Advanced pharmaceutical bulletin. 12(1):17-33.
doi: 10.34172/apb.2022.007
Review Article
A Recent Update: Solid Lipid Nanoparticles for Effective Drug Delivery
Sonia Pandey 1, *  , Farhinbanu Shaikh 1, Arti Gupta 1
, Farhinbanu Shaikh 1, Arti Gupta 1  , Purnima Tripathi 2, Jitendra Singh Yadav 3
, Purnima Tripathi 2, Jitendra Singh Yadav 3 
Author information:
1Maliba Pharmacy College, Uka tarsadia University, Bardoli Mahuva Road, Surat 394350, Gujarat, India.
2Department of Pharmaceutics, Bundelkhand University, Jhansi, U.P India.
3Shree Naranjibhai Lalbhai Patel College of Pharmacy, Umarkh, Surat 394350, Gujarat, India.
Abstract
Solid lipid nanoparticles (SLNs) are one of the developed technologies for addressing the bioavailability and targeting issues of drug delivery. In this review article, we attempted to incorporate all the essential details of SLNs like various methods of preparation, different models of SLNs, updated characterization methods, in vivo behavior (uptake), their applications, route of administration as well as advancements taken place in the field of delivery of biological drugs like gene vector, new adjuvant for vaccines, protein, and peptide with SLNs. Surface modified SLNs hold excellent potential for targeted and controlled drug delivery which is discussed and summarized. Based on the available data, the future success of SLNs is widened because they could be easily fabricated with various functionalities which would display enormous potential for targeting and diagnosing various diseases. This review would help the budding researchers to find out the unexplored areas of SLNs with the present discussion that reframes the potential of SLNs by gathering the various research findings of SLNs in tabular form along with the approved patent technologies of SLNs.
Keywords: Solid lipid nanoparticles (SLNs), Method of preparation, Route of administration, Biological drugs, Surface modified SLNs, Patents
Copyright and License Information
©2022 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
In the most recent years, nanotechnology has influenced all technical fields, including drug delivery systems. Modern drug delivery technology is growing rapidly. For the deepest interpretation and association with biotechnology, biomedical engineering and nanotechnology solid lipid nanoparticles (SLNs) extends their application in care and diagnosis.
1
Formulation scientists are facing challenges in improving the solubility and bioavailability of the newly invented drugs. Lipid nanoparticle presents a successful approach in resolving the solubility and bioavailability issues.Nanotechnological applications in medicine
2
as compared to other colloidal carriers, lipids are biocompatible, biodegradable, and mostly they comprise physiological components which are generally regarded as safe (GRAS).Insoluble drug delivery strategies: review of recent advances and business prospects
3,4
SLNs as a colloidal carrier have proved their potential by surpassing the limitations of other carriers from the early 1990s.
5
Several potent formulations do not show success in therapy, leading to an increase in the rejection rate of API from the FDA. Factors that contribute to treatment failure include low absorption and fast metabolism, indiscriminate drug distribution leading to insufficient drug concentration (e.g. peptides, proteins), BCS class II and IV drugs (excluding I.V aqueous injectable solution), and unpredictable bioavailability.
6,7
To improves the therapy success rate, instead of developing or focusing on a new molecule, it would be cost-effective to do the suitable modification in drug molecule with an existing colloidal carrier like SLNs. The SLNs structure (Figure 1) is made up of lipid, which may contain triglycerides, glyceride blends, or waxes that are solid at both room temperature and human body temperature.
8
SLNs also contain different surfactants and co-surfactants to enhance the stability in the concentration range of 0.5% to 5%. Commonly used lipids are listed in Table 1. Due to the presence of solid lipid and submicron-sized nanoparticles, SLNs show less toxicity and easily attain sustained release.
9,10
The reticuloendothelial system cells are not taken up immediately, particularly those between 50–200 nm, and thus bypass the liver and spleen filtration.
11
SLNs also offer the advantage of controlled and targeted release because the surface of solid lipid can be easily tailored with suitable ligands and polymers.
12
Incorporation of active compounds into the solid matrix of SLNs offers stability against chemical degradation and environmental factors.
13
Both hydrophilic and lipophilic drugs can be easily incorporated into the matrix of solid lipid.
14,15
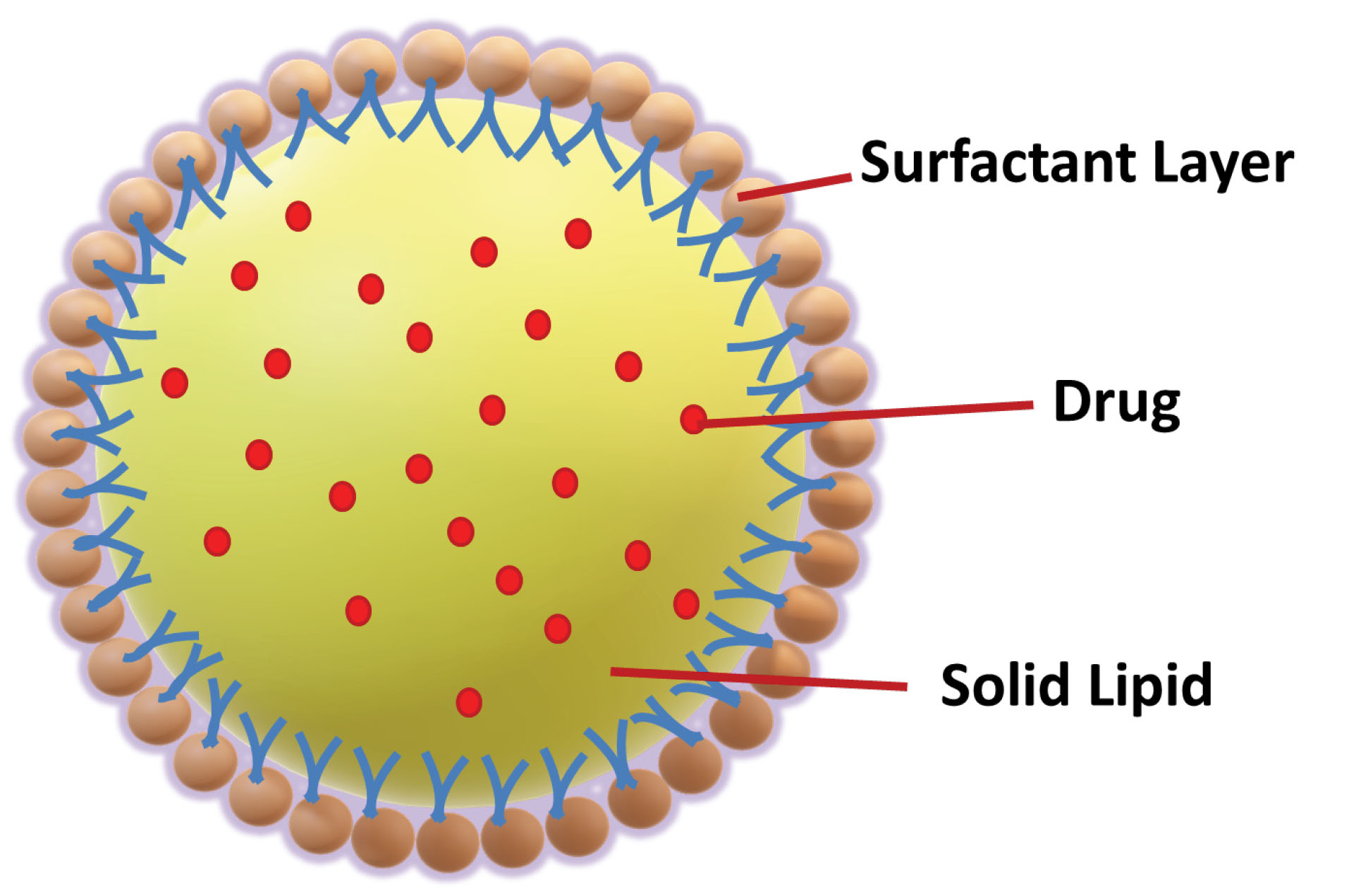
Figure 1.
Schematic figure of solid lipoid nanoparticles.
.
Schematic figure of solid lipoid nanoparticles.
Table 1.
Lipid used for solid lipid nanoparticle preparation
|
Lipids
|
Examples
|
|
Triglycerides
16,17
|
Trilaurin, Tricaprin, Hydrogenated coco glycerides (Softisan®142), Tripalmitin [Dynasan® 116, Tristearin [Dynasan® 118, Trimyristin [Dynasan®114 |
|
Fatty Acids
18
|
Dodecanoic acid, Myristic acid, Palmitic acid, Stearic acid |
|
Monoglycerides
18
|
Glyceryl monostearate, Glyceryl hydroxyl stearate, Glycerylbehenate |
|
Waxes
17
|
Cetyl palmitate, Beeswax, Carnauba wax |
Despite several advantages, certain drawbacks are also reported for SLNs which include (a) poor drug loading capacity especially for the hydrophilic drug (b) limited solubility of drugs in the lipid melt (c) chances of drug expulsion and particle aggregation after polymeric transition during storage.
19-21
A comparison of benefits of SLNs over liposomes and other polymeric systems is summarized in Table 2.
Table 2.
Benefits of SLNs with respect to liposome and polymeric nano-systems
|
Points to consider
|
Benefits of SLNs over liposomes
|
Benefits of SLNs over polymeric Nano-systems
|
| Organ Distribution |
SLNs High bioactivity is in the spleen while Liposomes are more active in the liver due to the flexibility difference of both formulations.
22
|
SLNs do not have undesirable effects unlike polymeric nanoparticles such as accumulation in various organs like the spleen, liver, etc. which leads to unwanted effects.
23
|
| Flexibility in the selection of preparation method |
The use of organic solvents can be avoided by the selection of a suitable method with scale-up and reproducible properties. |
Homogenization is an aqueous-based scalable method available for the production of SLNs.
24
|
| Target ability |
Both Surfaces modified liposomes and SLNs can be used for site-specific delivery but very less work is reported on gene delivery with liposomes due to various cellular barriers like the liposome-cargo-barrier interaction, binding of the liposome to the cell surface, liposome entry into the cells by endocytosis, or direct traversing of the plasma membrane, escape of the liposome from the endosome and dissociation of the liposome to release the nucleic acid payload.
25
|
Surface modified SLNs offer site-specific delivery for the drugs as well as protein, DNA, and RNA while polymeric nanoparticles may produce nonspecific drug delivery and still more work is to be done on a tailored synthetic approach for gene delivery.
26
|
In vivo behavior of SLNs
The portal circulation facilitates accessibility of the administered drug into the systemic circulation. To understand the lipid digestion and absorption processes associated with the delivery of lipophilic drugs which play a crucial role in the transport of drugs to the lymphatic system we should understand the physiology of lipid digestion and absorption.
27
In Vivo behavior of SLNs is reflected in Figure 2.
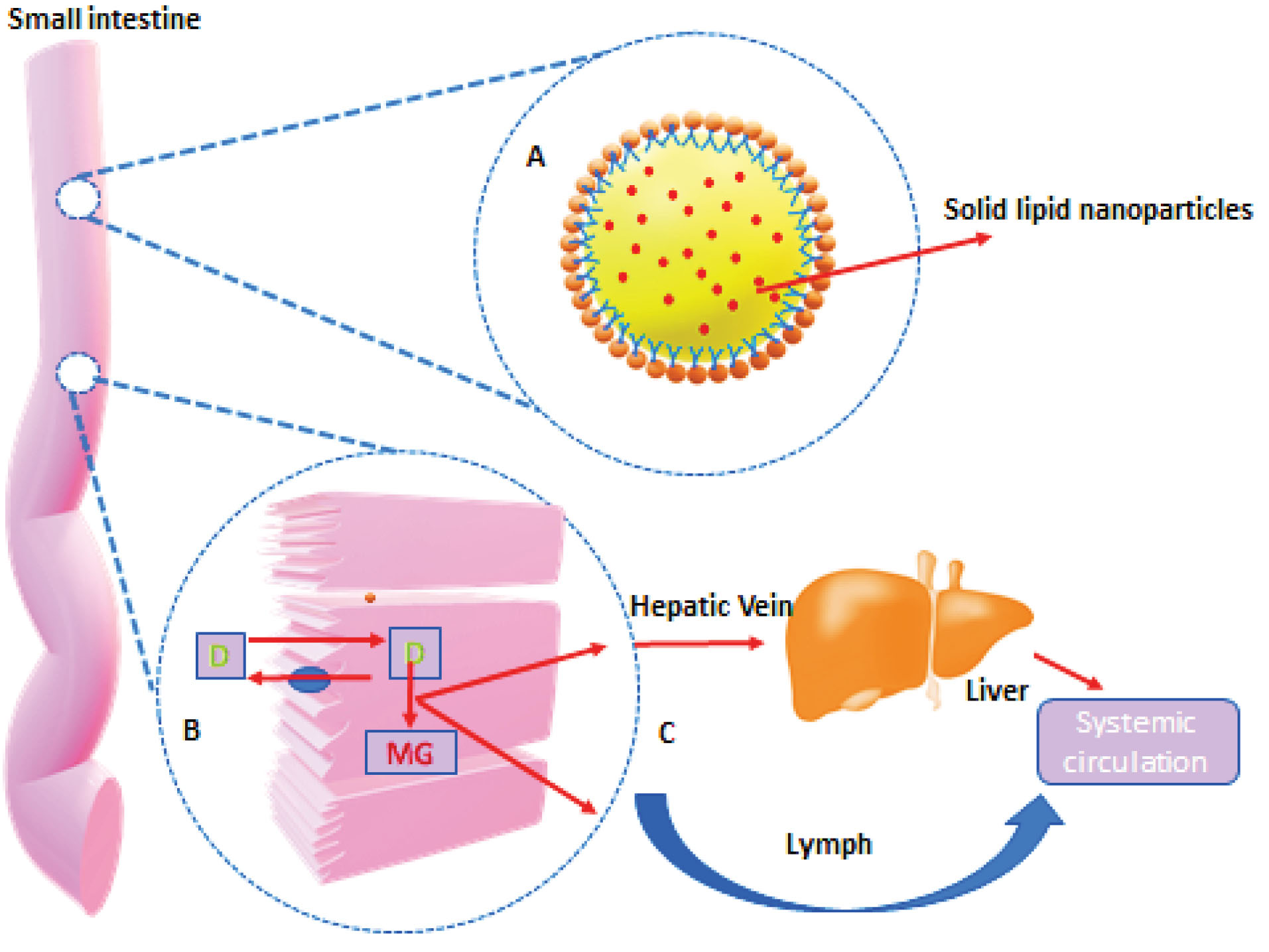
Figure 2.
In vivobehavior of SLNs.
.
In vivobehavior of SLNs.
Lipid digestion starts in the oral cavity by the action of lingual lipases. Digestion continues in the stomach by the action of both lingual and gastric enzymes. Initially formed lipid emulsion of lipid enters in duodenum in the form of fine droplets and undergoes various chemical and physical changes by the actions of bile and pancreatic juices. Bile and pancreatic juices provide pancreatic lipase, bile salts, and colipase for the effective digestion and absorption of lipids. In the duodenum micellization along with emulsification and hydrolysis continues to promote absorption through the intestinal wall.
28
Digestion and absorption of triacylglycerides (TAGs)
TAGs are primarily digested by the pancreatic lipase in the upper part of the jejunum. Pancreatic lipase acts on the surface of emulsion particles and converts TAGs into 2-monoacylglycerol (2-MAG) and free fatty acids (FFAs). 2-MAG is the major form in which MAG is absorbed from the small intestine. FFAs are absorbed from the intestinal lumen into the enterocytes. Here it is used to biosynthesize the neutral fats. A number of proteins are involved in the uptake and transport of FFAs.
Biosynthesis of TAGs
Once inside the enterocytes, specific binding proteins carry fatty acids and MAG to the intracellular site, for the biosynthesis of TAG.
In the case of SLNs, drug absorption through the lymphatic system is assisted by the lipid core of SLNs, which stimulates the formation of lipoprotein (chylomicrons) and absorbs free drugs associated with the lipoprotein. The lipoprotein (like chylomicrons) associated with hydrophobic drugs with a size <1 µm in diameter facilitates selective lymph transport in the intestines. The compound was also exposed during the absorption process to Cytochrome P450 3A4 (CYP 3A4) enzymes found in enterocytes at higher concentrations and studies proved the role of these enzymes to improve drug bioavailability in the use of lipids.
29,30
Suzanne M. Calip has reported in her research that lymphatic transport of extreme lipophilic drugs (log P > 5, solubility in triglycerides (TG) > 50 mg/mL) was strongly correlated with the TG content of the lymph.
31
Drugs with limited solubility (BCS II & IV) are suitable candidates for SLNs. Due to the presence of lipids, SLNs showed increased bioavailability because lipids are consumed by intestinal lymph (dietary or lipid dependent formula) and in combination with long-chain TGs transported (Lipid core formed into enterocytes of the intestinal lipoprotein after FA and MG re-esterification). Co-administration of lipid with drug promotes the synthesis of lipoprotein and therefore it enhances the lymphatic drug transport of drug.
32,33
Lymphatic fluid (average 3 L a day) is pumped into the subclavian vein through a thoracic duct to shield this medicament from first-pass hepatic metabolism. Dispersed structures such as micelles or mixed micelles may be available in a circulatory system in their free form. When combined with significant quantities of blood/lymph, the concentration of the surfactant will decrease below its critical micelles concentration and micelles may dissociate into monomers through it helps the drug transported as lipid vesicles in intact form over an extended period, and it leads to prolonging the release of the entrapped drug.
34,35
Drug loading model and release pattern from SLNs
Based on the various production methods of SLNs and as described by Müller et al, three types of SLNs are reported for drug incorporation.
18,34-39
Details of all types of SLNs with their properties and applications are summarized in Table 3.
Table 3.
Summary of drug loading models in SLNs
|
Model
|
Properties
|
Applications
|
References
|
| Drug-enriched shell model |
The lipid center is surrounded by a drug-enriched outer shell. |
(a) Suitable for potent drugs.
(b) Suitable model for burst release
|
18,34-37
|
| Drug-enriched core model |
The drug is concentrated in the core of SLNs. |
(a) Suitable for high-dose drugs.
(b) This SLN model is desirable for burst release as in the case of dermal preparation along with occlusive effect.
|
38-39
|
| Homogenous matrix model or solid solution model |
Drugs within the melted lipid are dispersed in the core of SLNs in amorphous clusters or molecularly dispersed phases. |
The model is suitable for a highly lipophilic drug. |
40
|
The SLNs are composed of physiological lipids in the submicron size range (50–1000 nm) and at room temperature, the particles are in the solid-state, which helps to reduce the mobility of entrapped drugs, which is a prerequisite for controlled drug release.
41,42
The common ideology of drug release from any nanoparticle reflects that the release is affected by particle size and the type of drug entrapment model of SLNs. The release of drugs can be affected by parameters like drug solution and its relationship with the lipid matrix.
43
The release profile of SLNs can be modified in response to external and internal stimuli by temperature transition. Chen et al investigated the pH-sensitive release profile of doxorubicin-loaded cholesterol-PEG coated SLNs and found the accelerated drug release of doxorubicin at pH 4.7 compared to pH 7.4. The author had concluded that the protonation of negatively charged lipid core lauric acid to the positively charged doxorubicin leads to depletion of electrostatic attractions which promotes the release profile at lower pH microenvironment of cancerous tissue.
44
Generally burst release was observed with SLNs.
35
The burst release of the drug could be reduced with increasing particle size and prolonged release will be achieved.
45
zur Mühlen et al had taken tetracaine, etomidate, and prednisolone as a model drug and reported that due to large surface area and drug augmentation in the outer shell, tetracaine and etomidate SLNs were detected with a burst drug release (100 % release <1 minute). In contrary to this data, 5 weeks prolonged release was reported with prednisolone-loaded SLNs. Due to the different chemical behavior of the lipid matrix-like cholesterol and compritol, burst (83.8%) and controlled releases (37.1%) were achieved respectively.
46
Olbrich and Muller
47
reported that lipid matrix degraded by lipases requires a lipid interface for enzyme activation. To modify the release and increase the stability of SLNs appropriate steric stabilizers and other surfactants should be optimized and therefore surface modification with the hydrophilic carrier (like PEG) is suggested so that SLNs surface will not be recognized easily by lipase enzymes. Savla et al had recently mentioned in their review that drugs with a Log P value of 2 and high melting point (numerically not defined) are usually poor candidates for lipid systems.
48,49
Lipid-based formulations are an excellent carrier for the highly lipophilic drug (Log P>5) (BCS Class-II). In support of this Chen et al also proposed
50
the following drug profile for lipid formulations: hydrophilicity (water solubility) <10 mcg/mL; Log P >5; solubility in oils and lipids >25 mg/mL; relatively low melting point; and good chemical stability. However, there are inadequate studies reported especially relative studies for the group of drugs having Log P 2-5.
Method of preparation
High energy approaches
High-pressure homogenization (HPH)
HPH includes two types of methods one is hot homogenization, and another one is cold homogenization. Both hot and cold method involves a preliminary step of dissolving or dispersing the drug in solid lipid melt.
51
The HPH method includes a high-pressure chamber piston and a narrow gap. The pressure piston can make the pressure of 10–500 mPa. A narrow gap in the HPH is the place from where the primary emulsion will be forced to go through the valve and in the valve’s limited territory the emulsion drops will be reduced into small sizes.
52,53
Hot homogenization method:At the lab-scale, this is the most accepted method to formulate the SLNs. By the addition of lipid melt holding the drug to an aqueous phase containing emulsifier with the addition of energy of high shear homogenizer at 500-1500 bar pressure, a pre-emulsion is formed with reduced size. The hot colloidal O/W emulsion forms which lead to the forming of SLNs after cooling the lipid melt dispersion in the globules.
Cold homogenization method:To address problems with hot homogenization processes like (a) thermolabile drugs cannot handle with high temperature, (b) drug loss during its distribution in the aqueous phase, and (c) complex crystalline structure of lipid
54-56
cold homogenization mechanism has been introduced
Ultrasonication technique
This method requires the addition of homogenization or stirring steps to avoid the particle size growth due to broader particle size distribution.
57
Ultrasonication method is also known as the High-speed Homogenization method.
4,18
Electrospray technique
With the electrospray technique to date, more than 30 polymers have been effectively electrospun. The fundamental setup for electrostatic atomization includes a spout associated with a high-voltage control supply, provided with a fluid to be atomized.
58-60
In general the solution of the matrix is filled in the syringe with a metal capillary, which is attached to an electrode with high power supply. A collector, made up of foil is placed opposite the metal capillary to act as a counter electrode.
60
Low energy approaches
Microemulsion method
The word microemulsion was initially proposed by Schulman et al.
61
Microemulsions are the two-phase systems. An emulsifier (e.g. polysorbate 80), a co-emulsifier (e.g. butanol), and water are an important parts of typical micro-emulsions. They are an optically transparent mixture.
62
Membrane contractor method
An effective module, including a Kerasep clay film (0.1, 0.2, 0.45 μm pore estimate), has been recognized for this process., which isolates the water phase, allowed the digression into the layer surface, and lipid phase, allows it to move digressively to the layer surface. The lipid phase is heated above its melting point by a pressure vessel, passed through the module through a cylinder, and squeezed via membrane pores to allow smaller particles to form. After cooling, SLNs are formed in an aqueous phase.
60,63
The particle size can be managed by controlling the process parameter like lipid content, lipid phase pressure, and aqueous cross-flow velocity. Smaller size SLNs are obtained by keeping the aqueous phase temperature below the lipid’s melting point, and this is because the lipidic phase solidifies suddenly in an aqueous phase.
64
Charcosset et al used membrane contactors for the formulation of SLNs. The merit of this new process of SLNs appeared to be its feasibility of utilization, and control of particle size can be achieved with suitable process parameters and easy scale-up ability.
63
Phase inversion temperature (PIT) method
The essential elements of the phase inversion temperature method are mechanical emulsification at the Phase inversion temperature followed by sudden cooling to room temperature, where an emulsion with the large number of small droplets is found.
65
In this method, two main components are used one is the oil phase containing solid lipids and nonionic surfactant, and another is an aqueous phase containing NaCl. Both phases are heated at ~90°C (above phase transition temperature). With constant stirring and temperature, an aqueous phase is added drop-wise to the oily phase to obtain W/O Emulsion. Then the mixture is allowed to cool at room temperature under continuous stirring. At the phase inversion temperature, turbid mixture gets cleared and below the PIT O/W nanoemulsion is formed. The stability of the lipid nanoparticles after fabrication depends on the storage temperature relative to the PIT and melting/crystallization points.
66
Coacervation method
This is the solvent-free technique for the production of SLNs by the acidification of salt of micelles. When pH is low fatty acids start precipitating as a result of proton transfer between the solution of acid and soap. This method is widely used to formulate polymeric nanoparticles. Nanoparticles in the range of 250-500 nm size with spherical shape are obtained with this method.
67,68
Double emulsion method
This method is mainly used for hydrophilic drugs. The drug is dissolved in an aqueous phase and emulsified in melted lipid. Primary emulsion is formed, and that primary emulsion is stabilized by using appropriate surfactants and co-surfactants. Then the primary emulsion will be dispersed in an aqueous phase containing an aqueous emulsifier like PVA.
69
Approaches with organic solvents
Solvent emulsification evaporation technique
In this technique, lipid and drug are dissolved in an organic solvent (e.g. cyclohexane, dichloromethane, toluene, chloroform) followed by emulsification using high-speed homogenizers in an aqueous process. The coarse emulsion was quickly passed through a microfluidizer to increase the efficiency of emulsification. By mechanical mixing at room temperature and reduced pressures (e.g., rotatory evaporators), the naturally solubilized content disappears leaving SLNs lipid precipitates.
11,34
Solvent emulsification diffusion technique
In this method, water-miscible organic solvent is used (e.g., methyl acetate, isopropyl acetate, benzyl alcohol, ethyl acetate, butyl lactate). The initial saturation of both aqueous and oil phases maintains the initial thermodynamic balance of both phases.
70
Supercritical fluid (SCF) technique
Being productive and environment friendly, the supercritical liquid-based technique proved its efficiency and an efficient substitute over the conventional techniques for the production of SLNs for molecule generation. The supercritical liquid innovation removes the wide setting and assembly constraints for the production of SLNs related to other techniques but to produce the nanometer scale SLNs has been challenging.
71
SCF is defined as a substance that existed above its critical temperature (TC) and critical pressure (PC). The critical point represents the highest temperature and pressure at which the substance can exist as a vapour and liquid in equilibrium. The SCF has unique thermo-physical properties which can be changed easily by small changes in the pressure since the pressure increases the power of the fluid to dissolve compounds increases while the viscosity remains constant. In the supercritical range under high pressure and a sufficient temperature, the fluid can act as an alternative to organic solvents and dissolve different drugs and lipids.
72
SCF like carbon dioxide is safe, cheap, non-irritable, and generally inactive, and has a low critical point. The strategy frequently yields particles in the micrometer run and is regularly joined with another homogenization system like ultrasound.
73
Solvent injection technique
A fundamental principle of this method is the precipitation of dissolved lipid in a solution.
74
Solid lipid is dissolved in an organic solvent, and the mixture is injected with a syringe into the stirred aqueous phase having surfactant. Obtained dispersion is filtered to remove any excess amount of lipid. The aqueous emulsifier helps to produce lipid droplets at the injection site and also assists in stabilizing the SLNs by reducing the surface tension between the water and lipid phase.
75
Spray drying
It is a less expensive and alternative procedure of lyophilization. This strategy causes aggregation of molecules because of high temperature, shear force, and partial melting of the particles.
9
The effect of spray drying on the W/O/W double emulsion of methyl testosterone loaded stearic acid matrix has been stated by Mlalila et al.
76
The lipid usage with a melting point >700°C for spray drying was recommended by Freitas and Muller. SLNs have provided the best results with 1% solution of trehalose in water or 20% in ethanol-water mixtures (10/90 v/v).
77
SLNs characterization
Characterization of SLNs is a key parameter for the successful development of drug delivery. The physiochemical parameters like size, surface charge, molecular weight, and solubility have a profound effect on the uptake and distribution of lipid-based nano-formulations by the lymphatic system, so all these parameters need to be critically characterized.
Surface charge and particle size
The most frequently used methods for calculating particle size are photon correlation spectroscopy (PCS) and laser diffraction (LD).
5,9
PCS was previously known as quasi-elastic light scattering and currently known as dynamic light scattering. PCS measures the scattered light intensity, fluctuated by the mobile molecules.
78,79
PCS can be used to detect only nanoparticles; limitation arises with more considerable micro particles determination. In light scattering (LD), the diffraction angle of the particle radius is measured. Larger particles cause less scattering of light as compared to smaller particles. LD covers a broad range of particle size. Zeta potential of electrokinetic properties of particles is the ability of colloids to move under an electric field.
80
The colloidal suspension can be stabilized by electric repulsion at higher zeta potential (e.g. more than 30 mV or less than -30 mV). Electric repulsion normally leads to less interaction and lower aggregation of particles.
Crystallinity and lipid modifications
The crystallization of solid lipid leads to gelling or expulsion of the incorporated drug, so this parameter needs to be critically evaluated. Crystallization behavior and kinetic energy of lipids after polymorphic modifications in the scattered state differ from their mass material.
81
Basic methods which are used to analyze radiation geometric scattering from planes of crystal within a solid permitting degree of crystallinity to be assessed with X-ray diffraction and differential scanning calorimetry (DSC).
6,7,82
DSC works on the fact that different lipids modifications have different melting points and melting energy.
5
Infrared Radiation spectroscopy and Raman spectroscopy both techniques are used to find out the structural properties of lipids.
83
Where CrI is the relative degree of crystallinity, I002 is the maximum intensity (in arbitrary units) of the I002 lattice diffraction and lam is the intensity of diffraction in the same units at 28 = 18°.
84
Powder X-ray diffraction
X-ray is a result of constructive interference between the monochromatic X-rays and sample x-rays are generated by cathode tube filtered to produce the monochromatic waves and directed towards the sample.
85,86
Entrapment efficiency and loading capacity
Lipid and aqueous phase separation is the key parameter to determine the amount of drug entrapped per unit weight of lipid nano-carrier. Ultrafiltration,
87
Centrifugation filtration,
88
and dialysis
89
are employed for the separation. Drug loading (%DL) capacity of lipid nanoparticles depends on various factors like; solubility and polymorphic state of lipid material.
90
The high lipid solubility of the drug is the requirement for adequate loading capability to be achieved. The drug solubility in lipid must be higher than desired because it decreases during the cooling step of the process. Mono and diglyceride components of used lipid facilitate the drug solubilization. Lipids that form the crystalline particles with defined lattice leads to drug expulsion.
36,45,91
zur Mühlen et al have studied the effect of drug loading and drug incorporation over the release profile and lipid matrix structure of SLNs with three model drugs (tetracaine, etomidate, and prednisolone). In the first two drugs (tetracaine and etomidate) 10% drug loading was achieved with Compritol 888 ATO, but prednisolone SLNs with cholesterol and compritol could incorporate only up to 3.6% and 1.67% respectively.
46
Generally only 5-10% drugs can usually be incorporated despite this fact as in the case of ubidecarenone a coenzyme Q10, 40% drug loading was reported and more than 50% higher concentration can also be incorporated in the dispersed phase.
92
Morphological characterization
Direct imaging (shape) and dimensional analysis (size) of nanoparticles can be accomplished by transmission electron microscope (TEM) and scanning electron microscope (SEM) methods because of the higher resolution power and pace. Transmission electron microscopy has a higher resolution power than SEM because of its electron energy at above 100 KeV.
93,94
TEM allows visualization of nanoparticles after freeze fracturing and freeze substitution.
95,96
Atomic force microscopy has drawn attention in imaging, for instance, imaging of fibrinogen polymerization, imaging of growing infection in an infected cell, and imaging of in-vitro degradation of polymer surfaces and polymer nanoparticles were performed.
6,7
Structure and drug distribution of SLNs
To find out the qualitative nature and size of nanoparticle nuclear magnetic resonance can be used. The selectivity of this method is due to chemical shift which gives the sensitivity to the molecular mobility which provides physicochemical properties of components within the nanoparticles.
45
In vitro drug release study
From the lipid matrix, the drug release occurred by the diffusion mechanism. The critical factors influencing drug release from SLNs are the method of preparation, drug solubility in the lipid, drug/lipid interactions, type of surfactant, composition of lipid matrix, and particle size. The in-vitro release profile helps to uncover the mechanism of drug release and its kinetic behavior.
95,96
Typically SLNs show biphasic release profile, burst release followed by controlled. Immediate release effect was observed in SLNs during the beginning of release profile because the adherent drug on the SLNs surface will disperse from the nanoparticle, after that the lipid matrix starts to degrade and release the drug in a controlled manner.
46
Dialysis tubing
In the pre-washed dialysis tubing, the stable lipid nanoparticle dispersion may be hermetically set. At room temperature the dialysis sac dialyzed with an appropriate medium; The samples are at reasonable intervals pulled back from the dissolution medium, centrifuged, and observed for the drug content utilizing an appropriate analytical method.
19,70
In the normal dialysis technique, samples are taken from the outer compartment to find out the drug release from the nanoparticles. However, in contrast, in reverse dialysis samples are taken from the inner compartment to analyze the release profile, and nanoparticles are placed in the outer compartment with agitation to minimize the unstirred water layer.
19,64,97
Delivery of SLNs by different route of administration
Parenteral route of administration
Parenteral administration is the most suitable and studied route to deliver the SLNs, particularly for targeted cancer therapy.
98
For the efficient delivery of biotechnological products like protein and peptides parenteral route is most commonly preferred due to their enzymatic degradation in the gastrointestinal tract.
36
The injectable SLNs that have been studied so far were encapsulated with different therapeutic classes of drugs like anticancer agents, imaging agents, anti-parkinsonism, antibiotics, etc. First in vivostudy of SLNs loaded with anticancer drugs was carried out by Yang et al. They used camptothecin as an anti-cancer drug and studied its anticancer activity with SLNs, administered by Intravenous injection. The author concluded that SLNs have a higher residence time in the brain, heart, and reticuloendothelial cells.
99,100
After intravenous administration, doxorubicin-loaded stealth SLNs were detected only in the brain. On the other hand, after the injection of non-stealth stable lipid nanoparticles in rabbit mononuclear tissues (liver, lungs, spleen, kidney, and heart), the volume of doxorubicin present was always smaller.
101
Wang et al reported the chitosan nano layered cisplatin loaded SLNs to enhance cisplatin’s anti-cancer activity for the treatment of HeLa cell carcinoma. Results showed that the incorporation of cisplatin in solid lipid leads to an increase in its activity as evident from MTT cell assay. Data suggests that SLNs formulation is a better choice for cervical cancer.
102
Oral route of administration
The lipid structure of SLNs makes it suitable and interesting for the oral route of administration to increase the bioavailability by protecting the drug from chemical as well as enzymatic degradation, thereby delaying the in vivo metabolism.
103
Aqueous dispersion or conventional dosage forms, such as pellets, capsules, or tablets, are the oral dosage forms of SLNs. The conditions of gastric parts lead to particle aggregation due to the high concentration of acid and ionic strength present in the stomach.
50,104
Along with this fact influence of stomach and pancreatic lipase on SLNs degradation remains a question. Sarmento et al developed insulin-loaded SLNs for oral drug delivery by modified solvent emulsification evaporation method. The investigator noted that the hypoglycemic effect was observed in diabetic rats after oral administration of insulin-loaded SLNs and also it could be said that SLNs can promote the oral absorption of insulin.
105
Transdermal route of administration
The highest amount of lipid is found in the uppermost (epidermis) layer of the skin; therefore, All lipid nanoparticles quickly bind themselves to the surface of the skin and facilitate lipid exchange between the stratum corneum’s outer layers; and for topical and transdermal distribution, the carrier appears promising.
46,106
For the effective delivery of SLNs carrier to the skin, lipid amount must be kept at a low level.
9
A drug which undergoes the first-pass metabolism with high molecular weight is an ideal candidate for transdermal drug delivery. This route can provide drug release up to one week in a controlled manner.
107
Kurakula et al formulated and optimized avanafil (AVA) loaded SLNs with subsequent loading into hydrogel films for the transdermal delivery of AVA. The results suggested that transdermal drug delivery of AVA can be used as an alternative to peroral dosage form with increased bioavailability.
108
Nasal route of administration
The nasal route is a great alternative route for the systemic delivery of the drug, when it is restricted by the I.V. route, because of the higher surface area and presence of porous epithelial layers.
109
Nasal drug delivery system is an effective technique because of the following reasons: (a) Nose has a larger surface area for absorption of drugs due to the microvilli present on the surface of the nose (b) the subepithelial layer of the nasal mucosa is highly vascularized, and the blood flows directly from nose to systemic circulation.
110
SLNs could be an efficient delivery system for the treatment of CNS diseases like Parkinson’s and Alzheimer’s diseases. CNS bioactive compounds have the limitations like hydrophobicity, poor intestinal solubility/absorption, poor bioavailability, less effectiveness, and limitation to cross BBB (blood-brain barrier). To overcome all these limitations, the nanotechnology-based nasal route approach proposed the appropriate field for research.
111-114
Md et al have prepared bromocriptine (BRC) loaded chitosan nanoparticles (CS NPs) intended for the nose to brain delivery. The brain/blood ratio of BRC solution (i.n.), BRC loaded CS NPs (i.n.) and (i.v.) were found to be 0.47 ± 0.04, 0.69 ± 0.031, and 0.05 ± 0.01 respectively. The drug transport percentage and drug targeting efficiency for BRC loaded CS NP after the intranasal route was 84.2% ± 1.9% and 6.3 ± 0.8 respectively, which is very promising. Favorable results are suggestive of the direct nose to brain transport bypassing the BBB as compared with BRC solution i.n. and i.v.
115
Gupta et al recently reported the SLNs of non-nucleoside reverse transcriptase inhibitor efavirenz, used in HIV infections via intranasal delivery. Promising pharmacokinetic studies showed 70 times better relative bioavailability for the efavirenz loaded SLNs dispersion via i.v. route as compared to the orally administered powder drug which indicates its potential towards the complete eradication of HIV in infected patients.
116
Fatouh et al adopted a nasal route to avoid the first-pass metabolism of agomelatine to increase the bioavailability of the drug and to achieve the targeted nose to brain delivery. Results are supported with the data like peak plasma concentration, AUC (0-360 minutes), and absolute bioavailability as compared to that of the marketed oral product (Valdoxan®) with the values of 759.00 ng/mL, 7805.69 ng⋅min/mL, and 44.44%, respectively.
117
Pulmonary administrations
Among the pharmaceutical researches, pulmonary drug delivery is one of the most explored delivery systems. When a foreign particle enters in the lung, macrophages attack that particle and try to damage it. To prevent such damage, the most effective approaches include a stealth approach such as PEGylation or the usage of the endogenous compound which occurs in lung dipalmitoyl phosphatidylcholine (DPPC).
118
Ghanshyam et al reported triamcinolone acetonide loaded SLNs dry powder inhaler.
119
Bakhtiary et al demonstrated the formulation of dry powder inhaler of erlotinib (ETB)-loaded SLNs through hot homogenization method with compritol 888 ATO® and Tween 80 as the surfactant. The advanced formulation showed <100 nm particle size, PDI 0.367, and 78.21% encapsulation efficiency. Higher cytotoxicity was found with A549 cells. Finally, spray-dried microparticles with 1-5 µm aerodynamic size were produced for deep lung delivery.
120
Ocular route of administration
The eyes are among the most sensitive organs of our body, and hence drug delivery to eye tissues is especially dangerous.
16
SLNs displayed outstanding optical conveyance penetration properties. The discharge of the drug may be assisted or regulated into the ocular mucosa, which in contrast to conventional ophthalmic arrangements increased the pre-corneal maintenance time of the medication. The nanoscopic size of SLNs does not bring out any obscured vision. SLNs went for visual conveyance ought to need to meet explicit criteria, similar to visual safety (Draize rabbit eye test), sterility, isotonicity, and pH of suspension (like lachrymal liquid).
121
Chetoni et al had developed tobramycin (Tobra) loaded SLNs for ophthalmic treatment. The author had found profound results as compared with Tobral® commercial preparation. In aqueous humour tobramycin concentration is reported to increase by two and fivefold (P < 0.01) after 1 and 3 hours respectively. Due to small particle size and high viscosity, accumulation of the drug in the retina even after 1 hour was 17.2 μg/g which is three times more than that achieved with instillation (4.74 μg/g).
122
Tatke et al developed triamcinolone acetonide-loaded
SLNs in Situ Gel (TA-SLN-IG) for enhanced topical ocular delivery. The rheological and trans-corneal permeability for TA-SLN and TA-SLN-IG was 10.2 and 9.3 folds higher as compared to TA-control along with this higher tear concentration of 13.3 μg/mL at 2 hours is found which reflects an enhanced precorneal residence time (Table 4).
123
Table 4.
Various research findings of SLN formulations with their lipid, method of preparation, route of administration
|
Drug
|
Route of administration
|
Lipid
|
Size
|
%Entrapment Efficiency
|
Reference
|
| Curcumin |
I.V |
Compritol 888 ATO |
9.51nm |
- |
124
|
| CdSEe/ZnS |
I.V |
- |
- |
- |
125
|
| - |
I.V |
Stearic acid |
159-239 nm |
|
126
|
| Doxorubicin |
I.V |
Stearic acid |
80-90 ± 5 nm |
|
101
|
| Paclitaxel (PTX) and TOs-Cisplatin |
I.V |
Glyceride monostearate |
108.6 ± 3.1 nm |
90.3 ± 3.2% |
127
|
| Methotrexate (MTX) |
I.V |
Stearyl amine |
174.51± 5.1 nm |
84.3 ± 1.24 % |
128
|
| Nitrendipine (NDP) |
I.V and Intraduodenal |
Trimyristin, tripalmitin, tristearin, soy phosphatidylcholine 95% |
101.9 ± 3 nm |
99.8 ± 0.23 % |
129
|
| Idebenone |
I.V. route |
Cetyl palmitate |
30 -95 nm |
|
66
|
| Repaglinide (RG) |
Oral |
Stearic acid |
360± 2.5 nm (Solvent injection) 281±5.3 nm
(Ultrasonication)
|
62.14 ± 1.29% |
121
|
| Carbamazepine |
Oral |
Tristearin,
Phospholipon 80 H
|
168±1.8 nm |
62.14 % |
130
|
| Elvitegravir |
Oral |
Gelucire 44/14 |
151.0±2.4- 199.1±2.7 nm |
89.7±0.27% |
131
|
| Insulin |
Oral |
Cetyl palmitate |
361±30 nm |
46±6 % |
105
|
| Ramipril |
Oral |
Glyceryl monostearate, glyceryl monooleate |
104-334 nm |
72.5 ± 86.40% |
132
|
| Glibenclamide (GLI) |
Oral |
Precirol and compritol |
105.1±2.9-183.1±3.2 nm |
80±5% |
133
|
| Carvedilol (CVD) |
Oral |
Precirol ATO5 |
20±0.009 –
58±2.09 nm
|
78±5.17-94±3.71% |
134
|
| Buspirone HCl |
Oral |
Cetyl Alcohol |
345.7 nm |
---- |
135
|
| Donepezil (DPL) |
Intranasal |
Glyceryl monostearate |
121.0 nm |
67.95% |
136
|
| Agomelatine |
Intranasal |
Glyceryl tripalmitate, Gelucire 43/01, Glyceryl tristeratae, Stearic acid, Precirol, and Galeol |
220.90 ± 1.55-515.30±2.40 nm |
58.19± 8.10-93.68 ±3.4% |
117
|
| Rifabutin (RFB) |
P.A |
Glyceryl dibehenate, glyceryl tristearate |
92 ± 1 nm |
91.2±3.6% |
137
|
| Ethambutol (EMB) |
P.A |
Compritol |
56.25±2.05- 81.86±3.20 nm |
98.16±0.66-99.04±0.4% |
138
|
| Triamcinolone acetonide |
P.A |
Soya lecithin |
339.2 ± 1.85 nm |
58.23±1.8% |
119
|
| Naringenin (NRG) |
P.A |
Glyceryl monostearate |
98 nm |
79.11% |
139
|
| Paclitaxel (PTX) |
P.A |
|
|
|
140
|
| Avanafil (AVA) |
T.D |
Compritol 888, Cholesterol, Castor oil |
86 nm |
85.01% |
108
|
| Diclofenac Sodium (DS) |
T.D |
|
|
89% |
141
|
| Triptolide(TPL) |
T.D |
Compritol 888 ATO |
104 ± 1.82 nm |
92.8± 8.52% |
142
|
| Ivermectin (IVM) |
T.D |
Palmitic acid |
312.8 ±2.4 nm |
98.48± 0.052% |
143
|
| Isoniazid(INH) |
O.D |
Compritol 888:
Stearic acid(4:1)
|
316.5± 8.7 nm |
65.2± 2.2% |
144
|
| Natamycin (NAT) |
O.D |
Precirol ATO5 |
21.8- 47.48 nm |
41.06-83.2% |
145
|
| Cyclosporine |
O.D |
|
355±11- 487±32 nm |
71±1-100±1% |
146
|
| Alendronate |
P.A |
Compritol 888: |
<100 nm |
- |
147
|
| Triamcinolone Acetonide-(TA) |
O.D |
Stearic acid |
80±11.1 nm |
100% |
122
|
T.D=Transdermal Delivery, O.D= Ocular Delivery, P.A = Pulmonary administration
SLN: carrier for biological drug
The biological drugs do not hold the required physicochemical properties to get absorbed and enter target cells; therefore, there is a strict need for the delivery system (carrier) to overcome the hurdles of conventional delivery systems and to improve drug performance.
SLNs as a gene vector carrier
In recent days gene delivery is considered an attractive therapeutic technique that utilizes viral and non-viral vectors. Because of the stability and safety profile, non-viral vectors are more commonly used as a vector to transfer gene. Non-viral gene easily passes through biological barriers as compared to viral vectors.
148,149
Botto et al recently reported the potential of cationic SLNs (cSLNs) as non-viral vectors for shNUPR1 plasmid delivery in Hepatic cell Carcinoma gene therapy. The author also obtained the highest in vitro transfection efficiency and biocompatibility for cSLNs, so they proposed cSLNs as an excellent transfection vector for HCC gene treatment.
150
Bondi et al focused on the suitability of SLNs as a carrier (non-viral) for the delivery of genes. Promising results showed that SLNs were successfully developed using the microemulsion method and they can bind efficiently with DNA, and this type of vector can be used frequently due to its safety, and it can efficiently deliver DNA by maintaining the efficacy.
151
Penumarthi et al
152
demonstrated the formulation of DNA-SLNs complex for non-viral delivery of plasmid DNA to dendritic cells. Large particle size (758.7 nm) was reported due to the strong electrostatic interaction between negatively charged DNA and positively charged SLNs. The most efficient proportions for the formation of such complex were 1:10 (DNA: SLNs). The cytotoxicity of 10 μg/mL DNA–SLNs complexes was significantly low as compared to plain SLNs over 72 hours and cell viability, which might be due to the increase in cell division by lipids available from nanoparticles. Development of protamine (P) attached DNA loaded cholesteryl oleate SLNs (P:DNA: CO-SLNs) were recently reported by Limeres et al
153
to deliver the non-viral vector nucleic acid delivery. They found the suitable proportion 2:1:7 of P:DNA: CO-SLNs for efficient delivery and reported that the presence of protamine facilitates the binding efficiency and nucleic acid delivery to the cytoplasm. In another study, DNA delivery by the incorporation of cationic lipid (Precirol ATO and stearyl amine) in SLNs was achieved by Carrillo et al.
154
DNA delivery via cationic SLNs. Authors had found that at 1: 1.25 ratio of stearylamine: poloxamer, SLNs were smaller in size but carry higher zeta potential (342.3 ± 0.076 nm, 43.98 ± 1.58). The most efficient binding found from 15:1–5: 1 ratio of SLNs: DNA and lyophilization with the 5% trehalose cryoprotectant does not alter the quality of the product. Yu et al
155
has developed the surface modified with mannan, phosphatidylethanolamine-grafted DNA loaded SLNs for the targeted gene delivery. Targeting potential had been checked with MTT assay in RAW 264.7 cells and found the least cytotoxicity with Man-SLNs and highest transfection efficiency with Man-SLNs–DNA. The results proposed Man-SLNs-DNA as a promising non-viral vector with efficient active targeting potential for gene delivery.
SLN as a potential new adjuvant for vaccines
An adjuvant is required for subunit and single antigen-based vaccines to provide sufficient immunogenicity. Adjuvant helps to reduce the frequency of immunization and the antigen amount. Emulsion-based adjuvant systems had been widely applied for the development of successful vaccines.
53,156
Mishra et al explored the capacity of SLNs as a vector for the surface antigen of hepatitis B (HBsAg) by modifying the surface of SLNs for improvement of loading capacity and cellular uptake by subcutaneous route. By comparing the results with soluble HBsAg, SLNs, and mannosylated carrier, the author concluded that SLNs carrier showed better cellular uptake and it also induced more significant TH1 immune response.
157
Stelzner et al have investigated the potential of squalene containing SLNs a promising adjuvant system for yeast vaccines. Supporting results revealed an excellent immune-stimulating effect that was comparable to that of commercially available (AddaVaxTM) adjuvant in terms of size, sterility as well as stability obtained. These data suggested squalene SLNs as an excellent adjuvant candidate that could be used in future vaccine trials.
158
Protein and peptide drug delivery
SLNs are based on dispersed phase technologies/ because of their hydrophilic nature, and many proteins are expected to be poorly encapsulated into the lipophilic matrix of the solid lipid core, leading to the partition of the aqueous phase during the preparation which can be further increased by the use of surfactants as emulsion and stabilizers.
159
Gallarate et al
68
concluded in the research that lyophilic ion coupling of leuprolide and insulin permitted the entrapment of these molecules in SLNs. As demonstrated with leuprolide stoichiometry of the ion pair could be used as a determinant for encapsulation efficiency. Different peptide and protein also used in treatment of various cancer with SLNs which offers various advantages like low toxicity, high bioavailability of and can incorporate both hydrophilic and hudrophobic drug.
160
Ezzati Nazhad Dolatabadi and Omidi
161
in his review had discussed the various aspects of targeted delivery of drug and gene with DNA and RNA. Authors had concluded that cationic SLNs surface DNA loaded and decorated with tumor-specific target showed an improved therapeutic targeted potential for drug and gene delivery.
Surface engineered solid lipid nanoparticles
New approaches and polymers had been reported to modify the surface of the SLNs with target active moieties which improve biocompatibility, stability, and target ability. Recently Arana et al modified the SLNs with phosphatidylethanolamine polyethylene glycol (PE–PEG) and observed that the presence of PE–PEG improved targeting ability in an oral adenocarcinoma cell line and concluded that surface modification with PE–PEG improves the efficiency and discriminates the distribution of the SLNs-loaded drug in comparison to non-coated SLNs.
162
Cho et al developed Tween 80-emulsified and TPGS 1000-emulsified tristearin-based lipidic nanoparticles and by comparing both the formulations they concluded that the intestinal absorption and relative oral bioavailability of docetaxel in rats were further improved in TPGS 1000-emulsified SLNs as compared to Tween 80-emulsified SLNs, probably due to better inhibition of drug efflux by TPGS 1000, along with intestinal lymphatic uptake.
163
Zhou et al developed hyaluronic acid-coated solid lipid nanoparticles (HA-SLNs) of prednisolone(PD) HA-SLNs/PD. In mice with collagen-induced arthritis (CIA), the developed HA-SLNs/PD particles were injected through I.V and particles get accumulated in affected joint tissues only. HA-SLNs/PD showed increased circulation time and preserved bones and cartilages better than free drug or drug encapsulated in SLNs without HA. Promising results suggest that encapsulating PD in HA-coated SLNs may present as an excellent carrier for treating inflammatory disorders.
164
Some of the tailored surfaces of SLNs with active moieties are enlisted in Table 5.
Table 5.
Examples of surface tailored solid lipid nanoparticles
|
Drug
|
Surface Modifier
|
Modification Rational
|
Reference
|
| Curcumin |
N-trimethyl chitosan |
Burst release of curcumin SLN in an acidic environment was the main obstacle. N-trimethyl chitosan is used as an acid protective coat to prevent the burst release of curcumin SLNs. |
165
|
| Triamcinolone acetonide |
a pH-sensitive derivative of phosphatidylethanolamine |
Tumor and inflamed tissues are having leaky vasculature structures, and also that region is having different acidic pH than normal vasculature. To control the drug release behavior of drug pH, the sensitive coat is done. |
166
|
| Resveratrol |
N-trimethyl chitosan-g -palmitic acid |
The potential application of resveratrol is limited due to its poor aqueous solubility, its photosensitivity, poor absorption properties, and rapid first-pass metabolism. To overcome the problems, it is coated with the N-trimethyl chitosan-g-palmitic acid. |
167
|
| Docetaxel |
Hydroxypropyl trimethylammonium chloride chitosan |
To reduce its first-pass metabolism and increase its solubility SLN of Docetaxel is formulated. However, a negative charge on the SLNs is an obstacle in drug absorption because of the electrostatic repulsion between the cell membrane and SLNs it is coated with positively charged chitosan to reduce the repulsion. |
168
|
| Rifampicin |
Methyl α-D-mannopyranoside |
To increase the targeting of Rifampicin SLN formulation, it is coated with methyl α-D-mannopyranoside |
169
|
| Ifosfamide |
Crosslinked with sodium tripolyphosphate |
Ifosfamide gets degraded in the acidic medium, which is pH-dependent on reducing the degradation of the drug; it is coated and crosslinked with tripolyphosphate. |
170
|
| Retinyl palmitate |
Diacetyl phosphate (DCP) |
Diacetyl phosphate has a negative charge on its surface. This type of charge is known to affect the delivery efficiencies of modified carriers also DCP is considered as a safe excipient to use in a topical preparation. |
171
|
| Paclitaxel |
Hyaluronic acid |
CD44 receptors are present on cancer stem cell (CSCs) which specifically binds to the Hyaluronic acid. |
172
|
| Paclitaxel |
Folate-grafted copolymer of PEG and chitosan |
To increase the circulation time and stability. |
140
|
| Prednisolone |
Hyaluronic acid |
To target, the CD44 receptors are present on synovial lymphocytes in arthritis. |
164
|
Current scenario of patent for SLNs
Rationally designed, ease of surface tailoring, long-term stability, feasible scale-up potential, and promising in vivo result studies with SLNs have resulted in a large number of patents being filed. Diorio and Lokhnauth received a patent of curcumin SLNs, and the inventor claimed solid lipid particles comprising of a hydrophobic matrix from 5 wt. % to about 30 wt. % of curcumin, wherein lipid hydrophobic matrix is substantially free of water and curcumin loaded SLNs had an average particle size diameter ranging from 100 um to 1500 um and lipid matrix melting range from 15°C to 85°C and 30°C to 45°C to get the stable SLN formulations.
173
A summary of some patents of SLNs is given in Table 6.
Table 6.
Patents on solid lipid nanoparticles
|
Title
|
Patent no.
|
Reference No
|
| Nano pellets as a carrier system for medicinal products for peroral use |
EP0167825A2 |
174
|
| Lipid particles based on mixtures of liquid and solid lipids and method for producing same |
US8663692 |
175
|
| Topical preparation containing a suspension of solid lipid particles |
EP0506197B2 |
176
|
| Polymerized solid lipid nanoparticles for oral or mucosal delivery of therapeutic proteins and peptides |
US20080311214 |
177
|
| Formulation of UV absorbers by incorporation in solid lipid nanoparticles |
US20030235540 |
178
|
| Manufacture of lipid-based nanoparticles using a dual asymmetric centrifuge |
US20080193511 |
179
|
| Microemulsion as precursors to solid nanoparticles |
US7153525 |
180
|
| Solid lipid nanoparticles (ii) |
US20160030305 |
181
|
| Solid lipid nanoparticles (I) |
US20160022550 |
182
|
| The lipid nanoparticle or polymyxin |
US20160113995 |
183
|
| Lipid nanoparticle capsules |
US20130017239 |
184
|
| Curcumin solid lipid particles and methods for their preparation and use |
US20180036248 |
173
|
Conclusion and Future Prospects
The SLNs have the potential to maintain high stability during their storage period. A varied range of lipids (oils) and fatty acids are accessible for tuning the release kinetics. SLNs are very flexible lipid carriers that can be easily tailored with the terminal groups of solid lipid to attain efficient improvement for a given treatment. Drug expulsion and targeting problems can be efficiently addressed by surface modification. SLNs are not only used for treatments, imaging agent or diagnostic agent potential are also explored. A front line of research should merely be focused on the development of surface-modified SLNs for future perspectives. It would have great potential in imaging, active and specific delivery in various tissue regions. Researchers have already filed and received many patents related to SLNs, and young researchers can anticipate more patented SLNs-based (surface-modified SLN) delivery systems soon for the treatment and diagnosis of various diseases especially for targeting by tailoring the surface. If properly explored, a very well-designed, SLNs seems to be a promising carrier that may open a new benchmark in treatment, diagnosis, and as a carrier for biological drugs.
Ethical Issues
Not applicable.
Conflict of Interest
Authors have no conflict of interest.
Acknowledgments
Authors are thankful to the Uka Tarsdia University and Maliba Pharmacy College for the continuous support and motivation.
References
- Gasco MR. Lipid nanoparticles: perspectives and challenges. Adv Drug Deliv Rev 2007; 59(6):377-8. doi: 10.1016/j.addr.2007.05.004 [Crossref] [ Google Scholar]
- Caruthers SD, Wickline SA, Lanza GM. Nanotechnological applications in medicine. Curr Opin Biotechnol 2007; 18(1):26-30. doi: 10.1016/j.copbio.2007.01.006 [Crossref] [ Google Scholar]
- Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B 2015; 5(5):442-53. doi: 10.1016/j.apsb.2015.07.003 [Crossref] [ Google Scholar]
- Yadav N, Khatak S, Sara US. Solid lipid nanoparticles-a review. Int J Appl Pharm 2013; 5(2):8-18. [ Google Scholar]
- Akash C, Sudheer P, Sogali BS. Solid lipid nanoparticles-an innovative approach for improving the solubility and bioavailability. J Pharm Res 2017; 16(2):148-53. doi: 10.18579/jpcrkc/2017/16/2/116433 [Crossref] [ Google Scholar]
- Patidar A, Thakur DS, Kumar P, Verma J. A review on novel lipid based nanocarriers. Int J Pharm Pharm Sci 2010; 2(4):30-5. [ Google Scholar]
- Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev 2001; 47(2-3):165-96. doi: 10.1016/s0169-409x(01)00105-3 [Crossref] [ Google Scholar]
- Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst 2009; 26(6):523-80. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10 [Crossref] [ Google Scholar]
- Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci 2009; 71(4):349-58. doi: 10.4103/0250-474x.57282 [Crossref] [ Google Scholar]
- Surender V, Deepika M. Solid lipid nanoparticles: a comprehensive review. J Chem Pharm Res 2016; 8(8):102-14. [ Google Scholar]
- Yuan H, Chen J, Du YZ, Hu FQ, Zeng S, Zhao HL. Studies on oral absorption of stearic acid SLN by a novel fluorometric method. Colloids Surf B Biointerfaces 2007; 58(2):157-64. doi: 10.1016/j.colsurfb.2007.03.002 [Crossref] [ Google Scholar]
- Attama AA, Kenechukwu FC, Onuigbo EB, Nnamani PO, Obitte N, Finke JH. Solid lipid nanoparticles encapsulating a fluorescent marker (coumarin 6) and antimalarials – artemether and lumefantrine: evaluation of cellular uptake and antimalarial activity. Eur J Nanomed 2016; 8(3):129-38. doi: 10.1515/ejnm-2016-0009 [Crossref] [ Google Scholar]
- Jain A, Agarwal A, Majumder S, Lariya N, Khaya A, Agrawal H. Mannosylated solid lipid nanoparticles as vectors for site-specific delivery of an anti-cancer drug. J Control Release 2010; 148(3):359-67. doi: 10.1016/j.jconrel.2010.09.003 [Crossref] [ Google Scholar]
-
Vyas SP, Khar RK. Targeted & Controlled Drug Delivery: Novel Carrier Systems. CBS Publishers & Distributors; 2004.
- Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull 2015; 5(3):305-13. doi: 10.15171/apb.2015.043 [Crossref] [ Google Scholar]
- Pardeshi C, Rajput P, Belgamwar V, Tekade A, Patil G, Chaudhary K. Solid lipid based nanocarriers: an overview. Acta Pharm 2012; 62(4):433-72. doi: 10.2478/v10007-012-0040-z [Crossref] [ Google Scholar]
- Pouton CW, Porter CJ. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv Drug Deliv Rev 2008; 60(6):625-37. doi: 10.1016/j.addr.2007.10.010 [Crossref] [ Google Scholar]
-
Shah R, Eldridge D, Palombo E, Harding I. Lipid Nanoparticles: Production, Characterization and Stability. New York, NY: Springer; 2015.
- Sarangi B, Jana U, Palei NN, Mohanta GP, Manna PK. Solid lipid nanoparticles: a potential approach for drug delivery system. Nanoscience & Nanotechnology-Asia 2019; 9(2):142-56. doi: 10.2174/2210681208666180321144536 [Crossref] [ Google Scholar]
- Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 2011; 12(1):62-76. doi: 10.1208/s12249-010-9563-0 [Crossref] [ Google Scholar]
- Üner M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): their benefits as colloidal drug carrier systems. Pharmazie 2006; 61(5):375-86. [ Google Scholar]
- Lopes RM, Gaspar MM, Pereira J, Eleutério CV, Carvalheiro M, Almeida AJ. Liposomes versus lipid nanoparticles: comparative study of lipid-based systems as oryzalin carriers for the treatment of leishmaniasis. J Biomed Nanotechnol 2014; 10(12):3647-57. doi: 10.1166/jbn.2014.1874 [Crossref] [ Google Scholar]
- Duan Y, Dhar A, Patel C, Khimani M, Neogi S, Sharma P. A brief review on solid lipid nanoparticles: part and parcel of contemporary drug delivery systems. RSC Adv 2020; 10(45):26777-91. doi: 10.1039/d0ra03491f [Crossref] [ Google Scholar]
- Gohla SH, Dingler A. Scaling up feasibility of the production of solid lipid nanoparticles (SLN). Pharmazie 2001; 56(1):61-3. [ Google Scholar]
- Saffari M, Moghimi HR, Dass CR. Barriers to liposomal gene delivery: from application site to the target. Iran J Pharm Res 2016; 15(Suppl):3-17. [ Google Scholar]
- Rai R, Alwani S, Badea I. Polymeric nanoparticles in gene therapy: new avenues of design and optimization for delivery applications. Polymers (Basel) 2019; 11(4):745. doi: 10.3390/polym11040745 [Crossref] [ Google Scholar]
- Kim H, Kim Y, Lee J. Liposomal formulations for enhanced lymphatic drug delivery. Asian J Pharm Sci 2013; 8(2):96-103. doi: 10.1016/j.ajps.2013.07.012 [Crossref] [ Google Scholar]
- Erlanson-Albertsson C. Pancreatic colipase Structural and physiological aspects. Biochim Biophys Acta 1992; 1125(1):1-7. doi: 10.1016/0005-2760(92)90147-n [Crossref] [ Google Scholar]
- Cai S, Yang Q, Bagby TR, Forrest ML. Lymphatic drug delivery using engineered liposomes and solid lipid nanoparticles. Adv Drug Deliv Rev 2011; 63(10-11):901-8. doi: 10.1016/j.addr.2011.05.017 [Crossref] [ Google Scholar]
- Jawahar N, Meyyanathan SN, Reddy G, Sood S. Solid lipid nanoparticles for oral delivery of poorly soluble drugs. J Pharm Sci Res 2012; 4(7):1848-55. [ Google Scholar]
- Caliph SM, Charman WN, Porter CJ. Effect of short-, medium-, and long-chain fatty acid-based vehicles on the absolute oral bioavailability and intestinal lymphatic transport of halofantrine and assessment of mass balance in lymph-cannulated and non-cannulated rats. J Pharm Sci 2000; 89(8):1073-84. [ Google Scholar]
- Pragati S, Kuldeep S, Ashok S, Satheesh M. Solid lipid nanoparticles: a promising drug delivery technology. Int J Pharm Sci Nanotechnol 2009; 2(2):509-16. [ Google Scholar]
- Trevaskis NL, Charman WN, Porter CJ. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliv Rev 2008; 60(6):702-16. doi: 10.1016/j.addr.2007.09.007 [Crossref] [ Google Scholar]
- Poovi G, Damodharan N. Lipid nanoparticles: a challenging approach for oral delivery of BCS Class-II drugs. Futur J Pharm Sci 2018; 4(2):191-205. doi: 10.1016/j.fjps.2018.04.001 [Crossref] [ Google Scholar]
- Ali Khan A, Mudassir J, Mohtar N, Darwis Y. Advanced drug delivery to the lymphatic system: lipid-based nanoformulations. Int J Nanomedicine 2013; 8:2733-44. doi: 10.2147/ijn.s41521 [Crossref] [ Google Scholar]
- Üner M, Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomedicine 2007; 2(3):289-300. [ Google Scholar]
-
Mousavi AK, Alaie S, Leseman ZC. Encyclopedia of Nanotechnology. Netherlands: Springer; 2016.
- Ganesan P, Narayanasamy D. Lipid nanoparticles: different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain Chem Pharm 2017; 6:37-56. doi: 10.1016/j.scp.2017.07.002 [Crossref] [ Google Scholar]
-
Kammari R, Das NG, Das SK. Nanoparticulate systems for therapeutic and diagnostic applications. In: Mitra AK, Cholkar K, Mandal A, eds. Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices. Boston: Elsevier; 2017. p. 105-44. 10.1016/b978-0-323-42978-8.00006-1
- Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci 2018; 13(4):288-303. doi: 10.4103/1735-5362.235156 [Crossref] [ Google Scholar]
-
Luks S, Muller R. Medication vehicles made of solid lipid particles. EP0605497 A1 1992;16.
- Scheffel U, Rhodes BA, Natarajan TK, Wagner HN Jr. Albumin microspheres for study of the reticuloendothelial system. J Nucl Med 1972; 13(7):498-503. [ Google Scholar]
- Bagul US, Pisal VV, Solanki NV, Karnavat A. Current status of solid lipid nanoparticles: a review. Modern Applications of Bioequivalence & Bioavailability 2018; 3(4):555617. [ Google Scholar]
- Chen HH, Huang WC, Chiang WH, Liu TI, Shen MY, Hsu YH. pH-Responsive therapeutic solid lipid nanoparticles for reducing P-glycoprotein-mediated drug efflux of multidrug resistant cancer cells. Int J Nanomedicine 2015; 10:5035-48. doi: 10.2147/ijn.s86053 [Crossref] [ Google Scholar]
- Mishra V, Bansal KK, Verma A, Yadav N, Thakur S, Sudhakar K. Solid lipid nanoparticles: emerging colloidal nano drug delivery systems. Pharmaceutics 2018; 10(4):191. doi: 10.3390/pharmaceutics10040191 [Crossref] [ Google Scholar]
- zur Mühlen A, Schwarz C, Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery--drug release and release mechanism. Eur J Pharm Biopharm 1998; 45(2):149-55. doi: 10.1016/s0939-6411(97)00150-1 [Crossref] [ Google Scholar]
- Olbrich C, Müller RH. Enzymatic degradation of SLN-effect of surfactant and surfactant mixtures. Int J Pharm 1999; 180(1):31-9. doi: 10.1016/s0378-5173(98)00404-9 [Crossref] [ Google Scholar]
- Shrestha H, Bala R, Arora S. Lipid-based drug delivery systems. J Pharm (Cairo) 2014; 2014:801820. doi: 10.1155/2014/801820 [Crossref] [ Google Scholar]
- Savla R, Browne J, Plassat V, Wasan KM, Wasan EK. Review and analysis of FDA approved drugs using lipid-based formulations. Drug Dev Ind Pharm 2017; 43(11):1743-58. doi: 10.1080/03639045.2017.1342654 [Crossref] [ Google Scholar]
- Chen XQ, Gudmundsson OS, Hageman MJ. Application of lipid-based formulations in drug discovery. J Med Chem 2012; 55(18):7945-56. doi: 10.1021/jm3006433 [Crossref] [ Google Scholar]
-
Kumar R. Lipid-based nanoparticles for drug-delivery systems. In: Mohapatra SS, Ranjan S, Dasgupta N, Mishra RK, Thomas S, eds. Nanocarriers for Drug Delivery. Elsevier; 2019. p. 249-84. 10.1016/b978-0-12-814033-8.00008-4
- Yong AP, Islam MA, Hasan N. The effect of pH and high-pressure homogenization on droplet size. Int J Eng Mater Manuf 2017; 2(4):110-22. doi: 10.26776/ijemm.02.04.2017.05 [Crossref] [ Google Scholar]
- Teja VC, Chowdary VH, Raju YP, Surendra N, Vardhan RV, Reddy BK. A glimpse on solid lipid nanoparticles as drug delivery systems. J Glob Trends Pharm Sci 2014; 5(2):1649-57. [ Google Scholar]
- Shylaja P, Mathew M. Preparation and characterization of alpha tocopherol loaded solid lipid nanoparticles by hot homogenization method. Int J Pharm Pharm Res 2016; 7(1):437-48. [ Google Scholar]
- Pallerla SM, Prabhak B. A review on solid lipid nanoparticles. ChemInform 2014; 45(21):196-206. [ Google Scholar]
- Garud A, Singh D, Garud N. Solid lipid nanoparticles (SLN): method, characterization and applications. Int Curr Pharm J 2012; 1(11):384-93. doi: 10.3329/icpj.v1i11.12065 [Crossref] [ Google Scholar]
- Lingayat VJ, Zarekar NS, Shendge RS. Solid lipid nanoparticles: a review. Nanoscience and Nanotechnology Research 2017; 4(2):67-72. doi: 10.12691/nnr-4-2-5 [Crossref] [ Google Scholar]
- Trotta M, Cavalli R, Trotta C, Bussano R, Costa L. Electrospray technique for solid lipid-based particle production. Drug Dev Ind Pharm 2010; 36(4):431-8. doi: 10.3109/03639040903241817 [Crossref] [ Google Scholar]
- Jaworek A. Micro- and nanoparticle production by electrospraying. Powder Technol 2007; 176(1):18-35. doi: 10.1016/j.powtec.2007.01.035 [Crossref] [ Google Scholar]
- Battaglia L, Gallarate M, Panciani PP, Ugazio E, Sapino S, Peira E. Techniques for the preparation of solid lipid nano and microparticles In: Application of Nanotechnology in Drug Delivery. IntechOpen 2014. doi: 10.5772/58405 [Crossref]
- Malik MA, Wani MY, Hashim MA. Microemulsion method: a novel route to synthesize organic and inorganic nanomaterials: 1st Nano Update. Arab J Chem 2012; 5(4):397-417. doi: 10.1016/j.arabjc.2010.09.027 [Crossref] [ Google Scholar]
- Prieto C, Calvo L. Performance of the biocompatible surfactant Tween 80, for the formation of microemulsions suitable for new pharmaceutical processing. J Appl Chem 2013; 2013:930356. doi: 10.1155/2013/930356 [Crossref] [ Google Scholar]
- Charcosset C, El-Harati A, Fessi H. Preparation of solid lipid nanoparticles using a membrane contactor. J Control Release 2005; 108(1):112-20. doi: 10.1016/j.jconrel.2005.07.023 [Crossref] [ Google Scholar]
-
Charcosset C, Bernard S, Fiaty K, Bechelany M, Cornu D. Membrane techniques for the preparation of nanomaterials: nanotubes, nanowires and nanoparticles: a review. In: Teixeira da Silva JA, ed. Dynamic Biochemistry, Process Biotechnology and Molecular Biology. Global Science Books; 2007. p. 15-23.
- Friberg SE, Corkery RW, Blute IA. Phase inversion temperature (PIT) emulsification process. J Chem Eng Data 2011; 56(12):4282-90. doi: 10.1021/je101179s [Crossref] [ Google Scholar]
- Montenegro L, Campisi A, Sarpietro MG, Carbone C, Acquaviva R, Raciti G. In vitro evaluation of idebenone-loaded solid lipid nanoparticles for drug delivery to the brain. Drug Dev Ind Pharm 2011; 37(6):737-46. doi: 10.3109/03639045.2010.539231 [Crossref] [ Google Scholar]
- Battaglia L, Gallarate M, Cavalli R, Trotta M. Solid lipid nanoparticles produced through a coacervation method. J Microencapsul 2010; 27(1):78-85. doi: 10.3109/02652040903031279 [Crossref] [ Google Scholar]
- Gallarate M, Battaglia L, Peira E, Trotta M. Peptide-loaded solid lipid nanoparticles prepared through coacervation technique. Int J Chem Eng 2011; 2011:132435. doi: 10.1155/2011/132435 [Crossref] [ Google Scholar]
- Ramteke KH, Joshi SA, Dhole SN. Solid lipid nanoparticle: a review. IOSR J Pharm 2012; 2(6):34-44. [ Google Scholar]
- Reddy AP, Parthiban S, Vikneswari A, Senthilkumar GP. A modern review on solid lipid nanoparticles as novel controlled drug delivery system. Int J Res Pharm Nano Sci 2014; 3:313-25. [ Google Scholar]
- Santo IE, Pedro AS, Fialho R, Cabral-Albuquerque E. Characteristics of lipid micro- and nanoparticles based on supercritical formation for potential pharmaceutical application. Nanoscale Res Lett 2013; 8(1):386. doi: 10.1186/1556-276x-8-386 [Crossref] [ Google Scholar]
- Kankala RK, Zhang YS, Wang SB, Lee CH, Chen AZ. Supercritical fluid technology: an emphasis on drug delivery and related biomedical applications. Adv Healthc Mater 2017; 6(16):1700433. doi: 10.1002/adhm.201700433 [Crossref] [ Google Scholar]
- Akbari Z, Amanlou M, Karimi-Sabet J, Golestani A, Shariaty Niassar M. Preparation and characterization of solid lipid nanoparticles through rapid expansion of supercritical solution. Int J Pharm Sci Res 2013; 5(5):1693-704. doi: 10.13040/ijpsr.0975-8232.5(5).1693-04 [Crossref] [ Google Scholar]
- Patil J, Gurav P, Kulkarni R, Jadhav S, Mandave S, Shete M. Applications of solid lipid nanoparticle in novel drug delivery system. Br Biomed Bull 2013; 1(2):103-8. [ Google Scholar]
- Schubert MA, Müller-Goymann CC. Solvent injection as a new approach for manufacturing lipid nanoparticles--evaluation of the method and process parameters. Eur J Pharm Biopharm 2003; 55(1):125-31. doi: 10.1016/s0939-6411(02)00130-3 [Crossref] [ Google Scholar]
- Mlalila N, Swai H, Kalombo L, Hilonga A. Effects of spray-drying on w/o/w multiple emulsions prepared from a stearic acid matrix. Nanotechnol Sci Appl 2014; 7:105-12. doi: 10.2147/nsa.s72083 [Crossref] [ Google Scholar]
- Freitas C, Müllerä RH. Spray-drying of solid lipid nanoparticles (SLN TM). Eur J Pharm Biopharm 1998; 46(2):145-51. doi: 10.1016/s0939-6411(97)00172-0 [Crossref] [ Google Scholar]
-
Tscharnuter W. Photon correlation spectroscopy in particle sizing. In: Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation. Wiley; 2006.
- Lee SP, Tscharnuter W, Chu B. Calibration of an optical self-beating spectrometer by polystyrene latex spheres and confirmation of the stokes-einstein formula. J Polym Sci B Polym Phys 1972; 10(12):2453-9. doi: 10.1002/pol.1972.180101213 [Crossref] [ Google Scholar]
- Bhattacharjee S. DLS and zeta potential - what they are and what they are not?. J Control Release 2016; 235:337-51. doi: 10.1016/j.jconrel.2016.06.017 [Crossref] [ Google Scholar]
- Thukral DK, Dumoga S, Mishra AK. Solid lipid nanoparticles: promising therapeutic nanocarriers for drug delivery. Curr Drug Deliv 2014; 11(6):771-91. doi: 10.2174/156720181106141202122335 [Crossref] [ Google Scholar]
- Gill P, Moghadam TT, Ranjbar B. Differential scanning calorimetry techniques: applications in biology and nanoscience. J Biomol Tech 2010; 21(4):167-93. [ Google Scholar]
- Blume A. Properties of lipid vesicles: FT-IR spectroscopy and fluorescence probe studies. Curr Opin Colloid Interface Sci 1996; 1(1):64-77. doi: 10.1016/s1359-0294(96)80046-x [Crossref] [ Google Scholar]
- Segal L, Creely JJ, Martin AE, Conrad CM. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 1959; 29(10):786-94. doi: 10.1177/004051755902901003 [Crossref] [ Google Scholar]
- Bunaciu AA, Udriştioiu EG, Aboul-Enein HY. X-ray diffraction: instrumentation and applications. Crit Rev Anal Chem 2015; 45(4):289-99. doi: 10.1080/10408347.2014.949616 [Crossref] [ Google Scholar]
- Sadati Behbahani E, Ghaedi M, Abbaspour M, Rostamizadeh K. Optimization and characterization of ultrasound assisted preparation of curcumin-loaded solid lipid nanoparticles: application of central composite design, thermal analysis and X-ray diffraction techniques. Ultrason Sonochem 2017; 38:271-80. doi: 10.1016/j.ultsonch.2017.03.013 [Crossref] [ Google Scholar]
- Yang SC, Zhu JB. Preparation and characterization of camptothecin solid lipid nanoparticles. Drug Dev Ind Pharm 2002; 28(3):265-74. doi: 10.1081/ddc-120002842 [Crossref] [ Google Scholar]
- Jain SK, Chourasia MK, Masuriha R, Soni V, Jain A, Jain NK. Solid lipid nanoparticles bearing flurbiprofen for transdermal delivery. Drug Deliv 2005; 12(4):207-15. doi: 10.1080/10717540590952591 [Crossref] [ Google Scholar]
- Heiati H, Tawashi R, Phillips NC. Drug retention and stability of solid lipid nanoparticles containing azidothymidine palmitate after autoclaving, storage and lyophilization. J Microencapsul 1998; 15(2):173-84. doi: 10.3109/02652049809006847 [Crossref] [ Google Scholar]
- Pardeshi CV, Rajput PV, Belgamwar VS, Tekade AR, Surana SJ. Novel surface modified solid lipid nanoparticles as intranasal carriers for ropinirole hydrochloride: application of factorial design approach. Drug Deliv 2013; 20(1):47-56. doi: 10.3109/10717544.2012.752421 [Crossref] [ Google Scholar]
- Prajapati HN, Dalrymple DM, Serajuddin AT. A comparative evaluation of mono-, di- and triglyceride of medium chain fatty acids by lipid/surfactant/water phase diagram, solubility determination and dispersion testing for application in pharmaceutical dosage form development. Pharm Res 2012; 29(1):285-305. doi: 10.1007/s11095-011-0541-3 [Crossref] [ Google Scholar]
- Bunjes H, Drechsler M, Koch MH, Westesen K. Incorporation of the model drug ubidecarenone into solid lipid nanoparticles. Pharm Res 2001; 18(3):287-93. doi: 10.1023/a:1011042627714 [Crossref] [ Google Scholar]
- Severs NJ. Freeze-fracture electron microscopy. Nat Protoc 2007; 2(3):547-76. doi: 10.1038/nprot.2007.55 [Crossref] [ Google Scholar]
- Manjunath K, Reddy JS, Venkateswarlu V. Solid lipid nanoparticles as drug delivery systems. Methods Find Exp Clin Pharmacol 2005; 27(2):127-44. doi: 10.1358/mf.2005.27.2.876286 [Crossref] [ Google Scholar]
- Sawant KK, Dodiya SS. Recent advances and patents on solid lipid nanoparticles. Recent Pat Drug Deliv Formul 2008; 2(2):120-35. doi: 10.2174/187221108784534081 [Crossref] [ Google Scholar]
- Prasad D. Nanoparticulate drug delivery systems, in vitro drug release test methods. Int J Pharma Bio Sci 2017; 8(3):103-19. doi: 10.22376/ijpbs.2017.8.3 [Crossref] [ Google Scholar]
- D’Souza S. A review of in vitro drug release test methods for nano-sized dosage forms. Adv Pharm 2014; 2014:304757. doi: 10.1155/2014/304757 [Crossref] [ Google Scholar]
- Harms M, Müller-Goymann CC. Solid lipid nanoparticles for drug delivery. J Drug Deliv Sci Technol 2011; 21(1):89-99. doi: 10.1016/S1773-2247(11)50008-5 [Crossref] [ Google Scholar]
- Yang SC, Lu LF, Cai Y, Zhu JB, Liang BW, Yang CZ. Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brain. J Control Release 1999; 59(3):299-307. doi: 10.1016/s0168-3659(99)00007-3 [Crossref] [ Google Scholar]
- Shaji J, Jain V. Solid lipid nanoparticles: a novel carrier for chemotherapy. Int J Pharm Pharm Sci 2010; 2(3):8-17. [ Google Scholar]
- Fundarò A, Cavalli R, Bargoni A, Vighetto D, Zara GP, Gasco MR. Non-stealth and stealth solid lipid nanoparticles (SLN) carrying doxorubicin: pharmacokinetics and tissue distribution after iv administration to rats. Pharmacol Res 2000; 42(4):337-43. doi: 10.1006/phrs.2000.0695 [Crossref] [ Google Scholar]
- Wang JY, Wang Y, Meng X. Chitosan nanolayered cisplatin-loaded lipid nanoparticles for enhanced anticancer efficacy in cervical cancer. Nanoscale Res Lett 2016; 11(1):524. doi: 10.1186/s11671-016-1698-9 [Crossref] [ Google Scholar]
- Müller RH, Runge S, Ravelli V, Mehnert W, Thünemann AF, Souto EB. Oral bioavailability of cyclosporine: solid lipid nanoparticles (SLN) versus drug nanocrystals. Int J Pharm 2006; 317(1):82-9. doi: 10.1016/j.ijpharm.2006.02.045 [Crossref] [ Google Scholar]
- Muraleetharan V, Mantaj J, Swedrowska M, Vllasaliu D. Nanoparticle modification in biological media: implications for oral nanomedicines. RSC Adv 2019; 9(69):40487-97. doi: 10.1039/c9ra08403g [Crossref] [ Google Scholar]
- Sarmento B, Martins S, Ferreira D, Souto EB. Oral insulin delivery by means of solid lipid nanoparticles. Int J Nanomedicine 2007; 2(4):743-9. [ Google Scholar]
- Schäfer-Korting M, Mehnert W, Korting HC. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv Drug Deliv Rev 2007; 59(6):427-43. doi: 10.1016/j.addr.2007.04.006 [Crossref] [ Google Scholar]
- Langer R. Transdermal drug delivery: past progress, current status, and future prospects. Adv Drug Deliv Rev 2004; 56(5):557-8. doi: 10.1016/j.addr.2003.10.021 [Crossref] [ Google Scholar]
- Kurakula M, Ahmed OA, Fahmy UA, Ahmed TA. Solid lipid nanoparticles for transdermal delivery of avanafil: optimization, formulation, in-vitro and ex-vivo studies. J Liposome Res 2016; 26(4):288-96. doi: 10.3109/08982104.2015.1117490 [Crossref] [ Google Scholar]
- Alagusundaram M, Chengaiah B, Gnanaprakash K, Ramkanth S, Chetty CM, Dhachinamoorthi D. Nasal drug delivery system-an overview. Int J Res Pharm Sci 2010; 1(4):454-65. [ Google Scholar]
- Türker S, Onur E, Ózer Y. Nasal route and drug delivery systems. Pharm World Sci 2004; 26(3):137-42. doi: 10.1023/b:phar.0000026823.82950.ff [Crossref] [ Google Scholar]
- Cacciatore I, Ciulla M, Fornasari E, Marinelli L, Di Stefano A. Solid lipid nanoparticles as a drug delivery system for the treatment of neurodegenerative diseases. Expert Opin Drug Deliv 2016; 13(8):1121-31. doi: 10.1080/17425247.2016.1178237 [Crossref] [ Google Scholar]
- Rajput AH. Frequency and cause of Parkinson’s disease. Can J Neurol Sci 1992; 19(1 Suppl):103-7. [ Google Scholar]
- Mori NM, Sheth NR, Mendapara VP, Ashara KC, Paun JS. SLN brain targeting drug delivery for CNS: a novel approach. Int Res J Pharm 2014; 5(9):658-62. doi: 10.7897/2230-8407.0509134 [Crossref] [ Google Scholar]
- Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev 2007; 59(6):454-77. doi: 10.1016/j.addr.2007.04.011 [Crossref] [ Google Scholar]
- Md S, Khan RA, Mustafa G, Chuttani K, Baboota S, Sahni JK. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: pharmacodynamic, pharmacokinetic and scintigraphy study in mice model. Eur J Pharm Sci 2013; 48(3):393-405. doi: 10.1016/j.ejps.2012.12.007 [Crossref] [ Google Scholar]
- Gupta S, Kesarla R, Chotai N, Misra A, Omri A. Systematic approach for the formulation and optimization of solid lipid nanoparticles of efavirenz by high pressure homogenization using design of experiments for brain targeting and enhanced bioavailability. Biomed Res Int 2017; 2017:5984014. doi: 10.1155/2017/5984014 [Crossref] [ Google Scholar]
- Fatouh AM, Elshafeey AH, Abdelbary A. Intranasal agomelatine solid lipid nanoparticles to enhance brain delivery: formulation, optimization and in vivo pharmacokinetics. Drug Des Devel Ther 2017; 11:1815-25. doi: 10.2147/dddt.s102500 [Crossref] [ Google Scholar]
- Smola M, Vandamme T, Sokolowski A. Nanocarriers as pulmonary drug delivery systems to treat and to diagnose respiratory and non respiratory diseases. Int J Nanomedicine 2008; 3(1):1-19. [ Google Scholar]
- Ghanshyam U, Patel P, Jayvadan P. Formulation and characterization of solid lipid nanoparticles dry powder inhaler containing triamcinolone acetonide. Int J Res Pharm Chem 2011; 1(3):662-73. [ Google Scholar]
- Bakhtiary Z, Barar J, Aghanejad A, Saei AA, Nemati E, Ezzati Nazhad Dolatabadi J. Microparticles containing erlotinib-loaded solid lipid nanoparticles for treatment of non-small cell lung cancer. Drug Dev Ind Pharm 2017; 43(8):1244-53. doi: 10.1080/03639045.2017.1310223 [Crossref] [ Google Scholar]
- Rawat MK, Jain A, Mishra A, Muthu MS, Singh S. Development of repaglinide loaded solid lipid nanocarrier: selection of fabrication method. Curr Drug Deliv 2010; 7(1):44-50. doi: 10.2174/156720110790396472 [Crossref] [ Google Scholar]
- Chetoni P, Burgalassi S, Monti D, Tampucci S, Tullio V, Cuffini AM. Solid lipid nanoparticles as promising tool for intraocular tobramycin delivery: pharmacokinetic studies on rabbits. Eur J Pharm Biopharm 2016; 109:214-23. doi: 10.1016/j.ejpb.2016.10.006 [Crossref] [ Google Scholar]
- Tatke A, Dudhipala N, Janga KY, Balguri SP, Avula B, Jablonski MM. In situ gel of triamcinolone acetonide-loaded solid lipid nanoparticles for improved topical ocular delivery: tear kinetics and ocular disposition studies. Nanomaterials (Basel) 2018; 9(1):33. doi: 10.3390/nano9010033 [Crossref] [ Google Scholar]
- Ayan AK, Yenilmez A, Eroglu H. Evaluation of radiolabeled curcumin-loaded solid lipid nanoparticles usage as an imaging agent in liver-spleen scintigraphy. Mater Sci Eng C Mater Biol Appl 2017; 75:663-70. doi: 10.1016/j.msec.2017.02.114 [Crossref] [ Google Scholar]
- Weyhers H, Löbenberg R, Mehnert W, Souto EB, Kreuter J, Mueller RH. In vivo distribution of 125I-radiolabelled solid lipid nanoparticles. Pharm Ind 2006; 68(7):889-94. [ Google Scholar]
- Peira E, Marzola P, Podio V, Aime S, Sbarbati A, Gasco MR. In vitro and in vivo study of solid lipid nanoparticles loaded with superparamagnetic iron oxide. J Drug Target 2003; 11(1):19-24. doi: 10.1080/1061186031000086108 [Crossref] [ Google Scholar]
- Liu B, Han L, Liu J, Han S, Chen Z, Jiang L. Co-delivery of paclitaxel and TOS-cisplatin via TAT-targeted solid lipid nanoparticles with synergistic antitumor activity against cervical cancer. Int J Nanomedicine 2017; 12:955-68. doi: 10.2147/ijn.s115136 [Crossref] [ Google Scholar]
- Garg NK, Singh B, Jain A, Nirbhavane P, Sharma R, Tyagi RK. Fucose decorated solid-lipid nanocarriers mediate efficient delivery of methotrexate in breast cancer therapeutics. Colloids Surf B Biointerfaces 2016; 146:114-26. doi: 10.1016/j.colsurfb.2016.05.051 [Crossref] [ Google Scholar]
- Manjunath K, Venkateswarlu V. Pharmacokinetics, tissue distribution and bioavailability of nitrendipine solid lipid nanoparticles after intravenous and intraduodenal administration. J Drug Target 2006; 14(9):632-45. doi: 10.1080/10611860600888850 [Crossref] [ Google Scholar]
- Nair R, Kumar AC, Priya VK, Yadav CM, Raju PY. Formulation and evaluation of chitosan solid lipid nanoparticles of carbamazepine. Lipids Health Dis 2012; 11:72. doi: 10.1186/1476-511x-11-72 [Crossref] [ Google Scholar]
- Kommavarapu P, Maruthapillai A, Palanisamy K. Preparation, characterization and evaluation of elvitegravir-loaded solid lipid nanoparticles for enhanced solubility and dissolution rate. Trop J Pharm Res 2015; 14(9):1549-56. doi: 10.4314/tjpr.v14i9.2 [Crossref] [ Google Scholar]
- Ekambaram P, Sathali AA. Formulation and evaluation of solid lipid nanoparticles of ramipril. J Young Pharm 2011; 3(3):216-20. doi: 10.4103/0975-1483.83765 [Crossref] [ Google Scholar]
- Gonçalves LM, Maestrelli F, Di Cesare Mannelli L, Ghelardini C, Almeida AJ, Mura P. Development of solid lipid nanoparticles as carriers for improving oral bioavailability of glibenclamide. Eur J Pharm Biopharm 2016; 102:41-50. doi: 10.1016/j.ejpb.2016.02.012 [Crossref] [ Google Scholar]
- El-Say KM, Hosny KM. Optimization of carvedilol solid lipid nanoparticles: an approach to control the release and enhance the oral bioavailability on rabbits. PLoS One 2018; 13(8):e0203405. doi: 10.1371/journal.pone.0203405 [Crossref] [ Google Scholar]
- Varshosaz J, Tabbakhian M, Mohammadi MY. Formulation and optimization of solid lipid nanoparticles of buspirone HCl for enhancement of its oral bioavailability. J Liposome Res 2010; 20(4):286-96. doi: 10.3109/08982100903443065 [Crossref] [ Google Scholar]
- Yasir M, Sara UVS, Chauhan I, Gaur PK, Singh AP, Puri D. Solid lipid nanoparticles for nose to brain delivery of donepezil: formulation, optimization by Box–Behnken design, in vitro and in vivo evaluation. Artif Cells Nanomed Biotechnol 2018; 46(8):1838-51. doi: 10.1080/21691401.2017.1394872 [Crossref] [ Google Scholar]
- Gaspar DP, Faria V, Gonçalves LM, Taboada P, Remuñán-López C, Almeida AJ. Rifabutin-loaded solid lipid nanoparticles for inhaled antitubercular therapy: physicochemical and in vitro studies. Int J Pharm 2016; 497(1-2):199-209. doi: 10.1016/j.ijpharm.2015.11.050 [Crossref] [ Google Scholar]
- Nemati E, Mokhtarzadeh A, Panahi-Azar V, Mohammadi A, Hamishehkar H, Mesgari-Abbasi M. Ethambutol-loaded solid lipid nanoparticles as dry powder inhalable formulation for tuberculosis therapy. AAPS PharmSciTech 2019; 20(3):120. doi: 10.1208/s12249-019-1334-y [Crossref] [ Google Scholar]
- Ji P, Yu T, Liu Y, Jiang J, Xu J, Zhao Y. Naringenin-loaded solid lipid nanoparticles: preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics. Drug Des Devel Ther 2016; 10:911-25. doi: 10.2147/dddt.s97738 [Crossref] [ Google Scholar]
- Rosière R, Van Woensel M, Gelbcke M, Mathieu V, Hecq J, Mathivet T. New folate-grafted chitosan derivative to improve delivery of paclitaxel-loaded solid lipid nanoparticles for lung tumor therapy by inhalation. Mol Pharm 2018; 15(3):899-910. doi: 10.1021/acs.molpharmaceut.7b00846 [Crossref] [ Google Scholar]
- Liu D, Ge Y, Tang Y, Yuan Y, Zhang Q, Li R. Solid lipid nanoparticles for transdermal delivery of diclofenac sodium: preparation, characterization and in vitro studies. J Microencapsul 2010; 27(8):726-34. doi: 10.3109/02652048.2010.513456 [Crossref] [ Google Scholar]
- Gu Y, Yang M, Tang X, Wang T, Yang D, Zhai G. Lipid nanoparticles loading triptolide for transdermal delivery: mechanisms of penetration enhancement and transport properties. J Nanobiotechnology 2018; 16(1):68. doi: 10.1186/s12951-018-0389-3 [Crossref] [ Google Scholar]
- Guo D, Dou D, Li X, Zhang Q, Bhutto ZA, Wang L. Ivermection-loaded solid lipid nanoparticles: preparation, characterisation, stability and transdermal behaviour. Artif Cells Nanomed Biotechnol 2018; 46(2):255-62. doi: 10.1080/21691401.2017.1307207 [Crossref] [ Google Scholar]
- Singh M, Guzman-Aranguez A, Hussain A, Srinivas CS, Kaur IP. Solid lipid nanoparticles for ocular delivery of isoniazid: evaluation, proof of concept and in vivo safety & kinetics. Nanomedicine (Lond) 2019; 14(4):465-91. doi: 10.2217/nnm-2018-0278 [Crossref] [ Google Scholar]
- Khames A, Khaleel MA, El-Badawy MF, El-Nezhawy AOH. Natamycin solid lipid nanoparticles - sustained ocular delivery system of higher corneal penetration against deep fungal keratitis: preparation and optimization. Int J Nanomedicine 2019; 14:2515-31. doi: 10.2147/ijn.s190502 [Crossref] [ Google Scholar]
- Battaglia L, D’Addino I, Peira E, Trotta M, Gallarate M. Solid lipid nanoparticles prepared by coacervation method as vehicles for ocular cyclosporine. J Drug Deliv Sci Technol 2012; 22(2):125-30. doi: 10.1016/s1773-2247(12)50016-x [Crossref] [ Google Scholar]
- Ezzati Nazhad Dolatabadi J, Hamishehkar H, Valizadeh H. Development of dry powder inhaler formulation loaded with alendronate solid lipid nanoparticles: solid-state characterization and aerosol dispersion performance. Drug Dev Ind Pharm 2015; 41(9):1431-7. doi: 10.3109/03639045.2014.956111 [Crossref] [ Google Scholar]
- Aravalli RN, Steer CJ. Gene editing technology as an approach to the treatment of liver diseases. Expert Opin Biol Ther 2016; 16(5):595-608. doi: 10.1517/14712598.2016.1158808 [Crossref] [ Google Scholar]
- Rudolph C, Rosenecker J. Formation of solid lipid nanoparticle (SLN)-gene vector complexes for transfection of mammalian cells in vitro. Cold Spring Harb Protoc 2012; 2012(3):357-60. doi: 10.1101/pdb.prot068122 [Crossref] [ Google Scholar]
- Botto C, Augello G, Amore E, Emma MR, Azzolina A, Cavallaro G. Cationic solid lipid nanoparticles as non viral vectors for the inhibition of hepatocellular carcinoma growth by RNA interference. J Biomed Nanotechnol 2018; 14(5):1009-16. doi: 10.1166/jbn.2018.2557 [Crossref] [ Google Scholar]
- Bondi ML, Azzolina A, Craparo EF, Lampiasi N, Capuano G, Giammona G. Novel cationic solid-lipid nanoparticles as non-viral vectors for gene delivery. J Drug Target 2007; 15(4):295-301. doi: 10.1080/10611860701324698 [Crossref] [ Google Scholar]
- Penumarthi A, Parashar D, Abraham AN, Dekiwadia C, Macreadie I, Shukla R. Solid lipid nanoparticles mediate non-viral delivery of plasmid DNA to dendritic cells. J Nanopart Res 2017; 19(6):210. doi: 10.1007/s11051-017-3902-y [Crossref] [ Google Scholar]
- Limeres MJ, Suñé-Pou M, Prieto-Sánchez S, Moreno-Castro C, Nusblat AD, Hernández-Munain C. Development and characterization of an improved formulation of cholesteryl oleate-loaded cationic solid-lipid nanoparticles as an efficient non-viral gene delivery system. Colloids Surf B Biointerfaces 2019; 184:110533. doi: 10.1016/j.colsurfb.2019.110533 [Crossref] [ Google Scholar]
- Carrillo C, Sánchez-Hernández N, García-Montoya E, Pérez-Lozano P, Suñé-Negre JM, Ticó JR. DNA delivery via cationic solid lipid nanoparticles (SLNs). Eur J Pharm Sci 2013; 49(2):157-65. doi: 10.1016/j.ejps.2013.02.011 [Crossref] [ Google Scholar]
- Yu W, Liu C, Liu Y, Zhang N, Xu W. Mannan-modified solid lipid nanoparticles for targeted gene delivery to alveolar macrophages. Pharm Res 2010; 27(8):1584-96. doi: 10.1007/s11095-010-0149-z [Crossref] [ Google Scholar]
- Mahajan PS, Mahajan KB, Darekar AB. A review on solid lipid nanoparticle (SLN): an advanced treatment modality. Int J Pharm Sci Res 2015; 6(9):3698-712. [ Google Scholar]
- Mishra H, Mishra D, Mishra PK, Nahar M, Dubey V, Jain NK. Evaluation of solid lipid nanoparticles as carriers for delivery of hepatitis B surface antigen for vaccination using subcutaneous route. J Pharm Pharm Sci 2010; 13(4):495-509. doi: 10.18433/j3xk53 [Crossref] [ Google Scholar]
- Stelzner JJ, Behrens M, Behrens SE, Mäder K. Squalene containing solid lipid nanoparticles, a promising adjuvant system for yeast vaccines. Vaccine 2018; 36(17):2314-20. doi: 10.1016/j.vaccine.2018.03.019 [Crossref] [ Google Scholar]
- Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev 2007; 59(6):478-90. doi: 10.1016/j.addr.2007.04.007 [Crossref] [ Google Scholar]
- Bayón-Cordero L, Alkorta I, Arana L. Application of solid lipid nanoparticles to improve the efficiency of anticancer drugs. Nanomaterials (Basel) 2019; 9(3):474. doi: 10.3390/nano9030474 [Crossref] [ Google Scholar]
- Ezzati Nazhad Dolatabadi J, Omidi Y. Solid lipid-based nanocarriers as efficient targeted drug and gene delivery systems. TrAC Trends Anal Chem 2016; 77:100-8. doi: 10.1016/j.trac.2015.12.016 [Crossref] [ Google Scholar]
- Arana L, Bayón-Cordero L, Sarasola LI, Berasategi M, Ruiz S, Alkorta I. Solid lipid nanoparticles surface modification modulates cell internalization and improves chemotoxic treatment in an oral carcinoma cell line. Nanomaterials (Basel) 2019; 9(3):464. doi: 10.3390/nano9030464 [Crossref] [ Google Scholar]
- Cho HJ, Park JW, Yoon IS, Kim DD. Surface-modified solid lipid nanoparticles for oral delivery of docetaxel: enhanced intestinal absorption and lymphatic uptake. Int J Nanomedicine 2014; 9:495-504. doi: 10.2147/ijn.s56648 [Crossref] [ Google Scholar]
- Zhou M, Hou J, Zhong Z, Hao N, Lin Y, Li C. Targeted delivery of hyaluronic acid-coated solid lipid nanoparticles for rheumatoid arthritis therapy. Drug Deliv 2018; 25(1):716-22. doi: 10.1080/10717544.2018.1447050 [Crossref] [ Google Scholar]
- Ramalingam P, Ko YT. Enhanced oral delivery of curcumin from N-trimethyl chitosan surface-modified solid lipid nanoparticles: pharmacokinetic and brain distribution evaluations. Pharm Res 2015; 32(2):389-402. doi: 10.1007/s11095-014-1469-1 [Crossref] [ Google Scholar]
- Kashanian S, Hemati Azandaryani A, Derakhshandeh K. New surface-modified solid lipid nanoparticles using N-glutaryl phosphatidylethanolamine as the outer shell. Int J Nanomedicine 2011; 6:2393-401. doi: 10.2147/ijn.s20849 [Crossref] [ Google Scholar]
- Ramalingam P, Ko YT. Improved oral delivery of resveratrol from N-trimethyl chitosan-g-palmitic acid surface-modified solid lipid nanoparticles. Colloids Surf B Biointerfaces 2016; 139:52-61. doi: 10.1016/j.colsurfb.2015.11.050 [Crossref] [ Google Scholar]
- Shi LL, Xie H, Lu J, Cao Y, Liu JY, Zhang XX. Positively charged surface-modified solid lipid nanoparticles promote the intestinal transport of docetaxel through multifunctional mechanisms in rats. Mol Pharm 2016; 13(8):2667-76. doi: 10.1021/acs.molpharmaceut.6b00226 [Crossref] [ Google Scholar]
- Maretti E, Costantino L, Rustichelli C, Leo E, Croce MA, Buttini F. Surface engineering of Solid Lipid Nanoparticle assemblies by methyl α-d-mannopyranoside for the active targeting to macrophages in anti-tuberculosis inhalation therapy. Int J Pharm 2017; 528(1-2):440-51. doi: 10.1016/j.ijpharm.2017.06.045 [Crossref] [ Google Scholar]
- Pandit AA, Dash AK. Surface-modified solid lipid nanoparticulate formulation for ifosfamide: development and characterization. Nanomedicine (Lond) 2011; 6(8):1397-412. doi: 10.2217/nnm.11.57 [Crossref] [ Google Scholar]
- Jeon HS, Seo JE, Kim MS, Kang MH, Oh DH, Jeon SO. A retinyl palmitate-loaded solid lipid nanoparticle system: effect of surface modification with dicetyl phosphate on skin permeation in vitro and anti-wrinkle effect in vivo. Int J Pharm 2013; 452(1-2):311-20. doi: 10.1016/j.ijpharm.2013.05.023 [Crossref] [ Google Scholar]
- Shen H, Shi S, Zhang Z, Gong T, Sun X. Coating solid lipid nanoparticles with hyaluronic acid enhances antitumor activity against melanoma stem-like cells. Theranostics 2015; 5(7):755-71. doi: 10.7150/thno.10804 [Crossref] [ Google Scholar]
-
Diorio C, Lokhnauth J. Curcumin Solid Lipid Particles and Methods for Their Preparation and Use. Google Patents; 2016. US 2016/0000714.
-
Speiser P. Lipid Nano Pellets as Drug Carriers for Oral Administration. Google Patents; 1986. EP0167825.
-
Müller RH, Jenning V, Mader K, Lippacher A. Lipid Particles on the Basis of Mixtures of Liquid and Solid Lipids and Method for Producing Same. Google Patents; 2014. US 8,663,692.
-
De Vringer T. Topical Preparation Containing a Suspension of Solid Lipid Particles. Google Patents; 1997. JPH05262641A.
-
Rao K. Polymerized Solid Lipid Nanoparticles for Oral or Mucosal Delivery of Therapeutic Proteins and Peptides. Google Patents; 2007. WO 2007/113665 A2.
-
Herzog B. Formulation of UV Absorbers by Incorporation in Solid Lipid Nanoparticles. Google Patents; 2006. US 7,147,841 B2.
-
Massing U. Manufacture of Lipid-Based Nanoparticles Using a Dual Asymmetric Centrifuge. Google Patents; 2008. US 2008/0193511.
-
Mumper RJ, Jay M. Microemulsions as Precursors to Solid Nanoparticles. Google Patents; 2006. US Patent 7,153,525.
-
Weiss J MC, Kessler A, Tedeschi C. Inventor Solid Lipid Nanoparticles (II). U.S.A. patent US 2016 0030305.
-
Weiss J SC, Leuenberger B, Novotny M. Inventor Solid Lipid Nanoparticles(I). 2017. U.S.A patent. US 9,616,001 B2.
-
Lafuente EG, Perez ADP, Lucea GG, Moreno OI, Rincon SV, Plagaro RF, et al. Lipid nanoparticle of polymyxin. 2016. US 2016/0113995.
-
Petit JV, Gonzalez RD, Botello AF. Lipid Nanoparticle Capsules. Google Patents; 2013. US 2013/0017239.