Advanced pharmaceutical bulletin. 10(3):359-369.
doi: 10.34172/apb.2020.044
Review Article
A Review on Solid Dispersion and Carriers Used Therein for Solubility Enhancement of Poorly Water Soluble Drugs
Avinash Ramrao Tekade *  , Jyoti Narayan Yadav
, Jyoti Narayan Yadav
Author information:
Department of Pharmaceutics, Marathwada Mitra Mandal’s College of Pharmacy, Thergaon, Pune, Maharashtra- 411033, India.
Abstract
A large number of hydrophilic and hydrophobic carriers in pharmaceutical excipients are available today which are used for formulation of solid dispersions. Depending on nature of carriers the immediate release solid dispersions and/or controlled release solid dispersions can be formulated. Initially crystalline carriers were used which are transformed into amorphous solid dispersions with enhanced properties. The carriers used previously were mostly synthetic one. Recent trend towards the use of natural carriers have replaced the use of synthetic carriers. This review is the overview of various synthetic, natural, semisynthetic, modified natural hydrophilic carriers used for formulation of solid dispersions.
Keywords: Hydrophillic carriers, Mucilage’s, Natural gums, Solid dispersions, Solubility, Solubility enhancement
Copyright and License Information
© 2020 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Absorption of drug and its therapeutic effectiveness get affected by solubility which is a significant physicochemical factor. Poor aqueous solubility can leads to failure in formulation development process. The main reason behind inadequate bioavailability of drug is its low dissolution rate and low solubility in aqueous medium.1 A large number of hydrophilic carriers are explored today which have shown significant results for solubility enhancement. Nowadays, most of the drug substances were innovated but the venture to improve the solubility and dissolution of hydrophobic drug substances remain one of the trickiest tasks in drug development. Dissolution of drug in aqueous medium like gastric fluid is important to get better absorption and bioavailability for orally administered drug. Therefore, to progress bioavailability of poorly water soluble compounds like biopharmaceutical classification system class II and IV drugs, polymer matrix of various origin can be used. Various solubility enhancement methods have been introduced to triumph over this problem.1 There are several techniques for solubility enhancement which can be categorized into physical modification, chemical modifications for the drug substance, and other techniques2 which are listed in Table 1.
Table 1.
Techniques for solubility enhancement of poorly water soluble drugs
|
Techniques for solubility enhancement
2-5
|
| Physical modification |
A) Reduction Particle size |
| a) Micronization |
| b) Nanosuspension, |
| B) Modification of the crystal habit |
| C) Solid dispersions |
| a) Eutectic mixtures |
| b) Solid solutions |
| c) Amorphous solid solutions |
| d) Glass solutions and glass suspension |
| e) Cryogenic techniques. |
| Chemical modification |
A) Change of pH, |
| B) Use of buffer, |
| C) Derivatization, |
| D) Complexation, |
| E) Salt formation. |
| Miscellaneous methods |
A) Supercritical fluid process, |
| B) Use of adjuvant like surfactant, solubilizers, cosolvency, hydrotropy, and novel excipients. |
One of the most promising and efficient techniques for solubility enhancement is solid dispersion formulation. According to Chiou and Riegelman, solid dispersion systems can be defined as ‘the dispersion of one or more active ingredients in an inert carrier or matrix at solid state prepared by the melting [fusion], solvent, or melting-solvent method’. The drug is hydrophobic in nature whereas matrix is hydrophilic. Solid dispersion can be classified as simple eutectic mixtures, solid solutions, glass solutions and glass suspensions, amorphous precipitation in a crystalline carrier, compound or complex formations.3 However, several modifications have been done in classification systems by various researchers which will be discussed in the following.
Classification of solid dispersions
On the basis of recent advancement in solid dispersion, they can be classified as:
First generation solid dispersion
The solid dispersions which could be prepared by using crystalline carriers are categorized as the first generation solid dispersions.6 Examples of used crystalline carriers are urea and sugars.7 In this type, thermodynamically stable crystalline solid dispersion get formed which releases the drug slowly.6 The dissolution rate is faster in case of amorphous solid dispersions (ASDs) as compared to crystalline sold dispersions. The first reported solid dispersion was eutectic mixture or monotectic mixture. In case of eutectic mixture melting point of dispersion is lower than the melting point of carrier and drug the melting point of drug and carrier are constant in case of monotectic mixture. In cooling process of eutectic mixture, the drug and carrier will crystallize simultaneously therefore it is preferred over the monotectic mixture.8 At the specific composition in eutectic mixture where drug crystallizes out is referred as eutectic point, and the mixture consists of fine crystals of two components.6 Small particle size will results in increased specific surface area which generally improves rate of dissolution and oral absorption of poorly water soluble drugs.3 Moreover, the number of studies having exact eutectic composition in solid dispersion is very limited.8
Based on the extent of miscibility between the two components or the crystalline structure of solid solution they are of two kinds. One is continuous [or isomorphous, complete, unlimited] solid solutions and the other discontinuous [or restricted, partial, limited, complete] solid solutions. They can also be classified into two groups- substitutional solid solutions in which the solute molecule substitutes the solvent molecule in the crystal lattice of the solid solvent. Whereas, in case of interstitial solid solutions, the solute molecule occupies the interstitial space of the solvent lattice.3 The disadvantage of first generation solid dispersion is forming crystalline solid dispersion as they were prepared using crystalline carriers like urea and sugars. Crystalline solid dispersions were more thermodynamically stable which lowers their dissolution rate as compared to amorphous one.9 Okonogi et al10 has studied the effect of urea and mannitol on crystallinity of ofloxacin. The higher solubility and dissolution rate were observed in case of urea based solid dispersions than mannitol based solid dispersions because urea reduced the crystallinity of ofloxacin more than mannitol proved by PXRD and DSC results.
Second generation solid dispersion
These contain amorphous carriers like PVP, PEG, cellulose derivatives, etc.7 Second generation solid dispersions were found more effective than first generation solid dispersions (SD) because of their thermodynamic stability.9 According to the physical state of drug, ASDs can be classified as amorphous solid suspensions and amorphous solid solutions [glass solutions]. Amorphous solid suspensions consist of two separate phases while amorphous solid solutions contain molecularly homogenous mixture of both the drug and amorphous carriers. Amorphous carriers can be synthetic polymer or natural polymer.8 Amorphous solid suspensions can be formulated in case of drugs with limited carrier solubility or high melting point. In case of second generation solid dispersions because of forced solubilization of the drug in the carrier the drug is in its supersaturated state.9 Due to increase in chain length or molecular weights of polymers the aqueous solubility of polymers get decrease and viscosity get increased. Prevention of recrystallization of drugs in manufacturing, storage and dissolution process can be achieved by using high viscosity polymers. Moreover, the use of high viscosity polymer can delay the dissolution rate of drug in aqueous medium. The major problem regarding second generation solid dispersion is drug precipitation and recrystallization which affect the in vitro or in vivo drug release.8
Third generation solid dispersion
The dissolution profile of drug can be improved using third generation solid dispersions which consists of carriers having surface activity or emulsifying properties.9 Use of special type of carrier for formulation of solid dispersions will overcome precipitation and recrystallization problems. Use of surfactant or emulsifiers not only improve the dissolution profile of drug but also improves physical and chemical stability of drug in solid dispersion. Examples of these carriers are inulin, Gelucire, poloxamer, etc.8 The physical and chemical stability of solid dispersion get enhanced by preventing nucleation and agglomeration.9 The selection of surface active agent or another polymer is based on dissolution or stability profile of drug, i.e. surfactant is used when faster dissolution is required while polymer with higher Tg may be used when prevention of re-crystallization is needed.8,11
Fourth generation solid dispersion
These type of dispersions can be referred as controlled release solid dispersions (CRSD). It contain poorly water soluble drug with a short biological half life.8 The carrier used are either water soluble carrier or water insoluble carrier.7 Solubility enhancement and extended release of drug in controlled manner are the two targets in CRSD.8 The water soluble carriers used in CRSD are ethyl cellulose, Eudragit RS, Eudragit RL, HPC, etc.7
On the basis of physical state and molecular arrangement of active pharmaceutical ingredient (API) and carrier, binary solid dispersions can be divided into six distinct systems as follows:
Meng et al have classified solid dispersions into six groups Class C–C, Class C–A, Class A–C, Class A–A, Class M–C, Class M–A based on physical state and molecular arrangement of both API and carrier (Table 2). Further efforts are needed to shape a clear classification system and correlate it with the performance of solid dispersion in terms of solubility and stability.12
Table 2.
Classification According to Physical State and Molecular Arrangement of API and Carrier
|
Class
|
API
|
Carrier
|
| C-C |
Crystalline |
Crystalline |
| C-A |
Crystalline |
Amorphous |
| A-C |
Amorphous |
Crystalline |
| A-A |
Amorphous |
Amorphous |
| M-C |
Molecularly dispersed |
Crystalline |
| M-A |
Molecularly dispersed |
Amorphous |
The aim of this article is to update on information regarding hydrophilic carriers used in solubility enhancement by using various solid dispersion methods. Here, hydrophilic carriers were classified on the basis of its origin like synthetic hydrophilic carrier, natural hydrophilic carriers, modified natural hydrophilic carriers and semi-synthetic hydrophilic carriers. Initially crystalline carriers like urea, sugars etc. were used in formulation of solid dispersions which have been changed to amorphous carriers including polymers. Therefore, mostly used form of solid dispersions is the ASDs. Use of various polymeric carriers affects dissolution characteristics of dispersed drug. Water soluble carrier results in a fast drug release while poorly soluble or insoluble carrier will retard the drug release from the matrix.8
Mechanisms of incorporation of drug into polymer
Carriers used in solid dispersions are polymers. When drug and polymer are in intimate contact then drug occupy void spaces between polymeric chain and makes polymer chain relatively flexible. For example, in case of hot melt extrusion process, polymer is allowed to heat up to some extent that the heat given is responsible for loosening of polymer chain and incorporation of drug molecule into it. While in spray drying method the solvent used in process is responsible for weak cohesive inter and intra molecular interactions of polymer chain and resulting in formation of solvent polymer interactions. After this, drug molecules dissolved in solvent are incorporated into the loosened polymer chains.
Antiplasticization effect is observed when the mechanical properties of substance changes into stiff and brittle when another substance is added. In another way it can be explained as, compound with low Tg of resulting mixture would fall somewhere in between the Tg’s of both compounds. In this case drug undergoes antiplasticization. Where as polymer undergoes plasticization as its Tg decreases.13
Drug release mechanism from solid dispersion
Dissolution performance of solid dispersion after its oral administration in the form of tablet, capsules, etc. will give proper idea about ultimate success. One of the successful approaches for enhancement of solubility of poorly soluble drug is conversion of crystalline form of drug to an amorphous from. For successful solid dispersion formulation major keys are supersaturation state maintenance and amorphous form stabilization. The problem regarding solid dispersions is precipitation of supersaturated drug which will ultimately affect its bioavailability.13 Increases stability and solubility of drug in medium is observed due to particle size reduction and reduced agglomeration.14 In supersaturating drug delivery system such as solid dispersion spring like effect is observed due to enhancement of dissolution rate of drug. At the stage of supersaturation decrease in dissolution rate is observed due to drug precipitation. Furthermore in such system parachute like effect is observed on dissolution profile of drugs when precipitation inhibitors are added.12 Drug controlled release and carrier controlled release are two types of mechanisms involved in drug release from immediate release solid dispersions. While in case of CRSD diffusion and erosion drug release mechanisms are observed depending on characteristics of polymer and the miscibility of the drug and carrier.8 If the carrier is soluble in the dissolution medium then the release of ASD is dissolution controlled mechanism while in case of insoluble carrier diffusion controlled mechanism is observed.15
Carriers used in solid dispersion
Carriers plays major role in formulation of solid dispersion.16 They can be hydrophilic or hydrophobic or water swellable. Depending on their characteristics they can be used as release retardant or release enhancers.8 Also the dissolution characteristics of drug molecules are depend on nature of carriers.17 The criteria for selection of carrier are as follows:
-
It should be water soluble or swellable, soluble in variety of solvents.
-
It should be economical, pharmacologically inert, non-toxic.
-
It should be heat stable.
-
Chemically compatible with drug.
The better chemical stability of solid dispersion was observed in case of solid dispersions which are formulated using solvent based methods as compared to fusion based methods. Solvent based methods include spray drying, co-precipitation, etc. While hot melt extrusion, KinetiSol® dispersing technology, etc. comes under fusion based methods.18 Hydrophilic carriers used in solid dispersion are classified on the basis of their origin. Table 3 shows list of synthetic hydrophilic carriers.
Table 3.
Synthetic Hydrophilic Carriers
|
Name of hydrophilic carrier
|
Method used for preparation of SDs
|
Drug used
|
Conclusion
|
| Brij35 and Pluronic F-127 19 |
Solvent evaporation method |
Progesterone |
Progesterone incorporation into Pluronic F-127 and Brij 35 micelles improved its aqueous solubility by ~20 folds |
| Carboxymethylcellulose and sodium alginate (ALG)20 |
Solvent evaporation method |
Praziquantel |
Improved solubility |
| Compritol 888 ATO21 |
Solvent evaporation method, Sustained release solid dispersions |
Metformin HCL |
Solid dispersion(SD) for controlled release of metformin were developed using compritol |
| Dextrin22 |
Spray-drying technique |
Micronized amlodipine |
Amlo-SD with and without SLS provided 2.8- and 2.0-fold increase in AUC respectively |
| Eudragit E, Plasdone S and Soluplus23 |
Hot melt extrusion (HME), freeze-drying (FD), and supercritical fluid (SF) |
Theobromine |
Improved dissolution profile |
| Eudragit S10024 |
Solvent evaporation method |
Berberine HCL |
Improved efficacy |
| Gelucire and Sorbitol25 |
Solvent method, Melt method |
Ritonavir |
Release of ritonavir from solid dispersion is more than that of pure drug |
| HPMC and PVA-PEG grafted copolymer26 |
Solvent evaporation method |
Kaempferia parviflora
|
Solid dispersion technique can be applied for the production of herbal extracts |
| Mannitol27 |
Spray drying method |
Diazepam |
Improved dissolution behaviour of drug |
| Palm stearin based polyesteramide (PSPEA)28 |
Hot melt method |
Mefenamic acid |
Improved the release and solubility |
| Pectin: poly (vinyl pyrrolidone)29 |
Spray drying technique |
Curcumin |
The in vitro dissolution data showed many fold increase in dissolution rate |
| Poloxamer 18830 |
Hot melt method |
Losartan potassium |
A dissolution rate much higher than that of pure drug |
| Poloxamer 188, Poloxamer 40731 |
Kneading method, solvent evaporation method |
Boswellic acid |
Improved solubility of poorly water soluble boswellic acid |
| Poloxamer + d-α-tocopherol polyethylene glycol 1000 succinate (TPGS)32 |
Solvent evaporation method |
Febuxostat |
Solid dispersion exhibited a drug release rate that was 1.94 times greater than plain drug |
| Poly(2-ethyl-2-oxazoline)33 |
Solvent evaporation method |
Glipizide |
Glipizide solubility improved by 2.5 times with poly[2-ethyl-2-oxazoline] compared to about 1.8 times with pure drug |
| Poly(ethylene oxide) (PEO) (3400, 10000, 20000)34 |
Solvent evaporation method |
Griseofulvin |
Increase the dissolution rate and the bioavailability of poorly water-soluble drug |
| Polyethylene glycol (PEG) 400035 |
Fusion method |
Gliclazide |
Dissolution rate improved 90% compared to pure drug |
| Polyethylene glycol (PEG) 400036 |
Microwave induced solid dispersion |
Glipizide |
The solid dispersion matrix tablet displayed retarded drug release up to 99.320% in 12 h. |
| Polyethylene glycol 600037 |
Microwave induced fusion method |
Atorvastatin calcium |
An increase in the solubility of atorvastatin was observed with increasing concentration of PEG 6000 |
| Polyethylene glycol 8000 and polyethylene glycol 1000038 |
Solvent evaporation method |
Flurbiprofen |
Significant increase in drug release |
| Polyoxyethylene 40 stearate39 |
Solvent melt method |
Cyclosporine A |
Improved dissolution and bioavailability |
| Polyvinylpolypyrrolidone (PVPP)40 |
Solvent evaporation method |
Oleanolic acid |
The optimized SD's system could improve about 15 times of dissolution rate than that of free OLA in 5 min. |
| Polyvinylpyrrolidone (PVP)41 |
Melt quenching |
Celecoxib |
Improved in vitro and in vivo performance |
| Polyvinylpyrrolidone (PVP) K30: isomalt (Galen IQ 810)42 |
Spray drying method |
Celecoxib |
Increased dissolution rate and
saturation solubility of celecoxib |
| PVP K-12, PVP K-17, PVP K-30, Copovidone43 |
Solvent evaporation method, Spray drying method |
Chlortetracycline hydrochloride |
The effect of preparation methods, polymer types and polymer concentrations were evaluated |
| PVP-VA64 + POLOXAMER 40744 |
Spray drying method |
Rebamipide |
Bioavailability and efficacy of rebamipide were increased significantly by solubility enhancement of the drug. |
| Sodium acetate45 |
Freeze drying |
Docetaxel |
Enhanced dissolution rate resulting in enhanced bioavailability |
| Soluplus46 |
Hot Melt Extrusion, Spray Drying |
Telmisartan |
Results demonstrated an improvement in solubility of TEL by 99 times as compared to pure drug in buffer pH 7.4 |
| Soluplus47 |
Solvent evaporation technique |
Edaravone |
Self-nanomicellizing solid dispersion (SNMSD) of edaravone give 17.53 fold improve in aqueous solubility. |
| Tocopheryl PEG 1000-succinate (TPGS)48 |
Solvent evaporation method |
Dutasteride |
Dutasteride solid dispersion formulations improved the dissolution and oral bioavailability of drug because of hydrogen interactions between carrier and drug |
| Vinylpyrrolidone-vinyl acetate (VA 64), PVP, HPMC49 |
Solvent evaporation method |
Dronedarone HCL |
Improved dissolution rate and intestinal absorption |
| Vinylpyrrolidone-Vinyl Acetate Copolymer-64+ Sucrose laurate50 |
Surface modified SD's prepared by Spray drying method |
Oxcarbazepine |
Enhanced solubility |
| Urea51 |
Fusion method |
Rofecoxib |
The mean dissolution time (MDT) of rofecoxib decreased which results in enhanced dissolution rate. |
In recent years, polymers those are derived from plant origin are getting tremendous interest because of their diverse pharmaceutical applications and also easy availability, biocompatibility, non-toxic nature, chemically inertness they are preferred over the synthetic ones. Demand for these substances is increasing and new sources are being developed. Polysaccharide, one of the most abundant industrial raw materials and have been the subject of intensive research due to their sustainability, bio-safety and bio-degradability. The natural gums are metabolic by-products of plants obtained from various parts of plant like seed, fruit, incised trunk (gummy exudate), etc.52 Various natural hydrophilic carriers used till date are given in Table 4.
Table 4.
Natural hydrophilic carriers
|
Name of hydrophilic carrier
|
Method used for preparation of solid dispersions
|
Drug used
|
Conclusion
|
|
Aegle marmelos Gum53 |
Microwave induced fusion method, lyophilisation Technique. |
Atorvastatin calcium |
The SD's prepared using the lyophilization method displays faster dissolution rates compared with those prepared using other method |
| Alginate54 |
Solvent evaporation method |
Lovastatin and Indomethacin |
Significant improvement in drug dissolution and stability |
| Arginine55 |
Freeze drying |
Simvastatin |
Simvastatin-arginine (SMV-ARG) complex exhibited solubility enhancement by 12 000 fold in both acidic and alkaline dissolution media |
| Caffeine56 |
Solubility method |
Celecoxib |
Enhancement of solubility and dissolution rate was observed |
| Chitosan57 |
Solvent evaporation method |
Abietic acid |
Improved activity |
| Low molecular weight chitosan58 |
Solvent evaporation method |
Tanshinone llA |
Dissolution of Tanshinone llA increased about 368.2% compared with the pure drug |
|
Elaeagnus angustifolia fruit powder, crospovidone, microcrystalline cellulose59 |
Cogrinding method |
Piroxicam |
Increased dissolution rate was observed |
| Skimmed milk60 |
Lyophilization |
Simvastatin |
In-vitro drug release studies exhibited a cumulative release of 86.69% as compared to 25.19% for the pure drug |
| Soybean seeds61 |
NA |
Pioglitazone HCl |
Increase in drug solubility |
|
Daucus carota
62
|
Solvent evaporation and kneading method |
Ambrisentan |
Solid dispersion by kneading method showed better results than the solvent evaporation method. |
| Water soluble gelatin and egg albumin63 |
Kneading method |
Nifedipine |
Improved the wettability of the drug, and consequently enhanced dissolution rate |
| Gelatin 50PS64 |
Freeze drying |
12 Active pharmaceutical ingredients |
Gelatin 50PS was screened for its feasibility as carrier
in the formulation of solid dispersions by lyophilization |
| Neem gum65 |
Solvent evaporation and kneading method |
Atorvastatin |
Enhanced dissolution rate and bioavailability |
| Oleaster powder, microcrystalline cellulose and crospovidone66 |
Cogrinding method |
Ibuprofen |
Oleaster powder is suggested as a potential hydrophilic carrier for increasing the drug release |
| Sericin67 |
Spray drying, solvent evaporation,
ball milling |
Lornoxicam, meloxicam and felodipine |
Spray drying as an efficient technique for improvement in solubility [8 to 10 fold] and dissolution of drugs |
| Sodium alginate68 |
Grinding method |
Telmisartan |
The highest release rates were achieved by SD with higher proportions of sodium alginate about 19.5 fold increase in dissolution profile were observed. |
| Tamarind seed polysaccharide69 |
Co-grinding method, kneading method, solvent deposition method |
Celecoxib |
Enhanced dissolution rate |
|
Vigna radiata extract70 |
Solvent evaporation method |
Clopidogrel bisulfate |
The maximum drug release was found in formulation was 96.12% |
Natural gum polysaccharides are promising biodegradable, biocompatible materials for use in drug delivery systems. However, these materials have certain limitations, like uncontrolled rate of hydration, change in viscosity during shelf life, microbial contamination. Therefore to overcome this problems some modification have done. Modifications can be done in terms of physical modifications and chemical modifications. Physical methods for modification of involves use of dry heat, microwave technology, UV and gamma radiations. While chemical modifications involves carboxymethylation/carbomoylethylation in which free –OH groups replacement were done which leads to improved aqueous drug solubility.71 Generally natural carriers on modification using heating method will leads to change in its physical characteristics like viscosity, density, swelling index, water holding capacity, flow properties etc. Due to changes in these characteristics the improved results were obtained in case of modified natural carriers.4 Carboxymethylation of natural carriers increases their hydrophilicity and makes them more soluble in aqueous systems.71 Various modified hydrophilic carriers and semi synthetic carriers used are shown in Table 5 and Table 6.
Table 5.
Modified natural hydrophilic carriers
|
Name of hydrophilic carrier
|
Method used for preparation of SDs
|
Drug used
|
Conclusion
|
| Locust bean gum, Modified LBG (LBG/ MLBG)72 |
Kneading method, spray drying method, solvent wetting method, modified solvent evaporation method |
Lovastatin |
Increase in apparent solubility and increase in dissolution rate |
| Modified gum Karaya73 |
Solvent evaporation method |
Glimepiride |
Enhanced dissolution rate |
| Modified gum Karaya74 |
Co-grinding mixture, kneading mixture, solvent evaporation method |
Nimodipine |
Improvement of dissolution rate |
| Locust bean gum (LBG), modified LBG, Guar gum, Modified guar gum75 |
Solvent evaporation method |
Glibenclamide |
Modified forms of natural carriers could be potential carriers in dissolution rate enhancement of poorly soluble drugs |
| Guar gum, Modified guar gum76 |
Cogrinding mixtures |
Licofelone |
Enhanced dissolution rate and decreased particle agglomeration |
| Guar gum, Modified guar gum77 |
Microwave induced solid dispersions |
Simvastatin |
Optimized formulation showed 99.35±3.3.05% drug release, which is higher than that of MKT [97.77±2.51%] |
| Hupu gum, modified hupu gum78 |
Gel entrapment technique |
Clopidogrel Bisulphate |
Enhancement in solubility of Clopidogrel bisulphate |
| Hupu gum, modified hupu gum79 |
Cogrinding method |
Pioglitazone HCL |
Enhanced solubility & bioavailability |
| Modified fenugreek gum80 |
Co solvent precipitation method |
Simvastatin |
Used as solubility enhancer and stabilizer in solid dispersion preparation |
| Modified xanthan gum81 |
Kneading method |
Pioglitazone hydrochloride |
Cumulative drug release in optimized formulations of xanthan gum and modified xanthan gum was 85.37% and 99.21% respectively |
Table 6.
Semi-synthetic hydrophilic carriers
|
Name of hydrophilic carrier
|
Method used for Preparation of SD's
|
Drug used
|
Conclusion
|
| Chito-oligosaccharide82 |
Spray drying method. |
Hesperidin |
Enhanced solubility and antioxidant activity |
| Ethyl cellulose, hydroxypropyl methylcellulose83 |
Solvent evaporation method |
Hydrochlorothiazide |
Improved dissolution rate |
| Hydroxypropyl methylcellulose (HPMC E5 LV)84 |
Spray drying method |
Irbesartan |
Enhancing solubility and dissolution rate |
| Hydroxypropyl methylcellulose (HPMC E5 LV)85 |
Microwave induced fusion method |
Raloxifene HCL |
Increased solubility as well as in vitro drug dissolution |
| HPMC: MESOPOROUS SILICA (ternary ASD)86 |
Hot melt extrusion |
Indomethacin |
Enhanced dissolution rate and physical stability |
Advantages of solid dispersions
The main objective behind formulation of solid dispersions is to enhance solubility of drug and thereby enhancement of its in vitro dissolution rate and bioavailability as well as developing controlled release solid dispersions.87 The factors affecting drug solubility are its particle size, porosity, wettability, etc.9 Various advantageous properties of solid dispersions are showed in Figure 1.
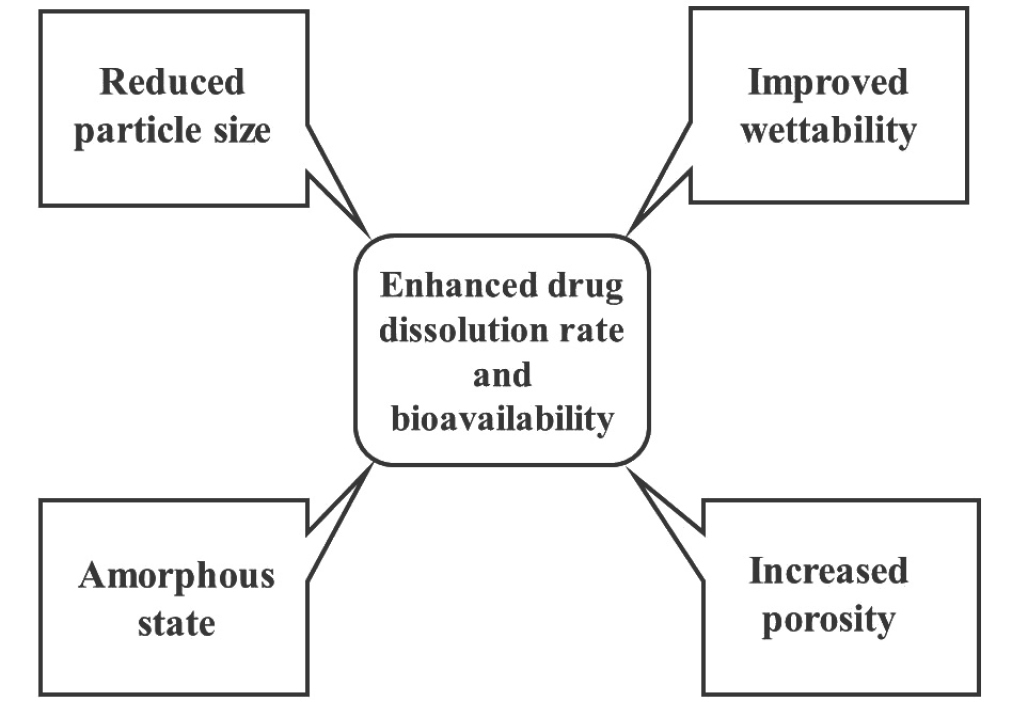
Figure 1.
Advantages of solid dispersions.
.
Advantages of solid dispersions.
Characterization of solid dispersions
Solid dispersions are mainly known for their use in dissolution rate and bioavailability enhancement. The enhanced dissolution rate can be examined by using standard dissolution methods which involves use of USP dissolution test apparatus. Another parameter studied in case of solid dispersion is to detect the physical state of drug and polymer, like state of material (amorphous or crystalline) and degree of crystallinity.8 Many analytical and instrumental technique are used to characterize solid dispersions. Techniques used for characterization are can be thermal methods, spectroscopic methods, microscopic methods, microthermal analysis, macroscopic techniques, etc.88 Their significance and characteristic features are listed in Table 7.
Table 7.
Various methods for characterization of solid dispersions and its significance
|
Characteristics
|
Methods used
|
Significance
|
| Physical state examination |
Differential scanning calorimetry, Powder X-ray diffraction, Hot stage microscopy, Humidity stage microscopy |
To find out physical state of sample, crystallinity and degree of crystallinity of drug, polymer, solid dispersion. |
| Surface microscopy |
Scanning electron microscopy, hot stage microscopy, polarized light optical microscopy |
To examine microscopy and crystallinity |
| Structure elucidation |
Solid state nuclear magnetic resonance spectroscopy, Fourier transform infrared spectroscopy |
To investigate bonding between drug and carrier e.g. hydrogen bonding |
| Drug carrier interactions |
Differential scanning calorimetry, Nuclear magnetic resonance spectroscopy, Fourier transform infrared spectroscopy |
To study physical and chemical interactions between drug and polymer |
| Dissolution rate |
Dissolution studies, dynamic solubility studies |
To study rate and extent of drug release |
| Stability |
Differential scanning calorimetry, nuclear magnetic resonance spectroscopy, Fourier transform infrared spectroscopy |
To study physical and chemical interactions between drug and polymer during its manufacturing and storage period |
Conclusion
New chemical entities are mostly the poorly water soluble drugs. To overcome poor solubility problem, solid dispersions can be prepared using hydrophilic carriers. These carriers can be of synthetic or of natural origin. Major problem regarding solid dispersions is its stability which can also be overcome by using newly coming carriers and use of optimized manufacturing techniques. Industrial and academic research work have solving the scalability problem of solid dispersions. There are still several carriers which are not investigated so far. Therefore, studies on such carrier materials should be done for solubility enhancement.
Ethical Issues
Not applicable.
Conflict of interests
There is no conflict of interest.
Acknowledgments
Authors are thankful to the Management and Principal of Marathwada Mitra Mandal’s College of Pharmacy, Thergaon, Pune, for motivation and infrastructural facilities for completing this review.
References
- Akbarpour Nikghalb L, Singh G, Singh G, Fazaeli Kahkeshan K. Solid dispersion: methods and polymers to increase the solubility of poorly soluble drugs. J Appl Pharm Sci 2012; 2(10):170-5. doi: 10.7324/JAPS.2012.21031 [Crossref] [ Google Scholar]
- Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharm 2012; 2012:195727. doi: 10.5402/2012/195727 [Crossref] [ Google Scholar]
- Chiou WL, Riegelman S. Pharmaceutical applications of solid dispersion systems. J Pharm Sci 1971; 60(9):1281-302. doi: 10.1002/jps.2600600902 [Crossref] [ Google Scholar]
- Shejul AA, Deshmane S, Biyani K. Modified natural carrier in solid dispersion for enhancement of solubility of poorly water soluble drugs. J Drug Deliv Ther 2014; 4(1):111-6. doi: 10.22270/jddt.v4i1.749 [Crossref] [ Google Scholar]
- Kumar B. Solid dispersion- a review. PharmaTutor 2017; 5(2):24-9. [ Google Scholar]
- Kim KT, Lee JY, Lee MY, Song CK, Choi J, Kim DD. Solid dispersions as a drug delivery system. J Pharm Investig 2011; 41(3):125-42. doi: 10.4333/KPS.2011.41.3.125 [Crossref] [ Google Scholar]
- Bindhani S, Mohapatra S. Recent approaches of solid dispersion: a new concept toward oral bioavailability. Asian J Pharm Clin Res 2018; 11(2):72-8. doi: 10.22159/ajpcr.2018.v11i2.23161 [Crossref] [ Google Scholar]
- Vo CL, Park C, Lee BJ. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharm Biopharm 2013; 85(3 Pt B):799-813. doi: 10.1016/j.ejpb.2013.09.007 [Crossref] [ Google Scholar]
- Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today 2007; 12(23-24):1068-75. doi: 10.1016/j.drudis.2007.09.005 [Crossref] [ Google Scholar]
- Okonogi S, Oguchi T, Yonemochi E, Puttipipatkhachorn S, Yamamoto K. Improved dissolution of ofloxacin via solid dispersion. Int J Pharm 1997; 156(2):175-80. doi: 10.1016/S0378-5173(97)00196-8 [Crossref] [ Google Scholar]
- Prasad D, Lande J, Chauhan H, Chauhan H. Ternary amorphous solid dispersions. J Dev Drugs 2017; 6(3):181. doi: 10.4172/2329-6631.1000181 [Crossref] [ Google Scholar]
- Meng F, Gala U, Chauhan H. Classification of solid dispersions: correlation to (i) stability and solubility (ii) preparation and characterization techniques. Drug Dev Ind Pharm 2015; 41(9):1401-15. doi: 10.3109/03639045.2015.1018274 [Crossref] [ Google Scholar]
- Tejaa SB, Patil SP, Shete G, Patel S, Bansal AK. Drug-excipient behavior in polymeric amorphous solid dispersions. J Excip Food Chem 2013; 4(3):70-94. [ Google Scholar]
- Craig DQ. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm 2002; 231(2):131-44. doi: 10.1016/s0378-5173(01)00891-2 [Crossref] [ Google Scholar]
- Maincent J, Williams RO 3rd. Sustained-release amorphous solid dispersions. Drug Deliv Transl Res 2018; 8(6):1714-25. doi: 10.1007/s13346-018-0494-8 [Crossref] [ Google Scholar]
- Shahi SR, Khan A, Bhalerao P, Ade P. A review on formulation aspects of solid dispersions. Eur J Pharm Med Res 2017; 4(12):148-60. [ Google Scholar]
- Singh N, Sarangi MK. Solid dispersion-a novel approach for enhancement of bioavailability of poorly soluble drugs in oral drug delivery system. Glob J Pharm Pharm Sci 2017; 3(2):1-8. doi: 10.19080/GJPPS.2017.03.555608 [Crossref] [ Google Scholar]
- Huang S, Williams RO 3rd. Effects of the preparation process on the properties of amorphous solid dispersions. AAPS PharmSciTech 2018; 19(5):1971-84. doi: 10.1208/s12249-017-0861-7 [Crossref] [ Google Scholar]
- Hassan AS, Soliman GM, El-Mahdy MM, El-Gindy GEA. Solubilization and enhancement of ex vivo vaginal delivery of progesterone using solid dispersions, inclusion complexes and micellar solubilization. Curr Drug Deliv 2018; 15(1):110-21. doi: 10.2174/1567201814666170320142136 [Crossref] [ Google Scholar]
- Marques CSF, Rezende P, Andrade LN, Mendes TMF, Allegretti SM, Bani C. Solid dispersion of praziquantel enhanced solubility and improve the efficacy of the schistosomiasis treatment. J Drug Deliv Sci Technol 2018; 45:124-34. doi: 10.1016/j.jddst.2018.03.009 [Crossref] [ Google Scholar]
- Jagdale S, Patil S, Kuchekar B, Chabukswar A. Preparation and characterization of Metformin hydrochloride - Compritol 888 ATO solid dispersion. J Young Pharm 2011; 3(3):197-204. doi: 10.4103/0975-1483.83758 [Crossref] [ Google Scholar]
- Jang DJ, Sim T, Oh E. Formulation and optimization of spray-dried amlodipine solid dispersion for enhanced oral absorption. Drug Dev Ind Pharm 2013; 39(7):1133-41. doi: 10.3109/03639045.2012.723218 [Crossref] [ Google Scholar]
- Pinho LAG, Lima SGB, Malaquias LFB, de Pires FQ, Sá-Barreto LL, Cardozo-Filho L. Improvements of theobromine pharmaceutical properties using solid dispersions prepared with newfound technologies. Chem Eng Res Des 2018; 132:1193-201. doi: 10.1016/j.cherd.2017.10.019 [Crossref] [ Google Scholar]
- Guo S, Wang G, Wu T, Bai F, Xu J, Zhang X. Solid dispersion of berberine hydrochloride and Eudragit® S100: formulation, physicochemical characterization and cytotoxicity evaluation. J Drug Deliv Sci Technol 2017; 40:21-7. doi: 10.1016/j.jddst.2017.02.003 [Crossref] [ Google Scholar]
- Sinha S, Ali M, Baboota S, Ahuja A, Kumar A, Ali J. Solid dispersion as an approach for bioavailability enhancement of poorly water-soluble drug ritonavir. AAPS PharmSciTech 2010; 11(2):518-27. doi: 10.1208/s12249-010-9404-1 [Crossref] [ Google Scholar]
- Weerapol Y, Tubtimsri S, Jansakul C, Sriamornsak P. Improved dissolution of Kaempferia parviflora extract for oral administration by preparing solid dispersion via solvent evaporation. Asian J Pharm Sci 2017; 12(2):124-33. doi: 10.1016/j.ajps.2016.09.005 [Crossref] [ Google Scholar]
- Kauppinen A, Broekhuis J, Grasmeijer N, Tonnis W, Ketolainen J, Frijlink HW. Efficient production of solid dispersions by spray drying solutions of high solid content using a 3-fluid nozzle. Eur J Pharm Biopharm 2018; 123:50-8. doi: 10.1016/j.ejpb.2017.11.009 [Crossref] [ Google Scholar]
- Wong WS, Lee CS, Er HM, Lim WH, Wong SF. Biocompatible palm stearin-based polyesteramide as polymer carrier for solid dispersion. J Appl Polym Sci 2018; 135(8):45892. doi: 10.1002/app.45892 [Crossref] [ Google Scholar]
- Gaikwad D, Shewale R, Patil V, Mali D, Gaikwad U, Jadhav N. Enhancement in in vitro anti-angiogenesis activity and cytotoxicity in lung cancer cell by pectin-PVP based curcumin particulates. Int J Biol Macromol 2017; 104(Pt A):656-64. doi: 10.1016/j.ijbiomac.2017.05.170 [Crossref] [ Google Scholar]
- Tran HT, Park JB, Hong KH, Choi HG, Han HK, Lee J. Preparation and characterization of pH-independent sustained release tablet containing solid dispersion granules of a poorly water-soluble drug. Int J Pharm 2011; 415(1-2):83-8. doi: 10.1016/j.ijpharm.2011.05.052 [Crossref] [ Google Scholar]
- Tambe A, Pandita N. Enhanced solubility and drug release profile of boswellic acid using a poloxamer-based solid dispersion technique. J Drug Deliv Sci Technol 2018; 44:172-80. doi: 10.1016/j.jddst.2017.11.025 [Crossref] [ Google Scholar]
- Sheng X, Tang J, Bao J, Shi X, Su W. Enhancement of in vitro dissolution and in vivo performance/oral absorption of FEB-poloxamer-TPGS solid dispersion. J Drug Deliv Sci Technol 2018; 46:408-15. doi: 10.1016/j.jddst.2018.06.005 [Crossref] [ Google Scholar]
- Fael H, Ràfols C, Demirel AL. Poly(2-ethyl-2-oxazoline) as an alternative to poly(vinylpyrrolidone) in solid dispersions for solubility and dissolution rate enhancement of drugs. J Pharm Sci 2018; 107(9):2428-38. doi: 10.1016/j.xphs.2018.05.015 [Crossref] [ Google Scholar]
- Stachurek I, Pielichowski K. Preparation and thermal characterization of poly(ethylene oxide)/griseofulvin solid dispersions for biomedical applications. J Appl Polym Sci 2009; 111(4):1690-6. doi: 10.1002/app.29181 [Crossref] [ Google Scholar]
- Patil MP, Gaikwad NJ. Preparation and characterization of gliclazide-polyethylene glycol 4000 solid dispersions. Acta Pharm 2009; 59(1):57-65. doi: 10.2478/v10007-009-0001-3 [Crossref] [ Google Scholar]
- Isaac J, Kaity S, Ganguly S, Ghosh A. Microwave-induced solid dispersion technology to improve bioavailability of glipizide. J Pharm Pharmacol 2013; 65(2):219-29. doi: 10.1111/j.2042-7158.2012.01595.x [Crossref] [ Google Scholar]
- Maurya D, Belgamwar V, Tekade A. Microwave induced solubility enhancement of poorly water soluble atorvastatin calcium. J Pharm Pharmacol 2010; 62(11):1599-606. doi: 10.1111/j.2042-7158.2010.01187.x [Crossref] [ Google Scholar]
- Daravath B, Naveen C, Vemula SK, Tadikonda RR. Solubility and dissolution enhancement of flurbiprofen by solid dispersion using hydrophilic carriers. Braz J Pharm Sci 2018; 53(4):e00010. doi: 10.1590/s2175-97902017000400010 [Crossref] [ Google Scholar]
- Liu C, Wu J, Shi B, Zhang Y, Gao T, Pei Y. Enhancing the bioavailability of cyclosporine a using solid dispersion containing polyoxyethylene (40) stearate. Drug Dev Ind Pharm 2006; 32(1):115-23. doi: 10.1080/03639040500388573 [Crossref] [ Google Scholar]
- Wang W, Cui C, Li M, Zhang Z, Lv H. Study of a novel disintegrable oleanolic acid-polyvinylpolypyrrolidone solid dispersion. Drug Dev Ind Pharm 2017; 43(7):1178-85. doi: 10.1080/03639045.2017.1301950 [Crossref] [ Google Scholar]
- Knopp MM, Wendelboe J, Holm R, Rades T. Effect of amorphous phase separation and crystallization on the in vitro and in vivo performance of an amorphous solid dispersion. Eur J Pharm Biopharm 2018; 130:290-5. doi: 10.1016/j.ejpb.2018.07.005 [Crossref] [ Google Scholar]
- Motallae S, Taheri A, Homayouni A. Preparation and characterization of solid dispersions of celecoxib obtained by spray-drying ethanolic suspensions containing PVP-K30 or isomalt. J Drug Deliv Sci Technol 2018; 46:188-96. doi: 10.1016/j.jddst.2018.05.020 [Crossref] [ Google Scholar]
- Apiwongngam J, Limwikrant W, Jintapattanakit A, Jaturanpinyo M. Enhanced supersaturation of chlortetracycline hydrochloride by amorphous solid dispersion. J Drug Deliv Sci Technol 2018; 47:417-26. doi: 10.1016/j.jddst.2018.08.007 [Crossref] [ Google Scholar]
- Tung NT, Park CW, Oh TO, Kim JY, Ha JM, Rhee YS. Formulation of solid dispersion of rebamipide evaluated in a rat model for improved bioavailability and efficacy. J Pharm Pharmacol 2011; 63(12):1539-47. doi: 10.1111/j.2042-7158.2011.01360.x [Crossref] [ Google Scholar]
- Ngo AN, Thomas D, Murowchick J, Ayon NJ, Jaiswal A, Youan BC. Engineering fast dissolving sodium acetate mediated crystalline solid dispersion of docetaxel. Int J Pharm 2018; 545(1-2):329-41. doi: 10.1016/j.ijpharm.2018.04.045 [Crossref] [ Google Scholar]
- Enose AA, Dasan P, Sivaramakrishanan H, Kakkar V. Formulation, characterization and pharmacokinetic evaluation of telmisartan solid dispersions. J Mol Pharm Org Process Res 2016; 4(1):131. doi: 10.4172/2329-9053.1000131 [Crossref] [ Google Scholar]
- Parikh A, Kathawala K, Tan CC, Garg S, Zhou XF. Self-nanomicellizing solid dispersion of edaravone: part I - oral bioavailability improvement. Drug Des Devel Ther 2018; 12:2051-69. doi: 10.2147/dddt.s161940 [Crossref] [ Google Scholar]
- Choi JS, Lee SE, Jang WS, Byeon JC, Park JS. Solid dispersion of dutasteride using the solvent evaporation method: approaches to improve dissolution rate and oral bioavailability in rats. Mater Sci Eng C Mater Biol Appl 2018; 90:387-96. doi: 10.1016/j.msec.2018.04.074 [Crossref] [ Google Scholar]
- Jung HJ, Han SD, Kang MJ. Enhanced dissolution rate of dronedarone hydrochloride via preparation of solid dispersion using vinylpyrrolidone-vinyl acetate copolymer (Kollidone® VA 64). Bull Korean Chem Soc 2015; 36(9):2320-4. doi: 10.1002/bkcs.10455 [Crossref] [ Google Scholar]
- Shin KH, Lee HJ, Ha ES, Sim WY, Kim MS, Cho CW. Improvement of dissolution rate of oxcarbazepine using surface-modified solid dispersion with vinylpyrrolidone-vinyl acetate copolymer and sucrose laurate. Bull Korean Chem Soc 2018; 39(8):995-8. doi: 10.1002/bkcs.11500 [Crossref] [ Google Scholar]
- Liu C, Desai KGH, Liu C, Park HJ. Enhancement of dissolution rate of rofecoxib using solid dispersions with urea. Drug Dev Res 2004; 63(4):181-9. doi: 10.1002/ddr.10412 [Crossref] [ Google Scholar]
- Puri V, Sharma P, Nagpal M. An Update on some recent solubility enhancers as pharmaceutical excipients. J Pharm Technol Res Manag 2016; 4(1):45-62. doi: 10.15415/jptrm.2016.41004 [Crossref] [ Google Scholar]
- Ratnaparkhi MP, Chaudhari PD. Solubility enhancement of poorly water soluble drug using natural carrier. Int J Life Sci Pharma Res 2017; 7(3):9-18. [ Google Scholar]
- Guan J, Liu Q, Zhang X, Zhang Y, Chokshi R, Wu H. Alginate as a potential diphase solid dispersion carrier with enhanced drug dissolution and improved storage stability. Eur J Pharm Sci 2018; 114:346-55. doi: 10.1016/j.ejps.2017.12.028 [Crossref] [ Google Scholar]
- Meor Mohd Affandi MMR, Tripathy M, Abdul Majeed AB. Arginine complexes with simvastatin: apparent solubility, in vitro dissolution and solid state characterization. Curr Drug Deliv 2018; 15(1):77-86. doi: 10.2174/1567201814666170320144259 [Crossref] [ Google Scholar]
- Shakeel F, Faisal MS. Caffeine: a potential complexing agent for solubility and dissolution enhancement of celecoxib. Pharm Biol 2010; 48(1):113-5. doi: 10.3109/13880200903030074 [Crossref] [ Google Scholar]
- Cuzzucoli Crucitti V, Migneco LM, Piozzi A, Taresco V, Garnett M, Argent RH. Intermolecular interaction and solid state characterization of abietic acid/chitosan solid dispersions possessing antimicrobial and antioxidant properties. Eur J Pharm Biopharm 2018; 125:114-23. doi: 10.1016/j.ejpb.2018.01.012 [Crossref] [ Google Scholar]
- Liu QY, Zhang ZH, Jin X, Jiang YR, Jia XB. Enhanced dissolution and oral bioavailability of tanshinone IIA base by solid dispersion system with low-molecular-weight chitosan. J Pharm Pharmacol 2013; 65(6):839-46. doi: 10.1111/jphp.12047 [Crossref] [ Google Scholar]
- Barzegar-Jalali M, Ghanbarzadeh S, Adibkia K, Valizadeh H, Bibak S, Mohammadi G. Development and characterization of solid dispersion of piroxicam for improvement of dissolution rate using hydrophilic carriers. Bioimpacts 2014; 4(3):141-8. doi: 10.15171/bi.2014.007 [Crossref] [ Google Scholar]
- Sonar PA, Behera AL, Banerjee SK, Gaikwad DD, Harer SL. Preparation and characterization of Simvastatin solid dispersion using skimmed milk. Drug Dev Ind Pharm 2015; 41(1):22-7. doi: 10.3109/03639045.2013.845836 [Crossref] [ Google Scholar]
- Deshmane SV, Biyani KR. Evaluation of hot water extracted polysaccharides from soybean seeds ASA drug carrier. International Journal of Drug Formulation and Research 2014; 5(3):60-6. [ Google Scholar]
- Deshmane S, Deshmane S, Shelke S, Biyani K. Enhancement of solubility and bioavailability of ambrisentan by solid dispersion using Daucus carota as a drug carrier: formulation, characterization, in vitro, and in vivo study. Drug Dev Ind Pharm 2018; 44(6):1001-11. doi: 10.1080/03639045.2018.1428339 [Crossref] [ Google Scholar]
- Acartürk F, Kişlal Ö, Çelebi N. The effect of some natural polymers on the solubility and dissolution characteristics of nifedipine. Int J Pharm 1992; 85(1-3):1-6. doi: 10.1016/0378-5173(92)90127-N [Crossref] [ Google Scholar]
- Pas T, Vergauwen B, Van den Mooter G. Exploring the feasibility of the use of biopolymers as a carrier in the formulation of amorphous solid dispersions - Part I: Gelatin. Int J Pharm 2018; 535(1-2):47-58. doi: 10.1016/j.ijpharm.2017.10.050 [Crossref] [ Google Scholar]
- Rodde MS, Divase GT, Devkar TB, Tekade AR. Solubility and bioavailability enhancement of poorly aqueous soluble atorvastatin: in vitro, ex vivo, and in vivo studies. Biomed Res Int 2014; 2014:463895. doi: 10.1155/2014/463895 [Crossref] [ Google Scholar]
- Mohammadi G, Barzegar-Jalali M, Valizadeh H, Nazemiyeh H, Barzegar-Jalali A, Siahi Shadbad MR. Reciprocal powered time model for release kinetic analysis of ibuprofen solid dispersions in oleaster powder, microcrystalline cellulose and crospovidone. J Pharm Pharm Sci 2010; 13(2):152-61. doi: 10.18433/j3jg61 [Crossref] [ Google Scholar]
- Salunkhe NH, Jadhav NR, More HN, Jadhav AD. Screening of drug-sericin solid dispersions for improved solubility and dissolution. Int J Biol Macromol 2018; 107(Pt B):1683-91. doi: 10.1016/j.ijbiomac.2017.10.035 [Crossref] [ Google Scholar]
- Borba PAA, Pinotti M, de Campos CEM, Pezzini BR, Stulzer HK. Sodium alginate as a potential carrier in solid dispersion formulations to enhance dissolution rate and apparent water solubility of BCS II drugs. Carbohydr Polym 2016; 137:350-9. doi: 10.1016/j.carbpol.2015.10.070 [Crossref] [ Google Scholar]
- Satle A, Agrawal S. Solubility enhancement potential of tamarind seed polysaccharide as pharmaceutical excipient. Int J Pharm Biol Sci Arch 2012; 3(3):456-9. [ Google Scholar]
- Sawale R, Deshmane S, Biyani K. Preparation and characterization of clopidogrel bisulfate solid dispersion using vigna radiata extract as a natural drug carrier. Asian J Pharm 2016; 10(2):108-12. [ Google Scholar]
- Prajapati VD, Jani GK, Moradiya NG, Randeria NP. Pharmaceutical applications of various natural gums, mucilages and their modified forms. Carbohydr Polym 2013; 92(2):1685-99. doi: 10.1016/j.carbpol.2012.11.021 [Crossref] [ Google Scholar]
- Patel M, Tekade A, Gattani S, Surana S. Solubility enhancement of lovastatin by modified locust bean gum using solid dispersion techniques. AAPS PharmSciTech 2008; 9(4):1262-9. doi: 10.1208/s12249-008-9171-4 [Crossref] [ Google Scholar]
- Nagpal M, Rajera R, Nagpal K, Rakha P, Singh S, Mishra D. Dissolution enhancement of glimepiride using modified gum karaya as a carrier. Int J Pharm Investig 2012; 2(1):42-7. doi: 10.4103/2230-973x.96925 [Crossref] [ Google Scholar]
- Murali Mohan Babu GV, Prasad Ch D, Ramana Murthy KV. Evaluation of modified gum karaya as carrier for the dissolution enhancement of poorly water-soluble drug nimodipine. Int J Pharm 2002; 234(1-2):1-17. doi: 10.1016/s0378-5173(01)00925-5 [Crossref] [ Google Scholar]
- Sharma J, Nagpal M, Arora S. Glibenclamide solubility enhancement by modified natural carriers using the solid dispersion technique. Farmacia 2012; 60(6):822-38. [ Google Scholar]
- Shah V, Patel D, Mane S, Upadhyay U. Solubility and dissolution rate enhancement of licofelone by using modified Guar gum. Int J Pharmtech Res 2010; 2(3):1847-54. [ Google Scholar]
- Salappa A, Jaychandran E, Srinivasa Rao D, Kushare S. Solubility enhancement of poorly water soluble drug Simvastatin by solid dispersion technique using natural polymer Guar gum. J Chem Pharm Sci 2015; 8(3):547-57. [ Google Scholar]
- Shejul A, Deshmane S, Biyani K. Modification and physical characterization of hupu gum as a carrier in solid dispersion containing clopidogrel bisulphate. Am J Pharm Health Res 2014; 2(7):228-36. [ Google Scholar]
- Shingne NS, Nagpure SV, Deshmane S, Biyani K. Modified Hupu gum: a novel application in solid dispersion containing pioglitazone HCl. Am J Pharmtech Res 2013; 3(4):462-72. [ Google Scholar]
- Sav AK, Fule R, Moravkar K, Amin PD. Modified Fenugreek gum for solubility and dissolution rate enhancement of simvastatin. World J Pharm Pharm Sci 2013; 2(5):4019-29. [ Google Scholar]
- Deshmane S, Shingne N, Sheikh AA, Shingne P, Biyani K. Solubility ENHANCEMENT OF PIOGLITAZONE HCl using plain and modified xanthan gum in solid dispersion. Inventi Impact: Novel Excipients 2015; 2:42-7. [ Google Scholar]
- Cao R, Zhao Y, Zhou Z, Zhao X. Enhancement of the water solubility and antioxidant activity of hesperidin by chitooligosaccharide. J Sci Food Agric 2018; 98(6):2422-7. doi: 10.1002/jsfa.8734 [Crossref] [ Google Scholar]
- Ganesan P, Soundararajan R, Shanmugam U, Ramu V. Development, characterization and solubility enhancement of comparative dissolution study of second generation of solid dispersions and microspheres for poorly water soluble drug. Asian J Pharm Sci 2015; 10(5):433-41. doi: 10.1016/j.ajps.2015.05.001 [Crossref] [ Google Scholar]
- Boghra RJ, Kothawade PC, Belgamwar VS, Nerkar PP, Tekade AR, Surana SJ. Solubility, dissolution rate and bioavailability enhancement of irbesartan by solid dispersion technique. Chem Pharm Bull (Tokyo) 2011; 59(4):438-41. doi: 10.1248/cpb.59.438 [Crossref] [ Google Scholar]
- Patil PH, Belgamwar VS, Patil PR, Surana SJ. Enhancement of solubility and dissolution rate of poorly water soluble raloxifene using microwave induced fusion method. Braz J Pharm Sci 2013; 49(3):571-8. doi: 10.1590/S1984-82502013000300019 [Crossref] [ Google Scholar]
- Hanada M, Jermain SV, Lu X, Su Y, Williams RO, 3rd 3rd. Predicting physical stability of ternary amorphous solid dispersions using specific mechanical energy in a hot melt extrusion process. Int J Pharm 2018; 548(1):571-85. doi: 10.1016/j.ijpharm.2018.07.029 [Crossref] [ Google Scholar]
- Singh G, Kaur L, Sharma S, Gupta GD. Enhancement of the Solubility of poorly water soluble drugs through solid dispersion: a comprehensive review. Indian J Pharm Sci 2017; 79(5):674-87. doi: 10.4172/pharmaceutical-sciences.1000279 [Crossref] [ Google Scholar]
- Hallouard F, Mehenni L, Lahiani-Skiba M, Anouar Y, Skiba M. Solid dispersions for oral administration: an overview of the methods for their preparation. Curr Pharm Des 2016; 22(32):4942-58. doi: 10.2174/1381612822666160726095916 [Crossref] [ Google Scholar]