Advanced pharmaceutical bulletin. 14(2):302-313.
doi: 10.34172/apb.2024.035
Review Article
Notch Signaling Suppression by Golden Phytochemicals: Potential for Cancer Therapy
Masoumeh Kaveh Zenjanab Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, 1 
Nastaran Hashemzadeh Investigation, 2
Sajjad Alimohammadvand Investigation, Software, 3
Masoumeh Sharifi-Azad Investigation, 3
Elaheh Dalir Abdolahinia Project administration, Resources, 4, * 
Rana Jahanban-Esfahlan Conceptualization, Data curation, Formal analysis, Supervision, Validation, Writing – review & editing, 1, 3, * 
Author information:
1Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
2Pharmaceutical Analysis Research Center and Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran.
3Department of Medical Biotechnology, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
4Department of Oral Science and Translation Research, College of Dental Medicine, Nova Southeastern University, Fort Lauderdale, FL 33314, US.
Abstract
Cancer is one of the main causes of mortality worldwide. Cancer cells are characterized by unregulated cellular processes, including proliferation, progression, and angiogenesis. The occurrence of these processes is due to the dysregulation of various signaling pathways such as NF-κB (nuclear factor-κB), Wnt/beta-catenin, Notch signaling and MAPK (mitogen-activated protein kinases). Notch signaling pathways cause the progression of various types of malignant tumors. Among the phytochemicals for cancer therapy, several have attracted great interest, including curcumin, genistein, quercetin, silibinin, resveratrol, cucurbitacin and glycyrrhizin. Given the great cellular and molecular heterogeneity within tumors and the high toxicity and side effects of synthetic chemotherapeutics, natural products with pleiotropic effects that simultaneously target numerous signaling pathways appear to be ideal substitutes for cancer therapy. With this in mind, we take a look at the current status, impact and potential of known compounds as golden phytochemicals on key signaling pathways in tumors, focusing on the Notch pathway. This review may be useful for discovering new molecular targets for safe and efficient cancer therapy with natural chemotherapeutics.
Keywords: Cancer, Cell signaling, Notch signaling pathway, Natural compounds, Phytochemicals
Copyright and License Information
©2024 The Authors.
This is an open access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Funding Statement
RJE is supported by Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran, grant number 62674.
Introduction
Cancer is one of the most important diseases in industrialized and developing countries. In 2018, cancer contributed to 9.6 million deaths worldwide.1 Various environmental and genetic factors trigger mutations in susceptible cells, leading to growth, progression and ultimately cancer.2 Normal cells have mechanisms to suppress tumors. However, problems arise when these mechanisms and their functions are limited by a mutation in a gene that suppresses cancer.3 The body’s homeostasis is necessary for survival, and cell death is essential to control cell turnover. However, uncontrolled growth and tumors occur when cells cannot maintain the balance between survival and death.4 Signaling pathways play a critical role in maintaining the balance between proliferation, survival, and apoptosis of cells, and dysregulation of these pathways leads to uncontrolled growth of cells and metastasis to other parts of the body.5,6 Due to the high incidence of cancer, its treatment has been relatively unsuccessful. Current cancer treatment options include surgical removal and radiotherapy, usually followed by systemic chemotherapy.7,8 Available chemotherapeutic agents include anti-tubulin agents (taxanes), DNA-interacting agents (e.g., doxorubicin, cisplatin), antimetabolites (e.g., methotrexate), molecular targeting agents and hormones.9,10 While chemotherapy is one of the major cancer treatments, it has many disadvantages such as cancer recurrence, toxicity to nontargeted tissues, and drug resistance. These drawbacks can limit the use of chemotherapeutic agents. The search for new promising anticancer drugs with fewer side effects and better efficacy is essential to overcome the problems of current treatments.8,11-13
Plant derivatives and phytochemicals are promising options for improving the efficacy of cancer patient treatment and eliciting minimum adverse effects.14,15 Some of these phytochemicals have significant anticancer effects.16 Many of the phytochemical components are part of the human diet. Thus, a long history of these phytochemical components including human exposure, good tolerance, low toxicity, and documented biological activities, is currently being evaluated to eliminate cancer.17-22
Dysregulation of numerous signaling pathways, such as Notch, contributes to cancer progression and recurrence. The Notch signaling pathway plays an important role in stem cell survival, proliferation, renewal, cell differentiation, and cell fate determination during morphogenesis and development.23 Studies have shown that disruption of Notch pathway regulation contributes to carcinogenesis, angiogenesis, cancer stem cell (CSC) renewal, and resistance to chemotherapy. High levels of Notch ligands and receptors correlate with cancer progression and poor survival.24 In addition, the transcriptional activity of important target genes is regulated by the Notch signaling pathway via interactions with numerous other signaling pathways. The Notch signaling pathway has been shown to be a suitable therapeutic target for the treatment of various types of cancer. Researchers have demonstrated the anti-tumor properties of Notch inhibitors in various types of cancer.25
The current review has classified a variety of phytochemicals and focuses on their effects on the different signaling pathways, with special attention to the Notch pathway. Also, the connection between the different signaling pathways, in particular the Notch pathway, with other signaling pathways is explained. In addition, the potential of these natural compounds for cancer therapy is discussed, which can be useful for the discovery of new molecular targets for safe and efficient cancer therapy with natural chemotherapeutics.
Notch signaling pathway
The Notch signaling pathway plays a critical role in cell fate, and mutations in this pathway lead to malignancy and drug resistance.26 The Notch signaling structure consists of the Notch receptors in humans (Notch 1-4), a transmembrane protein, and their ligands (Jagged1, 2 and delta-like ligands 1, 3 and 4).27 The adjacent cell ligands bind to the Notch receptors and γ-Secretase cleavages the receptor of Notch, then Notch intracellular domain (NICD) release. NICD translocates towards the nucleus, binds to numerous transcriptional regulators and induces apoptosis, and induces cell proliferation.28-30 In addition, Notch signaling during embryonic development plays an important role in lineage decision and stem cell maintenance. Depending on the organ and tissue, Notch signaling may play a different role, such as hematopoiesis in the fetus and in adulthood, or initiate terminal differentiation.28
Notch genes encode highly conserved transmembrane receptors that are involved in cell fate decisions (Figure 1).31-33 Signaling through this pathway depends on direct contact between neighboring cells expressing Notch receptors and ligands. Finally, downstream signaling leads to differentiation, proliferation, survival and regulation of cell fate specification.33,34
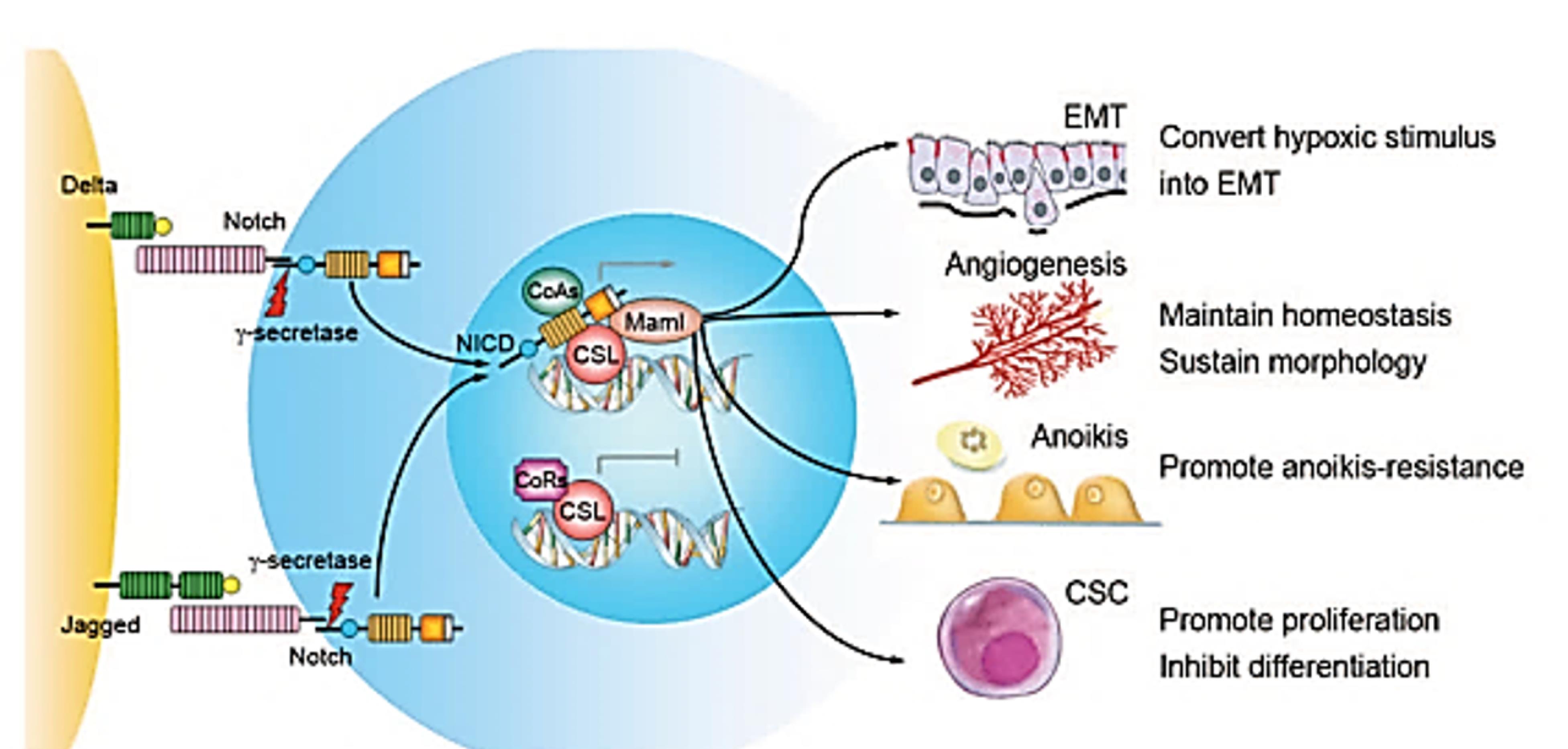
Figure 1.
The pathway of Notch signaling and its roles in metastasis of cancers. Delta-like and Jagged families of ligands activate Notch receptors. NICD proteins, after proteolysis by γ-secretase, are transferred to the nucleus, bind to the DNA binding protein CSL, and take the place of corepressors (CoRs). NICD forms a complex with the coactivators (CoAs) and DNA binding protein CSL, which activate the transcription of Notch target genes. Notch signaling activation in the microenvironment of a tumor can increase tumor cell resistance to anoikis, epithelial-mesenchymal transition (EMT), maintenance of angiogenic homeostasis, vascular morphology, and self-renewal of cancer stem cells (CSCs). Adopted from Hu et al31 with permission from Springer
.
The pathway of Notch signaling and its roles in metastasis of cancers. Delta-like and Jagged families of ligands activate Notch receptors. NICD proteins, after proteolysis by γ-secretase, are transferred to the nucleus, bind to the DNA binding protein CSL, and take the place of corepressors (CoRs). NICD forms a complex with the coactivators (CoAs) and DNA binding protein CSL, which activate the transcription of Notch target genes. Notch signaling activation in the microenvironment of a tumor can increase tumor cell resistance to anoikis, epithelial-mesenchymal transition (EMT), maintenance of angiogenic homeostasis, vascular morphology, and self-renewal of cancer stem cells (CSCs). Adopted from Hu et al31 with permission from Springer
Recent studies have shown that mutations in protein-coding genes lead to abnormal protein expression.35 Notch is one of the complex networks that play a key role in cell survival or death and also promote cancer cell growth and malignancy. The effects of this pathway on the tumor microenvironment (TME), such as matrix remodeling, are also known.36 Other signaling pathways, such as WNT, contribute to the promotion of Notch signaling pathways that can be targeted simultaneously.37 Several phytochemical and non-phytochemical compounds targeting the Notch signaling pathway have been reported. Some of these compounds are currently in clinical trials for a wide range of diseases, including γ-secretase inhibitors (RO4929097, MRK-003, MK-0752, PF-03084014, etc), immunotherapy (OMP-59R5, OMP-21M18, NRR1, NRR2, NRR3, A5226A, DLL1-Fc & JAG1-Fc) and MAM peptide antagonists (SAHM1).38-40 Milk phospholipids, Lactobacillus acidophilus, L. rhamnosus GG, γ-secretase inhibitors (dibenzoazepine, DAPT), 6-formylindolo(3,2-b)carbazole are examples of drug regulation of Notch whose effects on inflammatory bowel disease and colon cancer have been studied.41 Important phytochemicals in cancer therapy via Notch signaling suppression is presented in Table 1.
Table 1.
Golden phytochemicals with potential to inhibit notch signaling for cancer therapy
|
Phytochemicals
|
Chemical structure
|
Plant origin
|
group of natural substances
|
Biological effect
|
Ref
|
| Curcumin |
|
Curcuma longa
|
Diarylheptanoid |
Treatment of several chronic diseases such as liver disease, inflammation, arthritis, metabolic syndrome, liver disease, obesity, neurological diseases, and also several cancers due to its anti-inflammatory and antioxidant actions |
42
|
| Genistein |
|
Genista tinctoria
|
Isoflavonoids |
anti-tumor activity
, impairment of angiogenesis
in cancer cells, improvement of glucose metabolism, decrease of peri-menopausal and
postmenopausal hot flashes, and modulation of antioxidant actions. |
43
|
| Glycyrrhizin |
|
Glycyrrhiza glabra
|
Triterpene glycoside (saponin) |
Anti-inflammatory, anti-cancer, anti-viral, and anti-oxidant activity |
44
|
| Cucurbitacin |
|
Cucurbitaceae
|
Triterpene |
Anti-inflammatory and anti-cancer effects, treatment of cardiovascular diseases, and diabetes |
45
|
| Resveratrol |
|
Veratrum grandiflorum
|
Stilbene |
Antitumor, antioxidant, anti-viral, and phytoestrogenic effects |
46
|
| Silibinin |
|
Silybum marianum
|
Flavonolignans |
Anticancer, hepatoprotective, anti-inflammatory, and anti-fibrotic effects |
47
|
| Quercetin |
|
Quercetum (oak forest) |
Flavonoid |
Anti-oxidant, anti-cancer, anticarcinogenic, and antimicrobial activities |
48
|
|
Honokiol
|
|
Magnolia
|
Lignan |
Antitumorigenic, Antithrombotic, Anti-inflammatory, Anti-oxidant
Anti-viral |
41
|
Phytochemicals’s effects against various cancers
Since chemotherapeutic drugs are associated with significant side effects and toxicity, natural compounds such as phytochemicals show therapeutic benefits in various diseases such as cancer.14,49 The phytochemicals can be extracted from fruits and vegetables, and their antitumor activities influence the metabolism, proliferation and epigenetic modification of cancer cells.16
About 50 % of the anticancer drugs approved in 2014 are derived from or directly extracted from natural products.50 Some important anticancer phytochemicals have been tested in vitro and in vivo for their anticancer activity. Phytochemicals have overlapping and complementary mechanisms to slow carcinogenesis by suppressing cancer cell survival and proliferation,51 reducing tumor invasion and angiogenesis,52 and scavenging free radicals.53 Phytochemicals exert a complex and wide-ranging influence on signal transduction pathways and various molecular targets such as suppressor proteins or downstream tumor activators,54 microRNAs,55 membrane receptors,56 transcription factors,57 kinases,58 cyclins and caspases.51
Exciting research is related to endoplasmic reticulum (ER) stress, and the effect of natural compounds on apoptosis through ER stress is considered a suitable anti-cancer strategy.59 In addition, curcumin, and gallic acid have been used in breast, colon and ovarian cancer.60 The properties of important phytochemicals in cancer therapy that suppress Notch signaling are presented.
Curcumin
Curcumin has been extracted from the rhizomes of Curcuma longa, the turmeric, since 1815 and has been taken into account by many scientists ever since. Due to its antioxidant and anti-inflammatory effects, curcumin may be beneficial in the treatment of various chronic diseases such as inflammation, arthritis, liver disease, metabolic syndrome, liver disease, obesity, neurological diseases and various cancers.61,62 Curcumin influences cell proliferation, growth, survival, apoptosis, migration, invasion and angiogenesis.63,64
Another anti-cancer effect of curcumin is its influence on cyclin D1 levels, an important regulator of cell cycle progression. High levels of cyclin D1 are associated with the development and progression of cancer. Curcumin suppresses cyclin D1 by inhibiting nuclear factor-κB (NF-κB).56 NF-κB, a proinflammatory transcription factor, promotes the proliferation of breast cancer cells and controls the regulation of more than 500 different genes and protein expressions involved in the signaling pathway.65,66 In squamous cell carcinoma of the head and neck, curcumin has been reported to inhibit cell growth and stimulate apoptosis by suppressing NF-κB activity and expressing NF-κB-regulatory genes such as Bcl-2, cyclin D1, Cox-2 and MMP-9.67 Curcumin inhibits oral squamous cell carcinoma cell invasion by suppressing NF-κB activation, and studies suggest that NF-κB is regulated by the Notch signaling pathway in oral cancer.68 Notch signaling pathways are essential for regulating the balance between cell growth, differentiation, and apoptosis.69 Notch signaling pathways are involved in the development of cancers such as oral, pancreatic, prostate, breast, and many other cancers.70-72 Reducing Notch activity can be considered a promising approach to combating cancer in vitro and in vivo.73 Notch1 activity is essential for maintaining of NF-κB activity. Notch1 signaling pathways induce promoter activity and expression of multiple NF-κB subunits, as shown in mice in which decreased NF-κB activity leads to downregulation of Notch signaling pathways.74 Wang et al. investigated the effect of curcumin on the Notch1 signaling pathway in pancreatic cancer (BxPC-3 and PANC-1 cells). The results showed that curcumin reduced the transcription and translation of Notch1 as well as the expression of Hes-1, cyclin D1 and Bcl-X genes, which showed a decreasing trend compared to the control groups. In addition, this study showed that curcumin not only stopped cell growth but also induced apoptosis of BxPC-3 and PANC-1 cells. In this study, BxPC-3 cells were transfected with small-interfering RNA (siRNA) as a positive control, which inhibited the expression of the Notch1 signaling pathway. The NF-κB signaling pathway was measured by electrophoretic mobility shift assay (EMSA), which showed that Notch1 siRNA inhibited the DNA-binding activity of NF-κB and enhanced the effect of curcumin on NF-κB inhibition. This result proves the interaction between Notch1 and the NF-κB signaling pathway.72 Studies in oral cancer cells have shown that curcumin reduces Notch1 activity, leading to downregulation of NF-κB and its target genes such as Bcl-2, cyclin D1, VEGF, and MMP-9.71 The expression of HES1 proteins, which are the target genes of Notch1 and influence cell fate, was also decreased.75 Since curcumin has photodynamic properties, a combination of photodynamic therapy (PDT) and Notch receptor blockers (DAPT) was used. The results showed decreased proliferation, induced apoptosis, and blocked Notch signaling pathway by downregulating NF-κB and Notch1 expression.76
The translation of messenger RNA (mRNA) is regulated by the eIF4F complex, which consists of the eukaryotic translation initiation factors eIF4A, eIF4E and eIF4G. A study has shown that the use of small interfering RNAs to knock down eIF4E in head and neck carcinomas leads to a decrease in cyclin D1 protein levels and inhibition of cell growth.77 When studying immortalized normal cells, Chakravarti et al. showed that all eIF4E proteins were downregulated after treatment with curcumin, which correlated with decreased cyclin D1 protein expression and inhibition of cell growth. They also found that immortalized normal and cancer cells had high levels of components of the eIF4F complex as well as proteins that activate eIF4F (Mnk1) or low levels of eIF4F inhibitory proteins (4E-BP1), as curcumin inhibits the growth of oral cancer cells.78
One study investigated the effect of curcumin on esophageal cancer cells. Curcumin reduced the size and number of esophageal spheres. In addition, treatment with curcumin resulted in reduced activation of Notch-1, the expression of Jagged-1 and its downstream target HES1. This reduced activation of Notch-1 was found to be due to the downregulation of key components of γ-secretase complex proteins such as nicastrin and presenilin 1. A combination of curcumin and a known γ-secretase inhibitor DAPT induced apoptosis and further reduced proliferation in esophageal cancer cells (Figure 2). Finally, curcumin decreased the expression of the microRNAs Notch1 miR-34a and miR-21 and upregulated the tumor suppressor miRNA let-7a.79
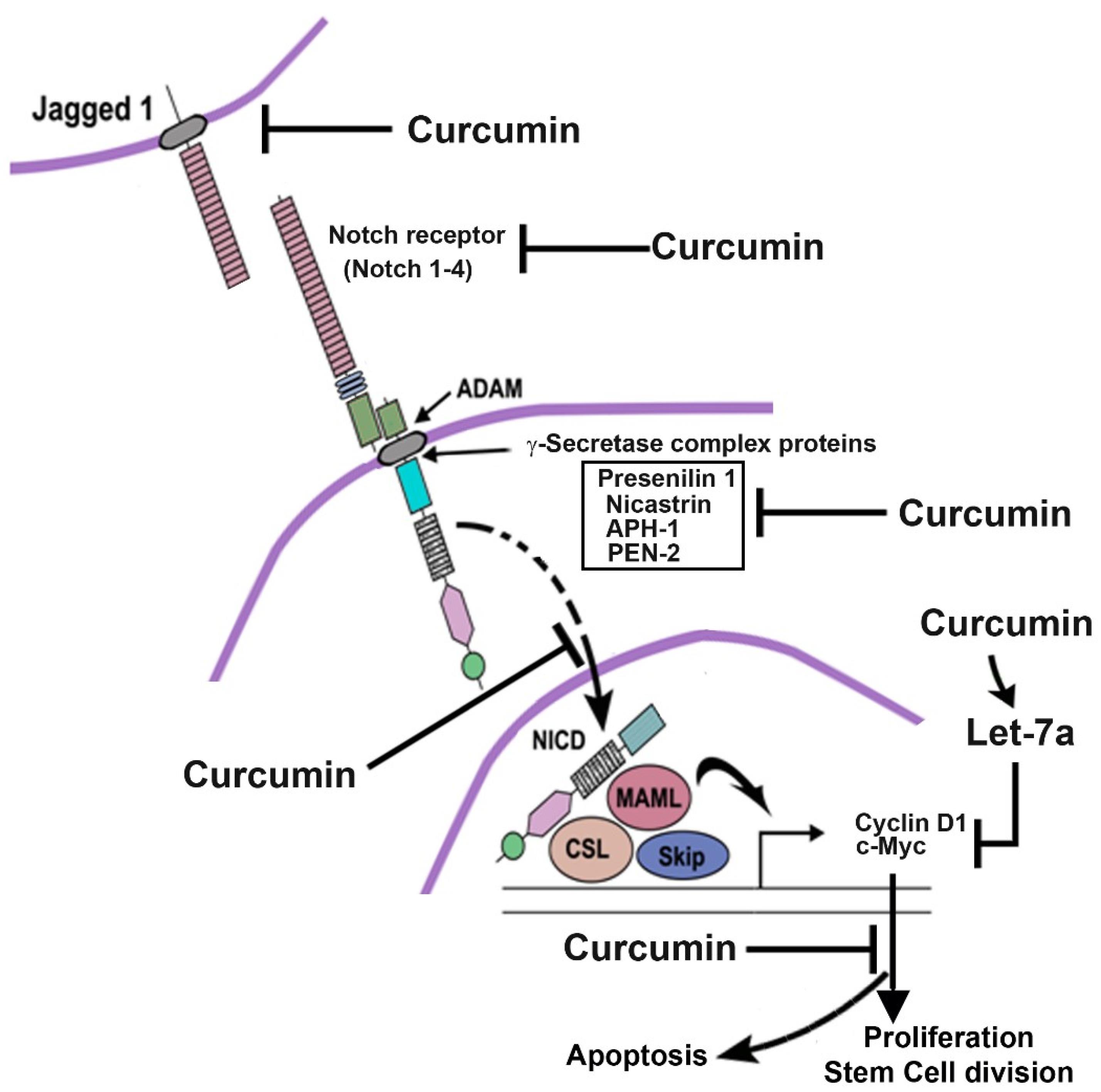
Figure 2.
Influence of curcumin on Notch signaling in esophageal cancer. Curcumin inhibits Notch-1 and Jagged-1 receptor expression. Curcumin also prevents proteins of the γ-secretase complex and thus prevents the cleavage of the Notch receptor. Consequently, the NICD is not released and therefore does not translocate to the nucleus where it activates cyclin D1 and c-myc as the downstream target genes. This leads to the inhibition of cell proliferation and regeneration of stem cells while inducing apoptosis. Reprinted from Subramaniam et al79 under the terms of the Creative Commons Attribution License 4.0
.
Influence of curcumin on Notch signaling in esophageal cancer. Curcumin inhibits Notch-1 and Jagged-1 receptor expression. Curcumin also prevents proteins of the γ-secretase complex and thus prevents the cleavage of the Notch receptor. Consequently, the NICD is not released and therefore does not translocate to the nucleus where it activates cyclin D1 and c-myc as the downstream target genes. This leads to the inhibition of cell proliferation and regeneration of stem cells while inducing apoptosis. Reprinted from Subramaniam et al79 under the terms of the Creative Commons Attribution License 4.0
Genistein
Genistein is a phytoestrogen that belongs to the isoflavone family and is found in soybeans, chickpeas and other soy-based foods that are also used in herbal medicine.80-82 The daily intake of isoflavones is higher in people in Asian countries than in the Western world, and studies have shown that some specific cancers such as breast and prostate cancer are less common in Asian countries than in the Western world.83 A wide range of anti-cancer properties of genistein have been reported in numerous experiments, including interference with cell cycle control and apoptosis.84 However, genistein has many other beneficial effects on a variety of diseases. Due to its anti-inflammatory effects, it can cure diseases such as allergies.85 Receptor tyrosine kinases (RTKs) are activated by various peptide growth factors such as IGF (insulin-like growth factor), EGF (epidermal growth factor) and NGF (nerve growth factor) etc. RTKs cause phosphorylation of downstream pathway proteins leading to complicated cytoplasmic and nuclear events, such as phosphorylation of other proteins and activation of enzymes involved in cell growth, survival and differentiation.86,87
The property of genistein to modulate PTKs was demonstrated in 1987. In vitro studies have shown that genistein inhibits EGF receptor activity by interacting with tyrosine–specific protein kinase-like EGF receptor and histone H2B as a phosphate receptor.88,89 MiR-34a is a miRNA involved in the suppression of cell growth by inhibiting Notch1 expression in pancreatic cancer.90 Xia et al revealed that treatment of pancreatic cancer cells with genistein resulted in increased re-expression of miR-34a in cancer cells and down-regulation of Notch1. They also found that treatment of pancreatic cancer cells with miR-34a and genistein significantly reduced Notch1 levels compared to genistein alone.91 Zhou et al reported that genistein decreased the expression of Notch1 signaling protein and also induced the expression of Bax/Bcl-2, caspase-8 and caspase-3 in HT-29 cells (colon cancer), which decreased cancer cell growth and increased apoptosis.92 Some studies have shown that genistein suppresses the activity of Akt, leading to inactivation of the downstream signaling pathways, NF-κB. In addition, genistein directly inactivates NF-κB and subsequently reduces cancer cell growth.93
Quercetin
Quercetin is a subclass of polyphenolic flavonoids that are abundant in natural products such as vegetables, fruits, and cereals, mostly in the form of glycosides.94 There is evidence that quercetin has numerous biochemical effects such as antioxidant, anticancer, anticarcinogenic, and antimicrobial activities.95 CSCs have unique properties such as self-renewal and differentiation potential that may be very similar to the properties of stem cells, such that many cancer cells arise from normal stem cell transformation.96,97 Extensive research has also shown that CSCs lead to cancer cell resistance to treatment, recurrence after treatment, and tumor regeneration.98 Li et al. documented that quercetin has a dramatic anticancer effect by suppressing the Notch signaling pathway in colon CSCs. These studies showed that protein levels of Jagged-1, all five proteins of the γ-secretase complex, and cleaved Notch1 decreased dramatically in colon cancer cells treated with quercetin plus radiotherapy.99 Experiments with human colon cancer xenografts in the BALB/c mouse model also showed that synergistic treatment of CSCs with quercetin and radiation was more efficient in reducing tumor volume than quercetin or radiation alone.99 The division of CSCs by self-renewal leads to two identical daughter cells (symmetric cell division) or two abnormal daughter cells (asymmetric cell division).100 Symmetric cell division leads to the growth and regeneration of tumor cells, whereas asymmetric cell division leads to the maintenance of the number of CSCs.101,102 The upregulation of Notch signaling is a trigger for symmetric division and eventually leads to cancer.103 Clifford et al reported that quercetin remarkably upregulates miR-200b-3p, a non-coding RNA that directly targets the 3’UTR of Notch1, and causes inhibition of Notch signaling by performing a dual-luciferase reporter assay in pancreatic ductal adenocarcinoma CSCs. In addition, they transfected miR-200b-3p into cancer cells, and the result of this experiment showed that treatment of CSCs with miR-200b-3p and quercetin caused the symmetric division of CSCs to change to asymmetric and inhibited not only the self-renewal but also the differentiation of CSCs by inhibiting Notch signaling.104
Treatment of the AGS human gastric adenocarcinoma cell line with quercetin in combination with a low dose of SN-38 (inhibitor of DNA topoisomerase I) enhanced the anticancer effect of SN-38 by decreasing β-catenin protein levels and increasing apoptosis. In addition, treatment with a combination of SN-38 and quercetin in the AGS xenograft mouse model showed that the expression of cyclooxygenase-2 and markers of epithelial-mesenchymal transition, such as ITGβ6 and Twist1, was remarkably reduced compared to SN-38 treatment.105
Silibinin
The natural flavonoid silibinin or silybin is the main bioactive compound of silymarin, which is isolated from the milk thistle plant (Silybum marianum), and the extraction of silibinin is used in traditional medicine to treat various diseases.106,107 The numerous beneficial properties of silibinin include hepatoprotective, antioxidant, anti-inflammatory, chemopreventive and many other properties.108 In addition, many studies have shown the anti-cancer effects of silibinin against various cancers such as lung, bladder, prostate, breast, lung, skin, colon and ovarian cancer.109 Kim et al reported that silibinin has a promising anticancer effect on breast cancer. According to their experiments, silibinin treatment induced apoptosis in MCF7 and MDA-MB-231 cells by increasing the formation of ROS and downregulating the expression of Notch1 mRNA in both cancer cells.110 Zhang et al investigated the effects of silibinin on human HepG2 cells (hepatocellular carcinoma). The result showed that silibinin treatment decreased the migration and adhesion ability of HepG2 cells, increased the generation of ROS, caspase3 activity and apoptosis. In addition, the combined treatment with silibinin and transfection of Notch1 siRNA onto HepG2 cells was found to decrease NICD levels and Notch signaling. In addition, Bax was upregulated and the survivin gene was downregulated compared to cells treated with silibinin or transfected with Notch1 siRNA.111 Chang et al treated line 786-O renal carcinoma with silibinin, and their results showed that urokinase plasminogen activator (u-PA), metalloproteinase (MMP) -2, -9 and MAPK pathway protein expression decreased and tumor weight was reduced.112 Studies on human ovarian cancer cells showed that silibinin inhibits ERK and Akt and reduces tumor growth.113 Non-small cell lung cancer was treated with silibinin. The result showed that silibinin disrupts cell proliferation by stimulating G0/G1 cell cycle arrest and apoptosis, and prevents tumor angiogenesis, invasion and migration. Silibinin regulated phosphorylated EGFR expression by binding to its receptor and then inhibited downstream targets and JAK2/STAT5 and PI3K/AKT signaling pathways, leading to a reduction in cancer rate.114,115
Resveratrol
Some spermatophytes, such as grapevine, produce resveratrol, a natural phytoalexin. Resveratrol is a stilbenoid found in various plants such as grapes, peanuts, and berries that protects them from environmental stress and pathogenic attack when damaged; red wine also contains high levels of resveratrol.116-118 Many studies have shown that resveratrol has important pharmacological properties, such as prevention of heart disease, slowing the aging process, alleviating diabetes, reducing inflammatory stress, lowering blood lipids, resistance to lipid peroxidation, usefulness in curing coughs and asthma, resistance to pathogenic microorganisms, and the unique ability to fight various types of cancer by suppressing the initiation, growth and progression of cancer and inducing apoptosis.119-121 In addition, resveratrol has antioxidant potential due to the three hydroxyl groups that interfere with intracellular redox signaling.122 Dong et al found that MDA-MB-231 cells (breast cancer cells) showed significantly decreased expression of Notch1 protein and decreased expression of Jagged1 ligand after treatment with different concentrations of resveratrol. In addition, the expression of the Hes5 protein was significantly decreased compared to the control group, and a downregulation of the Delta-like 4 ligand (Dll4) was also observed.123 Dll4/Notch signaling plays an important role in embryonic vascular development. Downregulation of DLL4 leads to decreased expression of EphrinB2 and HEY1 and prevents proliferation, migration, and network formation of endothelial cells, all of which play important roles in tumor angiogenesis.124 The study on human osteosarcoma cells treated with resveratrol showed it led to the inhibition of JAK2/STAT3 signaling reduced cell viability, self-renewal ability, and tumorigenesis. In addition, inhibition of JAK2/STAT3 signaling led to a decrease in the osteosarcoma stem cell marker CD133. The expression of Bcl-2 was increased, so resveratrol led to a decrease in the CSC subpopulation of osteosarcoma and inhibited the self-renewal ability of these cells.125 Li et al reported that resveratrol in combination with artemisinin has a stronger anti-cancer effect compared to treatment with artemisinin and resveratrol alone. In their experiment, cell apoptosis was examined and it was found that the number of apoptotic cells in HeLa cells increased after synergistic treatment with resveratrol and artemisinin compared to the treatment group alone. In addition, DCF fluorescence staining showed that HeLa cells treated with the combination of artemisinin and resveratrol increased ROS production and the migration rate decreased significantly in HeLa cells treated with artemisinin and resveratrol.126
Cucurbitacin
Cucurbitacin (cucurbitacin A-T) is one of the natural compounds isolated from some cucurbits such as bitter melons, zucchini, and pumpkins.127 Cucurbitacin acts on cancer cells by inducing apoptosis in cancer cells, especially in CSC, and arresting the cell cycle in G2/M, which inhibits proliferation.128 One of the unique properties of cucurbitacin is to affect the expression of CD44, one of the specific markers for cancer cells, and to increase apoptosis in head and neck cancer by decreasing STAT3 signaling, whereby STAT3 contributes to tumor cell progression, migration and survival.129 It has been suggested that cucurbitacin E suppresses MAPK kinases, a signaling pathway, and inhibits angiogenesis in cancer cells Cucurbitacin B was reported to inhibit signaling pathways such as Wnt/β-catenin and Hippo- YAP in lung cancer and colon cancer, respectively.130 It was shown that treatment with cucurbitacin B and I resulted in the downregulation of Notch signaling pathway including receptors, ligands, γ-secretase, and target genes. The decrease in Notch signaling expression led to a decrease in invasive behavior and poorer survival, which correlates with the decrease in epithelial-to-mesenchymal transition (EMT) in colon cancer cells.131 Dysregulation of Notch1,2 and their ligands is associated with colon cancer, and Notch3 and the ligands Jagged-1 and Dll-4 are also dysregulated in the aggressive phenotype of xenografts.132 Colon cancer cells exhibiting high Notch signaling activity were treated with Bcl-2 N-[N-3,5-difluorophenacetyl]-l-alanyl-S-phenylglycine methyl ester (DAPM) inhibitors of γ-secretase and showed a loss of subpopulations of cancer cells in xenografts.133 The interaction of cucurbitacin I and B with Notch1 has been reported to alter protein conformation and suppress the growth of colon cancer cells and stem cells.128
Glycyrrhizin
Glycyrrhizin is extracted from licorice (Glycyrrhiza glabra Linn), one of the most important medicinal plants known for its antioxidant, antiviral, antidiabetic and anti-inflammatory properties.134 In addition, the antitumor role of glycyrrhizin is confirmed by slowing down the cell cycle in G0/G1, increasing the production of intracellular ROS and inducing apoptosis in cervical cancer. Glycyrrhizin modulates Notch signaling through the action of Notch1 and the ligand Jagged-1, which reduces the expression of its downstream target gene HES-1 and cyclin D1, which is one of the important proteins that activate NICD in cervical cancer. This study also showed that down-regulation of Notch signaling by glycyrrhizin resulted in increased up-regulation of pro-apoptotic proteins such as Bad and Bax and down-regulated expression of anti-apoptotic proteins such as Bcl-2.135 Treatment of CaSki cervical cancer cells with glycyrrhizin has been shown to suppress proliferation, lead to mitochondrial dysfunction and decrease Notch1 mRNA expression.136 High mobility group box 1 (HMGB1), which triggers EMT, was targeted by glycyrrhizin in prostate cancer and glycyrrhizin was shown to inhibit HMGB1.137
Conclusion
Cancer is considered a critical health condition that is highly associated with death. Therefore, it is clear that the development of the best treatment strategies seems necessary. Among the various cancer treatment approaches, targeting a different part of the signaling pathways that play a key role in maintaining the balance between survival and death by somatic cells could be promising for cancer therapy. These cancer therapies targeting hormone receptor signaling, RTKs, the MAPK pathway, NF-κB, cyclin-dependent kinases, etc. are applicable and have received regulatory approval. As discussed in this review, the Notch signaling pathway has special properties for targeting different types of cancer and can be targeted with different strategies, such as cleavage inhibitors, γ-secretase inhibitors, γ-secretase modulators, antibodies against ligands and receptors, and transcription blockers. Natural products with unique properties are potential substitutes for chemotherapeutic agents and provide adequate therapeutic results. They can induce cell death mediated by signaling pathways, autophagy and apoptosis, reduce chemoresistance and inhibit the drug efflux pump. Although these natural agents are beneficial, there are still some concerns, such as the narrow therapeutic window and liver toxicity, which need to be further investigated.
In the last century, cancer for which there is no definitive treatment has been the most common factor leading to human death. Therefore, there is an urgent need for therapeutically effective drugs that are less toxic to the body, such as natural products. This review provides a rationale and a potential target for the treatment of cancer by herbal anticancer agents.
Acknowledgments
RJE is supported by Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran, grant number 62674.
Competing Interests
The authors declare no conflict of interest.
Ethical Approval
Not applicable.
References
- Aruoma OI, Bahorun T, Agnihotri AK. Cancer risks and perspectives: molecular mechanisms. Mutat Res 2014; 768:1-5. doi: 10.1016/j.mrfmmm.2014.09.001 [Crossref] [ Google Scholar]
- Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol 2017; 71(1):96-108. doi: 10.1016/j.eururo.2016.06.010 [Crossref] [ Google Scholar]
- Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal 2020; 18(1):59. doi: 10.1186/s12964-020-0530-4 [Crossref] [ Google Scholar]
- Sharifi-Azad M, Fathi M, Cho WC, Barzegari A, Dadashi H, Dadashpour M. Recent advances in targeted drug delivery systems for resistant colorectal cancer. Cancer Cell Int 2022; 22(1):196. doi: 10.1186/s12935-022-02605-y [Crossref] [ Google Scholar]
- Mehrgou A, Ebadollahi S, Seidi K, Ayoubi-Joshaghani MH, Ahmadieh Yazdi A, Zare P. Roles of miRNAs in colorectal cancer: therapeutic implications and clinical opportunities. Adv Pharm Bull 2021; 11(2):233-47. doi: 10.34172/apb.2021.029 [Crossref] [ Google Scholar]
- Doustmihan A, Fathi M, Mazloomi M, Salemi A, Hamblin MR, Jahanban-Esfahlan R. Molecular targets, therapeutic agents and multitasking nanoparticles to deal with cancer stem cells: a narrative review. J Control Release 2023; 363:57-83. doi: 10.1016/j.jconrel.2023.09.029 [Crossref] [ Google Scholar]
- Jahanban-Esfahlan R, Seidi K, Banimohamad-Shotorbani B, Jahanban-Esfahlan A, Yousefi B. Combination of nanotechnology with vascular targeting agents for effective cancer therapy. J Cell Physiol 2018; 233(4):2982-92. doi: 10.1002/jcp.26051 [Crossref] [ Google Scholar]
- Mahmudi H, Adili-Aghdam MA, Shahpouri M, Jaymand M, Amoozgar Z, Jahanban-Esfahlan R. Tumor microenvironment penetrating chitosan nanoparticles for elimination of cancer relapse and minimal residual disease. Front Oncol 2022; 12:1054029. doi: 10.3389/fonc.2022.1054029 [Crossref] [ Google Scholar]
- Nussbaumer S, Bonnabry P, Veuthey JL, Fleury-Souverain S. Analysis of anticancer drugs: a review. Talanta 2011; 85(5):2265-89. doi: 10.1016/j.talanta.2011.08.034 [Crossref] [ Google Scholar]
- Mesgari Abbasi M, Moradzadeh Khiavi M, Monfaredan A, Hamishehkar H, Seidi K, Jahanban-Esfahlan R. DOX-MTX-NPs augment p53 mRNA expression in OSCC model in rat: effects of IV and oral routes. Asian Pac J Cancer Prev 2014; 15(19):8377-82. doi: 10.7314/apjcp.2014.15.19.8377 [Crossref] [ Google Scholar]
- Dadashi H, Eskandani M, Roshangar L, Sharifi-Azad M, Shahpouri M, Cho WC. Remotely-controlled hydrogel platforms for recurrent cancer therapy. J Drug Deliv Sci Technol 2023; 82:104354. doi: 10.1016/j.jddst.2023.104354 [Crossref] [ Google Scholar]
- Jahanban-Esfahlan R, de la Guardia M, Ahmadi D, Yousefi B. Modulating tumor hypoxia by nanomedicine for effective cancer therapy. J Cell Physiol 2018; 233(3):2019-31. doi: 10.1002/jcp.25859 [Crossref] [ Google Scholar]
- Jahanban-Esfahlan R, Seidi K, Manjili MH, Jahanban-Esfahlan A, Javaheri T, Zare P. Tumor cell dormancy: threat or opportunity in the fight against cancer. Cancers (Basel) 2019; 11(8):1207. doi: 10.3390/cancers11081207 [Crossref] [ Google Scholar]
- Dutta S, Mahalanobish S, Saha S, Ghosh S, Sil PC. Natural products: an upcoming therapeutic approach to cancer. Food Chem Toxicol 2019; 128:240-55. doi: 10.1016/j.fct.2019.04.012 [Crossref] [ Google Scholar]
- Jahanban-Esfahlan A, Jamei R, Jahanban-Esfahlan R. The importance of almond (Prunus amygdalus L) and its by-products. Food Chem 2010; 120(2):349-60. doi: 10.1016/j.foodchem.2009.09.063 [Crossref] [ Google Scholar]
- Kubczak M, Szustka A, Rogalińska M. Molecular targets of natural compounds with anti-cancer properties. Int J Mol Sci 2021; 22(24):13659. doi: 10.3390/ijms222413659 [Crossref] [ Google Scholar]
- Mesgari Abbasi M, Mehdipour M, Monfaredan A, Jahanban-Esfahlan R. HESA-a down-regulates erb/B2 oncogene expression and improves outcome of oral carcinoma in a rat model. Asian Pac J Cancer Prev 2015; 16(16):6947-51. doi: 10.7314/apjcp.2015.16.16.6947 [Crossref] [ Google Scholar]
- Jahanban-Esfahlan A, Modaeinama S, Abasi M, Mesgari Abbasi M, Jahanban-Esfahlan R. Anti proliferative properties of Melissa officinalis in different human cancer cells. Asian Pac J Cancer Prev 2015; 16(14):5703-7. doi: 10.7314/apjcp.2015.16.14.5703 [Crossref] [ Google Scholar]
- Jahanban-Esfahlan R, Seidi K, Monfaredan A, Shafie-Irannejad V, Mesgari Abbasi M, Karimian A. The herbal medicine Melissa officinalis extract effects on gene expression of p53, Bcl-2, Her2, VEGF-A and hTERT in human lung, breast and prostate cancer cell lines. Gene 2017; 613:14-9. doi: 10.1016/j.gene.2017.02.034 [Crossref] [ Google Scholar]
- Nasiri M, Zarghami N, Nejati-Koshki K, Mollazadeh M, Pourhassan-Moghaddam M, Rahmati-Yamchi M. Curcumin and silibinin inhibit telomerase expression in T47D human breast cancer cells. Asian Pac J Cancer Prev 2013; 14(6):3449-53. doi: 10.7314/apjcp.2013.14.6.3449 [Crossref] [ Google Scholar]
- Nejati-Koshki K, Zarghami N, Pourhassan-Moghaddam M, Rahmati-Yamchi M, Mollazade M, Nasiri M. Inhibition of leptin gene expression and secretion by silibinin: possible role of estrogen receptors. Cytotechnology 2012; 64(6):719-26. doi: 10.1007/s10616-012-9452-3 [Crossref] [ Google Scholar]
- Sayadnia S, Arkan E, Jahanban‐Esfahlan R, Sayadnia S, Jaymand M. Tragacanth gum‐based pH‐responsive magnetic hydrogels for “smart” chemo/hyperthermia therapy of solid tumors. Polym Adv Technol 2021; 32(1):262-71. doi: 10.1002/pat.5082 [Crossref] [ Google Scholar]
- Majidinia M, Ghazizadeh Darband S, Kaviani M, Nabavi SM, Jahanban-Esfahlan R, Yousefi B. Cross-regulation between Notch signaling pathway and miRNA machinery in cancer. DNA Repair (Amst) 2018; 66-67:30-41. doi: 10.1016/j.dnarep.2018.04.002 [Crossref] [ Google Scholar]
- Shahpouri M, Adili-Aghdam MA, Mahmudi H, Jaymand M, Amoozgar Z, Akbari M. Prospects for hypoxia-based drug delivery platforms for the elimination of advanced metastatic tumors: from 3D modeling to clinical concepts. J Control Release 2023; 353:1002-22. doi: 10.1016/j.jconrel.2022.12.009 [Crossref] [ Google Scholar]
- Krishna BM, Jana S, Singhal J, Horne D, Awasthi S, Salgia R. Notch signaling in breast cancer: from pathway analysis to therapy. Cancer Lett 2019; 461:123-31. doi: 10.1016/j.canlet.2019.07.012 [Crossref] [ Google Scholar]
- Miele L, Golde T, Osborne B. Notch signaling in cancer. Curr Mol Med 2006; 6(8):905-18. doi: 10.2174/156652406779010830 [Crossref] [ Google Scholar]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 2006; 7(9):678-89. doi: 10.1038/nrm2009 [Crossref] [ Google Scholar]
- Borggrefe T, Oswald F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci 2009; 66(10):1631-46. doi: 10.1007/s00018-009-8668-7 [Crossref] [ Google Scholar]
- Borggrefe T, Liefke R. Fine-tuning of the intracellular canonical Notch signaling pathway. Cell Cycle 2012; 11(2):264-76. doi: 10.4161/cc.11.2.18995 [Crossref] [ Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 2009; 137(2):216-33. doi: 10.1016/j.cell.2009.03.045 [Crossref] [ Google Scholar]
- Hu YY, Zheng MH, Zhang R, Liang YM, Han H. Notch signaling pathway and cancer metastasis. Adv Exp Med Biol 2012; 727:186-98. doi: 10.1007/978-1-4614-0899-4_14 [Crossref] [ Google Scholar]
- Guo S, Liu M, Gonzalez-Perez RR. Role of Notch and its oncogenic signaling crosstalk in breast cancer. BiochimBiophys Acta Revi Cancer 2011; 1815(2):197-213. doi: 10.1016/j.bbcan.2010.12.002 [Crossref] [ Google Scholar]
- Suresh S, Irvine AE. The Notch signaling pathway in normal and malignant blood cell production. J Cell Commun Signal 2015; 9(1):5-13. doi: 10.1007/s12079-015-0271-0 [Crossref] [ Google Scholar]
- Weinmaster G. The ins and outs of Notch signaling. Mol Cell Neurosci 1997; 9(2):91-102. doi: 10.1006/mcne.1997.0612 [Crossref] [ Google Scholar]
- Nik-Zainal S, Davies H, Staaf J, Ramakrishna M, Glodzik D, Zou X. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 2016; 534(7605):47-54. doi: 10.1038/nature17676 [Crossref] [ Google Scholar]
- Shen Q, Reedijk M. Notch signaling and the breast cancer microenvironment. Adv Exp Med Biol 2021; 1287:183-200. doi: 10.1007/978-3-030-55031-8_12 [Crossref] [ Google Scholar]
- Fendler A, Bauer D, Busch J, Jung K, Wulf-Goldenberg A, Kunz S. Inhibiting WNT and Notch in renal cancer stem cells and the implications for human patients. Nat Commun 2020; 11(1):929. doi: 10.1038/s41467-020-14700-7 [Crossref] [ Google Scholar]
- Groth C, Fortini ME. Therapeutic approaches to modulating Notch signaling: current challenges and future prospects. Semin Cell Dev Biol 2012; 23(4):465-72. doi: 10.1016/j.semcdb.2012.01.016 [Crossref] [ Google Scholar]
- Dong Z, Huo J, Liang A, Chen J, Chen G, Liu D. Gamma-Secretase Inhibitor (DAPT), a potential therapeutic target drug, caused neurotoxicity in planarian regeneration by inhibiting Notch signaling pathway. Sci Total Environ 2021; 781:146735. doi: 10.1016/j.scitotenv.2021.146735 [Crossref] [ Google Scholar]
- Smyth IM, Bertram JF. Seminars in cell and developmental biology. Semin Cell Dev Biol 2019; 91:84-5. doi: 10.1016/j.semcdb.2018.11.003 [Crossref] [ Google Scholar]
- Pu Z, Yang F, Wang L, Diao Y, Chen D. Advancements of compounds targeting Wnt and Notch signalling pathways in the treatment of inflammatory bowel disease and colon cancer. J Drug Target 2021; 29(5):507-19. doi: 10.1080/1061186x.2020.1864741 [Crossref] [ Google Scholar]
- Kotha RR, Luthria DL. Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019; 24(16):2930. doi: 10.3390/molecules24162930 [Crossref] [ Google Scholar]
- Chae HS, Xu R, Won JY, Chin YW, Yim H. Molecular targets of genistein and its related flavonoids to exert anticancer effects. Int J Mol Sci 2019; 20(10):2420. doi: 10.3390/ijms20102420 [Crossref] [ Google Scholar]
- Bakr AF, Shao P, Farag MA. Recent advances in glycyrrhizin metabolism, health benefits, clinical effects and drug delivery systems for efficacy improvement; a comprehensive review. Phytomedicine 2022; 99:153999. doi: 10.1016/j.phymed.2022.153999 [Crossref] [ Google Scholar]
- Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX. Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat Prod Rep 2005; 22(3):386-99. doi: 10.1039/b418841c [Crossref] [ Google Scholar]
- Risuleo G, La Mesa C. Resveratrol: Biological Activities and Potential Use in Health and Disease. In: Gupta, R, Srivastava A, Lall, R. (eds) Nutraceuticals in Veterinary Medicine. Springer, Cham 2019; 215-226. 10.1007/978-3-030-04624-8_15.
- Mashhadi Akbar Boojar M, Mashhadi Akbar Boojar M, Golmohammad S. Overview of silibinin anti-tumor effects. J Herb Med 2020; 23:100375. doi: 10.1016/j.hermed.2020.100375 [Crossref] [ Google Scholar]
- Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 2008; 585(2-3):325-37. doi: 10.1016/j.ejphar.2008.03.008 [Crossref] [ Google Scholar]
- Sharifi S, Arablouye Moghaddam F, Abedi A, Maleki Dizaj S, Ahmadian S, Abdolahinia ED. Phytochemicals impact on osteogenic differentiation of mesenchymal stem cells. Biofactors 2020; 46(6):874-93. doi: 10.1002/biof.1682 [Crossref] [ Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 2016; 79(3):629-61. doi: 10.1021/acs.jnatprod.5b01055 [Crossref] [ Google Scholar]
- Yan XB, Xie T, Wang SD, Wang Z, Li HY, Ye ZM. Apigenin inhibits proliferation of human chondrosarcoma cells via cell cycle arrest and mitochondrial apoptosis induced by ROS generation-an in vitro and in vivo study. Int J Clin Exp Med 2018; 11(3):1615-31. [ Google Scholar]
- Lu L, Zhao Z, Liu L, Gong W, Dong J. Combination of baicalein and docetaxel additively inhibits the growth of non-small cell lung cancer in vivo. Tradit Med Mod Med 2018; 1(3):213-8. doi: 10.1142/s2575900018500131 [Crossref] [ Google Scholar]
- Lee WL, Huang JY, Shyur LF. Phytoagents for cancer management: regulation of nucleic acid oxidation, ROS, and related mechanisms. Oxid Med Cell Longev 2013; 2013:925804. doi: 10.1155/2013/925804 [Crossref] [ Google Scholar]
- Adams LS, Phung S, Yee N, Seeram NP, Li L, Chen S. Blueberry phytochemicals inhibit growth and metastatic potential of MDA-MB-231 breast cancer cells through modulation of the phosphatidylinositol 3-kinase pathway. Cancer Res 2010; 70(9):3594-605. doi: 10.1158/0008-5472.can-09-3565 [Crossref] [ Google Scholar]
- Cojocneanu Petric R, Braicu C, Raduly L, Zanoaga O, Dragos N, Monroig P. Phytochemicals modulate carcinogenic signaling pathways in breast and hormone-related cancers. Onco Targets Ther 2015; 8:2053-66. doi: 10.2147/ott.s83597 [Crossref] [ Google Scholar]
- Deng QP, Wang MJ, Zeng X, Chen GG, Huang RY. Effects of glycyrrhizin in a mouse model of lung adenocarcinoma. Cell PhysiolBiochem 2017; 41(4):1383-92. doi: 10.1159/000467897 [Crossref] [ Google Scholar]
- Zhang W, Su J, Xu H, Yu S, Liu Y, Zhang Y. Dicumarol inhibits PDK1 and targets multiple malignant behaviors of ovarian cancer cells. PLoS One 2017; 12(6):e0179672. doi: 10.1371/journal.pone.0179672 [Crossref] [ Google Scholar]
- Dou J, Wang Z, Ma L, Peng B, Mao K, Li C. Baicalein and baicalin inhibit colon cancer using two distinct fashions of apoptosis and senescence. Oncotarget 2018; 9(28):20089-102. doi: 10.18632/oncotarget.24015 [Crossref] [ Google Scholar]
- Kim C, Kim B. Anti-cancer natural products and their bioactive compounds inducing er stress-mediated apoptosis: a review. Nutrients 2018; 10(8):1021. doi: 10.3390/nu10081021 [Crossref] [ Google Scholar]
- Subramaniam S, Selvaduray KR, Radhakrishnan AK. Bioactive compounds: natural defense against cancer?. Biomolecules 2019; 9(12):758. doi: 10.3390/biom9120758 [Crossref] [ Google Scholar]
- Negahdari R, Sharifi S, Ghavimi MA, Memar MY, Khaneshi B, Maleki Dizaj S. Curcumin nanocrystals: production, physicochemical assessment, and in vitro evaluation of the antimicrobial effects against bacterial loading of the implant fixture. Appl Sci 2020; 10(23):8356. doi: 10.3390/app10238356 [Crossref] [ Google Scholar]
- Maleki Dizaj S, Alipour M, Dalir Abdolahinia E, Ahmadian E, Eftekhari A, Forouhandeh H. Curcumin nanoformulations: beneficial nanomedicine against cancer. Phytother Res 2022; 36(3):1156-81. doi: 10.1002/ptr.7389 [Crossref] [ Google Scholar]
- Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett 2008; 267(1):133-64. doi: 10.1016/j.canlet.2008.03.025 [Crossref] [ Google Scholar]
- Sharifi S, Zununi Vahed S, Ahmadian E, Maleki Dizaj S, Abedi A, Hosseiniyan Khatibi SM. Stem cell therapy: curcumin does the trick. Phytother Res 2019; 33(11):2927-37. doi: 10.1002/ptr.6482 [Crossref] [ Google Scholar]
- Liu Q, Loo WT, Sze SC, Tong Y. Curcumin inhibits cell proliferation of MDA-MB-231 and BT-483 breast cancer cells mediated by down-regulation of NFkappaB, cyclinD and MMP-1 transcription. Phytomedicine 2009; 16(10):916-22. doi: 10.1016/j.phymed.2009.04.008 [Crossref] [ Google Scholar]
- Kim JM, Noh EM, Kwon KB, Kim JS, You YO, Hwang JK. Curcumin suppresses the TPA-induced invasion through inhibition of PKCα-dependent MMP-expression in MCF-7 human breast cancer cells. Phytomedicine 2012; 19(12):1085-92. doi: 10.1016/j.phymed.2012.07.002 [Crossref] [ Google Scholar]
- Aggarwal S, Takada Y, Singh S, Myers JN, Aggarwal BB. Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor-kappaB signaling. Int J Cancer 2004; 111(5):679-92. doi: 10.1002/ijc.20333 [Crossref] [ Google Scholar]
- Yao J, Duan L, Fan M, Wu X. Gamma-secretase inhibitors exerts antitumor activity via down-regulation of Notch and Nuclear factor kappa B in human tongue carcinoma cells. Oral Dis 2007; 13(6):555-63. doi: 10.1111/j.1601-0825.2006.01334.x [Crossref] [ Google Scholar]
- Ohishi K, Katayama N, Shiku H, Varnum-Finney B, Bernstein ID. Notch signalling in hematopoiesis. Semin Cell Dev Biol 2003; 14(2):143-50. doi: 10.1016/s1084-9521(02)00183-0 [Crossref] [ Google Scholar]
- Wang Z, Li Y, Banerjee S, Kong D, Ahmad A, Nogueira V. Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-kappaB signaling pathways. J Cell Biochem 2010; 109(4):726-36. doi: 10.1002/jcb.22451 [Crossref] [ Google Scholar]
- Liao S, Xia J, Chen Z, Zhang S, Ahmad A, Miele L. Inhibitory effect of curcumin on oral carcinoma CAL-27 cells via suppression of Notch-1 and NF-κB signaling pathways. J Cell Biochem 2011; 112(4):1055-65. doi: 10.1002/jcb.23019 [Crossref] [ Google Scholar]
- Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer 2006; 106(11):2503-13. doi: 10.1002/cncr.21904 [Crossref] [ Google Scholar]
- Miele L, Osborne B. Arbiter of differentiation and death: Notch signaling meets apoptosis. J Cell Physiol 1999; 181(3):393-409. doi: 10.1002/(sici)1097-4652(199912)181:3<393::aidjcp3>3.0.co;2-6 [Crossref] [ Google Scholar]
- Shin HM, Minter LM, Cho OH, Gottipati S, Fauq AH, Golde TE. Notch1 augments NF-kappaB activity by facilitating its nuclear retention. EMBO J 2006; 25(1):129-38. doi: 10.1038/sj.emboj.7600902 [Crossref] [ Google Scholar]
- Zhao NJ, Liao MJ, Wu JJ, Chu KX. Curcumin suppresses Notch-1 signaling: improvements in fatty liver and insulin resistance in rats. Mol Med Rep 2018; 17(1):819-26. doi: 10.3892/mmr.2017.7980 [Crossref] [ Google Scholar]
- He G, Mu T, Yuan Y, Yang W, Zhang Y, Chen Q. Effects of Notch signaling pathway in cervical cancer by curcumin mediated photodynamic therapy and its possible mechanisms in vitro and in vivo. J Cancer 2019; 10(17):4114-22. doi: 10.7150/jca.30690 [Crossref] [ Google Scholar]
- Oridate N, Kim HJ, Xu X, Lotan R. Growth inhibition of head and neck squamous carcinoma cells by small interfering RNAs targeting eIF4E or cyclin D1 alone or combined with cisplatin. Cancer Biol Ther 2005; 4(3):318-23. doi: 10.4161/cbt.4.3.1504 [Crossref] [ Google Scholar]
- Chakravarti N, Kadara H, Yoon DJ, Shay JW, Myers JN, Lotan D. Differential inhibition of protein translation machinery by curcumin in normal, immortalized, and malignant oral epithelial cells. Cancer Prev Res (Phila) 2010; 3(3):331-8. doi: 10.1158/1940-6207.capr-09-0076 [Crossref] [ Google Scholar]
- Subramaniam D, Ponnurangam S, Ramamoorthy P, Standing D, Battafarano RJ, Anant S. Curcumin induces cell death in esophageal cancer cells through modulating Notch signaling. PLoS One 2012; 7(2):e30590. doi: 10.1371/journal.pone.0030590 [Crossref] [ Google Scholar]
- Khongsti K, Das KB, Das B. MAPK pathway and SIRT1 are involved in the down-regulation of secreted osteopontin expression by genistein in metastatic cancer cells. Life Sci 2021; 265:118787. doi: 10.1016/j.lfs.2020.118787 [Crossref] [ Google Scholar]
- Price KR, Fenwick GR. Naturally occurring oestrogens in foods--a review. Food AdditContam 1985; 2(2):73-106. doi: 10.1080/02652038509373531 [Crossref] [ Google Scholar]
- Szkudelska K, Nogowski L. Genistein--a dietary compound inducing hormonal and metabolic changes. J Steroid Biochem Mol Biol 2007; 105(1-5):37-45. doi: 10.1016/j.jsbmb.2007.01.005 [Crossref] [ Google Scholar]
- Sak K. Epidemiological evidences on dietary flavonoids and breast cancer risk: a narrative review. Asian Pac J Cancer Prev 2017; 18(9):2309-28. doi: 10.22034/apjcp.2017.18.9.2309 [Crossref] [ Google Scholar]
- Dixon RA, Ferreira D. Genistein. Phytochemistry 2002; 60(3):205-11. doi: 10.1016/s0031-9422(02)00116-4 [Crossref] [ Google Scholar]
- Xu J, Xiao C, Xu H, Yang S, Chen Z, Wang H. Anti-inflammatory effects of Ganoderma lucidum sterols via attenuation of the p38 MAPK and NF-κB pathways in LPS-induced RAW 2647 macrophages. Food Chem Toxicol 2021; 150:112073. doi: 10.1016/j.fct.2021.112073 [Crossref] [ Google Scholar]
- Moshrefiravasjani R, Kamrani A, Nazari N, Jafari F, Nasiri H, Jahanban-Esfahlan R. Exosome-mediated tumor metastasis: biology, molecular targets and immuno-therapeutic options. Pathol Res Pract 2024; 254:155083. doi: 10.1016/j.prp.2023.155083 [Crossref] [ Google Scholar]
- Jahanban-Esfahlan R, Seidi K, Zarghami N. Tumor vascular infarction: prospects and challenges. Int J Hematol 2017; 105(3):244-56. doi: 10.1007/s12185-016-2171-3 [Crossref] [ Google Scholar]
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 1987; 262(12):5592-5. doi: 10.1016/s0021-9258(18)45614-1 [Crossref] [ Google Scholar]
- Polkowski K, Mazurek AP. Biological properties of genistein A review of in vitro and in vivo data. Acta Pol Pharm 2000; 57(2):135-55. [ Google Scholar]
- Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One 2009; 4(8):e6816. doi: 10.1371/journal.pone.0006816 [Crossref] [ Google Scholar]
- Xia J, Duan Q, Ahmad A, Bao B, Banerjee S, Shi Y. Genistein inhibits cell growth and induces apoptosis through up-regulation of miR-34a in pancreatic cancer cells. Curr Drug Targets 2012; 13(14):1750-6. doi: 10.2174/138945012804545597 [Crossref] [ Google Scholar]
- Zhou P, Wang C, Hu Z, Chen W, Qi W, Li A. Genistein induces apoptosis of colon cancer cells by reversal of epithelial-to-mesenchymal via a Notch1/NF-κB/slug/E-cadherin pathway. BMC Cancer 2017; 17(1):813. doi: 10.1186/s12885-017-3829-9 [Crossref] [ Google Scholar]
- Javed Z, Khan K, Herrera-Bravo J, Naeem S, Iqbal MJ, Sadia H. Genistein as a regulator of signaling pathways and microRNAs in different types of cancers. Cancer Cell Int 2021; 21(1):388. doi: 10.1186/s12935-021-02091-8 [Crossref] [ Google Scholar]
- Kawabata K, Mukai R, Ishisaka A. Quercetin and related polyphenols: new insights and implications for their bioactivity and bioavailability. Food Funct 2015; 6(5):1399-417. doi: 10.1039/c4fo01178c [Crossref] [ Google Scholar]
- Yarahmadi A, Khademi F, Mostafavi-Pour Z, Zal F. In-vitro analysis of glucose and quercetin effects on m-TOR and Nrf-2 expression in HepG2 cell line (diabetes and cancer connection). Nutr Cancer 2018; 70(5):770-5. doi: 10.1080/01635581.2018.1470654 [Crossref] [ Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 2001; 414(6859):105-11. doi: 10.1038/35102167 [Crossref] [ Google Scholar]
- Dianat-Moghadam H, Heidarifard M, Jahanban-Esfahlan R, Panahi Y, Hamishehkar H, Pouremamali F. Cancer stem cells-emanated therapy resistance: implications for liposomal drug delivery systems. J Control Release 2018; 288:62-83. doi: 10.1016/j.jconrel.2018.08.043 [Crossref] [ Google Scholar]
- Pham TND, Stempel S, Shields MA, Spaulding C, Kumar K, Bentrem DJ. Quercetin enhances the anti-tumor effects of BET inhibitors by suppressing hnRNPA1. Int J Mol Sci 2019; 20(17):4293. doi: 10.3390/ijms20174293 [Crossref] [ Google Scholar]
- Li Y, Wang Z, Jin J, Zhu SX, He GQ, Li SH. Quercetin pretreatment enhances the radiosensitivity of colon cancer cells by targeting Notch-1 pathway. BiochemBiophys Res Commun 2020; 523(4):947-53. doi: 10.1016/j.bbrc.2020.01.048 [Crossref] [ Google Scholar]
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 2006; 441(7097):1068-74. doi: 10.1038/nature04956 [Crossref] [ Google Scholar]
- Sugiarto S, Persson AI, Munoz EG, Waldhuber M, Lamagna C, Andor N. Asymmetry-defective oligodendrocyte progenitors are glioma precursors. Cancer Cell 2011; 20(3):328-40. doi: 10.1016/j.ccr.2011.08.011 [Crossref] [ Google Scholar]
- Tang Y, Hou J, Li G, Song Z, Li X, Yang C. ABCG2 regulates the pattern of self-renewing divisions in cisplatin-resistant non-small cell lung cancer cell lines. Oncol Rep 2014; 32(5):2168-74. doi: 10.3892/or.2014.3470 [Crossref] [ Google Scholar]
- Egger B, Gold KS, Brand AH. Notch regulates the switch from symmetric to asymmetric neural stem cell division in the Drosophila optic lobe. Development 2010; 137(18):2981-7. doi: 10.1242/dev.051250 [Crossref] [ Google Scholar]
- Nwaeburu CC, Abukiwan A, Zhao Z, Herr I. Quercetin-induced miR-200b-3p regulates the mode of self-renewing divisions in pancreatic cancer. Mol Cancer 2017; 16(1):23. doi: 10.1186/s12943-017-0589-8 [Crossref] [ Google Scholar]
- Lei CS, Hou YC, Pai MH, Lin MT, Yeh SL. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: in vitro and in vivo studies. J NutrBiochem 2018; 51:105-13. doi: 10.1016/j.jnutbio.2017.09.011 [Crossref] [ Google Scholar]
- Kauntz H, Bousserouel S, Gosse F, Marescaux J, Raul F. Silibinin, a natural flavonoid, modulates the early expression of chemoprevention biomarkers in a preclinical model of colon carcinogenesis. Int J Oncol 2012; 41(3):849-54. doi: 10.3892/ijo.2012.1526 [Crossref] [ Google Scholar]
- Deep G, Gangar SC, Rajamanickam S, Raina K, Gu M, Agarwal C. Angiopreventive efficacy of pure flavonolignans from milk thistle extract against prostate cancer: targeting VEGF-VEGFR signaling. PLoS One 2012; 7(4):e34630. doi: 10.1371/journal.pone.0034630 [Crossref] [ Google Scholar]
- Tiwari P, Mishra K. Silibinin in cancer therapy: a promising prospect. Cancer Res Front 2015; 1(3):303-18. doi: 10.17980/2015.303 [Crossref] [ Google Scholar]
- Chhabra N, Buzarbaruah S, Singh R, Kaur J. Silibinin: a promising anti-neoplastic agent for the future? A critical reappraisal. Int J NutrPharmacol Neurol Dis 2013; 3(3):206-18. doi: 10.4103/2231-0738.114836 [Crossref] [ Google Scholar]
- Kim TH, Woo JS, Kim YK, Kim KH. Silibinin induces cell death through reactive oxygen species-dependent downregulation of Notch-1/ERK/Akt signaling in human breast cancer cells. J Pharmacol Exp Ther 2014; 349(2):268-78. doi: 10.1124/jpet.113.207563 [Crossref] [ Google Scholar]
- Zhang S, Yang Y, Liang Z, Duan W, Yang J, Yan J. Silybin-mediated inhibition of Notch signaling exerts antitumor activity in human hepatocellular carcinoma cells. PLoS One 2013; 8(12):e83699. doi: 10.1371/journal.pone.0083699 [Crossref] [ Google Scholar]
- Chang HR, Chen PN, Yang SF, Sun YS, Wu SW, Hung TW. Silibinin inhibits the invasion and migration of renal carcinoma 786-O cells in vitro, inhibits the growth of xenografts in vivo and enhances chemosensitivity to 5-fluorouracil and paclitaxel. Mol Carcinog 2011; 50(10):811-23. doi: 10.1002/mc.20756 [Crossref] [ Google Scholar]
- Cho HJ, Suh DS, Moon SH, Song YJ, Yoon MS, Park DY. Silibinin inhibits tumor growth through downregulation of extracellular signal-regulated kinase and Akt in vitro and in vivo in human ovarian cancer cells. J Agric Food Chem 2013; 61(17):4089-96. doi: 10.1021/jf400192v [Crossref] [ Google Scholar]
- Rugamba A, Kang DY, Sp N, Jo ES, Lee JM, Bae SW. Silibinin regulates tumor progression and tumorsphere formation by suppressing PD-L1 expression in non-small cell lung cancer (NSCLC) cells. Cells 2021; 10(7):1632. doi: 10.3390/cells10071632 [Crossref] [ Google Scholar]
- Thomas SJ, Snowden JA, Zeidler MP, Danson SJ. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br J Cancer 2015; 113(3):365-71. doi: 10.1038/bjc.2015.233 [Crossref] [ Google Scholar]
- Soleas GJ, Diamandis EP, Goldberg DM. Resveratrol: a molecule whose time has come? And gone?. Clin Biochem 1997; 30(2):91-113. doi: 10.1016/s0009-9120(96)00155-5 [Crossref] [ Google Scholar]
- Frémont L. Biological effects of resveratrol. Life Sci 2000; 66(8):663-73. doi: 10.1016/s0024-3205(99)00410-5 [Crossref] [ Google Scholar]
- Langcake P, Pryce RJ. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol Plant Pathol 1976; 9(1):77-86. doi: 10.1016/0048-4059(76)90077-1 [Crossref] [ Google Scholar]
- Serrero G, Lu R. Effect of resveratrol on the expression of autocrine growth modulators in human breast cancer cells. Antioxid Redox Signal 2001; 3(6):969-79. doi: 10.1089/152308601317203512 [Crossref] [ Google Scholar]
- Fontecave M, Lepoivre M, Elleingand E, Gerez C, Guittet O. Resveratrol, a remarkable inhibitor of ribonucleotide reductase. FEBS Lett 1998; 421(3):277-9. doi: 10.1016/s0014-5793(97)01572-x [Crossref] [ Google Scholar]
- Wahab A, Gao K, Jia C, Zhang F, Tian G, Murtaza G. Significance of resveratrol in clinical management of chronic diseases. Molecules 2017; 22(8):1329. doi: 10.3390/molecules22081329 [Crossref] [ Google Scholar]
- Bhullar KS, Hubbard BP. Lifespan and healthspan extension by resveratrol. BiochimBiophys Acta 2015; 1852(6):1209-18. doi: 10.1016/j.bbadis.2015.01.012 [Crossref] [ Google Scholar]
- Dong J, Yang W, Han J, Cheng R, Li L. Effects of Notch signaling components from breast cancer cells treated in culture with resveratrol. Res Vet Sci 2020; 132:369-78. doi: 10.1016/j.rvsc.2020.07.017 [Crossref] [ Google Scholar]
- Patel NS, Li JL, Generali D, Poulsom R, Cranston DW, Harris AL. Up-regulation of delta-like 4 ligand in human tumor vasculature and the role of basal expression in endothelial cell function. Cancer Res 2005; 65(19):8690-7. doi: 10.1158/0008-5472.can-05-1208 [Crossref] [ Google Scholar]
- Peng L, Jiang D. Resveratrol eliminates cancer stem cells of osteosarcoma by STAT3 pathway inhibition. PLoS One 2018; 13(10):e0205918. doi: 10.1371/journal.pone.0205918 [Crossref] [ Google Scholar]
- Li P, Yang S, Dou M, Chen Y, Zhang J, Zhao X. Synergic effects of artemisinin and resveratrol in cancer cells. J Cancer Res Clin Oncol 2014; 140(12):2065-75. doi: 10.1007/s00432-014-1771-7 [Crossref] [ Google Scholar]
- Kaushik U, Aeri V, Mir SR. Cucurbitacins - an insight into medicinal leads from nature. Pharmacogn Rev 2015; 9(17):12-8. doi: 10.4103/0973-7847.156314 [Crossref] [ Google Scholar]
- Dandawate P, Subramaniam D, Panovich P, Standing D, Krishnamachary B, Kaushik G. Cucurbitacin B and I inhibits colon cancer growth by targeting the Notch signaling pathway. Sci Rep 2020; 10(1):1290. doi: 10.1038/s41598-020-57940-9 [Crossref] [ Google Scholar]
- Chen YW, Chen KH, Huang PI, Chen YC, Chiou GY, Lo WL. Cucurbitacin I suppressed stem-like property and enhanced radiation-induced apoptosis in head and neck squamous carcinoma--derived CD44( + )ALDH1( + ) cells. Mol Cancer Ther 2010; 9(11):2879-92. doi: 10.1158/1535-7163.mct-10-0504 [Crossref] [ Google Scholar]
- Shukla S, Sinha S, Khan S, Kumar S, Singh K, Mitra K. Cucurbitacin B inhibits the stemness and metastatic abilities of NSCLC via downregulation of canonical Wnt/β-catenin signaling axis. Sci Rep 2016; 6:21860. doi: 10.1038/srep21860 [Crossref] [ Google Scholar]
- Yuan R, Ke J, Sun L, He Z, Zou Y, He X. HES1 promotes metastasis and predicts poor survival in patients with colorectal cancer. Clin Exp Metastasis 2015; 32(2):169-79. doi: 10.1007/s10585-015-9700-y [Crossref] [ Google Scholar]
- Wu WK, Wang XJ, Cheng AS, Luo MX, Ng SS, To KF. Dysregulation and crosstalk of cellular signaling pathways in colon carcinogenesis. Crit Rev Oncol Hematol 2013; 86(3):251-77. doi: 10.1016/j.critrevonc.2012.11.009 [Crossref] [ Google Scholar]
- Miyamoto S, Nakanishi M, Rosenberg DW. Suppression of colon carcinogenesis by targeting Notch signaling. Carcinogenesis 2013; 34(10):2415-23. doi: 10.1093/carcin/bgt191 [Crossref] [ Google Scholar]
- Akamatsu H, Komura J, Asada Y, Niwa Y. Mechanism of anti-inflammatory action of glycyrrhizin: effect on neutrophil functions including reactive oxygen species generation. Planta Med 1991; 57(2):119-21. doi: 10.1055/s-2006-960045 [Crossref] [ Google Scholar]
- Ahmad A, Tiwari RK, Saeed M, Ahmad I, Ansari IA. Glycyrrhizin mediates downregulation of Notch pathway resulting in initiation of apoptosis and disruption in the cell cycle progression in cervical cancer cells. Nutr Cancer 2022; 74(2):622-39. doi: 10.1080/01635581.2021.1895234 [Crossref] [ Google Scholar]
- Ahmad A, Tiwari RK, Mishra P, Alkhathami AG, Almeleebia TM, Alshahrani MY. Antiproliferative and apoptotic potential of glycyrrhizin against HPV16 + Caski cervical cancer cells: a plausible association with downreguation of HPV E6 and E7 oncogenes and Notch signaling pathway. Saudi J Biol Sci 2022; 29(5):3264-75. doi: 10.1016/j.sjbs.2022.01.054 [Crossref] [ Google Scholar]
- Chang HY, Chen SY, Wu CH, Lu CC, Yen GC. Glycyrrhizin attenuates the process of epithelial-to-mesenchymal transition by modulating HMGB1 initiated novel signaling pathway in prostate cancer cells. J Agric Food Chem 2019; 67(12):3323-32. doi: 10.1021/acs.jafc.9b00251 [Crossref] [ Google Scholar]