Advanced pharmaceutical bulletin. 11(4):618-623.
doi: 10.34172/apb.2021.070
Mini Review
DR-5 and DLL-4 mAb Functionalized SLNs of Gamma-Secretase Inhibitors- An Approach for TNBC Treatment
Mamta Kumari  , Praveen T. Krishnamurthy *
, Praveen T. Krishnamurthy *  , Sai kiran S. S. Pinduprolu , Piyongsola Sola
, Sai kiran S. S. Pinduprolu , Piyongsola Sola
Author information:
Department of Pharmacology, JSS College of Pharmacy, JSS Academy of Higher Education & Research, Ooty, The Nilgiris, Tamil Nadu, India.
Abstract
Triple-negative breast cancer (TNBC) is the most aggressive and heterogeneous cancer subtypes. High rates of metastasis, poor prognosis, and drug resistance are the major problems associated with TNBC. The current chemotherapeutics eliminate only the bulk tumor cells (non-BCSCs) and do not affect breast cancer stem cells (BCSCs). The BCSCs which are left behind after chemotherapy is reported to promote recurrence and metastasis of TNBC. Death receptor-5 (DR-5) is exclusively expressed in TNBCs and mediates the extrinsic pathway of apoptosis. DR-5, therefore, can be exploited for targeted drug delivery and to induce apoptosis. Gamma-secretase mediated Notch signaling in BCSCs regulates its proliferation, differentiation, and metastasis. The endogenous ligand, Delta-like ligand 4 (DLL4), is reported to activate this Notch signaling in TNBC. Blocking this signaling pathway using both gamma-secretase inhibitors (GSIs) and DLL4 monoclonal antibody (mAb) may produce synergistic benefits. Further, the GSIs (DAPT, LY-411575, RO4929097, MK0752, etc.) suffer from poor bioavailability and off-target side effects such as diarrhea, suppression of lymphopoiesis, headache, hypertension, fatigue, and ventricular dysfunctions. In this hypothesis, we discuss Solid lipid nanoparticles (SLNs) based drug delivery systems containing GSIs and surface modified with DR-5 and DLL4 monoclonal antibodies (mAb) to effectivity target and treat TNBC. The delivery system is designed to deliver the drug cargo precisely to TNBCs through its DR-5 receptors and hence expected to reduce the off-target side effects of GSIs. Further, DLL4 mAb and GSIs are expected to act synergistically to block the Notch signal mediated BCSCs proliferation, differentiation, and metastasis.
Keywords: Triple negative breast cancer (TNBC), Death receptor 5 (DR-5), Delta-like ligand 4 (DLL4), Notch Signaling, Gamma secretase inhibitors (GSIs), Dual targeting
Copyright and License Information
© 2021 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Triple-negative breast cancer (TNBC) is characterized by the lack of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER2).1 TNBC grows spread, and recur after treatment because of its aggressive pathological features.2,3 TNBC accounts for approximately 10–15% of all invasive breast cancer with a short overall survival.4 Prevalence of TNBC in India (27% to 35%) is significantly higher compared to western population (12% to 17%).5 Existing chemotherapies for TNBC treatment can eradicate the rapidly dividing bulk tumor breast cancer cells (non-BCSCs), and they do not affect small subpopulation of breast cancer stem cells (BCSCs).6 Available evidence suggests that the leftover BCSCs are the leading cause of metastasis through epithelial to mesenchymal transition (EMT) process. In the EMT process, the epithelial BCSCs cells lose their inter-cellular adherence by gaining invasion and migration capabilities.7,8 There is a need, therefore, to eliminate BCSCs in addition to non-BCSCs.
The Notch signaling pathway is a fundamental regulator of angiogenesis in non-BCSCS and self-renewal & maintenance in BCSCs.9-11 The above findings has prompted scientists to evaluate possible benefits of gamma-secretase inhibitors (GSIs) in TNBC to control growth, prevent self-renewal, and suppress drug resistance of TNBCs.12-16 Some studies suggest that, although GSIs, like DAPT, LY-411575, RO4929097, MK0752 are not significantly cytotoxic, several studies indicate that they will be useful in potentiating the cytotoxic effects of other anticancer agents and helpful in eliminating BCSCs. However, the GSIs are associated with severe off-target side effects such as diarrhea, suppression of lymphopoiesis, headache, hypertension, fatigue and ventricular dysfunctions, which limit their clinical use.17-19 Targeted delivery of GSIs to TNBCs could be one of the strategies to overcome this problem.15,20
Activation of Notch1 and 4 receptors by delta-like ligand 4 (DLL4) endogenous ligand in TNBC results in aberrant activation of Notch signaling.21 DLL4 binding to Notch1/Notch4 receptors ensures the cleavage of the Notch intracellular domain (NICD) by the enzyme gamma-secretase. The NICD translocates to the nucleus and activates the expression of notch target genes involved in angiogenesis, apoptosis, metastasis, and chemoresistance.22,23 DLL4 and gamma-secretase enzyme are highly expressed in TNBC cells and are, therefore, considered as unique targets of TNBC.24,25
Recent studies have reported that GSIs are useful in the eradication of BCSCs, inhibition of EMT, angiogenesis, and tumor growth.19,26,27 Besides, GSIs also potentiate the effect of chemotherapeutic agents by inhibiting the genes involved in chemoresistance (Hes and Hey).28,29 Existing evidence also suggests that the inhibition of DLL4 mediated activation of Notch receptors by anti-DLL4 mAb also produce anti-angiogenic, proapoptotic and chemo-sensitizing effects on TNBC cells.30-32 It was recently reported that a combination of DLL4 mAb with GSIs has synergetic proapoptotic effects.33
Death receptor 5 (DR-5) is a member of tumor necrosis factor receptor superfamily, overexpressed explicitly on the surface of TNBC cells. Therefore, it can be used as a target for effective drug delivery in TNBCs.34,35 Besides, activation of DR-5 leads to activation of extrinsic apoptotic signaling and hence proapoptotic effects.36
Solid liquid nanoparticles (SLNs) are widely used for targeted drug delivery to minimize off-target effects and improve the bioavailability of drugs.37 Also, SLNs offer several advantages such as improved stability of the drug, higher entrapment efficacy, and biocompatibility over other nanoparticle-based delivery systems. SLNs with appropriate stealth properties will have reduced clearance and improved drug cargo delivery to the tumor site through enhanced permeation and retention (EPR) effect.38,39 However, accumulating evidence suggest that the EPR effect alone` is not sufficient to achieve site-specific delivery of drug cargo to the tumor site. Alternatively, unique cancer surface proteins have been targeted using monoclonal antibodies (mAbs) to improve target-specific delivery of drug cargo.40
Hypothesis
TNBC targeted GSI-SLNs surface modified with DR-5, and DLL4 mAbs can be an effective strategy to treat TNBC.
The SLN delivery system will deliver the GSIs drug cargo precisely to TNBCs through membrane DR-5 receptors and hence reduce the off-target side effects of GSIs. DLL4 mAb and GSIs are expected to act synergistically to block the Notch signal mediated BCSCs proliferation, differentiation, and metastasis. Further, when combined conventional chemotherapy, the formulation will effectively eliminate both BCSCs and non-BCSCs and, thus, help in achieving the complete cure of TNBC.
Explanation of the hypothesis
Notch signaling is one of the critical pathological pathways implicated in TNBCs. This pathway regulates the expression of target genes such as HES1, HEY2, MYC, CCND1, HES4, NRAR, etc., and involved in BCSCs proliferation, differentiation, and apoptosis.41,42 Notch signaling is activated by the binding of transmembrane ligands [Delta-like (DLL) 1, 4, and Jagged (JAG) 1, 2] to the Notch receptors present on the cell surface. This binding results in the proteolytic invasion of Notch by a presenilin-dependent gamma-secretase complex to release of NICD, which later translocates to the nucleus and heterodimerizes with a transcription factor, CSL (suppressor of hairless), and activates various target genes (Figure 1).43-45 Several studies conclude that Notch signaling pathway activation in BCSCs predominantly activates the expression of HES1 and HEY2 genes, which participates in the perpetuation of self-renewal of BCSCs.46-48 Notch signaling also reported to regulate cyclinD1, c-Myc, p21, Survivin, Slug, CCNA, CCNB, CCNDI, HER2 expression, and stimulate the nuclear factor-kappa B (NF-κB) pathway.49,50 The Notch-mediated activation of all these factors promotes cell proliferation, angiogenesis, resistance to apoptosis, and self-renewal of BCSCs.
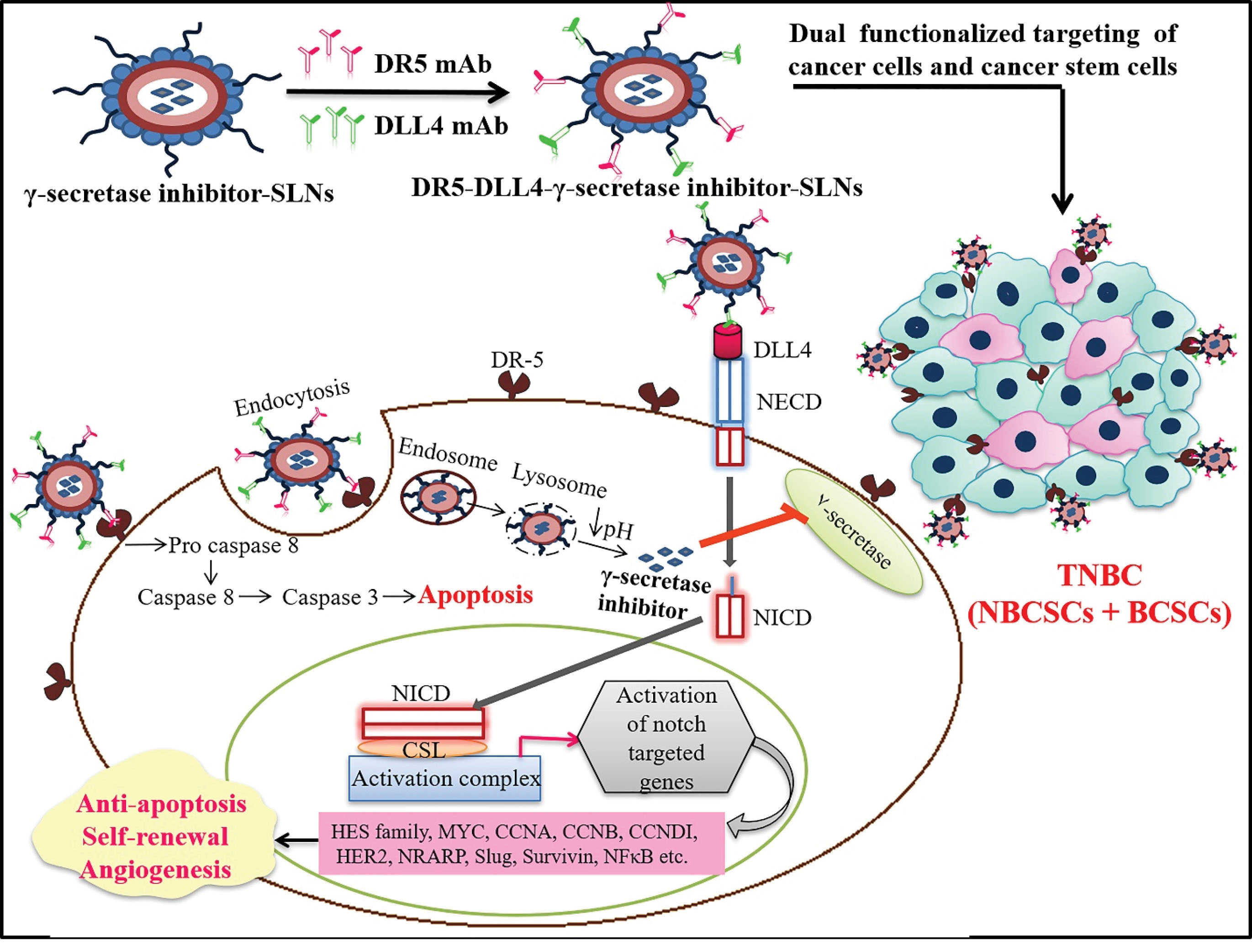
Figure 1.
SLNs of Gamma-secretase inhibitor (GSI) surface modified with dual mAbs for the site specific delivery of GSI to breast cancer cells and breast Cancer Stem Cells (BCSCs). Silencing of Notch signaling by this apporach inhibits cell proliferation, angiogenesis, apoptotic resistance and self-renewal of BCSCs.
.
SLNs of Gamma-secretase inhibitor (GSI) surface modified with dual mAbs for the site specific delivery of GSI to breast cancer cells and breast Cancer Stem Cells (BCSCs). Silencing of Notch signaling by this apporach inhibits cell proliferation, angiogenesis, apoptotic resistance and self-renewal of BCSCs.
Surface modified SLNs with DR-5 mAb expected to bind selectively to DR-5 receptors present on TNBC and therefore help in active targeting through receptor-mediated endocytosis, further binding with DR-5 may also initiate the extrinsic pathway of apoptosis and hence proapoptotic effects.51 Ding et al demonstrated that anti-DR-5 mAb-mediated delivery of dacarbazine (DTIC) nanoparticles show an improved antitumor and proapoptotic activity when compared to the bulk drug.52 Tummala et al, report that, gold nanoparticles of oxaliplatin conjugating with anti-DR-5 mAb show enhanced anticancer activity and site-specific delivery of drug cargo.53 Zhang et al reported that anti-DR-5 (Zaptuzumab) antibody-drug conjugate show increased therapeutic efficacy and safety when compared to naked antibody Zaptuzumab treatment.54
The expression of Notch ligand (DLL4) in tumor vasculature is high as compared to healthy tissues.55 In TNBC, DLL4 binding to the Notch receptors promotes the transcription activation of genes regulating tumor angiogenesis and growth.55,56 DLL4 mediated activation of Notch signaling, therefore, improves vascular function and promotes tumor growth.57 Jia et al demonstrated that a humanized anti-DLL4 mAb inhibits breast tumor growth in an MDA-MB-231 xenograft model in mice. The results indicate that anti-DLL4 mAb prevents tumor growth by blocking the DLL4-Notch signaling pathway.58 Hoey et al report that anti-DLL4 treatment blocks the Notch pathway mediated expression of anti-apoptotic genes (HSPA6 and BIRC3) and hence sensitize the tumor cells towards chemotherapy.59
Yen et al establish that the combination of anti-DLL4 antibody and paclitaxel decrease BCSCs frequency and retard the tumor relapse in paclitaxel-resistant TNBCs.60 Wang et al successfully developed antibody-drug conjugates of Monomethyl auristatin E and anti-DLL4 antibody to promote tumor cell death and to achieve site-specific delivery.61
Gamma-secretase enzyme regulates tumor-promoting Notch signaling in TNBC by controlling NICD levels. Inhibition of this enzyme through GSIs, therefore, a promising strategy.62,63 Accumulating pieces of evidence suggest that, the GSIs are associated with clinical limitations such as poor bioavailability and off-target side effects include diarrhea, suppression of lymphopoiesis, headache, hypertension, fatigue, and ventricular dysfunctions.17,19,63 To overcome these limitations, researchers focused on nanocarriers based drug delivery which improves bioavailability and delivers the drug cargo specifically to tumor sites.15,64
Mamaeva et al developed a glucose functionalized Mesoporous silica nanoparticles of GSI (DAPT). They report that the developed nanoformulation efficiently delivered the drug to TNBC and reduced the BCSCs population by inhibiting the Notch signaling pathway.15 Kang et al establish that the concurrent treatment of GSIs with anti-DLL4 increases the anticancer and proapoptotic efficiency of GSIs in gastrointestinal cancer. The combined therapy of GSIs with anti-DLL4 boosted the expression of BAX and P53 and reduced the expression of Bcl-2. On the other hand, naïve GSI only enhanced the expression of BAX and P53, suggesting that the reduced Bcl-2 expression had a significant function in synergistic antitumor and proapoptotic effects.33
Drug delivery using SLNs is an accepted approach for targeted drug delivery to improve efficacy and reduce off-target side effects. SLNs as a nanocarrier, enhance the bioavailability, and provide control release. Besides, SLNs also modulate the release kinetics, minimize systemic toxicity and increase the therapeutic efficacy of chemotherapeutic agents.65,66 Pindiprolu et al reported that, SLNs of niclosamide improve the anticancer efficiency by increasing the site-specific delivery of niclosamide to the TNBC.67 Wang et al successfully demonstrate the improved anticancer efficacy of Curcumin SLNs in SKBR3 cells as compared to naïve curcumin.68
Dominguez et al successfully conjugated anti-RNEU and anti-CD40 antibodies on the surface of PLA-(poly dl-lactic acid)-biodegradable nanoparticles. They establish that dual antibody conjugated nanoformulation boosts the antitumor response and also results in complete tumor eradication.69 Kosmides et al developed nanoparticles surface modified with two different mAbs (anti-PD-1 and anti-CTLA-4 mAb), which concurrently impede the inhibitory PD-L1 signal and activate T cells through the 4-1BB co-stimulatory pathway.70 The combination of mAbs on the surface of nanoparticles has significantly increased their efficacy when compared to individual mAbs.70 Chen et al developed dual antibodies (anti-CD44 and anti-CD133) conjugated transretinoic acid-loaded poly(lactide-co-glycolide)-lecithin-PEG nanoparticles to target cancer stem cells (CSCs). They report that dual-targeted nanoparticles effectively inhibit the tumor growth and eradicate the CSC population.71
Conclusion
In this article, we propose to prepare DR-5, and DLL-4 mAbs functionalized SLNs of GSIs to enhance their bioavailability, provide TNBC specific delivery, and to reduce off-target side effects. Further, anti-DLL-4 mAb and GSIs may synergistically act to eliminate the resistant BCSCs of TNBC. Besides, when combined with anticancer chemotherapeutics, the formulation may enhance their overall anticancer efficacy.
Ethical Issues
The study does not involve any human subjects and/or animals.
Conflict of Interest
The authors confirm that this article content has no conflict of interest.
Acknowledgments
The authors would like to thank the Department of Science and Technology- Fund for Improvement of Science and Technology Infrastructure in Universities and Higher Educational Institutions (DST-FIST), New Delhi, for their infrastructure support to our department (Grant No. SR/FST/LSI-574/2013). MK acknowledges AICTE-NDF (Application No.- 52198) for her SRF.
References
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010; 363(20):1938-48. doi: 10.1056/NEJMra1001389 [Crossref] [ Google Scholar]
- Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur J Cancer 2009; 45 Suppl 1:27-40. doi: 10.1016/s0959-8049(09)70013-9 [Crossref] [ Google Scholar]
- Aysola K, Desai A, Welch C, Xu J, Qin Y, Reddy V. Triple negative breast cancer - an overview. Hereditary Genet 2013; 2013(Suppl 2). doi: 10.4172/2161-1041.s2-001 [Crossref]
- Collignon J, Lousberg L, Schroeder H, Jerusalem G. Triple-negative breast cancer: treatment challenges and solutions. Breast Cancer (Dove Med Press) 2016; 8:93-107. doi: 10.2147/bctt.s69488 [Crossref] [ Google Scholar]
- Sandhu GS, Erqou S, Patterson H, Mathew A. Prevalence of triple-negative breast cancer in India: systematic review and meta-analysis. J Glob Oncol 2016; 2(6):412-21. doi: 10.1200/jgo.2016.005397 [Crossref] [ Google Scholar]
- Nedeljković M, Damjanović A. Mechanisms of chemotherapy resistance in triple-negative breast cancer-how we can rise to the challenge. Cells 2019; 8(9):957. doi: 10.3390/cells8090957 [Crossref] [ Google Scholar]
- Bai X, Ni J, Beretov J, Graham P, Li Y. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat Rev 2018; 69:152-63. doi: 10.1016/j.ctrv.2018.07.004 [Crossref] [ Google Scholar]
- Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010; 29(34):4741-51. doi: 10.1038/onc.2010.215 [Crossref] [ Google Scholar]
- Stylianou S, Clarke RB, Brennan K. Aberrant activation of Notch signaling in human breast cancer. Cancer Res 2006; 66(3):1517-25. doi: 10.1158/0008-5472.can-05-3054 [Crossref] [ Google Scholar]
- Farnie G, Clarke RB. Mammary stem cells and breast cancer--role of Notch signalling. Stem Cell Rev 2007; 3(2):169-75. doi: 10.1007/s12015-007-0023-5 [Crossref] [ Google Scholar]
- Nickoloff BJ, Osborne BA, Miele L. Notch signaling as a therapeutic target in cancer: a new approach to the development of cell fate modifying agents. Oncogene 2003; 22(42):6598-608. doi: 10.1038/sj.onc.1206758 [Crossref] [ Google Scholar]
- Li ZL, Chen C, Yang Y, Wang C, Yang T, Yang X. Gamma secretase inhibitor enhances sensitivity to doxorubicin in MDA-MB-231 cells. Int J Clin Exp Pathol 2015; 8(5):4378-87. [ Google Scholar]
- Azzam DJ, Zhao D, Sun J, Minn AJ, Ranganathan P, Drews-Elger K. Triple negative breast cancer initiating cell subsets differ in functional and molecular characteristics and in γ-secretase inhibitor drug responses. EMBO Mol Med 2013; 5(10):1502-22. doi: 10.1002/emmm.201302558 [Crossref] [ Google Scholar]
- Locatelli MA, Aftimos P, Dees EC, LoRusso PM, Pegram MD, Awada A. Phase I study of the gamma secretase inhibitor PF-03084014 in combination with docetaxel in patients with advanced triple-negative breast cancer. Oncotarget 2017; 8(2):2320-8. doi: 10.18632/oncotarget.13727 [Crossref] [ Google Scholar]
- Mamaeva V, Niemi R, Beck M, Özliseli E, Desai D, Landor S. Inhibiting Notch activity in breast cancer stem cells by glucose functionalized nanoparticles carrying γ-secretase inhibitors. Mol Ther 2016; 24(5):926-36. doi: 10.1038/mt.2016.42 [Crossref] [ Google Scholar]
- Shih IM, Wang TL. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res 2007; 67(5):1879-82. doi: 10.1158/0008-5472.can-06-3958 [Crossref] [ Google Scholar]
- Rizzo P, Osipo C, Foreman K, Golde T, Osborne B, Miele L. Rational targeting of Notch signaling in cancer. Oncogene 2008; 27(38):5124-31. doi: 10.1038/onc.2008.226 [Crossref] [ Google Scholar]
- Takebe N, Nguyen D, Yang SX. Targeting Notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther 2014; 141(2):140-9. doi: 10.1016/j.pharmthera.2013.09.005 [Crossref] [ Google Scholar]
- Olsauskas-Kuprys R, Zlobin A, Osipo C. Gamma secretase inhibitors of Notch signaling. Onco Targets Ther 2013; 6:943-55. doi: 10.2147/ott.s33766 [Crossref] [ Google Scholar]
- Nasrin A, Hassan M, Ye P. Inhibition of notch signaling pathway using γ-secretase inhibitor delivered by a low dose of Triton-X100 in cultured oral cancer cells. Biochem Biophys Res Commun 2018; 495(3):2118-24. doi: 10.1016/j.bbrc.2017.12.082 [Crossref] [ Google Scholar]
- Briot A, Iruela-Arispe ML. Blockade of specific notch ligands: a new promising approach in cancer therapy. Cancer Discov 2015; 5(2):112-4. doi: 10.1158/2159-8290.cd-14-1501 [Crossref] [ Google Scholar]
- Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 2006; 444(7122):1032-7. doi: 10.1038/nature05355 [Crossref] [ Google Scholar]
- Aster JC, Pear WS, Blacklow SC. The varied roles of notch in cancer. Annu Rev Pathol 2017; 12:245-75. doi: 10.1146/annurev-pathol-052016-100127 [Crossref] [ Google Scholar]
- Jiang X, Zhang QL, Liu TG, Zhao WP, Yang M, Wang LN. Evaluation of local injection of bevacizumab against triple-negative breast cancer xenograft tumors. Curr Pharm Des 2019; 25(8):862-70. doi: 10.2174/1381612825666190306164157 [Crossref] [ Google Scholar]
- Hernandez F, Peluffo MC, Stouffer RL, Irusta G, Tesone M. Role of the DLL4-notch system in PGF2alpha-induced luteolysis in the pregnant rat. Biol Reprod 2011; 84(5):859-65. doi: 10.1095/biolreprod.110.088708 [Crossref] [ Google Scholar]
- Schott AF, Landis MD, Dontu G, Griffith KA, Layman RM, Krop I. Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin Cancer Res 2013; 19(6):1512-24. doi: 10.1158/1078-0432.ccr-11-3326 [Crossref] [ Google Scholar]
- Choi S, Yu J, Park A, Dubon MJ, Do J, Kim Y. BMP-4 enhances epithelial mesenchymal transition and cancer stem cell properties of breast cancer cells via Notch signaling. Sci Rep 2019; 9(1):11724. doi: 10.1038/s41598-019-48190-5 [Crossref] [ Google Scholar]
- Wang Z, Li Y, Ahmad A, Azmi AS, Banerjee S, Kong D. Targeting Notch signaling pathway to overcome drug resistance for cancer therapy. Biochim Biophys Acta 2010; 1806(2):258-67. doi: 10.1016/j.bbcan.2010.06.001 [Crossref] [ Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 2009; 137(2):216-33. doi: 10.1016/j.cell.2009.03.045 [Crossref] [ Google Scholar]
- Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther 2020; 5(1):1-35. doi: 10.1038/s41392-020-0110-5 [Crossref] [ Google Scholar]
- Kuhnert F, Kirshner JR, Thurston G. Dll4-Notch signaling as a therapeutic target in tumor angiogenesis. Vasc Cell 2011; 3(1):20. doi: 10.1186/2045-824x-3-20 [Crossref] [ Google Scholar]
- Xu Z, Wang Z, Jia X, Wang L, Chen Z, Wang S. MMGZ01, an anti-DLL4 monoclonal antibody, promotes nonfunctional vessels and inhibits breast tumor growth. Cancer Lett 2016; 372(1):118-27. doi: 10.1016/j.canlet.2015.12.025 [Crossref] [ Google Scholar]
- Kang M, Zhang Y, Jin X, Chen G, Huang Y, Wu D. Concurrent treatment with anti-DLL4 enhances antitumor and proapoptotic efficacy of a γ-secretase inhibitor in gastric cancer. Transl Oncol 2018; 11(3):599-608. doi: 10.1016/j.tranon.2018.02.016 [Crossref] [ Google Scholar]
- Elrod HA, Sun SY. Modulation of death receptors by cancer therapeutic agents. Cancer Biol Ther 2008; 7(2):163-73. doi: 10.4161/cbt.7.2.5335 [Crossref] [ Google Scholar]
- Wang G, Wang X, Yu H, Wei S, Williams N, Holmes DL. Small-molecule activation of the TRAIL receptor DR5 in human cancer cells. Nat Chem Biol 2013; 9(2):84-9. doi: 10.1038/nchembio.1153 [Crossref] [ Google Scholar]
- Sun SY. Understanding the role of the death receptor 5/FADD/caspase-8 death signaling in cancer metastasis. Mol Cell Pharmacol 2011; 3(1):31-4. [ Google Scholar]
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2007; 2(12):751-60. doi: 10.1038/nnano.2007.387 [Crossref] [ Google Scholar]
- Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev 2011; 63(3):131-5. doi: 10.1016/j.addr.2010.03.011 [Crossref] [ Google Scholar]
- ud Din F, Aman W, Ullah I, Qureshi OS, Mustapha O, Shafique S. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomedicine 2017; 12:7291-309. doi: 10.2147/ijn.s146315 [Crossref] [ Google Scholar]
- Muhamad N, Plengsuriyakarn T, Na-Bangchang K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: a systematic review. Int J Nanomedicine 2018; 13:3921-35. doi: 10.2147/ijn.s165210 [Crossref] [ Google Scholar]
- Suman S, Das TP, Damodaran C. Silencing NOTCH signaling causes growth arrest in both breast cancer stem cells and breast cancer cells. Br J Cancer 2013; 109(10):2587-96. doi: 10.1038/bjc.2013.642 [Crossref] [ Google Scholar]
- Olsauskas-Kuprys R, Zlobin A, Osipo C. Gamma secretase inhibitors of Notch signaling. Onco Targets Ther 2013; 6:943-55. doi: 10.2147/ott.s33766 [Crossref] [ Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 2001; 414(6859):105-11. doi: 10.1038/35102167 [Crossref] [ Google Scholar]
- Sorensen EB, Conner SD. γ-secretase-dependent cleavage initiates Notch signaling from the plasma membrane. Traffic 2010; 11(9):1234-45. doi: 10.1111/j.1600-0854.2010.01090.x [Crossref] [ Google Scholar]
- Bray SJ. Notch signalling in context. Nat Rev Mol Cell Biol 2016; 17(11):722-35. doi: 10.1038/nrm.2016.94 [Crossref] [ Google Scholar]
- Zanotti S, Canalis E. Notch signaling and the skeleton. Endocr Rev 2016; 37(3):223-53. doi: 10.1210/er.2016-1002 [Crossref] [ Google Scholar]
- Schweisguth F. Regulation of Notch signaling activity. Curr Biol 2004; 14(3):R129-38. [ Google Scholar]
- Acar A, Simões BM, Clarke RB, Brennan K. A role for Notch signalling in breast cancer and endocrine resistance. Stem Cells Int 2016; 2016:2498764. doi: 10.1155/2016/2498764 [Crossref] [ Google Scholar]
- Venkatesh V, Nataraj R, Thangaraj GS, Karthikeyan M, Gnanasekaran A, Kaginelli SB. Targeting Notch signalling pathway of cancer stem cells. Stem Cell Investig 2018; 5:5. doi: 10.21037/sci.2018.02.02 [Crossref] [ Google Scholar]
- Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell 2000; 5(2):197-206. doi: 10.1016/s1097-2765(00)80416-5 [Crossref] [ Google Scholar]
- Fay F, McLaughlin KM, Small DM, Fennell DA, Johnston PG, Longley DB. Conatumumab (AMG 655) coated nanoparticles for targeted pro-apoptotic drug delivery. Biomaterials 2011; 32(33):8645-53. doi: 10.1016/j.biomaterials.2011.07.065 [Crossref] [ Google Scholar]
- Ding B, Wu X, Fan W, Wu Z, Gao J, Zhang W. Anti-DR5 monoclonal antibody-mediated DTIC-loaded nanoparticles combining chemotherapy and immunotherapy for malignant melanoma: target formulation development and in vitro anticancer activity. Int J Nanomedicine 2011; 6:1991-2005. doi: 10.2147/ijn.s24094 [Crossref] [ Google Scholar]
- Tummala S, Kumar MN, Pindiprolu SK. Improved anti-tumor activity of oxaliplatin by encapsulating in anti-DR5 targeted gold nanoparticles. Drug Deliv 2016; 23(9):3505-19. doi: 10.1080/10717544.2016.1199606 [Crossref] [ Google Scholar]
- Zhang S, Zheng C, Zhu W, Xiong P, Zhou D, Huang C. A novel anti-DR5 antibody-drug conjugate possesses a high-potential therapeutic efficacy for leukemia and solid tumors. Theranostics 2019; 9(18):5412-23. doi: 10.7150/thno.33598 [Crossref] [ Google Scholar]
- Sainson RC, Harris AL. Anti-Dll4 therapy: can we block tumour growth by increasing angiogenesis?. Trends Mol Med 2007; 13(9):389-95. doi: 10.1016/j.molmed.2007.07.002 [Crossref] [ Google Scholar]
- Brzozowa M, Wojnicz R, Kowalczyk-Ziomek G, Helewski K. The Notch ligand Delta-like 4 (DLL4) as a target in angiogenesis-based cancer therapy?. Contemp Oncol (Pozn) 2013; 17(3):234-7. doi: 10.5114/wo.2013.35588 [Crossref] [ Google Scholar]
- Li JL, Sainson RC, Shi W, Leek R, Harrington LS, Preusser M. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res 2007; 67(23):11244-53. doi: 10.1158/0008-5472.can-07-0969 [Crossref] [ Google Scholar]
- Jia X, Wang W, Xu Z, Wang S, Wang T, Wang M. A humanized anti-DLL4 antibody promotes dysfunctional angiogenesis and inhibits breast tumor growth. Sci Rep 2016; 6:27985. doi: 10.1038/srep27985 [Crossref] [ Google Scholar]
- Hoey T, Yen WC, Axelrod F, Basi J, Donigian L, Dylla S. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell 2009; 5(2):168-77. doi: 10.1016/j.stem.2009.05.019 [Crossref] [ Google Scholar]
- Yen W, Fischer M, Lewicki J, Gurney A, Hoey T. Targeting cancer stem cells and vasculature by a novel anti-Delta-like 4 ligand (DLL4) antibody for treatment of triple negative breast cancer. Cancer Res 2010; 69(24 Suppl):5071. doi: 10.1158/0008-5472.sabcs-09-5071 [Crossref] [ Google Scholar]
- Wang S, Zhou R, Sun F, Li R, Wang M, Wu M. The two novel DLL4-targeting antibody-drug conjugates MvM03 and MGD03 show potent anti-tumour activity in breast cancer xenograft models. Cancer Lett 2017; 409:125-36. doi: 10.1016/j.canlet.2017.09.004 [Crossref] [ Google Scholar]
- Sail V, Hadden MK. Notch pathway modulators as anticancer chemotherapeutics. Annu Rep Med Chem 2012; 47:267-280. doi: 10.1016/b978-0-12-396492-2.00018-7 [Crossref] [ Google Scholar]
- Akiyoshi T, Nakamura M, Yanai K, Nagai S, Wada J, Koga K. Gamma-secretase inhibitors enhance taxane-induced mitotic arrest and apoptosis in colon cancer cells. Gastroenterology 2008; 134(1):131-44. doi: 10.1053/j.gastro.2007.10.008 [Crossref] [ Google Scholar]
- El-Nemr A, El-Sikaily A, Makkey A, Darwish A, Ismail MN. Magnetoelectric chitosan nanoparticles loaded with DAPT, a secretase inhibitor effectively inhibits NOTCH1 signalling pathway in breast cancer stem cells. Biochem Mol Biol J 2018; 4:45-56. doi: 10.21767/2471-8084-c4-018 [Crossref] [ Google Scholar]
- Bayón-Cordero L, Alkorta I, Arana L. Application of solid lipid nanoparticles to improve the efficiency of anticancer drugs. Nanomaterials (Basel) 2019; 9(3):474. doi: 10.3390/nano9030474 [Crossref] [ Google Scholar]
- Stella B, Peira E, Dianzani C, Gallarate M, Battaglia L, Gigliotti CL. Development and characterization of solid lipid nanoparticles loaded with a highly active doxorubicin derivative. Nanomaterials (Basel) 2018; 8(2):110. doi: 10.3390/nano8020110 [Crossref] [ Google Scholar]
- Pindiprolu S, Chintamaneni PK, Krishnamurthy PT, Ratna Sree Ganapathineedi K. Formulation-optimization of solid lipid nanocarrier system of STAT3 inhibitor to improve its activity in triple negative breast cancer cells. Drug Dev Ind Pharm 2019; 45(2):304-13. doi: 10.1080/03639045.2018.1539496 [Crossref] [ Google Scholar]
- Wang W, Chen T, Xu H, Ren B, Cheng X, Qi R. Curcumin-loaded solid lipid nanoparticles enhanced anticancer efficiency in breast cancer. Molecules 2018; 23(7):1578. doi: 10.3390/molecules23071578 [Crossref] [ Google Scholar]
- Dominguez AL, Lustgarten J. Targeting the tumor microenvironment with anti-neu/anti-CD40 conjugated nanoparticles for the induction of antitumor immune responses. Vaccine 2010; 28(5):1383-90. doi: 10.1016/j.vaccine.2009.10.153 [Crossref] [ Google Scholar]
- Kosmides AK, Sidhom JW, Fraser A, Bessell CA, Schneck JP. Dual targeting nanoparticle stimulates the immune system to inhibit tumor growth. ACS Nano 2017; 11(6):5417-29. doi: 10.1021/acsnano.6b08152 [Crossref] [ Google Scholar]
- Chen H, Lin J, Shan Y, Zhengmao L. The promotion of nanoparticle delivery to two populations of gastric cancer stem cells by CD133 and CD44 antibodies. Biomed Pharmacother 2019; 115:108857. doi: 10.1016/j.biopha.2019.108857 [Crossref] [ Google Scholar]