Advanced pharmaceutical bulletin. 11(3):514-521.
doi: 10.34172/apb.2021.059
Research Article
Evaluation of Design and Fabrication of Food-Grade Nanofibers from Chitosan-Gelatin for Nanoencapsulation of Stigmasterol Using the Electrospinning Method
Mir-Michael Mousavi 1, 2  , Mohammadali Torbati 3, Parastou Farshi 4, Hedayat Hosseini 5, Masoud Aman Mohammadi 1, 2
, Mohammadali Torbati 3, Parastou Farshi 4, Hedayat Hosseini 5, Masoud Aman Mohammadi 1, 2  , Seyede Marzieh Hosseini 5, *
, Seyede Marzieh Hosseini 5, *  , Simzar Hosseinzadeh 6, *
, Simzar Hosseinzadeh 6, * 
Author information:
1(Department) National Nutrition and Food Technology Research Institute, Faculty of Nutrition Sciences and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2Students’ Research Committee, National Nutrition and Food Technology Research Institute, Faculty of Nutrition Sciences and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3Department of Food Science and Technology, Faculty of Nutrition and Food Sciences, Nutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
4Food Science Institute, Kansas State University, Manhattan, KS, USA.
5Department of Food Science and Technology, National Nutrition and Food Technology Research Institute, Faculty of Nutrition Sciences and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
6School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Abstract
Purpose:
In this research, electrospinning method was employed to fabricate food-grade nanofibers (NFs) from chitosan-gelatin combination for stigmasterol encapsulation. The spinnability of mixed chitosan and gelatin solutions was investigated at different polymer ratios, and the physicochemical properties of the NFs were evaluated.
Methods:
The mixture solution of chitosan (1.5 % w/v) and gelatin (20 % w/v) in acetic acid indicated spinnability under the following conditions: the ratio of 25:75, voltage of 17 kV, and 15 cm capillary collector distance with a flow rate of 0.2 mL/min. Stigmasterol (0.04 % w/v) was incorporated into NFs of chitosan-gelatin at a respective ratio of 25:75.
Results:
Encapsulation efficiency (EE) of loaded stigmasterol was found to be 87 ± 5 %. The antioxidant ability of loaded stigmasterol was considerably higher than that observed for free stigmasterol. Scanning electron microscopy (SEM) results demonstrated the formation of the ultrathin fibers with no bead (with diameters of 217 ± 43 nm). The concentration of polymeric solution and viscosity had a notable effect on the electrospinning efficiency of the chitosan-gelatin-based NFs. The thermal stability of chitosan and gelatin fibers was more than that of native gelatin and chitosan. The in vitro stigmasterol release from these NFs followed a controlled-release pattern. The released phytosterol from chitosan formula was less than from those without chitosan formula (46 ± 3 % and 96 ± 4 % respectively).
Conclusion:
The obtained results suggested that gelatin had a high potential for enhancing the spinnability of chitosan under acidic conditions at optimized concentrations.
Keywords: Electrospinning, Chitosan, Gelatin, Nanofibers, Stigmasterol
Copyright and License Information
©2021 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Phytosterols (such as stigmasterol) are bioactive compounds of vegetables which have many biological activities. The chemical structure of phytosterols consists of three rings of six-carbon atoms, one ring of five-carbon atoms, and a 3-β-hydroxyl group linked to an aliphatic chain. Phytosterols have diverse beneficial effects on human health such as reducing the cholesterol serum levels, anti-oxidative effects,
1
anti-inflammatory effects, anti-atherosclerotic properties, and anti-cancer activities
2-4
such as the ability to inhibit the development of colon cancer.
5
However, water insolubility, instability, high melting temperature, chalky taste, susceptibility to oxidation, and poor absorption by the human body are some properties that have limited their applications in food fortification. Stigmasterol a plant sterol with several pharmacological activities is susceptible to oxidation when exposed to air, a process enhanced by heat and humidity. In this context, microencapsulation is a way of preventing oxidation, allowing stigmasterol to be incorporated into various pharmaceutical forms while increasing its absorption.
6-8
Thus, microencapsulation technology has been applied to overcome some of the mentioned limitations. Encapsulation is a method which has been used in the form of micro- and nanoparticles for various food applications such as food protection to avoid adverse effects of some factors including heat, moisture, oxidation, formation of off-flavors, and evaporation or loss of volatile compounds.
9
Polysaccharides and proteins are biopolymers which can join together through electrostatic attraction producing molecular complexes such as protein-protein, polysaccharide-polysaccharide, and polysaccharide-protein (nano) complexes.
10
Protein-polysaccharide complexes can be formed by different approaches such as covalent bound formation through Maillard reaction or ionic interactions in polyelectrolyte complexes.
11,12
These complex structures can be used to encapsulate hydrophobic and hydrophilic nutraceuticals such as vitamins, essential oils, fatty acids, flavors, etc.
13
polysaccharide and protein interaction affects on functional and physicochemical characteristics of the final mixture including thermal stability, solubility, foaming ability, and surface tension.
14
Two biopolymers that were used in this study were gelatin and chitosan. Gelatin is a protein which can be produced from the basic hydrolysisof collagen and can also be obtained from various sources such as fish (1.5%), cowhide (29.4%) pig skin (46%), and pig bone (23.1%). The surface charge of gelatin above its isoelectric point is negative while below it is positive.
15-17
Chitosan is soluble at low pH values which can limit its function as an encapsulating compound. However, because of mucoadhesive properties of this polymer, it has been used frequently in controlled-released delivery systems. Further, the efficiency of microencapsulation method can be enhanced using a combination of two or more shell materials.
18-21
Recently, there were more general attraction towards fabrication of nanofibers (NFs) from mixed biopolymers for nanoencapsulation of bioactive compounds using the electrospinning method.
22
Electrospinning technique uses electrostatic force to produce fibers with diameters from micrometer to nanometer.
23
The electrospinning procedure has various advantages such as the absence of heat and high encapsulation efficacy of bioactive constituents upon processing storage. NFs that are obtained from electrospinning process have advantages such as high porosity, large surface areas, low density, and small pore sizes.
24
The spinnability of polymers can be affected by some factors including, viscosity, electrical conductivity, conformational shape, and surface tension of polymers.
25
Generally, proteins are known as biopolymers which cannot be spun due to their complex secondary and tertiary structures and weak internal interactions.
26
Thus, synthetic polymers including polyvinyl alcohol and polyethylene oxide can be used to increase their spinnability.
27
To the best of our knowledge, there is no report published on fabrication of chitosan-gelatin electrospun fibers without adding synthetic polymers. Thus, in this study the spinnability of mixed chitosan and gelatin solutions was investigated at different polymer ratios, and the physicochemical properties of the fabricated electrospun fibers were evaluated.
Materials and Methods
Stigmasterol (assay purity 97%) and medium molecular-weight chitosan with >75 % degree of deacetylation was purchased from Sigma Aldrich (USA). Gelatin (obtained from cold water fish skin) was bought from Sigma Chemical Co. (St. Louis, MO). Acetic acid was acquired from Merck Co. (Germany). For preparation of all solutions, deionized water was used.
Preparation of target biopolymer solutions
To choose the optimum concentration of gelatin spinnability, the tests were accomplished at various concentrations of gelatin. Firstly, different concentrations (12-20 % w/v) of gelatin was solubilized in aqueous acetic acid (90% v/v) and stirred for 2 hours to hydrate it completely. In this study, the concentration of chitosan was considered constant. A chitosan solution (1.5 % (w/v)) was prepared in 90% v/v aqueous acetic acid for 2 hours under magnetic stirring at room temperature. In the next step, chitosan solution was added to the gelatin solutions at various ratios under stirring (Table 1). The final solutions were used for spinnability examination. The electrospun fiber obtained from the examination was placed in a 25°C vacuum oven for one day to remove any remaining solvent. The fabricated fiber was chosen according to results from scanning electron microscope (SEM) and spinnability examinations. For preparing stigmasterol-loaded NFs, the ethanolic solution of stigmasterol was added to the polymers’ solution. The concentration of stigmasterol in the mixture was set at 0.04% w/v.
Table 1.
Physicochemical properties of chitosan-gelatin solutionsa
|
Chitosan (%w/v)
|
Gelatin (%w/v)
|
Ratio
|
Spinnability
|
Viscosity (mPa.s)
|
| 1.5 |
20 |
100:0 |
-
|
109.00±2.83d
|
| 1.5 |
20 |
75:25 |
-
|
126.00±4.24c
|
| 1.5 |
20 |
50:50 |
++
|
140.00±1.41b
|
| 1.5 |
20 |
25:75 |
++++
|
154.00±2.82a
|
aData reported are average values ± standard deviations. Values within each column with different letters are significantly different (P < 0.05)
Electrospinning of biopolymer solutions
High voltage power of 17 kV with a flow rate of 0.2 mL h−1 was applied to the biopolymer solutions, and were then sucked up in a 10 ml plastic syringe attached to a blunt-tipped 21-gauge needle. In order to collect the NFs, an aluminum foil with a nozzle-collector distance of 15 cm was used. The electrospinning protocol was accomplished under ambient conditions at 25 ± 3°C and 30 ± 2% RH.
Viscosity measurement of solutions
The viscosity of solutions was determined using Physica MCR 301 rheometer (Anton-Paar GmbH, Graz, Austria) after transferring 2 mL of the samples into cone and plate cylinder geometry with the shear rate range from 0.1 to 1000 s−1 at 25°C. (Measuring System: CP50-1-SN14598; d=0.099 mm). The viscosity of biopolymer solutions was identified at 100 s−1.
Antioxidant activity of chitosan and chitosan-gelatin NFs
Antioxidant activities ofchitosan and chitosan-gelatin NFs, in the presence of stigmasterol or without it, were evaluated by 2,2-diphenyl-picrylhydrazyl (DPPH) radical scavenging analysis as defined previously with minor changes.
28
Briefly, 40 mg of each sample was dissolved in 2 mL ethanol by consistently stirring at 200 rpm/min. After adding 2 mL (0.01mM) of DPPH solution into the test tube, it was incubated for 50 min, and then the solutions were centrifuged at 4000 × g (Hettich ROTFIX 32A). The absorbance of the supernatant was determined at the wavelength of 517 nm using a spectrophotometer (Perkin Elmer, Lambda2).
Encapsulation efficiency
In order to determine the encapsulation efficiency (EE %), 5 mg of stigmasterol-loaded fibers was taken in 1 mL of ethanol and then the mixture was centrifuged at 4000 rpm for 6 minutes at ambient temperature to remove any stigmasterol attached on the surface of the encapsulated structure. Further, 2.5 mL of ethanol was added to the precipitate from the centrifuge and the mixture was centrifuged again at 8000 rpm for 8 minutes. The supernatant was then used for HPLC injection. HPLC-UV analysis was conducted on a high performance liquid chromatography apparatus (Cecil CE-4900, Cambridge, England) consisting of multiple solvent delivery units, two CE-4100 pumps, mixing chamber, six-port valve (Rheodyne, USA), vacuum degasser, and CE-4200 i=UV–Vis detector (Cambridge, England). The used column for separation was an analytical C18 column (4.6 mm ID × 25 cm, 5 µm particle diameter) (Knauer, Germany) and the analysis was done at room temperature. The mobile phase was 20:80 (V/V) ratio of methanol and phosphate buffer solution (10 mM) with the pH of 6.8 set for the final solution. The method was isocratic and the flow rate was 1 mL/min. The stigmasterol quantification was carried out at 346 nm, and the calibration curve was plotted as wavelength versus 1-30 ppm concentrations of stigmasterol in ethanol. The EE was calculated using the following equation:
Scanning electron microscopy (SEM) analysis
Firstly, the samples were sputtered with a gold–palladium mixture in vacuum and then the structure and morphology of NFs were evaluated by SEM (HITACHI model SU3500). The acceleration voltage of 25 kV was applied. The distribution of the diameters of fibers was determined through randomly measuring the diameter of approximately 100 fibers from SEM images at magnification of 5000×, using Image J software.
Fourier transform-infrared spectrometry (FT-IR) analysis
Chitosan and gelatin powders andelectrospinning fibers were pressed with KBr pellets and then FT-IR analysis was conducted using a spectrometer (Agilent Technologies Cary 630 FTIR). Interferograms were collected over a spectral range of 4000–500 cm−1 with a nominal resolution of 2 cm−1 and 100 scans.
Thermo gravimetric analysis (TGA)
Thermogravimetric analyzer (TGA 50, Shimadzu) was used for TGA and calcium oxalate monohydrate was used for apparatus calibration. Approximately 15 mg of samples was heated from 25 to 600°C at a scanning rate of 10°C per minute under nitrogen atmosphere.
In vitro
release
Dialysis bag diffusion technique was used for evaluating the in-vitro cumulative release of stigmasterol from the gelatin-chitosan NFs (cut off value =11000 Da) by imitating oral delivery to a specific site in the small intestine, where the NFs transit gastric condition is pH 1.2 for 2 hours in stomach, and intestinal condition is pH 6.8 for 3 hours in small intestine.
29
To imitate stomach environment with and without enzyme, 1 mL of the delivery system containing stigmasterol was added into the dialysis bag and placed in 20 mL of HCl (0.1 M)-ethanol w/v (80:20 ratio) in the presence and absence of pepsin. Then, the dialysis bag was put in 20 mL phosphate buffer-ethanol (80:20 ratio) under magnetic stirring (50 rpm and 37°C) to simulate small intestine environment. The stigmasterol amount was determined at the end of simulation time.
Results and Discussion
Electrospinning and physicochemical properties of chitosan-gelatin solutions (characterization of NFs)
The spun formation capability of the polymer solutions was primarily evaluated through visual observation. SEM images of samples were used to determine the diameter and morphology of fibers. SEM images of chitosan and gelatin solutions at various ratios are shown at Figure 1. According to the images, the quality as well as the diameters of chitosan fibers in acetic acid increased with gelation concentration rise, where the fibers formed with higher concentrations of gelatin (20 % w/v) were more uniform and they had lower amount of bead in them (Figure 1d). A solution of 1.5% chitosan alone as well as gelatin-chitosan solution at the ratio of 3 to 1 could not form a NFs, but with the decrease in the amount of chitosan and increase in gelatin concentration, uniform fibers were formed (Figure 1c and 1d). Fibers were composed of 50: 50 ratios of chitosan-gelatin containing beads Figure 1c. The best and most uniform fiber was formed at the chitosan-gelatin ratio of 1 to 3, Figure 1d.
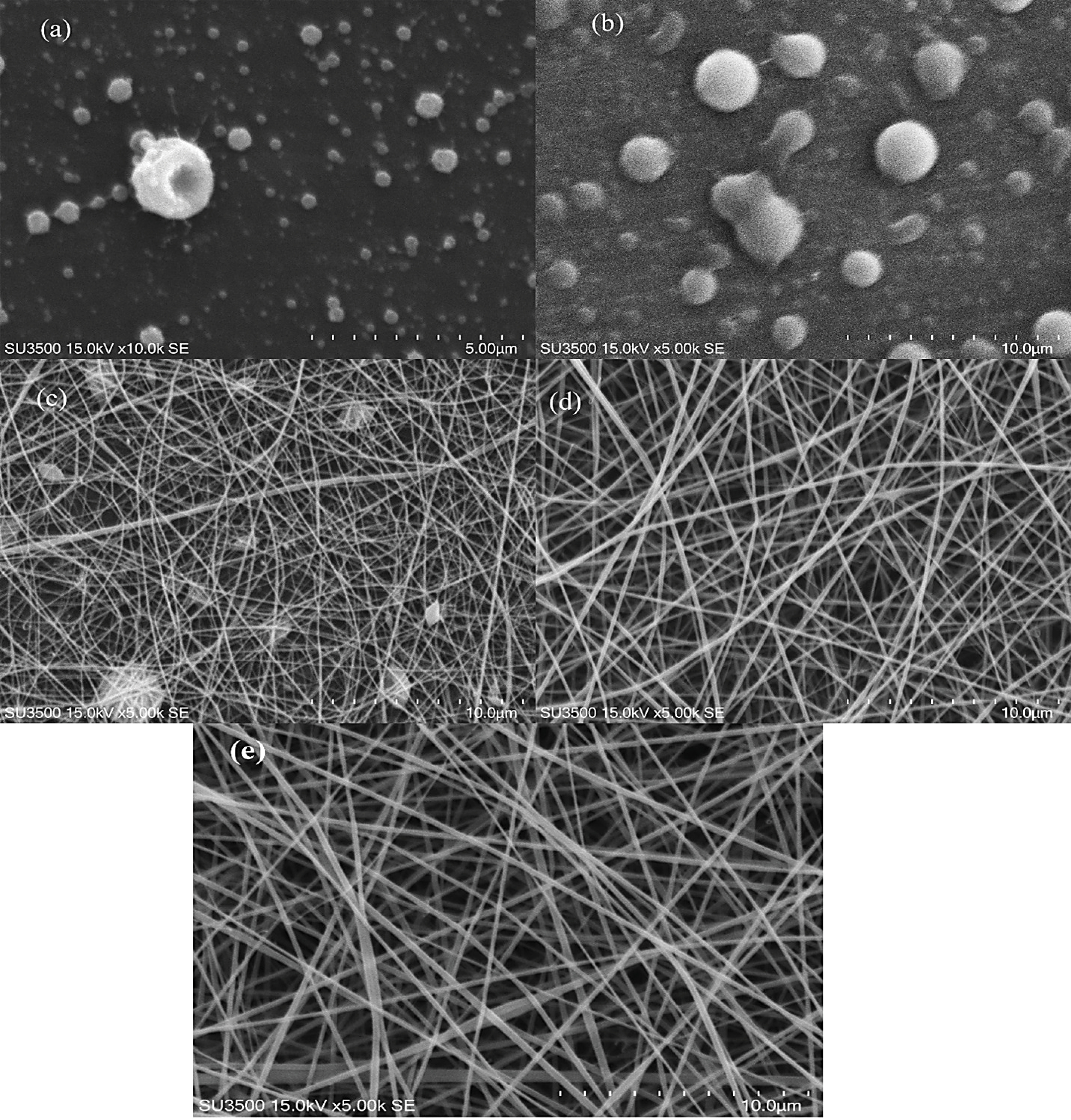
Figure 1.
SEM image of chitosan-gelatin nanofibers (NFs) (1.5-20%w/v) at ratio of 100:0 (a), 75:25(b), 50:50 (c), 25:75(d) and stigmasterol loaded 25:75 at 0.04 %w/v (e).
.
SEM image of chitosan-gelatin nanofibers (NFs) (1.5-20%w/v) at ratio of 100:0 (a), 75:25(b), 50:50 (c), 25:75(d) and stigmasterol loaded 25:75 at 0.04 %w/v (e).
According to the former studies that used acetic acid as the solvent for the formation of chitosan fibers, spark was formed during electrospinning procedure which was due to the high conductivity of acetic acid and its effect on the viscosity of chitosan solution.
30
Gelatin addition to chitosan solution had a positive effect on the electrospinning protocol and the quality of NFs. As mentioned in previous studies, the physicochemical properties of biopolymer solutions such as viscosity have a significant role in the formation of uniform and continuous fibers.
25,31
The viscosity of chitosan solution increased in the presence of gelatin (Table 1). Generally, it can be realized (Table 1) that increase in the amount of the protein polymer in the mixture solutions causes an increase in the apparent viscosity (thus it can be approved that the addition of gelatin improves chain entanglement for fiber formation). As shown in SEM images (Figure 1c and 1e), by increasing the apparent viscosity, bead free and more uniform fibers were formed at the ratio of 25:75 of chitosan-gelatin solutions.
The measured diameters of fibers in the chitosan-gelatin at the ratio of 75:25 were zero (the fibers were not formed), the mean diameter of fibers at the ratio of 50:50 were 105 ± 35 nm, while the diameter of fibers at ratio of 25:75 (chitosan-gelatin) meaningfully increased to 217 ± 43 nm, which was comparable to 15 % w/v gelatin in formulation (Figure 2). As observed in Figure 1d and 1e, increase in the concentration ratio of gelatin in the blend (25:75 of chitosan-gelatin) has a positive effect on the morphology of fibers and the spinnability of biopolymer solution.
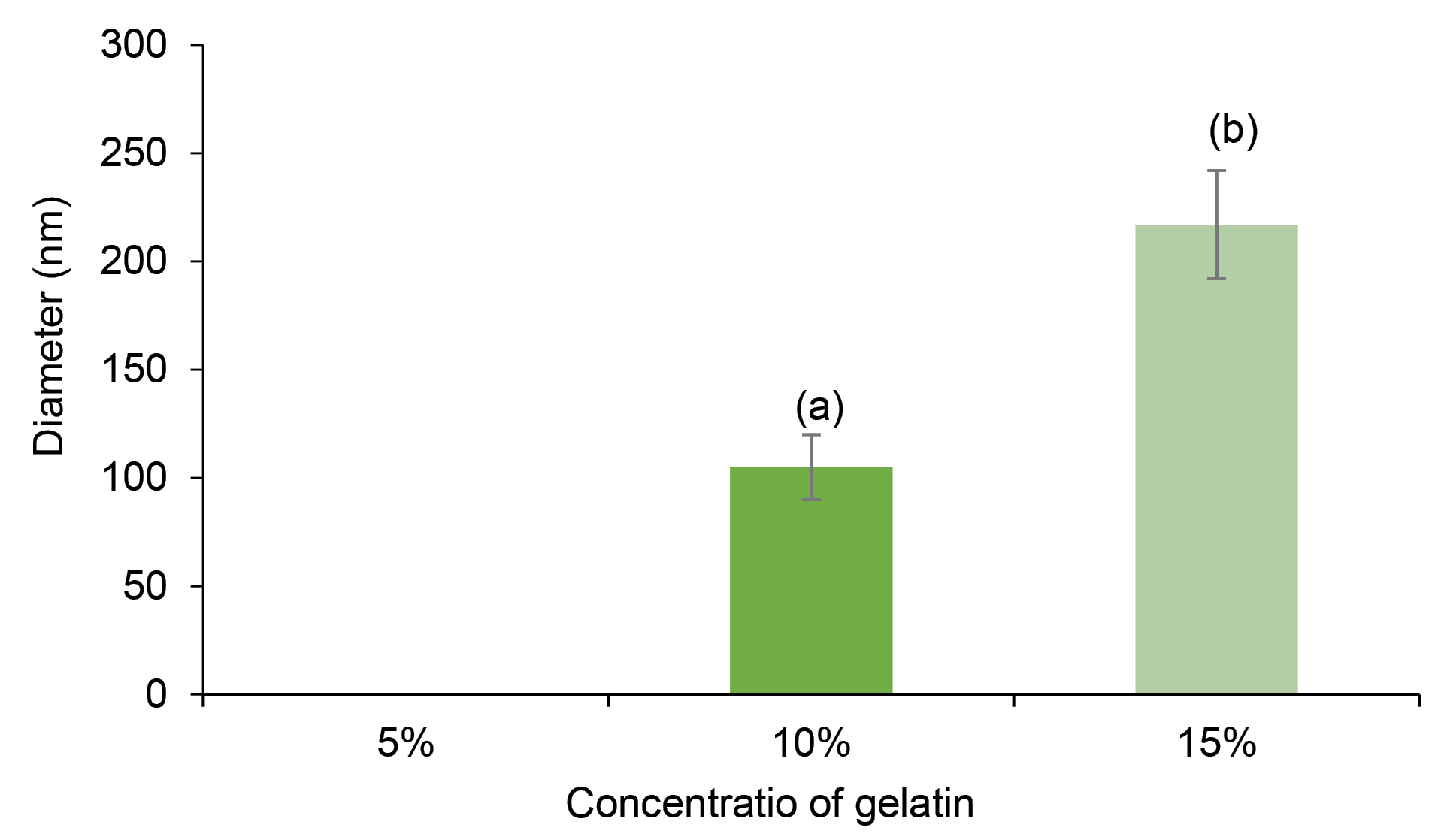
Figure 2.
Diameter of fibers of chitosan–gelatin. (a): 50-50 chitosan–gelatin, (b): 25-75 chitosan–gelatin. At concentertion 5% of gelatin the fibers were not formed.
.
Diameter of fibers of chitosan–gelatin. (a): 50-50 chitosan–gelatin, (b): 25-75 chitosan–gelatin. At concentertion 5% of gelatin the fibers were not formed.
According to the previous investigations in electrospinning method, the viscosity and surface tension of solutions play an important role in the protocol control.
32
The surface tension determines the spinnability of polymer when the viscosity of polymer is low; however, in the case of a viscose biopolymer solution such as 20 % w/v gelatin, the viscosity is a factor that controls the productivity of electrospinning. Moreover, gelatin implicitly reduces the conductivity of biopolymer solutions, which limits spark creation during electrospinning process. There are limited studies on the electrospinning of mixed polymers (chitosan and agarose – trifluoroacetic acid solvent, chitosan, and gellan-trifluoroacetic acid solvent).
33-35
The obtained result of this study about spinnability is in agreement with other studies in which the spinnability of chitosan increased in the presence of agarose and gellan. Thus, electrospinning productivity improved with optimum values of conductivity and chitosan-gelatin solution’s viscosity, which was attained at a defined concentration ratio. Addition of stigmasterol to chitosan-gelatin solution (at the ratio of 25:75) resulted in more suitable formulations according to SEM and spinnability results. The SEM image of 0.04 % w/v stigmasterol loaded NF is displayed in Figure 1e. Uniform fibers without any beads were produced with certain diameters 208 ± 32 nm. The increase in stigmasterol level up to more than 0.04% w/v revealed a negative effect on fibers’ morphology and electrospinning yield.
Antioxidant activity
The antioxidant activity results indicated that addition of stigmasterol significantly increased the antioxidant activity of NFs (Figure 3). According to earlier investigations, a growth in surface area, specifically in NFs, can cause increase in antioxidant capacity of bioactive components.
36
Furthermore, due to the active amino and hydroxyl groups in the chitosan, the polymer chains show antioxidant activity, which are the reasons that chitosan has hydrogen-donating ability.
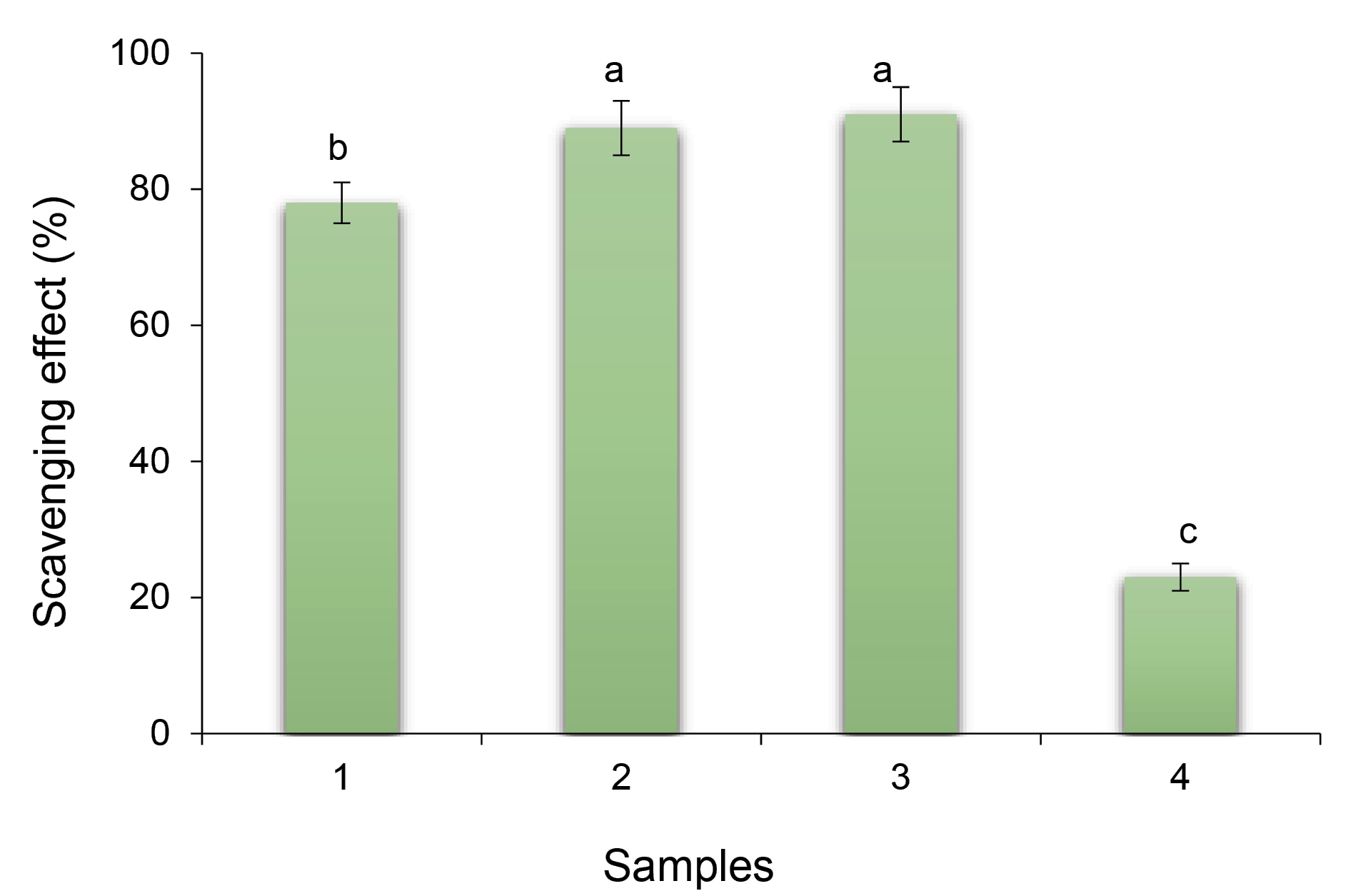
Figure 3.
Antioxidant activity of stigmasterol loaded nanofibers (NFs) against free stigmasterol and gelatin NFs. 1) Stigmasterol (40 uM), 2) Gelatin-stigmassterol NFs, 3) Chitosan-gelatin-stigmasterol NFs, 4) Gelatin NFs. Different letters in each column represent significant differences (P < 0.05).
.
Antioxidant activity of stigmasterol loaded nanofibers (NFs) against free stigmasterol and gelatin NFs. 1) Stigmasterol (40 uM), 2) Gelatin-stigmassterol NFs, 3) Chitosan-gelatin-stigmasterol NFs, 4) Gelatin NFs. Different letters in each column represent significant differences (P < 0.05).
Encapsulation efficiency
EE of stigmasterol in gelatin fibers was 62 ± 4%, but it increased to 87 ± 5% in fibers formulated using chitosan-gelatin at the ratio of 25:75 (Figure 4). The chitosan has a significant role in improving the quantity of physical entrapment of stigmasterol during electrospinning. During EE measurement, the physical form of the electrospun fibers was determined. After adding the fibers to the ethanolic solution the fragmented to visible particles were obvious in the solution, and after centrifugation step the undissolved particles could be seen in the bottom of the tube.
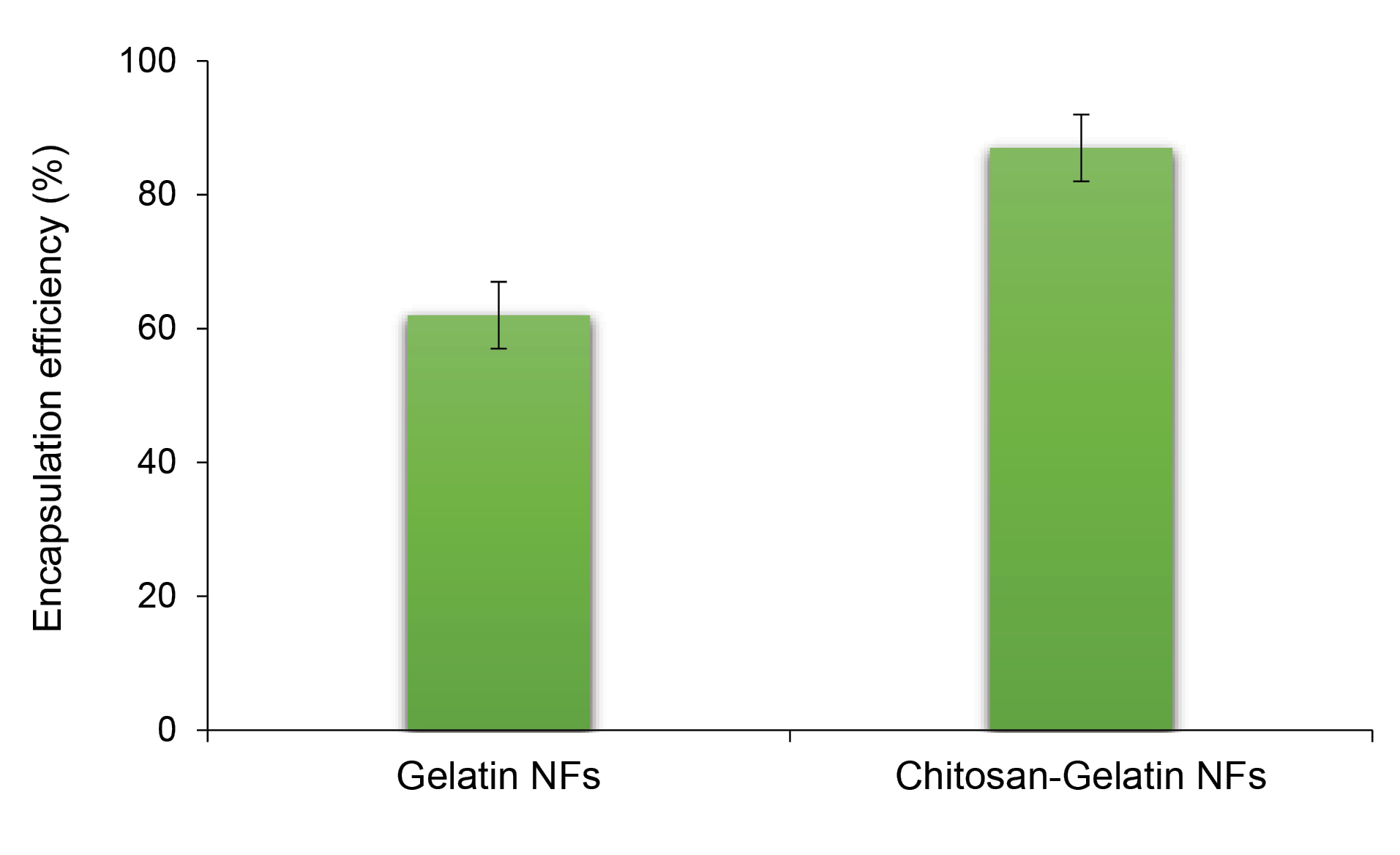
Figure 4.
Encapsulation efficiency of stigmaserol in gelatin NFs and chitosan-gelatin NFs (at ratio of 25:75).
.
Encapsulation efficiency of stigmaserol in gelatin NFs and chitosan-gelatin NFs (at ratio of 25:75).
FT-IR
The FT-IR spectra of pure and chitosan NFs are shown in Figure 5. In all spectra, the absorption peak appeared at around 3400 cm−1 and 1629 cm−1 associated with the asymmetric and symmetric stretching vibrations of the hydroxyl group (O-H). The patterns related to the gelatin structure and vibrations of C=O and N-H bonds in amide I, as well as vibrations of C≡N and N-H groups in amides II and III, also appeared at around 1633 cm-1, 1583 cm-1, and 1238 cm-1.
35
The peaks which appeared in 1518 cm-1 were attributed to the vibration mode of the N-H band in amide II, and the peak appearing at shorter wavelengths indicated N-H vibration of NH3 group in the chitosan structure.
37
The change in the intensity and location of the peaks is 5 to 10 cm-1, which is an evidence for the presence of both materials and the successful preparation of the two-component NFs.
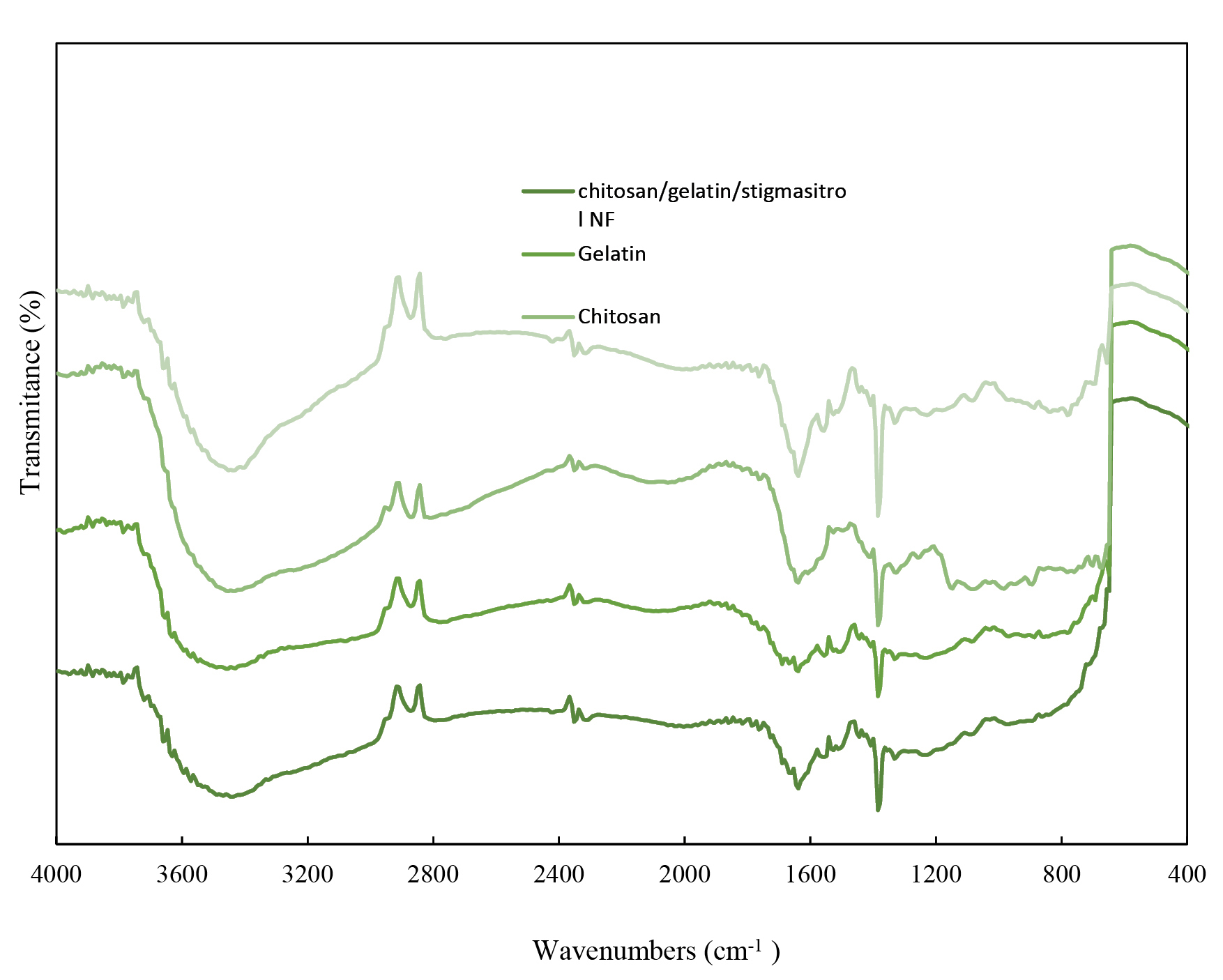
Figure 5.
Fourier transform infrared spectroscopy (FT-IR) spectra of chitosan,gelatin, Stigmasterol-loaded chitosan-gelatinNFs and chitosan-gelatin NFs. (a) Chitosan-Gelatin-Stigmasterol NFs, (b) Gelatin, (c) Chitosan, (d) Chitosan-Gelatin NFs.
.
Fourier transform infrared spectroscopy (FT-IR) spectra of chitosan,gelatin, Stigmasterol-loaded chitosan-gelatinNFs and chitosan-gelatin NFs. (a) Chitosan-Gelatin-Stigmasterol NFs, (b) Gelatin, (c) Chitosan, (d) Chitosan-Gelatin NFs.
TGA analysis
TGA graphs of chitosan, gelatin, and their two-component NFs are shown in Figure 6. As can be seen, less weight loss occurred at around 70°C for all samples and more weight loss occurred at the temperatures of around 180°C and 300°C, respectively, which are related to the removal of adsorbed water, deacetylation, and decomposition of chitosan above its melting point. At 600°C, all samples are completely decomposed. Note that the stability of NFs increased due to the addition of gelatin to chitosan.
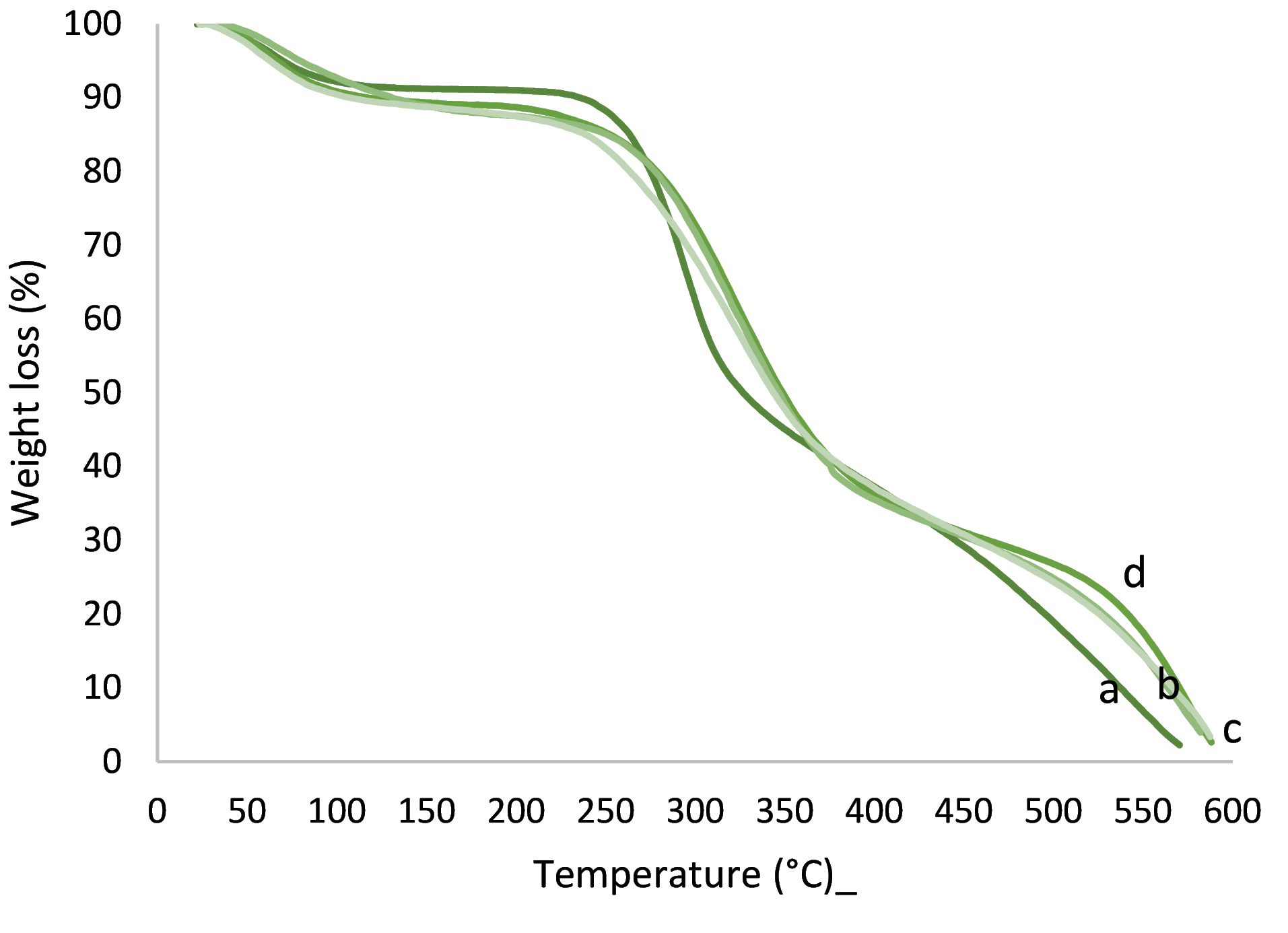
Figure 6.
Thermogravimetric spectra of (a) chitosan, (b) chitosan-gelatin-stigmasterol, (c) gelatin, (d) chitosan-gelatin NFs.
.
Thermogravimetric spectra of (a) chitosan, (b) chitosan-gelatin-stigmasterol, (c) gelatin, (d) chitosan-gelatin NFs.
Release behavior in gastrointestinal
Figure 7 depicts the release behavior of stigmasterol from gelatin-chitosan colloidal NFs. The amounts of stigmasterol release in small intestine in the presence of chitosan formula were significantly less than those without chitosan formula (46 ± 3 % and 96 ± 4 % respectively). Note that the contents of stigmasterol delivered to small intestine in formulations with and without chitosan were between 71 ± 3 % and 10 ± 5 % of the initial load of stigmasterol. Thus, this newly colloidal system (gelatin-chitosan colloidal NFs) has a promising potential for small intestine target delivery of stigmasterol.
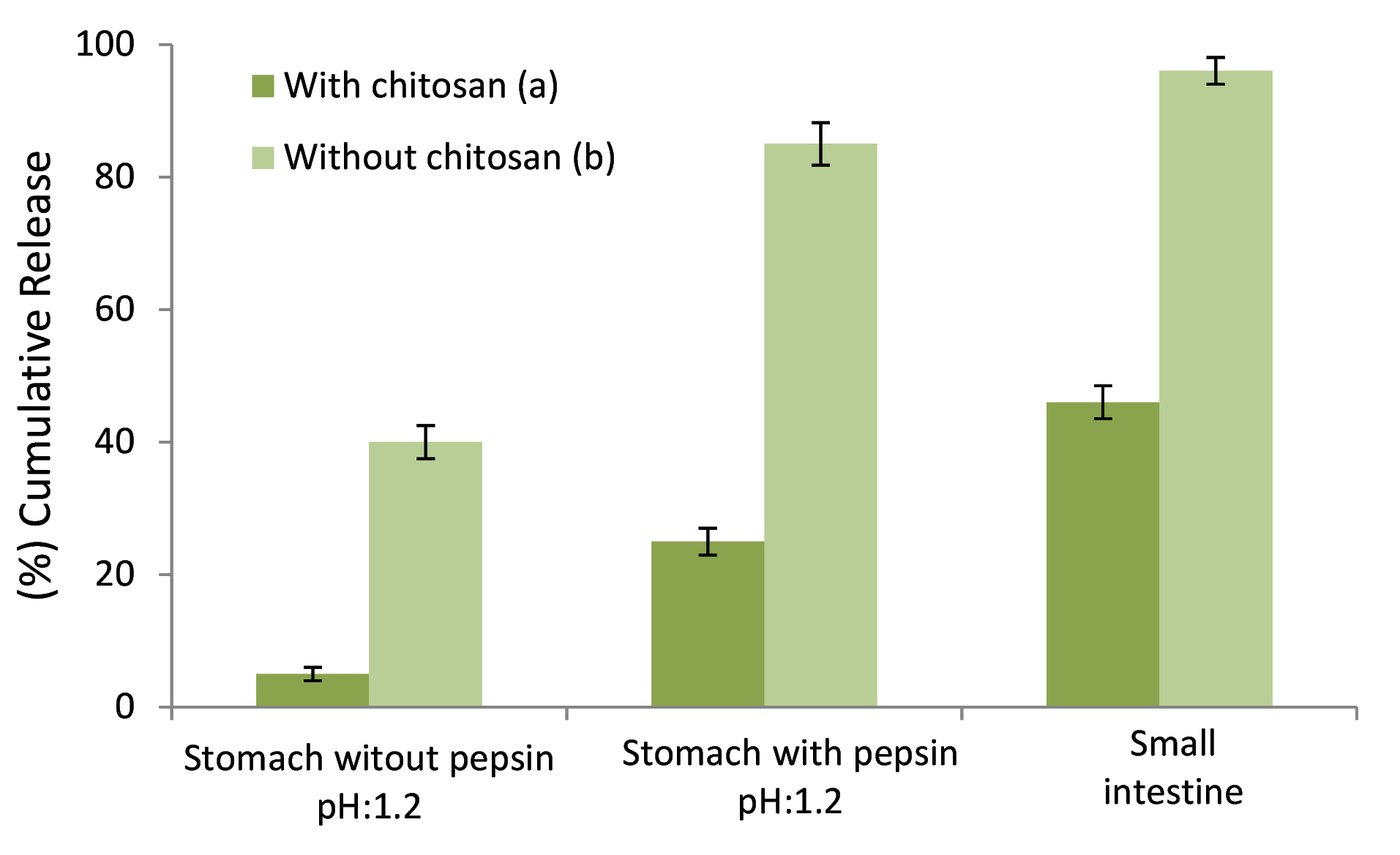
Figure 7.
Cumulative release behavior of stigmasterol loaded NPs in mimic of gastroin-testinal conditions. (a) with chitosan, (b) without chitosan.
.
Cumulative release behavior of stigmasterol loaded NPs in mimic of gastroin-testinal conditions. (a) with chitosan, (b) without chitosan.
In each of the stigmasterol loaded systems, in the presence of pepsin, the release at pH 6.8 was quicker than that of pH 1.2. The mechanism of pH-sensitive release is that amine groups of chitosan are being protonated at low pH, and hydroxyl groups of gelatin are being deprotonated at high pH. In the presence of pepsin at pH 1.2, the release was far faster than at pH 6.8 which might be due to the protease property of pepsin.
Conclusion
In this study, stigmasterol loaded food-grade NFs with aqueous solutions of chitosan-gelatin were successfully fabricated using electrospinning method without using any synthetic polymers. Ultra-thin beads free NFs were prepared with a high concentration of gelatin (20% w/v) and chitosan (1.5 % w/v) at 25:75 (chitosan-gelatin) and 0.04 % w/v stigmasterol. Electrospinning productivity was enhanced upon the addition of gelatin. The concentration of polymeric solution and viscosity had a notable effect on the electrospinning efficiency of the chitosan-gelatin-based NFs.The results of this study suggested that gelatin has a high potential for enhancing the spinnability of chitosan under acidic conditions at optimized concentrations.
Ethical Issue
Not applicable
Conflict of Interest
The authors have declared that there is no conflict of interest.
Acknowledgments
The authors gratefully acknowledge Miss. Mohammadian (PhD candidate of physical chemistry in the Shahid Beheshti University) for her help in the analysis and characterization of the samples. Furthermore, this research was a part of PhD dissertation at Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- van Rensburg SJ, Daniels WM, van Zyl JM, Taljaard JJ. A comparative study of the effects of cholesterol, beta-sitosterol, beta-sitosterol glucoside, dehydroepiandrosterone sulphate and melatonin on in vitro lipid peroxidation. Metab Brain Dis 2000; 15(4):257-65. doi: 10.1023/a:1011167023695 [Crossref] [ Google Scholar]
- Rao AV, Janezic SA. The role of dietary phytosterols in colon carcinogenesis. Nutr Cancer 1992; 18(1):43-52. doi: 10.1080/01635589209514203 [Crossref] [ Google Scholar]
- Awad AB, von Holtz RL, Cone JP, Fink CS, Chen YC. beta-Sitosterol inhibits growth of HT-29 human colon cancer cells by activating the sphingomyelin cycle. Anticancer Res 1998; 18(1A):471-3. [ Google Scholar]
- Awad AB, Roy R, Fink CS. Beta-sitosterol, a plant sterol, induces apoptosis and activates key caspases in MDA-MB-231 human breast cancer cells. Oncol Rep 2003; 10(2):497-500. [ Google Scholar]
- Bouic PJ. The role of phytosterols and phytosterolins in immune modulation: a review of the past 10 years. Curr Opin Clin Nutr Metab Care 2001; 4(6):471-5. doi: 10.1097/00075197-200111000-00001 [Crossref] [ Google Scholar]
- Botelho PB, Galasso M, Dias V, Mandrioli M, Lobato LP, Rodriguez-Estrada MT. Oxidative stability of functional phytosterol-enriched dark chocolate. LWT Food Sci Technol 2014; 55(2):444-51. doi: 10.1016/j.lwt.2013.09.002 [Crossref] [ Google Scholar]
- Fujiwara GM, Campos R, Costa CK, de Fátima Gaspari Dias J, Miguel OG, Miguel MD. Production and characterization of alginate-starch-chitosan microparticles containing stigmasterol through the external ionic gelation technique. Braz J Pharm Sci 2013; 49(3):537-47. doi: 10.1590/s1984-82502013000300015 [Crossref] [ Google Scholar]
- Tolve R, Condelli N, Can A, Tchuenbou-Magaia FL. Development and characterization of phytosterol-enriched oil microcapsules for foodstuff application. Food Bioproc Tech 2018; 11(1):152-63. doi: 10.1007/s11947-017-1990-4 [Crossref] [ Google Scholar]
- Ozkan G, Franco P, De Marco I, Xiao J, Capanoglu E. A review of microencapsulation methods for food antioxidants: principles, advantages, drawbacks and applications. Food Chem 2019; 272:494-506. doi: 10.1016/j.foodchem.2018.07.205 [Crossref] [ Google Scholar]
- de Kruif CG, Tuinier R. Polysaccharide protein interactions. Food Hydrocoll 2001; 15(4-6):555-63. doi: 10.1016/s0268-005x(01)00076-5 [Crossref] [ Google Scholar]
- Turgeon SL, Schmitt C, Sanchez C. Protein–polysaccharide complexes and coacervates. Curr Opin Colloid Interface Sci 2007; 12(4-5):166-78. doi: 10.1016/j.cocis.2007.07.007 [Crossref] [ Google Scholar]
- Martinez-Alvarenga MS, Martinez-Rodriguez EY, Garcia-Amezquita LE, Olivas GI, Zamudio-Flores PB, Acosta-Muniz CH. Effect of Maillard reaction conditions on the degree of glycation and functional properties of whey protein isolate – Maltodextrin conjugates. Food Hydrocoll 2014; 38:110-8. doi: 10.1016/j.foodhyd.2013.11.006 [Crossref] [ Google Scholar]
- Zimet P, Livney YD. Beta-lactoglobulin and its nanocomplexes with pectin as vehicles for ω-3 polyunsaturated fatty acids. Food Hydrocoll 2009; 23(4):1120-6. doi: 10.1016/j.foodhyd.2008.10.008 [Crossref] [ Google Scholar]
- Turgeon SL, Beaulieu M, Schmitt C, Sanchez C. Protein–polysaccharide interactions: phase-ordering kinetics, thermodynamic and structural aspects. Curr Opin Colloid Interface Sci 2003; 8(4-5):401-14. doi: 10.1016/s1359-0294(03)00093-1 [Crossref] [ Google Scholar]
-
Yan M. Microencapsulation with coacervation. In: Handbook of Encapsulation and Controlled Release. CRC Press; 2015. p. 248-58.
- Comunian TA, Favaro-Trindade CS. Microencapsulation using biopolymers as an alternative to produce food enhanced with phytosterols and omega-3 fatty acids: a review. Food Hydrocoll 2016; 61:442-57. doi: 10.1016/j.foodhyd.2016.06.003 [Crossref] [ Google Scholar]
- Díaz-Calderón P, Caballero L, Melo F, Enrione J. Molecular configuration of gelatin–water suspensions at low concentration. Food Hydrocoll 2014; 39:171-9. doi: 10.1016/j.foodhyd.2013.12.019 [Crossref] [ Google Scholar]
- Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release 2004; 100(1):5-28. doi: 10.1016/j.jconrel.2004.08.010 [Crossref] [ Google Scholar]
- Luo Y, Wang Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int J Biol Macromol 2014; 64:353-67. doi: 10.1016/j.ijbiomac.2013.12.017 [Crossref] [ Google Scholar]
- Fathi M, Martín Á, McClements DJ. Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends Food Sci Technol 2014; 39(1):18-39. doi: 10.1016/j.tifs.2014.06.007 [Crossref] [ Google Scholar]
- Das S, Chaudhury A, Ng KY. Preparation and evaluation of zinc-pectin-chitosan composite particles for drug delivery to the colon: role of chitosan in modifying in vitro and in vivo drug release. Int J Pharm 2011; 406(1-2):11-20. doi: 10.1016/j.ijpharm.2010.12.015 [Crossref] [ Google Scholar]
-
Ramakrishna S. An Introduction to Electrospinning and Nanofibers. World Scientific; 2005.
- Frenot A, Chronakis IS. Polymer nanofibers assembled by electrospinning. Curr Opin Colloid Interface Sci 2003; 8(1):64-75. doi: 10.1016/s1359-0294(03)00004-9 [Crossref] [ Google Scholar]
- Mendes AC, Stephansen K, Chronakis IS. Electrospinning of food proteins and polysaccharides. Food Hydrocoll 2017; 68:53-68. doi: 10.1016/j.foodhyd.2016.10.022 [Crossref] [ Google Scholar]
- Kriegel C, Arecchi A, Kit K, McClements DJ, Weiss J. Fabrication, functionalization, and application of electrospun biopolymer nanofibers. Crit Rev Food Sci Nutr 2008; 48(8):775-97. doi: 10.1080/10408390802241325 [Crossref] [ Google Scholar]
- Nieuwland M, Geerdink P, Brier P, van den Eijnden P, Henket JT, Langelaan ML. Food-grade electrospinning of proteins. Innov Food Sci Emerg Technol 2013; 20:269-75. doi: 10.1016/j.ifset.2013.09.004 [Crossref] [ Google Scholar]
- Colín-Orozco J, Zapata-Torres M, Rodríguez-Gattorno G, Pedroza-Islas R. Properties of poly (ethylene oxide)/ whey protein isolate nanofibers prepared by electrospinning. Food Biophys 2015; 10(2):134-44. doi: 10.1007/s11483-014-9372-1 [Crossref] [ Google Scholar]
- Charernsriwilaiwat N, Rojanarata T, Ngawhirunpat T, Sukma M, Opanasopit P. Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int J Pharm 2013; 452(1-2):333-43. doi: 10.1016/j.ijpharm.2013.05.012 [Crossref] [ Google Scholar]
- Krivorotova T, Cirkovas A, Maciulyte S, Staneviciene R, Budriene S, Serviene E. Nisin-loaded pectin nanoparticles for food preservation. Food Hydrocoll 2016; 54(Pt A):49-56. doi: 10.1016/j.foodhyd.2015.09.015 [Crossref] [ Google Scholar]
- Geng X, Kwon OH, Jang J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005; 26(27):5427-32. doi: 10.1016/j.biomaterials.2005.01.066 [Crossref] [ Google Scholar]
- Wongsasulak S, Kit KM, McClements DJ, Yoovidhya T, Weiss J. The effect of solution properties on the morphology of ultrafine electrospun egg albumen–PEO composite fibers. Polymer 2007; 48(2):448-57. doi: 10.1016/j.polymer.2006.11.025 [Crossref] [ Google Scholar]
- Deitzel JM, Kleinmeyer J, Harris D, Beck Tan NC. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001; 42(1):261-72. doi: 10.1016/S0032-3861(00)00250-0 [Crossref] [ Google Scholar]
- Teng SH, Wang P, Kim HE. Blend fibers of chitosan–agarose by electrospinning. Mater Lett 2009; 63(28):2510-2. doi: 10.1016/j.matlet.2009.08.051 [Crossref] [ Google Scholar]
- Raeisi M, Mohammadi MA, Coban OE, Ramezani S, Ghorbani M, Tabibiazar M. Physicochemical and antibacterial effect of Soy Protein Isolate/Gelatin electrospun nanofibres incorporated with Zataria multiflora and Cinnamon zeylanicum essential oils. Journal of Food Measurement and Characterization 2021; 15(2):1116-1126. [ Google Scholar]
- Rostami M, Ghorbani M, Aman Mohammadi M, Delavar M, Tabibiazar M, Ramezani S. Development of resveratrol loaded chitosan-gellan nanofiber as a novel gastrointestinal delivery system. Int J Biol Macromol 2019; 135:698-705. doi: 10.1016/j.ijbiomac.2019.05.187 [Crossref] [ Google Scholar]
- Neves AR, Lúcio M, Martins S, Lima JL, Reis S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int J Nanomedicine 2013; 8:177-87. doi: 10.2147/ijn.s37840 [Crossref] [ Google Scholar]
- Staroszczyk H, Sztuka K, Wolska J, Wojtasz-Pająk A, Kołodziejska I. Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with EDC films: FT-IR study. Spectrochim Acta A Mol Biomol Spectrosc 2014; 117:707-12. doi: 10.1016/j.saa.2013.09.044 [Crossref] [ Google Scholar]