Advanced pharmaceutical bulletin. 11(3):426-438.
doi: 10.34172/apb.2021.050
Review Article
Understanding Hyaluronan Receptor (CD44) Interaction, HA-CD44 Activated Potential Targets in Cancer Therapeutics
Gul-e-Saba Chaudhry 1, *  , Abdah Akim 2, Muhammad Naveed Zafar 3, Naila Safdar 4, Yeong Yik Sung 1, Tengku Sifzizul Tengku Muhammad 1
, Abdah Akim 2, Muhammad Naveed Zafar 3, Naila Safdar 4, Yeong Yik Sung 1, Tengku Sifzizul Tengku Muhammad 1
Author information:
1Institute of Marine Biotechnology, Universiti Malaysia Terengganu, 21030 Kuala Terengganu, Malaysia.
2Department of Biomedical Sciences, Universiti Putra Malaysia, Seri Kembangan, Selangor, Malaysia.
3Department of Chemistry, Quaid-i-Azam University, Islamabad, 45320, Pakistan.
4Department of Environmental Sciences, Fatima Jinnah University, Rawalpindi, Pakistan.
*Corresponding Author: Gul-e-Saba Chaudhry, Tel: +609-6683810, Fax: +609-6683810, Email:
gul.saba@umt.edu.my
Abstract
Cancer is a complex mechanism involving a series of cellular events. The glycoproteins such as hyaluronan (HA) are a significant element of extracellular matrix (ECM), involve in the onset of cancer developmental process. The pivotal roles of HA in cancer progression depend on dysregulated expression in various cancer. HA, also gain attention due to consideration as a primary ligand of CD44 receptor. The CD44, complex transmembrane receptor protein, due to alternative splicing in the transcription process, various CD44 isoforms predominantly exist. The overexpression of distinct CD44 isoforms (CD44v) standard (CD44s) depends on the tumour type and stage. The receptor proteins, CD44 engage in a variety of biological processes, including cell growth, apoptosis, migration, and angiogenesis. HA-CD44 interaction trigger survival pathways that result in cell proliferation, invasion ultimately complex metastasis. The interaction and binding of ligand-receptor HA-CD44 regulate the downstream cytoskeleton pathways involve in cell survival or cell death. Thus, targeting HA, CD44 (variant and standard) isoform, and HA-CD44 binding consider as an attractive and useful approach towards cancer therapeutics. The use of various inhibitors of HA, hyaluronidases (HYALs), and utilizing targeted Nano-delivery of anticancer agents and antibodies against CD44, peptides gives promising results in vitro and in vivo. However, they are in clinical trials with favourable and unfavourable outcomes, which reflects the need for various modifications in targeting agents and a better understanding of potential targets in tumour progression pathways.
Keywords: Apoptosis, Cancer pathways, CD44, Cell proliferation, Hyaluronan, Targeted therapeutics, Tumor progression, Nanotechnology
Copyright and License Information
©2021 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
ECM, a potential source of cancer progression initiators
The extracellular environment is surrounding the cells considered as extracellular matrices (ECMs) necessary for support and communication between cells (Figure 1). The extracellular matrices composed of various glycol-proteins, which play an essential role in structure as well as functional aspects. The components include; i) proteoglycans, ii) proteins including collagens (fibrillar), iii) elastins proteins, iv) fibronectins proteins and v) laminins and the particular class of glycosaminoglycans (GAGs).
1,2
These ECM components could act as ligand molecules, and due to ligand-receptor binding, the signal transduction pathways triggered that lead to a variety of disease propagation and cytoskeleton and chromatin structures organization aswell.
3-7
Hyaluronan (HA) belongs to glycosaminoglycans, an integral hydrophilic part of the matrix. The surrounding proteins interact with integrin protein receptors where CD44 and RHAMM receptors got special attention due to cancer development and growth.
8,9
The components in matrix regulate the fundamental process in the cell, any dysregulation considered as essential hallmarks of cancer.
10-17
Additionally, ECM regulates the expression of stromal cells and indirectly the tumorigenic microenvironment. Any dysregulation leads to the development of inflammation and the generation of a disease microenvironment.
13
The integrin-FAK signaling due to modification of the ECM results in reactivating dormant tumour cells.
18
However, alteration in ECM is quite often due to variation in the expression level of matrix-degrading enzymes
19
. The alterations in ECM promotes the formation of a tumorigenic microenvironment leads to metastasis.
20
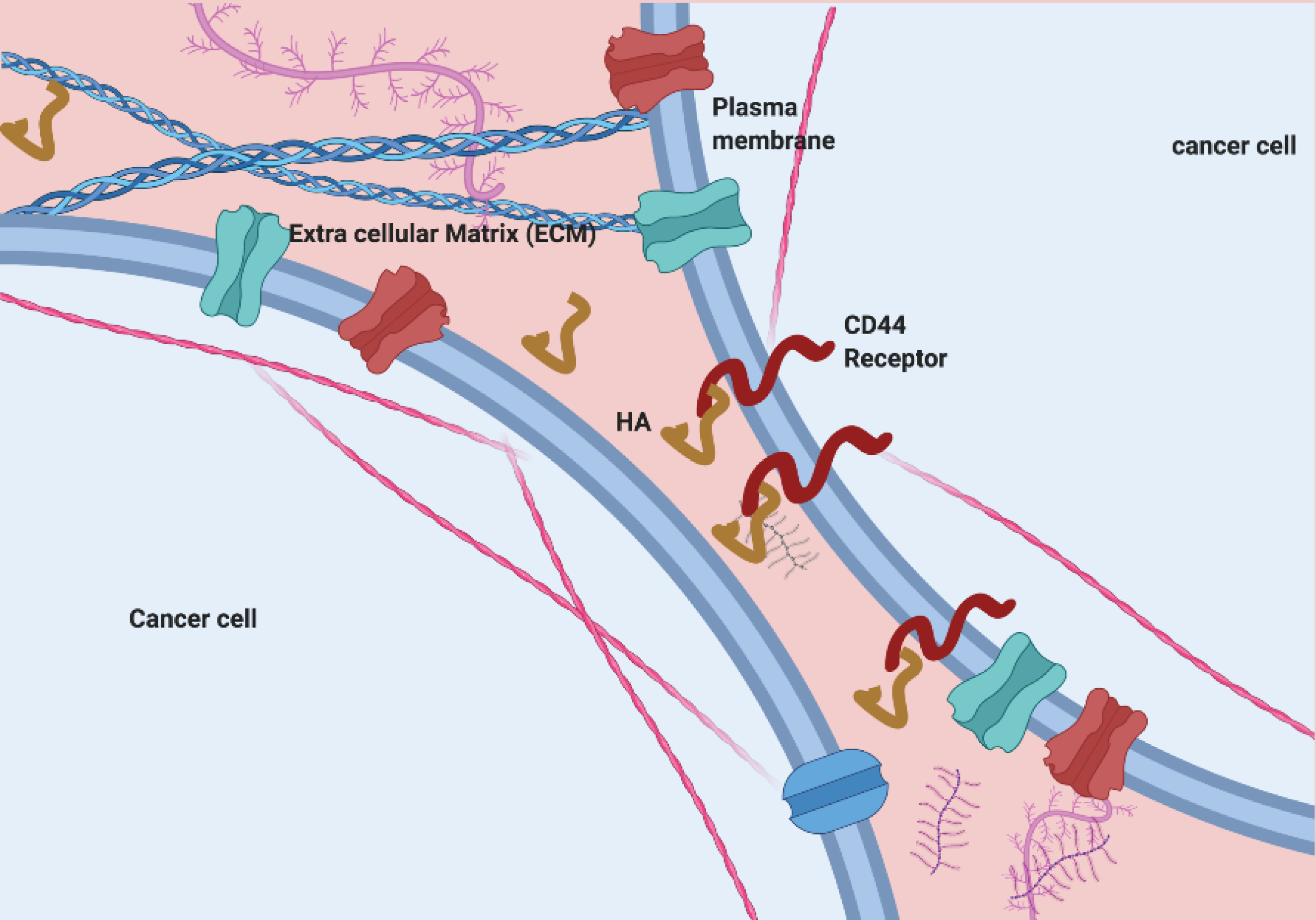
Figure 1.
ECM, potential site for HA-CD44 communication
.
ECM, potential site for HA-CD44 communication
A major component of ECM, Hyaluronan
HA is linear repeating units of β glucuronic acid and β-N-acetyl glucosamine. HA, a principal component of the ECM in cancer cells, as well as normal cells, involved in various developmental process.
21
Various enzymes involved in the synthesis of different molecular weight (MW) of HA. HA produced by HA synthases (HAS1-3). The disaccharides unit of N-acetyl-D-glucosamine and D-glucuronic acid polymerize via β-(1-3)-glucuronidic bond.
22
Similarly, HA synthases HAS2 produces the larger HA more than 2 MDa; and HAS1 and HAS3 produces smaller HA with a range of less than 2 MDa.
23
Along with synthases, hyaluronidases (HYAL1-3) fragment the higher MW HA to medium MMW (via HYAL2) and lower LMW (via HYAL1) into oHA.
24,25
Alteration in a post-translational modification in the HAS2 produce mutations involve the replacement of one amino acid asparagines by another amino acid serines. Also, hyaluronidases (HYALs) mutations have linked to tumour resistance in the naked mole-rat.
26,27
HA involve in various developmental processes such as cell growth, proliferation, migration, tissue development, embryogenesis, and wound healing.
28
HA is the primary ligand of CD44 receptors.
30
Various modifications in the splicing mechanism and glycosylation process produce of CD44 variants. These variants are differentiated by structural and functional aspects mainly responsible for a pro-inflammatory activity via cell-ECM component interacts.
22-25
The HA-receptor mediated signal transduction occurs via CD44, the primary receptor for HA-binding and RHAMM (receptor for HA mediated mobility), toll-like receptors (TLR2, TLR4), HA receptor for endocytosis (HARE), HA-binding protein 1 (HABP1), and lymphatic vessel endothelial receptor for HA 1 (LYVE1).
28-32
CD44 receptor
CD44 transmembrane protein, glycoprotein, encoded by 19 exons compromised gene positioned on chromosome 11 in humans.
33
The protein is a transmembrane protein with three visible domains includes i) outermost extracellular domain, ii) middle transmembrane domain, and iii) innermost intracellular domain. The N-terminal region is essential for cellular interaction with ECM components such as ligand HA and due to the presence of area responsible for the formation of the various variant. During post-transcription modifications, alternative splicing occurs in variant exons (nine exons) results in the formation of variant isoforms (CD44v). However, constant exons encode the CD44 standard (CD44s). A-part from hyaluronic acid (HA), CD44 receptor have an affinity towards other ligands including osteopontin, collagen, chondroitin, fibronectin, and sulfated proteoglycan.
34-38
The HA major ECM component is having a more binding affinity towards all isoforms of the CD44 receptor protein. The HA binds to CD44 (Figure 2) at extracellular domain causes conformational changes, which leads to activation of CD44 and recruitment of various other cytoplasmic and membrane-bound cytoplasmic proteins (adaptor molecules) trigger the downstream cell survival, growth and tumour progression pathways.
39
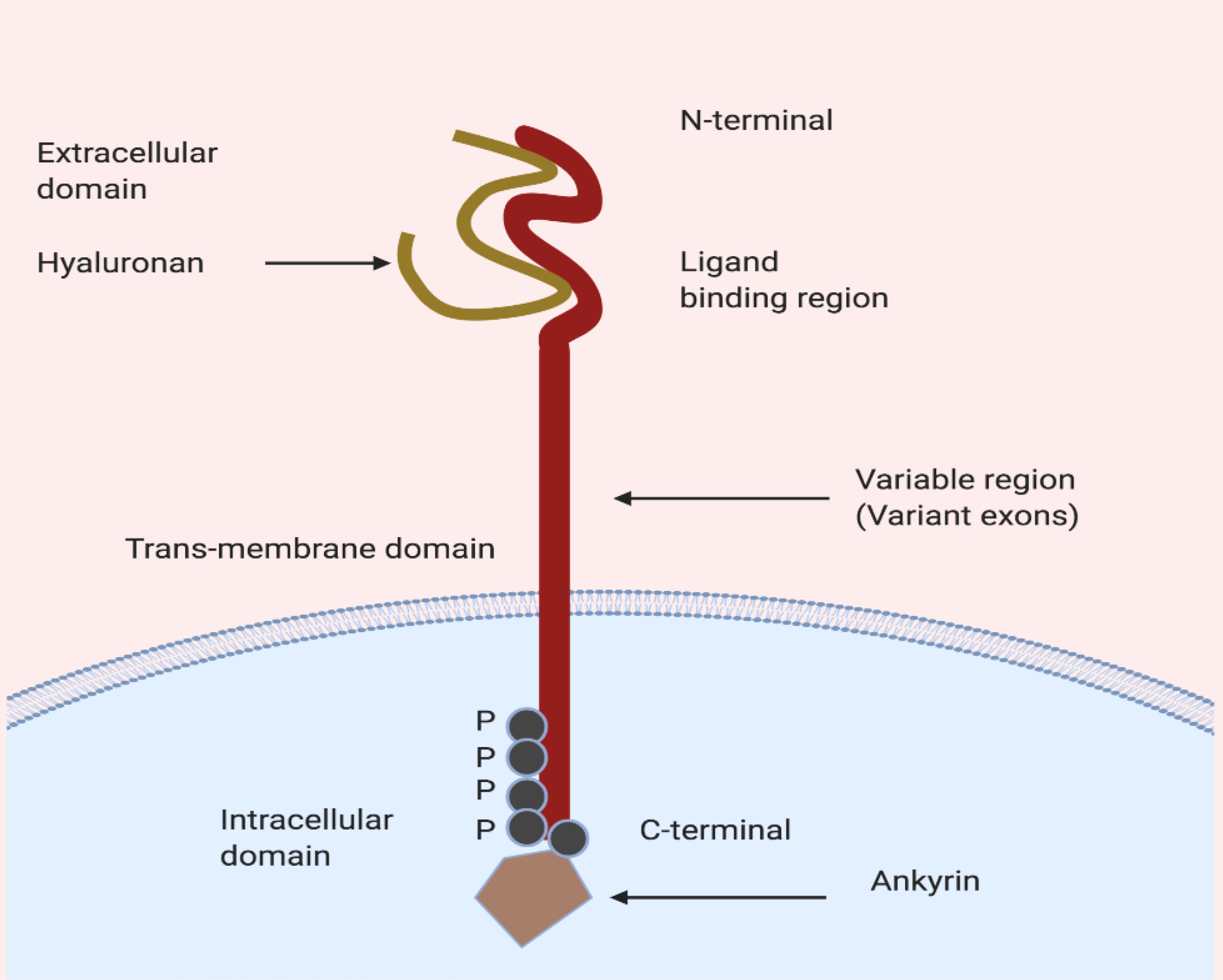
Figure 2.
Schematic presentation of HA binding to cytoplasmic domain of CD44.
.
Schematic presentation of HA binding to cytoplasmic domain of CD44.
HA-CD44 interaction pathways in cancer progression
HA interaction with CD44 receptor leads to cell survival, cell growth, invasion, and metastasis via signaling networks include; RhoGTPases and PI3K/AKT pathway
40
and other chemoresistance pathways via ROK activation (Figure 3). CD44 receptor triggers cell survival promoted the tumour metastasis. The HA ligand upon binding with CD44 triggers signal cascade via activating the cytoplasmic domain of CD44 and which results in the recruitment of various protein a series of cell signaling events. The CD44-cytoplasmic domain activated proteins such as ankyrin, merlin, and ERM involve in actin polymerization mediated by ERM proteins results in cytoskeleton rearrangements for tumour invasion and migration.
41
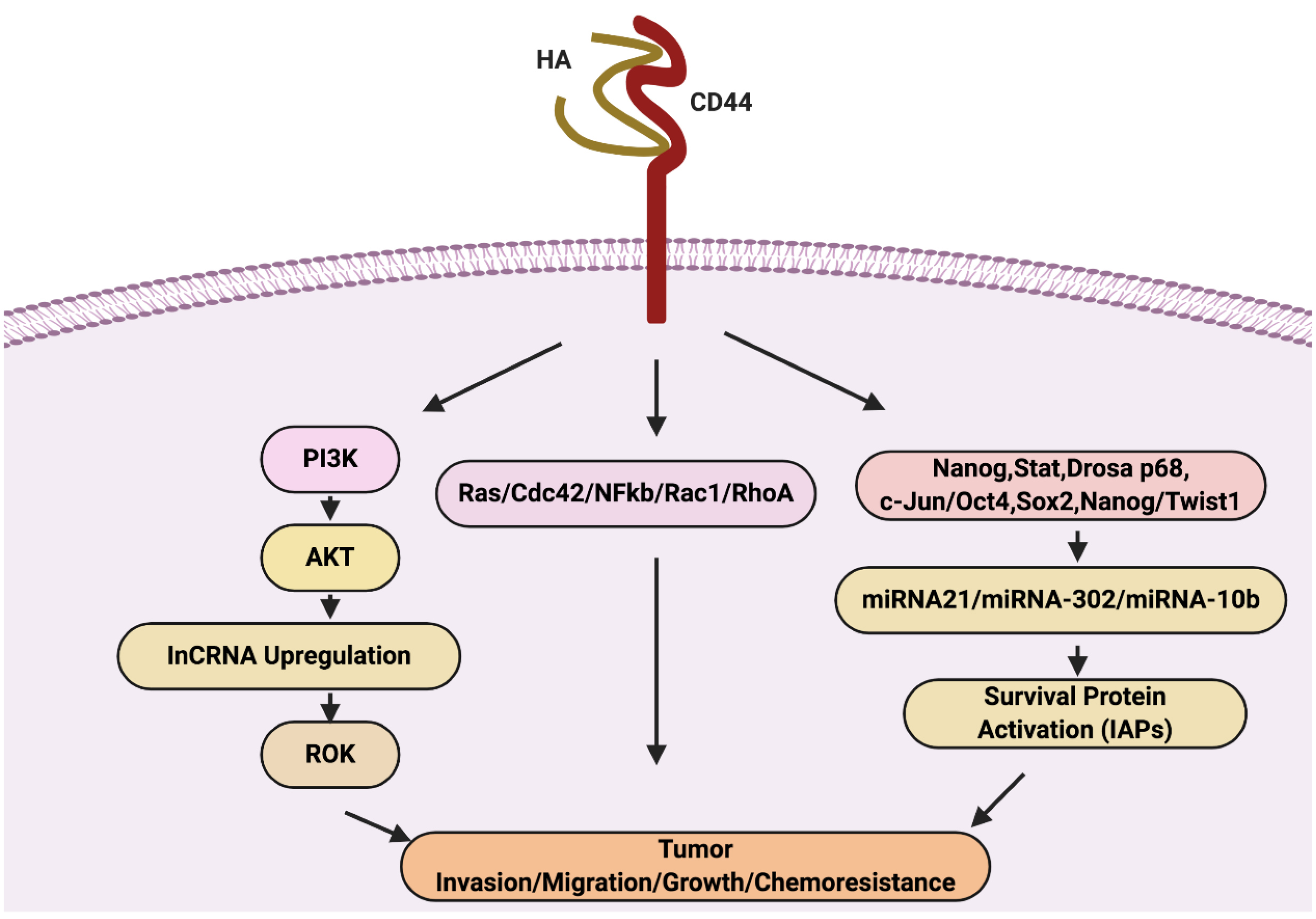
Figure 3.
Therapeutic targets of tumor growth, invasion, metastasis and chemo resistance.
.
Therapeutic targets of tumor growth, invasion, metastasis and chemo resistance.
CD44 interaction is associated with metastasis in several tumours.
42,43
The role of CD44 in tumour progression quite clear; however, its involvement in trigger apoptosis inhibits tumour invasion and progression. CD44 in inducing apoptosis and reducing cell invasion is HA MW dependable but still contradictory which show the response differ in differ tissue also along with MW.
44,45
The high molecular weight HA blocked invasion and small molecular weight oligomerized HA to initiate apoptosis and via inhibition the activation of cell survival and growth pathway (AKT) in breast, colon and mammary cancer cells.
46,47
Besides AKT, RhoGTPases triggers a cascade of events from cell surface receptors to downstream signals. RhoGTPases acts as response molecules which act in the response of signal or external stimuli and regulates the various process of cytoskeleton reorganization results in cell invasion and metastasis. RhoGTPases belong to Ras superfamily, includes RhoA, Rac1, and Cdc42. These Rho proteins exist in an inactive form and convert to active form upon binding of GTP molecules. The RhoGTPase involve in normal cell invasion and development. However, the overexpression in various human cancers link them to cancer progression and poor prognosis.
48-51
Additionally, an important kinase as Rho kinase (ROK) regulates the process of tumour invasion and metastasis via myosin light chain phosphatase. The myosin light chain phosphatase promotes the secretion of ECM degrading proteins as matrix metalloproteins (MMPs). The activation of MMPs via CD44 receptor protein help in invasion and also angiogenesis via degradation of collagen.
52,53
The MMPs include MMP-2 (type IV collagenase), MMP-9 (type V collagenase), MMP-3 (stromelysins) and MMP-1 (interstitial fibroblast-type collagenase) degrade ECM materials, crucial in tumor invasion and metastases.
HA/CD44 interaction mediates cytoskeleton activation via critical RhoA signaling. RhoA regulation cellular functions via alteration of intracellular Ca
2
levels. The intracellular Ca
2
mobilization mediated via essential phospholipase C (PLC). Upon activated, PLCs converted to inositol trisphosphate (IP3) via PIP2 (hydrolyzed), which results in the release of Ca
2
from deposit storage. This released Ca
2
stimulates cytoskeleton rearrangement and eventually tumour progression.
54-56
In breast tumour cells107-109, HA activation of CD44 leads to the expression of multidrug resistance gene (P-glycoprotein) and anti-apoptotic gene Bcl expression, which promotes tumour cell proliferation and survival.
57-59
Apoptosis is a highly regulated mechanism of cell death characterized by morphological and biochemical changes, including various enzymes proteins that lead to cell death and maintain cell growth.
60
However, the recent proposed HA-CD44 induced tumour progression signaling events in cancer by Bourguignon 2019.
61
The interaction and binding of HA to CD44v upregulate Ras/Cdc42/Rac1/RhoA/NFkB expression, and various cytokines production involve in tumour cell survival, growth, invasion, and migration. As we already discussed, HA-AKT activation stimulates PI3K/AKT activation also the production of LncRNA (UCA1). LncRNA triggered tumour cell migration and invasion via ROK. Moreover, HA/CD44 also responsible for chemoresistance via activation of transcriptional factor-induced miR-10b, miRNA-21, and miR-302 expression. The JNK activity causes activation of c-Jun via phosphorylation. The activated c-Jun binds to the miR-21 promoter and induces the up-regulation of miR-21 expression. Similarly, HA-CD44 interaction triggers the complex formation include; Nanog, Stat-3, DROSHA, and p68. This complex bind to the miR-21 promoter region, resulting in the expression of miR-21. The miRNA expression upregulates IAP protein, involved in tumour cell survival, and chemoresistance. Activated miR-21 stimulates upregulation in RhoC and ROK-regulated tumour cell migration and invasion. In contrast, activated miRNA-302 expression involves in chemoresistance also due to HA-CD44 interaction. HA binding to CD44 promotes the recruitment and build association between cytoplasmic domain of CD44v3 and OCT4/SOX2/Nanog. This OCT4/SOX2/Nanog induce IAP (cIAP-1, cIAP-2, and XIAP) expression via the upregulation of miR-302 gene expression. IAPs involve in tumour cell growth and chemoresistance in tumour cells. Moreover, the cytoskeleton activation by miRNA-10b expression trigger via HA-CD44 interaction. HA binding to CD44 promotes c-Src activation via phosphorylation, the activated c-Src causes phosphorylation of Twist. The activated Twist acts as TF, activate the mR-10b expression, promotes tumour cell migration, and invasion.
HA and CD44 role in Cancer regulation
The microenvironment outside the cell is a critical regulator of the fundamental growth of the cell. Alteration in cell growth trigger rapid growth and migration causes metastasis.
62
HA is biopolymer and the primary component of ECM, provides a hydrophilic part to the matrix and cellular support but gain special attention when it comes to cell survival, growth, and differentiation.
63
However, HA contributes to abrupt growth and invasion of cancer cells. Due to its hydrophilic nature, HA can form a protective lining around the tumour cell to protect the tumour cells from immune attack.
64,65
Any transcriptional or translational disruption in HA synthases or hyaluronidases can lead to over or fragmented production of HA. In several tumours, HAS and HYAL regulate the production of HA, which linked to tumour development and metastasis. Besides, HYAL fragmented HA some ROS-fragmented HA also found to be involved in overproduction of HA in tumours growth, invasion and spreading.
66
The over-expression of synthase HAS2 and HAS3 results in enhancing HA overproduction, which triggers the production sarcomas and other melanoma cells.
67,68
HA trigger the formation of new blood vessels and also circulation of tumour cells via growth and invasion seen in some studies. HA induce angiogenesis and lymphatic invasion in tumour transgenic mouse models.
69,70
Apart from the above concern, interstitial fluid pressure cause by HA also highlighted in drug delivery. By using HYALs, it degrades HA, reduces the pressure, and improve the efficacy of therapeutics.
71
The CD44 exists in various isoforms and gain much importance due to involvement in the pathogenesis of cancer. The variant form of CD44v could use as a biomarker for disease (cancer) prognosis. Several isoforms linked to specific cancer and play a role in cell growth and metastasis. The CD44v found to be involved in tumour progression and invasion. The overexpressed form of CD44v6 linked to invasion in endocrine-resistant breast cancer cells via activation of epidermal growth factor receptor signalling.
72
As cancer is a multistage disease, and the CD44v expression also have an association with various stages which ensure the precise involvement of CD44v in tumour progression. CD44v6v appearance only noticed in advanced stages of pancreatic cancer during the metastatic behaviour of cells and not visible in a benign tumour.
73
Increased expression of CD44v6 in pancreatic cancer link to metastasis.
74,75
The CD44v6 role in metastasis confirmed by transfection study confirms the involvement of CD44v6 in rat pancreatic adenocarcinoma.
76
Various treatment against CD44v6 linked metastasis include bFGF, EGF, TNFα, and TGF-β1(growth factors and cytokines) unable to reduce the surface expression of CD44v6 receptor. Similarly, interferon (IFN) γ also not wholly inhibit the expression of CD44v6.
77
The studies also confirm the CD44v as a survival marker. The presence of enhancing expression of CD44v6 link to shorter survival time as compared to tumours deprived of CD44v6.
78
Also, the positive expression of CD44v6 and CD44v9 together link to metastasis with low survival rate.
79,80
Also, the standard form of CD44 regulates the metastasis in tumour cells. Studies show that down-regulation of CD44s detected in human metastasis tissue.
Silencing the expression of CD44v9 (via short hairpin RNA) overturned the gathering of various proteins (HSP90, PI3K, and others) into lipid raft-like structures and attenuate the downstream signal cascade of tumour progression.
81
The isoforms CD44v6 and CD44v9 overexpressed in prostate cancer (PC3-epithelial cells).
82
The function of CD44v6 evaluated using small interfering RNA (siRNA) in prostate cancer cells and Knock-down of CD44v6 linked to increasing chemo/radiosensitivity.
83
Similarly, overexpression of CD44v3 isoform triggered a substantial increase in cell migration. In neck squamous cell carcinoma (HNSCC), elevated CD44v3 levels noticed.
72
The inhibitor study using, anti- CD44v3 antibody shows the reverse cisplatin resistance and decrease cell proliferation.
84
In colorectal cancer, CD44v6 and CD44v10 overexpression detected which linked to poor prognosis, cell migration and metastasis.
85-87
Various cytokines regulate CD44v6 expression, which activates Wnt/β-catenin pathway and promotes migration and metastasis.
88
Previous study shows that CD44v operates in a specific and overlapping manner and by studying CD44v help to understand the various variant as a potential target in drug delivery. The variant form CD44v8-10 involve in the protection of gastrointestinal cancer cells against ROS (reactive oxygen species), CD44v4-10 and CD44v8-10 promoted adenoma and gastric tumour initiation.
89,90
Similarly, another variant CD44v9 found to be overexpression gastric adenocarcinoma.
91
Overexpression of CD44v6 activate the phosphorylation of AKT, autophagy flux, and ERKs which result in cell survival and growth. The colon cancer cells treated with chemotherapy drugs with enhancing the expression of CD44v6 reduce cytotoxicity.
92
Strategies to target HA and CD44 interaction
HA as a therapeutic target
The rich level HA in various tumours and metastatic cancers responsible for cell growth proliferation, invasion, angiogenesis, metastasis along with acquiring resistance in response to anticancer agents. HA abundance in pancreatic ductal adenocarcinoma links to a lower survival rate. HA interaction with CD44 results in cell growth, invasion, and metastasis. To prevent the interaction and downstream pathways of cancer propagation, HA ligand, CD44 receptor with its variants, and HA-CD44 interaction could consider as important targets in drug development. In the case of HA, the strategies are i) hyaluronidases along with chemotherapeutics, ii) use of HA oligosaccharides (oHA) to induce apoptosis, iii) inhibitors of HA and iv) HA-base drugs.
HYALs are well-known enzymes that can fragment HA. Previous studies demonstrate that the expression of wild type HYAL-1 (HYAL-1-v1) induces apoptosis via G2-M arrest in bladder cancer cells.
60
However, due to adverse impact as an anti-adhesive on normal tissue, HYALs have limitations and side effects.
93
Interestingly, commercial bovine testicular or bacterial HYAL act as an anti-adhesive compound on EMT-6 tumour spheroids improve the chemosensitivity by increasing the accessibility.
94
In regards to drug therapeutics, HA also increase the intestinal fluid pressure and hinder the drug penetration in pancreatic ductal adenocarcinomas. The application of PEGPH20 increases vessel size and recover the increased interstitial fluid. The treatment of Gem+PEGPH20 in placebo control trials in KPC improves the survival rate and significant reduction of metastatic tumour burden.
95
Interestingly, HA oligosaccharides (oHA) trigger the induction of apoptosis in lymphoma cell lines via inhibition of downstream cancer survival pathways. The oligosaccharides (oHA) inhibited PIP3 production, involve in PI3-K activation and results in deactivation of Akt (via reduced phosphorylation) without involving NF-kB activation in lymphoma cells.
HA found to be abundant in most cancers. Previous studies show that 4-methylumbelliferone (MU) inhibitor of HA synthesis, deplete uridine diphosphate glucuronic acid, and the down-regulation of HAS human skin fibroblasts. Similarly, MU inhibits HA production in pancreatic cancer cells (KP1-NL) and decreases liver metastases in mice injected with pancreatic cancer cells.
96
4-Methylumbelliferone was then later shown to inhibit HA production and to have anticancer activities in mouse models of various cancers. However, the inhibition depends on MW of HA, as HMW-HA revoke the inhibitory and non-metastatic effects of MU on MIA PaCa-2 cells. Studies have shown that MU inhibits HA synthesis in at least two ways. First, MU depletes UDP-GlcUA, which is a precursor of HA.
97
Second, MU reduces the expression of HAS mRNA.
98
However, no studies have described the mechanisms through which MU reduces HAS mRNA expression. However, more studies need to do as the MU in inhibition of overall HAS mRNA and effect on CD44 expression still not clear yet. Some reports have indicated that MU suppresses HAS mRNA expression,
99-101
whereas another report stated that HAS mRNA expression upregulated.
102
However, its right to be said that the effect of MU on expression depends on various cell types with specific up-regulation or down-regulation.
HA, a versatile biopolymer, increases the solubility of poorly soluble anticancer drugs. HA improve the efficacy of anticancer drugs with improving solubility and biocompatibility via various mode as follows; i) HA-conjugate drugs, ii) HA-encapsulated drugs, iii) gagomers, iv) micelles and v) Nanocarriers. The paclitaxel (PTX), the potent antimitotic agent inhibits cell growth, but due to poor water solubility limits its application in drug therapeutics. The HA-PTX conjugated drug name as HYTAD1-p20 with improve solubility and biocompatibility in the bladder and other cancers.
103,104
Similarly, HA-conjugated-PTX-N-hydroxysuccinimide ester (PTX-NHS) and HA-SN-38 (CPT11 (irinotecan) metabolite) used against CD44 expressing cell line (colon, breast, gastric and others) reduced tumour cell growth and metastasis.
105-109
The side effects of potential anticancer also hinder the application of potential compounds in drug delivery. However, in-vitro and in-vivo HA encapsulated drugs, HA-5-fluorouracil (HyFIVETM) and HA-doxorubicin (5- FU) show reasonable cytotoxic efficacy, undergoing clinical trials.
110,111
Self-assemble Lipid molecule coated with hyaluronan (HMW) as liposomes or lipoplexes loaded drug deliver plasmid DNA and siRNA (small
interfering RNA) also used against several cancers including head and cancer, expressed CD44 receptor.
112-117
The HA micelles are self-organized structure, HA polymer (carboxyl group) conjugate to another polymer, i.e., PEG (polyethylene) amino group. HA-micelles with paclitaxel (HA-PTX), doxorubicin (HA-DOX) HA-DOX, and HA-salinomycin produced effective cytotoxicity on DC44 expressing cells.
118-120
Nanoparticles act as Nanocarriers for delivery the therapeutic agent to target site hold promising features include; dots (quantum and nanodots, nanodots graphene,
121-123
carbon nanotubes,
124
and nanoparticles gold and iron oxide nanoparticles.
125,126
and silica nanoparticles,
127
and they have found to acquire novel characteristics after their conjugation with HA.
128
Due to improve the efficacy of HA conjugated nanocarriers delivered anticancer drugs such as HA-epirubicin,
129
HA-DOX,
115
HA-PTX,
130,131
and HA-MMC,
129
as well as HA-siRNA make HA attractive polymer in drug delivery. Similarly, drug-resistant tumor cell treatment improved via combination therapy of HA (HA–CUR/DOX-NPs). The improve efficacy was achieved via down-regulate the expression of P-gp and induce apoptosis (regulating Bax/Bcl-2),
132
thus increasing the therapeutic effect.
CD44 as a therapeutic target
The CD44 essential receptor protein involves in cancer cell growth, survival, and metastasis in the majority of cancer. HA is the primary ligand of CD44 receptor; upon binding, to the receptor, the ligand-receptor interaction activates the down-stream cancer pathways.
133
CD44 do exist in various isoforms of CD44v along with CD44s play a significant role progression of cancer and prognosis.
134-136
Several studies regarding the role of CD44s and CD44v in the onset of cancer propagation. However, the function of CD44 isoforms differs in various cancers and different outcomes CD44 isoforms. As in one study, the CD44s (standard isoform) aberrant expression or loss is linked to an increased risk of metastasis, as observed in malignant breast cancer.
135
Along with this, CD44s indorsed invasion in breast tumour and liver metastasis.
136
Amongst isoforms of CD44, CD44v (3, 6 and, 7 and 8) linked to the propagation of breast cancer.
137
In lymph node metastasis, CD44v6 expression was upregulated, while both CD44v (v3 and v6) were upregulated invasive cribriform breast tumours. However, the downregulation in CD44v4 in the invasive cribriform breast but upregulated in trans-endothelial cell metastasis. More research needs to done further to clarify the specific role of CD44s and CD44v variants.
Antibodies to inhibit the CD44 mediated cancer progression also show remarkable results in inhibition of HA-CD44 downstream pathways. The binding of antibodies (anti-CD44) to CD44 variants that expressed in a variety of cancers interfere with the binding of HA to CD44 receptor cells and disrupt CD44 matrix interactions. This interruption cause alteration in CD44, triggered cancer progression pathways as well as induce apoptosis.
138
The use of several peptides, The Pep-1 and BH-P, inhibit the proliferation of melanoma tumour cells in nude mice xenograft models.
139
The CD44v6 isoforms identified as markers of CSCs in colon cancer. As a receptor CD44v6 involve in matrix assembly and link with metastasis.
140,141
Therefore, targeting CD44v6 in colon cancer is a promising therapeutic approach. The siRNA/shRNA use in the inhibition of CD44 receptor expression successfully blocks the interaction (HA-CD44) and induction of cancer progression pathways. The using cell-specific shRNA delivery approach to inhibit HA-CD44v6 interaction inhibited distant growth in colon tumour and downstream tumour progression pathways.
142,143
The double approach in targeting CD44 by using an antibody as well as cytotoxic drugs have studied. The cytotoxic drug (mertansine) conjugated to an antibody against CD44v6 used in early phase clinical trials.
144,145
Similarly, another drug bivatuzumab (anti-CD44v6 monoclonal antibody) along with combinational drug mertansine also undergoing clinical trials.
146,147
However, due to toxicity towards healthy cells (CD44v6 positive expression), the phase I clinical trials did not consider as successful. By studying this limitation of antibody therapy, the quest for alternative therapies increased.
Natural products show the remarkable result in cancer therapeutics. Besides various synthetic inhibitor, different natural potential compounds such as silibinin, zerumbone, gemini, curcumin, epigallocatechin gallate, and apigenin down-regulate the expression of CD44 isoforms.
148
Silibinin bioactive component, isolated from plant Silybum marianum used in phase II clinical trials in prostate cancer and. Silibinin inhibits cell growth in a time-dependent manner in BxPC3 and Panc1 pancreatic cancer cell lines. The active compound (silibinin) acts as a transcription regulator in CD44 expression decreased the expression of levels of CD44v7-10 RNA and well as translated protein in prostate cancer cells.
149-151
Another potential regulator of the CD44 receptor cell, Zerumbone, belongs to the terpene class of phytochemicals. The expression level of CD44 in breast cancer cells downregulated by Zerumbone via inhibiting phosphorylation of STAT3 which involve in EGF-induced CD44 expression.
152-154
Similarly, members of vitamin D family Gemini also act as a transcription regulator. As a transcription regulator, it downregulates the expression of CD44 protein level, inhibited the invasion and metastasis in mice model.
155,156
The enhance synergistic effect of curcumin (potential active compound) and epigallocatechin gallate (catechin) inhibit the expression of the CD44 receptor on cancer cells. The combine approaches successfully reduced CD44- positive (CSC) and downregulate the phosphorylation of STAT3 expression.
157
Also, potent phytochemical apigenin belongs to flavonoid group improve the cell survival in a prostate cancer patient by dose-dependent inhibition in expression of CD44 in CSC by upregulating the expression of p21 and p27.
158
Along with previous reports and our research various natural extract and fractions rich of potential phytochemicals and targeted delivery produce effective cytotoxicity via induction of apoptosis (in-vitro).
159-167
However, the study of receptor-ligand molecular interactions required to understand the inhibition of anti-apoptosis pathways involves in cancer progression.
Conclusion
HA versatile biopolymer, the primary ligand of CD44 receptor cells. HA-CD44 interaction and binding can regulate cytoskeleton protein activation in various tumour cells. The cytoskeleton activation triggers different downstream pathways involved in tumour propagation. The HA and CD44, along with signaling proteins in the cytoskeleton could be suitable markers for cancer drug therapeutics. The CD44 exists in various isoforms and regulate the cell survival signaling pathways in a variety of cancers. The alternative splicing triggers the variant isoform of CD44, and the expression level is a critical regulator in various cancers. Here, we discussed the understanding of the HA-CD44 interaction/binding and discussed different activated pathways involve in cancer cell growth, survival, and metastasis via signaling networks include; RhoGTPases and PI3K/AKT pathway. HA-CD44 activation stimulates AKT activation via ROK (kinase) triggered tumour cell migration and invasion. Also, HA/CD44 also responsible for chemoresistance via the up-regulation of miR-10b, miRNA-21, and miR-302 expression. The overexpression of various CD44 isoforms depends on different cell types and stages—the fate of cancer cells regulated by isoforms, CD44v, and standard CD44s. Thus, targeting HA by using HYALs, targeting HA synthase and HA-inhibitors, useful strategy to inhibit HA trigger CD44-signaling pathways. Similarly, targeting CD44(variant) by application of CD44 anti-bodies, peptides HA-Nano carries and natural CD44 negative regulator showing optimal success. Thus, extensive studies show that CD44 has the potential to be an effective drug therapeutics.
Ethical Issue
Not applicable.
Conflict of Interest
Authors have no conflict of interest.
Acknowledgments
The authors would like to acknowledge the postdoctoral fellowship provided to Dr Gul-e-Saba Chaudhry. The Figures were created by using Biorender.
References
- Theocharis AD, Skandalis SS, Tzanakakis GN, Karamanos NK. Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J 2010; 277(19):3904-23. doi: 10.1111/j.1742-4658.2010.07800.x [Crossref] [ Google Scholar]
- Schaefer L, Schaefer RM. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res 2010; 339(1):237-46. doi: 10.1007/s00441-009-0821-y [Crossref] [ Google Scholar]
- Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev 2009; 28(1-2):167-76. doi: 10.1007/s10555-008-9178-z [Crossref] [ Google Scholar]
- Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol 2003; 162(6):1123-33. doi: 10.1083/jcb.200302090 [Crossref] [ Google Scholar]
- Stickens D, Behonick DJ, Ortega N, Heyer B, Hartenstein B, Yu Y. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development 2004; 131(23):5883-95. doi: 10.1242/dev.01461 [Crossref] [ Google Scholar]
- Rebustini IT, Myers C, Lassiter KS, Surmak A, Szabova L, Holmbeck K. MT2-MMP-dependent release of collagen IV NC1 domains regulates submandibular gland branching morphogenesis. Dev Cell 2009; 17(4):482-93. doi: 10.1016/j.devcel.2009.07.016 [Crossref] [ Google Scholar]
- Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 2011; 3(12). doi: 10.1101/cshperspect.a005058 [Crossref]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002; 110(6):673-87. doi: 10.1016/s0092-8674(02)00971-6 [Crossref] [ Google Scholar]
- Maxwell CA, McCarthy J, Turley E. Cell-surface and mitotic-spindle RHAMM: moonlighting or dual oncogenic functions?. J Cell Sci 2008; 121(Pt 7):925-32. doi: 10.1242/jcs.022038 [Crossref] [ Google Scholar]
- Nikitovic D, Kouvidi K, Karamanos NK, Tzanakakis GN. The roles of hyaluronan/RHAMM/CD44 and their respective interactions along the insidious pathways of fibrosarcoma progression. Biomed Res Int 2013; 2013:929531. doi: 10.1155/2013/929531 [Crossref] [ Google Scholar]
- Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med 2006; 4(1):38. doi: 10.1186/1741-7015-4-38 [Crossref] [ Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009; 139(5):891-906. doi: 10.1016/j.cell.2009.10.027 [Crossref] [ Google Scholar]
- Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 2012; 196(4):395-406. doi: 10.1083/jcb.201102147 [Crossref] [ Google Scholar]
- Nikitovic D, Chatzinikolaou G, Tsiaoussis J, Tsatsakis A, Karamanos NK, Tzanakakis GN. Insights into targeting colon cancer cell fate at the level of proteoglycans / glycosaminoglycans. Curr Med Chem 2012; 19(25):4247-58. doi: 10.2174/092986712802884268 [Crossref] [ Google Scholar]
- Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 2011; 278(1):16-27. doi: 10.1111/j.1742-4658.2010.07919.x [Crossref] [ Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature 2004; 432(7015):332-7. doi: 10.1038/nature03096 [Crossref] [ Google Scholar]
- Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev 2012; 31(1-2):195-208. doi: 10.1007/s10555-011-9340-x [Crossref] [ Google Scholar]
- Barkan D, Green JE, Chambers AF. Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur J Cancer 2010; 46(7):1181-8. doi: 10.1016/j.ejca.2010.02.027 [Crossref] [ Google Scholar]
- Ilan N, Elkin M, Vlodavsky I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol 2006; 38(12):2018-39. doi: 10.1016/j.biocel.2006.06.004 [Crossref] [ Google Scholar]
- Skandalis SS, Aletras AJ, Gialeli C, Theocharis AD, Afratis N, Tzanakakis GN. Targeting the tumor proteasome as a mechanism to control the synthesis and bioactivity of matrix macromolecules. Curr Mol Med 2012; 12(8):1068-82. doi: 10.2174/156652412802480943 [Crossref] [ Google Scholar]
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990; 61(7):1303-13. doi: 10.1016/0092-8674(90)90694-a [Crossref] [ Google Scholar]
- Vigetti D, Viola M, Karousou E, De Luca G, Passi A. Metabolic control of hyaluronan synthases. Matrix Biol 2014; 35:8-13. doi: 10.1016/j.matbio.2013.10.002 [Crossref] [ Google Scholar]
- Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem 1999; 274(35):25085-92. doi: 10.1074/jbc.274.35.25085 [Crossref] [ Google Scholar]
- Maharjan AS, Pilling D, Gomer RH. High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation. PLoS One 2011; 6(10):e26078. doi: 10.1371/journal.pone.0026078 [Crossref] [ Google Scholar]
- Stern R. Devising a pathway for hyaluronan catabolism: are we there yet?. Glycobiology 2003; 13(12):105R-15R. doi: 10.1093/glycob/cwg112 [Crossref] [ Google Scholar]
- Bohaumilitzky L, Huber AK, Stork EM, Wengert S, Woelfl F, Boehm H. A trickster in disguise: hyaluronan’s ambivalent roles in the matrix. Front Oncol 2017; 7:242. doi: 10.3389/fonc.2017.00242 [Crossref] [ Google Scholar]
- Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 2013; 499(7458):346-9. doi: 10.1038/nature12234 [Crossref] [ Google Scholar]
- Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation 2002; 70(9-10):537-46. doi: 10.1046/j.1432-0436.2002.700907.x [Crossref] [ Google Scholar]
- Misra S, Heldin P, Hascall VC, Karamanos NK, Skandalis SS, Markwald RR. Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J 2011; 278(9):1429-43. doi: 10.1111/j.1742-4658.2011.08071.x [Crossref] [ Google Scholar]
- Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 2003; 4(1):33-45. doi: 10.1038/nrm1004 [Crossref] [ Google Scholar]
- Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem 2001; 276(22):19420-30. doi: 10.1074/jbc.M011004200 [Crossref] [ Google Scholar]
- Naor D, Sionov RV, Ish-Shalom D. CD44: structure, function, and association with the malignant process. Adv Cancer Res 1997; 71:241-319. doi: 10.1016/s0065-230x(08)60101-3 [Crossref] [ Google Scholar]
- Azevedo R, Gaiteiro C, Peixoto A, Relvas-Santos M, Lima L, Santos LL, Ferreira JA. CD44 glycoprotein in cancer: a molecular conundrum hampering clinical applications. Clin Proteomics 2018; 15:22. doi: 10.1186/s12014-018-9198-9 [Crossref] [ Google Scholar]
- Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science 1996; 271(5248):509-12. doi: 10.1126/science.271.5248.509 [Crossref] [ Google Scholar]
- Faassen AE, Schrager JA, Klein DJ, Oegema TR, Couchman JR, McCarthy JB. A cell surface chondroitin sulfate proteoglycan, immunologically related to CD44, is involved in type I collagen-mediated melanoma cell motility and invasion. J Cell Biol 1992; 116(2):521-31. doi: 10.1083/jcb.116.2.521 [Crossref] [ Google Scholar]
- Knutson JR, Iida J, Fields GB, McCarthy JB. CD44/chondroitin sulfate proteoglycan and alpha 2 beta 1 integrin mediate human melanoma cell migration on type IV collagen and invasion of basement membranes. Mol Biol Cell 1996; 7(3):383-96. doi: 10.1091/mbc.7.3.383 [Crossref] [ Google Scholar]
- Jalkanen M, Elenius K, Salmivirta M. Syndecan--a cell surface proteoglycan that selectively binds extracellular effector molecules. Adv Exp Med Biol 1992; 313:79-85. doi: 10.1007/978-1-4899-2444-5_8 [Crossref] [ Google Scholar]
- Toyama-Sorimachi N, Sorimachi H, Tobita Y, Kitamura F, Yagita H, Suzuki K. A novel ligand for CD44 is serglycin, a hematopoietic cell lineage-specific proteoglycan Possible involvement in lymphoid cell adherence and activation. J Biol Chem 1995; 270(13):7437-44. doi: 10.1074/jbc.270.13.7437 [Crossref] [ Google Scholar]
- Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 2003; 4(1):33-45. doi: 10.1038/nrm1004 [Crossref] [ Google Scholar]
- Okamoto I, Kawano Y, Murakami D, Sasayama T, Araki N, Miki T. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J Cell Biol 2001; 155(5):755-62. doi: 10.1083/jcb.200108159 [Crossref] [ Google Scholar]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 2002; 3(8):586-99. doi: 10.1038/nrm882 [Crossref] [ Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 2000; 14(2):163-76. [ Google Scholar]
- Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev 1999; 13(1):35-48. doi: 10.1101/gad.13.1.35 [Crossref] [ Google Scholar]
- Deed R, Rooney P, Kumar P, Norton JD, Smith J, Freemont AJ. Early-response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells Inhibition by non-angiogenic, high-molecular-weight hyaluronan. Int J Cancer 1997; 71(2):251-6. doi: 10.1002/(sici)1097-0215(19970410)71:2<251::aid-ijc21>3.0.co;2-j [Crossref] [ Google Scholar]
- Naor D, Nedvetzki S, Golan I, Melnik L, Faitelson Y. CD44 in cancer. Crit Rev Clin Lab Sci 2002; 39(6):527-79. doi: 10.1080/10408360290795574 [Crossref] [ Google Scholar]
- Lopez JI, Camenisch TD, Stevens MV, Sands BJ, McDonald J, Schroeder JA. CD44 attenuates metastatic invasion during breast cancer progression. Cancer Res 2005; 65(15):6755-63. doi: 10.1158/0008-5472.can-05-0863 [Crossref] [ Google Scholar]
- Ghatak S, Misra S, Toole BP. Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem 2002; 277(41):38013-20. doi: 10.1074/jbc.M202404200 [Crossref] [ Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science 1998; 279(5350):509-14. doi: 10.1126/science.279.5350.509 [Crossref] [ Google Scholar]
- Li X, Lim B. RhoGTPases and their role in cancer. Oncol Res 2003; 13(6-10):323-31. doi: 10.3727/096504003108748528 [Crossref] [ Google Scholar]
- Suwa H, Ohshio G, Imamura T, Watanabe G, Arii S, Imamura M. Overexpression of the rhoC gene correlates with progression of ductal adenocarcinoma of the pancreas. Br J Cancer 1998; 77(1):147-52. doi: 10.1038/bjc.1998.23 [Crossref] [ Google Scholar]
- Bourguignon LY, Peyrollier K, Xia W, Gilad E. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J Biol Chem 2008; 283(25):17635-51. doi: 10.1074/jbc.M800109200 [Crossref] [ Google Scholar]
- Chen WT. Proteases associated with invadopodia, and their role in degradation of extracellular matrix. Enzyme Protein 1996; 49(1-3):59-71. doi: 10.1159/000468616 [Crossref] [ Google Scholar]
- Gum R, Lengyel E, Juarez J, Chen JH, Sato H, Seiki M. Stimulation of 92-kDa gelatinase B promoter activity by ras is mitogen-activated protein kinase kinase 1-independent and requires multiple transcription factor binding sites including closely spaced PEA3/ets and AP-1 sequences. J Biol Chem 1996; 271(18):10672-80. doi: 10.1074/jbc.271.18.10672 [Crossref] [ Google Scholar]
- Bourguignon LY, Gilad E, Brightman A, Diedrich F, Singleton P. Hyaluronan-CD44 interaction with leukemia-associated RhoGEF and epidermal growth factor receptor promotes Rho/Ras co-activation, phospholipase C epsilon-Ca2+ signaling, and cytoskeleton modification in head and neck squamous cell carcinoma cells. J Biol Chem 2006; 281(20):14026-40. doi: 10.1074/jbc.M507734200 [Crossref] [ Google Scholar]
- Bourguignon LY, Zhu H, Shao L, Zhu D, Chen YW. Rho-kinase (ROK) promotes CD44v(3,8-10)-ankyrin interaction and tumor cell migration in metastatic breast cancer cells. Cell Motil Cytoskeleton 1999; 43(4):269-87. doi: 10.1002/(sici)1097-0169(1999)43:4<269::aid-cm1>3.0.co;2-5 [Crossref] [ Google Scholar]
- Singleton PA, Bourguignon LY. CD44v10 interaction with Rho-kinase (ROK) activates inositol 1,4,5-triphosphate (IP3) receptor-mediated Ca2+ signaling during hyaluronan (HA)-induced endothelial cell migration. Cell Motil Cytoskeleton 2002; 53(4):293-316. doi: 10.1002/cm.10078 [Crossref] [ Google Scholar]
- Lakshman M, Subramaniam V, Rubenthiran U, Jothy S. CD44 promotes resistance to apoptosis in human colon cancer cells. Exp Mol Pathol 2004; 77(1):18-25. doi: 10.1016/j.yexmp.2004.03.002 [Crossref] [ Google Scholar]
- Yasuda M, Tanaka Y, Fujii K, Yasumoto K. CD44 stimulation down-regulates Fas expression and Fas-mediated apoptosis of lung cancer cells. Int Immunol 2001; 13(10):1309-19. doi: 10.1093/intimm/13.10.1309 [Crossref] [ Google Scholar]
- Park YS, Huh JW, Lee JH, Kim HR. shRNA against CD44 inhibits cell proliferation, invasion and migration, and promotes apoptosis of colon carcinoma cells. Oncol Rep 2012; 27(2):339-46. doi: 10.3892/or.2011.1532 [Crossref] [ Google Scholar]
- Jan R, Chaudhry GE. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv Pharm Bull 2019; 9(2):205-18. doi: 10.15171/apb.2019.024 [Crossref] [ Google Scholar]
- Bourguignon LYW. Matrix hyaluronan-CD44 interaction activates MicroRNA and LncRNA signaling associated with chemoresistance, invasion, and tumor progression. Front Oncol 2019; 9:492. doi: 10.3389/fonc.2019.00492 [Crossref] [ Google Scholar]
- Jiang WG, Sanders AJ, Katoh M, Ungefroren H, Gieseler F, Prince M. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin Cancer Biol 2015; 35 Suppl:S244-S75. doi: 10.1016/j.semcancer.2015.03.008 [Crossref] [ Google Scholar]
- Laurent TC, Fraser JR. Hyaluronan. FASEB J 1992; 6(7):2397-404. [ Google Scholar]
- McBride WH, Bard JB. Hyaluronidase-sensitive halos around adherent cells Their role in blocking lymphocyte-mediated cytolysis. J Exp Med 1979; 149(2):507-15. doi: 10.1084/jem.149.2.507 [Crossref] [ Google Scholar]
- Gately CL, Muul LM, Greenwood MA, Papazoglou S, Dick SJ, Kornblith PL. In vitro studies on the cell-mediated immune response to human brain tumors II Leukocyte-induced coats of glycosaminoglycan increase the resistance of glioma cells to cellular immune attack. J Immunol 1984; 133(6):3387-95. [ Google Scholar]
- Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev 2006; 106(3):818-39. doi: 10.1021/cr050247k [Crossref] [ Google Scholar]
- Kosaki R, Watanabe K, Yamaguchi Y. Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Res 1999; 59(5):1141-5. [ Google Scholar]
- Liu N, Gao F, Han Z, Xu X, Underhill CB, Zhang L. Hyaluronan synthase 3 overexpression promotes the growth of TSU prostate cancer cells. Cancer Res 2001; 61(13):5207-14. [ Google Scholar]
- Itano N, Zhuo L, Kimata K. Impact of the hyaluronan-rich tumor microenvironment on cancer initiation and progression. Cancer Sci 2008; 99(9):1720-5. doi: 10.1111/j.1349-7006.2008.00885.x [Crossref] [ Google Scholar]
- West DC, Kumar S. The effect of hyaluronate and its oligosaccharides on endothelial cell proliferation and monolayer integrity. Exp Cell Res 1989; 183(1):179-96. doi: 10.1016/0014-4827(89)90428-x [Crossref] [ Google Scholar]
- Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012; 21(3):418-29. doi: 10.1016/j.ccr.2012.01.007 [Crossref] [ Google Scholar]
- Bellerby R, Smith C, Kyme S, Gee J, Günthert U, Green A. Overexpression of specific CD44 isoforms is associated with aggressive cell features in acquired endocrine resistance. Front Oncol 2016; 6:145. doi: 10.3389/fonc.2016.00145 [Crossref] [ Google Scholar]
- Wielenga VJ, Heider KH, Offerhaus GJ, Adolf GR, van den Berg FM, Ponta H. Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res 1993; 53(20):4754-6. [ Google Scholar]
- Rall CJ, Rustgi AK. CD44 isoform expression in primary and metastatic pancreatic adenocarcinoma. Cancer Res 1995; 55(9):1831-5. [ Google Scholar]
- Castellà EM, Ariza A, Ojanguren I, Mate JL, Roca X, Fernández-Vasalo A. Differential expression of CD44v6 in adenocarcinoma of the pancreas: an immunohistochemical study. Virchows Arch 1996; 429(4-5):191-5. doi: 10.1007/bf00198333 [Crossref] [ Google Scholar]
- Günthert U, Hofmann M, Rudy W, Reber S, Zöller M, Haussmann I. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 1991; 65(1):13-24. doi: 10.1016/0092-8674(91)90403-l [Crossref] [ Google Scholar]
- Ringel J, Jesnowski R, Schmidt C, Ringel J, Köhler HJ, Rychly J. CD44 in normal human pancreas and pancreatic carcinoma cell lines. Teratog Carcinog Mutagen 2001; 21(1):97-106. doi: 10.1002/1520-6866(2001)21:1<97::aid-tcm9>3.0.co;2-o [Crossref] [ Google Scholar]
- Gotoda T, Matsumura Y, Kondo H, Saitoh D, Shimada Y, Kosuge T. Expression of CD44 variants and its association with survival in pancreatic cancer. Jpn J Cancer Res 1998; 89(10):1033-40. doi: 10.1111/j.1349-7006.1998.tb00493.x [Crossref] [ Google Scholar]
- Zhou G, Chiu D, Qin D, Niu L, Cai J, He L. Detection and clinical significance of CD44v6 and integrin-β1 in pancreatic cancer patients using a triplex real-time RT-PCR assay. Appl Biochem Biotechnol 2012; 167(8):2257-68. doi: 10.1007/s12010-012-9752-2 [Crossref] [ Google Scholar]
- Li Z, Chen K, Jiang P, Zhang X, Li X, Li Z. CD44v/CD44s expression patterns are associated with the survival of pancreatic carcinoma patients. Diagn Pathol 2014; 9:79. doi: 10.1186/1746-1596-9-79 [Crossref] [ Google Scholar]
- Ghatak S, Hascall VC, Markwald RR, Misra S. Stromal hyaluronan interaction with epithelial CD44 variants promotes prostate cancer invasiveness by augmenting expression and function of hepatocyte growth factor and androgen receptor. J Biol Chem 2010; 285(26):19821-32. doi: 10.1074/jbc.M110.104273 [Crossref] [ Google Scholar]
- Hernandez JR, Kim JJ, Verdone JE, Liu X, Torga G, Pienta KJ. Alternative CD44 splicing identifies epithelial prostate cancer cells from the mesenchymal counterparts. Med Oncol 2015; 32(5):159. doi: 10.1007/s12032-015-0593-z [Crossref] [ Google Scholar]
- Ni J, Cozzi PJ, Hao JL, Beretov J, Chang L, Duan W. CD44 variant 6 is associated with prostate cancer metastasis and chemo-/radioresistance. Prostate 2014; 74(6):602-17. doi: 10.1002/pros.22775 [Crossref] [ Google Scholar]
- Wang SJ, Wreesmann VB, Bourguignon LY. Association of CD44 V3-containing isoforms with tumor cell growth, migration, matrix metalloproteinase expression, and lymph node metastasis in head and neck cancer. Head Neck 2007; 29(6):550-8. doi: 10.1002/hed.20544 [Crossref] [ Google Scholar]
- Mulder JW, Kruyt PM, Sewnath M, Oosting J, Seldenrijk CA, Weidema WF. Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet 1994; 344(8935):1470-2. doi: 10.1016/s0140-6736(94)90290-9 [Crossref] [ Google Scholar]
- Kaufmann M, Heider KH, Sinn HP, von Minckwitz G, Ponta H, Herrlich P. CD44 variant exon epitopes in primary breast cancer and length of survival. Lancet 1995; 345(8950):615-9. doi: 10.1016/s0140-6736(95)90521-9 [Crossref] [ Google Scholar]
- Yamao T, Matsumura Y, Shimada Y, Moriya Y, Sugihara K, Akasu T. Abnormal expression of CD44 variants in the exfoliated cells in the feces of patients with colorectal cancer. Gastroenterology 1998; 114(6):1196-205. doi: 10.1016/s0016-5085(98)70425-1 [Crossref] [ Google Scholar]
- Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell 2014; 14(3):342-56. doi: 10.1016/j.stem.2014.01.009 [Crossref] [ Google Scholar]
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19(3):387-400. doi: 10.1016/j.ccr.2011.01.038 [Crossref] [ Google Scholar]
- Zeilstra J, Joosten SP, van Andel H, Tolg C, Berns A, Snoek M. Stem cell CD44v isoforms promote intestinal cancer formation in Apc(min) mice downstream of Wnt signaling. Oncogene 2014; 33(5):665-70. doi: 10.1038/onc.2012.611 [Crossref] [ Google Scholar]
- Mayer B, Jauch KW, Günthert U, Figdor CG, Schildberg FW, Funke I. De-novo expression of CD44 and survival in gastric cancer. Lancet 1993; 342(8878):1019-22. doi: 10.1016/0140-6736(93)92879-x [Crossref] [ Google Scholar]
- Lv L, Liu HG, Dong SY, Yang F, Wang QX, Guo GL. Upregulation of CD44v6 contributes to acquired chemoresistance via the modulation of autophagy in colon cancer SW480 cells. Tumour Biol 2016; 37(7):8811-24. doi: 10.1007/s13277-015-4755-6 [Crossref] [ Google Scholar]
- Lokeshwar VB, Estrella V, Lopez L, Kramer M, Gomez P, Soloway MS. HYAL1-v1, an alternatively spliced variant of HYAL1 hyaluronidase: a negative regulator of bladder cancer. Cancer Res 2006; 66(23):11219-27. doi: 10.1158/0008-5472.can-06-1121 [Crossref] [ Google Scholar]
- St Croix B, Man S, Kerbel RS. Reversal of intrinsic and acquired forms of drug resistance by hyaluronidase treatment of solid tumors. Cancer Lett 1998; 131(1):35-44. doi: 10.1016/s0304-3835(98)00199-2 [Crossref] [ Google Scholar]
- St Croix B, Rak JW, Kapitain S, Sheehan C, Graham CH, Kerbel RS. Reversal by hyaluronidase of adhesion-dependent multicellular drug resistance in mammary carcinoma cells. J Natl Cancer Inst 1996; 88(18):1285-96. doi: 10.1093/jnci/88.18.1285 [Crossref] [ Google Scholar]
- Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012; 21(3):418-29. doi: 10.1016/j.ccr.2012.01.007 [Crossref] [ Google Scholar]
- Nagase H, Kudo D, Suto A, Yoshida E, Suto S, Negishi M. 4-Methylumbelliferone suppresses hyaluronan synthesis and tumor progression in SCID mice intra-abdominally inoculated with pancreatic cancer cells. Pancreas 2017; 46(2):190-7. doi: 10.1097/mpa.0000000000000741 [Crossref] [ Google Scholar]
- Kakizaki I, Kojima K, Takagaki K, Endo M, Kannagi R, Ito M. A novel mechanism for the inhibition of hyaluronan biosynthesis by 4-methylumbelliferone. J Biol Chem 2004; 279(32):33281-9. doi: 10.1074/jbc.M405918200 [Crossref] [ Google Scholar]
- Kultti A, Pasonen-Seppänen S, Jauhiainen M, Rilla KJ, Kärnä R, Pyöriä E. 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp Cell Res 2009; 315(11):1914-23. doi: 10.1016/j.yexcr.2009.03.002 [Crossref] [ Google Scholar]
- Lokeshwar VB, Lopez LE, Munoz D, Chi A, Shirodkar SP, Lokeshwar SD. Antitumor activity of hyaluronic acid synthesis inhibitor 4-methylumbelliferone in prostate cancer cells. Cancer Res 2010; 70(7):2613-23. doi: 10.1158/0008-5472.can-09-3185 [Crossref] [ Google Scholar]
- Urakawa H, Nishida Y, Wasa J, Arai E, Zhuo L, Kimata K. Inhibition of hyaluronan synthesis in breast cancer cells by 4-methylumbelliferone suppresses tumorigenicity in vitro and metastatic lesions of bone in vivo. Int J Cancer 2012; 130(2):454-66. doi: 10.1002/ijc.26014 [Crossref] [ Google Scholar]
- Arai E, Nishida Y, Wasa J, Urakawa H, Zhuo L, Kimata K. Inhibition of hyaluronan retention by 4-methylumbelliferone suppresses osteosarcoma cells in vitro and lung metastasis in vivo. Br J Cancer 2011; 105(12):1839-49. doi: 10.1038/bjc.2011.459 [Crossref] [ Google Scholar]
- Luo Y, Ziebell MR, Prestwich GD. A hyaluronic acid-taxol antitumor bioconjugate targeted to cancer cells. Biomacromolecules 2000; 1(2):208-18. doi: 10.1021/bm000283n [Crossref] [ Google Scholar]
- Rosato A, Banzato A, De Luca G, Renier D, Bettella F, Pagano C. HYTAD1-p20: a new paclitaxel-hyaluronic acid hydrosoluble bioconjugate for treatment of superficial bladder cancer. Urol Oncol 2006; 24(3):207-15. doi: 10.1016/j.urolonc.2005.08.020 [Crossref] [ Google Scholar]
- Luo Y, Prestwich GD. Synthesis and selective cytotoxicity of a hyaluronic acid-antitumor bioconjugate. Bioconjug Chem 1999; 10(5):755-63. doi: 10.1021/bc9900338 [Crossref] [ Google Scholar]
- Luo Y, Kirker KR, Prestwich GD. Cross-linked hyaluronic acid hydrogel films: new biomaterials for drug delivery. J Control Release 2000; 69(1):169-84. doi: 10.1016/s0168-3659(00)00300-x [Crossref] [ Google Scholar]
- Serafino A, Zonfrillo M, Andreola F, Psaila R, Mercuri L, Moroni N. CD44-targeting for antitumor drug delivery: a new SN-38-hyaluronan bioconjugate for locoregional treatment of peritoneal carcinomatosis. Curr Cancer Drug Targets 2011; 11(5):572-85. doi: 10.2174/156800911795655976 [Crossref] [ Google Scholar]
- Bassi PF, Volpe A, D’Agostino D, Palermo G, Renier D, Franchini S. Paclitaxel-hyaluronic acid for intravesical therapy of bacillus Calmette-Guérin refractory carcinoma in situ of the bladder: results of a phase I study. J Urol 2011; 185(2):445-9. doi: 10.1016/j.juro.2010.09.073 [Crossref] [ Google Scholar]
- Montagner IM, Banzato A, Zuccolotto G, Renier D, Campisi M, Bassi P. Paclitaxel-hyaluronan hydrosoluble bioconjugate: mechanism of action in human bladder cancer cell lines. Urol Oncol 2013; 31(7):1261-9. doi: 10.1016/j.urolonc.2012.01.005 [Crossref] [ Google Scholar]
- Rosenthal MA, Gibbs P, Brown TJ, Wong S, Uren S, Ellis A. Phase I and pharmacokinetic evaluation of intravenous hyaluronic acid in combination with doxorubicin or 5-fluorouracil. Chemotherapy 2005; 51(2-3):132-41. doi: 10.1159/000085621 [Crossref] [ Google Scholar]
- Gibbs P, Brown TJ, Ng R, Jennens R, Cinc E, Pho M. A pilot human evaluation of a formulation of irinotecan and hyaluronic acid in 5-fluorouracil-refractory metastatic colorectal cancer patients. Chemotherapy 2009; 55(1):49-59. doi: 10.1159/000180339 [Crossref] [ Google Scholar]
- Peer D, Margalit R. Loading mitomycin C inside long circulating hyaluronan targeted nano-liposomes increases its antitumor activity in three mice tumor models. Int J Cancer 2004; 108(5):780-9. doi: 10.1002/ijc.11615 [Crossref] [ Google Scholar]
- Peer D, Margalit R. Tumor-targeted hyaluronan nanoliposomes increase the antitumor activity of liposomal doxorubicin in syngeneic and human xenograft mouse tumor models. Neoplasia 2004; 6(4):343-53. doi: 10.1593/neo.03460 [Crossref] [ Google Scholar]
- Surace C, Arpicco S, Dufaÿ-Wojcicki A, Marsaud V, Bouclier C, Clay D. Lipoplexes targeting the CD44 hyaluronic acid receptor for efficient transfection of breast cancer cells. Mol Pharm 2009; 6(4):1062-73. doi: 10.1021/mp800215d [Crossref] [ Google Scholar]
- Eliaz RE, Szoka FC Jr. Liposome-encapsulated doxorubicin targeted to CD44: a strategy to kill CD44-overexpressing tumor cells. Cancer Res 2001; 61(6):2592-601. [ Google Scholar]
- Ruhela D, Riviere K, Szoka FC Jr. Efficient synthesis of an aldehyde functionalized hyaluronic acid and its application in the preparation of hyaluronan-lipid conjugates. Bioconjug Chem 2006; 17(5):1360-3. doi: 10.1021/bc0600721 [Crossref] [ Google Scholar]
- Dufaÿ Wojcicki A, Hillaireau H, Nascimento TL, Arpicco S, Taverna M, Ribes S. Hyaluronic acid-bearing lipoplexes: physico-chemical characterization and in vitro targeting of the CD44 receptor. J Control Release 2012; 162(3):545-52. doi: 10.1016/j.jconrel.2012.07.015 [Crossref] [ Google Scholar]
- Liu Y, Sun J, Cao W, Yang J, Lian H, Li X. Dual targeting folate-conjugated hyaluronic acid polymeric micelles for paclitaxel delivery. Int J Pharm 2011; 421(1):160-9. doi: 10.1016/j.ijpharm.2011.09.006 [Crossref] [ Google Scholar]
- Qiu L, Li Z, Qiao M, Long M, Wang M, Zhang X. Self-assembled pH-responsive hyaluronic acid-g-poly((L)-histidine) copolymer micelles for targeted intracellular delivery of doxorubicin. Acta Biomater 2014; 10(5):2024-35. doi: 10.1016/j.actbio.2013.12.025 [Crossref] [ Google Scholar]
- Zhang Y, Zhang H, Wang X, Wang J, Zhang X, Zhang Q. The eradication of breast cancer and cancer stem cells using octreotide modified paclitaxel active targeting micelles and salinomycin passive targeting micelles. Biomaterials 2012; 33(2):679-91. doi: 10.1016/j.biomaterials.2011.09.072 [Crossref] [ Google Scholar]
- Pellegrino T, Kudera S, Liedl T, Muñoz Javier A, Manna L, Parak WJ. On the development of colloidal nanoparticles towards multifunctional structures and their possible use for biological applications. Small 2005; 1(1):48-63. doi: 10.1002/smll.200400071 [Crossref] [ Google Scholar]
- Geim AK. Graphene: status and prospects. Science 2009; 324(5934):1530-4. doi: 10.1126/science.1158877 [Crossref] [ Google Scholar]
- Baker SN, Baker GA. Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed Engl 2010; 49(38):6726-44. doi: 10.1002/anie.200906623 [Crossref] [ Google Scholar]
- Tenne R. Inorganic nanotubes and fullerene-like nanoparticles. Nat Nanotechnol 2006; 1(2):103-11. doi: 10.1038/nnano.2006.62 [Crossref] [ Google Scholar]
- Lee MY, Yang JA, Jung HS, Beack S, Choi JE, Hur W. Hyaluronic acid-gold nanoparticle/interferon α complex for targeted treatment of hepatitis C virus infection. ACS Nano 2012; 6(11):9522-31. doi: 10.1021/nn302538y [Crossref] [ Google Scholar]
- Kumar A, Sahoo B, Montpetit A, Behera S, Lockey RF, Mohapatra SS. Development of hyaluronic acid-Fe2O3 hybrid magnetic nanoparticles for targeted delivery of peptides. Nanomedicine 2007; 3(2):132-7. doi: 10.1016/j.nano.2007.03.001 [Crossref] [ Google Scholar]
- Lu J, Liong M, Li Z, Zink JI, Tamanoi F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small 2010; 6(16):1794-805. doi: 10.1002/smll.20100053 [Crossref] [ Google Scholar]
- Cho HJ, Yoon HY, Koo H, Ko SH, Shim JS, Lee JH. Self-assembled nanoparticles based on hyaluronic acid-ceramide (HA-CE) and Pluronic® for tumor-targeted delivery of docetaxel. Biomaterials 2011; 32(29):7181-90. doi: 10.1016/j.biomaterials.2011.06.028 [Crossref] [ Google Scholar]
- Akima K, Ito H, Iwata Y, Matsuo K, Watari N, Yanagi M. Evaluation of antitumor activities of hyaluronate binding antitumor drugs: synthesis, characterization and antitumor activity. J Drug Target 1996; 4(1):1-8. doi: 10.3109/10611869609046255 [Crossref] [ Google Scholar]
- Abdullah MA, Chaudhry GE, Abdah A, Abdullah MA. Cytotoxic effects of drug-loaded hyaluronanglutaraldehyde cross-linked nanoparticles and the release kinetics modeling. J Adv Chem Eng 2014; 4(1):104. doi: 10.4172/2090-4568.1000104 [Crossref] [ Google Scholar]
-
Chaudhry GE, Abdullah MA. Polymeric nanoparticle mediated targeted drug delivery to cancer cells. In: Thangadurai D, Sangeetha J, eds. Biotechnology and Bioinformatics: Advances and Applications for Bioenergy, Bioremediation, and Biopharmaceutical Research. Waretown, NJ: Apple Academic Press; 2015. p. 1-34.
- Zhao MD, Li JQ, Chen FY, Dong W, Wen LJ, Fei WD. Co-delivery of curcumin and paclitaxel by “core-shell” targeting amphiphilic copolymer to reverse resistance in the treatment of ovarian cancer. Int J Nanomedicine 2019; 14:9453-67. doi: 10.2147/ijn.s224579 [Crossref] [ Google Scholar]
- Hrabarova E, Juranek I, Soltes L. Pro-oxidative effect of peroxynitrite regarding biological systems: a special focus on high-molar-mass hyaluronan degradation. Gen Physiol Biophys 2011; 30(3):223-38. doi: 10.4149/gpb_2011_03_223 [Crossref] [ Google Scholar]
- Ghatak S, Misra S, Norris RA, Moreno-Rodriguez RA, Hoffman S, Levine RA. Periostin induces intracellular cross-talk between kinases and hyaluronan in atrioventricular valvulogenesis. J Biol Chem 2014; 289(12):8545-61. doi: 10.1074/jbc.M113.539882 [Crossref] [ Google Scholar]
- Hrabárová E, Valachová K, Juránek I, Soltés L. Free-radical degradation of high-molar-mass hyaluronan induced by ascorbate plus cupric ions: evaluation of antioxidative effect of cysteine-derived compounds. Chem Biodivers 2012; 9(2):309-17. doi: 10.1002/cbdv.201100046 [Crossref] [ Google Scholar]
- Soltés L, Mendichi R, Kogan G, Schiller J, Stankovska M, Arnhold J. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules 2006; 7(3):659-68. doi: 10.1021/bm050867v [Crossref] [ Google Scholar]
- Thapa R, George D. Wilson, “The Importance of CD44 as a Stem Cell Biomarker and Therapeutic Target in Cancer”. Stem Cells International 2016; 15:2016. doi: 10.1155/2016/2087204 [Crossref] [ Google Scholar]
- Song G, Liao X, Zhou L, Wu L, Feng Y, Han ZC. HI44a, an anti-CD44 monoclonal antibody, induces differentiation and apoptosis of human acute myeloid leukemia cells. Leuk Res 2004; 28(10):1089-96. doi: 10.1016/j.leukres.2004.02.005 [Crossref] [ Google Scholar]
- Mummert ME, Mummert DI, Ellinger L, Takashima A. Functional roles of hyaluronan in B16-F10 melanoma growth and experimental metastasis in mice. Mol Cancer Ther 2003; 2(3):295-300. [ Google Scholar]
- Klingbeil P, Marhaba R, Jung T, Kirmse R, Ludwig T, Zöller M. CD44 variant isoforms promote metastasis formation by a tumor cell-matrix cross-talk that supports adhesion and apoptosis resistance. Mol Cancer Res 2009; 7(2):168-79. doi: 10.1158/1541-7786.mcr-08-0207 [Crossref] [ Google Scholar]
- Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell 2014; 14(3):342-56. doi: 10.1016/j.stem.2014.01.009 [Crossref] [ Google Scholar]
- Paul CP, Good PD, Winer I, Engelke DR. Effective expression of small interfering RNA in human cells. Nat Biotechnol 2002; 20(5):505-8. doi: 10.1038/nbt0502-505 [Crossref] [ Google Scholar]
- Misra S, Hascall VC, Markwald RR, Ghatak S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front Immunol 2015; 6:201. doi: 10.3389/fimmu.2015.00201 [Crossref] [ Google Scholar]
- de Bree R, Roos JC, Quak JJ, den Hollander W, Wilhelm AJ, van Lingen A. Biodistribution of radiolabeled monoclonal antibody E48 IgG and F(ab’)2 in patients with head and neck cancer. Clin Cancer Res 1995; 1(3):277-86. [ Google Scholar]
- Börjesson PK, Postema EJ, Roos JC, Colnot DR, Marres HA, van Schie MH. Phase I therapy study with (186)Re-labeled humanized monoclonal antibody BIWA 4 (bivatuzumab) in patients with head and neck squamous cell carcinoma. Clin Cancer Res 2003; 9(10 Pt 2):3961S-72S. [ Google Scholar]
- Heider KH, Sproll M, Susani S, Patzelt E, Beaumier P, Ostermann E. Characterization of a high-affinity monoclonal antibody specific for CD44v6 as candidate for immunotherapy of squamous cell carcinomas. Cancer Immunol Immunother 1996; 43(4):245-53. doi: 10.1007/s002620050329 [Crossref] [ Google Scholar]
- Schrijvers AH, Quak JJ, Uyterlinde AM, van Walsum M, Meijer CJ, Snow GB. MAb U36, a novel monoclonal antibody successful in immunotargeting of squamous cell carcinoma of the head and neck. Cancer Res 1993; 53(18):4383-90. [ Google Scholar]
- Stauder R, Eisterer W, Thaler J, Günthert U. CD44 variant isoforms in non-Hodgkin’s lymphoma: a new independent prognostic factor. Blood 1995; 85(10):2885-99. [ Google Scholar]
- Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell 2007; 1(4):389-402. doi: 10.1016/j.stem.2007.08.001 [Crossref] [ Google Scholar]
- Guo W, Frenette PS. Alternative CD44 splicing in intestinal stem cells and tumorigenesis. Oncogene 2014; 33(5):537-8. doi: 10.1038/onc.2013.260 [Crossref] [ Google Scholar]
- Kainz C, Kohlberger P, Sliutz G, Tempfer C, Heinzl H, Reinthaller A. Splice variants of CD44 in human cervical cancer stage IB to IIB. Gynecol Oncol 1995; 57(3):383-7. doi: 10.1006/gyno.1995.1159 [Crossref] [ Google Scholar]
- Kainz C, Kohlberger P, Tempfer C, Sliutz G, Gitsch G, Reinthaller A. Prognostic value of CD44 splice variants in human stage III cervical cancer. Eur J Cancer 1995; 31A(10):1706-9. doi: 10.1016/0959-8049(95)00353-k [Crossref] [ Google Scholar]
- Hsieh HF, Yu JC, Ho LI, Chiu SC, Harn HJ. Molecular studies into the role of CD44 variants in metastasis in gastric cancer. Mol Pathol 1999; 52(1):25-8. doi: 10.1136/mp.52.1.25 [Crossref] [ Google Scholar]
- Shtivelman E, Bishop JM. Expression of CD44 is repressed in neuroblastoma cells. Mol Cell Biol 1991; 11(11):5446-53. doi: 10.1128/mcb.11.11.5446 [Crossref] [ Google Scholar]
- Lesley J, Hyman R, English N, Catterall JB, Turner GA. CD44 in inflammation and metastasis. Glycoconj J 1997; 14(5):611-22. doi: 10.1023/a:1018540610858 [Crossref] [ Google Scholar]
- De Marzo AM, Bradshaw C, Sauvageot J, Epstein JI, Miller GJ. CD44 and CD44v6 downregulation in clinical prostatic carcinoma: relation to Gleason grade and cytoarchitecture. Prostate 1998; 34(3):162-8. doi: 10.1002/(sici)1097-0045(19980215)34:3<162::aid-pros2>3.0.co;2-k [Crossref] [ Google Scholar]
- Liao HX, Lee DM, Levesque MC, Haynes BF. N-terminal and central regions of the human CD44 extracellular domain participate in cell surface hyaluronan binding. J Immunol 1995; 155(8):3938-45. [ Google Scholar]
- Hsieh HF, Yu JC, Ho LI, Chiu SC, Harn HJ. Molecular studies into the role of CD44 variants in metastasis in gastric cancer. Mol Pathol 1999; 52(1):25-8. doi: 10.1136/mp.52.1.25 [Crossref] [ Google Scholar]
- Chaudhry GE, Islamiah M, Ismail N, Mohamad H, Sung YY, Muhammad TST. Induction of apoptosis by Aaptos sp, fractions in human breast cancer cell line, MCF-7. Int J Res Pharm Sci 2018; 9(2):328-37. [ Google Scholar]
- Chaudhry GE, Jan R, Mohamad H, Muhammad TST. Vitex rotundifolia fractions induce apoptosis in human breast cancer cell line, MCF-7, via extrinsic and intrinsic pathways. Res Pharm Sci 2019; 14(3):273-85. doi: 10.4103/1735-5362.258496 [Crossref] [ Google Scholar]
- Chaudhry GE, Jan R, Naveed Zafar M, Mohammad H, Muhammad TST. Vitex rotundifolia fractions induced apoptosis in human breast cancer T-47D cell line via activation of extrinsic and intrinsic pathway. Asian Pac J Cancer Prev 2019; 20(12):3555-62. doi: 10.31557/apjcp.2019.20.12.3555 [Crossref] [ Google Scholar]
- Hudaya T, Chaudhry GE, Taib M, Ismail N, Mohammad TST. Methanol extracts of four selected marine sponges induce apoptosis in human breast cancer cell line, MCF-7. Int J Res Pharm Sci 2017; 8(4):667-75. [ Google Scholar]
- Chaudhry GS, Murni NIK, Zafar MN, Mohamad H, Andriani Y, Ismail N. Induction of apoptosis by Stichopus chloronotus and Holothuria nobilis fractions in human cervical cancer cell line, HeLa. Int J Res Pharm Sci 2020; 11(1):1238-47. doi: 10.26452/ijrps.v11i1.1964 [Crossref] [ Google Scholar]
- Chaudhry GE, Akim A, Zafar MN, Abdullah MA, Sung YY, Muhammad TST. Induction of apoptosis and role of paclitaxel-loaded hyaluronic acid-crosslinked nanoparticles in the regulation of AKT and RhoA. J Adv Pharm Technol Res 2020; 11(3):101-6. doi: 10.4103/japtr.JAPTR_26_20 [Crossref] [ Google Scholar]
- Chaudhry GE, Sohimi NKA, Mohamad H, Zafar MN, Ahmed A, Sung YY. Xylocarpus moluccensis induces cytotoxicity in human hepatocellular carcinoma HepG2 cell line via activation of the extrinsic pathway. Asian Pac J Cancer Prev 2021; 22(S1):17-24. doi: 10.31557/apjcp.2021.22.s1.17 [Crossref] [ Google Scholar]
- Chaudhry GS, Zafar MN, Sung YY, Muhammad TST. Phytochemistry and biological activity of Vitex rotundifolia L. Res J Pharm Technol 2020; 13(11):5534-8. doi: 10.5958/0974-360x.2020.00966.x [Crossref] [ Google Scholar]
- Yunus U, Zulfiqar MA, Ajmal M, Bhatti MH, Chaudhry GE, Muhammad TST. Targeted drug delivery systems: synthesis and in vitro bioactivity and apoptosis studies of gemcitabine-carbon dot conjugates. Biomed Mater 2020; 15(6):065004. doi: 10.1088/1748-605X/ab95e1 [Crossref] [ Google Scholar]