Advanced pharmaceutical bulletin. 13(1):7-23.
doi: 10.34172/apb.2023.009
Review Article
What We Need to Know about Liposomes as Drug Nanocarriers: An Updated Review
Hanieh Abbasi 1, 2  , Maryam Kouchak 2, 3, Zohreh Mirveis 1, 2, Fatemeh Hajipour 1, Mohsen Khodarahmi 1, 2, Nadereh Rahbar 2, *
, Maryam Kouchak 2, 3, Zohreh Mirveis 1, 2, Fatemeh Hajipour 1, Mohsen Khodarahmi 1, 2, Nadereh Rahbar 2, *  , Somayeh Handali 4, *
, Somayeh Handali 4, * 
Author information:
1Department of Medicinal Chemistry, School of Pharmacy, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
2Nanotechnology Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
3Department of Pharmaceutics, School of Pharmacy, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
4Medical Biomaterials Research Center (MBRC), Tehran University of Medical Sciences, Tehran, Iran.
Abstract
Liposomes have been attracted considerable attention as phospholipid spherical vesicles, over the past 40 years. These lipid vesicles are valued in biomedical application due to their ability to carry both hydrophobic and hydrophilic agents, high biocompatibility and biodegradability. Various methods have been used for the synthesis of liposomes, so far and numerous modifications have been performed to introduce liposomes with different characteristics like surface charge, size, number of their layers, and length of circulation in biological fluids. This article provides an overview of the significant advances in synthesis of liposomes via active or passive drug loading methods, as well as describes some strategies developed to fabricate their targeted formulations to overcome limitations of the "first-generation" liposomes.
Keywords: Liposome, Liposome synthesis, Liposome targeting methods, Liposome loading methods, Liposome applications
Copyright and License Information
©2023 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Nowadays, the use of nanomaterials as a system for drug-delivery has been widely considered, specially, in cancer therapy.1 It has been proved that materials in nanoscale (˂ 200 nm) can prolong the circulation time in body as well as entering the cells via endocytosis; consequently, cause intracellular absorption.2,3 Different nanomaterials such as micelles,4 dendrimers,5,6 superparamagnetic iron oxide nanoparticles (SPIONs),7 mesoporous silica nanoparticles,8 gold nanoparticles (GNPs),9 quantum dots,10 carbon nanotubes,11 and liposomes have been used in drug delivery systems.12 Among them liposomes are the most common nanocarriers due to their inherent advantages such as high biocompatibility, low immunogenicity, cell-like membrane, low toxicity, and ability to protect drugs from hydrolysis and prolong their biological half-life. They are able to encapsulate either hydrophobic or hydrophilic molecules and control the drug release.3,13,14 Besides, many efforts have been made in developing of smart drug carriers that deliver their cargo in response to an external or internal trigger. In this regard, liposomes are recognized as one of the most successful drug delivery systems.15,16
In general, liposomes are sphere-shaped microscopic vesicles with the hydrophilic portion completely enclosed by one or more phospholipid bilayers (Figure 1).17 Due to the amphiphilic nature of phospholipids, they favor to assemble as closed bilayer structures in such a way that minimize the confrontation between aqueous and hydrophobic domains. So, the lowest free energy state and the maximum stability to self-assembled structures are achieved. Besides, the hydrodynamic and other destabilizing forces can cause the fragmentation of the bilayer to form the smaller liposomes.18,19In vivo and in vitro stability of liposomes are controlled by their physical and chemical characteristics such as lipid composition, size, charge, number of lamellae and surface modifications.20 Up to now, numerous researches related to liposomes have been performed owing to their importance in the nanomedicine field. Loaded drugs on liposomes can include a wide range of anti-cancer drugs, antibiotics, small interfering RNAs (siRNA), antisense oligonucleotides, and bacterial plasmids carrying therapeutic genes.21 Similarity of phospholipids to cell membrane facilitates passage of liposome through some membrane barriers for distribution in tissues and removal from the elimination organs. Besides, modification of liposomes with various ligands and polymers improves drug uptake and increases circulation time of drug in the blood.12,22 After the clinical approval of PEGylated liposomal doxorubicin (Doxil®) as the first nanodrug by US FDA in 1995, 19 liposomal formulations have been clinically approved for the treatment of various diseases. Nevertheless, there are major concerns about their stability, controlled and predictable pharmacokinetics and pharmacodynamics as well as reproducible production in large scale that needs improvement.14,20,23 One of the challenges in application of liposomes in clinical use is the interaction of liposome constituents with the immune system. Liposome components can induce antibody production which leads to reduction of their efficacy.24 In addition, the lack of established techniques for large-scale production of liposomes, and suitable models that exactly imitate tumor heterogeneity, are limitations for clinical development of liposomes.25
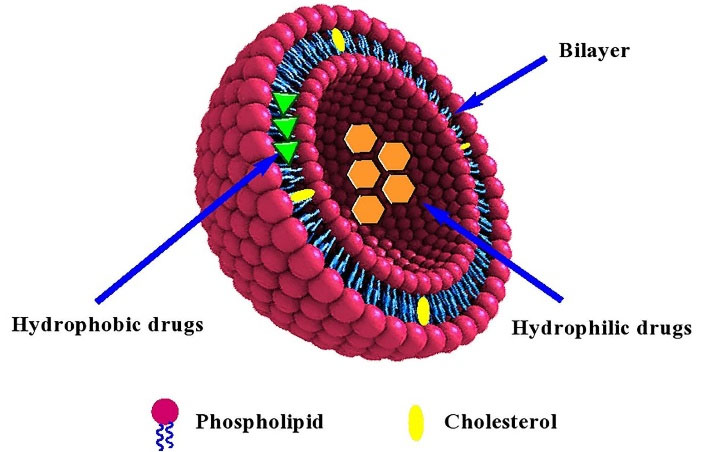
Figure 1.
Schematic presentation of structure of a typical liposome loaded with hydrophilic and hydrophobic drugs
.
Schematic presentation of structure of a typical liposome loaded with hydrophilic and hydrophobic drugs
Several review articles have been published describing liposomal structures, preparation methods and their application.12,15,17,19,24-31 The present review, besides the general aspects liposomes, focuses on the significant advances in their synthesis via active or passive drug loading methods, as well as describing some strategies developed to fabricate new-generation liposomes with target-specificity and stimuli-sensitivity.
Structural units of liposomes
In general, the structure of liposomes consists of two parts; phospholipid and cholesterol. Phospholipids are the major component of liposomal structure and cholesterol improves their stability. The hydrophilic head of these fats is a phosphate group that is joined to hydrophobic components by a water-soluble molecule like glycerol and can be natural or synthetic.18,22 A list of different types of phospholipids is presented in Table 1. Choosing the proper phospholipid for achieving the desired therapeutic goals is essential.32-34 Cholesterol incorporates within phospholipid bilayer because it cannot form liposomes alone. It is essential for the consolidation of bilayers, increasing the packaging of phospholipid molecules, controlling drug retention, and reducing the permeability of the bilayers.17,32,35,36
Table 1.
Various types of phospholipids used in the preparation of liposomes
|
Chemical name
|
Abbrev.
|
Formula
|
Source
|
Ref.
|
| Phosphatidylcholine |
PC |
C42H82NO8P |
Egg yolk, soybeans |
37
|
| Phosphatidylethanolamine |
PE |
C7H12NO8PR2 |
Chocolate, soybean milk |
38
|
| 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine |
DSPE |
C41H82NO8P |
Synthetic |
39
|
| Dimyristoyl phosphatidylcholine |
DMPC |
C36H72NO8P |
synthetic |
29
|
| Dimyristoyl phosphatidylglycerol |
DMPG |
C34H67O10P |
synthetic |
29
|
| Dipalmitoylphosphatidylcholine |
DPPC |
C40H80NO8P |
cell membranes, pulmonary surfactant |
29
|
| Dioleoyphosphatidyl choline |
DOPC |
C44H84NO8P |
synthetic |
29
|
| dipalmitoyl phosphatidyl glycerol |
DPPG |
C38H75O10P |
mitochondrial membranes, pulmonary surfactant |
29
|
| 2,3-dioleyloxy-N-[2(sperminecarbox amido)ethyl]-N,N-dimethyl-1-propanammonium trifluoroacetate |
DOSPA |
C56H111F3N6O5 |
synthetic |
29
|
| 1,2-bis(oleoyloxy)-3-(trim ethylammonio)propane |
DOTAP |
C10H25N3 |
synthetic |
29
|
| 1,2-dimystyloxypropyl-3-dimethyl hydroxyethyl ammonium bromide |
DMRE |
C20H29BrN2 |
synthetic |
29
|
| 3β[N-(N',N'-dimethylaminoethane)-carbomoyl] cholesterol |
DC-CHOL |
C32H56N2O2 |
synthetic |
29
|
| Dioctadecylamino-glycyl-spermine |
DOGS |
C10H26N4 |
As polycation in eukaryotic cells |
29
|
| Phosphatidylinositol |
PI |
C47H83O13P |
Endoplasmic reticulum |
40
|
| Phosphatidylserine |
PS |
C13H24NO10P |
Soy, white beans, egg yolks, chicken liver, beef liver |
41
|
| Phosphatidic acid |
PA |
C39H77O8P |
Cabbage and radish leaves, Mallotus japonicas |
42
|
| Phosphatidylglycerol |
PG |
C40H77O10P |
Live amniotic fluid surfactant |
43
|
| Cardiolipin |
CL |
C81H158O17P2 |
Mammalian and plant cells, inner mitochondrial membrane |
44
|
Characteristics of liposomes
The performance of liposomes depends on their size, number of layers, shape, and surface charge. Therefore, estimating and characterizing these properties is essential for clinical application as well as in determining their half-life.19 These colloidal vesicles have different number of layers are classified based on size and number of bilayers (Figure S1). They can be unilamellar (UV) or multilayer. UVs are also classified into four subgroups of small (SUVs), medium (MUVs), large (LUVs), and giant (GUVs) vesicles according to their size. The multilayer liposomes are divided to oligolamellar (OLVs), multilamellar (MLVs), and multivesicular (MVVs) vesicles.45,46 The zeta potential is the electrostatic charge of the particle surface that prevents the proximity and aggregation of particles.47 Zeta potential can provide perception about circulation times, stability, circulation times, and biocompatibility of nanoparticles.48 Moreover, Zeta potential is important factor in the initial adsorption of liposome onto the surface of cells.49
Synthesis methods of liposomes
Up to now, many methods have been reported for the production of liposomes which can be divided to conventional and novel techniques. Conventional strategies include thin-film hydration (Figure S2),50 reverse phase evaporation (Figure S3),26 ethanol injection (Figure S4),51 ether injection (Figure S5, Supplementary file 1),52 electro-formation,53 and detergent depletion methods.54 These methods are easy to implement and do not require complicated equipment; however, scale-up for industrial manufacture and scale-down for point-of-care applications are challenging issues of them.19,55 In addition, limitations in process control, poor reproducibility, and inefficient use of materials and reagents are other significant problems.56 For overcoming these problems, some new methods have been developed for preparation of liposome.
Microfluidic methods
Microfluidic techniques refer to the strategies in which the procedures are performed in a small volume, typically in sub-millimeter scales and low Reynolds Numbers. By exploiting these microfluidic techniques the laboratory procedures can be performed in planar chips or other small devices result in reducing cost of chemical and biological experimentation.56,57 A number of microfluidic methods have been developed called modified Electro-formation,58 lipid hydration,59 micro hydrodynamic focusing,60 Pulsed jetting,61 double emulsion templates,62 lipid coated ice droplet hydration,63 transient membrane ejection56,64 modified droplet emulsion transfer65 either as modification of macro-scale techniques or as completely novel methods.
Supercritical fluid (SCF) based methods
Some new methods exploit SCF which is increasingly replacing organic solvents due to its ability for efficient separation and purification. There are several strategies for liposome preparation using SCF method. In a technique, a compressed mixture of the lipids, SCF and organic co-solvent is injected into the aqueous phase, and sprayed into water to form liposomes (injection method).
Whereas, in another approach, the compressed phase composed of lipid, SCF as well as aqueous phase is sprayed into air through a nozzle (decompression method). The size of the obtained vesicles is related to the rate of depressurization. It has been claimed that through these methods sterile, solvent free and pharmaceutical grade liposomes having a narrow particle size distribution can be produced. The incorporation of aqueous phase is the major difference between these approaches.19,54 In another method, supercritical reverse phase evaporation (scRPE), a mixture of lipid, organic co-solvent and compressed gas are put in a stirred, variable volume cell above the lipid phase transition temperature, and then an aqueous solution is slowly introduced to the cell. The liposomes are formed upon the pressure is reduced by the release of the compressed gas. The principle of the scRPE method is similar to the decompression method. However, in this method the depressurization occurs by the release of the dense gas from a variable volume cell.54,66 In another method called supercritical anti-solvent precipitation, the phospholipid dissolved in an organic co-solvent is sprayed into the SCF as an anti-solvent, resulting in formation of micronized particles. The size of the particles depends on the droplet size of the spray and the concentration of the lipid in the co-solvent. After hydration of the particles in an aqueous buffer the liposomes are formed. It was reported that increase in the pressure of the system or the SCF/co-solvent ratio causes the reduction in the fraction of small liposomes in the system.67,68 It has been claimed that the scaling-up of the SCF methods can be implemented with less problems.19
Other new methods
In the method called “freeze-drying double emulsions”, preparation of liposomes is accomplished by the lyophilization of double emulsions (W1/O/W2) containing disaccharides as lyoprotectants in both the inner and outer aqueous phase, by a two-step emulsification procedure at room temperature.69 “Membrane contactor” is a modified ethanol injection method in which phospholipid solution in alcohol was extruded through a membrane contactor into an aqueous solution and the liposomes are formed.70 In the method “hydration of deposited phospholipids on nanostructures” phospholipids are deposited on amphiphilic electrospun nanofibres composed of polyvinylpyrrolidone and soybean lecithin. The liposomes self-assembled upon addition of the nanofibers into water.71 In the other method namely “Curvature-tuning”, the phenomenon of spontaneous vesiculation and theory of curvature have been taken into consideration in solvent-free liposome preparation procedure. In this method, rapid pH change followed by a defined period of equilibration is exploited for the preparation of stable, monodisperse, and unilamellar liposomes. Further, by direct addition of the lipids into an aqueous buffer, there is no need to first preparation of MLVs suspension. The size, shape, and dispersity of the liposomes are affected by some critical factors such as time interval of pH increase, time of equilibration, temperature, and type of lipid.19,72,73
Large-scale techniques for liposome production
Application of liposomal formulation in industrial scale has two challenging issues including; poor capability of transferring from academic bench to highly regulated technology and stability of liposomes.74,75 Ethanol injection method is one of the interesting methods for scaling-up production of liposomes owing to reproducibility, fast implementation, and simplicity. Moreover, this technique did not cause oxidation and degradation of lipids. It has been reported that by use of this method, 0.5 to 12 kg of liposomes can be obtained from batches.76 Microfluidic isanother effective reproducible method for scale up of liposomes. This method has a high potential to achieve more control over the physical properties of the end product, especially in terms of size distribution, lamellarity, and high encapsulation efficiency.75,77,78
Modifications to conventional liposomes
Vesicles with simple structure including; cholesterol and phospholipid are named conventional liposomes or “first-generation liposomes”. These liposomes have some drawbacks like fast release of drug, rapid elimination from the blood, capture by the mononuclear phagocyte system, and low entrapment efficiency of water–soluble drugs.45,79 To overcome the mentioned deficiencies some new strategies have been developed in liposome preparations and novel generations of these vesicles have been emerged.30,80
Fusogenic liposomes (FLs)
Conventional liposomes are usually taken up into cells by phagocytosis or endocytosis and the main part of their content such as macromolecules might be degraded before reaching the cytoplasm.79,81 The induction of membrane fusion between liposomes and the cell membrane can overcome to this problem. FLs are nanocarriers which may fuse with biological membranes, thereby increasing drug contact and delivery into cells. FLs are composed of lipids, such as dioleoyl-phosphatidylethanolamine (DOPE) and cholesterylhemisuccinate (CHEMS), which cause increased fluidity in the lipid bilayer and can destabilize biological membranes.82 Due to their composition, the bilayer structure of FLs is efficiently fused with the cellular plasma membrane of cell to deliver the content of liposomes into the cytoplasm without degradation.79,83
One of the most interesting types of FLs is virosomes. These FLs are prepared by incorporation of conventional liposomes-based phospholipid with UV-disabled Sendai virus. The presence of the Sendai virus allows liposomes to rapidly and directly transfer their contents into the cells by membrane fusion. Therefore, these liposomes can be used as drug carriers for specific purposes.45,84,85
pH-sensitive liposomes
To date, various triggered releasemodels are widely researched and reported in order to increase the therapeutic index of pharmaceutical or other materials encapsulated within liposomes. Liposome composition can be modified to obtain triggered release in response to environmental conditions. The pH-sensitive liposomes are designed to control the release of their contents in response to acidic pH of the endosomal system. These liposomes have obviously improved the intracellular delivery of a variety of materials such as anti-cancer drugs, toxins, proteins, and DNA.86-88 The typical lipids used to prepare pH-sensitive liposomes are phosphatidylethanolamine (PE) and its derivatives including; diacetylenic phosphatidylethanolamine (DAPE), phosphatidylethanolamine (POPE) and DOPE. They are mixed with the compounds containing an acidic group that acts as a stabilizer at neutral pH. DOPE is usually combined with gently acidic amphiphiles such as oleic acid, CHEMS, and palmitoyl homocysteine.89,90 The most commonly used lipid combination is DOPE with CHEMS. Recently, a pH-responsive liposome has been prepared from 3ß-[N-(N’,N’-dimethylaminoethane)-carbamoyl]cholesterol hydrochloride (DC-liposome) for endosomal escape mediated drug delivery. Doxorubicin-loaded DC-liposome has exhibited higher cytotoxicity effect than free drug which supporting the endosomal escape of pH-responsive DC-liposome.91
The pH-sensitive liposomes are stable at neutral pH. In this condition, amphiphilic acid molecules cause the electrostatic repulsion between carboxylate and phosphate groups resulting in the formation of lamellar phases. However, an acidic medium (in pH less than the normal physiological value), either in endosomal vesicles or in the extracellular tumor environment, causes the protonation of the carboxylate groups triggering a transition from lamellar to hexagonal phase leading the release of loaded drugs.89,92 The surface of pH-sensitive liposomes can be coated by PEG to prolong the circulation time. Therefore, the liposomes are prevented from rapid clearance via the reticuloendothelial system (RES).93
Cationic liposomes
Cationic liposomes are vesicles that are constructed from positively charged lipids and have increasingly been used in gene therapy because of their interactions with negatively charged DNA.94-97 It is notable that the negatively charged genetic material is not encapsulated in liposomes but form complex with cationic empty liposomes by electrostatic interactions whereas total surface charge of DNA/liposome remains positive.81 DNA-cationic liposome complexes (lipoplexes) enter the cell by fusion with the plasma or endosome membrane. Conventional liposomes are negatively charged and may release their contents in the circulation and/or extracellularly after interaction with blood components and tissues due to their weak affinity for cell membrane. However, unlike these vesicles, cationic liposomes with positive charge are highly interactive with cells (with negatively charged biological membrane) and can deliver contents into cells by fusion with cell membranes. They are usually constructed from a neutral phospholipid (DOPE) and a positive derivative such as stearylamine, dimethyldioctadecylammonium bromide, dimethyl-aminoethane carbamoyl cholesterol (DC-chol), and Dioleoyl-3-trimethylammonium propane (DOTAP).98,99
Temperature-sensitive liposomes
The temperature-sensitive liposomes (TSLs) are vesicles that their content release behavior is controlled with temperature changes. TSLs rapidly release the loaded drug at few degrees above physiological temperature or hyperthermic conditions.100-103 The release of encapsulated hydrophilic drugs is related to the melting phase transition temperature (Tm) of the lipid bilayer; the temperature that the structure of the lipid bilayer changes from solid gel phase to liquid-crystalline phase. In the liquid-crystalline phase, the membrane of TSLs is more permeable to water and hydrophilic drugs than in the gel phase. In the most TSLs, the major component for liposome formulation is 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) with Tm of 41.4°C. To prevent the drug leakage at body temperature, DPPC can be mixed with small amounts of other phospholipids, such as 1, 2-distearoyl-sn-glycero-3-phosphocholine (DSPC; Tm = 54.9°C). The composition of the mixed phospholipids specifies the Tm of the formulation. Further, TSLs can be constructed by modification of conventional liposomes with thermosensitive polymers.104-106 TSLs in combination with local hyperthermia or high intensity focused ultrasound are concerned as effective route for external targeting of anti-cancer drugs to solid tumors.107,108
Stealth liposomes
Stealth liposomes (long circulating liposomes) namely “second-generation liposomes” are obtained by modifying the surface of the vesicles with an inert molecule.29 At first, liposomes with modified surfaces were developed using several molecules, such as glycolipids or sialic acids. However, with inclusion of the synthetic polymer polyethylene glycol (PEG) in the liposome composition, the long-circulating pegylated liposomes as a new generation were emerged. It has been proved that such surface modification extends blood-circulation time of liposomes and stabilizes these nanocarriers by minimizing their interaction with the RES.45,109 So far, this technology has been used to formulate a large number of liposomes containing various drugs or other biomolecules with high efficiency and activity. Moreover, by combining of the terminal PEG with appropriate compound, long-circulating liposomes can be synthesized to target on specified cells.109
Magnetoliposomes
Combination of liposomes and SPIONs or other magnetic nanoparticles,110,111 that called magnetoliposomes (MLs) is an interesting strategy creating vesicles with the potential for the application in controlled drug delivery systems and diagnostic imaging. They are promising nanocarriers for the development of the selective and site-specific drug delivery systems in the cancer therapy which can effectively deliver the drug towards tumor cells by applying a magnetic field.112,113 MLs are widely exploited as contrast agents in magnetic resonance imaging and as chemotherapeutic agents.114 There are three different approaches in associating the SPIONs to liposomes: (i) encapsulation of magnetic nanoparticles directly within the liposome lumen,115-117 (ii) embedding them in between the lipid bilayer,118-120 and (iii) directly conjugating magnetic nanoparticles to the liposome surface.121 The different types of MLs are depicted in Figure 2.
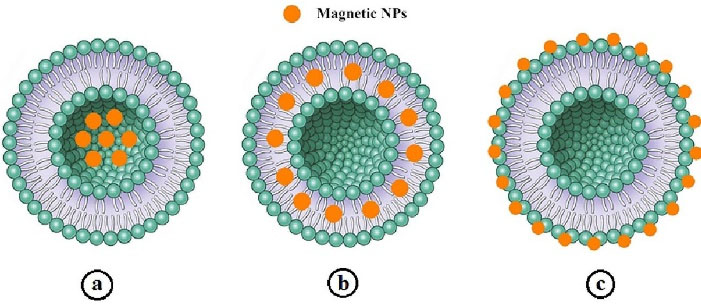
Figure 2.
Schematic illustration of three approaches in associating of SPIONs to the liposomes: a) Encapsulation directly within liposome lumen, b) embedding in between the lipid bilayer, and (c) directly conjugating to the liposome surface115
.
Schematic illustration of three approaches in associating of SPIONs to the liposomes: a) Encapsulation directly within liposome lumen, b) embedding in between the lipid bilayer, and (c) directly conjugating to the liposome surface115
Photosensitive liposomes
In the contrary to internal triggerable liposomes like pH-sensitive liposomes, external triggering system exploit the outside factors such as light, heat or magnetic field to release the liposomal cargo. On the other hand, the thermo-sensitive liposomes undergo phase transition of the phospholipids, while photo-triggerable liposomes are composed of a light-sensitive group engineered into the vesicle. Function of light-triggering liposome is based on two approaches: (i) photo-destabilization of liposome membrane to promote cargo release and (ii) light absorption of metal nanoparticles such as gold nanoparticles.122 Different photosensitive molecules can induce membrane destabilization and permeabilization. They can locate in the liposomal structure according to their intrinsic polarity. Phospholipid molecule modification can be performed in potential sites, namely, head group, glycerol backbone and fatty acyl chains.123-126 The various mechanisms for cargo release from photosensitive liposomes including; light-induced oxidation, photocrosslinking, photoisomerization, photocleavage, and photothermal release have been extensively reviewed by Miranda and Lovell.124
In case of incorporation of metal nanoparticles like GNPs in liposomal structure, they can localize within lipid bilayer, into the lumen, and on the surface of liposomes, aggregate with liposomes or be free in liposome solution (Figure 3). With irradiation of liposomes, GNPs convert the photo energy to thermal energy, inducing the instability of liposome membrane; therefore, the entrapped drug is released. Photo-responsive liposomes are powerful carriers for topical and transdermal drug delivery to superficial tissues like skin, eyes, and mucous membranes.16,127
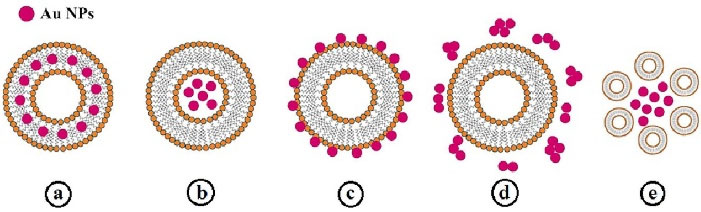
Figure 3.
Different types of GNPs incorporation in liposome structure: (a) in the lipid bilayer, (b) in the lumen, (c) on the surface of liposomes, (d) free in the liposome solution, and (e) aggregate with liposomes16
.
Different types of GNPs incorporation in liposome structure: (a) in the lipid bilayer, (b) in the lumen, (c) on the surface of liposomes, (d) free in the liposome solution, and (e) aggregate with liposomes16
Liposomes as targeting nanocarriers
Cancer therapy using liposomes can be accomplished through two main approaches including; passive and active targeting.
Passive targeting of liposomes
In passive targeting, the nanocarriers are transported into the tumor interstitium and cells through leaky tumor capillary fenestrations by convection or passive diffusion.128 In general, all nanoparticle-based drug delivery systems use the tumor characteristics for targeting. The angiogenesis phenomenon in tumor tissues causes the irregularity of endothelial cells with pore sizes of 100 nm to 2 μm. The different pore sizes between the endothelial cells of the tumor microvasculature and the tighter structures of normal cells causes the nanoparticles such as liposomes have better and more access to the cancerous sites. The “enhanced permeability and retention effect” (EPR) causes the increased drug delivery to the affected tissues with a much less return of the fluids to the lymphatic circulation.129,130 All nanocarriers benefit from the EPR effect in passive targeting so that of drug-loaded nanocarriers in tumor site are 10-50 folds higher than in normal tissue within 1-2 days.131 The nanocarriers must have at least three characteristics to exploit in passive drug delivery system: (i) The size of nanocarrier should be much less than 400 nm, and being in the range of 10-100 nm which is ideal for efficient extravasation to tumor site, (ii) having neutral or anionic charge for the nanocarriers is necessary to avoid the renal elimination, and (iii) the nanocarriers should be protected from the RES.128,131,132 The mechanism of passive targeting is illustrated in Figure 4. A number of successful results have been obtained from passive targeting property of liposomes in cancer therapy.133-138
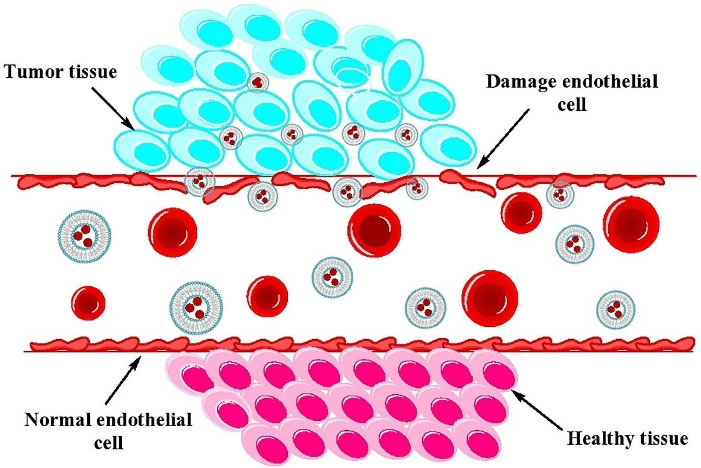
Figure 4.
Schematic illustration of the mechanism of passive targeting
.
Schematic illustration of the mechanism of passive targeting
Active targeting of liposomes
The reduction of drug toxicity and increase the therapeutic index can be implemented by the site-specific delivery. Nanocarriers can reach tumor microenvironment passively through the EPR effect, whereas the surface engineered nanomedicine acts through binding to the receptors over-expressed by cancer or angiogenic endothelial cells such as epidermal growth factor, fibroblast growth factor, folate, transferrin, and nuleolin receptors (Figure 5). Targeting these overexpressed receptors to increase the anti-cancer agents up taken by cancer cell as well as accumulation in cancer microenvironment is a vital approach.31 Surface modification of a variety of nanocarriers such as liposomes with antibodies specified for cancer cells, is a more common method. In addition to antibodies, the other molecules or biomaterials with the various strategies for the conjugation have been attached to PEGylated liposomes which enabling them to be actively taken up by the target cells via receptor-mediated endocytosis.128,139,140 Active targeting and efficient ligand receptor interaction are related to some factors such as the extent of expression of the receptor on tumor cells relative to non-target cells, availability of the receptor on the surface of target cells, the internalization rate, and heterogeneous expression of tumor receptor.141 Monoclonal antibodies, transferrin,1 folic acid,34 and aptamers142,143 have been frequently used for surface modification of nanocarriers such as liposomes.128,130,144,145 A schematic presentation of entrance of these ligands into the cell is shown in Figure 6. A number of researches have been performed in this area. Recently, functionalization of liposomal surface and targeting strategies in treatment of solid tumors are extensively investigated. Recently, mannosylated liposomes have been developed to encapsulate Chlorogenic acid as a targeted delivery system to tumor-associated macrophages (TAMs) for cancer immunotherapy. It has been reported that chlorogenic acid-loaded liposomes conjugated with mannose exhibited superior accumulation in tumors through the mannose receptor-mediated TAMs-targeting effects.146 Cancer cells overexpress α5β1, αvβ3 and αvβ5 integrins and it has been observed that the cyclic RGD (cRGD) can strongly attach to αvβ3 and αvβ5 integrins on cancer cells. cRGD-PEG liposomes loaded miR-34a have been developed for suppressing microRNA in breast cancer cells.147 In one study, it has been reported that tyrosine-modified irinotecan-loaded liposomes exhibited more cellular uptake in MCF-7 and BxPC-3 cells due to highly expressed ATB0,+ and LAT1 in cancer cells.148 In another study, the liposomes modified by glutamic hexapeptide and folic acid were designed for bone metastatic breast cancer. The results showed that paclitaxel loaded in co-modified liposomes presented high stability, more hydroxyapatite binding efficiency and also improved cytotoxic activity of the drug.149 Some of the more recent published works in this field are summarized in Table 2.
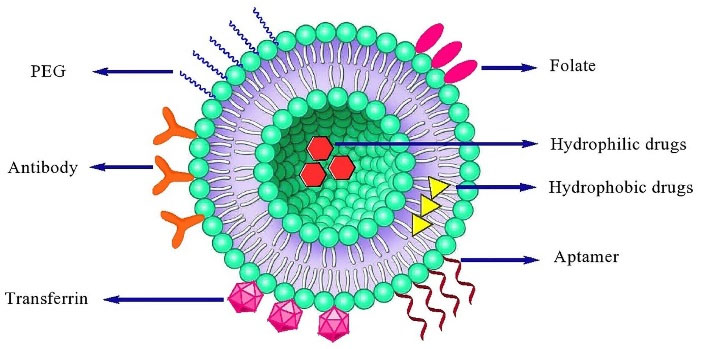
Figure 5.
A schematic presentation of various ligands used in liposome targeting
.
A schematic presentation of various ligands used in liposome targeting
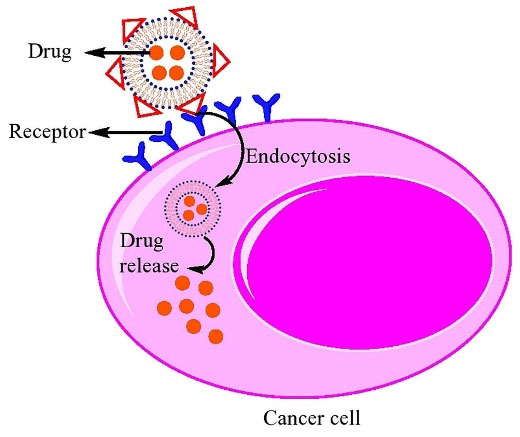
Figure 6.
Schematic representation of active targeting using surface engineered liposomes
.
Schematic representation of active targeting using surface engineered liposomes
Table 2.
Some recently published researches in active targeting of liposomes for cancer therapy
|
Targeting ligand
|
Drug
|
Liposome type
|
Preparation method
|
Loading method
|
Cancer treated
|
Ref.
|
| Monoclonal antibodies |
Glycosylated paclitaxel |
Immunoliposomes |
Thin Film |
Passive |
Ovarian |
150
|
| Monoclonal antibodies |
Curcumin |
Cationic liposome |
Thin Film |
Passive |
Breast |
151
|
| Monoclonal antibodies |
Doxorubicin |
PEG-liposome |
Ethanol injection |
Passive |
Breast |
152
|
| Monoclonal antibodies |
Doxorubicin |
PEG-liposome |
Thin Film |
Passive |
Breast |
153
|
| Folate |
Oleuropein |
PEG-liposome |
Thin Film |
Passive |
Prostate |
154
|
| Folate |
Gold nanorods and doxorubicin |
PEG-liposome |
Thin Film |
Passive |
Breast |
155
|
| Folate |
Rapamycin |
PEG-liposome |
Thin Film |
Passive |
Bladder |
156
|
| Folate |
Arsenic trioxide |
PEG-liposome |
Thin Film |
Active |
Cervical |
157
|
| Transferrin |
Doxorubicin
and Sorafenib tosylate |
PEG-liposome |
Thin Film |
Passive |
Breast |
158
|
| Transferrin |
Doxorubicin |
Cationic liposome |
Ethanol injection |
Passive |
Glioma |
159
|
| Transferrin |
Plumbagin |
PEG-liposome |
Thin Film |
Passive |
Carcinoma, glioblastoma |
160
|
| Transferrin |
Resveratrol |
PEG-liposome |
Thin Film |
Passive |
Glioblastoma |
161
|
| Aptamer |
A-particle
generator 225Ac |
PEG-liposome |
Thin Film |
Passive |
Prostate |
162
|
| Aptamer |
All-trans retinoic acid |
PEG-liposome |
Thin Film |
Passive |
Bone |
163
|
| Aptamer |
MiR-139-5p |
Cationic Liposome |
Thin Film |
Passive |
Colorectal |
164
|
| Aptamer |
Paclitaxel and siRNA |
Cationic Liposome |
Thin Film |
Passive |
Breast |
165
|
| Mannose |
chlorogenic acid |
PEG-liposome |
Thin Film |
- |
glioblastoma |
146
|
| RGD |
microRNA |
PEG liposomes |
Thin Film |
- |
Breast |
147
|
| Amino acid |
irinotecan |
Liposome |
Thin Film |
- |
Breast |
148
|
| Glutamic hexapeptide and folic acid |
paclitaxel |
liposome |
Thin Film |
- |
Bone metastatic breast cancer |
149
|
Methods for encapsulating materials into liposomes
Various methods of liposome loading largely depend on the physicochemical characteristics of the loaded agents. In general, the encapsulation strategies are divided in two categories: passive and active loading.166,167
Passive loading
In the all techniques where the lipids and encapsulating agents are introduced in an aqueous buffer solution and the entrapment is achieved while the liposomes are being formed, a passive trapping is occurred. Passive loading of pharmaceutical agents into the liposomes are implemented by two ways including (i) entrapment in liposomal membrane (bilayer) by hydrophobic interaction, electrostatic interaction or combination of these two mechanisms, or (ii) entrapment of hydrophilic substances such as salts (ionic compounds), amino acids, antibiotics, and proteins in intra-liposome aqueous phase (shown in Figure 1). However, the lipophilic substances are added in the organic solvent containing lipids, before formation of liposomes.168 In both routes, encapsulation is occurred simultaneously with the lipid self-assembly and liposome formation.169 In this method the loading efficiency, and therefore, drug/lipid ratio (D/L) is usually low (10-50%). However, some additional procedures such as freeze-thaw and dehydration-rehydration can provide higher encapsulation efficiencies. Besides, encapsulation efficiency depends on some factors such as the lipid amount and concentration, and the solubility of the entrapping agent in aqueous phase.166,170-172
Active loading
Passive encapsulation method presents some disadvantages including; low entrapment efficiency (20-30%), non-loaded drug loss, organic solvent impurities, and fast release of drug. Hydrophilic small molecules are usually passively loaded during the phospholipid self-assembly. However, amphiphilic substances can be actively loaded into liposomes after liposome formation without diffusing back out. In active encapsulation, the molecules cross through the lipid bilayer into the internal aqueous compartment of liposomes, and cannot diffuse back out or return into the external aqueous solution. The active loading methods are typically based on two phenomena: (i) a given lipophilic molecule diffuse the lipid bilayer and gain a charge upon entering the liposomal core and (ii) the molecule as an ion cannot be able to cross the bilayer and accumulation of the encapsulated agent is achieved.167,173 Some frequently used active drug loading methods are summarized in the following sections.
The pH gradient method
In this method, penetration of the drug into the preformed liposomes is driven by a transmembrane pH gradient. In order to achieve the efficient active loading, the aqueous solubility of the encapsulating drug and the presence of ionizable functional groups in its structure (e.g. amine group in weak bases) are necessary.174 In the pH of the extra-liposome aqueous phase the drug exists in the unionized form and is able to migrate across the liposome bilayer. Upon translocation into the liposomal core, the drug changes to ionized form due to the differing pH and retained there. Thus, for amphiphilic drugs which are weak bases or acids, a pH gradient can be the driving force to translocate and retain in liposomes. A pH gradient of 3 units can cause a 1000-fold higher concentration of a substance within the liposome core in comparison to the external aqueous phase.167,173-175
Citrate method
In this approach pH of intra-liposomal core is 4 owing to the presence of citrate buffer and extra-liposome aqueous phase has a pH equal to 7.4 which is adjusted with HEPES buffer.176 Therefore, a proton gradient is observed when substances such as biogenic amines and anti-cancer doxorubicin are present in extra-liposomal aqueous solution. In the presence of HEPES buffer (pH 7.4), the compounds containing the amine groups are in the neutral form and therefore, are able to cross the lipid bilayer. By entering the amines inside the liposome, they produce low-soluble citrate salts due to the presence of citrate ions. This method has been used for remote loading of anthracycline into the liposomes with coffee bean liposome appearance.177,178 Citrate method was successfully exploited in commercially manufacturing of doxorubicin and daunorubicin products namely Myocet and DaunoXome, respectively.173
Ammonium sulfate gradient method
The remote loading strategy that called transmembrane ammonium sulfate gradient method was introduced by Haran et al. for the encapsulation of amines.179 In this method, there is no need to prepare the liposomes in the acidic pH and alkalinize of extra-liposomal aqueous phase.180 Ammonium ion gradient is generated via its counter-ion sulfate which stabilizes anthracyclines by aggregation and gelation as anthracycline sulfate salt. Firstly, the empty liposomes are formed in ammonium sulfate solution using thin-layer method. Then, the liposomes are dialyzed in PBS solution to form ammonium sulfate gradient inside and outside of liposomes. After the formation of ammonium sulfate gradient, remote encapsulation is carried out by incubation of liposomes with drug solution. The neutral ammonia molecules (permeability coefficient 0.13 cm/s) are diffused towards extra-liposomal solution and left behind a proton due to the higher concentration of ammonium ions in aqueous core of liposome; therefore, the pH gradient is formed. The drug including; amine group in its neutral form (in pH 7.4 buffer) penetrates the bilayer and precipitation of the drug as sulfate salt is occurred. Doxil as the first commercially available long circulating liposomal doxorubicin was produced using ammonium sulfate gradient method.173,179-181
Calcium acetate method
The transmembrane calcium acetate method is based on different permeability coefficients of acetic acid and calcium ions. In this technique, the blank liposomes are prepared in calcium acetate solution. Whereas the calcium ions remain in the liposomal aqueous core, the acetic acid molecules act as proton shuttles. This generates a pH gradient, with higher pH value inside the liposomes, results in entrapping the weak amphiphilic acid molecules inside the liposomes in a way similar to that of weak amphiphilic bases.173,181,182 In the another method, similar to the aforementioned strategy, arsenic trioxide as an anti-cancer agent has been actively loaded into the liposomes that contain acetate salts of bivalent cations such as Co(II), Ni(II), Cu(II), and Zn(II). The external neutral As (OH)3 penetrates across the bilayer and forms the low-soluble heavy metal- arsenite complex in the liposomal core. On the other hand, the released protons associate with the acetate anions produce the weak acid (HOAC) which diffuses out of the liposome. Both phenomena: the formation of insoluble nickel (II) arsenite compound and the diffusion of the acetic acid out of the liposome drive drug uptake.183
Ionophore-mediated method
This protocol involved the use of ionophore agents such as antibiotic nigericin or A23187 which mediate the exchange of K + and H + across the liposomal bilayer generating pH gradient of about 2 units. When, loaded liposomes with K2SO4 are placed in the K + -free aqueous phase containing an ionophore, the pH of the intra-liposomal aqueous solution decreases due to the release of potassium and the entry of protons.184 This pH decrease results in the active encapsulation of weak bases. It is notable that nigericin cause a one-for-one exchange of K + for H +, whereas A23187 makes it possible to move two protons per every divalent metal cation such as Ca2 +, Mn2 +, or Mg2 +. In the use of divalent cations, the presence of EDTA as external chelator is required to bind with the released cations and complete the uptake process as well as prevent aggregation of liposomes.173,185
EDTA gradient method
It has been reported that EDTA can form low soluble precipitates with anthracyclines or other weak bases and the formation of low soluble EDTA-drug complexes inside of liposomes can lead to increased drug encapsulation and retention. The protocol is especially suggested for the encapsulation of idarubicin because of its very low solubility in EDTA solutions at acidic pHs. In this case, the liposomes are formed via hydration of the lipid film with EDTA disodium salt or EDTA di-ammonium salt solution and then, idarubicin hydrochloride solution is added to the liposomal suspension. The accumulation of idarubicin in liposomes is pH-dependent, so that in higher external pH (8.5) and lower internal pH (4) the drug is better accumulated and higher encapsulation efficiency is achieved.173,186
Phosphate gradient method
The main concept in transmembrane phosphate gradient strategy is the same as in the case of other pH gradient methods. In this case, the liposomes are prepared in (NH4)2HPO4 solution. It is reported that a near 100% doxorubicin accumulation inside the liposomes via both protonation and precipitation of drug have been observed as in the case of other gradients. Besides, in pHs close to physiological level no drug leakage is observed from the liposomes. However, in acidic extra-liposomal medium accelerated drug leakage is achieved. It was suggested that doxorubicin can be retained in the hydrophilic liposomal core by protonation and precipitation, or incorporated in the bilayer. This remote loading process depends on various factors such as intra-liposomal salt concentration and pH value.173,187
Solvent-assisted active loading method
In all the aforementioned remote loading methods the compounds with high solubility and membrane permeability are solubilized in the exterior aqueous phase and penetrate through the liposome bilayer into the internal aqueous phase. However, a large number of drugs have low-water solubility and are passively encapsulated in the lipid bilayer of liposomes. The solvent-assisted active loading technology (SALT) has been used for remote encapsulating of poorly soluble drugs in the liposomal core in order to achieve the better loading efficiency and formulation stability. In this technique, a small volume (~5 v/v %) of a water miscible solvent is added to the loading solution for complete dissolution of the compound. Then, the dissolved compound diffuses into the internal compartment of liposome and interacts with a precipitating agent to form low-soluble precipitate. The solvent can be eliminated using dialysis or gel filtration techniques. It is reported that a rapid and complete encapsulation with high D/L ratio and improved circulation half-life are achieved by exploiting this method.188
Medical applications of liposomes
Liposomes can be used in the various clinical fields including; therapeutic systems, medical imaging, and cosmetic products which have been summarized below13:
Therapeutic systems
Liposomes are used in many fields of medical treatment such as cancer therapy, anti-infective therapy, protein or peptide drug delivery, gene delivery, macrophage activation, and vaccination.13,15,27,45 The first application of liposomes as drug delivery system was the delivery of chemotherapeutic small molecules.15 A large number of drugs are formulated into the liposomes taking advantage of high therapeutic efficiency and low systemic toxicity compared with the free drug. Anti-infective drugs and anti-tumor drugs are two major classes of small molecules that can be loaded in liposomal formulations. After approval of liposomal systems for delivery of small molecules, delivery of macromolecules such as nucleic acid-based therapeutics (gene therapy agents) was noticed. Nucleic acid-based materials with high-molecular weight and highly charged molecules cannot cross cell membranes by passive diffusion. Moreover, applications of these materials as therapeutic agents are limited by their rapid enzymatic degradation, low selectivity, and poor cellular uptake. Lipoplex is a complex between negatively charged nucleic acid-based material and cationic liposome which can enter the cell via fusion with the plasma membrane. Thereby, gene therapy is performed through these liposomal formulations. Protein or peptide based therapeutic agents including; enzymes, peptide hormones, and cytokines are the other class of drugs that can be encapsulated in liposomes.189,190 Incorporation of these agents into the liposomes resulted in some advantages such as improving therapeutic activity of protein and peptide drugs, reducing their side effects and modulating the immune response towards these proteins and peptides.45
Diagnostic imaging
Apart from the method used, in diagnostic imaging the appropriate intensity of signal from an area of interest is needed to differentiate specified structures from surrounding tissues. On the other hand, molecular imaging has an important role in diagnosis and treatment tracing of diseases. Liposomes can be targeted to specified disease tissues by combining with specific targeting ligands and imaging molecular probes. These probes are loaded with liposomes in four ways: (i) incorporating into the liposome during its formation, (ii) penetration into the lipid bilayer of preformed liposome, (iii) encapsulation into the preformed liposome via various active methods, and (iv) attaching on the surface of preformed liposome.45,191,192
Cosmetics
Liposomes have been considered in the delivery of ingredients in cosmetics due to their unique physicochemical properties. Incorporation of liposomes in cosmetic formulations has shown some advantages such as increasing in skin moisture, improving the cell membrane fluidity, and causing deep penetration of oil or water-soluble cosmetic ingredients through the skin. Liposomal cosmetics have been manufactured by famous company namely Christian Dior, for first time in 1987. After that, some other liposomal cosmetic formulations are reported and manufactured.13,45
Future prospective of liposomal formulations
Today, many approved liposomal and nano-liposomal products entered the commercial market. Some of these products are presented in Table 3.193-196 However, there are huge challenges in their clinical translation. It seems that the most important field of research ahead is related to solving problems related to the targeted liposomal formulations. Many of these nanocarriers have shown more efficiency in animal and in vitro studies; however; a few of them have been entered to clinical trials, and there is not any investigationand evaluation guideline for active targeting liposomal formulations in cancer treatment.20 To achieve the successful clinical use of these formulations, many problems have to be solved. It has been shown that a number of factors such as liposome size and charge, type and amount of ligand, the ligand binding with the serum proteins, and the elimination by the body immune system can influence on the function of targeted liposomes.197-202 Owing to the excellent clinical potential of targeted liposomes to improve the therapeutic index of drugs, further research is required for their clinical applications.
Table 3.
List of some approved liposomal drugs
|
Trade name
|
Chemical name
|
Clinical uses
|
Ref.
|
| Ambisome |
Amphotericin B |
Sever fungal infections |
26
|
| DaunoXome |
Daunorubicin |
Kaposi's sarcoma |
26
|
| Myocet |
Doxorubicin |
Breast cancer |
13
|
| DepoDur |
Morphine |
Pain following surgery |
13
|
| DepoCyt |
Cytarabine |
Neoplastic meningitis, lymphomatous meningitis |
13
|
| Visudyne |
Vertporfin |
Macular degeneration, pathologic myopia, ocular histoplasmosis |
170
|
| Abelcet |
Amphotericin B |
Sever fungal infections |
26
|
| Amphotec |
Amphotericin B |
Sever fungal infections |
26
|
| Doxil |
Doxorubicin |
Kaposi's sarcoma, ovarian and breast cancer |
24
|
| Onivyde |
Irinotecan |
Pancreatic cancer |
13
|
| ELA-Max |
Lidocaine |
Skin diseases |
144
|
| Newcastle |
Disease vaccine |
Chicken |
144
|
| Epaxal |
A vaccine |
Hepatitis |
144
|
| Lipodox |
Doxorubicin |
Anti-cancer |
170
|
| Marqibo |
Vincristine |
Acute lymphoblastic leukemia |
13
|
| EvacetTM |
Doxorubicin |
Metastatic breast cancer |
203
|
| Avian retrovirus vaccine |
Killed avian retrovirus |
Chicken pox |
203
|
| Novasome® |
Smallpox vaccine |
Smallpox |
203
|
| Topex-Br |
Terbutaline sulphate |
Asthma |
203
|
| Alectm |
Dry protein free powder of DPPC-PG |
Expanding lung diseases in babies |
203
|
| Ventustm |
Prostaglandin-E1 |
Systemic inflammatory diseases |
203
|
| Fungizone® |
Amphotericin-B |
Fungal infections, Leishmaniasis |
203
|
| VincaXome |
Vincristine |
Solid Tumors |
204
|
| Autragen |
Tretinoin |
Kaposi’s sarcoma |
204
|
| Shigella Flexneri 2A vaccine |
Shigella flexneri 2A |
Shigella flexneri 2A infections |
204
|
| Nyotran |
Nystatin |
Systemic fungal infections |
204
|
Conclusion
Liposomes have been recognized as therapeutic carriers in very diverse clinical fields because of their unique physicochemical properties. They are the first nano-delivery systems that some of them are already successfully translated into the clinical use, and some liposomal formulations are approved or under clinical trials. Employing of liposomes as drug delivery systems provide a platform for delivering of drugs with reducing side effects and increasing their efficacy, solubility, and bioavailability. Despite the improvements made to these carriers to reduce their adverse effects and increase the therapeutic index of the cargo, investigations to fabricate the liposomes with fewer deficiencies are ongoing. A number of synthesis methods have been developed to obtain liposomes with various structure, size, and polydispersity. Ethanol injection technique is one of the interesting methods for scaling-up production of liposomes due to simplicity, fast implementation, and reproducibility. To overcome some practical challenges such as precise process control, poor reproducibility, and inefficient use of materials and reagents novel strategies such as microfluidic and SCF based methods have been designed for preparation of liposomes. Microfluidic iseffective reproducible method for scale-up of liposomes owing to achieve more control over the physical properties including; size distribution, lamellarity, and high encapsulation efficiency. Moreover, solvent free and pharmaceutical grade liposomes having a narrow particle size distribution can be produced by SCF method. Fabrication of the PEGylated liposomes is the important modification to solve the problem of uptake by RES and rapid clearance from bloodstream. However, the selective delivery of these liposomes to the action site is limited. Today, research in fabrication of stimuli-sensitive and functionalized liposomes are two forefront fields to increase of their target specificity. Besides, to overcome to the passive loading drawbacks such as low entrapment efficiency, non-loaded drug loss, and fast release of drug, several active loading methods have been developed. Furthermore, in order to enhance internalization of liposomes by the specified tissues, their surfaces can be modified with targeting ligands such as transferrin, integrins, polysaccharides, folic acid, aptamers and antibodies. However surely, liposomes have found their place in the modern pharmaceutics and their use is increasing day by day. Nowadays, many liposomal anti-cancer drugs have been used in the treatment of breast, ovarian cancers, and sarcoma. Due to the potential clinical applications of liposomes, challenges such as therapeutic and loading efficiency, stability, and scale up of industrial production with more clinical success, needs further investigation.
Acknowledgments
This work was supported by Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Author Contributions
Conceptualization: Hanieh Abbasi, Maryam Kouchak, Zohreh Mirveis, Fatemeh Hajipour, Mohsen Khodarahmi.
Formal Analysis: Nadereh Rahbar, Hanieh Abbasi.
Investigation: Hanieh AbbasiD, Maryam Kouchak, Zohreh Mirveis, Fatemeh Hajipour, Mohsen Khodarahmi.
Methodology: Nadereh Rahbar, Hanieh Abbasi.
Project administration: Nadereh Rahbar, Somayeh Handali.
Software: Nadereh Rahbar, Hanieh Abbasi.
Supervision: Nadereh Rahbar, Somayeh Handali.
Validation: Nadereh Rahbar, Hanieh Abbasi.
Visualization: Nadereh Rahbar, Somayeh Handali.
Writing – original draft: Hanieh Abbasi. Nadereh Rahbar.
Writing – review & editing: Nadereh Rahbar, Somayeh Handali.
Ethical Issues
Not applicable.
Conflict of Interest
The authors declare that they have no competing interests.
Supplementary Files
Supplementary file 1 contains Figures S1-S5.
(pdf)
References
- Moghimipour E, Rezaei M, Kouchak M, Ramezani Z, Amini M, Ahmadi Angali K. A mechanistic study of the effect of transferrin conjugation on cytotoxicity of targeted liposomes. J Microencapsul 2018; 35(6):548-58. doi: 10.1080/02652048.2018.1547325 [Crossref] [ Google Scholar]
- Singh R, Lillard JW Jr. Nanoparticle-based targeted drug delivery. Exp Mol Pathol 2009; 86(3):215-23. doi: 10.1016/j.yexmp.2008.12.004 [Crossref] [ Google Scholar]
- Wu PT, Lin CL, Lin CW, Chang NC, Tsai WB, Yu J. Methylene-blue-encapsulated liposomes as photodynamic therapy nano agents for breast cancer cells. Nanomaterials (Basel) 2018; 9(1):14. doi: 10.3390/nano9010014 [Crossref] [ Google Scholar]
- Letchford K, Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm 2007; 65(3):259-69. doi: 10.1016/j.ejpb.2006.11.009 [Crossref] [ Google Scholar]
- Lee CC, MacKay JA, Fréchet JM, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol 2005; 23(12):1517-26. doi: 10.1038/nbt1171 [Crossref] [ Google Scholar]
- Bosman AW, Janssen HM, Meijer EW. About dendrimers: structure, physical properties, and applications. Chem Rev 1999; 99(7):1665-88. doi: 10.1021/cr970069y [Crossref] [ Google Scholar]
- Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev 2011; 63(1-2):24-46. doi: 10.1016/j.addr.2010.05.006 [Crossref] [ Google Scholar]
- Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater 2012; 24(12):1504-34. doi: 10.1002/adma.201104763 [Crossref] [ Google Scholar]
- Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev 2008; 60(11):1307-15. doi: 10.1016/j.addr.2008.03.016 [Crossref] [ Google Scholar]
- Juzenas P, Chen W, Sun YP, Coelho MA, Generalov R, Generalova N. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv Drug Deliv Rev 2008; 60(15):1600-14. doi: 10.1016/j.addr.2008.08.004 [Crossref] [ Google Scholar]
- Belin T, Epron F. Characterization methods of carbon nanotubes: a review. Mater Sci Eng B 2005; 119(2):105-18. doi: 10.1016/j.mseb.2005.02.046 [Crossref] [ Google Scholar]
- Hossen S, Hossain MK, Basher MK, Mia MNH, Rahman MT, Uddin MJ. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: a review. J Adv Res 2019; 15:1-18. doi: 10.1016/j.jare.2018.06.005 [Crossref] [ Google Scholar]
- Li M, Du C, Guo N, Teng Y, Meng X, Sun H. Composition design and medical application of liposomes. Eur J Med Chem 2019; 164:640-53. doi: 10.1016/j.ejmech.2019.01.007 [Crossref] [ Google Scholar]
- Sousa I, Rodrigues F, Prazeres H, Lima RT, Soares P. Liposomal therapies in oncology: does one size fit all?. Cancer Chemother Pharmacol 2018; 82(5):741-55. doi: 10.1007/s00280-018-3668-7 [Crossref] [ Google Scholar]
- Abu Lila AS, Ishida T. Liposomal delivery systems: design optimization and current applications. Biol Pharm Bull 2017; 40(1):1-10. doi: 10.1248/bpb.b16-00624 [Crossref] [ Google Scholar]
- Mathiyazhakan M, Wiraja C, Xu C. A concise review of gold nanoparticles-based photo-responsive liposomes for controlled drug delivery. Nanomicro Lett 2018; 10(1):10. doi: 10.1007/s40820-017-0166-0 [Crossref] [ Google Scholar]
- Anwekar H, Patel S, Singhai AK. Liposome-as drug carriers. Int J Pharm Life Sci 2011; 2(7):945-51. [ Google Scholar]
- Verma P, Ram A, Jha AK, Mishra A, Thakur A. Phosphatidylcholine: a revolution in drug delivery technology. Int J Pharm Sci Res 2010; 1(2):1-12. [ Google Scholar]
- Patil YP, Jadhav S. Novel methods for liposome preparation. Chem Phys Lipids 2014; 177:8-18. doi: 10.1016/j.chemphyslip.2013.10.011 [Crossref] [ Google Scholar]
- Yan W, Leung SS, To KK. Updates on the use of liposomes for active tumor targeting in cancer therapy. Nanomedicine (Lond) 2020; 15(3):303-18. doi: 10.2217/nnm-2019-0308 [Crossref] [ Google Scholar]
- Fenske DB, Cullis PR. Liposomal nanomedicines. Expert Opin Drug Deliv 2008; 5(1):25-44. doi: 10.1517/17425247.5.1.25 [Crossref] [ Google Scholar]
- Xu X, Ho W, Zhang X, Bertrand N, Farokhzad O. Cancer nanomedicine: from targeted delivery to combination therapy. Trends Mol Med 2015; 21(4):223-32. doi: 10.1016/j.molmed.2015.01.001 [Crossref] [ Google Scholar]
- He H, Lu Y, Qi J, Zhu Q, Chen Z, Wu W. Adapting liposomes for oral drug delivery. Acta Pharm Sin B 2019; 9(1):36-48. doi: 10.1016/j.apsb.2018.06.005 [Crossref] [ Google Scholar]
- Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol 2015; 6:286. doi: 10.3389/fphar.2015.00286 [Crossref] [ Google Scholar]
- Belfiore L, Saunders DN, Ranson M, Thurecht KJ, Storm G, Vine KL. Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: challenges and opportunities. J Control Release 2018; 277:1-13. doi: 10.1016/j.jconrel.2018.02.040 [Crossref] [ Google Scholar]
- Dua JS, Rana AC, Bhandari AK. Liposome: methods of preparation and applications. Int J Pharm Stud Res 2012; 3(2):14-20. [ Google Scholar]
- Daraee H, Etemadi A, Kouhi M, Alimirzalu S, Akbarzadeh A. Application of liposomes in medicine and drug delivery. Artif Cells NanomedBiotechnol 2016; 44(1):381-91. doi: 10.3109/21691401.2014.953633 [Crossref] [ Google Scholar]
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y. Liposome: classification, preparation, and applications. Nanoscale Res Lett 2013; 8(1):102. doi: 10.1186/1556-276x-8-102 [Crossref] [ Google Scholar]
- Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine 2006; 1(3):297-315. [ Google Scholar]
- Arias JL. Liposomes in drug delivery: a patent review (2007-present). Expert OpinTher Pat 2013; 23(11):1399-414. doi: 10.1517/13543776.2013.828035 [Crossref] [ Google Scholar]
- Riaz MK, Riaz MA, Zhang X, Lin C, Wong KH, Chen X. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: a review. Int J Mol Sci 2018; 19(1):195. doi: 10.3390/ijms19010195 [Crossref] [ Google Scholar]
- Kirby C, Clarke J, Gregoriadis G. Effect of the cholesterol content of small unilamellar liposomes on their stability in vivo and in vitro. Biochem J 1980; 186(2):591-8. doi: 10.1042/bj1860591 [Crossref] [ Google Scholar]
- Handali S, Moghimipour E, Kouchak M, Ramezani Z, Amini M, Angali KA. New folate receptor targeted nano liposomes for delivery of 5-fluorouracil to cancer cells: strong implication for enhanced potency and safety. Life Sci 2019; 227:39-50. doi: 10.1016/j.lfs.2019.04.030 [Crossref] [ Google Scholar]
- Moghimipour E, Rezaei M, Ramezani Z, Kouchak M, Amini M, Angali KA. Folic acid-modified liposomal drug delivery strategy for tumor targeting of 5-fluorouracil. Eur J Pharm Sci 2018; 114:166-74. doi: 10.1016/j.ejps.2017.12.011 [Crossref] [ Google Scholar]
- Handali S, Moghimipour E, Rezaei M, Ramezani Z, Kouchak M, Amini M. A novel 5-Fluorouracil targeted delivery to colon cancer using folic acid conjugated liposomes. Biomed Pharmacother 2018; 108:1259-73. doi: 10.1016/j.biopha.2018.09.128 [Crossref] [ Google Scholar]
- Sułkowski WW, Pentak D, Nowak K, Sułkowska A. The influence of temperature, cholesterol content and pH on liposome stability. J Mol Struct 2005; 744-747:737-47. doi: 10.1016/j.molstruc.2004.11.075 [Crossref] [ Google Scholar]
- Ichihara K, Iwasaki H, Ueda K, Takizawa R, Naito H, Tomosugi M. Synthesis of phosphatidylcholine: an improved method without using the cadmium chloride complex of sn-glycero-3-phosphocholine. Chem Phys Lipids 2005; 137(1-2):94-9. doi: 10.1016/j.chemphyslip.2005.06.001 [Crossref] [ Google Scholar]
- Bouchet AM, Frías MA, Lairion F, Martini F, Almaleck H, Gordillo G. Structural and dynamical surface properties of phosphatidylethanolamine containing membranes. BiochimBiophys Acta 2009; 1788(5):918-25. doi: 10.1016/j.bbamem.2009.02.012 [Crossref] [ Google Scholar]
- Allen C, Dos Santos N, Gallagher R, Chiu GN, Shu Y, Li WM. Controlling the physical behavior and biological performance of liposome formulations through use of surface grafted poly(ethylene glycol). Biosci Rep 2002; 22(2):225-50. doi: 10.1023/a:1020186505848 [Crossref] [ Google Scholar]
- Garigapati VR, Roberts MF. Synthesis of short chain phosphatidylinositols. Tetrahedron Lett 1993; 34(5):769-72. doi: 10.1016/0040-4039(93)89007-d [Crossref] [ Google Scholar]
- Demel RA, Paltauf F, Hauser H. Monolayer characteristics and thermal behavior of natural and synthetic phosphatidylserines. Biochemistry 1987; 26(26):8659-65. doi: 10.1021/bi00400a025 [Crossref] [ Google Scholar]
- Wang X, Devaiah SP, Zhang W, Welti R. Signaling functions of phosphatidic acid. Prog Lipid Res 2006; 45(3):250-78. doi: 10.1016/j.plipres.2006.01.005 [Crossref] [ Google Scholar]
- Kiyasu JY, Pieringer RA, Paulus H, Kennedy EP. The biosynthesis of phosphatidylglycerol. J Biol Chem 1963; 238:2293-8. [ Google Scholar]
- Schlame M, Greenberg ML. Cardiolipin synthase from yeast. BiochimBiophys Acta 1997; 1348(1-2):201-6. doi: 10.1016/s0005-2760(97)00117-3 [Crossref] [ Google Scholar]
- Tripathi G, Chaurasiya K, Katare P. Liposomal current status, evaluation and recent advances. Int J Curr Pharm Res 2013; 5:4-14. [ Google Scholar]
- Ashara KC, Paun JS, Soniwala MM, Chavda JR, Nathawani SV, Mori NM. Vesicular drug delivery system: a novel approach. Mintage J Pharm Med Sci 2014; 3(Suppl 3):1-14. [ Google Scholar]
- Lowry GV, Hill RJ, Harper S, Rawle AF, Hendren CO, Klaessig F. Guidance to improve the scientific value of zeta-potential measurements in nanoEHS. Environ Sci Nano 2016; 3(5):953-65. doi: 10.1039/c6en00136j [Crossref] [ Google Scholar]
- Smith MC, Crist RM, Clogston JD, McNeil SE. Zeta potential: a case study of cationic, anionic, and neutral liposomes. Anal Bioanal Chem 2017; 409(24):5779-87. doi: 10.1007/s00216-017-0527-z [Crossref] [ Google Scholar]
- Rasmussen MK, Pedersen JN, Marie R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat Commun 2020; 11(1):2337. doi: 10.1038/s41467-020-15889-3 [Crossref] [ Google Scholar]
- Samad A, Sultana Y, Aqil M. Liposomal drug delivery systems: an update review. Curr Drug Deliv 2007; 4(4):297-305. doi: 10.2174/156720107782151269 [Crossref] [ Google Scholar]
- Jaafar-Maalej C, Diab R, Andrieu V, Elaissari A, Fessi H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J Liposome Res 2010; 20(3):228-43. doi: 10.3109/08982100903347923 [Crossref] [ Google Scholar]
- Deamer D, Bangham AD. Large volume liposomes by an ether vaporization method. BiochimBiophys Acta 1976; 443(3):629-34. doi: 10.1016/0005-2736(76)90483-1 [Crossref] [ Google Scholar]
- Rodriguez N, Pincet F, Cribier S. Giant vesicles formed by gentle hydration and electroformation: a comparison by fluorescence microscopy. Colloids Surf B Biointerfaces 2005; 42(2):125-30. doi: 10.1016/j.colsurfb.2005.01.010 [Crossref] [ Google Scholar]
- Meure LA, Foster NR, Dehghani F. Conventional and dense gas techniques for the production of liposomes: a review. AAPS PharmSciTech 2008; 9(3):798-809. doi: 10.1208/s12249-008-9097-x [Crossref] [ Google Scholar]
- Mozafari MR. Liposomes: an overview of manufacturing techniques. Cell Mol Biol Lett 2005; 10(4):711-9. [ Google Scholar]
- van Swaay D, deMello A. Microfluidic methods for forming liposomes. Lab Chip 2013; 13(5):752-67. doi: 10.1039/c2lc41121k [Crossref] [ Google Scholar]
- Lou G, Anderluzzi G, Woods S, Roberts CW, Perrie Y. A novel microfluidic-based approach to formulate size-tuneable large unilamellar cationic liposomes: formulation, cellular uptake and biodistribution investigations. Eur J Pharm Biopharm 2019; 143:51-60. doi: 10.1016/j.ejpb.2019.08.013 [Crossref] [ Google Scholar]
- Shaklee PM, Semrau S, Malkus M, Kubick S, Dogterom M, Schmidt T. Protein incorporation in giant lipid vesicles under physiological conditions. Chembiochem 2010; 11(2):175-9. doi: 10.1002/cbic.200900669 [Crossref] [ Google Scholar]
- Lin YC, Huang KS, Chiang JT, Yang CH, Lai TH. Manipulating self-assembled phospholipid microtubes using microfluidic technology. Sens Actuators B Chem 2006; 117(2):464-71. doi: 10.1016/j.snb.2005.12.054 [Crossref] [ Google Scholar]
- Jahn A, Vreeland WN, Gaitan M, Locascio LE. Controlled vesicle self-assembly in microfluidic channels with hydrodynamic focusing. J Am Chem Soc 2004; 126(9):2674-5. doi: 10.1021/ja0318030 [Crossref] [ Google Scholar]
- Funakoshi K, Suzuki H, Takeuchi S. Formation of giant lipid vesiclelike compartments from a planar lipid membrane by a pulsed jet flow. J Am Chem Soc 2007; 129(42):12608-9. doi: 10.1021/ja074029f [Crossref] [ Google Scholar]
- Shum HC, Lee D, Yoon I, Kodger T, Weitz DA. Double emulsion templated monodisperse phospholipid vesicles. Langmuir 2008; 24(15):7651-3. doi: 10.1021/la801833a [Crossref] [ Google Scholar]
- Sugiura S, Kuroiwa T, Kagota T, Nakajima M, Sato S, Mukataka S. Novel method for obtaining homogeneous giant vesicles from a monodisperse water-in-oil emulsion prepared with a microfluidic device. Langmuir 2008; 24(9):4581-8. doi: 10.1021/la703509r [Crossref] [ Google Scholar]
- Ota S, Yoshizawa S, Takeuchi S. Microfluidic formation of monodisperse, cell-sized, and unilamellar vesicles. Angew Chem Int Ed Engl 2009; 48(35):6533-7. doi: 10.1002/anie.200902182 [Crossref] [ Google Scholar]
- Matosevic S, Paegel BM. Stepwise synthesis of giant unilamellar vesicles on a microfluidic assembly line. J Am Chem Soc 2011; 133(9):2798-800. doi: 10.1021/ja109137s [Crossref] [ Google Scholar]
- Otake K, Imura T, Sakai H, Abe M. Development of a new preparation method of liposomes using supercritical carbon dioxide. Langmuir 2001; 17(13):3898-901. doi: 10.1021/la010122k [Crossref] [ Google Scholar]
- Magnan C, Badens E, Commenges N, Charbit G. Soy lecithin micronization by precipitation with a compressed fluid antisolvent-influence of process parameters. J Supercrit Fluids 2000; 19(1):69-77. doi: 10.1016/s0896-8446(00)00076-0 [Crossref] [ Google Scholar]
- Lesoin L, Crampon C, Boutin O, Badens E. Preparation of liposomes using the supercritical anti-solvent (SAS) process and comparison with a conventional method. J Supercrit Fluids 2011; 57(2):162-74. doi: 10.1016/j.supflu.2011.01.006 [Crossref] [ Google Scholar]
- Wang T, Deng Y, Geng Y, Gao Z, Zou J, Wang Z. Preparation of submicron unilamellar liposomes by freeze-drying double emulsions. BiochimBiophys Acta 2006; 1758(2):222-31. doi: 10.1016/j.bbamem.2006.01.023 [Crossref] [ Google Scholar]
- Laouini A, Jaafar-Maalej C, Sfar S, Charcosset C, Fessi H. Liposome preparation using a hollow fiber membrane contactor--application to spironolactone encapsulation. Int J Pharm 2011; 415(1-2):53-61. doi: 10.1016/j.ijpharm.2011.05.034 [Crossref] [ Google Scholar]
- Yu DG, Branford-White C, Williams GR, Bligh SW, White K, Zhu LM. Self-assembled liposomes from amphiphilic electrospun nanofibers. Soft Matter 2011; 7(18):8239-47. doi: 10.1039/c1sm05961k [Crossref] [ Google Scholar]
- Genç R, Ortiz M, O’Sullivan CK. Curvature-tuned preparation of nanoliposomes. Langmuir 2009; 25(21):12604-13. doi: 10.1021/la901789h [Crossref] [ Google Scholar]
- Mouritsen OG. Lipids, curvature, and nano-medicine. Eur J Lipid Sci Technol 2011; 113(10):1174-87. doi: 10.1002/ejlt.201100050 [Crossref] [ Google Scholar]
- Wiggenhorn M. Scale-Up of Liposome Manufacturing: Combining High Pressure Liposome Extrusion with Drying Technologies. Cuvillier Verlag; 2008.
- Shah VM, Nguyen DX, Patel P, Cote B, Al-Fatease A, Pham Y. Liposomes produced by microfluidics and extrusion: a comparison for scale-up purposes. Nanomedicine 2019; 18:146-56. doi: 10.1016/j.nano.2019.02.019 [Crossref] [ Google Scholar]
- Charcosset C, Juban A, Valour JP, Urbaniak S, Fessi H. Preparation of liposomes at large scale using the ethanol injection method: effect of scale-up and injection devices. Chem Eng Res Des 2015; 94:508-15. doi: 10.1016/j.cherd.2014.09.008 [Crossref] [ Google Scholar]
- Carugo D, Bottaro E, Owen J, Stride E, Nastruzzi C. Liposome production by microfluidics: potential and limiting factors. Sci Rep 2016; 6:25876. doi: 10.1038/srep25876 [Crossref] [ Google Scholar]
- Penoy N, Grignard B, Evrard B, Piel G. A supercritical fluid technology for liposome production and comparison with the film hydration method. Int J Pharm 2021; 592:120093. doi: 10.1016/j.ijpharm.2020.120093 [Crossref] [ Google Scholar]
- Kube S, Hersch N, Naumovska E, Gensch T, Hendriks J, Franzen A. Fusogenic Liposomes as Nanocarriers for the Delivery of Intracellular Proteins. Langmuir 2017; 33(4):1051-9. doi: 10.1021/acs.langmuir.6b04304 [Crossref] [ Google Scholar]
- Madni A, Sarfraz M, Rehman M, Ahmad M, Akhtar N, Ahmad S. Liposomal drug delivery: a versatile platform for challenging clinical applications. J Pharm Pharm Sci 2014; 17(3):401-26. doi: 10.18433/j3cp55 [Crossref] [ Google Scholar]
- Kaneda Y. Virosomes: evolution of the liposome as a targeted drug delivery system. Adv Drug Deliv Rev 2000; 43(2-3):197-205. doi: 10.1016/s0169-409x(00)00069-7 [Crossref] [ Google Scholar]
- Scriboni AB, Couto VM, Ribeiro LNM, Freires IA, Groppo FC, de Paula E. Fusogenic liposomes increase the antimicrobial activity of vancomycin against Staphylococcus aureus biofilm. Front Pharmacol 2019; 10:1401. doi: 10.3389/fphar.2019.01401 [Crossref] [ Google Scholar]
- Csiszár A, Hersch N, Dieluweit S, Biehl R, Merkel R, Hoffmann B. Novel fusogenic liposomes for fluorescent cell labeling and membrane modification. Bioconjug Chem 2010; 21(3):537-43. doi: 10.1021/bc900470y [Crossref] [ Google Scholar]
- Yoshikawa T, Okada N, Nakagawa S. Fusogenic liposomes and their suitability for gene delivery. Future Lipidol 2006; 1(6):735-42. doi: 10.2217/17460875.1.6.735 [Crossref] [ Google Scholar]
- Kunisawa J, Masuda T, Katayama K, Yoshikawa T, Tsutsumi Y, Akashi M. Fusogenic liposome delivers encapsulated nanoparticles for cytosolic controlled gene release. J Control Release 2005; 105(3):344-53. doi: 10.1016/j.jconrel.2005.03.020 [Crossref] [ Google Scholar]
- Paliwal SR, Paliwal R, Vyas SP. A review of mechanistic insight and application of pH-sensitive liposomes in drug delivery. Drug Deliv 2015; 22(3):231-42. doi: 10.3109/10717544.2014.882469 [Crossref] [ Google Scholar]
- Chen Y, Du Q, Guo Q, Huang J, Liu L, Shen X. A W/O emulsion mediated film dispersion method for curcumin encapsulated pH-sensitive liposomes in the colon tumor treatment. Drug Dev Ind Pharm 2019; 45(2):282-91. doi: 10.1080/03639045.2018.1539099 [Crossref] [ Google Scholar]
- Rehman AU, Omran Z, Anton H, Mély Y, Akram S, Vandamme TF. Development of doxorubicin hydrochloride loaded pH-sensitive liposomes: investigation on the impact of chemical nature of lipids and liposome composition on pH-sensitivity. Eur J Pharm Biopharm 2018; 133:331-8. doi: 10.1016/j.ejpb.2018.11.001 [Crossref] [ Google Scholar]
- Liu X, Huang G. Formation strategies, mechanism of intracellular delivery and potential clinical applications of pH-sensitive liposomes. Asian J Pharm Sci 2013; 8(6):319-28. doi: 10.1016/j.ajps.2013.11.002 [Crossref] [ Google Scholar]
- Karanth H, Murthy RS. pH-sensitive liposomes--principle and application in cancer therapy. J Pharm Pharmacol 2007; 59(4):469-83. doi: 10.1211/jpp.59.4.0001 [Crossref] [ Google Scholar]
- Rayamajhi S, Marchitto J, Nguyen TDT, Marasini R, Celia C, Aryal S. pH-responsive cationic liposome for endosomal escape mediated drug delivery. Colloids Surf B Biointerfaces 2020; 188:110804. doi: 10.1016/j.colsurfb.2020.110804 [Crossref] [ Google Scholar]
- Monteiro LOF, Malachias Â, Pound-Lana G, Magalhães-Paniago R, Mosqueira VCF, Oliveira MC. Paclitaxel-loaded pH-sensitive liposome: new insights on structural and physicochemical characterization. Langmuir 2018; 34(20):5728-37. doi: 10.1021/acs.langmuir.8b00411 [Crossref] [ Google Scholar]
- Kanamala M, Palmer BD, Jamieson SM, Wilson WR, Wu Z. Dual pH-sensitive liposomes with low pH-triggered sheddable PEG for enhanced tumor-targeted drug delivery. Nanomedicine (Lond) 2019; 14(15):1971-89. doi: 10.2217/nnm-2018-0510 [Crossref] [ Google Scholar]
- Zou Y, Zong G, Ling YH, Perez-Soler R. Development of cationic liposome formulations for intratracheal gene therapy of early lung cancer. Cancer Gene Ther 2000; 7(5):683-96. doi: 10.1038/sj.cgt.7700156 [Crossref] [ Google Scholar]
- Heuts J, Varypataki EM, van der Maaden K, Romeijn S, Drijfhout JW, van Scheltinga AT. Cationic liposomes: a flexible vaccine delivery system for physicochemically diverse antigenic peptides. Pharm Res 2018; 35(11):207. doi: 10.1007/s11095-018-2490-6 [Crossref] [ Google Scholar]
- Shim G, Kim MG, Park JY, Oh YK. Application of cationic liposomes for delivery of nucleic acids. Asian J Pharm Sci 2013; 8(2):72-80. doi: 10.1016/j.ajps.2013.07.009 [Crossref] [ Google Scholar]
- Simões S, Filipe A, Faneca H, Mano M, Penacho N, Düzgünes N. Cationic liposomes for gene delivery. Expert Opin Drug Deliv 2005; 2(2):237-54. doi: 10.1517/17425247.2.2.237 [Crossref] [ Google Scholar]
- Sharma A, Sharma US. Liposomes in drug delivery: progress and limitations. Int J Pharm 1997; 154(2):123-40. doi: 10.1016/s0378-5173(97)00135-x [Crossref] [ Google Scholar]
- Wrobel I, Collins D. Fusion of cationic liposomes with mammalian cells occurs after endocytosis. BiochimBiophys Acta 1995; 1235(2):296-304. doi: 10.1016/0005-2736(95)80017-a [Crossref] [ Google Scholar]
- Needham D, Dewhirst MW. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv Drug Deliv Rev 2001; 53(3):285-305. doi: 10.1016/s0169-409x(01)00233-2 [Crossref] [ Google Scholar]
- Wagner A, Vorauer-Uhl K. Liposome technology for industrial purposes. J Drug Deliv 2011; 2011:591325. doi: 10.1155/2011/591325 [Crossref] [ Google Scholar]
- Rossmann C, McCrackin MA, Armeson KE, Haemmerich D. Temperature sensitive liposomes combined with thermal ablation: effects of duration and timing of heating in mathematical models and in vivo. PLoS One 2017; 12(6):e0179131. doi: 10.1371/journal.pone.0179131 [Crossref] [ Google Scholar]
- Lyu Y, Xiao Q, Yin L, Yang L, He W. Potent delivery of an MMP inhibitor to the tumor microenvironment with thermosensitive liposomes for the suppression of metastasis and angiogenesis. Signal Transduct Target Ther 2019; 4:26. doi: 10.1038/s41392-019-0054-9 [Crossref] [ Google Scholar]
- Kono K, Ozawa T, Yoshida T, Ozaki F, Ishizaka Y, Maruyama K. Highly temperature-sensitive liposomes based on a thermosensitive block copolymer for tumor-specific chemotherapy. Biomaterials 2010; 31(27):7096-105. doi: 10.1016/j.biomaterials.2010.05.045 [Crossref] [ Google Scholar]
- van Elk M, Deckers R, Oerlemans C, Shi Y, Storm G, Vermonden T. Triggered release of doxorubicin from temperature-sensitive poly(N-(2-hydroxypropyl)-methacrylamide mono/dilactate) grafted liposomes. Biomacromolecules 2014; 15(3):1002-9. doi: 10.1021/bm401904u [Crossref] [ Google Scholar]
- van Elk M, Ozbakir B, Barten-Rijbroek AD, Storm G, Nijsen F, Hennink WE. Alginate microspheres containing temperature sensitive liposomes (TSL) for MR-guided embolization and triggered release of doxorubicin. PLoS One 2015; 10(11):e0141626. doi: 10.1371/journal.pone.0141626 [Crossref] [ Google Scholar]
- Kneidl B, Peller M, Winter G, Lindner LH, Hossann M. Thermosensitive liposomal drug delivery systems: state of the art review. Int J Nanomedicine 2014; 9:4387-98. doi: 10.2147/ijn.s49297 [Crossref] [ Google Scholar]
- Lindner LH, Eichhorn ME, Eibl H, Teichert N, Schmitt-Sody M, Issels RD. Novel temperature-sensitive liposomes with prolonged circulation time. Clin Cancer Res 2004; 10(6):2168-78. doi: 10.1158/1078-0432.ccr-03-0035 [Crossref] [ Google Scholar]
- Kataria S, Sandhu P, Bilandi A, Akanksha M, Kapoor B. Stealth liposomes: a review. Int J Res Ayurveda Pharm 2011; 2(5):1534-8. [ Google Scholar]
- Pereira DSM, Cardoso BD, Rodrigues ARO, Amorim CO, Amaral VS, Almeida BG. Magnetoliposomes containing calcium ferrite nanoparticles for applications in breast cancer therapy. Pharmaceutics 2019; 11(9):477. doi: 10.3390/pharmaceutics11090477 [Crossref] [ Google Scholar]
- Cardoso BD, Rio ISR, Rodrigues ARO, Fernandes FCT, Almeida BG, Pires A. Magnetoliposomes containing magnesium ferrite nanoparticles as nanocarriers for the model drug curcumin. R Soc Open Sci 2018; 5(10):181017. doi: 10.1098/rsos.181017 [Crossref] [ Google Scholar]
- Lorente C, Cabeza L, Clares B, Ortiz R, Halbaut L, Delgado Á V. Formulation and in vitro evaluation of magnetoliposomes as a potential nanotool in colorectal cancer therapy. Colloids Surf B Biointerfaces 2018; 171:553-65. doi: 10.1016/j.colsurfb.2018.07.070 [Crossref] [ Google Scholar]
- Bakandritsos A, Fatourou AG, Fatouros DG. Magnetoliposomes and their potential in the intelligent drug-delivery field. TherDeliv 2012; 3(12):1469-82. doi: 10.4155/tde.12.129 [Crossref] [ Google Scholar]
- Joniec A, Sek S, Krysinski P. Magnetoliposomes as potential carriers of doxorubicin to tumours. Chemistry 2016; 22(49):17715-24. doi: 10.1002/chem.201602809 [Crossref] [ Google Scholar]
- da Costa e Silva RM, Lara LR, López JL, Andrade ÂL, Oliveira JA, Takahashi JA. Preparation of magnetoliposomes with a green, low-cost, fast and scalable methodology and activity study against S. aureus and C freundii bacterial strains. J Braz Chem Soc 2018; 29(12):2636-45. doi: 10.21577/0103-5053.20180144 [Crossref] [ Google Scholar]
- Nappini S, Bombelli FB, Bonini M, Nordèn B, Baglioni P. Magnetoliposomes for controlled drug release in the presence of low-frequency magnetic field. Soft Matter 2010; 6(1):154-62. doi: 10.1039/b915651h [Crossref] [ Google Scholar]
- Sabaté R, Barnadas-Rodríguez R, Callejas-Fernández J, Hidalgo-Alvarez R, Estelrich J. Preparation and characterization of extruded magnetoliposomes. Int J Pharm 2008; 347(1-2):156-62. doi: 10.1016/j.ijpharm.2007.06.047 [Crossref] [ Google Scholar]
- Choi WI, Sahu A, Wurm FR, Jo SM. Magnetoliposomes with size controllable insertion of magnetic nanoparticles for efficient targeting of cancer cells. RSC Adv 2019; 9(26):15053-60. doi: 10.1039/c9ra02529d [Crossref] [ Google Scholar]
- Szuplewska A, Rękorajska Joniec A, Pocztańska E, Krysiński P, Dybko A, Chudy M. Magnetic field-assisted selective delivery of doxorubicin to cancer cells using magnetoliposomes as drug nanocarriers. Nanotechnology 2019; 30(31):315101. doi: 10.1088/1361-6528/ab19d3 [Crossref] [ Google Scholar]
- Shirmardi Shaghasemi B, Virk MM, Reimhult E. Optimization of magneto-thermally controlled release kinetics by tuning of magnetoliposome composition and structure. Sci Rep 2017; 7(1):7474. doi: 10.1038/s41598-017-06980-9 [Crossref] [ Google Scholar]
- Monnier CA, Burnand D, Rothen-Rutishauser B, Lattuada M, Petri-Fink A. Magnetoliposomes: opportunities and challenges. Eur J Nanomed 2014; 6(4):201-15. doi: 10.1515/ejnm-2014-0042 [Crossref] [ Google Scholar]
- Yavlovich A, Smith B, Gupta K, Blumenthal R, Puri A. Light-sensitive lipid-based nanoparticles for drug delivery: design principles and future considerations for biological applications. Mol Membr Biol 2010; 27(7):364-81. doi: 10.3109/09687688.2010.507788 [Crossref] [ Google Scholar]
- Puri A. Phototriggerable liposomes: current research and future perspectives. Pharmaceutics 2013; 6(1):1-25. doi: 10.3390/pharmaceutics6010001 [Crossref] [ Google Scholar]
- Miranda D, Lovell JF. Mechanisms of light-induced liposome permeabilization. BioengTransl Med 2016; 1(3):267-76. doi: 10.1002/btm2.10032 [Crossref] [ Google Scholar]
- Yavlovich A, Singh A, Blumenthal R, Puri A. A novel class of photo-triggerable liposomes containing DPPC:DC(8,9)PC as vehicles for delivery of doxorubcin to cells. BiochimBiophys Acta 2011; 1808(1):117-26. doi: 10.1016/j.bbamem.2010.07.030 [Crossref] [ Google Scholar]
- Juarranz Á, Jaén P, Sanz-Rodríguez F, Cuevas J, González S. Photodynamic therapy of cancer Basic principles and applications. Clin Transl Oncol 2008; 10(3):148-54. doi: 10.1007/s12094-008-0172-2 [Crossref] [ Google Scholar]
- Paasonen L, Sipilä T, Subrizi A, Laurinmäki P, Butcher SJ, Rappolt M. Gold-embedded photosensitive liposomes for drug delivery: triggering mechanism and intracellular release. J Control Release 2010; 147(1):136-43. doi: 10.1016/j.jconrel.2010.07.095 [Crossref] [ Google Scholar]
- Danhier F, Feron O, Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release 2010; 148(2):135-46. doi: 10.1016/j.jconrel.2010.08.027 [Crossref] [ Google Scholar]
- Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today 2006; 11(17-18):812-8. doi: 10.1016/j.drudis.2006.07.005 [Crossref] [ Google Scholar]
- Alavi M, Hamidi M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab Pers Ther 2019;34(1). 10.1515/dmpt-2018-0032.
- Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm 2009; 71(3):409-19. doi: 10.1016/j.ejpb.2008.11.010 [Crossref] [ Google Scholar]
- Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci 2009; 30(11):592-9. doi: 10.1016/j.tips.2009.08.004 [Crossref] [ Google Scholar]
- Okumura M, Ichihara H, Matsumoto Y. Hybrid liposomes showing enhanced accumulation in tumors as theranostic agents in the orthotopic graft model mouse of colorectal cancer. Drug Deliv 2018; 25(1):1192-9. doi: 10.1080/10717544.2018.1475517 [Crossref] [ Google Scholar]
- Wu G, Li J, Yue J, Zhang S, Yunusi K. Liposome encapsulated luteolin showed enhanced antitumor efficacy to colorectal carcinoma. Mol Med Rep 2018; 17(2):2456-64. doi: 10.3892/mmr.2017.8185 [Crossref] [ Google Scholar]
- Zhang Y, Sriraman SK, Kenny HA, Luther E, Torchilin V, Lengyel E. Reversal of chemoresistance in ovarian cancer by co-delivery of a P-glycoprotein inhibitor and paclitaxel in a liposomal platform. Mol Cancer Ther 2016; 15(10):2282-93. doi: 10.1158/1535-7163.mct-15-0986 [Crossref] [ Google Scholar]
- Iwamoto Y, Matsumoto Y, Ueoka R. Induction of apoptosis of human lung carcinoma cells by hybrid liposomes containing polyoxyethylenedodecyl ether. Int J Pharm 2005; 292(1-2):231-9. doi: 10.1016/j.ijpharm.2004.11.034 [Crossref] [ Google Scholar]
- Liu Y, Lu WL, Guo J, Du J, Li T, Wu JW. A potential target associated with both cancer and cancer stem cells: a combination therapy for eradication of breast cancer using vinorelbine stealthy liposomes plus parthenolide stealthy liposomes. J Control Release 2008; 129(1):18-25. doi: 10.1016/j.jconrel.2008.03.022 [Crossref] [ Google Scholar]
- Guo L, Fan L, Ren J, Pang Z, Ren Y, Li J. Combination of TRAIL and actinomycin D liposomes enhances antitumor effect in non-small cell lung cancer. Int J Nanomedicine 2012; 7:1449-60. doi: 10.2147/ijn.s24711 [Crossref] [ Google Scholar]
- Li X, Ding L, Xu Y, Wang Y, Ping Q. Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int J Pharm 2009; 373(1-2):116-23. doi: 10.1016/j.ijpharm.2009.01.023 [Crossref] [ Google Scholar]
- Baek SE, Lee KH, Park YS, Oh DK, Oh S, Kim KS. RNA aptamer-conjugated liposome as an efficient anticancer drug delivery vehicle targeting cancer cells in vivo. J Control Release 2014; 196:234-42. doi: 10.1016/j.jconrel.2014.10.018 [Crossref] [ Google Scholar]
- Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release 2011; 153(3):198-205. doi: 10.1016/j.jconrel.2011.06.001 [Crossref] [ Google Scholar]
- Alshaer W, Hillaireau H, Vergnaud J, Ismail S, Fattal E. Functionalizing liposomes with anti-CD44 aptamer for selective targeting of cancer cells. Bioconjug Chem 2015; 26(7):1307-13. doi: 10.1021/bc5004313 [Crossref] [ Google Scholar]
- Liao ZX, Chuang EY, Lin CC, Ho YC, Lin KJ, Cheng PY. An AS1411 aptamer-conjugated liposomal system containing a bubble-generating agent for tumor-specific chemotherapy that overcomes multidrug resistance. J Control Release 2015; 208:42-51. doi: 10.1016/j.jconrel.2015.01.032 [Crossref] [ Google Scholar]
- Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev 2008; 60(15):1615-26. doi: 10.1016/j.addr.2008.08.005 [Crossref] [ Google Scholar]
- Lian T, Ho RJ. Trends and developments in liposome drug delivery systems. J Pharm Sci 2001; 90(6):667-80. doi: 10.1002/jps.1023 [Crossref] [ Google Scholar]
- Ye J, Yang Y, Jin J, Ji M, Gao Y, Feng Y. Targeted delivery of chlorogenic acid by mannosylated liposomes to effectively promote the polarization of TAMs for the treatment of glioblastoma. Bioact Mater 2020; 5(3):694-708. doi: 10.1016/j.bioactmat.2020.05.001 [Crossref] [ Google Scholar]
- Vakhshiteh F, Khabazian E, Atyabi F, Ostad SN, Madjd Z, Dinarvand R. Peptide-conjugated liposomes for targeted miR-34a delivery to suppress breast cancer and cancer stem-like population. J Drug Deliv Sci Technol 2020; 57:101687. doi: 10.1016/j.jddst.2020.101687 [Crossref] [ Google Scholar]
- Wang Z, Chi D, Wu X, Wang Y, Lin X, Xu Z. Tyrosine modified irinotecan-loaded liposomes capable of simultaneously targeting LAT1 and ATB(0, + ) for efficient tumor therapy. J Control Release 2019; 316:22-33. doi: 10.1016/j.jconrel.2019.10.037 [Crossref] [ Google Scholar]
- Yang Y, Zhao Z, Xie C, Zhao Y. Dual-targeting liposome modified by glutamic hexapeptide and folic acid for bone metastatic breast cancer. Chem Phys Lipids 2020; 228:104882. doi: 10.1016/j.chemphyslip.2020.104882 [Crossref] [ Google Scholar]
- Khayrani AC, Mahmud H, Oo AKK, Zahra MH, Oze M, Du J. Targeting ovarian cancer cells overexpressing CD44 with immunoliposomes encapsulating glycosylated paclitaxel. Int J Mol Sci 2019; 20(5):1042. doi: 10.3390/ijms20051042 [Crossref] [ Google Scholar]
- Lin YL, Tsai NM, Chen CH, Liu YK, Lee CJ, Chan YL. Specific drug delivery efficiently induced human breast tumor regression using a lipoplex by non-covalent association with anti-tumor antibodies. J Nanobiotechnology 2019; 17(1):25. doi: 10.1186/s12951-019-0457-3 [Crossref] [ Google Scholar]
- Wöll S, Dickgiesser S, Rasche N, Schiller S, Scherließ R. Sortagged anti-EGFR immunoliposomes exhibit increased cytotoxicity on target cells. Eur J Pharm Biopharm 2019; 136:203-12. doi: 10.1016/j.ejpb.2019.01.020 [Crossref] [ Google Scholar]
- Dumont N, Merrigan S, Turpin J, Lavoie C, Papavasiliou V, Geretti E. Nanoliposome targeting in breast cancer is influenced by the tumor microenvironment. Nanomedicine 2019; 17:71-81. doi: 10.1016/j.nano.2018.12.010 [Crossref] [ Google Scholar]
- Nassir AM, Ibrahim IAA, Md S, Waris M, Tanuja Tanuja, Ain MR. Surface functionalized folate targeted oleuropein nano-liposomes for prostate tumor targeting: in vitro and in vivo activity. Life Sci 2019; 220:136-46. doi: 10.1016/j.lfs.2019.01.053 [Crossref] [ Google Scholar]
- Nguyen VD, Min HK, Kim CS, Han J, Park JO, Choi E. Folate receptor-targeted liposomal nanocomplex for effective synergistic photothermal-chemotherapy of breast cancer in vivo. Colloids Surf B Biointerfaces 2019; 173:539-48. doi: 10.1016/j.colsurfb.2018.10.013 [Crossref] [ Google Scholar]
- Yoon HY, Chang IH, Goo YT, Kim CH, Kang TH, Kim SY. Intravesical delivery of rapamycin via folate-modified liposomes dispersed in thermo-reversible hydrogel. Int J Nanomedicine 2019; 14:6249-68. doi: 10.2147/ijn.s216432 [Crossref] [ Google Scholar]
- Akhtar A, Ghali L, Wang SX, Bell C, Li D, Wen X. Optimisation of folate-mediated liposomal encapsulated arsenic trioxide for treating HPV-positive cervical cancer cells in vitro. Int J Mol Sci 2019; 20(9):2156. doi: 10.3390/ijms20092156 [Crossref] [ Google Scholar]
- Fu J, Li W, Xin X, Chen D, Hu H. Transferrin-modified nanoliposome codelivery strategies for enhancing the cancer therapy. J Pharm Sci 2020; 109(8):2426-36. doi: 10.1016/j.xphs.2019.11.013 [Crossref] [ Google Scholar]
- Wang X, Zhao Y, Dong S, Lee RJ, Yang D, Zhang H. Cell-penetrating peptide and transferrin co-modified liposomes for targeted therapy of glioma. Molecules 2019; 24(19):3540. doi: 10.3390/molecules24193540 [Crossref] [ Google Scholar]
- Sakpakdeejaroen I, Somani S, Laskar P, Mullin M, Dufès C. Transferrin-bearing liposomes entrapping plumbagin for targeted cancer therapy. J InterdiscipNanomed 2019; 4(2):54-71. doi: 10.1002/jin2.56 [Crossref] [ Google Scholar]
- Jhaveri A, Deshpande P, Pattni B, Torchilin V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J Control Release 2018; 277:89-101. doi: 10.1016/j.jconrel.2018.03.006 [Crossref] [ Google Scholar]
- Bandekar A, Zhu C, Jindal R, Bruchertseifer F, Morgenstern A, Sofou S. Anti-prostate-specific membrane antigen liposomes loaded with 225Ac for potential targeted antivascular α-particle therapy of cancer. J Nucl Med 2014; 55(1):107-14. doi: 10.2967/jnumed.113.125476 [Crossref] [ Google Scholar]
- Gui K, Zhang X, Chen F, Ge Z, Zhang S, Qi X. Lipid-polymer nanoparticles with CD133 aptamers for targeted delivery of all-trans retinoic acid to osteosarcoma initiating cells. Biomed Pharmacother 2019; 111:751-64. doi: 10.1016/j.biopha.2018.11.118 [Crossref] [ Google Scholar]
- Zhao Y, Xu J, Le VM, Gong Q, Li S, Gao F. EpCAM aptamer-functionalized cationic liposome-based nanoparticles loaded with miR-139-5p for targeted therapy in colorectal cancer. Mol Pharm 2019; 16(11):4696-710. doi: 10.1021/acs.molpharmaceut.9b00867 [Crossref] [ Google Scholar]
- Yu S, Bi X, Yang L, Wu S, Yu Y, Jiang B. Co-delivery of paclitaxel and PLK1-targeted siRNA using aptamer-functionalized cationic liposome for synergistic anti-breast cancer effects in vivo. J Biomed Nanotechnol 2019; 15(6):1135-48. doi: 10.1166/jbn.2019.2751 [Crossref] [ Google Scholar]
- Kulkarni SB, Betageri GV, Singh M. Factors affecting microencapsulation of drugs in liposomes. J Microencapsul 1995; 12(3):229-46. doi: 10.3109/02652049509010292 [Crossref] [ Google Scholar]
- Wang Y, Tu S, Pinchuk AN, Xiong MP. Active drug encapsulation and release kinetics from hydrogel-in-liposome nanoparticles. J Colloid Interface Sci 2013; 406:247-55. doi: 10.1016/j.jcis.2013.05.081 [Crossref] [ Google Scholar]
- Alavi M, Karimi N, Safaei M. Application of various types of liposomes in drug delivery systems. Adv Pharm Bull 2017; 7(1):3-9. doi: 10.15171/apb.2017.002 [Crossref] [ Google Scholar]
- Saraf S, Jain A, Hurkat P, Jain SK. Topotecan liposomes: a visit from a molecular to a therapeutic platform. Crit Rev Ther Drug Carrier Syst 2016; 33(5):401-32. doi: 10.1615/CritRevTherDrugCarrierSyst.2016015926 [Crossref] [ Google Scholar]
- Petrilli R, Eloy JO, Saggioro FP, Chesca DL, de Souza MC, Dias MVS. Skin cancer treatment effectiveness is improved by iontophoresis of EGFR-targeted liposomes containing 5-FU compared with subcutaneous injection. J Control Release 2018; 283:151-62. doi: 10.1016/j.jconrel.2018.05.038 [Crossref] [ Google Scholar]
- Cullis PR, Mayer LD, Bally MB, Madden TD, Hope MJ. Generating and loading of liposomal systems for drug-delivery applications. Adv Drug Deliv Rev 1989; 3(3):267-82. doi: 10.1016/0169-409x(89)90024-0 [Crossref] [ Google Scholar]
- Brusa P, Immordino ML, Rocco F, Cattel L. Antitumor activity and pharmacokinetics of liposomes containing lipophilic gemcitabine prodrugs. Anticancer Res 2007; 27(1a):195-9. [ Google Scholar]
- Gubernator J. Active methods of drug loading into liposomes: recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin Drug Deliv 2011; 8(5):565-80. doi: 10.1517/17425247.2011.566552 [Crossref] [ Google Scholar]
- Odeh F, Nsairat H, Alshaer W, Alsotari S, Buqaien R, Ismail S. Remote loading of curcumin-in-modified β-cyclodextrins into liposomes using a transmembrane pH gradient. RSC Adv 2019; 9(64):37148-61. doi: 10.1039/c9ra07560g [Crossref] [ Google Scholar]
- Sur S, Fries AC, Kinzler KW, Zhou S, Vogelstein B. Remote loading of preencapsulated drugs into stealth liposomes. Proc Natl Acad Sci U S A 2014; 111(6):2283-8. doi: 10.1073/pnas.1324135111 [Crossref] [ Google Scholar]
- Bally MB, Mayer LD, Loughrey H, Redelmeier T, Madden TD, Wong K. Dopamine accumulation in large unilamellar vesicle systems induced by transmembrane ion gradients. Chem Phys Lipids 1988; 47(2):97-107. doi: 10.1016/0009-3084(88)90078-3 [Crossref] [ Google Scholar]
- Dos Santos N, Cox KA, McKenzie CA, van Baarda F, Gallagher RC, Karlsson G. pH gradient loading of anthracyclines into cholesterol-free liposomes: enhancing drug loading rates through use of ethanol. BiochimBiophys Acta 2004; 1661(1):47-60. doi: 10.1016/j.bbamem.2003.11.016 [Crossref] [ Google Scholar]
- Dos Santos N, Mayer LD, Abraham SA, Gallagher RC, Cox KA, Tardi PG. Improved retention of idarubicin after intravenous injection obtained for cholesterol-free liposomes. BiochimBiophys Acta 2002; 1561(2):188-201. doi: 10.1016/s0005-2736(02)00345-0 [Crossref] [ Google Scholar]
- Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. BiochimBiophys Acta 1993; 1151(2):201-15. doi: 10.1016/0005-2736(93)90105-9 [Crossref] [ Google Scholar]
- Wei H, Song J, Li H, Li Y, Zhu S, Zhou X. Active loading liposomal irinotecan hydrochloride: preparation, in vitro and in vivo evaluation. Asian J Pharm Sci 2013; 8(5):303-11. doi: 10.1016/j.ajps.2013.10.006 [Crossref] [ Google Scholar]
- Zucker D, Marcus D, Barenholz Y, Goldblum A. Liposome drugs’ loading efficiency: a working model based on loading conditions and drug’s physicochemical properties. J Control Release 2009; 139(1):73-80. doi: 10.1016/j.jconrel.2009.05.036 [Crossref] [ Google Scholar]
- Clerc S, Barenholz Y. Loading of amphipathic weak acids into liposomes in response to transmembrane calcium acetate gradients. BiochimBiophys Acta 1995; 1240(2):257-65. doi: 10.1016/0005-2736(95)00214-6 [Crossref] [ Google Scholar]
- Chen H, MacDonald RC, Li S, Krett NL, Rosen ST, O’Halloran TV. Lipid encapsulation of arsenic trioxide attenuates cytotoxicity and allows for controlled anticancer drug release. J Am Chem Soc 2006; 128(41):13348-9. doi: 10.1021/ja064864h [Crossref] [ Google Scholar]
- Deamer DW, Prince RC, Crofts AR. The response of fluorescent amines to pH gradients across liposome membranes. BiochimBiophys Acta 1972; 274(2):323-35. doi: 10.1016/0005-2736(72)90180-0 [Crossref] [ Google Scholar]
- Fenske DB, Wong KF, Maurer E, Maurer N, Leenhouts JM, Boman N. Ionophore-mediateduptake of ciprofloxacin and vincristine into large unilamellar vesicles exhibiting transmembrane ion gradients. BiochimBiophys Acta 1998; 1414(1-2):188-204. doi: 10.1016/s0005-2736(98)00166-7 [Crossref] [ Google Scholar]
- Gubernator J, Chwastek G, Korycińska M, Stasiuk M, Grynkiewicz G, Lewrick F. The encapsulation of idarubicin within liposomes using the novel EDTA ion gradient method ensures improved drug retention in vitro and in vivo. J Control Release 2010; 146(1):68-75. doi: 10.1016/j.jconrel.2010.05.021 [Crossref] [ Google Scholar]
- Fritze A, Hens F, Kimpfler A, Schubert R, Peschka-Süss R. Remote loading of doxorubicin into liposomes driven by a transmembrane phosphate gradient. BiochimBiophys Acta 2006; 1758(10):1633-40. doi: 10.1016/j.bbamem.2006.05.028 [Crossref] [ Google Scholar]
- Tang WL, Tang WH, Szeitz A, Kulkarni J, Cullis P, Li SD. Systemic study of solvent-assisted active loading of gambogic acid into liposomes and its formulation optimization for improved delivery. Biomaterials 2018; 166:13-26. doi: 10.1016/j.biomaterials.2018.03.004 [Crossref] [ Google Scholar]
- Hwang H, Jeong HS, Oh PS, Kim M, Lee TK, Kwon J. PEGylated nanoliposomes encapsulating angiogenic peptides improve perfusion defects: radionuclide imaging-based study. Nucl Med Biol 2016; 43(9):552-8. doi: 10.1016/j.nucmedbio.2016.05.010 [Crossref] [ Google Scholar]
- Lin C, Zheng H, Sun M, Guo Y, Luo F, Guo L. Highly sensitive colorimetric aptasensor for ochratoxin A detection based on enzyme-encapsulated liposome. Anal Chim Acta 2018; 1002:90-6. doi: 10.1016/j.aca.2017.11.061 [Crossref] [ Google Scholar]
- Chen Q, Shang W, Zeng C, Wang K, Liang X, Chi C. Theranostic imaging of liver cancer using targeted optical/MRI dual-modal probes. Oncotarget 2017; 8(20):32741-51. doi: 10.18632/oncotarget.15642 [Crossref] [ Google Scholar]
- Silindir M, Erdoğan S, Özer AY, Maia S. Liposomes and their applications in molecular imaging. J Drug Target 2012; 20(5):401-15. doi: 10.3109/1061186x.2012.685477 [Crossref] [ Google Scholar]
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin PharmacolTher 2008; 83(5):761-9. doi: 10.1038/sj.clpt.6100400 [Crossref] [ Google Scholar]
- Zylberberg C, Matosevic S. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Deliv 2016; 23(9):3319-29. doi: 10.1080/10717544.2016.1177136 [Crossref] [ Google Scholar]
- Moghimipour E, Handali S. Liposomes as drug delivery systems: properties and applications. Res J Pharm Biol Chem Sci 2013; 4(1):169-85. [ Google Scholar]
- Sen R, Satpathy S. Liposomes as drug delivery system: a brief review. Int J Res Pharm Sci 2014; 5(4):309-21. [ Google Scholar]
- Abbasi H, Rahbar N, Kouchak M, Khalil Dezfuli P, Handali S. Functionalized liposomes as drug nanocarriers for active targeted cancer therapy: a systematic review. J Liposome Res 2022; 32(2):195-210. doi: 10.1080/08982104.2021.1903035 [Crossref] [ Google Scholar]
- Cedervall T, Lynch I, Foy M, Berggård T, Donnelly SC, Cagney G. Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew Chem Int Ed Engl 2007; 46(30):5754-6. doi: 10.1002/anie.200700465 [Crossref] [ Google Scholar]
- Mustafa Khidir A, Saeed AA. Ligand-targeted liposomes. Health Prim Care 2020; 4:1-4. doi: 10.15761/hpc.1000188 [Crossref] [ Google Scholar]
- Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci U S A 2008; 105(38):14265-70. doi: 10.1073/pnas.0805135105 [Crossref] [ Google Scholar]
- Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release 2012; 161(2):175-87. doi: 10.1016/j.jconrel.2011.09.063 [Crossref] [ Google Scholar]
- Fathi S, Oyelere AK. Liposomal drug delivery systems for targeted cancer therapy: is active targeting the best choice?. Future Med Chem 2016; 8(17):2091-112. doi: 10.4155/fmc-2016-0135 [Crossref] [ Google Scholar]
- Dwivedi C, Verma S. Review on preparation and characterization of liposomes with application. Int J Sci Innov Res 2013; 2(2):486-508. [ Google Scholar]
- Zalba S, Garrido MJ. Liposomes, a promising strategy for clinical application of platinum derivatives. Expert Opin Drug Deliv 2013; 10(6):829-44. doi: 10.1517/17425247.2013.778240 [Crossref] [ Google Scholar]