Advanced pharmaceutical bulletin. 12(2):336-345.
doi: 10.34172/apb.2022.032
Research Article
Carrier Effect in Development of Rifampin Loaded Proliposome for Pulmonary Delivery: A Quality by Design Study
Elahehnaz Parhizkar 1  , Delaram Sadeghinia 1, Hamed Hamishehkar 2, Shadi Yaqoubi 3, Ali Nokhodchi 4
, Delaram Sadeghinia 1, Hamed Hamishehkar 2, Shadi Yaqoubi 3, Ali Nokhodchi 4  , Shohreh Alipour 5, *
, Shohreh Alipour 5, * 
Author information:
1Department of Pharmaceutics, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran.
2Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
3Biotechnology Research Center, Student Research Committee and Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran.
4Pharmaceutics Research Laboratory, School of Life Sciences, University of Sussex, Brighton, BN1 9QJ, UK. 5 Pharmaceutical Sciences Research Center and Department of Quality Control, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran.
5Pharmaceutical Sciences Research Center and Department of Quality Control, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran.
Abstract
Purpose:
Pulmonary tuberculosis (TB) is a worldwide life-threatening infection. Therecommended anti-TB regimen contains oral administration of classical first-line drugs suchas rifampin for 6-24 months which often leads to low patient compliance due to high adverseeffects; therefore, lung localized pulmonary delivery of anti-TB agents may be a suitablealternative. Proliposomes free-flowing powders are well-known carriers for lung delivery sincethey can form liposomes by hydration. Liposomes are safe and useful carriers for lung deliverydue to their phospholipid structure.
Methods:
Porous lactose and mannitol as proliposome carriers were prepared by spray dryingtechnique using sucrose and citric acid as templating agents. Design Expert® software wasused to develop forty formulations based on the porous and non-porous carriers, which werecharacterized with respect to their weight yield, density, and flowability. Rifampin-loadedhydrated liposomes were produced and evaluated for size, morphology, loading capacityand encapsulation efficiency. The optimized proliposomes in vitro release and aerosolizationproperties were evaluated. Solid-state analysis was confirmed by differential scanningcalorimetry (DSC).
Results:
Porous lactose surface area was 80 folds higher than non-porous one, respectively.Optimized porous-based proliposome indicated the acceptable aerosolization properties,including mass median aerodynamic diameter (MMAD) of 6.21 ± 0.36 μm and fine particlefraction (FPF) of 9.17 ± 0.18% with a fast rifampin release (80%) within one hour. DSC resultsproved that there was no change in the solid-state of rifampin during the production process.
Conclusion:
Hence, it seems; rifampin loaded inhalable proliposomes may be a suitable systemfor delivering liposomal rifampin into the lungs.
Keywords: aerosolization properties, carrier porosity, dry powder, proliposome, rifampin
Copyright and License Information
©2022 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Tuberculosis (TB) is Mycobacterium tuberculosis induced debilitating infectious disease which caused 1.8 million deaths in 2015
1
and is recognized as the second factor of mortality and morbidity in acquired immune deficiency syndrome (AIDS) patients.
2
Therefore, it seems that the treatment and eradication of this life-threatening infection are very important. Pulmonary TB which involves the lungs, contributes more than 80% of TB pathology reported cases. Different approaches in global eradication of TB infection are achieving, but due to the slow production process and approval of new anti-TB drugs, there are a few new drugs close to reaching the market.
3
Therefore, the currently recommended TB treatment regimen contains the classical combination of first-line drugs, including oral and parenteral administration isoniazid, rifampin, ethambutol and pyrazinamide which indicated more than 90% cure rates in drug-susceptible cases.
4,5
However, this regimen is associated to some disadvantages, including poor bioavailability and high rates of the first-pass metabolism. Besides, long treatment duration (6 to 24 months) associated to unwanted side effects for patients, not only leads to low patient compliance and therapeutic failure
6-8
but also it may emerge the new bacterial resistance.
9-11
As a result, the new inhalable formulation design for available conventional drugs may be an effective strategy to overcome mentioned obstacles of TB treatment since it can localize the drug in the lung, which is the site of action, in addition, can decrease drug administered dose and systemic side effects while it may accelerate drugs’ onset of action.
7,12
Among available three inhalation delivery systems, dry powder inhalers (DPIs) were rapidly improved due to their propellant-free nature with higher stability and dose capacity which leads to higher patients’ compliance.
8,13
During past decades, many investigations have focused on three main categories of inhalable formulations for anti-TB drugs to enhance localized drug targeting, which include liposomes, microparticles and nanoparticles.
12,14
Liposomes are lipid-containing vesicles formulated with exogenous phospholipids similar to lung surfactant that represent a promising drug carrier for pulmonary delivery.
1,12
This carrier is stored in mononuclear phagocytic system cells, mainly macrophages where the Mycobacterium tuberculosis resides. Therefore, the carrier improves the effectiveness of anti-TB drugs.
4,15
Besides, liposomes offer other advantages such as reduction of drug toxicity and side effects, high encapsulation efficacy, controlled drug release over a long period, and drug protection in destructive conditions, such as pH and enzyme.
16,17
Despite liposomal benefits, these carriers have serious limitations like phospholipid oxidation, aggregation, sedimentation, instability and drug leakage. To eliminate the mentioned drawbacks, proliposomes were introduced, which were free-flowing powders that contained a hydrophilic carrier that was layered with phospholipids and form liposomal dispersion on hydration. The solid-state of proliposomes ensures chemical and physical stability, and improves the shelf-life of encapsulated drugs.
18-20
Proliposomes production is a simple and reproducible manufacturing technique for large-scale production of liposomes.
19
Proliposome carriers are water-soluble materials such as mannitol, sorbitol, maltodextrin, etc. Carriers with higher surface area and porosity may regulate the lipid amount required to make proliposomes.
19,21
Proliposomes’ ingredients are very similar to mammalian membrane structures that result in higher biocompatibility and biodegradability and lead to suitable properties as pulmonary delivery of drugs.
20
Proliposomes as DPIs are beneficial for pulmonary administration considering their reduced toxicity, enhanced potency, controlled drug delivery ability, and uniform lung deposition.
13
Different inhalable proliposomes as the pulmonary carrier of different drugs including budesonide,
22
ketotifen,
23
amikacin,
24
dapsone
13
and levofloxacin
25
were developed in previous studies. These proliposomes prepared using different phospholipids (hydrogenated soya phosphatidylcholine, egg phosphatidyl choline, dipalmitoyl phosphatidylcholine, cholesterol) and carriers (sucrose, lactose, mannitol), have shown proper aerosolization properties with successful in vitro lung deposition.
One of the most important parameters in aerosol delivery is aerodynamic diameter, which plays a key role in the deposition of particles within the respiratory system. Most of the dense powder aerosols indicate a density of 1 ± 0.5 g/cm3; however, porous particles with lower density (< 0.4 g/cm3) may deposit in lung deeper
26
Considering the direct proportional of the aerodynamic diameter with the square root of particle density, the lower particle density leads to the smaller aerodynamic diameter. Porous carriers show low density with improved aerodynamic properties.
27
The objective of this study was first to produce porous carriers using different porogen agents and then design and evaluate carrier porosity effect on inhalable rifampin-loaded proliposomes properties.
Materials and Methods
Materials
Rifampin was purchased from Hakim pharmaceutical company, Iran. Lactose, sucrose, mannitol, cholesterol, and acetic acid were obtained from Merck Chemicals Co., Germany. L-α-lecithin ((3-sn-phosphatidylcholine) from Soybean, Type IV-S, ≥30 enzymatic) was obtained from Sigma, USA. All the other chemicals and reagents were of analytical grade.
Rifampin analysis validation
UV-vis spectrophotometer (T80, Germany) was applied at a maximum absorbance wavelength for rifampin quantification. Two analytical curves were plotted in water: ethanol (30:70) and phosphate buffer solution pH 7.4 as medium solutions. All rifampin concentrations were prepared on three different days, and every concentration was tested three times a day. The curves were validated by linearity, inter-day, and intra-day precision, and accuracy.
Porous carrier preparation
To produce porous carrier, lactose (L) and mannitol (M) aqueous solutions (10% w/v) were used as main carriers, and sucrose and citric acid (1 and 2% w/v) were used as templating agents to induce porosity.
28
Eight prepared solutions (L1-L4 and M1-M4) were spray dried using Dorsa spray drier, Iran, with pump rate 50%, aspirator 90 %, and inlet temperature 100 °C. The resultant powders were dispersed in ethanol to remove the templating agents. Carrier formulations are reported in Table 1.
Table 1.
Selected experiments by software for proliposome formulations
|
|
F1
|
F2
|
F3
|
F4
|
F5
|
F6
|
F7
|
F8
|
F9
|
F10
|
F11
|
F12
|
F13
|
F14
|
F15
|
F16
|
F17
|
F18
|
F19
|
F20
|
| Rifampin (mg) |
4.9 |
30.1 |
17.5 |
25 |
17.5 |
25 |
17.5 |
25 |
17.5 |
10 |
25 |
17.5 |
17.5 |
10 |
10 |
17.5 |
17.5 |
10 |
17.5 |
17.5 |
| Lipid (mM) |
350 |
350 |
350 |
500 |
350 |
200 |
350 |
500 |
97.7 |
500 |
200 |
350 |
350 |
200 |
500 |
602.3 |
350 |
200 |
350 |
350 |
| Carrier (mg) |
575 |
575 |
575 |
250 |
575 |
900 |
575 |
900 |
575 |
900 |
250 |
575 |
575 |
250 |
250 |
575 |
28.4 |
900 |
1121.6 |
575 |
| Carrier/rifampin |
117 |
19 |
33 |
10 |
33 |
36 |
33 |
36 |
33 |
90 |
10 |
33 |
33 |
25 |
25 |
33 |
2 |
90 |
64 |
33 |
| Lipid/rifampin |
71 |
12 |
20 |
20 |
20 |
8 |
20 |
20 |
6 |
50 |
8 |
20 |
20 |
20 |
50 |
34 |
20 |
20 |
20 |
20 |
| Carrier/lipid |
1.6 |
1.6 |
1.6 |
0.5 |
1.6 |
4.5 |
1.6 |
1.8 |
5.9 |
1.8 |
1.3 |
1.6 |
1.6 |
1.3 |
0.5 |
1.0 |
0.1 |
4.5 |
3.2 |
1.6 |
Porous carrier characterization
Surface area
The surface area of powders was determined using ChemBet-3000, USA. Powders previously were degassed at 100°C for 3 hours and surface area values were reported as BET numbers. Non-porous lactose and mannitol were also analyzed as reference powders for the following comparisons.
Morphology
Selected porous powder morphology was analyzed using Scanning Emission Microscope (SEM) (Cambridge S-360, USA, 13Kv). Samples were spread on an aluminum stub, and then particles were golden sputtered. For proper comparison, the non-porous powder was also analyzed by this method.
Proliposome preparation
Proliposomes were prepared using the slurry method. In brief, the lipid phase and rifampin were dissolved in chloroform and then mixed with carrier powder (in porous and non-porous forms). The obtained dispersion was transferred to the rotary evaporator (80 rpm, 40°C, IKA, Germany) to remove chloroform under vacuum. The final dry powder was passed through mesh 60 and maintained in a desiccator for complete drying. Rifampin-loaded proliposomes were optimized using quality by design method. Based on preliminary formulations, four factors of carrier type (porous and non-porous), carrier amount (200-900 mg), rifampin amount (10-25 mg), and lipid phase amount (200-500 mM) were selected as variables. Powder formulations were prepared by applying optimization design using Design Expert 10® software (Stat-Ease, Inc.; Trial version). A randomized central composite response surface method was used for proliposome optimization. Different responses were analyzed, including proliposome powder weight yield, density, flowability and hydrated liposomes size, loading capacity and encapsulation efficiency. The software suggested 40 experiments (Table 1, 20 experiments for porous and 20 for non-porous carrier), including six center points to identify any curvature.
Proliposome characterization
Weight yield
The yield was calculated by weighing the obtained powder divided by the theoretical weight. The weight yield was reported as a percentage, and the samples were examined in triplicate.
Flowability
Due to USP guidelines, a defined weight of proliposome powders was transferred to Erweka®Granule Flow tester, and their angle of repose was measured. All tests were repeated three times.
Bulk density
To determine the bulk density, a defined weight of the powders was transferred to a graduated cylinder. Then, the density of powders was calculated by the division of weight over the volume. Samples were tested in triplicate.
Drug content
Content uniformity was defined by measuring the total amount of rifampin in proliposomal particles. Definite amounts of proliposome formulations were dissolved in absolute ethanol until the lipid phase was dissolved completely. Then, the samples were centrifuged 30 minutes (4°C, 18 000 rpm). The supernatant rifampin content was analyzed using the validated analysis method. The process was repeated 3 times.
Hydrated liposomes characterization
Hydrated liposomes were obtained by adding water to proliposomes. Typical characteristics of hydrated liposomes, including size, loading capacity and encapsulation efficiency were evaluated.
Size
The hydrated liposome size was analyzed using particle size analyzer (Shimadzu, SALD-2101, Japan). Mean volume and number diameters of liposomes were determined.
Loading capacity and encapsulation efficiency
A certain amount of proliposome was dispersed in distilled water and was shaken to obtain a colloidal dispersion, followed by centrifugation for 30 minutes at 18 000 rpm, 4°C. The supernatant was analyzed using the analysis method to quantify the amount of rifampin. The absorbance demonstrates the un-encapsulated drug content. Hence, the amount of trapped drug was calculated by subtracting the un-encapsulated value from the total amount of rifampin added to the formulation. Loading capacity is the amount of drug which was encapsulated in hydrated liposomes. Loading capacity (DL) and encapsulation efficiency (EE) were assessed using the following equations
29
:
Final optimized proliposome characterization
Considering powder characterization for pulmonary delivery, powder density and flowability were the main parameters for optimization. In a more step, due to software results, the most significant effective parameter for hydrated liposome was extracted to determine the optimized formulation.
In vitro release
A known amount of final optimized proliposomes was dispersed in phosphate buffer solution pH 7.4 to create sink condition and incubated in a shaker incubator at 50 rpm for 8 hours at 37 ± 1°C. Samples were taken at 0.5, 1, 2, 4, 6, and 8 hours (n = 3) and analyzed using the analysis method described earlier.
In vitro aerosolization
Selected formulations were assessed for the aerosolization properties using a Next Generation Impactor (NGI, Copley Scientific, UK) at room temperature. The instrument was equipped with a USP induction port and pre-separator. Airflow of the instrument was retained at 60 L/min by a flow meter (DFM 2000, COPLEY scientific, UK). Prior to the study, all collection cups were coated using a solution of Tween 80 in ethanol (1% W/V). The optimized formulations were delivered to a size 3 capsule and were actuated to the NGI using an Aerolizer®. Finally, deposited drug concentration in each stage was determined using the validated UV-VIS analysis method. Mass median aerodynamic diameter (MMAD), geometric standard deviation (GSD), fine particle fraction (FPF), and powder recovery% were determined. All experiments were repeated three times.
Morphology
Morphology of selected proliposome formulations was evaluated by SEM (TESCAN-VEGA3 (Czech Republic), 10 kV). The selected samples were spread on an aluminum stub, and then particles were golden sputtered. Liposomes were formed by hydration in water, and their shape was examined by transmission electron microscope (TEM) (LEO 906E, Philips, Germanys). The sample was added on a formvar-coated grid and stained by 2% (w/w) uranyl acetate.
Differential scanning calorimetry (DSC)
DSC method (BAHR thermo analyzer, GmbH, Germany) was applied to study the solid-state and stability of rifampin in the proliposome particles. Rifampin, rifampin encapsulated proliposome and blank proliposome were set in aluminum pans and heated up to 350°C (heating rate of 10°C/min). The void aluminum pan was sealed as a reference sample.
Statistical analysis
All experiments were done in triplicate (n = 3) and data were expressed as the mean ± standard deviations. Analysis of variance (ANOVA) via SPSS v.15 software was used for statistical analysis in which P value of <0.05 was considered to denote a statistically significant difference.
Results and Discussion
Rifampin analysis validation
Rifampin analysis was evaluated in water: ethanol (30:70) and phosphate buffer solution pH 7.4 at ƛmax 475 nm. The data of curve validation containing the regression equation, the correlation coefficient (r
2
) of the standard curve, and the precise and accurate validation of the results of analytical curves are presented in Table 2. The validation results implied acceptable correlation coefficient, precision, and accuracy which approved the applied method for evaluation of rifampin in the rest of the study is valid.
Table 2.
Validation parameters of different analytical curves of rifampin (n = 9)
|
Solvent
|
Equation
|
r2
|
Precision% (Intraday)
|
Precision% (Interday)
|
Accuracy%
|
| Water: Ethanol (30:70) |
y = 0.015x+0.009 |
0.999 |
99.6 ± 0.2 |
98.6 ± 1.2 |
98.2 ± 2.9 |
| Phosphate buffer pH 7.4 |
y = 0.018x-0.014 |
0.999 |
98.4 ± 1.9 |
98.1 ± 1.4 |
97.7 ± 2.8 |
Porous carrier characterization
Surface area
Porous particles with high void spaces and low density may promote aerosol powder performance.
30
As previously reported, the templating method was used to prepare powder with higher porosity and surface area. Porous lactose and mannitol carriers were prepared by spray drying method using different templating agents, and the surface area of powders was determined and compared. As it is shown in Table 3, non-porous lactose and mannitol had surface areas of 0.30 ± 0.09 and 0.71 ± 0.1 m2/g, respectively. The highest area (24.95 m2/g) was determined for lactose samples containing sucrose 2% w/v as templating agent, which was 80 times higher than intact lactose, respectively. Similar results were reported in a previous study that examined different templating agents in the preparation of porous lactose carrier which indicated the highest BET number (20 ± 1 m2/g) for sucrose as a templating agent.
28
Citric acid inefficiency in making porous carrier can be explained due to the Williams-Landel-Ferry theory which indicates that the lower glass transition temperatures (Tg) of templating agent molecules leads to higher crystallization (aggregation) rates of templating agent during spray drying. Therefore, citric acid with glass transition temperatures of 11°C would be crystallized rapidly while sucrose acid with glass transition temperatures of 62°C crystallized slowly.
28,31
However, citric acid pH effect on the degree of crystallinity in the powder is an important factor. Citric acid with low pH may increase lactose crystallization due to the increasing the mutarotation and orientation rate of lactose molecules into the crystals, which may form more aggregates.
32
Due to BET results, it seems that lactose with 2% sucrose as the templating agent may be a more appropriate carrier for proliposomes production due to its higher surface area and porosity.
Table 3.
Different spray dried carrier formulations surface area determined by BET test (m2/g) (n = 3)
|
|
Carrier (Lactose)
|
Templating agent % (w/v)
|
Surface area (m
2
/g)
|
|
Carrier (Mannitol)
|
Templating agent % (w/v)
|
Surface area (m
2
/g)
|
| L0 |
Non porous |
- |
0.30 ± 0.09 |
M0 |
Non porous |
- |
0.71 ± 0.1 |
| L1 |
Porous |
Sucrose 1 |
13.67 ± 0.95 |
M1 |
Porous |
Sucrose 1 |
3.14 ± 0.14 |
| L2 |
Porous |
Sucrose 2 |
24.95 ± 1.13 |
M2 |
Porous |
Sucrose 2 |
4.64 ± 0.30 |
| L3 |
Porous |
Citric acid 1 |
2.69 ± 0.34 |
M3 |
Porous |
Citric acid 1 |
3.02 ± 0.11 |
| L4 |
Porous |
Citric acid 2 |
3.35 ± 0.23 |
M4 |
Porous |
Citric acid 2 |
3.36 ± 0.57 |
Morphology
Porous and non-porous powders morphologies were studied by SEM (Figure 1). As it is shown, non-porous powders (A) had smooth and un-textured surfaces with no assignable pore. In comparison, the porous powder had a high intensity of asperities on the surface (B) by the uniform distribution of pores; which can be ascribed to the proper dispersion of templating molecules in the lactose structure. The data is based on the BET data and approves the creation of a high surface area in lactose after ethanol washing.
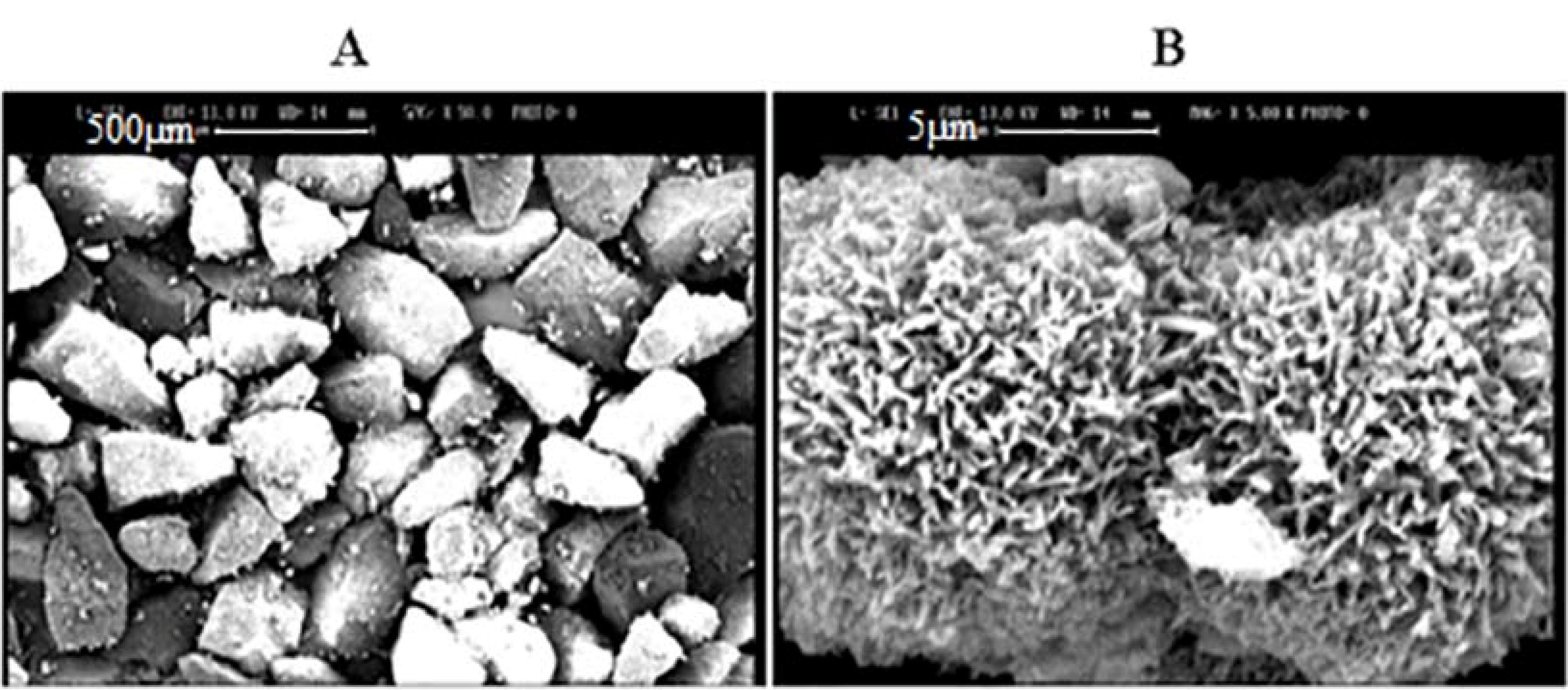
Figure 1.
SEM graphs of powders from (A) lactose (scale bar 500µm) and (B) porous lactose (scale bar 5 µm).
.
SEM graphs of powders from (A) lactose (scale bar 500µm) and (B) porous lactose (scale bar 5 µm).
Proliposome preparation
Different methods were employed for proliposome production on a large scale, including spray drying and fluidized bed coating; however, traditionally feed-line method utilizing rotary evaporator is achieved for the small-scale process. Considering lipid losses in the feeding tube and long-lasting process, finding a simple alternative production method would be highly advantageous.
33
Therefore, in the present study slurry method was used for proliposome production.
Based on the software, 40 formulations containing 20 formulations for porous and 20 formulations for non-porous carriers were designed and prepared. Powders weight yield, flowability and density were determined. The best-fitting models were selected based on the statistical parameters including lack of fit (shows the fitness of the model), the multiple correlation coefficients (R2, approves the correlation coefficient and reaches 1 as the results become better), predicted multiple correlation coefficients (predicted R2, measurement of the predictive capability of the model), and adjusted multiple correlation coefficients (adjusted R2, adjustment of the number of model parameters relative to the number of runs). The difference between predicted R2 and adjusted R2 values should be less than 0.2 for the proper prediction of the model.
Proliposome characterization
Weight yield
Weight yield for the prepared proliposome is shown in Table 4. Non-porous lactose powders weight yield was in a lower range of 47- 78%, and just NP18 showed 83%, while porous lactose powders weight yield was in a higher range of 53-85%. Formulations P17 and NP17 had the lowest weight yield, which would be due to the lowest carrier/lipid and carrier/rifampin ratio that caused stickiness to the rotary evaporator flask. Therefore, it was omitted for further evaluation.
Table 4.
Characterization of proliposome powders
|
|
Weight yield (%)
|
Angle of repose
|
Density
|
|
Weight Yield (%)
|
Angle of repose
|
Density
|
| P1 |
77 ± 3.1 |
19.6 ± 0.3 |
0.35 ± 0.03 |
NP1 |
71 ± 2.2 |
23 ± 0.7 |
0.51 ± 0.03 |
| P2 |
65 ± 2.9 |
22.1 ± 0.6 |
0.34 ± 0.02 |
NP2 |
65 ± 3.1 |
24.4 ± 0.8 |
0.52 ± 0.04 |
| P3 |
72 ± 2.3 |
16.2 ± 0.4 |
0.43 ± 0.01 |
NP3 |
66 ± 2.1 |
23 ± 0.3 |
0.54 ± 0.01 |
| P4 |
53 ± 2.1 |
40.3 ± 1.0 |
0.36 ± 0.02 |
NP4 |
52 ± 1.4 |
22.5 ± 0.5 |
0.47 ± 0.03 |
| P5 |
71 ± 4.1 |
16.0 ± 0.3 |
0.40 ± 0.03 |
NP5 |
67 ± 2.8 |
23.2 ± 0.2 |
0.53 ± 0.02 |
| P6 |
82 ± 6.0 |
15.3 ± 1.0 |
0.40 ± 0.01 |
NP6 |
76 ± 3.8 |
22.8 ± 0.6 |
0.55 ± 0.02 |
| P7 |
70 ± 5.4 |
15.8 ± 0.9 |
0.42 ± 0.02 |
NP7 |
64 ± 4.1 |
22.8 ± 0.6 |
0.53 ± 0.01 |
| P8 |
78 ± 6.3 |
14.5 ± 0.8 |
0.38 ± 0.03 |
NP8 |
70 ± 3.6 |
24.5 ± 0.3 |
0.52 ± 0.01 |
| P9 |
79 ± 2.4 |
20.4 ± 0.4 |
0.31 ± 0.03 |
NP9 |
67 ± 3.4 |
19.7 ± 0.7 |
0.54 ± 0.01 |
| P10 |
85 ± 3.2 |
12.5 ± 0.9 |
0.42 ± 0.01 |
NP10 |
71 ± 2.5 |
22.7 ± 0.3 |
0.47 ± 0.01 |
| P11 |
66 ± 1.8 |
25.0 ± 0.5 |
0.36 ± 0.03 |
NP11 |
54 ± 2.3 |
25.5 ± 0.3 |
0.46 ± 0.02 |
| P12 |
70 ± 1.4 |
15.5 ± 0.5 |
0.42 ± 0.02 |
NP12 |
61 ± 3.9 |
22.1 ± 0.3 |
0.52 ± 0.03 |
| P13 |
71 ± 3.5 |
13.5 ± 0.7 |
0.40 ± 0.03 |
NP13 |
60 ± 3.1 |
22.5 ± 0.5 |
0.54 ± 0.03 |
| P14 |
72 ± 2.7 |
19.8 ± 0.3 |
0.29 ± 0.02 |
NP14 |
59 ± 1.9 |
27.9 ± 0.4 |
0.46 ± 0.01 |
| P15 |
63 ± 3.1 |
30 ± 0.7 |
0.45 ± 0.03 |
NP15 |
47 ± 2.2 |
23.5 ± 0.5 |
0.46 ± 0.02 |
| P16 |
71 ± 3.8 |
21.2 ± 0.7 |
0.38 ± 0.01 |
NP16 |
60 ± 2.7 |
20.6 ± 0.7 |
0.51 ± 0.03 |
| P17 |
- |
- |
- |
NP17 |
- |
- |
- |
| P18 |
85 ± 2.9 |
11.9 ± 0.4 |
0.27 ± 0.03 |
NP18 |
83 ± 2.4 |
24.3 ± 0.3 |
0.53 ± 0.02 |
| P19 |
77 ± 4.1 |
16.0 ± 0.6 |
0.40 ± 0.02 |
NP19 |
78 ± 3.6 |
22.9 ± 0.5 |
0.47 ± 0.03 |
| P20 |
66 ± 1.5 |
19.6 ± 0.5 |
0.42 ± 0.04 |
NP20 |
64 ± 2.7 |
23.0 ± 0.7 |
0.54 ± 0.01 |
(P: porous, NP: non-porous).
Flowability
The flowability of powders (Table 4) was evaluated by measuring the angle of repose and comparing it with mentioned USP indexes. The angle of repose describes the flow characteristics and powder friction. The small angle of repose (< 30) is an indication of lower powder internal friction and cohesiveness.
34
The angle of repose for non-porous formulations showed a narrower range 19.7-27.9, compared to porous formulations (12.5-40) with a wider range. All porous formulations’ angle of repose was in the acceptable range except P4 and P15 with higher amounts which may be related to the lowest carrier/lipid ratio, which contains the highest lipid with the lowest carrier amount in the formulation. The excellent flow properties were obtained when the angle of repose was between 25 to 30, and it was fair flowability property when the range was between 36 to 40.
Due to the software, the lack of fit of this criterion was not significant. R-squared was 0.97, and the difference between Pred R2 and Adj R2 was less than 0.2. Adeq precision of 22.06 indicates an adequate signal.
Angle of repose = +31.57278-0.8594*Drug-0.018695*Lipid-9.00735E-003*Carrier +8.96667E-004*Drug*Lipid-7.42051E004*Drug*Carrier-7.72564E-005*Lipid*Carrier +0.032838*Drug2+8.14485E-005*Lipid2+2.47549E-005*Carrier2
Bulk density
The bulk density of prepared powders is mentioned in Table 4. The density of porous formulations was in the range of 0.27 to 0.45 g/mL, while in non- porous formulations, this factor was in the range of 0.46 to 0.56 g/mL; therefore, as it was expected, the porous particles with higher surface area showed lower density. P18 with the higher carrier/lipid and carrier/rifampin ratio had the lowest bulk density. Due to the previous studies, particles with a density lower than 0.4 g/ mL could enter the lower parts of the respiratory tract containing alveoli and be more effective in therapeutic regimens.
26
Due to the software, the lack of fit of this criterion was not significant. R-squared was 0.97 and the difference between Pred R2 and Adj R2 was less than 0.2. Adeq precision of 19.56 indicates an adequate signal. Based on the mentioned results, non- porous formulations were eliminated, and formulations with porous carriers were examined in further assays.
Density = -0.088085 +0.025227*Drug +1.65059E-003*Lipid -8.16685E005*Carrier -3.33333E-005*Drug*Lipid+7.17949E-006*Drug*Carrier+1.00176E021*Lipid*Carrier-4.97979E004*Drug2-1.26136E006*Lipid2 -4.91113E-008*Carrier2
Drug content
As it was mentioned, porous proliposomes were selected for further assays. The drug content of the prepared formulations is mentioned in Table 5. The indirect method was applied to estimate the amount of rifampin in proliposome powders. All samples were in the acceptable range.
Table 5.
Characterization of hydrated liposome vesicles
|
|
Drug Content (%)
|
Volume diameter (μm)
|
Encapsulation efficacy (%)
|
Loading capacity (%)
|
| P1 |
93.9 ± 0.32 |
39.50 ± 0.5 |
72.3 ± 2.6 |
1.40 ± 0.03 |
| P2 |
97.4 ± 0.21 |
7.21 ± 0.55 |
34.1 ± 3.4 |
4.90 ± 0.41 |
| P3 |
105.4 ± 0.45 |
4.04 ± 0.48 |
53.0 ± 1.6 |
3.30 ± 0.28 |
| P4 |
100.6 ± 0.76 |
4.27 ± 0.50 |
52.0 ± 3.9 |
6.31 ± 0.19 |
| P5 |
105.4 ± 0.89 |
4.10 ± 0.67 |
43 ± 2.3 |
2.80 ± 0.14 |
| P6 |
103.5 ± 0.31 |
11.81 ± 0.50 |
26 ± 1.5 |
5.80 ± 0.30 |
| P7 |
101.0 ± 0.65 |
4.85 ± 0.64 |
53 ± 2.1 |
3.90 ± 0.51 |
| P8 |
106.5 ± 0.74 |
3.85 ± 0.47 |
39.8 ± 4.7 |
3.10 ± 0.12 |
| P9 |
101.0 ± 0.49 |
10.78 ± 0.54 |
28.5 ± 1.9 |
6.40 ± 0.43 |
| P10 |
106.5 ± 0.44 |
11.78 ± 0.60 |
58.5 ± 3.6 |
1.71 ± 0.07 |
| P11 |
110.7 ± 0.39 |
4.21 ± 0.56 |
47.6 ± 2.3 |
6.91 ± 0.34 |
| P12 |
101.1 ± 0.26 |
4.83 ± 0.72 |
54.7 ± 1.7 |
2.91 ± 0.19 |
| P13 |
101.0 ± 0.12 |
4.16 ± 0.34 |
48.7 ± 2.1 |
3.04 ± 0.27 |
| P14 |
103.1 ± 0.65 |
3.93 ± 0.51 |
81.8 ± 3.1 |
4.53 ± 0.32 |
| P15 |
86.0 ± 0.90 |
3.21 ± 0.49 |
99.3 ± 3.3 |
2.70 ± 0.15 |
| P16 |
104.6 ± 0.12 |
3.41 ± 0.47 |
78.8 ± 2.6 |
3.24 ± 0.25 |
| P18 |
113.1 ± 0.43 |
7.89 ± 0.56 |
60.3 ± 2.2 |
3.70 ± 0.19 |
| P19 |
101.0 ± 0.91 |
19.47 ± 0.75 |
28.3 ± 3.8 |
2.41 ± 0.36 |
| P20 |
93.9 ± 0.67 |
4.84 ± 0.56 |
50.7 ± 1.6 |
3.22 ± 0.32 |
Hydrated liposome characterization
Size
The mean volume diameter of hydrated liposomal vesicles was in the range of 3.21 to 7.8 μm except for P1, P10 (Table 5), which contain higher lipid/drug ratio and P6, P9 with the highest carrier/lipid ratio. Due to the software modeling, this criterion was not significant in the optimization of formulations. As reported in previous studies, the slurry method resulted in smaller particles size and variability in comparison with other methods. Besides, hydrated liposomes with 4-5.5 μm size range showed proper inhalation properties in volunteers that inhaled the liposomes by jet nebulizers.
30,33
Results indicated a similar size of 3-4 micron for all formulations except P2, P18 (7-8 micron), P1, P6, P9, P10, and P19 (11-39.5 micron), which may attribute to higher carrier/lipid, carrier/rifampin and lipid/ rifampin ratios of mentioned formulations.
30
Loading capacity and encapsulation efficiency
Liposomal encapsulation efficacy was between 21.5, and 78% and rifampin loading was 1.4 to 6.9% in different formulations (Table 5). Previous reports indicated higher encapsulation efficiency for liposomes prepared using the slurry method.
33
P4, P9, and P11 had the highest amount of rifampin loading that would be due to the highest ratio of drug to lipid. The results indicated that a reduction in the lipid/carrier ratio that indicated a negative effect on encapsulation efficiency, especially in the cases of P6 and P9, while increasing this ratio in P4, P14, P15, and P16 led to positive effects on encapsulation efficiency. Lack of fit for loading capacity criteria was not significant. R-squared values were 0.97 for loading capacity, and the difference between Adj and Pred R2 was less than 0.2. Adeq precision of 19.49 for loading capacity indicates an adequate signal
Loading capacity = +7.00687+0.16764*Drug- 0.024048*Lipid-1.86723E-003* Carrier+6.32413E-005*Drug*Lipid-1.28377E-004*Drug*Carrier-6.03694E-006*Lipid*Carrier+9.74574E-004*Drug2+2.88901E-005*Lipid2+3.15089E-006*Carrier2
Final optimized proliposome characterization
The selection of final formulations, prepared with the porous carriers, was based on the obtained results in terms of proliposome density, flowability, and hydrated liposomes loading capacity (Table 6). Using the lowest powder density in the software, it proposed P18 as the optimized formulation (Figure 2). P18 was selected formulation considering density and flowability (angle of repose) as the main factors in combination. In the next step, in addition to powder density and flowability, liposomes loading capacity was also proposed as another main factor and the overlay counterplot as illustrated in Figure 2 showed P11 as the best formulation. Density was selected as the main factor in proliposome characteristics since density can affect powder aerosolization characteristics.
35
Therefore, P11 and P18 formulations were evaluated for further controls.
Table 6.
Factorial experimental design analysis of variance obtained
|
|
Angle of repose
|
Density
|
Drug loading
|
| R-squared |
0.9713 |
0.9890 |
0.9672 |
| Adj R-squared |
0.9426 |
0.9774 |
0.9344 |
| Pred R-squared |
0.7629 |
0.9216 |
0.7670 |
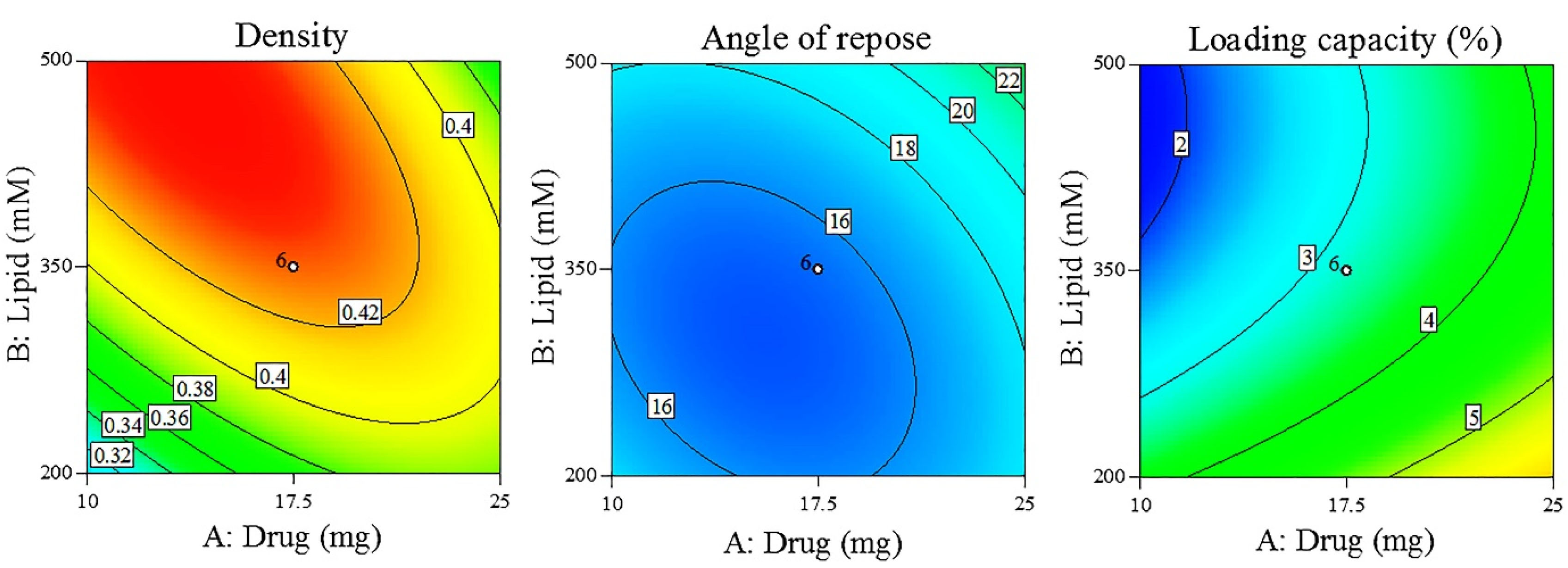
Figure 2.
The contour plots of density, angle of repose and loading capacity.
.
The contour plots of density, angle of repose and loading capacity.
In vitro release
As presented in Figure 3, 85% of Free rifampin was dissolved in release media within 1 hour and it was completely dissolved after 2 hours while it was last 6 hours for both optimized formulations to release rifampin; however, P18 showed the faster rifampin release compared to P11. Rifampin release rate was 8.5, 2.5, and 1.7 times higher in P18 after 0.5, 1, and 2 hours, which could be attributed to high carrier/rifampin, lipid/rifampin, and carrier/lipid in P18. Higher ratios of the carrier increase the probability of rifampin entrance into the carrier pores that could lead to the burst release
30
of rifampin that is shown in P18 formulation.
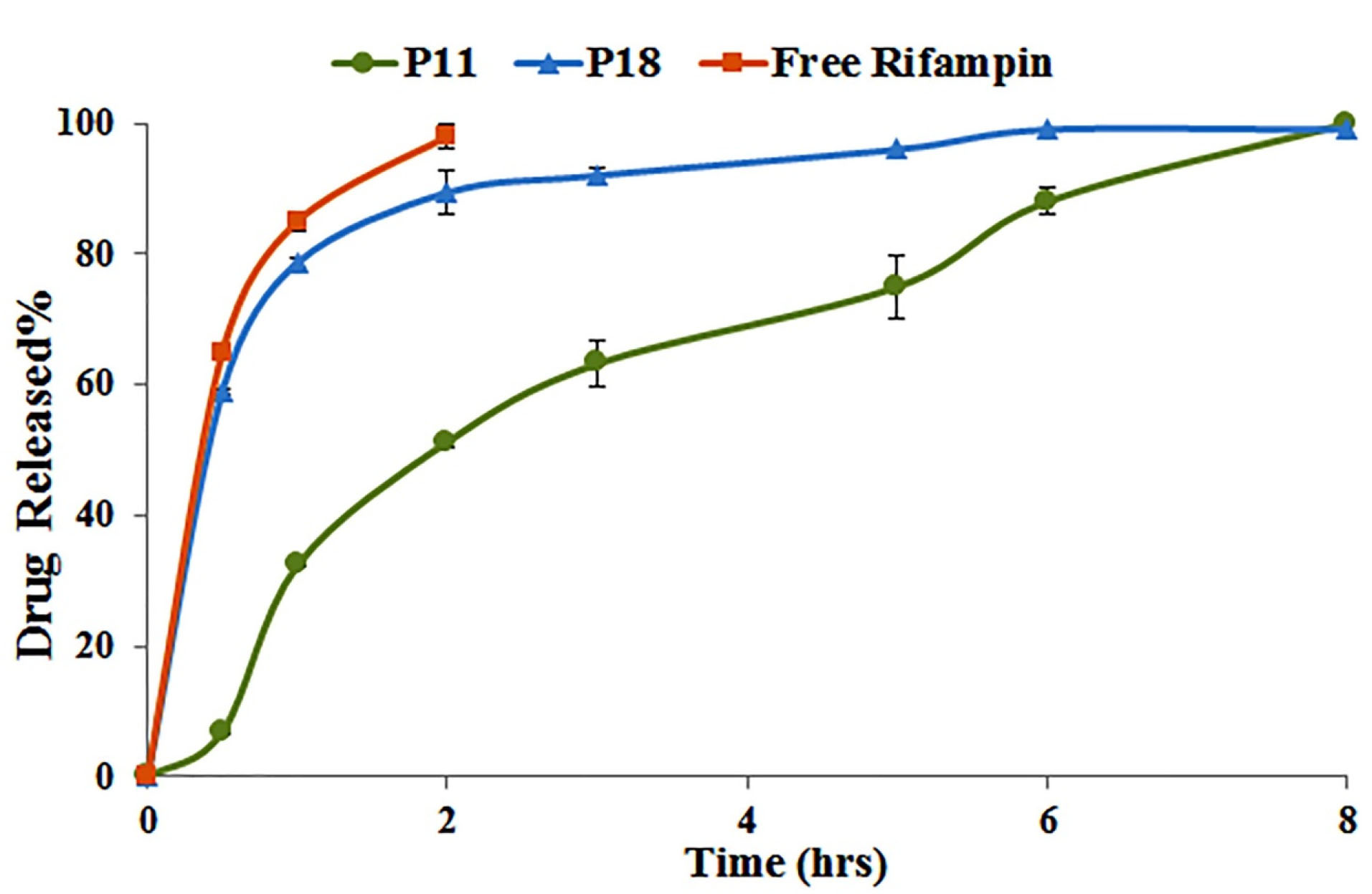
Figure 3.
In vitro release profile of selected formulations in phosphate buffer solution pH 7.4 (n = 3).
.
In vitro release profile of selected formulations in phosphate buffer solution pH 7.4 (n = 3).
In vitro aerosolization
NGI evaluated inhalation properties of the proliposome powders are mentioned in Table 7. NGI deposition pattern presented in Figure 4. The aerodynamic diameter of P11 and P18 was in the acceptable range (1-10 μm), while P18 showed a little larger size. GSD was in an acceptable range for P18 formulation that was less than 2.5.
36
Both formulations had high sedimentation in the upper parts of the NGI. This was predictable since the lipid nature of proliposomes would enhance the sedimentation rate. These results were approximately similar to previous reports that showed almost low FPF% of 15% for non-porous and 19-29% for the porous carriers.
25,27
Moreover, another study reported low FPF (0 to 3.99%) for proliposomes with lactose as the carrier.
30
The low FPF% for proliposomes may be related to the agglomeration of particles that were coated with lipid superficially.
27
Higher FPF% and powder recovery% of P18 may related to the higher carrier/lipid and carrier/rifampin ratio in the formulation.
30
Due to the obtained results, P18 was selected as the final formulation with the best powder characteristics.
Table 7.
Optimized proliposomes in vitro aerosol assessment
|
|
P11
|
P18
|
| MMAD |
5.25 ± 0.02 |
6.21 ± 0.36 |
| GSD |
5.42 ± 0.80 |
2.36 ± 0.25 |
| FPF% |
0.71 ± 0.06 |
9.17 ± 0.18 |
| Emitted dose (mg) |
0.95 ± 0.21 |
1.63 ± 0.25 |
| Powder recovery% |
75.91 ± 3.52 |
90.15 ± 3.01 |

Figure 4.
Deposition patterns of optimized proliposome powder in the NGI.
.
Deposition patterns of optimized proliposome powder in the NGI.
Morphology
SEM evaluated P18 formulation morphology. To confirm a liposome formation in the next step, the formulation was in contact with water to form liposomal vesicle and was evaluated by TEM. As it is presented in Figure 5, proliposome powders have a linear structure that could form into oily droplets in a liposomal vesicle state. The comparison of proliposome (P18) and porous lactose structure in Figure 1 showed the lipid phase clearly. Due to previous reports, decreasing the carrier ratio to less than 80% would form linear sticky particles.
27
TEM picture in Figure 5 showed liposomal vesicles as oily droplets, which confirmed liposome formation.
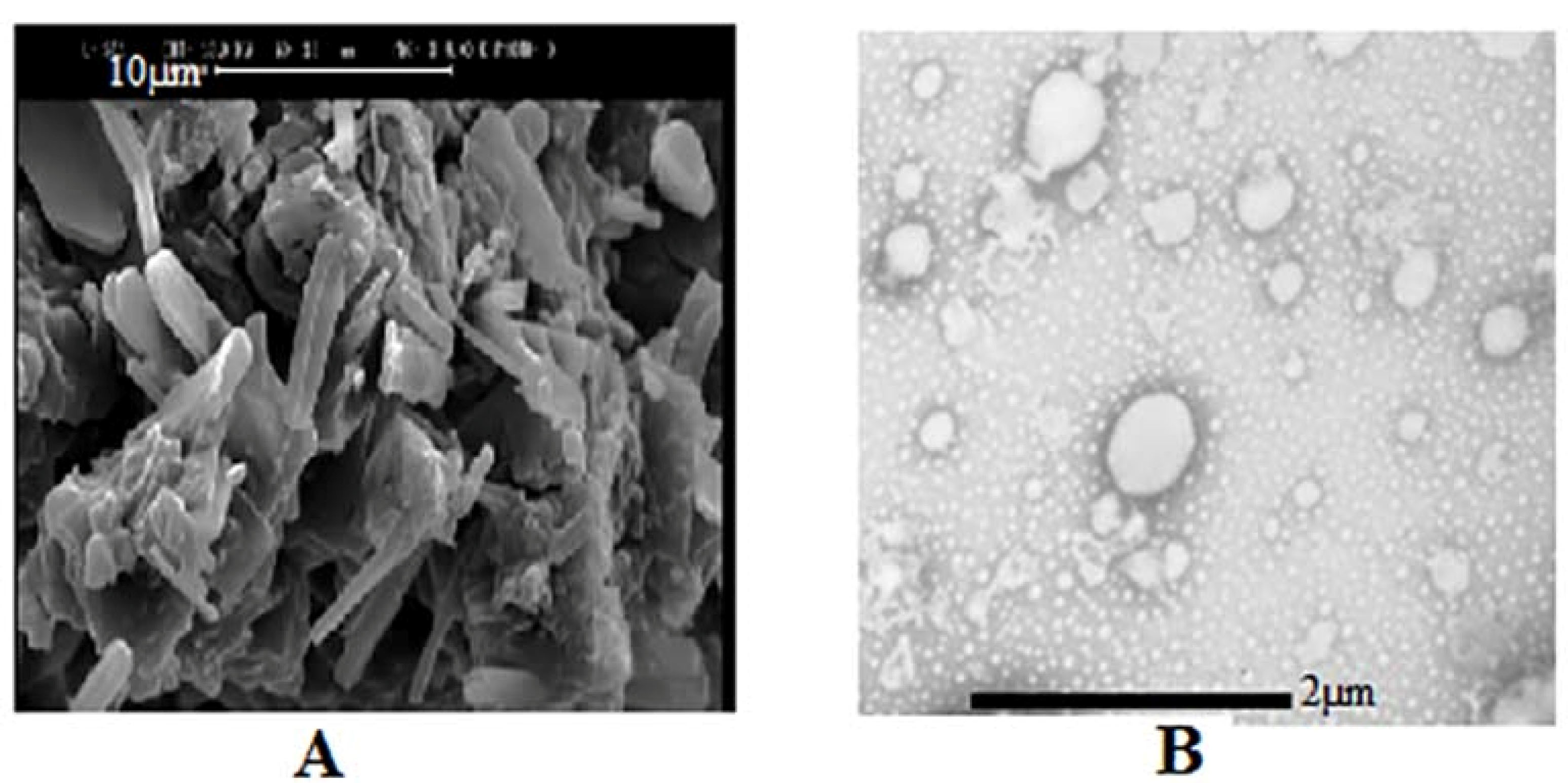
Figure 5.
(A) SEM of proliposome P18 (scale bar 10µm), (B) TEM of hydrated liposomes of P18 (scale bar 2 µm).
.
(A) SEM of proliposome P18 (scale bar 10µm), (B) TEM of hydrated liposomes of P18 (scale bar 2 µm).
Differential scanning calorimetry
Thermograms of rifampin, blank proliposome and P18 proliposome are in Figure 6. Rifampin is a polymorphic compound that showed the endothermic peak of melting point (195.2°C) and the exothermic peak (211.6°C) that is related to the recrystallization and converting to type I polymorph. The exothermic peak at 258.5°C is related to the destruction of rifampin.
37,38
In the P18 formulation thermogram, rifampin exothermic peak at 225°C is obvious that confirms the presence of intact rifampin in proliposome powder.
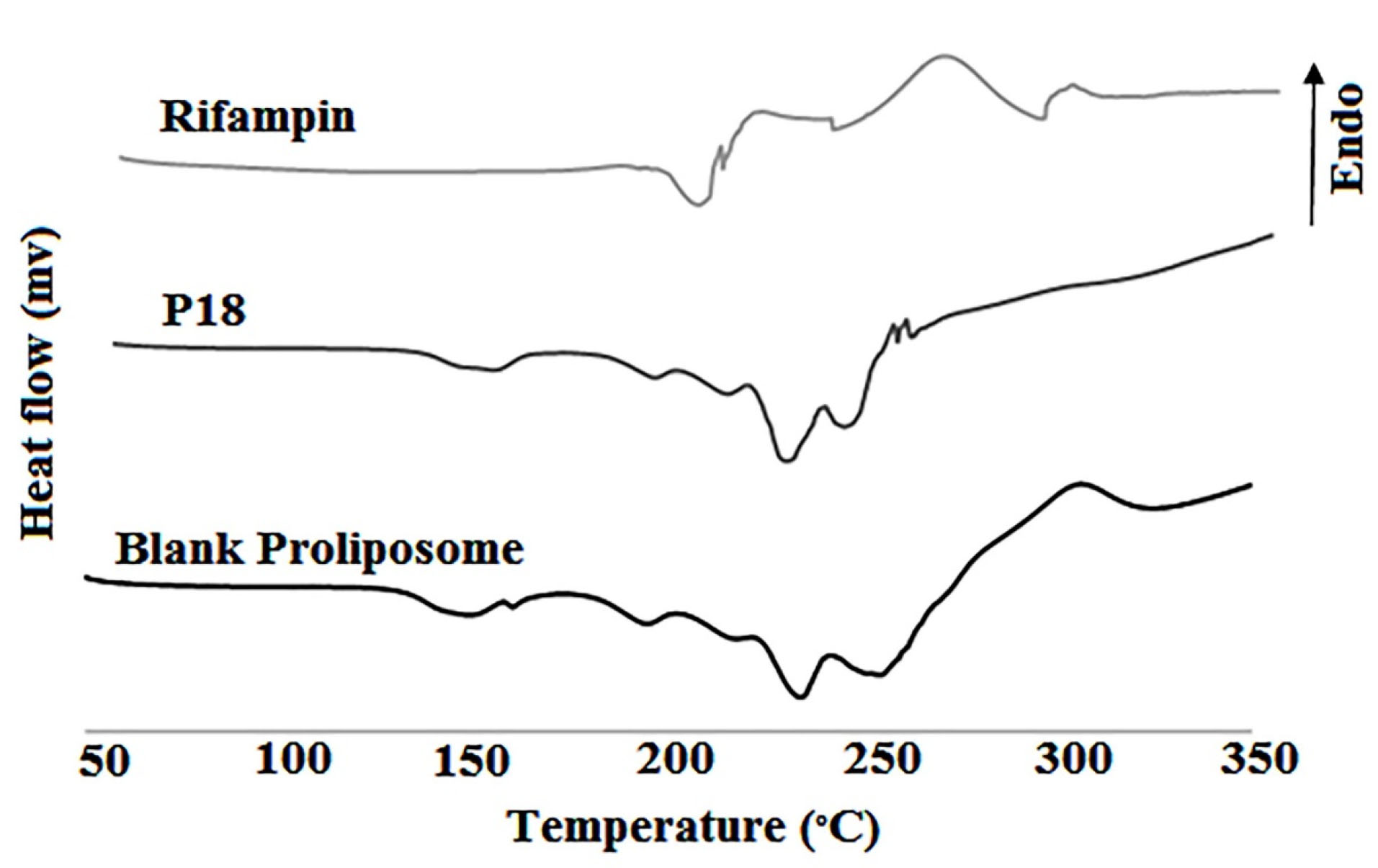
Figure 6.
DSC thermograms of rifampin, blank proliposome and P18 formulation
.
DSC thermograms of rifampin, blank proliposome and P18 formulation
Conclusion
The effect of different factors such as carrier type, lipid phase amount and drug amount was evaluated on the powder characteristics of rifampin inhalable proliposome powder. The results of present study indicated that sucrose could enhance the porosity of lactose and its surface area. Besides, proliposome preparation by the slurry method was a proper method in laboratory scale to load rifampin in liposomes without destruction. The selected formulation showed almost acceptable in vitro aerosolization properties such as MMAD, GSD and emitted dose while FPF% was low which was related to the lipid nature of proliposome powder. Considering the optimized formulation suitable properties further studies with other different lipids may be needed to improve FPF% of rifampin-loaded proliposomes. Rifampin-loaded proliposomes with porous carriers showed acceptable aerosolization properties comparing non-porous carriers. Therefore, carrier porosity was an important parameter specially in aerosolization properties.
Funding
This study was part of the Pharm.D project of Delaram Sadeghinia and was financially supported by Shiraz University of Medical Sciences.
Ethical Issues
Not applicable.
Conflict of Interest
Authors declare no conflict of interest.
References
- Nkanga CI, Krause RW, Noundou XS, Walker RB. Preparation and characterization of isoniazid-loaded crude soybean lecithin liposomes. Int J Pharm 2017; 526(1-2):466-73. doi: 10.1016/j.ijpharm.2017.04.074 [Crossref] [ Google Scholar]
- du Toit LC, Pillay V, Danckwerts MP. Tuberculosis chemotherapy: current drug delivery approaches. Respir Res 2006; 7(1):118. doi: 10.1186/1465-9921-7-118 [Crossref] [ Google Scholar]
- Garcia Contreras L, Sung J, Ibrahim M, Elbert K, Edwards D, Hickey A. Pharmacokinetics of inhaled rifampicin porous particles for tuberculosis treatment: insight into rifampicin absorption from the lungs of guinea pigs. Mol Pharm 2015; 12(8):2642-50. doi: 10.1021/acs.molpharmaceut.5b00046 [Crossref] [ Google Scholar]
- Pham DD, Fattal E, Tsapis N. Pulmonary drug delivery systems for tuberculosis treatment. Int J Pharm 2015; 478(2):517-29. doi: 10.1016/j.ijpharm.2014.12.009 [Crossref] [ Google Scholar]
- Traini D, Young PM. Drug delivery for tuberculosis: is inhaled therapy the key to success?. Ther Deliv 2017; 8(10):819-21. doi: 10.4155/tde-2017-0050 [Crossref] [ Google Scholar]
- Blomberg B, Spinaci S, Fourie B, Laing R. The rationale for recommending fixed-dose combination tablets for treatment of tuberculosis. Bull World Health Organ 2001; 79(1):61-8. [ Google Scholar]
- Rawal T, Parmar R, Tyagi RK, Butani S. Rifampicin loaded chitosan nanoparticle dry powder presents an improved therapeutic approach for alveolar tuberculosis. Colloids Surf B Biointerfaces 2017; 154:321-30. doi: 10.1016/j.colsurfb.2017.03.044 [Crossref] [ Google Scholar]
- Patil-Gadhe A, Pokharkar V. Single step spray drying method to develop proliposomes for inhalation: a systematic study based on quality by design approach. Pulm Pharmacol Ther 2014; 27(2):197-207. doi: 10.1016/j.pupt.2013.07.006 [Crossref] [ Google Scholar]
- Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med 2007; 4(7):e238. doi: 10.1371/journal.pmed.0040238 [Crossref] [ Google Scholar]
- Joshi JM. Tuberculosis chemotherapy in the 21 century: back to the basics. Lung India 2011; 28(3):193-200. doi: 10.4103/0970-2113.83977 [Crossref] [ Google Scholar]
- Sahbazian B, Weis SE. Treatment of active tuberculosis: challenges and prospects. Clin Chest Med 2005; 26(2):273-82. doi: 10.1016/j.ccm.2005.02.011 [Crossref] [ Google Scholar]
- Pandey R, Khuller GK. Antitubercular inhaled therapy: opportunities, progress and challenges. J Antimicrob Chemother 2005; 55(4):430-5. doi: 10.1093/jac/dki027 [Crossref] [ Google Scholar]
- Chougule M, Padhi B, Misra A. Development of spray dried liposomal dry powder inhaler of dapsone. AAPS PharmSciTech 2008; 9(1):47-53. doi: 10.1208/s12249-007-9024-6 [Crossref] [ Google Scholar]
- Ahsan F, Rivas IP, Khan MA, Torres Suarez AI. Targeting to macrophages: role of physicochemical properties of particulate carriers--liposomes and microspheres--on the phagocytosis by macrophages. J Control Release 2002; 79(1-3):29-40. doi: 10.1016/s0168-3659(01)00549-1 [Crossref] [ Google Scholar]
- Thomas DA, Myers MA, Wichert B, Schreier H, Gonzalez-Rothi RJ. Acute effects of liposome aerosol inhalation on pulmonary function in healthy human volunteers. Chest 1991; 99(5):1268-70. doi: 10.1378/chest.99.5.1268 [Crossref] [ Google Scholar]
- Desai TR, Wong JP, Hancock RE, Finlay WH. A novel approach to the pulmonary delivery of liposomes in dry powder form to eliminate the deleterious effects of milling. J Pharm Sci 2002; 91(2):482-91. doi: 10.1002/jps.10021 [Crossref] [ Google Scholar]
- Chimote G, Banerjee R. In vitro evaluation of inhalable isoniazid-loaded surfactant liposomes as an adjunct therapy in pulmonary tuberculosis. J Biomed Mater Res B Appl Biomater 2010; 94(1):1-10. doi: 10.1002/jbm.b.31608 [Crossref] [ Google Scholar]
- Mehta PP, Ghoshal D, Pawar AP, Kadam SS, Dhapte-Pawar VS. Recent advances in inhalable liposomes for treatment of pulmonary diseases: concept to clinical stance. J Drug Deliv Sci Technol 2020; 56(Pt A):101509. doi: 10.1016/j.jddst.2020.101509 [Crossref] [ Google Scholar]
- Singh N, Kushwaha P, Ahmad U, Abdullah M. Proliposomes: an approach for the development of stable liposome. Ars Pharm 2019; 60(4):231-40. doi: 10.30827/ars.v60i4.8517 [Crossref] [ Google Scholar]
- Khan I, Yousaf S, Subramanian S, Alhnan MA, Ahmed W, Elhissi A. Proliposome powders for the generation of liposomes: the influence of carbohydrate carrier and separation conditions on crystallinity and entrapment of a model antiasthma steroid. AAPS PharmSciTech 2018; 19(1):262-74. doi: 10.1208/s12249-017-0793-2 [Crossref] [ Google Scholar]
- Kumara BC, Parthiban S, Senthil kumar GP, Tamiz Mani T. Proliposome: a novel approach to carrier drug delivery system. Int J Biopharm 2015; 6(2):98-106. [ Google Scholar]
- Joshi MR, Misra A. Liposomal budesonide for dry powder inhaler: preparation and stabilization. AAPS PharmSciTech 2001; 2(4):44-53. doi: 10.1007/bf02830565 [Crossref] [ Google Scholar]
- Joshi M, Misra A. Dry powder inhalation of liposomal Ketotifen fumarate: formulation and characterization. Int J Pharm 2001; 223(1-2):15-27. doi: 10.1016/s0378-5173(01)00705-0 [Crossref] [ Google Scholar]
- Shah SP, Misra A. Liposomal amikacin dry powder inhaler: effect of fines on in vitro performance. AAPS PharmSciTech 2004; 5(4):e65. doi: 10.1208/pt050465 [Crossref] [ Google Scholar]
- Rojanarat W, Nakpheng T, Thawithong E, Yanyium N, Srichana T. Levofloxacin-proliposomes: opportunities for use in lung tuberculosis. Pharmaceutics 2012; 4(3):385-412. doi: 10.3390/pharmaceutics4030385 [Crossref] [ Google Scholar]
- Edwards DA, Hanes J, Caponetti G, Hrkach J, Ben-Jebria A, Eskew ML. Large porous particles for pulmonary drug delivery. Science 1997; 276(5320):1868-71. doi: 10.1126/science.276.5320.1868 [Crossref] [ Google Scholar]
- Rojanarat W, Nakpheng T, Thawithong E, Yanyium N, Srichana T. Inhaled pyrazinamide proliposome for targeting alveolar macrophages. Drug Deliv 2012; 19(7):334-45. doi: 10.3109/10717544.2012.721144 [Crossref] [ Google Scholar]
- Ebrahimi A, Saffari M, Langrish T. Spray drying and post-processing production of highly-porous lactose particles using sugars as templating agents. Powder Technol 2015; 283:171-7. doi: 10.1016/j.powtec.2015.05.026 [Crossref] [ Google Scholar]
- Ahmadi F, Bahmyari M, Akbarizadeh A, Alipour S. Doxorubicin-verapamil dual loaded PLGA nanoparticles for overcoming P-glycoprotein mediated resistance in cancer: effect of verapamil concentration. J Drug Deliv Sci Technol 2019; 53:101206. doi: 10.1016/j.jddst.2019.101206 [Crossref] [ Google Scholar]
-
Omer HK. Spray-Dried Bioadhesive Formulations for Pulmonary Delivery [dissertation]. England: University of Central Lancashire; 2014.
- Tan S, Ebrahimi A, Liu X, Langrish T. Role of templating agents in the spray drying and postcrystallization of lactose for the production of highly porous powders. Dry Technol 2018; 36(15):1882-91. doi: 10.1080/07373937.2018.1445096 [Crossref] [ Google Scholar]
- Ebrahimi A, Saffari M, Langrish T. Developing a new production process for high-porosity lactose particles with high degrees of crystallinity. Powder Technol 2015; 272:45-53. doi: 10.1016/j.powtec.2014.11.033 [Crossref] [ Google Scholar]
- Khan I, Yousaf S, Subramanian S, Korale O, Alhnan MA, Ahmed W. Proliposome powders prepared using a slurry method for the generation of beclometasone dipropionate liposomes. Int J Pharm 2015; 496(2):342-50. doi: 10.1016/j.ijpharm.2015.10.002 [Crossref] [ Google Scholar]
- Bobbala SK, Veerareddy PR. Formulation, evaluation, and pharmacokinetics of isradipine proliposomes for oral delivery. J Liposome Res 2012; 22(4):285-94. doi: 10.3109/08982104.2012.697067 [Crossref] [ Google Scholar]
- Brunaugh AD, Wu T, Kanapuram SR, Smyth HDC. Effect of particle formation process on characteristics and aerosol performance of respirable protein powders. Mol Pharm 2019; 16(10):4165-80. doi: 10.1021/acs.molpharmaceut.9b00496 [Crossref] [ Google Scholar]
- Alipour S, Montaseri H, Khalili A, Tafaghodi M. Non-invasive endotracheal delivery of paclitaxel-loaded alginate microparticles. J Chemother 2016; 28(5):411-6. doi: 10.1080/1120009x.2015.1105624 [Crossref] [ Google Scholar]
- Alves R, da Silva Reis TV, da Silva LC, Storpírtis S, Mercuri LP, do Rosário Matos J. Thermal behavior and decomposition kinetics of rifampicin polymorphs under isothermal and non-isothermal conditions. Braz J Pharm Sci 2010; 46(2):343-51. doi: 10.1590/s1984-82502010000200022 [Crossref] [ Google Scholar]
- Bhise SB, More AB, Malayandi R. Formulation and in vitro evaluation of rifampicin loaded porous microspheres. Sci Pharm 2010; 78(2):291-302. doi: 10.3797/scipharm.0910-09 [Crossref] [ Google Scholar]