Advanced pharmaceutical bulletin. 10(3):370-378.
doi: 10.34172/apb.2020.045
Review Article
Medicinal Plants Extracts with Antiangiogenic Activity: Where Is the Link?
Zohreh Hoseinkhani 1  , Fathemeh Norooznezhad 1, Mohsen Rastegari-Pouyani 2, Kamran Mansouri 1, *
, Fathemeh Norooznezhad 1, Mohsen Rastegari-Pouyani 2, Kamran Mansouri 1, * 
Author information:
1Medical Biology Research Center Medical Sciences, Health Technology Institute, Kermanshah, Iran.
2Student Research Committee, Department of Immunology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
*
Corresponding Author: Kamran Mansouri, Tel: +98 8334276473; Fax: +98 8334276471, Email:
kmansouri@kums.ac.ir
Abstract
Angiogenesis is a strictly controlled process defined as the formation of new blood vessels essential for certain physiologic and pathologic conditions where the latter includes tumor growth, development, and metastasis. Thus, inhibiting angiogenesis along with other anticancer strategies such as chemotherapy seems to be invaluable for reaching an optimal outcome in cancer patients. It has been shown that some natural plant-derived compounds are capable of preventing the formation of these new blood vessels in the tumor and also inhibit the proliferation and growth of the cancer cells. In this review, we intend to introduce plants with anti-angiogenic properties and discuss their related features.
Keywords: Angiogenesis, Plant extract, Natural compounds, Chemotherapy
Copyright and License Information
© 2020 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Man has long worked to fight the difficulties he encounters through his life and has tried various methods to control diseases. These efforts include investigating medicinal herbs which have long been used to cure diseases and still continue to keep their advantages despite the technological and scientific advances and the ever-growing use of chemical substances in modern medicine.1,2 Regarding the medicinal value of these herbs, their abundance, as well as the fact that most of them are unknown; the need for more research on these compounds is well tangible.3 Angiogenesis is the creation of new blood vessels from preformed ones in order to provide cellular needs. Angiogenesis plays an unquestionable role in some (certain) physiologic processes such as wound healing, diabetes, as well as pathologic conditions of tumor growth and metastasis.4,5 According to the significance of this cascade in cancers, researchers seek to find derivative compounds from herbal sources to inhibit angiogenesis. Due to fewer side-effects of herbal compounds in the treatment of angiogenesis-related disorders, investigations on plants in order to identify and discover such compounds could be a quite promising therapeutic approach. Use of herbs as treatment for cancer has a long history; so that herbs have been the primary source of traditional medications for treatment of various diseases. Although the real compounds derived from plants might not usually be utilized as medications, plants are still considered important sources for development of novel therapeutic factors for researchers. Herbal-rich food regimen not only provides the body with necessary vitamins and minerals, but also there are over 25 000 chemical compounds in different herbs; most of which hold biological effects and characteristics.6,7 The molecules in herbal compounds are able to bind the therapeutic agents to carrier molecules. These plant-derived tumor-targeting complexes shape the hope for producing natural drugs more effectively and highly toxic to tumors, but not normal tissues. After the identification of new proteins with important regulatory effects on the tumors’ cell cycle advancement, researchers have proven that the molecules derived from plants and other organisms could be among important inhibitors sources of synthesis or function of these key proteins. Therefore, these herbs have the potential to bring about developing novel anti-cancer agents and medications.7-9
Angiogenesis is the formation of new capillaries from primary blood vessels and is involved in some pathologic processes such as tumor growth and metastasis. It is also play role in certain physiologic processes like organ growth and development as well as wound healing. Angiogenesis is a necessary procedure in natural physiology of the body and if the equilibrium between its inducers and inhibitors is disturbed, opportunity will be provided for some diseases to arise.10-13 Angiogenesis in tumors is intervened by targeting several molecules including nitric oxide (NO) which is a critical intermediate in angiogenesis that enhances endothelial cell survival, proliferation, and migration; so, it is basically considered a pro-angiogenic factor.14,15 On the other hand, some of the growth factors such as vascular endothelial growth factor (VEGF) are highly specific for endothelial cells while some others such as matrix metalloproteinases (MMPs), and basic fibroblast growth factor (bFGF) have a broader spectrum of target cells. Activating factors can be secreted by tumor cells, the surrounding tissues, fibroblasts, or by macrophages which enter the tumor microenvironment. Currently, the use of VEGF pathway inhibitors in angiogenesis is considered as an anti-cancer treatment strategy with clinical credit.16-23
The role of herbs in the inhibition of angiogenesis
Plants contain several active chemical compounds simultaneously and unlike chemical drugs, they can have synergistic effects and therefore, influence different aspects of disease pathology at the same time. In another words, plant extracts rich in biologically active compounds can slow down the growth of cancer cells and induce apoptosis in them at the same time which leads to tumor eradication by hindering angiogenesis and therefore, metastasis. Interactions of the active ingredients in plants extracts with tumors can give this opportunity to the immune system to identify and respond to the tumor cell.24-26
Nowadays, the importance of food of plant origins in preventing various diseases such as cancer, which depends on angiogenesis for growth, has been well proven. However, further studies are still required in order to study and discover more therapeutic plants with anti-angiogenic effects. Table 1 illustrates a list of plants and their major derivatives which inhibit the function of cyclo-oxygenase (COX) enzyme (one of the most important active enzymes in the angiogenesis process pathway).12,27,28 Table 2 shows some of the discovered plants and their derivatives which inhibit VEGF.27 Studies have shown, fewer side-effects of herbal compounds could be imagined compared to the chemicals and anti-cancer agents. However, there are diverse unwanted effects regarding the application of crude plant extract in in vivo condition. The use of medicinal herbs should be standardized since their direct use could sometimes lead to severe poisoning, allergic reactions, bleeding, and cases of death. Herbal remedies may interfere with the absorption of certain necessary nutrients. Other types also increase or decrease the effect of a particular drug along with exhibiting serious side effects.28
Table 1.
Plants and their major derivative compounds with anti-COX effects
21,27
|
Plant
|
| Ginger |
| Aloe vera |
| Epigallocatechin-3 gallate/green tea |
| Resveratrol |
| Liquorice |
| Milk thistle |
| Antioxidants present in plants (vitamins A, C, E, Se, Zn: carotenoids, flavonoids) |
| Boswellia |
| Bromelain |
| Garlic |
| Chinese skullcap |
| Bilberry |
| Grape seed extract proanthocyanidins |
| Panax ginseng |
| Curcumin |
Table 2.
Plants and their derivatives with specifically VEGF inhibitory effects
27
|
Plant
|
Plant Derivative
|
| Magnolia seed cones |
Contains 90% honokiol |
|
Taxus brevifolia (pacific yew ) |
Contains Taxol |
|
Polygonum cuspidatum (Japanese knotweed) |
Contains 20% resveratrol |
|
Vsicum album (European mistletoe) |
Contains mistletoe lectin III (ML3A) |
|
Artemisia annua (Chinese worm wood) |
Contains 95% artemisinin, and other related terpenes and flavonoids |
|
Curcuma longa (turmeric) |
Contains 95% curcumin |
|
Camellia sinensis (green tea) |
Contains 95 % phenols; 50% epigallocatechin |
|
Vitis vinifera (grape seed extract) |
Contains 95% proanthocyanidins |
|
Scutellaria baicalensis (Chinese skullcap) |
Contains 95% baicalin and flavonoids |
|
Silybum marianum (milk thistle) |
Contains 80% silymarin (silybin) |
|
Angelica sinensis (dong quai) |
Contains 4-hydroxyderricin |
Allium ascalonicum
Allium ascalonicum (Figure 1a) is considered an important species of the genus Allium which has long been used medicinally in many countries, including Iran. Allium is also used in the traditional foods. The plant has been known to retain properties like being effective on hematological indices; anti-oxidant, anti-fungal, and anti-bacterial potentials. In addition, a study on its chemical composition shows that it contains compounds such as organosulphons and polyphenols. Based on the results of the studies performed in Medical Biology Research Center, Kermanshah, Iran, on anti-angiogenic properties of Allium, it was found that the shallot rhizome extract has a significant inhibitory effect on angiogenesis. These useful features of Allium plant reveal its importance more than ever. Thus, given that Allium is routinely consumed in different communities and regarding its inhibitory effect on angiogenesis, it could be among the most convincing plant candidates for consideration in cancer treatment.29-31

Figure 1.
a) Allium ascalonicum, b) Black rice, c) Cinnamon, d)Oak, e)Peganum harmala, f)Cucumis melo seeds, g)Nigella sativa, h)Marsdenia tenacissina, i )Curcuma longa, j)Silybum marianum. k) Wheatgrass, l)Teucrium polium .
.
a) Allium ascalonicum, b) Black rice, c) Cinnamon, d)Oak, e)Peganum harmala, f)Cucumis melo seeds, g)Nigella sativa, h)Marsdenia tenacissina, i )Curcuma longa, j)Silybum marianum. k) Wheatgrass, l)Teucrium polium .
Black rice
Black rice (Figure 1b) contains a high level of anthocyanin and is widely used as a health-promoting food in some parts of the world. Studies have shown that black rice extract has beneficial effects against breast cancer in laboratory conditions. Anthocyanin-rich extract from black rice (AEBR) increases cytochrome C secretion which induces cell apoptosis, reduces the stability of cancer cells and also has cytotoxic effects. AEBR reduces matrix metalloproteinase 2 (MMP2), matrix metalloproteinase 9 (MMP9), and urokinase plasminogen activator (uPA) expression in mouse tumor tissues and also restrains VEGF activity and thus angiogenesis in the tumor tissue.32-34
Cinnamon
Cinnamon (Figure 1c) is a spice obtained from the inner bark of trees called Cinnamomum. It is used in foods as well as medicine. Procyanidin oligomers, as the active ingredients of cinnamon, inhibit kinase activity of purified VEGFR2. CE reduces the proliferation of cancer cells by increasing the expression of tumor necrosis factor and interferon gamma and reducing the expression of HIF which is involved in angiogenesis. EC significantly prevents transcription and translation of growth factors (EFG, VEGF, TGF-β).35 In a study by Kwon et al in 2010 on human melanoma cells and mouse melanoma, it was shown that cinnamon extract down-regulated activator protein 1(AP1) and nuclear factor kappa B(NF-KB) levels and also increased apoptosis rate in various cancer cells such as lymphoma, cervical cancer, and colorectal cancer.
Oral administration of cinnamon extract in a melanoma model, exerted a significant anti-tumor effect and inhibited tumor growth.36-39 According to available information, the plant and its derivatives have antiseptic activity and play a role in regulating apoptosis.
Oak
Oak (Figure 1d) is a shrub from the family of beech. Oak is a plant extensively used in pharmacy. The hydro-alcoholic extract from oak’s corm shell possesses antibacterial properties. In addition, this extract reduces MMP9 expression and also inhibits VEGF secretion from tumor cells.40 Therefore, oak can exert inhibitory effects on tumor growth by inhibiting factors involved in angiogenesis.
Peganum harmala
Peganum harmala (Figure 1e) is a plant of the family Nitrariaceae usually used in traditional Iranian medicine as a treatment for various types of cancers. It also has antiviral, anti-microbial, anti-nociceptive, and anti-inflammatory activities. The hydroalcoholic extract of this plant has strong anti-angiogenic effects as well; achieved through inhibiting VEGF secretion. Harmane is a naturally occurring ß-carboline extracted from Peganum harmala that can significantly decrease the expression of pro-inflammatory cytokines and pro-angiogenic factors such as NO and VEGF. The P. harmala extract can induce apoptosis and inhibit tumor growth in vitro by affecting BCL-2 and P-Akt genes expression. Moreover, harmane has been shown to decrease NF-KB, MMP2, and MMP9 expression. These results show that HM acts as an anti-angiogenic factor in preventing cancer.41-43
Cucumis melo seeds
Melon (Figure 1f) is a native Iranian plant with cytotoxic, antioxidant, anti-inflammatory, and anti-fungal effects. Trypsin inhibitors from C. melo seeds (TICMS) inhibit endothelial cell migration and cell proliferation of human umbilical vein endothelial cells (HUVECs). TICMS affect the secretion of MMP2, MMP9 and VEGF from HUVEC and prevents their function. Therefore, it could be considered as an angiogenesis inhibitor.44,45
Nigella sativa
Nigella sativa (black caraway) (Figure 1g) is an annual flowering plant in the family Ranunculaceae. It is known for its antioxidant, anti-inflammatory,46 immunomodulatory,47 and neuroprotective48 properties. Thymoquinone is a phytochemical compound found in Nigella sativa capable of inhibiting NF-KB activation and also the expression of MMPs, VEGF, and cyclin D1.
Other studies have also shown that this plant prevents transcription of the angiogenesis factors of VEGF and HIF1α. In addition, it decreases the activity level of the enzymes MMP2 and MMP9.49,50
Marsdenia tenacissima
The stem of Marsdenia tenacissima (Figure 1h), also known as ‘Tong-guan-teng’ in traditional Chinese medicine (TCM),51 is often used to treat cough, expectorant, asthma, esophageal cancer, lung cancer, gastric cancer, and hepatocellular carcinoma.52,53 Laboratory studies indicate that the compounds found in this plant inhibit angiogenesis by reducing VEGF and MMP2,9 expressions. Moreover, it induces apoptosis in cancer cells. The use of this plant on A20 mouse lymphoma shows that Marsdenia tenacissima extract (MTE) associates with suppressed tumor growth and decreased angiogenesis in A20 mouse lymphoma model.53,54
Curcuma longa
Curcumin is a compound extracted from the Curcuma longa (Figure 1i) that interacts with cancer cells in different levels. Its anti-metastatic effects are partly due to decreased MMP expression and increased TIMP1 expression. Studies have also shown that this compound inhibits the transcription of angiogenic factors of VEGF and bFGF and, in addition, inhibits NO production (in endothelial cells, which plays an important role in tumor angiogenesis and growth).55,56
Other activities of this combination include binding to CD13 antibody expressed by components of blood vessels and inhibiting its activity, down expression of VEGF genes, 9-MMP, and inhibition of VEGF and EGF receptors. It is also counteract the intracellular signaling pathway of tyrosine kinases.57
Silybum marianum
Silymarin are polyphenolic flavonoids isolated from fruits and seeds of Silybum marianum (Figure 1j). Researchers have concluded that silymarin has antitumor activity by reducing VEGF and EGFR expression.
Silymarin inhibits angiogenesis and metastasis due to the accumulation of phenols via PI3. These results suggest that silymarin may be a candidate for cancer prevention.58,59
Wheatgrass
Wheatgrass (Figure 1k) is a young tender grass of common wheat (Triticum aestivum ). Its anti-metastasis effects is partly mediated through decreasing expression of the enzymes MMP2,9 and COX-2 and also increasing the enzyme tissue inhibitor of metalloproteinases 1 (TIMP1). Studies have also demonstrated that this compound prevents the transcription of the angiogenesis factor VEGF.60 Wheatgrass inhibits the process of angiogenesis through accumulation of polyphenols via PI3K/AKT pathway. Thus, it could help to restrain angiogenesis and metastasis.61
Teucrium polium
The Teucrium polium (Figure 1l) is a wild-growing flowering plant found in Europe and southwestern Asia. T. polium has been applied in Iranian traditional medicine for treating multitude of diseases due to its pharmacological properties. This plant has been reported to have hypolipidemic62 hypoglycaemic 63 anti-nociceptive64 anti-oxidant, anti-bacterial, anti-fungal, anti-septic, and anti-inflammatory65 potentials. There has also been an anti-angiogenic feature of T. polium extract reported which is attributed to a decreased NO secretion by HUVECs. Aside from blocking NO secretion and proliferation inhibition, T. polium can also trigger apoptosis by increasing Bax (a pro-apoptotic factor) expression and decreasing Bcl-2 expression (an anti-apoptotic factor).63 In 2019, Askari et al reported that the T. polium extract inhibited HUVEC cell growth in vitro and also VEGF secretion. These results show that T. polium could be a candidate for angiogenesis prevention.66
Plants rich of quercetin
Quercetin is a powerful flavonoid that has a wide range of benefits to human health including its ability to reduce inflammation, relieving pain, protection against cardiovascular diseases, preventing certain cancers, and boosting the immune system. Perhaps the most important of all could be its potent antioxidant activity. This compound, like other flavonoids, binds free radicals in the body and neutralizes them before they can cause any damages. Most importantly, it inhibits angiogenesis by interacting with VEGF, cyclooxygenase 2, and lipoxygenase 5. It could also induce cancer cell death through inhibiting Akt, mTOR, and HIF-1. A study on the other hand, shows that the antioxidant property of quercetin protects endothelial cells against high glucose levels which activates an autophagy response in them. However, the precise acting mechanism of quercetin has not been fully elucidated.67
Plants rich of carvacrol
Carvacrol is a phenol, a derivative of natural monoterpene with antioxidant and anticancer effects found in many plants such as wild bergamot, thyme, and tomato. Carvacrol has a potent anti-inflammatory effect exerted through inhibiting COX-2 enzyme and plays a role in reducing oxidative stress.68 This compound, by activating apoptosis, has an anti-proliferative effect on lung and breast cancer cells. Carvacrol significantly inhibits cell migration by blocking the phosphorylation of FAK and MMP-9 and MMP-2 and inhibited angiogenesis. In contrast, other studies have reported results of a VEGF-related angiogenesis induction by this compound. The reason behind such inconsistency could be related to the dose of carvacrol used as it reduces and inhibits MMP2 and 9 levels at high doses while increasing migration and stimulating angiogenesis at low doses.69
Extracts of Chinese herbs
So far, numerous herbs that have been traditionally administered as anti-cancer medications in China are screened in various laboratory model settings to find whether they have anti-angiogenesis properties. Table 3 includes a list of plants whose anti-angiogenesis effects have been shown using bovine aortic endothelial cell culture assay as well as chicken chorioallantoic membrane model of angiogenesis.12,70
Table 3.
Chinese herbs with anti-angiogenesis effects
12,58
|
Name
|
Part used
|
% Inhibition BAECC
|
% Inhibition CAM
|
|
Taxus chinensis
|
Bark |
26 |
- |
|
Catharanthus roseus
|
Leaf |
30 |
27 |
|
Scrophularia ningpoensis
|
Root |
34 |
20 |
|
Polygonum cuspidatum
|
Whole plant |
28 |
- |
|
Coptis chinensis
|
Rhizome |
37 |
25 |
|
Berberis paraspecta
|
Root |
38 |
25 |
|
Scutellaria baicalensis
|
Root |
41 |
2 |
CAM: chick embryo chorioallantoic membrane assay; BAECC: bovine aortic endothelial cell culture assay.
Molecular mechanisms of angiogenesis
Angiogenesis process is initiated by the activation of growth factors such as VEGF, Platelet-derived growth factor, bFGF, transforming growth factor-β (TGF-β), keratinocyte growth factor, hepatocyte growth factor, ephrin-B2, and angiopoietin. Hypoxia and the activated signaling pathway of HIF in tumor cells is an important stimulator for angiogenesis. HIF-1α and HIF-2α regulate the expression of pro-angiogenic genes including TIE-2, Ang1, Ang2, and VEGF. As already mentioned, VEGF is a target gene of HIF-1α which induced-expression by HIF-1α in endothelium leads to the activation of a VEGF-related autocrine signaling pathway. This cascade is involved in the survival and proliferation of endothelial cells. VEGF on the other hand, increases the permeability of blood vessels while modulating the secretion of extracellular matrix degrading enzymes. The latter in turn leads to the expansion of the vascular system. VEGF exerts its physiologic effects through binding homologues receptors of VEGFR1 and VEGEFR2 on endothelial cells to finally activate them. Upon activation, endothelial cells secret certain types of metalloproteinases that break down the basement membrane to facilitate endothelial cell’s migration. Once the extracellular matrix is degraded and being rearranged, tubulogenesis and therefore angiogenesis are triggered by angiopoietin TIE-2, a regulator of VEGF.66,71,72
Conclusion
The resistance of cancers to common treatments have always been a troublesome matter for specialists. Thus, researchers have focused a major part of their efforts on discovering and identifying new anti-cancer agents that could increase the sensitivity of cancer cells to drugs. Resistance of cancer cells to chemical medications has led to a reduction in their response to medications and as a result, failure of therapeutics. Therefore, investigating and development of more effective medications or those with fewer and less intense side-effects are of great necessity nowadays. So far, several chemical medications have been directly or indirectly derived from natural compounds found in extracts of plants. Some of the cytotoxic chemotherapy agents that are currently being administered have been designed to inhibit angiogenesis and leave the minimum toxicity in low doses. This strategy may provide a chance for patients with advanced cancers to have a longer life of a higher quality. This kind of treatment with low doses is termed metronomic dose. Metronomic model of traditional chemotherapy shows that it is also possible to administer herbal compounds reacting with the angiogenesis process to likely reinforce the positive effects of conventional chemotherapy. In other words, targeting the endothelium of vessels using non-toxic therapeutic agents with low and continuous doses can control the propagation of tumor without causing extra toxicity. The potential role of such treatments in order to extend patients’ survival and improve their quality of life needs further thorough investigation and research in clinical trials. Thus, clinical experts are also interested in identifying compounds that specifically fight different stages of angiogenesis when administered in low doses. These agents may somehow have lower toxicity in lower doses and they will most probably result in better therapeutic outcomes. The number of researches on herbs as treatments for various cancers is extensively growing because of their long-term therapeutic effects. Furthermore, researches on new herbs with anti-cancer effects look quite promising and will hopefully lead to the discovery of novel anti-cancer medications with herbal origin in the near future which would be a significant achievement in the field.
Several plants have been reported to exhibit anti-angiogenic properties through various molecular pathways (Figure 2 and Table 4).
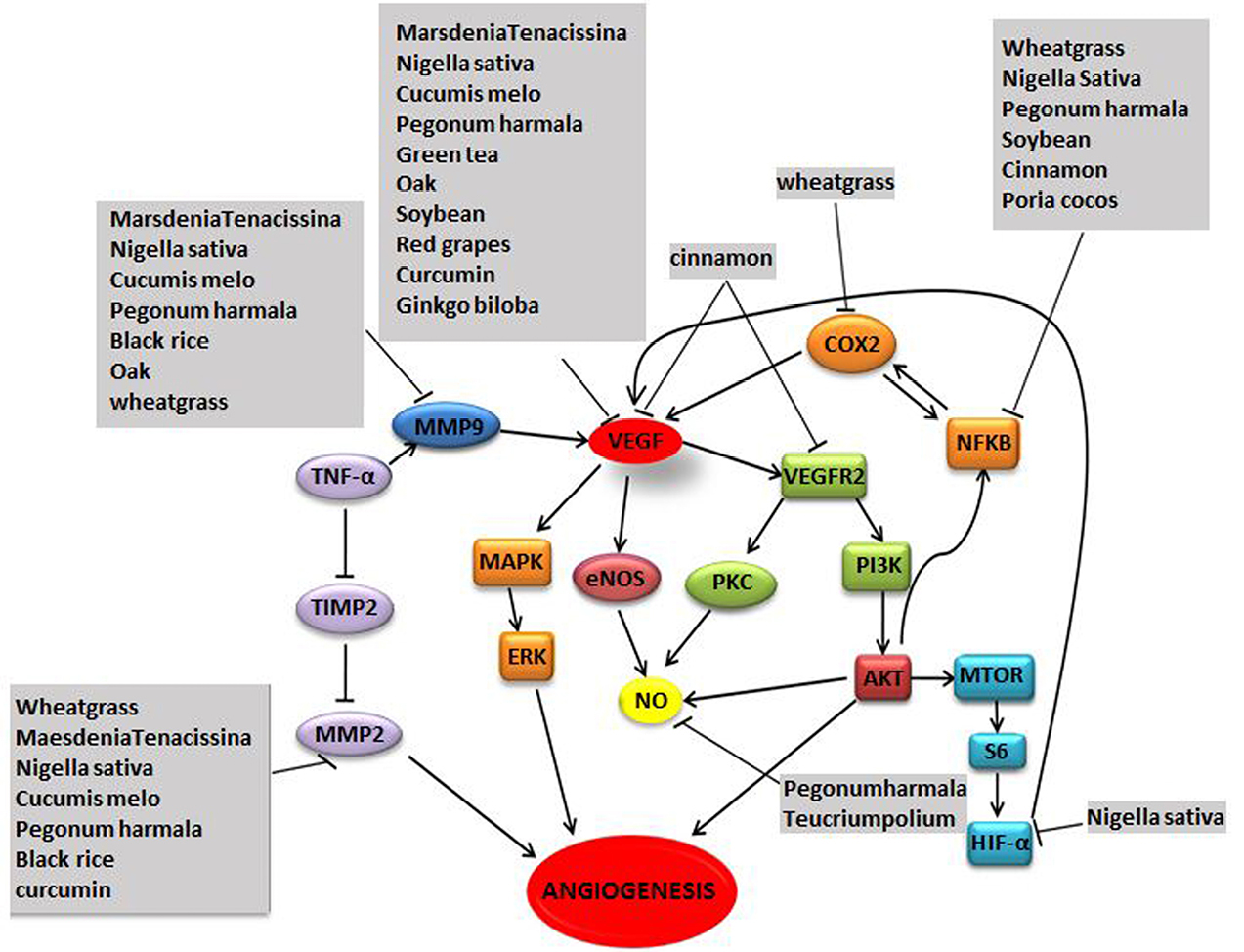
Figure 2.
Angiogenesis pathways targeted by plants.
.
Angiogenesis pathways targeted by plants.
Table 4.
Angiogenesis inhibitory plants
|
Plant
|
In Vitro
|
In Vivo
|
Possible mechanism
|
| Black rice |
MCF-7, MDA-MB-231, MDA-MB-453 |
Xenografted MDA-MB-453 Cells in Athymic Mic |
Suppresses MMP2, MMP9 and uPA expression 31 |
| Cinnamon |
Lymphoma, Melanoma, Cervix cancer, colorectal cancer and HUVEC |
Mouse melanoma model |
Suppresses VEGF and VEGFR2 expression, inhibits receptor tyrosine kinase, inhibits activation of NF-kB and AP1 signalling pathways 34,36 |
| Oak |
Endothelial cells |
Unknown |
Decreases VEGF secretion from tumor cells, Inhibits MMP9 expression in tumor cells 38 |
|
Pegonum harmala
|
Endothelial cells, MDA-MB-231 |
Unknown |
Suppresses VEGF and MMP2,9 expression, inhibits activation of NF-kB40 |
|
Cucumis melo seeds |
Endothelial cells |
Wistar rats with gastric ulcer73 |
Inhibits VEGF and MMP2, 9 expression42 |
|
Nigella sativa
|
Unknown |
Spinal cord injury in rats, Wistar with thickness burn rat with wound |
Decreases HIF1α and VEGF expression, inhibits activation of NF-kB, decreases enzymatic activity of MMP2, 947,48 |
|
Marsdenia tenacissima
|
Huvecs |
Chick embryo chorioallantoic membrane (CAM) |
Inhibits VEGF and MMP2, 9 expression, induces apoptosis in cancer cells51 |
| Wheatgrass |
HEp-2 cell |
Unknown |
Inhibits COX2 and MMP2, 953 |
| Teucrium polium |
Huvecs |
Visceral pain model in mice |
Causes EC apoptosis by reducing Bcl-2 expression, reduces NO secretion by HUVECs55 |
|
Poria cocos
|
RAW 264.7 cells |
Unknown |
Inhibits activation of NF-kB74 |
| Green tea |
MDA-MB231, MCF7, HUVEC |
C57Bl6 mice |
Abrogates VEGF signaling by interfering with VEGF formation75,76 |
|
Silybum marianum
|
MCF-7 and MDA-MB-468, HUVEC |
Ovarian cancer xenografts |
Decreases EGFR and VEGF expression58 |
|
Curcuma longa
|
Endothelial cells macrophage |
HepG2 xenografts, mouse corneal |
Inhibits the transcription of angiogenic factors VEGF, EGFR and BFGF, decreased MMP expression and increased TIMP55 |
Ethical Issues
Not applicable.
Conflict of Interest
None declared.
Acknowledgments
We specially thank Dr Hamid Rza Mohammadimotlagh, Rezvan Asgari and Azadeh mahnam for their valuable discussions and help with the manuscript preparation.
References
- Ichikawa H, Nakamura Y, Kashiwada Y, Aggarwal BB. Anticancer drugs designed by mother nature: ancient drugs but modern targets. Curr Pharm Des 2007; 13(33):3400-16. doi: 10.2174/138161207782360492 [Crossref] [ Google Scholar]
- Asadi-Samani M, Kooti W, Aslani E, Shirzad H. A systematic review of Iran’s medicinal plants with anticancer effects. J Evid Based Complementary Altern Med 2016; 21(2):143-53. doi: 10.1177/2156587215600873 [Crossref] [ Google Scholar]
- Shariatifar N, Chamanzari H, Ghanay M S. The study of flos plant on progmastigote in culture. The Horizon of Medical Sciences 2006; 11(4):5-9. [ Google Scholar]
- Karamysheva AF. Mechanisms of angiogenesis. Biochemistry (Mosc) 2008; 73(7):751-62. doi: 10.1134/s0006297908070031 [Crossref] [ Google Scholar]
- Abdelrahim M, Konduri S, Basha R, Philip PA, Baker CH. Angiogenesis: an update and potential drug approaches (review). Int J Oncol 2010; 36(1):5-18. [ Google Scholar]
- Rao BN. Bioactive phytochemicals in Indian foods and their potential in health promotion and disease prevention. Asia Pac J Clin Nutr 2003; 12(1):9-22. [ Google Scholar]
- Ingber D, Fujita T, Kishimoto S, Sudo K, Kanamaru T, Brem H. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 1990; 348(6301):555-7. doi: 10.1038/348555a0 [Crossref] [ Google Scholar]
- Kruger EA, Duray PH, Price DK, Pluda JM, Figg WD. Approaches to preclinical screening of antiangiogenic agents. Semin Oncol 2001; 28(6):570-6. doi: 10.1016/s0093-7754(01)90026-0 [Crossref] [ Google Scholar]
- Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol 2005; 100(1-2):72-9. doi: 10.1016/j.jep.2005.05.011 [Crossref] [ Google Scholar]
- Mukherjee AK, Basu S, Sarkar N, Ghosh AC. Advances in cancer therapy with plant based natural products. Curr Med Chem 2001; 8(12):1467-86. doi: 10.2174/0929867013372094 [Crossref] [ Google Scholar]
- Appelmann I, Liersch R, Kessler T, Mesters RM, Berdel WE. Angiogenesis inhibition in cancer therapy: platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) and their receptors: biological functions and role in malignancy. Recent Results Cancer Res 2010; 180:51-81. doi: 10.1007/978-3-540-78281-0_5 [Crossref] [ Google Scholar]
- Mohammadi-Motlagh HR, Mansouri K, Mostafaie A. Plants as useful agents for angiogenesis and tumor growth prevention. Physiol Pharmacol 2010; 14(3):297-312. [ Google Scholar]
- Mostafaie A, Mohammadi-Motlagh HR, Mansouri K. Angiogenesis and the models to study angiogenesis. Yakhteh Medical Journal 2010; 11(4):374-81. [ Google Scholar]
- Cooke JP. NO and angiogenesis. Atheroscler Suppl 2003; 4(4):53-60. doi: 10.1016/S1567-5688(03)00034-5 [Crossref] [ Google Scholar]
- Pratheeshkumar P, Kuttan G. Nomilin inhibits tumor-specific angiogenesis by downregulating VEGF, NO and proinflammatory cytokine profile and also by inhibiting the activation of MMP-2 and MMP-9. Eur J Pharmacol 2011; 668(3):450-8. doi: 10.1016/j.ejphar.2011.07.029 [Crossref] [ Google Scholar]
- Ruhrberg C. Endogenous inhibitors of angiogenesis. J Cell Sci 2001; 114(Pt 18):3215-6. [ Google Scholar]
- Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem 1998; 273(21):13313-6. doi: 10.1074/jbc.273.21.13313 [Crossref] [ Google Scholar]
- Yoon SO, Park SJ, Yun CH, Chung AS. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J Biochem Mol Biol 2003; 36(1):128-37. doi: 10.5483/bmbrep.2003.36.1.128 [Crossref] [ Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore!. Curr Opin Cell Biol 2001; 13(5):534-40. doi: 10.1016/s0955-0674(00)00248-9 [Crossref] [ Google Scholar]
- Angiogenesis markers in gynecological tumors and patents for anti-angiogenic approach: review. Recent Pat Anticancer Drug Discov 2015; 10(3):298-307. doi: 10.2174/1574892810999150827153642 [Crossref] [ Google Scholar]
- Mousa L, Salem ME, Mikhail S. Biomarkers of angiogenesis in colorectal cancer. Biomark Cancer 2015; 7(S1):13-9. doi: 10.4137/bic.s25250 [Crossref] [ Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 1996; 16(9):4604-13. doi: 10.1128/mcb.16.9.4604 [Crossref] [ Google Scholar]
- Ghavamipour F, Shahangian SS, Sajedi RH, Arab SS, Mansouri K, Aghamaali MR. Development of a highly-potent anti-angiogenic VEGF8-109 heterodimer by directed blocking of its VEGFR-2 binding site. FEBS J 2014; 281(19):4479-94. doi: 10.1111/febs.12956 [Crossref] [ Google Scholar]
- Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem 2006; 97(4):654-60. doi: 10.1016/j.foodchem.2005.04.028 [Crossref] [ Google Scholar]
- Yari K, Afzali S, Mozafari H, Mansouri K, Mostafaie A. Molecular cloning, expression and purification of recombinant soluble mouse endostatin as an anti-angiogenic protein in Escherichia coli. Mol Biol Rep 2013; 40(2):1027-33. doi: 10.1007/s11033-012-2144-4 [Crossref] [ Google Scholar]
- Mansouri K, Mirshahi M, Pourfathollah A, Hassan ZM, Taheripak R. Anti-plasminogen monoclonal antibody (MC2B8) inhibites angiogenesis. Pak J Biol Sci 2007; 10(19):3450-3. doi: 10.3923/pjbs.2007.3450.3453 [Crossref] [ Google Scholar]
- Gordaliza M. Natural products as leads to anticancer drugs. Clin Transl Oncol 2007; 9(12):767-76. doi: 10.1007/s12094-007-0138-9 [Crossref] [ Google Scholar]
- Ernst E. Harmless herbs? a review of the recent literature. Am J Med 1998; 104(2):170-8. doi: 10.1016/s0002-9343(97)00397-5 [Crossref] [ Google Scholar]
- Mohammadi-Motlagh HR, Mostafaie A, Mansouri K. Anticancer and anti-inflammatory activities of shallot (Allium ascalonicum) extract. Arch Med Sci 2011; 7(1):38-44. doi: 10.5114/aoms.2011.20602 [Crossref] [ Google Scholar]
- Mohammadi-Motlagh HR, Mansouri K, Shakiba Y, Keshavarz M, Khodarahmi R, Siami A. Anti-angiogenic effect of aqueous extract of shallot (Allium ascalonicum) bulbs in rat aorta ring model. Yakhteh Medical Journal 2009; 11(2):190-5. [ Google Scholar]
-
Mohammadi-Motlagh HR. The study of anti-angiogenic effects of shallot (Allium hirtifolium) extract and isolation of effective fraction [thesis]. Tabriz: Azarbaijan University of Tarbiat Moallem; 2008.
- Hui C, Bin Y, Xiaoping Y, Long Y, Chunye C, Mantian M. Anticancer activities of an anthocyanin-rich extract from black rice against breast cancer cells in vitro and in vivo. Nutr Cancer 2010; 62(8):1128-36. doi: 10.1080/01635581.2010.494821 [Crossref] [ Google Scholar]
- Phetpornpaisan P, Tippayawat P, Jay M, Sutthanut K. A local Thai cultivar glutinous black rice bran: a source of functional compounds in immunomodulation, cell viability and collagen synthesis, and matrix metalloproteinase-2 and -9 inhibition. J Funct Foods 2014; 7:650-61. doi: 10.1016/j.jff.2013.12.020 [Crossref] [ Google Scholar]
- Banjerdpongchai R, Wudtiwai B, Sringarm K. Cytotoxic and apoptotic-inducing effects of purple rice extracts and chemotherapeutic drugs on human cancer cell lines. Asian Pac J Cancer Prev 2014; 14(11):6541-8. doi: 10.7314/apjcp.2013.14.11.6541 [Crossref] [ Google Scholar]
- Finney-Brown T. Cinnamon extract suppresses tumor progression. Australian Journal of Medical Herbalism 2009; 21(2):49-50. [ Google Scholar]
- Lu J, Zhang K, Nam S, Anderson RA, Jove R, Wen W. Novel angiogenesis inhibitory activity in cinnamon extract blocks VEGFR2 kinase and downstream signaling. Carcinogenesis 2010; 31(3):481-8. doi: 10.1093/carcin/bgp292 [Crossref] [ Google Scholar]
- Cao H, Polansky MM, Anderson RA. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch Biochem Biophys 2007; 459(2):214-22. doi: 10.1016/j.abb.2006.12.034 [Crossref] [ Google Scholar]
- Kwon HK, Hwang JS, So JS, Lee CG, Sahoo A, Ryu JH. Cinnamon extract induces tumor cell death through inhibition of NFkappaB and AP1. BMC Cancer 2010; 10:392. doi: 10.1186/1471-2407-10-392 [Crossref] [ Google Scholar]
- Bansode RR, Leung T, Randolph P, Williams LL, Ahmedna M. Cinnamon extract inhibits angiogenesis in zebrafish and human endothelial cells by suppressing VEGFR1, VEGFR2, and PKC-mediated MAP kinase. Food Sci Nutr 2013; 1(1):74-82. doi: 10.1002/fsn3.13 [Crossref] [ Google Scholar]
- Yarani R, Mansouri K, Mohammadi-Motlagh HR, Mahnam A, Emami Aleagha MS. In vitro inhibition of angiogenesis by hydroalcoholic extract of oak (Quercus infectoria) acorn shell via suppressing VEGF, MMP-2, and MMP-9 secretion. Pharm Biol 2013; 51(3):361-8. doi: 10.3109/13880209.2012.729147 [Crossref] [ Google Scholar]
- Derakhshanfar A, Oloumi MM, Mirzaie M. Study on the effect of Peganum harmala extract on experimental skin wound healing in rat: pathological and biomechanical findings. Comp Clin Path 2010; 19(2):169-72. doi: 10.1007/s00580-009-0848-1 [Crossref] [ Google Scholar]
- Hamsa TP, Kuttan G. Harmine inhibits tumour specific neo-vessel formation by regulating VEGF, MMP, TIMP and pro-inflammatory mediators both in vivo and in vitro. Eur J Pharmacol 2010; 649(1-3):64-73. doi: 10.1016/j.ejphar.2010.09.010 [Crossref] [ Google Scholar]
- Yavari N, Emamian F, Yarani R, Mohammadi-Motlagh HR, Mansouri K, Mostafaie A. In vitro inhibition of angiogenesis by heat and low pH stable hydroalcoholic extract of Peganum harmala seeds via inhibition of cell proliferation and suppression of VEGF secretion. Pharm Biol 2015; 53(6):855-61. doi: 10.3109/13880209.2014.946057 [Crossref] [ Google Scholar]
- Rasouli H, Parvaneh S, Mahnam A, Rastegari-Pouyani M, Hoseinkhani Z, Mansouri K. Anti-angiogenic potential of trypsin inhibitor purified from Cucumis melo seeds: homology modeling and molecular docking perspective. Int J Biol Macromol 2017; 96:118-28. doi: 10.1016/j.ijbiomac.2016.12.027 [Crossref] [ Google Scholar]
- Mansouri K, Parvaneh S, Mostafaie A, Mahnam A, Mohammadi-Motlagh HR. Cancer: P-27: trypsin inhibitor purified from Cucumis melo seeds: a possible therapeutic perspective for tumor and angiogenesis related disease. Yakhteh Medical Journal 2011; 12 Suppl 1:51-2. [ Google Scholar]
- Al-Ghamdi MS. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J Ethnopharmacol 2001; 76(1):45-8. doi: 10.1016/s0378-8741(01)00216-1 [Crossref] [ Google Scholar]
- Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L seed. Int Immunopharmacol 2005; 5(13-14):1749-70. doi: 10.1016/j.intimp.2005.06.008 [Crossref] [ Google Scholar]
- Kanter M, Coskun O, Kalayci M, Buyukbas S, Cagavi F. Neuroprotective effects of Nigella sativa on experimental spinal cord injury in rats. Hum Exp Toxicol 2006; 25(3):127-33. doi: 10.1191/0960327106ht608oa [Crossref] [ Google Scholar]
- Randhawa MA, Alghamdi MS. Anticancer activity of Nigella sativa (black seed) - a review. Am J Chin Med 2011; 39(6):1075-91. doi: 10.1142/s0192415x1100941x [Crossref] [ Google Scholar]
- Bahramsoltani R, Farzaei MH, Rahimi R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: an integrative review. Arch Dermatol Res 2014; 306(7):601-17. doi: 10.1007/s00403-014-1474-6 [Crossref] [ Google Scholar]
- Lee H, Lin JY. Antimutagenic activity of extracts from anticancer drugs in Chinese medicine. Mutat Res 1988; 204(2):229-34. doi: 10.1016/0165-1218(88)90093-6 [Crossref] [ Google Scholar]
- Deng J, Shen F, Chen D. Quantitation of seven polyoxypregnane glycosides in Marsdenia tenacissima using reversed-phase high-performance liquid chromatography-evaporative light-scattering detection. J Chromatogr A 2006; 1116(1-2):83-8. doi: 10.1016/j.chroma.2006.03.021 [Crossref] [ Google Scholar]
- Huang Z, Lin H, Wang Y, Cao Z, Lin W, Chen Q. Studies on the anti-angiogenic effect of Marsdenia tenacissima extract in vitro and in vivo. Oncol Lett 2013; 5(3):917-22. doi: 10.3892/ol.2013.1105 [Crossref] [ Google Scholar]
- Hu YL, Wang SH, He CW, Liu TX. Research progress on anti-tumor properties of Marsdenia tenacissima. Tradit Med Res 2018; 3(4):202-13. doi: 10.12032/tmr201812079 [Crossref] [ Google Scholar]
- Hassan ZK, Daghestani MH. Curcumin effect on MMPs and TIMPs genes in a breast cancer cell line. Asian Pac J Cancer Prev 2012; 13(7):3259-64. doi: 10.7314/apjcp.2012.13.7.3259 [Crossref] [ Google Scholar]
- Jantaratnotai N, Utaisincharoen P, Piyachaturawat P, Chongthammakun S, Sanvarinda Y. Inhibitory effect of Curcuma comosa on NO production and cytokine expression in LPS-activated microglia. Life Sci 2006; 78(6):571-7. doi: 10.1016/j.lfs.2005.04.065 [Crossref] [ Google Scholar]
- Gururaj AE, Belakavadi M, Venkatesh DA, Marmé D, Salimath BP. Molecular mechanisms of anti-angiogenic effect of curcumin. Biochem Biophys Res Commun 2002; 297(4):934-42. doi: 10.1016/s0006-291x(02)02306-9 [Crossref] [ Google Scholar]
- Jiang C, Agarwal R, Lü J. Anti-angiogenic potential of a cancer chemopreventive flavonoid antioxidant, silymarin: inhibition of key attributes of vascular endothelial cells and angiogenic cytokine secretion by cancer epithelial cells. Biochem Biophys Res Commun 2000; 276(1):371-8. doi: 10.1006/bbrc.2000.3474 [Crossref] [ Google Scholar]
- Gallo D, Giacomelli S, Ferlini C, Raspaglio G, Apollonio P, Prislei S. Antitumour activity of the silybin-phosphatidylcholine complex, IdB 1016, against human ovarian cancer. Eur J Cancer 2003; 39(16):2403-10. doi: 10.1016/s0959-8049(03)00624-5 [Crossref] [ Google Scholar]
- Shakya G, Balasubramanian S, Hoda M, Rajagopalan R. Inhibition of metastasis and angiogenesis in Hep-2 cells by wheatgrass extract - an in vitro and in silico approach. Toxicol Mech Methods 2018; 28(3):205-18. doi: 10.1080/15376516.2017.1388460 [Crossref] [ Google Scholar]
-
Shakya G. Pleiotropic Effects of Wheatgrass on Cancer a Study to Unravel the Underlying Mechanisms and to Delineate the Potential Molecular Targets [thesis]. India: Pondicherry University; 2015.
- Sharififar F, Dehghn-Nudeh G, Mirtajaldini M. Major flavonoids with antioxidant activity from Teucrium polium L. Food Chem 2009; 112(4):885-8. doi: 10.1016/j.foodchem.2008.06.064 [Crossref] [ Google Scholar]
- Sheikhbahaei F, Khazaei M, Nematollahi-Mahani SN. Teucrium polium extract enhances the anti-angiogenesis effect of tranilast on human umbilical vein endothelial cells. Adv Pharm Bull 2018; 8(1):131-9. doi: 10.15171/apb.2018.016 [Crossref] [ Google Scholar]
- Abdollahi M, Karimpour H, Monsef-Esfehani HR. Antinociceptive effects of Teucrium polium L total extract and essential oil in mouse writhing test. Pharmacol Res 2003; 48(1):31-5. doi: 10.1016/s1043-6618(03)00059-8 [Crossref] [ Google Scholar]
- Tariq M, Ageel AM, al-Yahya MA, Mossa JS, al-Said MS. Anti-inflammatory activity of Teucrium polium. Int J Tissue React 1989; 11(4):185-8. [ Google Scholar]
- Askari V, Shamlou S, Mostafaie A, Khaleqi S. Ethyl acetate fraction of Teucrium polium extract abolishes human umbilical vein endothelial cells (HUVEC) tubulogenesis in collagen bed through suppression of cell proliferation/VEGF secretion. Iran J Allergy Asthma Immunol 2019; 18(3):281-8. doi: 10.18502/ijaai.v18i3.1121 [Crossref] [ Google Scholar]
- Rezabakhsh A, Rahbarghazi R, Malekinejad H, Fathi F, Montaseri A, Garjani A. Quercetin alleviates high glucose-induced damage on human umbilical vein endothelial cells by promoting autophagy. Phytomedicine 2019; 56:183-93. doi: 10.1016/j.phymed.2018.11.008 [Crossref] [ Google Scholar]
- Khan I, Bhardwaj M, Shukla S, Lee H, Oh MH, Bajpai VK. Carvacrol encapsulated nanocarrier/ nanoemulsion abrogates angiogenesis by downregulating COX-2, VEGF and CD31 in vitro and in vivo in a lung adenocarcinoma model. Colloids Surf B Biointerfaces 2019; 181:612-22. doi: 10.1016/j.colsurfb.2019.06.016 [Crossref] [ Google Scholar]
- Matluobi D, Araghi A, Faramarzian Azimi Maragheh B, Rezabakhsh A, Soltani S, Khaksar M. Carvacrol promotes angiogenic paracrine potential and endothelial differentiation of human mesenchymal stem cells at low concentrations. Microvasc Res 2018; 115:20-7. doi: 10.1016/j.mvr.2017.08.003 [Crossref] [ Google Scholar]
- Wang S, Zheng Z, Weng Y, Yu Y, Zhang D, Fan W. Angiogenesis and anti-angiogenesis activity of Chinese medicinal herbal extracts. Life Sci 2004; 74(20):2467-78. doi: 10.1016/j.lfs.2003.03.005 [Crossref] [ Google Scholar]
- Choi JY, Jang YS, Min SY, Song JY. Overexpression of MMP-9 and HIF-1alpha in breast cancer cells under hypoxic conditions. J Breast Cancer 2011; 14(2):88-95. doi: 10.4048/jbc.2011.14.2.88 [Crossref] [ Google Scholar]
- Lewis CE, De Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res 2007; 67(18):8429-32. doi: 10.1158/0008-5472.can-07-1684 [Crossref] [ Google Scholar]
-
Adebayo-Gege GI, Okoli BJ, Oluwayinka PO, Ajayi AF, Fanyana M, editors. Antiulcer and Cluster of Differentiation-31 Properties of Cucumis melo L. on Indomethacin-Induced Gastric Ulceration in Male Wistar Rats. In: Ramasami P, Gupta Bhowon M, Jhaumeer Laulloo S, Li Kam Wah H, eds. International Conference on Pure and Applied Chemistry. Cham: Springer; 2018. p. 501-16. 10.1007/978-3-030-20283-5_29
- Lee KY, You HJ, Jeong HG, Kang JS, Kim HM, Rhee SD. Polysaccharide isolated from Poria cocos sclerotium induces NF-kappaB/Rel activation and iNOS expression through the activation of p38 kinase in murine macrophages. Int Immunopharmacol 2004; 4(8):1029-38. doi: 10.1016/j.intimp.2004.03.014 [Crossref] [ Google Scholar]
- Leong H, Mathur PS, Greene GL. Green tea catechins inhibit angiogenesis through suppression of STAT3 activation. Breast Cancer Res Treat 2009; 117(3):505-15. doi: 10.1007/s10549-008-0196-x [Crossref] [ Google Scholar]
- Sartippour MR, Shao ZM, Heber D, Beatty P, Zhang L, Liu C. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr 2002; 132(8):2307-11. doi: 10.1093/jn/132.8.2307 [Crossref] [ Google Scholar]