Advanced pharmaceutical bulletin. 15(2):268-283.
doi: 10.34172/apb.025.45122
Systematic Review
Therapeutic Potential of Endothelial Progenitor Cells in Angiogenesis and Cardiac Regeneration: A Systematic Review and Meta-Analysis of Rodent Models
Samaneh Narimani Data curation, Investigation, Writing – original draft, 1 
Reza Rahbarghazi Conceptualization, Data curation, Funding acquisition, Resources, Supervision, Writing – review & editing, 1, 2, * 
Hanieh Salehipourmehr Data curation, Formal analysis, Software, Validation, Visualization, 3
Maryam Taghavi Narmi Investigation, Writing – original draft, 2
Hamid Lotfimehr Investigation, Writing – original draft, 2
Robab Mehdipour Investigation, Methodology, Project administration, Software, Writing – original draft, 3
Author information:
1Department of Applied Cell Sciences, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
2Stem Cell Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Research Center for Evidence-Based Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Purpose:
Myocardial infarction (MI), the leading cause of human mortality, is induced by a sudden interruption of blood supply. Among various stem cell types, endothelial progenitor cells (EPCs) are novel and valid cell sources for the restoration of vascularization in the ischemic tissue. The present study aimed to evaluate the regenerative properties of EPCs in rodent models of MI.
Methods:
A comprehensive systematic search was implemented in Cochrane Library, Embase, PubMed, Scopus, and Web of Science databases without language limitation in Sep 2024. Of the 67 papers pooled, 42 met the inclusion criteria and were subjected to multiple analyses.
Results:
Compared to the MI group, the overall effect size was confirmed in the groups receiving EPC with enhanced angiogenesis (SMD: 2.02, CI 95%: 1.51-2.54, P<0.00001; I2: 82%), reduced fibrosis (SMD: -1.48; 95% CI−2.15, -0.81; P<0.0001; I2: 88%), improved ejection fraction (EF; SMD: 1.72; 95% CI−1.21, 2.23; P<0.00001; I2: 87%), and fractional shortening (FS; SMD: 1.58; 95% CI−1.13, 2.03; P<0.00001; I2: 82%). Data confirmed significant improvements in the cardiac tissue parameters after intramyocardial injection of EPCs.
Conclusion:
These data showed that EPC transplantation is an alternative therapy to ameliorate ischemic myocardium in rodents via the stimulation of angiogenesis, reduction of fibrosis, and improvement of fractional shortening and ejection fraction.
Keywords: Endothelial progenitor cells, Myocardial infarction, Rodents, Regenerative outcomes
Copyright and License Information
© 2025 The Author (s).
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Funding Statement
Research reported in this publication was supported by the Elite Researcher Grant Committee under award number (IR.NIMAD.REC.1402.031) and grant No. of 4010084 from the National Institute for Medical Research Development (NIMAD), Tehran, Iran, and Tabriz University of Medical Sciences (IR.TBZMED.VCR.REC.1402.198).
Introduction
Ischemic heart disease (IHD) is a global leading cause of human mortality and disability in the clinical setting.1 Typically, myocardial infarction (MI) occurs following partial or complete occlusion of a coronary artery leading to massive cardiomyocyte damage, inflammation, and subsequent fibrotic changes.2 Notably, the contraction of fibroblasts and collagen fibers at the healing site can contribute to the thinning of the left ventricle (LV). Over time, the reduction of ejection fraction (EF) and lethal arrhythmias in an ischemic heart can be life-threatening.3 Currently, percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) are clinical modalities for the restoration of blood and reduction of cardiomyocyte injury.4 Unfortunately, these approaches are not fully effective, and the development and application of de novo therapeutic strategies are highly recommended.5
In recent decades, the discovery and application of stem cells in various pathological conditions have revolutionized regenerative medicine.6 It has been shown that stem cells can promote the healing of ischemic myocardium via the release of cytokines, growth factors, and direct differentiation into cardiomyocytes.6,7 Besides, these cells can accelerate the regeneration of injured myocardium via juxtacrine interaction and production of pro-angiogenesis factors.8,9
According to recent data, it has been confirmed that endothelial progenitor cells (EPCs) are valid cell sources for restoring dysfunctional endothelium via various reparative functions, especially promoting angiogenesis and vasculogenesis.10 In this regard, EPCs alone or in combination with other stem cells or mature cells have been used in different studies to accelerate regenerative outcomes and circumvent limitations associated with the administration of single stem cell type alone.11,12 Proteomic analyses have proved the existence of common specific surface molecules such as CD133, CD34, vascular endothelial growth factor-2 (VEGFR-2), Tie-2, and Sca-1 between EPCs and hematopoietic stem cells.13 Following various pathologies and hypoxic conditions, EPCs are recruited from the bone marrow niche, the primary storage site in adults, to the circulation system.14 Circulating EPCs migrate toward the injury sites in a cytokine gradient manner where they gradually lose their stemness features (CD133↓, and CD34↓) and mature into endothelial cells (ECs; CD31↑ and vWF↑).15 Besides differentiation capacity, EPCs release several proangiogenesis factors (IGF-1, VEGF, HGF, FGF-2, etc.) to expedite the formation of new blood vessels in the hypoxic areas.16 Data have indicated that the injection of EPCs in several animal models of MI can improve the healing of myocardium through the stimulation of angiogenesis, regulation of inflammation, and control of extracellular matrix (ECM) remodeling.12
In the present systematic review, the application of EPCs in the rodent model of MI and their potential in the restoration of injured myocardium mainly via angiogenesis was explored. To the best of our knowledge, there are few reports related to systematic review and metanalysis of EPCs in humans and different animal models of MI. Most of the studies have investigated the diagnostic properties of EPCs under certain pathological conditions such as ischemic diseases in humans or there are several reposts related to separate applications of EPCs in certain MI models in animals.17-19 Although the reparative properties of EPCs have been proved in different MI animal models, it is imperative that data from various experiments with similar objectives be combined and assessed to minimize the possible bias and make logic in the interpretation of the obtained data.20 In the last decades, rodents have been widely used for different experiments related to the MI model due to inherent advantages like small body mass and easy handling pre- and post-MI induction with minimal space and resources. Besides, researchers can have access to various rodents with similar genetic characteristics which facilitates high repeatability.21 It seems that data from this study can provide invaluable data about the eligibility of EPC application in the alleviation of MI in the clinical setting.
Material and Methods
The current systematic review and meta-analysis were conducted based on the PRISMA 2020 statement guideline. The used protocol was registered in the PROSPERO database (CRD42024571517).
Search strategy
A comprehensive systematic search was implemented in Cochrane Library, Embase, PubMed, Scopus, and Web of Science databases without the limitations of language and date in Sep 2024. After the completion of the systematic search, collected articles, experiments, and contacted authors were carefully monitored and validated for subsequent evaluations. The abstracts from the international congresses were also monitored. The strategy used in this study is shown in Table S1 (see Supplementary file 1).
Study design considerations
All preclinical studies associated with the application of EPCs in rodent models of MI, including mice and rats were reviewed. Rodents with experimentally induced MI in any age in both genders were included. EPCs transplantation in human counterparts, and other species (i.e., rabbits, porcine, canines, etc.), and in vitro experiments were excluded from the present analysis. Data related to the administration of EPCs alone, but not in combination with other stem cell types, were collected. Also, studies related to the use of EPC exosomes in rodent models of MI were not included. Articles with no access to their full texts were not considered. In Table 1, inclusion and exclusion criteria are outlined.
Table 1.
Inclusion and exclusion criteria
|
Inclusion criteria
|
Exclusion criteria
|
-
Preclinical studies about EPCs as therapy on rodent models (mice and rats), with cardiac infarction in any age or gender
-
Endothelial progenitor cells
-
Studies including the combination therapy with EPC such as scaffold, miRNA, growth factors, and other type of stem cells
-
Studies with CD34 + cells transplantation
-
All experimental studies (preclinical)
|
-
Not an animal study
-
Other animal study rather than rodents
-
Not a myocardial infarction model
-
Clinical studies on humans
-
In vitro studies
-
Other types of stem cells
-
Studies with CD133 + cells transplantation
-
Studies including combination therapy with EPC and other types of stem cells
-
Not transplantation of EPC and just mobilization investigation
-
EPCs-derived exosome transplantation
-
Other study type
-
In vitro studies
-
Studies without any access to the full text, or studies in the other languages, and retracted studies
|
The primary outcome indicators were “angiogenesis”, and “infarct size”. The secondary outcome indicators were “LVEF”, and “fractional shortening (FS)”. For the meta-analysis, the data containing at least one of the outcomes measured between 1- and 8 weeks post-EPC transplantation were used. If studies contained more than one set of data for primary or secondary outcome analysis, the selection was done based on the more relevant and common data.
Study selection
Once the databases were searched for the relevant papers, all collected citations were uploaded to EndNote 18 software with duplicate studies being deleted. Two separate reviewers blindly screened the titles and abstracts to ensure the eligibility of the studies in terms of the inclusion and exclusion criteria. Any discrepancy was re-checked again by a third blind reviewer.
Data collection
The collected data from multiple search databases were organized using PRISMA guidelines. For this purpose, articles were entered into an Excel spreadsheet. The process was continued by an independent review of the selected abstracts by the same reviewers. Any disagreements were critically assessed until a precise decision was made and the opinion of a third reviewer was obtained if it was required.
Evaluation of methodological quality
Using the modified CAMARADES checklist, two independent reviewers monitored the methodological validity of the quantitative publications selected for retrieval before their inclusion in the systematic review. Again, any disagreements were resolved through consultation with a third reviewer.
Statistical analysis
The results of the selected data were analyzed using RevMan 5.4.1. Data are presented as mean ± SD with a 95% confidence interval (CI). Statistical heterogeneity was analyzed using the I2 value and the chi-square test. In this study, P < 0.05 and I2 > 50% were considered statistical heterogeneity. Fixed and mixed models were used for low and high heterogeneity in the parameters analysis. The subgroup analysis was performed if needed. Publication bias was assessed using funnel plots and more formally with both Begg and Mazumdar’s rank correlation test (Kendall’s tau) and Egger’s regression test. Begg’s test assesses the correlation between the effect estimates and their variances, while Egger’s test examines the relationship between the effect estimates and their standard errors. A P value of less than 0.05 was indicative of statistically significant publication bias.
Results
Description of studies and risk of bias
The flow chart for data selection and handling is presented in Figure 1. Here, a modified CAMARADES quality checklist was used to assess the collected experiments. Of all peer-reviewed articles, 67 declared compliances with animal welfare regulations. It is worth mentioning that random allocation to different groups was detected in 28 studies and 42 experiments expressed a conflict-of-interest statement. Furthermore, 6 articles had blinded induction of MI in the rodent models and 30 studies benefitted from both animal exclusion criteria and blind outcome assessment based on our evaluation. In the selected articles, no study declared the methodology related to sample size calculation (Figure 2). All articles were included for quality synthesis (Table S2; See Supplementary file 2).
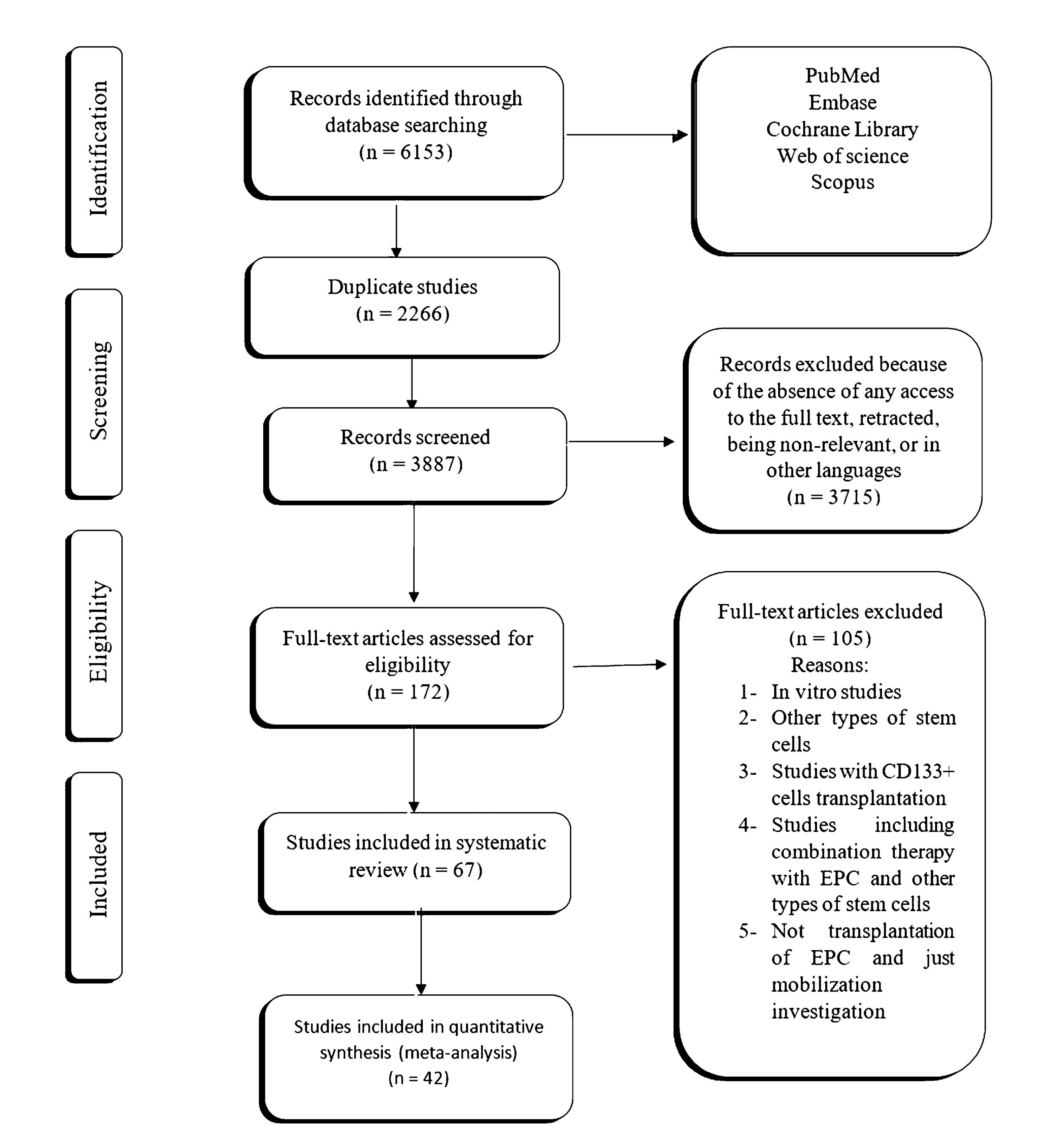
Figure 1.
PRISMA diagram of the review process for the meta-analysis
.
PRISMA diagram of the review process for the meta-analysis
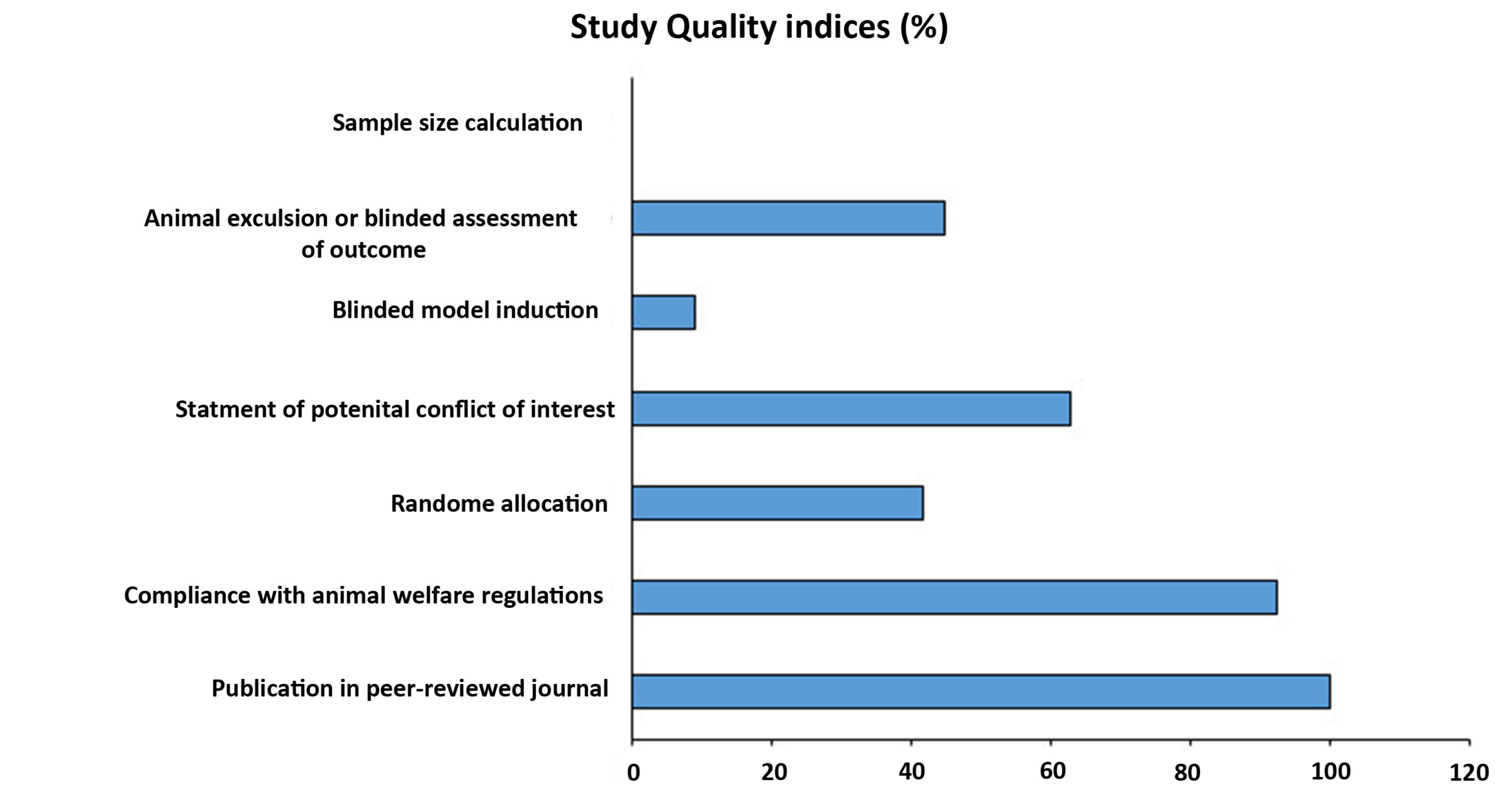
Figure 2.
Percentage of selected experiments for each item in the modified version of the CAMARADES (Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies) quality checklist
.
Percentage of selected experiments for each item in the modified version of the CAMARADES (Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies) quality checklist
Characteristics of studies
All the included studies from 2004 to 2021 with access to full text were selected. The systematic review focused on rodent models of MI consisting of rat (N = 37; 55.22%) and mouse (N = 30; 44.78%) models of MI (Table S2). Data indicated that a greater number of experiments were done on male rats/mice (N = 41; 61.19%), while 17 (25.37%) studies were conducted on female models Interestingly, in one study both genders were used. Rodents in 36 studies (53.73%) aged between 4 to 20 weeks. In 8 experiments (11.94%), the term “adult” was used to describe rodent age. In just one experiment (1.49%), “at least 9-week-old” rodents were used for the MI model. Rats and mice subjected to MI models weighed 80-350, and 18-250 grams, respectively. 58.21% of rats and mice were in healthy status (N = 39). Nude animals constituted 25.37% (N = 17) of the experiments. In 3 studies (4.48%), MI was inducted on diabetic models. Animals with severe combined immunodeficiency including NOD-SCID (N = 2; 2.99%), SCID (N = 1; 1.49%), and a combination of Nude/J or NOD-SCID (N = 1; 1.49%) were employed. Immunocompetent experimental models were 1.49% of collected studies (N = 1). In one study (1.49%), the models underwent ovariectomy together with splenectomy; while in one experiment just ovariectomy was conducted (1.49%). Experiments with both wild-type and IL-10 knockout models comprised 1.49% (N = 1) of the studies. Protocols consisting direct left anterior descending coronary artery (LAD) ligation (N = 64; 95.52%); injection of vitamin D3 in high-fat diet-fed rodents (N = 1; 1.49%), intramyocardial administration of microembolism suspension following the occlusion of the ascending aorta (N = 1; 1.49%), and LAD ligation followed by reperfusion besides aorta cross-clamping (N = 1; 1.49%) were used to induce experimental MI models. Based on the analysis, MI (N = 63; 94.03%), progressive MI to cardiomyopathy (N = 1; 1.49%), MI with ischemic reperfusion (N = 1; 1.49%), coronary artery microembolization (CME) (N = 1; 1.49%), and ICM (ischemic cardiomyopathy model) (N = 1; 1.49%) were pathological conditions in rodent models. In the selected articles, EPCs were collected from different sources as follows; Bone marrow (N = 32; 47.76%), peripheral blood (N = 19; 28.36%), umbilical cord blood (N = 11; 16.42%), direct cardio-puncture (N = 1; 1.49%), spleen (N = 1; 1.49%), dental pulp (N = 1; 1.49%), and both peripheral blood and bone marrow (N = 1; 1.49%). EPCs were administrated as doses between 2 × 102 and 2 × 107 in most of the experiments (N = 59; 88.06%). In contrast to studies using single EPC injection, 8 experiments (11.94%) were conducted based on multiple EPC administrations. Timing of EPC injection varied from immediate to delayed administration (until 4 weeks) following MI induction. Different introduction approaches and terms were found in different studies such as intramyocardial injection (N = 44; 65.67%), intravenous injection (N = 9; 13.43%), intramyocardial injection and subsequent treatment with the construct (N = 3; 4.48%), simultaneous intramyocardial and intracoronary injections (N = 2; 2.99%), injection into the LV (N = 2; 2.99%), transepicardial injection (N = 1; 1.49%), anterolateral LV surface suture (N = 1; 1.49%), implantation (N = 1; 1.49%), intracoronary injection (N = 1; 1.49%), percutaneously injection into LV (N = 1; 1.49%), injection to the border of occluded region (N = 1; 1.49%), and intramyocardial (intramuscular) or systemic injection (N = 1; 1.49%).
EPC transplantation effect on angiogenesis potential
A random-effects model was applied to find differences in angiogenesis potential in 32 eligible studies (Figure 3a; SMD: 2.02, 95% CI: 1.51-2.54, P < 0.00001; I2: 82%). The subgroup analysis of EPC injection in different time points (1, 2, 3, 4, 6, and 8) indicated an improved angiogenesis potential after MI induction. Of note, these changes reached statistically significant levels post EPC injection after one week (SMD: 1.29, 95% CI: 0.27-2.31, P = 0.01; I2: 0%; N = 2), two weeks (SMD: 2.61, 95% CI: 1.95-3.27, P < 0.00001; I2: 0%; N = 4), four (SMD: 1.72, 95% CI: 1.19-2.26, P < 0.00001; I2: 73%; N = 18), and eight weeks (SMD: 5.98, 95% CI: 0.25-11.70, P = 0.04; I2: 89%; N = 2). The other features were not statistically significant compared to the control group.
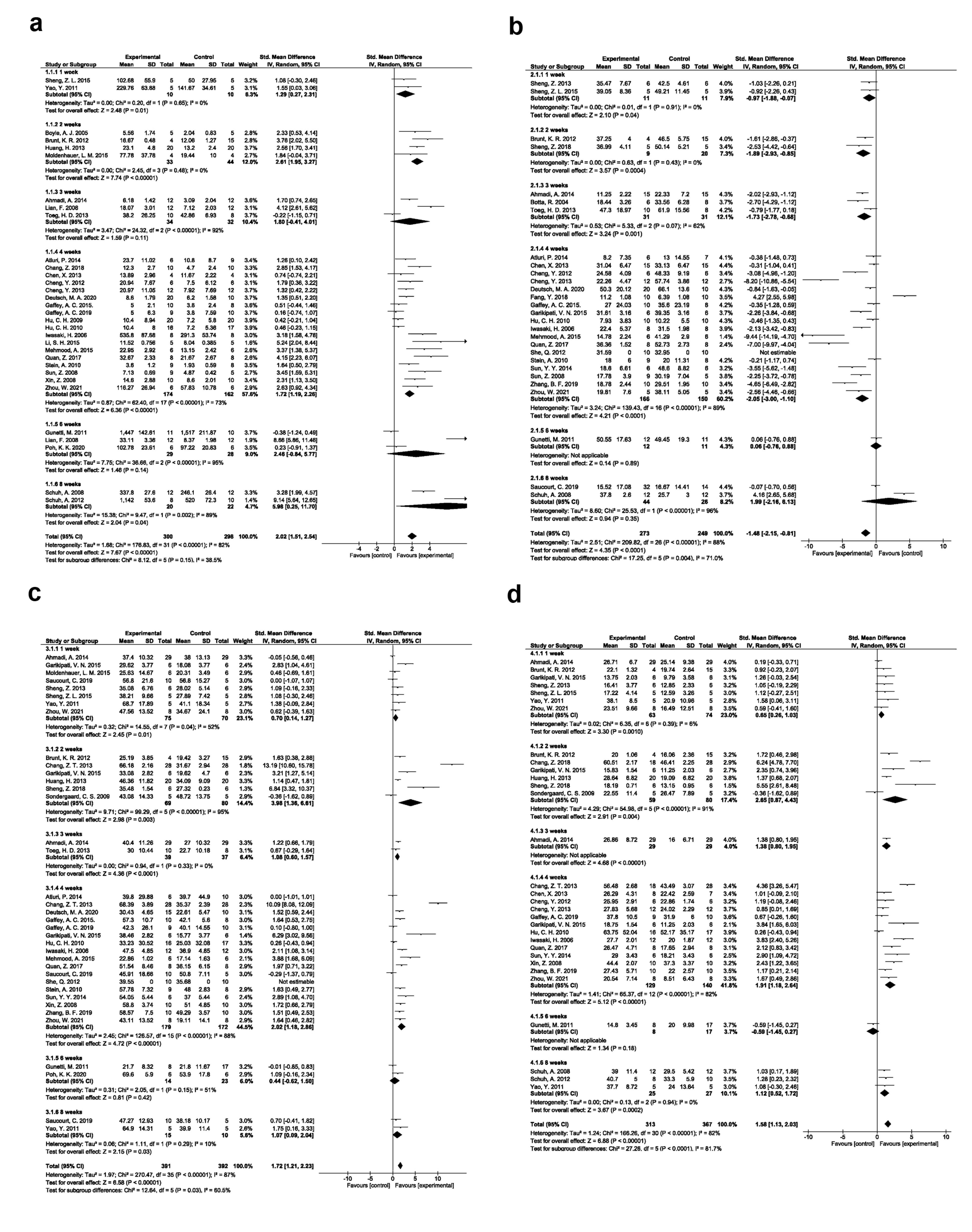
Figure 3.
Angiogenesis improvement based on the time of results assessment (a). Fibrosis improvement based on the time of results assessment (b). Ejection fraction improvement based on the time of results assessment (c). Fractional shortening improvement based on the time of results assessment (d)
.
Angiogenesis improvement based on the time of results assessment (a). Fibrosis improvement based on the time of results assessment (b). Ejection fraction improvement based on the time of results assessment (c). Fractional shortening improvement based on the time of results assessment (d)
EPC transplantation effect on myocardial fibrosis
Data confirmed the reduction of myocardial fibrosis in 28 studies after EPC transplantation compared to the control group (SMD: -1.48; 95% CI − 2.15, -0.81; P < 0.0001; I2: 88%). Subgroup analysis revealed significant differences of post-EPC administration after one week (SMD: -0.97; 95% CI − 1.88, -0.07; P-0.04; I2: 0%; N = 2), two weeks (SMD: − 1.89; 95% CI − 2.93, -0.85; P = 0.0004; I2: 0%; N = 2), three weeks (SMD: − 1.73; 95% CI − 2.78, -0.68; P = 0.001; I2: 62%; N = 3), and four weeks (SMD: -2.05; 95% CI, − 3.00, -1.10; P < 0.0001; I2: 89%; N = 18) (Figure 3b).
EPC transplantation effect on cardiac ejection fraction
Random-effects model for differences in LVEF values is shown in Figure 3c. Data showed the efficiency of EPC transplantation in the improvement of LVEF after one week (SMD: 0.70; 95% CI − 0.14, 1.27; P = 0.01; I2: 52%; N = 8), two weeks (SMD: 3.98; 95% CI 1.36, 6.61; P= 0.003; I2: 95%; N = 6), three weeks (SMD: 1.08; 95% CI 0.60, 1.57; P < 0.0001; I2: 0%; N = 2), four weeks (SMD: 2.02; 95% CI 1.18, 2.86; P < 0.00001; I2: 88%; N = 17), and eight weeks (SMD: 1.07; 95% CI 0.09, 2.04; P = 0.03; I2: 10%; N = 2) compared to the control group. Despite these results, two experiments reported the lack of statistically significant differences in LVEF parameters after 6 weeks post-EPC administration between the control and EPC groups.
EPC transplantation effect on cardiac FS
Data obtained from a random-effects model indicated significant differences in cardiac FS following EPC therapy in rodent models of MI. To be specific, statistically significant differences were found in FS parameter after one week (SMD: 0.65; 95% CI 0.26, 1.03; P = 0.0010; I2: 6%; N = 7), two weeks (SMD: 2.65; 95% CI 0.87, 4.43; P = 0.004; I2: 91%; N = 6), four weeks (SMD: 1.91; 95% CI 1.18, 2.64; P < 0.00001; I2: 82%; N = 13), and eight weeks (SMD: 1.12; 95% CI 0.52, 1.72; P= 0.0002; I2: 0%; N = 3) in EPC group as compared with the control group (Figure 3d).
Different EPC injection approaches
The regenerative efficacy of the EPC injection route was also assessed in rodent MI models. Intramyocardial route is the commonly used approach for the introduction of EPCs into the ischemic myocardium with the angiogenesis potential (SMD 1.91, 95% CI- 1.39-2.43, P < 0.00001, I2: 80%; N = 27; Figure 4a); reduction of fibrosis (SMD -1.16, 95% CI- -1.96, -0.36, P = 0.004, I2: 90%; N = 25; Figure 4b); improving EF (SMD:1.53, 95% CI- 0.92-2.15, P < 0.00001, I2: 86%; N = 24; Figure 4c), and FS values (SMD:1.58, 95% CI- 1.04-2.12, P < 0.00001, I2: 80%; N = 21; Figure 4d).
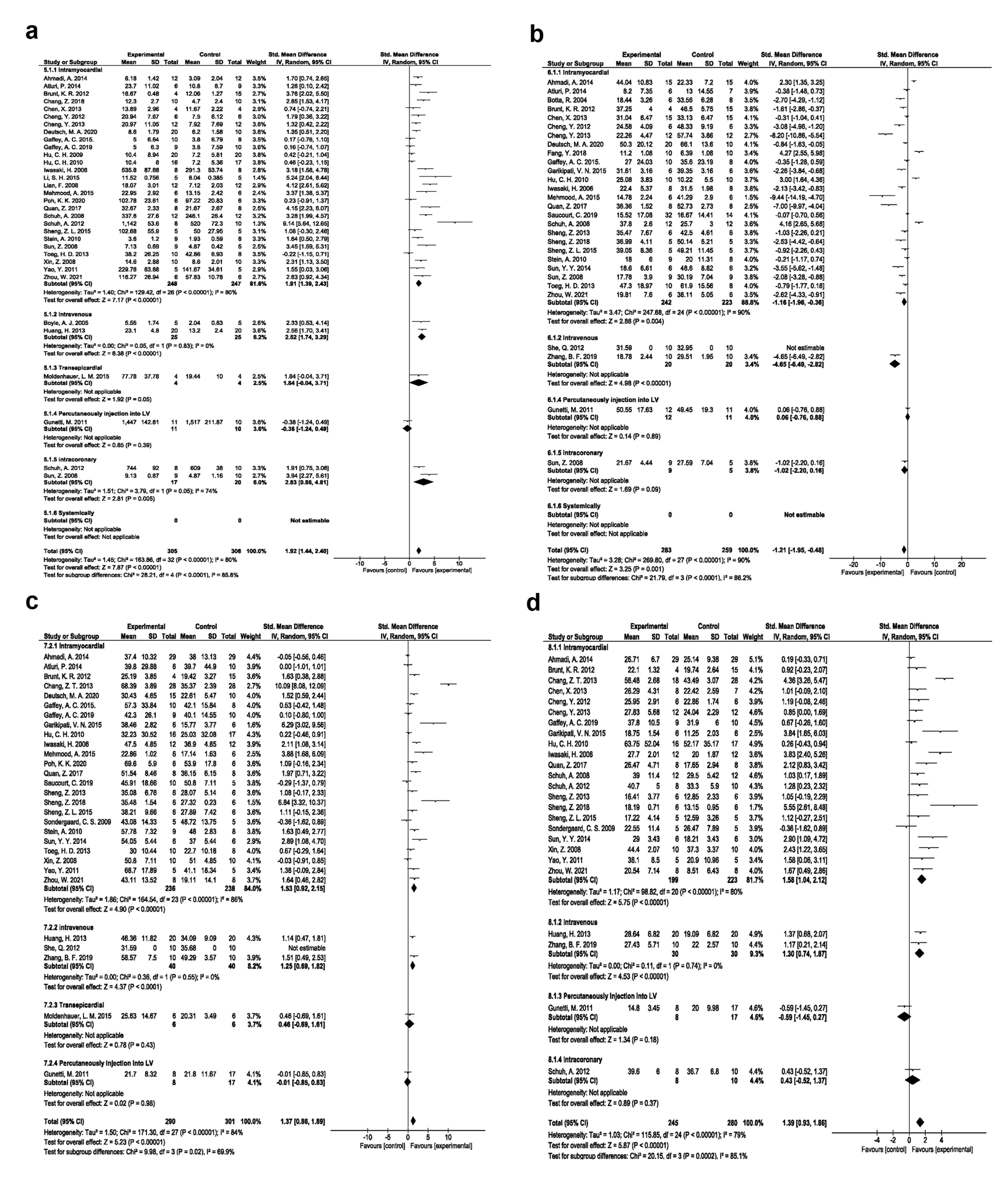
Figure 4.
Angiogenesis improvement based on injection method (a). Fibrosis improvement based on injection method (b). Ejection fraction improvement based on injection method (c). Fractional shortening improvement based on injection method (d)
.
Angiogenesis improvement based on injection method (a). Fibrosis improvement based on injection method (b). Ejection fraction improvement based on injection method (c). Fractional shortening improvement based on injection method (d)
Various EPC doses
Based on EPC dose, studies were categorized into 5 groups as follows; up to 0.5 × 106, 0.5 to 1 × 106, 1 to 2 × 106, 2 to 5 × 106, and more than 5 × 106 groups. The weighted applied dose to EPC transplantation is dose 1 (up to 0.5 × 106), which demonstrated significant angiogenesis effects (SMD 7.16, 95% CI- 4.30-10.01, P < 0.00001, I2: 92%; N = 12; Figure 5a), reduced fibrosis (SMD -10.31, 95% CI- -18.72, -1.90, P = 0.02, I2: 98%; N = 15; Figure 5b), improved EF (SMD 11.33, 95% CI- 2.44-20.22, P = 0.01, I2: 98%; N = 11; Figure 5c), and FS (SMD 5.58, 95% CI- 2.80-8.37, P < 0.0001, I2: 92%; N = 11; Figure 5d) compared to the other doses.
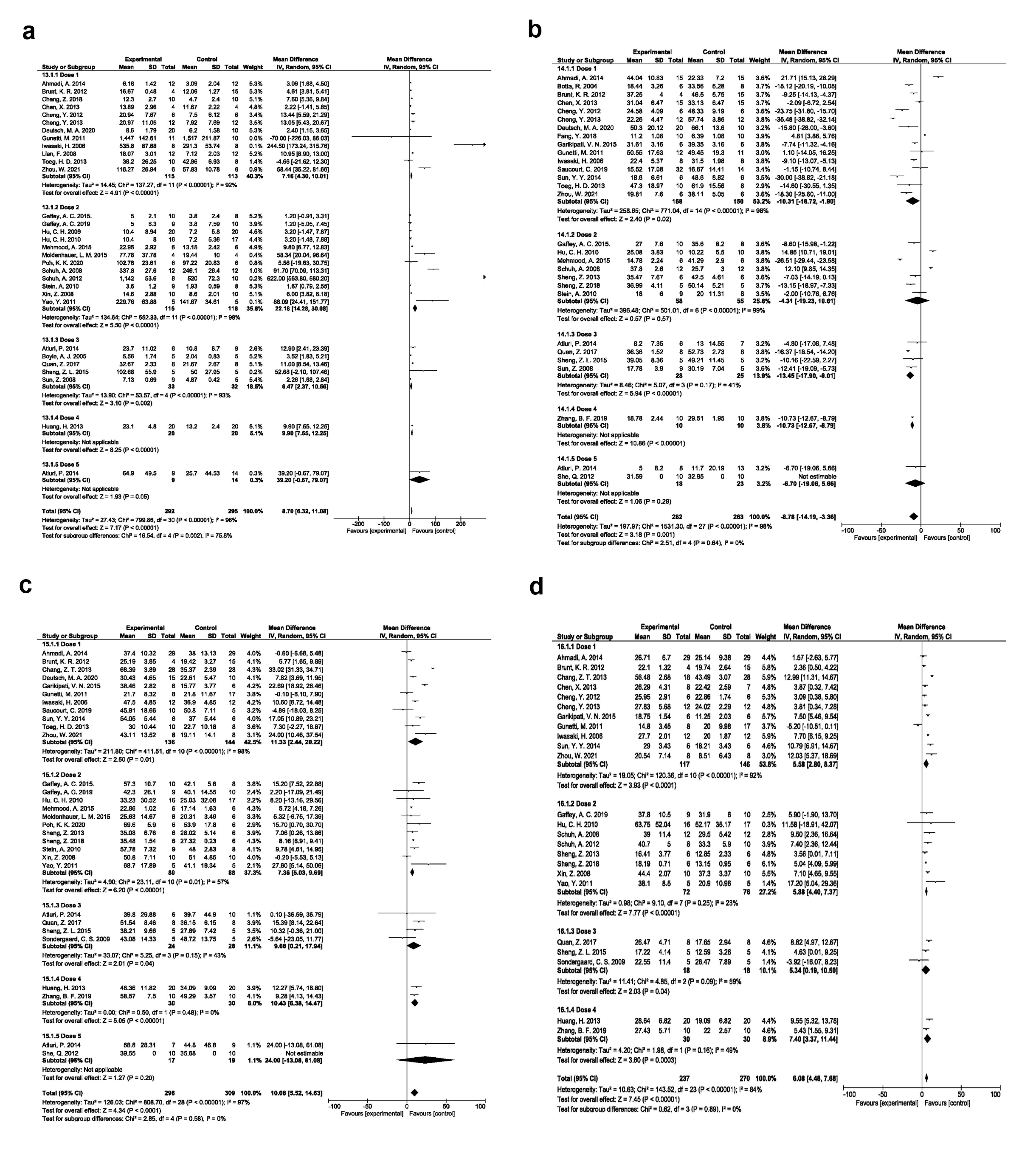
Figure 5.
Angiogenesis improvement based on EPC dose (a). Fibrosis improvement based on EPC dose (b). Ejection fraction improvement based on EPC dose (c). Fractional shortening improvement based on EPC dose (d)
.
Angiogenesis improvement based on EPC dose (a). Fibrosis improvement based on EPC dose (b). Ejection fraction improvement based on EPC dose (c). Fractional shortening improvement based on EPC dose (d)
Various EPC sources
Based on our data, it was confirmed that bone marrow EPCs exerted significant angiogenesis effects (SMD 6.88, 95% CI- 4.57-9.19, P < 0.00001, I2: 88%; N = 16; Figure 6a); reduced fibrosis (SMD -15.28, 95% CI- -20.40, -10.16, P < 0.00001, I2: 95%; N = 16; Figure 6b); improved EF (SMD:10.63, 95% CI- 7.53-13.73, P < 0.00001, I2: 86%; N = 17; Figure 6c), and FS (SMD: 6.93, 95% CI- 4.25-9.61, P < 0.00001, I2: 89%; N = 15; Figure 6d) in comparison with EPC types.
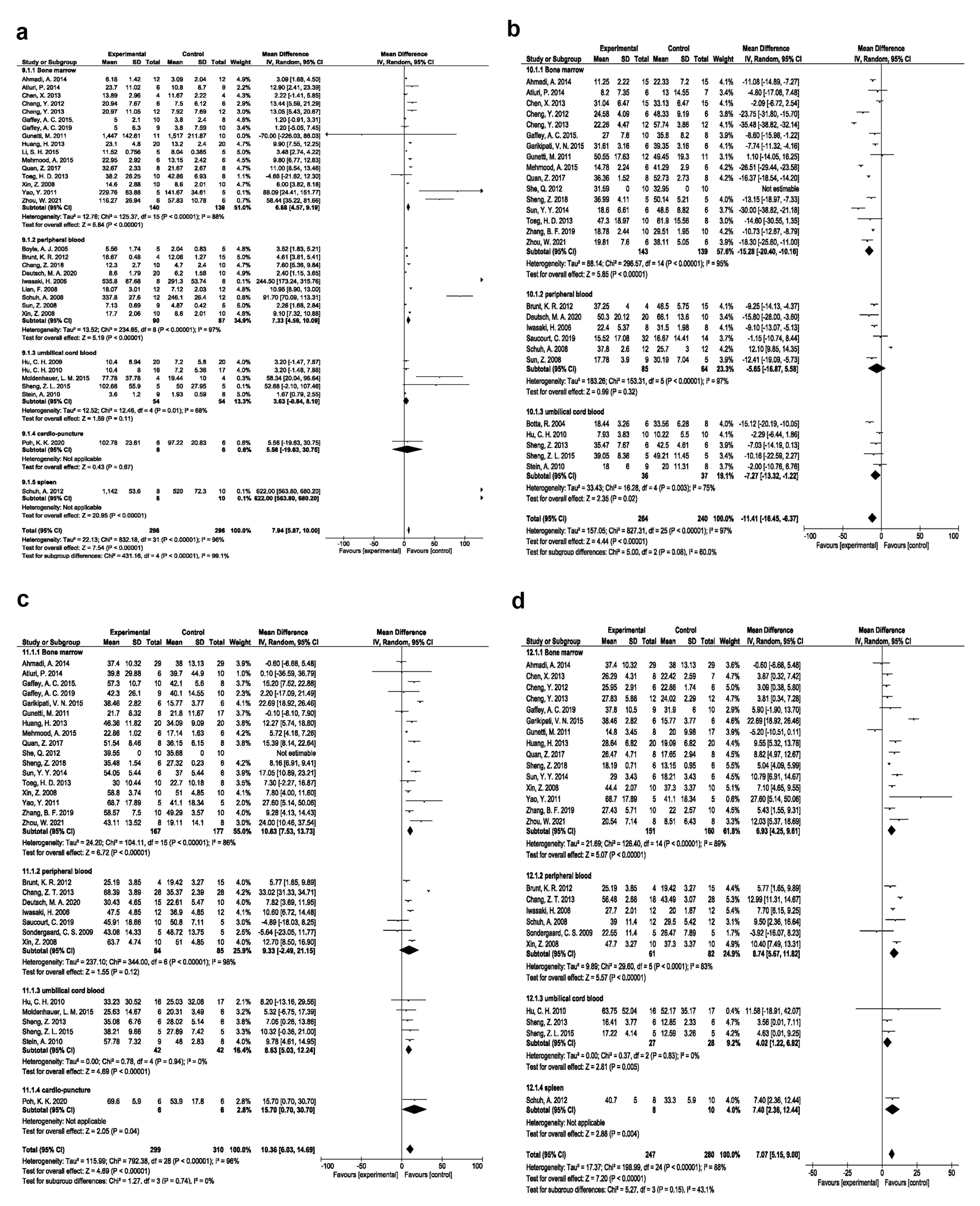
Figure 6.
Angiogenesis improvement based on EPC source (a). Fibrosis improvement based on EPC source (b). Ejection fraction improvement based on EPC source (c). Fractional shortening improvement based on EPC source (d)
.
Angiogenesis improvement based on EPC source (a). Fibrosis improvement based on EPC source (b). Ejection fraction improvement based on EPC source (c). Fractional shortening improvement based on EPC source (d)
Publication bias
Four funnel plots were developed using RevMan 5.4.1 to assess the publication bias among the selected experiments on each outcome (Figures 7a-d). For angiogenesis potential,Begg and Mazumdar’s test revealed Kendall’s tau of 0.546 (z = 4.395, P = 0.00001), and Egger’s regression test indicated a significant intercept of 5.79 (SE = 0.855, P = 0.00000). For anti-fibrosis properties,Begg and Mazumdar’s test yielded Kendall’s tau of -0.576 (z = 4.211, P = 0.00003) with Egger’s regression of -4.62 (SE = 1.407, P = 0.00300). In terms of EF,Kendall’s tau of 0.522 (z = 4.481, P = 0.00001) was obtained by Begg and Mazumdar’s test, and a noteworthy intercept of 5.43 (SE = 1.011, P = 0.00001) was evaluated by Egger’s regression test. Finally, in the FS parameter,Begg and Mazumdar’s test demonstrated Kendall’s tau of 0.460 (z = 3.637, P = 0.00028) and an intercept of 4.27 (SE = 1,113, P = 0.00062) after Egger’s regression test. These results suggest publication bias based on both the visual inspection of the funnel plot and the statistical tests in all outcomes.
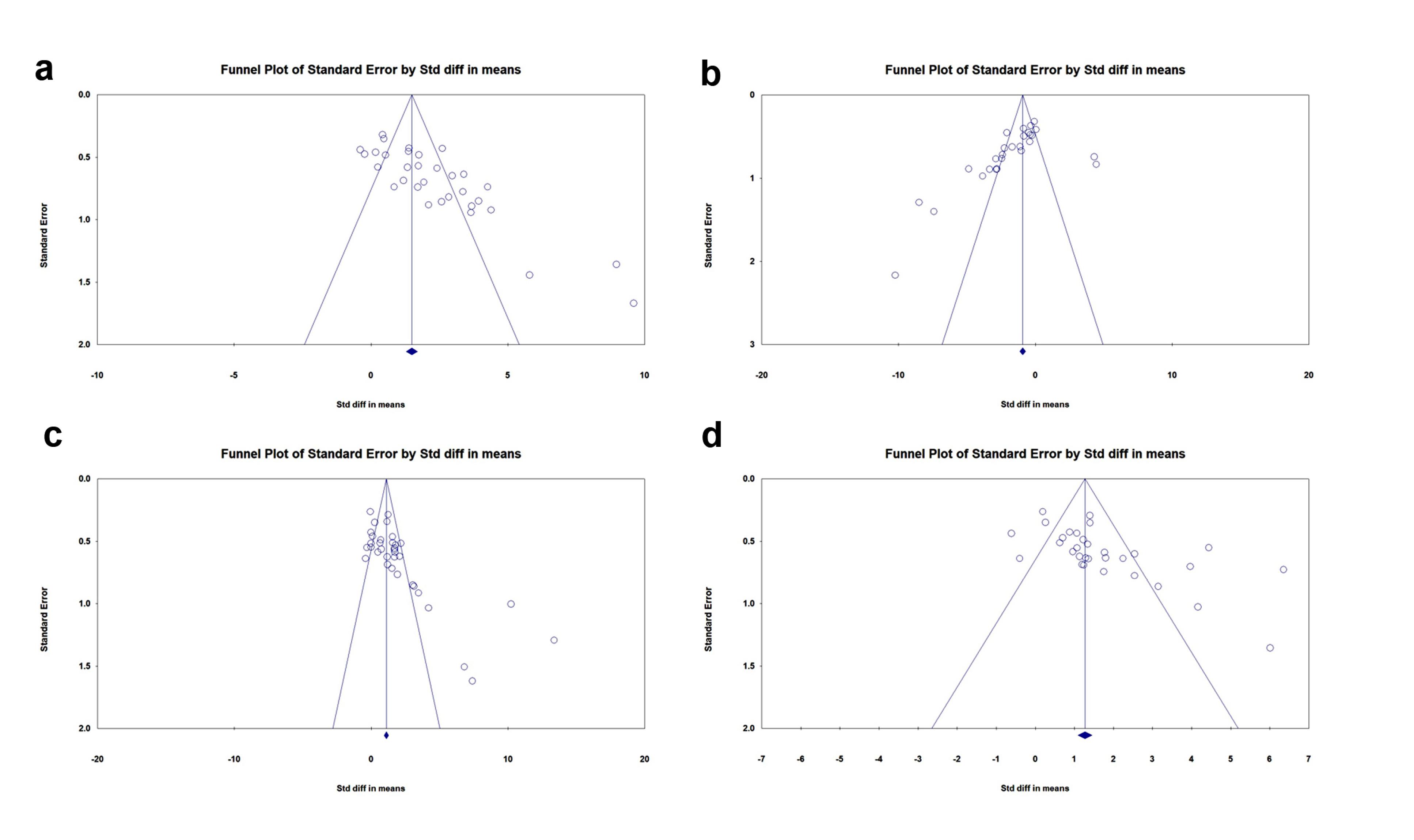
Figure 7.
Funnel plot of standard error by the standard difference. Angiogenesis (a); Fibrosis (b); Ejection fraction (c); and Fractional shortening (d)
.
Funnel plot of standard error by the standard difference. Angiogenesis (a); Fibrosis (b); Ejection fraction (c); and Fractional shortening (d)
Discussion
MI is a debilitating pathological condition with a high rate of mortality in societies.89 Therapeutic strategies targeting the increase of vascularization and blood perfusion are beneficial to alleviate the adverse effects of MI. In this regard, in-time blood vessel formation can significantly reduce scar formation, abnormal LV remodeling, and massive cardiomyocyte damage.90 Emerging in vitro, preclinical, and clinical data have indicated the potency of various stem cell types, especially EPCs, in the restoration of vascularization into the ischemic sites. It was suggested that both maturation into functional ECs, and the release of several proangiogenesis factors can expedite the process of healing in the ischemic sites.13 Of note, in vitro, ex vivo experiments, preclinical studies, and in silico analyses are required to evaluate the efficacy and safety of cells or drug candidates before application in the human counterpart.91 In this regard, the current systemic review and meta-analysis included preclinical experiments and aimed to explore the effectiveness of EPCs in rodent (rat and mouse) models of MI. Features such as angiogenesis, fibrosis, EF, and SF were monitored in MI animals following the administration of EPCs and compared to the control MI group.
The present data noted that EPC transplantation can influence primary outcomes such as angiogenesis and fibrosis in MI groups receiving only cell-free phosphate-buffered saline (PBS) or culture medium. Along with these changes, EPC administration led to improvements in cardiac function parameters, such as FS, and EF following MI induction. It has been assumed that several underlying molecular mechanisms are stimulated after the injection of EPCs into ischemic tissues.92 For example, EPCs are capable of ensuring cardiac tissue regeneration via the reduction of oxidative stress.93 Xue et al found that moderate-to-high doses of EPCs blunt the oxidative stress (8-iso-prostaglandin F2α↓, and SOD↑), and endoplasmic reticulum stress (GRP78 and CHOP) in a rat model of acute MI.94 Of course, prolonged exposure to insulting conditions contributes to the induction of oxidative stress in EPCs. Under such conditions, the function of EPCs and angiogenesis potential are fundamentally influenced. Hamed and co-workers found that diabetic circulating EPCs produce higher oxygen free radicals and exhibit higher SOD, NADPH oxidase activity with reduced NO bioavailability compared to normal EPCs.95 Therefore, attention should be given to the selection of appropriate EPCs to achieve optimal regenerative outcomes under varying pathological conditions.
It is hypothesized that direct physical contact between the EPCs and cardiac cells can stimulate several healing processes related to angiogenesis, ECM remodeling, and ventricular function.12 Multiple cell death modes such as cardiomyocyte apoptosis, excessive autophagic death, and necrosis are diminished following the administration of EPCs.96,97 Besides, EPCs exert anti-fibrotic properties through the modulation of the TGF-β signaling pathway and regulation of Smads.98 Of course, the regenerative potential of EPCs is not limited to the above-mentioned mechanisms, and these cells can affect the bioactivity of multiple cardiac cells in a paracrine and juxtacrine manner.99 For instance, the EPC secretome contains various signaling factors affecting the function of ECs after injury. In response to the EPC paracrine activity, the angiogenesis potential of ECs is promoted while simultaneously inflammatory damage is reduced in ECs.100 One possible explanation for this effect is that the EPC-derived extracellular vesicles harbor high levels of pro-angiogenesis factors, such as VEGF and miR-183, which have the potential to activate the biological activity of ECs at the site of injury.101 More interestingly, the differentiation of cardiac cells increases toward endothelial lineage once certain signaling pathways such as Shh are stimulated.12 Abd El Aziz et al found that intramyocardial transplantation of 5 × 106 human cord blood EPCs improves cardiac tissue function in a canine model of infarction via localization in the vascular units and direct differentiation into troponin I+ cardiomyocytes.102 The increase of endothelial nitric oxide synthetase and NO inside ECs is also associated with the paracrine activity of EPCs.103 Likewise, both superoxide dismutase and catalase stimulation and the expression of Bcl-2 increase EC resistance to oxidative stress juxtaposed to ischemic myocardium.12 Li et al found that shortly after ischemia induction in mice, donor EPCs can rapidly be recruited into the myocardium and elevate the local NO contents via the production of endothelial (eNOS) and inducible nitric oxide synthetase (iNOS).104 In line with this, Cristóvão and co-workers indicated lower CD34+/KDR+ EPC levels in ischemic cardiomyopathy patients compared to healthy counterparts, indicating fast and appropriate recruitment of EPCs in response to hypoxic/ischemic conditions.105
Data have confirmed that the direct juxtacrine activity of EPCs can promote neointima formation via the regulation of pericyte migration, secretion capacity, and phenotypic switching.106 Notably, EPCs can be genetically modified before transplantation to increase their regenerative potential.107 For instance, miR-214 expressing EPCs efficiently can control calcium hemostasis in stressed cardiomyocytes and enhance survival rate.12 Exosomal miR-1246 and miR-1290 driven EPCs upregulate ELF5 and SP1 in cardiac fibroblasts and increase endothelial differentiation.108
In addition to reducing fibrosis, the promotion of angiogenesis, activation of local cardiac progenitor cells, and increase in circulating progenitors within the infarcted myocardium collectively accelerate the healing process.109 Therefore, EPC administration appears to promote cardiac tissues through both endogenous and exogenous mechanisms.110
Recent data affirm that the administration route influences the healing capacity and regenerative outcomes by affecting the on-target delivery, stem cell survival rate, and bioactivities.111 According to the search we conducted, the direct intramyocardial injection yields better healing properties compared to the other administration routes. The systemic administration could lead to the sequestration of EPCs in certain tissues such as the liver, spleen, and lungs due to massive vascular beds while direct injection into the target tissues provides a higher delivery rate and retention time.112 Therefore, the homing of systemically administrated EPCs into the myocardium is less due to low retention time and certain anatomical features of cardiac tissue.113 Like intramyocardial injection, the intracoronary EPC infusion is considered to be widely administered. However, this modality requires higher cell volume compared to direct intramyocardial injection. It is worth remembering that the intracoronary route can increase the probability of cell clustering, and embolism, resulting in the occlusion of supporting blood vessels into the affected sites.114,115 Although intramyocardial injection ascertains higher cell delivery into the ischemic sites, this approach leads to the loss of a fraction of transplanted cells due to mechanical stress in solid organs such as cardiac tissue. Besides, iatrogenic inflammation and secondary tissue injuries can also occur when the cells are directly administrated into the myocardium.116 Like transepicardial and intracoronary routes, the intramyocardial injection essentially requires thoracotomies, which is an invasive surgical approach and cannot be performed when multiple cell doses are required.117 Despite the low targeting efficiency of EPC therapy via the systemic route, this approach is suitable for multiple-dose injection purposes.110,117 Using special advanced technologies such as ultrasound-guided percutaneous injection, the high cell doses can be directly delivered into different parts of LV in a relatively non-invasive manner. To standardize this approach with minimum side effects, various studies must be conducted
The statistically significant results of Egger’s and Begg’s tests suggest the possibility of publication bias, implying that studies with statistically significant results may be more likely to be published than studies with null or negative findings. This could lead to an overestimation of the true effect size. Therefore, the results of this meta-analysis should be interpreted with caution. Future research, including studies with negative or null findings, would be valuable to clarify the true effect of EPCs in the restoration of cardiac function following experimentally induced MI in rodents.
This study has several limitations and future experiments should address them as much as possible. Even though this study made an effort to synthesize the available evidence rigorously, the high heterogeneity observed for most outcomes (I2 > 80%) suggests considerable variability between the included studies. Despite the conduction of subgroup analyses, it was not feasible to fully explore the potential sources of this heterogeneity due to limitations in the reported data of the original publications. Due to these features, it was not possible to draw firm conclusions about the specific factors influencing the effectiveness of EPC therapy. In addition, a small sample size related to some parameters would make the interpretation problematic. These limitations highlight the necessity of further experiments to address the gaps and flaws. Specifically, future studies should report detailed data in a more standardized and comprehensive manner in terms of EPC source, dosage, administration route, experimental conditions, and relevant outcome measures.
The micro-, and microanatomy structure of cardiac tissue and its kinetics profoundly vary in rodents compared to their human counterparts. It is estimated that rodents have high heart rates and short lifespans. Meanwhile, the expression of genes and factors in cardiac cells can in part but not completely differ as compared to the other mammals.21 For instance, alpha isoform is the dominant type of myosin heavy chain in humans and large mammals atrium while this protein type is highly expressed in ventricles of mice and rats.21 The prominent difference in cardiac tissue kinetics and parameters can lead to relatively incomparable outcomes in rodents receiving stem cells and progenitors compared to large-size mammal models and humans.118 EPCs display high similarity with other cell lineages such as hematopoietic stem cells, thus the precise characterization, isolation, and purification of EPCs seem problematic. Besides, EPCs constitute 0.01 to 0.0001% of total bone marrow mononuclear cells, and in vitro expansion using different growth factors and supporting ECM components are necessary to yield EPCs in high quantities.13,119 Regarding the limited number of EPCs in freshly collected samples, serial passages and prolonged culture time can contribute to the loss of EPC phenotype and functionality.120 Although cryopreservation in part preserves the phenotype and biological activity, attention should be given to optimizing the cryopreservation protocols using suitable cryoprotectants to minimize the adverse effects of storage temperature.121 Based on the recent data, EPC type and maturation stage can influence the angiogenesis outcomes. Sieveking and co-workers found that later outgrowth EPCs can directly participate in the structure of vascular units better than that of early EPCs. It seems that early EPCs can promote the angiogenesis phenomenon indirectly via the release of angiogenesis factors at the site of injury.122 The mobilization of EPCs in response to cytokine gradient increases simultaneous maturation and functional activity compared to the resident progenitors inside the bone marrow niche.13 The circulating EPCs can lose their stemness features (CD133↓, and CD34↓) accompanied with the expression of certain markers such as CD31, and vWF with the reaching to the injured site.13 These data confirm that bone marrow EPCs are putative progenitor cells in the induction of angiogenesis in the ischemic regions. Besides cell source, the number of graft stem cells can predetermine the angiogenesis outcomes, especially in tissue with chronic injuries. However, less and excessive stem cells can cause the disruption of the healing process via an imbalance in immune cell activity and normal development of resident cells and transplanted stem cells.123 Taken together, the number and source of EPCs can be effective in the induction of angiogenesis in the ischemic myocardium.
Conclusion
The current systemic review and meta-analysis showed the eligibility of EPCs in the restoration of cardiac function following experimentally induced MI rodents, either rats or mice. The stimulation of angiogenesis and reduction of fibrosis along with the improvement of cardiac functional parameters (EF, and FS) are the main outcomes following EPC transplantation. Taken together, the current data provide new insights into the potential clinical application of EPCs and their regenerative properties in patients with MI.
Competing Interests
The authors have declared that no competing interests exist.
Ethical Approval
All phases of this study were approved by local ethics committee of Tabriz University of Medical Sciences (ethical code: IR.TBZMED.VCR.REC.1402.198 Approval date: 2023-10-16) and NIMAD (National Institute for Medical Research Development) (ethical code: IR.NIMAD.REC.1402.031; Approval date: 2023-12-30) under research proposal entitled “Application of endothelial progenitor cells in the alleviation of cardiac infarction in rodent models”.
Supplementary File
Supplementary file contains Table S1 and S2.
(pdf)
Acknowledgements
We thank Dr. Solmaz Saghebasl, Miss Saba Habibi, Ms. Narges Mardi, Dr. Fatemeh Sadeghsoltani, and Dr. Afshin Rahbarghazi for helping us with data extraction. The authors declare that artificial intelligence is not used in this study.
References
- Tsao CW, Aday AW, Almarzooq ZI, Anderson CA, Arora P, Avery CL. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation 2023; 147(8):e93-621. doi: 10.1161/cir.0000000000001123 [Crossref] [ Google Scholar]
- Brazile BL, Butler JR, Patnaik SS, Claude A, Prabhu R, Williams LN. Biomechanical properties of acellular scar ECM during the acute to chronic stages of myocardial infarction. J Mech Behav Biomed Mater 2021; 116:104342. doi: 10.1016/j.jmbbm.2021.104342 [Crossref] [ Google Scholar]
- Leancă SA, Crișu D, Petriș AO, Afrăsânie I, Genes A, Costache AD. Left ventricular remodeling after myocardial infarction: from physiopathology to treatment. Life (Basel) 2022; 12(8):1111. doi: 10.3390/life12081111 [Crossref] [ Google Scholar]
- Hassanpour P, Sadeghsoltani F, Haiaty S, Zakeri Z, Saghebasl S, Izadpanah M. Mitochondria-loaded alginate-based hydrogel accelerated angiogenesis in a rat model of acute myocardial infarction. Int J Biol Macromol 2024; 260(Pt 2):129633. doi: 10.1016/j.ijbiomac.2024.129633 [Crossref] [ Google Scholar]
- Doenst T, Haverich A, Serruys P, Bonow RO, Kappetein P, Falk V. PCI and CABG for treating stable coronary artery disease: JACC review topic of the week. J Am Coll Cardiol 2019; 73(8):964-76. doi: 10.1016/j.jacc.2018.11.053 [Crossref] [ Google Scholar]
- Yuan HL, Chang L, Fan WW, Liu X, Li Q, Tian C. Application and challenges of stem cells in cardiovascular aging. Regen Ther 2024; 25:1-9. doi: 10.1016/j.reth.2023.11.009 [Crossref] [ Google Scholar]
- Kawaguchi N, Nakanishi T. Stem cell studies in cardiovascular biology and medicine: a possible key role of macrophages. Biology (Basel) 2022; 11(1):122. doi: 10.3390/biology11010122 [Crossref] [ Google Scholar]
- Sid-Otmane C, Perrault LP, Ly HQ. Mesenchymal stem cell mediates cardiac repair through autocrine, paracrine and endocrine axes. J Transl Med 2020; 18(1):336. doi: 10.1186/s12967-020-02504-8 [Crossref] [ Google Scholar]
- Rahbarghazi R, Nassiri SM, Khazraiinia P, Kajbafzadeh AM, Ahmadi SH, Mohammadi E. Juxtacrine and paracrine interactions of rat marrow-derived mesenchymal stem cells, muscle-derived satellite cells, and neonatal cardiomyocytes with endothelial cells in angiogenesis dynamics. Stem Cells Dev 2013; 22(6):855-65. doi: 10.1089/scd.2012.0377 [Crossref] [ Google Scholar]
- Shi H, Zhao Z, Jiang W, Zhu P, Zhou N, Huang X. A review into the insights of the role of endothelial progenitor cells on bone biology. Front Cell Dev Biol 2022; 10:878697. doi: 10.3389/fcell.2022.878697 [Crossref] [ Google Scholar]
- Sun K, Zhou Z, Ju X, Zhou Y, Lan J, Chen D. Combined transplantation of mesenchymal stem cells and endothelial progenitor cells for tissue engineering: a systematic review and meta-analysis. Stem Cell Res Ther 2016; 7(1):151. doi: 10.1186/s13287-016-0390-4 [Crossref] [ Google Scholar]
- Huang H, Huang W. Regulation of endothelial progenitor cell functions in ischemic heart disease: new therapeutic targets for cardiac remodeling and repair. Front Cardiovasc Med 2022; 9:896782. doi: 10.3389/fcvm.2022.896782 [Crossref] [ Google Scholar]
- Rashidi S, Bagherpour G, Abbasi-Malati Z, Didar Khosrowshahi N, Aghakhani Chegeni S, Roozbahani G. Endothelial progenitor cells for fabrication of engineered vascular units and angiogenesis induction. Cell Prolif 2024; 57(9):e13716. doi: 10.1111/cpr.13716 [Crossref] [ Google Scholar]
- Shi X, Simms KJ, Ewing TJ, Lin YP, Chen YL, Melvan JN. The bone marrow endothelial progenitor cell response to septic infection. Front Immunol 2024; 15:1368099. doi: 10.3389/fimmu.2024.1368099 [Crossref] [ Google Scholar]
- Salybekov AA, Kobayashi S, Asahara T. Characterization of endothelial progenitor cell: past, present, and future. Int J Mol Sci 2022; 23(14):7697. doi: 10.3390/ijms23147697 [Crossref] [ Google Scholar]
- Liu ZL, Chen HH, Zheng LL, Sun LP, Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther 2023; 8(1):198. doi: 10.1038/s41392-023-01460-1 [Crossref] [ Google Scholar]
- Pelliccia F, Pasceri V, Zimarino M, De Luca G, De Caterina R, Mehran R. Endothelial progenitor cells in coronary atherosclerosis and percutaneous coronary intervention: a systematic review and meta-analysis. Cardiovasc Revasc Med 2022; 42:94-9. doi: 10.1016/j.carrev.2022.02.025 [Crossref] [ Google Scholar]
- García Granado JF, Rodríguez Esparragón FJ, González Martín JM, Cazorla Rivero SE, González Hernández AN. Endothelial and circulating progenitor cells as prognostic biomarkers of stroke: a systematic review and meta-analysis. Thromb Res 2025; 245:109224. doi: 10.1016/j.thromres.2024.109224 [Crossref] [ Google Scholar]
- Cavalcante SL, Lopes S, Bohn L, Cavero-Redondo I, Álvarez-Bueno C, Viamonte S. Effects of exercise on endothelial progenitor cells in patients with cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Rev Port Cardiol (Engl Ed) 2019; 38(11):817-27. doi: 10.1016/j.repc.2019.02.016 [Crossref] [ Google Scholar]
- Li Y, Wang Z, Mao M, Zhao M, Xiao X, Sun W. Velvet antler mobilizes endothelial progenitor cells to promote angiogenesis and repair vascular endothelial injury in rats following myocardial infarction. Front Physiol 2018; 9:1940. doi: 10.3389/fphys.2018.01940 [Crossref] [ Google Scholar]
- Shin HS, Shin HH, Shudo Y. Current status and limitations of myocardial infarction large animal models in cardiovascular translational research. Front BioengBiotechnol 2021; 9:673683. doi: 10.3389/fbioe.2021.673683 [Crossref] [ Google Scholar]
- Atluri P, Trubelja A, Fairman AS, Hsiao P, MacArthur JW, Cohen JE. Normalization of postinfarct biomechanics using a novel tissue-engineered angiogenic construct. Circulation 2013; 128(11 Suppl 1):S95-104. doi: 10.1161/circulationaha.112.000368 [Crossref] [ Google Scholar]
- Burghoff S, Ding Z, Blaszczyk A, Wirrwar A, Buchholz D, Müller HW. Cross-linking enhances deposition of human endothelial progenitor cells in the rat heart after intracoronary transplantation. Cell Transplant 2010; 19(1):113-7. doi: 10.3727/096368909x474834 [Crossref] [ Google Scholar]
- Chang Z, Yang G, Sheng G, Zhang X. Endothelial progenitor cell (EPC) transplantation improves myocardial infarction via up-regulation of vascular endothelial growth factor and gap junction protein connexin 43 in rats. Int J Clin Exp Med 2018; 11(3):1805-14. [ Google Scholar]
- Fang Y, Chen S, Liu Z, Ai W, He X, Wang L. Endothelial stem cells attenuate cardiac apoptosis via downregulating cardiac microRNA-146a in a rat model of coronary heart disease. Exp Ther Med 2018; 16(5):4246-52. doi: 10.3892/etm.2018.6702 [Crossref] [ Google Scholar]
- Gaffey AC, Chen MH, Venkataraman CM, Trubelja A, Rodell CB, Dinh PV. Injectable shear-thinning hydrogels used to deliver endothelial progenitor cells, enhance cell engraftment, and improve ischemic myocardium. J Thorac Cardiovasc Surg 2015; 150(5):1268-76. doi: 10.1016/j.jtcvs.2015.07.035 [Crossref] [ Google Scholar]
- Gaffey AC, Chen MH, Trubelja A, Venkataraman CM, Chen CW, Chung JJ. Delivery of progenitor cells with injectable shear-thinning hydrogel maintains geometry and normalizes strain to stabilize cardiac function after ischemia. J Thorac Cardiovasc Surg 2019; 157(4):1479-90. doi: 10.1016/j.jtcvs.2018.07.117 [Crossref] [ Google Scholar]
- Quan Z, Wang QL, Zhou P, Wang GD, Tan YZ, Wang HJ. Thymosin β4 promotes the survival and angiogenesis of transplanted endothelial progenitor cells in the infarcted myocardium. Int J Mol Med 2017; 39(6):1347-56. doi: 10.3892/ijmm.2017.2950 [Crossref] [ Google Scholar]
- Schuh A, Kroh A, Konschalla S, Liehn EA, Sobota RM, Biessen EA. Myocardial regeneration by transplantation of modified endothelial progenitor cells expressing SDF-1 in a rat model. J Cell Mol Med 2012; 16(10):2311-20. doi: 10.1111/j.1582-4934.2012.01539.x [Crossref] [ Google Scholar]
- Schuh A, Liehn EA, Sasse A, Hristov M, Sobota R, Kelm M. Transplantation of endothelial progenitor cells improves neovascularization and left ventricular function after myocardial infarction in a rat model. Basic Res Cardiol 2008; 103(1):69-77. doi: 10.1007/s00395-007-0685-9 [Crossref] [ Google Scholar]
- Sen S, Merchan J, Dean J, Ii M, Gavin M, Silver M. Autologous transplantation of endothelial progenitor cells genetically modified by adeno-associated viral vector delivering insulin-like growth factor-1 gene after myocardial infarction. Hum Gene Ther 2010; 21(10):1327-34. doi: 10.1089/hum.2010.006 [Crossref] [ Google Scholar]
- She Q, Xia S, Deng SB, Du JL, Li YQ, He L. Angiogenesis in a rat model following myocardial infarction induced by hypoxic regulation of VEGF₁₆₅ gene-transfected EPCs. Mol Med Rep 2012; 6(6):1281-7. doi: 10.3892/mmr.2012.1112 [Crossref] [ Google Scholar]
- Zhao Y, Song J, Bi X, Gao J, Shen Z, Zhu J. Thymosin β4 promotes endothelial progenitor cell angiogenesis via a vascular endothelial growth factor-dependent mechanism. Mol Med Rep 2018; 18(2):2314-20. doi: 10.3892/mmr.2018.9199 [Crossref] [ Google Scholar]
- Boyle AJ, Schuster M, Witkowski P, Xiang G, Seki T, Way K. Additive effects of endothelial progenitor cells combined with ACE inhibition and beta-blockade on left ventricular function following acute myocardial infarction. J Renin Angiotensin Aldosterone Syst 2005; 6(1):33-7. doi: 10.3317/jraas.2005.004 [Crossref] [ Google Scholar]
- Chaudeurge A, Wilhelm C, Chen-Tournoux A, Farahmand P, Bellamy V, Autret G. Can magnetic targeting of magnetically labeled circulating cells optimize intramyocardial cell retention?. Cell Transplant 2012; 21(4):679-91. doi: 10.3727/096368911x612440 [Crossref] [ Google Scholar]
- Demetz G, Oostendorp RAJ, Boxberg AM, Sitz W, Farrell E, Steppich B. Overexpression of insulin-like growth factor-2 in expanded endothelial progenitor cells improves left ventricular function in experimental myocardial infarction. J Vasc Res 2017; 54(6):321-8. doi: 10.1159/000479872 [Crossref] [ Google Scholar]
- Frederick JR, Fitzpatrick JR, 3rd 3rd, McCormick RC, Harris DA, Kim AY, Muenzer JR. Stromal cell-derived factor-1alpha activation of tissue-engineered endothelial progenitor cell matrix enhances ventricular function after myocardial infarction by inducing neovasculogenesis. Circulation 2010; 122(11 Suppl):S107-17. doi: 10.1161/circulationaha.109.930404 [Crossref] [ Google Scholar]
- Li H, Liu Q, Wang N, Xu Y, Kang L, Ren Y. Transplantation of endothelial progenitor cells overexpressing miR-126-3p improves heart function in ischemic cardiomyopathy. Circ J 2018; 82(9):2332-41. doi: 10.1253/circj.CJ-17-1251 [Crossref] [ Google Scholar]
- Mehmood A, Ali M, Khan SN, Riazuddin S. Diazoxide preconditioning of endothelial progenitor cells improves their ability to repair the infarcted myocardium. Cell Biol Int 2015; 39(11):1251-63. doi: 10.1002/cbin.10498 [Crossref] [ Google Scholar]
- Li SH, Wang DD, Xu YJ, Ma GD, Li XY, Liang WJ. Exogenous hTERT gene transfected endothelial progenitor cells from bone marrow promoted angiogenesis in ischemic myocardium of rats. Int J Clin Exp Med 2015; 8(8):14447-53. [ Google Scholar]
- Lian F, Xue S, Gu P, Zhu HS. The long-term effect of autologous endothelial progenitor cells from peripheral blood implantation on infarcted myocardial contractile force. J Int Med Res 2008; 36(1):40-6. doi: 10.1177/147323000803600106 [Crossref] [ Google Scholar]
- Poh KK, Lee PS, Djohan AH, Galupo MJ, Songco GG, Yeo TC. Transplantation of endothelial progenitor cells in obese diabetic rats following myocardial infarction: role of thymosin beta-4. Cells 2020; 9(4):949. doi: 10.3390/cells9040949 [Crossref] [ Google Scholar]
- Yao Y, Li Y, Ma G, Liu N, Ju S, Jin J. In vivo magnetic resonance imaging of injected endothelial progenitor cells after myocardial infarction in rats. Mol Imaging Biol 2011; 13(2):303-13. doi: 10.1007/s11307-010-0359-0 [Crossref] [ Google Scholar]
- Garikipati VN, Krishnamurthy P, Verma SK, Khan M, Abramova T, Mackie AR. Negative regulation of miR-375 by interleukin-10 enhances bone marrow-derived progenitor cell-mediated myocardial repair and function after myocardial infarction. Stem Cells 2015; 33(12):3519-29. doi: 10.1002/stem.2121 [Crossref] [ Google Scholar]
- Ahmadi A, McNeill B, Vulesevic B, Kordos M, Mesana L, Thorn S. The role of integrin α2 in cell and matrix therapy that improves perfusion, viability and function of infarcted myocardium. Biomaterials 2014; 35(17):4749-58. doi: 10.1016/j.biomaterials.2014.02.028 [Crossref] [ Google Scholar]
- Brunt KR, Wu J, Chen Z, Poeckel D, Dercho RA, Melo LG. Ex vivo Akt/HO-1 gene therapy to human endothelial progenitor cells enhances myocardial infarction recovery. Cell Transplant 2012; 21(7):1443-61. doi: 10.3727/096368912x653002 [Crossref] [ Google Scholar]
- Chen X, Gu M, Zhao X, Zheng X, Qin Y, You X. Deterioration of cardiac function after acute myocardial infarction is prevented by transplantation of modified endothelial progenitor cells overexpressing endothelial NO synthases. Cell PhysiolBiochem 2013; 31(2-3):355-65. doi: 10.1159/000343373 [Crossref] [ Google Scholar]
- Cheng Y, Hu R, Lv L, Ling L, Jiang S. Erythropoietin improves the efficiency of endothelial progenitor cell therapy after myocardial infarction in mice: effects on transplanted cell survival and autologous endothelial progenitor cell mobilization. J Surg Res 2012; 176(1):e47-55. doi: 10.1016/j.jss.2012.04.047 [Crossref] [ Google Scholar]
- Hu CH, Li ZM, Du ZM, Zhang AX, Rana JS, Liu DH. Expanded human cord blood-derived endothelial progenitor cells salvage infarcted myocardium in rats with acute myocardial infarction. Clin Exp PharmacolPhysiol 2010; 37(5-6):551-6. doi: 10.1111/j.1440-1681.2010.05347.x [Crossref] [ Google Scholar]
- Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation 2006; 113(10):1311-25. doi: 10.1161/circulationaha.105.541268 [Crossref] [ Google Scholar]
- Li H, Liu J, Ye X, Zhang X, Wang Z, Chen A. 17β-estradiol enhances the recruitment of bone marrow-derived endothelial progenitor cells into infarcted myocardium by inducing CXCR4 expression. Int J Cardiol 2013; 162(2):100-6. doi: 10.1016/j.ijcard.2011.05.074 [Crossref] [ Google Scholar]
- Cheng Y, Jiang S, Hu R, Lv L. Potential mechanism for endothelial progenitor cell therapy in acute myocardial infarction: activation of VEGF- PI3K/Akte-NOS pathway. Ann Clin Lab Sci 2013; 43(4):395-401. [ Google Scholar]
- Hamada H, Kim MK, Iwakura A, Ii M, Thorne T, Qin G. Estrogen receptors alpha and beta mediate contribution of bone marrow-derived endothelial progenitor cells to functional recovery after myocardial infarction. Circulation 2006; 114(21):2261-70. doi: 10.1161/circulationaha.106.631465 [Crossref] [ Google Scholar]
- Botta R, Gao E, Stassi G, Bonci D, Pelosi E, Zwas D. Heart infarct in NOD-SCID mice: therapeutic vasculogenesis by transplantation of human CD34 + cells and low dose CD34 + KDR + cells. FASEB J 2004; 18(12):1392-4. doi: 10.1096/fj.03-0879fje [Crossref] [ Google Scholar]
- Deutsch MA, Brunner S, Grabmaier U, David R, Ott I, Huber BC. Cardioprotective potential of human endothelial-colony forming cells from diabetic and nondiabetic donors. Cells 2020; 9(3):588. doi: 10.3390/cells9030588 [Crossref] [ Google Scholar]
- Moldenhauer LM, Cockshell MP, Frost L, Parham KA, Tvorogov D, Tan LY. Interleukin-3 greatly expands non-adherent endothelial forming cells with pro-angiogenic properties. Stem Cell Res 2015; 14(3):380-95. doi: 10.1016/j.scr.2015.04.002 [Crossref] [ Google Scholar]
- Gunetti M, Noghero A, Molla F, Staszewsky LI, de Angelis N, Soldo A. Ex vivo-expanded bone marrow CD34 + for acute myocardial infarction treatment: in vitro and in vivo studies. Cytotherapy 2011; 13(9):1140-52. doi: 10.3109/14653249.2011.597559 [Crossref] [ Google Scholar]
- Saucourt C, Vogt S, Merlin A, Valat C, Criquet A, Harmand L. Design and validation of an automated process for the expansion of peripheral blood-derived CD34 + cells for clinical use after myocardial infarction. Stem Cells Transl Med 2019; 8(8):822-32. doi: 10.1002/sctm.17-0277 [Crossref] [ Google Scholar]
- Sheng Z, Ju C, Li B, Chen Z, Pan X, Yan G. TWEAK promotes endothelial progenitor cell vasculogenesis to alleviate acute myocardial infarction via the Fn14-NF-κB signaling pathway. Exp Ther Med 2018; 16(5):4019-29. doi: 10.3892/etm.2018.6703 [Crossref] [ Google Scholar]
- Sheng Z, Yao Y, Li Y, Yan F, Huang J, Ma G. Bradykinin preconditioning improves therapeutic potential of human endothelial progenitor cells in infarcted myocardium. PLoS One 2013; 8(12):e81505. doi: 10.1371/journal.pone.0081505 [Crossref] [ Google Scholar]
- Sheng ZL, Yao YY, Li YF, Fu C, Ma GS. Transplantation of bradykinin-preconditioned human endothelial progenitor cells improves cardiac function via enhanced Akt/eNOS phosphorylation and angiogenesis. Am J Transl Res 2015; 7(6):1045-57. [ Google Scholar]
- Shintani S, Kusano K, Ii M, Iwakura A, Heyd L, Curry C. Synergistic effect of combined intramyocardial CD34 + cells and VEGF2 gene therapy after MI. Nat Clin Pract Cardiovasc Med 2006; 3 Suppl 1:S123-8. doi: 10.1038/ncpcardio0430 [Crossref] [ Google Scholar]
- Sondergaard CS, Bonde J, Dagnaes-Hansen F, Nielsen JM, Zachar V, Holm M. Minimal engraftment of human CD34 + cells mobilized from healthy donors in the infarcted heart of athymic nude rats. Stem Cells Dev 2009; 18(6):845-56. doi: 10.1089/scd.2008.0006 [Crossref] [ Google Scholar]
- Stein A, Knödler M, Makowski M, Kühnel S, Nekolla S, Keithahn A. Local erythropoietin and endothelial progenitor cells improve regional cardiac function in acute myocardial infarction. BMC Cardiovasc Disord 2010; 10:43. doi: 10.1186/1471-2261-10-43 [Crossref] [ Google Scholar]
- Sun Z, Wu J, Fujii H, Wu J, Li SH, Porozov S. Human angiogenic cell precursors restore function in the infarcted rat heart: a comparison of cell delivery routes. Eur J Heart Fail 2008; 10(6):525-33. doi: 10.1016/j.ejheart.2008.04.004 [Crossref] [ Google Scholar]
- Thal MA, Krishnamurthy P, Mackie AR, Hoxha E, Lambers E, Verma S. Enhanced angiogenic and cardiomyocyte differentiation capacity of epigenetically reprogrammed mouse and human endothelial progenitor cells augments their efficacy for ischemic myocardial repair. Circ Res 2012; 111(2):180-90. doi: 10.1161/circresaha.112.270462 [Crossref] [ Google Scholar]
- Xin Z, Meng W, Ya-Ping H, Wei Z. Different biological properties of circulating and bone marrow endothelial progenitor cells in acute myocardial infarction rats. Thorac Cardiovasc Surg 2008; 56(8):441-8. doi: 10.1055/s-2008-1038879 [Crossref] [ Google Scholar]
- Xue Y, Zhou B, Wu J, Miao G, Li K, Li S. Transplantation of endothelial progenitor cells in the treatment of coronary artery microembolism in rats. Cell Transplant 2020; 29:963689720912688. doi: 10.1177/0963689720912688 [Crossref] [ Google Scholar]
- Yang K, Liu X, Lin W, Zhang Y, Peng C. Upregulation of microRNA-125b leads to the resistance to inflammatory injury in endothelial progenitor cells. Cardiol Res Pract 2020; 2020:6210847. doi: 10.1155/2020/6210847 [Crossref] [ Google Scholar]
- Yao Y, Sheng Z, Li Y, Fu C, Ma G, Liu N. Tissue kallikrein-modified human endothelial progenitor cell implantation improves cardiac function via enhanced activation of Akt and increased angiogenesis. Lab Invest 2013; 93(5):577-91. doi: 10.1038/labinvest.2013.48 [Crossref] [ Google Scholar]
- Yoo CH, Na HJ, Lee DS, Heo SC, An Y, Cha J. Endothelial progenitor cells from human dental pulp-derived iPS cells as a therapeutic target for ischemic vascular diseases. Biomaterials 2013; 34(33):8149-60. doi: 10.1016/j.biomaterials.2013.07.001 [Crossref] [ Google Scholar]
- Yuan Z, Kang L, Wang Z, Chen A, Zhao Q, Li H. 17β-estradiol promotes recovery after myocardial infarction by enhancing homing and angiogenic capacity of bone marrow-derived endothelial progenitor cells through ERα-SDF-1/CXCR4 crosstalking. Acta BiochimBiophys Sin (Shanghai) 2018; 50(12):1247-56. doi: 10.1093/abbs/gmy127 [Crossref] [ Google Scholar]
- Zhou W, Zheng X, Cheng C, Guo G, Zhong Y, Liu W. Rab27a deletion impairs the therapeutic potential of endothelial progenitor cells for myocardial infarction. Mol Cell Biochem 2021; 476(2):797-807. doi: 10.1007/s11010-020-03945-x [Crossref] [ Google Scholar]
- Atluri P, Miller JS, Emery RJ, Hung G, Trubelja A, Cohen JE. Tissue-engineered, hydrogel-based endothelial progenitor cell therapy robustly revascularizes ischemic myocardium and preserves ventricular function. J Thorac Cardiovasc Surg 2014; 148(3):1090-8. doi: 10.1016/j.jtcvs.2014.06.038 [Crossref] [ Google Scholar]
- Yang J, Ii M, Kamei N, Alev C, Kwon SM, Kawamoto A. CD34 + cells represent highly functional endothelial progenitor cells in murine bone marrow. PLoS One 2011; 6(5):e20219. doi: 10.1371/journal.pone.0020219 [Crossref] [ Google Scholar]
- Park JH, Yoon JY, Ko SM, Jin SA, Kim JH, Cho CH. Endothelial progenitor cell transplantation decreases lymphangiogenesis and adverse myocardial remodeling in a mouse model of acute myocardial infarction. Exp Mol Med 2011; 43(8):479-85. doi: 10.3858/emm.2011.43.8.054 [Crossref] [ Google Scholar]
- Huang H, Huang F, Huang JP. Transplantation of bone marrow-derived endothelial progenitor cells overexpressing Delta-like-4 enhances functional neovascularization in ischemic myocardium. Mol Med Rep 2013; 8(5):1556-62. doi: 10.3892/mmr.2013.1657 [Crossref] [ Google Scholar]
- Li X, Xue X, Sun Y, Chen L, Zhao T, Yang W. MicroRNA-326-5p enhances therapeutic potential of endothelial progenitor cells for myocardial infarction. Stem Cell Res Ther 2019; 10(1):323. doi: 10.1186/s13287-019-1413-8 [Crossref] [ Google Scholar]
- Zhang BF, Jiang H, Chen J, Hu Q, Yang S, Liu XP. Silica-coated magnetic nanoparticles labeled endothelial progenitor cells alleviate ischemic myocardial injury and improve long-term cardiac function with magnetic field guidance in rats with myocardial infarction. J Cell Physiol 2019; 234(10):18544-59. doi: 10.1002/jcp.28492 [Crossref] [ Google Scholar]
- Xiao Q, Zhao XY, Jiang RC, Chen XH, Zhu X, Chen KF. Increased expression of Sonic hedgehog restores diabetic endothelial progenitor cells and improves cardiac repair after acute myocardial infarction in diabetic mice. Int J Mol Med 2019; 44(3):1091-105. doi: 10.3892/ijmm.2019.4277 [Crossref] [ Google Scholar]
- Sun YY, Bai WW, Wang B, Lu XT, Xing YF, Cheng W. Period 2 is essential to maintain early endothelial progenitor cell function in vitro and angiogenesis after myocardial infarction in mice. J Cell Mol Med 2014; 18(5):907-18. doi: 10.1111/jcmm.12241 [Crossref] [ Google Scholar]
- Wu Y, Ip JE, Huang J, Zhang L, Matsushita K, Liew CC. Essential role of ICAM-1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circ Res 2006; 99(3):315-22. doi: 10.1161/01.RES.0000235986.35957.a3 [Crossref] [ Google Scholar]
- Chang ZT, Hong L, Wang H, Lai HL, Li LF, Yin QL. Application of peripheral-blood-derived endothelial progenitor cell for treating ischemia-reperfusion injury and infarction: a preclinical study in rat models. J Cardiothorac Surg 2013; 8:33. doi: 10.1186/1749-8090-8-33 [Crossref] [ Google Scholar]
- Hu CH, Li ZM, Du ZM, Zhang AX, Yang DY, Wu GF. Human umbilical cord-derived endothelial progenitor cells promote growth cytokines-mediated neorevascularization in rat myocardial infarction. Chin Med J (Engl) 2009; 122(5):548-55. [ Google Scholar]
- Mackie AR, Klyachko E, Thorne T, Schultz KM, Millay M, Ito A. Sonic hedgehog-modified human CD34 + cells preserve cardiac function after acute myocardial infarction. Circ Res 2012; 111(3):312-21. doi: 10.1161/circresaha.112.266015 [Crossref] [ Google Scholar]
- Murasawa S, Kawamoto A, Horii M, Nakamori S, Asahara T. Niche-dependent translineage commitment of endothelial progenitor cells, not cell fusion in general, into myocardial lineage cells. ArteriosclerThrombVasc Biol 2005; 25(7):1388-94. doi: 10.1161/01.ATV.0000168409.69960.e9 [Crossref] [ Google Scholar]
- Rong Q, Huang J, Su E, Li J, Li J, Zhang L. Infection of hepatitis B virus in extrahepatic endothelial tissues mediated by endothelial progenitor cells. Virol J 2007; 4:36. doi: 10.1186/1743-422x-4-36 [Crossref] [ Google Scholar]
- Toeg HD, Tiwari-Pandey R, Seymour R, Ahmadi A, Crowe S, Vulesevic B. Injectable small intestine submucosal extracellular matrix in an acute myocardial infarction model. Ann Thorac Surg 2013; 96(5):1686-94. doi: 10.1016/j.athoracsur.2013.06.063 [Crossref] [ Google Scholar]
- Salari N, Morddarvanjoghi F, Abdolmaleki A, Rasoulpoor S, Khaleghi AA, Afshar Hezarkhani L. The global prevalence of myocardial infarction: a systematic review and meta-analysis. BMC Cardiovasc Disord 2023; 23(1):206. doi: 10.1186/s12872-023-03231-w [Crossref] [ Google Scholar]
- Li J, Zhao Y, Zhu W. Targeting angiogenesis in myocardial infarction: novel therapeutics (review). Exp Ther Med 2022; 23(1):64. doi: 10.3892/etm.2021.10986 [Crossref] [ Google Scholar]
- Huang W, Percie du Sert N, Vollert J, Rice AS. General principles of preclinical study design. Handb Exp Pharmacol 2020; 257:55-69. doi: 10.1007/164_2019_277 [Crossref] [ Google Scholar]
- Li J, Ma Y, Miao XH, Guo JD, Li DW. Neovascularization and tissue regeneration by endothelial progenitor cells in ischemic stroke. Neurol Sci 2021; 42(9):3585-93. doi: 10.1007/s10072-021-05428-3 [Crossref] [ Google Scholar]
- Shen X, Wang M, Bi X, Zhang J, Wen S, Fu G. Resveratrol prevents endothelial progenitor cells from senescence and reduces the oxidative reaction via PPAR-γ/HO-1 pathways. Mol Med Rep 2016; 14(6):5528-34. doi: 10.3892/mmr.2016.5929 [Crossref] [ Google Scholar]
- Xue M, Liu M, Zhu X, Yang L, Miao Y, Shi D. Effective components of Panax quinquefolius and Corydalis tuber protect myocardium through attenuating oxidative stress and endoplasmic reticulum stress. Evid Based Complement Alternat Med 2013; 2013:482318. doi: 10.1155/2013/482318 [Crossref] [ Google Scholar]
- Hamed S, Brenner B, Aharon A, Daoud D, Roguin A. Nitric oxide and superoxide dismutase modulate endothelial progenitor cell function in type 2 diabetes mellitus. Cardiovasc Diabetol 2009; 8:56. doi: 10.1186/1475-2840-8-56 [Crossref] [ Google Scholar]
- Kim SM, Vetrivel P, Ha SE, Kim HH, Kim JA, Kim GS. Apigetrin induces extrinsic apoptosis, autophagy and G2/M phase cell cycle arrest through PI3K/AKT/mTOR pathway in AGS human gastric cancer cell. J NutrBiochem 2020; 83:108427. doi: 10.1016/j.jnutbio.2020.108427 [Crossref] [ Google Scholar]
- Deng X, Zhao W, Song L, Ying W, Guo X. Pro-apoptotic effect of TRAIL-transfected endothelial progenitor cells on glioma cells. Oncol Lett 2018; 15(4):5004-12. doi: 10.3892/ol.2018.7977 [Crossref] [ Google Scholar]
- Liang Q, Pan F, Qiu H, Zhou X, Cai J, Luo R. CLC-3 regulates TGF-β/smad signaling pathway to inhibit the process of fibrosis in hypertrophic scar. Heliyon 2024; 10(3):e24984. doi: 10.1016/j.heliyon.2024.e24984 [Crossref] [ Google Scholar]
- Emontzpohl C, Simons D, Kraemer S, Goetzenich A, Marx G, Bernhagen J, et al. Isolation of endothelial progenitor cells from healthy volunteers and their migratory potential influenced by serum samples after cardiac surgery. J Vis Exp 2017(120):55192. doi: 10.3791/55192.
- Ma X, Wang J, Li J, Ma C, Chen S, Lei W. Loading miR-210 in endothelial progenitor cells derived exosomes boosts their beneficial effects on hypoxia/reoxygeneation-injured human endothelial cells via protecting mitochondrial function. Cell PhysiolBiochem 2018; 46(2):664-75. doi: 10.1159/000488635 [Crossref] [ Google Scholar]
- Ngo NH, Chang YH, Vuong CK, Yamashita T, Obata-Yasuoka M, Hamada H. Transformed extracellular vesicles with high angiogenic ability as therapeutics of distal ischemic tissues. Front Cell Dev Biol 2022; 10:869850. doi: 10.3389/fcell.2022.869850 [Crossref] [ Google Scholar]
- Abd El Aziz MT, Abd El Nabi EA, Abd El Hamid M, Sabry D, Atta HM, Rahed LA. Endothelial progenitor cells regenerate infracted myocardium with neovascularisation development. J Adv Res 2015; 6(2):133-44. doi: 10.1016/j.jare.2013.12.006 [Crossref] [ Google Scholar]
- Chen Z, Haus JM, Chen L, Wu SC, Urao N, Koh TJ. CCL28-induced CCR10/eNOS interaction in angiogenesis and skin wound healing. FASEB J 2020; 34(4):5838-50. doi: 10.1096/fj.201902060R [Crossref] [ Google Scholar]
- Ii M, Nishimura H, Iwakura A, Wecker A, Eaton E, Asahara T. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation 2005; 111(9):1114-20. doi: 10.1161/01.Cir.0000157144.24888.7e [Crossref] [ Google Scholar]
- Cristóvão G, Milner J, Sousa P, Ventura M, Cristóvão J, Elvas L. Improvement in circulating endothelial progenitor cells pool after cardiac resynchronization therapy: increasing the list of benefits. Stem Cell Res Ther 2020; 11(1):194. doi: 10.1186/s13287-020-01713-8 [Crossref] [ Google Scholar]
- Mause SF, Ritzel E, Deck A, Vogt F, Liehn EA. Endothelial progenitor cells modulate the phenotype of smooth muscle cells and increase their neointimal accumulation following vascular injury. ThrombHaemost 2022; 122(3):456-69. doi: 10.1055/s-0041-1731663 [Crossref] [ Google Scholar]
- Yan F, Li J, Zhang W. Transplantation of endothelial progenitor cells: summary and prospect. Acta Histochem 2023; 125(1):151990. doi: 10.1016/j.acthis.2022.151990 [Crossref] [ Google Scholar]
- Huang Y, Chen L, Feng Z, Chen W, Yan S, Yang R. EPC-derived exosomal miR-1246 and miR-1290 regulate phenotypic changes of fibroblasts to endothelial cells to exert protective effects on myocardial infarction by targeting ELF5 and SP1. Front Cell Dev Biol 2021; 9:647763. doi: 10.3389/fcell.2021.647763 [Crossref] [ Google Scholar]
- Hong X, Luo AC, Doulamis I, Oh N, Im GB, Lin CY. Photopolymerizable hydrogel for enhanced intramyocardial vascular progenitor cell delivery and post-myocardial infarction healing. Adv Healthc Mater 2023; 12(29):e2301581. doi: 10.1002/adhm.202301581 [Crossref] [ Google Scholar]
- Sun R, Wang X, Nie Y, Hu A, Liu H, Zhang K. Targeted trapping of endogenous endothelial progenitor cells for myocardial ischemic injury repair through neutrophil-mediated SPIO nanoparticle-conjugated CD34 antibody delivery and imaging. Acta Biomater 2022; 146:421-33. doi: 10.1016/j.actbio.2022.05.003 [Crossref] [ Google Scholar]
- Zhou T, Yuan Z, Weng J, Pei D, Du X, He C. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol 2021; 14(1):24. doi: 10.1186/s13045-021-01037-x [Crossref] [ Google Scholar]
- Srinivasan RC, Kannisto K, Strom SC, Gramignoli R. Evaluation of different routes of administration and biodistribution of human amnion epithelial cells in mice. Cytotherapy 2019; 21(1):113-24. doi: 10.1016/j.jcyt.2018.10.007 [Crossref] [ Google Scholar]
- Liu Z, Mikrani R, Zubair HM, Taleb A, Naveed M, Baig M. Systemic and local delivery of mesenchymal stem cells for heart renovation: challenges and innovations. Eur J Pharmacol 2020; 876:173049. doi: 10.1016/j.ejphar.2020.173049 [Crossref] [ Google Scholar]
- Vekstein AM, Wendell DC, DeLuca S, Yan R, Chen Y, Bishawi M. Targeted delivery for cardiac regeneration: comparison of intra-coronary infusion and intra-myocardial injection in porcine hearts. Front Cardiovasc Med 2022; 9:833335. doi: 10.3389/fcvm.2022.833335 [Crossref] [ Google Scholar]
- Gathier WA, van Ginkel DJ, van der Naald M, van Slochteren FJ, Doevendans PA, Chamuleau SAJ. Retrograde coronary venous infusion as a delivery strategy in regenerative cardiac therapy: an overview of preclinical and clinical data. J Cardiovasc Transl Res 2018; 11(3):173-81. doi: 10.1007/s12265-018-9785-1 [Crossref] [ Google Scholar]
- Xu CM, Sabe SA, Brinck-Teixeira R, Sabra M, Sellke FW, Abid MR. Visualization of cardiac uptake of bone marrow mesenchymal stem cell-derived extracellular vesicles after intramyocardial or intravenous injection in murine myocardial infarction. Physiol Rep 2023; 11(6):e15568. doi: 10.14814/phy2.15568 [Crossref] [ Google Scholar]
- Li J, Hu S, Zhu D, Huang K, Mei X, López de Juan Abad B. All roads lead to Rome (the heart): cell retention and outcomes from various delivery routes of cell therapy products to the heart. J Am Heart Assoc 2021; 10(8):e020402. doi: 10.1161/jaha.120.020402 [Crossref] [ Google Scholar]
- Romagnuolo R, Masoudpour H, Porta-Sánchez A, Qiang B, Barry J, Laskary A. Human embryonic stem cell-derived cardiomyocytes regenerate the infarcted pig heart but induce ventricular tachyarrhythmias. Stem Cell Reports 2019; 12(5):967-81. doi: 10.1016/j.stemcr.2019.04.005 [Crossref] [ Google Scholar]
- Jiménez-Beltrán MA, Gómez-Calderón AJ, Quintanar-Zúñiga RE, Santillán-Cortez D, Téllez-González MA, Suárez-Cuenca JA. Electrospinning-generated nanofiber scaffolds suitable for integration of primary human circulating endothelial progenitor cells. Polymers (Basel) 2022; 14(12):2448. doi: 10.3390/polym14122448 [Crossref] [ Google Scholar]
- Gong X, Li B, Yang Y, Huang Y, Sun Y, Liu M. Bone marrow derived endothelial progenitor cells retain their phenotype and functions after a limited number of culture passages and cryopreservation. Cytotechnology 2019; 71(1):1-14. doi: 10.1007/s10616-018-0234-4 [Crossref] [ Google Scholar]
- Wang J, Li R. Effects, methods and limits of the cryopreservation on mesenchymal stem cells. Stem Cell Res Ther 2024; 15(1):337. doi: 10.1186/s13287-024-03954-3 [Crossref] [ Google Scholar]
- Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol 2008; 51(6):660-8. doi: 10.1016/j.jacc.2007.09.059 [Crossref] [ Google Scholar]
- Guillamat-Prats R. The role of MSC in wound healing, scarring and regeneration. Cells 2021; 10(7):1729. doi: 10.3390/cells10071729 [Crossref] [ Google Scholar]