Advanced pharmaceutical bulletin. 14(3):574-590.
doi: 10.34172/apb.2024.052
Review Article
Harnessing the Therapeutic Potential of Mesenchymal Stem Cells in Cancer Treatment
Parisa Kangari Conceptualization, Data curation, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, 1, 2, * 
Reza Salahlou Resources, Validation, Writing – review & editing, 3, 4
Somayeh Vandghanooni Data curation, Resources, Supervision, Validation, Writing – review & editing, 2, * 
Author information:
1Department of Applied Cell Sciences, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
2Hematology and Oncology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
3Department of Medical Biotechnology, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
4Research Center for Pharmaceutical Nanotechnology, Biomedicine Institute, Tabriz University of Medical Sciences, Tabriz, Iran.
Abstract
Cancer, as a complicated disease, is considered to be one of the major leading causes of death globally. Although various cancer therapeutic strategies have been established, however, some issues confine the efficacies of the treatments. In recent decades researchers for finding efficient therapeutic solutions have extensively focused on the abilities of stem cells in cancer inhibition. Mesenchymal stem cells (MSCs) are multipotent stromal cells that can the most widely extracted from various sources such as the bone marrow (BM), placenta, umbilical cord (UC), menses blood, Wharton’s jelly (WJ), adipose tissue and dental pulp (DP). These cells are capable of differentiating into the osteoblasts, chondrocytes, and adipocytes. Due to the unique characteristics of MSCs such as paracrine effects, immunomodulation, tumor-tropism, and migration, they are considered promising candidates for cancer therapeutics. Currently, MSCs are an excellent living carrier for delivery of therapeutic genes and chemical agents to target tumor sites. Also, exosomes, the most important extracellular vesicle released from MSCs, act as a strong cell-free tool for cancer therapeutics. MSCs can prevent cancer progression by inhibiting several signaling pathways, such as wnt/β-catenin and PI3K/AKT/mTOR. However, there are several challenges associated with the use of MSCs and their exosomes in the field of therapy that need to be considered. This review explores the significance of MSCs in cell-based therapy, focusing on their homing properties and immunomodulatory characteristics. It also examines the potential of using MSCs as carriers for delivery of anticancer agents and their role in modulating the signal transduction pathways of cancer cells.
Keywords: Mesenchymal stem cells, Cancer therapy, Cell therapy, Exosomes, Immunomodulation, Chemotherapeutic agents
Copyright and License Information
©2024 The Author (s).
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Funding Statement
This work was supported by a grant from the Hematology and Oncology Research Center at Tabriz University of Medical Sciences (#71247).
Introduction
Currently, cancer is a major and widespread health concern, contributing significantly to global mortality and morbidity across various populations.1 According to the latest global cancer statistics in 2022, the most prevalent malignancies around the world include breast cancer in women (11.7%), lung cancer (11.4%), colorectal cancer (10.0%), prostate cancer (7.3%), and stomach cancer (5.6 %).2 The conventional cancer treatment methods include surgery, chemotherapy, immunotherapy, and radiation therapy that can be used alone or in combination. However, multiple deficiencies, such as significant adverse effects, off-target effects of therapeutic agents, and drug resistance restrict the efficacies of the therapeutic modalities.3,4 Recently, to dominate the barriers and disadvantages correlated with traditional treatments in cancer, cell therapy as one of the most prominent emerging medical treatments has been extensively considered.5 Mesenchymal stem cells (MSCs) are an appealing resource for cell-based therapy in a wide range of diseases including cancer due to their distinct characteristics.6 MSCs are fibroblast-like multipotent adult stem cells that can easily be derived, without ethical conflicts, from different tissues including bone marrow (BM), placenta, umbilical cord (UC), UC blood, menses blood, endometrium, Wharton’s jelly (WJ), adipose tissue, amnion, dental pulp (DP), etc.7 According to the definition of the International Society for Cell Therapy (ISCT), MSCs are characterized as plastic-adherent cells, with trilineage differentiation ability (osteoblasts, adipocytes, and chondroblasts), positive for CD105, CD73, and CD90, whereas negative for hematopoietic markers, e.g., CD45, CD34, CD14, CD19 and MHC class II (Figure 1).8 In addition, MSCs-based therapeutic approaches provide a promising platform for the treatment of incurable diseases including cancer due to their pleiotropic activities such as paracrine effects, immunomodulation, tumor-tropism, tumor-homing, and migration.9 Lazarus et al performed the first clinical trial of culture-expanded MSCs in patients with hematologic malignancies. They showed that expansion and following infusion of human bone marrow-derived stromal progenitor cells (BM-MSCs) in patients caused no intensive side effects.10 Because of the many benefits of MSCs, extensive preclinical (Table 1) and clinical studies have been conducted in a variety of cancer (Table 2). The present review has focused on the mechanisms of MSCs in inhibiting the progression of cancer and has explored the therapeutic approaches for treating cancer using MSCs.
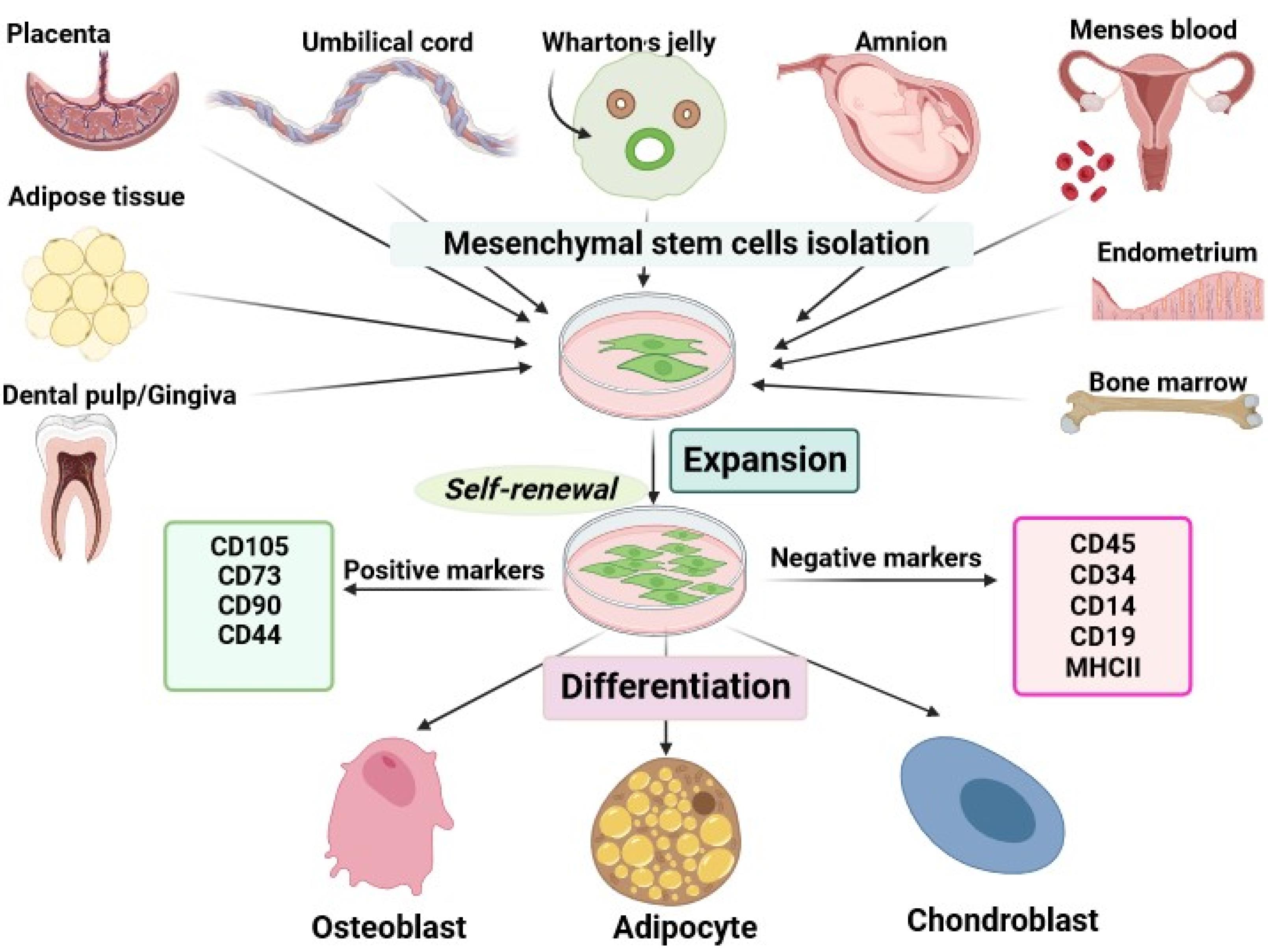
Figure 1.
Mesenchymal stem cells sources and characterization. The web-based application BioRender was employed to design the figure.MSCs are isolated from various tissue sources such as dental pulp, adipose tissue, placenta, umbilical cord, Wharton’s jelly, menses blood, endometrium, and bone marrow. These plastic adherent cells are identified by their ability for trilineage differentiation, consistent expression of nonhematopoietic cell markers such as CD105, and CD73, and absence of hematopoietic cell markers such as CD34, CD 45, etc
.
Mesenchymal stem cells sources and characterization. The web-based application BioRender was employed to design the figure.MSCs are isolated from various tissue sources such as dental pulp, adipose tissue, placenta, umbilical cord, Wharton’s jelly, menses blood, endometrium, and bone marrow. These plastic adherent cells are identified by their ability for trilineage differentiation, consistent expression of nonhematopoietic cell markers such as CD105, and CD73, and absence of hematopoietic cell markers such as CD34, CD 45, etc
Table 1.
Preclinical studies of MSCs for the treatment of cancer
|
Condition
|
Model
|
Treatment agent
|
Findings
|
| Breast cancer |
Nude Mice |
rBM-MSCs + PS-SiO 2 NPs |
The suppression of tumor growth, resulting from injecting PS-loaded MSCs, is due to the naturally high affinity of MSCs toward tumors.11 |
| Lung cancer |
Nude Mice Rabbit Monkey |
MSCs/NP/DTX |
The lung targeting ability of MSC in different animal models is highly efficient for MSCs/NP/DTX systems in tumor inhibition rather than NP/DTX.12 |
| Breast cancer |
Mice |
Mouse BM-MSCs-derived exosomes |
Downregulation of VEGF expression in tumor cells, inhibition of angiogenesis, and tumor growth.13 |
| Multiple myeloma |
SCID Mice |
pIL6-TRAIL-engineered hUC-MSC |
Induction of apoptosis in MM cells.14 |
| Kaposi's sarcoma |
Nude mouse |
hMSCs-CXCR4/Fluc2 |
Inhibition of Akt activity within KS cells and tumor-suppression.15 |
| Hepatoma |
SCID Mice |
HMSCs |
Inhibition of cancer cell phenotypes.16 |
rBM-MSCs: Rat bone marrow-derived mesenchymal stem cells, PS: Photo-sensitizer, SiO 2 NPs: Silica nanoparticles, MSCs: Mesenchymal stem cells, BM-MSCs: Bone marrow-derived mesenchymal stem cell, DTX: Docetaxel, NP: Nanoparticle, VEGF: vascular endothelial growth factor, SCID: Severe combined immunodeficiency, pIL6: Interleukin-6 promoter, TRAIL: transduced to express the tumor necrosis factor-related apoptosis-inducing ligand, hUC-MSCs: Human umbilical cord-derived mesenchymal stem cells, MM: Multiple myeloma, KS: Kaposi's sarcoma, CXCR4/Fluc2: CXC chemokine receptor 4/ firefly luciferase2, hMSCs: Human mesenchymal stem cells.
Table 2.
Clinical studies of cancer treatment using MSCs
|
Trial NCT number |
Condition
|
Treatment agent
|
Phase
|
Start date
|
Status
|
Results
|
| NCT01129739 |
Myelodysplastic syndromes |
hUC/PL-MSCs |
II |
2010 |
Unknown |
No results available |
| NCT01092026 |
Hematological malignancies |
UCB-HSCT with MSCs |
I, II |
2010 |
Completed |
No results available |
| NCT01045382 |
Hematological malignancies |
MSCs |
II |
2010 |
Terminated |
No results available |
| NCT01844661 |
Solid tumors metastases |
CELYVIR (BM- MSCs infected with ICOVIR5 (oncolytic virus) |
I, II |
2013 |
Completed |
A reasonably safe and well-tolerated medication that may help patients with advanced malignancies experience a therapeutic response |
| NCT01983709 |
Prostate cancer |
Allogeneic BM-MSC |
I |
2013 |
Terminated |
No results available |
| NCT02270307 |
Hematological malignancies |
MSCs and Cyclophosphamide |
II, III |
2014 |
Unknown |
No results available |
| NCT02079324 |
Head and neck cancer |
Genetically Modified Mesenchymal Stem Cells (GX-051) |
I |
2014 |
Unknown |
No results available |
| NCT03106662 |
Hematological malignancies |
MSCs |
III |
2014 |
Completed |
No results available |
| NCT02513238 |
head and neck cancer |
ASCs |
II |
2015 |
Completed |
Primary results include changes in salivary flow rate, with secondary outcomes focusing on safety, quality of life, and gland evaluations using MRI and core-needle samples. |
| NCT02530047 |
Ovarian cancer |
BM-MSCs-INFβ |
I |
2016 |
Completed |
No results available |
| NCT02648386 |
Rectal cancer |
HUC-MSCs |
I, II |
2016 |
Unknown |
No results available |
| NCT03896568 |
Glioma |
Allogeneic BM- MSCs loaded with the oncolytic adenovirus DNX-2401(BM-MSCs-DNX2401) |
I |
2019 |
Recruiting |
Study is ongoing |
| NCT03298763 |
Adenocarcinoma of lung |
MSC-TRAIL |
I, II |
2019 |
Recruiting |
Study is ongoing |
| NCT04657315 |
Recurrent glioblastoma |
MSC11FCD the suicide gene, cytosine deaminase |
I, II |
2020 |
Completed |
No results available |
| NCT03608631 |
Pancreatic cancer |
MSCs-derived Exosomes with KRAS G12D siRNA (iExosomes) |
I |
2021 |
Recruiting |
Reduction of STAT3 levels, Inhibition of ECM deposition, and improving liver function in mice with liver fibrosis, presenting a promising anti-fibrotic therapeutic approach. |
| NCT03184935 |
Myelodysplastic syndromes |
Allogeneic hUC-MSCs |
I, II |
2023 |
Suspended |
Study is ongoing |
MSCs: Mesenchymal stem cells, hUC/PL-MSCs: Human umbilical cord/placenta-derived MSCs, UCB-HSCT: Umbilical cord blood-hematopoietic stem cell transplantation, hUC-MSCs: Human umbilical cord-derived mesenchymal stem cells, BM-MSCs: Bone marrow-derived mesenchymal stem cells, ASCs: Adipose tissue-derived mesenchymal stem cells
Mechanisms of MSCs function in cancer management
The tumor tropism and migration, homing properties of MSCs
For juxtacrine effects (cell-to-cell or cell-to-ECM signaling) to occur, the migration and homing of MSCs to the damaged tissue are essential initial stages for the treatment of cancer in MSCs-based therapy. Numerous studies have demonstrated the natural affinity of MSCs for tumors, as well as their ability to migrate and home in on tumor tissues. This makes stem cells an exceptional therapeutic approach for targeting cancer cells. It has been found that the recruitment of MSCs towards tumor cells is thought to be due paracrine signaling loop between the chemoattractants from the tumor microenvironment (TME) and the corresponding receptors on MSCs.17,18 The inflammatory microenvironment of malignant cells due to the secretion of factors including growth factors, chemokines, and cytokines plays a substantial role in stimulating tumor tropism of MSCs.19 The most plentiful secreted chemokines in the TME are interleukin-6 (IL-6), monocyte chemotactic protein(MCP-1/CCL2), CCL15, macrophage inflammatory protein-3 (MIP3A/CCL20), CCL25, C-X-C motif chemokine ligand 1 (CXCL1), interleukin-8 (IL-8/CXCL8), stromal-derived factor 1(SDF-1/CXCL12) that contribute to the recruitment of MSCs derived from different sources toward cancer cells through interaction with their specific chemokine receptors at their surface.20-22 Further, several other trophic factors released from tumor cells and stroma such as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), tumor necrosis factor (TNF-α), granulocyte colony-stimulating factor (G-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF) induce MSCs attraction and homing in tumor sites.23,24 Furthermore, MSCs exhibit a remarkable tendency to migrate toward cancer through the expression of a large number of molecules including chemokines and their receptors, growth factors, toll-like receptors (TLRs), adhesion molecules, and growth factors.25 MSCs can express a variety range of functional chemokine receptors such as CCR1, CCR4, CCR7, CXCR4, CXCR6 which have been extensively related to tumors tropism.26,27 Based on a growing body of evidence, CXCR4 is one of the most important chemokine receptors in MSCs that plays a pivotal role in targeted homing of MSCs through interaction with stromal cell-derived factor 1 (SDF-1).28 In addition, the expression of different growth factor receptors on MSCs is involved in the migration and homing process. For example, c-met (HGF-R) is expressed on MSCs derived from cord blood, bone marrow, adipose tissue, cord blood, and skin and can induce cell migration and homing to the target site by binding to hepatocyte growth factor (HGF). To date, a multitude of studies have shown that the expression of PDGFR α and β, EGF-R, and IGF-R1 in BM-MSCs allows homing of these cells.29-31 MSCs recruitment into injured tissue is dependent on the expression of a variety of adhesion molecules including integrins. MSCs have been shown to express various integrin subunits such as α1, α2, α3, α4, α5, αv, β1, β3, and β4.32 Integrin heterodimer α4/β1, composed of CD49d and CD29, plays a crucial role in MSCs rolling and migration into targeted sites via binding to the vascular cell adhesion molecule 1 (VCAM-1) or CD106 expressed on endothelial cells.33 Matrix metalloproteinases (MMPs), zinc-dependent proteolytic enzymes, play a pronounced role in regulating the migratory activity of MSCs.34 Ho and colleagues reported for the first time that the expression of MMP-1 on BM-MSCs is most vital for the migration of these cells onto human glioma via the MMP1/PAR1 axis.35 MMP-1 can be considered as an IGFBP2 protease which may regulate MSCs tropism and migration to tumor through cleavage of IGF‐2/IGFBP 2 complex and extracellular release of free IGF‐2.36 Furthermore, it has been shown that blocking MMP-2 in tumor cells leads to the inhibition of human UC blood-derived MSCs attraction to tumor sites by preventing SDF1/CXCR4 signaling (Figure 2).37 While the recruitment of MSCs into the TME through growth factors and cytokines released by cancerous cells implicates tumor suppression, the interaction between MSCs and tumor cells may also contribute to the advancement of cancer.38 For instance, MSCs can differentiate into cancer-associated fibroblasts (CAFs) by cancer microenvironment-derived TGF-β, WNT, and IL-6/STAT3 signaling, increasing tumor cell heterogeneity and directly being involved in the progression of cancer.39-41 Accumulating evidence has indicated that MSCs, by upregulating EMT markers including vimentin, Twist, N-cadherin, and Snail and downregulating E-cadherin, could trigger cancer cell metastasis.42,43 It is also reported that MSCs have the ability to accelerate cancer progression by the secretion of multiple growth factors such as VEGF and bFGF and the prevention of apoptosis in tumor cells.17 In addition, MSCs were found to play a supportive role in tumor proliferation and promotion via the release of IL8 and the recruitment of leukocytes, such as macrophages and neutrophils.44,45 Consequently, the presence of MSCs in TME can have contradictory effects.46 Even though the precise mechanisms of MSCs migration to sites of tumor are not yet completely understood, the tumor-seeking behavior of MSCs has been utilized to develop more specific and efficient anticancer therapeutic strategies.
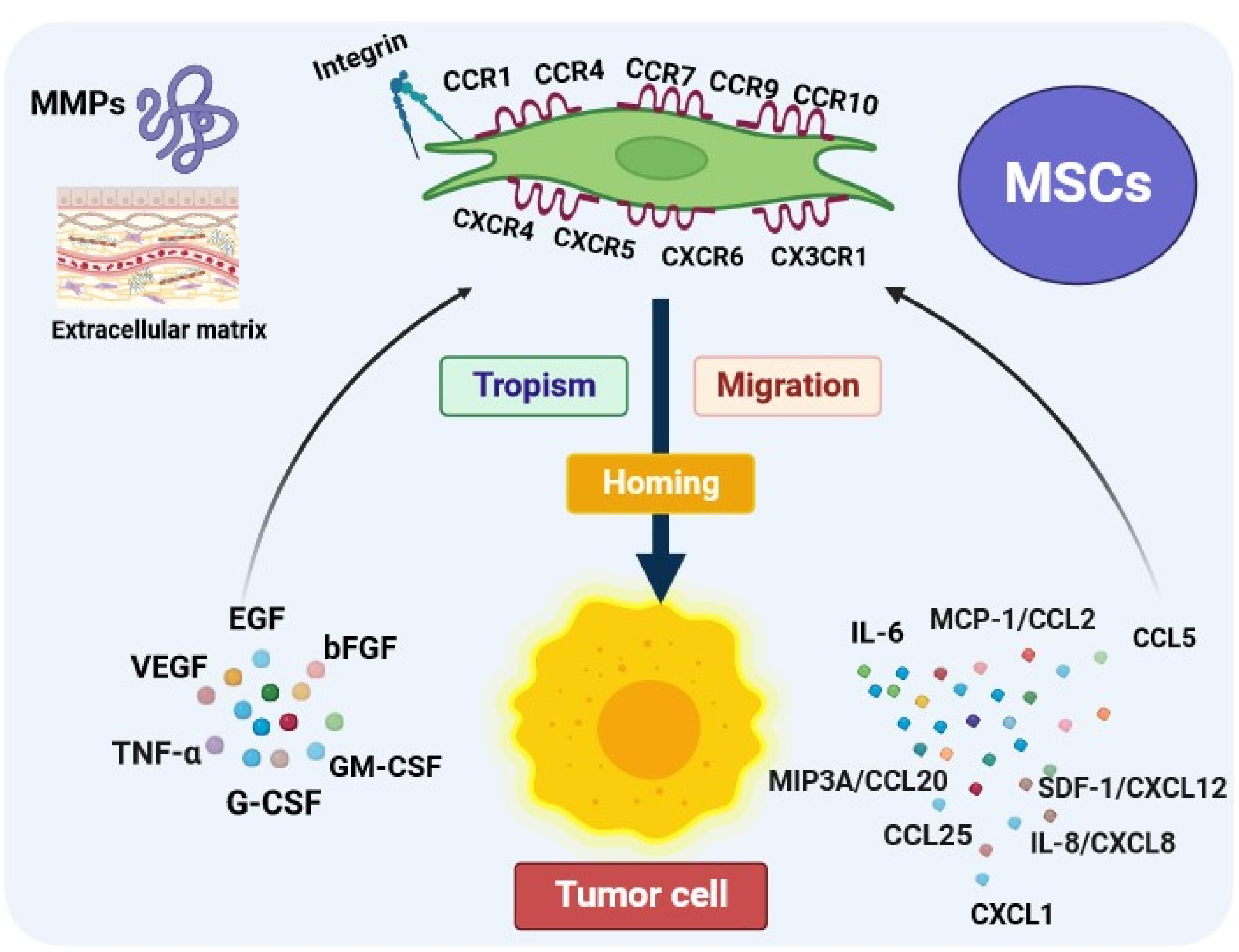
Figure 2.
The mechanisms of mesenchymal stem cells tropism, migration, and homing. The web-based application BioRender was employed to design the figure.MSCs areattracted into the tumor microenvironment by interacting with multiple factors including chemokines, cytokines, and growth factors released by tumor cells. Moreover, the expression of some proteins such as integrin and matrix metalloproteinases (MMPs) in MSCs can favor tropism/migration and homing features of them. interleukin-6 (IL-6), monocyte chemotactic protein1(MCP-1/CCL2), Chemokine (C-C motif) ligand 25 (CCL25), macrophage inflammatory protein-3 (MIP3A/CCL20), C-X-C motif chemokine ligand 1 (CXCL1), interleukin-8 (IL-8/CXCL8), stromal-derived factor 1(SDF-1/CXCL12), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), tumor necrosis factor (TNF-α), granulocyte colony-stimulating factor(G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF)
.
The mechanisms of mesenchymal stem cells tropism, migration, and homing. The web-based application BioRender was employed to design the figure.MSCs areattracted into the tumor microenvironment by interacting with multiple factors including chemokines, cytokines, and growth factors released by tumor cells. Moreover, the expression of some proteins such as integrin and matrix metalloproteinases (MMPs) in MSCs can favor tropism/migration and homing features of them. interleukin-6 (IL-6), monocyte chemotactic protein1(MCP-1/CCL2), Chemokine (C-C motif) ligand 25 (CCL25), macrophage inflammatory protein-3 (MIP3A/CCL20), C-X-C motif chemokine ligand 1 (CXCL1), interleukin-8 (IL-8/CXCL8), stromal-derived factor 1(SDF-1/CXCL12), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), tumor necrosis factor (TNF-α), granulocyte colony-stimulating factor(G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF)
Immunomodulatory
MSCs mainly exert low immunogenicity and reveal a remarkable capacity to modulate immune responses.47 In a study by Bartholomew and colleagues, the immunomodulatory potential of baboon-derived MSCs was demonstrated through the inhibition of allogeneic peripheral blood lymphocyte proliferation and also the prevention of rejection in a baboon skin allograft model in vivo.48 It is well proven that MSCs elicit the immunomodulation functions through various mechanisms including direct interaction with immune cells and mediation of paracrine activity.47 The different kinds of innate immune cells such as dendritic cells (DCs), natural killer (NK) cells, monocytes/ macrophages, neutrophils, and adaptive immune cells such as T cells and B cells are suppressed or activated by MSCs (Figure 3).49 A significant focus has been placed on the paracrine effects of MSCs in this context as multiple factors derived from MSCs including cytokines and exosomes are related to their immunomodulatory capacity.50 For example, MSCs-derived indoleamine 2, 3-dioxygenase (IDO) and prostaglandin E2 (PGE2) inhibit the polarization of pro-inflammatory macrophage, proliferation of T cells, and cytotoxic activity of NK cells. The MSC-derived transforming growth factor-β (TGF-β) plays an indispensable role in maintaining systemic immune tolerance by promoting the induction of regulatory T cells (T reg).51 Additionally, MSCs can inhibit the maturation of DCs by releasing IL-10 and activating the signal transducer and activator of transcription (STAT) 3 signaling. This leads to reduced IL-12 production by DCs, which in turn prevents the proliferation and activation of NK cells, cytotoxic T cells (CTLs), and type 1 T helper (Th1) cells.47 The immunomodulatory effects of MSCs are primarily influenced by the solid tumor environment. This suggests that the immune regulatory responses induced by MSCs may vary or change depending on the surrounding microenvironment in which they are present.52,53 In addition to the ability of MSCs to modulate immune responses, they can regulate immunocompetence via stimulating immune cells to recruit into inflammatory conditions.54 MSCs can exhibit either pro-inflammatory or anti-inflammatory functions in an inflammatory environment. This is based on the levels or amounts of various factors they secrete or release into the surrounding environment.55 Accordingly, Waterman et al, suggested a new type of MSCs in which MSCs-1 express toll-like receptor 4 (TLR 4), and exert antitumor activity, while, due to the expression of TLR3, MSCs-2 inhibit immune cell activity and support tumor growth.56 The investigations have shown that MSCs adopt a pro-inflammatory phenotype or characteristics in the presence of low levels of the IFN-γ and TNF-α. In such environments, MSCs secret certain soluble factors such as MIP-1α/β, RANTES, CXCL9, CXCL10, and CXCL-11 to further activate T cells. Furthermore, in the absence of the IL-6, but in the presence of IFN-γ and IL-1, MSCs can activate M1 macrophages. These macrophages then further release high levels of IFN-γ and TNF-α within the damaged tissue environment.57 The findings of an in vivo study carried out by Ohlsson et al showed that co-administration of cancer cells and MSCs facilitated infiltration of monocytes and granulocytes in contrast to tumor cells or mesenchymal progenitor cells alone. This research demonstrated that MSCs are capable of preventing colon carcinoma growth.58 Further, it has been found that MSCs can cause cancer cell death through the production of unique immunomodulatory cytokines. For example, it was demonstrated that the overexpression of IL-12 in MSCs increased antitumor responses of T cells and repressed tumor growth.59 Due to the immunomodulatory trait of MSCs, they are recognized as an excellent focal point in anticancer therapeutic strategies.
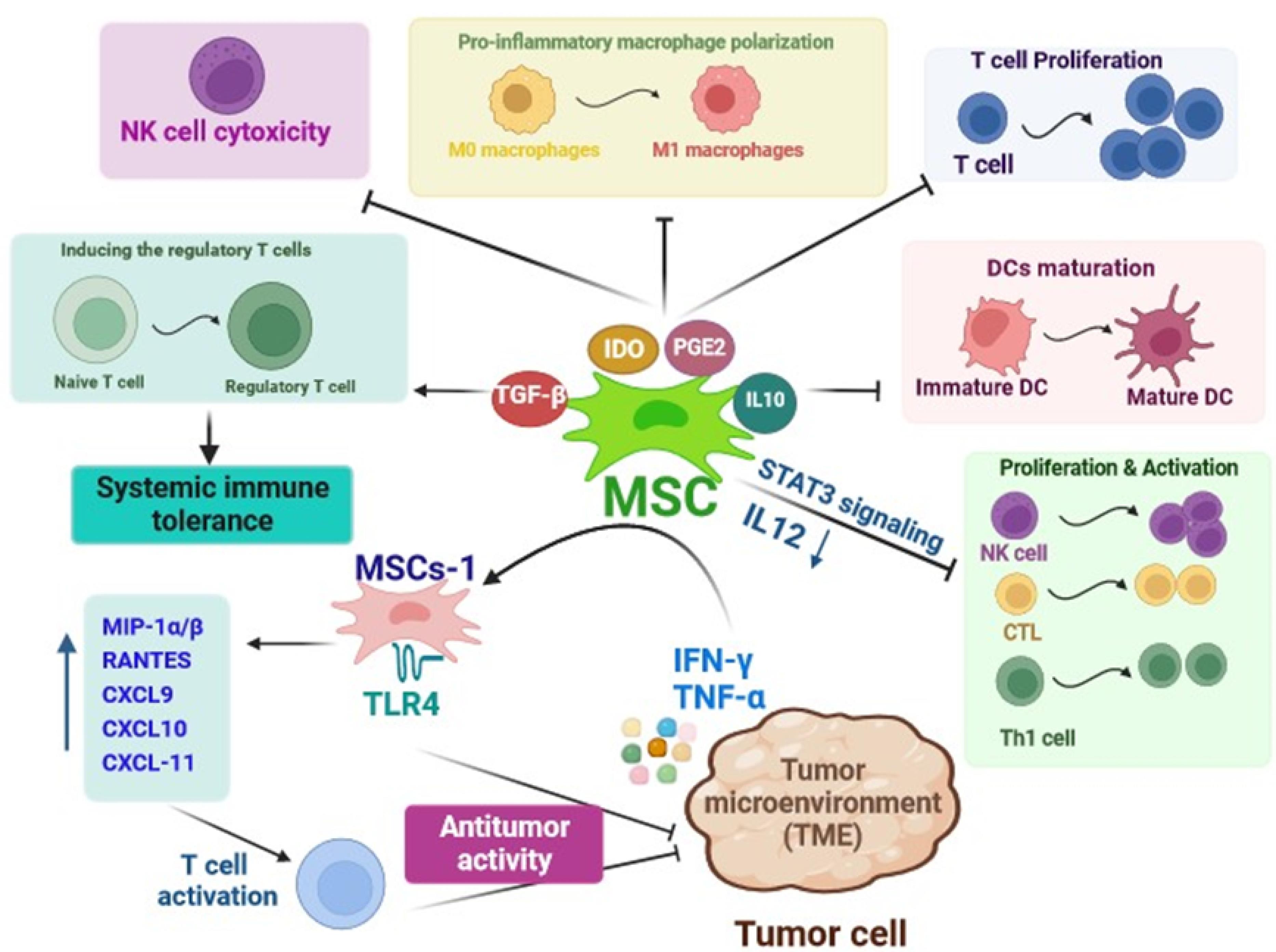
Figure 3.
Schematic summarizing the immunomodulatory mechanisms of mesenchymal stem cells in cancer therapy. The web-based application BioRender was employed to design the figure. MSCs produce a variety of soluble factors that by interaction with immune cells activate or suppress their function and display immunomodulatory effects. macrophage Inflammatory Protein -1α/β(MIP-1α/β), regulated upon activation, normal T cell expressed and presumably secreted(RANTES), C-X-C Motif Chemokine Ligand 1(CXCL), toll-like receptor 4(TLR 4), interferon‐gamma (IFN‐γ), Tumor necrosis factor α (TNFα) indoleamine 2,3-dioxygenase(IDO), transforming growth factor-β (TGF-β), prostaglandin E2 (PGE2), Interleukin(IL), dendritic cell (DC), natural killer cells(NK cell), cytotoxic T lymphocytes (CTL), T helper cells (Th1 cell)
.
Schematic summarizing the immunomodulatory mechanisms of mesenchymal stem cells in cancer therapy. The web-based application BioRender was employed to design the figure. MSCs produce a variety of soluble factors that by interaction with immune cells activate or suppress their function and display immunomodulatory effects. macrophage Inflammatory Protein -1α/β(MIP-1α/β), regulated upon activation, normal T cell expressed and presumably secreted(RANTES), C-X-C Motif Chemokine Ligand 1(CXCL), toll-like receptor 4(TLR 4), interferon‐gamma (IFN‐γ), Tumor necrosis factor α (TNFα) indoleamine 2,3-dioxygenase(IDO), transforming growth factor-β (TGF-β), prostaglandin E2 (PGE2), Interleukin(IL), dendritic cell (DC), natural killer cells(NK cell), cytotoxic T lymphocytes (CTL), T helper cells (Th1 cell)
Current strategies in MSCs-based cancer therapy
Exploitation of innate abilities of MSCs including tumor tropism, homing, and immunomodulatory endows new and innovative applications for inflammatory disorders including cancer. Recently, significant research attempts have concentrated on the use of stem cells as the vehicle for to targeted delivery of anti-cancer agents to tumor cells. Moreover, many studies have highlighted that MSCs-derived exosomes can serve as a potent cell-free tool for cancer treatment.60 Resistance against anti-cancer payloads is one of the most substantial barriers in the treatment of divergent types of solid tumors. Resistance develops due to the continuing utilization and elevated concentration of anti-cancer compounds, which augment the toxicity of anti-cancer drugs in noncancerous proliferating cells.61 Inadequate selectivity of a variety of anticancer therapeutic agents could be responsible for the problem.62 To overcome the mentioned problems, MSCs have been considered an appropriate vehicle for the targeted delivery of chemotherapeutic drugs, suicide genes, oncolytic viruses (OVs), cytokines, and growth factors to tumor cells, because of their intrinsic ability in tumor tropism and deep migration into the TME.63
MSCs as a delivery system for chemotherapeutic agents
In this regard, research teams have investigated the potential of MSCs as a delivery system for widely recognized chemotherapeutic drugs, such as paclitaxel (PTX), doxorubicin (DOX), sorafenib, and gemcitabine. After loading with drugs MSCs can locally release their consignment through passive diffusion in the tumor stroma thereby leading to the death of cancer cells.64 The previous studies showed the effectiveness of PTX-loaded BM-MSCs and DP-MSCs in inhibiting the growth of certain malignant conditions including glioblastoma,65 and breast cancer66 through mitigation of the cell proliferation and inducing apoptosis. Moreover, conditioned medium obtained from ASCs-PTX significantly inhibited the proliferation of ovarian cancer cells compared with free PTX, and diminished PTX resistance in cancer cells.67 Likewise, the assessment of the potential antitumor activity of human DOX-loaded BM-MSCs in xenograft mouse models of thyroid or breast cancer demonstrated significant cytotoxic effects on tumor cells.68 Additionally, ASCs loaded with DOX showed impressive antitumor effects in l- B16F10 melanoma lung metastasis in vivo.69 A study has shown that human MSCs isolated from gingival papilla can serve as a reliable delivery system for gemcitabine release in an active form and in appropriate quantities to inhibit the proliferation of oral squamous cells.70 Another experimental study indicated that gemcitabine-loaded human BM-MSCs could dramatically repress the growth of human pancreatic malignant cells (Figure 4A).71
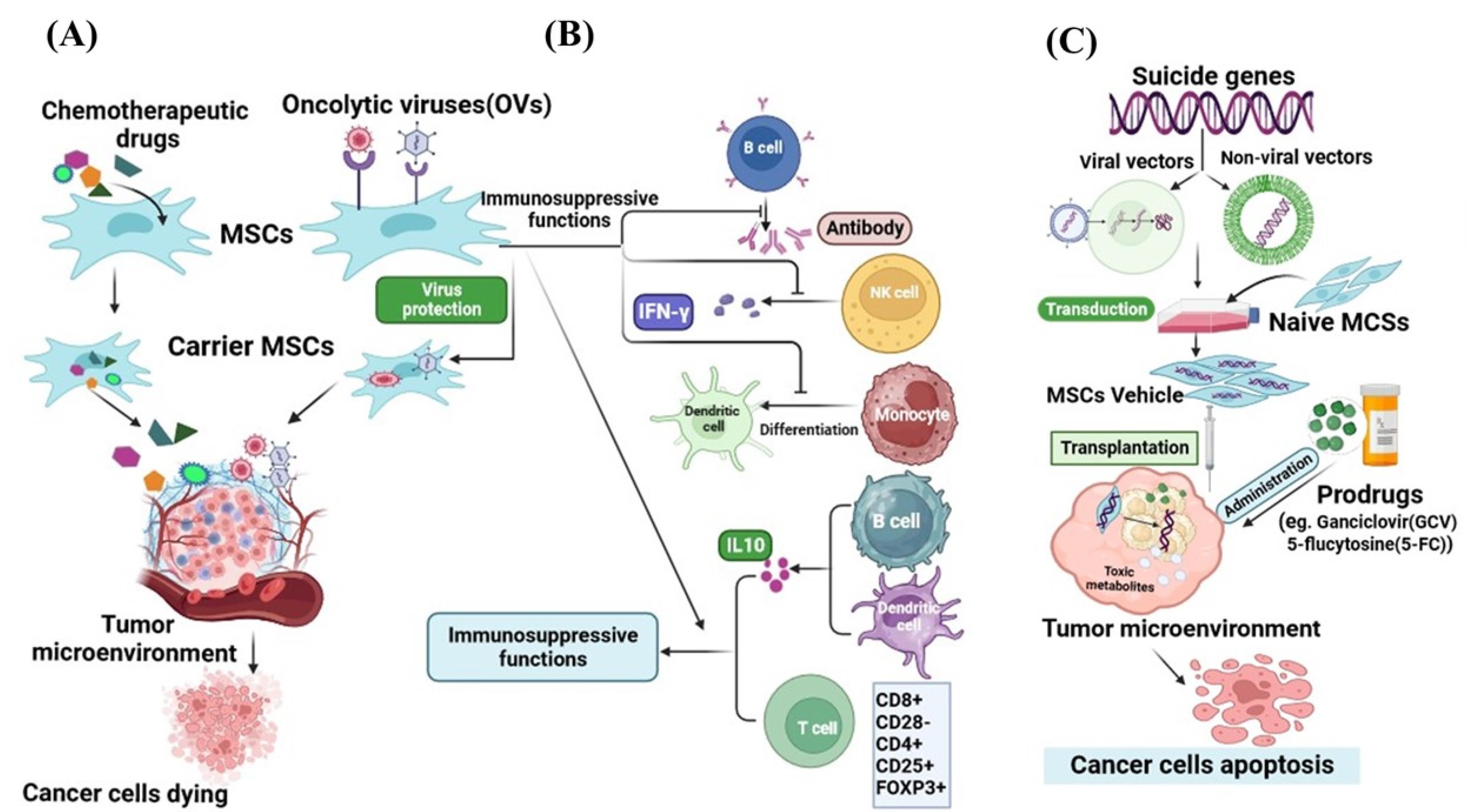
Figure 4.
Mesenchymal stem cells as an appropriate carrier for drug agents, viruses, and gene delivery to tumor microenvironment. The web-based application BioRender was employed to design the figure. MSCs due to inherent tropism and high migration potential to cancer cells are convincing vehicles to load (A) chemotherapeutic drugs, (B) oncolytic viruses, and (C) suicide genes. MSCs with immunosuppressive ability protect from oncolytic viruses against immune cells (B). Various suicide genes have been transferred into MSCs by viral and non-viral vectors and expressed at tumor sites for therapy (C)
.
Mesenchymal stem cells as an appropriate carrier for drug agents, viruses, and gene delivery to tumor microenvironment. The web-based application BioRender was employed to design the figure. MSCs due to inherent tropism and high migration potential to cancer cells are convincing vehicles to load (A) chemotherapeutic drugs, (B) oncolytic viruses, and (C) suicide genes. MSCs with immunosuppressive ability protect from oncolytic viruses against immune cells (B). Various suicide genes have been transferred into MSCs by viral and non-viral vectors and expressed at tumor sites for therapy (C)
MSCs as a protective tool for the delivery of oncolytic viruses
Oncolytic viruses, as anti-tumor biological compounds, are considered an innovative and promising therapeutic strategy for the amelioration of malignancies, which can selectively kill infected cancer cells by apoptosis induction.72 Oncolytic viruses with a natural affinity for tumor cells, can selectively target the malignant cells and lead to their lysis.73 Talimogene laherparepvec (T-VEC) is a genetically modified form of human herpes simplex virus type 1 (HSV-1). In 2015, T-VEC was approved by the United States Food and Drug Administration (US-FDA) as the first OV for melanoma treatment.74 In a late study, Zhang and colleagues reported that oncolytic HSV-1 can influence TME by decreasing the proportion of anti-inflammatory macrophages and elevating the presence of tumor-infiltrating lymphocytes. Furthermore, they represented that the combination of oncolytic HSV-1 and immune checkpoint modulators significantly extended the lifespan of the pancreatic tumor-bearing mice.75 However numerous factors contribute to the effectiveness of the virus in spreading within a cancerous tissue, including the quick elimination of the virus by the immune system and viral captivation by tissues and organs.76,77 Several research studies have indicated that MSCs can serve as eligible vehicles to protect OVs from neutralizing host effects, facilitate the targeted delivery of OVs, and improve their capacity to infect and eliminate cancer cells.78 The experimental findings demonstrated that MSCs loaded with oncolytic adenovirus promote virus replication leading to increased production of virus particles and a high accumulation of virions in tumors. Ultimately MSCs infected with oncolytic adenoviruses were able to effectively destroy hepatocellular carcinoma cells in vitro.79 Additionally, in vivo findings displayed that MSCs can prevent immune response by suppressing the release of interferon-γ (IFN-γ) from activated T cells. Also, MSCs enhanced the distribution and persistence of adenovirus in comparison to the injection of the virus alone in vivo.80 For enveloped OVs, MSCs can transport these viruses to tumor tissues through a process that involves hetero-cellular fusion. Ong et al introduced the oncolytic measles virus into bone marrow-derived MSCs and carried out in vitro co-culture experiments with human hepatocellular carcinoma cells.72 The findings indicated that the number of syncytia (cell fusions) increases when MSCs carry the measles virus, whereas this effect is not observed with non-enveloped viruses. Moreover, when high-titer anti-measles virus antibodies are present, virus-infected MSCs notably induce the formation of heterocellular structures when compared to the naked virus. These results align with the observations reported by of Castleton et al, who studied the use of MSCs to deliver the measles virus in a model of acute lymphoblastic leukemia. They suggested that using MSCs for OVs delivery could substantially extend survival and improve the effectiveness of anti-tumor interventions compared to using the virus alone.81 The different investigations demonstrated that MSCs with their unique abilities such as tumor tropism and immunosuppression help the virus to precisely reach the tumor site and enhance the virus persistence.82,83 In recent years, an increasing amount of evidence from both preclinical and clinical studies has highlighted the immunosuppressive abilities of MSCs, as they can inhibit the activity of specific immune cell types, such as T and B lymphocytes, as well as NK cells. Consequently, this modulation extends to affecting the function of monocytes, DCs, and macrophages.84-87 MSCs influence the activation, growth, maturation, cytokine release, and cytotoxic capabilities of both innate and adaptive immune cells.88 Certainly, MSCs can decrease cytokine production by helper T cells, diminish the cytotoxic effects of effector T lymphocytes,89 impede the differentiation of B lymphocytes, and hinder their capacity to release immunoglobulins.90,91 Additionally, they can confine INF-γ secretion by NK cells and attenuate their cytotoxic potential. Furthermore, MSCs hinder the differentiation of CD14 + monocytes and CD34 + progenitor cells into fully mature DCs.92 Crucially, MSCs foster the development of regulatory immune subgroups, such as CD8 + CD28− T lymphocytes,93 CD4 + CD25 + FOXP3 + T lymphocytes,94 IL-10 producing B lymphocytes and DCs (Figure 4B).95,96 Hence, the inhibition of immune cell activities and the promotion of regulatory immune cell subsets may play beneficial roles in enhancing immunosuppressive capabilities of MSCs. These functions are essential MSCs features in protecting OVs from immune system clearance guaranteeing enhanced OV spread and increased viral persistence.80 It has been acknowledged that MSCs-mediated delivery of OVs in animal models of solid tumors has shown successful outcomes in improving hepatocellular carcinoma,97 glioblastoma,98 glioma,99 colorectal cancer,100 prostate cancer,100 and lung metastases of breast carcinoma.101
MSCs as a new platform for the delivery of suicide genes
Another modality for cancer treatment is using suicide genes which provide the possibility for selective destruction of malignant cells without harming the surrounding normal cells.102 Suicide gene therapy or gene-directed enzyme prodrug therapy, is based on the transfer of a foreign gene that encodes an enzyme into cancer cells. This enzyme converts a prodrug into toxic metabolites leading to the death of the cancer cells.103 The bystander effect is an intriguing characteristic of the suicide gene resulting in the elimination of both cancerous cells in which the toxic metabolites are formed and the adjacent non-transgenic cancer cells.104 The major limitation that restricts the success of suicide gene cancer therapy is the low efficiency in delivering and expressing the therapeutic genes.105 To address the challenges associated with using suicide gene therapy for treating tumors, scientists have identified the beneficial role of MSCs due to their homing ability to target cancerous cells, as the appropriate cellular carriers for suicide genes.106 Expression of the suicide genes by the MSCs at the tumor local converts the administered non-toxic prodrug to an active toxic compound that is fatal to tumor cells.107 The herpes simplex virus thymidine kinase complexed with ganciclovir (HSV-TK/GCV system) and yeast cytosine deaminase (CD) with 5-fluorocytosine (5-FC) are the most common enzyme-prodrug complexes that in combination with MSCs can target different types of tumors. 108 For example, the injection of hMSCs transfected with the suicide gene CD, which was followed by the administration of 5-FU in a mouse model with gastric cancer was found to suppress the growth of the tumor.109 Furthermore, in vitro and in vivo experiments demonstrated that MSCs expressing cytosine deaminase::uracil phosphoribosyl transferase (CD::UPRT) can trigger complete tumor regression in prostate cancer models.110 CD::UPRT can convert the non-toxic 5-fluorocytosine into the cytotoxic anti-tumor drug known as 5-fluorouracil.111,112 Recent findings showed the safety of adipose tissue–derived allogeneic MSCs which carry herpes simplex virus-thymidine kinase (HSV-TK) gene, as a suicide gene therapy in patients with recurrent glioblastoma.113 Another investigation displayed that when MSCs expressing the HSV-TK were used in conjunction with the ganciclovir (GCV) prodrug, it exhibited its possible anti-tumor effectiveness both in laboratory settings and in mice models using the human glioblastoma cell line U87TK. During this, MSCs preserved cell proliferation, karyotype stability, and retained their MSCs characteristics. Moreover, genetic modification had a notable impact on their secretory profile, leading to a substantial increase in various anti-tumor immune soluble factors such as IFN-γ, IL-2, MCP-1, and IL12p40 (Figure 4C).114 Several studies in the recent decade have shown that anticancer drug-conjugated nanoparticle-loaded MSCs, due to the increasing migration activity of MSCs and controlled and gradual drug- release in target tissue, can be introduced as a novel tool in cancer therapy.115-117
MSCs-derived exosomes for cancer therapy
Extracellular vesicles (EVs) are a diverse group of small, membrane-enclosed structures ranging from approximately 30 to 1000 nanometers in diameter. These EVs are actively released by all types of cells into the extracellular space and plentifully found in different body biofluids such as saliva, synovial fluid, amniotic fluid, ejaculate, cerebral spinal fluid, milk, and even urine.118-121 These spherical, bilayered particles are rich in proteins, lipids, nucleic acids, and other bioactive metabolites.122,123 EVs, as a modern messaging system, can mediate cell-to-cell contact and intercellular crosstalk transfer via transferring their bioactive cargo to recipient cells.124 Different types of EVs can be broadly categorized into three major groups based on their mode of biogenesis: exosomes, ectosomes, and apoptotic bodies.125 Exosomes generally constitute the smallest EVs, less than 150 nm in size. They are produced as intraluminal vesicles in the endosomal system through the fusion of multivesicular bodies with the plasma membrane.126-128 In contrast, ectosomes, or microvesicles, are larger, varying from approximately 100–1000 nm, and are secreted by direct outward budding and shedding from the plasma membrane.129 Similarly, exosomes and ectosomes play crucial roles in intercellular communications.130 Tetraspanins form a diverse superfamily of small transmembrane proteins that are present in both types of EV and are accepted as a critical cellular effector during the biogenesis of these EVs.131,132 Apoptotic bodies are a peculiar type of EV with a large size (1000–5000 nm) secreted by cells that have undergone apoptosis, or programmed cell death.133 MSCs from different tissue sources possess the ability to generate and release various types of EVs.134 Exosomes are the most important secreted extracellular particles from MSCs with a diameter of 30–100 nm that not only express common surface biomarkers such as CD81 and CD9, but also express MSCs surface markers, such as CD29, CD44, CD73, and CD90.135,136 MSCs-derived exosomes owing to containing multiple therapeutic cargoes including proteins, lipids, nucleic acids (DNAs and RNAs), and metabolites, have distinct effects on cell interactions through various mechanisms.137 Furthermore, MSCs-derived exosomes have been proposed as a prospective and powerful cell-free-based tool for combatting cancer due to having numerous exclusive features such as low immunogenicity, biosafety, biocompatibility, prolonged circulation time, sustained release, and tumor-particular homing.138-140 Angiogenesis, as a process involved in new vessel formation, can accelerate tumor growth, while exosomes derived from MSCs can prevent angiogenesis by regulating VEGF expression. The study by Miranda and colleagues found that exosomes derived from MSCs can inhibit angiogenesis in prostate cancer (PC3) cells. This inhibition is achieved by reducing the secretion of the pro-angiogenic factor VEGF, suppressing the activity of the transcription factor NF-κB, and promoting the production of reactive oxygen species within the cancer cells.141 The researchers further investigated the anticancer features of exosomes by examining how MSCs-derived exosomes affect the expression of genes involved in angiogenesis and apoptosis in several cancer cell lines. They indicated that MSCs-released exosomes can induce apoptosis by enhancing p53 gene expression and decreasing BCL2 gene expression, meanwhile impeding the proliferation of cancer cells.142 The available evidence suggests that MSCs exosomes can serve as effective nano-carriers for the delivery of antitumor drugs/ genes (miRNA or siRNA), facilitate tumor-targeted drug delivery, and enhance the bioavailability and efficacy of drugs.143 Ono et al found that MSCs-derived exosomes can suppress the cell cycle and promote dormancy in breast cancer cells via secretion of miR-23b, leading to the inhibition of migration and metastases of breast cancer cells.144 Besides, it has been presented that MSCs-derived exosomes containing miR-379 can inhibit growth of T47D breast cell lines expressing miR-379, indicating their potential as an effective cell-based therapy for targeted therapy of breast cancer.145 Small interfering ribonucleic acid (siRNA), which selectively inhibits a target gene, possesses great features in cancer treatment. Recently, a group of researchers found that MSCs-released exosomes carrying polo-like kinase 1 (PLK-1) siRNA leading to apoptosis and necrosis in bladder cancer cells.146 Pascucci et al were the first to investigate the ability of MSCs-derived exosomes to encapsulate and deliver PTX as a chemotherapeutic agent. The obtained data from this study showed that exosomes have good efficacy to uptake/release PTX, indicating that MSCs-derived exosomes can be a new method for drug delivery.147 The results of another investigation confirmed the anticancer function of exosomes loaded with PXT by reducing the tumor size and inhibiting the distant metastasis of breast cancer cells in the liver, spleen, and kidneys.148 Further, it has been reported that the uptake and cytotoxicity of MSCs-derived exosomes loaded with DOX are significantly higher than the free DOX in the osteosarcoma MG63 cell line. Therefore combined exosome-DOX was introduced as a super candidate for osteosarcoma treatment.149 Collectively, MSCs-derived exosomes exhibit distinct characteristics such as paracrine effects, immunomodulatory capabilities, gene transfer potential, biocompatibility, and stability. These attributes make them valuable biological tools for enhancing the efficacy and safety of conventional anticancer therapies (Figure 5).150,151
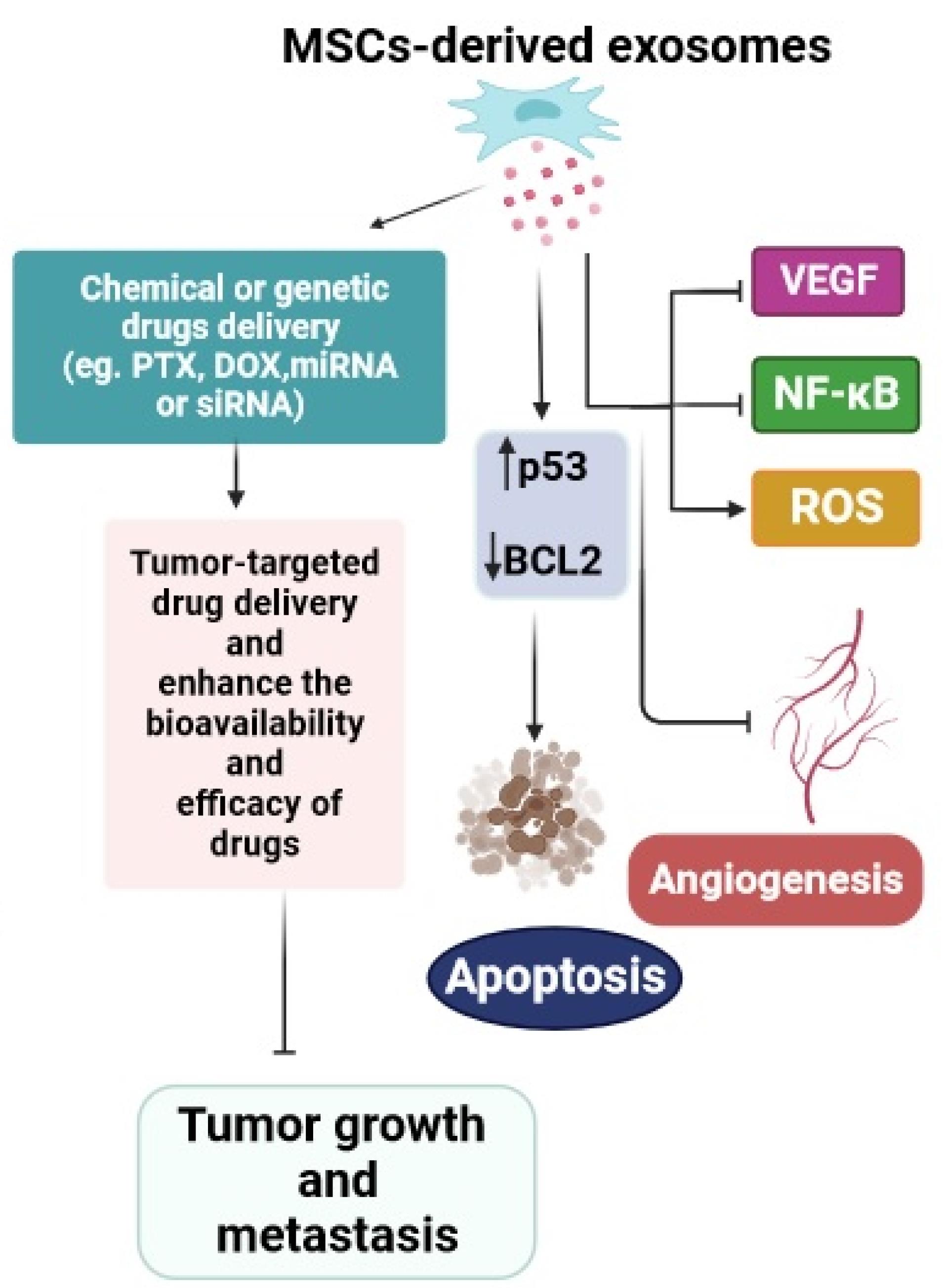
Figure 5.
Mesenchymal stem cells-derived exosomes as an attractive cell-free approach in cancer therapy. The web-based application BioRender was employed to design the figure. MSCs-derived exosomes as an attractive cell-free approach in cancer therapy. MSCs-exosomes can be loaded with various types of compounds such as chemical or genetic drugs to target tumor tissues and inhibit tumor growth and metastasis. Exosomes can also influence cancer cells by promoting apoptosis and preventing angiogenesis
.
Mesenchymal stem cells-derived exosomes as an attractive cell-free approach in cancer therapy. The web-based application BioRender was employed to design the figure. MSCs-derived exosomes as an attractive cell-free approach in cancer therapy. MSCs-exosomes can be loaded with various types of compounds such as chemical or genetic drugs to target tumor tissues and inhibit tumor growth and metastasis. Exosomes can also influence cancer cells by promoting apoptosis and preventing angiogenesis
Signaling pathways regulated by MSCs
Numerous studies in recent decades have suggested that different signaling pathways are implicated in the development and progression of cancer.152 Based on different evidence, MSCs show a high capability to inhibit cancer through the modulation of diverse signaling pathways in the TME.153 In this section, several primary cancer-related signaling pathways influenced by MSCs were chosen for a thorough assessment of their anti-tumor effects. The Wnt signaling pathway, as one of the most important pathways in controlling the processes of cell growth and specialization, plays a prominent role in cancer progression.154 MSCs can suppress the growth of cancer cells by overexpressing P21CIP1 and P27KIP1, which in turn inhibit the Wnt signaling pathway by down-regulating c-Myc and cyclinD2 and promoting the production of the tumor suppressor Dickkopf-related protein 1.155 The wnt/β-catenin pathway promotes tumorigenesis in various types of cancer.156 The research conducted by Visweswaran et al has proven that factors derived from ASCs can inhibit cancer cell growth by reducing the expression of activated protein β-catenin and cyclin D1, and key target proteins of the Wnt pathway, and also can induce apoptosis via inhibition of the anti-apoptotic protein expression such as Bcl-XL.157 Blocking the β-catenin signaling pathway had a profound impact on preventing both tumor formation and metastasis in breast cancer cells overexpressing HER2.158 The PI3K/AKT/mTOR signaling pathway, as one of the most common intracellular pathways, can influence various downstream target proteins and is implicated in tumorigenesis, proliferation, drug resistance, the emergence of stem-cell-like traits, invasion, and metastasis of malignant cells.159,160 MSCs can induce cell cycle arrest and reduce cancer growth by inhibiting proliferation-related signaling pathways, such as the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT).161 Inhibition of AKT was showed in a Kaposi’s sarcoma model in which intravenously injected MSCs migrated to tumors and significantly suppressed tumor growth. The JAK/STAT signaling pathway is a key factor in the progression of cancer, serving as a driver of cancer growth and metastasis within tumors, or as a regulator of immune surveillance.162 Thus, suppressing the JAK/STAT pathway is encouraging for remedying various illnesses. He et al have presented that the MSCs-conditioned medium impedes the STAT3 signaling pathway in breast cancer cells and inhibits tumor progression. This finding indicates that paracrine-soluble factors secreted by MSCs could regulate JAK/STAT signaling and suppress the growth of breast tumors.163
Limitations and disadvantages of MSCs-and exosome-based therapies for cancer
During the past decades, there has been a discernible advancement in MSCs-based therapies for different cancer type.164 In spite of the amazing therapeutic potential of MSCs, there have been some inconsistent results from the use of MSCs in preclinical and clinical studies that may be caused by the heterogeneity of them.165 The heterogeneity of MSCs depends on different factors, including cell origin (tissue), the conditions of donors (age, diseases, or unknown factors), dosage, administration route, expansion protocol, and culture passage number of cells.166,167 Therefore, strategies and methods are needed that can manage these challenging issues. The use of standardized procedures for MSCs isolation, characterization, and expansion is critical to mitigating variability and improving the clinical efficacy of MSCs.168 Also, the dosage, route, and timing of administration should be optimized.169 Although the ideal MSCs dosage is still unknown, systematic intravenous injection (IV) of MSCs at a dose of 100–150 million cells per patient has been recommended to be beneficial for cancer therapy approaches.170 Meanwhile, many studies have shown that IV infusion of MSCs leads to the entrapment of cells in the lung, resulting in a reduction in the population of cells and the homing of less than 1% of them to target sites.171,172 Another of the most important reasons for MSCs utilization in the therapeutic area is their differentiation potential and immunomodulatory potency.173 These properties are affected by the specific tissue source from which the MSCs are derived, the age and health status of the donor, and the culture conditions and environment in which the MSCs were grown and expanded outside the body before being administered.174 So, MSCs derived from different tissues have some divergence in their proliferative and differentiation capacities and levels of secreted immunoregulatory cytokines. Furthermore, multiple studies provide evidence that aging causes a considerable reduction in the differentiation ability and immunomodulatory function of hMSCs.175-177 Among the major challenges to the application of MSCs to treat diverse pathologies is the need for large and sufficient amounts of cells that can only be obtained through long-term ex vivo expansion.178 Genomic instability and chromosomal aberrations are recognized as the most important occurrences during long-term culture that elevate the risk of tumorigenicity of MSCs after transplantation in patients. Therefore, MSCs can be unsafe for clinical use.179,180 In this regard, a number of studies have suggested exosomes derived from MSCs as an appropriate substitute option for overcoming the restrictions and disadvantages associated with cell-based therapy.181,182 Exosomes, the natural nanocarriers of bioactive signals, due to their hydrophilicity and small size, can even cross the blood-brain barrier and placental barrier, exerting favorable therapeutic effects in different types of disease.183,184 So far, several clinical trials have confirmed the helpful effects of MSCs-exosomes on the improvement of patients with cancer.185 Nevertheless, employing exosomes in clinical trials encounters challenges and limitations. The outstanding obstacles include the absence of a standardized exosome extraction and purification procedure, weak characterization, low yield of exosomes, sterility and biosafety, long-term maintenance, optimal therapeutic dosage, injection root,, and a short half-life.186,187 Exosome isolation is a determining process for getting a pure and uncontaminated sample with a high concentration, which facilitates precise evaluations of the functions and characteristics unique to exosomes.188 The heterogenicity of exosomes, arising from differences in their size, contents, and surface markers, poses a significant problem for efficient isolation, purification, and characterization of them.189 In order to overcome the heterogeneity of exosomes, it is necessary to recruit an efficient separation strategy, enabling the distinction of exosomes from various sample matrices.190 Currently, several techniques have been established for the sorting of exosomes based on their density, size, and surface proteins, including ultracentrifugation, size-exclusion chromatography, immunoaffinity, and polymer-based precipitation. These procedures, along with analysis methods such as nanoparticle tracking, electron microscopy, flow cytometry, and western blots, have helped advance the use of purified exosomes as an efficient drug delivery vehicle for cancer therapy.188,190-192 Nevertheless, the approaches used to extract, purify, and store exosomes need to conform to Good Manufacturing Practice (GMP) standards in order to generate a product with high biosafety to enter clinical settings.193 Determining the optimal dose, as one of the impressionable factors in exosome-based therapy, is affected by some considerations, such as the administration route and half-life.194 Due to the short circulation half-lives of exosomes and rapid fluid turnover (blood, sweat, or tears), systemically and locally administered injections cause rapid clearance from blood circulation and cumulation of the exosomes in the spleen, liver, and lung.195,196 Thus, the short half-life is an important limitation for the effective transfer of exosomes to damaged tissue and the continuity of their presence in the target location.197 Despite considerable advances in the study of MSCs and their derived exosomes, there are numerous issues and restrictions that have hindered their clinical use and should be addressed more in future research.
Conclusion
Cancer is one of the most significant causes of people life-threatening worldwide. Even though the disease conditions are highly progressive, there is no definitive cure, and nearly all current therapeutic approaches aim to control the advancement and progression of cancer. MSCs-associated cell therapies are considered promising treatment candidates with potential ameliorating effects on disease progression. MSCs due to having robust tumor-tropic capacity can migrate to tumor tissues, therefore these cells are a good option for targeted delivery of different chemical and genetic agents to tumor sites, reducing the side effects of various drugs on healthy tissues. They also can modulate inflammation conditions in the TME via producing higher levels of paracrine factors and suppression of T cell proliferation, NK cells activation, and DCs maturation, as a result, can be the favorite therapy for controlling cancer. In addition, MSCs-derived exosomes, as a cell-free tool, offer unique advantages for use in cancer therapy and are notable for the delivery of several therapeutic molecules including chemotherapeutic drugs, miRNAs, specific siRNAs, and suicide gene mRNAs. Overall, the numerous experimental studies and clinical trials provide promising results regarding the use of MSCs in cancer therapy and confirm the potential of MSCs to combat cancer. In conclusion, hopeful progressions have been made in oncology research, so MSCs-based therapies can be a surprising revolution in medicine and the treatment of patients suffering from cancer.
Acknowledgments
We acknowledge the Hematology and Oncology Research Center at Tabriz University of Medical Sciences, Iran for their support.
Competing Interests
The authors declare that there are no conflicts of interest.
Consent for publication
The authors consent for the publication of the manuscript.
Ethical Approval
Not applicable.
References
- Song Y, Liu X, Cheng W, Li H, Zhang D. The global, regional and national burden of stomach cancer and its attributable risk factors from 1990 to 2019. Sci Rep 2022; 12(1):11542. doi: 10.1038/s41598-022-15839-7 [Crossref] [ Google Scholar]
- Deo SV, Sharma J, Kumar S. GLOBOCAN 2020 report on global cancer burden: challenges and opportunities for surgical oncologists. Ann Surg Oncol 2022; 29(11):6497-500. doi: 10.1245/s10434-022-12151-6 [Crossref] [ Google Scholar]
- Debela DT, Muzazu SG, Heraro KD, Ndalama MT, Mesele BW, Haile DC. New approaches and procedures for cancer treatment: current perspectives. SAGE Open Med 2021; 9:20503121211034366. doi: 10.1177/20503121211034366 [Crossref] [ Google Scholar]
- Takayama Y, Kusamori K, Nishikawa M. Mesenchymal stem/stromal cells as next-generation drug delivery vehicles for cancer therapeutics. Expert Opin Drug Deliv 2021; 18(11):1627-42. doi: 10.1080/17425247.2021.1960309 [Crossref] [ Google Scholar]
- Zhuang WZ, Lin YH, Su LJ, Wu MS, Jeng HY, Chang HC. Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications. J Biomed Sci 2021; 28(1):28. doi: 10.1186/s12929-021-00725-7 [Crossref] [ Google Scholar]
- Marofi F, Vahedi G, Biglari A, Esmaeilzadeh A, Athari SS. Mesenchymal stromal/stem cells: a new era in the cell-based targeted gene therapy of cancer. Front Immunol 2017; 8:1770. doi: 10.3389/fimmu.2017.01770 [Crossref] [ Google Scholar]
- Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 2011; 9:12. doi: 10.1186/1478-811x-9-12 [Crossref] [ Google Scholar]
- Wright A, Arthaud-Day ML, Weiss ML. Therapeutic use of mesenchymal stromal cells: the need for inclusive characterization guidelines to accommodate all tissue sources and species. Front Cell Dev Biol 2021; 9:632717. doi: 10.3389/fcell.2021.632717 [Crossref] [ Google Scholar]
- Aravindhan S, Ejam SS, Lafta MH, Markov A, Yumashev AV, Ahmadi M. Mesenchymal stem cells and cancer therapy: insights into targeting the tumour vasculature. Cancer Cell Int 2021; 21(1):158. doi: 10.1186/s12935-021-01836-9 [Crossref] [ Google Scholar]
- Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant 1995; 16(4):557-64. [ Google Scholar]
- Cao B, Yang M, Zhu Y, Qu X, Mao C. Stem cells loaded with nanoparticles as a drug carrier for in vivo breast cancer therapy. Adv Mater 2014; 26(27):4627-31. doi: 10.1002/adma.201401550 [Crossref] [ Google Scholar]
- Wang X, Chen H, Zeng X, Guo W, Jin Y, Wang S. Efficient lung cancer-targeted drug delivery via a nanoparticle/MSC system. Acta Pharm Sin B 2019; 9(1):167-76. doi: 10.1016/j.apsb.2018.08.006 [Crossref] [ Google Scholar]
- Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One 2013; 8(12):e84256. doi: 10.1371/journal.pone.0084256 [Crossref] [ Google Scholar]
- Cafforio P, Viggiano L, Mannavola F, Pellè E, Caporusso C, Maiorano E. pIL6-TRAIL-engineered umbilical cord mesenchymal/stromal stem cells are highly cytotoxic for myeloma cells both in vitro and in vivo. Stem Cell Res Ther 2017; 8(1):206. doi: 10.1186/s13287-017-0655-6 [Crossref] [ Google Scholar]
- Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira Rovira, II II. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med 2006; 203(5):1235-47. doi: 10.1084/jem.20051921 [Crossref] [ Google Scholar]
- Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res 2008; 18(4):500-7. doi: 10.1038/cr.2008.40 [Crossref] [ Google Scholar]
- Hmadcha A, Martin-Montalvo A, Gauthier BR, Soria B, Capilla-Gonzalez V. Therapeutic potential of mesenchymal stem cells for cancer therapy. Front BioengBiotechnol 2020; 8:43. doi: 10.3389/fbioe.2020.00043 [Crossref] [ Google Scholar]
- Rosu A, Ghaemi B, Bulte JWM, Shakeri-Zadeh A. Tumor-tropic Trojan horses: using mesenchymal stem cells as cellular nanotheranostics. Theranostics 2024; 14(2):571-91. doi: 10.7150/thno.90187 [Crossref] [ Google Scholar]
- Chulpanova DS, Kitaeva KV, Tazetdinova LG, James V, Rizvanov AA, Solovyeva VV. Application of mesenchymal stem cells for therapeutic agent delivery in anti-tumor treatment. Front Pharmacol 2018; 9:259. doi: 10.3389/fphar.2018.00259 [Crossref] [ Google Scholar]
- de Araújo Farias V, Carrillo-Gálvez AB, Martín F, Anderson P. TGF-β and mesenchymal stromal cells in regenerative medicine, autoimmunity and cancer. Cytokine Growth Factor Rev 2018; 43:25-37. doi: 10.1016/j.cytogfr.2018.06.002 [Crossref] [ Google Scholar]
- Pavon LF, Sibov TT, de Souza AV, da Cruz EF, Malheiros SMF, Cabral FR. Tropism of mesenchymal stem cell toward CD133 + stem cell of glioblastoma in vitro and promote tumor proliferation in vivo. Stem Cell Res Ther 2018; 9(1):310. doi: 10.1186/s13287-018-1049-0 [Crossref] [ Google Scholar]
- Moskaleva EY, Zhorova ES, Semochkina YP, Shuvatova VG, Rodina AV, Saprykin VP. Mechanisms of stimulation of the growth of mouse mammary adenocarcinoma by mesenchymal stem cells. Bull Exp Biol Med 2021; 171(1):141-9. doi: 10.1007/s10517-021-05186-4 [Crossref] [ Google Scholar]
- Li M, Zhang F, Chen K, Wang C, Su Y, Liu Y. Nanoparticles and mesenchymal stem cells: a win-win alliance for anticancer drug delivery. RSC Adv 2016; 6(43):36910-22. doi: 10.1039/c6ra00398b [Crossref] [ Google Scholar]
- Nwabo Kamdje AH, Kamga PT, Tagne Simo R, Vecchio L, Seke Etet PF, Muller JM. Mesenchymal stromal cells’ role in tumor microenvironment: involvement of signaling pathways. Cancer Biol Med 2017; 14(2):129-41. doi: 10.20892/j.issn.2095-3941.2016.0033 [Crossref] [ Google Scholar]
- Liang W, Chen X, Zhang S, Fang J, Chen M, Xu Y. Mesenchymal stem cells as a double-edged sword in tumor growth: focusing on MSC-derived cytokines. Cell Mol Biol Lett 2021; 26(1):3. doi: 10.1186/s11658-020-00246-5 [Crossref] [ Google Scholar]
- Dwyer RM, Khan S, Barry FP, O’Brien T, Kerin MJ. Advances in mesenchymal stem cell-mediated gene therapy for cancer. Stem Cell Res Ther 2010; 1(3):25. doi: 10.1186/scrt25 [Crossref] [ Google Scholar]
- Ho IA, Lam PY. Signaling molecules and pathways involved in MSC tumor tropism. HistolHistopathol 2013; 28(11):1427-38. doi: 10.14670/hh-28.1427 [Crossref] [ Google Scholar]
- Ling L, Hou J, Liu D, Tang D, Zhang Y, Zeng Q. Important role of the SDF-1/CXCR4 axis in the homing of systemically transplanted human amnion-derived mesenchymal stem cells (hAD-MSCs) to ovaries in rats with chemotherapy-induced premature ovarian insufficiency (POI). Stem Cell Res Ther 2022; 13(1):79. doi: 10.1186/s13287-022-02759-6 [Crossref] [ Google Scholar]
- Baek SJ, Kang SK, Ra JC. In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp Mol Med 2011; 43(10):596-603. doi: 10.3858/emm.2011.43.10.069 [Crossref] [ Google Scholar]
- Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal stem cell migration and tissue repair. Cells 2019; 8(8):784. doi: 10.3390/cells8080784 [Crossref] [ Google Scholar]
- Zimolag E, Borowczyk-Michalowska J, Kedracka-Krok S, Skupien-Rabian B, Karnas E, Lasota S. Electric field as a potential directional cue in homing of bone marrow-derived mesenchymal stem cells to cutaneous wounds. BiochimBiophys Acta Mol Cell Res 2017; 1864(2):267-79. doi: 10.1016/j.bbamcr.2016.11.011 [Crossref] [ Google Scholar]
- Ghaffari-Nazari H. The known molecules involved in MSC homing and migration. J Stem Cell Res Med 2018; 3(1):1-4. doi: 10.15761/jscrm.1000127 [Crossref] [ Google Scholar]
- De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy?. World J Stem Cells 2016; 8(3):73-87. doi: 10.4252/wjsc.v8.i3.73 [Crossref] [ Google Scholar]
- Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int 2013; 2013:130763. doi: 10.1155/2013/130763 [Crossref] [ Google Scholar]
- Ho IA, Chan KY, Ng WH, Guo CM, Hui KM, Cheang P. Matrix metalloproteinase 1 is necessary for the migration of human bone marrow-derived mesenchymal stem cells toward human glioma. Stem Cells 2009; 27(6):1366-75. doi: 10.1002/stem.50 [Crossref] [ Google Scholar]
- Guan SP, Lam AT, Newman JP, Chua KLM, Kok CY, Chong ST. Matrix metalloproteinase-1 facilitates MSC migration via cleavage of IGF-2/IGFBP2 complex. FEBS Open Bio 2018; 8(1):15-26. doi: 10.1002/2211-5463.12330 [Crossref] [ Google Scholar]
- Bhoopathi P, Chetty C, Gogineni VR, Gujrati M, Dinh DH, Rao JS. MMP-2 mediates mesenchymal stem cell tropism towards medulloblastoma tumors. Gene Ther 2011; 18(7):692-701. doi: 10.1038/gt.2011.14 [Crossref] [ Google Scholar]
- Slama Y, Ah-Pine F, Khettab M, Arcambal A, Begue M, Dutheil F. The dual role of mesenchymal stem cells in cancer pathophysiology: pro-tumorigenic effects versus therapeutic potential. Int J Mol Sci 2023; 24(17):13511. doi: 10.3390/ijms241713511 [Crossref] [ Google Scholar]
- Yang D, Liu J, Qian H, Zhuang Q. Cancer-associated fibroblasts: from basic science to anticancer therapy. Exp Mol Med 2023; 55(7):1322-32. doi: 10.1038/s12276-023-01013-0 [Crossref] [ Google Scholar]
- Barcellos-de-Souza P, Comito G, Pons-Segura C, Taddei ML, Gori V, Becherucci V. Mesenchymal stem cells are recruited and activated into carcinoma-associated fibroblasts by prostate cancer microenvironment-derived TGF-β1. Stem Cells 2016; 34(10):2536-47. doi: 10.1002/stem.2412 [Crossref] [ Google Scholar]
- Aoto K, Ito K, Aoki S. Complex formation between platelet-derived growth factor receptor β and transforming growth factor β receptor regulates the differentiation of mesenchymal stem cells into cancer-associated fibroblasts. Oncotarget 2018; 9(75):34090-102. doi: 10.18632/oncotarget.26124 [Crossref] [ Google Scholar]
- Martin FT, Dwyer RM, Kelly J, Khan S, Murphy JM, Curran C. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res Treat 2010; 124(2):317-26. doi: 10.1007/s10549-010-0734-1 [Crossref] [ Google Scholar]
- Xue Z, Wu X, Chen X, Liu Y, Wang X, Wu K. Mesenchymal stem cells promote epithelial to mesenchymal transition and metastasis in gastric cancer though paracrine cues and close physical contact. J Cell Biochem 2015; 116(4):618-27. doi: 10.1002/jcb.25013 [Crossref] [ Google Scholar]
- Powell D, Lou M, Barros Becker F, Huttenlocher A. Cxcr1 mediates recruitment of neutrophils and supports proliferation of tumor-initiating astrocytes in vivo. Sci Rep 2018; 8(1):13285. doi: 10.1038/s41598-018-31675-0 [Crossref] [ Google Scholar]
- Guo X, Zhao Y, Yan H, Yang Y, Shen S, Dai X. Single tumor-initiating cells evade immune clearance by recruiting type II macrophages. Genes Dev 2017; 31(3):247-59. doi: 10.1101/gad.294348.116 [Crossref] [ Google Scholar]
- Ramírez Idarraga JA, Restrepo Múnera LM. Mesenchymal stem cells: their role in the tumor microenvironment. Tissue Eng Part B Rev 2023; 29(6):681-91. doi: 10.1089/ten.TEB.2023.0048 [Crossref] [ Google Scholar]
- Rivera-Cruz CM, Shearer JJ, Figueiredo Neto M, Figueiredo ML. The immunomodulatory effects of mesenchymal stem cell polarization within the tumor microenvironment niche. Stem Cells Int 2017; 2017:4015039. doi: 10.1155/2017/4015039 [Crossref] [ Google Scholar]
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30(1):42-8. doi: 10.1016/s0301-472x(01)00769-x [Crossref] [ Google Scholar]
- Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif 2020; 53(1):e12712. doi: 10.1111/cpr.12712 [Crossref] [ Google Scholar]
- Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol Sci 2020; 41(9):653-64. doi: 10.1016/j.tips.2020.06.009 [Crossref] [ Google Scholar]
- Seo Y, Kang MJ, Kim HS. Strategies to potentiate paracrine therapeutic efficacy of mesenchymal stem cells in inflammatory diseases. Int J Mol Sci 2021; 22(7):3397. doi: 10.3390/ijms22073397 [Crossref] [ Google Scholar]
- Taeb S, Rostamzadeh D, Mafi S, Mofatteh M, Zarrabi A, Hushmandi K. Update on mesenchymal stem cells: a crucial player in cancer immunotherapy. Curr Mol Med 2024; 24(1):98-113. doi: 10.2174/1566524023666221226143814 [Crossref] [ Google Scholar]
- Poggi A, Giuliani M. Mesenchymal stromal cells can regulate the immune response in the tumor microenvironment. Vaccines (Basel) 2016; 4(4):41. doi: 10.3390/vaccines4040041 [Crossref] [ Google Scholar]
- Harrell CR, Volarevic A, Djonov VG, Jovicic N, Volarevic V. Mesenchymal stem cell: a friend or foe in anti-tumor immunity. Int J Mol Sci 2021; 22(22):12429. doi: 10.3390/ijms222212429 [Crossref] [ Google Scholar]
- Sarsenova M, Kim Y, Raziyeva K, Kazybay B, Ogay V, Saparov A. Recent advances to enhance the immunomodulatory potential of mesenchymal stem cells. Front Immunol 2022; 13:1010399. doi: 10.3389/fimmu.2022.1010399 [Crossref] [ Google Scholar]
- Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One 2010; 5(4):e10088. doi: 10.1371/journal.pone.0010088 [Crossref] [ Google Scholar]
- Mishra VK, Shih HH, Parveen F, Lenzen D, Ito E, Chan TF. Identifying the therapeutic significance of mesenchymal stem cells. Cells 2020; 9(5):1145. doi: 10.3390/cells9051145 [Crossref] [ Google Scholar]
- Ohlsson LB, Varas L, Kjellman C, Edvardsen K, Lindvall M. Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp Mol Pathol 2003; 75(3):248-55. doi: 10.1016/j.yexmp.2003.06.001 [Crossref] [ Google Scholar]
- Seo SH, Kim KS, Park SH, Suh YS, Kim SJ, Jeun SS. The effects of mesenchymal stem cells injected via different routes on modified IL-12-mediated antitumor activity. Gene Ther 2011; 18(5):488-95. doi: 10.1038/gt.2010.170 [Crossref] [ Google Scholar]
- Szewc M, Radzikowska-Bűchner E, Wdowiak P, Kozak J, Kuszta P, Niezabitowska E. MSCs as tumor-specific vectors for the delivery of anticancer agents-a potential therapeutic strategy in cancer diseases: perspectives for quinazoline derivatives. Int J Mol Sci 2022; 23(5):2745. doi: 10.3390/ijms23052745 [Crossref] [ Google Scholar]
- Xu C, Feng Q, Yang H, Wang G, Huang L, Bai Q. A light-triggered mesenchymal stem cell delivery system for photoacoustic imaging and chemo-photothermal therapy of triple negative breast cancer. Adv Sci (Weinh) 2018; 5(10):1800382. doi: 10.1002/advs.201800382 [Crossref] [ Google Scholar]
- Thu KL, Soria-Bretones I, Mak TW, Cescon DW. Targeting the cell cycle in breast cancer: towards the next phase. Cell Cycle 2018; 17(15):1871-85. doi: 10.1080/15384101.2018.1502567 [Crossref] [ Google Scholar]
- Almeida-Porada G, Atala AJ, Porada CD. Therapeutic mesenchymal stromal cells for immunotherapy and for gene and drug delivery. Mol Ther Methods Clin Dev 2020; 16:204-24. doi: 10.1016/j.omtm.2020.01.005 [Crossref] [ Google Scholar]
- Petrella F, Rimoldi I, Rizzo S, Spaggiari L. Mesenchymal stromal cells for antineoplastic drug loading and delivery. Medicines (Basel) 2017; 4(4):87. doi: 10.3390/medicines4040087 [Crossref] [ Google Scholar]
- Pessina A, Bonomi A, Coccè V, Invernici G, Navone S, Cavicchini L. Mesenchymal stromal cells primed with paclitaxel provide a new approach for cancer therapy. PLoS One 2011; 6(12):e28321. doi: 10.1371/journal.pone.0028321 [Crossref] [ Google Scholar]
- Salehi H, Al-Arag S, Middendorp E, Gergely C, Cuisinier F, Orti V. Dental pulp stem cells used to deliver the anticancer drug paclitaxel. Stem Cell Res Ther 2018; 9(1):103. doi: 10.1186/s13287-018-0831-3 [Crossref] [ Google Scholar]
- Borghese C, Casagrande N, Corona G, Aldinucci D. Adipose-derived stem cells primed with paclitaxel inhibit ovarian cancer spheroid growth and overcome paclitaxel resistance. Pharmaceutics 2020; 12(5):401. doi: 10.3390/pharmaceutics12050401 [Crossref] [ Google Scholar]
- Kalimuthu S, Zhu L, Oh JM, Gangadaran P, Lee HW, Baek SH. Migration of mesenchymal stem cells to tumor xenograft models and in vitro drug delivery by doxorubicin. Int J Med Sci 2018; 15(10):1051-61. doi: 10.7150/ijms.25760 [Crossref] [ Google Scholar]
- Zhao Y, Tang S, Guo J, Alahdal M, Cao S, Yang Z. Targeted delivery of doxorubicin by nano-loaded mesenchymal stem cells for lung melanoma metastases therapy. Sci Rep 2017; 7:44758. doi: 10.1038/srep44758 [Crossref] [ Google Scholar]
- Coccè V, Farronato D, Brini AT, Masia C, Giannì AB, Piovani G. Drug loaded gingival mesenchymal stromal cells (GinPa-MSCs) inhibit in vitro proliferation of oral squamous cell carcinoma. Sci Rep 2017; 7(1):9376. doi: 10.1038/s41598-017-09175-4 [Crossref] [ Google Scholar]
- Bonomi A, Sordi V, Dugnani E, Ceserani V, Dossena M, Coccè V. Gemcitabine-releasing mesenchymal stromal cells inhibit in vitro proliferation of human pancreatic carcinoma cells. Cytotherapy 2015; 17(12):1687-95. doi: 10.1016/j.jcyt.2015.09.005 [Crossref] [ Google Scholar]
- Bartlett DL, Liu Z, Sathaiah M, Ravindranathan R, Guo Z, He Y. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer 2013; 12(1):103. doi: 10.1186/1476-4598-12-103 [Crossref] [ Google Scholar]
- Hemminki O, Dos Santos JM, Hemminki A. Oncolytic viruses for cancer immunotherapy. J Hematol Oncol 2020; 13(1):84. doi: 10.1186/s13045-020-00922-1 [Crossref] [ Google Scholar]
- Rehman H, Silk AW, Kane MP, Kaufman HL. Into the clinic: talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J Immunother Cancer 2016; 4:53. doi: 10.1186/s40425-016-0158-5 [Crossref] [ Google Scholar]
- Zhang L, Wang W, Wang R, Zhang N, Shang H, Bi Y. Reshaping the immune microenvironment by oncolytic herpes simplex virus in murine pancreatic ductal adenocarcinoma. Mol Ther 2021; 29(2):744-61. doi: 10.1016/j.ymthe.2020.10.027 [Crossref] [ Google Scholar]
- Schirrmacher V, van Gool S, Stuecker W. Breaking therapy resistance: an update on oncolytic Newcastle disease virus for improvements of cancer therapy. Biomedicines 2019; 7(3):66. doi: 10.3390/biomedicines7030066 [Crossref] [ Google Scholar]
- Roy DG, Bell JC, Bourgeois-Daigneault MC. Magnetic targeting of oncolytic VSV-based therapies improves infection of tumor cells in the presence of virus-specific neutralizing antibodies in vitro. BiochemBiophys Res Commun 2020; 526(3):641-6. doi: 10.1016/j.bbrc.2020.03.135 [Crossref] [ Google Scholar]
- Choi S, Hong JA, Choi HJ, Song JJ. Enhanced tumor targeting and timely viral release of mesenchymal stem cells/oncolytic virus complex due to GRP78 and inducible E1B55K expressions greatly increase the antitumor effect of systemic treatment. Mol Ther Oncolytics 2022; 27:26-47. doi: 10.1016/j.omto.2022.09.004 [Crossref] [ Google Scholar]
- Yoon AR, Hong J, Li Y, Shin HC, Lee H, Kim HS. Mesenchymal stem cell-mediated delivery of an oncolytic adenovirus enhances antitumor efficacy in hepatocellular carcinoma. Cancer Res 2019; 79(17):4503-14. doi: 10.1158/0008-5472.can-18-3900 [Crossref] [ Google Scholar]
- Ahmed AU, Rolle CE, Tyler MA, Han Y, Sengupta S, Wainwright DA. Bone marrow mesenchymal stem cells loaded with an oncolytic adenovirus suppress the anti-adenoviral immune response in the cotton rat model. Mol Ther 2010; 18(10):1846-56. doi: 10.1038/mt.2010.131 [Crossref] [ Google Scholar]
- Castleton A, Dey A, Beaton B, Patel B, Aucher A, Davis DM. Human mesenchymal stromal cells deliver systemic oncolytic measles virus to treat acute lymphoblastic leukemia in the presence of humoral immunity. Blood 2014; 123(9):1327-35. doi: 10.1182/blood-2013-09-528851 [Crossref] [ Google Scholar]
- Hadryś A, Sochanik A, McFadden G, Jazowiecka-Rakus J. Mesenchymal stem cells as carriers for systemic delivery of oncolytic viruses. Eur J Pharmacol 2020; 874:172991. doi: 10.1016/j.ejphar.2020.172991 [Crossref] [ Google Scholar]
- Russell L, Peng KW, Russell SJ, Diaz RM. Oncolytic viruses: priming time for cancer immunotherapy. BioDrugs 2019; 33(5):485-501. doi: 10.1007/s40259-019-00367-0 [Crossref] [ Google Scholar]
- Carreras-Planella L, Monguió-Tortajada M, Borràs FE, Franquesa M. Immunomodulatory effect of MSC on B cells is independent of secreted extracellular vesicles. Front Immunol 2019; 10:1288. doi: 10.3389/fimmu.2019.01288 [Crossref] [ Google Scholar]
- Wilson A, Chee M, Butler P, Boyd AS. Isolation and characterisation of human adipose-derived stem cells. Methods Mol Biol 2019; 1899:3-13. doi: 10.1007/978-1-4939-8938-6_1 [Crossref] [ Google Scholar]
- Zhang F, Wang C, Wen X, Chen Y, Mao R, Cui D. Mesenchymal stem cells alleviate rat diabetic nephropathy by suppressing CD103 + DCs-mediated CD8 + T cell responses. J Cell Mol Med 2020; 24(10):5817-31. doi: 10.1111/jcmm.15250 [Crossref] [ Google Scholar]
- Haddad R, Saldanha-Araujo F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: what do we know so far?. Biomed Res Int 2014; 2014:216806. doi: 10.1155/2014/216806 [Crossref] [ Google Scholar]
- Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis 2016; 7(1):e2062. doi: 10.1038/cddis.2015.327 [Crossref] [ Google Scholar]
- Rozenberg A, Rezk A, Boivin MN, Darlington PJ, Nyirenda M, Li R. Human mesenchymal stem cells impact Th17 and Th1 responses through a prostaglandin E2 and myeloid-dependent mechanism. Stem Cells Transl Med 2016; 5(11):1506-14. doi: 10.5966/sctm.2015-0243 [Crossref] [ Google Scholar]
- Khare D, Or R, Resnick I, Barkatz C, Almogi-Hazan O, Avni B. Mesenchymal stromal cell-derived exosomes affect mRNA expression and function of B-lymphocytes. Front Immunol 2018; 9:3053. doi: 10.3389/fimmu.2018.03053 [Crossref] [ Google Scholar]
- Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F. Human mesenchymal stem cells modulate B-cell functions. Blood 2006; 107(1):367-72. doi: 10.1182/blood-2005-07-2657 [Crossref] [ Google Scholar]
- Xu LL, Fu HX, Zhang JM, Feng FE, Wang QM, Zhu XL. Impaired function of bone marrow mesenchymal stem cells from immune thrombocytopenia patients in inducing regulatory dendritic cell differentiation through the Notch-1/Jagged-1 signaling pathway. Stem Cells Dev 2017; 26(22):1648-61. doi: 10.1089/scd.2017.0078 [Crossref] [ Google Scholar]
- Liu Q, Zheng H, Chen X, Peng Y, Huang W, Li X. Human mesenchymal stromal cells enhance the immunomodulatory function of CD8 + CD28- regulatory T cells. Cell Mol Immunol 2015; 12(6):708-18. doi: 10.1038/cmi.2014.118 [Crossref] [ Google Scholar]
- El Omar R, Xiong Y, Dostert G, Louis H, Gentils M, Menu P. Immunomodulation of endothelial differentiated mesenchymal stromal cells: impact on T and NK cells. Immunol Cell Biol 2016; 94(4):342-56. doi: 10.1038/icb.2015.94 [Crossref] [ Google Scholar]
- Cho KA, Lee JK, Kim YH, Park M, Woo SY, Ryu KH. Mesenchymal stem cells ameliorate B-cell-mediated immune responses and increase IL-10-expressing regulatory B cells in an EBI3-dependent manner. Cell Mol Immunol 2017; 14(11):895-908. doi: 10.1038/cmi.2016.59 [Crossref] [ Google Scholar]
- Liu X, Qu X, Chen Y, Liao L, Cheng K, Shao C. Mesenchymal stem/stromal cells induce the generation of novel IL-10-dependent regulatory dendritic cells by SOCS3 activation. J Immunol 2012; 189(3):1182-92. doi: 10.4049/jimmunol.1102996 [Crossref] [ Google Scholar]
- Yoon AR, Hong J, Li Y, Shin HC, Lee H, Kim HS. Mesenchymal stem cell-mediated delivery of an oncolytic adenovirus enhances antitumor efficacy in hepatocellular carcinoma. Cancer Res 2019; 79(17):4503-14. doi: 10.1158/0008-5472.can-18-3900 [Crossref] [ Google Scholar]
- Duebgen M, Martinez-Quintanilla J, Tamura K, Hingtgen S, Redjal N, Wakimoto H. Stem cells loaded with multimechanistic oncolytic herpes simplex virus variants for brain tumor therapy. J Natl Cancer Inst 2014; 106(6):dju090. doi: 10.1093/jnci/dju090 [Crossref] [ Google Scholar]
- Chastkofsky M, Pituch KC, Katagi H, Ilut L, Xiao T, Han Y, et al. MSCs successfully deliver oncolytic virotherapy to diffuse intrinsic pontine glioma. bioRxiv [Preprint]. May 10, 2020. Available from: https://www.biorxiv.org/content/10.1101/2020.05.09.085837v1.full.
- Guo Y, Zhang Z, Xu X, Xu Z, Wang S, Huang D. Menstrual blood-derived stem cells as delivery vehicles for oncolytic adenovirus virotherapy for colorectal cancer. Stem Cells Dev 2019; 28(13):882-96. doi: 10.1089/scd.2018.0222 [Crossref] [ Google Scholar]
- Zhang Y, Liu C, Wang T, Kong F, Zhang H, Yi J. Therapeutic effects of mesenchymal stem cells loaded with oncolytic adenovirus carrying decorin on a breast cancer lung metastatic mouse model. Mol TherOncolytics 2022; 24:486-96. doi: 10.1016/j.omto.2022.01.007 [Crossref] [ Google Scholar]
- Belete TM. The current status of gene therapy for the treatment of cancer. Biologics 2021; 15:67-77. doi: 10.2147/btt.s302095 [Crossref] [ Google Scholar]
- Düzgüneş N. Origins of suicide gene therapy. Methods Mol Biol 2019; 1895:1-9. doi: 10.1007/978-1-4939-8922-5_1 [Crossref] [ Google Scholar]
- Zarogoulidis P, Darwiche K, Sakkas A, Yarmus L, Huang H, Li Q. Suicide gene therapy for cancer - current strategies. J Genet Syndr Gene Ther 2013; 4:16849. doi: 10.4172/2157-7412.1000139 [Crossref] [ Google Scholar]
- Amara I, Touati W, Beaune P, de Waziers I. Mesenchymal stem cells as cellular vehicles for prodrug gene therapy against tumors. Biochimie 2014; 105:4-11. doi: 10.1016/j.biochi.2014.06.016 [Crossref] [ Google Scholar]
- Pawitan JA, Bui TA, Mubarok W, Antarianto RD, Nurhayati RW, Dilogo IH. Enhancement of the therapeutic capacity of mesenchymal stem cells by genetic modification: a systematic review. Front Cell Dev Biol 2020; 8:587776. doi: 10.3389/fcell.2020.587776 [Crossref] [ Google Scholar]
- Niess H, Thomas MN, Schiergens TS, Kleespies A, Jauch KW, Bruns C. Genetic engineering of mesenchymal stromal cells for cancer therapy: turning partners in crime into Trojan horses. Innov Surg Sci 2016; 1(1):19-32. doi: 10.1515/iss-2016-0005 [Crossref] [ Google Scholar]
- Golinelli G, Mastrolia I, Aramini B, Masciale V, Pinelli M, Pacchioni L. Arming mesenchymal stromal/stem cells against cancer: has the time come?. Front Pharmacol 2020; 11:529921. doi: 10.3389/fphar.2020.529921 [Crossref] [ Google Scholar]
- You MH, Kim WJ, Shim W, Lee SR, Lee G, Choi S. Cytosine deaminase-producing human mesenchymal stem cells mediate an antitumor effect in a mouse xenograft model. J Gastroenterol Hepatol 2009; 24(8):1393-400. doi: 10.1111/j.1440-1746.2009.05862.x [Crossref] [ Google Scholar]
- Cavarretta IT, Altanerova V, Matuskova M, Kucerova L, Culig Z, Altaner C. Adipose tissue-derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth. Mol Ther 2010; 18(1):223-31. doi: 10.1038/mt.2009.237 [Crossref] [ Google Scholar]
- Altaner C. Prodrug cancer gene therapy. Cancer Lett 2008; 270(2):191-201. doi: 10.1016/j.canlet.2008.04.023 [Crossref] [ Google Scholar]
- Durinikova E, Plava J, Tyciakova S, Skvara P, Vojs Stanova A, Kozovska Z. Cytotoxic response of 5-fluorouracil-resistant cells to gene- and cell-directed enzyme/prodrug treatment. Cancer Gene Ther 2018; 25(11-12):285-99. doi: 10.1038/s41417-018-0030-5 [Crossref] [ Google Scholar]
- Oraee-Yazdani S, Tavanaei R, Rostami F, Hajarizadeh A, Mehrabadi M, Akhlaghpasand M. Suicide gene therapy using allogeneic adipose tissue-derived mesenchymal stem cell gene delivery vehicles in recurrent glioblastoma multiforme: a first-in-human, dose-escalation, phase I clinical trial. J Transl Med 2023; 21(1):350. doi: 10.1186/s12967-023-04213-4 [Crossref] [ Google Scholar]
- Villatoro AJ, Alcoholado C, Del Carmen Martín-Astorga M, Rubio N, Blanco J, Garrido CP. Suicide gene therapy by canine mesenchymal stem cell transduced with thymidine kinase in a u-87 glioblastoma murine model: secretory profile and antitumor activity. PLoS One 2022; 17(2):e0264001. doi: 10.1371/journal.pone.0264001 [Crossref] [ Google Scholar]
- Gao Z, Zhang L, Hu J, Sun Y. Mesenchymal stem cells: a potential targeted-delivery vehicle for anti-cancer drug, loaded nanoparticles. Nanomedicine 2013; 9(2):174-84. doi: 10.1016/j.nano.2012.06.003 [Crossref] [ Google Scholar]
- Aoki M, Kakimoto K, Goto M, Higuchi K. Novel therapeutic approach using drug-loaded adipose-derived stem cells for pancreatic cancer. Sci Rep 2019; 9(1):17971. doi: 10.1038/s41598-019-53807-w [Crossref] [ Google Scholar]
- Shah K. Mesenchymal stem cells engineered for cancer therapy. Adv Drug Deliv Rev 2012; 64(8):739-48. doi: 10.1016/j.addr.2011.06.010 [Crossref] [ Google Scholar]
- Uddin MJ, Mohite P, Munde S, Ade N, Oladosu TA, Chidrawar VR. Extracellular vesicles: the future of therapeutics and drug delivery systems. Intelligent Pharmacy 2024; 2(3):312-28. doi: 10.1016/j.ipha.2024.02.004 [Crossref] [ Google Scholar]
- van de Wakker SI, Meijers FM, Sluijter JPG, Vader P. Extracellular vesicle heterogeneity and its impact for regenerative medicine applications. Pharmacol Rev 2023; 75(5):1043-61. doi: 10.1124/pharmrev.123.000841 [Crossref] [ Google Scholar]
- Tian Y, Yan X. Flow cytometry for single extracellular vesicle analysis. In: Wang Q, Zheng L, eds. Extracellular Vesicles: From Bench to Bedside. Singapore: Springer; 2024. p. 111-24. 10.1007/978-981-99-8365-0_8.
- Yang C, Xue Y, Duan Y, Mao C, Wan M. Extracellular vesicles and their engineering strategies, delivery systems, and biomedical applications. J Control Release 2024; 365:1089-123. doi: 10.1016/j.jconrel.2023.11.057 [Crossref] [ Google Scholar]
- Clos-Sansalvador M, Monguió-Tortajada M, Roura S, Franquesa M, Borràs FE. Commonly used methods for extracellular vesicles’ enrichment: Implications in downstream analyses and use. Eur J Cell Biol 2022; 101(3):151227. doi: 10.1016/j.ejcb.2022.151227 [Crossref] [ Google Scholar]
- Wu P, Jiang J, Xu W. Biological functions of extracellular vesicles in various systems. In: Wang Q, Zheng L, ed. Extracellular Vesicles: From Bench to Bedside. Singapore: Springer; 2024. p. 45-52. 10.1007/978-981-99-8365-0_4.
- Goryunov K, Ivanov M, Kulikov A, Shevtsova Y, Burov A, Podurovskaya Y. A review of the use of extracellular vesicles in the treatment of neonatal diseases: current state and problems with translation to the clinic. Int J Mol Sci 2024; 25(5):2879. doi: 10.3390/ijms25052879 [Crossref] [ Google Scholar]
- Hánělová K, Raudenská M, Masařík M, Balvan J. Protein cargo in extracellular vesicles as the key mediator in the progression of cancer. Cell Commun Signal 2024; 22(1):25. doi: 10.1186/s12964-023-01408-6 [Crossref] [ Google Scholar]
- Parameshwar K, Anitha K, Mounika N, Parameshwar R, Narayana NA. Exosomes: biogenesis, composition, and synthesis. In: Mishra N, Ashique S, Garg A, Chithravel V, Anand K, eds. Exosomes Based Drug Delivery Strategies for Brain Disorders. Singapore: Springer; 2024. p. 37-53. 10.1007/978-981-99-8373-5_2.
- Mondal J, Pillarisetti S, Junnuthula V, Surwase SS, Hwang SR, Park IK. Extracellular vesicles and exosome-like nanovesicles as pioneering oral drug delivery systems. Front BioengBiotechnol 2023; 11:1307878. doi: 10.3389/fbioe.2023.1307878 [Crossref] [ Google Scholar]
- Skotland T, Hessvik NP, Sandvig K, Llorente A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J Lipid Res 2019; 60(1):9-18. doi: 10.1194/jlr.R084343 [Crossref] [ Google Scholar]
- Le Vo KL. Analytical Approaches to Study Vesicular and Exosomal Release [dissertation]. University of Gothenburg; 2024.
- Meldolesi J. Exosomes and ectosomes in intercellular communication. Curr Biol 2018; 28(8):R435-44. doi: 10.1016/j.cub.2018.01.059 [Crossref] [ Google Scholar]
- Rojas A, Regev-Rudzki N. Biogenesis of extracellular vesicles from the pathogen perspective: transkingdom strategies for delivering messages. CurrOpin Cell Biol 2024; 88:102366. doi: 10.1016/j.ceb.2024.102366 [Crossref] [ Google Scholar]
- Andreu Z, Yáñez-Mó M. Tetraspanins in extracellular vesicle formation and function. Front Immunol 2014; 5:442. doi: 10.3389/fimmu.2014.00442 [Crossref] [ Google Scholar]
- Amin S, Massoumi H, Tewari D, Roy A, Chaudhuri M, Jazayerli C. Cell type-specific extracellular vesicles and their impact on health and disease. Int J Mol Sci 2024; 25(5):2730. doi: 10.3390/ijms25052730 [Crossref] [ Google Scholar]
- Bhat A, Malik A, Yadav P, Ware W-PJ, Kakalij P, Chand S. Mesenchymal stem cell-derived extracellular vesicles: recent therapeutics and targeted drug delivery advances. J Extracell Biol 2024; 3(5):e156. doi: 10.1002/jex2.156 [Crossref] [ Google Scholar]
- Sun Y, Liu G, Zhang K, Cao Q, Liu T, Li J. Mesenchymal stem cells-derived exosomes for drug delivery. Stem Cell Res Ther 2021; 12(1):561. doi: 10.1186/s13287-021-02629-7 [Crossref] [ Google Scholar]
- Lin Z, Wu Y, Xu Y, Li G, Li Z, Liu T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: recent advances and therapeutic potential. Mol Cancer 2022; 21(1):179. doi: 10.1186/s12943-022-01650-5 [Crossref] [ Google Scholar]
- Oveili E, Vafaei S, Bazavar H, Eslami Y, Mamaghanizadeh E, Yasamineh S. The potential use of mesenchymal stem cells-derived exosomes as microRNAs delivery systems in different diseases. Cell Commun Signal 2023; 21(1):20. doi: 10.1186/s12964-022-01017-9 [Crossref] [ Google Scholar]
- Lyu T, Wang Y, Li D, Yang H, Qin B, Zhang W. Exosomes from BM-MSCs promote acute myeloid leukemia cell proliferation, invasion and chemoresistance via upregulation of S100A4. Exp Hematol Oncol 2021; 10(1):24. doi: 10.1186/s40164-021-00220-7 [Crossref] [ Google Scholar]
- Weng Z, Zhang B, Wu C, Yu F, Han B, Li B. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J Hematol Oncol 2021; 14(1):136. doi: 10.1186/s13045-021-01141-y [Crossref] [ Google Scholar]
- Tan F, Li X, Wang Z, Li J, Shahzad K, Zheng J. Clinical applications of stem cell-derived exosomes. Signal Transduct Target Ther 2024; 9(1):17. doi: 10.1038/s41392-023-01704-0 [Crossref] [ Google Scholar]
- Alcayaga-Miranda F, González PL, Lopez-Verrilli A, Varas-Godoy M, Aguila-Díaz C, Contreras L. Prostate tumor-induced angiogenesis is blocked by exosomes derived from menstrual stem cells through the inhibition of reactive oxygen species. Oncotarget 2016; 7(28):44462-77. doi: 10.18632/oncotarget.9852 [Crossref] [ Google Scholar]
- Rezaeian A, Khatami F, Heidari Keshel S, Akbari MR, Mirzaei A, Gholami K. The effect of mesenchymal stem cells-derived exosomes on the prostate, bladder, and renal cancer cell lines. Sci Rep 2022; 12(1):20924. doi: 10.1038/s41598-022-23204-x [Crossref] [ Google Scholar]
- Li T, Li X, Han G, Liang M, Yang Z, Zhang C. The therapeutic potential and clinical significance of exosomes as carriers of drug delivery system. Pharmaceutics 2022; 15(1):21. doi: 10.3390/pharmaceutics15010021 [Crossref] [ Google Scholar]
- Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal 2014; 7(332):ra63. doi: 10.1126/scisignal.2005231 [Crossref] [ Google Scholar]
- O’Brien KP, Khan S, Gilligan KE, Zafar H, Lalor P, Glynn C. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene 2018; 37(16):2137-49. doi: 10.1038/s41388-017-0116-9 [Crossref] [ Google Scholar]
- Greco KA, Franzen CA, Foreman KE, Flanigan RC, Kuo PC, Gupta GN. PLK-1 silencing in bladder cancer by siRNA delivered with exosomes. Urology 2016;91:241.e1-241.e7. 10.1016/j.urology.2016.01.028.
- Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release 2014; 192:262-70. doi: 10.1016/j.jconrel.2014.07.042 [Crossref] [ Google Scholar]
- Melzer C, Rehn V, Yang Y, Bähre H, von der Ohe J, Hass R. Taxol-loaded MSC-derived exosomes provide a therapeutic vehicle to target metastatic breast cancer and other carcinoma cells. Cancers (Basel) 2019; 11(6):798. doi: 10.3390/cancers11060798 [Crossref] [ Google Scholar]
- Wei H, Chen J, Wang S, Fu F, Zhu X, Wu C. A nanodrug consisting of doxorubicin and exosome derived from mesenchymal stem cells for osteosarcoma treatment in vitro. Int J Nanomedicine 2019; 14:8603-10. doi: 10.2147/ijn.s218988 [Crossref] [ Google Scholar]
- Wang N, Pei B, Yuan X, Yi C, Wiredu Ocansey DK, Qian H. Emerging roles of mesenchymal stem cell-derived exosomes in gastrointestinal cancers. Front BioengBiotechnol 2022; 10:1019459. doi: 10.3389/fbioe.2022.1019459 [Crossref] [ Google Scholar]
- Dalmizrak A, Dalmizrak O. Mesenchymal stem cell-derived exosomes as new tools for delivery of miRNAs in the treatment of cancer. Front BioengBiotechnol 2022; 10:956563. doi: 10.3389/fbioe.2022.956563 [Crossref] [ Google Scholar]
- Yip HY, Papa A. Signaling pathways in cancer: therapeutic targets, combinatorial treatments, and new developments. Cells 2021; 10(3):659. doi: 10.3390/cells10030659 [Crossref] [ Google Scholar]
- Ramuta TŽ, Kreft ME. Mesenchymal stem/stromal cells may decrease success of cancer treatment by inducing resistance to chemotherapy in cancer cells. Cancers (Basel) 2022; 14(15):3761. doi: 10.3390/cancers14153761 [Crossref] [ Google Scholar]
- Koni M, Pinnarò V, Brizzi MF. The Wnt signalling pathway: a tailored target in cancer. Int J Mol Sci 2020; 21(20):7697. doi: 10.3390/ijms21207697 [Crossref] [ Google Scholar]
- Zhu Y, Sun Z, Han Q, Liao L, Wang J, Bian C. Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia 2009; 23(5):925-33. doi: 10.1038/leu.2008.384 [Crossref] [ Google Scholar]
- Gangrade A, Pathak V, Augelli-Szafran CE, Wei HX, Oliver P, Suto M. Preferential inhibition of Wnt/β-catenin signaling by novel benzimidazole compounds in triple-negative breast cancer. Int J Mol Sci 2018; 19(5):1524. doi: 10.3390/ijms19051524 [Crossref] [ Google Scholar]
- Visweswaran M, Arfuso F, Dilley RJ, Newsholme P, Dharmarajan A. The inhibitory influence of adipose tissue-derived mesenchymal stem cell environment and Wnt antagonism on breast tumour cell lines. Int J Biochem Cell Biol 2018; 95:63-72. doi: 10.1016/j.biocel.2017.12.013 [Crossref] [ Google Scholar]
- Otsu K, Das S, Houser SD, Quadri SK, Bhattacharya S, Bhattacharya J. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood 2009; 113(18):4197-205. doi: 10.1182/blood-2008-09-176198 [Crossref] [ Google Scholar]
- Lv X, Li CY, Han P, Xu XY. MicroRNA-520a-3p inhibits cell growth and metastasis of non-small cell lung cancer through PI3K/AKT/mTOR signaling pathway. Eur Rev Med Pharmacol Sci 2018; 22(8):2321-7. doi: 10.26355/eurrev_201804_14822 [Crossref] [ Google Scholar]
- Jiang N, Dai Q, Su X, Fu J, Feng X, Peng J. Role of PI3K/AKT pathway in cancer: the framework of malignant behavior. Mol Biol Rep 2020; 47(6):4587-629. doi: 10.1007/s11033-020-05435-1 [Crossref] [ Google Scholar]
- Lu L, Chen G, Yang J, Ma Z, Yang Y, Hu Y. Bone marrow mesenchymal stem cells suppress growth and promote the apoptosis of glioma U251 cells through downregulation of the PI3K/AKT signaling pathway. Biomed Pharmacother 2019; 112:108625. doi: 10.1016/j.biopha.2019.108625 [Crossref] [ Google Scholar]
- Brooks AJ, Putoczki T. JAK-STAT signalling pathway in cancer. Cancers (Basel) 2020; 12(7):1971. doi: 10.3390/cancers12071971 [Crossref] [ Google Scholar]
- He N, Kong Y, Lei X, Liu Y, Wang J, Xu C. MSCs inhibit tumor progression and enhance radiosensitivity of breast cancer cells by down-regulating Stat3 signaling pathway. Cell Death Dis 2018; 9(10):1026. doi: 10.1038/s41419-018-0949-3 [Crossref] [ Google Scholar]
- Aravindhan S, Ejam SS, Lafta MH, Markov A, Yumashev AV, Ahmadi M. Mesenchymal stem cells and cancer therapy: insights into targeting the tumour vasculature. Cancer Cell Int 2021; 21(1):158. doi: 10.1186/s12935-021-01836-9 [Crossref] [ Google Scholar]
- Li J, Wu Z, Zhao L, Liu Y, Su Y, Gong X. The heterogeneity of mesenchymal stem cells: an important issue to be addressed in cell therapy. Stem Cell Res Ther 2023; 14(1):381. doi: 10.1186/s13287-023-03587-y [Crossref] [ Google Scholar]
- Jovic D, Yu Y, Wang D, Wang K, Li H, Xu F. A brief overview of global trends in MSC-based cell therapy. Stem Cell Rev Rep 2022; 18(5):1525-45. doi: 10.1007/s12015-022-10369-1 [Crossref] [ Google Scholar]
- Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci 2020; 77(14):2771-94. doi: 10.1007/s00018-020-03454-6 [Crossref] [ Google Scholar]
- Todtenhaupt P, Franken LA, Groene SG, van Hoolwerff M, van der Meeren LE, van Klink JM. A robust and standardized method to isolate and expand mesenchymal stromal cells from human umbilical cord. Cytotherapy 2023; 25(10):1057-68. doi: 10.1016/j.jcyt.2023.07.004 [Crossref] [ Google Scholar]
- Lavi Arab F, Hoseinzadeh A, Hafezi F, Mohammadi FA, Zeynali F, Hadad Tehran M. Mesenchymal stem cell-derived exosomes for management of prostate cancer: an updated view. Int Immunopharmacol 2024; 134:112171. doi: 10.1016/j.intimp.2024.112171 [Crossref] [ Google Scholar]
- Kabat M, Bobkov I, Kumar S, Grumet M. Trends in mesenchymal stem cell clinical trials 2004-2018: is efficacy optimal in a narrow dose range?. Stem Cells Transl Med 2020; 9(1):17-27. doi: 10.1002/sctm.19-0202 [Crossref] [ Google Scholar]
- Karimi-Shahri M, Javid H, Sharbaf Mashhad A, Yazdani S, Hashemy SI. Mesenchymal stem cells in cancer therapy; the art of harnessing a foe to a friend. Iran J Basic Med Sci 2021; 24(10):1307-23. doi: 10.22038/ijbms.2021.58227.12934 [Crossref] [ Google Scholar]
- Moll G, Ankrum JA, Kamhieh-Milz J, Bieback K, Ringdén O, Volk HD. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med 2019; 25(2):149-63. doi: 10.1016/j.molmed.2018.12.006 [Crossref] [ Google Scholar]
- Maged G, Abdelsamed MA, Wang H, Lotfy A. The potency of mesenchymal stem/stromal cells: does donor sex matter?. Stem Cell Res Ther 2024; 15(1):112. doi: 10.1186/s13287-024-03722-3 [Crossref] [ Google Scholar]
- Levy O, Kuai R, Siren EM, Bhere D, Milton Y, Nissar N. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv 2020; 6(30):eaba6884. doi: 10.1126/sciadv.aba6884 [Crossref] [ Google Scholar]
- Siegel G, Kluba T, Hermanutz-Klein U, Bieback K, Northoff H, Schäfer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med 2013; 11:146. doi: 10.1186/1741-7015-11-146 [Crossref] [ Google Scholar]
- Kizilay Mancini Ö, Lora M, Shum-Tim D, Nadeau S, Rodier F, Colmegna I. A proinflammatory secretome mediates the impaired immunopotency of human mesenchymal stromal cells in elderly patients with atherosclerosis. Stem Cells Transl Med 2017; 6(4):1132-40. doi: 10.1002/sctm.16-0221 [Crossref] [ Google Scholar]
- Zhang Y, Ravikumar M, Ling L, Nurcombe V, Cool SM. Age-related changes in the inflammatory status of human mesenchymal stem cells: implications for cell therapy. Stem Cell Reports 2021; 16(4):694-707. doi: 10.1016/j.stemcr.2021.01.021 [Crossref] [ Google Scholar]
- Ma CY, Zhai Y, Li CT, Liu J, Xu X, Chen H. Translating mesenchymal stem cell and their exosome research into GMP compliant advanced therapy products: promises, problems and prospects. Med Res Rev 2024; 44(3):919-38. doi: 10.1002/med.22002 [Crossref] [ Google Scholar]
- Arefnezhad R, Helfi M, Okhravijouybari R, Goleij P, Sargolzaeimoghaddam M, Mohammadi H. Umbilical cord mesenchymal stem cells and lung cancer: we should be hopeful or hopeless?. Tissue Cell 2024; 88:102410. doi: 10.1016/j.tice.2024.102410 [Crossref] [ Google Scholar]
- Rasouli M, Naeimzadeh Y, Hashemi N, Hosseinzadeh S. Age-related alterations in mesenchymal stem cell function: understanding mechanisms and seeking opportunities to bypass the cellular aging. Curr Stem Cell Res Ther 2024; 19(1):15-32. doi: 10.2174/1574888x18666230113144016 [Crossref] [ Google Scholar]
- Janockova J, Slovinska L, Harvanova D, Spakova T, Rosocha J. New therapeutic approaches of mesenchymal stem cells-derived exosomes. J Biomed Sci 2021; 28(1):39. doi: 10.1186/s12929-021-00736-4 [Crossref] [ Google Scholar]
- Wei W, Ao Q, Wang X, Cao Y, Liu Y, Zheng SG. Mesenchymal stem cell-derived exosomes: a promising biological tool in nanomedicine. Front Pharmacol 2020; 11:590470. doi: 10.3389/fphar.2020.590470 [Crossref] [ Google Scholar]
- Müller Bark J, Kulasinghe A, Chua B, Day BW, Punyadeera C. Circulating biomarkers in patients with glioblastoma. Br J Cancer 2020; 122(3):295-305. doi: 10.1038/s41416-019-0603-6 [Crossref] [ Google Scholar]
- Chen C, Zhang Z, Gu X, Sheng X, Xiao L, Wang X. Exosomes: new regulators of reproductive development. Mater Today Bio 2023; 19:100608. doi: 10.1016/j.mtbio.2023.100608 [Crossref] [ Google Scholar]
- Rezaie J, Feghhi M, Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun Signal 2022; 20(1):145. doi: 10.1186/s12964-022-00959-4 [Crossref] [ Google Scholar]
- Zhou Y, Dong Y, Zhang A, Wu J, Sun Q. The role of mesenchymal stem cells derived exosomes as a novel nanobiotechnology target in the diagnosis and treatment of cancer. Front BioengBiotechnol 2023; 11:1214190. doi: 10.3389/fbioe.2023.1214190 [Crossref] [ Google Scholar]
- Lone SN, Nisar S, Masoodi T, Singh M, Rizwan A, Hashem S. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol Cancer 2022; 21(1):79. doi: 10.1186/s12943-022-01543-7 [Crossref] [ Google Scholar]
- Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 2019; 8(4):307. doi: 10.3390/cells8040307 [Crossref] [ Google Scholar]
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020; 367(6478):eaau6977. doi: 10.1126/science.aau6977 [Crossref] [ Google Scholar]
- Hussen BM, Faraj GS, Rasul MF, Hidayat HJ, Salihi A, Baniahmad A. Strategies to overcome the main challenges of the use of exosomes as drug carrier for cancer therapy. Cancer Cell Int 2022; 22(1):323. doi: 10.1186/s12935-022-02743-3 [Crossref] [ Google Scholar]
- Sadeghi S, Ramezani Tehrani F, Tahmasebi S, Shafiee A, Hashemi SM. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology 2023; 31(1):145-69. doi: 10.1007/s10787-022-01115-7 [Crossref] [ Google Scholar]
- Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal 2021; 19(1):47. doi: 10.1186/s12964-021-00730-1 [Crossref] [ Google Scholar]
- Lin Z, Wu Y, Xu Y, Li G, Li Z, Liu T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: recent advances and therapeutic potential. Mol Cancer 2022; 21(1):179. doi: 10.1186/s12943-022-01650-5 [Crossref] [ Google Scholar]
- Harrell CR, Jovicic N, Djonov V, Volarevic V. Therapeutic use of mesenchymal stem cell-derived exosomes: from basic science to clinics. Pharmaceutics 2020; 12(5):474. doi: 10.3390/pharmaceutics12050474 [Crossref] [ Google Scholar]
- Zhai Z, Cui T, Chen J, Mao X, Zhang T. Advancements in engineered mesenchymal stem cell exosomes for chronic lung disease treatment. J Transl Med 2023; 21(1):895. doi: 10.1186/s12967-023-04729-9 [Crossref] [ Google Scholar]
- Deng D, Li X, Zhang JJ, Yin Y, Tian Y, Gan D. Biotin-avidin system-based delivery enhances the therapeutic performance of MSC-derived exosomes. ACS Nano 2023; 17(9):8530-50. doi: 10.1021/acsnano.3c00839 [Crossref] [ Google Scholar]
- Tzng E, Bayardo N, Yang PC. Current challenges surrounding exosome treatments. Extracell Vesicle 2023; 2:100023. doi: 10.1016/j.vesic.2023.100023 [Crossref] [ Google Scholar]