Advanced pharmaceutical bulletin. 11(4):580-594.
doi: 10.34172/apb.2021.068
Review Article
Breast Cancer: A Global Concern, Diagnostic and Therapeutic Perspectives, Mechanistic Targets in Drug Development
Gul-e-Saba Chaudhry 1, *  , Rehmat Jan 2, Abdah Akim 3, Muhammad Naveed Zafar 4, Yeong Yik Sung 1, Tengku Sifzizul Tengku Muhammad 1
, Rehmat Jan 2, Abdah Akim 3, Muhammad Naveed Zafar 4, Yeong Yik Sung 1, Tengku Sifzizul Tengku Muhammad 1
Author information:
1Institute of Marine Biotechnology, University Malaysia Terengganu, 21030 Kuala Terengganu, Malaysia.
2Department of Environmental Sciences, Fatima Jinnah University, Rawalpindi, Pakistan.
3Department of Biomedical Sciences, Universiti Putra Malaysia, Seri Kembangan, Selangor, Malaysia.
4Department of Chemistry, Quaid-i-Azam University, Islamabad, 45320, Pakistan.
*
Corresponding Author: Gul-e-Saba Chaudhry, Tel: +609-6683810, Fax: +609-6683810, Email:
gul.saba@umt.edu.my
Abstract
Cancer is a complex multifactorial process, unchecked and abrupt division, and cell growth—conventional chemotherapy, along with radiotherapy, is used to treat breast cancer. Due to reduce efficacy and less survival rate, there is a particular need for the discovery of new active anticancer agents. Natural resources such as terrestrial/marine plants or organisms are a promising source for the generation of new therapeutics with improving efficacy. The screening of natural plant extracts and fractions, isolations of phytochemicals, and mechanistic study of those potential compounds play a remarkable role in the development of new therapeutic drugs with increased efficacy. Cancer is a multistage disease with complex signaling cascades. The initial study of screening whole extracts or fractions and later the isolation of secondary compounds and their mechanism of action study gives a clue of potential therapeutic agents for future drug development. The phytochemicals present in extracts/fractions produce remarkable effects due to synergistically targeting multiple signals. In this review, the molecular targets of extracts/ fractions and isolated compounds highlighted. The therapeutic agent's mechanistic targets in drug development focused involves; i) Induction of Apoptosis, ii) modulating cell cycle arrest, iii) Inhibition or suppression of invasion and metastasis and iv) various other pro-survival signaling pathways. The phytochemicals and their modified analogs identified as future potential candidates for anticancer chemotherapy.
Keywords: Apoptosis, Breast cancer, Cell cycle arrest, Drug development, Natural products, Mechanism of action, Phytochemicals, Plant anticancer drugs
Copyright and License Information
© 2021 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Breast cancer is the second leading cause of death (11.6% of the total cancer deaths), followed by colorectal and lung cancer.
1
Cancer is a complex disease concerning pathology and biochemistry. It begins when cells in the body start to grow in an uncontrolled and abnormal manner, which may also cause disturbances and alter the structure of surrounding tissues.
2
The evasion of apoptosis, limitless replicative potential, evading growth suppressors, sustaining proliferative signaling, inducing angiogenesis and activating tissue invasion and metastasis are critical features of cancer which contribute towards tumor development.
3,4
Alterations in cellular DNA and transcriptional/translational processes causes irregularity in the gene expressions and results in cancer cell proliferation. Primary entities involved in carcinogenesis are oncogenes and tumor suppressor genes. Defects in tumor suppressor genes and mutations in the proto-oncogenes results in uncontrolled multiplication of cells leading to cancer.
5,6
Breast cancer, lung cancer, and colorectal cancer are frequently occurring cancer in both men and women.
7
Breast cancer: a global concern
Breast cancer triggers due to the uncontrolled multiplication of cells. It is the most frequently occurring cancer type and the leading cause of death in women over the last few years.
8
The cancer trigger due to mutations in genes responsible for the production of pro-apoptotic/anti-apoptotic proteins, tumor suppressers proteins, and growth factors. According to the United States, cancer statistics report 2018, an estimate of about 268 670 new breast cancer cases and a total of 41 400 deaths cases due to breast cancer in the United States in 2018.
9
Breast cancer broadly categorized into two types, invasive breast cancer, and non-invasive breast cancer. However, other types of breast cancer include medullary and tubular carcinoma, inflammatory breast cancer,Paget’s disease (PD), and phyllodes tumor (PT).Generally, in invasive breast cancer, cells are not only confined to ducts and lobular walls but also spread to surrounding areas of breast (connective and fatty tissues). The infiltrating-lobular-carcinoma and infiltrating-ductal-carcinoma are frequently occurring invasive-breast cancer. The lobular carcinoma isinitiated in the milk glands, while ductal carcinoma began in the breast’s milk ducts. Medullary breast carcinoma and tubular carcinomas are the subtypes of invasive breast carcinoma.
10
Furthermore, inflammatory breast cancer type is characterized by inflamed breasts with indentation and thick ridges. Only 1% to 2% of all invasive breast cancers and 1% of all breast cancers are inflammatory breast cancer with low survival rates at all stages. However, non-invasive breast cancer cells restricted to ducts only (do not penetrate surrounding tissues) of the breast. Ductal carcinoma in situ and lobular carcinoma in situ are the two forms of non-invasive breast cancer.
10-13
PD pharmaceutically described by the infiltration of the nipple epidermis by destructive breast epithelial cells. PD of the breast defined as a skin alteration in the nipple-areola region. It is less common and generally linked with in-situ or invasive carcinoma.
14
Breast PT, is a rare tumor, and shows different behavior, as it could be benign (non-cancerous) or malignant (cancerous).
15
PT can cause uncommon fibroepithelial lesions to account for around 0.3% to 0.5% of breast tumors diagnosed in women and has an occurrence of about 2.1 per million.
16
Breast cancer usually classified as two types i) estrogen receptor-positive (ER+) and ii) estrogen receptor-negative (ER-) breast cancer. Estrogen receptor-positive cell lines include MCF-7 and T-47D, while MDA-MB-231, MDA- MB-453 and MDA-MB-468 are estrogen negative receptor cell lines. Which, further characterized as luminal A (ER+, PR+, HER2-), luminal B (ER+, PR+, HER2+), HER2-enriched, basal-like, and normal-like based on progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER2) status.
17
The MCF-7 and T-47D cell line together with other breast cancer cell type MDA-MB-231, have been studied in above two-third of the total publications in Medline.
18
Risk factors, diagnostic and therapeutic perspective for breast cancer
Common risk factors associated, such as age, gender, family history, breast density, radiation exposure, reproductive factors, genetic mutations, and diabetes.
19
Early screening, detection, and diagnosis, significantly affect the occurrence and survival rate of breast cancer.Several diagnostic approaches include mammograms, ultrasound, magnetic resonance imaging, breast self-examination, positron emission tomography scan, computerized tomography, bone scintigraphy, chest X-ray, and biopsy.
20
However, due to some limitations of these approaches, such as high cost, time consumption, and age restriction, the development of highly sensitive and early-stage diagnostic techniques required. Different biomarkers such as proteomic biomarkers, gene biomarkers, and various imaging techniques are a useful analytical tool for fast and economic early-stage breast cancer diagnosis.
21
Breast cancer conventional treatment approach involves; (i) surgical removal of cancer cells. (ii) use of chemotherapy coupled with hormonal therapy and gene therapy; and (iii) radiation therapy.
22
Surgery is considered as the earliest method and used for most of the solid tumors.
22
The surgical treatment depends on the stage and tumor form; involves removal of the only lump (lumpectomy) or surgical removal of the entire breast (mastectomy). Breast-conserving surgery includes lumpectomy (removal of lump only or a small number of surrounding tissues), wide excision (partial mastectomy), and quadrantectomy (removal of about one-quarter of the breast.
12
Currently, sentinel lymph node dissection has become a well-known suitable technique as it necessitates the excision of very few lymph nodes, causing very few or no side effects. Over the past decade, advances in sentinel lymph node mapping have enhanced the precision of detecting sentinel lymph nodes from 80% to 92%-98% using different combined modalities.
12,22
Chemotherapy is the most conventional therapy available for malignant cancers.
22
In chemotherapy, anticancer drugs, orally or intravenously given to patients, might cause severe side effects due to non-specific killing of cancer cells. However, radiotherapy is a conventional approach used in the treatment of cancer, along with chemotherapy.
22
For the treatment of HER2-neu positive tumors, trastuzumab, in combination with radiotherapy, is needed.
23
Hormonal therapy studied for the treatment of ER+ breast cancer for several decades. The anticancer drug tamoxifen behaves as an antagonist in the breast, causing a delay in thetranscription of estrogen-regulated genes and interrupting in the proliferative effects of estrogen in the breast. Similarly, fulvestrant acts as tamoxifen, but it causes degradation of the ER protein and loss of estrogen and progesterone receptor expression.
24
Menopausal hormone therapy usually restrained from breast cancer survivors because of the risk of reoccurrence. Menopausal hormone therapy provides adequate assistance from climacteric symptoms, but few are associated with enhanced risk of stroke and also breast, ovarian, and endometrial cancers.
25
Gene therapies have developed as promising new treatments for breast cancer. Proto-oncogene and tumor suppressor genes have shown accelerated improvement in gene therapy approaches.
12
Various clinical trials are ongoing to deliver p53 to cancer cells. The viral vectors have employed to transfer a breast cancer gene BRCA1, as a mutation in BRCA genes is also responsible for breast cancer cases. Also, the use of antisense strategies in clinical trials considered the most common approach. Adenoviral gene E1A that interferes with the transcription of erbB-2 can use to inhibit the transcription of overexpressed oncogenes in the treatment of ovarian and breast cancer.
12
Plant-derived anti-breast cancer therapeutic agents
Natural products played a remarkable role in the prevention and treatment of cancer and remained a focus of research in drug discovery.
26
Over 3000 plant species reported having anticancer properties.
27
Plant-derived natural products have significant efficacy in cancer treatment due to reduced adverse side effects as compared to conventional chemotherapy.
28
This review article’s primary objective was to study the therapeutic potential of natural products in whole plant extracts/fractions or isolated secondary metabolites in breast cancer treatment. The initial stage in drug discovery is to screen the potential extracts and fractions, which gives the clue of the presence of novel phytochemicals. The knowledge provides a better understanding of the presence of various phytochemicals and their synergistic approach, which nowadays played a remarkable role in combination drug therapy. The extracts/fractions screening reduces the cost of isolation of phytochemicals by revealing a potential agent’s presence. The isolated secondary metabolites from various natural sources, mechanistic study (in-vitro), give better molecular fundamental knowledge of the future therapeutic agent. Figure 1 describes the preparation of phytochemical compounds isolated from plants and their use in breast cancer therapy.
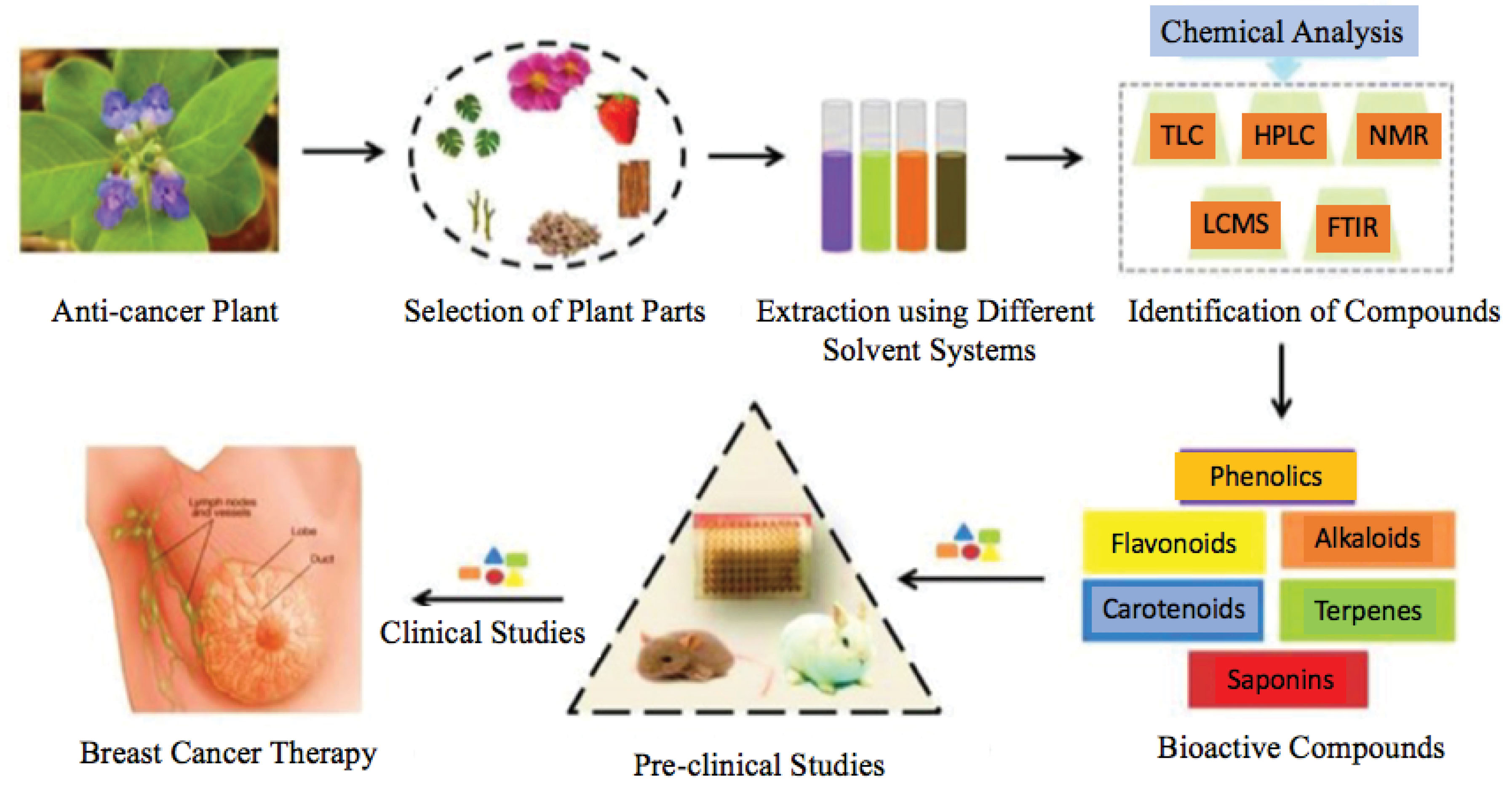
Figure 1.
Schematic illustration of plant-derived anti-breast cancer therapeutic agent
.
Schematic illustration of plant-derived anti-breast cancer therapeutic agent
Anti-breast cancer therapeutic agents and their molecular mechanistic targets
Several crude extracts/fractions possessing potential natural products have tested on a variety of breast cancer cell lines. The potential phytochemicals induced cytotoxicity on breast cancer through several mechanisms. Such as via induction of apoptosis, cell cycle arrest in cancer cells, inhibition of metastatic potential, obstructing the process of angiogenesis, pro-survival signaling, and autophagy activation. Figures 2 and 2 provide detailed information about potential therapeutic approaches of several anti-breast cancer plant species and their mechanism of action, respectively.
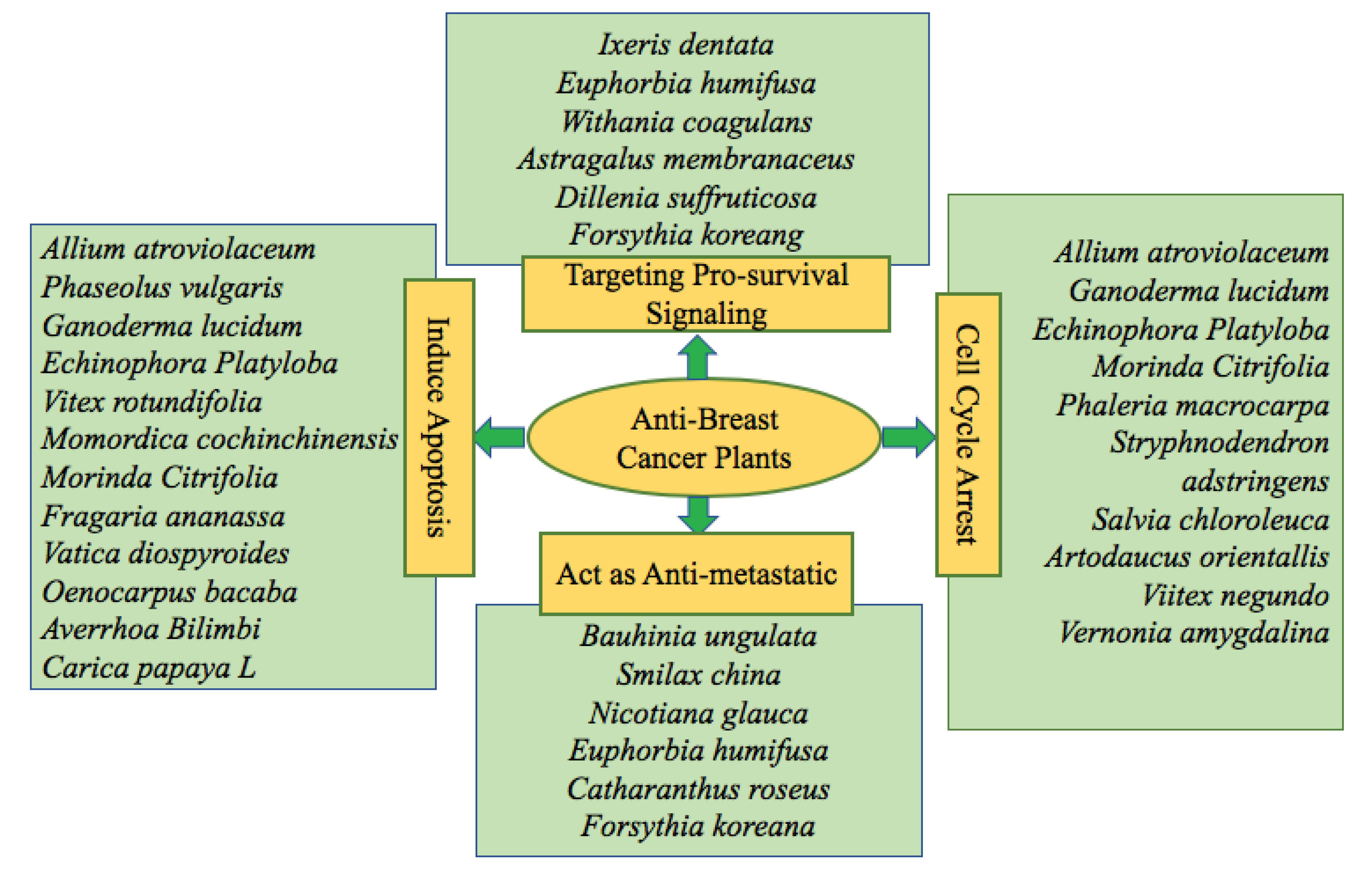
Figure 2.
Anti-breast cancer plants and their therapeutic approaches
.
Anti-breast cancer plants and their therapeutic approaches
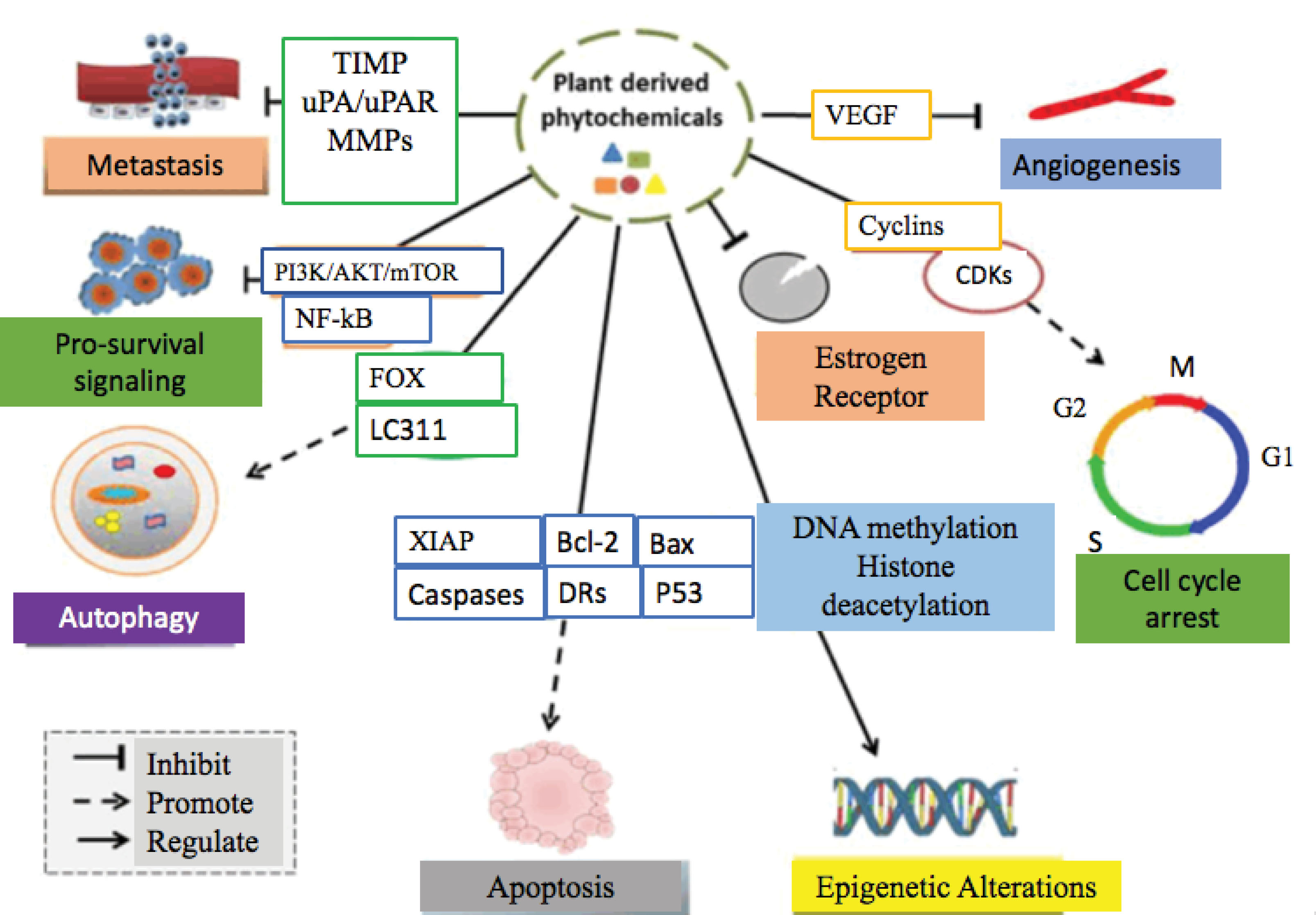
Figure 3.
Therapeutic targets of plant derived anti-breast cancer agents on breast cancer cells
.
Therapeutic targets of plant derived anti-breast cancer agents on breast cancer cells
Induction of apoptosis
Apoptosis, tightly regulated mechanism of cell death as a result of signal cascades involved during healthy development and morphogenesis.
3,29
The enzymatic proteins caspases are prominent initiators and executioners in the process of apoptosis. Along with caspases, various pro and anti-apoptotic proteins such as Bcl-2 family proteins, tumor suppressor proteins (p53), cytochrome c release from mitochondria, activation of several death receptors involved in the trigger of apoptosis. Besides, various apoptosis proteins (IAPs) play a vital role in the induction and regulation of apoptosis.
30,31
Apoptosis can occur via both the extrinsic pathway (death receptor-mediated pathway) and the intrinsic pathway (mitochondrial-mediated), and these pathways converge at the execution pathway of apoptosis.
3
Death receptors, DR4, DR5 trigger the extrinsic pathway of apoptosis, up-regulation of pro-apoptotic protein (Bax) and down-regulation of an anti-apoptotic member of Bcl-2, which is essential for the activation of the intrinsic pathway of apoptosis.
3,32
Extract of Phaseolus vulgaris (family Fabaceae) induces apoptosis in MCF-7 and MDA-MB-231 via up-regulating pro-apoptotic protein (Bax) and down-regulated anti-apoptotic protein (Bcl-2, Bcl-xL).
33
Similarly, fruit extract of Momordica cochinchinensis causes the induction of apoptosis in breast cancer (MCF-7) cells via the up-regulation of Bax and enhanced caspase 6, 8, and 9 activity.
34
Also, Fragaria ananassa (Strawberry) methanolic extract induced apoptosis by an intrinsic pathway in T-47D by the up-regulation of Bax, Bid, p73, and down-regulation of BCL-xL.
35
The aqueous extract fraction of Oenocarpus bacaba also induced apoptosis in MCF-7 cells by both extrinsic and intrinsic pathways through activation of caspases-6, -8, and -9.
36
Moreover, the methanolic fractions of Scrophularia oxysepala cause caspase-dependent apoptosis in MCF-7 cells.
37
The up-regulation of Bax induces apoptosis in MDA-MB-468 cells, treated with acetone and methanolic extracts of Vatica diospyroides.
38
Modulating cell cycle arrest
Cell cycle, remarkable role in cellular genomic integrity, and timely progression of cells.
39
Different phases such as (i) G1-phase (gap 1), (ii) S-phase (DNA synthesis), (iii) G2-phase (gap 2), and (iv) M-phase (mitosis) involve in the cell cycle. In S-phase, DNA synthesis and genome replication occur, required for the transmission of genetic information between generations. The M-phase causes segregation of genetic information, sister chromatids, and cell division. G1 is the gap between M and S phase, while G2 is the gap between S and M phase. These intervals (G1 and G2), essential to ensure that each phase is complete before moving to the next phase.
40,41
Activation of cell cycle check-points usually occurs as a response to replication stress and DNA damage. The activation and inactivation of cyclin-dependent kinases and cyclins play a vital role in the cellular progression and cell cycle regulation.
39,41
The methanolic extract prepared from Allium atroviolaceum (family Amaryllidaceae) induces apoptosis by modulating cell cycle arrest in caspase-dependent and p53-independent pathway in the breast cancer cell (MCF-7, MDA-MB-231).
42
Similarly, ethanol extract of Ganoderma lucidum chipped fruiting bodies causes cell cycle arrest in MCF-7 cells by up‐regulation of p21/Waf1 and down‐regulation of cyclin D1.
43
The crude extracts of Echinophora platyloba, Vernonia amygdalina, Morinda Citrifolia induces apoptosis in MCF-7 and MDA-MB-231 cell lines via G0/G1/S phase cell cycle arrest.
12,44,45
The diethyl-ether extract of Artocarpus altilis and hexane and methylene chloride fractions from roots of Salvia chloroleuca induced apoptosis and sub-G1 peak in T-47D and MCF-7 cells respectively.
46,47
Also, ethyl acetate fraction from Phaleria macrocarpa (fruit) induce G0/G1 and G2/M cell cycle arrest in MDA-MB-231.
48
Inhibition of invasion and metastasis suppression
The conventional therapeutic approaches quite challenging, especially in metastasized cancer. The mechanism of metastasis involves invasion, intravasation, and extravasation. The process of invasion characterizes by the spreading of cancer cells to distant sites via the circulatory system. However, extravasation requires the penetration of cancer cells to the endothelium and the basement membrane. At the point of extravasation, cancer cells can grow at secondary focus.
49,50
The matrix metalloproteinases (MMPs), critical proteins involved in metastasis of tumor cells. The inhibition or blocking of MMPs is an essential target in the suppression of metastatic potential. Other than MMPs, metastasis suppressor genes, MKK4 (mitogen-activated protein kinase 4), BRMS1 (breast cancer metastasis suppressor 1) and NM23 (non-metastatic gene 23) also play a remarkable role in the inhibition of metastasis.
50,51
Similarly, modulation of uPA, uPAR, and TIMP expression also plays a vital role in the suppression of metastasis.
52
The crude extracts of Catharanthus roseus, Origanum majorana, and Brassica oleracea possess anti-invasive and anti-metastatic activities in breast cancer cell line, MDA-MB-231. Anti-invasive and anti-metastatic activities via suppression of MMPs (MMP-2 and MMP-9) activities.
53-55
Similarly, ethanol extract of Smilax china causes suppression of metastasis via modulation of uPA, uPAR, and TIMP expression in MDA MB 231 cells.
56
Also, different fractions from stem of Bauhinia ungulata anti-metastatic decrease the activity of potential target of metastasis MMP-2.
57
Pro-survival signaling pathway
Several pro-survival signaling pathways were determining the fate of a cancer cell and mainly transduced by a complex net of signaling molecule cascade. Pro-survival signaling cascades, IP3K-PKB/Akt, and MAPK, activated by several cytokines and growth factors. The nuclear factor-κB (NFκB) plays an essential role in the regulation of inflammation and immune responses.
58
Blocking of these pro-survival signaling pathways has been widely studied, crucial for the treatment of breast cancer. The previous study shows that methanol extract of Ixeris dentata induced apoptosis in T-47D, MCF-7, SK-BR-3, and MDA-MB-231 via inhibiting Akt and NF-κB signaling pathway.
59
Similarly, ethyl acetate fraction of Euphorbia humifusa causes inhibition of NF-κB activity in MDA-MB-231 cell line.
60
Water- ethanol extract of Astragalus membranaceus induced apoptosis in MCF-7, SK-BR-3, and MDA-MB-231 through inhibition of PI3K, Akt and mTOR signaling pathways.
61
Also, ethyl acetate extract from Roots of Dillenia suffruticosa induces apoptosis in MCF-7 via inhibition of AKT and ERK, and activation of JNK.
62
Other potential pathways
Various signal cascades induce cytotoxicity of breast cancer cell lines via regulation of angiogenesis, autophagy, suppression of ERα expression, down-regulation of intracellular ROS generation, and mitochondrial membrane potential activated. The ethanol crude extract of Salvia triloba possesses angiogenesis activities in MCF-7 that is mediated by the inhibition of VEGF expression at both mRNA and protein levels.
63
Similarly, ethyl acetate fractions of Eugenia jambolana and Musa paradisiaca causes suppression of VEGF-induced angiogenesis in MCF-7 and MDA-MB-231 cells.
64
The extract of Buxus sempervirens induces autophagic cell death in MCF7, T47D, MCF10CA1a, and BT-20.
65
However, ROS mediated apoptosis in MCF-7 and MDA-MB-231 noticeable after treatment with chloroform fraction of Tinospora cordifolia.
66
Similarly, the hexane and methylene chloride fractions of Salvia chloroleuca also induce ROS-mediated pathway in MCF-7 cells.
46
Also, Morinda citrifolia (ethyl-acetate) extract downregulates intracellular ROS generation and mitochondrial membrane potential in MCF-7, and MDA-MB-231.
45
The Acanthopanax sessiliflorus (hexane fraction) causes mitochondria associated with both ROS dependent and independent pathways in MDA-MB-231 and MCF-7.
67
Phytochemicals in anti-breast cancer drug development
Plants possess different phytochemical compounds, and they classified based on the functional group, structures, and biosynthetic origins. Phytochemicals in medicinal plants include phenolics, flavonoids, alkaloids, terpenoids, carotenoids, saponins, steroids, and antioxidants induces cell death in MCF-7 cell lines.
68-86
Among all phytochemicals, phenolics are the most structurally diverse.
86
Here, we discuss potential phytochemicals as future anti-breast cancer therapeutic agents and drug development.
Phenolics
Phenolic compounds, widely occurring secondary metabolites isolated from plants and are most structurally diverse among all phytochemicals.
87
Plant-derived phenolic compounds classified as; (i) simple phenols, (ii) flavonoids, (iii) lignins, (iv) lignans, (v) tannins, (vi) xanthones, and (vii) coumarins. Previous studies show that various phenolic compounds inhibit the initiation and progression of a variety of cancers by inducing cell cycle arrest, angiogenesis and apoptosis, modulating ROS levels and inhibiting oncogenic signaling cascades controlling cell proliferation.
88
The quercetin induces apoptosis in MCF-7, T47D, MDA-MB-453 and MDA-MB-231 cell line by up-regulation of Bax, down-regulation of Bcl-2 and activation of caspase-3. Similarly, quercetin also results in cell cycle arrest via modulation of Foxo3a activity in breast cancer.
89-94
Interestingly, luteolin causes cytotoxicity in breast cancer cell line, MDA-MB-231 via suppression of epidermal growth factor receptor-mediated pathway IGF-1 pathway-dependent ERα.
95
Moreover, different phenolic acids such as ferulic acid, caffeic acid, and gallic acid also induces apoptosis in ER+ and ER– breast cancer cell lines.
88,96,97
Alkaloids
Plant-derived alkaloids possess oncogenesis suppression via modulating critical signaling pathways in human cancer. The paclitaxel possesses anticancer activity against breast cancer, ovarian cancer, prostate cancer, and lung cancer and is in clinical use.
98
Similarly, vinca alkaloids clinically used to treat human cancers. The vinca alkaloids (VA), from the Madagascar periwinkle plant (Catharanthus roseus G. Don), possess hypoglycaemic and cytotoxic properties. The VA considered as cancer fighters, second-most-certified class of cancer drugs. The four major vinca alkaloids include; (i) vincristine, (ii) vinblastine, (iii) vinorelbine, and (iv) vindesine are in clinical use. The vinflunine, a new synthetic vinca alkaloid, is used in treatment for carcinomas and other malignancies.
99
The vinca alkaloids interaction with tubulin protein, interfere with the assembly of microtubules, leads to cell division arrest in metaphase.
Similarly, vinflunine, a potent inhibitor of tubulin, causes hindrance in microtubule assembly and induces apoptosis. Moreover, vinflunine apoptosis mechanism involved activation of caspases 3 and 7 and c-Jun N-terminal kinase 1.
100
Other than vinca alkaloids; another alkaloid compound berberine induces cell cycle arrest and mitochondrial or intrinsic pathway in MCF-7 and MDA-MB-231 cells.
101,102
Similarly, noscapine induces apoptosis in breast cancer cells via intrinsic and extrinsic pathways by upregulation of Bax, downregulation of Bcl-2 and activation of caspases.
103-105
The hirsutine causes cell death in MDA-MB-231 cells by activating the intrinsic pathway of apoptosis and targeting NF-κB signaling pathway.
106,107
Moreover, the treatment of MCF-7 cells by procaine decreases DNA methylation and RARβ2 promoter methylation.
108
Terpenes
Terpenes or terpenoids classified based on the number of C5 units or cyclic structures present in the molecule.
109
The terpenoids can exert a broad spectrum of biological activities such as antioxidation, anti-inflammation, and anticancer activities. Numerous terpenoid compounds are known to possess anticancer potential in a verity of human cancers by causing inhibition of cancer cell proliferation and inducing apoptosis. Monoterpenoids such as D-limonene, have demonstrated antitumor and anticancer activities against breast cancer.
110
Several diterpenoids also possess anticancer activity against breast cancers and are involved in the induction of apoptosis. These include triptolide, oridonin, and ponicidin.
111
The triptolide also possesses antiproliferative activity and down-regulates the expression of ERα in different breast cancer cell lines.
112-114
The triterpenoids are close to steroids in structure and evoke apoptosis in a variety of cancer such as prostate and breast cancer. Different triterpenoids, like cucurbitacins, dammaranes, friedelanes, limonoids, lanostanes, lupanes, oleananes, tirucallanes, and ursanes, have been isolated from plants and studied for anticancer efficacy in breast cancer cells.
115
Ursolic acid, a triterpene acid causes DNA fragmentation induced apoptosis in MCF-7 cells by downregulation of Bcl-2 and activation of caspase -3.
116,117
Tetraterpenes also was known as carotenoids broadly categorized as acyclic tetraterpenoids and bicyclic tetraterpenoids. Carotenoids or tetraterpenoids such as lycopene and lutein are also known to possess anticancer activities in breast cancer cell lines.
111
Saponins
Saponins are natural glycosides widely distributed in plants classified into; triterpenoid, saponins, and steroid saponins.
118
Saponins possess potential biological activities includes; anti-inflammatory, antiproliferative, immunomodulatory, and anticancer activities.
119
Several saponins possess anticancer activities against various cancer cell lines.
120
For example, Avicin D, a triterpenoid glycoside compound, induces apoptosis in cutaneous T-cell lymphoma cells via downregulation of p-STAT-3 and bcl-2.
121
Similarly, tubeimoside-1 exhibits anticancer effects via mitochondrial dysfunction and endoplasmic reticulum stress pathways in HeLa cells.
122
The steroid saponins, degalactotigonin, and Polyphyllin D, possess cytotoxicity activity in ER+ human breast cancer cell line, MCF-7.
123,124
Moreover, the triterpene saponins such as gummiferaoside B and C possess antiproliferative activity in MDA-MB-435 cells.
125
Also, Avicins D, G induce apoptosis and cell cycle arrest in MDA-MB-435 cell line.
126
The phenolics, alkaloids, terpenoids and saponins derived from other sources also possess anticancer activity in different breast cancer cell lines.
127-142
Moreover, plant extracts, phytochemicals, and their potential mechanism of action against breast cancer are enlisted in Tables 1 and 2, respectively. Also, our various studies show the induction of cell death (apoptosis) in other cell lines.
143
The induction of cell death mainly due to presence of potential phytochemicals such as phenolics, saponins, terpenoids. The more screening and mechanistic studies need to be done to fully explore the potent phytochemicals in field of cancer therapeutics.
Table 1.
Plant extracts and their potential mechanism of action against breast cancer
|
Plant name
|
Extract / Fraction
|
Part used
|
Target cell lines
|
Mechanism of cell death
|
References
|
|
Allium atroviolaceum
|
Methanolic Extract |
Flower |
MCF-7, MDA-MB-231 |
- Induces apoptosis
- Modulating cell cycle arrest
- Caspase-dependent and p53-independent Pathway
|
42
|
Phaseolus vulgaris
(black turtle bean)
|
Extract |
Seeds |
MCF-7 and MDA-MB231 |
- Upregulation of Bax and downregulation of Bcl-2 and Bcl-xL
- Activation of caspase -3/7
|
33
|
|
Ganoderma lucidum
|
Ethanol extract |
Chipped fruiting bodies |
MCF-7 |
- Induces cell cycle arrest and apoptosis
- Up‐regulation of p21/Waf1 and down‐regulation of cyclin D1
- Up‐regulation of pro‐apoptotic Bax protein
|
43
|
|
Echinophora Platyloba
|
Methanol Extract |
Leaves |
MDA-MB-231 |
- Induces apoptosis and cell cycle arrest at S-phase
- Up-regulation of bax and p27
- Down-regulation of bcl-2
|
44
|
|
Momordica cochinchinensis
|
Aril Extract |
Fruit |
MCF-7 |
- Induces apoptosis
- Increased bax enhanced caspase 6, 8 and 9 activity
|
34
|
|
Morinda Citrifolia
|
Ethyl-acetate extract |
Fruit |
MCF-7, MDA-MB-231 |
- Arrested the cell cycle in the G1/S phase in MCF-7 and G0/G1 phase in MDA-MB-231 cells
- Downregulation of intracellular ROS generation and mitochondrial membrane potential
|
45
|
Fragaria ananassa
Strawberry
|
Methanolic extract |
Fruit |
T-47D |
- Cleavage of MCL-1
- downregulation of BCL-xL
- Upregulation of expression of proapoptotic proteins such as BAX and BID
- Upregulation of p73
- Activation of CASPASE 3 and CASPASE 9
|
35
|
|
Vatica diospyroides
|
Acetone and methanolic extracts |
Fruit |
MDA-MB-468 |
- Induces apoptosis
- Up-regulation of Bax
|
38
|
|
Oenocarpus bacaba
|
Phenolic extract |
Fruit |
MCF-7 |
- Induces apoptosis
- Caspases-6, -8 and -9 activated
|
36
|
|
Averrhoa Bilimbi
|
Methanolic extract |
Fruit, Leaves |
MCF-7 |
- Anticancer activity |
68
|
|
Carica papaya L
|
Aqueous Extract |
Leaves |
MCF-7 |
- Anti-proliferation and Apoptosis induction |
69
|
|
Mimosa caesalpiniifolia
|
Ethanolic extract |
Leaf |
MCF-7 |
- Induces apoptosis
- DNA fragmentation
|
70
|
|
Annona muricata
|
Aqueous extract |
Leaves |
MCF-7, MDA-MB-231 |
- Induces apoptosis |
71
|
|
Acanthopanax sessiliflorus
|
Hexane fraction |
Stem bark |
MDA-MB-231 and MCF-7 |
- Non-apoptotic cell death via mitochondria associated with both ROS dependent and independent pathways |
67
|
|
Phaleria macrocarpa
|
Ethyl acetate fraction |
Fruit |
MDA-MB-231 |
- Induce G0/G1 and G2/M cell cycle arrest
- Activation of caspase -8,9 and 3
- Upregulation of Bax, Bid
- cytochrome c, p21, p27, p53 and SMAC
- Downregulation of Bcl-2, Bcl-w, XIAP and survivin
|
48
|
|
Stryphnodendron adstringen
|
Aqueous extract fraction |
Leaves |
MCF-7, MDA-MB-435 |
- Upregulation of Bax, caspase-9, active caspase-3, - caspase-8, LC-3, and beclin-1
- Downregulation of Bcl-2
|
72
|
|
Avicennia Marina
|
Crude methanol extract and fraction |
Leaves |
MDA-MB 231 |
- DNA fragmentation
- Decreased mRNA expression level of Bcl-2 and increased p53
|
73
|
|
Salvia chloroleuca
|
Hexane and methylene chloride fractions |
Roots |
MCF-7 |
- Induced a sub-G1 peak
- DNA fragmentation
- ROS-mediated pathway
|
46
|
|
Scrophularia oxysepala
|
Methanolic subfractions |
Aerial parts |
MCF-7 |
- Activation of caspase-3
- Downregulation of Bcl-2
|
36
|
|
Artocarpus altilis
|
Diethyl ether extract |
Wood |
T-47D |
- Induced apoptosis and sub-G1 phase formation |
47
|
|
Piper crocatum
|
Methanol extract |
Leaves |
T-47D |
- Inhibition of p44/p42 phosphorylation |
74
|
|
Pistacia atlanticasubkurdica
|
Methanol |
Fruits skin |
T-47D |
- Activation of caspase 3
- Poly ADP ribose polymerase (PARP) cleavage
|
75
|
|
Vitex rotundifolia
|
fraction |
leave |
MCF-7 |
- extrinsic and intrinsic pathway |
76
|
|
Vitex rotundifolia
|
fraction |
leave |
T47D |
- extrinsic and intrinsic pathway |
77
|
|
Aaptos sp., marine
|
fraction |
whole |
MCF-7 |
- DNA fragmentation |
78
|
|
Marine sponges
|
Methanol extract |
whole |
MCF-7 |
- DNA fragmentation |
79
|
|
Vitex negundo
|
Aqueous and Ethanolic extract |
Leaves |
MCF-7 |
- Induced apoptosis |
80
|
|
Jatropha curcas
|
Ethanol extract |
Root bark |
MCF-7 |
- Inducing anoikis |
81
|
|
Vernonia amygdalina
|
Ethanol extract |
Leaves |
MCF-7 and MDA-MB-231 |
- Induced apoptosis
- G1/S phase cell cycle arrest
- Caspase-dependent
|
45
|
|
Strobilanthes crispa
|
Hexane extract |
Stem |
MDA-MB-231 |
- Induced apoptosis |
82
|
|
Ixeris dentata
|
Methanol extract |
-
|
T-47D, MCF-7, SK-BR-3, and MDA-MB-231 |
- Induced apoptosis
- via Akt-NF-κB signaling
|
59
|
|
Tinospora cordifolia
|
Chloroform
fraction
|
Stems |
MCF-7 and MDA-MB-231 |
- ROS mediated apoptosis |
66
|
|
Smilax china
|
Ethanol extract |
Bark |
MDA-MB-231 |
- Suppression of metastasis
Modulation of uPA, uPAR and TIMP expression
|
56
|
|
Bauhinia ungulata
|
Different fractions |
Stem |
4T1 |
- Anti-tumor
- Antimetastatic
- decreasing the MMP-2 activity
|
57
|
|
Nicotiana glauca
|
Dichloromethane fraction |
Stem |
MCF-7 |
- Anti-Metastatic |
83
|
|
Euphorbia humifusa
|
Ethyl acetate fraction |
Whole plant |
MDA-MB-231 |
- Inhibition of NF-κB activity
- Induced matrix metalloproteinase (MMP)-9 mRNA expression
|
60
|
|
Withania coagulans
|
Ethyl acetate |
Aerial with fruit |
MCF-7, MDA-MB-231 |
- Inhibited TNF-α induced NFκB activity |
84
|
|
Astragalus membranaceus
|
Water- ethanol extract |
Roots |
MCF-7, SK-BR-3 and MDA-MB-231 |
- Anti-proliferative
- Induced apoptosis
- Inhibition of PI3K/AKT/mTOR signaling pathway
|
61
|
|
Dillenia suffruticosa
|
Ethyl acetate extract |
Roots |
MCF-7 |
- Induces apoptosis via inhibition of AKT and ERK, and activation of JNK |
62
|
|
Catharanthus roseus
|
Methanol extract |
Leaves |
MDA-MB-231 |
- Anti-invasive
- Suppressed the MMP-2 and MMP-9 activity
|
53
|
|
Forsythia koreana
|
Methanol extract |
Fruit and leaves |
MDA-MB-231 |
- Suppressed invasion and MMPs activities
- Inhibited the receptor activator of nuclear factor kappa-B
|
85
|
|
Origanum majorana
|
Ethanolic extract |
Leaves |
MDA-MB-231 |
- Anti-invasive and anti-metastatic
- Downregulates the phosphorylation of IκB, nuclear level of NFκB and Nitric Oxide (NO) production
|
54
|
|
Brassica oleracea
|
Extract |
- |
MDA-MB-231 |
- Anti-invasive
- Suppressed TPA-induced MMP-9 activity
|
55
|
|
Salvia triloba
|
Ethanolic crude extracts |
Whole plant |
MCF 7 |
- Antiangiogenesis
- Inhibited the expression of VEGF at the mRNA and protein level
|
63
|
|
Eugenia jambolana
|
Ethyl acetate fractions |
Seeds |
MCF-7 and MDA-MB-231 |
- Suppression of VEGF-induced angiogenesis |
64
|
|
Musa paradisiaca
|
Ethyl acetate fractions |
Roots |
MCF-7 and MDA-MB-231 |
- Suppression of VEGF-induced angiogenesis |
64
|
|
Buxus sempervirens
|
Acetonic extract |
Leaves and flowers |
MCF7, T47D, MCF10CA1a and BT-20 |
- Induces apoptosis,
Cell cycle arrest, autophagy
|
65
|
Table 2.
Plant derived phytochemicals and their potential mechanism of action against breast cancer
|
Phytochemicals
|
Compound name, type
|
Target cell lines
|
Mechanism of cell death
|
References
|
| Phenolics |
Quercetin
(Flavonoid)
|
MCF-7, T47D, MDA-MB-453, MDA-MB-231 |
- Induces apoptosis
- Through suppression of Twist via p38 MAPK pathway
- Increased Bax expression and decreased Bcl-2 expression
- Increased cleaved caspase-3 and PARP expression
- Cell cycle arrest through modulation of Foxo3a activity
|
89-94
|
Casticin
(flavonoid)
|
MCF-7, MDA-MB-231 |
- Induces apoptosis
- Inhibiting the expression of forkhead box protein M1
|
127
|
Luteolin
(flavonoid)
|
MDA-MB-231, MCF-7 |
- Suppression of epidermal growth factor receptor-mediated pathway
- IGF-1 pathway dependent ERα
|
95,128
|
Ferulic acid
(phenolic acid)
|
MDA-MB-231,
T47D,
MCF-7
|
- Induces apoptosis
- Suppression of metastatic potential
- Anti-proliferative
|
96,97,129
|
Caffeic acid
(phenolic acid)
|
T47D,
MCF-7
|
- Anti-proliferative,
- Induces apoptosis
- Inhibition of NFκB and activation of Fas
|
97,130
|
Gallic acid
(Phenolic acid)
|
MDA-MB-231 |
- Induces apoptosis |
88
|
| Alkaloids |
Berberine |
MCF-7, MDA-MB-231 |
- Inducing cell cycle arrest
- Increasing levels of cytoplasmic cytochrome c, caspase-9, p53 and p27
- Cleavage of PARP
- Decreasing levels of Bcl-2
|
101,102,131
|
| Noscapine |
MCF-7,
MDA-MB-231, T47D
|
- Activation of caspase-8 and caspase-9
- Upregulation of Bax, downregulation of Bcl-2
- Anti-neoplastic
|
103-105
|
| Pretazettine |
MCF-7 |
- Anti-tumor activity |
132
|
| Piperlongumine |
MDA-MB-453,
MCF-7, T-47D
|
- STAT3 Inhibitor |
133
|
| Hirsutine |
MDA-MB-453, MDA-MB-231, 4T1 |
- DNA damage response
- NF-κB and Akt pathways
- Activation of the p38 MAPK pathway
- Upregulation of Bax, downregulation of Bcl-2
- Activating caspase 9 and caspase 3
|
106-107,134
|
|
|
Procaine |
MCF-7 |
- Decrease global DNA methylation
- Decrease RARβ2 promoter methylation
|
108
|
|
|
Benzyl Isothiocyanate |
MDA-MB-231, MCF-7, MDA-MB-468, BT-474, and BRI-JM04 |
- FoxO1-mediated autophagic cell death |
135
|
| Terpenoid |
D-Limonene
(Monoterpene)
|
|
|
|
Triptolide
(Diterpene)
|
MDA-MB-435,
MDA-MB-231,
MCF7
|
- Anti-proliferative
- Suppression of phospholipase D expression
- Down-regulate the expression of ERα
|
112-114
|
Ursolic acid
(triterpene acid)
|
MCF-7 |
- Triggers apoptosis
- DNA fragmentation
- Downregulation of Bcl-2
- Activation of caspase -3
|
116-117
|
Betulinic acid
(triterpene)
|
MDAMB- 231, MDL13E, BT438, BT474, BT549, T47D |
- Exhibited cytotoxicity
- Induces apoptosis
- Down-regulation of Bcl-2 and cyclin D1
|
136-137
|
Lupeol
(triterpene)
|
MDA-MB-231 |
- Suppressed the proliferation |
138
|
Lycopene
(tetraterpenoids, carotenoids)
|
MCF 7, MDA-MB-231 |
- Trigger G2/M arrest and suppress Bcl-2 expression
- Induce apoptosis
|
139
|
|
|
Parthenolide |
ZR-75-1,
MDA-MB-231
|
- Inhibits HDAC1 increases global H3 acetylation,
- Induces p21
|
140-141
|
| Saponins |
Gummiferaoside B, C
(triterpene saponins)
|
MDA-MB-435 |
- Anti-proliferative |
125
|
Degalactotigonin
(steroid saponins)
|
MCF-7 |
- Cytotoxic |
123
|
Polyphyllin D
(Steroid saponins)
|
MCF-7 |
- Cytotoxic |
124
|
Avicins D, G
(triterpenoid saponins)
|
MDA-MB-435 |
- Apoptosis,
- Cell cycle (G1) arrest
|
126
|
Ginsenoside Rh2
(dammarane-type saponins)
|
MCF7 |
- Cytotoxic |
142
|
Conclusion
Cancer is a complex disease, leading cause of death worldwide. Despite the development of many synthetic anticancer drugs, toxicity remains the main problem, which reduces the survival rate. Therefore, there is an increase in demand for alternative treatments. Amongst the alternative approaches, the natural product derived anticancer agents are a practical choice. The secondary metabolites, as potential anticancer agents with understandable anticancer mechanisms of action, leads to the development of novel therapeutic drugs. Additionally, the plant extracts are an excellent source of lead compounds. The isolated lead compound can either used or undergoes some structural modifications to increase the effectiveness in term of their pharmacological potential.
Ethical Issues
Not applicable.
Conflict of Interest
The authors have no conflict of interest.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6):394-424. doi: 10.3322/caac.21492 [Crossref] [ Google Scholar]
- Nithya M, Ambikapathy V, Panneerselvam A, Thajuddin N. Anti tumour activity of different extracts of Ganoderma lucidum (Curt: Fr) P karst. World J Pharm Res 2014; 3(4):2204-14. [ Google Scholar]
- Jan R, Chaudhry GE. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv Pharm Bull 2019; 9(2):205-18. doi: 10.15171/apb.2019.024 [Crossref] [ Google Scholar]
- Tonini T, Rossi F, Claudio PP. Molecular basis of angiogenesis and cancer. Oncogene 2003; 22(42):6549-56. doi: 10.1038/sj.onc.1206816 [Crossref] [ Google Scholar]
- Rice H, Bryant S, Handley C, Hall M. Oncogenes and tumor suppressor genes: an essential building block of cancer. Chemist 2014; 87(2):15-8. [ Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66(1):7-30. doi: 10.3322/caac.21332 [Crossref] [ Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67(1):7-30. doi: 10.3322/caac.21387 [Crossref] [ Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68(1):7-30. doi: 10.3322/caac.21442 [Crossref] [ Google Scholar]
- Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res 2004; 6(3):R149-56. doi: 10.1186/bcr767 [Crossref] [ Google Scholar]
- Raghav K, Morris V, Tang C, Morelli P, Amin HM, Chen K. MET amplification in metastatic colorectal cancer: an acquired response to EGFR inhibition, not a de novo phenomenon. Oncotarget 2016; 7(34):54627-31. doi: 10.18632/oncotarget.10559 [Crossref] [ Google Scholar]
- Karakas C. Paget’s disease of the breast. J Carcinog 2011; 10:31. doi: 10.4103/1477-3163.90676 [Crossref] [ Google Scholar]
- Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 2010; 141(1):69-80. doi: 10.1016/j.cell.2010.02.027 [Crossref] [ Google Scholar]
- Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst 2010; 102(3):170-8. doi: 10.1093/jnci/djp482 [Crossref] [ Google Scholar]
- Roberts N, Runk DM. Aggressive malignant phyllodes tumor. Int J Surg Case Rep 2015; 8c:161-5. doi: 10.1016/j.ijscr.2014.12.041 [Crossref] [ Google Scholar]
- Mishra SP, Tiwary SK, Mishra M, Khanna AK. Phyllodes tumor of breast: a review article. ISRN Surg 2013; 2013:361469. doi: 10.1155/2013/361469 [Crossref] [ Google Scholar]
- Taherian-Fard A, Srihari S, Ragan MA. Breast cancer classification: linking molecular mechanisms to disease prognosis. Brief Bioinform 2015; 16(3):461-74. doi: 10.1093/bib/bbu020 [Crossref] [ Google Scholar]
- Dai X, Cheng H, Bai Z, Li J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J Cancer 2017; 8(16):3131-41. doi: 10.7150/jca.18457 [Crossref] [ Google Scholar]
- Fenga C. Occupational exposure and risk of breast cancer. Biomed Rep 2016; 4(3):282-92. doi: 10.3892/br.2016.575 [Crossref] [ Google Scholar]
- McDonald ES, Clark AS, Tchou J, Zhang P, Freedman GM. Clinical diagnosis and management of breast cancer. J Nucl Med 2016; 57 Suppl 1:9S-16S. doi: 10.2967/jnumed.115.157834 [Crossref] [ Google Scholar]
- Wang L. Early diagnosis of breast cancer. Sensors (Basel) 2017; 17(7):1572. doi: 10.3390/s17071572 [Crossref] [ Google Scholar]
- Roy PS, Saikia BJ. Cancer and cure: a critical analysis. Indian J Cancer 2016; 53(3):441-2. doi: 10.4103/0019-509x.200658 [Crossref] [ Google Scholar]
- van Uden DJ, van Laarhoven HW, Westenberg AH, de Wilt JH, Blanken-Peeters CF. Inflammatory breast cancer: an overview. Crit Rev Oncol Hematol 2015; 93(2):116-26. doi: 10.1016/j.critrevonc.2014.09.003 [Crossref] [ Google Scholar]
- Puhalla S, Bhattacharya S, Davidson NE. Hormonal therapy in breast cancer: a model disease for the personalization of cancer care. Mol Oncol 2012; 6(2):222-36. doi: 10.1016/j.molonc.2012.02.003 [Crossref] [ Google Scholar]
- Jones ME, Schoemaker MJ, Wright L, McFadden E, Griffin J, Thomas D. Menopausal hormone therapy and breast cancer: what is the true size of the increased risk?. Br J Cancer 2016; 115(5):607-15. doi: 10.1038/bjc.2016.231 [Crossref] [ Google Scholar]
- Valli M, Pivatto M, Danuello A, Castro-Gamboa I, Silva DH, Cavalheiro AJ. Tropical biodiversity: has it been a potential source of secondary metabolites useful for medicinal chemistry?. Quím Nova 2012; 35(11):2278-87. doi: 10.1590/s0100-40422012001100036 [Crossref] [ Google Scholar]
- Habli Z, Toumieh G, Fatfat M, Rahal ON, Gali-Muhtasib H. Emerging cytotoxic alkaloids in the battle against cancer: overview of molecular mechanisms. Molecules 2017; 22(2):250. doi: 10.3390/molecules22020250 [Crossref] [ Google Scholar]
- Desai AG, Qazi GN, Ganju RK, El-Tamer M, Singh J, Saxena AK. Medicinal plants and cancer chemoprevention. Curr Drug Metab 2008; 9(7):581-91. doi: 10.2174/138920008785821657 [Crossref] [ Google Scholar]
- Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta 2013; 1833(12):3448-59. doi: 10.1016/j.bbamcr.2013.06.001 [Crossref] [ Google Scholar]
- Proskuryakov SY, Gabai VL, Konoplyannikov AG. Necrosis is an active and controlled form of programmed cell death. Biochemistry (Mosc) 2002; 67(4):387-408. doi: 10.1023/a:1015289521275 [Crossref] [ Google Scholar]
- Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature 2012; 491(7425):554-9. doi: 10.1038/nature11581 [Crossref] [ Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 2007; 35(4):495-516. doi: 10.1080/01926230701320337 [Crossref] [ Google Scholar]
- Kumar S, Sharma VK, Yadav S, Dey S. Antiproliferative and apoptotic effects of black turtle bean extracts on human breast cancer cell line through extrinsic and intrinsic pathway. Chem Cent J 2017; 11(1):56. doi: 10.1186/s13065-017-0281-5 [Crossref] [ Google Scholar]
- Petchsak P, Sripanidkulchai B. Momordica cochinchinensis aril extract induced apoptosis in human MCF-7 breast cancer cells. Asian Pac J Cancer Prev 2015; 16(13):5507-13. doi: 10.7314/apjcp.2015.16.13.5507 [Crossref] [ Google Scholar]
- Somasagara RR, Hegde M, Chiruvella KK, Musini A, Choudhary B, Raghavan SC. Extracts of strawberry fruits induce intrinsic pathway of apoptosis in breast cancer cells and inhibits tumor progression in mice. PLoS One 2012; 7(10):e47021. doi: 10.1371/journal.pone.0047021 [Crossref] [ Google Scholar]
- Abadio Finco FDB, Kloss L, Graeve L. Bacaba (Oenocarpus bacaba) phenolic extract induces apoptosis in the MCF-7 breast cancer cell line via the mitochondria-dependent pathway. NFS J 2016; 5:5-15. doi: 10.1016/j.nfs.2016.11.001 [Crossref] [ Google Scholar]
- Orangi M, Pasdaran A, Shanehbandi D, Kazemi T, Yousefi B, Hosseini BA. Cytotoxic and apoptotic activities of methanolic subfractions of Scrophularia oxysepala against human breast cancer cell line. Evid Based Complement Alternat Med 2016; 2016:8540640. doi: 10.1155/2016/8540640 [Crossref] [ Google Scholar]
- Srisawat T, Sukpondma Y, Chimplee S, Kanokwiroon K, Tedasen A, Graidist P. Extracts from Vatica diospyroides type SS fruit show low dose activity against MDA-MB-468 breast cancer cell-line via apoptotic action. Biomed Res Int 2014; 2014:479602. doi: 10.1155/2014/479602 [Crossref] [ Google Scholar]
- Azzopardi M, Farrugia G, Balzan R. Cell-cycle involvement in autophagy and apoptosis in yeast. Mech Ageing Dev 2017; 161(Pt B):211-24. doi: 10.1016/j.mad.2016.07.006 [Crossref] [ Google Scholar]
- Israels ED, Israels LG. The cell cycle. Oncologist 2000; 5(6):510-3. doi: 10.1634/theoncologist.5-6-510 [Crossref] [ Google Scholar]
- Pucci B, Kasten M, Giordano A. Cell cycle and apoptosis. Neoplasia 2000; 2(4):291-9. doi: 10.1038/sj.neo.7900101 [Crossref] [ Google Scholar]
- Khazaei S, Abdul Hamid R, Ramachandran V, Mohd Esa N, Pandurangan AK, Danazadeh F. Cytotoxicity and proapoptotic effects of Allium atroviolaceum flower extract by modulating cell cycle arrest and caspase-dependent and p53-independent pathway in breast cancer cell lines. Evid Based Complement Alternat Med 2017; 2017:1468957. doi: 10.1155/2017/1468957 [Crossref] [ Google Scholar]
- Hu H, Ahn NS, Yang X, Lee YS, Kang KS. Ganoderma lucidum extract induces cell cycle arrest and apoptosis in MCF-7 human breast cancer cell. Int J Cancer 2002; 102(3):250-3. doi: 10.1002/ijc.10707 [Crossref] [ Google Scholar]
- Birjandian E, Motamed N, Yassa N. Crude methanol extract of Echinophora platyloba induces apoptosis and cell cycle arrest at S-phase in human breast cancer cells. Iran J Pharm Res 2018; 17(1):307-16. [ Google Scholar]
- Sharma K, Pachauri SD, Khandelwal K, Ahmad H, Arya A, Biala P. Anticancer effects of extracts from the fruit of Morinda citrifolia (Noni) in breast cancer cell lines. Drug Res (Stuttg) 2016; 66(3):141-7. doi: 10.1055/s-0035-1555804 [Crossref] [ Google Scholar]
- Wong FC, Woo CC, Hsu A, Tan BK. The anti-cancer activities of Vernonia amygdalina extract in human breast cancer cell lines are mediated through caspase-dependent and p53-independent pathways. PLoS One 2013; 8(10):e78021. doi: 10.1371/journal.pone.0078021 [Crossref] [ Google Scholar]
- Tayarani-Najaran Z, Asili J, Aioubi E, Emami SA. Growth inhibition and apoptosis induction of Salvia chloroleuca on MCF-7 breast cancer cell line. Iran J Pharm Res 2013; 12(4):789-99. [ Google Scholar]
- Arung ET, Wicaksono BD, Handoko YA, Kusuma IW, Yulia D, Sandra F. Anti-cancer properties of diethylether extract of wood from sukun (Artocarpus altilis) in human breast cancer (T47D) cells. Trop J Pharm Res 2009; 8(4):317-24. doi: 10.4314/tjpr.v8i4.45223 [Crossref] [ Google Scholar]
- Kavitha N, Ein Oon C, Chen Y, Kanwar JR, Sasidharan S. Phaleria macrocarpa (Boerl) fruit induce G(0)/G(1) and G(2)/M cell cycle arrest and apoptosis through mitochondria-mediated pathway in MDA-MB-231 human breast cancer cell. J Ethnopharmacol 2017; 201:42-55. doi: 10.1016/j.jep.2017.02.041 [Crossref] [ Google Scholar]
- Carr I, Orr FW. Invasion and metastasis. Can Med Assoc J 1983; 128(10):1164-7. [ Google Scholar]
-
Martin TA, Ye L, Sanders AJ, Lane J, Jiang WG. Cancer invasion and metastasis: molecular and cellular perspective. In: Blagosklonny MV, Pardee AB, eds. Madame Curie Bioscience Database. Austin, TX: Landes Bioscience; 2000-2013.
- Decock J, Thirkettle S, Wagstaff L, Edwards DR. Matrix metalloproteinases: protective roles in cancer. J Cell Mol Med 2011; 15(6):1254-65. doi: 10.1111/j.1582-4934.2011.01302.x [Crossref] [ Google Scholar]
- Cox G, Steward WP, O’Byrne KJ. The plasmin cascade and matrix metalloproteinases in non-small cell lung cancer. Thorax 1999; 54(2):169-79. doi: 10.1136/thx.54.2.169 [Crossref] [ Google Scholar]
- Eltayeb NM, Ng SY, Ismail Z, Salhimi SM. Anti-invasive effect of Catharanthus roseus extract on highly metastatic human breast cancer MDA-MB-231 cells. J Teknol 2016; 78(11-3):35-40. doi: 10.11113/jt.v78.9870 [Crossref] [ Google Scholar]
- Al Dhaheri Y, Attoub S, Arafat K, Abuqamar S, Viallet J, Saleh A. Anti-metastatic and anti-tumor growth effects of Origanum majorana on highly metastatic human breast cancer cells: inhibition of NFκB signaling and reduction of nitric oxide production. PLoS One 2013; 8(7):e68808. doi: 10.1371/journal.pone.0068808 [Crossref] [ Google Scholar]
- Rose P, Huang Q, Ong CN, Whiteman M. Broccoli and watercress suppress matrix metalloproteinase-9 activity and invasiveness of human MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol 2005; 209(2):105-13. doi: 10.1016/j.taap.2005.04.010 [Crossref] [ Google Scholar]
- Nho KJ, Chun JM, Kim HK. Anti-metastatic effect of Smilax china L extract on MDA-MB-231 cells. Mol Med Rep 2015; 11(1):499-502. doi: 10.3892/mmr.2014.2698 [Crossref] [ Google Scholar]
- Santos KM, Gomes INF, Romao W, Ribeiro RIMA, Silva-Oliveira RJ, Pinto FE. Bauhinia stem extracts as a possible new treatment for breast cancer and metastasis: inhibition of migration, invasion and of the activity of matrix metalloproteinases. J Biotechnol Biomater 2017; 7(6 Suppl):59. doi: 10.4172/2155-952x-c1-085 [Crossref] [ Google Scholar]
- Klener P Jr, Andera L, Klener P, Necas E, Zivný J. Cell death signalling pathways in the pathogenesis and therapy of haematologic malignancies: overview of apoptotic pathways. Folia Biol (Praha) 2006; 52(1-2):34-44. [ Google Scholar]
- Shin SA, Lee HN, Choo GS, Kim HJ, Che JH, Jung JY. Ixeris dentata (Thunb Ex Thunb) Nakai extract inhibits proliferation and induces apoptosis in breast cancer cells through Akt/NF-κB pathways. Int J Mol Sci 2017; 18(2). doi: 10.3390/ijms18020275 [Crossref]
- Shin WS, Han J, Kumar R, Lee GG, Sessler JL, Kim JH. Programmed activation of cancer cell apoptosis: a tumor-targeted phototherapeutic topoisomerase I inhibitor. Sci Rep 2016; 6:29018. doi: 10.1038/srep29018 [Crossref] [ Google Scholar]
- Zhou R, Chen H, Chen J, Chen X, Wen Y, Xu L. Extract from Astragalus membranaceus inhibit breast cancer cells proliferation via PI3K/AKT/mTOR signaling pathway. BMC Complement Altern Med 2018; 18(1):83. doi: 10.1186/s12906-018-2148-2 [Crossref] [ Google Scholar]
- Tor YS, Yazan LS, Foo JB, Wibowo A, Ismail N, Cheah YK. Induction of apoptosis in MCF-7 cells via oxidative stress generation, mitochondria-dependent and caspase-independent pathway by ethyl acetate extract of Dillenia suffruticosa and its chemical profile. PLoS One 2015; 10(6):e0127441. doi: 10.1371/journal.pone.0127441 [Crossref] [ Google Scholar]
- Zihlif M, Afifi F, Abu-Dahab R, Abdul Majid AM, Sumrein H, Saleh MM. The antiangiogenic activities of ethanolic crude extracts of four Salvia species. BMC Complement Altern Med 2013; 13:358. doi: 10.1186/1472-6882-13-358 [Crossref] [ Google Scholar]
- Harsha Raj M, Ghosh D, Banerjee R, Salimath BP. Suppression of VEGF-induced angiogenesis and tumor growth by Eugenia jambolana, Musa paradisiaca, and Coccinia indica extracts. Pharm Biol 2017; 55(1):1489-99. doi: 10.1080/13880209.2017.1307422 [Crossref] [ Google Scholar]
- Ait-Mohamed O, Battisti V, Joliot V, Fritsch L, Pontis J, Medjkane S. Acetonic extract of Buxus sempervirens induces cell cycle arrest, apoptosis and autophagy in breast cancer cells. PLoS One 2011; 6(9):e24537. doi: 10.1371/journal.pone.0024537 [Crossref] [ Google Scholar]
- Ansari JA, Rastogi N, Ahmad MK, Mahdi AA, Khan AR, Thakur R. ROS mediated pro-apoptotic effects of Tinospora cordifolia on breast cancer cells. Front Biosci (Elite Ed) 2017; 9:89-100. doi: 10.2741/e788 [Crossref] [ Google Scholar]
- Thamizhiniyan V, Young-Woong C, Young-Kyoon K. The cytotoxic nature of Acanthopanax sessiliflorus stem bark extracts in human breast cancer cells. Saudi J Biol Sci 2015; 22(6):752-9. doi: 10.1016/j.sjbs.2015.04.004 [Crossref] [ Google Scholar]
- Nair MS, Soren K, Singh V, Boro B. Anticancer activity of fruit and leaf extracts of Averrhoa bilimbi on MCF-7 human breast cancer cell lines: a preliminary study. Austin J Pharmacol Ther 2016; 4(2):1082. [ Google Scholar]
- Zuhrotun Nisa F, Astuti M, Murdiati A, Mubarika Haryana S. Anti-proliferation and apoptosis induction of aqueous leaf extract of Carica papaya L on human breast cancer cells MCF-7. Pak J Biol Sci 2017; 20(1):36-41. doi: 10.3923/pjbs.2017.36.41 [Crossref] [ Google Scholar]
- Silva MJD, Carvalho AJS, Rocha CQ, Vilegas W, Silva MA, Gouvêa CMCP. Ethanolic extract of Mimosa caesalpiniifolia leaves: chemical characterization and cytotoxic effect on human breast cancer MCF-7 cell line. S Afr J Bot 2014; 93:64-9. doi: 10.1016/j.sajb.2014.03.011 [Crossref] [ Google Scholar]
- Kim JY, Dao TTP, Song K, Park SB, Jang H, Park MK. Annona muricata leaf extract triggered intrinsic apoptotic pathway to attenuate cancerous features of triple negative breast cancer MDA-MB-231 cells. Evid Based Complement Alternat Med 2018; 2018:7972916. doi: 10.1155/2018/7972916 [Crossref] [ Google Scholar]
- Sabino APL, Eustáquio LMS, Miranda ACF, Biojone C, Mariosa TN, Gouvêa C. Stryphnodendron adstringens (“Barbatimão”) leaf fraction: chemical characterization, antioxidant activity, and cytotoxicity towards human breast cancer cell lines. Appl Biochem Biotechnol 2018; 184(4):1375-89. doi: 10.1007/s12010-017-2632-z [Crossref] [ Google Scholar]
- Momtazi-Borojeni AA, Behbahani M, Sadeghi-Aliabadi H. Antiproliferative activity and apoptosis induction of crude extract and fractions of Avicennia marina. Iran J Basic Med Sci 2013; 16(11):1203-8. [ Google Scholar]
- Wicaksono BD, Handoko YA, Arung ET, Kusuma IW, Yulia D, Pancaputra AN. Antiproliferative effect of the methanol extract of Piper crocatum Ruiz & Pav leaves on human breast (T47D) cells in-vitro. Trop J Pharm Res 2009; 8(4):345-52. doi: 10.4314/tjpr.v8i4.45229 [Crossref] [ Google Scholar]
- Rezaei PF, Fouladdel S, Cristofanon S, Ghaffari SM, Amin GR, Azizi E. Comparative cellular and molecular analysis of cytotoxicity and apoptosis induction by doxorubicin and Baneh in human breast cancer T47D cells. Cytotechnology 2011; 63(5):503-12. doi: 10.1007/s10616-011-9373-6 [Crossref] [ Google Scholar]
- Chaudhry GE, Jan R, Mohammad H, Muhammad TST. Vitex rotundifolia fractions induce apoptosis in human breast cancer cell line, MCF-7, via extrinsic and intrinsic pathways. Res Pharm Sci 2019; 14(3):273-85. doi: 10.4103/1735-5362.258496 [Crossref] [ Google Scholar]
- Chaudhry GE, Jan R, Zafar MN, Mohammad H, Muhammad TST. Vitex rotundifolia fractions induced apoptosis in human breast cancer T-47D cell line via activation of extrinsic and intrinsic pathway. Asian Pac J Cancer Prev 2019; 20(12):3555-62. doi: 10.31557/apjcp.2019.20.12.3555 [Crossref] [ Google Scholar]
- Chaudhry GE, Kassim MNI, Ismail N, Mohammad H, Sung YY, Muhammad TST. Induction of apoptosis by Aaptos sp, fractions in human breast cancer cell line, MCF-7. Int J Res Pharm Sci 2018; 9(2):328-37. [ Google Scholar]
- Hudaya T, Chaudhry GE, Taib M, Ismail N, Mohammad TST. Methanol extracts of four selected marine sponges induce apoptosis in human breast cancer cell line, MCF-7. Int J Res Pharm Sci 2017; 8(4):667-75. [ Google Scholar]
- Arulvasu C, Prabhu D, Manikandan R, Srinivasan P, Dinesh D, Babu G. Induction of apoptosis by the aqueous and ethanolic leaf extract of Vitex negundo L in MCF-7 human breast cancer cells. Int J Drug Discov 2010; 2(1):1-7. doi: 10.9735/0975-4423.2.1.1-7 [Crossref] [ Google Scholar]
- Engel N, Falodun A, Kühn J, Kragl U, Langer P, Nebe B. Pro-apoptotic and anti-adhesive effects of four African plant extracts on the breast cancer cell line MCF-7. BMC Complement Altern Med 2014; 14:334. doi: 10.1186/1472-6882-14-334 [Crossref] [ Google Scholar]
- Koh RY, Lim FP, Ling LSY, Ng CPL, Liew SF, Yew MY. Anticancer mechanisms of Strobilanthes crispa Blume hexane extract on liver and breast cancer cell lines. Oncol Lett 2017; 14(4):4957-64. doi: 10.3892/ol.2017.6821 [Crossref] [ Google Scholar]
- Tabana YM, Dahham SS, Ahmed Hassan LE, Al-Mansoub MA, Taleb-Agha M, Abdul Majid AM. In vitro anti-metastatic and antioxidant activity of Nicotiana glauca fraction against breast cancer cells. Adv Biol Res 2015; 9(2):95-102. doi: 10.5829/idosi.abr.2015.9.2.9521 [Crossref] [ Google Scholar]
- Ihsan-ul-Haq Ihsan-ul-Haq, Mirza B, Kondratyuk TP, Park EJ, Burns BE, Marler LE. Preliminary evaluation for cancer chemopreventive and cytotoxic potential of naturally growing ethnobotanically selected plants of Pakistan. Pharm Biol 2013; 51(3):316-28. doi: 10.3109/13880209.2012.728612 [Crossref] [ Google Scholar]
- Kim YL, Lee SK, Park KK, Chung WY. The inhibitory effects of Forsythia koreana extracts on the metastatic ability of breast cancer cells and bone resorption by osteoclasts. J Cancer Prev 2016; 21(2):88-94. doi: 10.15430/jcp.2016.21.2.88 [Crossref] [ Google Scholar]
- Saxena M, Saxena J, Nema R, Singh D, Gupta A. Phytochemistry of medicinal plants. J Pharmacogn Phytochem 2013; 1(6):168-82. [ Google Scholar]
- Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem 2006; 99(1):191-203. doi: 10.1016/j.foodchem.2005.07.042 [Crossref] [ Google Scholar]
- Anantharaju PG, Gowda PC, Vimalambike MG, Madhunapantula SV. An overview on the role of dietary phenolics for the treatment of cancers. Nutr J 2016; 15(1):99. doi: 10.1186/s12937-016-0217-2 [Crossref] [ Google Scholar]
- Somasagara RR, Hegde M, Chiruvella KK, Musini A, Choudhary B, Raghavan SC. Extracts of strawberry fruits induce intrinsic pathway of apoptosis in breast cancer cells and inhibits tumor progression in mice. PLoS One 2012; 7(10):e47021. doi: 10.1371/journal.pone.0047021 [Crossref] [ Google Scholar]
- Duo J, Ying GG, Wang GW, Zhang L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol Med Rep 2012; 5(6):1453-6. doi: 10.3892/mmr.2012.845 [Crossref] [ Google Scholar]
- Rahimifard M, Sadeghi F, Asadi-Samani M, Nejati-Koshki K. Effect of quercetin on secretion and gene expression of leptin in breast cancer. J Tradit Chin Med 2017; 37(3):321-5. [ Google Scholar]
- Choi EJ, Bae SM, Ahn WS. Antiproliferative effects of quercetin through cell cycle arrest and apoptosis in human breast cancer MDA-MB-453 cells. Arch Pharm Res 2008; 31(10):1281-5. doi: 10.1007/s12272-001-2107-0 [Crossref] [ Google Scholar]
- Chien SY, Wu YC, Chung JG, Yang JS, Lu HF, Tsou MF. Quercetin-induced apoptosis acts through mitochondrial- and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum Exp Toxicol 2009; 28(8):493-503. doi: 10.1177/0960327109107002 [Crossref] [ Google Scholar]
- Nguyen LT, Lee YH, Sharma AR, Park JB, Jagga S, Sharma G. Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity. Korean J Physiol Pharmacol 2017; 21(2):205-13. doi: 10.4196/kjpp.2017.21.2.205 [Crossref] [ Google Scholar]
- Lee EJ, Oh SY, Sung MK. Luteolin exerts anti-tumor activity through the suppression of epidermal growth factor receptor-mediated pathway in MDA-MB-231 ER-negative breast cancer cells. Food Chem Toxicol 2012; 50(11):4136-43. doi: 10.1016/j.fct.2012.08.025 [Crossref] [ Google Scholar]
- Zhang X, Lin D, Jiang R, Li H, Wan J, Li H. Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol Rep 2016; 36(1):271-8. doi: 10.3892/or.2016.4804 [Crossref] [ Google Scholar]
- Kampa M, Alexaki VI, Notas G, Nifli AP, Nistikaki A, Hatzoglou A. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action. Breast Cancer Res 2004; 6(2):R63-74. doi: 10.1186/bcr752 [Crossref] [ Google Scholar]
- Habli Z, Toumieh G, Fatfat M, Rahal ON, Gali-Muhtasib H. Emerging cytotoxic alkaloids in the battle against cancer: overview of molecular mechanisms. Molecules 2017; 22(2):250. doi: 10.3390/molecules22020250 [Crossref] [ Google Scholar]
- Moudi M, Go R, Yien CY, Nazre M. Vinca alkaloids. Int J Prev Med 2013; 4(11):1231-5. [ Google Scholar]
- Ali R, Mirza Z, Ashraf GM, Kamal MA, Ansari SA, Damanhouri GA. New anticancer agents: recent developments in tumor therapy. Anticancer Res 2012; 32(7):2999-3005. [ Google Scholar]
- Kim JB, Yu JH, Ko E, Lee KW, Song AK, Park SY. The alkaloid Berberine inhibits the growth of Anoikis-resistant MCF-7 and MDA-MB-231 breast cancer cell lines by inducing cell cycle arrest. Phytomedicine 2010; 17(6):436-40. doi: 10.1016/j.phymed.2009.08.012 [Crossref] [ Google Scholar]
- Patil JB, Kim J, Jayaprakasha GK. Berberine induces apoptosis in breast cancer cells (MCF-7) through mitochondrial-dependent pathway. Eur J Pharmacol 2010; 645(1-3):70-8. doi: 10.1016/j.ejphar.2010.07.037 [Crossref] [ Google Scholar]
- Quisbert-Valenzuela EO, Calaf GM. Apoptotic effect of noscapine in breast cancer cell lines. Int J Oncol 2016; 48(6):2666-74. doi: 10.3892/ijo.2016.3476 [Crossref] [ Google Scholar]
- Afzali M, Ghaeli P, Khanavi M, Parsa M, Montazeri H, Ghahremani MH. Non-addictive opium alkaloids selectively induce apoptosis in cancer cells compared to normal cells. Daru 2015; 23(1):16. doi: 10.1186/s40199-015-0101-1 [Crossref] [ Google Scholar]
- DeBono A, Capuano B, Scammells PJ. Progress toward the development of noscapine and derivatives as anticancer agents. J Med Chem 2015; 58(15):5699-727. doi: 10.1021/jm501180v [Crossref] [ Google Scholar]
- Huang QW, Zhai NN, Huang T, Li DM. [Hirsutine induces apoptosis of human breast cancer MDA-MB-231 cells through mitochondrial pathway]. Sheng Li Xue Bao 2018; 70(1):40-6. [ Google Scholar]
- Lou C, Takahashi K, Irimura T, Saiki I, Hayakawa Y. Identification of Hirsutine as an anti-metastatic phytochemical by targeting NF-κB activation. Int J Oncol 2014; 45(5):2085-91. doi: 10.3892/ijo.2014.2624 [Crossref] [ Google Scholar]
- Villar-Garea A, Fraga MF, Espada J, Esteller M. Procaine is a DNA-demethylating agent with growth-inhibitory effects in human cancer cells. Cancer Res 2003; 63(16):4984-9. [ Google Scholar]
- Rajeshwari CU, Shobha RI, Andallu B. Phytochemicals in diet and human health with special reference to polyphenols. Ann Phytomed 2014; 3(2):4-25. [ Google Scholar]
- Crowell PL. Monoterpenes in breast cancer chemoprevention. Breast Cancer Res Treat 1997; 46(2-3):191-7. doi: 10.1023/a:1005939806591 [Crossref] [ Google Scholar]
- Yang H, Dou QP. Targeting apoptosis pathway with natural terpenoids: implications for treatment of breast and prostate cancer. Curr Drug Targets 2010; 11(6):733-44. doi: 10.2174/138945010791170842 [Crossref] [ Google Scholar]
- Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF. Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther 2003; 2(1):65-72. [ Google Scholar]
- Kang DW, Lee JY, Oh DH, Park SY, Woo TM, Kim MK. Triptolide-induced suppression of phospholipase D expression inhibits proliferation of MDA-MB-231 breast cancer cells. Exp Mol Med 2009; 41(9):678-85. doi: 10.3858/emm.2009.41.9.074 [Crossref] [ Google Scholar]
- Liang M, Fu J. Triptolide inhibits interferon-gamma-induced programmed death-1-ligand 1 surface expression in breast cancer cells. Cancer Lett 2008; 270(2):337-41. doi: 10.1016/j.canlet.2008.05.025 [Crossref] [ Google Scholar]
- Bishayee A, Ahmed S, Brankov N, Perloff M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front Biosci (Landmark Ed) 2011; 16:980-96. doi: 10.2741/3730 [Crossref] [ Google Scholar]
- Zhang GP, Lu YY, Lv JC, Ou HJ. [Effect of ursolic acid on caspase-3 and PARP expression of human MCF-7 cells]. Zhongguo Zhong Yao Za Zhi 2006; 31(2):141-4. [ Google Scholar]
- Kassi E, Sourlingas TG, Spiliotaki M, Papoutsi Z, Pratsinis H, Aligiannis N. Ursolic acid triggers apoptosis and Bcl-2 downregulation in MCF-7 breast cancer cells. Cancer Invest 2009; 27(7):723-33. doi: 10.1080/07357900802672712 [Crossref] [ Google Scholar]
- Cheok CY, Salman HA, Sulaiman R. Extraction and quantification of saponins: a review. Food Res Int 2014; 59:16-40. doi: 10.1016/j.foodres.2014.01.057 [Crossref] [ Google Scholar]
- Podolak I, Galanty A, Sobolewska D. Saponins as cytotoxic agents: a review. Phytochem Rev 2010; 9(3):425-74. doi: 10.1007/s11101-010-9183-z [Crossref] [ Google Scholar]
- Yokosuka A, Jitsuno M, Yui S, Yamazaki M, Mimaki Y. Steroidal glycosides from Agave utahensis and their cytotoxic activity. J Nat Prod 2009; 72(8):1399-404. doi: 10.1021/np900168d [Crossref] [ Google Scholar]
- Zhang C, Li B, Gaikwad AS, Haridas V, Xu Z, Gutterman JU. Avicin D selectively induces apoptosis and downregulates p-STAT-3, bcl-2, and survivin in cutaneous T-cell lymphoma cells. J Invest Dermatol 2008; 128(11):2728-35. doi: 10.1038/jid.2008.138 [Crossref] [ Google Scholar]
- Xu Y, Chiu JF, He QY, Chen F. Tubeimoside-1 exerts cytotoxicity in HeLa cells through mitochondrial dysfunction and endoplasmic reticulum stress pathways. J Proteome Res 2009; 8(3):1585-93. doi: 10.1021/pr801001j [Crossref] [ Google Scholar]
- Zhou X, He X, Wang G, Gao H, Zhou G, Ye W. Steroidal saponins from Solanum nigrum. J Nat Prod 2006; 69(8):1158-63. doi: 10.1021/np060091z [Crossref] [ Google Scholar]
- Siu FM, Ma DL, Cheung YW, Lok CN, Yan K, Yang Z. Proteomic and transcriptomic study on the action of a cytotoxic saponin (Polyphyllin D): induction of endoplasmic reticulum stress and mitochondria-mediated apoptotic pathways. Proteomics 2008; 8(15):3105-17. doi: 10.1002/pmic.200700829 [Crossref] [ Google Scholar]
- Cao S, Norris A, Miller JS, Ratovoson F, Razafitsalama J, Andriantsiferana R. Cytotoxic triterpenoid saponins of Albizia gummifera from the Madagascar rain forest. J Nat Prod 2007; 70(3):361-6. doi: 10.1021/np060506g [Crossref] [ Google Scholar]
- Mujoo K, Haridas V, Hoffmann JJ, Wächter GA, Hutter LK, Lu Y. Triterpenoid saponins from Acacia victoriae (Bentham) decrease tumor cell proliferation and induce apoptosis. Cancer Res 2001; 61(14):5486-90. [ Google Scholar]
- Liu LP, Cao XC, Liu F, Quan MF, Sheng XF, Ren KQ. Casticin induces breast cancer cell apoptosis by inhibiting the expression of forkhead box protein M1. Oncol Lett 2014; 7(5):1711-7. doi: 10.3892/ol.2014.1911 [Crossref] [ Google Scholar]
- Wang LM, Xie KP, Huo HN, Shang F, Zou W, Xie MJ. Luteolin inhibits proliferation induced by IGF-1 pathway dependent ERα in human breast cancer MCF-7 cells. Asian Pac J Cancer Prev 2012; 13(4):1431-7. doi: 10.7314/apjcp.2012.13.4.1431 [Crossref] [ Google Scholar]
- Vashisth P, Kumar N, Sharma M, Pruthi V. Biomedical applications of ferulic acid encapsulated electrospun nanofibers. Biotechnol Rep (Amst) 2015; 8:36-44. doi: 10.1016/j.btre.2015.08.008 [Crossref] [ Google Scholar]
- Watabe M, Hishikawa K, Takayanagi A, Shimizu N, Nakaki T. Caffeic acid phenethyl ester induces apoptosis by inhibition of NFkappaB and activation of Fas in human breast cancer MCF-7 cells. J Biol Chem 2004; 279(7):6017-26. doi: 10.1074/jbc.M306040200 [Crossref] [ Google Scholar]
- Xie J, Xu Y, Huang X, Chen Y, Fu J, Xi M. Berberine-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species generation and mitochondrial-related apoptotic pathway. Tumour Biol 2015; 36(2):1279-88. doi: 10.1007/s13277-014-2754-7 [Crossref] [ Google Scholar]
- Zupkó I, Réthy B, Hohmann J, Molnár J, Ocsovszki I, Falkay G. Antitumor activity of alkaloids derived from Amaryllidaceae species. In Vivo 2009; 23(1):41-8. [ Google Scholar]
- Bharadwaj U, Eckols TK, Kolosov M, Kasembeli MM, Adam A, Torres D. Drug-repositioning screening identified piperlongumine as a direct STAT3 inhibitor with potent activity against breast cancer. Oncogene 2015; 34(11):1341-53. doi: 10.1038/onc.2014.72 [Crossref] [ Google Scholar]
- Lou C, Yokoyama S, Saiki I, Hayakawa Y. Selective anticancer activity of hirsutine against HER2-positive breast cancer cells by inducing DNA damage. Oncol Rep 2015; 33(4):2072-6. doi: 10.3892/or.2015.3796 [Crossref] [ Google Scholar]
- Xiao D, Bommareddy A, Kim SH, Sehrawat A, Hahm ER, Singh SV. Benzyl isothiocyanate causes FoxO1-mediated autophagic death in human breast cancer cells. PLoS One 2012; 7(3):e32597. doi: 10.1371/journal.pone.0032597 [Crossref] [ Google Scholar]
- Kessler JH, Mullauer FB, de Roo GM, Medema JP. Broad in vitro efficacy of plant-derived betulinic acid against cell lines derived from the most prevalent human cancer types. Cancer Lett 2007; 251(1):132-45. doi: 10.1016/j.canlet.2006.11.003 [Crossref] [ Google Scholar]
- Hordyjewska A, Ostapiuk A, Horecka A, Kurzepa J. Betulin and betulinic acid: triterpenoids derivatives with a powerful biological potential. Phytochem Rev 2019; 18(3):929-51. doi: 10.1007/s11101-019-09623-1 [Crossref] [ Google Scholar]
- Lambertini E, Lampronti I, Penolazzi L, Khan MT, Ather A, Giorgi G. Expression of estrogen receptor alpha gene in breast cancer cells treated with transcription factor decoy is modulated by Bangladeshi natural plant extracts. Oncol Res 2005; 15(2):69-79. [ Google Scholar]
- Li Z, Wang Y, Mo B. [The effects of carotenoids on the proliferation of human breast cancer cell and gene expression of bcl-2]. Zhonghua Yu Fang Yi Xue Za Zhi 2002; 36(4):254-7. [ Google Scholar]
- Gopal YN, Arora TS, Van Dyke MW. Parthenolide specifically depletes histone deacetylase 1 protein and induces cell death through ataxia telangiectasia mutated. Chem Biol 2007; 14(7):813-23. doi: 10.1016/j.chembiol.2007.06.007 [Crossref] [ Google Scholar]
- Salisbury CM, Cravatt BF. Optimization of activity-based probes for proteomic profiling of histone deacetylase complexes. J Am Chem Soc 2008; 130(7):2184-94. doi: 10.1021/ja074138u [Crossref] [ Google Scholar]
- Qiu YK, Dou DQ, Cai LP, Jiang HP, Kang TG, Yang BY. Dammarane-type saponins from Panax quinquefolium and their inhibition activity on human breast cancer MCF-7 cells. Fitoterapia 2009; 80(4):219-22. doi: 10.1016/j.fitote.2009.01.011 [Crossref] [ Google Scholar]
- Chaudhry GE, Kassim MNI, Zafar MN, Mohammad H, Andriani Y, Ismail N. Induction of apoptosis by Stichopus chloronotus and Holothuria nobilis fractions in human cervical cancer cell line, HeLa. Int J Res Pharm Sci 2020; 11(1):1238-47. doi: 10.26452/ijrps.v11i1.1964 [Crossref] [ Google Scholar]