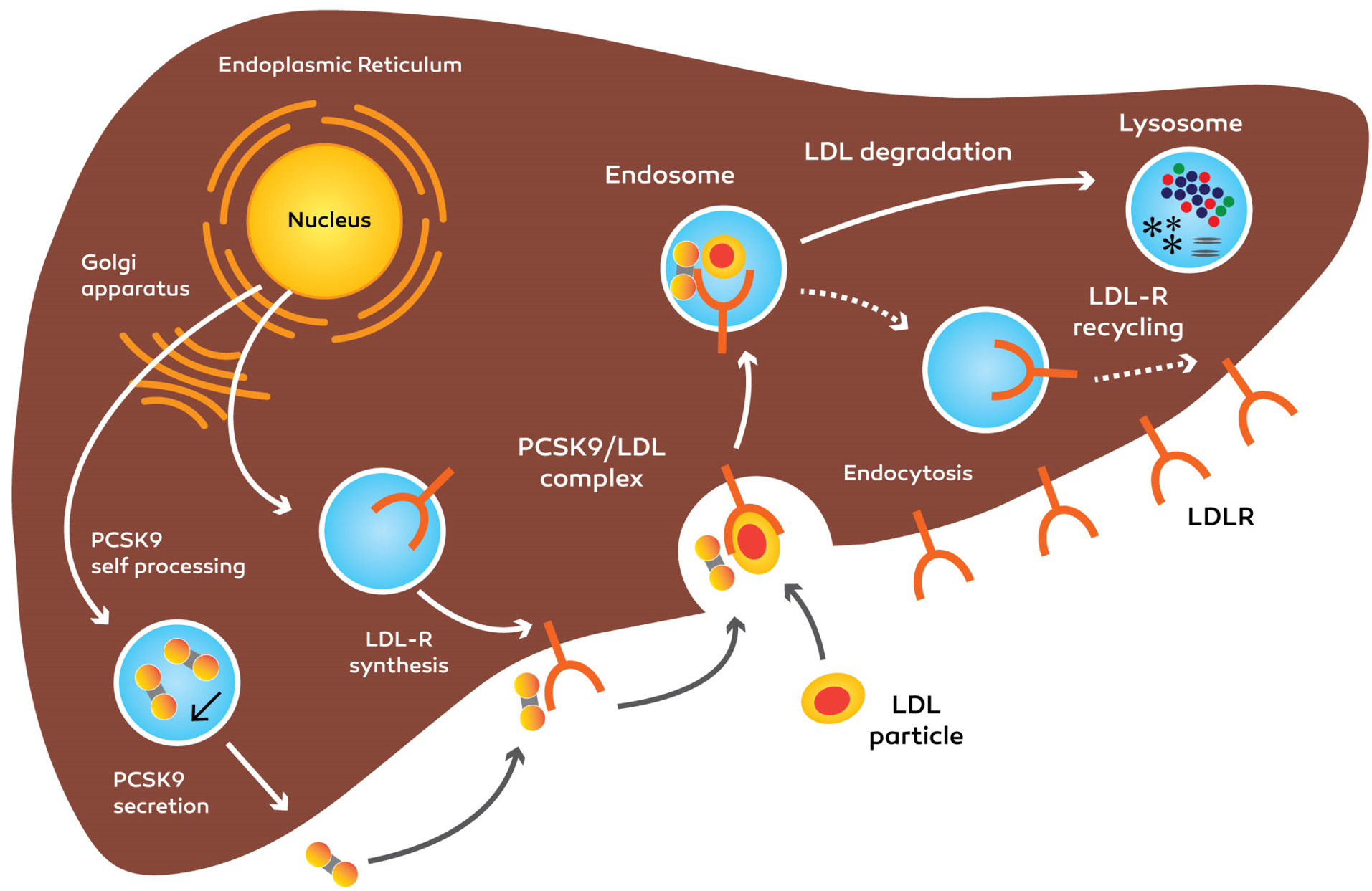
Figure 3.
Synthesis, secretion of PCSK9 and impact on LDLR.PCSK9 binds to LDLR and upon internalization, directs LDLR to lysosomal degradation, reducing the quantity of LDLRs at the cellular surface. LDLR: low-density lipoprotein cholesterol receptor, PCSK9: Proprotein convertase, subtilisin/kexin type 9.
.
Synthesis, secretion of PCSK9 and impact on LDLR.PCSK9 binds to LDLR and upon internalization, directs LDLR to lysosomal degradation, reducing the quantity of LDLRs at the cellular surface. LDLR: low-density lipoprotein cholesterol receptor, PCSK9: Proprotein convertase, subtilisin/kexin type 9.
After PCSK9 is secreted, the cleaved pro domain keeps the connection with the catalytic domain and help mature PCSK9 molecule move out of the ER into the secretion procedure.
5,41
PCSK9 entered to blood circulation as a phosphoprotein and has no other substrates.
29
After PCSK9 is discharged from the cell, whether it attaches to the peripheral LDL receptor and is endocytosed collectively with the receptor, or the protein can stay in the plasma.
42,43
The PCSK9 accomplishes plasma and entering the tissues such as the liver, kidneys, intestines, lungs, pancreas and adipose tissue, and modulate LDL-receptor recycling.
44
One feasible reason for this apparently ineffective controlling cycle is the potential of PCSK9 as a ‘brake’ to help the gradual absorb the cholesterol while LDLRs destroyed once they have internalized LDL. PCSK9 can doubtlessly avoid extreme cellular cholesterol buildup by preventing the LDLRs recycling in the cell surface of different organs.
25
PCSK9 attaches to the LDL receptor on the cytoplasm membrane at the first epidermal growth factor-like (EGF A) domain. The PCSK9-LDL receptor complex moves into endosomal or lysosomal space and is degraded that results in reduced LDL receptors at the surface of the cell.
45
PCSK9 binds the extracellular domain of the LDLR
The PCSK9 reduces the level of LDLR in hepatocytes. That observation was confirmed in mouse models for the first time then, was verified in patients affected with PCSK9 mutations through genetic tests.
26,46
The molecular mechanism of this regression was revealed after it was shown that PCSK9 secrete by different cells and this secreted PCSK9 after attached to LDLR, undergoes internalization that speeds up its destroying.
47,48
The internalization method is associated with the presence of ARH adaptor protein.
49
The location of the interplay between discharged PCSK9 and the extracellular domain of the LDLR became the first epidermal growth factor-like repeat homology domain (EGF-A) inside the LDLR in the human being.
If in the EGFA domain of the LDLR a molecular change (mutation) occur, cleavage of LDLR via PCSK9 will be arrested, it is because that interacts with PCSK9 in this domain happened of the receptor. According to the current crystal structure of interaction in PCSK9/EGFA, it demonstrates that the EGF-a domain of the LDLR attaches to PCSK9 on top of the catalytic domain.
28
Apparently, such attaching requires calcium and it is done with a 1:1 stoichiometry at a k d of 170–750 nM, at neutral pH of plasma.
29,50
The interaction of the EGFA domain of the receptor is only with the catalytic domain of PCSK9 at the cell membrane (i.e., at neutral pH).
50
The binding affinity for attaching the PCSK9 to LDLR is negatively regulated by the acidic stretch positioned inside the prodomain.
51
However, the affinity between the receptor and PCSK9 is boosted (k d of 1–8 n M) than that of the neutral pH following endocytosis (i.e., at the acidic pH of endosomes).
52
In acidic pH, salts bridges are formed by the prodomain of PCSK9 with the propeller domain of the LDLR. It was argued that charged C-terminal domain of PCSK9 attaches to the domain of the LDLR with negative charge.
53
Clinical significance
It demonstrated that PCSK9 substitutes are capable of decreasing or increasing upsurge of the circulating cholesterol. LDL-C is removed from the blood when it attaches to the surface of liver cells and is moved into the cells. While PCSK9 attaches to an LDLR, the receptor is wrecked along with the LDL molecule. LDLR degrades by PCSK9 through suppression of the hairpin structural modification of LDLR.
54,55
Additional variants are associated with an uncommon autosomal dominant familial hypercholesterolemia (HCHOLA3) disease.
56,57
Its protease activity is boosted by mutations, the amount of LDLR is reduced, and LDL cholesterol will not be absorbed into the cells.
56
PCSK9 protein in human first undergoes transformation and settle down within the brain. However, also found in other tissue like liver, kidney, pancreas, and in small intestine.
58
Studies have shown that PCSK9 is expressed significantly in arterial wall components such as endothelial cells, smooth muscle cells and also macrophages. Along with side effects like atherosclerosis, it would regulate homeostasis.
59,60
Thus, it is evident that PCSK9 will involve in the pro-atherosclerotic procedure and in addition regulates lipoprotein synthesis.
30
PCSK9 attaches to LDLR and suppress the removal of LDLC from the blood. Researchers have documents that PCSK9 inhibitors could be used for treating hypercholesterolemia conditions.
13,61-63
Besides, loss-of-function mutations in the PCSK9 gene reduce LDL levels and safety in cardiovascular disease cases.
26,46,64
In addition to its effect on lipoprotein synthetic procedure and its pro atherosclerotic property, PCSK9 acts as a mediator in glucose metabolism, weight complications and so on.
65-67
The presence of PCSK9 has been observed in various types of infections (bacterial or viral) and sepsis.
68,69
The role and features of PCSK9 in the brain are unclear; in nervous system development, it might be both pro-apoptotic and protective agent.
5
PCSK9 and atherosclerotic plaques
Although the majority of researches was done on the position of PCSK9 on expression of LDLR in the liver, there is increasing evidences about the expression of PCSK9 and its function in greater-hepatic tissues.
44,70
Most recent studies show that PCSK9 as well expressed in the artery wall tissue, including macrophages, endothelium cell (EC) and smooth muscle cellular (SMC) that accompanied by localized activities that probably influence on the vascular homeostasis and formation of sclerosis in affected arthritis.
64,59
The PCSK9 that secreted by SMC is able to degrade LDLR by macrophages; hence, it could be said that PCSK9 effects on the LDLR level inside the wall of the artery might play a role in the biology of lesion.
71
Under the hyper lipidemic state, peripheral blood monocytes are entered into the lesion site and developed into macrophages, it leading to the development of atherosclerosis.
72
It is believed that foam cell formation, as a main symptom of atherosclerosis, is caused by the absorption of lipoprotein by macrophages that settle down in lesion. The intake of local and unmodified LDL is facilitated by LDLR, this phenomenon only little influences on legion improvement because its feedback is regulated quickly and tightly in comparison to the huge lipid access available to scavenger receptors.
73
Nevertheless, it was confirmed, that in C57BL/6 mouse null for macrophage LDLR that feed with specific diet causing hyperlipidemia, foam cell formation was decreased; hence, it is likely that macrophage LDLR is involved in the development of the procedure of atherosclerosis.
74
It was shown that low stress triggers the increased PCSK9 expression in vascular EC and SMC accompanied by the production of reactive oxygen species (ROS), with phosphorylated NF-jB making a strong connection to PCSK9 expression with the mediation of LOX-1.
75
Consequently, LDLR is detached at the surface of arterial macrophages by PCSK9 derived from SMC. This process usually triggers LDL to accumulate in the artery wall and contributes to the formation of oxidized LDL (OxLDL). Then a feed-ahead loop is set off that produces extra ROS within the cells which, in turn, causes further changes in LDL and their attachment to LOX-1.
64,75
According to the results of the study on PCSK9 knockout mice that received PCSK9-expressing bone marrow cells, macrophage produce the PCSK9 and released into the circulation; it should be noted that less than 1% of total plasma PCSK9 is produced by macrophages.
58,59
Moreover, secreted PCSK9 from macrophages and/or SMC are able to reduce the expression of LDLR in macrophages having a likely positional paracrine and autocrine degrade the LDLR in atheroma cells. It was reported that C57BL6 mice with macrophages expressing or missing LDLR have enhanced level of macrophage LDLR on lipid buildup in the artery wall and atherosclerotic lesion progress.
74
Significant more small lesions have been observed in animals receiving LDLR bone marrow in comparison with those receiving normal bone marrow; hence, it could be said that the number of foam cell formation inside the artery wall is affected by macrophage LDLR, leading to atherosclerosis development ultimately. It was observed that decreased LDLR level by PCSK9 in macrophages influences on the reduced number of foam cell development inside the artery wall and causing decreased atherosclerosis manifestation.
58,76
Hence, it could be said that PCSK9 secreted through macrophages released into the plasma and the atheroma, becomes gathered in the lesion that affects the composition of plaque regardless of the amount of serum lipid. Therefore, this could be another cardiovascular advantage of anti-PCSK9 treatment options.
60
Strategies for inhibition of PCSK9
Several healing methods to inhibit PCSK9 were proposed, which concentrated on both extracellular and intracellular PCSK9.
77-79
These techniques include suppression of PCSK9 synthesis by gene silencing agents, suppression of PCSK9 attachment to LDLR by monoclonal antibodies (mAbs), and suppression of PCSK9 autocatalytic processing by small-molecule suppressors.
15,80-82
Antisense oligonucleotides
A common procedure to suppress the secretion of PCSK9 is to control the mRNA of PCSK9; this could be done by applying antisense oligonucleotides (ASOs); it is a quick sequence of nucleotides that attaches to the mRNA and suppress the translation procedure.
83
Murine hepatic Pcsk9 mRNA secretion and the LDL-C level were reduced to 92% and 32% respectively by the 2nd-generation ASO for PCSK9. In addition, this kind of ASO is also capable of overexpression of Apo bec1 mRNA, a three-fold reduction within the stage of Apo B48, and a 50% reduction in the level of Apo B100.
84
Besides, after ASOs are injected into monkeys, the serum level of PCSK9 was reduced by 85%, and LDL C level was decreased by 50%.
85
This study demonstrated no discouraging results have been observed from the ASOs.
86
At present, ASOs targeting PCSK9 is not being tested; a section I trial in 2011 was ended in advance because of secret reasons.
Small interfering RNA
Other techniques for suppressing mRNA are use the single-stranded RNA or small interfering RNA (siRNA) that are probably carried out intravenously by small lipoid nanoparticles.
87
Experiments conducted on mice models show that specific siRNA of liver concentrated on PCSK9 was able to suppress mRNA 50%-60% maximally that decreased the LDL level of plasma by 30%. In nonhuman experiments, single-dose management of five mg of the drug reduced the LDL C by 56%-70% after 72 hours and it was kept constant within some weeks.
88
Phase I clinical trial carried out through Alnylam prescribed drugs with ALN-pcs siRNA has been done.
89
Monoclonal antibodies
The production process of mAbs starts in so-called hybridoma cells. The first generations of therapeutic mAbs had been produced in mouse origin cells, regularly developed the more specific human anti-mouse antibodies and ensuing reduced multiplied hazard of hypersensitive reaction.
90,91
In the end, chimeric, humanized mAbs developed in a human consistent location, which every kind has growing extents of human sequence inside the variable location and less immunogenicity.
92,93
Clinical administration of mAbs well-tolerated normally through intravenous and subcutaneous.
8
The mAbs have several applications including Cancer Immunotherapy,
91,94
Bone Marrow and Organ Transplantation,
95,96
Protein Purification,
97-99
Disease Diagnosis in immunoassay tests including enzyme-linked immune sorbent assay (ELISA), western blotting, immunohistochemistry and immunofluorescence tests.
100,101
Our team has been developed the mAbs against a wide range of antigens including TNF-α,
102
CD20,
103
CD34,
104,105
receptor tyrosine kinase-like orphan receptor proteins,
106
human epidermal growth factor receptor,
107
heat shock protein 70,
108
vascular endothelial growth factor receptor 2,
77,78
human IgG and IgA.
109,110
The mAbs against PCSK9 categorized as anti-lipid drugs. Three monoclonal antibodies have been tested in terms of their efficacy and protection including Praluent (Alirocumab; REGN 727) (Evolocumab; AMBG 1451) and Bococizumab (RN 316) Alirocumab and Evolocumab, which are the main suppressor of PCSK9 determined by the FDA and EU medical researchers.
77,78,82
On 24th of July 2015, the FDA confirmed the use of Alirocumab for diet and maximally tolerated statin therapy in adults with HeFH or patients suffering from current medical ASCVD accompanied by coronary heart attack or stroke who should undergo excessive LDLC reduction. Likewise, on the 27th of August 2015, the FDA verified the use of Evolocumab for diet and maximally tolerable statin remedy in adults with HeFH and HoFH or patients diagnosed for medical ASCVD inclusive of coronary heart attack or stroke who should undergo excessive LDLC reduction.
Bococizumab, which was developed by Pfizer, was not confirmed by the FDA. It underwent through phase two trial.
111
Suppression of PCSK9 can be investigated based on some of the finished scientific trials such as the mAbs method as the main objective for decreasing the level of atherogenic lipoproteins. Monoclonal antibodies are capable of decreasing LDL-C, non-HDL-C and Apo B levels significantly accompanied by desirable effects on Lp(a) and sometimes TG stages.
Studies show that PCSK9 suppressors in humans influence HDL-C and Apo AI degrees minimally.
It has been shown that PCSK9 is greatly influenced by mAbs; it has also been used for statins in hypercholesterolemic patients without or with familial hypercholesterolemia, in patients intolerant to statin remedy and additionally in monotherapy.
1
It is believed that statins are the main options for treating heightened levels of LDLC. They reduce LDLC by suppressing the enzyme that is responsible for the synthesis of cholesterol in the liver. Likewise, statins increase the expression of LDLR (as well as of PCSK9). It is believed that statin is able to decrease LDLC by 30–40% (based on the dose of statin). Nevertheless, the absolute value of LDL-C is seldom lower that <60 mg/dL. Monoclonal antibodies suppress PCSK9 and stop the destruction of LDL(R). They can decrease LDLC to 25–40 mg/dL (50%–75% reduction) and outperform statins and ezetimibe in terms of strength, and power.
111
Recent findings show that a thoroughly completely human mAb focused on PCSK9 is probably able to decrease concentrations of atherogenic lipoproteins significantly accompanied by a considerable reduction in LDLC, Apo B, non-HDL-C and besides Lp (a) concentrations.
Up to now, the efficacy of statins has been verified in hypercholesterolaemic patients with and without FH, in patients intolerant to statin treatment and in monotherapy (Figure 4). It was reported that patients with statin intolerance are influenced by PCSK9 suppressor and ezetimibe. The ability of on-goal results of PCSK9 suppressors has not been specified by modern records. It is predictable that the effects of mAbs on PCSK9 will have fewer side effects in comparison to maximum statin doses. The brief-time (12-week) protection problems have not been specified from phase II trials and the mAbs tested so far were well tolerated in comparison with the moderate injection-site reactions.
6
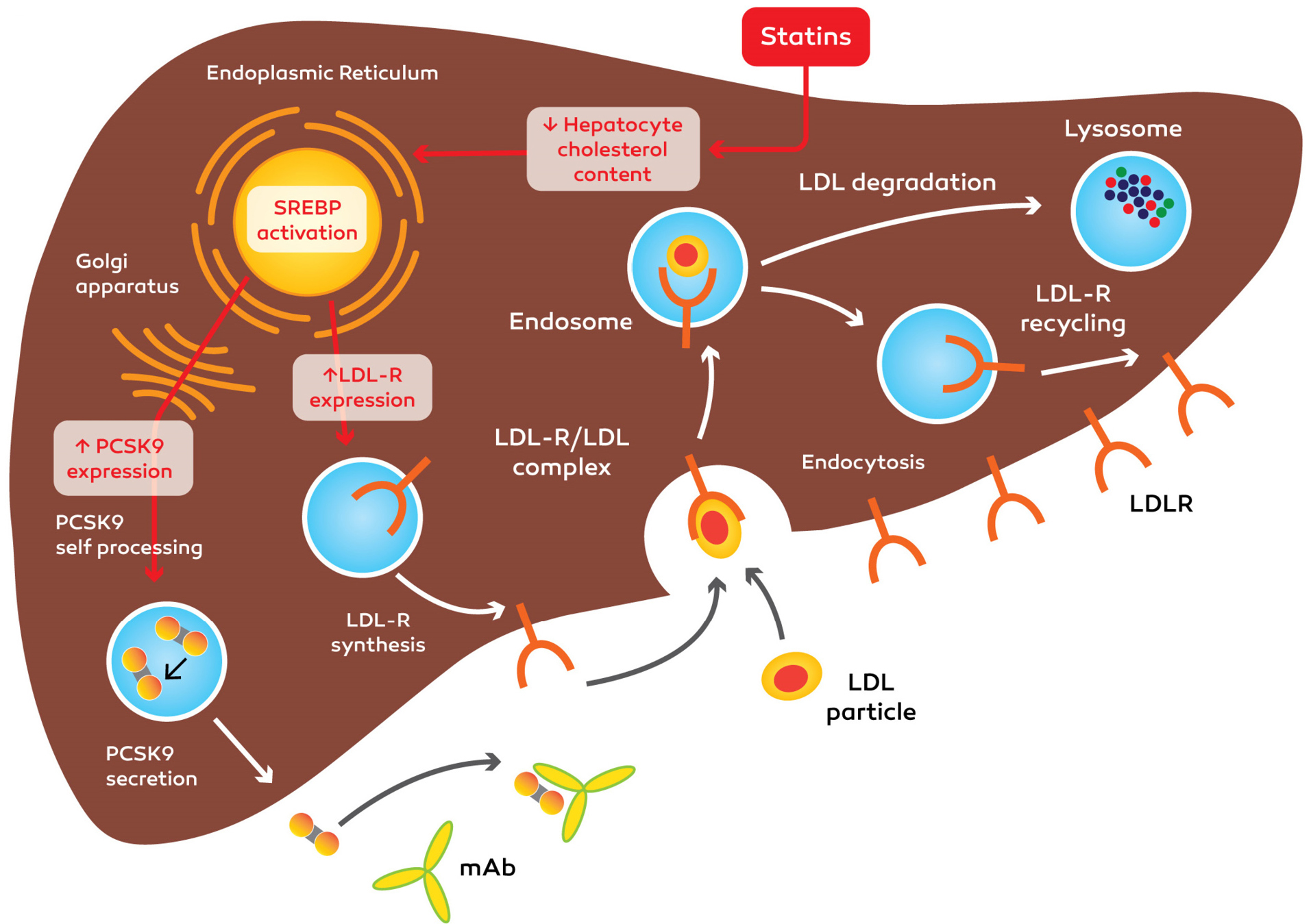
Figure 4.
Mechanism action of anti-PCSK9 monoclonal antibody ( mAb ) and statins. Anti-PCSK9 mAb prevents binding of PCSK9 to the LDLR/LDL complex. PCSK9: Proprotein convertase, subtilisin/kexin type 9, LDL: low-density lipoprotein and LDLR: low-density lipoprotein cholesterol receptor.
.
Mechanism action of anti-PCSK9 monoclonal antibody ( mAb ) and statins. Anti-PCSK9 mAb prevents binding of PCSK9 to the LDLR/LDL complex. PCSK9: Proprotein convertase, subtilisin/kexin type 9, LDL: low-density lipoprotein and LDLR: low-density lipoprotein cholesterol receptor.
Conclusion
PCSK9 is known to be the main player in the metabolism of LDL, particularly when it boosts the degradation of LDLR in the liver. The reduced number of cardiovascular diseases in patients diagnosed with PCSK9 LOF mutations justifies the development of PCSK9 suppressors. It is believed that PCSK9 suppression is a promising objective for treatment. The main suppressers of PCSK9 are monoclonal antibodies that impede the destruction of LDL(R) accompanied by a significant decrease of LDL c in comparison statin and ezetimibe.
Ethical Issues
This work does not contain any studies with animals or human participants conducted by any of the authors.
Conflict of Interest
Authors declare no conflict of interest in this study.
Acknowledgments
This study was financially supported by a grant from Tabriz University of Medical Sciences (58092).
References
- Farnier M. PCSK9: from discovery to therapeutic applications. Arch Cardiovasc Dis 2014; 107(1):58-66. doi: 10.1016/j.acvd.2013.10.007 [Crossref] [ Google Scholar]
- Marduel M, Carrié A, Sassolas A, Devillers M, Carreau V, Di Filippo M. Molecular spectrum of autosomal dominant hypercholesterolemia in France. Hum Mutat 2010; 31(11):E1811-24. doi: 10.1002/humu.21348 [Crossref] [ Google Scholar]
- Mehta A, Mahtta D, Gulati M, Sperling LS, Blumenthal RS, Virani SS. Cardiovascular disease prevention in focus: highlights from the 2019 american heart association scientific sessions. Curr Atheroscler Rep 2020; 22(1):3. doi: 10.1007/s11883-020-0822-6 [Crossref] [ Google Scholar]
-
Musunuru K, Qasim AN, Reilly MP. Genetics and genomics of atherosclerotic cardiovascular disease. In: Pyeritz RE, Korf BR, Grody WW, eds. Emery and Rimoin’s Principles and Practice of Medical Genetics and Genomics. 7th ed. Elsevier; 2020. p. 209-30.
- Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A 2003; 100(3):928-33. doi: 10.1073/pnas.0335507100 [Crossref] [ Google Scholar]
- Wu C, Macleod I, Su AI. BioGPS and MyGeneinfo: organizing online, gene-centric information. Nucleic Acids Res 2013; 41(Database issue):D561-5. doi: 10.1093/nar/gks1114 [Crossref] [ Google Scholar]
- Weinreich M, Frishman WH. Antihyperlipidemic therapies targeting PCSK9. Cardiol Rev 2014; 22(3):140-6. doi: 10.1097/crd.0000000000000014 [Crossref] [ Google Scholar]
- Joseph L, Robinson JG. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition and the future of lipid lowering therapy. Prog Cardiovasc Dis 2015; 58(1):19-31. doi: 10.1016/j.pcad.2015.04.004 [Crossref] [ Google Scholar]
- Reklou A, Katsiki N, Karagiannis A, Athyros V. Effects of lipid lowering drugs on arterial stiffness: one more way to reduce cardiovascular risk?. Curr Vasc Pharmacol 2020; 18(1):38-42. doi: 10.2174/1570161117666190121102323 [Crossref] [ Google Scholar]
- Steffens D, Bramlage P, Scheeff C, Kasner M, Hassanein A, Friebel J. PCSK9 inhibitors and cardiovascular outcomes. Expert Opin Biol Ther 2020; 20(1):35-47. doi: 10.1080/14712598.2020.1677604 [Crossref] [ Google Scholar]
- Everett BM, Smith RJ, Hiatt WR. Reducing LDL with PCSK9 Inhibitors--the clinical benefit of lipid drugs. N Engl J Med 2015; 373(17):1588-91. doi: 10.1056/NEJMp1508120 [Crossref] [ Google Scholar]
-
Doggrell SA, Lynch KA. Is there enough evidence with
evolocumab and alirocumab (antibodies to proprotein
convertase substilisin-kexin type, PCSK9) on cardiovascular
outcomes to use them widely? Evaluation of Sabatine MS,
Giugliano RP, Wiviott SD et al. Efficacy and safety of
evolocumab in reducing lipids and cardiovascular events. N
Engl J Med 2015;372:1500-1509, and Robinson JG, Farnier
M, Krempf M et al. Efficacy and safety of alirocumab in
reducing lipids and cardiovascular events. N Engl J Med
2015;372:1488-99. Expert Opin Biol Ther 2015;15(12):1671-
5. 10.1517/14712598.2015.1093109
- Kazi DS, Moran AE, Coxson PG, Penko J, Ollendorf DA, Pearson SD. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA 2016; 316(7):743-53. doi: 10.1001/jama.2016.11004 [Crossref] [ Google Scholar]
- Sabatine MS. PCSK9 inhibitors: clinical evidence and implementation. Nat Rev Cardiol 2019; 16(3):155-65. doi: 10.1038/s41569-018-0107-8 [Crossref] [ Google Scholar]
- Reeskamp LF, Tromp TR, Hovingh GK. PCSK9 as predictor for recurrent cardiovascular disease in familial hypercholesterolemia. Eur J Prev Cardiol 2019:2047487319886140. doi: 10.1177/2047487319886140 [Crossref]
- Maxwell KN, Soccio RE, Duncan EM, Sehayek E, Breslow JL. Novel putative SREBP and LXR target genes identified by microarray analysis in liver of cholesterol-fed mice. J Lipid Res 2003; 44(11):2109-19. doi: 10.1194/jlr.M300203-JLR200 [Crossref] [ Google Scholar]
- Abifadel M, Elbitar S, El Khoury P, Ghaleb Y, Chémaly M, Moussalli ML. Living the PCSK9 adventure: from the identification of a new gene in familial hypercholesterolemia towards a potential new class of anticholesterol drugs. Curr Atheroscler Rep 2014; 16(9):439. doi: 10.1007/s11883-014-0439-8 [Crossref] [ Google Scholar]
- Urban D, Pöss J, Böhm M, Laufs U. Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J Am Coll Cardiol 2013; 62(16):1401-8. doi: 10.1016/j.jacc.2013.07.056 [Crossref] [ Google Scholar]
- Mayne J, Dewpura T, Raymond A, Bernier L, Cousins M, Ooi TC. Novel loss-of-function PCSK9 variant is associated with low plasma LDL cholesterol in a French-Canadian family and with impaired processing and secretion in cell culture. Clin Chem 2011; 57(10):1415-23. doi: 10.1373/clinchem.2011.165191 [Crossref] [ Google Scholar]
- Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov 2012; 11(5):367-83. doi: 10.1038/nrd3699 [Crossref] [ Google Scholar]
- Seidah NG, Awan Z, Chrétien M, Mbikay M. PCSK9: a key modulator of cardiovascular health. Circ Res 2014; 114(6):1022-36. doi: 10.1161/circresaha.114.301621 [Crossref] [ Google Scholar]
- Kotowski IK, Pertsemlidis A, Luke A, Cooper RS, Vega GL, Cohen JC. A spectrum of PCSK9 alleles contributes to plasma levels of low-density lipoprotein cholesterol. Am J Hum Genet 2006; 78(3):410-22. doi: 10.1086/500615 [Crossref] [ Google Scholar]
- Cicero AF, Tartagni E, Ertek S. Efficacy and safety profile of evolocumab (AMG145), an injectable inhibitor of the proprotein convertase subtilisin/kexin type 9: the available clinical evidence. Expert Opin Biol Ther 2014; 14(6):863-8. doi: 10.1517/14712598.2014.902929 [Crossref] [ Google Scholar]
- Naureckiene S, Ma L, Sreekumar K, Purandare U, Lo CF, Huang Y. Functional characterization of Narc 1, a novel proteinase related to proteinase K. Arch Biochem Biophys 2003; 420(1):55-67. doi: 10.1016/j.abb.2003.09.011 [Crossref] [ Google Scholar]
- Horton JD, Cohen JC, Hobbs HH. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem Sci 2007; 32(2):71-7. doi: 10.1016/j.tibs.2006.12.008 [Crossref] [ Google Scholar]
- Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet 2005; 37(2):161-5. doi: 10.1038/ng1509 [Crossref] [ Google Scholar]
- Fasano T, Cefalù AB, Di Leo E, Noto D, Pollaccia D, Bocchi L. A novel loss of function mutation of PCSK9 gene in white subjects with low-plasma low-density lipoprotein cholesterol. Arterioscler Thromb Vasc Biol 2007; 27(3):677-81. doi: 10.1161/01.ATV.0000255311.26383.2f [Crossref] [ Google Scholar]
- Kwon HJ, Lagace TA, McNutt MC, Horton JD, Deisenhofer J. Molecular basis for LDL receptor recognition by PCSK9. Proc Natl Acad Sci U S A 2008; 105(6):1820-5. doi: 10.1073/pnas.0712064105 [Crossref] [ Google Scholar]
- Cunningham D, Danley DE, Geoghegan KF, Griffor MC, Hawkins JL, Subashi TA. Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat Struct Mol Biol 2007; 14(5):413-9. doi: 10.1038/nsmb1235 [Crossref] [ Google Scholar]
- Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354(12):1264-72. doi: 10.1056/NEJMoa054013 [Crossref] [ Google Scholar]
- Jeong HJ, Lee HS, Kim KS, Kim YK, Yoon D, Park SW. Sterol-dependent regulation of proprotein convertase subtilisin/kexin type 9 expression by sterol-regulatory element binding protein-2. J Lipid Res 2008; 49(2):399-409. doi: 10.1194/jlr.M700443-JLR200 [Crossref] [ Google Scholar]
- Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 1997; 89(3):331-40. doi: 10.1016/s0092-8674(00)80213-5 [Crossref] [ Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 2002; 109(9):1125-31. doi: 10.1172/jci15593 [Crossref] [ Google Scholar]
- Lagace TA. PCSK9 and LDLR degradation: regulatory mechanisms in circulation and in cells. Curr Opin Lipidol 2014; 25(5):387-93. doi: 10.1097/mol.0000000000000114 [Crossref] [ Google Scholar]
- Browning JD, Horton JD. Fasting reduces plasma proprotein convertase, subtilisin/kexin type 9 and cholesterol biosynthesis in humans. J Lipid Res 2010; 51(11):3359-63. doi: 10.1194/jlr.P009860 [Crossref] [ Google Scholar]
- Persson L, Cao G, Ståhle L, Sjöberg BG, Troutt JS, Konrad RJ. Circulating proprotein convertase subtilisin kexin type 9 has a diurnal rhythm synchronous with cholesterol synthesis and is reduced by fasting in humans. Arterioscler Thromb Vasc Biol 2010; 30(12):2666-72. doi: 10.1161/atvbaha.110.214130 [Crossref] [ Google Scholar]
- Richard C, Couture P, Desroches S, Benjannet S, Seidah NG, Lichtenstein AH. Effect of the Mediterranean diet with and without weight loss on surrogate markers of cholesterol homeostasis in men with the metabolic syndrome. Br J Nutr 2012; 107(5):705-11. doi: 10.1017/s0007114511003436 [Crossref] [ Google Scholar]
- Rodríguez-Pérez C, Ramprasath VR, Pu S, Sabra A, Quirantes-Piné R, Segura-Carretero A. Docosahexaenoic acid attenuates cardiovascular risk factors via a decline in proprotein convertase subtilisin/kexin type 9 (PCSK9) plasma levels. Lipids 2016; 51(1):75-83. doi: 10.1007/s11745-015-4099-4 [Crossref] [ Google Scholar]
- Bjermo H, Iggman D, Kullberg J, Dahlman I, Johansson L, Persson L. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr 2012; 95(5):1003-12. doi: 10.3945/ajcn.111.030114 [Crossref] [ Google Scholar]
- Zaid A, Roubtsova A, Essalmani R, Marcinkiewicz J, Chamberland A, Hamelin J. Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology 2008; 48(2):646-54. doi: 10.1002/hep.22354 [Crossref] [ Google Scholar]
- Benjannet S, Rhainds D, Essalmani R, Mayne J, Wickham L, Jin W. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem 2004; 279(47):48865-75. doi: 10.1074/jbc.M409699200 [Crossref] [ Google Scholar]
- Cariou B, Le May C, Costet P. Clinical aspects of PCSK9. Atherosclerosis 2011; 216(2):258-65. doi: 10.1016/j.atherosclerosis.2011.04.018 [Crossref] [ Google Scholar]
- Tibolla G, Norata GD, Artali R, Meneghetti F, Catapano AL. Proprotein convertase subtilisin/kexin type 9 (PCSK9): from structure-function relation to therapeutic inhibition. Nutr Metab Cardiovasc Dis 2011; 21(11):835-43. doi: 10.1016/j.numecd.2011.06.002 [Crossref] [ Google Scholar]
- Schmidt RJ, Beyer TP, Bensch WR, Qian YW, Lin A, Kowala M. Secreted proprotein convertase subtilisin/kexin type 9 reduces both hepatic and extrahepatic low-density lipoprotein receptors in vivo. Biochem Biophys Res Commun 2008; 370(4):634-40. doi: 10.1016/j.bbrc.2008.04.004 [Crossref] [ Google Scholar]
- Poirier S, Mayer G, Benjannet S, Bergeron E, Marcinkiewicz J, Nassoury N. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J Biol Chem 2008; 283(4):2363-72. doi: 10.1074/jbc.M708098200 [Crossref] [ Google Scholar]
- Kathiresan S. A PCSK9 missense variant associated with a reduced risk of early-onset myocardial infarction. N Engl J Med 2008; 358(21):2299-300. doi: 10.1056/NEJMc0707445 [Crossref] [ Google Scholar]
- Lagace TA, Curtis DE, Garuti R, McNutt MC, Park SW, Prather HB. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J Clin Invest 2006; 116(11):2995-3005. doi: 10.1172/jci29383 [Crossref] [ Google Scholar]
- Lambert G, Charlton F, Rye KA, Piper DE. Molecular basis of PCSK9 function. Atherosclerosis 2009; 203(1):1-7. doi: 10.1016/j.atherosclerosis.2008.06.010 [Crossref] [ Google Scholar]
- Qian YW, Schmidt RJ, Zhang Y, Chu S, Lin A, Wang H. Secreted PCSK9 downregulates low density lipoprotein receptor through receptor-mediated endocytosis. J Lipid Res 2007; 48(7):1488-98. doi: 10.1194/jlr.M700071-JLR200 [Crossref] [ Google Scholar]
- Fisher TS, Lo Surdo P, Pandit S, Mattu M, Santoro JC, Wisniewski D. Effects of pH and low density lipoprotein (LDL) on PCSK9-dependent LDL receptor regulation. J Biol Chem 2007; 282(28):20502-12. doi: 10.1074/jbc.M701634200 [Crossref] [ Google Scholar]
- Benjannet S, Saavedra YG, Hamelin J, Asselin MC, Essalmani R, Pasquato A. Effects of the prosegment and pH on the activity of PCSK9: evidence for additional processing events. J Biol Chem 2010; 285(52):40965-78. doi: 10.1074/jbc.M110.154815 [Crossref] [ Google Scholar]
- Lo Surdo P, Bottomley MJ, Calzetta A, Settembre EC, Cirillo A, Pandit S. Mechanistic implications for LDL receptor degradation from the PCSK9/LDLR structure at neutral pH. EMBO Rep 2011; 12(12):1300-5. doi: 10.1038/embor.2011.205 [Crossref] [ Google Scholar]
- Yamamoto T, Lu C, Ryan RO. A two-step binding model of PCSK9 interaction with the low density lipoprotein receptor. J Biol Chem 2011; 286(7):5464-70. doi: 10.1074/jbc.M110.199042 [Crossref] [ Google Scholar]
- Zhang DW, Garuti R, Tang WJ, Cohen JC, Hobbs HH. Structural requirements for PCSK9-mediated degradation of the low-density lipoprotein receptor. Proc Natl Acad Sci U S A 2008; 105(35):13045-50. doi: 10.1073/pnas.0806312105 [Crossref] [ Google Scholar]
- Monami M, Sesti G, Mannucci E. PCSK9 inhibitor therapy: a systematic review and meta-analysis of metabolic and cardiovascular outcomes in patients with diabetes. Diabetes Obes Metab 2019; 21(4):903-8. doi: 10.1111/dom.13599 [Crossref] [ Google Scholar]
- Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003; 34(2):154-6. doi: 10.1038/ng1161 [Crossref] [ Google Scholar]
- Dubuc G, Chamberland A, Wassef H, Davignon J, Seidah NG, Bernier L. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 2004; 24(8):1454-9. doi: 10.1161/01.atv.0000134621.14315.43 [Crossref] [ Google Scholar]
- Norata GD, Tavori H, Pirillo A, Fazio S, Catapano AL. Biology of proprotein convertase subtilisin kexin 9: beyond low-density lipoprotein cholesterol lowering. Cardiovasc Res 2016; 112(1):429-42. doi: 10.1093/cvr/cvw194 [Crossref] [ Google Scholar]
- Giunzioni I, Tavori H, Covarrubias R, Major AS, Ding L, Zhang Y. Local effects of human PCSK9 on the atherosclerotic lesion. J Pathol 2016; 238(1):52-62. doi: 10.1002/path.4630 [Crossref] [ Google Scholar]
- Wu CY, Tang ZH, Jiang L, Li XF, Jiang ZS, Liu LS. PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL via Bcl/Bax-caspase9-caspase3 pathway. Mol Cell Biochem 2012; 359(1-2):347-58. doi: 10.1007/s11010-011-1028-6 [Crossref] [ Google Scholar]
- Peng W, Qiang F, Peng W, Qian Z, Ke Z, Yi L. Therapeutic efficacy of PCSK9 monoclonal antibodies in statin-nonresponsive patients with hypercholesterolemia and dyslipidemia: A systematic review and meta-analysis. Int J Cardiol 2016; 222:119-29. doi: 10.1016/j.ijcard.2016.07.239 [Crossref] [ Google Scholar]
- Colivicchi F, Massimo Gulizia M, Arca M, Luigi Temporelli P, Gonzini L, Venturelli V. Lipid lowering treatment and eligibility for PCSK9 inhibition in post-myocardial infarction patients in Italy: insights from two contemporary Nationwide registries. Cardiovasc Ther 2020; 2020:3856242. doi: 10.1155/2020/3856242 [Crossref] [ Google Scholar]
- Cariou B, Dijk W. EGF-A peptides: a promising strategy for PCSK9 inhibition. Atherosclerosis 2020; 292:204-6. doi: 10.1016/j.atherosclerosis.2019.11.010 [Crossref] [ Google Scholar]
- Ferri N, Tibolla G, Pirillo A, Cipollone F, Mezzetti A, Pacia S. Proprotein convertase subtilisin kexin type 9 (PCSK9) secreted by cultured smooth muscle cells reduces macrophages LDLR levels. Atherosclerosis 2012; 220(2):381-6. doi: 10.1016/j.atherosclerosis.2011.11.026 [Crossref] [ Google Scholar]
- Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 2012; 380(9841):565-71. doi: 10.1016/s0140-6736(12)61190-8 [Crossref] [ Google Scholar]
- Robinson JG. Nonstatins and Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitors: Role in Non-Familial Hypercholesterolemia. Prog Cardiovasc Dis 2016; 59(2):165-71. doi: 10.1016/j.pcad.2016.07.011 [Crossref] [ Google Scholar]
- Sharotri V, Collier DM, Olson DR, Zhou R, Snyder PM. Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexin type 9 (PCSK9). J Biol Chem 2012; 287(23):19266-74. doi: 10.1074/jbc.M112.363382 [Crossref] [ Google Scholar]
- Norata GD, Pirillo A, Ammirati E, Catapano AL. Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis 2012; 220(1):11-21. doi: 10.1016/j.atherosclerosis.2011.06.045 [Crossref] [ Google Scholar]
- Diedrich G.
. How does hepatitis C virus enter cells? FEBS J 2006; 273(17):3871-85. doi: 10.1111/j.1742-4658.2006.05379.x [Crossref] [ Google Scholar]
- Rousselet E, Marcinkiewicz J, Kriz J, Zhou A, Hatten ME, Prat A. PCSK9 reduces the protein levels of the LDL receptor in mouse brain during development and after ischemic stroke. J Lipid Res 2011; 52(7):1383-91. doi: 10.1194/jlr.M014118 [Crossref] [ Google Scholar]
- Momtazi-Borojeni AA, Sabouri-Rad S, Gotto AM, Pirro M, Banach M, Awan Z. PCSK9 and inflammation: a review of experimental and clinical evidence. Eur Heart J Cardiovasc Pharmacother 2019; 5(4):237-45. doi: 10.1093/ehjcvp/pvz022 [Crossref] [ Google Scholar]
- Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A 2006; 103(27):10340-5. doi: 10.1073/pnas.0604260103 [Crossref] [ Google Scholar]
- Hiltunen TP, Ylä-Herttuala S. Expression of lipoprotein receptors in atherosclerotic lesions. Atherosclerosis 1998; 137 Suppl:S81-8. doi: 10.1016/s0021-9150(97)00307-9 [Crossref] [ Google Scholar]
- Linton MF, Babaev VR, Gleaves LA, Fazio S. A direct role for the macrophage low density lipoprotein receptor in atherosclerotic lesion formation. J Biol Chem 1999; 274(27):19204-10. doi: 10.1074/jbc.274.27.19204 [Crossref] [ Google Scholar]
- Ding Z, Liu S, Wang X, Deng X, Fan Y, Sun C. Hemodynamic shear stress via ROS modulates PCSK9 expression in human vascular endothelial and smooth muscle cells and along the mouse aorta. Antioxid Redox Signal 2015; 22(9):760-71. doi: 10.1089/ars.2014.6054 [Crossref] [ Google Scholar]
- Herijgers N, Van Eck M, Groot PH, Hoogerbrugge PM, Van Berkel TJ. Low density lipoprotein receptor of macrophages facilitates atherosclerotic lesion formation in C57Bl/6 mice. Arterioscler Thromb Vasc Biol 2000; 20(8):1961-7. doi: 10.1161/01.atv.20.8.1961 [Crossref] [ Google Scholar]
- Kordi S, Rahmati-Yamchi M, Asghari Vostakolaei M, Barzegari A, Abdolalizadeh J. Purification of a novel anti-vegfr2 single chain antibody fragment and evaluation of binding affinity by surface plasmon resonance. Adv Pharm Bull 2019; 9(1):64-9. doi: 10.15171/apb.2019.008 [Crossref] [ Google Scholar]
- Kordi S, Rahmati-Yamchi M, Asghari Vostakolaei M, Etemadie A, Barzegari A, Abdolalizadeh J. Isolation of a novel anti-kdr3 single-chain variable fragment antibody from a phage display library. Iran J Allergy Asthma Immunol 2019; 18(3):289-99. doi: 10.18502/ijaai.v18i3.1122 [Crossref] [ Google Scholar]
- Navar AM, Mulder HM, Wojdyla DM, Peterson ED. Have the major cardiovascular outcomes trials impacted payer approval rates for PCSK9 inhibitors?. Circ Cardiovasc Qual Outcomes 2020; 13(1):e006019. doi: 10.1161/circoutcomes.119.006019 [Crossref] [ Google Scholar]
- Dadu RT, Ballantyne CM. Lipid lowering with PCSK9 inhibitors. Nat Rev Cardiol 2014; 11(10):563-75. doi: 10.1038/nrcardio.2014.84 [Crossref] [ Google Scholar]
- Wilson A. PCSK9 inhibitors, the most significant advance in lipid lowering therapy since statins? a literature review. Osteopathic Family Physician 2019; 11(4):24-30. [ Google Scholar]
- Chen B, Shi X, Cui Y, Hou A, Zhao P. A review of PCSK9 inhibitors and their effects on cardiovascular diseases. Curr Top Med Chem 2019; 19(20):1790-817. doi: 10.2174/1568026619666190809094203 [Crossref] [ Google Scholar]
- Gupta N, Fisker N, Asselin MC, Lindholm M, Rosenbohm C, Ørum H. A locked nucleic acid antisense oligonucleotide (LNA) silences PCSK9 and enhances LDLR expression in vitro and in vivo. PLoS One 2010; 5(5):e10682. doi: 10.1371/journal.pone.0010682 [Crossref] [ Google Scholar]
- Graham MJ, Lemonidis KM, Whipple CP, Subramaniam A, Monia BP, Crooke ST. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J Lipid Res 2007; 48(4):763-7. doi: 10.1194/jlr.C600025-JLR200 [Crossref] [ Google Scholar]
- Lindholm MW, Elmén J, Fisker N, Hansen HF, Persson R, Møller MR. PCSK9 LNA antisense oligonucleotides induce sustained reduction of LDL cholesterol in nonhuman primates. Mol Ther 2012; 20(2):376-81. doi: 10.1038/mt.2011.260 [Crossref] [ Google Scholar]
- Brautbar A, Ballantyne CM. Pharmacological strategies for lowering LDL cholesterol: statins and beyond. Nat Rev Cardiol 2011; 8(5):253-65. doi: 10.1038/nrcardio.2011.2 [Crossref] [ Google Scholar]
- Eivazy P, Atyabi F, Jadidi-Niaragh F, Aghebati Maleki L, Miahipour A, Abdolalizadeh J. The impact of the codelivery of drug-siRNA by trimethyl chitosan nanoparticles on the efficacy of chemotherapy for metastatic breast cancer cell line (MDA-MB-231). Artif Cells Nanomed Biotechnol 2017; 45(5):889-96. doi: 10.1080/21691401.2016.1185727 [Crossref] [ Google Scholar]
- Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci U S A 2008; 105(33):11915-20. doi: 10.1073/pnas.0805434105 [Crossref] [ Google Scholar]
- Hooper AJ, Burnett JR. Anti-PCSK9 therapies for the treatment of hypercholesterolemia. Expert Opin Biol Ther 2013; 13(3):429-35. doi: 10.1517/14712598.2012.748743 [Crossref] [ Google Scholar]
- Catapano AL, Papadopoulos N. The safety of therapeutic monoclonal antibodies: implications for cardiovascular disease and targeting the PCSK9 pathway. Atherosclerosis 2013; 228(1):18-28. doi: 10.1016/j.atherosclerosis.2013.01.044 [Crossref] [ Google Scholar]
- Majidi J, Barar J, Baradaran B, Abdolalizadeh J, Omidi Y. Target therapy of cancer: implementation of monoclonal antibodies and nanobodies. Hum Antibodies 2009; 18(3):81-100. doi: 10.3233/hab-2009-0204 [Crossref] [ Google Scholar]
- Abdolalizadeh J, Nouri M, Zolbanin JM, Baradaran B, Barzegari A, Omidi Y. Downstream characterization of anti-TNF-α single chain variable fragment antibodies. Hum Antibodies 2012; 21(1-2):41-8. doi: 10.3233/hab-2012-0260 [Crossref] [ Google Scholar]
- Abdolalizadeh J, Nouri M, Zolbanin JM, Barzegari A, Baradaran B, Barar J. Targeting cytokines: production and characterization of anti-TNF-α scFvs by phage display technology. Curr Pharm Des 2013; 19(15):2839-47. doi: 10.2174/1381612811319150019 [Crossref] [ Google Scholar]
- Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 2010; 10(5):317-27. doi: 10.1038/nri2744 [Crossref] [ Google Scholar]
- Lonial S, Durie B, Palumbo A, San-Miguel J. Monoclonal antibodies in the treatment of multiple myeloma: current status and future perspectives. Leukemia 2016; 30(3):526-35. doi: 10.1038/leu.2015.223 [Crossref] [ Google Scholar]
- Ishida JH, Patel A, Mehta AK, Gatault P, McBride JM, Burgess T. Phase 2 randomized, double-blind, placebo-controlled trial of RG7667, a combination monoclonal antibody, for prevention of cytomegalovirus infection in high-risk kidney transplant recipients. Antimicrob Agents Chemother 2017; 61(2). doi: 10.1128/aac.01794-16 [Crossref]
- Raoufinia R, Mota A, Nozari S, Aghebati Maleki L, Balkani S, Abdolalizadeh J. A methodological approach for purification and characterization of human serum albumin. J Immunoassay Immunochem 2016; 37(6):623-35. doi: 10.1080/15321819.2016.1184163 [Crossref] [ Google Scholar]
- Balkani S, Shamekhi S, Raoufinia R, Parvan R, Abdolalizadeh J. Purification and characterization of bovine serum albumin using chromatographic method. Adv Pharm Bull 2016; 6(4):651-4. doi: 10.15171/apb.2016.080 [Crossref] [ Google Scholar]
- Raoufinia R, Balkani S, Keyhanvar N, Mahdavipor B, Abdolalizadeh J. Human albumin purification: a modified and concise method. J Immunoassay Immunochem 2018; 39(6):687-95. doi: 10.1080/15321819.2018.1531884 [Crossref] [ Google Scholar]
- Kong D, Wu X, Li Y, Liu L, Song S, Zheng Q. Ultrasensitive and eco-friendly immunoassays based monoclonal antibody for detection of deoxynivalenol in cereal and feed samples. Food Chem 2019; 270:130-7. doi: 10.1016/j.foodchem.2018.07.075 [Crossref] [ Google Scholar]
- Esteve-Turrillas FA, Mercader JV, Agulló C, Abad-Somovilla A, Abad-Fuentes A. Highly sensitive monoclonal antibody-based immunoassays for boscalid analysis in strawberries. Food Chem 2018; 267:2-9. doi: 10.1016/j.foodchem.2017.06.013 [Crossref] [ Google Scholar]
- Abdolalizadeh J, Majidi Zolbanin J, Nouri M, Baradaran B, Barzegari A, Omidi Y. Isolation of anti-tumor necrosis factor-alpha (TNF-α) scFvs antibody from phage antibody library. Journal of Babol University of Medical Sciences 2013; 15(3):79-87. [ Google Scholar]
- Sineh Sepehr K, Baradaran B, Majidi J, Abdolalizadeh J, Aghebati L, Zare Shahneh F. Mass-production and characterization of anti-CD20 monoclonal antibody in peritoneum of Balb/c mice. Adv Pharm Bull 2013; 3(1):109-13. doi: 10.5681/apb.2013.018 [Crossref] [ Google Scholar]
- Aghebati Maleki L, Majidi J, Baradaran B, Abdolalizadeh J, Akbari AM. Production and characterization of murine monoclonal antibody against synthetic peptide of CD34. Hum Antibodies 2013; 22(1-2):1-8. doi: 10.3233/hab-130265 [Crossref] [ Google Scholar]
- Aghebati Maleki L, Majidi J, Baradaran B, Abdolalizadeh J, Kazemi T, Aghebati Maleki A. Large scale generation and characterization of anti-human CD34 monoclonal antibody in ascetic fluid of Balb/c mice. Adv Pharm Bull 2013; 3(1):211-6. doi: 10.5681/apb.2013.035 [Crossref] [ Google Scholar]
- Aghebati Maleki L, Younesi V, Baradaran B, Abdolalizadeh J, Motallebnezhad M, Nickho H. Antiproliferative and apoptotic effects of novel anti-ROR1 single-chain antibodies in hematological malignancies. SLAS Discov 2017; 22(4):408-17. doi: 10.1177/2472555216689659 [Crossref] [ Google Scholar]
- Baradaran B, Hosseini AZ, Majidi J, Farajnia S, Barar J, Saraf ZH. Development and characterization of monoclonal antibodies against human epidermal growth factor receptor in Balb/c mice. Hum Antibodies 2009; 18(1-2):11-6. doi: 10.3233/hab-2009-0195 [Crossref] [ Google Scholar]
- Asghari Vostakolaei M, Molavi O, Hejazi MS, Kordi S, Rahmati S, Barzegari A. Isolation and characterization of a novel scFv antibody fragments specific for Hsp70 as a tumor biomarker. J Cell Biochem 2019; 120(9):14711-24. doi: 10.1002/jcb.28732 [Crossref] [ Google Scholar]
- Aghebati Maleki L, Baradaran B, Abdolalizadeh J, Ezzatifar F, Majidi J. A unique report: development of super anti-human IgG monoclone with optical density over than 3. Adv Pharm Bull 2013; 3(2):333-7. doi: 10.5681/apb.2013.054 [Crossref] [ Google Scholar]
- Ezzatifar F, Majidi J, Baradaran B, Aghebati Maleki L, Abdolalizadeh J, Yousefi M. Large scale generation and characterization of anti-human IgA monoclonal antibody in ascitic fluid of Balb/c mice. Adv Pharm Bull 2015; 5(1):97-102. doi: 10.5681/apb.2015.013 [Crossref] [ Google Scholar]
- Gupta S. Development of proprotein convertase subtilisin/kexin type 9 inhibitors and the clinical potential of monoclonal antibodies in the management of lipid disorders. Vasc Health Risk Manag 2016; 12:421-33. doi: 10.2147/vhrm.s83719 [Crossref] [ Google Scholar]