Advanced pharmaceutical bulletin. 11(1):1-5.
doi: 10.34172/apb.2021.007
Editorial
Postbiotics as Promising Tools for Cancer Adjuvant Therapy
Aziz Homayouni Rad 1  , Leili Aghebati Maleki 2
, Leili Aghebati Maleki 2  , Hossein Samadi Kafil 3
, Hossein Samadi Kafil 3  , Hamideh Fathi Zavoshti 4
, Hamideh Fathi Zavoshti 4  , Amin Abbasi 5, *
, Amin Abbasi 5, * 
Author information:
1Nutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
2Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
3Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
4Department of Food Hygiene and Aquatics, Faculty of Veterinary Medicine, Tabriz University, Tabriz, Iran.
5Student’s Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran.
Abstract
As many investigations have reported, there is a complicated relation between fermented foods, lactic acid bacteria (LAB), and human health. It seems that bioactive components such as prebiotics, probiotics, and postbiotics are key mediators of the complex and direct association between these factors. LAB activity in the matrix of fermented foods and improving their growth by prebiotic compounds ultimately results in the production of bioactive molecules (postbiotics), which possess specific biological and physiological properties. The term "postbiotics" refers to a complex of biological micro- and macromolecules, if consumed in adequate amounts, provides the host with different health-promoting effects. Different reports have suggested that postbiotics possess the ability to moderate the effectiveness of cancer treatment and reduce the side-effects of conventional therapies in cancer patients due to their anti-proliferative, anti-inflammatory and anti-cancer properties. Consequently, postbiotics, for their unique characteristics, have gained great scientific attention and are considered as a novel approach for adjuvant therapy in patients with cancer.
Keywords: Cancer, Postbiotic, Probiotic, Health, Treatment
Copyright and License Information
© 2021 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Lactic acid bacteria (LAB) are an integral part of foods and have been present in the matrix of fermented foods as safe and functional compounds whose consumption is directly linked to health.1 LAB in the matrix of fermented foods, utilizes appropriate substrates (prebiotics) and subsequently, generates a wide range of bioactive substances such as organic acids and bacteriocins, which possess key biological and physiological properties.2
The main pro-health effects of LAB and its derived bioactive metabolites include (a) improving gastrointestinal disorders such as inflammatory bowel diseases (IBD), (b) improving urogenital disorders, (c) improving lactose metabolism, (d) antidiabetic activities, (e) immunomodulation properties, (f) anticarcinogenic activities, (g) improving lactose metabolism, and (h) promoting cancer therapies.3-5 In this regard, a body of epidemiological pieces of evidence confirms the positive effects of LAB and its derived metabolites on the colon, bladder, liver, breast, and gastric cancers.6
The anticarcinogenic effect of LAB is mediated through different mechanisms including gut microbiota modification and dominance of beneficial microbiota, improvement of the immune system function/response, and possessing significant antioxidative and anti-proliferative properties.7 Moreover, other clinical health merits of LAB and its metabolites associated with the cancer therapies include the establishment of eubiosis conditions in the gut ecosystem, contribution to the recovery (after cancer surgery) and decreasing hospitalization period,8 eliminating superficial incisional surgical position infection,9 and preventing some side-effects of conventional cancer therapies (chemotherapy and antibiotic-induced diarrhea).10,11 Several factors such as virulence factor and antibiotic resistance genes transfer to host’s (humans, animals) pathogenic microbes,12 stimulating acute inflammatory responses13 and significant difference between the declared levels with the actual amount of live probiotic cells in commercial products14 have made great interests in probiotic health effects mediated by beneficial microbial metabolites which are characterized as postbiotics.
The term of postbiotics refers to a complex of micro- and macromolecules such as inactivated microbial cells (non-viable cells), cell fractions (muropeptides, teichoic acids, endo- and exopolysaccharides, and surface-layer proteins) or cell metabolites (short-chain fatty acids, SCFAs), organic acids, bacteriocins, and enzymes) that are naturally made by live probiotic cells in fermentation process and/or made synthetically by laboratory procedures. If they are consumed in sufficient quantities, they can leave different physiological health-promoting effects on the consumer.15,16 On the other hand, postbiotics are known as multi-functional agents owning anti-microbial, anti-inflammatory, anti-oxidant, immunomodulation, anti-hypertensive, anti-diabetic, anti-obesogenic, and anti-proliferative activities, which are attributed to the presence of surface and intracellular bioactive molecules.17-20 In this regard, Gao et al21 evaluated the biological role of gut beneficial microbiota-derived postbiotics in preserving gut health and function. They established that postbiotics can act as their parent live cells and can be considered as a safe alternative to live probiotic cells.
The application of postbiotics as an adjunct for cancer prevention and treatment was strongly associated with the function/response of the host immune system. Different investigations confirmed the potential role of postbiotics in the prevention and treatment of cancer, particularly in gastrointestinal (GI) cancer cases22,23 (Table 1). In this regard, Motevaseli et al33 reported the selectively anti-proliferative effects of various postbiotics (whole inactivated cell, cell-wall, and cytoplasmic extracts, culture supernatants) derived from vaginal-origin Lactobacillus crispatus and L. gasseri on normal and cervical tumor cells. The most highlighted feature of postbiotics is their ability to distinguish between normal and cancer cells, which modulates the proliferation of normal cells but suppresses angiogenesis and drives apoptosis in cancerous cells.34 Ou et al35 investigated the effect of diet on colon cancer risk. They studied the gut microbiota through their metabolites in humans with high (African Americans) and low risk (rural native Africans) of colon cancer. They found notable associations between reduced generations of SCFAs, increased secondary bile acid metabolites, and increased risk of colon cancer. Their findings confirmed that colon cancer risk can be affected by the balance between the microbial generation of potentially health-promoting and carcinogenic metabolites. Besides, the postbiotic of exopolysaccharide (EPS) derived from Lactobacillus spp is reported to exert significant anti-proliferative activities against colonic carcinoma cell lines.36 The cell-free supernatants (CFS) derived from human breast milk L. casei and L. paracasei have anti-carcinogenic effects against cervical cancer cell lines.37 Shyu et al.38 evaluated the cytotoxic effects of Lactobacillus spp-derived CFS (isolated from dairy products) on colon cancer cells (HCT116 and HT-29), leukemia cells (THP-1), and normal human dermal fibroblasts (HDFn) with PrestoBlue. They established that all studied probiotic CFSs have cytotoxic effects on HCT-116 and HT-29 colon cancer cell lines. They also considerably up-regulated the expression of early apoptotic-promoting cfos, cjun and down-regulated the proinflammatory cytokine IL-β, TNF- α genes in treated cancer cells with CFSs. The outcomes clearly supported the potential application of postbiotics in the modulation of inflammatory responses (as a precursor to carcinogenesis) and anticancer therapy. Further mechanisms involved in the anti-cancer activities of postbiotics include regulating immune responses, reducing cell viability, binding to mutagenic and carcinogenic constituents, triggering pro-apoptotic cell death pathways, reducing microbial translocation, increasing apoptosis and necrosis, increasing tumor cell death via autophagy, anti-proliferative activity against cancer cells, decreasing metalloproteinase-9 activity, and inhibiting cancer invasion.39-42 Therefore, it is clear that gut beneficial microbes-derived postbiotic components have significant anti-proliferative properties due to their potentiality in regulating cell cycle, stimulating differentiation, and up-regulating the pro-apoptotic pathways in different cancer cells. These biological properties are mainly based on the phenotypic mood of cells, the parent microbial cell strains, methods applied to the preparation of postbiotics, and the presence of bioactive micro- and macromolecules43 (Figure 1).
Table 1.
Postbiotics and their potential anti-cancer activities
|
Bacteria
|
Inactivation method
|
Postbiotic
|
Cell line(s)
|
Main mechanism(s)
|
Reference
|
|
Lactobacillus rhamnosus SHA111, SHA112, and SHA113 |
S* |
CFS |
HeLa |
Induction of apoptosis by up-regulation of BAD, BAX, Caspase-3, Caspase-8, Caspase-9, and down-regulation of BCL-2 genes |
24
|
|
Lactobacillus fermentum sp |
S |
CFS |
HCT-116, HT-29 |
Induction of apoptosis by up-regulation of Caspase-3, Bax, Bak, Noxa, and Bid mRNA expressions |
25
|
|
Lactobacillus casei ATCC334 |
S |
CFS, Ferrichrome |
SW-620 |
Induction of apoptosis by the activation of c-jun N-terminal kinase |
26
|
|
Bifidobacterium spp |
S |
CFS |
SW-742 |
Decrease cell proliferation |
27
|
|
Lactobacillus plantarum GD2, Lactobacillus rhamnosus E9, Lactobacillus brevis LB63, and Lactobacillus delbrueckii ssp. bulgaricus B3 |
TT** |
EPS |
HT-29 |
Induction of apoptosis via increasing the expression of Bax, Caspase-3, Caspase-9 and decreasing the expression of Bcl-2 and Survivin |
28
|
|
Faecalibacterium prausnitzii A2–165 |
S |
CFS, EV |
A549 |
Up-regulate anti-inflammatory cytokines (IL-10, TGF-β2 and IL-1Ra) and down-regulate some of the important pro-inflammatory cytokines such as IL-6, TNF-𝛼 and TNF-β |
29
|
|
Lactobacillus paracasei sp |
S |
CWP |
Caco-2 |
Decrease cell proliferation
Induction of apoptosis |
30
|
|
Lactobacillus plantarum sp |
S |
CFS |
MCF-7 |
Induction of apoptosis via increasing the expression of DECAY, FADD, RAS64B apoptotic genes and decreasing the expression of BCL-2 and BUFFY genes |
31
|
|
Clostridium butyricum sp |
S |
SCFA |
HCT-116, Caco-2, and HCT-8 |
Suppresses the Wnt/β-catenin signaling pathway and modulate the gut microbiota composition |
32
|
CFS: cell-free supernatant, EPS: exopolysaccharide, EV: extracellular vesicles, CWP: cell wall protein, SCFA: short-chain fatty acid. Cervical cancer cells: HeLa. Colon cancer cells: HT-29, Caco-2, SW-620, SW-742, HCT-8, HCT-116. Breast cancer cells: MCF-7. Lung adenocarcinoma epithelial cells: A549.
*Sonication, **Thermal treatment.
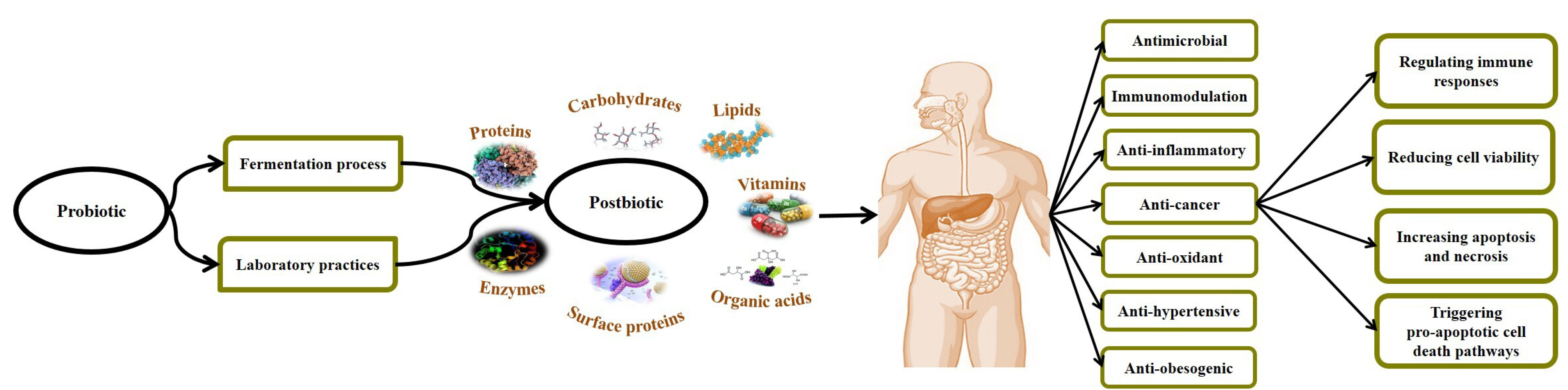
Figure 1.
Postbiotics and their potential anti-cancer effects in the host.
.
Postbiotics and their potential anti-cancer effects in the host.
In conclusion, due to their unique characteristics (safe origin, more shelf-life, low preparation cost, without toxic effect), postbiotics have received much scientific attention and are considered as a novel approach for adjuvant therapy in patients with cancer. Randomized double-blind clinical trials are necessary to specify the optimal dose and administration frequency of postbiotic supplements for cancer patients.
Ethical Issues
Not applicable
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors gratefully acknowledge the financial support of this study by the Tabriz University of Medical Sciences, Tabriz, Iran.
References
- Karovičová J, Kohajdová Z. Lactic acid fermentation of various vegetable juices. Acta Aliment 2005; 34(3):237-46. doi: 10.1556/aalim.34.2005.3.5 [Crossref] [ Google Scholar]
- Jama YH, Varadaraj MC. Antibacterial effect of plantaricin LP84 on foodborne pathogenic bacteria occurring as contaminants during idli batter fermentation. World J Microbiol Biotechnol 1999; 15(1):27-32. doi: 10.1023/a:1008887201516 [Crossref] [ Google Scholar]
- Vaghef-Mehrabany E, Vaghef-Mehrabany L, Asghari-Jafarabadi M, Homayouni-Rad A, Issazadeh K, Alipour B. Effects of probiotic supplementation on lipid profile of women with rheumatoid arthritis: a randomized placebo-controlled clinical trial. Health Promot Perspect 2017; 7(2):95-101. doi: 10.15171/hpp.2017.17 [Crossref] [ Google Scholar]
- Vaghef-Mehrabani E, Homayouni Rad A, Alipour B, Vaghef-Mehrabany L, Saghafi-Asl M. Formulation and design of probiotic supplements for rheumatoid arthritis patients. Pharm Sci 2018; 24(1):44-51. doi: 10.15171/ps.2018.08 [Crossref] [ Google Scholar]
- Mohamadshahi M, Veissi M, Haidari F, Shahbazian H, Kaydani GA, Mohammadi F. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts 2014; 4(2):83-8. doi: 10.5681/bi.2014.007 [Crossref] [ Google Scholar]
- Chuah LO, Foo HL, Loh TC, Mohammed Alitheen NB, Yeap SK, Abdul Mutalib NE. Postbiotic metabolites produced by Lactobacillus plantarum strains exert selective cytotoxicity effects on cancer cells. BMC Complement Altern Med 2019; 19(1):114. doi: 10.1186/s12906-019-2528-2 [Crossref] [ Google Scholar]
- Zhong L, Zhang X, Covasa M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J Gastroenterol 2014; 20(24):7878-86. doi: 10.3748/wjg.v20.i24.7878 [Crossref] [ Google Scholar]
- Tan CK, Said S, Rajandram R, Wang Z, Roslani AC, Chin KF. Pre-surgical administration of microbial cell preparation in colorectal cancer patients: a randomized controlled trial. World J Surg 2016; 40(8):1985-92. doi: 10.1007/s00268-016-3499-9 [Crossref] [ Google Scholar]
- Aisu N, Tanimura S, Yamashita Y, Yamashita K, Maki K, Yoshida Y. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Exp Ther Med 2015; 10(3):966-72. doi: 10.3892/etm.2015.2640 [Crossref] [ Google Scholar]
- Dietrich CG, Kottmann T, Alavi M. Commercially available probiotic drinks containing Lactobacillus casei DN-114001 reduce antibiotic-associated diarrhea. World J Gastroenterol 2014; 20(42):15837-44. doi: 10.3748/wjg.v20.i42.15837 [Crossref] [ Google Scholar]
- Homayouni Rad A, Aghebati-Maleki L, Samadi Kafil H, Abbasi A. Molecular mechanisms of postbiotics in colorectal cancer prevention and treatment. Crit Rev Food Sci Nutr 2020:1-17. doi: 10.1080/10408398.2020.1765310 [Crossref]
- Fasoli S, Marzotto M, Rizzotti L, Rossi F, Dellaglio F, Torriani S. Bacterial composition of commercial probiotic products as evaluated by PCR-DGGE analysis. Int J Food Microbiol 2003; 82(1):59-70. doi: 10.1016/s0168-1605(02)00259-3 [Crossref] [ Google Scholar]
- Tsilingiri K, Barbosa T, Penna G, Caprioli F, Sonzogni A, Viale G. Probiotic and postbiotic activity in health and disease: comparison on a novel polarised ex-vivo organ culture model. Gut 2012; 61(7):1007-15. doi: 10.1136/gutjnl-2011-300971 [Crossref] [ Google Scholar]
- Vinderola G, Binetti A, Burns P, Reinheimer J. Cell viability and functionality of probiotic bacteria in dairy products. Front Microbiol 2011; 2:70. doi: 10.3389/fmicb.2011.00070 [Crossref] [ Google Scholar]
- Rad AH, Maleki LA, Kafil HS, Zavoshti HF, Abbasi A. Postbiotics as novel health-promoting ingredients in functional foods. Health Promot Perspect 2020; 10(1):3-4. doi: 10.15171/hpp.2020.02 [Crossref] [ Google Scholar]
- Homayouni Rad A, Aghebati Maleki L, Samadi Kafil H, Abbasi A. Postbiotics: a novel strategy in food allergy treatment. Crit Rev Food Sci Nutr 2020:1-8. doi: 10.1080/10408398.2020.1738333 [Crossref]
- Patel RM, Denning PW. Therapeutic use of prebiotics, probiotics, and postbiotics to prevent necrotizing enterocolitis: what is the current evidence?. Clin Perinatol 2013; 40(1):11-25. doi: 10.1016/j.clp.2012.12.002 [Crossref] [ Google Scholar]
- Izuddin WI, Loh TC, Foo HL, Samsudin AA, Humam AM. Postbiotic L plantarum RG14 improves ruminal epithelium growth, immune status and upregulates the intestinal barrier function in post-weaning lambs. Sci Rep 2019; 9(1):9938. doi: 10.1038/s41598-019-46076-0 [Crossref] [ Google Scholar]
- Delgado S, Sánchez B, Margolles A, Ruas-Madiedo P, Ruiz L. Molecules produced by probiotics and intestinal microorganisms with immunomodulatory activity. Nutrients 2020; 12(2). doi: 10.3390/nu12020391 [Crossref]
- Homayouni Rad A, Abbasi A, Samadi Kafil H, Ganbarov K. Potential pharmaceutical and food applications of postbiotics: a review. Curr Pharm Biotechnol 2020. doi: 10.2174/1389201021666200516154833 [Crossref]
- Gao J, Li Y, Wan Y, Hu T, Liu L, Yang S. A novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front Microbiol 2019; 10:477. doi: 10.3389/fmicb.2019.00477 [Crossref] [ Google Scholar]
- Schwartz DJ, Rebeck ON, Dantas G. Complex interactions between the microbiome and cancer immune therapy. Crit Rev Clin Lab Sci 2019; 56(8):567-85. doi: 10.1080/10408363.2019.1660303 [Crossref] [ Google Scholar]
- Klemashevich C, Wu C, Howsmon D, Alaniz RC, Lee K, Jayaraman A. Rational identification of diet-derived postbiotics for improving intestinal microbiota function. Curr Opin Biotechnol 2014; 26:85-90. doi: 10.1016/j.copbio.2013.10.006 [Crossref] [ Google Scholar]
- Riaz Rajoka MS, Zhao H, Mehwish HM, Li N, Lu Y, Lian Z. Anti-tumor potential of cell free culture supernatant of Lactobacillus rhamnosus strains isolated from human breast milk. Food Res Int 2019; 123:286-97. doi: 10.1016/j.foodres.2019.05.002 [Crossref] [ Google Scholar]
- Lee JE, Lee J, Kim JH, Cho N, Lee SH, Park SB. Characterization of the anti-cancer activity of the probiotic bacterium Lactobacillus fermentum using 2D vs 3D culture in colorectal cancer cells. Biomolecules 2019; 9(10). doi: 10.3390/biom9100557 [Crossref]
- Konishi H, Fujiya M, Tanaka H, Ueno N, Moriichi K, Sasajima J. Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis. Nat Commun 2016; 7:12365. doi: 10.1038/ncomms12365 [Crossref] [ Google Scholar]
- Bahmani S, Azarpira N, Moazamian E. Anti-colon cancer activity of Bifidobacterium metabolites on colon cancer cell line SW742. Turk J Gastroenterol 2019; 30(9):835-42. doi: 10.5152/tjg.2019.18451 [Crossref] [ Google Scholar]
- Tukenmez U, Aktas B, Aslim B, Yavuz S. The relationship between the structural characteristics of lactobacilli-EPS and its ability to induce apoptosis in colon cancer cells in vitro. Sci Rep 2019; 9(1):8268. doi: 10.1038/s41598-019-44753-8 [Crossref] [ Google Scholar]
- Jafari B, Khavari Nejad RA, Vaziri F, Siadat SD. Evaluation of the effects of extracellular vesicles derived from Faecalibacterium prausnitzii on lung cancer cell line. Biologia 2019; 74(7):889-98. doi: 10.2478/s11756-019-00229-8 [Crossref] [ Google Scholar]
- Nozari S, Faridvand Y, Etesami A, Ahmad Khan Beiki M, Miresmaeili Mazrakhondi SA, Abdolalizadeh J. Potential anticancer effects of cell wall protein fractions from Lactobacillus paracasei on human intestinal Caco-2 cell line. Lett Appl Microbiol 2019; 69(3):148-54. doi: 10.1111/lam.13198 [Crossref] [ Google Scholar]
- Sentürk M, Ercan F, Yalcin S. The secondary metabolites produced by Lactobacillus plantarum downregulate BCL-2 and BUFFY genes on breast cancer cell line and model organism Drosophila melanogaster: molecular docking approach. Cancer Chemother Pharmacol 2020; 85(1):33-45. doi: 10.1007/s00280-019-03978-0 [Crossref] [ Google Scholar]
- Chen D, Jin D, Huang S, Wu J, Xu M, Liu T. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett 2020; 469:456-67. doi: 10.1016/j.canlet.2019.11.019 [Crossref] [ Google Scholar]
- Motevaseli E, Shirzad M, Akrami SM, Mousavi AS, Mirsalehian A, Modarressi MH. Normal and tumour cervical cells respond differently to vaginal lactobacilli, independent of pH and lactate. J Med Microbiol 2013; 62(Pt 7):1065-72. doi: 10.1099/jmm.0.057521-0 [Crossref] [ Google Scholar]
- Davis CD, Milner JA. Gastrointestinal microflora, food components and colon cancer prevention. J Nutr Biochem 2009; 20(10):743-52. doi: 10.1016/j.jnutbio.2009.06.001 [Crossref] [ Google Scholar]
- Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr 2013; 98(1):111-20. doi: 10.3945/ajcn.112.056689 [Crossref] [ Google Scholar]
- Wang K, Li W, Rui X, Chen X, Jiang M, Dong M. Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int J Biol Macromol 2014; 63:133-9. doi: 10.1016/j.ijbiomac.2013.10.036 [Crossref] [ Google Scholar]
- Riaz Rajoka MS, Zhao H, Lu Y, Lian Z, Li N, Hussain N. Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct 2018; 9(5):2705-15. doi: 10.1039/c8fo00547h [Crossref] [ Google Scholar]
- Shyu PT, Oyong GG, Cabrera EC. Cytotoxicity of probiotics from Philippine commercial dairy products on cancer cells and the effect on expression of cfos and cjun early apoptotic-promoting genes and Interleukin-1 β and Tumor necrosis factor-α proinflammatory cytokine genes. Biomed Res Int 2014; 2014:491740. doi: 10.1155/2014/491740 [Crossref] [ Google Scholar]
- Kamrani A, Mehdizadeh A, Ahmadi M, Aghebati-Maleki L, Yousefi M. Therapeutic approaches for targeting receptor tyrosine kinase like orphan receptor-1 in cancer cells. Expert Opin Ther Targets 2019; 23(5):447-56. doi: 10.1080/14728222.2019.1602608 [Crossref] [ Google Scholar]
- Song H, Wang W, Shen B, Jia H, Hou Z, Chen P. Pretreatment with probiotic Bifico ameliorates colitis-associated cancer in mice: transcriptome and gut flora profiling. Cancer Sci 2018; 109(3):666-77. doi: 10.1111/cas.13497 [Crossref] [ Google Scholar]
- Kurata N, Tokashiki N, Fukushima K, Misao T, Hasuoka N, Kitagawa K. Short chain fatty acid butyrate uptake reduces expressions of prostanoid EP(4) receptors and their mediation of cyclooxygenase-2 induction in HCA-7 human colon cancer cells. Eur J Pharmacol 2019; 853:308-15. doi: 10.1016/j.ejphar.2019.04.014 [Crossref] [ Google Scholar]
- Ambalam P, Dave JM, Nair BM, Vyas BR. In vitro mutagen binding and antimutagenic activity of human Lactobacillus rhamnosus 231. Anaerobe 2011; 17(5):217-22. doi: 10.1016/j.anaerobe.2011.07.001 [Crossref] [ Google Scholar]
- Sharma M, Shukla G. Metabiotics: one step ahead of probiotics; an insight into mechanisms involved in anticancerous effect in colorectal cancer. Front Microbiol 2016; 7:1940. doi: 10.3389/fmicb.2016.01940 [Crossref] [ Google Scholar]