Advanced pharmaceutical bulletin. 14(1):120-131.
doi: 10.34172/apb.2024.013
Review Article
AMPK Signaling Pathway as a Potential Therapeutic Target for Parkinson’s Disease
Seyed Zanyar Athari Conceptualization, Data curation, Formal analysis, Validation, Writing – original draft, Writing – review & editing, 1, 2 
Fereshteh Farajdokht Formal analysis, Validation, Writing – original draft, Writing – review & editing, 2, * 
Rana Keyhanmanesh Supervision, Validation, Writing – review & editing, 1 
Gisou Mohaddes Conceptualization, Project administration, Supervision, Validation, Writing – review & editing, 2, 3, * 
Author information:
1Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
2Neurosciences Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
3Department of Biomedical Education, California Health Sciences University, College of Osteopathic Medicine, Clovis, CA, USA.
Abstract
Parkinson’s disease (PD) is the second most common neurodegenerative disease caused by the loss of dopaminergic neurons. Genetic factors, inflammatory responses, oxidative stress, metabolic disorders, cytotoxic factors, and mitochondrial dysfunction are all involved in neuronal death in neurodegenerative diseases. The risk of PD can be higher in aging individuals due to decreased mitochondrial function, energy metabolism, and AMP-activated protein kinase (AMPK) function. The potential of AMPK to regulate neurodegenerative disorders lies in its ability to enhance antioxidant capacity, reduce oxidative stress, improve mitochondrial function, decrease mitophagy and macroautophagy, and inhibit inflammation. In addition, it has been shown that modulating the catalytic activity of AMPK can protect the nervous system. This article reviews the mechanisms by which AMPK activation can modulate PD.
Keywords: Parkinson's disease, AMPK, α-Synuclein, Oxidative stress, Inflammation
Copyright and License Information
©2024 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease caused by the loss of dopaminergic neurons.1-3 In 2016, it was estimated that PD affected 6.1 million people worldwide, up from 2.5 million in 1990, and this figure is predicted to more than double by 2040.4 Moreover, PD is present in approximately 3% of individuals aged 65 and above, with the largest number of cases reported in those over 70 years old.5
Clinically, symptoms of PD can be categorized as non-motor signs and motor symptoms. The non-motor symptoms are more common and emerge years before motor symptoms.6 Non-motor symptoms comprise loss of sense of smell, sensory disturbances (such as pain), sleep disorders, autonomic disorders (orthostatic hypotension), gastrointestinal disorders (constipation), urogenital disorders, sexual dysfunction, as well as cognitive deficits and dementia.7 Motor symptoms include bradykinesia, tremors at rest, rigid muscles, impaired posture, and imbalance. In addition to the main symptoms, patients may show other motor symptoms like micrography, freezing, masked face, decreased blink rate, dysphagia, and softened voice.8
Pathologically, the key characteristics of PD are the damage to dopaminergic neurons in substantia nigra pars compacta (SNpc) and ventral tegmental area (VTA), depletion of dopamine in the striatum, and the presence of Lewy bodies in the cytoplasm formed mainly by the α-synuclein (α-syn) protein. PD affects various neurotransmitters aside from the dopamine system, such as noradrenaline, serotonin, glutamate, γ-aminobutyric acid (GABA), acetylcholine, and neuropeptides. The development of PD may also be caused by the degeneration of cholinergic neurons in the meynert nucleus, norepinephrinergic neurons in the locus cereus, and serotoninergic neurons in the raphe nuclei.9 Non-motor symptoms caused by non-dopaminergic neurotransmitter system dysfunction are unresponsive to dopaminergic therapy.10
Along with genetic factors, inflammation, oxidative stress, mitochondrial dysfunction, and cytotoxic factors,11,12 metabolism-related dysfunction is also involved in the pathophysiology of PD.13 Evidence shows that impaired regulation of glucose metabolism, which occurs in early PD, reduces antioxidant capacity and neuronal survival.14 Furthermore, during the initial stages of PD, oxidative stress, a crucial characteristic of metabolic syndrome, leads to mitochondrial structural abnormalities and mutations in mitochondrial DNA, which worsen oxidative stress and ultimately cause neuronal death.15
Energy dysregulation is implicated as a possible trigger for PD, indicating that a deeper understanding of the molecular pathways controlling energy balance could lead to identifying therapeutic targets. The AMP-activated protein kinase (AMPK) signaling pathway regulates metabolism, cell growth, and autophagy,16 and serves as a metabolic energy sensor and controls both lipid and carbohydrate metabolism inside the cell.17,18 Moreover, inhibiting AMPK expression or activity results in an increase in pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α,19 whereas stimulating AMPK pathway has been shown to boost neuroprotection.20 AMPK is also involved in regulating macroautophagy,21 mitochondrial biogenesis,22 and gene expression.23
Energy balance in cells is maintained by AMPK, which inhibits energy consumption and activates energy production processes in response to specific conditions to restore ATP levels.24 The mitochondrial oxidative-phosphorylation (OxPh) pathway is commonly used to produce ATP from glucose. Hence, increasing AMPK activity is a viable strategy to avoid bioenergetics failure and boost energy levels in vulnerable neurons.25 AMPK stimulates glucose transport through glucose membrane vectors and the breakdown of stored glycogen in the cytoplasm.25,26 AMPK also provides substrates for other OxPh sources like fatty acids (FA) and glutamine.27 During calorie restriction, AMPK acutely increases the uptake and transfer of FAs to the mitochondria for catabolism, oxidation, and energy production. Long-term activation of AMPK can influence energy metabolism by activating regulatory factors like forkhead box transcription factors (FOXO) and peroxisomal proliferator-activated receptor-gamma coactivator (PGC)-1 α for energy production and consumption.28-30
Furthermore, AMPK regulates cellular ATP production and energy levels by restricting anabolic processes.24 AMPK inhibits processes that require ATP, like new protein production and cell growth, to maintain the ATP level in energy-constrained conditions.31 The mammalian target of rapamycin complex (mTORC)-1 is an essential cellular protein that promotes protein synthesis and growth and induces nutrient signals.24 Evidence shows that AMPK inhibits mTORC-1 through activating tuberous sclerosis complex (TSC)-2 and inhibiting regulatory-associated protein of mTOR (RAPTOR).32,33 Also, AMPK has the ability to decrease protein production by inhibiting the synthesis of ribosomal RNA.34
AMPK effect on mitochondrial function
Cell metabolism relies on organelles called mitochondria, which provide energy through the OxPh process. The OxPh generates additional substances, particularly reactive oxygen species (ROS), that can negatively affect mitochondrial function when produced excessively. The decrease in cellular energy production following mitochondrial dysfunction creates a vicious cycle of chronic ROS production and worsens mitochondrial dysfunction.35 Therefore, cells’ essential functions are to control mitochondrial health, biogenesis, fission-fusion dynamics, and mitochondrial autophagy (mitophagy).36 The process of mitochondrial quality control declines with age, particularly in those with PD.37,38 Additionally, accumulated α-syn may be the reason for mitochondrial damage in PD.39,40
Mitochondria can change their structure, size, and shape through repetitive cycles of fission and fusion.41 Mitochondrial dynamics can be influenced by calcium homeostasis, apoptosis, and respiration. Genetic mutations or exposure to toxins can lead to changes in mitochondrial dynamics, causing neurodegenerative disorders. The fusion of mitochondria is accomplished by two groups of GTPases: mitofusins (MFN1 and MFN2) located in the outer mitochondrial membrane and optic atrophy (OPA)-1 located in the inner mitochondrial membrane.42 Fission is another alteration in mitochondrial dynamics where dynamin-related protein (DRP)-1 is the key factor.43
Dopaminergic neurons in the SNpc have limited mitochondrial content and rely heavily on energy balance for survival.44 Sporadic and familial forms of PD affect diverse aspects of mitochondria, including their bioenergy capacity, quality control, life cycle, morphology (fission and fusion), transportation, and control of cellular apoptosis pathways.45 Furthermore, PINK1 and PARKIN genes play a key role in mitochondrial function and quality control as they detect damage in mitochondria and facilitate mitophagy to eliminate and replace dysfunctional mitochondrial components.46,47 Ubiquitination of MFN1 and MFN2 proteins, which are involved in mitochondria fusion, depends on the Parkin/PINK1 pathway, wherein PINK1 phosphorylates MFN2, resulting in Parkin recruitment and protein ubiquitination.48 This process is essential to identify mitochondria for degradation through mitophagy and prevent them from reintegrating into the mitochondrial network. However, this process is disturbed by PD, leading to the accumulation of abnormal mitochondria and respiratory dysfunction. Moreover, loss of DRP-1 in dopaminergic neurons leads to the degeneration of SN neurons in mice and a Parkinson’s-like phenotype due to depletion of axonal mitochondria.49
One of the primary regulators of mitochondrial biogenesis is a transcriptional activator called PGC-1.50 According to prior studies, PD causes a decline in the expression of PGC-1 and its downstream genes responsible for controlling cellular bioenergy and mitochondrial biogenesis.51,52 Interestingly, overexpression of PGC-1 can prevent dopaminergic neuron death caused by α-syn overexpression or rotenone-induced damage, potentially improving PD-like pathologies.52
As AMPK is vital for intracellular energy metabolism in response to energy depletion, it is expected that AMPK has a significant impact on mitochondrial homeostasis. An in vitro study has shown that α-syn overexpression reduces AMPK activity, leading to a decrease in cellular resistance to α-syn.53 A deficiency in AMPK activity can lead to reduced mitochondria and abnormal mitochondrial biogenesis due to disruption of the AMPK/PGC-1 axis, putting dopaminergic neurons at risk of degeneration and causing symptoms similar to PD.54,55 However, pharmacological AMPK activation provides neuroprotection.55
Through activating PGC-1α, AMPK promotes mitochondrial biogenesis, activating mitochondrial transcription factor A (TFAM), leading to increased transcription and replication of mitochondrial DNAs.56,57 Furthermore, AMPK enhances mitochondrial fusion, leading to the development of extensive and highly branched mitochondrial networks in a PGC-1-dependent way.58,59 Besides, AMPK activates mitochondrial fission factor (MFF) to promote mitochondrial fission but inhibits mTORC1 to suppress it.60,61 Therefore, it seems that the role of AMPK in intervening mitochondrial homeostasis is context-dependent based on cellular energy status. In mild energy depletion, it may stimulate fusion to boost energy production, but under prolonged and intense cellular stress, it may trigger fission to promote mitophagy and initiate mitochondrial biogenesis to substitute the impaired ones.
AMPK also facilitates mitochondrial function by controlling the direct phosphorylation of target proteins and transcriptional regulation of the relevant genes.62 Mitophagy is a physiological process that eliminates damaged mitochondria while promoting mitochondrial biogenesis pathways to replenish mitochondrial levels.63,64 Through the phosphorylation of Unc-51-like autophagy activating kinase (ULK)-1, AMPK promotes mitophagy by facilitating autophagosome formation and directing damaged mitochondria to lysosomes.65 AMPK activation also couples mitochondria fission with mitophagy by phosphorylating MFF and activating DRP-1 to maintain energy bioavailability and high-quality mitochondria.66,67
The mitochondrial electron transport chain is the major source of ROS, and cells rely on antioxidant mechanisms to prevent damage from ROS and maintain redox homeostasis. Proper cellular function and metabolic stress adaptation necessitate the regulation of ROS generated by mitochondria.68,69 Damage to essential cellular components caused by excessive free radical production and impaired redox balance in neurons contributes to the degeneration of dopaminergic neurons in the SN. The low glutathione levels, high levels of oligomeric α-syn, high iron and calcium contents, mitochondrial dysfunction, and dopamine degradation and oxidation are responsible for ROS production in PD.70,71 Genetic mutations in SNCA, PARKIN, PINK1, LRRK2, FBXO7, ATP13A2, GIGYF2 and HTRA2 are also responsible for impairing mitochondrial function and morphology, leading to ROS formation.72 The connection between oxidative stress and PD pathogenesis is backed up by neurotoxin-induced animal models (6-hydroxydopamine (6-OHDA), rotenone, and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)), which result in ROS generation and gradual loss of nigrostriatal dopaminergic system.73-76
Aberrant production of ROS and imbalanced redox status activate AMPK to maintain redox homeostasis. AMPK promotes the expression of antioxidant enzymes such as glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) to mitigate ROS generation by activating Sirt1/PGC-1α/FOXO-1 pathway (Figure 1). However, pharmacological or genetic inactivation of AMPK leads to elevated mitochondrial ROS levels, promoting cytotoxicity.69 Nuclear factor E2-related factor 2 (Nrf2) maintains redox balance and protects cells from oxidative damage. Nrf2 is usually kept in the cytoplasm during stress-free conditions, but it translocates to the nucleus on exposure to oxidative stress. Once bound to the antioxidant response element, it activates the expression of several antioxidative enzymes, including heme oxygenase-1 (HO-1), SOD, and GPx, which help to detoxify free radicals. Through phosphorylation, AMPK also enhances Nrf2 nuclear translocation, thus reducing ROS levels and inhibiting oxidative stress.77,78 Therefore, activating the AMPK pathway may serve as a therapeutic approach for inhibiting oxidative stress in PD.
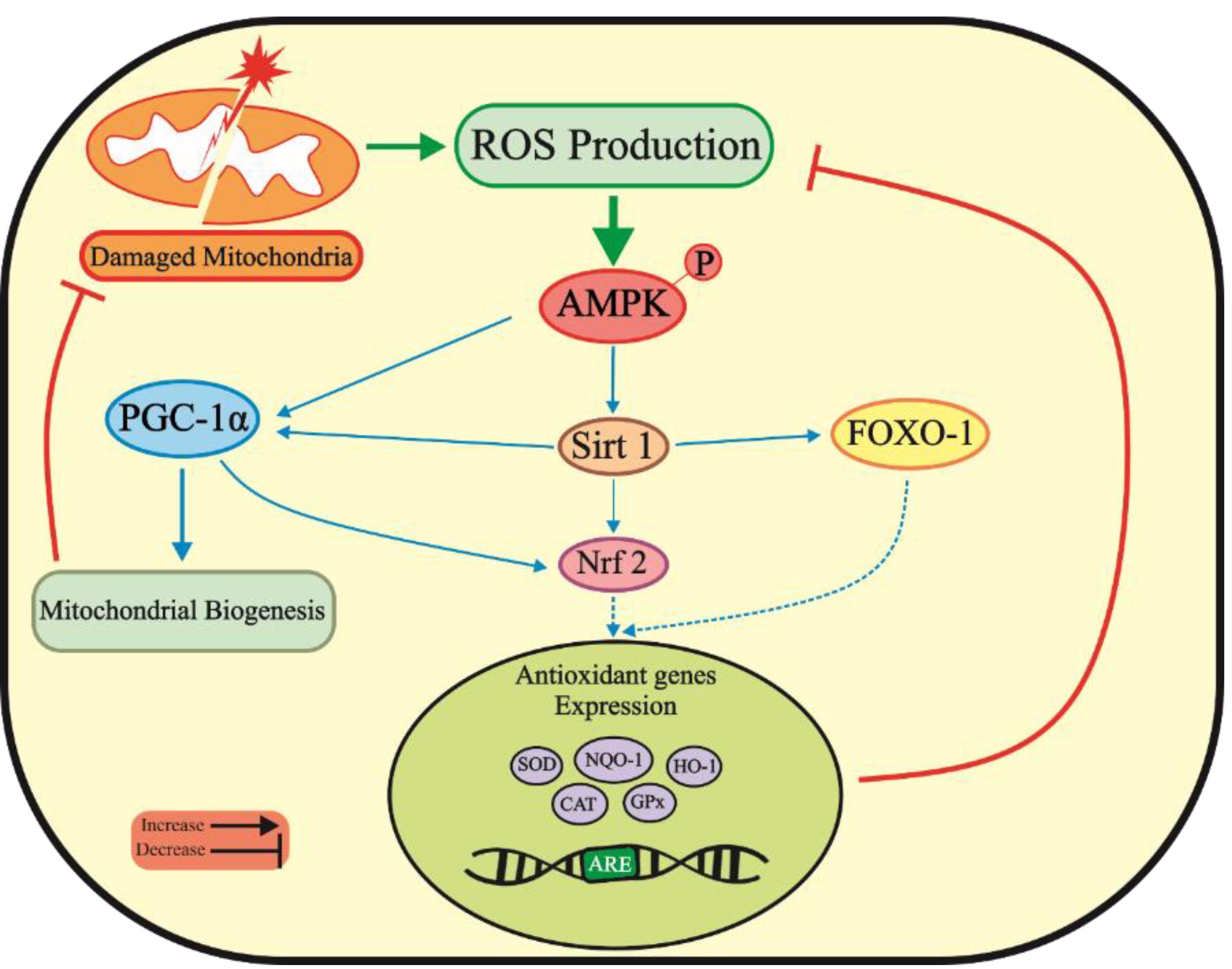
Figure 1.
Activated AMPK suppresses oxidative stress pathways related to PD by activating PGC-1α and Sirt 1 pathways, resulting in increased antioxidant gene expression and inhibition of mitochondrial damages. ROS, Reactive oxygen species; AMPK, AMP-activated protein kinase; FOXO-1, Forkhead box class O family member proteins-1; Sirt 1, Sirtuin 1; PGC-1α, Peroxisome Proliferator-activated receptor-gamma coactivator-1; Nrf2, Nuclear factor E2-related factor 2; SOD, Superoxide dismutase; NQO-1, NAD(P)H quinone dehydrogenase 1; HO-1, Heme oxygenase-1; CAT, Catalase; GPx, Glutathione peroxidase
.
Activated AMPK suppresses oxidative stress pathways related to PD by activating PGC-1α and Sirt 1 pathways, resulting in increased antioxidant gene expression and inhibition of mitochondrial damages. ROS, Reactive oxygen species; AMPK, AMP-activated protein kinase; FOXO-1, Forkhead box class O family member proteins-1; Sirt 1, Sirtuin 1; PGC-1α, Peroxisome Proliferator-activated receptor-gamma coactivator-1; Nrf2, Nuclear factor E2-related factor 2; SOD, Superoxide dismutase; NQO-1, NAD(P)H quinone dehydrogenase 1; HO-1, Heme oxygenase-1; CAT, Catalase; GPx, Glutathione peroxidase
Effect of AMPK on macroautophagy
Autophagy is a process that transfers waste products, cellular components, and large molecules to the lysosome for decomposition and ingestion.24 Autophagy disturbance is one of the etiologies of PD, leading to α-syn accumulation in the brain.79 Moreover, deleting essential genes involved in autophagy, such as autophagy-related gene-7 (ATG-7), can induce neurodegeneration similar to PD in mice.80 A recent study has shown that tricin, a natural flavonoid, can improve autophagy and ATG-7-dependent clearance of α-syn via an AMPK/mTOR pathway.81
There are three main ways to remove α-syn from neurons: the ubiquitin-proteasome system (UPS), chaperone-mediated autophagy (CMA), and macroautophagy.82 Removing α-syn oligomers requires macroautophagy-mediated degradation because UPS and CMA are ineffective. To accomplish this, autophagosomes are formed to separate cytoplasmic components and carry them to lysosomes.83,84 In both PD patients and animal models, macroautophagy is stimulated by transcription factor EB (TFEB), which mediates lysosomal biogenesis and macroautophagy development due to increased α-syn levels.85,86 In the PD mice model, overexpression of α-syn causes macroautophagy dysfunction and increases dopaminergic neuron degeneration in SNpc and movement disorders. These defects can be improved by overexpression of TFEB or Beclin-1 (another autophagy regulator), suggesting that macroautophagy regulation can be helpful in the PD to reduce α-syn accumulation and neuronal damage.86,87
Autophagy initiation is mainly driven by ULK-1, while the inhibition of ATG-13 phosphorylation by mTORC-1 leads to a decrease in the ULK-1 complex activity, ultimately suppressing autophagy.88,89 ULK-1 factor initiates the formation and maturation of autophagosomes through the Beclin-1 phosphorylation.90 Evidence suggests that AMPK boosts autophagic degradation by activating ULK-1 through phosphorylation and inhibiting mTORC1 and blocking its inhibitory effect on ULK-1.91 Moreover, AMPK promotes lysosomal biogenesis by increasing the activity of TFEB92 and improving the transcription of proteins required for macroautophagy by FOXO-3.93 Preclinical studies indicate that autophagy-promoting agents can improve α-syn clearance and provide neuroprotection.94 Metformin has been shown to stimulate autophagy and protect nigrostriatal neurons in PD models by activating the AMPK/FOXO-3 pathway.53,95,96 Moreover, resveratrol exhibits neuroprotective properties in PD models by inducing autophagy via AMPK activation and mTOR inhibition.97,98 Therefore, AMPK-dependent stimulation of autophagy may hold promising potential for developing new therapeutic strategies in PD.
Effect of AMPK on genetic PD
Genetic PD is rare; however, several types are identified that account for almost 30% of familial cases.99 Genetic mutations in LRRK2, PARK2, PARK7, PINK1, or the SNCA gene can lead to familial cases of PD. Accumulating studies show that mutations in SNCA, GBA, and LRRK2 genes result in overexpression of α-syn and increased secretion of pro-inflammatory cytokines, leading to the development of motor dysfunction.92,100-102
PARK7 (also known as DJ-1) is the gene responsible for the expression of DJ-1 protein, and its mutation causes genetic form and early onset of PD.103 A critical function of DJ-1 is nuclear communication with mitochondria.104 The wild-type DJ-1 enzyme prevents glycolysis metabolite damage in cells metabolizing carbohydrates.105 It protects cells from oxidative stress-induced cytotoxicity by enhancing Nrf2 transcriptional activity and preventing Nrf2 inactivation.106,107 Moreover, DJ-1 is one of the influential factors in cellular signals, including transcription of tyrosine hydroxylase, dopamine receptor, and p53 signaling pathway.104 PINK1 is also transcriptionally up-regulated by Nrf2, which shields dopamine neurons from neurotoxicity induced by oxidative stress.106,107 AMPK can enhance Nrf2 nuclear translocation through phosphorylation and inhibiting oxidative stress.77,78
PARKIN, PINK1, LRRK2, and PARK7 genetic mutations cause mitochondrial morphology and function abnormalities.108 Point mutations in the PARK7 (NM_007262.5) gene include p.Leu166Pro(c.497T > C), p.Ala104Thr (c.310G > A), p.Met26Ile(c.78G > A), p.Asp149Ala (c.446A > C), p.Glu64Asp(c.192G > C), p.Leu10Pro(c.29T > C), and p.Pro158del(c.471_473del).109 Activation of AMPK by adaptor protein phosphotyrosine interacting with PH domain and leucine zipper (APPL)-1, an endosomal adapter protein, can protect against the p.Leu166Pro(c.497T > C) mutation of the PARK7 gene.110
Effect of AMPK on inflammation
Both preclinical and clinical PD studies have proved that the onset and progression of PD involve neuroinflammation and immune dysfunction.111 The causes of inflammation in PD include exposure to heavy metals, environmental toxins, bacterial and viral infections, and pesticides.112
Microglia, a part of the innate immune system in the central nervous system (CNS), are categorized into M1 and M2 subtypes. The M2 phenotype has anti-inflammatory and cytoprotective properties, essential for maintaining CNS homeostasis. Upon microglia activation, the M2 subtype is transformed into the M1 subtype, known to be cytotoxic and pro-inflammatory.113-115 In the pathology of PD, the accumulation of α-syn and the increase of ROS in dopaminergic cells promote neuronal death, followed by the release of damage-associated molecular patterns (DAMPs) from neurons, resulting in an increase in the activity of M1 microglia in the CNS.116 Preclinical PD models have shown that microglial activation and secretion of pro-inflammatory cytokines, particularly IL-6 and IL-1β, precede the degeneration of dopamine neurons.117,118 Additionally, there is a connection between pathological α-syn accumulation and the PD brain’s heightened inflammation.119,120
The blood-brain barrier becomes weaker when inflammation increases in the brain, leading to the penetration of harmful substances like ROS and NO, which cause further damage.121 In a 6-OHDA-induced PD model, the amount of pro-inflammatory cytokines such as IL-1, IL-6, TNF-α, and INF-γ were increased, while anti-inflammatory cytokines such as IL-10 was decreased, indicating dysregulation in the immune system and the occurrence of inflammation in the CNS.122 In basal condition, nuclear factor kappa B (NF-κB) is inactive, localizes in the cytoplasm, and tightly bound to an inhibitor of nuclear κB (IκB). Upon activation by DAMPs, IκB kinase (IKK) targets IκB for degradation, resulting in translocation of NF-κB to the nucleus, pro-inflammatory gene expression, and damage to dopaminergic neurons through impaired mitochondrial function and autophagy by suppressing Sirt1/FOXO-PGC-1α pathway.123,124
On the other hand, nuclear receptor-related protein 1 (NURR1) controls the expression of genes essential for the survival of dopaminergic neurons and has the potential to inhibit NF-κB activity when activated.125 Nrf2 transcription factor not only boosts antioxidative defense but also plays a critical role in regulating inflammation and has been substantiated to obstruct inflammatory responses prompted by inflammatory factors. Typically, Nrf2 is expressed at high levels in glial cells, and its activation reduces neuroinflammation.121 The survival of dopaminergic neurons is influenced by Nrf2 and NF-κB, which behave as antagonistic transcription factors. Nrf2 negates NF-κB signaling, while NF-κB silences Nrf2 target genes and deprives it of necessary co-transcription factors. However, a lack of Nrf2 results in an increase in NF-κB levels through proteasome-mediated IκB degradation. Therefore, activation of the Nrf2 pathway can alleviate PD symptoms by reducing cellular damage from oxidative stress and neuroinflammation, as well as improving mitochondrial function.126,127
Evidence suggests that chronic inflammation leads to a gradual decrease in AMPK function,128 while an increase in AMPK activity encourages microglial anti-inflammatory M2 polarization.129 Furthermore, AMPK suppresses NF-κB activation in the brain to inhibit inflammatory responses.130,131 In an MPTP-induced PD model, liraglutide was shown to modulate the AMPK/NF-κB pathway, leading to improvements in PD-related motor symptoms, rescue of dopaminergic neurons, and diminished activated microglia in the SN.132 Another pathway by which AMPK regulates inflammation is sirtuin1 (Sirt1). Indole-3-carbinol was reported to activate the AMPK/Sirt1 pathway and reduce nervous system inflammation in PD model mice.133 Moreover, AMPK reduces inflammation by inhibiting NOX-mediated ROS production and decreasing nitric oxide synthase (iNOS)-mediated nitric oxide (NO) production.134–137 AMPK also acts as a cofactor for Sirt1 activity and Sirt1 activation protects dopaminergic neurons through inhibiting iNOS, p53, and NF-κB expression, and increasing FOXO-3/PGC-1α pathway.138–141 The next target of AMPK in nerve cells to deal with neuroinflammation is activation of Nrf2.142 As shown in Figure 2, AMPK activation through modulation of several pathways can protect dopaminergic neurons from inflammation.
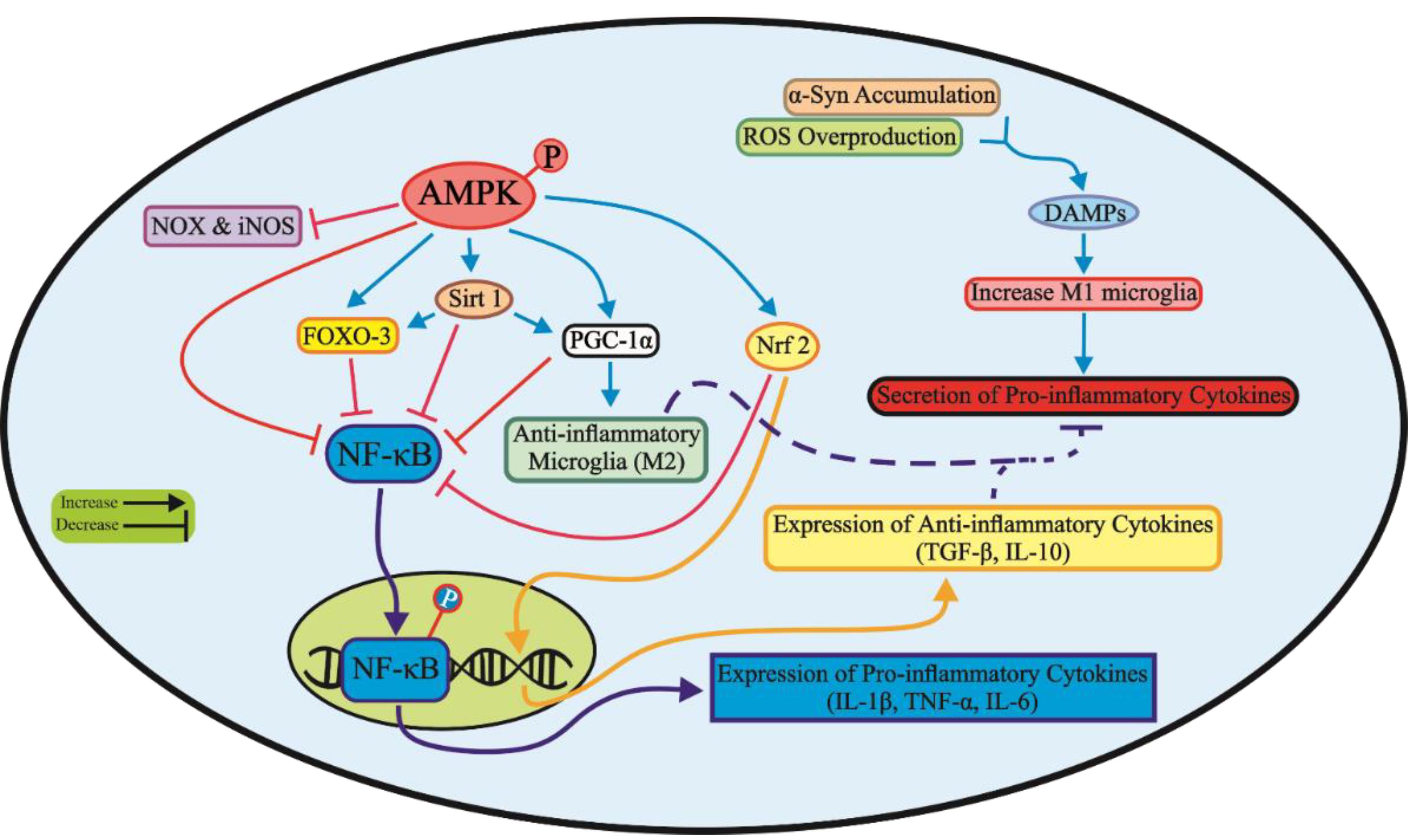
Figure 2.
The phosphorylation of AMPK targets multiple signaling pathways to inhibit neuroinflammation responses related to PD. This results in the suppression of NF-κB translocation to the nucleus and expression of pro-inflammatory cytokines while enhancing the expression of anti-inflammatory cytokines. AMPK, AMP-activated protein kinase; NOX, NADPH oxidase; iNOS, Induciblenitric oxide synthase; FOXO-3, Forkhead box class O family member proteins-3; Sirt 1, Sirtuin 1; PGC-1α, Peroxisome proliferator-activated receptor-gamma coactivator-1; Nrf2, Nuclear factor E2-related factor 2; NF-κB, Nuclear factor kappa B; IL, Interleukin; TNF-α, Tumor necrosis factor-α; DAMP, Damage-associated molecular patterns; ROS, Reactive oxygen species; α-Syn: α-Synuclein
.
The phosphorylation of AMPK targets multiple signaling pathways to inhibit neuroinflammation responses related to PD. This results in the suppression of NF-κB translocation to the nucleus and expression of pro-inflammatory cytokines while enhancing the expression of anti-inflammatory cytokines. AMPK, AMP-activated protein kinase; NOX, NADPH oxidase; iNOS, Induciblenitric oxide synthase; FOXO-3, Forkhead box class O family member proteins-3; Sirt 1, Sirtuin 1; PGC-1α, Peroxisome proliferator-activated receptor-gamma coactivator-1; Nrf2, Nuclear factor E2-related factor 2; NF-κB, Nuclear factor kappa B; IL, Interleukin; TNF-α, Tumor necrosis factor-α; DAMP, Damage-associated molecular patterns; ROS, Reactive oxygen species; α-Syn: α-Synuclein
Effect of AMPK on cell survival and apoptosis
In PD, the activation of the intrinsic apoptotic pathway induces the death of dopaminergic neurons in the SNpc. Many studies suggest that PD is connected with mitochondrial-mediated apoptosis, leading to an increase in pro-apoptotic factors like BAX and cytochrome c, caspase-9, and caspase-3, and a decrease in anti-apoptotic factors such as Bcl-2 and Bcl-XL. As mentioned, PD is associated with a chain of events that drive cells toward apoptosis, including genetic mutation, accumulation of α-syn, neuroinflammation, ROS production, and mitochondrial dysfunction.48,143-145 Besides, genetic mutation of PD-related genes, namely Parkin, LRRK2, PINK1, and PARK7, contribute to mitochondrial impairment and apoptosis.99,143
AMPK plays a dual role in regulating cell death and survival, depending on the type of stress and cells, and duration of exposure.146-148 Some studies have shown that the activation of AMPK for a prolonged duration can activate c-Jun N-terminal protein kinase (JNK), leading to apoptosis in liver cells and pancreas beta cells.149,150 However, another study showed that activation of AMPK inhibited dexamethasone-induced apoptosis in thymocytes.151 Conversely, some studies suggest that the activation of AMPK-related pathways could prevent the apoptosis pathway, particularly in neurons, by correcting mitochondrial abnormalities. Furthermore, 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) triggers AMPK activation that prevents apoptosis while inhibiting AMPK activity induces cell apoptosis.152-156 Additionally, an in vitro study demonstrated that disruption of the AMPK/Sirt1 signaling pathway by sevoflurane caused an increase in the apoptosis rate in neural cells while promoting AMPK level can improve apoptosis.157 In the intracerebral hemorrhage model, activating the αVβ5/AMPK pathway by Irisin, a myokine, inhibited apoptosis in the brain.158 In an MPTP-induced PD model, activation of the AMPK/MAPK pathway by osmotin administration reduced α-syn and apoptosis-related proteins.159 Besides, treatment with the AMPK agonist, GSK621, attenuated mitochondrial dysfunction and apoptotic neuronal death in the SNpc in the MPTP-induced PD mice model by activating the AMPK/GSK-3β/PP2A.160 Therefore, the regulation of apoptosis by AMPK is a controversial topic that requires more study.
AMPK activation provides a significant neuroprotective effect and enhances cell survival against several cytotoxic agents. The mechanisms that AMPK activation may use to regulate PD-related pathology were summarized in this review (Table 1).
Table 1.
The AMPK downstream pathways to protect dopaminergic neurons.
|
Signaling pathways
|
Outcome
|
References
|
AMPK/mTORC-1
AMPK/mTOR/ULK-1
AMPK/Sirt1/PGC-1α |
Increases Autophagy
Increases Mitophagy |
161-163
|
|
164,165
|
AMPK/Nrf2
AMPK/Sirt1/PGC-1α
AMPK/Sirt1/Nrf2
AMPK/Nrf2/TXNIP
AMPK/Sirt1/FOXO-1 |
Reduces Oxidative Stress |
130,133,164-167
|
AMPK/PGC-1α/NF-κB
AMPK/Sirt1/FOXO-3
AMPK/Sirt1/NF-κB
AMPK/AKT/NF-κB
AMPK/Nrf2/TXNIP |
Reduces Inflammation |
130,133,166,167
|
AMPK/Akt/mTOR
AMPK/Sirt1/NF-κB
AMPK/FOXO-3
AMPK/Sirt1/mTOR
AMPK/MAPK/mTOR
AMPK/GSK-3β/PP2A |
Inhibits apoptosis |
95,133,161-163,168,169
|
Conclusion
The pathophysiology of PD is complex and multifactorial, involving abnormalities in mitochondrial function and morphology, impaired energy metabolism, genetic mutation, aggregation of α-syn, resulting in loss of dopaminergic neurons. AMPK can regulate multiple biological functions, including mitochondrial homeostasis, mitophagy, autophagy, oxidative stress, inflammation, and apoptosis, by which effectively prevents PD-related pathology (Figure 3). To treat PD effectively, conducting additional preclinical research is necessary to gain a better understanding of the potential benefits and drawbacks of AMPK activation. This will help identify specific downstream pathways of AMPK and avoid activating any detrimental pathways.
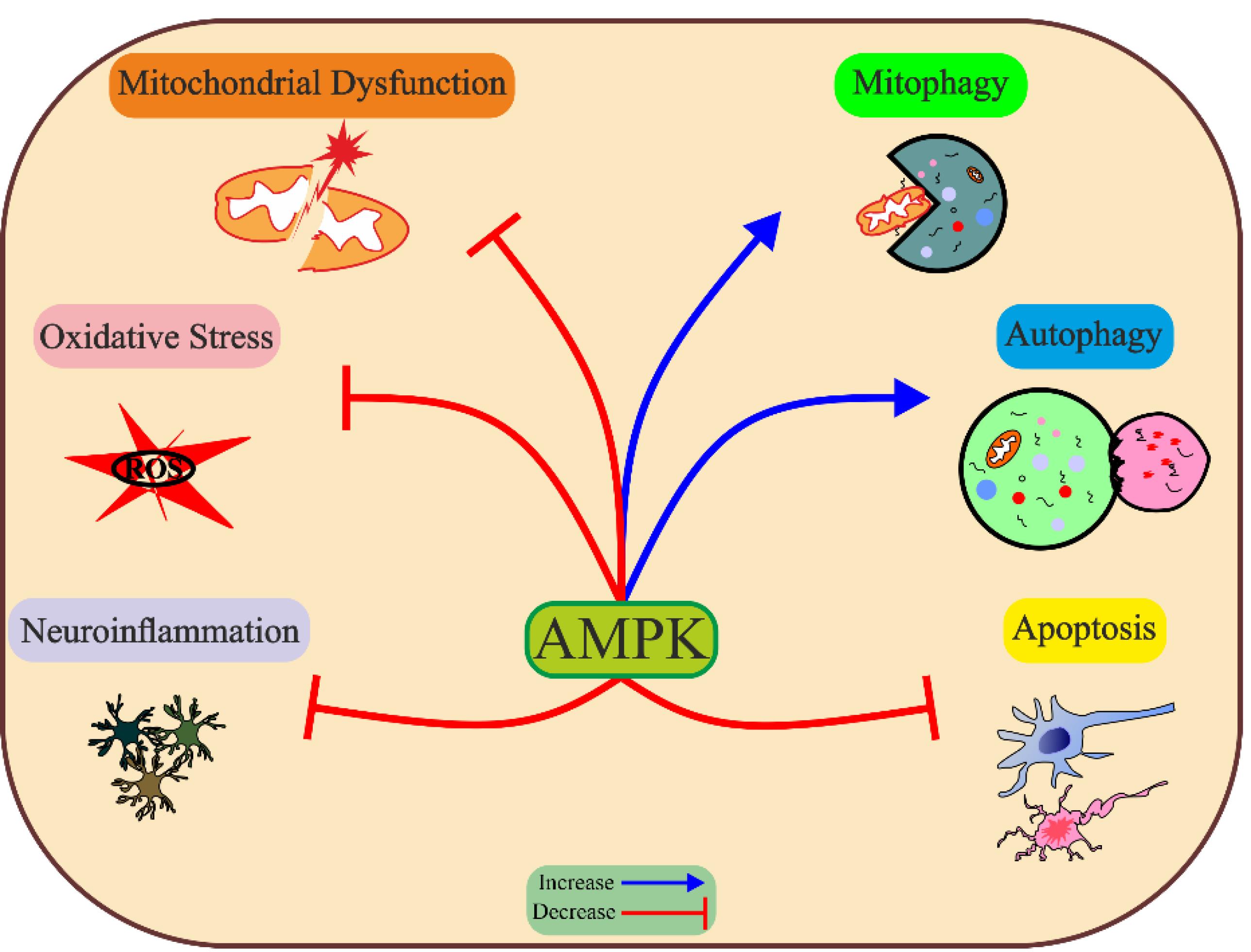
Figure 3.
Protective effect of AMPK against Parkinson’s disease etiologies. Activated AMPK target Parkinson’s disease-related etiology by decreasing neuroinflammation, oxidative stress and apoptosis, and improving mitochondrial function, mitophagy, and autophagy. ROS, reactive oxygen species
.
Protective effect of AMPK against Parkinson’s disease etiologies. Activated AMPK target Parkinson’s disease-related etiology by decreasing neuroinflammation, oxidative stress and apoptosis, and improving mitochondrial function, mitophagy, and autophagy. ROS, reactive oxygen species
Acknowledgments
The authors would like to appreciate Dr. Mahnaz Motayar and Mr. Habib Zakerani for their kind support of this study. This article aligns with Seyed Zanyar Athari’s Ph.D. thesis with ethical approval no. IR.TBZMED.AEC.1401.032.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
No funds, grants, or other support were received.
References
- Aarsland D, Batzu L, Halliday GM, Geurtsen GJ, Ballard C, Ray Chaudhuri K. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers 2021; 7(1):47. doi: 10.1038/s41572-021-00280-3 [Crossref] [ Google Scholar]
- Hu LF, Lu M, Tiong CX, Dawe GS, Hu G, Bian JS. Neuroprotective effects of hydrogen sulfide on Parkinson’s disease rat models. Aging Cell 2010; 9(2):135-46. doi: 10.1111/j.1474-9726.2009.00543.x [Crossref] [ Google Scholar]
- Hashempour-Baltork F, Farshi P, Mirza Alizadeh A, Eskandarzadeh S, Abedinzadeh S, Azadmard-Damirchi S. Effect of refined edible oils on neurodegenerative disorders. Adv Pharm Bull 2023; 13(3):461-8. doi: 10.34172/apb.2023.060 [Crossref] [ Google Scholar]
- Dorsey ER, Elbaz A, Nichols E, Abbasi N, Abd-Allah F, Abdelalim A. Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018; 17(11):939-53. doi: 10.1016/s1474-4422(18)30295-3 [Crossref] [ Google Scholar]
- Armstrong MJ, Okun MS. Time for a new image of Parkinson disease. JAMA Neurol 2020; 77(11):1345-6. doi: 10.1001/jamaneurol.2020.2412 [Crossref] [ Google Scholar]
- Elfil M, Kamel S, Kandil M, Koo BB, Schaefer SM. Implications of the gut microbiome in Parkinson’s disease. Mov Disord 2020; 35(6):921-33. doi: 10.1002/mds.28004 [Crossref] [ Google Scholar]
- Sveinbjornsdottir S. The clinical symptoms of Parkinson’s disease. J Neurochem 2016; 139 Suppl 1:318-24. doi: 10.1111/jnc.13691 [Crossref] [ Google Scholar]
- Mazzoni P, Shabbott B, Cortés JC. Motor control abnormalities in Parkinson’s disease. Cold Spring HarbPerspect Med 2012; 2(6):a009282. doi: 10.1101/cshperspect.a009282 [Crossref] [ Google Scholar]
- Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J. Harrison’s Principles of Internal Medicine. 20th ed. New York, NY: McGraw-Hill Education; 2018.
- Bonnet A-M. Involvement of non-dopaminergic pathways in Parkinson’s disease. CNS Drugs 2000; 13(5):351-64. doi: 10.2165/00023210-200013050-00005 [Crossref] [ Google Scholar]
- Spatola M, Wider C. Genetics of Parkinson’s disease: the yield. Parkinsonism RelatDisord 2014; 20 Suppl 1:S35-8. doi: 10.1016/s1353-8020(13)70011-7 [Crossref] [ Google Scholar]
- Surmeier DJ. Calcium, ageing, and neuronal vulnerability in Parkinson’s disease. Lancet Neurol 2007; 6(10):933-8. doi: 10.1016/s1474-4422(07)70246-6 [Crossref] [ Google Scholar]
- Zhang P, Tian B. Metabolic syndrome: an important risk factor for Parkinson’s disease. Oxid Med Cell Longev 2014; 2014:729194. doi: 10.1155/2014/729194 [Crossref] [ Google Scholar]
- Pagano G, Polychronis S, Wilson H, Giordano B, Ferrara N, Niccolini F. Diabetes mellitus and Parkinson disease. Neurology 2018; 90(19):e1654-e62. doi: 10.1212/wnl.0000000000005475 [Crossref] [ Google Scholar]
- Yan MH, Wang X, Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med 2013; 62:90-101. doi: 10.1016/j.freeradbiomed.2012.11.014 [Crossref] [ Google Scholar]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 2011; 13(9):1016-23. doi: 10.1038/ncb2329 [Crossref] [ Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 2012; 13(4):251-62. doi: 10.1038/nrm3311 [Crossref] [ Google Scholar]
- Srivastava RA, Pinkosky SL, Filippov S, Hanselman JC, Cramer CT, Newton RS. AMP-activated protein kinase: an emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J Lipid Res 2012; 53(12):2490-514. doi: 10.1194/jlr.R025882 [Crossref] [ Google Scholar]
- Sag D, Carling D, Stout RD, Suttles J. Adenosine 5’-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol 2008; 181(12):8633-41. doi: 10.4049/jimmunol.181.12.8633 [Crossref] [ Google Scholar]
- Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. AMPK in the brain: its roles in energy balance and neuroprotection. J Neurochem 2009; 109(Suppl 1):17-23. doi: 10.1111/j.1471-4159.2009.05916.x [Crossref] [ Google Scholar]
- Curry DW, Stutz B, Andrews ZB, Elsworth JD. Targeting AMPK signaling as a neuroprotective strategy in Parkinson’s disease. J Parkinsons Dis 2018; 8(2):161-81. doi: 10.3233/jpd-171296 [Crossref] [ Google Scholar]
- Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 2018; 19(2):121-35. doi: 10.1038/nrm.2017.95 [Crossref] [ Google Scholar]
- Sukumaran A, Choi K, Dasgupta B. Insight on transcriptional regulation of the energy sensing AMPK and biosynthetic mTOR pathway genes. Front Cell Dev Biol 2020; 8:671. doi: 10.3389/fcell.2020.00671 [Crossref] [ Google Scholar]
- Garcia D, Shaw RJ. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell 2017; 66(6):789-800. doi: 10.1016/j.molcel.2017.05.032 [Crossref] [ Google Scholar]
- Berndt N, Bulik S, Holzhütter HG. Kinetic modeling of the mitochondrial energy metabolism of neuronal cells: the impact of reduced α-ketoglutarate dehydrogenase activities on ATP production and generation of reactive oxygen species. Int J Cell Biol 2012; 2012:757594. doi: 10.1155/2012/757594 [Crossref] [ Google Scholar]
- Wu N, Zheng B, Shaywitz A, Dagon Y, Tower C, Bellinger G. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol Cell 2013; 49(6):1167-75. doi: 10.1016/j.molcel.2013.01.035 [Crossref] [ Google Scholar]
- Chaube B, Bhat MK. AMPK, a key regulator of metabolic/energy homeostasis and mitochondrial biogenesis in cancer cells. Cell Death Dis 2016; 7(1):e2044. doi: 10.1038/cddis.2015.404 [Crossref] [ Google Scholar]
- Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans 2002; 30(Pt 6):1064-70. doi: 10.1042/bst0301064 [Crossref] [ Google Scholar]
- Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC. AMPK regulates energy expenditure by modulating NAD + metabolism and SIRT1 activity. Nature 2009; 458(7241):1056-60. doi: 10.1038/nature07813 [Crossref] [ Google Scholar]
- Cantó C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci 2010; 67(20):3407-23. doi: 10.1007/s00018-010-0454-z [Crossref] [ Google Scholar]
- Tarasiuk O, Miceli M, Di Domizio A, Nicolini G. AMPK and diseases: state of the art regulation by AMPK-targeting molecules. Biology (Basel) 2022; 11(7):1041. doi: 10.3390/biology11071041 [Crossref] [ Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003; 115(5):577-90. doi: 10.1016/s0092-8674(03)00929-2 [Crossref] [ Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 2008; 30(2):214-26. doi: 10.1016/j.molcel.2008.03.003 [Crossref] [ Google Scholar]
- Hoppe S, Bierhoff H, Cado I, Weber A, Tiebe M, Grummt I. AMP-activated protein kinase adapts rRNA synthesis to cellular energy supply. Proc Natl Acad Sci U S A 2009; 106(42):17781-6. doi: 10.1073/pnas.0909873106 [Crossref] [ Google Scholar]
- Franco-Iborra S, Vila M, Perier C. The Parkinson disease mitochondrial hypothesis: where are we at?. Neuroscientist 2016; 22(3):266-77. doi: 10.1177/1073858415574600 [Crossref] [ Google Scholar]
- Fischer F, Hamann A, Osiewacz HD. Mitochondrial quality control: an integrated network of pathways. Trends Biochem Sci 2012; 37(7):284-92. doi: 10.1016/j.tibs.2012.02.004 [Crossref] [ Google Scholar]
- Shi R, Guberman M, Kirshenbaum LA. Mitochondrial quality control: the role of mitophagy in aging. Trends Cardiovasc Med 2018; 28(4):246-60. doi: 10.1016/j.tcm.2017.11.008 [Crossref] [ Google Scholar]
- de Castro IP, Martins LM, Loh SH. Mitochondrial quality control and Parkinson’s disease: a pathway unfolds. Mol Neurobiol 2011; 43(2):80-6. doi: 10.1007/s12035-010-8150-4 [Crossref] [ Google Scholar]
- Kamp F, Exner N, Lutz AK, Wender N, Hegermann J, Brunner B. Inhibition of mitochondrial fusion by α-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J 2010; 29(20):3571-89. doi: 10.1038/emboj.2010.223 [Crossref] [ Google Scholar]
- Ordonez DG, Lee MK, Feany MB. α-synuclein induces mitochondrial dysfunction through spectrin and the actin cytoskeleton. Neuron 2018;97(1):108-24.e6. 10.1016/j.neuron.2017.11.036.
- Twig G, Shirihai OS. The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal 2011; 14(10):1939-51. doi: 10.1089/ars.2010.3779 [Crossref] [ Google Scholar]
- Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 2006; 126(1):177-89. doi: 10.1016/j.cell.2006.06.025 [Crossref] [ Google Scholar]
- Clinton RW, Francy CA, Ramachandran R, Qi X, Mears JA. Dynamin-related protein 1 oligomerization in solution impairs functional interactions with membrane-anchored mitochondrial fission factor. J Biol Chem 2016; 291(1):478-92. doi: 10.1074/jbc.M115.680025 [Crossref] [ Google Scholar]
- Liang CL, Wang TT, Luby-Phelps K, German DC. Mitochondria mass is low in mouse substantia nigra dopamine neurons: implications for Parkinson’s disease. Exp Neurol 2007; 203(2):370-80. doi: 10.1016/j.expneurol.2006.08.015 [Crossref] [ Google Scholar]
- Park JS, Davis RL, Sue CM. Mitochondrial dysfunction in Parkinson’s disease: new mechanistic insights and therapeutic perspectives. Curr Neurol Neurosci Rep 2018; 18(5):21. doi: 10.1007/s11910-018-0829-3 [Crossref] [ Google Scholar]
- Ge P, Dawson VL, Dawson TM. PINK1 and Parkin mitochondrial quality control: a source of regional vulnerability in Parkinson’s disease. Mol Neurodegener 2020; 15(1):20. doi: 10.1186/s13024-020-00367-7 [Crossref] [ Google Scholar]
- Jin SM, Youle RJ. PINK1- and Parkin-mediated mitophagy at a glance. J Cell Sci 2012; 125(Pt 4):795-9. doi: 10.1242/jcs.093849 [Crossref] [ Google Scholar]
- Bose A, Beal MF. Mitochondrial dysfunction in Parkinson’s disease. J Neurochem 2016; 139 Suppl 1:216-31. doi: 10.1111/jnc.13731 [Crossref] [ Google Scholar]
- Berthet A, Margolis EB, Zhang J, Hsieh I, Zhang J, Hnasko TS. Loss of mitochondrial fission depletes axonal mitochondria in midbrain dopamine neurons. J Neurosci 2014; 34(43):14304-17. doi: 10.1523/jneurosci.0930-14.2014 [Crossref] [ Google Scholar]
- Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. BiochimBiophys Acta 2011; 1813(7):1269-78. doi: 10.1016/j.bbamcr.2010.09.019 [Crossref] [ Google Scholar]
- Corona JC, Duchen MR. PPARγ and PGC-1α as therapeutic targets in Parkinson’s. Neurochem Res 2015; 40(2):308-16. doi: 10.1007/s11064-014-1377-0 [Crossref] [ Google Scholar]
- Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML. PGC-1α, a potential therapeutic target for early intervention in Parkinson’s disease. Sci Transl Med 2010; 2(52):52ra73. doi: 10.1126/scitranslmed.3001059 [Crossref] [ Google Scholar]
- Dulovic M, Jovanovic M, Xilouri M, Stefanis L, Harhaji-Trajkovic L, Kravic-Stevovic T. The protective role of AMP-activated protein kinase in alpha-synuclein neurotoxicity in vitro. Neurobiol Dis 2014; 63:1-11. doi: 10.1016/j.nbd.2013.11.002 [Crossref] [ Google Scholar]
- Hang L, Wang Z, Foo ASC, Goh GWY, Choong HC, Thundyil J. Conditional disruption of AMP kinase in dopaminergic neurons promotes Parkinson’s disease-associated phenotypes in vivo. Neurobiol Dis 2021; 161:105560. doi: 10.1016/j.nbd.2021.105560 [Crossref] [ Google Scholar]
- Hang L, Thundyil J, Goh GWY, Lim KL. AMP kinase activation is selectively disrupted in the ventral midbrain of mice deficient in Parkin or PINK1 expression. Neuromolecular Med 2019; 21(1):25-32. doi: 10.1007/s12017-018-8517-7 [Crossref] [ Google Scholar]
- Marcinko K, Steinberg GR. The role of AMPK in controlling metabolism and mitochondrial biogenesis during exercise. Exp Physiol 2014; 99(12):1581-5. doi: 10.1113/expphysiol.2014.082255 [Crossref] [ Google Scholar]
- Kang C, Li Ji L. Role of PGC-1α signaling in skeletal muscle health and disease. Ann N Y Acad Sci 2012; 1271(1):110-7. doi: 10.1111/j.1749-6632.2012.06738.x [Crossref] [ Google Scholar]
- Peng K, Yang L, Wang J, Ye F, Dan G, Zhao Y. The interaction of mitochondrial biogenesis and fission/fusion mediated by PGC-1α regulates rotenone-induced dopaminergic neurotoxicity. Mol Neurobiol 2017; 54(5):3783-97. doi: 10.1007/s12035-016-9944-9 [Crossref] [ Google Scholar]
- Robb EL, Moradi F, Maddalena LA, Valente AJF, Fonseca J, Stuart JA. Resveratrol stimulates mitochondrial fusion by a mechanism requiring mitofusin-2. BiochemBiophys Res Commun 2017; 485(2):249-54. doi: 10.1016/j.bbrc.2017.02.102 [Crossref] [ Google Scholar]
- Morita M, Prudent J, Basu K, Goyon V, Katsumura S, Hulea L, et al. mTOR controls mitochondrial dynamics and cell survival via MTFP1. Mol Cell 2017;67(6):922-35.e5. 10.1016/j.molcel.2017.08.013.
- Toyama EQ, Herzig S, Courchet J, Lewis TL Jr, Losón OC, Hellberg K. Metabolism AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 2016; 351(6270):275-81. doi: 10.1126/science.aab4138 [Crossref] [ Google Scholar]
- Marin TL, Gongol B, Zhang F, Martin M, Johnson DA, Xiao H. AMPK promotes mitochondrial biogenesis and function by phosphorylating the epigenetic factors DNMT1, RBBP7, and HAT1. Sci Signal 2017; 10(464):eaaf7478. doi: 10.1126/scisignal.aaf7478 [Crossref] [ Google Scholar]
- Ma K, Chen G, Li W, Kepp O, Zhu Y, Chen Q. Mitophagy, mitochondrial homeostasis, and cell fate. Front Cell Dev Biol 2020; 8:467. doi: 10.3389/fcell.2020.00467 [Crossref] [ Google Scholar]
- Zhu J, Wang KZ, Chu CT. After the banquet: mitochondrial biogenesis, mitophagy, and cell survival. Autophagy 2013; 9(11):1663-76. doi: 10.4161/auto.24135 [Crossref] [ Google Scholar]
- Park JS, Lee DH, Lee YS, Oh E, Bae KH, Oh KJ. Dual roles of ULK1 (unc-51 like autophagy activating kinase 1) in cytoprotection against lipotoxicity. Autophagy 2020; 16(1):86-105. doi: 10.1080/15548627.2019.1598751 [Crossref] [ Google Scholar]
- Wang S, Tan J, Miao Y, Zhang Q. Mitochondrial dynamics, mitophagy, and mitochondria-endoplasmic reticulum contact sites crosstalk under hypoxia. Front Cell Dev Biol 2022; 10:848214. doi: 10.3389/fcell.2022.848214 [Crossref] [ Google Scholar]
- Laker RC, Drake JC, Wilson RJ, Lira VA, Lewellen BM, Ryall KA. Ampk phosphorylation of ULK1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun 2017; 8(1):548. doi: 10.1038/s41467-017-00520-9 [Crossref] [ Google Scholar]
- Diebold L, Chandel NS. Mitochondrial ROS regulation of proliferating cells. Free Radic Biol Med 2016; 100:86-93. doi: 10.1016/j.freeradbiomed.2016.04.198 [Crossref] [ Google Scholar]
- Rabinovitch RC, Samborska B, Faubert B, Ma EH, Gravel SP, Andrzejewski S. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep 2017; 21(1):1-9. doi: 10.1016/j.celrep.2017.09.026 [Crossref] [ Google Scholar]
- Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis 2013; 3(4):461-91. doi: 10.3233/jpd-130230 [Crossref] [ Google Scholar]
- Wei Z, Li X, Li X, Liu Q, Cheng Y. Oxidative stress in Parkinson’s disease: a systematic review and meta-analysis. Front Mol Neurosci 2018; 11:236. doi: 10.3389/fnmol.2018.00236 [Crossref] [ Google Scholar]
- Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR. Oxidative stress and Parkinson’s disease. Front Neuroanat 2015; 9:91. doi: 10.3389/fnana.2015.00091 [Crossref] [ Google Scholar]
- Guo JD, Zhao X, Li Y, Li GR, Liu XL. Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease (review). Int J Mol Med 2018; 41(4):1817-25. doi: 10.3892/ijmm.2018.3406 [Crossref] [ Google Scholar]
- Greenamyre JT, Cannon JR, Drolet R, Mastroberardino PG. Lessons from the rotenone model of Parkinson’s disease. Trends Pharmacol Sci 2010; 31(4):141-2. doi: 10.1016/j.tips.2009.12.006 [Crossref] [ Google Scholar]
- Blesa J, Przedborski S. Parkinson’s disease: animal models and dopaminergic cell vulnerability. Front Neuroanat 2014; 8:155. doi: 10.3389/fnana.2014.00155 [Crossref] [ Google Scholar]
- Stott SRW, Barker RA. Time course of dopamine neuron loss and glial response in the 6-OHDA striatal mouse model of Parkinson’s disease. Eur J Neurosci 2014; 39(6):1042-56. doi: 10.1111/ejn.12459 [Crossref] [ Google Scholar]
- Zimmermann K, Baldinger J, Mayerhofer B, Atanasov AG, Dirsch VM, Heiss EH. Activated AMPK boosts the Nrf2/HO-1 signaling axis--a role for the unfolded protein response. Free Radic Biol Med 2015; 88(Pt B):417-26. doi: 10.1016/j.freeradbiomed.2015.03.030 [Crossref] [ Google Scholar]
- Tebay LE, Robertson H, Durant ST, Vitale SR, Penning TM, Dinkova-Kostova AT. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol Med 2015; 88(Pt B):108-46. doi: 10.1016/j.freeradbiomed.2015.06.021 [Crossref] [ Google Scholar]
- Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ. The role of autophagy in Parkinson’s disease. Cold Spring HarbPerspect Med 2012; 2(4):a009357. doi: 10.1101/cshperspect.a009357 [Crossref] [ Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006; 441(7095):880-4. doi: 10.1038/nature04723 [Crossref] [ Google Scholar]
- Wang X, Hu W, Qu L, Wang J, Wu A, Lo HH. Tricin promoted ATG-7 dependent autophagic degradation of α-synuclein and dopamine release for improving cognitive and motor deficits in Parkinson’s disease. Pharmacol Res 2023; 196:106874. doi: 10.1016/j.phrs.2023.106874 [Crossref] [ Google Scholar]
- Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L. Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Biol Chem 2008; 283(35):23542-56. doi: 10.1074/jbc.M801992200 [Crossref] [ Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 2004; 305(5688):1292-5. doi: 10.1126/science.1101738 [Crossref] [ Google Scholar]
- Ciechanover A, Kwon YT. Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Exp Mol Med 2015; 47(3):e147. doi: 10.1038/emm.2014.117 [Crossref] [ Google Scholar]
- Xilouri M, Stefanis L. Autophagic pathways in Parkinson disease and related disorders. Expert Rev Mol Med 2011; 13:e8. doi: 10.1017/s1462399411001803 [Crossref] [ Google Scholar]
- Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Björklund A. TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc Natl Acad Sci U S A 2013; 110(19):E1817-26. doi: 10.1073/pnas.1305623110 [Crossref] [ Google Scholar]
- Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci 2009; 29(43):13578-88. doi: 10.1523/jneurosci.4390-09.2009 [Crossref] [ Google Scholar]
- Egan DF, Chun MG, Vamos M, Zou H, Rong J, Miller CJ. Small molecule inhibition of the autophagy kinase ULK1 and Identification of ULK1 substrates. Mol Cell 2015; 59(2):285-97. doi: 10.1016/j.molcel.2015.05.031 [Crossref] [ Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of ULK1. Nat Cell Biol 2011; 13(2):132-41. doi: 10.1038/ncb2152 [Crossref] [ Google Scholar]
- Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol 2013; 15(7):741-50. doi: 10.1038/ncb2757 [Crossref] [ Google Scholar]
- Wang S, Li H, Yuan M, Fan H, Cai Z. Role of AMPK in autophagy. Front Physiol 2022; 13:1015500. doi: 10.3389/fphys.2022.1015500 [Crossref] [ Google Scholar]
- Lyra P, Machado V, Rota S, Chaudhuri KR, Botelho J, Mendes JJ. Revisiting Alpha-Synuclein Pathways to Inflammation. Int J Mol Sci 2023; 24(8):7137. doi: 10.3390/ijms24087137 [Crossref] [ Google Scholar]
- Rosso P, Fioramonti M, Fracassi A, Marangoni M, Taglietti V, Siteni S. AMPK in the central nervous system: physiological roles and pathological implications. Res Rep Biol 2016; 7:1-13. doi: 10.2147/rrb.s90858 [Crossref] [ Google Scholar]
- Moors TE, Hoozemans JJ, Ingrassia A, Beccari T, Parnetti L, Chartier-Harlin MC. Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Mol Neurodegener 2017; 12(1):11. doi: 10.1186/s13024-017-0154-3 [Crossref] [ Google Scholar]
- El-Ghaiesh SH, Bahr HI, Ibrahiem AT, Ghorab D, Alomar SY, Farag NE. Metformin protects from rotenone-induced nigrostriatal neuronal death in adult mice by activating AMPK-FOXO3 signaling and mitigation of angiogenesis. Front Mol Neurosci 2020; 13:84. doi: 10.3389/fnmol.2020.00084 [Crossref] [ Google Scholar]
- Ng CH, Guan MS, Koh C, Ouyang X, Yu F, Tan EK. AMP kinase activation mitigates dopaminergic dysfunction and mitochondrial abnormalities in Drosophila models of Parkinson’s disease. J Neurosci 2012; 32(41):14311-7. doi: 10.1523/jneurosci.0499-12.2012 [Crossref] [ Google Scholar]
- Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals 2011; 19(3):163-74. doi: 10.1159/000328516 [Crossref] [ Google Scholar]
- Ferretta A, Gaballo A, Tanzarella P, Piccoli C, Capitanio N, Nico B. Effect of resveratrol on mitochondrial function: implications in parkin-associated familiar Parkinson’s disease. BiochimBiophys Acta 2014; 1842(7):902-15. doi: 10.1016/j.bbadis.2014.02.010 [Crossref] [ Google Scholar]
- Klein C, Westenberger A. Genetics of Parkinson’s disease. Cold Spring HarbPerspect Med 2012; 2(1):a008888. doi: 10.1101/cshperspect.a008888 [Crossref] [ Google Scholar]
- Srinivasan E, Chandrasekhar G, Chandrasekar P, Anbarasu K, Vickram AS, Karunakaran R. Alpha-synuclein aggregation in Parkinson’s disease. Front Med (Lausanne) 2021; 8:736978. doi: 10.3389/fmed.2021.736978 [Crossref] [ Google Scholar]
- Kalia LV, Lang AE. Parkinson’s disease. Lancet 2015; 386(9996):896-912. doi: 10.1016/s0140-6736(14)61393-3 [Crossref] [ Google Scholar]
- Phillipson OT. Alpha-synuclein, epigenetics, mitochondria, metabolism, calcium traffic, & circadian dysfunction in Parkinson’s disease An integrated strategy for management. Ageing Res Rev 2017; 40:149-67. doi: 10.1016/j.arr.2017.09.006 [Crossref] [ Google Scholar]
- Deeg S, Gralle M, Sroka K, Bähr M, Wouters FS, Kermer P. BAG1 restores formation of functional DJ-1 L166P dimers and DJ-1 chaperone activity. J Cell Biol 2010; 188(4):505-13. doi: 10.1083/jcb.200904103 [Crossref] [ Google Scholar]
- Cookson MR. DJ-1, PINK1, and their effects on mitochondrial pathways. Mov Disord 2010; 25(Suppl 1):S44-8. doi: 10.1002/mds.22713 [Crossref] [ Google Scholar]
- Heremans IP, Caligiore F, Gerin I, Bury M, Lutz M, Graff J. Parkinson’s disease protein PARK7 prevents metabolite and protein damage caused by a glycolytic metabolite. Proc Natl Acad Sci U S A 2022; 119(4):e2111338119. doi: 10.1073/pnas.2111338119 [Crossref] [ Google Scholar]
- Meulener M, Whitworth AJ, Armstrong-Gold CE, Rizzu P, Heutink P, Wes PD. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson’s disease. Curr Biol 2005; 15(17):1572-7. doi: 10.1016/j.cub.2005.07.064 [Crossref] [ Google Scholar]
- Murata H, Takamatsu H, Liu S, Kataoka K, Huh NH, Sakaguchi M. NRF2 regulates PINK1 expression under oxidative stress conditions. PLoS One 2015; 10(11):e0142438. doi: 10.1371/journal.pone.0142438 [Crossref] [ Google Scholar]
- Moon HE, Paek SH. Mitochondrial dysfunction in Parkinson’s disease. Exp Neurobiol 2015; 24(2):103-16. doi: 10.5607/en.2015.24.2.103 [Crossref] [ Google Scholar]
- Cookson MR. Parkinsonism due to mutations in PINK1, Parkin, and DJ-1 and oxidative stress and mitochondrial pathways. Cold Spring HarbPerspect Med 2012; 2(9):a009415. doi: 10.1101/cshperspect.a009415 [Crossref] [ Google Scholar]
- Li X, Geng J, Liu J. Adiponectin offers protection against L166P mutant DJ-1-induced neuronal cytotoxicity mediated by APPL1-dependent AMPK activation. Int J Neurosci 2014; 124(5):350-61. doi: 10.3109/00207454.2013.846340 [Crossref] [ Google Scholar]
- Gundersen V. Parkinson’s disease: can targeting inflammation be an effective neuroprotective strategy?. Front Neurosci 2020; 14:580311. doi: 10.3389/fnins.2020.580311 [Crossref] [ Google Scholar]
- Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol 2011; 26 Suppl 1:S1-58. doi: 10.1007/s10654-011-9581-6 [Crossref] [ Google Scholar]
- O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013; 493(7432):346-55. doi: 10.1038/nature11862 [Crossref] [ Google Scholar]
- Yin J, Valin KL, Dixon ML, Leavenworth JW. The role of microglia and macrophages in CNS homeostasis, autoimmunity, and cancer. J Immunol Res 2017; 2017:5150678. doi: 10.1155/2017/5150678 [Crossref] [ Google Scholar]
- Kofler J, Wiley CA. Microglia: key innate immune cells of the brain. ToxicolPathol 2011; 39(1):103-14. doi: 10.1177/0192623310387619 [Crossref] [ Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988; 38(8):1285-91. doi: 10.1212/wnl.38.8.1285 [Crossref] [ Google Scholar]
- Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS One 2010; 5(1):e8784. doi: 10.1371/journal.pone.0008784 [Crossref] [ Google Scholar]
- Chung CY, Koprich JB, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci 2009; 29(11):3365-73. doi: 10.1523/jneurosci.5427-08.2009 [Crossref] [ Google Scholar]
- Sanchez-Guajardo V, Tentillier N, Romero-Ramos M. The relation between α-synuclein and microglia in Parkinson’s disease: recent developments. Neuroscience 2015; 302:47-58. doi: 10.1016/j.neuroscience.2015.02.008 [Crossref] [ Google Scholar]
- Dzamko N, Gysbers A, Perera G, Bahar A, Shankar A, Gao J. Toll-like receptor 2 is increased in neurons in Parkinson’s disease brain and may contribute to alpha-synuclein pathology. Acta Neuropathol 2017; 133(2):303-19. doi: 10.1007/s00401-016-1648-8 [Crossref] [ Google Scholar]
- Pajares M, Rojo AI, Manda G, Boscá L, Cuadrado A. Inflammation in Parkinson’s disease: mechanisms and therapeutic implications. Cells 2020; 9(7):1687. doi: 10.3390/cells9071687 [Crossref] [ Google Scholar]
- Na SJ, DiLella AG, Lis EV, Jones K, Levine DM, Stone DJ. Molecular profiling of a 6-hydroxydopamine model of Parkinson’s disease. Neurochem Res 2010; 35(5):761-72. doi: 10.1007/s11064-010-0133-3 [Crossref] [ Google Scholar]
- Singh SS, Rai SN, Birla H, Zahra W, Rathore AS, Singh SP. NF-κB-mediated neuroinflammation in Parkinson’s disease and potential therapeutic effect of polyphenols. Neurotox Res 2020; 37(3):491-507. doi: 10.1007/s12640-019-00147-2 [Crossref] [ Google Scholar]
- Li Y, Xia Y, Yin S, Wan F, Hu J, Kou L. Targeting microglial α-synuclein/TLRs/NF-kappaB/NLRP3 inflammasome axis in Parkinson’s disease. Front Immunol 2021; 12:719807. doi: 10.3389/fimmu.2021.719807 [Crossref] [ Google Scholar]
- Al-Nusaif M, Yang Y, Li S, Cheng C, Le W. The role of NURR1 in metabolic abnormalities of Parkinson’s disease. Mol Neurodegener 2022; 17(1):46. doi: 10.1186/s13024-022-00544-w [Crossref] [ Google Scholar]
- Yang XX, Yang R, Zhang F. Role of Nrf2 in Parkinson’s disease: toward new perspectives. Front Pharmacol 2022; 13:919233. doi: 10.3389/fphar.2022.919233 [Crossref] [ Google Scholar]
- Parga JA, Rodriguez-Perez AI, Garcia-Garrote M, Rodriguez-Pallares J, Labandeira-Garcia JL. NRF2 activation and downstream effects: focus on Parkinson’s disease and brain angiotensin. Antioxidants (Basel) 2021; 10(11):1649. doi: 10.3390/antiox10111649 [Crossref] [ Google Scholar]
- Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (Berl) 2011; 89(7):667-76. doi: 10.1007/s00109-011-0748-0 [Crossref] [ Google Scholar]
- Chu X, Cao L, Yu Z, Xin D, Li T, Ma W. Hydrogen-rich saline promotes microglia M2 polarization and complement-mediated synapse loss to restore behavioral deficits following hypoxia-ischemic in neonatal mice via AMPK activation. J Neuroinflammation 2019; 16(1):104. doi: 10.1186/s12974-019-1488-2 [Crossref] [ Google Scholar]
- Salama A, Elgohary R. L-carnitine and Co Q10 ameliorate potassium dichromate -induced acute brain injury in rats targeting AMPK/AKT/NF-κβ. Int Immunopharmacol 2021; 101(Pt B):107867. doi: 10.1016/j.intimp.2021.107867 [Crossref] [ Google Scholar]
- Li YQ, Chen Y, Jiang SQ, Shi YY, Jiang XL, Wu SS. An inhibitor of NF-κB and an agonist of AMPK: network prediction and multi-omics integration to derive signaling pathways for acteoside against Alzheimer’s disease. Front Cell Dev Biol 2021; 9:652310. doi: 10.3389/fcell.2021.652310 [Crossref] [ Google Scholar]
- Cao B, Zhang Y, Chen J, Wu P, Dong Y, Wang Y. Neuroprotective effects of liraglutide against inflammation through the AMPK/NF-κB pathway in a mouse model of Parkinson’s disease. Metab Brain Dis 2022; 37(2):451-62. doi: 10.1007/s11011-021-00879-1 [Crossref] [ Google Scholar]
- Mohamad KA, El-Naga RN, Wahdan SA. Neuroprotective effects of indole-3-carbinol on the rotenone rat model of Parkinson’s disease: impact of the SIRT1-AMPK signaling pathway. Toxicol Appl Pharmacol 2022; 435:115853. doi: 10.1016/j.taap.2021.115853 [Crossref] [ Google Scholar]
- Skonieczna M, Hejmo T, Poterala-Hejmo A, Cieslar-Pobuda A, Buldak RJ. NADPH oxidases: insights into selected functions and mechanisms of action in cancer and stem cells. Oxid Med Cell Longev 2017; 2017:9420539. doi: 10.1155/2017/9420539 [Crossref] [ Google Scholar]
- Balteau M, Van Steenbergen A, Timmermans AD, Dessy C, Behets-Wydemans G, Tajeddine N. AMPK activation by glucagon-like peptide-1 prevents NADPH oxidase activation induced by hyperglycemia in adult cardiomyocytes. Am J Physiol Heart Circ Physiol 2014; 307(8):H1120-33. doi: 10.1152/ajpheart.00210.2014 [Crossref] [ Google Scholar]
- Pilon G, Dallaire P, Marette A. Inhibition of inducible nitric-oxide synthase by activators of AMP-activated protein kinase: a new mechanism of action of insulin-sensitizing drugs. J Biol Chem 2004; 279(20):20767-74. doi: 10.1074/jbc.M401390200 [Crossref] [ Google Scholar]
- Guma M, Wang Y, Viollet B, Liu-Bryan R. AMPK activation by A-769662 controls IL-6 expression in inflammatory arthritis. PLoS One 2015; 10(10):e0140452. doi: 10.1371/journal.pone.0140452 [Crossref] [ Google Scholar]
- Zhang Q, Zhang P, Qi GJ, Zhang Z, He F, Lv ZX. Cdk5 suppression blocks SIRT1 degradation via the ubiquitin-proteasome pathway in Parkinson’s disease models. BiochimBiophys Acta Gen Subj 2018; 1862(6):1443-51. doi: 10.1016/j.bbagen.2018.03.021 [Crossref] [ Google Scholar]
- Rodriguez L, Marano MM, Tandon A. Import and export of misfolded α-synuclein. Front Neurosci 2018; 12:344. doi: 10.3389/fnins.2018.00344 [Crossref] [ Google Scholar]
- Batiha GE, Al-Kuraishy HM, Al-Gareeb AI, Elekhnawy E. SIRT1 pathway in Parkinson’s disease: a faraway snapshot but so close. Inflammopharmacology 2023; 31(1):37-56. doi: 10.1007/s10787-022-01125-5 [Crossref] [ Google Scholar]
- Li X, Feng Y, Wang XX, Truong D, Wu YC. The critical role of SIRT1 in Parkinson’s disease: mechanism and therapeutic considerations. Aging Dis 2020; 11(6):1608-22. doi: 10.14336/ad.2020.0216 [Crossref] [ Google Scholar]
- Xu H, Shen J, Xiao J, Chen F, Wang M. Neuroprotective effect of cajaninstilbene acid against cerebral ischemia and reperfusion damages by activating AMPK/Nrf2 pathway. J Adv Res 2021; 34:199-210. doi: 10.1016/j.jare.2020.07.011 [Crossref] [ Google Scholar]
- Erekat NS. Apoptosis and its role in Parkinson’s disease. In: Stoker TB, Greenland JC, eds. Parkinson’s Disease: Pathogenesis and Clinical Aspects. Brisbane, AU: Codon Publications. 2018. p. 65-82. 10.15586/codonpublications.parkinsonsdisease.2018.ch4.
- Hartmann A, Michel PP, Troadec JD, Mouatt-Prigent A, Faucheux BA, Ruberg M. Is Bax a mitochondrial mediator in apoptotic death of dopaminergic neurons in Parkinson’s disease?. J Neurochem 2001; 76(6):1785-93. doi: 10.1046/j.1471-4159.2001.00160.x [Crossref] [ Google Scholar]
- Chang KH, Chen CM. The role of oxidative stress in Parkinson’s disease. Antioxidants (Basel) 2020; 9(7):597. doi: 10.3390/antiox9070597 [Crossref] [ Google Scholar]
- Villanueva-Paz M, Cotán D, Garrido-Maraver J, Oropesa-Ávila M, de la Mata M, Delgado-Pavón A. AMPK regulation of cell growth, apoptosis, autophagy, and bioenergetics. Exp Suppl 2016; 107:45-71. doi: 10.1007/978-3-319-43589-3_3 [Crossref] [ Google Scholar]
- Bonini MG, Gantner BN. The multifaceted activities of AMPK in tumor progression--why the “one size fits all” definition does not fit at all?. IUBMB Life 2013; 65(11):889-96. doi: 10.1002/iub.1213 [Crossref] [ Google Scholar]
- Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally?. J Physiol 2006; 574(Pt 1):95-112. doi: 10.1113/jphysiol.2006.109389 [Crossref] [ Google Scholar]
- Meisse D, Van de Casteele M, Beauloye C, Hainault I, Kefas BA, Rider MH. Sustained activation of AMP-activated protein kinase induces c-Jun N-terminal kinase activation and apoptosis in liver cells. FEBS Lett 2002; 526(1-3):38-42. doi: 10.1016/s0014-5793(02)03110-1 [Crossref] [ Google Scholar]
- Kefas BA, Cai Y, Ling Z, Heimberg H, Hue L, Pipeleers D. AMP-activated protein kinase can induce apoptosis of insulin-producing MIN6 cells through stimulation of c-Jun-N-terminal kinase. J Mol Endocrinol 2003; 30(2):151-61. doi: 10.1677/jme.0.0300151 [Crossref] [ Google Scholar]
- Stefanelli C, Stanic I, Bonavita F, Flamigni F, Pignatti C, Guarnieri C. Inhibition of glucocorticoid-induced apoptosis with 5-aminoimidazole-4-carboxamide ribonucleoside, a cell-permeable activator of AMP-activated protein kinase. BiochemBiophys Res Commun 1998; 243(3):821-6. doi: 10.1006/bbrc.1998.8154 [Crossref] [ Google Scholar]
- Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci 2001; 17(1):45-58. doi: 10.1385/jmn:17:1:45 [Crossref] [ Google Scholar]
- Russell RR 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest 2004; 114(4):495-503. doi: 10.1172/jci19297 [Crossref] [ Google Scholar]
- Kim J, Park YJ, Jang Y, Kwon YH. AMPK activation inhibits apoptosis and tau hyperphosphorylation mediated by palmitate in SH-SY5Y cells. Brain Res 2011; 1418:42-51. doi: 10.1016/j.brainres.2011.08.059 [Crossref] [ Google Scholar]
- Shaw MM, Gurr WK, McCrimmon RJ, Schorderet DF, Sherwin RS. 5’AMP-activated protein kinase alpha deficiency enhances stress-induced apoptosis in BHK and PC12 cells. J Cell Mol Med 2007; 11(2):286-98. doi: 10.1111/j.1582-4934.2007.00023.x [Crossref] [ Google Scholar]
- Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem 2010; 285(48):37503-12. doi: 10.1074/jbc.M110.136796 [Crossref] [ Google Scholar]
- Liu L, Liu C, Fang L. AMPK-SIRT1 pathway dysfunction contributes to neuron apoptosis and cognitive impairment induced by sevoflurane. Mol Med Rep 2021; 23(1):56. doi: 10.3892/mmr.2020.11694 [Crossref] [ Google Scholar]
- Wang Y, Tian M, Tan J, Pei X, Lu C, Xin Y. Irisin ameliorates neuroinflammation and neuronal apoptosis through integrin αVβ5/AMPK signaling pathway after intracerebral hemorrhage in mice. J Neuroinflammation 2022; 19(1):82. doi: 10.1186/s12974-022-02438-6 [Crossref] [ Google Scholar]
- Park JS, Choe K, Lee HJ, Park TJ, Kim MO. Neuroprotective effects of osmotin in Parkinson’s disease-associated pathology via the AdipoR1/MAPK/AMPK/mTOR signaling pathways. J Biomed Sci 2023; 30(1):66. doi: 10.1186/s12929-023-00961-z [Crossref] [ Google Scholar]
- Su J, Zhang J, Bao R, Xia C, Zhang Y, Zhu Z. Mitochondrial dysfunction and apoptosis are attenuated through activation of AMPK/GSK-3β/PP2A pathway in Parkinson’s disease. Eur J Pharmacol 2021; 907:174202. doi: 10.1016/j.ejphar.2021.174202 [Crossref] [ Google Scholar]
- Peixoto CA, de Oliveira WH, da Racho Araújo SM, Nunes AKS. AMPK activation: role in the signaling pathways of neuroinflammation and neurodegeneration. Exp Neurol 2017; 298(Pt A):31-41. doi: 10.1016/j.expneurol.2017.08.013 [Crossref] [ Google Scholar]
- Chen P, Wang Y, Chen L, Song N, Xie J. Apelin-13 protects dopaminergic neurons against rotenone-induced neurotoxicity through the AMPK/mTOR/ULK-1 mediated autophagy activation. Int J Mol Sci 2020; 21(21):8376. doi: 10.3390/ijms21218376 [Crossref] [ Google Scholar]
- Chen M, Peng L, Gong P, Zheng X, Sun T, Zhang X. Baicalein induces mitochondrial autophagy to prevent Parkinson’s disease in rats via miR-30b and the SIRT1/AMPK/mTOR pathway. Front Neurol 2021; 12:646817. doi: 10.3389/fneur.2021.646817 [Crossref] [ Google Scholar]
- Wang H, Dou S, Zhu J, Shao Z, Wang C, Xu X. Ghrelin protects against rotenone-induced cytotoxicity: Involvement of mitophagy and the AMPK/SIRT1/PGC1α pathway. Neuropeptides 2021; 87:102134. doi: 10.1016/j.npep.2021.102134 [Crossref] [ Google Scholar]
- Huang Y, Wu H, Hu Y, Zhou C, Wu J, Wu Y. Puerarin attenuates oxidative stress and ferroptosis via AMPK/PGC1α/Nrf2 pathway after subarachnoid hemorrhage in rats. Antioxidants (Basel) 2022; 11(7):1259. doi: 10.3390/antiox11071259 [Crossref] [ Google Scholar]
- de Gregorio E, Colell A, Morales A, Marí M. Relevance of SIRT1-NF-κB axis as therapeutic target to ameliorate inflammation in liver disease. Int J Mol Sci 2020; 21(11):3858. doi: 10.3390/ijms21113858 [Crossref] [ Google Scholar]
- Yu J, Wang WN, Matei N, Li X, Pang JW, Mo J. Ezetimibe attenuates oxidative stress and neuroinflammation via the AMPK/Nrf2/TXNIP pathway after MCAO in rats. Oxid Med Cell Longev 2020; 2020:4717258. doi: 10.1155/2020/4717258 [Crossref] [ Google Scholar]
- Li J, Chen L, Qin Q, Wang D, Zhao J, Gao H. Upregulated hexokinase 2 expression induces the apoptosis of dopaminergic neurons by promoting lactate production in Parkinson’s disease. Neurobiol Dis 2022; 163:105605. doi: 10.1016/j.nbd.2021.105605 [Crossref] [ Google Scholar]
- Park JS, Choe K, Lee HJ, Park TJ, Kim MO. Neuroprotective effects of osmotin in Parkinson’s disease-associated pathology via the AdipoR1/MAPK/AMPK/mTOR signaling pathways. J Biomed Sci 2023; 30(1):66. doi: 10.1186/s12929-023-00961-z [Crossref] [ Google Scholar]