Advanced pharmaceutical bulletin. 14(3):537-542.
doi: 10.34172/apb.2024.056
Review Article
Sex Differences on the Pharmacokinetics of Drugs for Children with Chronic Kidney Disease: A Narrative Review
Toktam Faghihi Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – review & editing, 1 
Farahnak Assadi Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, 2, * 
Author information:
1Department of Clinical Pharmacy, School of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran, and Pediatrics Center of Excellence, Children’s Medical Center, Tehran University of Medical Sciences, Tehran, Iran.
2Department of Pediatrics, Division of Nephrology, Rush University Medical Center, Chicago, Illinois USA.
Abstract
Effective optimal pharmacotherapy requires a comprehensive understanding of the drug’s pharmacokinetic properties. Chronic kidney disease (CKD) influences medication pharmacokinetics. However, whether sex differences exist in the pharmacokinetics of drugs for children with CKD is unknown. The primary aim of this article was to evaluate the effect of sex on pharmacokinetics of drugs commonly used for CKD treatment in children. Secondary outcome was to address the impact of sex in CKD disease progression. Electronic databases, PubMed, EMBASE, Google Scholar, and Web of Science were searched from inception, using Mesh terms in English for sex differences in the pharmacokinetics of drugs in children with CKD. No studies have documented sex-related differences in the pharmacokinetics of drugs for the treatment of CKD in children. As a consequence, it is difficult to predict the effect of sex on pharmacokinetics by extrapolating data from adult studies to children. Evidence to date suggests that girls generally have a higher prevalence and disease progression of CKD when compared to boys regardless of age. Understanding the pharmacokinetics and pharmacodynamics of drugs provides practical consideration for dosing optimal medication regimens. Future kinetic studies are needed evaluating the effect of sex on the pharmacokinetics and pharmacodynamics of drugs in children with CKD.
Keywords: Children, Chronic kidney disease, Pharmacotherapy, Pharmacokinetics, Sex differences
Copyright and License Information
©2024 The Author (s).
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Introduction
It has been illuminated that CKD influences drug pharmacokinetics.1 Interestingly, accumulating evidence from epidemiological and clinical studies suggests that sex-related differences exist in the prevalence, course, and progression of chronic kidney disease (CKD) in adults.2 In adults, the prevalence, incidence, and disease progression are higher in men than in women.3 Among children, girls have been reported to have a higher incidence rate of CKD, defined as estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2, than boys because of a faster decline in GFR compared with boys. Certain risk factors for the development and progression of CKD including frequent urinary tract infection (UTI) and autoimmune-related glomerular diseases such as lupus nephritis are also higher in girls than in boys.4-6 Similarly, the mortality rate is also substantially higher in girls treated with renal replacement therapy (RRT) compared to boys.7-9 Moreover, significant sex differences also exist in the pharmacokinetics and pharmacodynamics of drugs between men and women,10-13 but data on sex differences in pediatric pharmacokinetics and drug dosing are lacking.
Children’s doses cannot be extrapolated directly from adult studies as the pharmacokinetics of many drugs are different in children compared to adults. Pharmacokinetics vary with sex, age, rapid changes in size, body composition, and organ function, particularly during early development.14,15 A change in the pharmacokinetics during development can affect drug elimination and exposure predisposing the child to over or under medication leading to severe adverse events or treatment failure.2,3
The lack of pharmacokinetics knowledge complicates finding the correct dosing for the management of children with CKD. This review addresses the influence of sex differences on the pharmacokinetics of drugs and on the incidence of disease progression in children with CKD.
Electronic databases, PubMed, Embase, and Web of Science have been searched since inception, using Mesh terms English for sex differences in the management and pharmacokinetics of drugs in children and adolescents with CKD. All studies included were published from inception. Of the 70 reviewed articles, 36 met the inclusion criteria and were included in analyses.
Sex differences in genetic and epigenetic process
Studies have documented genetic dispositions for certain diseases associated with CKD in females.16-18 Skewed X chromosome inactivation has been shown to predispose females to several autoimmune diseases including lupus nephritis, scleroderma, rheumatoid arthritis, Sjogren’s disease, and myasthenia gravis.16 Many genes possess hormone response elements in the region of their target genes, which can interfere with the transcription of target genes. The expression of DNA methylation is also under sex hormones (estrogens and androgens) control.19 Hormonal and genetic factors have also been shown to play a relevant role in explaining these differences in CKD, as demonstrated by several experimental studies, showing an overall protective role for estrogens and progesterone.20-22
Environmental exposures to toxins, air pollution, chemical, microbial, allergens, and unhealthy dietary habits can increase the risk of developing CKD through DNA methylation, suggesting this environmental interaction or epigenetic dysregulation may play an important role in the sex-related differences in CKD amongst children.18,19
Current screening standards including measurement of eGFR and urinary biomarkers such as beta-2 microglobulin and kidney injury molecule (KIM-1) are widely used for CDK detection, but it is difficult to predict CKD precisely.23 Evidence-based medicine requires gender heterogeneity to be taken into account in CKD deterioration to inform the risk assessment, monitoring, and prognosis.
Sex differences in susceptibility to CKD
The majority of glomerular diseases show a male bias, except lupus nephritis, which is strongly female-predominant.24 Studies have shown that collagen-vascular diseases and autoimmune disorders affect females three to ten times more than males.25 Environmental stressors-induced epigenetic alteration such as chemical exposures, drugs, and infections during pregnancy, ureter-placental hypoxia, and maternal under-nutrition may lead to prematurity and low birth weight infants and the risk of long-term hypertension and metabolic syndrome leading to the development of CKD, in children and adults.18,19,26,27
UTI is also strongly sex-biased, with girls being 20 to 40 times to have a UTI than boys of the same age.5,6,28 Being female significantly influences immune response to diseases at mucosal surfaces. Sex hormones, sex differences, sex chromosomes, and sexual dimorphism all contribute to the development of CKD.16,17
Sex differences also exist in susceptibility to metabolic syndrome with female children being at a higher risk of developing obesity, dyslipidemia, type 2 diabetes, and CKD.29-31 Variable epigenetic background, diet, levels of physical activity, and levels of estrogens may influence the higher prevalence of metabolic syndrome in females than in men.32
Sex differences in renal physiology
Clinical studies have demonstrated sex differences in kidney size morphology and hemodynamic functions. Total renal mass and the renal cortex and proximal tubules are larger in males than in females.20 The contribution of these structural differences to sex-related variation in renal physiology may also account for a higher incidence rate of CKD progression in female children.
Sex differences in the pharmacokinetics of drugs frequently used to treat CKD in children
In contrast to adults, there is no information in the literature about sex-related differences in the pharmacokinetics of drugs for the treatment of CKD in children. As a consequence, it is difficult to predict the impact of sex on pharmacokinetics by extrapolating data from adult studies in children.
Influence of CKD on medication pharmacokinetics
CKD influences multiple pharmacokinetic parameters, which needs to be considered for commonly used medications in this population.1 Understanding the pharmacokinetics (PK) properties and the study of the absorption, distribution, metabolism, and excretion (ADME) processes of a drug is essential for effective optimal pharmacotherapy in patients with CKD.
The pharmacokinetics of many drugs in children is different compared to adults.12 Children have a lower body weight and organ size and faster growth and development during early development, which can significantly affect drug absorption, distribution, and elimination.12
In general, the renal drug clearance depends on GFR, tubular reabsorption capacity, and tubular excretion. In CKD, in the absence of pharmacokinetics properties, drugs that are primarily excreted by GFR such as aminoglycosides, dose adjustment can be made by either decreasing the initial dose or increasing the dosing interval. However, in some patients with active infection, a higher initial loading dose is required to rapidly achieve therapeutic concentration.33 A loading dose decreases the time to achieve the target concentration. The plasma drugs’ half-time (t1/2) is often prolonged in adult patients with CKD because of reduced GFR. Increasing the t1/2 delays the time to achieve steady-state plasma concentrations and results in higher plasma concentrations. Loading dose decreases the time to achieve the target therapeutic plasma concentration (Figure 1). An increase in a drug’s t1/2 prolongs the time to achieve steady-state plasma concentrations with maintenance dosing. Failure to reduce the maintenance dose or frequency in CKD patients with the longer t ½ may predispose them to adverse drug reactions (Figure 2).
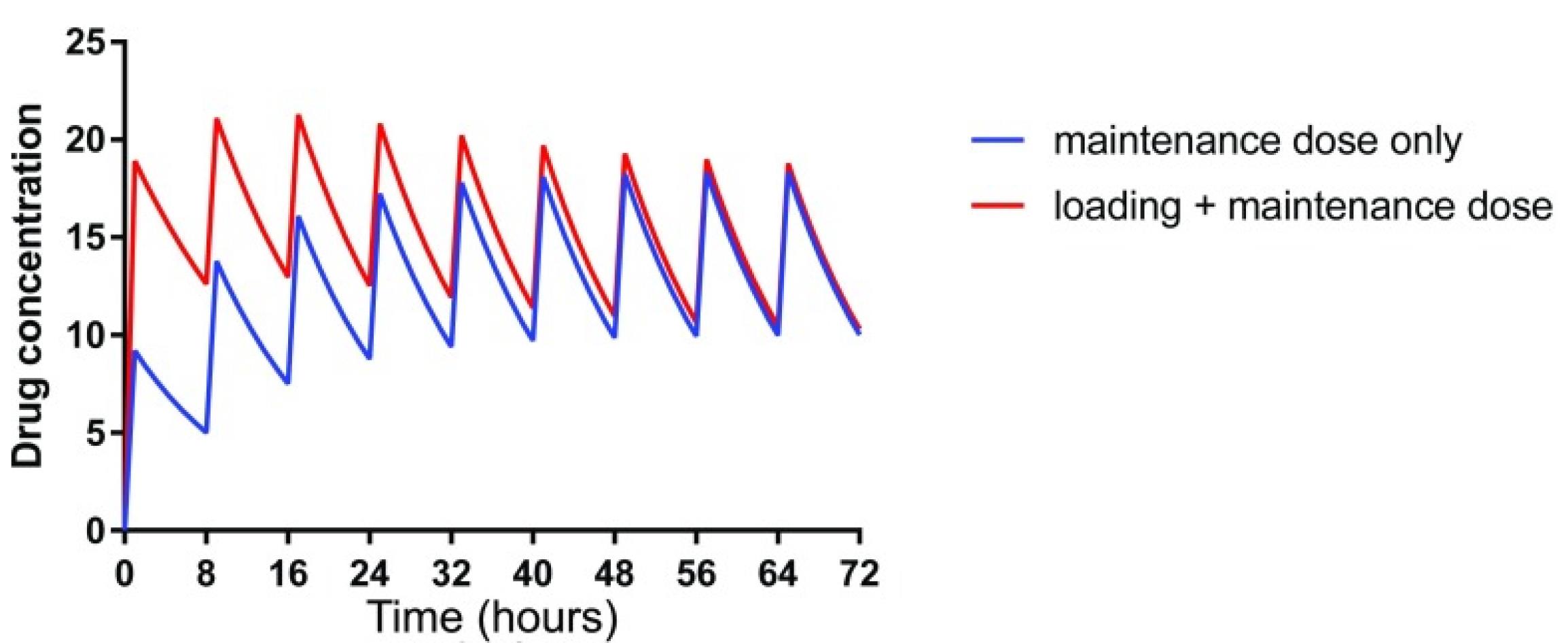
Figure 1.
Loading dose decreases the time to achieve target concentration (Used with permission, Robert et al.7
.
Loading dose decreases the time to achieve target concentration (Used with permission, Robert et al.7
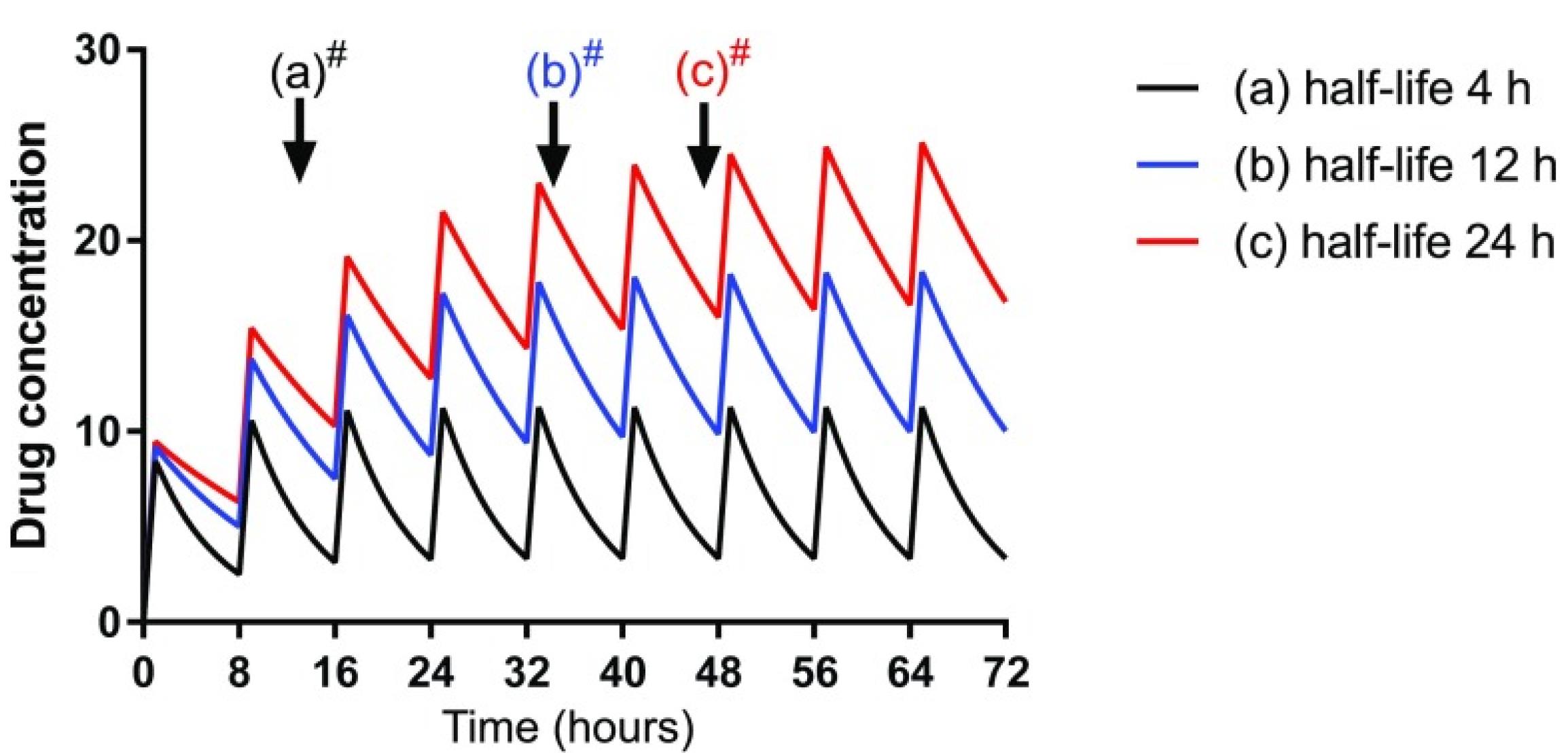
Figure 2.
An increase in a drug’s t1/2 prolongs the time to achieve steady-state plasma concentrations with maintenance dosing (Used with permission, Robert et al.7
.
An increase in a drug’s t1/2 prolongs the time to achieve steady-state plasma concentrations with maintenance dosing (Used with permission, Robert et al.7
The volume of distribution (Vd) and clearance (CL) are the two most important parameters of the pharmacokinetics of drugs. Both Vd and CL also changes in patients with CKD.1
In general, drug distribution is dependent on the extent of protein binding, lipophilicity or water solubility, renal blood flow, membrane permeability, and tissue uptake.9-12,16 Increased protein binding decreases the free concentration and fraction of drugs, thereby limiting the capacity of the active drug to diffuse more easily across the cell membranes. In CKD, uremic toxins displace some drugs from protein binding sites leading to increased unbound fraction. Likewise, phenytoin does not need dose adjustment in patients with reduced GFR, however free drug concentration should be monitored instead of total drug concentration in CKD patients.34
Table 1 summarizes the pharmacokinetic parameters and the impact of sex on pharmacokinetics of commonly administered drugs in adults with stage 1-4 CKD.35-58
Table 1.
Sex-related differences in the pharmacokinetics of frequently prescribed drugs in patients with CKD
|
Drug class
|
BA (%)
|
PB (%)
|
Vd (L/kg)
|
t1/2 (h)
|
Adults
|
Children
|
| Antihypertensives |
|
|
|
|
|
|
| Captopril |
40-90 |
25-30 |
0.7 |
6-12 |
No sex-related effect35 |
Unknown |
| Enalapril |
40 |
50 |
21 |
42-55 |
Women may have higher oral bioavailability, lower clearance, and smaller Vd36 |
Unknown |
| Metoprolol |
43-69 |
10-12 |
3.2-5.6 |
2-3 |
Faster clearance in women than in men37 |
Unknown |
| Propranolol |
25 |
90 |
4 |
6-8 |
No sex-related effect38 |
Unknown |
| Nifedipine |
56-77 |
92-98 |
0.6-0.8 |
2-3 |
No sex-related effect39 |
Unknown |
| Amlodipine |
65-90 |
85-90 |
20-22 |
30-50 |
No sex-related effect40 |
Unknown |
| Prazocin |
43-82 |
92-96 |
0.5 |
2-3 |
No sex-related effect41 |
Unknown |
| Labetolol |
20-30 |
50 |
3.3-7.9 |
5-6 |
No sex-related effect42 |
Unknown |
| Diuretics |
|
|
|
|
|
|
| Furosemide |
50-70 |
95-99 |
0.2-0.5 |
> 8 |
No sex-related effect43 |
Unknown |
| Chlorothiazide |
Poor |
40-68 |
4-13 |
40-60 |
No sex-related effect44 |
Unknown |
| Spironolactone |
80-90 |
> 90 |
5-7 |
2-26 |
No sex-related effect45 |
Unknown |
| Digoxin |
70-80 |
20-30 |
2.5-7.5 |
30-120 |
Lower clearance and smaller Vdin women46 |
Unknown |
| Antibiotics |
|
|
|
|
|
|
| Cefotaxime |
- |
32-44 |
0.2-0.4 |
1.9-5.8 |
No sex-related effect47 |
Unknown |
| Ciprofloxacin |
60-66 |
20-40 |
2-3 |
4.2-5.1 |
Lower clearance and longer t ½ in females48 |
Unknown |
| Erythromycin |
30-65 |
80-90 |
0.64 |
5-6 |
Lower bioavailability, larger Vd, and longer t1/2 in females49 |
Unknown |
| Quinolones |
70-90 |
20-40 |
1.8-2.1 |
13-17 |
Lower Vdand longer t½ in females during50 pregnancy |
Unknown |
| Vancomycin |
< 10 |
40-60 |
0.7-0.9 |
28-36 |
No sex-related effect51 |
Unknown |
| Anticonvulsants |
|
|
|
|
|
|
| Phenobarbital |
95-100 |
20-45 |
0.63 |
> 120 |
No sex-related effect52 |
Unknown |
| Phenytoin |
40-60 |
87.8-91.9 |
0.4-0.6 |
0.5-0.8 |
No sex-related effect53 |
Unknown |
| Anticoagulants |
|
|
|
|
|
|
| Heparin |
- |
|
|
|
Lower clearance and Vdin women54 |
Unknown |
| Warfarin |
- |
99 |
0.14 |
|
Lower clearance and smallerVd in women55 |
Unknown |
| Immunosuppressives |
|
|
|
|
|
|
| Cyclosporine |
5-89 |
96-99 |
4-6 |
6.2-15.8 |
Lower clearance in women, suggesting higher rate of nephrotoxicity compared to men56 |
Unknown |
| Methylprednisolone |
81-110 |
40-60 |
24 |
2.6-4.3 |
Clearance is higher and as a consequence, half-life is lower in women, suggesting higher rate of nephrotoxicity compared to men57 |
Unknown |
| Mycophenolate |
75-87 |
82 |
3.6-4 |
14-18 |
Slower clearance and highert ½ in females58 |
Unknown |
Pharmacokinetic data presents adult data. CKD, chronic kidney disease; BA, bioavailability for oral formulations; PB, protein binding; vd, volume of distribution; t1/2, half-life; CCB, calcium channel blockers
Relevance to patient care and clinical practice
Impaired renal function can significantly alter the pharmacokinetics and pharmacodynamics of drugs, putting patients at risk for drug toxicity or treatment failure if appropriate dosing adjustments are not applied. The incidence rate and progression of CKD is higher in girls than in boys worldwide. There is no information in the literature about sex-related differences in the pharmacokinetics of drugs for the treatment of CKD in children.
Understanding the pharmacokinetics and pharmacodynamics of drugs provides practical consideration for dosing optimal medication regimens.
Discussion
The incidence rate and progression of CKDis higher in girls than in boys worldwide. There is no information in the literature about sex-related differences in the pharmacokinetics of drugs for the treatment of CKD in children.
Conclusion
The prevalence of CKD is higher in girls than boys but reverses when they reach adulthood, possibly owing to the protective effects of estrogens. More girls than boys start RRT because of faster CKD progression and because they are more likely to experience frequent UTIs. Girls are at greater risk of CKD progression and CVD disease.
In contrast to adults, there is no information in the literature about sex-related differences in the pharmacokinetics of drugs for the treatment of CKD in children.
The lack of pharmacokinetic studies in children with CKD makes it very difficult to predict the optimum therapeutic dosing. Prescribing drug doses extrapolated from adult studies increases the risk of adverse events due to overmedication or lead to treatment failure due to sub-therapeutic exposure.
Future kinetic studies are eagerly needed to anticipate better and model drug-response relationships in boys and girls.
Competing Interests
Theauthors have no relevant financial or non-financial interests to disclose.
Ethical Approval
Not applicable.
References
- Lea-Henry TN, Carland JE, Stocker SL, Sevastos J, Roberts DM. Clinical pharmacokinetics in kidney disease: fundamental principles. Clin J Am Soc Nephrol 2018; 13(7):1085-95. doi: 10.2215/cjn.00340118 [Crossref] [ Google Scholar]
- Ricardo AC, Yang W, Sha D, Appel LJ, Chen J, Krousel-Wood M. Sex-related disparities in CKD progression. J Am Soc Nephrol 2019; 30(1):137-46. doi: 10.1681/asn.2018030296 [Crossref] [ Google Scholar]
- Mayne KJ, Sullivan MK, Lees JS. Sex and gender differences in the management of chronic kidney disease and hypertension. J Hum Hypertens 2023; 37(8):649-53. doi: 10.1038/s41371-023-00843-9 [Crossref] [ Google Scholar]
- Bonnéric S, Karadkhele G, Couchoud C, Patzer RE, Greenbaum LA, Hogan J. Sex and glomerular filtration rate trajectories in children. Clin J Am Soc Nephrol 2020; 15(3):320-9. doi: 10.2215/cjn.08420719 [Crossref] [ Google Scholar]
- Deltourbe L, Lacerda Mariano L, Hreha TN, Hunstad DA, Ingersoll MA. The impact of biological sex on diseases of the urinary tract. Mucosal Immunol 2022; 15(5):857-66. doi: 10.1038/s41385-022-00549-0 [Crossref] [ Google Scholar]
- Harrington RD, Hooton TM. Urinary tract infection risk factors and gender. J GendSpecif Med 2000; 3(8):27-34. [ Google Scholar]
- Ahearn P, Johansen KL, McCulloch CE, Grimes BA, Ku E. Sex disparities in risk of mortality among children with ESRD. Am J Kidney Dis 2019; 73(2):156-62. doi: 10.1053/j.ajkd.2018.07.019 [Crossref] [ Google Scholar]
- Plumb L, Magadi W, Casula A, Reynolds BC, Convery M, Haq S. Advanced chronic kidney disease among UK children. Arch Dis Child 2022; 107(11):1043-5. doi: 10.1136/archdischild-2021-323686 [Crossref] [ Google Scholar]
- Sugianto RI, Memaran N, Schmidt BM, Doyon A, Thurn-Valsassina D, Alpay H. Findings from 4C-T Study demonstrate an increased cardiovascular burden in girls with end stage kidney disease and kidney transplantation. Kidney Int 2022; 101(3):585-96. doi: 10.1016/j.kint.2021.11.032 [Crossref] [ Google Scholar]
- Roberts DM, Sevastos J, Carland JE, Stocker SL, Lea-Henry TN. Clinical pharmacokinetics in kidney disease: application to rational design of dosing regimens. Clin J Am Soc Nephrol 2018; 13(8):1254-63. doi: 10.2215/cjn.05150418 [Crossref] [ Google Scholar]
- Nicolas JM, Bouzom F, Hugues C, Ungell AL. Oral drug absorption in pediatrics: the intestinal wall, its developmental changes and current tools for predictions. Biopharm Drug Dispos 2017; 38(3):209-30. doi: 10.1002/bdd.2052 [Crossref] [ Google Scholar]
- van den Anker J, Reed MD, Allegaert K, Kearns GL. Developmental changes in pharmacokinetics and pharmacodynamics. J Clin Pharmacol 2018; 58 Suppl 10:S10-25. doi: 10.1002/jcph.1284 [Crossref] [ Google Scholar]
- Batchelor HK, Marriott JF. Paediatric pharmacokinetics: key considerations. Br J Clin Pharmacol 2015; 79(3):395-404. doi: 10.1111/bcp.12267 [Crossref] [ Google Scholar]
- Karlsson Lind L, Rydberg DM, Schenck-Gustafsson K. Sex and gender differences in drug treatment: experiences from the knowledge database Janusmed Sex and Gender. Biol Sex Differ 2023; 14(1):28. doi: 10.1186/s13293-023-00511-0 [Crossref] [ Google Scholar]
- Anderson GD. Children versus adults: pharmacokinetic and adverse-effect differences. Epilepsia 2002; 43 Suppl 3:53-9. doi: 10.1046/j.1528-1157.43.s.3.5.x [Crossref] [ Google Scholar]
- Kanaan SB, Onat OE, Balandraud N, Martin GV, Nelson JL, Azzouz DF. Evaluation of X chromosome inactivation with respect to HLA genetic susceptibility in rheumatoid arthritis and systemic sclerosis. PLoS One 2016; 11(6):e0158550. doi: 10.1371/journal.pone.0158550 [Crossref] [ Google Scholar]
- Nicolì V, Tabano SM, Colapietro P, Maestri M, Ricciardi R, Stoccoro A. Preferential X chromosome inactivation as a mechanism to explain female preponderance in myasthenia gravis. Genes (Basel) 2022; 13(4):696. doi: 10.3390/genes13040696 [Crossref] [ Google Scholar]
- Migliore L, Nicolì V, Stoccoro A. Gender specific differences in disease susceptibility: the role of epigenetics. Biomedicines 2021; 9(6):652. doi: 10.3390/biomedicines9060652 [Crossref] [ Google Scholar]
- Tseng CC, Liao WT, Wong MC, Chen CJ, Lee SC, Yen JH. Cell lineage-specific methylome and genome alterations in gout. Aging (Albany NY) 2021; 13(3):3843-65. doi: 10.18632/aging.202353 [Crossref] [ Google Scholar]
- Bairey Merz CN, Dember LM, Ingelfinger JR, Vinson A, Neugarten J, Sandberg KL. Sex and the kidneys: current understanding and research opportunities. Nat Rev Nephrol 2019; 15(12):776-83. doi: 10.1038/s41581-019-0208-6 [Crossref] [ Google Scholar]
- Harris RC, Zhang MZ. The role of gender disparities in kidney injury. Ann Transl Med 2020; 8(7):514. doi: 10.21037/atm.2020.01.23 [Crossref] [ Google Scholar]
- Brar A, Markell M. Impact of gender and gender disparities in patients with kidney disease. CurrOpin Nephrol Hypertens 2019; 28(2):178-82. doi: 10.1097/mnh.0000000000000482 [Crossref] [ Google Scholar]
- Assadi F, Ghane Sharbaf F. Urine KIM-1 as a potential biomarker of acute renal injury after circulatory collapse in children. PediatrEmerg Care 2019; 35(2):104-7. doi: 10.1097/pec.0000000000000886 [Crossref] [ Google Scholar]
- Lin S, Gong J, Canas GC, Winkle P, Pelletier K, LaBadie RR. A phase I study to evaluate the pharmacokinetics and safety of lorlatinib in adults with mild, moderate, and severe renal impairment. Eur J Drug MetabPharmacokinet 2022; 47(2):235-45. doi: 10.1007/s13318-021-00747-4 [Crossref] [ Google Scholar]
- Mallén A, Rodriguez-Urquia R, Alvarez R, Dorca-Duch E, Navarro E, Hueso M. Sex differences in glomerular lesions, in atherosclerosis progression, and in the response to angiotensin-converting enzyme inhibitors in the ApoE(-/-) mice model. Int J Mol Sci 2023; 24(17):13442. doi: 10.3390/ijms241713442 [Crossref] [ Google Scholar]
- Maxwell A, Adzibolosu N, Hu A, You Y, Stemmer PM, Ruden DM. Intrinsic sexual dimorphism in the placenta determines the differential response to benzene exposure. iScience 2023; 26(4):106287. doi: 10.1016/j.isci.2023.106287 [Crossref] [ Google Scholar]
- Mordaunt CE, Jianu JM, Laufer BI, Zhu Y, Hwang H, Dunaway KW. Cord blood DNA methylome in newborns later diagnosed with autism spectrum disorder reflects early dysregulation of neurodevelopmental and X-linked genes. Genome Med 2020; 12(1):88. doi: 10.1186/s13073-020-00785-8 [Crossref] [ Google Scholar]
- Simões E Silva AC, Oliveira EA, Mak RH. Urinary tract infection in pediatrics: an overview. J Pediatr (Rio J) 2020; 96(Suppl 1):65-79. doi: 10.1016/j.jped.2019.10.006 [Crossref] [ Google Scholar]
- Anderson WD, Soh JY, Innis SE, Dimanche A, Ma L, Langefeld CD. Sex differences in human adipose tissue gene expression and genetic regulation involve adipogenesis. Genome Res 2020; 30(10):1379-92. doi: 10.1101/gr.264614.120 [Crossref] [ Google Scholar]
- Yi Y, An J. Sex differences in risk factors for metabolic syndrome in the Korean population. Int J Environ Res Public Health 2020; 17(24):9513. doi: 10.3390/ijerph17249513 [Crossref] [ Google Scholar]
- Chung FF, Herceg Z. The promises and challenges of toxico-epigenomics: environmental chemicals and their impacts on the epigenome. Environ Health Perspect 2020; 128(1):15001. doi: 10.1289/ehp6104 [Crossref] [ Google Scholar]
- Vondracek SF, Teitelbaum I, Kiser TH. Principles of kidney pharmacotherapy for the nephrologist: core curriculum 2021. Am J Kidney Dis 2021; 78(3):442-58. doi: 10.1053/j.ajkd.2021.02.342 [Crossref] [ Google Scholar]
- Zucker I, Prendergast BJ. Sex differences in pharmacokinetics. Handb Exp Pharmacol 2023; 282:25-39. doi: 10.1007/164_2023_669 [Crossref] [ Google Scholar]
- Liponi DF, Winter ME, Tozer TN. Renal function and therapeutic concentrations of phenytoin. Neurology 1984; 34(3):395-7. doi: 10.1212/wnl.34.3.395 [Crossref] [ Google Scholar]
- Rasool MF, Ali S, Khalid S, Khalid R, Majeed A, Imran I. Development and evaluation of physiologically based pharmacokinetic drug-disease models for predicting captopril pharmacokinetics in chronic diseases. Sci Rep 2021; 11(1):8589. doi: 10.1038/s41598-021-88154-2 [Crossref] [ Google Scholar]
- Faisal M, Cawello W, Laeer S. Clinical pharmacokinetics of enalapril and enalaprilat in pediatric patients-a systematic review. Front Pediatr 2021; 9:611322. doi: 10.3389/fped.2021.611322 [Crossref] [ Google Scholar]
- Zamir A, Hussain I, Ur Rehman A, Ashraf W, Imran I, Saeed H. Clinical pharmacokinetics of metoprolol: a systematic review. Clin Pharmacokinet 2022; 61(8):1095-114. doi: 10.1007/s40262-022-01145-y [Crossref] [ Google Scholar]
- Bianchetti G, Graziani G, Brancaccio D, Morganti A, Leonetti G, Manfrin M. Pharmacokinetics and effects of propranolol in terminal uraemic patients and in patients undergoing regular dialysis treatment. Clin Pharmacokinet 1976; 1(5):373-84. doi: 10.2165/00003088-197601050-00004 [Crossref] [ Google Scholar]
- Kleinbloesem CH, van Brummelen P, van Harten J, Danhof M, Breimer DD. Nifedipine: influence of renal function on pharmacokinetic/hemodynamic relationship. Clin PharmacolTher 1985; 37(5):563-74. doi: 10.1038/clpt.1985.89 [Crossref] [ Google Scholar]
- Doyle GD, Donohue J, Carmody M, Laher M, Greb H, Volz M. Pharmacokinetics of amlodipine in renal impairment. Eur J Clin Pharmacol 1989; 36(2):205-8. doi: 10.1007/bf00609197 [Crossref] [ Google Scholar]
- Vincent J, Meredith PA, Reid JL, Elliott HL, Rubin PC. Clinical pharmacokinetics of prazosin--1985. Clin Pharmacokinet 1985; 10(2):144-54. doi: 10.2165/00003088-198510020-00002 [Crossref] [ Google Scholar]
- Wood AJ, Ferry DG, Bailey RR. Elimination kinetics of labetalol in severe renal failure. Br J Clin Pharmacol 1982; 13(1 Suppl):81S-6S. doi: 10.1111/j.1365-2125.1982.tb01893.x [Crossref] [ Google Scholar]
- Khan TM, Patel R, Siddiqui AH. Furosemide. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023.
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic
kidney disease. Kidney Int 2021;99(3S):S1-87. 10.1016/j.kint.2020.11.003
- Rossello X, Ferreira JP, Pocock SJ, McMurray JJ, Solomon SD, Lam CS. Sex differences in mineralocorticoid receptor antagonist trials: a pooled analysis of three large clinical trials. Eur J Heart Fail 2020; 22(5):834-44. doi: 10.1002/ejhf.1740 [Crossref] [ Google Scholar]
- Koup JR, Jusko WJ, Elwood CM, Kohli RK. Digoxin pharmacokinetics: role of renal failure in dosage regimen design. Clin PharmacolTher 1975; 18(1):9-21. doi: 10.1002/cpt19751819 [Crossref] [ Google Scholar]
- Paap CM, Nahata MC, Mentser MA, Mahan JD, Puri SK, Hubbard JW. Pharmacokinetics of cefotaxime and its active metabolite in children with renal dysfunction. Antimicrob Agents Chemother 1991; 35(9):1879-83. doi: 10.1128/aac.35.9.1879 [Crossref] [ Google Scholar]
- Kim MK, Nightingale C, Nicolau D. Influence of sex on the pharmacokinetic interaction of fleroxacin and ciprofloxacin with caffeine. Clin Pharmacokinet 2003; 42(11):985-96. doi: 10.2165/00003088-200342110-00004 [Crossref] [ Google Scholar]
- Nikam Y. Pharmacokinetics and antibiotic activity of erythromycin. Pharm Anal Chem 2022; 7(5):163. doi: 10.35248/2471-2698.7.163 [Crossref] [ Google Scholar]
- Fillastre JP, Leroy A, Moulin B, Dhib M, Borsa-Lebas F, Humbert G. Pharmacokinetics of quinolones in renal insufficiency. J Antimicrob Chemother 1990; 26 Suppl B:51-60. doi: 10.1093/jac/26.suppl_b.51 [Crossref] [ Google Scholar]
- Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother 1984; 25(4):433-7. doi: 10.1128/aac.25.4.433 [Crossref] [ Google Scholar]
- Methaneethorn J, Leelakanok N. Pharmacokinetic variability of phenobarbital: a systematic review of population pharmacokinetic analysis. Eur J Clin Pharmacol 2021; 77(3):291-309. doi: 10.1007/s00228-020-03011-x [Crossref] [ Google Scholar]
- Richens A. Clinical pharmacokinetics of phenytoin. Clin Pharmacokinet 1979; 4(3):153-69. doi: 10.2165/00003088-197904030-00001 [Crossref] [ Google Scholar]
- Roosendaal LC, Wiersema AM, Smit JW, Doganer O, Blankensteijn JD, Jongkind V. Editor’s choice - sex differences in response to administration of heparin during non-cardiac arterial procedures. Eur J VascEndovasc Surg 2022; 64(5):557-65. doi: 10.1016/j.ejvs.2022.08.005 [Crossref] [ Google Scholar]
- Holford NH. Holford NHClinical pharmacokinetics and pharmacodynamics of warfarinUnderstanding the dose-effect relationship. Clin Pharmacokinet 1986; 11(6):483-504. doi: 10.2165/00003088-198611060-00005 [Crossref] [ Google Scholar]
- Tornatore KM, Brazeau D, Dole K, Danison R, Wilding G, Leca N. Sex differences in cyclosporine pharmacokinetics and ABCB1 gene expression in mononuclear blood cells in African American and Caucasian renal transplant recipients. J Clin Pharmacol 2013; 53(10):1039-47. doi: 10.1002/jcph.123 [Crossref] [ Google Scholar]
- Jusko WJ, Milad MA, Ludwig EA, Lew KH, Kohli RK. Methylprednisolone pharmacokinetics and pharmacodynamics in chronic renal failure. Clin Nephrol 1995; 43 Suppl 1:S16-9. [ Google Scholar]
- MacPhee IA, Spreafico S, Bewick M, Davis C, Eastwood JB, Johnston A. Pharmacokinetics of mycophenolate mofetil in patients with end-stage renal failure. Kidney Int 2000; 57(3):1164-8. doi: 10.1046/j.1523-1755.2000.00943.x [Crossref] [ Google Scholar]